Abstract
The coronavirus disease 2019 (COVID-19) pandemic has swept the whole world and brought about a public health crisis of unprecedented proportions. To combat the rapid transmission and possible deaths due to the disease, researchers and companies around the world are developing all possible strategies. Due to the advantages of safety, specificity, and fewer adverse effects, polypeptide and peptidomimetic drugs are considered promising strategies. This review comprehensively summarizes and discusses the progress in development of peptide drugs for use in the treatment of COVID-19. Based on the latest results in this field, we divided them into clinically approved drugs, clinical trial drugs, and clinically ineffective drugs, and outlined the molecular targets and mechanisms of action one by one to reveal their feasibility as promising therapeutic agents for COVID-19. Notably, monoclonal antibodies have shown beneficial effects in the early stages of infection, while Paxlovid can significantly reduce hospitalization and mortality among non-vaccinated patients. Among clinical experimental drugs, both the interleukin-1 receptor antagonist anakinra and the bradykinin B2 receptor antagonist icatibant are well tolerated and effective in patients with COVID-19, but long-term trials are needed to confirm the durability of efficacy.
Key Points
| Polypeptide and peptidomimetic drugs are key tools to reduce hospitalization and mortality in COVID-19 patients. |
| Therapeutic polypeptides and peptidomimetics can act through multiple targets, such as viral proteins, ACE2 receptors, and virus-induced pro-inflammatory cytokines. |
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has emerged as a threat to global health, social stability, and the global economy. After identification and isolation of the virus, previous studies confirmed that it belongs to the genus β-coronavirus and is approximately 79% similar to the severe acute respiratory syndrome coronavirus (SARS-CoV) at the nucleotide level [1]. Based on this, the Coronaviridae Study Group of the International Committee on Taxonomy of Viruses named the RNA virus as SARS-CoV-2 [2]. Although vaccines are considered to be the most powerful weapon to fight against viral invasion at present, they are usually used as a preventive measure for uninfected individuals rather than as a treatment. Therefore, there is an urgent requirement to develop effective antiviral therapeutics that can not only treat COVID-19 patients rapidly and effectively but also prevent the further spread of the virus [3]. Notably, the slow efficacy of conventional drugs has failed to address the need for timely treatment. As a result, conventional drugs are not considered an effective option in the case of an outbreak. Compared with traditional small molecule drugs, peptide drugs are smaller fragments of proteins that are more suitable for synthesis in terms of time efficiency and cost. In addition, peptide drugs present several advantages as therapeutic approaches, including increased specificity and affinity for the target and low toxicity [4]. Hence, polypeptide and peptidomimetic drugs may be appropriate as a treatment for COVID-19.
Recent studies on the molecular biology of SARS-CoV-2 have also revealed the possibility of using peptide drugs. Infection is initiated when the receptor binding domain (RBD) of SARS-CoV-2 binds to the human angiotensin-converting enzyme 2 (ACE2) receptor [5]. After the virus enters the host cell, its RNA is released. Subsequently, host ribosomes are hijacked to produce the two viral replicase polyproteins, which can further be processed into 16 mature non-structural proteins (NSPs) through two virus-encoding proteases: main protease (Mpro) and papain-like protease. These NSPs are able to assemble into the replication and transcription complex to initiate viral RNA replication and transcription [6]. Moreover, the progression of COVID-19 is associated with a severe increase in inflammatory factors [7]. All these mean that peptide drugs may play a role through multiple mechanisms, including inhibiting the protein–protein interactions between SARS-CoV-2 spike glycoprotein and cellular receptors, inhibiting the proteases associated with viral replication and transcription and preventing the release of inflammatory cytokines.
Methods of Literature Search
Literature sources included the PubMed and Google Scholar databases (date of last search: August 14, 2022). The keywords included in the search were ‘COVID-19’ and ‘drugs’, ‘monoclonal antibodies’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘bamlanivimab etesevimab’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘casirivimab imdevimab’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘regdanvimab’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘sotrovimab’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘Paxlovid’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘tocilizumab’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘aviptadil’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘dalbavancin’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘anakinra’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘icatibant’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘solnatide’ and ‘COVID-19’ or ‘SARS-CoV-2’, ‘lopinavir ritonavir’ and ‘COVID-19’ or ‘SARS-CoV-2’.
