Abstract
Purpose
The study aims to investigate first the presence of Syncytin 2 and its receptor, MFSD2, in human sperm, and second whether the expressions of Syncytin 1, Syncytin 2, and their receptors, SLC1A5 and MFSD2, differ between normozoospermic, asthenozoospermic, oligozoospermic, and oligoasthenozoospermic human sperm samples.
Methods
The localization patterns and expression levels of syncytins and their receptors were evaluated in normozoospermic (concentration = 88.9 ± 5.5 × 106, motility = 79.2 ± 3.15%, n = 30), asthenozoospermic (concentration = 51.7 ± 7.18 × 106, motility = 24.0 ± 3.12%, n = 15), mild oligozoospermic (concentration = 13.5 ± 2.17 × 106, motility = 72.1 ± 6.5%, n = 15), moderate oligozoospermic (concentration = 8.4 ± 3.21 × 106, motility = 65.1 ± 8.9%, n = 15), severe oligozoospermic (concentration = 2.1 ± 1.01 × 106, motility = 67.5 ± 3.2%, n = 15), and oligoasthenozoospermic (concentration = 5.5 ± 3.21 × 106, motility = 18.5 ± 1.2%, n = 15) samples by immunofluorescence staining and western blot.
Results
Syncytins and their receptors visualized by immunofluorescence showed similar staining patterns with slight staining of the tail in all spermatozoa regardless of normozoospermia, asthenozoospermia, oligozoospermia, or oligoasthenozoospermia. The localization patterns were categorized as equatorial segment, midpiece region, acrosome, and post-acrosomal areas. The combined staining patterns were also detected as acrosomal cap plus post acrosomal region, the midpiece plus equatorial segment, and midpiece plus acrosomal region. However, some sperm cells were categorized as non-stained. Both syncytin proteins were most intensely localized in the midpiece region, while their receptors were predominantly present in the midpiece plus acrosomal region. Conspicuously, syncytins and their receptors showed decreased expression in asthenozospermic, oligozoospermic, and oligoasthenozoospermic samples compared to normozoospermic samples.
Conclusion
The expression patterns of HERV-derived syncytins and their receptors were identical regardless of the spermatozoa in men with normozoospermia versus impaired semen quality. Further, asthenozoospermia, oligozoospermia, and oligoasthenozoospermia as male fertility issues are associated with decreased expression of both syncytins and their receptors.
Keywords: Syncytin 1, Syncytin 2, MFSD2, SLC1A5, Human sperm
Introduction
Membrane fusion is a fundamental process in multicellular organisms important for many events in reproduction, immune reactions, and neurotransmission [1]. Therefore, understanding the mechanism of membrane fusion is essential to understanding several biological processes that can lead to disease [2]. Despite the common features of membrane fusion across different organisms, tissue-specific mechanisms of membrane fusion may vary and have not been fully elucidated [1, 3, 4].
During fertilization, a series of membrane fusion events are required to ensure this process and placenta formation. It occurs through the interaction of spermatozoa and the cumulus-oocyte complex, adhesion, attachment, penetration of spermatozoa into the zona pellucida, and membrane fusion of oocyte and sperm cells. In fact, mammalian fertilization has been extensively studied for many years to discover key factors to elucidate the molecular mechanisms underlying sperm and oocyte fusion [3]. In recent years, especially the discovery of the clustered regularly interspaced short palindromic repeat-knock out (CRISPR-KO) system has enabled the efficient screening and investigation of genes responsible for male fertility in vivo [5]. Four essential factors of the sperm and oocyte fusion have been identified; CD9 and JUNO are expressed in oocytes while modulating female fertility, whereas IZUMO1 and fertilization-affecting membrane protein (FIMP) regulate fusion in sperm [6–8]. In addition to these molecules, other molecules have been identified that participate in membrane fusion on oolemma (integrins, GPI-1-associated protein) and spermatozoa (disintegrin and a metalloprotease (ADAM) [8–10]. Integrins have also been described to participate in sperm-oocyte adhesion [11, 12]. The use of CRISPR-Cas9 technology has led to the recent identification of six new factors as essential factors required for mammalian fertilization: sperm oocyte fusion required protein 1 (SOF1), sperm acrosome membrane–associated protein 6 (SPACA6), transmembrane protein 95 (TMEM95), fertilization influencing membrane protein (FIMP), and dendrocyte expressed seven transmembrane protein domain-containing 1 and 2 (DCST1/2) [5, 6, 13]. However, the details of sperm-oocyte interactions remain a relative mystery despite many decades of research and new discoveries of many candidate molecules.
Human endogenous retroviruses (HERVs) are RNA viruses that can infect the human germline [14–17]. They first entered the primate genome between 25 and 40 million years ago, reviewed by our group [18] and others [15, 16, 19–21]. The HERV family is a class 1 viral fusion protein, akin to those found in viruses such as coronaviruses, influenza viruses, Ebola virus, Lassa virus, and human immunodeficiency virus [22]. HERVs make up about 8% of the human genome [14]. Although most of these viruses have been eliminated through mutations and deletions, some members have been evolutionarily conserved and maintain their expressions. Among these genes, particularly two key genes have been determined to induce cell–cell fusion and placenta formation in humans [23]. These genes encode HERV-W Env glycoprotein (Syncytin 1) [19, 24–26] and HERV-FRD Env glycoprotein (Syncytin 2) [27]. Syncytin 1 is introduced in human syncytiotrophoblasts, and its fusogenic activity is demonstrated in cytotrophoblasts [24]. It induces syncytium formation in placenta by interacting with the D-Type mammalian retrovirus receptor (ASCT2, SLC1A5, neutral amino acid transporter) [25, 28]. Therefore, Syncytin 1 is increasingly expressed in syncytiotrophoblast cells throughout pregnancy [24, 25, 29]. On the other hand, Syncytin 2 (HERV-FRD) is another member of the HERV family with fusogenic activities as a placental membrane protein [27, 30]. While syncytins induce cell fusions, their physiologic roles may go beyond that, for instance Syncytin 1 may also regulate the production of inflammatory mediators [31]. Syncytin 2 differs from Syncytin 1 by having an immunosuppressive domain that potentially plays a role in the protection of fetus from the maternal immune system [27, 30] and by interacting with a different receptor, major facilitator superfamily domain containing 2 (MFSD2), to mediate cell–cell fusion [32].
Altered expressions of syncytin proteins and their receptors were reported in various placental pathologies such as preeclampsia [33, 34], intrauterine growth restriction (IUGR) [35–37], and gestational diabetes mellitus [38, 39]. In addition to their fusogenic activity in placenta, syncytins are involved in cell fusion events in bone marrow (osteoclasts) [40], various cancers [41–49], and in different cell lines such as muscle [50], endometrial [45], colorectal [51], and breast tumor [52].
Considering the well-studied fusogenic activity of syncytin proteins, Bjerregaard and colleagues investigated the presence of Syncytin 1 and its receptor in human gametes [53]. The study revealed that Syncytin 1 is expressed at both mRNA and protein levels in human spermatozoa. The localization of Syncytin 1 is mainly observed in the acrosomal region or equatorial segment of spermatozoa together with mild expression in the midpiece and the tail. Its receptor, SLC1A5, is expressed in the acrosomal and tail regions of spermatozoa. Interestingly, Syncytin 1 expression is not detected in human oocytes, while its receptor SLC1A5 is present in oocytes [53]. However, samples with only normal semen quality were included. In line with this study, Enoiu and colleagues investigated Syncytin 1 and other membrane fusion proteins in spermatozoa from men experiencing total fertilization failure during IVF by immunofluorescence [54]. The authors reported a similar localization pattern with previous study by Bjerregaard and colleagues that Syncytin 1 was present predominantly in the acrosomal cap region and also slightly in the midpiece and tail regions of human sperm [54]. Bergallo and colleagues also showed that there are several retroviral mRNAs including Syncytin 1 in the human sperm, although in low amounts [55]. However, to the best of our knowledge, there is only one study available regarding the presence of Syncytin 2 in human sperm showing that Syncytin 2 is transcribed in spermatozoa, although at lower levels than Syncytin 1 by real-time PCR experiments at mRNA level [55]. Thus, no information is currently available on the localization patterns of Syncytin 2 or the existence of its receptor in human gametes.
