Abstract
Heart diseases and related complications constitute a leading cause of death and socioeconomic threat worldwide. Despite intense efforts and research on the pathogenetic mechanisms of these diseases, the underlying cellular and molecular mechanisms are yet to be completely understood. Several lines of evidence indicate a critical role of inflammatory and oxidative stress responses in the development and progression of heart diseases. Nevertheless, the molecular machinery that drives cardiac inflammation and oxidative stress is not completely known. Recent data suggest an important role of cardiac bitter taste receptors (TAS2Rs) in the pathogenetic mechanism of heart diseases. Independent groups of researchers have demonstrated a central role of TAS2Rs in mediating inflammatory, oxidative stress responses, autophagy, impulse generation/propagation and contractile activities in the heart, suggesting that dysfunctional TAS2R signalling may predispose to cardiac inflammatory and oxidative stress disorders, characterised by contractile dysfunction and arrhythmia. Moreover, cardiac TAS2Rs act as gateway surveillance units that monitor and detect toxigenic or pathogenic molecules, including microbial components, and initiate responses that ultimately culminate in protection of the host against the aggression. Unfortunately, however, the molecular mechanisms that link TAS2R sensing of the cardiac milieu to inflammatory and oxidative stress responses are not clearly known. Therefore, we sought to review the possible role of TAS2R signalling in the pathophysiology of cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases. Potential therapeutic significance of targeting TAS2R or its downstream signalling molecules in cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction is also discussed.
Keywords: Cardiac bitter taste receptors, Cardiac inflammation, Contractile dysfunction, Arrhythmia, Novel therapeutics
Introduction
Heart diseases are a global epidemic (Gianluigi Savarese 2017; Khan et al. 2020) that pose an immense public health concern with a prevalence of over 500 million, affecting all age groups (Ahern et al. 2011), including children (Musa et al. 2017). Heart diseases are the leading cause of death worldwide (Ahern et al. 2011; Roth et al. 2017). In 2015 alone, mortality due to heart diseases was estimated at about 18 million, representing 32% of all deaths in the world (Roth et al. 2017). Alas, billions of dollars are spent in the management of heart diseases, causing a huge burden to the sufferers, relatives, caregivers and the health care system (Muka et al. 2015; Gheorghe et al. 2018; Roth et al. 2020). Though scientific advances, preventive measures and campaigns against heart diseases (Khan et al. 2020) as well as health care system reforms in the cardiovascular setting (Obama 2016; Weintraub and Boden 2017; León-Cortés et al. 2019) may have brought about higher quality healthcare (Clarke et al. 2017), regrettably, the incidence and mortality of heart diseases have constantly increased over the past years (Gianluigi Savarese 2017; Khan et al. 2020; Emmons-Bell and Johnson 2022). Even so, the prevalence and mortality of heart diseases are projected to rise substantially in the next decades, if adequate measures are not taken to address the growing menace of these diseases on the society (Kelly and Donovan 1995).
Over the past few years, accumulating data have consistently implicated cardiac inflammation in etiopathogenetic mechanism of a range of heart diseases, including myocarditis (Lu et al. 2015; Tschöpe et al. 2021), pericarditis, Kawasaki syndrome (Jui et al. 2021), cardiac vessel endotheliitis (Maccio et al. 2021), infective endocarditis (Jui et al. 2021), myocardial infarction/ischaemic heart disease (Moreira et al. 2015), cardiomyopathies (Meraviglia et al. 2021; Tschöpe et al. 2021), angina pectoris (Lu et al. 2015), coronary artery disease/atherosclerotic heart disease (Moreira et al. 2015), cardiac arrhythmias (Shenasa and Shenasa 2017), congestive heart failure (Murphy et al. 2020), hypertensive heart disease (Shenasa and Shenasa 2017), rheumatic and non-rheumatic heart valve disease (Lars et al. 2007; Lee and Choi 2018; Passos et al. 2021), cardiac amyloidosis (Siegismund et al. 2018; McVeigh and Tennyson 2020), and human heart senescence (de Arellano et al. 2019). In addition, inflammatory response plays a pivotal role in the pathogenic mechanism that drives cardiac involvement in systemic diseases (Knockaert 2007; Veinot 2010). Cardiac inflammation is a cellular and tissue response to adverse stimuli due to the activation of signalling cascades that control the secretion of inflammatory mediators in resident immunocytes and cardiac cells as well as recruitment of inflammatory cells, triggered by microbial agents, toxins, cellular debris or toxigenic metabolites. Though inflammatory response constitutes a key defence mechanism against pathogenic aggression or adverse environmental stimuli (Furman et al. 2019), inadequate or chronic inflammatory response can potentially lead to harmful consequences resulting in the development of diseases (Williams et al. 2019; Tsampasian et al. 2021). Despite intense efforts and research investigation directed towards the identification of the pathogenetic basis of cardiac inflammation, the underlying cellular and molecular mechanisms responsible for triggering the inflammatory responses in the cells and tissues of the heart are yet to be fully understood.
Interestingly, inflammatory responses usually occur concurrently with oxidative stress in several pathophysiological conditions, including heart diseases (Neri et al. 2015; Dludla et al. 2019). Oxidative stress is an adverse cellular and tissue response due to dysbalance between the production of reactive species and the endogenous antioxidant defence system (Pizzino et al. 2017) resulting in cell and tissue damage (van der Pol et al. 2019). Indeed, independent research groups have demonstrated an association between proinflammatory responses and the generation of reactive species that promotes oxidative stress (Ji and Li 2016; Dludla et al. 2019). Correspondingly, progress in cardiac research has identified oxidative stress cascades as fundamental pathophysiological pathways in the development and progression of heart diseases (van der Pol et al. 2019). In the heart, oxidative stress induces cardiomyocyte hypertrophy, apoptosis and Ca2+ overload via oxidation of membrane phospholipids, proteins and DNA molecules (Shibata et al. 2021). Regrettably, however, the mechanisms of development of oxidative stress in heart diseases are not completely known, thereby substantiating the need to step up research investigation against this global epidemic and search for new frontiers that may lead to the development of novel therapeutics for some heart diseases.
The relatively recent finding that bitter taste receptor (TAS2R)-expressing cells play a critical role as innate immune sentinels (Lee and Cohen 2013a) has sparked research interest on the functional role and the molecular mechanisms of these nodal signalling units of the plasma membrane (Welcome 2020a; Welcome and Mastorakis 2021; Welcome et al. 2021). TAS2Rs are transmembrane proteins of the G-protein-coupled receptors that sense “bitter” molecules to initiate intracellular signalling downstream multiple cytoplasmic acceptors, mediating cellular responses that ultimately lead to elimination of the aggression or protection of the cell or tissue from damage (Welcome 2020a). So far, 25 TAS2Rs have been discovered in humans (Meyerhof et al. 2010), 35 TAS2Rs in rat and mice (Wu et al. 2005), 3 TAS2Rs in chicken and 50 TAS2Rs in frog (Di Pizio and Niv 2015). The amino acid sequence of TAS2Rs from different species share a similarity of about 23–86% (Chandrashekar et al. 2000; Shi et al. 2003; Wu et al. 2005). TAS2Rs are activated by hundreds of different substances, including denatonium, amarogentin, caffeine, chloroquine, quinine (Bayer et al. 2021), N-phenylthiourea, phenylthiocarbamide, cycloheximide (Meyerhof et al. 2010; Gradinaru et al. 2022), xanthohumol, dextromethorphan, methimazole, and glimepiride (D’Urso and Drago 2021). These taste receptors are ubiquitously expressed in many cells and tissues of the body. TAS2Rs were first identified in taste bud cells of the oral cavity and were thought to only detect and prevent the ingestion of potentially poisonous “bitter” molecules (Adler et al. 2000; Chandrashekar et al. 2000). Thereafter, TAS2Rs were discovered in other regions of the gut—stomach, ileum and colon (Rozengurt 2006; Vegezzi et al. 2014), which suggested that these nodal signalling units may play a number of essential roles other than detecting poisonous “bitter” molecules (Rozengurt 2006). TAS2Rs were also discovered in the upper respiratory tract, where they were shown to sense toxigenic and microbial molecules to mediate responses that ultimately lead to elimination of the pathogenic molecules (Gulbransen et al. 2008; Tizzano et al. 2011), in part, by activating the innate immune system to confer protection on the respiratory epithelium (Kinnamon and Finger 2019). TAS2Rs are expressed in blood monocytes or monocyte-derived macrophages (Grassin-Delyle et al. 2019), neutrophils (Maurer et al. 2015), keratinocytes (Wölfle et al. 2015), thymus (Panneck et al. 2014; Soultanova et al. 2015), vascular smooth muscles (Lund et al. 2013), pancreas (Gaida et al. 2016), brain (Singh et al. 2011), thyroid gland (Clark et al. 2015), testis (Xu et al. 2013), spermatozoa (Governini et al. 2020), prostate (Dupre and Martin 2017; Martin et al. 2019), vagina, cervix, endometrium, myometrium, placenta, ovary (Dupre and Martin 2017; Welcome 2020a), urethra, ureter, bladder (Welcome 2020a; Welcome et al. 2021), and kidney (Liu et al. 2015b). Relatively more recently, independent groups of researchers have identified the expression of TAS2Rs in cells and tissues of the heart (Foster et al. 2013, 2014; Manson et al. 2014), indicating that these receptors may be involved in mediating the inflammatory, oxidative stress responses, autophagy (Hamdard et al. 2019b), cardiac rhythm (Yuan et al. 2020) and contractile activities (Manson et al. 2014; Bloxham et al. 2020; Yuan et al. 2020) in this vital organ.
Indeed, several lines of evidence suggest that cardiac TAS2Rs may play a role in both cardiac and vascular diseases (Foster et al. 2015; D’Urso and Drago 2021; Kamila and Agnieszka 2021). The role of cardiac TAS2Rs in mediating reduction in spontaneous beating rate of the sinoatrial node and left ventricular relaxation (Yuan et al. 2020) indicates that dysfunction in cardiac TAS2Rs may necessarily affect impulse generation or propagation and mechanical efficiency of the heart, thereby predisposing to contractile dysfunction and arrhythmia. Comparable data have also been reported by Manson et al. (2014). Moreover, cardiac TAS2Rs can act as gateway surveillance units that detect pathogenic components, including the microbial quorum-sensing signal molecules. Thus, under normal condition, stimulation of TAS2R by microbial molecules results in downstream signalling that activates the intracellular acceptors responsible for protecting the cell via secretion of anti-inflammatory and -microbial mediators. However, dysfunctional cardiac TAS2R signalling or excessive action of the pathogenic or danger molecules can result in collateral tissue damage, predisposing to disease development (Foster et al. 2015; D’Urso and Drago 2021; Kamila and Agnieszka 2021).
Therefore, defects in cardiac TAS2R signalling can initiate inflammatory and oxidative stress responses, as well as disorder of cardiac rhythm and contractile force of the heart in cardiac diseases. Unfortunately, the molecular mechanisms that link TAS2R sensing of the cardiac milieu to inflammatory and oxidative stress responses are not clearly known. Hence, we sought to review the possible role of TAS2R signalling in the pathophysiology of cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases. First, we discuss the contemporary view of the mechanisms of inflammation and oxidative stress and their roles in the development of heart diseases. Second, we discuss the expression of TAS2R, its ligands, functional roles and signal transduction mechanism in the heart. Third, we describe the role of dysfunctional TAS2R signalling in cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases. Further, we attempt to delineate the molecular pathways, linking TAS2R sensing of microbial and toxigenic molecules with inflammatory, oxidative stress responses, arrhythmia and contractile dysfunction in heart diseases. Potential therapeutic significance of targeting TAS2R or its downstream signalling molecules in cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction is also discussed.
Contemporary view of the mechanisms of cardiac inflammation and oxidative stress and their roles in disruption of cardiac rhythm and contractility in heart diseases
Cardiac inflammatory and oxidative stress responses are triggered by multiple pathogenetic factors
The inflammatory and oxidative stress responses in the heart are triggered by multiple factors, which include pathogens (bacteria, viruses, fungi, parasites) (Zhang et al. 2020), gut microbiota disorder (Mesquita et al. 2021), tissue injury (Chen et al. 2018), acute emotional and chronic psychological stress (Wirtz 2017), toxins and irritants (Lu et al. 2015; Chen et al. 2018). These factors mediate the release of pathogenic and danger molecules that stimulate the pattern-recognition receptors on local and distant immunocytes (macrophages, leukocytes, natural killer and cytotoxic CD8+ T cells (Jui et al. 2021)) as well as cardiac cells to initiate the inflammatory and oxidative stress response (Mesquita et al. 2021). Therefore, gut microbiota disorder, for example, causes gut epithelial barrier leakage with corresponding increase in circulating microbial particles that promote chronic low-grade inflammatory response, which is considered as a central player in cardiac failure, diastolic dysfunction, arrhythmia, ageing, and fibrotic heart disease (Mesquita et al. 2021). Interestingly, multiple pathogenetic factors have been reportedly shown to trigger cardiac pathology via gut microbiota disorder (Mesquita et al. 2021).
Indeed, cardiac diseases are associated with numerous inflammatory mediators, including interleukin (IL)-1β, IL-6, tumour necrosis factor-α (TNF-α) (Lu et al. 2015; Chen et al. 2018), macrophage chemoattractant protein-1 (MCP-1), and C-reactive protein (Jui et al. 2021). Similarly, reactive species play a crucial role in the development and progression of many heart diseases (Pashkow 2011). In addition, secretion of alarmins such as matrix metalloprotease (MMP)-2, -9 (Jui et al. 2021), high-mobility group box 1 (HMGB-1), cardiac myosin, heat shock protein (HSP)-60, HSP-70, hyaluronic acid, fibronectin-extra domain A, extracellular adenosine triphosphate (ATP), circulating RNA, nuclear and mitochondrial DNA (Silvis et al. 2020), Ca2+-binding S100 proteins (S100A7, S100A8, S100A9 and S100A12) (Yan 2014; Lu et al. 2019; Silvis et al. 2020) also contribute to the signalling cascades that promote the development of heart diseases. Accordingly, Zhang et al. (2020) showed that these factors promote aberrant cardiac metabolism and mitochondrial dysfunction that further worsen the abnormalities of cardiac rhythm and contractility in heart diseases (Zhang et al. 2020). Thus, the inflammatory mediators, reactive species, and alarmins represent fundamental drivers of the pathogenetic processes in cardiac pathology (Wadley et al. 2013; Zhang et al. 2017a; Papaconstantinou 2019).
Though the mechanisms are not exactly clear, cardiac inflammatory signalling is closely associated with oxidative stress response in the heart (Ooi et al. 2017; Zhang et al. 2017a; Wu et al. 2021b). Recent investigation has implicated reactive isolevuglandin, a toxic lipid peroxidation byproduct and γ-ketoaldehyde, as a possible molecular switch, connecting cardiac inflammation to oxidative stress. Ngwenyama et al. (2021) showed that myocardial oxidative stress triggers the generation of reactive isolevuglandin molecules that act as cardiac antigens to stimulate the T cell receptor (Ngwenyama et al. 2021) to promote the development of heart failure (May-Zhang et al. 2018), cardiac senescence, atherosclerotic and hypertensive heart disorders (Aschner et al. 2021), including high salt-induced heart disease (Ruggeri Barbaro et al. 2021). Data from both animal and human research (Ruggeri Barbaro et al. 2021) have revealed that reactive isolevuglandin formation due to high salt diet (Ruggeri Barbaro et al. 2021), myocardial oxidative stress (Ngwenyama et al. 2021), pressure overload (Shang et al. 2019), and lipopolysaccharide (LPS)-induced inflammation in mice (Mayorov et al. 2019) can initiate the activation of monocytes, dendritic cell, and secretion of the proinflammatory cytokines TNF-α, IL-1β, IL-6, IL-17A (Dikalova et al. 2020; Ruggeri Barbaro et al. 2021), and reactive species (superoxide anions, and reactive nitrogen species) (Dikalova et al. 2020). Thus, isolevuglandin may serve as a therapeutic target in the treatment of certain heart diseases. Indeed, the isolevuglandin scavengers, 2-hydroxybenzylamine (Shang et al. 2019; Ngwenyama et al. 2021) and (4-(4-aminomethyl)-3-hydroxyphenoxy)butyl)-triphenylphosphonium (Mayorov et al. 2019; Dikalova et al. 2020) have been shown to attenuate the pathological sequelae of left ventricular hypertrophy and heart failure in both animal and human cell lines.
Pathological activation and phenotype switching of resident cardiac immunocytes are critical to inflammatory and oxidative stress responses in the heart
Macrophages are primary resident immunocytes involved in both inflammatory and oxidative stress responses in several disorders, including heart diseases (Hu et al. 2020). These resident immunocytes play a central role in initiating, maintaining, and resolving the inflammatory and oxidative stress responses through the secretion of cytokines, chemokines and growth factors (Liu et al. 2021). The pathogenic factors that initiate inflammatory and oxidative stress responses in the heart concomitantly cause pathological activation of the cardiac immunocytes, initiating the phenotype switching of non-activated macrophage (M0) to the proinflammatory subtype M1 that propagates the inflammatory processes (Orekhov et al. 2019). Research data have shown that generation of M1 macrophage is triggered by LPS or interferon-gamma (IFN-γ), whereas IL-4 and IL-13 mediate the polarisation of M2 macrophage (Liu et al. 2021). Investigators have reported that the proinflammatory cytokines/chemokines released in the heart following the action of pathological factors activate the cell surface receptors of the resting resident macrophages with increased generation of the M1 proinflammatory over the M2 anti-inflammatory phenotype (Hu et al. 2019). The activated M1 macrophage secretes proinflammatory factors such as IL-1β, IL-6, IL-12, IL-23, TNF-α, nitric oxide (NO), inducible NO synthase (iNOS), MCP-1, IFNγ, prostaglandins (PGE2), and MMPs to promote inflammation (Aimo et al. 2020; Liu et al. 2021). However, the M2 phenotype expresses the cluster of differentiation (CD) 14, CD80, CD163, CD200, and CD206 receptors and secretes anti-inflammatory factors, including IL-10, C–C motif chemokine ligand 17 (CCL17), CCL22, and CCL24 to blunt the inflammatory reactions, thereby enhancing tissue repair (Aimo et al. 2020; Kishore and Petrek 2021). Moreover, the macrophage precursors—monocytes can directly polarise into M1- or M2-like phenotypes or their respective isoforms to control inflammatory response (Orekhov et al. 2019; Lu et al. 2020).
Therefore, data from both animal (Hu et al. 2019) and human (Dai et al. 2021) studies have revealed that pathological processes resulting in heart diseases are associated with M1 macrophage polarisation, concomitantly with inhibition of M2 macrophage recruitment in the heart. For instance, Liu et al. (2015a, b) demonstrated M1 polarisation in a rat model of myocardial infarction along with disordered Ca2+ waves, which stimulated the extracellular Ca2+ receptor, CaSR, which in turn activated the NLRP3 (nucleotide-binding oligomerisation domain, leucine-rich repeats, pyrin domain-containing protein 3) inflammasome via phospholipase C (PLC)—inositol 1,4,5-triphosphate (IP3) pathway (Liu et al. 2015a). The authors also reported the secretion of collagen, α-SMA (alpha smooth muscle actin) and MMP-2/-9 (Liu et al. 2015a), which are implicated in cardiac fibrosis—a crucial process in the pathogenetic mechanism of myocardial infarction (Talman and Ruskoaho 2016; CHEN et al. 2021). In contrast, TIMP-2 (tissue inhibitor of matrix metalloproteinase) expression was downregulated in cardiac fibroblasts via IL-1β/IL-1 receptor (Liu et al. 2015a). TIMP-2 is highly expressed in the myocardium, and required for pro-MMP-2 activation and MMP-2 inhibition. TIMP-2 plays multiple roles in cardiac physiology, including electrical coupling among myocardial cells. For instance, Givvimani et al. (2013) showed decreased expression of myocardial connexin (Cx) 37 and 43 in TIMP-2 knockout mice compared with control animals (Givvimani et al. 2013). ((Cx43 is required for the maintenance of electrical and mechanical synchrony in the heart (vide infra)). In addition, MMP-2 and TIMP-2 dysbalance plays an important role in the development of cardiomyopathy (Li et al. 2010) and heart failure (Kobusiak-Prokopowicz et al. 2018) in both animals and humans. Thus, TIMP-2/MMP-2 axis may serve as an important molecular target in the treatment of some cardiac diseases.
