Abstract
Cardiovascular and renal physiology are interrelated. More than a decade ago this was codified in guidelines defining the five subtypes of the cardiorenal syndrome. Morbidity and mortality for those with the cardiorenal syndrome is high compared to demographically matched individuals without cardiorenal disease, acute or chronic. The focus of this review will be the epidemiology, the impact of chronic kidney disease on cardiac structure and function, and associated clinical symptoms, outcomes, and potential treatments for patients with chronic reno-cardiac syndrome, or cardiorenal syndrome type 4. Cardiac structural changes can be profound and are described in detail both at a cellular and physiologic level. Integrating therapies for the treatment of causative or resulting comorbidities may ultimately slow progression of both cardiac and renal disease as well as minimize symptoms and death.
Keywords: Chronic renal insufficiency, Cardiorenal syndrome, Left ventricular hypertrophy, Fibrosis, Arterial stiffness
Methods
The predominant source of information was obtained through PubMed searching terms including mesh terms (keywords as listed above and here: Chronic renal insufficiency, Cardiorenal syndrome, Left ventricular hypertrophy, Fibrosis, Arterial stiffness. The latter three terms were linked via “and” to the first mesh terms and “renal disease.” The citations of individual papers were then reviewed for additional primary source documentation. In addition, for epidemiologic data, we utilized data directly from the United States Renal Data System (https://www.usrds.org/annual-data-report/).
Introduction
The relationship between the cardiovascular and renal systems has long been recognized. In 2008, the acute dialysis quality initiative (ADQI) released consensus guidelines formally defining cardiorenal syndrome (CRS) and outlining five subtypes based on chronicity, primary organ of injury, and resultant pathophysiology (Table 1) [1, 2]. Much research interest remains in studying the population with chronic reno-cardiac syndrome, also known as CRS type 4, which is defined as cardiac dysfunction secondary to primary chronic renal disease [2]. Epidemiologic studies suggest that not only do chronic kidney disease (CKD) patients have a ten-fold increase in mortality risk compared to the general population [3, 4], but among this group greater than 50% of deaths are from cardiovascular disease (CVD) causes, including coronary artery disease, valvular dysfunction, arrhythmias, and cardiomyopathies [2, 3, 5–7]. As research aims to better characterize the interface between these two organ systems, understanding the pathophysiologic consequences of renal disease and resultant structural changes within the heart remains an important area of interest.
Table 1.
Cardiorenal syndrome definitions.
Adapted from Ronco et al. 2010 [2]
| Syndrome | Definition |
|---|---|
| Type I (acute cardiorenal) | Sudden decline in heart function leading to acute kidney injury and dysfunction |
| Type II (chronic cardiorenal) | Chronic impairment in heart function leading to chronic kidney disease |
| Type III (acute reno-cardiac) | Sudden decline in kidney function leading to cardiac injury and dysfunction |
| Type IV (chronic reno-cardiac) | Chronic kidney disease leading to heart injury, disease, or dysfunction |
| Type V (secondary Cardiorenal syndrome) | Systemic illness leading to simultaneous cardiac and renal impairment |
Epidemiology
The prevalence of chronic kidney disease in the USA, based on the United States Renal Data System (USRDS) reporting from 2003 to 2018, has remained stable at 14.4%, representing roughly 37 million Americans [8, 9]. This has led to an average annual Medicare cost of $114 billion U.S. dollars to care for these patients. The prevalence of advanced CKD, defined as stages 3–5, decreased from 2003 to 2006 compared to 2015–2018, with the greatest reduction in stage 3 from 6.3 to 5.8%. Within this same period, prevalence of all stages declined the most among individuals greater than age 65, from 43.2 to 36.8%, while remaining constant at 8.6% among those less than age 65. Given an aging population, such trends represent overall improvement, likely as a result of better treatment options and improved management strategies of associated risk factors [10].
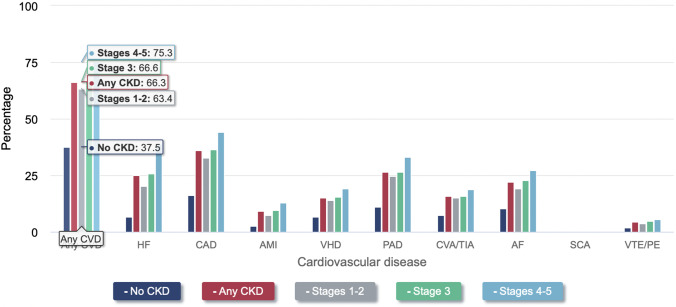
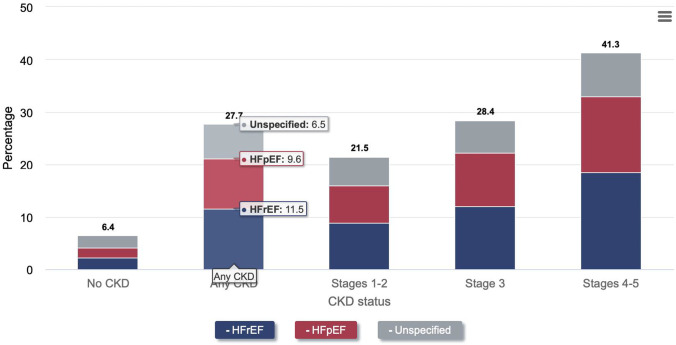
Presence of CVD (defined as acute myocardial infarction [AMI], cerebral vascular attacks [CVA], and heart failure [HF]), is much greater among the CKD population at 66.6%, compared to 37.5% among individuals without CKD (Fig. 1) [8, 11]. As the degree of CKD worsens, HF becomes more prevalent (Fig. 2), and is the most common cardiac manifestation within this population, with a prevalence in 2018 of 27.7% (11.5% HF with reduced ejection fraction [HFrEF], 9.6% HF with preserved ejection fraction [HFpEF], 6.5% undefined), which is roughly 4 times higher than in similar individuals without CKD [8, 12].