Currently Approved Drugs
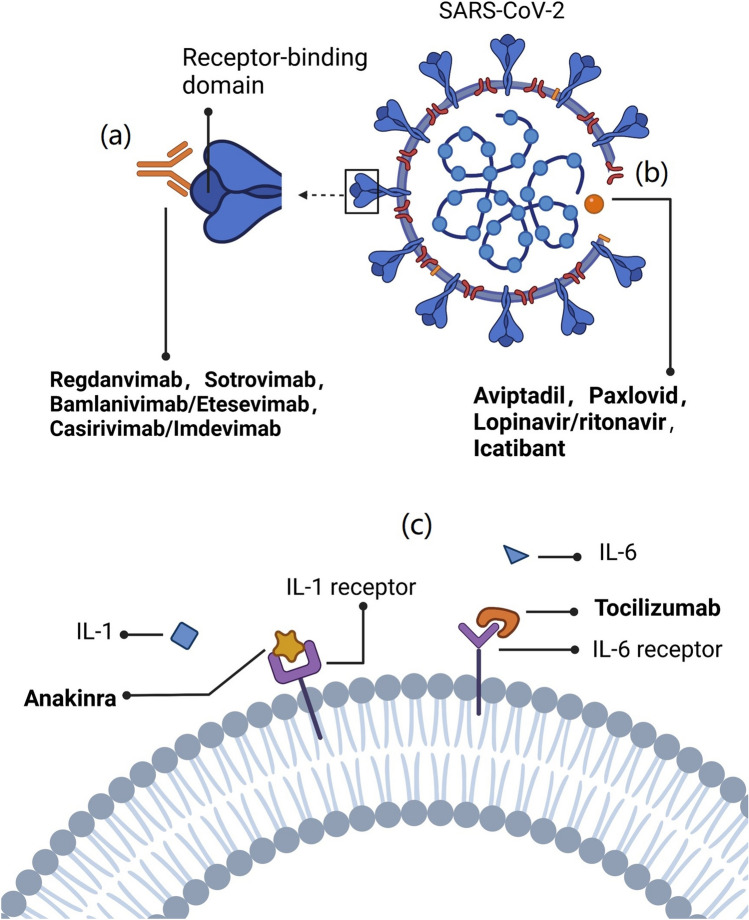
The use of monoclonal antibodies (mAbs) dates back more than three decades. The first approved mAb, muronomab, was used primarily for graft rejection in renal transplant patients [8]. Over the years, the use of mAbs has expanded from the treatment of autoimmune diseases and cancer therapeutics to communicable diseases [9]. A large number of mAbs for COVID-19 treatment have been developed at breakneck speed over the past 2 years. Currently, the following four SARS-CoV-2 RBD-specific mAbs are in clinical use: casirivimab (REGN10933) plus imdevimab (REGN10987) authorized by the US Food and Drug Administration (FDA) on November 21, 2020, sotrovimab (VIR-7831) authorized by the FDA on May 26, 2021, bamlanivimab (LY-CoV555) plus etesevimab (LY-CoV016) authorized by the FDA on February 9, 2021, and regdanvimab (CT-P59) approved in Korea on September 17, 2021 [10–13] (Table 1). These neutralizing mAbs can block the binding of ACE2 to the spike glycoprotein, thereby preventing the virus from infecting human cells (Fig. 1, created with biorender.com). Clinically, sotrovimab, bamlanivimab plus etesevimab, and casirivimab plus imdevimab have been demonstrated to be beneficial among outpatients with COVID-19 [14–16]. However, clinical studies of sotrovimab and casirivimab plus imdevimab have reported ineffective outcomes in the overall population of patients hospitalized with COVID-19 [17, 18]. In addition to trials of patients hospitalized with COVID-19 probably having a greater proportion of patients with endogenous anti-SARS-CoV-2 antibodies than outpatient trials, increased use of concomitant COVID-19 therapies for inpatients is considered to be an alternative or supplementary explanation for this phenomenon [17]. The additional antiviral activity from neutralizing mAb therapies might not provide incremental benefit above background therapy with other drugs. Thus, these therapies seem to be more suitable for use in the early infective phase of SARS-CoV-2 infection.
Table 1.