Furthermore, the M1 phenotype within the myocardium can mediate inflammatory processes by internalising and accumulating oxidised low-density lipoprotein, leading to the formation of “foam cells” in atherosclerotic plaques of the coronary vessels, which in turn may cause myocardial ischaemia and necrosis (Bonetti et al. 2021). However, phenotype conversion between M1 and M2 has been documented and depends on the activity of the dominant signalling molecules. For instance, M1 conversion to M2 occurs by selective apoptosis of M1 macrophages and maybe induced by iNOS/NO (Albina et al. 1989; Mills 2012; Lu et al. 2020), TNF/TNF receptor 1, and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathways (Lu et al. 2020). Interestingly, some pharmacological agents can attenuate cardiac inflammation and oxidative stress by promoting the polarisation of M2 over M1 phenotype or conversion of M1 to M2 phenotype. Correspondingly, Zhou et al. (2020) demonstrated specific inhibition of M1 polarisation by attenuating CD11c, iNOS, IL-6 and MCP-1 and augmenting CD206 and IL-10 expression in M2 via suppression of the nuclear factor κB (NF-κB) and Notch1 signalling upon treatment with recombinant human IL-37 in LPS- and IFN-γ-stimulated THP-1 monocytes (Zhou et al. 2020). Comparable results have been reported for other pharmacological agents (Zhou et al. 2015; Saqib et al. 2018; Davoodvandi et al. 2019), including drugs currently used in the clinic (He and Marneros 2014; He et al. 2021). For example, both preclinical (Garcia et al. 2007; Spaulding et al. 2018) and clinical (Cerisano et al. 2014) studies have shown that acute administration of the tetracycline-class antibiotic, doxycycline in myocardial infarction considerably ameliorates cardiac dysfunction by inhibiting the proinflammatory macrophage and mediators. In an open-label, randomised, phase II trial (ClinicalTrials.gov: NCT00469261), doxycycline (100 mg b.i.d. for 7 days) in addition to standard therapy substantially enhanced left ventricular function and reduced the infarct size in patients with acute ST-segment elevation myocardial infarction and low (< 40%) left ventricular ejection fraction (Cerisano et al. 2014). Several investigators have consistently shown that the beneficial effects of doxycycline on the heart are mainly due to the suppression of intracellular matrix metalloproteinase (MMP)-2 (Fan et al. 2014; Berry et al. 2015), MMP-9 (Fan et al. 2014), secretory phospholipase A2 activity (Berry et al. 2015), and M2-type macrophage polarisation (He and Marneros 2014b), while also upregulating and normalising the distribution of Cx43 in the infarct zone (Fan et al. 2014). (The role of Cx43 in cardiac physiology is discussed below). These data corroborate the essential role of this broad-spectrum antibiotic in abrogating the proinflammatory stress responses of the heart (Kandasamy et al. 2010).
Inflammatory and oxidative stress responses in the heart are responsible for disruption of cardiac rhythm and contractility via connexon and calcium signalling defects
Emerging data indicate that the detrimental effects of inflammation and oxidative stress in the heart are mediated via dysfunctional connexon, a major type of gap junction protein expressed in the heart. Research investigation has consistently shown that C×40, C×43, and C×45 are expressed in relative and distinct combination in different regions of the heart (Severs 2002; Severs et al. 2008). However, C×43 is the best characterised cardiac gap junction connexon protein with the most important role in cardiac physiology. In the myocardium of a healthy heart, the expression of C×43 is very high (Eckardt et al. 2004; Takanari et al. 2016), whereas in the cardiac conduction system, its expression is low (Eckardt et al. 2004). The myocardial Cx43 not only forms the gap junction that constitutes a critical component of the intercalated disc (Palatinus et al. 2012; Takanari et al. 2016), but also forms hemichannels, which represent crucial players in cardiac ionic homeostasis (Hirschhäuser et al. 2021). Therefore, myocardial Cx43 are required for direct cell-to-cell contact, movement of ions and signalling molecules to maintain cardiac electrical coupling (Takanari et al. 2016) and synchrony (Palatinus et al. 2012) that ensure a regulated contractile activity of the heart (Severs 2002).
Concomitantly with decreased C×43 protein expression in high glucose-stimulated AC16 human cardiomyocytes, Thakur et al. (2021) demonstrated upregulation of troponin-I, HSP-60, RAGE (receptor for advanced glycation endproducts), HMGB-1, toll-like receptor (TLR)-4, and CXC chemokine receptor (CXCR)-4 (Thakur et al. 2021). The reduction of Cx43 expression in left ventricular hypertrophy induced by type-2 diabetes mellitus in rats was associated with reduced expression of heme-oxygenase 1 (HO-1) and increased level of TNFα and asymmetric dimethylarginine (ADMA) (Leffler and Abdel-Rahman 2019, 2020). ADMA is a cardiac biomarker and risk factor for heart diseases that acts as an endogenous competitive inhibitor of NOS (Böger 2005; Hou et al. 2018), formed during proteolysis by protein methyl transferases via methylation of L-arginine sites of protein molecules (Bulau et al. 2007). ADMA has been demonstrated to inhibit the expression and heterogeneous localisation of C×43 (Jia et al. 2009; Tsang et al. 2013). Under normal condition, 10% of ADMA is formed daily and subsequently excreted in the urine. The remaining 90% is metabolised by dimethylarginine dimethylaminohydrolase (DDAH) to dimethylamine and L-citrulline (Tsikas 2020). Deficiency of DDAH due to cardiomyocyte injury or apoptosis is the primary cause of elevated circulating ADMA (Tsikas 2020). Interestingly, DDAH has been shown to attenuate left ventricular dysfunction after myocardial infarction by inhibiting oxidative stress and apoptotic biomarkers (Hou et al. 2018).
The secretion of inflammatory, oxidative stress and pro-fibrotic mediators due to C×43 dysfunction can trigger dysregulation of spontaneous generation of excitation by the cardiac pacemakers and impulse conduction via disruption of ionic homeostasis and redox potential, thereby predisposing to disorders of cardiac rhythm and contraction (Palatinus et al. 2012; Li et al. 2013; Andelova et al. 2021). Consistent with previous data (Takanari et al. 2016), several pieces of evidence have shown that C×43 breakdown, modification or remodelling in heart diseases essentially impairs the gap junction function through disruption of its charge, size, organisation and distribution—representing a distinct feature of cardiac arrhythmia in many heart diseases (Severs et al. 2008; Palatinus et al. 2012). Truly, several dysfunctions of the heart, including cardiac electrical instability have been associated with C×43 dysfunction in animal models of cardiac specific C×43 knockout (Gutstein et al. 2001; Eckardt et al. 2004). Similarly, a marked reduction in the expression of C×43 mRNA and protein levels in the left ventricle of ischaemic cardiomyopathy and idiopathic dilated cardiomyopathy was reported (Dupont et al. 2001). Though the mechanisms underlying connexon-induced elevation of proinflammatory, oxidative stress and pro-fibrotic molecules that subsequently results in cardiac pathology are not fully understood, researchers have previously demonstrated the association of endothelial C×43 knockout with hypotension, bradycardia, and elevation of angiotensin I and II in experimental animals (Liao et al. 2001). Indeed, the involvement of regional (organ-specific) angiotensin system in inflammatory and oxidative responses is extensively been discussed in the literature (Leung 2007; Rodriguez-Pallares et al. 2008; Labandeira-Garcia et al. 2017; Tsai et al. 2018; Milanesi et al. 2019). Regrettably, however, little is known about the role of intrinsic angiotensin system of the heart in cardiac inflammation, oxidative stress and fibrosis.
The cardiac C×43 also interacts with several ions, including Na+ (Delmar and Liang 2012) and Ca2+ (Takanari et al. 2016) to control cardiac rhythm via cross-talks in the ventricular cardiomyocytes. Thus, disorders in C×43 function can directly affect Na+ and Ca2+ transport across the sarcolemma (Delmar and Liang 2012; Takanari et al. 2016). Accordingly, dysfunctional Ca2+ signalling has been shown to mediate the detrimental effects of inflammatory and oxidative stress responses in the heart. Research data indicate that increased activity of Ca2+ signalling via calmodulin/Ca2+-calmodulin protein kinase II (CaM/CaMKII) regulates C×43 expression and impulse conduction in a diseased heart (Takanari et al. 2016). Dries et al. (2021) demonstrated CaMKII involvement in susceptibility to arrhythmia, via spontaneous Ca2+ release, in cryoinjured subendocardium of experimental rats (Dries et al. 2021). Therefore, investigators have reliably established that inhibition of CaMKII results in decreased tissue excitability (Procida et al. 2009) and susceptibility to arrhythmia (Dries et al. 2021). Similar findings were revealed by Takanari et al. (2016) who demonstrated augmentation of conduction velocity and C×43 expression in the intercalated disc of CaMKII knockout AC3-I mice subjected to pressure overload (Takanari et al. 2016). Researchers have demonstrated a heightened predisposition to inflammatory responses in the heart following experimental CaMKII activation. Thus, Suetomi et al. (2018) reported that CaMKIIδ actively participates in detection and transduction of pressure overload to trigger the activation of NF-κB and NLRP3 inflammasome (Suetomi et al. 2018). These molecular sensors of inflammation mediate an increased secretion of pro-fibrotic molecules, proinflammatory cytokines and chemokines in cardiomyocytes, along with macrophage activation, which predispose to arrhythmia and contractile dysfunction in heart diseases, including myocardial infarction (Suetomi et al. 2019). Similar findings are reported elsewhere (Ling et al. 2013; Willeford et al. 2018). As a matter of fact, inhibition of CaMKII reportedly attenuated the expression of proinflammatory and pro-fibrotic molecules (Suetomi et al. 2019). Likewise, Jiang et al. (2020) demonstrated attenuation of myocardial injury via inhibition of CaMKII by tilianin, a TAS2R agonist (Jiang et al. 2020). Though the role of CaMKII in cardiac pathology is not exactly clear, Gray and co-workers (2017) have previously demonstrated a differential regulation of CaMKII isoforms—CaMKIIδ, CaMKIIδB and CaMKIIδC in the development of myocardial ischaemia or infarction via NF-κB signalling (Gray et al. 2017). Further research on the role of specific isoforms of CaMKII in cardiac inflammation, oxidative stress, fibrosis and apoptotic cell death is important for understanding the underlying molecular mechanisms in some heart diseases.
Phosphorylation reactions at specific sites of the C×43 are also critical to the changes in its expression and functions in heart diseases (Procida et al. 2009). These reactions may be triggered by CaMKII (Procida et al. 2009), mitogen-activated protein kinase (MAPK) (Yang et al. 2019; Hirschhäuser et al. 2021), and protein kinase C (PKC) (Lampe et al. 2000; Hirschhäuser et al. 2021). Although certain phosphorylation of cardiac connexon may be protective, abnormal connexon phosphorylation can lead to serious abnormalities in electrical coupling and synchrony (Hirschhäuser et al. 2021). Thus, research on identification of novel pharmacological agents that can mediate favourable phosphorylation reactions of connexon in a region-dependent manner may provide important information for identification of new therapeutic options for some heart diseases. Correspondingly, a novel class of pharmacological agents—known as C×43 mimetic peptides, which stimulate favourable connexon phosphorylation are currently been developed for potential treatment of heart diseases. For instance, Jiang et al. (2019) reported a marked attenuation of left ventricular dysfunction and arrhythmia, accompanied by increased phosphorylation of C×43 at serine 368 by PKCε, following treatment with 25–amino acid α-carboxyl terminus 1 peptide (C×43 mimetic peptide αCT1) or a variant of αCT1 (known as αCT11) in a mouse model of myocardial ischaemia–reperfusion injury (Jiang et al. 2019).
Bitter taste receptors of the heart and their signal transduction mechanism
Cardiac bitter taste receptors: subtypes, ligands and functions
TAS2Rs were discovered in cardiomyocytes and fibroblasts of the rat and human heart by Foster and co-workers (Foster et al. 2013). The following year, same group of researchers established the expression of TAS2R subtypes 108, 137, and 143 in the mouse heart (Foster et al. 2014). Several subtypes of these receptors have been identified in the cells and tissues of both murine and human heart (Table 1).
Table 1.
Summary of bitter taste receptor expression in the heart
| Cells and tissues of the heart | Model | Types of taste receptors | Comments | Reference |
|---|---|---|---|---|
| Whole heart tissue | Mice | TAS2R108, TAS2R137, TAS2R143 | Concentration-dependent decrease in contraction | (Foster et al. 2014) |
| Left ventricle, sinoatrial node and cardiomyocytes | Rat | TAS2R108 | Concentration-dependent decrease in contraction | (Yuan et al. 2020) |
| Aorta and pulmonary arteries | Guinea-pig, mice, human | TAS2R3, TAS2R4, TAS2R10, TAS2R14 | Strong endothelium-independent relaxation | (Manson et al. 2014) |
| Aortic smooth muscle tissue | Rat | TAS2R and gustducin | Increased muscle tone | (Manson et al. 2014) |
| Whole heart tissue | Chinese Fast Yellow Chicken | TAS2R | – | (Hamdard et al. 2019a) |
| Whole heart tissue | Chinese fast yellow chicken | TAS2R1, 2, 7, α-gustducin | ↑PLCβ2, IP3R3, TRPM5, CALPN1, dyanin, GPX1, CAT, SOD1; ↓CASP, Bcl-2 (Bcl-2l1, Mcl, BID, NOXA), beclin-1* | (Hamdard et al. 2019b) |
| Cardiac myocytes and fibroblasts | Rat and human | TAS2R | – | (Foster et al. 2013) |
| Whole heart tissue | Human and mice | TAS2R | – | (Foster et al. 2015) |
| Whole heart tissue | Rodent | TAS2R14 | – | (D’Urso and Drago 2021) |
Note: *in acute administration at low-moderate doses (5 and 20 mg/kg), but not in chronic and large dose, Bcl-2 B cell lymphoma 2, Bcl-2l1 Bcl-2 like 1, BID—BH3-interacting domain death agonist, CALPN1 calpain 1, CASP caspase, CAT catalase, GPx1 glutathione peroxidase, Mcl Mantle cell lymphoma, NOXA NADPH (nicotinamide adenine dinucleotide phosphate) oxidase activator, SOD superoxide dismutase
The cardiac TAS2R mediates the sensation of bitter tasting (i.e. bitter tastants or bitterants), toxigenic or pathogenic molecules. There are thousands of TAS2R ligands. All currently known bitterants are available on the “database of bitter molecules”: BitterDB (http://bitterdb.agri.huji.ac.il) and PlantMolecularTasteDB (www.plantmoleculartastedb.org).
Similar to other extra-oral tissues, cardiac TAS2Rs may play multiple physiological roles, including immune defence against pathogens (D’Urso and Drago 2021) and local metabolic regulation, at least in part, by regulating cytoplasmic protein kinases and cyclic AMP levels (Manson et al. 2014; D’Urso and Drago 2021). TAS2Rs are also involved in the maintenance of endothelial homeostasis (D’Urso and Drago 2021).
Data also indicate a role of TAS2Rs in cardiac contractility (Foster et al. 2014; Bloxham et al. 2020) and vascular tone (Manson et al. 2014; Bloxham et al. 2020). Yuan et al. (2020) investigated the effect of the TAS2R agonists, quinine and chloroquine on Langendorff-perfused hearts in adult rat and demonstrated increased expression of TAS2R mRNA and α-gustducin in the left ventricle (Yuan et al. 2020). Furthermore, the researchers showed that stimulation of TAS2R with either quinine or chloroquine resulted in increased R-R interval and QRS duration (Yuan et al. 2020). Foster et al. (2014) demonstrated the involvement of mouse cardiac TAS2R 108, 137, and 143 in decreased force of ventricular contraction (Foster et al. 2014). Interestingly, previous investigation revealed this relaxation effect of TAS2R agonists in the two major arteries connecting the heart—aorta and pulmonary arteries (Manson et al. 2014). These results suggest that TAS2Rs may play an important role in the pathophysiology of cardiac and vascular diseases, including disorders of the coronary arteries (Manson et al. 2014; Foster et al. 2014).
Yuan et al. (2020) demonstrated increased expression of TAS2R and α-gustducin and reduced spontaneous beating rate in the sinoatrial node following treatment with the TAS2R agonists, quinine and chloroquine in Langendorff-perfused hearts of adult rats (Yuan et al. 2020), suggesting that cardiac TAS2R may play a crucial role in the regulation of cardiac rhythm. Furthermore, the involvement of cardiac TAS2R in the regulation of cardiac rhythm may be essential for the prevention of arrhythmia, in part, via regulation of C×43 activity (see “Bitter taste receptors, cardiac contractility and rhythm: bitter taste receptor agonists modulate cardiac contractility and pacemaker activity via Ca2+-, cyclic AMP- and PDE-dependent mechanisms” and “Molecular signalling pathways, linking bitter taste receptor sensing of pathogenic and toxigenic molecules with inflammatory, oxidative stress responses, arrhythmia and contractile dysfunction in heart diseases”).
The expression of TAS2Rs in the heart suggests that these nodal signalling units of the plasma membrane are critical for evaluating the chemical composition of blood and tissue fluid, serving as gateway surveillance units that sense and mobilise protective mechanisms against the transport of noxious or toxigenic molecules into the cells and tissues of the heart (D’Urso and Drago 2021). Thus, pharmacological agents that act on cardiac TAS2Rs can be harnessed for potential therapeutics in some cardiac diseases (Foster et al. 2015). Indeed, some phytochemicals and their derivatives acting as TAS2R agonists have been identified as promising therapeutic agents for potential treatment of several disorders, including heart diseases (vide infra) (Kamila and Agnieszka 2021). Therefore, research has revealed that denatonium benzoate, an agonist of TAS2R1, 2, 7, and α-gustducin, at low dose (5 mg/kg) and short period of treatment (i.e. 7 days) causes a decrease in the expression of apoptosis and autophagy-related genes—caspase (Casp) 2, Casp3, Casp7, Casp9, Bcl-2l1 (B cell lymphoma 2 like 1), Mcl (mantle cell lymphoma), Bid (BH3-interacting domain death agonist), and Noxa (NADPH oxidase activator), along with increased expression of antioxidant enzymes or mediators such as glutathione peroxidase 1 (Gpx1), catalase (CAT), superoxide dismutase 1 (SOD1) and calpain-1 in whole heart tissues of experimental animals (Hamdard et al. 2019b). However, at higher doses and chronic treatment with denatonium benzoate, Hamdard et al. (2019a, b) demonstrated increased expression of the apoptosis and autophagy-related genes, suggesting that TAS2R activation may be beneficial at low doses and acute treatment period (Hamdard et al. 2019b).
Furthermore, Burt and coauthors demonstrated that treatment of HL-1 mouse cardiac myocytes with flufenamic acid (10 μM) decreases Ca2+ oscillations followed by an overall increase in intracellular Ca2+ level as well as depolarisation of the mitochondrial membrane (Burt et al. 2013). Flufenamic acid is a TAS2R14 agonist (Meyerhof et al. 2010) and a member of the anthranilic acid derivative class of nonsteroidal anti-inflammatory drugs (Chi et al. 2011). Though it is not exactly clear whether the anti-inflammatory effect of flufenamic acid is due to its stimulatory action on TAS2R, available data suggest that this TAS2R14 agonist can modulate the expression of COX-2 gene via interaction with the NF-κB pathway (vide infra) or NADH oxidase signalling (Hamdard et al. 2019b). Indeed, activation of TAS2R1, 8, 10, 13, 14, and 38 by the human gut microbiota-derived quorum-sensing signal molecule, 3-oxo-C12:2-HSL in LPS- and IFNγ-stimulated RAW264.7 macrophages reportedly resulted in decreased expression of the proinflammatory cytokines IL-1β and TNFα via modulation of the NF-κB, Janus kinases/Signal transducer and activator of transcription (JAK/STAT) and TNF signalling pathways (Coquant et al. 2022). Similar cardioprotective effects have been reported for the TAS2R agonists genistein in preclinical studies (Bai and Wang 2019; Chen et al. 2019) and clinical trial (ClinicalTrials.gov: NCT00287690), and epigallocatechin-3-gallate in preclinical studies (Xuan and Jian 2016; Reddy et al. 2020), and clinical trials (ClinicalTrials.gov: NCT02015312; aus dem Siepen et al. 2015). However, it is not exactly clear how these agonists exert their protective effects on the heart via TAS2R-activated intracellular signalling.