Fig. 1.
Prevalence of various cardiovascular disease in CKD and non-CKD patient populations. Based on 2018 epidemiology data. Graph transcribed from USRDS Annual Data Report. 2020 [8]. Cardiovascular disease (CVD); heart failure (HF); acute myocardial infarction (AMI); valvular heart disease (VHD); peripheral artery disease (PAD); cerebral vascular attack (CVA); transient ischemic attack (TIA); atrial fibrillation (AF); sudden cardiac arrest (SCA); venous thromboembolism (VTE); pulmonary embolism (PE)
Fig. 2.
Increasing prevalence of heart failure in worsening stages of CKD. Based on 2018 epidemiology data. Graph from USRDS Annual Data Report. 2020 [8]
Patients with both CKD and CVD have increased risk of hospitalizations, need for intensive-unit level of care, and death [12]. For these reasons, cardiorenal syndromes remain a significant public health burden, and as such, gaining a comprehensive understanding of pathophysiology is all the more necessary towards developing new management strategies.
Cardiorenal Syndromes
Cardiac and renal dysfunction results in a nuanced spectrum of disease with several shared risk factors and whose biochemical and hemodynamic pathways still have much to be discerned. As a result of this fine interplay and limited mechanistic understanding, several challenges exist in formulating an accurate definition and classification system. The National Heart, Lung, and Blood Institute attempted to encompass cardiorenal disease with a broad definition, whereby worsening renal function leads to worsening cardiovascular outcomes [13]. This definition, however, failed to encompass all observations seen in clinical practice and describe a clear mechanistic pathway.
The ADQI consensus guidelines established in 2008 remain the principal definitions used in practice today [2]. Cardiorenal disease was classified into five subtypes, defined by primary organ injury, resultant secondary organ insult, and chronicity: either acute or chronic. This definition was appealing in that it encompassed clinical presentations of cardiorenal disease, which better identified affected populations that helped facilitate development of therapeutic strategies [1, 14, 15]. Several mechanistic questions still persist, however as the definitions did not elaborate on the exact underlying pathophysiologic mechanisms of each disease state [1, 14].
Type 4 Cardiorenal Syndrome
Given the large prevalence of CKD in the general population, and the associated high morbidity and mortality of CVD events described above, it is important to understand the features of Type 4 CRS, also known as chronic reno-cardiac syndrome, with the intent to better establish therapeutic options going forward.
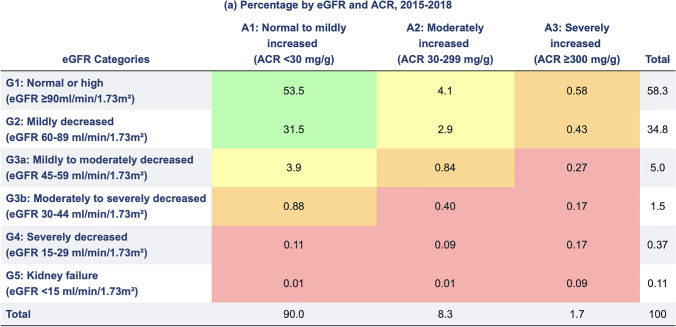
CRS type 4 is defined as longstanding CKD leading to cardiac injury and/or dysfunction [2]. According to the kidney disease improving global outcomes (KDIGO) guidelines in 2012 and 2019, CKD is defined as renal function with persistently reduced estimated glomerular filtration rate (eGFR) of < 60 ml/min/1.73 m2, or at least one indicator of kidney damage for greater than 3 months, including albuminuria, elevated urine sediment, or histological and structural renal abnormalities (Table 2) [12]. Physiologically, GFR is maintained at approximately 140 ml/min/ 1.73 m2 until age 40 (though epidemiologic cross-sectional analyses suggest decline begins as early as age 20), and gradually declines by 8 ml/min/1.73 m2 per decade afterwards [16, 17]. The presence of albuminuria, measured as a random urine albumin/Cr ratio (ACR) of 30 to 300 mg/g, also indicates CKD regardless of eGFR. Shared risk factors, including diabetes, hypertension, dyslipidemia, physical inactivity, smoking, and relevant family history harm both organ systems [8]. Both eGFR and degree of microalbuminuria have been shown to be independent contributors to risks of acute kidney injury (AKI), AMI, HF, CVA, or death.
Table 2.