One of the most serious social problems faced by developed countries today is the decreasing fertility rate. Impairment of male fertility may occur in the case of insufficient number of motile sperm, unsuccessful sperm-zona pellucida interaction, incomplete acrosome reactivity, and insufficiency of sperm function such as oocyte penetration [56]. However, assessment of male infertility potential is based on standard semen analysis (total sperm number, total and progressive motility, vitality, sperm concentration, sperm morphology) [57]. According to the World Health Organization, men whose sperm parameters are below normal values are considered to have male factor-related infertility [58]. Oligozoospermia is a male reproductive problem characterized by low sperm counts, and the sperm concentrations that drop below 15 million sperm per milliliter in the semen sample. Severe oligozoospermia is often accompanied by poor sperm motility, viability, and morphology reflecting qualitative and quantitative defects in spermatogenesis [59–61]. Natural fertility prospects are also poor with extreme oligozoospermia [59, 60]. In addition, there is an increased incidence of total fertilization failure and a low fertilization rate during conventional in vitro fertilization treatments involving men with poor sperm motility [62, 63]. Given the high fusogenic potential of syncytin proteins, our study aimed to investigate first the presence of Syncytin 2 and its receptor, MFSD2, in human sperm, and then, to evaluate whether the protein localization patterns and expression levels of syncytins and their receptors differ between normozoospermic, asthenozoospermic, oligozoospermic, and oligoasthenozoospermic human sperm samples.
Materials and methods
Selection of study samples
After routine semen analyses at the Andrology Laboratory, Department of Urology, Akdeniz University School of Medicine, Antalya, Turkey, the leftover de-identified semen samples were studied. Normozoospermic (concentration = 88.9 ± 5.5 × 106, motility = 79.2 ± 3.15, n = 30), asthenozoospermic (concentration = 51.7 ± 7.18 × 106, motility = 24.0 ± 3.12, n = 15), oligozoospermic, and oligoasthenozoospermic (concentration = 5.5 ± 3.21 × 106, motility = 18.5 ± 1.2%, n = 15) human sperm samples were included in the study. Oligozoospermia was further classified as mild oligozoospermia (between 10 and 15 million sperm/mL), moderate oligozoospermia (between 5 and 10 million sperm/mL), and severe oligozoospermia (less than 5 million sperm/mL) [64]. The comparisons of mild oligozoospermic (concentration = 13.5 ± 2.17 × 106, motility = 72.1 ± 6.50%, n = 15), moderate oligozoospermic (concentration = 8.4 ± 3.21 × 106, motility = 65.1 ± 8.9%, n = 15), and severe oligozoospermic (concentration = 2.1 ± 1.01 × 106, motility = 67.5 ± 3.2%), n = 15) sperm samples were also performed. The average ages of the patients in the normozoospermic, asthenozoospermic, mild oligozoospermic, moderate oligozoospermic, severe oligozoospermic, and oligoastenozoospermic groups were 38.00 ± 1.48, 36.00 ± 1.08, 37.00 ± 2.55, 35.00 ± 1.88, 38.00 ± 1.53, and 36.00 ± 1.15, respectively. However, there was no statistically significant age difference between the patient groups (p = 0.357). The samples were collected by masturbation into sterile wide mouth plastic jars following 2–5 days of sexual abstinence. Samples were allowed to liquefy at room temperature; then, sperm concentrations and motility were assessed according to WHO criteria 2010 [65]. Patients with ages of 18–40, sperm count not less than 1 million, the duration of sexual abstinence not less than 2 days or more than 5 days, have no known health problem, cancer, urogenital or genetic disease, no drug used continuously that can affect sperm parameters recently, those who have not received any treatment or surgery in the past due to the male factor, no chronical use of cigarettes, alcohol, and addictive substances were included in the study. All studies were approved by Ethical Committee of Akdeniz University School of Medicine (2012-KAEK-20).
Preparation of sperm samples and immunofluorescence staining
The preparation of sperm samples was carried out similarly as previously reported [66–68]. A sufficient amount of saline-imidazole (SAIM) solution was added to the fresh semen and centrifuged at 500 g for 18 min. After discarding the supernatant, 1–2 ml of SAIM solution was added to the pellet and resuspended. Small drops of suspension were placed on Poly-L-lysine-coated slides drawn with pap-pen, and 5% PB-sucrose (PBS + sucrose) solution was dripped onto it, paying attention to the absence of excessive sperm under the microscope. The slides were kept at + 4 °C overnight. The next day, the solution on the slides was removed, fixation solution was dripped onto the slides and kept at room temperature for 20 min. The excess solution was removed, and PB-sucrose solution was dripped 3 times on the slides. In the last step, the PB-sucrose solution was removed, and the slides were dried at room temperature.
Slides were washed three times with PB-sucrose at room temperature and blocked with 3% bovine serum albumin (BSA) for 1 h at room temperature. Primary antibodies (syncytin 1, syncytin 2, SLC1A5 and MFSD2) were diluted with 0.1% BSA and incubated at + 4 °C overnight (Table 1). Samples were washed three times with PB-sucrose at room temperature and incubated with secondary antibodies for 45 min at room temperature (Table 1). Following the washing step, slides were incubated with fluorescein isothiocyanate (FITC)-labeled Avidin D (Vector Laboratories) diluted in PB-sucrose for 30 min at room temperature. After washing with PB-sucrose, DAPI solution was added and the slides were kept in the dark for 10 min, washed with PB-sucrose solution three times for 5 min and mounted with antifade solution. Samples were left in the dark for 1–2 h and examined using a fluorescent microscope (Olympus BX61 Fully Motorized Fluorescence Microscope). At least 200 sperm cells were counted from each patient, and sperm cells were categorized according to their staining patterns as previously described [69].
Table 1.
Primary and secondary antibodies used for immunofluorescence (IF) staining and Western blot
Protein extraction from semen samples
The volume, concentration, and motility of freshly collected semen samples were calculated. Up to 10 ml of PB-sucrose solution was added to the tubes containing semen samples, centrifuged at + 4 °C, 2500 rpm for 10 min. The pellets of sperm samples were resuspended in lysis buffer and protease inhibitor cocktail (Sigma-Aldrich, MO, USA), further incubated, and homogenized by using a sonicator. After 1 h of incubation at + 4 °C, samples were centrifuged for 15.000 g at + 4 °C. The supernatants were collected, and stored at − 20 °C.
SDS-PAGE and Western blotting
Immunoblotting of semen samples was carried out as previously described [70]. The protein concentration was determined by a detergent compatible protein assay (Bicinchoninic Acid Kit for Protein Determination BCA1-1KT, Sigma). Samples (50 µg) were loaded on 10% Tris–HCl gels, electrophoretically separated, and electroblotted onto PVDF membrane (Bio-Rad Laboratories, CA, USA). The membranes were blocked with 5% non-fat dry milk in Tris buffered saline (TBS) containing 0.1% Tween 20 (TBS-T) for 1 h to reduce non-specific binding. The membranes were incubated at + 4 °C overnight with primary antibodies (Table 1), washed in TBS-T for 1 h then incubated with horseradish peroxidase (HRP) conjugated secondary antibodies for 1 h at room temperature. Following the final wash in TBS-T, the membranes were incubated with the Super Signal Chemiluminescence (CL)-HRP substrate system (Thermo Scientific, USA) for 5 min and the signals on the membranes were transferred to the hyperfilm (Amersham Biosciences; Buckinghamshire, England) in the dark room. Protein levels of syncytin 1, syncytin 2, SLC1A5, and MFSD2 were compared semiquantitatively with ImageJ 1.46 (NIH) analysis by normalizing to GAPDH.
Statistical analysis
All data was exported directly to the SigmaStat® 3.5 (Systat Software; San Jose, USA) program. The data obtained from patients with different impaired semen quality groups were compared with the data obtained from normozoospermic patient groups. The quantitative data was submitted to normality tests by the Kolmogorov–Smirnov test. Data were compared using one-way ANOVA followed by Holm–Sidak post hoc test for the data passed the normality test; otherwise, the Kruskal–Wallis test was followed by Dunn’s test. All data were expressed as mean ± SEM and statistical significance was defined as p < 0.05.
