Abstract
Although the Burkholderia cepacia complex consists of several genomovars, one highly transmissible strain of B. cepacia has been isolated from the sputa of cystic fibrosis (CF) patients throughout the United Kingdom and Canada. This strain expresses surface cable (Cbl) pili and is thought to be the major strain associated with the fatal “cepacia syndrome.” In the present report we characterize the specific 55-kDa buccal epithelial cell (BEC) protein that binds cable pilus-positive B. cepacia. N-terminal sequences of CNBr-generated internal peptides identified the protein as cytokeratin 13 (CK13). Western blots of BEC extracts probed with a specific monoclonal antibody to CK13 confirmed the identification. Mixed epidermal cytokeratins (which contain CK13), cytokeratin extract from BEC (which consists essentially of CK13 and CK4), and a polyclonal antibody to mixed cytokeratins inhibited B. cepacia binding to CK13 blots and to normal human bronchial epithelial (NHBE) cells. Preabsorption of the antikeratin antibody with the BEC cytokeratin fraction reversed the inhibitory effect of the antibody. A cytokeratin mixture lacking CK13 was ineffective as an inhibitor of binding. Colocalization of CK13 and B. cepacia by confocal microscopy demonstrated that intact nonpermeabilized NHBE cells express small amounts of surface CK13 and bind Cbl-positive B. cepacia in the same location. Binding to intact NHBE cells was dependent on bacterial concentration and was saturable, whereas a Cbl-negative isolate exhibited negligible binding. These findings raise the possibility that surface-accessible CK13 in respiratory epithelia may be a biologically relevant target for the binding of cable piliated B. cepacia.
Burkholderia cepacia is an opportunistic lung pathogen in cystic fibrosis (CF) patients, with prevalence varying from center to center (0 to 40%) (17, 22, 31). Although B. cepacia infects a relatively small proportion of CF patients (3% worldwide), it is associated with heightened morbidity and mortality (12, 22). However, the clinical outcome of individual infected patients is unpredictable. About one-third of patients show a rapid decline in their clinical condition and succumb to pneumonia, septicemia, and death (the “cepacia syndrome”) within weeks to months of acquisition. This pattern is not observed with the other major CF pathogens, S. aureus, H. influenzae, and P. aeruginosa (11, 12), which are more likely to produce signs of chronic low-grade infection and a slower, more gradual decline in lung function. A 1995 study from the Glasgow CF center indicated that pediatric patients infected with B. cepacia had a significantly increased mortality compared to patients infected with P. aeruginosa alone (48). A survey from U.S. CF centers suggested that the risk factor was two times higher for patients infected with both P. aeruginosa and B. cepacia compared to patients who are infected with only P. aeruginosa (33). The lower survival rate appears to be linked to the fact that B. cepacia are resistant to a wide range of antimicrobial agents (3).
Adult patients are generally much more susceptible to B. cepacia than pediatric-age CF patients. In the adult CF population of Ontario, Canada, for example, approximately 46% are colonized by B. cepacia (17), compared to approximately 7% of patients under the age of 16 years (M. Corey, personal communication). Chronic lung damage due to previous lung infections and/or inflammation is thought to be a major predisposing factor for B. cepacia colonization. Some putative B. cepacia virulence factors have been characterized, including a hemolysin (16), proteases (25), lipases (23), siderophores (4), lipopolysaccharide (15, 50), melanin-like pigment (51), and pili (36, 37), but proof of their role in the pathogenesis of CF lung disease has not been demonstrated. There is a pressing need, therefore, to elucidate the pathogenic mechanisms of B. cepacia infection and to develop an effective therapy.
By phenotypic and DNA-based typing methods, B. cepacia has been shown to cluster clonally in geographically separated CF centers, where epidemic-like spread occurs by person-to-person transmission (12, 24). One highly transmissible clonal lineage is very common in Canadian and United Kingdom CF centers and has been designated the ET (Edinburgh-Toronto) strain. Typing studies indicate that it belongs to randomly amplified polymorphic DNA (RAPD) type 2 and genomovar III (12, 24, 46). The ET strain has been shown to be associated with patients who developed the cepacia syndrome (20, 35). Isolates of this clone carry a DNA fragment known as the B. cepacia epidemic strain marker BCESM (24) and also the cblA gene that encodes the major subunit for surface cable (Cbl) pili (38, 43).