Peptide drugs for the treatment of COVID-19 infections
| Name | Discovery approach | Type | Latest status | Manufacturer | Route of administration | Adverse events | Treatment stage | Antiviral mechanisms | References |
|---|---|---|---|---|---|---|---|---|---|
| Bamlanivimab + etesevimab | Developed for SARS-CoV-2 | mAb | EUA (2/9/2021) | Eli Lilly | Intravenous infusion | Anaphylaxis and infusion-related reactionsa | Mild-to-moderate illness | They target the RBD and block the binding of spike protein to ACE2 | [10] |
| Casirivimab + imdevimab | Developed for SARS-CoV-2 | mAb | EUA (11/21/2020) | Regeneron Pharmaceuticals | Intravenous infusion or subcutaneous injection | Anaphylaxis and infusion-related reactionsa | Mild-to-moderate illness | They target the RBD and block the binding of spike protein to ACE2 | [11] |
| Regdanvimab | Developed for SARS-CoV-2 | mAb | Approved (9/17/2021) | Celltrion | Intravenous infusion | Elevated liver enzyme levels and hypertriglyceridemia | Elderly with mild illness and adults with moderate illness | It targets the RBD and blocks the binding of spike protein to ACE2 | [12] |
| Sotrovimab | Developed for SARS-CoV-2 | mAb |
EUA (5/26/2021) |
GSK and Vir Biotechnology | Intravenous infusion | Rash and diarrhea | Mild-to-moderate illness | It targets the RBD and blocks the binding of spike protein to ACE2 | [13] |
| Paxlovid | Developed for SARS-CoV-2 | Peptidomimetic | EUA (12/22/2021) | Pfizer | Oral | Impaired sense of taste, diarrhea, high blood pressure and muscle aches | Mild-to-moderate illness | It can inhibit viral replication by binding to the active site of Mpro | [24] |
| Tocilizumab | Repurposed IL-6 inhibitor | mAb | Approved (3/3/2020) | Roche | Intravenous infusion | Allergic reactions | Severe illness | It binds to IL-6 receptors, thereby inhibiting the cytokine release syndrome | [21] |
| Aviptadil | Repurposed VIP | Polypeptide | Granted Orphan Drug Designationb (7/14/2020) | NeuroRx and Relief Therapeutics | Intravenous infusion | Tachycardia, flushing, hypotension, diarrhea and alterations in electrocardiogram (bigeminy) | Severe illness | It protects alveolar type II cells by binding to the VPAC1 and prevents the apoptosis indicated by coronavirus | [30] |
| Dalbavancin | Repurposed glycopeptide antibiotic | Glycopeptide antibiotic | In animal models | Durata Therapeutics | N/A | N/A | N/A | It binds to human ACE2 and blocks the interaction with SARS-CoV-2 spike proteins | [55] |
| Anakinra | Repurposed IL-1 inhibitor | IL-1 receptor antagonist | Phase III | Swedish Orphan Biovitrum AB (publ) | Subcutaneous injection | In experiment | In experiment | It reduces the inflammatory response and tissue damage caused by IL-1 | [37] |
| Icatibant | Repurposed bradykinin B2 receptor inhibitor | Polypeptide | Phase II | Shire | Subcutaneous injection | In experiment | In experiment | It inhibits bradykinin and SARS-CoV-2 Mpro | [44] |
| Solnatide | Developed for the ARDS subjects | 17-mer cyclic peptide | Phase II | APEPTICO | Inhalation | In experiment | In experiment | It acts on the lower respiratory tract and activates the epithelial sodium ion channels | [53] |
| Lopinavir + ritonavir | Repurposed HIV-1 inhibitor | Peptidomimetic | Discontinued (6/29/2020) | AbbVie | Oral | Diarrhea, nausea and vomiting | N/A | They inhibit the activity of Mpro by occupying its active site, causing a competitive inhibition | [59] |
ACE2 angiotensin-converting enzyme 2, ARDS acute respiratory distress syndrome, EUA emergency use authorization, GSK GlaxoSmithKline, IL interleukin, mAb monoclonal antibody, Mpro main protease, N/A not applicable, RBD receptor binding domain, VIP vasoactive intestinal polypeptide, VPAC1 vasoactive intestinal peptide receptor 1
aSigns and symptoms of infusion-related reactions may include fever, difficulty breathing, reduced oxygen saturation, chills, fatigue, arrhythmia (e.g., atrial fibrillation, sinus tachycardia, bradycardia), chest pain or discomfort, weakness, altered mental status, nausea, headache, bronchospasm, hypotension, hypertension, angioedema, throat irritation, rash including urticaria, pruritus, myalgia, vasovagal reactions (e.g., presyncope, syncope), dizziness and diaphoresis
bFDA declined EUA for aviptadil for critically ill COVID-19 patients at immediate risk of death from respiratory failure despite receiving approved therapy
Fig. 1.
Key drug targets of SARS-CoV-2. a Neutralizing mAbs target the receptor binding domain to block the binding of ACE2 to the spike glycoprotein. b Inhibition of viral genome replication. c Inhibition of virus-induced pro-inflammatory cytokines. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, IL interleukin
Interleukin-6 (IL-6) is one of the key cytokines involved in infection-induced cytokine storm [19]. Tocilizumab, an IL-6 receptor antagonist, showed significant benefits in critically ill patients with COVID-19 [20]. It was officially added to the COVID-19 diagnosis and treatment program (7th edition) of the National Health Care Commission of China on March 3, 2020 [21] (Table 1). However, some trials did not show any mortality benefit [22, 23]. The conflicting results may be due to differences in timing, indication, dose of reinjection and the use of biomarkers. Thus, large-scale randomized controlled trials are still required to further confirm the clinical efficacy of tocilizumab in the treatment of patients with COVID-19.