Results
Immunolocalization of Syncytin 1 and its receptor SLC1A5 in normozoospermic, asthenozoospermic, oligozoospermic, and oligoasthenozoospermic human sperm samples
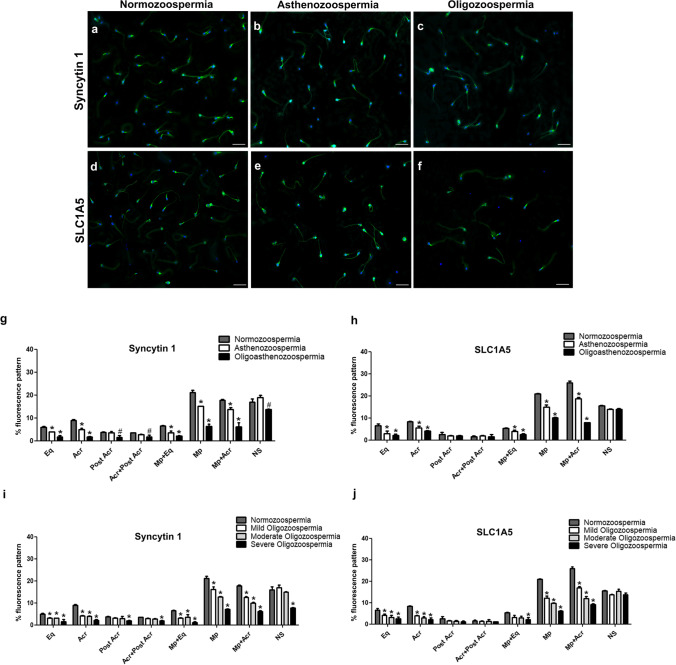
Detailed immunocytochemical analysis revealed similar localization pattern for Syncytin 1 and SLC1A5 with a slight staining of the tail in all spermatozoa. Both proteins were also localized in the regions of (1) the equatorial segment, (2) the acrosomal cap, (3) the post acrosomal region, (4) the acrosomal cap plus post acrosomal region, (5) the midpiece plus equatorial segment, (6) the midpiece region, and (7) the combined midpiece plus acrosomal region (Fig. 1). Comparison of the expression these patterns between normozoospermic, asthenozoospermic, oligozoospermic (mild, moderate, severe), and oligoasthenozoospermic samples revealed differences with varying percentages. There was a decreased percentage of Syncytin 1 immunoreactivity in the equatorial segment, the acrosomal cap, the midpiece plus equatorial segment, only the midpiece region, and the combined midpiece plus acrosomal region of asthenozoospermic and oligoasthenozoospermic sperm samples when compared to normozoospermic sperm samples (p < 0.001) (Fig. 2a, b, g). A significant decrease was detected in the percentage of sperm with the post acrosomal region and the acrosomal cap plus post acrosomal region staining patterns in the oligoasthenozoospermic sperm samples compared to the normozoospermic sperm samples (p < 0.05) (Fig. 2g). On the other hand, we found a significant decrease in the percentage of acrosomal cap, only the midpiece region, the combined midpiece plus acrosomal region staining patterns and unstained sperm in oligoasthenozoospermic sperm samples compared to asthenozoospermic sperm samples (p < 0.001) (Fig. 2g).
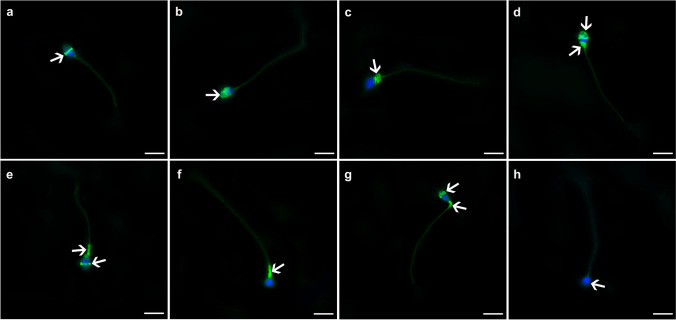
Fig. 1.
Immunofluorescence staining patterns of syncytins and their receptors. a The equatorial segment, b the acrosomal cap, c the post acrosomal region, d the acrosomal cap plus post acrosomal region, e the midpiece plus equatorial segment, f the midpiece region, g the combined midpiece plus acrosomal region, h non-stained sperm. Green: FITC; blue: DAPI. The scale bars represent 50 µm
Fig. 2.
Immunofluorescence localization of Syncytin 1 (a, b, c) and its receptor SLC1A5 (d, e, f) in normozoospermic, asthenozoospermic, and oligozoopermic human sperm samples. The percentage (%) of immunofluorescence staining patterns for Syncytin 1 (g, i) and SLC1A5 (h, j). Eq, equatorial segment; Acr, acrosomal cap; Post Acr, post acrosomal region; Acr + Post Acr, acrosomal cap and post acrosomal region; Mp + Eq, midpiece region and equatorial segment; Mp, midpiece region; Mp + Acr, midpiece region and acrosomal cap; NS, non-stained sperm. Green: FITC; blue: DAPI. The scale bars represent 50 µm. p values indicate the statistical comparisons between the sperm samples with normozoospermia and different patient categories. #p < 0.05, *p < 0.001
Regarding oligozoospermic sperm samples, we detected a decreased percentage of Syncytin 1 immunoreactivity in the equatorial segment, the acrosomal cap, the midpiece plus equatorial segment, only the midpiece region, and the combined midpiece plus acrosomal region of all oligozoospermic sperm samples when compared to normozoospermic sperm samples (p < 0.001) (Fig. 2a, c, i). On the other hand, a significant decrease was observed in both moderate and severe oligozoospermia groups compared to the mild oligozoospermia group especially in the midpiece region and combined midpiece plus acrosome region staining patterns (p < 0.001) (Fig. 2i). The decrease in the severe oligozoospermia was also statistically significant for the percentages of midpiece region and combined midpiece plus acrosome region staining compared to the percentages for moderate oligozoospermia (p < 0.001). In addition, a significant decrease was determined in the staining patterns of the midpiece plus equatorial segment, and the percentage of non-stained sperm in the severe oligozoospermia group compared to the mild and moderate oligozoospermia patient groups (p < 0.001) (Fig. 2i) even though no significant differences were found between the mild and moderate oligozoospermia groups for these localization patterns (p > 0.05) (Fig. 2i).
SLC1A5 immunoreaction was significantly decreased in the equatorial segment, the acrosomal cap, the midpiece plus equatorial segment, only the midpiece region, and the combined midpiece plus acrosomal region of asthenozoospermic and oligoasthenozoospermic sperm samples compared to the normozoopermic sperm samples (p < 0.001) (Fig. 2d, e, h). Moreover, there was a significant decrease in the percentage of the midpiece region and the combined midpiece plus acrosomal region staining patterns in oligoasthenozoospermic sperm samples compared to asthenozoospermic sperm samples (p < 0.001) (Fig. 2h). In contrast, no significant difference was observed in the percentage of the post acrosomal region, the acrosomal cap plus post acrosomal region staining patterns and the percentage of non-stained sperm between these patient categories (p > 0.05) (Fig. 2h).
Similarly, a statistically significant decrease in SLC1A5 expression was detected for the percentage of equatorial segment, the acrosomal cap, only the midpiece region, and the combined midpiece plus acrosomal region staining patterns in all of the oligozoospermic sperm samples compared to the normozoospermic samples (p < 0.001) (Fig. 2d, f, j). When the subcategories of oligozoospermic patients were compared, a significant decrease was determined in both moderate and severe oligozoospermia patient groups compared to the mild oligozoospermic patient groups in the midpiece region and combined midpiece plus acrosome region localization patterns (p < 0.001) (Fig. 2j). The decreased percentage of staining patterns in the midpiece region and combined midpiece plus acrosome region were also statistically significant in the severe oligozoospermia compared to moderate oligozoospermia (p < 0.001) (Fig. 2j). In fact, there were no statistically significant differences in the percentage of staining patterns for post acrosomal region, the acrosomal cap plus post acrosomal region, and the percentage of non-stained sperm (p > 0.05) (Fig. 2j).