Besides the TAS2R agonists, a few molecules have been identified to act as TAS2R antagonists in taste receptor-expressing cells. Probenecid ((p-(dipropylsulfamoyl)benzoic acid)) is an inhibitor of the multidrug resistance protein 1 transporter, approved by the United States Food and Drug Administration, and clinically used for the treatment of gout in humans. Probenecid was identified to inhibit the human type TAS2R16, -38, and -43 through an allosteric mechanism of action (Greene et al. 2011). Roland et al. (2014) demonstrated that 6,3'-dimethoxyflavanone, 4'-fluoro-6-methoxyflavanone, and 6-methoxyflavanone inhibit denatonium benzoate- and epigallocatechin-3-gallate-mediated activation of TAS2R14 and -39 by reversible insurmountable antagonism (Roland et al. 2014). Antagonists of TAS2Rs also include homoeriodictyol (TAS2R14, 39 and 43) (Tiroch et al. 2021), eriodictyol (TAS2R14, 39) (Ley et al. 2006), enterodiol (TAS2R10) (Ley et al. 2006, 2012), 2,4-dihydroxybenzoic acid vanillylamide (unknown TAS2R subtype) (Ley et al. 2006), [2]-gingerdione and its homologue [3]-gingerdione (unknown TAS2R subtype) (Ley et al. 2008), sakuranetin, 6-methoxysakuranetin, and jaceosidin (TAS2R31) (Fletcher et al. 2011). Research is required to explore and clarify the mechanisms of TAS2R antagonism, its implication on cellular signalling, the clinical significance and potential therapeutic effects of TAS2R antagonists in cardiac diseases.
It should be mentioned that the mechanism of TAS2R–ligand interactions is yet to be fully elucidated and the pharmacology of TAS2R is poorly defined (Devillier et al. 2015; Jaggupilli et al. 2016; Medapati et al. 2022). There is severe lack of data on the mechanism of deactivation of TAS2R after it has been activated by a noxious stimulant. Notwithstanding, available data indicate that the activation of TAS2R causes a conformational change in the receptor (Jaggupilli et al. 2016), which may lead to its deactivation, thereby preventing its continued stimulation. It is currently not clear whether the activation of this taste receptor causes it to subsequently undergo proteolysis or other mechanisms of deactivation. It is also not clear whether a feedback mechanism is involved in the control of TAS2R activity. Therefore, further research is required to unravel the pharmacology of TAS2R–ligand interactions.
Signal transduction mechanism of cardiac bitter taste receptors
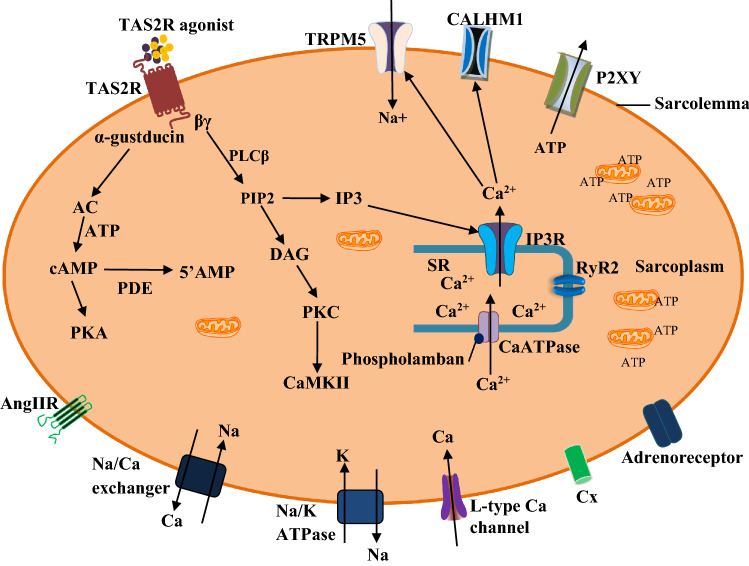
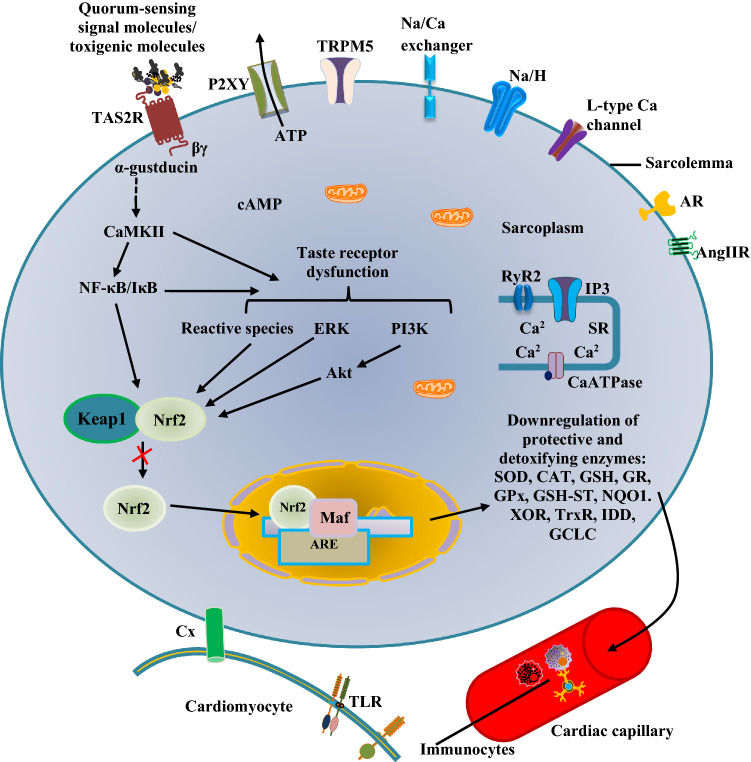
Upon activation by certain metabolites, toxigenic or microbial molecules, TAS2Rs relay their signal downstream the cytoplasm (Fig. 1), which under normal condition, is required to resolve cellular or tissue injury (Welcome 2020a; Welcome and Mastorakis 2021). However, disordered signalling or excessive activation of TAS2Rs by pathological molecules can initiate cellular or tissue damage, characterised by proinflammatory and oxidative stress responses (Welcome 2020a; Welcome and Mastorakis 2021; Welcome et al. 2021). Notwithstanding, however, there are peculiarities in the responses or mechanisms of signal transduction of TAS2Rs in some regions of the body. Therefore, in addition to activation and recruitment of macrophages and other immunocytes in the heart, the cardiac TAS2Rs respond to stimuli via transduction cascades involving the activation of phosphodiesterase (PDE)-dependent pathways and downregulation of cyclic AMP. The pathways of signal transduction of cardiac TAS2Rs are summarised in Fig. 1.
Fig. 1.
Signal transduction mechanisms of cardiac bitter taste receptors (TAS2R). The pathogenic or toxigenic molecules activate TAS2R (Freund et al. 2018) to trigger the dissociation of α-gustducin from the βγ subunit. The former stimulates the membrane bound enzyme, adenylate cyclase (AC). This enzyme produces cyclic adenosine monophosphate (AMP) in the presence of adenosine triphosphate (ATP) (Jeon et al. 2011; Workman et al. 2018; Xie et al. 2018). Cyclic AMP activates some ion channels and intracellular enzymes, including protein kinase A (PKA), which phosphorylates its downstream targets. However, cyclic AMP is hydrolysed by phosphodiesterases (PDE3, -4) to form 5’AMP, thereby decreasing the level of activated PKA (Foster et al. 2014). The βγ subunit stimulates phospholipase Cβ (PLCβ), which mediate the formation of diacylglycerol (DAG) and 1,4,5-inositol trisphosphate (IP3) from phosphatidylinositol 4,5-bisphosphate (PIP2). IP3 stimulates IP3 receptor (IP3R) to mediate cytosolic release of Ca2+. The increase in cytosolic Ca2+ activates transient receptor potential cation channel, subfamily M, member 5 (TRPM5), calcium homeostasis modulator 1 (CALHM1), and ryanodine receptor 2 (RyR2) of the sarcoplasmic reticulum (SR) (Chandrashekar et al. 2000; Jeon et al. 2011; Workman et al. 2018; Xie et al. 2018). Elevation of cytosolic Ca2+ may cause ATP secretion via P2XY channel (Welcome et al. 2015). However, increase in Ca2+ level is only transient and not able to cause contraction of the muscle cell. DAG activates protein kinase C (PKC), which phosphorylates other intracellular proteins and membrane receptors such as Ca2+-calmodulin protein kinase II (CaMKII), angiotensin II receptor (Ang IIR), connexins (Cx) (Manson et al. 2014; Welcome et al. 2015)
Emerging role of cardiac bitter taste receptors in cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction: cellular and molecular mechanisms
Data have consistently shown that TAS2Rs can act as immune sensors by detecting not only the danger-associated molecular patterns, but also microbial components to mobilise protective measures against the pathogenic invasion or aggression (Manson et al. 2014; Hamdard et al. 2019b; Welcome 2020a; D’Urso and Drago 2021; Welcome and Mastorakis 2021). Accordingly, TAS2Rs are now considered as integral components of the sensory (Palmer 2007; Barham et al. 2013) and innate immune system (Gulbransen et al. 2008; Tizzano et al. 2011). Therefore, cardiac TAS2Rs may function as immune cystocytes or sentinels that effectively monitor and maintain the cardiac immediate environment to ensure uninterrupted functioning of the heart. Furthermore, previous studies have revealed a potential role of these receptors as critical mediators of inflammatory and oxidative stress responses (Hamdard et al. 2019b; Welcome 2020a; Welcome and Mastorakis 2021). Very recent data suggest a possible role of cardiac TAS2Rs in the pathophysiology of arrhythmia and contractile dysfunction in heart diseases (vide infra).
Cardiac bitter taste receptors sense the “quorum” to mobilise defensive mechanisms against pathogenic aggression
Research has shown that TAS2Rs can sense the “quorum” to regulate the activities of pathogens by detecting the quorum-sensing signal molecules (Gulbransen et al. 2008; Tizzano et al. 2011). Quorum-sensing signal molecules are substances produced by pathogens that enable them communicate with each other, share information about cell density and adjust to gene expression until sufficient quantity of the pathogens is available to counteract the host defence mechanisms (Rajput et al. 2016). Quorum-sensing signal molecules are essential in secretion of virulence factors, biofilm formation, motility, etc. (Rajput et al. 2016). As a matter of fact, the virulence of pathogens, in part, depends on the quantity of quorum-sensing signal molecules synthesised in the host (Wu et al. 2021a). All currently identified quorum-sensing signal molecules are available in specialised repositories, namely SigMol Database (http://bioinfo.imtech.res.in/manojk/sigmol), which harbours about 1380 molecules (Rajput et al. 2016), and QSIdb (http://qsidb.lbci.net/), a quorum-sensing interference (QSI) database with several hundreds of quorum-sensing signal molecules (Wu et al. 2021a). Table 2 shows some quorum-sensing signal molecules produced by cardiotrophic pathogens.
Table 2.
Pathogens (bacteria, and parasites and viruses) and their quorum sensing signal molecules and evidence about their involvement in cardiac infection
| Pathogen | Genera/types | Quorum-sensing signal molecule | Evidence of involvement in cardiac infection |
|---|---|---|---|
| Gram-positive bacteria | Bacillus sp. (e.g. Bacillus cereus), Listeria sp., Enterococcus sp., Streptococcus pordonii, and Staphylococcus sp. (e.g. S. aureus) | Oligopeptides, thiolactone, PlcR, Npr, and PapR (autoinducing peptides) [166,167, 168] | Endocarditis (Fernández Guerrero et al. 2007; Guerrero et al. 2009; Thomas et al. 2012; Ferrand et al. 2013; Lamond et al. 2021); myocarditis (McGee et al. 2018; Strnadel et al. 2018) |
| Gram-negative bacteria | Escherichia coli | Autoinducer (AI)-3 (Zhou et al. 2016) | Endocarditis (Quiring and Burke 2021) |
| Stenotrophomonas Maltophilia (Xanthomonas maltophilia and Pseudomonas maltophilia), X. campestris | Homologue of farnesoic acid: cis-11-methyl-2-dodecenoic acid, α,β unsaturated fatty acid (diffusible signal factor, DSF) [178,179] | Endocarditis (Mehta et al. 2000) | |
| Acinetobacter sp. (A. baumannii), Aeromonas hydrophyla, Pseudomonas sp., Serratia marcescens, and Yersinia sp. | N-acyl-L-homoserine lactone, N-(3-oxoacyl)-L-homoserine lactone, N-(3-hydroxyacyl)-L-homoserine lactone; AI-1 (Deep et al. 2011; Zhou et al. 2016) | Endocarditis (Suri et al. 1971; Rodríguez-Hernández et al. 2004; Pugliese et al. 2016; Gürtler et al. 2019; Ioannou et al. 2021), myocarditis (Del-Pozo et al. 2011; Ranjani et al. 2015) | |
| Pseudomonas aeruginosa | Butyryl-homoserine lactone; AI-1(Deep et al. 2011; Zhou et al. 2016) | Endocarditis (Gürtler et al. 2019), myocarditis (Ranjani et al. 2015) | |
| Escherichia coli | Furanosyl borate diester (AI-2) (Li et al. 2007; Zhou et al. 2016) | Endocarditis (Quiring and Burke 2021) | |
| Escherichia coli, Salmonella enterica, and Shigella flexneri | 2-Methy-2,3,3,4-tetrahydroxytetrahydrofuran (AI-2) (Li et al. 2007; Zhou et al. 2016) | Endocarditis (Fernández Guerrero et al. 2004; Quiring and Burke 2021), pericarditis (Fernández Guerrero et al. 2004), myocarditis (Chehab et al. 2020), pancarditis (Guerrero Ortiz et al. 2003) | |
| Pseudomonas aeruginosa | 2-heptyl-3-hydroxy-4 quinolone, 3-oxododecanoyl-l-homoserine lactone (Rennemeier et al. 2009) | Vide supra | |
| Neisseria gonorrhoeae | NgAI-2 (AI) (Anderson et al. 2016; Edwards et al. 2016) | Endocarditis (Olayemi et al. 2017) | |
| Treponema pallidum | TpAI-2 (AI) (Babb et al. 2005; von Lackum et al. 2006; Arnold et al. 2015) | Endocarditis (Hijikata et al. 2019), septic cardiomyopathy (Guo and Guo 2021) | |
| Chlamydia trachomatis | CtAI, certain fatty acids (AI) (Bergsson et al. 1998; Barczak and Hung 2009; Rabin et al. 2015; Simon et al. 2015) | Endocarditis (Brearley and Hutchinson 1981), myocarditis (Ringel et al. 1982) | |
| Legionella sp. (e.g. Legionella pneumophila) | LAI-1 (Legionella autoinducer) (3-hydroxypentadecane-4-one—an α-hydroxyketone) (Tiaden and Hilbi 2012; Simon et al. 2015) | Endocarditis (Pearce et al. 2011), pericarditis, myocarditis (Burke et al. 2009) | |
| Vibrio vulnificus | cyclo-(l-Phe-l-Pro) (Kim et al. 2018) | Endocarditis (Truwit et al. 1987) | |
| Parasites | Candida albicans, C. auris, Aspergillus fumigatus, Cryptococcus | Farnesol, tyrosol, 3(R)-hydroxy-tetradecaenoic acid (β-oxidation metabolite of linoleic acid), phenylethanol, tryptophol (Rennemeier et al. 2009; Deep et al. 2011; Nigam et al. 2011) | Pacemaker site infection, cardiac failure (COHEN et al. 1991; Mullick et al. 2006) |
| Viruses | Coronaviruses (e.g. SARS-CoV-2), coxsackievirus, parvovirus B19, Epstein–Barr virus, cytomegalovirus, and varicella-zoster virus, human herpesvirus 6, etc | Rap-Phr, AimR-AimP, AimR-AimP-like, etc. (Bernard et al. 2021) | Endocarditis (Ouarradi et al. 2021), myocarditis (Martens and Accornero 2021) |
Note: QSS quorum-sensing signal, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, Gram-negative bacteria are called autoinducers, whereas Gram-positive bacteria produce autoinducing peptides
Under normal condition, the quorum-sensing signal molecules activate the TAS2Rs to initiate intracellular signalling that culminate in protection of the host against microbial invasion, at least in part, by suppressing the host immune responses (Grassin-Delyle et al. 2019; Kinnamon and Finger 2019). Though the mechanisms of suppression of the host immune system is not completely known, Lee et al. (2018) showed that quorum-sensing signal molecules subvert the host anti-viral, -bacteria and -fungi defences mainly by abrogating interferon (IFN)-β production via pathogen interaction with the activation of retinoic-acid-inducible gene-I (Lee et al. 2018). More so, evidence indicates that pathogens use quorum-sensing signal molecules to actively suppress the production of inflammatory cytokines, reactive species, including NO via inhibition of κB (IκB) kinase (IKK) phosphorylation, IκBα degradation, and nuclear translocation of NF-κB (Kim et al. 2015). In addition to activation of NF-κB, cardiotrophic quorum-sensing signal molecules (including those of the severe acute respiratory syndrome coronavirus 2) (Freeman and Swartz 2020; Kandasamy 2021) initiate intracellular signalling via TAS2Rs (Barham et al. 2021; Parsa et al. 2021; Sharma et al. 2021) to activate the cytoplasmic sensor of inflammatory signal—NLRP3 inflammasome (Freeman and Swartz 2020; Kandasamy 2021). However, under normal condition, cardiac TAS2Rs counteract these quorum-sensing signal molecules by binding to them and mediating responses that eventually result in the prevention of the pathogenic aggression, suggesting that TAS2Rs may serve as a novel type of pattern-recognition receptors (Lee et al. 2018; Zhou et al. 2021). Interestingly, recent data showed that TAS2R16 activation effectively suppresses LPS-induced secretion of proinflammatory cytokines (Zhou et al. 2021). Therefore, cardiac TAS2Rs detect quorum-sensing signal molecules to initiate microbicidal and other responses that lead to elimination of the pathogens. Nevertheless, dysfunctional signalling of TAS2Rs can predispose to pathologies (Welcome 2020a). Thus, investigating the mechanisms of detection of quorum-sensing signal molecules or other pathogenic molecules by TAS2Rs and their signal transduction cascades in health and disease can provide useful information for identification of novel frontiers in the treatment of some heart diseases.
Nitric oxide secretion contributes to the anti-inflammatory effects of cardiac bitter taste receptor activation
The synthesis and secretion of NO is a key mechanism involved in the anti-inflammatory effects of TAS2R activation. Kim et al. (2015) showed that pathogens use quorum-sensing signal molecules to actively suppress the production of NO (Kim et al. 2015). Indeed, NO has been repeatedly shown to exhibit a microbicidal effect on cardiotropic pathogens (Akaike and Maeda 2000; Uehara et al. 2015). TAS2Rs of the endocardium or cardiac vessel endothelium respond to quorum-sensing signal molecules, potentially dangerous microbial metabolites, toxins, pharmacological agents such as acetaminophen, chloramphenicol, chloroquine, quinine, noscapine (Tables 1 and 2) [BitterDB (http://bitterdb.agri.huji.ac.il)], and phytochemicals such as genistein and polyphenols (Gradinaru et al. 2022) to trigger NO secretion via the canonical pathway of taste receptor transduction (Gopallawa et al. 2021; Carey et al. 2021). Accordingly, in the canonical pathway, the microbicidal effects of activation of cardiac endothelial TAS2Rs are largely due to the rise in intracellular Ca2+, which forms complexes with CaM, and in turn activates CaM-dependent protein kinases to stimulate eNOS (endothelial NOS). eNOS is the enzyme that catalyses the formation of L-citrulline and NO from L-arginine (Welcome 2020a). Upon stimulation of α-gustducin by a higher concentration of bitterants or microbial quorum-sensing signal molecules in TAS2R-expressing endothelial cells, a rise in cytosolic Ca2+ activates a greater NO generation in a dose-dependent manner, thereby inducing substantial microbicidal effects on the pathogens (Gopallawa et al. 2021). Consistently, Grekov et al. (2017) reported significant destruction of Leishmania promastigotes, the parasite that causes leishmaniasis, following treatment with calcimycin (a calcium ionophore) via activation of NO secretion (Grekov et al. 2017). Comparable data are discussed elsewhere (Jeandroz et al. 2013).