Paxlovid attained emergency use authorization on December 22, 2021 (Table 1). The treatment includes nirmatrelvir and ritonavir [24]. The nitrile group of nirmatrelvir can covalently bind to the important Cys-145 residue of the Mpro through a Pinner-like reaction. Moreover, it is further stabilized by hydrophobic interactions and hydrogen bonding networks, which enhance its binding to the active site of SARS-CoV-2 Mpro [25, 26] (Fig. 1, created with biorender.com). Ritonavir is a potent inhibitor of microsomal cytochrome P450 3A4 enzyme that metabolizes several drugs. Therefore, the addition of ritonavir can reduce the degradation rate of nirmatrelvir [27] (Fig. 2, created with biorender.com). In non-vaccinated COVID-19 patients, treatment with Paxlovid resulted in a risk of progression to severe disease that was 89% lower than the risk with placebo [28]. Another study found that Paxlovid prescription within 5 days of diagnosis had a faster clearance of viral load and a shorter time to viral elimination in patients who are immunocompromised. Moreover, the correlation between timing of Paxlovid initiation and viral elimination is linear [29]. Thus, Paxlovid should be considered for introduction into primary care for high-risk patients who are immunocompromised, including those who are hospitalized, and unvaccinated in particular, in order to facilitate viral eradication.
Fig. 2.
Paxlovid’s mechanism of action against SARS-CoV-2. ACE2 angiotensin-converting enzyme 2, Mpro main protease, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
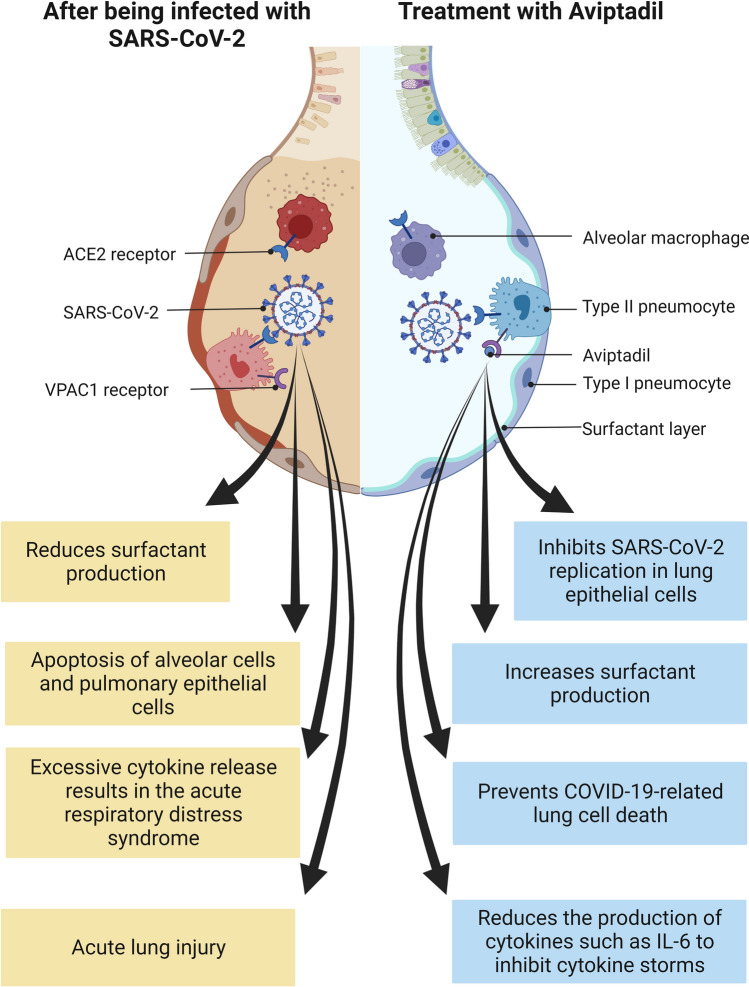
Aviptadil has been granted orphan drug designation by the FDA for the treatment of COVID-19 (Table 1). It is a synthetic form of human vasoactive intestinal polypeptide [30] and can bind to NSP16 at a specific site to inhibit the 2'-O-methyltransferase activity of the NSP10/NSP16 complex, which plays a role in evading the immune recognition process [31] (Fig. 1, created with biorender.com). Moreover, aviptadil could protect alveolar type II cells by binding to vasoactive intestinal peptide receptor 1 and preventing the action of caspase induced by the coronavirus [32]. In addition to this, aviptadil reduces the production of cytokines such as IL-6 to prevent acute respiratory distress syndrome (ARDS) in COVID-19 patients [30]. Furthermore, Li et al. (2007) found it can upregulate the expression of c-fos protein in alveolar type II cells, thereby increasing the synthesis of pulmonary surfactant phospholipids [33] (Fig. 3, created with biorender.com). In a prospective, multicenter, randomized, placebo-controlled trial of 196 patients admitted to intensive care at 10 US hospitals, it was observed that patients in the aviptadil group showed improvement which included the likelihood of recovering from failure, surviving to 60 days, and reducing hospital stay when compared with placebo [34]. In another case series, 21 confirmed SARS-CoV-2 patients treated with intravenous aviptadil showed significant radiological and clinical improvement. All of them had significantly lower inflammatory markers, such as ferritin, D-dimer, and IL-6 [35]. Notably, the FDA declined emergency use authorization for aviptadil for critically ill COVID-19 patients at immediate risk of death from respiratory failure [36]. Thus, more robust data on a larger target sample in different populations will be immensely helpful to confirm its safety and effectiveness in treating COVID-19 patients.