Immunolocalization of Syncytin 2 and its receptor MFSD2 in normozoospermic, asthenozoospermic, oligozoospermic, and oligoasthenozoospermic human sperm samples
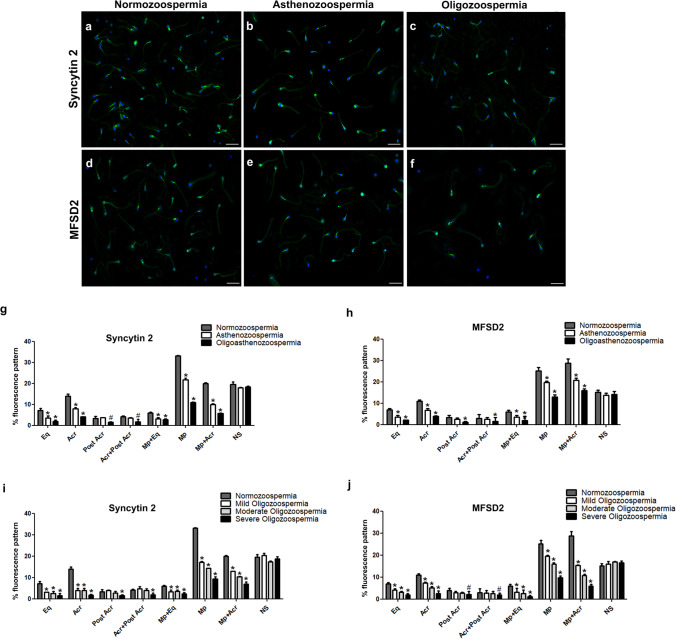
Syncytin 2 and its receptor MFSD2 proteins were detected in (1) the equatorial segment, (2) the acrosomal cap, (3) the post acrosomal region, (4) the acrosomal cap plus post acrosomal region, (5) the midpiece plus equatorial segment, (6) the midpiece region, and (7) the combined midpiece plus acrosomal region (Fig. 1) in the sperm samples from normozoospermic, asthenozoospermic, oligozoospermic (mild, moderate, severe), and oligoasthenozoospermic patients (Fig. 3). As observed in Syncytin 1 and SLC1A5, a slight staining of the tail were also noted for Syncytin 2 and its receptor MFSD2 in all spermatozoa. The immunoreaction of Syncytin 2 was found to be most prominent in the midpiece region of human sperm among the different staining patterns. In contrast, MFSD2 immunoreaction was predominantly observed in the combined midpiece plus acrosomal region as well as in the midpiece region of sperm.
Fig. 3.
Immunofluorescence localization of Syncytin 2 (a, b, c) and its receptor MFSD2 (d, e, f) in normozoospermic, asthenozoospermic, and oligozoopermic human sperm samples. The percentage (%) of immunofluorescence staining patterns for Syncytin 2 (g, i) and MFSD2 (h, j). Eq, equatorial segment; Acr, acrosomal cap; Post Acr, post acrosomal region; Acr + Post Acr, acrosomal cap and post acrosomal region; Mp + Eq, midpiece region and equatorial segment; Mp, midpiece region; Mp + Acr, midpiece region and acrosomal cap; NS, non-stained sperm. Green: FITC; blue: DAPI. The scale bars represent 50 µm. p values indicate the statistical comparisons between the sperm samples with normozoospermia and different patient categories. #p < 0.05, *p < 0.001
When asthenozoospermic and oligoasthenozoospermic sperm samples were compared to the normozoospermic sperm samples by means of Syncytin 2 immunoreactivity, there was a decreased percentage of staining patterns in the equatorial segment, the acrosomal cap, the midpiece plus equatorial segment, only the midpiece region, and the combined midpiece plus acrosomal region of asthenozoospermic and oligoasthenozoospermic sperm samples (p < 0.001) (Fig. 3a, b, g). A significant decrease was detected in the percentages of sperm stained for post acrosomal region (p = 0.003) and the acrosomal cap plus post acrosomal regions (p = 0.034) compared to the normozoospermic groups in the only oligoasthenozoospermic patient group (Fig. 3g). A significant decrease in the percentage of sperm with acrosomal cap, the midpiece region and the combined midpiece plus acrosomal region staining patterns was detected in oligoasthenozoospermic samples compared to asthenozoospermic sperm samples (p < 0.001) (Fig. 3g). In contrast, we observed no significant difference in the percentage of the non-stained sperm between these patient groups (p > 0.05) (Fig. 3g).
Syncytin 2 immunoreaction was significantly decreased in the equatorial segment, the acrosomal cap, the midpiece plus equatorial segment, only the midpiece region, and the combined midpiece plus acrosomal region of all oligozoospermic sperm samples with different subcategories when compared to normozoospermic sperm samples (p < 0.001) (Fig. 3a, c, i). However, a significant decrease was observed particularly in the percentages of midpiece region and combined midpiece plus acrosome region in both moderate and severe oligozoospermia groups compared to the mild oligozoospermia patient group (p < 0.001) (Fig. 3i). The statistical evaluation was also indicated a significant decrease in severe oligozoospermia samples compared to moderate oligozoospermia samples for these staining patterns (p < 0.001) (Fig. 3i). Furthermore, there was a significant decrease in percentage of the post acrosomal region and the acrosomal cap plus post acrosomal region in severe oligozoospermic sperm samples compared to normozoospermic sperm samples while no difference was detected in the mild or moderate oligozoospermic sperm samples (p < 0.001) (Fig. 3i). As observed with asthenozoospermic and oligoasthenozoospermic sperm samples, we found no statistically significant difference in the percentage of non-stained sperm between different oligozoospermic patient subcategories and normozoospermic patient groups (p > 0.05) (Fig. 3i).
A statistically significant decrease in MFSD2 immunoreaction was observed in the equatorial segment (p < 0.001), the acrosomal cap (p < 0.001), the midpiece plus equatorial segment (p < 0.001), only the midpiece region (p < 0.001), and the combined midpiece plus acrosomal region (p < 0.001) in asthenozoospermic and oligoasthenozoospermic sperm samples and all of the oligozoospermic patient subcategories compared to the normozoospermic sperm samples (Fig. 3d, e, f, h, j). On the other hand, there was a statistically significant decrease in the percentage of the acrosomal cap, the midpiece region and the combined midpiece plus acrosomal region staining patterns in oligoasthenozoospermic samples compared to asthenozoospermic sperm samples (p < 0.001) (Fig. 3h). A significant decrease was also determined in the acrosomal cap, the midpiece region and combined midpiece plus acrosome region staining in both moderate and severe oligozoospermia patient groups compared to patients with the mild oligozoospermia (p < 0.001) (Fig. 3j). In addition, there was a significant decrease in the percentage of equatorial segment and the midpiece plus equatorial segment in the sperm samples from severe oligozoospermia group compared to samples from mild oligozoospermia (p = 0.018, p = 0.0145, respectively). We found a significant decrease in the post acrosomal region (p = 0.015) and the acrosomal cap plus post acrosomal region staining patterns (p = 0.002) in severe oligozoospermic sperm samples compared to normozoospermic sperm samples while no statistical difference was analyzed in mild and moderate oligozoospermic sperm samples (p > 0.05) (Fig. 3j). In contrast, there was no statistically significant difference between patient groups in the percentage of non-stained sperm for either men with oligozoospermia, asthenozoospermia or oligoasthenozoospermia (p > 0.05) (Fig. 3h, j).
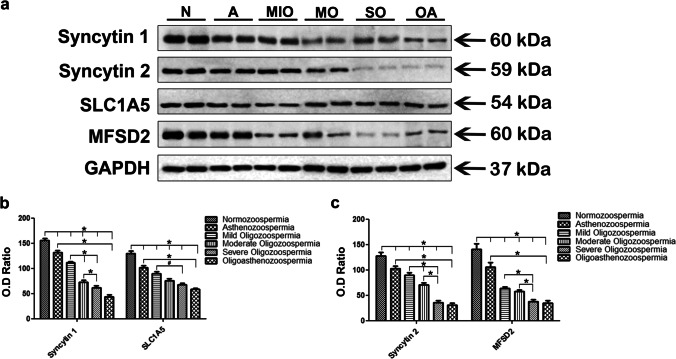
Protein expression levels of syncytin proteins and their receptors in normozoospermic, asthenozoospermic, oligozoospermic, and oligoasthenozoospermic human sperm samples
Western blot analysis of Syncytin 1 (60 kDa) and its receptor SLC1A5 (54 kDa) showed a statistically significant decrease in the asthenozoospermic, mild, moderate, and severe oligozoospermic and oligoasthenozoospermic sperm samples compared to the normozoospermic sperm samples by ImageJ analysis (p < 0.001) (Fig. 4a, b). When patient groups were compared to each other, we found a statistically significant decrease in both Syncytin 1 and SLC1A5 protein expression in oligoasthenozoospermic sperm samples compared to asthenozoospermic sperm samples (p < 0.001) (Fig. 4a, b). On the other hand, Syncytin 1 protein expression was significantly decreased in both moderate and severe oligozoospermic sperm samples compared to the mild oligozoospermic group (p < 0.001) (Fig. 4a, b). The statistically significance decrease was also observed between in severe oligozoospermic sperm samples compared to moderate oligozoospermic sperm samples (p < 0.001) (Fig. 4a, b). SLC1A5 protein expression was decreased only in the severe oligozoospermic sperm samples compared to the mild oligozoospermic sperm samples (p = 0.024), but no significant difference was found between severe oligozoospermic sperm samples versus moderate oligozoospermic sperm samples or moderate oligozoospermic sperm samples versus mild oligozoospermic sperm samples (p > 0.05).
Fig. 4.