CKD Stages one through five categorized according to eGFR and degree of albuminuria, with corresponding estimated percentages among the US general population surveyed between 2015 and 2018. From USRDS Annual Data Report. 2020 [8]
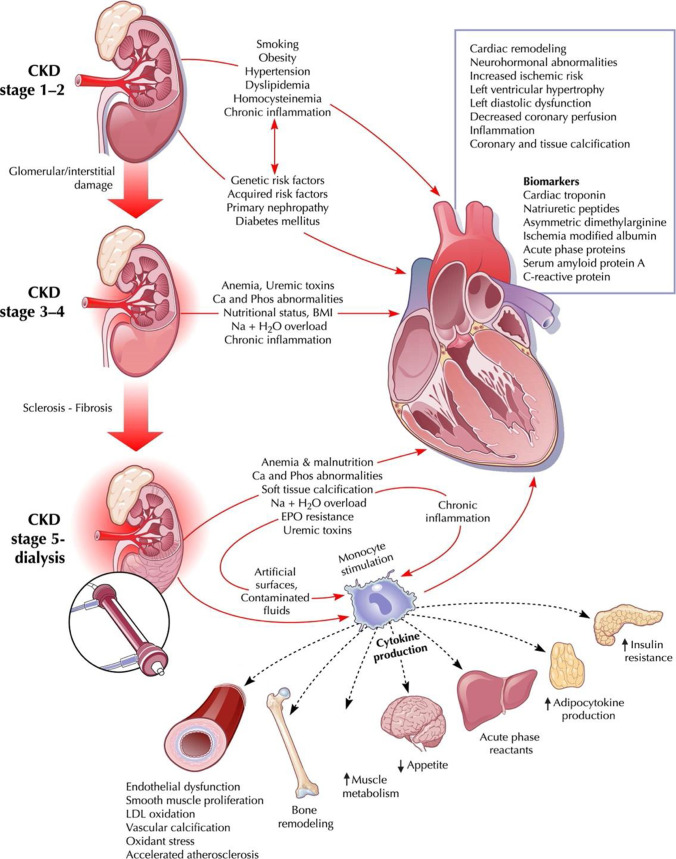
Cardiovascular risk factors such as hypertension, volume overload, anemia, iron-deficiency, nutritional deficiencies, bone disease, and uremic toxin buildup begin to develop once eGFR falls below 60 ml/min/1.73 m2 [4, 18]. Such factors trigger myocardial and endothelial dysfunction, resultant inflammation and oxidative stress, leading to vascular microcalcification and myocardial fibrosis. The cascade then manifests on a macrovascular level with reduced myocyte contractility, left ventricular hypertrophy (LVH) and/or dilation, ensuing heart failure (Fig. 3). At the level of the kidneys, glomerular sclerosis and renal parenchymal fibrosis are concomitantly observed [1, 3, 4, 7, 14, 19].
Fig. 3.
Overview of pathophysiologic mechanisms implicated in cardiovascular structural changes in the setting of CKD. Figure from Ronco et al. (2008) [20]
Microvascular Pathways and Changes
Cardiorenal syndrome can be thought of as a spectrum of disease with several microvascular pathways and mediators which all culminate in the final common denominator of fibrosis. An important aspect to consider is that many of these microvascular mechanisms are closely interlinked; thus, it is difficult to independently define a specific pathophysiologic role to each one individually.
The Renin–Angiotensin–Aldosterone System and Sympathetic Nervous System
The neurohormonal axis, composed of the renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system (SNS) is one such pathway. Chronic, persistent RAAS activation observed in renal disease patients occurs due to dysregulated renal blood flow. In addition to the net effect of sodium and water retention, elevated levels of aldosterone, and angiotensin II have numerous downstream implications within the inflammatory cascade and cardiac myocytes. Angiotensin II, a powerful vasoconstrictor, reduces coronary blood flow, and ensuing ischemia triggers an inflammatory cascade which then facilitates myocardial injury and necrosis, that then stimulates myocardial fibrosis and causes maladaptive remodeling and hypertrophy on the macrovascular level [21, 22]. Interestingly, in vivo studies on mice also suggested that circulating levels of angiotensin II may be cardioprotective following ischemic reperfusion injury [23], with one proposed mechanism stipulating that reduced coronary blood flow reduces the arrival of inflammatory mediators and thus limits inflammation; a hypothesis that will need validation in other models. Furthermore, several smaller peptides believed to be cleaved from angiotensin II, referred to angiotensin 1–7, can have cardioprotective effects [23]. Ongoing research is required to further delineate such mechanisms.
In conjunction with the RAAS, dysregulated and persistent SNS activation, a sequala of CKD, furthers maladaptive cardiac remodeling and dysfunction. Catecholamine release induces vasoconstriction within the kidneys, heart, and peripheral vasculature. Renal vasoconstriction triggers renin release and RAAS activation. Coronary vasoconstriction leads to myocyte ischemia. Catecholamines, in conjunction with angiotensin II and renin, stimulate tumor necrosis factors and interleukins, which in turn trigger endothelial dysfunction, myocyte apoptosis and necrosis, and fibroblast activation [23].