Fig. 3.
Mechanism of action of aviptadil for the treatment of COVID-19. ACE2 angiotensin-converting enzyme 2, COVID-19 coronavirus disease 2019, IL-6 interleukin-6, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, VPAC1 vasoactive intestinal peptide receptor 1
Drugs in Clinical Trials
Anakinra is a recombinant human interleukin-1 receptor antagonist (IL-1Ra) composed of 153 amino acid residues (Table 1) [37]. Unlike native human IL-1Ra, anakinra has an additional methionine residue at the amino terminus. Of note, severe COVID-19 patients may develop pulmonary edema [38]. This is due to the activation of bradykinin B1 receptor and bradykinin B2 receptor (B2R) on lung endothelial cells [39]. IL-1 can upregulate the expression of bradykinin B1 receptor protein [40]. However, anakinra can inhibit the activity of IL-1α and IL-1β by binding to IL-1R [41], thereby reducing the inflammatory response and tissue damage caused by IL-1 (Fig. 1, created with biorender.com). A retrospective cohort study in Italy (ClinicalTrials.gov identifier: NCT04341584) showed that high-dose anakinra treatment resulted in a 77% reduction in mortality at 21 days compared with a control group of patients with COVID-19 who received usual care in the same hospital [42]. Furthermore, anakinra improved overall survival and was well tolerated in patients with ARDS associated with COVID-19 [43]. However, these studies have small sample sizes. The results need to be validated by a longer-term controlled trial to examine the long-term efficacy.
Icatibant is a selective bradykinin B2 receptor antagonist with an affinity for the B2R as bradykinin itself (Table 1) [44]. The binding of bradykinin to B2R leads to more vasodilation and increased vascular permeability, resulting in fluid accumulation in the interstitial tissue [45, 46]. Based on the inhibitory effects of icatibant on bradykinin and Mpro, it has the potential to treat patients with COVID-19 [47]. Mansour et al. (2020) found that icatibant promoted significant improvement of lung computed tomography scores and increased blood eosinophils, which has been reported as an indicator of disease recovery [48]. In line with this, a case-control study in patients with COVID-19 also concluded that icatibant was excellently tolerated and improved the oxygenation [49]. Given the short half-life of icatibant [50], there is uncertainty regarding the most appropriate dose of icatibant to administer. Therefore, further research is still needed for clinical application.
Solnatide is a synthetic cyclic peptide composed of 17 amino acids that mimics the lectin-like structure of tumor necrosis factor-α. It can activate the lung epithelial sodium channel to directly affect alveolar liquid clearance and to reduce the leakage of blood and fluids from the capillaries in the airspace, that is, accelerate the resolution of alveolar edema and reduce barrier injury in the lung [51, 52]. Recently, based on the conformational studies and in the analysis of charge distribution of the peptide surface, Martin-Malpartida et al. (2022) described how solnatide interacts with the cytoplasmic C-terminal domain of the epithelial sodium channel α-subunit via electrostatic complementarity [53]. Additionally, as solnatide lacks pro-inflammatory activity, its intratracheal instillation does not stimulate chemokine production or increase neutrophil infiltration in lungs [51]. In 2013, APEPTICO, a privately held biotechnology company, fruitfully concluded a phase I clinical study in healthy subjects, verifying the safety of solnatide [54]. Furthermore, a phase IIb, randomized, placebo-controlled, double-blind, dose-escalation study (EUDRACT No. 2017-003855-47) is underway to access the safety and preliminary efficacy of sequential multiple ascending doses of solnatide in patients with moderate-to-severe ARDS and pulmonary permeability edema [51].