Isolates expressing the Cbl pilus phenotype are capable of binding to secretory mucins (35), and the adhesin responsible is a pilus-associated protein of 22 kDa (36). B. cepacia also bind to buccal epithelial cells (BEC) (37). Scatchard plot analyses and double reciprocal plots were used to establish that isolates possessing Cbl pili bound in a dose-dependent saturable fashion to a specific BEC protein receptor. Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of BEC homogenates and bacterial overlay experiments, we showed that the bacteria bound to the lower band of a 55-kDa doublet (37).
In the present study we provide biochemical identification of the 55-kDa receptor by its amino acid profile, N-terminal sequences of internal peptides, immunoreactivity, and binding inhibition assays. Concerns about its accessibility for B. cepacia binding led to a study of the distribution and function of the receptor in airway epithelial cells. Our findings may have significance for the initial stages of infection of CF lungs by Cbl-positive B. cepacia.
MATERIALS AND METHODS
Bacteria and growth conditions.
B. cepacia isolate BC7 (Cbl pilus positive) and BC45 (Cbl pilus negative) were isolated from sputa of Toronto CF patients and have been described in previous publications (35, 37, 44). By DNA ribotyping and biochemical analyses, these two isolates were determined to belong to the same hypertransmissible genomovar III, RAPD type 2 strain (the ET strain) of the B. cepacia complex (38, 43). PCR amplification (U. S. Sajjan et al., unpublished observations) confirmed that both these isolates contain the DNA “epidemic” strain marker BCESM (24). Although they both also contain the cblA gene for the major pilin subunit of cable pili, isolate BC45 does not express cable pili on the surface of the bacteria (36–38). In preparation for binding assays, BC isolates were subcultured on brain heart infusion agar for 48 h at 37°C, single colonies were inoculated into 10 ml of tryptic soy broth in the presence or absence of 0.1 mCi of [35S]methionine (specific activity, >1,000 Ci/mmol) (Amersham Canada, Ltd., Oakville, Ontario, Canada) and grown for 24 h on an orbital shaker at 37°C. Bacteria were collected by centrifugation and washed three times with 10 ml of phosphate-buffered saline (PBS). The final pellet was suspended in PBS containing 1% bovine serum albumin (BSA) to give 0.1 × 109 to 1 × 109 CFU/ml for bacterial overlay assays. For some binding experiments, bacteria were labeled with fluorescein isothiocyanate (FITC) as described by Falk et al. (7) and suspended at 107 CFU/ml.
Epithelial cell preparations.
Primary cultures of normal human bronchial epithelial (NHBE) cells and bronchial epithelial growth medium (BEGM) were purchased from Clonetics Corp. Walkersville, Md. NHBE cells grown in BEGM at passage 3 were used in these studies. A-431 (human epidermoid carcinoma), A-549 (human lung carcinoma), HEp-2 (human epidermoid carcinoma of larynx), and NCI-H520 (human lung squamous cell carcinoma) cell lines were purchased from the American Type Tissue Culture Collection, Rockville, Md. A-431 cells were grown at 37°C and 5% CO2 in high-glucose Dulbecco's modified Eagle's medium without sodium bicarbonate, supplemented with 10% fetal bovine serum (FBS). A-549 cells were grown in Ham's F-12K medium supplemented with 10% FBS. HEp-2 cells were grown in minimal essential medium, supplemented with 10% FBS, and NCI-H520 cells were grown in RPMI 1640 supplemented with 10% FBS. All culture media and FBS were purchased from Canadian Life Technologies (Burlington, Ontario, Canada). Binding experiments were carried out on cells that had reached 90 to 95% confluence.
BEC were obtained from 10 to 15 healthy volunteers by swabbing buccal surfaces gently with a sterile wooden spatula. Cells were washed three times with PBS (10 ml each time), resuspended to give 106 cells/ml and used immediately.
Isolation of the BEC receptor for cable pili.
BEC were extracted in Tris-HCl reducing buffer by heating at 100°C for 10 min and centrifuged, and the supernatant was stored at −20°C until use. Extracts were subjected to SDS-PAGE, transferred, and electrophoretically to Immobilon-P (Millipore), and the lower band of the (previously described [37]) doublet at 55 kDa was cut out and subjected to amino acid analysis by using a picotag high-performance liquid chromatography system (Pierce Chemical Co., Rockford, Ill.). For N-terminal amino acid sequences, immobilized proteins were analyzed by the Edman degradation procedure using a Porton gas phase microsequencer model 2090. To obtain internal peptides of the 55-kDa band, the immobilized protein (∼50 μg) was subjected in situ to CNBr cleavage by using the Probe-design peptide separation system of Promega (Madison, Wis.). Peptide fragments were separated by high-resolution polyacrylamide gels consisting of a 16.5% separating gel, a 10% spacer gel, and a 4% stacking gel. Peptides were transferred to Problott membranes (Promega), stained with Coomassie blue, and subjected to N-terminal sequence analysis. Sequence similarities to other proteins were checked by using The Wisconsin Sequence Analysis Package by GCG, Inc., Madison, Wis.