Inflammation and Cellular Mediators of Remodeling
The inflammatory cascade is important to consider as one of the principal drivers of structural heart changes observed in the setting of CKD. As previously discussed, reduction in eGFR below 60 ml/min/1.73m2 correlates with onset of a chronic inflammatory state [4, 7]. Neurohormonal activation, angiotensin II, catecholamines, uremic toxins activate pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα), interleukin-1 (IL-1), interleukin-6 (IL-6), and transforming growth factor beta (TGFβ) [1, 15, 21, 24, 25]. Renal impairment causes a systemic imbalance of superoxide and reactive oxygen species, which also contributes [22]. Such molecules trigger endothelial dysfunction and disrupt the delicate homeostasis of cellular membranes and mitochondria, leading to premature apoptosis and necrosis. Excessive endothelin-1 production within cardiomyocytes, for example, has been implicated in onset of LVH, as has persistent oxidative stress [1, 21, 24, 26, 27]. In particular, IL-6, a pro-inflammatory cytokine that has received significant attention as a result of its deleterious effects associated with severe COVID-19 infection, is also involved in regulating gene transcription among inflammatory, metabolic, and cell death pathways, and can have such deleterious effects on cardiac myocytes if left unregulated. Decreased nitric oxide availability due to endothelial dysfunction further propagates the above changes [15, 22].
Immune cells including macrophages, mast cells, neutrophils, and fibroblasts subsequently infiltrate the area of injured myocardium, remove the debris, and activate anti-inflammatory cytokines and fibroblasts, which, in mechanisms mediated by toll-like receptors (TLRs), damage-associated molecular patterns (DAMPs) among others, deposit collagen matrix, leading to fibrosis, reduced contractility, and hypertrophy [22]. While several molecules and mechanisms have been identified, the nuanced complexity carried among these systems remains an active area of research and must be further delineated before being effectively carried over to clinical treatments.
Fibroblast growth factor-23 (FGF23) is another mediator that has garnered interest, and, along with the transmembrane protein Klotho, are regarded as potentially having key roles in cardiac remodeling in the presence of CKD [28, 29]. Elevated levels of FGF23 have been implicated in the increased prevalence of LVH and mortality in CKD patients [30]. While the fibroblast growth factor proteins are involved in cellular development, proliferation, and differentiation, FGF23 works within the kidney to regulate phosphorus homeostasis by increasing phosphorus excretion within the proximal convoluted tubule and decreasing concentration of active vitamin D. CKD patients have impaired phosphorus excretion, thus elevated levels of FGF23 [30]. These pathologically elevated levels, in a dose-dependent manner, have been linked to greater risk of cardiovascular events and increased mortality. The study conducted by Faul et al. [30] sought to explain the pathophysiologic link associated with increased circulating FGF23 and CVD. In their proposed model, FGF23 acts upon the FGF receptor (FGFR) signaling cascade within cardiac myocytes, activating a host of tyrosine kinases implicated in signaling pathways that act upon the nucleus to upregulate gene transcription relating to growth and differentiation [30]. Similarly, there is research into the role of micro-RNA and myocyte development. In a model proposed by Bao et al. [31], FGF23, in addition to uremic toxins and TGFβ suppress cardiac micro-RNA 30 (miR-30) expression, a molecule abundant within cardiac myocytes that is implicated in regulation of cell hypertrophy [32–34]. Loss of miR-30 results in unregulated hypertrophy.
Klotho, a transmembrane protein principally expressed in the kidney and parathyroid glands, has been postulated as an important mediator of protective mechanisms against LVH. Klotho acts as a co-receptor which increases binding affinity of FGF23 to FGFR [30]. Though it was originally believed that Klotho and FGF23 acted in conjunction, Faul et al. demonstrated induction of LVH in mice where FGF23 levels were abnormally elevated, but Klotho was absent. Therefore, the role of Klotho ultimately remains to be further delineated for this reason [28]. However, longitudinal cohort studies among dialysis and pre-dialysis patients also did not detect a significant relationship between levels of circulating klotho and cardiovascular outcomes [35].
Fibrosis as the Final Common Pathway
While the initial cardiorenal syndrome classifications defined the different clinical presentations of disease, many questions remained about the underlying pathophysiology. Rather than think of each presentation as a distinct phenotype, cardiorenal syndrome is better described as a spectrum of disease, with tightly woven and interconnected pathways and mediators which ultimately result in tissue fibrosis on the microvascular level [14]. While the main intent of these mechanisms is to preserve cellular function and organ structure, the end result manifests as myocyte hypertrophy, increased stiffness with impaired contractility, chamber dilation, and subsequent HF symptoms. In the kidney, similar fibrotic pathophysiology occurs mediated by many of the same processes [14].