The glycopeptide antibiotic dalbavancin binds to human ACE2 with high affinity and blocks the interaction with SARS-CoV-2 spike protein (Table 1). Wang et al. (2021) found dalbavancin could effectively prevent SARS-CoV-2 replication in Vero E6 cells with a half maximal effective concentration of ~12 nM. In addition, histopathological injuries caused by SARS-CoV-2 infection in mouse and rhesus macaque models are significantly inhibited by its administration [55]. In combination with its good safety and long plasma half-life (5–7 days), dalbavancin should be considered as a promising anti-COVID-19 drug candidate.
Clinically Ineffective Drugs
Lopinavir/ritonavir (LPV/r) is a peptidomimetic molecule (Table 1) that inhibits the activity of Mpro by occupying its active site, causing a competitive inhibition [56] (Fig. 1, created with biorender.com). However, most human immunodeficiency virus protease inhibitors show poor bioavailability. This is because they can also be extensively metabolized by the P450 3A4 enzyme [57]. In the trial by Cao et al. (2020), adding LPV/r treatment did not reduce viral RNA loads or duration of viral RNA detectability as compared with the standard care group. Notably, a significant proportion of patients were unable to complete treatment due to severe gastrointestinal adverse effects [58]. In addition, the results of the Randomised Evaluation of COVID-19 Therapy (RECOVERY trial) showed that among patients admitted to hospital with COVID-19, LPV/r was not associated with reductions in 28-day mortality, length of hospital stay, or risk of progression to invasive mechanical ventilation or death. Subgroup analyses found no evidence for a time-to-treatment effect or benefit in those with less severe disease [59]. The World Health Organization has halted the LPV/r treatment groups involved in its Solidarity trial [60].
Conclusion
As previously mentioned, polypeptide and peptidomimetic drugs can act through multiple targets, such as the RBD, the Mpro, the ACE2 receptor, and virus-induced pro-inflammatory cytokines. Due to their satisfactory therapeutic efficacy, they appear to be effective drugs against SARS-CoV-2 at different stages of viral infection. In mild to moderate disease stages, neutralizing mAbs and Paxlovid have been shown to be effective drugs. Tocilizumab and aviptadil showed efficacy in patients with severe illness. Among these drugs, neutralizing mAbs can block protein–protein interactions between cellular receptors and SARS-CoV-2 spike glycoprotein. Paxlovid and aviptadil can inhibit proteases associated with viral replication. Several peptide drugs are still in small-sample, short-duration clinical trials. Therefore, many questions remain unanswered, including identifying the most effective drugs for mild, moderate, and severe disease; the best time to start treatment; ideal dosage; adverse effects; duration of treatment; and the most beneficial combination therapy. In summary, peptide drugs have many advantages in the prevention, control and treatment of COVID-19, and deserve further in-depth study.
Declarations
Authors’ Contribution
The first draft of the manuscript was written by Xinyu Liu, Jian Shi and Deyang Wang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (Grant no. ZR2020QC100).
Conflict of Interest
All authors have no financial interests or potential conflicts of interests to declare.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Code Availability
Not applicable.
Footnotes
Xinyu Liu, Jian Shi, Deyang Wang have contributed equally to this work and share first authorship.
References
- 1.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Pearce R, Zhang Y. De novo design of protein peptides to block association of the SARS-CoV-2 spike protein with human ACE2. Aging (Albany NY). 2020;12:11263. doi: 10.18632/aging.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanPatten S, He M, Altiti A, Cheng FK, Ghanem MH, Al-Abed Y. Evidence supporting the use of peptides and peptidomimetics as potential SARS-CoV-2 (COVID-19) therapeutics. Future Med Chem. 2020;12:1647–1656. doi: 10.4155/fmc-2020-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41:1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs, Taylor & Francis; 2015. pp. 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S, Choudhury S, Das D. An update of antispike severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) monoclonal antibodies. Indian J Pharmacol. 2022;54:51. doi: 10.4103/ijp.ijp_519_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0. Accessed 26 Sep 2022.
- 11.U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed 25 Sep 2022.
- 12.Ministry of Food and Drug Safety. Regdanivmab full Korean approval. 2021. https://www.mfds.go.kr/brd/m_99/view.do?seq=45778. Accessed 26 Sep 2022.
- 13.U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Monoclonal Antibody for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19. Accessed 24 Sep 2022.