Bitter taste receptors, cardiac contractility and rhythm: bitter taste receptor agonists modulate cardiac contractility and pacemaker activity via Ca2+-, cyclic AMP- and PDE-dependent mechanisms
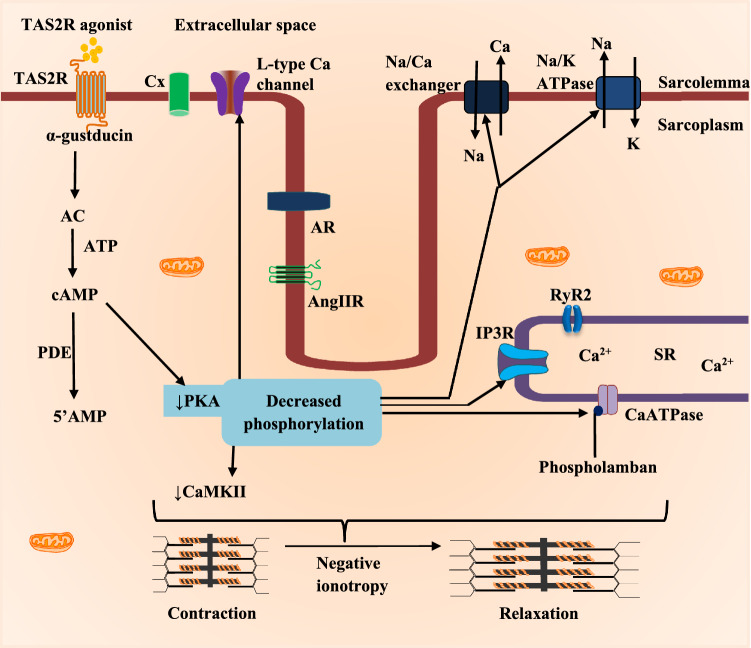
Since TAS2R signalling is associated with changes in cardiomyocyte cytosolic Ca2+ and cyclic AMP along with other signalling molecules (Manson et al. 2014; D’Urso and Drago 2021), activation of TAS2Rs will elicit corresponding changes in cardiac mechanics and pacemaking. Accordingly, administration of TAS2R108 and 137 agonists in Langendorff-perfused heart of C57BL/6 mice revealed a ∼40% decrease in left ventricular pressure and increase in the aortic pressure, respectively (Foster et al. 2014). However, these responses were abolished in the presence of pertussis toxin and gallein, which are, respectively, inhibitors of Gαi and Gβγ subunits of the G-protein, indicating a negative inotropic effect of TAS2R agonists on the heart (Foster et al. 2014). Thus, activation of cardiac TAS2Rs leads to negative ionotropy (Fig. 2).
Fig. 2.
The negative ionotropic effect of TAS2R activation in cardiac muscle cell. TAS2R activation initiates downstream signaling that culminates in reduced formation of cyclic AMP (adenosine monophosphate) in a PDE-dependent manner. This reduces the activity of protein kinase A (PKA) along with deregulated activity of protein kinase C (PKC) resulting in decreased phosphorylation of its targets (Foster et al. 2014; Manson et al. 2014; Welcome et al. 2015). These protein kinases reduce their stimulatory effect of Ca2+-calmodulin protein kinase II (CaMKII), thereby decreasing the activity of ion channels/receptors such as 1,4,5-inositol trisphosphate receptor (IP3R), transient receptor potential cation channel, subfamily M, member 5 (TRPM5), ryanodine receptor 2 (RyR2), CaATPase, angiotensin II receptor (AngIIR), adrenoreceptor (AR), etc. (Chandrashekar et al. 2000; Jeon et al. 2011; Workman et al. 2018; Xie et al. 2018). The resultant effect is reduced cytosolic calcium waves, decreased phosphorylation of the motor proteins that promote relaxation of the cardiac muscles (negative inotropy). Other abbreviations are similar to those in Fig. 1
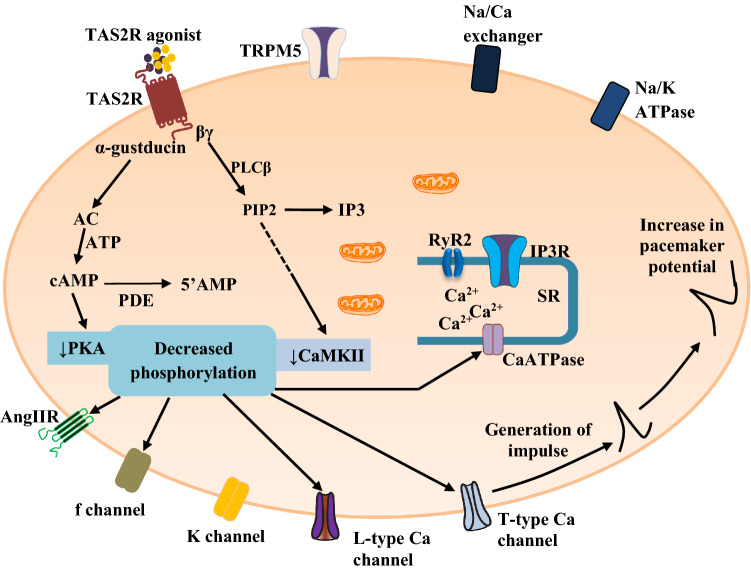
In a relatively recent study, Yuan et al. (2020) demonstrated a negative chronotropic effect of quinine and chloroquine on the sinoatrial node, which was abrogated via inhibition of TAS2R108 with abscisic acid. In addition, both TAS2R agonists suppressed the isoprenaline-induced tachycardia on the sinoatrial node (Yuan et al. 2020). In the same study, the authors showed that inhibition of phosphodiesterases (PDE3 and PDE4) with 3-isobutyl-1-methylxanthine resulted in a negative chronotropic effect on the sinoatrial node (Yuan et al. 2020). Thus, TAS2R agonists suppress the pacemaker activity of the sinoatrial node via PDE-induced cyclic AMP reduction (Fig. 3), suggesting that dysfunctional signalling of TAS2R may play a role in the development of cardiac arrhythmia via disordered generation of impulse.
Fig. 3.
The negative chronotropic effect of TAS2R activation on the sinoatrial node. The reduced activity of protein kinase A (PKA) and protein kinase C (PKC) leads to downregulation of the activity of Ca2+-calmodulin protein kinase II (CaMKII), funny (f) channel, L-type Ca channel, T-type Ca channel, 1,4,5-inositol trisphosphate receptor (IP3R), transient receptor potential cation channel, subfamily M, member 5 (TRPM5), ryanodine receptor 2 (RyR2), CaATPase, angiotensin II receptor (AngIIR), glucagon-like peptide 1 receptor (GLP1-R), adrenoreceptor (AR), etc. However, certain phosphorylation may increase channel activity (e.g. K channel). The overall effect is associated with increase in the slow depolarising (pacemaker) potential (negative chronotropic effect) mainly due to reduced if current, reduced Ca2+ waves, and increased K+ current (Foster et al. 2014; Manson et al. 2014; Welcome et al. 2015). Other abbreviations are similar to those in Fig. 1
Molecular signalling pathways, linking bitter taste receptor sensing of pathogenic and toxigenic molecules with inflammatory, oxidative stress responses, arrhythmia and contractile dysfunction in heart diseases
Accumulating research data have consistently shown that TAS2Rs participate in inflammatory and oxidative stress responses (Hamdard et al. 2019b; Welcome 2020a; Welcome et al. 2021; Welcome and Mastorakis 2021). Furthermore, available data indicate that TAS2Rs are involved in modulation of cardiac excitability and contractile activities, suggesting that disorders in TAS2R signalling might predispose to cardiac diseases, which are characterised by inflammation, oxidative stress, contractile dysfunction and possibly arrhythmia (Manson et al. 2014; Hamdard et al. 2019b; Yuan et al. 2020). Data also indicate a critical role of NLRP3 and NF-κB in mediating and establishing a molecular bridge between the immune-inflammatory system and TAS2R dysfunction (Welcome 2020a; Welcome and Mastorakis 2021). These molecular sensors of the immune system and inflammatory responses are responsible for cardiac inflammation due to defective TAS2R signalling.
The NF-κB and NLRP3 inflammasome are the primary regulators of inflammatory responses (Welcome 2020a; Welcome and Mastorakis 2021) and have been widely implicated in cardiac inflammation (Lu et al. 2015; Chen et al. 2018; Jui et al. 2021) and taste receptor signalling defects (Zhou et al. 2018; Welcome 2020a). In addition, it is widely acknowledged that the master oxidative stress sensor, Nrf-2 (nuclear factor erythroid 2-like 2) connects the inflammatory signalling cascades to oxidative stress responses and vice versa (Jui et al. 2021). Indeed, the Nrf-2 has been consistently implicated in the pathogenic mechanism of cardiac disorders (Jui et al. 2021). Thus, Nrf-2 may be responsible for mediating the signalling cascades that link oxi-inflammatory stress with taste receptor dysfunction (Hamdard et al. 2019b). Hence, an extensive cross-talk occurs between the inflammatory sensors (NLRP3 and NF-κB) and Nrf-2 to regulate the homeostasis of the immune system, inflammatory and oxidative stress profiles of the cells and tissues of the heart.
NF-κB signalling
The NF-κB is a transcription factor that regulates the expression of over 550 genes (nf-kb.org) involved in immune, inflammatory, oxidative stress responses, cell proliferation, differentiation, growth, survival, apoptosis and other cellular processes (Collins et al. 2016). The mammalian proteins of the NF-κB family consist of p105 (precursor protein of p50), p100 (precursor of p52), RelA (p65), RelB and c-Rel (Thu and Richmond 2010). The NF-κB signalling pathway is stimulated by pathogenic factors, cytokines, ROS, and reactive nitrogen species. Therefore, oxidative stress molecules such as ROS or the proinflammatory cytokine, IL-1β, produced by dysfunctional taste receptors (Zhou et al. 2018; Welcome and Mastorakis 2021) can activate the NF-κB signalling through the IκB kinase or TLR, respectively. In addition, abnormal stimulation of TAS2R by pathogenic components or quorum-sensing signal molecules can also activate the NF-κB (Welcome 2020a).
The NF-κB is abundantly expressed in cardiac endothelial cells, fibroblasts and cardiomyocytes (Sangeetha et al. 2011; Yin et al. 2021). This molecular sensor of inflammation is present in the cytosol of cardiac cells as an inactive protein bound to IκB to form the NF-κB/IκB complex. Upon activation by pathological stimuli, the IκB kinase phosphorylates the inhibitory kappa B (IκBs) protein of the cytoplasmic complex NF-κB/IκB to cause degradation of IκB and release of NF-κB protein heterodimers to translocate into the nucleus to activate gene transcription (Fig. 4). The IκB kinase is a primary target of reactive species, proinflammatory mediators such as IL-1β, TNFα and other pathogenic signals (Gloire and Legrand-Poels 2006; Thu and Richmond 2010; Collins et al. 2016; Jimi et al. 2019). Even so, a non-classical (non-canonical) or alternative pathway exists for the activation of NF-κB. The pathway involves activation of the NF-κB-inducing kinase (NIK) (Jimi et al. 2019; Pflug and Sitcheran 2020). In the alternative pathway, NIK is stimulated by ligands such as TNF receptor superfamily member 5 (CD40) ligand and TNF-related weak inducer of apoptosis (Thu and Richmond 2010) resulting in cleavage of the p100 to produce p52 with subsequent dimerisation of the latter with RelB. The complex formed translocates into the nucleus to initiate gene transcription (Thu and Richmond 2010). Recent evidence indicates that the NIK protein can interact with the canonical NF-κB pathway by activating the IκB kinase (Woronicz et al. 1997; Thu and Richmond 2010). The activated NF-κB induces the expression of caspase-1, IL-1β and NLRP3 (Welcome and Mastorakis 2021). NLRP3 is discussed in the next subsection.
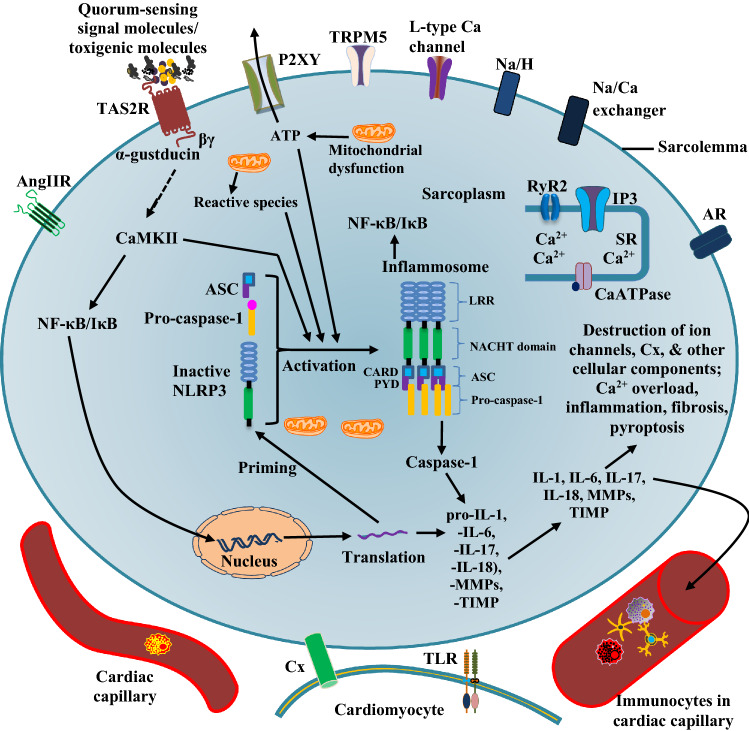
Fig. 4.
NF-κB/IκB and NLRP3 signaling in TAS2R dysfunction in cardiac cell. Dysfunctional signaling or pathological activation of TAS2R can trigger the activation of NLRP3 (NLR Family Pyrin Domain Containing 3) (Murakami et al. 2012; Zhong et al. 2016; Welcome and Mastorakis 2021) and nuclear factor κB (NF-κB) signaling, which mediate the production of proinflammatory cytokines and alarmins that cause inflammation, pyroptosis and fibrosis—constitute critical pathological processes in several heart diseases (Zhong et al. 2016; Chen et al.2017; Li et al. 2018). NF-κB/IκB and NLRP3 signaling in TAS2R dysfunction is also accompanied by Ca2+ overload, which worsens cardiac functions. The secreted interleukins can trigger proinflammatory responses in neighbouring cardiac cells and immunocytes. (See further explanation in text). TLR toll-like receptor, IL interleukin, MMPs Matrix metalloproteinases, TIMP tissue inhibitor of metalloproteinases, PYD pyrin domain, CARD caspase activation and recruitment domains, LRR leucine-rich repeat, ASC apoptosis-associated speck-like protein containing a caspase activation and recruitment domain—CARD, NACHT domain present in NAIP, CIITA, HETE and TP1. Other abbreviations are similar to those in Figs. 1 and 2
Though the exact mechanisms are not known, emerging data suggest that NF-κB activation is linked to TAS2R signalling. As a matter of fact, increased expression of taste receptors has been reported in inflammatory conditions (Shin et al. 2010), suggesting that taste receptor dysfunction can predispose to inflammatory conditions. Consistent with these findings, independent groups of researchers (Cui et al. 2019; Wu et al. 2020) have demonstrated the involvement of disorders of the bitter taste sensor, TRPM5 (transient receptor potential cation channel subfamily M member 5) in hypertensive heart disease. Zhou et al. (2021) reported that TAS2R16 activation by salicin effectively suppressed the release of LPS-induced proinflammatory cytokines, in part, by inhibiting the increase in cytosolic cyclic AMP and nuclear translocation of NF-κB p65 in human fibroblasts (Zhou et al. 2021). Though NF-κB p65 activation can mediate the secretion of alarmins and pore-forming proteins, which result in pyroptosis (a form of cell death ensuing from the formation of pores in the plasma membrane), evidence indicates that under normal condition, taste receptor signalling also leads to inhibition of autophagy in the heart through the activation of the mammalian rapamycin complex 1 (mTORC1) (Kokabu et al. 2015). In fact, rodents lacking taste receptors displayed increased rate of autophagy in the heart (Kokabu et al. 2015). Apparently, NF-κB activation provides a critical molecular nexus between inflammatory cascades and mTORC1 activity via interaction with IκBα. Moreover, the activation of mTORC1 is considered as a downstream event of NF-κB activation in immortalised NPE cells (Zhu et al. 2016). Therefore, NF-κB and mTORC1 exhibit an extensive cross-talk with each other (Li et al. 2019) and are both involved in regulating the inflammatory and host defence system against pathogenic aggression (Bao et al. 2015; Li et al. 2019).
Several pharmacological compounds activate the TAS2Rs to mediate downstream signalling that leads to the inhibition of NF-κB. For instance, the anti-microbial, -inflammatory and immunomodulatory activities of flavones are mediated via TA2R14 activation, which suppresses the release of proinflammatory cytokines (Hariri et al. 2017). Flavone-induced taste receptor activation also increases the TA2R14-driven Ca2+ flux that subsequently stimulate NO production in response to multiple inflammatory stimuli, in part, through inhibition of PKC, receptor tyrosine kinase (Hariri et al. 2017) and NF-κB (Panche et al. 2016; Yahfoufi et al. 2018; Choy et al. 2019). Some flavones may also activate a functional TA2R38 isoform, which closely co-localises with TA2R14 to mediate the anti-inflammatory effects of this class of phytochemicals (Hariri et al. 2017). Consistent with the findings of previous authors (Hariri et al. 2017), a recent investigation by Tiroch et al. (2021) revealed that the anti-inflammatory effect of trans-resveratrol was mediated via the activation of TAS2R50 as treatment with the TAS2R antagonist homoeriodictyol or small interfering RNA-mediated TAS2R50 knockdown completely abolished the anti-inflammatory effect of trans-resveratrol in LPS-induced IL-6 secretion in an in vitro model of human fibroblasts (Tiroch et al. 2021). Interestingly, the flavone and stilbenoid subclasses of polyphenolic compounds possess cardioprotective properties, which highlight a possible adjunct therapeutic role of these phytochemicals in heart diseases (Akinwumi et al. 2017; Khan et al. 2021).
NLRP3 signalling
The NLRP3 is a cytoplasmic sensor that detects pathogenic (Swanson et al. 2019; Ciążyńska et al. 2020) and endogenous danger molecules as well as environmental toxins, including ultraviolet radiation (Liu et al. 2014; Ciążyńska et al. 2020) resulting in the formation of the NLRP3 inflammasome (Swanson et al. 2019). The NLRP3 is also activated by metabolic toxins, extracellular ATP, reactive species, K+ efflux (Liu et al. 2014; Swanson et al. 2019; Wang et al. 2020) and disordered gut microbiota (Henao-Mejia et al. 2013).
Inflammasomes are cytosolic oligomeric multiprotein complexes of the innate immune system that assemble in response to pathogenic signals to mediate inflammatory responses via the activation of caspase-1, resulting in proteolytic cleavage of pro-IL-1β, -IL-18 and gasdermin-D into their active forms with corresponding initiation of proinflammatory responses and pyroptosis (Mariathasan et al. 2004; de Zoete et al. 2014; Broz and Dixit 2016). Out of the 22 proteins of the NLR ((nucleotide-binding oligomerisation domain (NOD) like receptor)) family currently discovered in humans, only 8 have been reported to form an inflammasome: NLRP-1, -2, -3, -6, -7, -12, NLRC4 (Nod-like receptor Card domain-containing protein 4), and AIM2 (Absent In Melanoma 2) (de Zoete et al. 2014). However, the NLRP3 is the most widely studied and best characterised inflammasome (Swanson et al. 2019).
The NLRP3 is normally autoinhibited in the absence of danger or pathogenic signals (Sharma and De Alba 2021). The presence of pathogenic signal leads to the activation of NF-κB to promote the transcription of NLRP3, pro-IL1β, pro-IL-18, and a 53-kDa gasdermin-D through a process known as priming (Brownlee et al. 2020). The signal subsequently activates the NLRP3 (Brownlee et al. 2020) to promote the formation of active NLRP3 (i.e. NLRP3 inflammasome), involving the assembly or oligomerisation of the inactive upstream sensor (NLRP3), the adapter protein ASC (apoptosis-associated speck-like protein), and the downstream effector procaspase-1 (Henao-Mejia et al. 2013; Brownlee et al. 2020). The formation of the inflammasome triggers an autocleavage of procaspase-1 to caspase-1, which mediates the proteolytic cleavage, maturation and release of IL-1β and IL-18 (Broz and Dixit 2016; Swanson et al. 2019). The downstream effector of the NLRP3 inflammasome also mediates the cleavage of gasdermin-D to produce a 22-kDa C-terminal and a 31-kDa N-terminal fragment. The latter mediates the formation of membrane pores resulting in a greater secretion of proinflammatory molecules and alarmins (Wang et al. 2020) and eventually, pyroptotic cell death (Broz and Dixit 2016; Swanson et al. 2019). Apart from caspase-1, other caspases (e.g. caspase-11 in rodents, caspase 4 and caspase 5 in humans) can cause the activation of inflammasome via the non-canonical pathway by direct sensing of microbial components (e.g. bacterial LPS) in the cytosol (Broz and Dixit 2016).