Macrovascular Pathways and Changes
Left Ventricular Hypertrophy and Increased Left Ventricular Mass
In conjunction with microvascular mechanisms, macrovascular changes also contribute to structural changes within the heart, kidneys, and peripheral vasculature. Post-mortem autopsies of CKD patients have identified LVH, diffuse coronary, valvular, and aortic calcifications, in addition to thickened, calcified pericardia [3]. In a study of 120 post-mortem evaluations of end-stage kidney disease (ESKD) patients, treated with hemodialysis (HD) with more than 1 year, 51% had dilated ventricles [36]. Similar findings have been observed using echocardiography. In a retrospective study of 567 ESKD patients initiating hemodialysis, the most common structural abnormality found at baseline was an increased left atrial volume index (81%), followed by grade 2 diastolic dysfunction (78%) and LVH (49%) [37]. Cardiac structural changes begin early during CKD. Individuals with stage 2 or 3 CKD, without prior cardiovascular heart disease, have subtle left ventricular dysfunction with reduced ventricular global systolic strain and higher left ventricular mass index, though overall LVEF remained preserved [38, 39]. Interestingly, the changes in cardiac structure and function with CKD is beyond the hemodynamic effects of concomitant disorders such as hypertension. In a study comparing 293 individuals with stages 2–5 CKD and hypertension to 289 individuals with hypertension without CKD. The CKD cohort had a significantly higher prevalence of echocardiography determined LVH and diastolic dysfunction of 62.8% versus 51.9% in those with only hypertension [40]. Finally, a cross-sectional study with cardiac magnetic resonance imaging of 134 nondiabetic, pre-dialysis patients with CKD stages 2–5 revealed increased myocardial T1 time, suggestive of increased interstitial fibrosis, with decreasing renal function, all in the setting of increased serum biomarker levels of fibrosis [41].
These studies underscore the observations that morphologic changes to the left ventricle begin to manifest in early stages of CKD, though LVEF remains preserved. Those progressing to ESKD carry a high prevalence of increased left ventricular mass, diastolic dysfunction, and impaired contractility by the time dialysis is initiated [21].
Hemodynamic Pathway
In terms of a mechanistic explanation of the abovementioned morphologic changes, several hemodynamic factors come into play [7, 21, 42–45]. As the RAAS system remains activated among CKD patients, this leads to a net retention of sodium and water, causing intravascular volume expansion. Resultant increase in central venous pressure causes elevated renal venous pressure, which reduces renal perfusion and further stimulates renin secretion and SNS activation. Interestingly, the right ventricle may play a greater role than previously thought, though the exact mechanism is unclear due to lack of studies highlighting presence of CRS in isolated right heart disease and absent left heart failure [46]. One proposed hypothesis is that increased RV afterload leads to a reduction in RV filling, thus reducing LV filling and output. Persistent RV pressure overload leads to RV wall dilation, which, within the confines of the pericardial sac can cause septal mechanical dyssynchrony with septal bowing into the LV, further reducing LV filling and stroke volume [1, 46]. Presence of RV dysfunction carries an independent association with increased mortality among a cohort of ESKD patients about to initiate hemodialysis [37], therefore further research is necessary on RV mechanistic properties.
At the microvascular level, peripheral venous congestion stresses vascular endothelium, causing phenotypic conversion of these cells into a pro-inflammatory state, accelerating calcification and fibrosis [1, 47]. Anemia of CKD is another contributor to hemodynamic imbalance, whereby reduced oxygen delivery to the endocardium perpetuates myocardial ischemia and triggers fibrosis, increased ventricular mass, and eventual reduced systolic function [48].
Afterload-related factors, such as chronic hypertension and decreased peripheral vascular compliance increase systemic vascular resistance. Arterio-venous fistula creation for hemodialysis can serve as high volume circulatory shunts, worsening intravascular volume overload and right ventricular workload [3, 12, 21]. Maladaptive myocardial cell thickening and concentric LVH in response to these factors may preserve LVEF during early course of CKD, but ultimately can lead to systolic failure [14, 21].
Peripheral Arterial Stiffness
Reduced peripheral vascular compliance also plays an important role in progressive left ventricular dysfunction in patients with CKD. It has been independently associated with increased cardiovascular risk, whereas reduced stiffness is associated with improved survival [25]. Arterial fibrosis occurs via similar microvascular pathways described above for the myocardium, leading to inflammation, calcification, and fibrosis of the artery vessel wall. In a mechanism set forth by Zanoli et al., elevated levels of circulating TNFα and decreased endothelial nitric oxide synthase (eNOS) expression allows for the local increase in oxygen radicals that trigger endothelial injury [25]. TNFα also upregulates low-density lipoprotein receptors, increases alkaline phosphatase expression, and reduces α-smooth muscle actin protein expression, ultimately stimulating inflammation and microcalcifications within vessel walls [25, 49]. Studies involving patients with inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease showed that anti-TNF agents may lead to reversal of inflammation-dependent aortic stiffening, and thus may represent a class of therapies to investigate in CKD patients as well [25].
Hemodialysis and Renal Transplant Implications on Cardiac Structure
Currently, medical options for ESKD patients aimed at reducing cardiovascular complications are limited. Evidence does exist that increasing the number of dialysis sessions reduces ventricular mass and lowers risk of cardiovascular-related hospitalizations and death [21, 50]. However, this potential benefit must be weighed against the risk of vascular access issues, complications, and infection [12]. Renal transplantation can also provide some degree of reversal in cardiac dysfunction, though this reversal is less prominent in those who have been undergoing hemodialysis for a longer period [51–54].