- 14.Gupta A, Gonzalez-Rojas Y, Juarez E, Casal MC, Moya J, Falci DR, Sarkis E, Solis J, Zheng H, Scott N. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry R, Boscia J, Heller B. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Self WH, Sandkovsky U, Reilly CS, Vock DM, Gottlieb RL, Mack M, Golden K, Dishner E, Vekstein A, Ko ER. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22:622–635. doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abani O, Abbas A, Abbas F, Abbas M, Abbasi S, Abbass H, Abbott A, Abdallah N, Abdelaziz A, Abdelfattah M. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Investigators R-C. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei P-F. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) Chin Med J. 2020;133:1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, Bruzzi P, Boni F, Braglia L, Turrà C. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19. Accessed 26 Sep 2022.
- 25.Menéndez JC. Approaches to the potential therapy of COVID-19: a general overview from the medicinal chemistry perspective. Molecules. 2022;27:658. doi: 10.3390/molecules27030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Fang C, Zhang Q, Zhang R, Zhao X, Duan Y, Wang H, Zhu Y, Feng L, Zhao J. Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332. Protein Cell. 2022;13:689–693. doi: 10.1007/s13238-021-00883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Extance A. Covid-19: what is the evidence for the antiviral Paxlovid? BMJ. 2022;377:o1037. doi: 10.1136/bmj.o1037. [DOI] [PubMed] [Google Scholar]
- 28.Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun F, Lin Y, Wang X, Gao Y, Ye S. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis. 2022;22:1279. doi: 10.1016/S1473-3099(22)00430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raveendran A, Al Dhuhli KS, Kumar GH. Role of Aviptadil in COVID-19. BMH Med J. 2021;8:77–83. [Google Scholar]
- 31.Alnomasy SF, Alotaibi BS, Aldosari ZM, Mujamammi AH, Anand P, Akhter YA, and Hasan MR. Inhibitory effects of aviptadil on the SARS-CoV-2 nsp10/nsp16 protein complex. Research Square [Preprint]. 2021. 10.21203/rs.3.rs-191980/v1.
- 32.Mukherjee T, Behl T, Sharma S, Sehgal A, Singh S, Sharma N, Mathew B, Kaur J, Kaur R, Das M. Anticipated pharmacological role of Aviptadil on COVID-19. Environ Sci Pollut Res. 2021;29:1–17. doi: 10.1007/s11356-021-17824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, She H, Yue S-J, Qin X-Q, Guan C-X, Liu H-J, Luo Z-Q. Role of c-fos gene in vasoactive intestinal peptide promoted synthesis of pulmonary surfactant phospholipids. Regul Pept. 2007;140:117–124. doi: 10.1016/j.regpep.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Jayaweera D, Park DJ. Recovery and survival in covid-19 respiratory failure , when treated with aviptadil. Age (years, mean). 2022;60:62–67. [Google Scholar]
- 35.Youssef JG, Al-Saadi M, Zahiruddin F, Beshay S, Bitar M, Javitt J. Rapid recovery from COVID-19 respiratory failure with comorbidity in 21 patients treated with vasoactive intestinal peptide. Social Science Electronic Publishing; 2020. [Google Scholar]
- 36.NRx Pharmaceuticals, Inc. FDA Declines Emergency Use Authorization for ZYESAMI (aviptadil) for subgroup of Patients with Critical COVID-19 at immediate risk of death from respiratory failure despite treatment with approved therapy, including remdesivir. https://www.prnewswire.com/news-releases/fda-declines-emergency-use-authorization-for-zyesami-aviptadil-for-subgroup-of-patients-with-critical-covid-19-at-immediate-risk-of-death-from-respiratory-failure-despite-treatment-with-approved-therapy-including-remdesivir-301579610.html. Accessed 28 Sep 2022.