Western blot analysis of Syncytin 1 (60 kDa), Syncytin 2 (59 kDa), SLC1A5 (54 kDa), and MFSD2 (60 kDa) proteins in the human sperm samples (a). The expression of GAPDH (37 kDa) was used to confirm equivalent amounts of total proteins loaded per lane. The optical density (OD) values of relevant proteins were normalized to the OD values of GAPDH bands and then graphed (b, c). N, normozoospermia; A, asthenozoospermia; MIO, mild oligozoospermia; MO, moderate oligozoospermia; SO, severe oligozoospermia; OA, oligoasthenozoospermia. #p < 0.05, *p < 0.001
Syncytin 2 (59 kDa) and MFSD2 (60 kDa) protein expressions were found to be significantly decreased in the asthenozoospermic, mild, moderate, and severe oligozoospermic and oligoasthenozoospermic sperm samples compared to the normozoospermic sperm samples as observed for Syncytin 1 and its receptor (p < 0.001) (Fig. 4a, c). A statistically significant decrease in both Syncytin 2 and MFSD2 protein expression was observed in oligoasthenozoospermic sperm samples compared to asthenozoospermic sperm samples (p < 0.001) (Fig. 4a, c). When oligozoospermic patient groups were compared among themselves, Syncytin 2 protein expression showed a significant decrease in both the moderate and severe oligozoospermic groups compared to the mild oligozoospermic group (p < 0.001) (Fig. 4a, c). In addition, a significant decrease was determined in the severe oligozoospermic sperm samples compared to the moderated oligozoospermic sperm samples (p < 0.001) (Fig. 4a, c). Even though MFSD2 protein expression was decreased significantly in severe oligozoospermic group compared to mild oligozoospermic group (p < 0.001), and also severe oligozoospermic sperm samples compared to moderate oligozoospermic sperm samples, no significant difference was observed between mild and moderate oligozoospermic patient groups (p > 0.05) (Fig. 4a, c).
Discussion
Here, we demonstrate the detailed expression profiles of syncytin proteins and their receptors in normozoospermic, asthenozoospermic, oligozoospermic (mild, moderate, severe), and oligoasthenozoospermic human sperm samples and revealed a significant decrease in the expression of syncytin 1, syncytin 2, and their receptors, SLC1A5 and MFSD2, in the patients with asthenozoospermia, oligozoospermia, and oligoasthenozoospermia. Although the presence of Syncytin 1 [53–55] and its receptor SLC1A5 [53] was reported in human sperm, the potential contributions of altered syncytin protein levels to sperm parameters such as reduced sperm motility and low sperm count or both were not demonstrated previously. Considering the dramatic increase of male infertility all over the World [71], unveiling the underlying mechanisms that could potentially contribute to reduced fertility is critical to understand, and then to improve the fertility outcomes.
Bjerregaard and colleagues indicated that a strong Syncytin 1 expression was present in the sperm acrosome or the equatorial segment and a weak staining pattern was observed in the midpiece and tail regions [53]. Recently, a similar expression pattern was also reported by Enoiu and colleagues with visible diffusion to the entire head in all sperm samples [54]. While the expression of Syncytin 1 in the acrosome, equatorial segment, midpiece, and tail regions of sperm is similar to the previous studies, here, we demonstrated that there is a more complex expression profile of Syncytin 1 suggesting a heterogeneity in human spermatozoa.
In addition to its crucial role in placentation, Syncytin 1 appears to play a role in fertilization and implantation. Among the other endogenous retroviruses, the higher level of Syncytin 1 transcript in human sperm [55] supports the potential role of Syncytin 1 in oocyte-sperm fusion. Syncytins and their receptors were present in the equatorial segment which has considerable functional importance to fertilization. The equatorial segment is crucial as it remains intact after the acrosome reaction, underlies the domain of the plasma membrane involved in fusion with the oocyte membrane, and is the site where breakdown of the sperm nuclear envelope is initiated after fertilization [72]. Interestingly, the percentage of the positively stained sperm samples for the equatorial segment staining pattern were significantly decreased in patients with asthenozoospermia, all oligozoospermia patient subcategories (mild, moderate, severe), and oligoasthenozoospermia for syncytins and their receptors. In fact, the molecular basis of sperm-egg recognition is unknown, but is likely to require interactions between receptor proteins displayed on their surface [7]. Since the percentages of the unstained sperm were also striking, the competence or development potential of syncytin-expressing or non-expressing sperm should be investigated further by utilizing mouse homologous conditional knockout models of syncytins and their receptors to investigate whether they will be functionally related to male infertility. Therefore, the best way to reveal these molecular mechanisms is possibly to design mouse/animal models with partial or complete absence of syncytin 1, syncytin 2, or their receptors in gametes, and test them for fertilization capacity.
Furthermore, the expression of Syncytin 1 in the trophectoderm directly beneath the inner cell mass of human blastocysts [73] suggests that Syncytin 1 may play role in embryo implantation. The decreased expression of Syncytin 1 and its receptor may cause failure by negatively affecting fertilization and embryo growth and can be used as important biomarker molecules in the development of new strategies in IVF treatments. A recent study by Enoiu and colleagues investigated the fusion proteins including Syncytin 1 in spermatozoa from men in couples experiencing total fertilization failure during IVF [54]. However, the study reported that there were not any significant changes in the expression of Syncytin 1 between the total fertilization failure and control groups [54]. However, the number of spermatozoa expressing Syncytin 1 was found to be higher in the control group compared with total fertilization failure even though it was not statistically significant. Thus, high expression profile of Syncytin 1 may contribute to successful gamete membrane fusion, an important step in fertilization.
To the best of our knowledge, no information was available on the localization pattern of Syncytin 2 or its receptor in human gametes. Previously, Syncytin 2 has been shown transcribed in spermatozoa, although the transcription level was lower than Syncytin 1 [55]. However, we report here for the first time that Syncytin 2 is preferentially expressed in the midpiece and combined midpiece plus acrosomal regions. This high expression pattern associated with the sperm midpiece where mitochondria are located might indicate a metabolism related function of these proteins. Taken together, our data demonstrating the presence of Syncytin 2 and its receptor in human sperm further support the idea that Syncytin 2 might also contribute to gamete fusion during fertilization.
Unfortunately, there is only one study in the existing literature related to syncytins and its receptors in the oocyte reporting that Syncytin 1 receptor, ASCT-2, was expressed in oocytes with different stages of oocyte maturation including germinal vesicle (GV) and MI and MII stages [53]. The study with 80 oocytes indicated that there was a significantly higher expression of Syncytin 1 receptor in mature MII stage oocytes compared to the immature GV stage. Although the expression of Syncytin 1 was not present in any of the oocytes examined by quantitative RT-PCR [53], it seems that the mRNA level of Syncytin 1 receptor increases as the oocytes mature from the GV to the MII stage. Thereafter, one can speculate that the presence of the Syncytin 1 receptor on the oocytes may be utilized in pharmaceutical or culture related interventions as an indicator of the developmental competence of the oocytes [53]. However, the question still remains whether the fusogen Syncytin 1 and its receptor could be involved in membrane fusion of the human gametes since Syncytin 1 is present and localized at the right place in spermatozoa and Syncytin 1 receptor is present in the mature oocytes.
Syncytins, as noted, belong to an endogenous retrovirus family and can function as a true retroviral envelope protein [74–76]. Syncytin therefore has the ability to block its own receptor if co-expressed with its receptor [76]. In this study, we found that syncytin 1, syncytin 2, and their receptors were expressed in similar localization patterns on spermatozoa from different patient groups, but their percentages in these patterns were different. Therefore, it can be speculated that co-expression of Syncytin 1 and its receptor SLC1A5 or Syncytin 2 and its receptor MFSD2 on spermatozoa inhibit membrane fusion at the designated sites and act as a regulator of syncytins.
The most important function of the acrosome reaction is to induce changes in the sperm membrane [77]. Although SPACA6, TMEM95, SOF1, FIMP, and DCST1/DCST2 have been shown among the new proteins required for sperm-oocyte fusion that occurs after the acrosome reaction, the importance and roles of these protein interactions are not clear [8]. However, studies suggested that the previously discovered tetraspanins CD9 and CD81 both play an important role in fertilization [78] and participate in membrane fusion as regulators of fusion platforms for membrane fusion to occur [79]. Cell–cell fusion following the acrosome reaction is facilitated by the interaction of the oocyte plasma membrane with a highly localized area of the sperm plasma membrane lining the equatorial segment [9]. The demonstration of syncytin 1, Syncytin 2 and their receptors on the equatorial segment of spermatozoa all together conforms to this model proposed by Nixon and colleagues [9].