Arrhythmogenicity and Sudden Cardiac Death
Compared to the general population, patients with CKD have increased risk of atrial fibrillation, ventricular arrhythmias, and a 4–20 times greater risk of sudden cardiac death (SCD), with risk increasing as renal function declines [55, 56]. The mechanism is thought to be due to cardiac fibrosis, which causes high-resistance pathways within the electrical conduction system that delay the physiologic action potential, favors re-entry pathways and induces arrhythmogenicity [21].
To date, no therapy has been shown to decrease the risk of SCD. In a recent prospective, randomized controlled trial 200 ESRD patients on hemodialysis with an LVEF of ≥ 35% underwent implantable cardioverter-defibrillator (ICD) insertion with the intent to prevent SCD; however, the study was stopped due to futility as both arms experienced similar rates of SCD and 5-year survival [19]. Prophylactic implantable cardioverter-defibrillator therapy did not reduce the rate of SCD suggesting that terminal tachy- or bradyarrhythmia’s may be more related to systemic processes than immediately reversible cardiac etiologies [19].
Atrial fibrillation is the most pervasive arrhythmia within the CKD population and stage progression. Although no distinct causative relationship has been demonstrated between CKD and atrial fibrillation, the association is strong as both diseases share several risk factors including inflammation, oxidative stress, and fibrosis [56]. With regard to treatment strategies, the risk of stroke must be weighed carefully against the risk of bleeding, particularly in ESKD, as the overall benefit in this population is offset due to a greater risk of bleeding. Currently, there are no proven approaches to guide clinical decision making within this population, further compounded by a lack of randomized controlled trials [57]. Though apixaban has been approved for use in end-stage renal disease, it has never been studied in randomized control trials with respect to efficacy [56].
Conclusion
Cardiorenal syndromes are a spectrum of diseases with many clinical phenotypes. Though fibrosis represents a common final mechanistic pathway resulting in symptoms of HF, arrhythmias, and death, preceding fibrosis are several microvascular mechanisms are at play. These can be finely interwoven with renal pathophysiology such that it is challenging to isolate a particular point to develop a therapeutic strategy. The RAAS system is perhaps the best understood, and pharmacologic control of this system via ACE or ARB blockade has been used to attenuate the maladaptive remodeling responses within the heart and kidneys and improve outcomes in HF patients as well as attenuate CKD progression. Finally, the recent successes with sodium-glucose cotransporter-2 (SGLT2) Inhibitors in diabetic patients with early stages of CKD to prevent both CVD and reduce CKD progression is an exciting development and with increased mechanistic insights may open the door to additional effective treatment paradigms for patients with type 4 cardiorenal syndrome [58, 59].
Abbreviations
- ACR
Albumin creatinine ratio
- ADQI
Acute dialysis quality initiative
- AKI
Acute kidney injury
- AMI
Acute myocardial infarction
- CKD
Chronic kidney disease
- CVA
Cerebral vascular accident
- CVD
Cardiovascular disease
- eGFR
Estimated glomerular filtration rate
- ESKD
End-stage kidney disease
- FGFR
Fibroblast growth factor receptor
- FGF23
Fibroblast growth factor-23
- HD
Hemodialysis
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- IL
Interleukin
- KDIGO
Kidney disease improving global outcomes
- LDL
Low-density lipoprotein
- LVEF
Left ventricular ejection fraction
- LVH
Left ventricular hypertrophy
- MI
Myocardial infarction
- MMP
Matrix metalloproteinases
- RAAS
Renin-angiotensin-aldosterone system
- SCD
Sudden cardiac death
- TGFβ
Transforming growth factor beta
- TIA
Transient ischemic attack
- TNFα
Tumor necrosis factor alpha
- USRDS
United States renal data system
Author Contribution
AM and LG were the primary authors who reviewed the literature and wrote the initial draft of this review manuscript. Cd reviewed the manuscript for accuracy and edited the contents prior to submission. All authors take responsibility for the accuracy and content of the manuscript.
Data Availability
All data used to create this review manuscript is publicly available and cited in the references.
Declarations
Conflict of Interest
None associated with this manuscript. Unrelated to the manuscript include Dr. deFilippi receives consulting income from Abbott Diagnostics, FujiRebio, Ortho Diagnostics, Quidel, Roche Diagnostics, and Siemens Healthineers. Dr. deFilippi receives royalties from UpToDate. Other authors have no COI or competing interests to declare.