- 37.Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahloul M, Ketata W, Lahyeni D, Mayoufi H, Kotti A, Smaoui F, Kallel N, Daoud E, Bouaziz M, Kammoun S. Pulmonary capillary leak syndrome following COVID-19 virus infection. J Med Virol. 2021;93:94. doi: 10.1002/jmv.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Veerdonk FL, Netea MG, van Deuren M, van der Meer JW, de Mast Q, Brüggemann RJ, and van der Hoeven H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife. 2020; 9. [DOI] [PMC free article] [PubMed]
- 40.Schanstra JP, Bataillé E, Castano MM, Barascud Y, Hirtz C, Pesquero JB, Pecher C, Gauthier F, Girolami J-P, Bascands J-L. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-kappaB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J Clin Investig. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinarello CA, van der Meer JW, Treating inflammation by blocking interleukin-1 in humans. Seminars in immunology. 2013;25:469–84. [DOI] [PMC free article] [PubMed]
- 42.Tharaux P-L, Pialoux G, Pavot A, Mariette X, Hermine O, Resche-Rigon M, Porcher R, Ravaud P, Bureau S, Dougados M. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franzetti M, Forastieri A, Borsa N, Pandolfo A, Molteni C, Borghesi L, Pontiggia S, Evasi G, Guiotto L, Erba M. IL-1 receptor antagonist anakinra in the treatment of COVID-19 acute respiratory distress syndrome: a retrospective, observational study. J Immunol. 2021;206:1569–1575. doi: 10.4049/jimmunol.2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malchair P, Otero A, Giol J, Solanich X, Carnaval T, Fernández-Nistal A, Sánchez-Gabriel A, Montoto C, Lleonart R, Videla S. A multicenter, open-label, randomized, proof-of-concept phase II clinical trial to assess the efficacy and safety of icatibant in patients infected with SARS-CoV-2 (COVID-19) and admitted to hospital units without invasive mechanical ventilation: study protocol (ICAT-COVID) Trials. 2022;23:1–15. doi: 10.1186/s13063-022-06219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarighi P, Eftekhari S, Chizari M, Sabernavaei M, Jafari D, Mirzabeigi P. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur J Pharmacol. 2021;895:173890. doi: 10.1016/j.ejphar.2021.173890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cockcroft J, Chowienczyk P, Brett S, Bender N, Ritter J. Inhibition of bradykinin-induced vasodilation in human forearm vasculature by icatibant, a potent B2-receptor antagonist. Br J Clin Pharmacol. 1994;38:317–321. doi: 10.1111/j.1365-2125.1994.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Wang X-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genom. 2020;47:119. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansour E, Palma AC, Ulaf RG, Ribeiro LC, Bernardes AF, Nunes TA, Agrela MV, Bombassaro B, Monfort-Pires M, Camargo RL. Pharmacological inhibition of the kinin-kallikrein system in severe COVID-19—a proof-of-concept study. MedRxiv. 2020;382:1787. doi: 10.3390/v13020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Veerdonk FL, Kouijzer IJ, de Nooijer AH, van der Hoeven HG, Maas C, Netea MG, Brüggemann RJ. Outcomes associated with use of a kinin B2 receptor antagonist among patients with COVID-19. JAMA Netw Open. 2020;3:e2017708–e2017708. doi: 10.1001/jamanetworkopen.2020.17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Medicines Agency. EPAR Assessment Report 09-020-2018. https://www.ema.europa.eu/en/medicines/human/EPAR/firazyr. Accessed 9 Nov 2022.
- 51.Schmid B, Kredel M, Ullrich R, Krenn K, Lucas R, Markstaller K, Fischer B, Kranke P, Meybohm P, Zwißler B. Safety and preliminary efficacy of sequential multiple ascending doses of solnatide to treat pulmonary permeability edema in patients with moderate-to-severe ARDS—a randomized, placebo-controlled, double-blind trial. Trials. 2021;22:1–21. doi: 10.1186/s13063-021-05588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shabbir W, Tzotzos S, Bedak M, Aufy M, Willam A, Kraihammer M, Holzner A, Czikora I, Scherbaum-Hazemi P, Fischer H. Glycosylation-dependent activation of epithelial sodium channel by solnatide. Biochem Pharmacol. 2015;98:740–753. doi: 10.1016/j.bcp.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Martin-Malpartida P, Arrastia-Casado S, Farrera-Sinfreu J, Lucas R, Fischer H, Fischer B, Eaton DC, Tzotzos S, Macias MJ. Conformational ensemble of the TNF-derived peptide solnatide in solution. Comput Struct Biotechnol J. 2022;20:2082–2090. doi: 10.1016/j.csbj.2022.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apetico, GmbH, Austria. Solnatide has been approved by the Austrian federal office for safety in health care (BASG) for the treatment of COVID-19 patients with severe pulmonary dysfunction. http://www.apeptico.com/index-news. Accessed 7 Nov 2022.
- 55.Wang G, Yang M-L, Duan Z-L, Liu F-L, Jin L, Long C-B, Zhang M, Tang X-P, Xu L, Li Y-C. Dalbavancin binds ACE2 to block its interaction with SARS-CoV-2 spike protein and is effective in inhibiting SARS-CoV-2 infection in animal models. Cell Res. 2021;31:17–24. doi: 10.1038/s41422-020-00450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nukoolkarn V, Lee VS, Malaisree M, Aruksakulwong O, Hannongbua S. Molecular dynamic simulations analysis of ritronavir and lopinavir as SARS-CoV 3CLpro inhibitors. J Theor Biol. 2008;254:861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharmacol Rep. 2020;72:1479–1508. doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horby PW, Mafham M, Bell JL, Linsell L, Staplin N, Emberson J, Palfreeman A, Raw J, Elmahi E, Prudon B. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19. Accessed 29 Sep 2022.