Furthermore, it is known that any structural or functional acrosomal abnormality can disrupt sperm fusion and ultimately cause infertility. Studies have shown that intra-cytoplasmic insemination with sperm containing acrosomal abnormalities does not lead to successful fertilization even in the absence of fertilization barriers, as the oocyte cannot be activated efficiently [80, 81]. Our immunofluorescence staining results showed that both syncytin 1, Syncytin 2 and their receptors were significantly reduced in the acrosome region in the asthenozoospermic, oligozoospermic and oligoasthenozoospermic sperm samples compared to normozoospermic sperm samples. Syncytin 1 has been previously reported to have a tendency to gather at the equatorial segment following progesterone-induction of the acrosome reaction suggesting a translocation of Syncytin 1 to the equatorial segment during the acrosome reaction and a potential involvement in this biological process [54]. It is likely that high expression of syncytins and their receptors may facilitate gamete membrane fusion, while low levels or absence of these proteins may suggest IVF treatments. Therefore, our study may have a significant impact on both clinical and research investigations of male fertility status and may advance our understanding of the role of these proteins in human health and fertility. The lower amount of these fusion proteins in patients with impaired semen quality may be an important marker for the evaluation of a man's reproductive potential with fertility implications.
Key events, including motility, capacitation, and acrosomal exocytosis, are important in the acquisition of fertilization ability by spermatozoa [82]. Low sperm count and/or quality is present in 90% of couples with fertility problems [83]. Oligozoospermic men have a very high rate of defective sperm-zona pellucida interactions, consistent with low natural fertility or low fertilization rates in conventional IVF [84]. On the other hand, the relationship between the degree of motility and the reproductive outcomes should also be emphasized. Sources of variability in sperm motility are found at different levels such as between sperm cells, ejaculates (from the same individual) and individuals [85]. It is also known that oxidative stress increases in samples with low sperm motility [86], and motility shows an inverse relationship with DNA fragmentation [87, 88]. Although controversial, extensive damage to sperm DNA can result in poor fertilization or embryo development rates and increased miscarriage rates [89–92]. In line with this, our data regarding the decreased expression of these fusogenic proteins and their receptors in the patients with asthenozoospermia, oligozoospermia, and oligoasthenozoospermia may lead to reduced fertilization success due to the inadequate interaction that occurs during the gamete membrane fusion. In fact, syncytins and their receptors were significantly decreased in the men with oligoasthenozoospermia compared to other groups. Therefore, the decrease in both concentration and motility may have also worsened the fertilization potential by causing a decrease in sperm function and its success in the female reproductive system. Thus, syncytins and their receptors might also clinically reflect the quality of semen samples.
It is also of note that even though Bjerregaard and colleagues reported that Syncytin 1 was present in all samples by quantitative RT-PCR analysis, protein levels varied between donors [53]. Therefore, further studies with large cohort sizes and specific patient groups such as male versus female factor infertility may offer further insight into whether differential expression of syncytins and their receptors adversely affects fertilization, implantation and embryo growth. On the other hand, we used immunofluorescence staining technique to visualize the different localization patterns in human sperm. The techniques itself include steps such as fixation and permeabilization that can affect cell morphology and/or produce artifacts. Since we used the washed semen samples, the sperm preparation does not reflect the whole sperm population, and in men with impaired semen quality, the number of mature spermatozoa thus selected is usually high. Moreover, the ejaculated semen contains not only spermatozoa but also round cells (mainly leucocytes and immature germ cells) whose concentration is often high in infertile patients with altered semen parameters [93]. Although western blot is validated as a highly specific method to detect the total proteins extracted from cells, the ability of this technique to detect syncytins and their receptors in such human semen samples may be limited. Thus, this possible interference needs to be further evaluated. In addition, it still remains to be answered whether or not syncytins and their receptors have important roles in oocyte-sperm fusion in humans, and whether these retroviral proteins alter pregnancy, implantation, IVF miscarriage rates, and male fertility potential in infertility treatments in the clinics. Therefore, our results may have a significant impact on clinical decision making in the context of reduced fertility, infertility, and sperm physiology studies as these proteins might potentially play a variety of roles in sperm biology and function.
Acknowledgements
We sincerely thank the patients for their participation and support in this study.
Author contribution
LS designed the experiment and interpreted the data; BS, GGT, OK, and LS performed the experiments, collected the data, and analyzed the results; GGT and LS wrote the manuscript. All authors edited the manuscript and have given approval for publication of the present version of this manuscript.
Funding
This work was supported by a research grant from the Akdeniz University, The Scientific Research Projects Coordination Unit (TSA-2021–5689).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethical Committee of Akdeniz University School of Medicine (2012-KAEK-20), and informed consents from patient were obtained before the initiation of the study. All the authors consented to participate in this study.
Consent for publication
All the authors consented for publication.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112(4):519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 2.Joardar A, Pattnaik GP, Chakraborty H. Mechanism of membrane fusion: interplay of lipid and peptide. J Membr Biol. 2022;255(2–3):211–224. doi: 10.1007/s00232-022-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlisle JA, Swanson WJ. Molecular mechanisms and evolution of fertilization proteins. J Exp Zool B Mol Dev Evol. 2021;336(8):652–665. doi: 10.1002/jez.b.23004. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Gou L, Chen S, et al. Membrane Fusion Involved in Neurotransmission: Glimpse from Electron Microscope and Molecular Simulation. Front Mol Neurosci. 2017;10(168). 10.3389/fnmol.2017.00168. [DOI] [PMC free article] [PubMed]
- 5.Noda T, Lu Y, Fujihara Y, et al. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm-oocyte fusion in mice. Proc Natl Acad Sci U S A. 2020;117(21):11493–11502. doi: 10.1073/pnas.1922650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujihara Y, Lu Y, Noda T, et al. Spermatozoa lacking fertilization influencing membrane protein (FIMP) fail to fuse with oocytes in mice. Proc Natl Acad Sci U S A. 2020;117(17):9393–9400. doi: 10.1073/pnas.1917060117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi E, Doe B, Goulding D, et al. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508(7497):483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siu KK, Serrao VHB, Ziyyat A, et al. The cell biology of fertilization: Gamete attachment and fusion. J Cell Biol. 2021;220(10). 10.1083/jcb.202102146. [DOI] [PMC free article] [PubMed]
- 9.Nixon B, Aitken RJ, McLaughlin EA. New insights into the molecular mechanisms of sperm-egg interaction. Cell Mol Life Sci. 2007;64(14):1805–1823. doi: 10.1007/s00018-007-6552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutovsky P. Sperm-egg adhesion and fusion in mammals. Expert Rev Mol Med. 2009;11(e11). 10.1017/S1462399409001045. [DOI] [PubMed]
- 11.Miyado K, Yamada G, Yamada S, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287(5451):321–324. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- 12.Merc V, Frolikova M, Komrskova K. Role of Integrins in Sperm Activation and Fertilization. Int J Mol Sci. 2021;22(21). 10.3390/ijms222111809. [DOI] [PMC free article] [PubMed]
- 13.Inoue N, Hagihara Y, Wada I. Evolutionarily conserved sperm factors, DCST1 and DCST2, are required for gamete fusion. Elife. 2021;10. 10.7554/eLife.66313. [DOI] [PMC free article] [PubMed]
- 14.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 15.Mao J, Zhang Q, Cong YS. Human endogenous retroviruses in development and disease. Comput Struct Biotechnol J. 2021;19:5978–5986. doi: 10.1016/j.csbj.2021.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng J, Wei Y, Han GZ. The diversity and evolution of retroviruses: perspectives from viral “fossils”. Virol Sin. 2022;37(1):11–18. doi: 10.1016/j.virs.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posso-Osorio I, Tobon GJ, Canas CA. Human endogenous retroviruses (HERV) and non-HERV viruses incorporated into the human genome and their role in the development of autoimmune diseases. J Transl Autoimmun. 2021;4(100137). 10.1016/j.jtauto.2021.100137. [DOI] [PMC free article] [PubMed]
- 18.Soygur B, Sati L. The role of syncytins in human reproduction and reproductive organ cancers. Reproduction. 2016;152(5):R167–R178. doi: 10.1530/REP-16-0031. [DOI] [PubMed] [Google Scholar]
- 19.Durnaoglu S, Lee SK, Ahnn J. Syncytin, envelope protein of human endogenous retrovirus (HERV): no longer 'fossil' in human genome. Anim Cells Syst (Seoul) 2021;25(6):358–368. doi: 10.1080/19768354.2021.2019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimenez-Orenga K, Oltra E. Human endogenous retrovirus as therapeutic targets in neurologic disease. Pharmaceuticals (Basel). 2021;14(6). 10.3390/ph14060495. [DOI] [PMC free article] [PubMed]