Under normal condition, the NLRP3 is expressed in the cytosol at low levels in healthy heart tissues (Huang et al. 2014; Ye et al. 2015). Liu et al. (2014) demonstrated sparse expression of NLRP3 in cardiomyocytes, but increased expression of the protein in cardiac microvascular endothelial cells, which may indicate a greater role of the cardiac endothelium in inflammatory and immune response (Liu et al. 2014). Correspondingly, the authors showed increased NLRP3 inflammasome activation in cardiac ischaemia/reperfusion injury in cardiac microvascular endothelial cells, but not in cardiomyocytes (Liu et al. 2014). New data indicate a fundamental role of the cardiac microvascular endothelial cells in triggering cardiomyocyte injury (Zhang et al. 2021). Consistent with these data, Mezzaroma et al. (2011), Sandanger et al. (2013), and Liu et al. (2014) previously showed high NLRP3 expression in myocardial leukocytes, fibroblasts, and endothelial cells in myocardial ischaemia. Similar findings have been reported in a recent investigation (Mesquita et al. 2021). Increased expression of NLRP3 has also been confirmed in heart failure and cardiomyopathy (Wang et al. 2020). This pathogenic sensor is believed to be a critical molecular target for some existing medications, including agents used for the treatment of cardiovascular diseases (Wang et al. 2020). The NLRP3 is also expressed in dendritic cells, monocytes, macrophages and neutrophils (Wang et al. 2018, 2020).
On the basis of available evidence, indicating that taste receptors are crucial components of the innate immune system, inflammatory (Gulbransen et al. 2008; Tizzano et al. 2011; Lee and Cohen 2013b) and oxidative stress (Hamdard et al. 2019b) responses, as well as data suggesting a critical role of taste receptor signalling in neuroinflammation (Welcome and Mastorakis 2021), respiratory tract infection (Lee and Cohen 2013b), gastrointestinal infection (Liszt et al. 2022) and urinary and reproductive tract inflammation (Welcome et al. 2021), we therefore suggest that activation of NLRP3 by reactive species and abnormal Ca2+ signal is involved in the proinflammatory and oxidative stress responses of dysfunctional taste receptor signalling in the heart (Fig. 4). The activation of NLRP3 contributes to several cardiac disorders, including atherosclerotic heart disease, hypertensive heart disease, myocardial infarction, cardiac infection, cardiomyopathy, heart failure (Wang et al. 2018), and ageing (Mesquita et al. 2021). Indeed, the NLRP3 inflammasome and elevated cytosolic ROS along with decreased intracellular Ca2+ level have been associated with taste receptor dysfunction involving downregulation of α-gustducin, TRPM5, and phospholipase C β2 (Zhou et al. 2018). In mammals, TRPM5 is coexpressed with phospholipase C β2 in taste receptor cells, and both molecules are essential elements in the signal transduction mechanism of TAS2R (Pérez et al. 2002). Again, disorders of these taste receptor components are involved in inflammation and cardiac disorders. For instance, it has been reported in animal and humans studies that TRPM5 dysfunction is associated with cardiac inflammation (Koc et al. 2022; Virginio et al. 2022) and conduction disorders (Syam et al. 2014), suggesting that specific cardiac isoforms of TRPM may serve as useful therapeutic targets in heart diseases. Accordingly, there is a growing interest on the use of pharmacological agents for targeting the TRPM in pathological conditions (Virginio et al. 2022). Therefore, Simard et al. (2012) demonstrated that 9-phenanthrol, an aromatic alcohol derived from phenanthrene and an inhibitor of TRPM4, substantially reduces the frequency of early afterdepolarisations in a mouse model of cardiac hypoxia/re-oxygenation injury (a model of cardiac ischaemia/arrhythmia), suggesting that 9-phenanthrol can act as an anti-arrhythmogenic agent (Simard et al. 2012). (Early afterdepolarisations are essential cause of life-threatening ventricular arrhythmias, which are associated with sudden cardiac death. The limited efficacy of current antiarrhythmic therapy and the associated toxicity Bhat et al. 2022; Zeppenfeld et al. 2022) substantiates the need to search for novel therapeutic agents for the treatment of ventricular arrhythmias). Furthermore, the phenanthrene-derived TRPM4 inhibitor has been demonstrated to abrogate cardiac contractile dysfunction and oxidative stress in preclinical studies. Wang et al. (2013) showed that treatment of isolated Langendorff-perfused rat hearts with 9-phenanthrol resulted in a dramatic recovery of contractile function, significant decrease in infarct size and ischaemia–reperfusion injury (Wang et al. 2013). The authors also revealed that 9-phenanthrol substantially reduced the activity of lactate dehydrogenase (LDH) (Wang et al. 2013), an important oxidative stress marker implicated in a range of cardiac diseases (Lin et al. 1991; Kopel et al. 2012; Dai et al. 2020). In contrast, control hearts had reduced contractile function, increased size of the infarct area and LDH activity (Wang et al. 2013). Piao et al. (2015) also reported that prior treatment with 9-phenanthrol or TRPM4 silencing substantially decreased the level of reactive species in ROS-induced injury in H9c2 cardiomyocytes subjected to hydrogen peroxide treatment (Piao et al. 2015). Flufenamic acid can also inhibit the TRPM4. Using a mouse model of cardiac hypoxia/re-oxygenation injury, Simard et al. (2012) showed that administration of flufenamic acid abolished cardiac arrhythmias and significantly improved cardiac contractility (Simard et al. 2012). The sulfonylurea antidiabetic drug, glibenclamide at a concentration of 100 μM completely inhibited TRPM4 activity in sinoatrial node cells (Demion et al. 2007). Interestingly, both flufenamic acid (Chi et al. 2011; Hamdard et al. 2019b) and glibenclamide (Zhang et al. 2017b; Hou et al. 2020) possess anti-inflammatory and antioxidative properties. However, only 9-phenanthrol specifically inhibits TRPM4 activity (Grand et al. 2008; Burris et al. 2015) as flufenamic acid and glibenclamide can also inhibit Ca2+ and ATP-dependent K+ channels, respectively (Ozhathil et al. 2018).
Research investigation with animal models has demonstrated cross-signalling between the NLRP3 inflammasome and cytosolic Ca2+ level, suggesting that Ca2+ is a critical modulator of inflammasome activity (Lee et al. 2012). Interestingly, the activation of TAS2Rs elicits a brief rise in cytosolic Ca2+ level (Tomás et al. 2016; Duarte et al. 2020), followed by a fall (Suetomi et al. 2018) in cardiac muscle cells. Notwithstanding, however, dysfunctional taste receptor signalling may result in an excessive increase in cytosolic Ca2+ level with associated cytotoxicity due to Ca2+-induced activation of inflammatory and apoptotic signalling cascades. Recent investigations have shown that the Ca2+-dependent protein kinase, CaMKIIδ plays a central role in initiating inflammatory responses through its interaction with the NLRP3 inflammasome in cardiomyocytes in response to pressure overload, thereby upregulating NLRP3, caspase-1, IL-1β, IL-18, and MCP-1 to trigger macrophage recruitment (Suetomi et al. 2018). These pathological processes are associated with cardiac fibrosis, apoptosis, necrosis along with ventricular dilation and contractile dysfunction in heart diseases (Suetomi et al. 2018). Thus, CaMKIIδ acts as an intracellular molecular switch that controls cardiac Ca2+ flux, contractility, and inflammation via interaction with NLRP3 (Suetomi et al. 2018) and NF-κB (Martin et al. 2018).
Moreover, ROS (Suetomi et al. 2018) and toxigenic metabolites formed during taste receptor disorder (Hamdard et al. 2019b) can activate the NLRP3 signalling pathway (Kong et al. 2016). Data obtained from animal experiment have shown that toxigenic metabolites induce the expression and release of peri- and intracellular degrading enzymes such as MMP-1, -3, -8, and -9 (Lee et al. 2012). Interestingly, MMP-3, -8, or -9 reportedly increased the cytosolic levels of ROS, NO, IL-1β, TNF-α, expression of NF-κB and protease-activated receptor-1 (Lee et al. 2010). Notably, MMPs have been shown to degrade cellular components by targeting proteinases, cell adhesion molecules, extracellular matrix proteins and cell surface receptors, including TAS2Rs (Sternlicht and Werb 2001). The secreted proinflammatory molecules promote inflammatory responses, oxidative stress, and downregulate the expression of connexon gap junction, anti-inflammatory mediators, pro-oxidants and antioxidant enzymes (Welcome 2020b; Welcome and Mastorakis 2020).
Nrf-2 signalling
The Nrf-2 is a transcription factor that acts as an oxidative stress sensor, which upon activation, translocates into the nucleus to bind with the antioxidant response element, stimulating a battery of cytoprotective, detoxifying and antioxidant genes (Fig. 5) (Vomund et al. 2017). Nrf-2 activity is mainly controlled by Kelch-like ECH-associated protein 1 (Li and Jia 2019) and protein kinases such as glycogen synthase kinase 3 beta and PI3K (Wu et al. 2014; Li and Jia 2019).
Fig. 5.
Nrf-2 and TAS2R signalling in cardiac cell. TAS2R dysfunction triggers the formation of intracellular molecules that inhibit the nuclear translocation of Nrf-2 to downregulate the synthesis and secretion of protective and detoxifying enzymes (see further explanation in text). Akt protein kinase B (PKB), ARE antioxidant response element, ERK extracellular signal-regulated kinase, Keap1 Kelch-like ECH-associated protein 1, Maf MAF transcription factor, Nrf-2 nuclear factor erythroid-derived 2-like 2, PI3K phosphoinositide 3-kinase, SOD superoxide dismutase, CAT catalase, GSH glutathione, GR glutathione reductase, GPx glutathione peroxidase, GSH-ST glutathione-S-transferase, NQO1 dehydrogenase quinone1, XOR xanthine oxidoreductase, TrxR thioredoxin reductase, IDD iodothyronine deiodinases, GCLC glutamate-cysteine ligase catalytic subunit. (See explanation in text)
The Nrf-2 transcription factor plays a major role in regulating the equilibrium between formation and removal of reactive species (Qin and Hou 2017). Nrf-2 interacts with inflammatory signalling pathways to increase the expression of anti-inflammatory mediators (Vomund et al. 2017). Thus, Nrf-2 activation is required to abrogate the progression of inflammatory and oxidative stress responses (Ahmed et al. 2017; Hennig et al. 2018). Truly, the Nrf-2 exhibits cross-talks with the NLRP3 (Ahmed et al. 2017; Hennig et al. 2018) and NF-κB signalling pathways (Lampiasi and Montana 2018). The IκB kinase mediates the molecular interaction between Nrf-2 and the proinflammatory NLRP3 and NF-κB. As an integration point, activation of the IκB kinase stimulates both Nrf-2 and NLRP3, and also serves as a potential mechanism for the activation of antioxidant enzymes such as SOD, CAT, GPx, glutathione, glutathione reductase, glutathione-S-transferase, dehydrogenase quinone1 (Tinggi 2008; Lampiasi and Montana 2018; Cecerska-Heryć et al. 2021), xanthine oxidoreductase, thioredoxin reductase, iodothyronine deiodinases, and glutamate-cysteine ligase catalytic subunit (Tinggi 2008; Cecerska-Heryć et al. 2021).
Regrettably, however, there is a severe scarcity of data on the interaction between TAS2Rs and Nrf-2. Nevertheless, it can be suggested that TAS2R signalling may activate downstream cytoplasmic acceptors that interact with this transcription factor. Indeed, TAS2R signalling has been associated with several oxidative stress markers (Hamdard et al. 2019b). Furthermore, several activators of TAS2R have been shown to interact with the Nrf-2 to mediate downregulation of oxidative stress and proinflammatory mediators (Zhai et al. 2018; Hamdard et al. 2019b; Welcome and Mastorakis 2021). For instance, salicin activates TAS2R to exert its anti-inflammatory and anti-oxidant effects by promoting Nrf-2 nuclear translocation, HO-1 expression and inhibition of p65 phosphorylation to reduce ROS, IL-6, TNF-α, MMP-1, and -3 in animal model of inflammation (Zhai et al. 2018).
Conclusion
Cardiac inflammatory and oxidative stress responses represent central pathophysiological processes in several diseases of the heart and are associated with dysfunctional cardiac TAS2R signalling. Under normal condition, cardiac TAS2R is required for endocardial and myocardial homeostasis, including regulation of impulse generation and propagation, cardiac rhythm, and contractile functions of the heart primarily via the activities of PDE3, PDE4, protein kinases, and Cx43. However, defects in cardiac TAS2R signal transduction essentially activate the molecular switches that connect cardiac inflammation and oxidative stress to contractile dysfunction and arrhythmia via Ca2+-calmodulin protein kinase II, NLRP3, NF-κB, and Nrf-2 signalling cascades. These molecular signalling pathways mediate the secretion of inflammatory, oxidative stress, apoptotic and fibrotic mediators, activation and recruitment of immunocytes as well as monocyte/macrophage polarisation, which initiate the development of cardiac arrhythmia and contractile dysfunction in heart diseases. Cardiac TAS2Rs also act as gateway surveillance units that monitor and detect toxigenic or pathogenic molecules, including microbial components, and initiate responses that ultimately culminate in protection of the host against pathogenic aggression. Several pharmacological agents can act as TAS2R agonists to attenuate cardiac inflammation, oxidative stress responses, apoptosis and fibrosis, thereby abrogating cardiac arrhythmia and contractile dysfunction via activation of Nrf-2 and inhibition of NLRP3 and NF-κB signalling cascades.
Future directions
Since TAS2R signalling defects are involved in the pathophysiology of heart diseases, investigating the molecular switches that connect cardiac inflammation and oxidative stress to contractile dysfunction and arrhythmia may provide important information for identifying the molecular culprits of some heart diseases. In addition, investigation of specific molecular bridges that link cardiac TAS2R signalling defects to cardiac inflammation, oxidative stress, contractile dysfunction and arrhythmia in heart diseases may lead to identification of novel molecular targets for potential therapeutics of heart diseases. There is need to investigate the potential therapeutic roles of pharmaceuticals in cardiac TAS2R dysfunction, inflammation, oxidative stress, fibrosis, and pyroptotic cell death in heart diseases.
Funding
Funding is not available for this paper.
Data availability
There is no competing interest regarding the publication of this article.