Footnotes
This article is part of the Topical Collection on Medicine
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139(16):e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31(6):703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullough PA, Roberts WC. Influence of chronic renal failure on cardiac structure. J Am Coll Cardiol. 2016;67(10):1183–1185. doi: 10.1016/j.jacc.2015.11.065. [DOI] [PubMed] [Google Scholar]
- 4.Clementi A, Virzì GM, Goh CY, et al. Cardiorenal syndrome type 4: a review. Cardiorenal Med. 2013;3(1):63–70. doi: 10.1159/000350397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuegel C, Bansal N. Heart failure in patients with kidney disease. Heart. 2017;103(23):1848–1853. doi: 10.1136/heartjnl-2016-310794. [DOI] [PubMed] [Google Scholar]
- 6.Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med. 2015;5(4):254–266. doi: 10.1159/000435838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Renal Data System. 2020 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://adr.usrds.org/2020/chronic-kidney-disease/4-cardiovascular-disease-in-patients-with-ckd (Accessed November 2, 2022)
- 9.Chronic Kidney Disease in the United States, 2021. Published March 9, 2021. https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html (Accessed November 2, 2022)
- 10.United States Renal Data System. 2021 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://adr.usrds.org/2021/chronic-kidney-disease/1-ckd-in-the-general-population (Accessed November 2, 2021)
- 11.Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. 2022;18(11):696–707. doi: 10.1038/s41581-022-00616-6. [DOI] [PubMed] [Google Scholar]
- 12.House AA, Wanner C, Sarnak MJ, et al. Heart failure in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;95(6):1304–1317. doi: 10.1016/j.kint.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Cardio-Renal Connections in Heart Failure and Cardiovascular Disease. Published August 20, 2004. https://www.nhlbi.nih.gov/events/2004/cardio-renal-connections-heart-failure-and-cardiovascular-disease (Accessed October 20, 2022)
- 14.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138(9):929–944. doi: 10.1161/CIRCULATIONAHA.117.028814. [DOI] [PubMed] [Google Scholar]
- 15.Braam B, Joles JA, Danishwar AH, Gaillard CA. Cardiorenal syndrome–current understanding and future perspectives. Nat Rev Nephrol. 2014;10(1):48–55. doi: 10.1038/nrneph.2013.250. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17(4):302–307. doi: 10.1053/j.ackd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Jukema JW, Timal RJ, Rotmans JI, et al. Prophylactic use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation. 2019;139(23):2628–2638. doi: 10.1161/CIRCULATIONAHA.119.039818. [DOI] [PubMed] [Google Scholar]
- 20.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S79–91. doi: 10.2215/CJN.04860709. [DOI] [PubMed] [Google Scholar]
- 22.Junho CVC, Trentin-Sonoda M, Panico K, et al. Cardiorenal syndrome: long road between kidney and heart. Heart Fail Rev. 2022;27(6):2137–2153. doi: 10.1007/s10741-022-10218-w. [DOI] [PubMed] [Google Scholar]
- 23.Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14(1):30–38. doi: 10.1038/nrcardio.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa T, Koeda M, Nitta K. Left ventricular diastolic dysfunction in end-stage kidney disease: pathogenesis, diagnosis, and treatment. Ther Apher Dial. 2015;19(5):427–435. doi: 10.1111/1744-9987.12301. [DOI] [PubMed] [Google Scholar]
- 25.Zanoli L, Lentini P, Briet M, et al. Arterial stiffness in the heart disease of CKD. J Am Soc Nephrol. 2019;30(6):918–928. doi: 10.1681/ASN.2019020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathaniel R. Left ventricular hypertrophy regression and allopurinol. J Am Coll Cardiol. 2013;62(24):2294–2296. doi: 10.1016/j.jacc.2013.08.695. [DOI] [PubMed] [Google Scholar]
- 27.Hutchings DC, Anderson SG, Caldwell JL, Trafford AW. Phosphodiesterase-5 inhibitors and the heart: compound cardioprotection? Heart. 2018;104(15):1244–1250. doi: 10.1136/heartjnl-2017-312865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slavic S, Ford K, Modert M, et al. Genetic ablation of Fgf23 or Klotho does not modulate experimental heart hypertrophy induced by pressure overload. Sci Rep. 2017;7(1):11298. doi: 10.1038/s41598-017-10140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho BB, Bergwitz C. FGF23 signalling and physiology. J Mol Endocrinol. 2021;66(2):R23–R32. doi: 10.1530/JME-20-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao J, Lu Y, She Q-Y, et al. MicroRNA-30 regulates left ventricular hypertrophy in chronic kidney disease. JCI Insight. Published online April 13, 2021. 10.1172/jci.insight.138027 [DOI] [PMC free article] [PubMed]
- 32.Mao L, Liu S, Hu L, et al. miR-30 family: a promising regulator in development and disease. Biomed Res Int. 2018;2018:9623412. doi: 10.1155/2018/9623412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganesan J, Ramanujam D, Sassi Y, et al. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation. 2013;127(21):2097–2106. doi: 10.1161/CIRCULATIONAHA.112.000882. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa Y, Nishikimi T, Kuwahara K, et al. MiR30‐GALNT1/2 axis‐mediated glycosylation contributes to the increased secretion of inactive human prohormone for brain natriuretic peptide (proBNP) from failing hearts. J Am Heart Assoc. 2017;6(2):003601. doi: 10.1161/JAHA.116.003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandenburg VM, Kleber ME, Vervloet MG, et al. Soluble Klotho and mortality: the Ludwigshafen risk and cardiovascular health study. Atherosclerosis. 2015;242(2):483–489. doi: 10.1016/j.atherosclerosis.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Roberts WC, Taylor MA, Shirani J. Cardiac findings at necropsy in patients with chronic kidney disease maintained on chronic hemodialysis. Medicine. 2012;91(3):165–178. doi: 10.1097/MD.0b013e318256e076. [DOI] [PubMed] [Google Scholar]