- 21.Jansz N, Faulkner GJ. Endogenous retroviruses in the origins and treatment of cancer. Genome Biol. 2021;22(1):147. doi: 10.1186/s13059-021-02357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vance TDR, Lee JE. Virus and eukaryote fusogen superfamilies. Curr Biol. 2020;30(13):R750–R754. doi: 10.1016/j.cub.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts RM, Ezashi T, Schulz LC, et al. Syncytins expressed in human placental trophoblast. Placenta. 2021;113(8-14). 10.1016/j.placenta.2021.01.006. [DOI] [PMC free article] [PubMed]
- 24.Mi S, Lee X, Li X, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403(6771):785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 25.Blond JL, Lavillette D, Cheynet V, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74(7):3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu SZ, Szeto VH, Bao M, et al. Pharmacological approaches promoting stem cell-based therapy following ischemic stroke insults. Acta Pharmacol Sin. 2018;39(5):695–712. doi: 10.1038/aps.2018.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaise S, Parseval N, Benit L, et al. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci U S A. 2003;100(22):13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stafl K, Travnicek M, Kucerova D, et al. Heterologous avian system for quantitative analysis of Syncytin-1 interaction with ASCT2 receptor. Retrovirology. 2021;18(1):15. doi: 10.1186/s12977-021-00558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupressoir A, Vernochet C, Bawa O, et al. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci U S A. 2009;106(29):12127–12132. doi: 10.1073/pnas.0902925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangeney M, Renard M, Schlecht-Louf G, et al. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc Natl Acad Sci U S A. 2007;104(51):20534–20539. doi: 10.1073/pnas.0707873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antony JM, Van MG, Opii W, et al. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7(10):1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- 32.Esnault C, Priet S, Ribet D, et al. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc Natl Acad Sci U S A. 2008;105(45):17532–17537. doi: 10.1073/pnas.0807413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas A, Zhou S, Ethier-Chiasson M, et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 2014;28(8):3703–3719. doi: 10.1096/fj.13-239053. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Peng B, Klausen C, et al. NPFF increases fusogenic proteins syncytin 1 and syncytin 2 via GCM1 in first trimester primary human cytotrophoblast cells. FASEB J. 2020;34(7):9419–9432. doi: 10.1096/fj.201902978R. [DOI] [PubMed] [Google Scholar]
- 35.Ruebner M, Strissel PL, Langbein M, et al. Impaired cell fusion and differentiation in placentae from patients with intrauterine growth restriction correlate with reduced levels of HERV envelope genes. J Mol Med (Berl) 2010;88(11):1143–1156. doi: 10.1007/s00109-010-0656-8. [DOI] [PubMed] [Google Scholar]
- 36.Langbein M, Strick R, Strissel PL, et al. Impaired cytotrophoblast cell-cell fusion is associated with reduced Syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75(1):175–183. doi: 10.1002/mrd.20729. [DOI] [PubMed] [Google Scholar]
- 37.Fahlbusch FB, Ruebner M, Volkert G, et al. Corticotropin-releasing hormone stimulates expression of leptin, 11beta-HSD2 and syncytin-1 in primary human trophoblasts. Reprod Biol Endocrinol. 2012;10(80). 10.1186/1477-7827-10-80. [DOI] [PMC free article] [PubMed]
- 38.Valent AM, Choi H, Kolahi KS, et al. Hyperglycemia and gestational diabetes suppress placental glycolysis and mitochondrial function and alter lipid processing. FASEB J. 2021;35(3):e21423. doi: 10.1096/fj.202000326RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soygur B, Sati L, Demir R. Altered expression of human endogenous retroviruses syncytin-1, syncytin-2 and their receptors in human normal and gestational diabetic placenta. Histol Histopathol. 2016;31(9):1037–47. doi: 10.14670/HH-11-735. [DOI] [PubMed] [Google Scholar]
- 40.Moller AM, Delaisse JM, Soe K. Osteoclast fusion: time-lapse reveals involvement of CD47 and syncytin-1 at different stages of nuclearity. J Cell Physiol. 2017;232(6):1396–1403. doi: 10.1002/jcp.25633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kristensen MK, Christensen T. Regulation of the expression of human endogenous retroviruses: elements in fetal development and a possible role in the development of cancer and neurological diseases. APMIS. 2021;129(5):241–253. doi: 10.1111/apm.13130. [DOI] [PubMed] [Google Scholar]
- 42.Dittmar T, Weiler J, Luo T, et al. Cell-Cell fusion mediated by viruses and HERV-derived fusogens in cancer initiation and progression. Cancers (Basel). 2021;13(21). 10.3390/cancers13215363. [DOI] [PMC free article] [PubMed]
- 43.Zhou Y, Liu L, Liu Y, et al. Implication of human endogenous retrovirus W family envelope in hepatocellular carcinoma promotes MEK/ERK-mediated metastatic invasiveness and doxorubicin resistance. Cell Death Discov. 2021;7(1):177. doi: 10.1038/s41420-021-00562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Liang JQ, Zheng S. Expressional activation and functional roles of human endogenous retroviruses in cancers. Rev Med Virol. 2019;29(2):e2025. doi: 10.1002/rmv.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Xu J, Wen F, et al. Upregulation of syncytin-1 promotes invasion and metastasis by activating epithelial-mesenchymal transition-related pathway in endometrial carcinoma. Onco Targets Ther. 2019;12:31–40. doi: 10.2147/OTT.S191041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu Y, Zhuang X, Xia X, et al. Correlation between promoter hypomethylation and increased expression of syncytin-1 in non-small cell lung cancer. Int J Gen Med. 2021;14:957–965. doi: 10.2147/IJGM.S294392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-Carballo D, Klein J, Acikelli AH, et al. Cytotoxic stress induces transfer of mitochondria-associated human endogenous retroviral RNA and proteins between cancer cells. Oncotarget. 2017;8(56):95945–95964. doi: 10.18632/oncotarget.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen JM, Christensen IJ, Nielsen HJ, et al. Syncytin immunoreactivity in colorectal cancer: potential prognostic impact. Cancer Lett. 2009;280(1):44–49. doi: 10.1016/j.canlet.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Strissel PL, Ruebner M, Thiel F, et al. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: emergence of new molecular targets. Oncotarget. 2012;3(10):1204–19. doi: 10.18632/oncotarget.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uygur B, Leikina E, Melikov K, et al. Interactions with muscle cells boost fusion, stemness, and drug resistance of prostate cancer cells. Mol Cancer Res. 2019;17(3):806–820. doi: 10.1158/1541-7786.MCR-18-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fei F, Li C, Wang X, et al. Syncytin 1, CD9, and CD47 regulating cell fusion to form PGCCs associated with cAMP/PKA and JNK signaling pathway. Cancer Med. 2019;8(6):3047–3058. doi: 10.1002/cam4.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chignola R, Sega M, Molesini B, et al. Collective radioresistance of T47D breast carcinoma cells is mediated by a Syncytin-1 homologous protein. PLoS ONE. 2019;14(1):e0206713. doi: 10.1371/journal.pone.0206713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjerregaard B, Lemmen JG, Petersen MR, et al. Syncytin-1 and its receptor is present in human gametes. J Assist Reprod Genet. 2014;31(5):533–539. doi: 10.1007/s10815-014-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enoiu SI, Nygaard MB, Bungum M, et al. Expression of membrane fusion proteins in spermatozoa and total fertilisation failure during in vitro fertilisation. Andrology. 2022 doi: 10.1111/andr.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergallo M, Canosa S, Galliano I, et al. Impaired transcription of human endogenous retroviruses in the sperm with exception of syncytin 1: short communication. Mol Biol Rep. 2021;48(7):5803–5808. doi: 10.1007/s11033-021-06577-6. [DOI] [PubMed] [Google Scholar]
- 56.Liu DY, Baker HW. Defective sperm-zona pellucida interaction: a major cause of failure of fertilization in clinical in-vitro fertilization. Hum Reprod. 2000;15(3):702–708. doi: 10.1093/humrep/15.3.702. [DOI] [PubMed] [Google Scholar]
- 57.Gill K, Jakubik J, Rosiak-Gill A, et al. Utility and Predictive Value of Human Standard Semen Parameters and Sperm DNA Dispersion for Fertility Potential. Int J Environ Res Public Health. 2019;16(11). 10.3390/ijerph16112004. [DOI] [PMC free article] [PubMed]
- 58.Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8(4):191–196. doi: 10.4103/0974-1208.170370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng J, Lu Y, Qu X, et al. Decreased sperm motility retarded ICSI fertilization rate in severe oligozoospermia but good-quality embryo transfer had achieved the prospective clinical outcomes. PLoS ONE. 2016;11(9):e0163524. doi: 10.1371/journal.pone.0163524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLachlan RI. Approach to the patient with oligozoospermia. J Clin Endocrinol Metab. 2013;98(3):873–880. doi: 10.1210/jc.2012-3650. [DOI] [PubMed] [Google Scholar]
- 61.Song SH, Bak CW, Lim JJ, et al. Natural course of severe oligozoospermia in infertile male: influence on future fertility potential. J Androl. 2010;31(6):536–539. doi: 10.2164/jandrol.110.010199. [DOI] [PubMed] [Google Scholar]
- 62.Tang L, Rao M, Yang W, et al. Predictive value of the sperm DNA fragmentation index for low or failed IVF fertilization in men with mild-to-moderate asthenozoospermia. J Gynecol Obstet Hum Reprod. 2021;50(6):101868. doi: 10.1016/j.jogoh.2020.101868. [DOI] [PubMed] [Google Scholar]
- 63.Vogiatzi P, Pouliakis A, Sakellariou M, et al. Male age and progressive sperm motility are critical factors affecting embryological and clinical outcomes in oocyte donor ICSI cycles. Reprod Sci. 2022;29(3):883–895. doi: 10.1007/s43032-021-00801-1. [DOI] [PubMed] [Google Scholar]
- 64.Shaw WPV, Daftary S, Howkins J, Bourne G. Infertility and sterility. In: Shaw’s Textbook of Gynaecology. New Delhi, India: Elsevier; 2015. p. 237–62.