Declarations
Conflict of interest
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adler E, Hoon MA, Mueller KL, et al. A novel family of mammalian taste receptors. Cell. 2000 doi: 10.1016/S0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Ahern RM, Lozano R, Naghavi M, et al. Improving the public health utility of global cardiovascular mortality data: The rise of ischemic heart disease. Popul Health Metr. 2011 doi: 10.1186/1478-7954-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SMU, Luo L, Namani A, et al. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Aimo A, Castiglione V, Borrelli C, et al. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27:494–510. doi: 10.1177/2047487319870344. [DOI] [PubMed] [Google Scholar]
- Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000 doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinwumi BC, Raj P, Lee DI, et al. Disparate effects of stilbenoid polyphenols on hypertrophic cardiomyocytes in Vitro vs. in the spontaneously hypertensive heart failure rat. Molecules. 2017 doi: 10.3390/molecules22020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albina JE, Caldwell MD, Henry WL, Mills CD. Regulation of macrophage functions by L-arginine. J Exp Med. 1989 doi: 10.1084/jem.169.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andelova K, Benova TE, Bacova BS, et al. Cardiac connexin-43 hemichannels and pannexin1 channels: Provocative antiarrhythmic targets. Int J Mol Sci. 2021;22:260. doi: 10.3390/ijms22010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MT, Byerly L, Apicella MA, Steven Seifert H. Seminal plasma promotes Neisseria gonorrhoeae aggregation and biofilm formation. J Bacteriol. 2016 doi: 10.1128/JB.00165-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Arellano MLB, Pozdniakova S, Kühl AA, et al (2019) Sex differences in the aging human heart: Decreased sirtuins, proinflammatory shift and reduced anti-oxidative defense. Aging (Albany NY). 10.18632/aging.101881 [DOI] [PMC free article] [PubMed]
- Arnold WK, Savage CR, Antonicello AD, Stevenson B. Apparent role for Borrelia burgdorferi LuxS during mammalian infection. Infect Immun. 2015 doi: 10.1128/IAI.00032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Nguyen TT, Sinitskii AI, et al. Isolevuglandins (isoLGs) as toxic lipid peroxidation byproducts and their pathogenetic role in human diseases. Free Radic Biol Med. 2021;162:266–273. doi: 10.1016/j.freeradbiomed.2020.10.024. [DOI] [PubMed] [Google Scholar]
- aus dem Siepen F, Buss SJ, Andre F, et al (2015) Extracellular remodeling in patients with wild-type amyloidosis consuming epigallocatechin-3-gallate: preliminary results of T1 mapping by cardiac magnetic resonance imaging in a small single center study. Clin Res Cardiol 10.1007/s00392-015-0826-3 [DOI] [PubMed]
- Babb K, Von Lackum K, Wattier RL, et al. Synthesis of autoinducer 2 by the lyme disease spirochete. Borrelia Burgdorferi J Bacteriol. 2005 doi: 10.1128/JB.187.9.3079-3087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Z, Wang Z. Genistein protects against doxorubicin-induced cardiotoxicity through Nrf-2/HO-1 signaling in mice model. Environ Toxicol. 2019 doi: 10.1002/tox.22730. [DOI] [PubMed] [Google Scholar]
- Bao W, Wang Y, Fu Y, et al. mTORC1 regulates flagellin-induced inflammatory response in macrophages. PLoS ONE. 2015 doi: 10.1371/journal.pone.0125910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczak AK, Hung DT. Productive steps toward an antimicrobial targeting virulence. Curr Opin Microbiol. 2009;12:490–496. doi: 10.1016/j.mib.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham HP, Cooper SE, Anderson CB, et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. 2013 doi: 10.1002/alr.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham HP, Taha MA, Broyles ST, et al. Association between bitter taste receptor phenotype and clinical outcomes among patients with COVID-19. JAMA Netw Open. 2021 doi: 10.1001/jamanetworkopen.2021.11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer S, Mayer AI, Borgonovo G, et al. Chemoinformatics view on bitter taste receptor agonists in food. J Agric Food Chem. 2021 doi: 10.1021/acs.jafc.1c05057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsson G, Arnfinnsson J, Karlsson SM, et al. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1998 doi: 10.1128/aac.42.9.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Li Y, Lopez P, Bapteste E (2021) Large-scale identification of viral quorum sensing systems reveal convergent evolution of density-dependent sporulation-hijacking in bacteriophages. bioRxiv 33
- Berry E, Hernandez-Anzaldo S, Ghomashchi F, et al. Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. J Am Heart Assoc. 2015 doi: 10.1161/JAHA.115.001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat SA, Gambril JA, Azali L, Chen ST, Rosen L, Palettas M, Wiczer T, Kalathoor S, Zhao Q, Rogers KA, Kittai AS, Grever MR, Awan FT, Ruz P, Byrd JC, Woyach JAAD. Ventricular arrhythmias and sudden death events following acalabrutinib initiation. Blood. 2022 doi: 10.1182/blood.2022016953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxham CJ, Foster SR, Thomas WG. A bitter taste in your heart. Front Physiol. 2020;11:431. doi: 10.3389/fphys.2020.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böger RH. Asymmetric dimethylarginine (ADMA) and cardiovascular disease: Insights from prospective clinical trials. Vasc Med. 2005 doi: 10.1191/1358863x05vm602oa. [DOI] [PubMed] [Google Scholar]
- Bonetti J, Corti A, Lerouge L, et al. Phenotypic modulation of macrophages and vascular smooth muscle cells in atherosclerosis-nitro-redox interconnections. Antioxidants. 2021 doi: 10.3390/antiox10040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brearley BF, Hutchinson DN. Endocarditis associated with Chlamydia trachomatis infection. Br Heart J. 1981 doi: 10.1136/hrt.46.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M, Lloyd P Aiello, Jennifer K Sun, Mark E Cooper, Eva L Feldman JP, AJMB (2020) Complications of Diabetes Mellitus. In: Melmed S (ed) Williams Textbook of Endocrinology, 37th edn. Elsevier, pp 1438–1524.e23
- Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- Bulau P, Zakrzewicz D, Kitowska K, et al. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am J Physiol Lung Cell Mol Physiol. 2007 doi: 10.1152/ajplung.00076.2006. [DOI] [PubMed] [Google Scholar]
- Burke PT, Shah R, Thabolingam R, Saba S. Suspected legionella-induced perimyocarditis in an adult in the absence of pneumonia: A rare clinical entity. Texas Hear Inst J. 2009;36:601. [PMC free article] [PubMed] [Google Scholar]
- Burris SK, Wang Q, Bulley S, et al. 9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels. Br J Pharmacol. 2015 doi: 10.1111/bph.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt R, Graves BM, Gao M, et al. 9-Phenanthrol and flufenamic acid inhibit calcium oscillations in HL-1 mouse cardiomyocytes. Cell Calcium. 2013 doi: 10.1016/j.ceca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecerska-Heryć E, Surowska O, Heryć R, et al. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients – A review. Clin Biochem. 2021;93:1–8. doi: 10.1016/j.clinbiochem.2021.03.008. [DOI] [PubMed] [Google Scholar]
- Cerisano G, Buonamici P, Valenti R, et al. Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: The TIPTOP trial. Eur Heart J. 2014 doi: 10.1093/eurheartj/eht420. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, et al. T2Rs function as bitter taste receptors. Cell. 2000 doi: 10.1016/S0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chehab O, McGuire E, Wani RLS, et al. Acute myopericarditis caused by Salmonella enterica serovar Enteritidis: A case report. Eur Hear J - Case Reports. 2020 doi: 10.1093/ehjcr/ytaa173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SP, Qin T, Seidel JL, et al. Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain. 2017 doi: 10.1093/brain/awx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Deng H, Cui H, et al (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9:7204-7218 10.18632/oncotarget.23208 [DOI] [PMC free article] [PubMed]
- Chen M, Samuel VP, Wu Y, Dang M, Lin Y, Sriramaneni R, Sah SK, Chinnaboina GKZG. Nrf2/HO-1 mediated protective activity of genistein against doxorubicin-induced cardiac toxicity. J Env Pathol Toxicol Oncol. 2019;38:143–152. doi: 10.1615/JEnvironPatholToxicolOncol.2019029341. [DOI] [PubMed] [Google Scholar]
- Chen R, Chen W, Huang X, RUI Q, Tanshinone IIA attenuates heart failure via inhibiting oxidative stress in myocardial infarction rats. Mol Med Rep. 2021 doi: 10.3892/mmr.2021.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Li K, Yan Q, et al. Nonsteroidal anti-inflammatory drug flufenamic acid is a potent activator of AMP-activated protein kinase. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.183020. [DOI] [PubMed] [Google Scholar]
- Choy KW, Murugan D, Leong XF, et al. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front Pharmacol. 2019 doi: 10.3389/fphar.2019.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciążyńska M, Bednarski IA, Wódz K, et al. NLRP1 and NLRP3 inflammasomes as a new approach to skin carcinogenesis (Review) Oncol Lett. 2020;19:1649–1656. doi: 10.3892/ol.2020.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AA, Dotson CD, Elson AET, et al. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015 doi: 10.1096/fj.14-262246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JL, Bourn S, Skoufalos A, et al. An innovative approach to health care delivery for patients with chronic conditions. Popul Health Manag. 2017 doi: 10.1089/pop.2016.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Pons VG, Schwartz J, Griffin JC. Candida Albicans pacemaker site infection. Pacing Clin Electrophysiol. 1991 doi: 10.1111/j.1540-8159.1991.tb05081.x. [DOI] [PubMed] [Google Scholar]
- Collins PE, Mitxitorena I, Carmody RJ. The ubiquitination of NF-κB subunits in the control of transcription. Cells. 2016;5:23. doi: 10.3390/cells5020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquant G, Aguanno D, Brot L, Belloir C, Delugeard J, Roger N, Pham HP, Briand L, Moreau M, de Sordi L, Carrière V, Grill JP, Thenet SSP. 3-oxo-C12:2-HSL, quorum sensing molecule from human intestinal microbiota, inhibits pro-inflammatory pathways in immune cells via bitter taste receptors. Sci Rep. 2022;12:9440. doi: 10.1038/s41598-022-13451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Wu H, Li Q, et al. Impairment of bitter taste sensor transient receptor potential channel M5-mediated aversion aggravates high-salt intake and hypertension. Hypertension. 2019 doi: 10.1161/HYPERTENSIONAHA.119.13358. [DOI] [PubMed] [Google Scholar]
- D’Urso O, Drago F. Pharmacological significance of extra-oral taste receptors. Eur J Pharmacol. 2021;910:174480. doi: 10.1016/j.ejphar.2021.174480. [DOI] [PubMed] [Google Scholar]
- Dai C, Li Q, May HI, et al. Lactate dehydrogenase a governs cardiac hypertrophic growth in response to hemodynamic stress. Cell Rep. 2020 doi: 10.1016/j.celrep.2020.108087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Liu J, Zhang Q, et al. Cathepsin C is involved in macrophage M1 polarization via p38/MAPK pathway in sudden cardiac death. Cardiovasc Ther. 2021 doi: 10.1155/2021/6139732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodvandi A, Sahebnasagh R, Mardanshah O, et al. Medicinal plants as natural polarizers of macrophages: phytochemicals and pharmacological effects. Curr Pharm Des. 2019 doi: 10.2174/1381612825666190829154934. [DOI] [PubMed] [Google Scholar]
- de Rossi BP, García C, Alcaraz E, Franco M. Stenotrophomonas maltophilia interferes via the DSF-mediated quorum sensing system with Candida albicans filamentation and its planktonic and biofilm modes of growth. Rev Argent Microbiol. 2014 doi: 10.1016/s0325-7541(14)70084-7. [DOI] [PubMed] [Google Scholar]
- de Zoete MR, Palm NW, Zhu SFR. Inflammasomes. Cold Spring Harb Perspect Biol. 2014 doi: 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep A, Chaudhary U, Gupta V. Quorum sensing and bacterial pathogenicity: from molecules to disease. J Lab Physicians. 2011 doi: 10.4103/0974-2727.78553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmar M, Liang FX. Connexin43 and the regulation of intercalated disc function. Hear Rhythm. 2012 doi: 10.1016/j.hrthm.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Pozo J, Girling S, Pizzi R, et al. Severe Necrotizing Myocarditis caused by Serratia marcescens Infection in an Axolotl (Ambystoma mexicanum) J Comp Pathol. 2011 doi: 10.1016/j.jcpa.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Demion M, Bois P, Launay P, Guinamard R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res. 2007 doi: 10.1016/j.cardiores.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Devillier P, Naline E, Grassin-Delyle S. The pharmacology of bitter taste receptors and their role in human airways. Pharmacol Ther. 2015;155:11–21. doi: 10.1016/j.pharmthera.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Di Pizio A, Niv MY. Promiscuity and selectivity of bitter molecules and their receptors. Bioorgan Med Chem. 2015 doi: 10.1016/j.bmc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Dikalova A, Mayorov V, Xiao L, et al. Mitochondrial isolevuglandins contribute to vascular oxidative stress and mitochondria-targeted scavenger of isolevuglandins reduces mitochondrial dysfunction and hypertension. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dludla PV, Nkambule BB, Jack B, et al. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients. 2019;11:23. doi: 10.3390/nu11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries E, Bardi I, Nunez-Toldra R, et al. Camkii inhibition reduces arrhythmogenic ca2+ events in subendocardial cryoinjured rat living myocardial slices. J Gen Physiol. 2021 doi: 10.1085/jgp.202012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte AC, Rosado T, Costa AR, et al. The bitter taste receptor TAS2R14 regulates resveratrol transport across the human blood-cerebrospinal fluid barrier. Biochem Pharmacol. 2020 doi: 10.1016/j.bcp.2020.113953. [DOI] [PubMed] [Google Scholar]
- Dupont E, Matsushita T, Kaba RA, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001 doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Louis Martin MN. Expression and functionality of bitter taste receptors in ovarian and prostate cancer. FASEB J. 2017 doi: 10.1096/fasebj.31.1_supplement.992.2. [DOI] [Google Scholar]
- Eckardt D, Theis M, Degen J, et al. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol. 2004 doi: 10.1016/j.yjmcc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Jennings MP, MAA& KLS, Is gonococcal disease preventable? The importance of understanding immunity andpathogenesis in vaccine development. Crit Rev Microbiol. 2016;42:928–941. doi: 10.3109/1040841X.2015.1105782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons-Bell S, Johnson CRG. Prevalence, incidence and survival of heart failure: a systematic review. Heart. 2022 doi: 10.1136/heartjnl-2021-320131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XZ, Zhu HJ, Wu X, et al. Effects of doxycycline on cx43 distribution and cardiac arrhythmia susceptibility of rats after myocardial infarction. Iran J Pharm Res. 2014 doi: 10.1136/heartjnl-2013-303992.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Guerrero ML, Aguado JM, Arribas A, et al. The spectrum of cardiovascular infections due to Salmonella enterica: a review of clinical features and factors determining outcome. Medicine (baltimore) 2004;83:123. doi: 10.1097/01.md.0000125652.75260.cf. [DOI] [PubMed] [Google Scholar]
- Fernández Guerrero ML, Goyenechea A, Verdejo C, et al. Enterococcal endocarditis on native and prosthetic valves: A review of clinical and prognostic factors with emphasis on hospital-acquired infections as a major determinant of outcome. Medicine (baltimore) 2007;86:363. doi: 10.1097/MD.0b013e31815d5386. [DOI] [PubMed] [Google Scholar]
- Ferrand J, Hadou T, Selton-Suty C, et al. Cardiac device-related endocarditis caused by Paenibacillus glucanolyticus. J Clin Microbiol. 2013 doi: 10.1128/JCM.00864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JN, Kinghorn AD, Slack JP, et al. In vitro evaluation of flavonoids from Eriodictyon californicum for antagonist activity against the bitterness receptor hTAS2R31. J Agric Food Chem. 2011 doi: 10.1021/jf204359q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SR, Porrello ER, Purdue B, et al. Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS ONE. 2013 doi: 10.1371/journal.pone.0064579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SR, Blank K, See Hoe LE, et al. Bitter taste receptor agonists elicit G-protein-dependent negative inotropy in the murine heart. FASEB J. 2014 doi: 10.1096/fj.14-256305. [DOI] [PubMed] [Google Scholar]
- Foster SR, Porrello ER, Stefani M, et al. Cardiac gene expression data and in silico analysis provide novel insights into human and mouse taste receptor gene regulation. Naunyn Schmiedebergs Arch Pharmacol. 2015 doi: 10.1007/s00210-015-1118-1. [DOI] [PubMed] [Google Scholar]
- Freeman TL, Swartz TH. Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol. 2020;11:1518. doi: 10.3389/fimmu.2020.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund JR, Mansfield CJ, Doghramji LJ, et al. Activation of airway epithelial bitter taste receptors by pseudomonas aeruginosa quinolones modulates calcium, cyclic-amp, and nitric oxide signaling. J Biol Chem. 2018 doi: 10.1074/jbc.RA117.001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019 doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaida MM, Mayer C, Dapunt U, et al (2016) Expression of the bitter receptor T2R38 in pancreatic cancer: Localization in lipid droplets and activation by a bacteria-derived quorum-sensing molecule. Oncotarget. 10.18632/oncotarget.7206 [DOI] [PMC free article] [PubMed]
- Garcia RA, Go KV, Villarreal FJ. Effects of timed administration of doxycycline or methylprednisolone on post-myocardial infarction inflammation and left ventricular remodeling in the rat heart. Mol Cell Biochem. 2007 doi: 10.1007/s11010-006-9379-0. [DOI] [PubMed] [Google Scholar]
- Gheorghe A, Griffiths U, Murphy A, et al. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: A systematic review. BMC Public Health. 2018 doi: 10.1186/s12889-018-5806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianluigi Savarese LHL (2017) Global public health burden of heart failure. Card Fail Rev 3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed]
- Givvimani S, Kundu S, Narayanan N, et al. TIMP-2 mutant decreases MMP-2 activity and augments pressure overload induced LV dysfunction and heart failure. Arch Physiol Biochem. 2013 doi: 10.3109/13813455.2012.755548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels SPJ. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Gopallawa I, Freund JR, Lee RJ. Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling. Cell Mol Life Sci. 2021 doi: 10.1007/s00018-020-03494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Governini L, Semplici B, Pavone V, et al. Expression of taste receptor 2 subtypes in human testis and sperm. J Clin Med. 2020 doi: 10.3390/jcm9010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru TC, Petran M, Dragos DGM. PlantMolecularTasteDB: a database of taste active phytochemicals. Front Pharmacol. 2022;12:751712. doi: 10.3389/fphar.2021.751712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand T, Demion M, Norez C, et al. 9-Phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol. 2008 doi: 10.1038/bjp.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassin-Delyle S, Salvator H, Mantov N, et al. Bitter taste receptors (TAS2Rs) in human lung macrophages: receptor expression and inhibitory effects of TAS2R agonists. Front Physiol. 2019 doi: 10.3389/fphys.2019.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CBB, Suetomi T, Xiang S, et al. CaMKIIδ subtypes differentially regulate infarct formation following ex vivo myocardial ischemia/reperfusion through NF-κB and TNF-α. J Mol Cell Cardiol. 2017 doi: 10.1016/j.yjmcc.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene TA, Alarcon S, Thomas A, et al. Probenecid inhibits the human bitter taste receptor TAS2R16 and suppresses bitter perception of salicin. PLoS ONE. 2011 doi: 10.1371/journal.pone.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grekov I, Pombinho AR, Kobets T, et al. Calcium ionophore, calcimycin, kills Leishmania promastigotes by activating parasite nitric oxide synthase. Biomed Res Int. 2017 doi: 10.1155/2017/1309485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero Ortiz M, Manrique Legaz A, Díaz Izquierdo L (2003) Pancarditis por salmonella entérica. Diagnóstico de localización mediante exploración con 67galio. Rev Esp Med Nucl 10.1157/13044680 [DOI] [PubMed]
- Guerrero MLF, López JJG, Goyenechea A, et al. Endocarditis caused by staphylococcus aureus a reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine (baltimore) 2009;88:1–22. doi: 10.1097/MD.0b013e318194da65. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol. 2008 doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Guo Q. Syphilis-associated septic cardiomyopathy: case report and review of the literature. BMC Infect Dis. 2021 doi: 10.1186/s12879-020-05722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler N, Osthoff M, Rueter F, et al. Prosthetic valve endocarditis caused by Pseudomonas aeruginosa with variable antibacterial resistance profiles: a diagnostic challenge. BMC Infect Dis. 2019 doi: 10.1186/s12879-019-4164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Vaidya D, et al. Heterogeneous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001 doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- Hamdard E, Lv Z, Jiang J, et al. Responsiveness expressions of bitter taste receptors against denatonium benzoate and genistein in the heart, spleen, lung, kidney, and bursa fabricius of chinese fast yellow chicken. Animals. 2019 doi: 10.3390/ani9080532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdard E, Shi Z, Lv Z, et al. Denatonium benzoate-induces oxidative stress in the heart and kidney of chinese fast yellow chickens by regulating apoptosis, autophagy, antioxidative activities and bitter taste receptor gene expressions. Animals. 2019 doi: 10.3390/ani9090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri BM, McMahon DB, Chen B, et al. Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J Biol Chem. 2017 doi: 10.1074/jbc.M116.771949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawver LA, Jung SA, Ng WL. Specificity and complexity in bacterial quorum-sensing systemsa. FEMS Microbiol Rev. 2016;40:738–752. doi: 10.1093/femsre/fuw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Marneros AG. Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization. J Biol Chem. 2014 doi: 10.1074/jbc.M113.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Jhong JH, Chen Q, Huang KY, Strittmatter K, Kreuzer J, DeRan M, Wu X, Lee TY, Slavov N, Haas WMA. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021;37:109955. doi: 10.1016/j.celrep.2021.109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Thaiss CA, Flavell RA. The intestinal microbiota in chronic liver disease. Adv Immunol. 2013;117:73–97. doi: 10.1016/B978-0-12-410524-9.00003-7. [DOI] [PubMed] [Google Scholar]