- 37.Hickson LJ, Negrotto SM, Onuigbo M, et al. Echocardiography criteria for structural heart disease in patients with end-stage renal disease initiating hemodialysis. J Am Coll Cardiol. 2016;67(10):1173–1182. doi: 10.1016/j.jacc.2015.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards NC, Hirth A, Ferro CJ, Townend JN, Steeds RP. Subclinical abnormalities of left ventricular myocardial deformation in early-stage chronic kidney disease: the precursor of uremic cardiomyopathy? J Am Soc Echocardiogr. 2008;21(12):1293–1298. doi: 10.1016/j.echo.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Hassanin N, Alkemary A. Early Detection of subclinical uremic cardiomyopathy using two-dimensional speckle tracking echocardiography. Echocardiography. 2016;33(4):527–536. doi: 10.1111/echo.13120. [DOI] [PubMed] [Google Scholar]
- 40.Nardi E, Mulè G, Giammanco A, et al. Left ventricular hypertrophy in chronic kidney disease: a diagnostic criteria comparison. Nutr Metab Cardiovasc Dis. 2021;31(1):137–144. doi: 10.1016/j.numecd.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 41.Hayer MK, Radhakrishnan A, Price AM, et al. Defining myocardial abnormalities across the stages of chronic kidney disease: a cardiac magnetic resonance imaging study. JACC Cardiovasc Imaging. 2020;13(11):2357–2367. doi: 10.1016/j.jcmg.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malík J, Tuka V, Mokrejšová M, Holaj R, Tesar V. Mechanisms of chronic heart failure development in end-stage renal disease patients on chronic hemodialysis. Physiol Res. 2009;58(5):613–621. doi: 10.33549/physiolres.931614. [DOI] [PubMed] [Google Scholar]
- 43.Ritz E, Wanner C. The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol. 2008;3(3):920–929. doi: 10.2215/CJN.04571007. [DOI] [PubMed] [Google Scholar]
- 44.Gross M-L, Ritz E. Hypertrophy and fibrosis in the cardiomyopathy of uremia–beyond coronary heart disease. Semin Dial. 2008;21(4):308–318. doi: 10.1111/j.1525-139X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 45.Ritz E. Left ventricular hypertrophy in renal disease: beyond preload and afterload. Kidney Int. 2009;75(8):771–773. doi: 10.1038/ki.2009.35. [DOI] [PubMed] [Google Scholar]
- 46.Bansal S, Prasad A, Linas S. Right heart failure-unrecognized cause of cardiorenal syndrome. J Am Soc Nephrol. 2018;29(7):1795–1798. doi: 10.1681/ASN.2018020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganda A, Onat D, Demmer RT, et al. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr Heart Fail Rep. 2010;7(2):66–74. doi: 10.1007/s11897-010-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiner DE, Tighiouart H, Vlagopoulos PT, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16(6):1803–1810. doi: 10.1681/ASN.2004070597. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Ma KL, Gao M, et al. Inflammation disrupts the LDL receptor pathway and accelerates the progression of vascular calcification in ESRD patients. PLoS ONE. 2012;7(10):e47217. doi: 10.1371/journal.pone.0047217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCullough PA, Chan CT, Weinhandl ED, Burkart JM, Bakris GL. Intensive hemodialysis, left ventricular hypertrophy, and cardiovascular disease. Am J Kidney Dis. 2016;68(5S1):S5–S14. doi: 10.1053/j.ajkd.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 51.Wali RK, Wang GS, Gottlieb SS, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005;45(7):1051–1060. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 52.Hawwa N, Shrestha K, Hammadah M, Yeo PSD, Fatica R, Tang WHW. Reverse remodeling and prognosis following kidney transplantation in contemporary patients with cardiac dysfunction. J Am Coll Cardiol. 2015;66(16):1779–1787. doi: 10.1016/j.jacc.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hewing B, Dehn AM, Staeck O, et al. Improved left ventricular structure and function after successful kidney transplantation. Kidney Blood Press Res. 2016;41(5):701–709. doi: 10.1159/000450559. [DOI] [PubMed] [Google Scholar]
- 54.Patel RK, Mark PB, Johnston N, McGregor E, Dargie HJ, Jardine AG. Renal transplantation is not associated with regression of left ventricular hypertrophy: a magnetic resonance study. Clin J Am Soc Nephrol. 2008;3(6):1807–1811. doi: 10.2215/CJN.01400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pun PH. The interplay between CKD, sudden cardiac death, and ventricular arrhythmias. Adv Chronic Kidney Dis. 2014;21(6):480–488. doi: 10.1053/j.ackd.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turakhia MP, Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and arrhythmias: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Eur Heart J. 2018;39(24):2314–2325. doi: 10.1093/eurheartj/ehy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belley-Cote EP, Eikelboom JW. Anticoagulation for stroke prevention in patients with atrial fibrillation and end-stage renal disease-first, do no harm. JAMA Netw Open. 2020;3(4):e202237. Published 2020 Apr 1. 10.1001/jamanetworkopen.2020.2237. [DOI] [PubMed]
- 58.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 59.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to create this review manuscript is publicly available and cited in the references.