- 65.WHO. WHO laboratory manual for the examination and processing of human semen. Fifth edition. Geneva: WHO Press; 2010.
- 66.Sati L, Huszar G. Methodology of aniline blue staining of chromatin and the assessment of the associated nuclear and cytoplasmic attributes in human sperm. Methods Mol Biol. 2013;927:425–36. doi: 10.1007/978-1-62703-038-0_36. [DOI] [PubMed] [Google Scholar]
- 67.Tiwari A, Tekcan M, Sati L, et al. A new media without animal component for sperm cryopreservation: motility and various attributes affecting paternal contribution of sperm. J Assist Reprod Genet. 2017;34(5):647–657. doi: 10.1007/s10815-017-0888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soygur B, Celik S, Celik-Ozenci C, et al. Effect of erythrocyte-sperm separation medium on nuclear, acrosomal, and membrane maturity parameters in human sperm. J Assist Reprod Genet. 2018;35(3):491–501. doi: 10.1007/s10815-017-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sati L, Cayli S, Delpiano E, et al. The pattern of tyrosine phosphorylation in human sperm in response to binding to zona pellucida or hyaluronic acid. Reprod Sci. 2014;21(5):573–581. doi: 10.1177/1933719113504467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kankavi O, Ata A, Celik-Ozenci C, et al. Presence and subcellular localizations of surfactant proteins A and D in human spermatozoa. Fertil Steril. 2008;90(5):1904–1909. doi: 10.1016/j.fertnstert.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 71.Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;(13-37). 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed]
- 72.Yanagimachi R, Noda YD. Ultrastructural changes in the hamster sperm head during fertilization. J Ultrastruct Res. 1970;31(5–6):465–485. doi: 10.1016/s0022-5320(70)90163-2. [DOI] [PubMed] [Google Scholar]
- 73.Soygur B, Moore H. Expression of Syncytin 1 (HERV-W), in the preimplantation human blastocyst, embryonic stem cells and trophoblast cells derived in vitro. Hum Reprod. 2016;31(7):1455–1461. doi: 10.1093/humrep/dew097. [DOI] [PubMed] [Google Scholar]
- 74.Weissenhorn W, Hinz A, Gaudin Y. Virus membrane fusion. FEBS Lett. 2007;581(11):2150–2155. doi: 10.1016/j.febslet.2007.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X, Huang J, Zhu F. Human endogenous retroviral envelope protein Syncytin-1 and inflammatory abnormalities in neuropsychological diseases. Front Psychiatry. 2018;9(422). https://doi.org/10.3389/fpsyt.2018.00422. [DOI] [PMC free article] [PubMed]
- 76.Potgens AJ, Drewlo S, Kokozidou M, et al. Syncytin: the major regulator of trophoblast fusion? Recent developments and hypotheses on its action. Hum Reprod Update. 2004;10(6):487–496. doi: 10.1093/humupd/dmh039. [DOI] [PubMed] [Google Scholar]
- 77.Okabe M. The acrosome reaction: a historical perspective. Adv Anat Embryol Cell Biol. 2016;220:1–13. doi: 10.1007/978-3-319-30567-7_1. [DOI] [PubMed] [Google Scholar]
- 78.Rubinstein E, Ziyyat A, Wolf JP, et al. The molecular players of sperm-egg fusion in mammals. Semin Cell Dev Biol. 2006;17(2):254–263. doi: 10.1016/j.semcdb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Gordon-Alonso M, Yanez-Mo M, Barreiro O, et al. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J Immunol. 2006;177(8):5129–5137. doi: 10.4049/jimmunol.177.8.5129. [DOI] [PubMed] [Google Scholar]
- 80.Nasr-Esfahani MH, Razavi S, Vahdati AA, et al. Evaluation of sperm selection procedure based on hyaluronic acid binding ability on ICSI outcome. J Assist Reprod Genet. 2008;25(5):197–203. doi: 10.1007/s10815-008-9223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nasr-Esfahani MH, Razavi S, Javdan Z, et al. Artificial oocyte activation in severe teratozoospermia undergoing intracytoplasmic sperm injection. Fertil Steril. 2008;90(6):2231–2237. doi: 10.1016/j.fertnstert.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 82.Allouche-Fitoussi D, Breitbart H. The role of zinc in male fertility. Int J Mol Sci. 2020;21(20). 10.3390/ijms21207796. [DOI] [PMC free article] [PubMed]
- 83.Leaver RB. Male infertility: an overview of causes and treatment options. Br J Nurs. 2016;25(18):S35–S40. doi: 10.12968/bjon.2016.25.18.S35. [DOI] [PubMed] [Google Scholar]
- 84.Liu DY, Baker HW. High frequency of defective sperm-zona pellucida interaction in oligozoospermic infertile men. Hum Reprod. 2004;19(2):228–233. doi: 10.1093/humrep/deh067. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez-Lopez P, Garriga J, Casas I, et al. Predicting fertility from sperm motility landscapes. Commun Biol. 2022;5(1):1027. doi: 10.1038/s42003-022-03954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meseguer M, Martinez-Conejero JA, O’Connor JE, et al. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008;89(5):1191–1199. doi: 10.1016/j.fertnstert.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 87.Palermo GD, Neri QV, Monahan D, et al. Development and current applications of assisted fertilization. Fertil Steril. 2012;97(2):248–259. doi: 10.1016/j.fertnstert.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 88.Chen C, Hu JCY, Neri QV, et al. Kinetic characteristics and DNA integrity of human spermatozoa. Hum Reproduction. 2011;19:i30. [Google Scholar]
- 89.Ribas-Maynou J, Benet J. Single and double strand sperm DNA damage: Different reproductive effects on male fertility. Genes (Basel). 2019;10(2). 10.3390/genes10020105. [DOI] [PMC free article] [PubMed]
- 90.Ribas-Maynou J, Garcia-Peiro A, Fernandez-Encinas A, et al. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS ONE. 2012;7(9):e44679. doi: 10.1371/journal.pone.0044679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Agarwal A, Barbarosie C, Ambar R, et al. The impact of single- and double-strand dna breaks in human spermatozoa on assisted reproduction. Int J Mol Sci. 2020;21(11). 10.3390/ijms21113882. [DOI] [PMC free article] [PubMed]
- 92.Casanovas A, Ribas-Maynou J, Lara-Cerrillo S, et al. Double-stranded sperm DNA damage is a cause of delay in embryo development and can impair implantation rates. Fertil Steril. 2019;111(4):699–707 e1. doi: 10.1016/j.fertnstert.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 93.Rodin DM, Larone D, Goldstein M. Relationship between semen cultures, leukospermia, and semen analysis in men undergoing fertility evaluation. Fertil Steril. 2003;79 Suppl 3(1555-8). 10.1016/s0015-0282(03)00340-6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.