- Hennig P, Garstkiewicz M, Grossi S, et al. The crosstalk between Nrf2 and inflammasomes. Int J Mol Sci. 2018;19:562. doi: 10.3390/ijms19020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata S, Hongo I, Nakayama S, et al. Infective endocarditis due to treponema pallidum: a case diagnosed using polymerase chain reaction analysis of aortic valve. Can J Cardiol. 2019 doi: 10.1016/j.cjca.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Hirschhäuser C, Lissoni A, Görge PM, et al. Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischemic preconditioning. Basic Res Cardiol. 2021 doi: 10.1007/s00395-021-00861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Guo J, Xu F, et al. Cardiomyocyte dimethylarginine dimethylaminohydrolase1 attenuates left-ventricular remodeling after acute myocardial infarction: involvement in oxidative stress and apoptosis. Basic Res Cardiol. 2018 doi: 10.1007/s00395-018-0685-y. [DOI] [PubMed] [Google Scholar]
- Hou L, Yang J, Li S, et al. Glibenclamide attenuates 2,5-hexanedione-induced neurotoxicity in the spinal cord of rats through mitigation of NLRP3 inflammasome activation, neuroinflammation and oxidative stress. Toxicol Lett. 2020 doi: 10.1016/j.toxlet.2020.06.002. [DOI] [PubMed] [Google Scholar]
- Hu C, Zhang X, Wei W, et al. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B. 2019 doi: 10.1016/j.apsb.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Zhang X, Zhang N, et al. Osteocrin attenuates inflammation, oxidative stress, apoptosis, and cardiac dysfunction in doxorubicin-induced cardiotoxicity. Clin Transl Med. 2020 doi: 10.1002/ctm2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZZ, Yu M, Tong S, et al. Tissue-specific expression of the NOD-like receptor protein 3 in BALB/c mice. J Vet Sci. 2014 doi: 10.4142/jvs.2014.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou P, Vougiouklakis G, Baliou S, et al. Infective endocarditis by yersinia species: A systematic review. Trop Med Infect Dis. 2021;6:19. doi: 10.3390/tropicalmed6010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggupilli A, Howard R, Upadhyaya JD, et al. Bitter taste receptors: Novel insights into the biochemistry and pharmacology. Int J Biochem Cell Biol. 2016 doi: 10.1016/j.biocel.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Jeandroz S, Lamotte O, Astier J, Rasul S, Trapet P, Besson-Bard A, Bourque S, Nicolas-Francès V, Ma W, Gerald A, Berkowitz DW. Focus issue on calcium signaling there’s more to the picture than meets the eye: nitric oxide cross talk with Ca2+ signaling. Plant Physiol. 2013;163:459–470. doi: 10.1104/pp.113.220624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon TI, Seo YK, Osborne TF. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J. 2011 doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Li XK (2016) Oxidative stress in atopic dermatitis. Oxid Med Cell Longev [DOI] [PMC free article] [PubMed]
- Jia SJ, Zhou Z, Zhang BK, et al. Asymmetric dimethylarginine damages connexin43-mediated endothelial gap junction intercellular communication. Biochem Cell Biol. 2009 doi: 10.1139/O09-042. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hoagland D, Palatinus JA, et al. Interaction of α carboxyl terminus 1 peptide with the connexin 43 carboxyl terminus preserves left ventricular function after ischemia-reperfusion injury. J Am Heart Assoc. 2019 doi: 10.1161/JAHA.119.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Xing J, Fang J, et al. Tilianin protects against ischemia/reperfusion-induced myocardial injury through the inhibition of the Ca2+/calmodulin-dependent protein kinase II-dependent apoptotic and inflammatory signaling pathways. Biomed Res Int. 2020 doi: 10.1155/2020/5939715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E, Fei H, Nakatomi C. Nf-κb signaling regulates physiological and pathological chondrogenesis. Int J Mol Sci. 2019;20:6275. doi: 10.3390/ijms20246275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jui E, Singampalli KL, Shani K, et al. The immune and inflammatory basis of acquired pediatric cardiac disease. Front Cardiovasc Med. 2021 doi: 10.3389/fcvm.2021.701224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamila T, Agnieszka K. An update on extra-oral bitter taste receptors. J Transl Med. 2021;19:1–33. doi: 10.1186/s12967-021-03067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy AD, Chow AK, Ali MAM, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: Beyond the matrix. Cardiovasc Res. 2010;85:413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2021 doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TM, Donovan K. Cardiac rehabilitation in the time of health-care reform. AACN Clin Issues. 1995 doi: 10.1097/00044067-199508000-00008. [DOI] [PubMed] [Google Scholar]
- Khan MA, Hashim MJ, Mustafa H, et al. Global epidemiology of ischemic heart disease: results from the global burden of disease study. Cureus. 2020 doi: 10.7759/cureus.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J, Deb PK, Priya S, et al. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules. 2021;26:4021. doi: 10.3390/molecules26134021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim NJ, Kim SY, et al. Cyclo(Phe-Pro) produced by the human pathogen Vibrio vulnificus inhibits host innate immune responses through the NF-κB pathway. Infect Immun. 2015 doi: 10.1128/IAI.02878-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Kim SY, Park NY, et al. Cyclo-(L-Phe-L-Pro), a quorum-sensing signal of Vibrio vulnificus, induces expression of hydroperoxidase through a ToxR-LeuO-HU-RpoS signaling pathway to confer resistance against oxidative stress. Infect Immun. 2018 doi: 10.1128/IAI.00932-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon SC, Finger TE (2019) Recent advances in taste transduction and signaling. F1000Research 8:2117 10.12688/f1000research.21099.1 [DOI] [PMC free article] [PubMed]
- Kishore A, Petrek M. Roles of macrophage polarization and macrophage-derived mirnas in pulmonary fibrosis. Front Immunol. 2021;12:678457. doi: 10.3389/fimmu.2021.678457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J. 2007;28:1797–1804. doi: 10.1093/eurheartj/ehm193. [DOI] [PubMed] [Google Scholar]
- Kobayashi J. Lifestyle-mediated nitric oxide boost to prevent SARS-CoV-2 infection: A perspective. Nitric Oxide Biol Chem. 2021;115:55–61. doi: 10.1016/j.niox.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobusiak-Prokopowicz M, Krzysztofik J, Kaaz K, et al. MMP-2 and TIMP-2 in patients with heart failure and chronic kidney disease. Open Med. 2018 doi: 10.1515/med-2018-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc G, Soyocak A, Duzgun Ergun D, et al. Association of TRPM5 Asn235Ser polymorphism and trace elements/minerals in chronic gastritis patients: a case-control study. Biol Trace Elem Res. 2022 doi: 10.1007/s12011-021-03002-8. [DOI] [PubMed] [Google Scholar]
- Kokabu S, Lowery JW, Toyono T, et al. Muscle regulatory factors regulate T1R3 taste receptor expression. Biochem Biophys Res Commun. 2015 doi: 10.1016/j.bbrc.2015.10.142. [DOI] [PubMed] [Google Scholar]
- Kong F, Ye B, Cao J, et al. Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB and P2X7R signaling in PMA-induced macrophages. Front Pharmacol. 2016 doi: 10.3389/fphar.2016.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopel E, Kivity S, Morag-Koren N, et al. Relation of serum lactate dehydrogenase to coronary artery disease. Am J Cardiol. 2012 doi: 10.1016/j.amjcard.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia JL, Rodríguez-Perez AI, Garrido-Gil P, et al. Brain renin-angiotensin system and microglial polarization: Implications for aging and neurodegeneration. Front Aging Neurosci. 2017;9:129. doi: 10.3389/fnagi.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond NM, McMullen PD, Paramasvaran D, et al. Cardiotropic isolates of listeria monocytogenes with enhanced vertical transmission dependent upon the bacterial surface protein InlB. Infect Immun. 2021 doi: 10.1128/IAI.00321-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, TenBroek EM, Burt JM, et al. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000 doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampiasi N, Montana G. An in vitro inflammation model to study the Nrf2 and NF-κB crosstalk in presence of ferulic acid as modulator. Immunobiology. 2018 doi: 10.1016/j.imbio.2017.10.046. [DOI] [PubMed] [Google Scholar]
- Lars W, Thora S, Mats B. Role of inflammation in nonrheumatic, regurgitant heart valve disease. A comparative, descriptive study regarding apolipoproteins and inflammatory cells in nonrheumatic heart valve disease. Cardiovasc Pathol. 2007 doi: 10.1016/j.carpath.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lee E-J, Woo M-S, Moon P-G, et al. α-Synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010 doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- Lee GS, Subramanian N, Kim AI, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Cohen NA. The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am J Rhinol Allergy. 2013 doi: 10.2500/ajra.2013.27.3911. [DOI] [PubMed] [Google Scholar]
- Lee SH, Choi JH. Involvement of inflammatory responses in the early development of calcific aortic valve disease: lessons from statin therapy. Animal Cells Syst. 2018;22:390–399. doi: 10.1080/19768354.2018.1528175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Lee SH, Kim M, et al. Vibrio vulnificus quorum-sensing molecule cyclo(Phe-Pro) inhibits RIG-I-mediated antiviral innate immunity. Nat Commun. 2018 doi: 10.1038/s41467-018-04075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler KE, Abdel-Rahman AA. Estrogen-dependent disruption of adiponectin-connexin43 signaling underlies exacerbated myocardial dysfunction in diabetic female rats. J Pharmacol Exp Ther. 2019 doi: 10.1124/jpet.118.254029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler KE, Abdel-Rahman AA. Restoration of adiponectin-connexin43 signaling mitigates myocardial inflammation and dysfunction in diabetic female rats. J Cardiovasc Pharmacol. 2020 doi: 10.1097/FJC.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Cortés JL, Leal Fernández G, Sánchez-Pérez HJ. Health reform in Mexico: Governance and potential outcomes. Int J Equity Health. 2019;18:1–6. doi: 10.1186/s12939-019-0929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol. 2007;580:31–37. doi: 10.1113/jphysiol.2006.126193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley JP, Blings M, Paetz S, et al. New bitter-masking compounds: Hydroxylated benzoic acid amides of aromatic amines as structural analogues of homoeriodictyol. J Agric Food Chem. 2006 doi: 10.1021/jf0617061. [DOI] [PubMed] [Google Scholar]
- Ley JP, Paetz S, Blings M, et al. Structural analogues of homoeriodictyol as flavor modifiers. Part III: Short chain gingerdione derivatives. J Agric Food Chem. 2008 doi: 10.1021/jf8006536. [DOI] [PubMed] [Google Scholar]
- Ley JP, Dessoy M, Paetz S, et al. Identification of enterodiol as a masker for caffeine bitterness by using a pharmacophore model based on structural analogues of homoeriodictyol. J Agric Food Chem. 2012 doi: 10.1021/jf301335z. [DOI] [PubMed] [Google Scholar]
- Li J, Attila C, Wang L, et al. Quorum sensing in Escherichia coli is signaled by AI-2/LsrR: effects on small RNA and biofilm architecture. J Bacteriol. 2007 doi: 10.1128/JB.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang Y, Sun SZ, et al. Effects of benazepril on cardiac fibrosis in STZ-induced diabetic rats. Acta Cardiol. 2010 doi: 10.2143/AC.65.4.2053902. [DOI] [PubMed] [Google Scholar]
- Li Y, Ge S, Peng Y, Chen X. Inflammation and cardiac dysfunction during sepsis, muscular dystrophy, and myocarditis. Burn Trauma. 2013;1(3):2321–3868. doi: 10.4103/2321-3868.123072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Feng X, Zhou Z, et al. Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr Biol. 2018 doi: 10.1016/j.cub.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Li R, Zhenquan Jia HZ. Regulation of Nrf2 Signaling. React Oxyg Species. 2019;8:312–322. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yang L, Dong L, et al. Crosstalk between the Akt/mTORC1 and NF-κB signaling pathways promotes hypoxia-induced pulmonary hypertension by increasing DPP4 expression in PASMCs. Acta Pharmacol Sin. 2019 doi: 10.1038/s41401-019-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci USA. 2001 doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sylvén C, Åström H, et al. Myocardial lactate dehydrogenase and its isoenzyme activities in transplanted human hearts. Scand Cardiovasc J. 1991 doi: 10.3109/14017439109098083. [DOI] [PubMed] [Google Scholar]
- Ling H, Gray CBB, Zambon AC, et al. Ca2+/calmodulin-dependent protein kinase ii δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κb. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.112.276915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszt KI, Wang Q, Farhadipour M, Segers A, Thijs T, Nys L, Deleus E, Van der Schueren B, Gerner C, Neuditschko B, Ceulemans LJ, Lannoo M, DI Tack J. Human intestinal bitter taste receptors regulate innate immune responses and metabolic regulators in obesity. J Clin Invest. 2022;132:e144828. doi: 10.1172/JCI144828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lian K, Zhang L, et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014 doi: 10.1007/s00395-014-0415-z. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhang X, Zhao M, et al. Activation in M1 but not M2 macrophages contributes to cardiac remodeling after myocardial infarction in rats: A critical role of the calcium sensing receptor/NRLP3 inflammasome. Cell Physiol Biochem. 2015 doi: 10.1159/000374048. [DOI] [PubMed] [Google Scholar]
- Liu X, Gu F, Jiang L, et al. Expression of bitter taste receptor Tas2r105 in mouse kidney. Biochem Biophys Res Commun. 2015 doi: 10.1016/j.bbrc.2015.01.089. [DOI] [PubMed] [Google Scholar]
- Liu J, Zong Z, Zhang W, et al. Nicotinamide mononucleotide alleviates LPS-induced inflammation and oxidative stress via decreasing COX-2 expression in macrophages. Front Mol Biosci. 2021 doi: 10.3389/fmolb.2021.702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Sun R, Liu M, Zheng YZP. The inflammatory heart diseases: causes, symptoms, and treatments. Cell Biochem Biophys. 2015;72:851–855. doi: 10.1007/s12013-015-0550-7. [DOI] [PubMed] [Google Scholar]
- Lu H, Zhang Z, Barnie PA, Su Z. Dual faced HMGB1 plays multiple roles in cardiomyocyte senescence and cardiac inflammatory injury. Cytokine Growth Factor Rev. 2019;47:74–82. doi: 10.1016/j.cytogfr.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Lu H, Chen R, Barnie PA, et al. Fibroblast transdifferentiation promotes conversion of M1 macrophages and replenishment of cardiac resident macrophages following cardiac injury in mice. Eur J Immunol. 2020 doi: 10.1002/eji.201948414. [DOI] [PubMed] [Google Scholar]
- Lund TC, Kobs AJ, Kramer A, et al. Bone marrow stromal and vascular smooth muscle cells have chemosensory capacity via bitter taste receptor expression. PLoS ONE. 2013 doi: 10.1371/journal.pone.0058945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccio U, Zinkernagel AS, Shambat SM, et al. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2020.103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson ML, Säfholm J, Al-Ameri M, et al. Bitter taste receptor agonists mediate relaxation of human and rodent vascular smooth muscle. Eur J Pharmacol. 2014 doi: 10.1016/j.ejphar.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Hewton K, Monack DM, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004 doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Martens CR, Accornero F. Viruses in the heart: Direct and indirect routes to myocarditis and heart failure. Viruses. 2021;13:1924. doi: 10.3390/v13101924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TP, McCluskey C, Cunningham MR, et al. CaMKIIδ interacts directly with IKKβ and modulates NF-κB signalling in adult cardiac fibroblasts. Cell Signal. 2018 doi: 10.1016/j.cellsig.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Martin LTP, Nachtigal MW, Selman T, et al. Bitter taste receptors are expressed in human epithelial ovarian and prostate cancers cells and noscapine stimulation impacts cell survival. Mol Cell Biochem. 2019 doi: 10.1007/s11010-018-3464-z. [DOI] [PubMed] [Google Scholar]
- Maurer S, Wabnitz GH, Kahle NA, et al. Tasting Pseudomonas aeruginosa biofilms: Human neutrophils express the bitter receptor T2R38 as sensor for the quorum sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. Front Immunol. 2015 doi: 10.3389/fimmu.2015.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorov V, Uchakin P, Amarnath V, et al. Targeting of reactive isolevuglandins in mitochondrial dysfunction and inflammation. Redox Biol. 2019 doi: 10.1016/j.redox.2019.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Zhang LS, Yermalitsky V, Huang J, et al. Modification by isolevuglandins, highly reactive γ-ketoaldehydes, deleteriously alters high-density lipoprotein structure and function. J Biol Chem. 2018 doi: 10.1074/jbc.RA117.001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee M, Shiel E, Brienesse S, et al. Staphylococcus aureus Myocarditis with Associated Left Ventricular Apical Thrombus. Case Reports Cardiol. 2018 doi: 10.1155/2018/7017286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh T, Tennyson C. Understanding and recognizing cardiac amyloidosis. JAAPA. 2020;33:16–20. doi: 10.1097/01.JAA.0000697236.11386.3a. [DOI] [PubMed] [Google Scholar]
- Medapati MR, Bhagirath AY, Singh N, Chelikani P. Pharmacology of T2R mediated host-microbe interactions. Handbook Experim Pharmacol. 2022 doi: 10.1007/164_2021_435. [DOI] [PubMed] [Google Scholar]
- Mehta NJ, Khan IA, Mehta RN, Gulati A. Stenotrophomonas maltophilia endocarditis of prosthetic aortic valve: Report of a case and review of literature. Hear Lung J Acute Crit Care. 2000 doi: 10.1067/mhl.2000.108362. [DOI] [PubMed] [Google Scholar]
- Meraviglia V, Alcalde MCO, BM, Inflammation in the pathogenesis of arrhythmogenic cardiomyopathy: secondary event or active driver? Front Cardiovasc Med. 2021;8:784715. doi: 10.3389/fcvm.2021.784715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita T, Lin YN, Ibrahim A. Chronic low-grade inflammation in heart failure with preserved ejection fraction. Aging Cell. 2021 doi: 10.1111/acel.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, et al (2010) The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors Present address : Department of Physiology Building 58, University of Saarland, Medical Chem Senses [DOI] [PubMed]
- Mezzaroma E, Toldo S, Farkas D, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanesi S, Verzola D, Cappadona F, et al. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J Cell Physiol. 2019 doi: 10.1002/jcp.27929. [DOI] [PubMed] [Google Scholar]
- Mills CD. M1 and M2 macrophages: Oracles of health and disease. Crit Rev Immunol. 2012 doi: 10.1615/CritRevImmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- Moreira DM, da Silva RL, Vieira JL, et al. Role of vascular inflammation in coronary artery disease: potential of anti-inflammatory drugs in the prevention of atherothrombosis: inflammation and anti-inflammatory drugs in coronary artery disease. Am J Cardiovasc Drugs. 2015 doi: 10.1007/s40256-014-0094-z. [DOI] [PubMed] [Google Scholar]
- Muka T, Imo D, Jaspers L, et al. The global impact of non-communicable diseases on healthcare spending and national income: a systematic review. Eur J Epidemiol. 2015;30:251–277. doi: 10.1007/s10654-014-9984-2. [DOI] [PubMed] [Google Scholar]
- Mullick A, Leon Z, Min-Oo G, et al. Cardiac failure in C5-deficient A/J mice after Candida albicans infection. Infect Immun. 2006 doi: 10.1128/IAI.00159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulya E, Waturangi DE. Screening and quantification of anti-quorum sensing and antibiofilm activity of Actinomycetes isolates against food spoilage biofilm-forming bacteria. BMC Microbiol. 2021 doi: 10.1186/s12866-020-02060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SP, Kakkar R, McCarthy CP, Januzzi JL. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1324–1340. doi: 10.1016/j.jacc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- Musa NL, Hjortdal V, Zheleva B, et al. The global burden of paediatric heart disease. Cardiol Young. 2017;27:S3–S8. doi: 10.1017/S1047951117002530. [DOI] [PubMed] [Google Scholar]
- Neri M, Fineschi V, Paolo M, et al. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. 2015 doi: 10.2174/15701611113119990003. [DOI] [PubMed] [Google Scholar]
- Ngwenyama N, Kirabo A, Aronovitz M, et al. Isolevuglandin-modified cardiac proteins drive CD4+ T-cell activation in the heart and promote cardiac dysfunction. Circulation. 2021 doi: 10.1161/CIRCULATIONAHA.120.051889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam S, Ciccoli R, Ivanov I, et al. On mechanism of quorum sensing in candida albicans by 3(R)-hydroxy-tetradecaenoic acid. Curr Microbiol. 2011 doi: 10.1007/s00284-010-9666-6. [DOI] [PubMed] [Google Scholar]
- Obama B. United States health care reform: Progress to date and next steps. JAMA. 2016;316:525–532. doi: 10.1001/jama.2016.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayemi G, Oferczak M, Elagizi A, El-Abbassi I, Eschete MCJ. Gonococcal endocarditis: The gift that stops giving! An uncommon presentation of a common disease. J La State Med Soc. 2017;169:47. [PubMed] [Google Scholar]
- Ooi BK, Goh BH, Yap WH. Oxidative stress in cardiovascular diseases: Involvement of Nrf2 antioxidant redox signaling in macrophage foam cells formation. Int J Mol Sci. 2017;18:2336. doi: 10.3390/ijms18112336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhov AN, Orekhova VA, Nikiforov NG, et al (2019) Monocyte differentiation and macrophage polarization. Vessel Plus 10.20517/2574-1209.2019.04
- Ouarradi AE, Kantri A, Agrad K, Bensahi I, Merzouk F, Guennoun Z, Makani S, Jebbari Y, Elkettani CSM (2021) Infective endocarditis following COVID-19 pneumonia: about two cases. Pan Afr Med J 40:152. 10.11604/pamj.2021.40.152.32071 [DOI] [PMC free article] [PubMed]
- Ozhathil LC, Delalande C, Bianchi B, et al. Identification of potent and selective small molecule inhibitors of the cation channel TRPM4. Br J Pharmacol. 2018 doi: 10.1111/bph.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatinus JA, Rhett JM, Gourdie RG. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta Biomembr. 2012;1818:1831–1843. doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RK. The pharmacology and signaling of bitter, sweet, and umami taste sensing. Mol Interv. 2007;7:87. doi: 10.1124/mi.7.2.9. [DOI] [PubMed] [Google Scholar]
- Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: An overview. J Nutr Sci 5 [DOI] [PMC free article] [PubMed]
- Panneck AR, Rafiq A, Schütz B, et al. Cholinergic epithelial cell with chemosensory traits in murine thymic medulla. Cell Tissue Res. 2014 doi: 10.1007/s00441-014-2002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaconstantinou J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells. 2019;8:1383. doi: 10.3390/cells8111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa S, Mogharab V, Ebrahimi M, et al. COVID-19 as a worldwide selective event and bitter taste receptor polymorphisms: An ecological correlational study. Int J Biol Macromol. 2021 doi: 10.1016/j.ijbiomac.2021.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashkow FJ. Oxidative stress and inflammation in heart disease: do antioxidants have a role in treatment and/or prevention? Int J Inflam. 2011 doi: 10.4061/2011/514623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos LSA, Nunes MCP, Aikawa E. Rheumatic heart valve disease pathophysiology and underlying mechanisms. Front Cardiovasc Med. 2021 doi: 10.3389/fcvm.2020.612716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce MM, Theodoropoulos N, Noskin GA, et al. Native valve endocarditis due to a novel strain of Legionella. J Clin Microbiol. 2011 doi: 10.1128/JCM.01066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez CA, Huang L, Rong M, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002 doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Pflug KM, Sitcheran R. Targeting NF-κB-inducing kinase (NIK) in immunity, inflammation, and cancer. Int J Mol Sci. 2020 doi: 10.3390/ijms21228470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao H, Takahashi K, Yamaguchi Y, et al. Transient receptor potential melastatin-4 is involved in hypoxia-reoxygenation injury in the cardiomyocytes. PLoS ONE. 2015 doi: 10.1371/journal.pone.0121703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzino G, Irrera N, Cucinotta M, et al (2017) Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev [DOI] [PMC free article] [PubMed]
- Procida K, Jørgensen L, Schmitt N, et al. Phosphorylation of connexin43 on serine 306 regulates electrical coupling. Hear Rhythm. 2009 doi: 10.1016/j.hrthm.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese ME, Falcone M, Oliva A, et al. Aeromonas hydrophila endocarditis with ruptured mycotic aneurysm of right renal artery. Infect Dis Rep. 2016 doi: 10.4081/idr.2016.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Hou DX. The biofunctions of phytochemicals and their applications in farm animals: The Nrf2/Keap1 system as a target. Engineering. 2017 doi: 10.1016/J.ENG.2017.03.011. [DOI] [Google Scholar]
- Quiring R, Burke V. Escherichia coli prosthetic valve endocarditis from a non-genitourinary source. Idcases. 2021 doi: 10.1016/j.idcr.2021.e01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin N, Zheng Y, Opoku-Temeng C, et al. Agents that inhibit bacterial biofilm formation. Future Med Chem. 2015;7:493–512. doi: 10.4155/fmc.15.6. [DOI] [PubMed] [Google Scholar]
- Rajput A, Kaur K, Kumar M. SigMol: Repertoire of quorum sensing signaling molecules in prokaryotes. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkv1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjani J, Pushpanathan M, Mahesh A, et al. Pseudomonas aeruginosa PAO1 induces distinct cell death mechanisms in H9C2 cells and its differentiated form. J Basic Microbiol. 2015 doi: 10.1002/jobm.201500037. [DOI] [PubMed] [Google Scholar]
- Reddy AT, Lakshmi SP, Maruthi Prasad E, et al. Epigallocatechin gallate suppresses inflammation in human coronary artery endothelial cells by inhibiting NF-κB. Life Sci. 2020 doi: 10.1016/j.lfs.2020.118136. [DOI] [PubMed] [Google Scholar]
- Rennemeier C, Frambach T, Hennicke F, et al. Microbial quorum-sensing molecules induce acrosome loss and cell death in human spermatozoa. Infect Immun. 2009 doi: 10.1128/IAI.00586-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel RE, Brenner JI, Rennels MB, et al. Serologic evidence for Chlamydia trachomatis myocarditis. Pediatrics. 1982 doi: 10.1542/peds.70.1.54. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Hernández MJ, Jiménez-Mejias ME, Pichardo C, et al. Colistin efficacy in an experimental model of Acinetobacter baumannii endocarditis. Clin Microbiol Infect. 2004 doi: 10.1111/j.1469-0691.2004.00910.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Rey P, Parga JA, et al. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008 doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Roland WSU, Gouka RJ, Gruppen H, et al. 6-Methoxyflavanones as bitter taste receptor blockers for hTAS2R39. PLoS ONE. 2014 doi: 10.1371/journal.pone.0094451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017 doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and α-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- Ruggeri Barbaro N, Van Beusecum J, Xiao L, et al. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc Res. 2021 doi: 10.1093/cvr/cvaa207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. Carey, Benjamin M. Hariri, Nithin D. Adappa, James N. Palmer RJL (2021) HSP90 modulates T2R bitter taste receptor nitric oxide production and innate immune responses in human airway epithelial cells and macrophages. bioRxiv. 10.1101/2021.11.16.468387 [DOI] [PMC free article] [PubMed]
- Sandanger Ø, Ranheim T, Vinge LE, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013 doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- Sangeetha M, Pillai MS, Philip L, et al. NF-κB inhibition compromises cardiac fibroblast viability under hypoxia. Exp Cell Res. 2011 doi: 10.1016/j.yexcr.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqib U, Sarkar S, Suk K, et al. Phytochemicals as modulators of M1–M2 macrophages in inflammation. Oncotarget. 2018;9:17937–17950. doi: 10.18632/oncotarget.24788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs NJ (2002) Gap junctions and connexin expression in human heart disease. In: Heart cell coupling and impulse propagation in health and disease. Basic Science for the Cardiologist, vol. 12. Springer, Boston, MA, pp 321–334
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Weng X, Wang D, et al. Isolevuglandin scavenger attenuates pressure overload-induced cardiac oxidative stress, cardiac hypertrophy, heart failure and lung remodeling. Free Radic Biol Med. 2019 doi: 10.1016/j.freeradbiomed.2019.06.029. [DOI] [PubMed] [Google Scholar]
- Sharma M, De Alba E. Structure, activation and regulation of NLRP3 and AIM2 inflammasomes. Int J Mol Sci. 2021 doi: 10.3390/ijms22020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, McAlinden KD, Ghavami S, Deshpande DA. Chloroquine: Autophagy inhibitor, antimalarial, bitter taste receptor agonist in fight against COVID-19, a reality check? Eur J Pharmacol. 2021 doi: 10.1016/j.ejphar.2021.173928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenasa M, Shenasa H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int J Cardiol. 2017 doi: 10.1016/j.ijcard.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J, Yang H, Zhang Y, ping, Adaptive diversification of bitter taste receptor genes in mammalian evolution. Mol Biol Evol. 2003 doi: 10.1093/molbev/msg083. [DOI] [PubMed] [Google Scholar]
- Shibata A, Izumiya Y, Yamaguchi Y, et al. Increased oxidative stress during exercise predicts poor prognosis in patients with acute decompensated heart failure. ESC Hear Fail. 2021 doi: 10.1002/ehf2.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YJ, Park JH, Choi JS, et al. Enhanced expression of the sweet taste receptors and alpha-gustducin in reactive astrocytes of the rat hippocampus following ischemic injury. Neurochem Res. 2010 doi: 10.1007/s11064-010-0223-2. [DOI] [PubMed] [Google Scholar]
- Siegismund CS, Escher F, Lassner D, et al. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur J Heart Fail. 2018 doi: 10.1002/ejhf.1039. [DOI] [PubMed] [Google Scholar]
- Silvis MJM, Kaffka genaamd Dengler SE, Odille CA, et al (2020) Damage-associated molecular patterns in myocardial infarction and heart transplantation: The Road to Translational Success. Front Immunol 11:599511 [DOI] [PMC free article] [PubMed]
- Simard C, Sallé L, Rouet R, Guinamard R. Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br J Pharmacol. 2012 doi: 10.1111/j.1476-5381.2011.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S, Schell U, Heuer N, et al. Inter-kingdom signaling by the legionella quorum sensing molecule LAI-1 modulates cell migration through an IQGAP1-Cdc42-ARHGEF9-dependent pathway. PLoS Pathog. 2015 doi: 10.1371/journal.ppat.1005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun. 2011 doi: 10.1016/j.bbrc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Soultanova A, Voigt A, Chubanov V, et al. Cholinergic chemosensory cells of the thymic medulla express the bitter receptor Tas2r131. Int Immunopharmacol. 2015 doi: 10.1016/j.intimp.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Spaulding K, Takaba K, Collins A, et al. Short term doxycycline treatment induces sustained improvement in myocardial infarction border zone contractility. PLoS ONE. 2018 doi: 10.1371/journal.pone.0192720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001 doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnadel M, Hnátek T, Havlíček R, et al. Listerial myocarditis as a complication of Listerial meningoencephalitis. Cor Vasa. 2018 doi: 10.1016/j.crvasa.2017.02.004. [DOI] [Google Scholar]
- Suetomi T, Willeford A, Brand CS, et al. Inflammation and NLRP3 Inflammasome activation initiated in response to pressure overload by Ca2+/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.118.034621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetomi T, Miyamoto S, Brown JH. Inflammation in nonischemic heart disease: Initiation by cardiomyocyte CaMKII and NLRP3 inflammasome signaling. Am J Physiol Hear Circ Physiol. 2019;317:H877–H890. doi: 10.1152/ajpheart.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri RK, Selby DA, Hawks GH, Baker CB. Serratia marcescens endocarditis. A report of a case involving Cross-Jones mitral valve prosthesis, with a review of the literature. Can Med Assoc J. 1971;104:1013. [PMC free article] [PubMed] [Google Scholar]
- Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syam N, Rougier JS, Abriel H. Glycosylation of TRPM4 and TRPM5 channels: Molecular determinants and functional aspects. Front Cell Neurosci. 2014 doi: 10.3389/fncel.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanari H, Bourgonje VJA, Fontes MSC, et al. Calmodulin/CaMKII inhibition improves intercellular communication and impulse propagation in the heart and is antiarrhythmic under conditions when fibrosis is absent. Cardiovasc Res. 2016 doi: 10.1093/cvr/cvw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res. 2016 doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V, Alcoreza N, Cazares J, Chattopadhyay M. Changes in stress-mediated markers in a human cardiomyocyte cell line under hyperglycemia. Int J Mol Sci. 2021 doi: 10.3390/ijms221910802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BS, Bankowski MJ, Lau WKK. Native valve Bacillus cereus endocarditis in a non-intravenous-drug-abusing patient. J Clin Microbiol. 2012 doi: 10.1128/JCM.00657-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu YM, Richmond A. NF-κB inducing kinase: A key regulator in the immune system and in cancer. Cytokine Growth Factor Rev. 2010 doi: 10.1016/j.cytogfr.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiaden A, Hilbi H. α-hydroxyketone synthesis and sensing by Legionella and Vibrio. Sensors. 2012 doi: 10.3390/s120302899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinggi U (2008) Selenium: Its role as antioxidant in human health. In: Environmental Health and Preventive Medicine [DOI] [PMC free article] [PubMed]
- Tiroch J, Sterneder S, Di Pizio A, et al. Bitter sensing TAS2R50 mediates the trans -resveratrol-induced anti-inflammatory effect on interleukin 6 release in HGF-1 cells in culture. J Agric Food Chem. 2021 doi: 10.1021/acs.jafc.0c07058. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in Solitary Chemosensory Cells of rodent airways. BMC Pulm Med. 2011 doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás J, Santos CRA, Quintela T, Gonçalves I. “Tasting” the cerebrospinal fluid: Another function of the choroid plexus? Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.01.057. [DOI] [PubMed] [Google Scholar]
- Truwit JD, Badesch DB, Savage AM, Shelton M. Vibrio vulnificus bacteremia with endocarditis. South Med J. 1987 doi: 10.1097/00007611-198711000-00032. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Liao MH, Shih CC, et al. Angiotensin-(1–7) attenuates organ injury and mortality in rats with polymicrobial sepsis. Crit Care. 2018 doi: 10.1186/s13054-018-2210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsampasian V, Swift AJ, Assadi H, et al. Myocardial inflammation and energetics by cardiac MRI: a review of emerging techniques. BMC Med Imaging. 2021;21:1–9. doi: 10.1186/s12880-021-00695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang H, Leiper J, Lao KH, et al. Role of asymmetric methylarginine and connexin 43 in the regulation of pulmonary endothelial function. Pulm Circ. 2013 doi: 10.1086/674440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;21:1–9. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsikas D. Urinary dimethylamine (DMA) and its precursor asymmetric dimethylarginine (ADMA) in clinical medicine, in the context of nitric oxide (no) and beyond. J Clin Med. 2020;9(6):1843. doi: 10.3390/jcm9061843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara EU, de Shida B, S, de Brito CA, Role of nitric oxide in immune responses against viruses: beyond microbicidal activity. Inflamm Res. 2015 doi: 10.1007/s00011-015-0857-2. [DOI] [PubMed] [Google Scholar]
- van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019 doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegezzi G, Anselmi L, Huynh J, et al. Diet-induced regulation of bitter taste receptor subtypes in the mouse gastrointestinal tract. PLoS ONE. 2014 doi: 10.1371/journal.pone.0107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinot K-LC, JP, Anatomic Basis of Echocardiographic Diagnosis. London: Springer; 2010. Cardiac Involvement by Systemic Diseases; pp. 439–454. [Google Scholar]
- Virginio C, Aldegheri L, Nola S, Brodbeck D, Brault L, Raveglia LF, Barilli A, Sabat MMR. Identification of positive modulators of TRPM5 channel from a high-throughput screen using a fluorescent membrane potential assay. SLAS Discov. 2022;27:55–64. doi: 10.1016/j.slasd.2021.10.004. [DOI] [PubMed] [Google Scholar]
- Vomund S, Schäfer A, Parnham MJ, et al. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017;18:2772. doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lackum K, Babb K, Riley SP, et al. Functionality of Borrelia burgdorferi LuxS: The Lyme disease spirochete produces and responds to the pheromone autoinducer-2 and lacks a complete activated-methyl cycle. Int J Med Microbiol. 2006 doi: 10.1016/j.ijmm.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Wadley AJ, Veldhuijzen Van Zanten JJCS, Aldred S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: The vascular health triad. Age (omaha) 2013 doi: 10.1007/s11357-012-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, He Y, Gao Y, et al. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004 doi: 10.1046/j.1365-2958.2003.03883.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Takahashi K, Piao H, et al. 9-Phenanthrol, a TRPM4 inhibitor, protects isolated rat hearts from ischemia-reperfusion injury. PLoS ONE. 2013 doi: 10.1371/journal.pone.0070587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hu W, Lu C, et al. Targeting NLRP3 (nucleotide-binding domain, leucine- rich-containing family, pyrin domain-containing-3) inflammasome in cardiovascular disorders. Arterioscler Thromb Vasc Biol. 2018;38:2765–2779. doi: 10.1161/ATVBAHA.118.311916. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu X, Shi H, et al. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin Transl Med. 2020 doi: 10.1002/ctm2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub WS, Boden WE. Making cardiovascular care more responsive to societal needs. Am J Med. 2017;130:1259–1261. doi: 10.1016/j.amjmed.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Welcome MO, Mastorakis NE, Pereverzev VA (2015) Sweet taste receptor signaling network: Possible implication for cognitive functioning. Neurol Res Int [DOI] [PMC free article] [PubMed]
- Welcome MO. The bitterness of genitourinary infections: Properties, ligands of genitourinary bitter taste receptors and mechanisms linking taste sensing to inflammatory processes in the genitourinary tract. Eur J Obstet Gynecol Reprod Biol. 2020;247:101–110. doi: 10.1016/j.ejogrb.2020.02.015. [DOI] [PubMed] [Google Scholar]
- Welcome MO. Cellular mechanisms and molecular signaling pathways in stress-induced anxiety, depression, and blood–brain barrier inflammation and leakage. Inflammopharmacology. 2020;28:643–665. doi: 10.1007/s10787-020-00712-8. [DOI] [PubMed] [Google Scholar]
- Welcome MO, Mastorakis NE. Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol Res. 2020 doi: 10.1016/j.phrs.2020.104769. [DOI] [PubMed] [Google Scholar]
- Welcome MO, Mastorakis NE. The taste of neuroinflammation: Molecular mechanisms linking taste sensing to neuroinflammatory responses. Pharmacol Res. 2021;167:105557. doi: 10.1016/j.phrs.2021.105557. [DOI] [PubMed] [Google Scholar]
- Welcome M, Jeremiah A, Allagoa D, et al. Etiopathogenesis of reproductive tract infections and the emerging role of bitter taste receptors: A scoping review. Asian Pacific J Reprod. 2021;10:145. doi: 10.4103/2305-0500.321122. [DOI] [Google Scholar]
- Willeford A, Suetomi T, Nickle A, et al. CaMKIIδ-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight. 2018 doi: 10.1172/jci.insight.97054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DWP, Koenig J, Carnevali L, et al. Heart rate variability and inflammation: A meta-analysis of human studies. Brain Behav Immun. 2019 doi: 10.1016/j.bbi.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, von KR (2017) Psychological stress, inflammation, and coronary heart disease. Curr Cardiol Rep 19:111. 10.1007/s11886-017-0919-x [DOI] [PubMed]
- Wölfle U, Elsholz FA, Kersten A, et al. Expression and functional activity of the bitter taste receptors TAS2R1 and TAS2R38 in human keratinocytes. Skin Pharmacol Physiol. 2015 doi: 10.1159/000367631. [DOI] [PubMed] [Google Scholar]
- Workman AD, Maina IW, Brooks SG, et al. The role of quinine-responsive taste receptor family 2 in airway immune defense and chronic rhinosinusitis. Front Immunol. 2018 doi: 10.3389/fimmu.2018.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woronicz JD, Gao X, Cao Z, et al. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK [see comments] Science. 1997;80–s:278. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genom. 2005 doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- Wu J, Williams D, Walter GA, et al. Estrogen increases Nrf2 activity through activation of the PI3K pathway in MCF-7 breast cancer cells. Exp Cell Res. 2014 doi: 10.1016/j.yexcr.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Wu H, Cui Y, He C, et al. Activation of the bitter taste sensor TRPM5 prevents high salt-induced cardiovascular dysfunction. Sci China Life Sci. 2020 doi: 10.1007/s11427-019-1649-9. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu C, Feng J, et al. QSIdb: Quorum sensing interference molecules. Brief Bioinform. 2021 doi: 10.1093/bib/bbaa218. [DOI] [PubMed] [Google Scholar]
- Wu X, Huang L, Liu J. Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (Review) Exp Ther Med. 2021 doi: 10.3892/etm.2021.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Wang X, Young RL, et al. Role of intestinal bitter sensing in enteroendocrine hormone secretion and metabolic control. Front Endocrinol. 2018 doi: 10.3389/fendo.2018.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Cao J, Iguchi N, et al. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod. 2013 doi: 10.1093/molehr/gas040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan F, Jian J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux. Int J Mol Med. 2016 doi: 10.3892/ijmm.2016.2615. [DOI] [PubMed] [Google Scholar]
- Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018 doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L (2014) Chronic sustained inflammation links to left ventricular hypertrophy and aortic valve sclerosis: a new link between S100/RAGE and FGF23. Inflamm Cell Signal 10.14800/ics.279 [DOI] [PMC free article] [PubMed]
- Yang Y, Yan X, Xue J, et al. Connexin43 dephosphorylation at serine 282 is associated with connexin43-mediated cardiomyocyte apoptosis. Cell Death Differ. 2019 doi: 10.1038/s41418-019-0277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Yu M, Zhang K, et al. Tissue-specific expression pattern and histological distribution of NLRP3 in Chinese yellow chicken. Vet Res Commun. 2015 doi: 10.1007/s11259-015-9641-6. [DOI] [PubMed] [Google Scholar]
- Yin C, Ye Z, Huang C, et al. Elevated Wnt2 and Wnt4 activate NF-κB signaling to promote cardiac fibrosis by cooperation of Fzd4/2 and LRP6 following myocardial infarction. SSRN Electron J. 2021 doi: 10.2139/ssrn.3861570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Jing Y, Wang T, et al. The bitter taste receptor agonist-induced negative chronotropic effects on the Langendorff-perfused isolated rat hearts. Eur J Pharmacol. 2020 doi: 10.1016/j.ejphar.2020.173063. [DOI] [PubMed] [Google Scholar]
- Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, de Chillou C, Eckardt L, Friede T, Haugaa KH, Hocini M, Lambiase PD, Marijon E, Merino JL, Peichl P, Priori SG, Reichlin T, Schulz-Menger J, Sticherling VMESDG. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Hear J. 2022 doi: 10.1093/eurheartj/ehac262. [DOI] [PubMed] [Google Scholar]
- Zhai KF, Duan H, Khan GJ, et al. Salicin from Alangium chinense ameliorates rheumatoid arthritis by modulating the Nrf2-HO-1-ROS pathways. J Agric Food Chem. 2018 doi: 10.1021/acs.jafc.8b02241. [DOI] [PubMed] [Google Scholar]
- Zhang B, Shen Q, Chen Y, et al. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci Rep. 2017 doi: 10.1038/srep44239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Lin X, Zhang S, et al. A protective role of glibenclamide in inflammation-associated injury. Mediat Inflamm. 2017 doi: 10.1155/2017/3578702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tian J, Diao S, et al. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction. Chem Biol Interact. 2020 doi: 10.1016/j.cbi.2020.109252. [DOI] [PubMed] [Google Scholar]
- Zhang X, Qu H, Yang T, et al. Regulation and functions of NLRP3 inflammasome in cardiac fibrosis: Current knowledge and clinical significance. Biomed Pharmacother. 2021;143:112219. doi: 10.1016/j.biopha.2021.112219. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Umemura A, Sanchez-Lopez E, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016 doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhang T, Wang X, et al. Curcumin modulates macrophage polarization through the inhibition of the toll-like receptor 4 expression and its signaling pathways. Cell Physiol Biochem. 2015 doi: 10.1159/000430126. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lyu Y, Richlen ML, et al. Quorum sensing is a language of chemical signals and plays an ecological role in algal-bacterial interactions. CRC Crit Rev Plant Sci. 2016 doi: 10.1080/07352689.2016.1172461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Huang W, Xu Y, et al. Sweet taste receptors mediated ROS-NLRP3 inflammasome signaling activation: Implications for diabetic nephropathy. J Diabetes Res. 2018 doi: 10.1155/2018/7078214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Li Q, Su S, et al. Interleukin 37 suppresses M1 macrophage polarization through inhibition of the Notch1 and nuclear factor Kappa B pathways. Front Cell Dev Biol. 2020 doi: 10.3389/fcell.2020.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Xi R, Liu J, Peng X, Zhao L, Zhou X, Li J, Xin Zheng XX. TAS2R16 activation suppresses LPS-induced cytokine expression in human gingival fibroblasts. Front Immunol. 2021;12:726546. doi: 10.3389/fimmu.2021.726546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DD, Zhang J, Deng W, et al. Significance of NF-κB activation in immortalization of nasopharyngeal epithelial cells. Int J Cancer. 2016 doi: 10.1002/ijc.29850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no competing interest regarding the publication of this article.