Preparation of cytosol plus membrane-rich (CM) and cytoskeleton-rich (CS) fractions.
The method of Franke et al. (8) was used with some modifications. Briefly, epithelial cells (106 cells) were incubated for 20 min at room temperature in 2 ml of PBS containing 1% Triton X-100, 5 mM EDTA, and 1.5 M KCl; the mixture was then centrifuged, and the supernatant (membrane and cytosolic proteins) was collected. The pellet was reextracted, and the combined supernatants were concentrated to 0.5 ml by ultrafiltration (millipore) using a 10K cutoff membrane. This fraction was designated the CM fraction. The pellet was washed twice with PBS, once with water, suspended in 0.5 ml of Tris-HCl reducing buffer, heated for 10 min in a boiling water bath, cooled to room temperature, and recentrifuged. The supernatant was designated the CS fraction (cytoskeleton rich). The CS fraction was dialyzed against water, and the precipitated cytoskeleton proteins were solubilized in 0.5 ml of keratin solubilization buffer.
The concentration of cytokeratin 13 (CK13) in the CS fraction of BEC was determined after SDS-PAGE by comparing the intensity of the Coomassie blue-stained lower band at 55 kDa (which reacts with a specific monoclonal antibody (MAb) for CK13 (see later) and serves as a receptor for B. cepacia) with BSA standards subjected to the same procedure. Color intensities were quantitated by densitometry of digitized images using the NIH Image Analysis software. By reference to BSA, the CK13 content was 8.35 to 10.1 μg/106 BEC (CS fraction). This amount constitutes 8.5 to 10% of the total protein of 106 BEC, as determined by the bincinchoninic acid (BCA) assay (Pierce, Rockford, Ill.) of two independent cell preparations.
Antibodies.
A rabbit polyclonal antibody to B. cepacia has been described earlier (44). MAbs to CK13 and CK4 were purchased from Boehringer Mannheim (Toronto, Canada), and a polyclonal antiserum to mixed epidermal cytokeratins was purchased from Calbiochem-Novabiochem Corp., La Jolla, Calif. A polyclonal antibody to chicken microtubulin (which recognizes human α- and β-tubulin) was purchased from Sigma Biochemicals, St. Louis, Mo.
Bacterial binding.
Three separate assays of B. cepacia binding were performed.
(i) Bacterial overlay assays on nitrocellulose blots.
Binding of B. cepacia to proteins in epithelial cell extracts was determined as described earlier (37). Briefly, CM or CS fractions isolated from 0.5 × 104 to 4 × 104 BEC (or other cells) were subjected to SDS-PAGE (12% Novex Gels; Novex, San Diego, Calif.), separated proteins were transferred electrophoretically to nitrocellulose, and the membrane was blocked with 1% gelatin, washed, and overlaid with 35S-labeled B. cepacia (0.1 × 109 to 1 × 109 CFU/ml) for 90 min. Nonbound bacteria were removed by washing five times with 20 ml of PBS, and bound bacteria were detected by autoradiography. In determining the inhibition of binding, either the blots or the bacteria (specified later) were preincubated with potential inhibitors for 1 h at 37°C, and the assay was then carried out as described above.
(ii) Bacterial binding to NHBE cells.
NHBE cells at passage 3 were seeded in 96-well plates and grown to 80 to 90% confluence. The wells were blocked with 3% BSA in BEGM for 1 h at 37°C, washed with PBS, and then incubated with isolate BC7 ranging in concentration from 105 to 108 CFU/ml for 1 h at 37°C. The wells were washed five times with PBS to remove nonbound bacteria, and the cells were fixed with 70% methanol containing 1% H2O2 for 15 min at 4°C. Wells were rinsed with PBS, blocked with 3% BSA in PBS for 1 h at room temperature, and incubated with 1:5,000-diluted polyclonal antibody to B. cepacia for 1 h at 37°C. After five washes with PBS to remove nonbound antibody, the wells were incubated with antirabbit goat immunoglobulin G (IgG) coupled to horseradish peroxidase (Bio-Rad) for 30 min. The wells were washed again, and the bound antibody was detected colorimetrically by using TNB substrate (Pierce) according to the manufacturer's instructions. Nonspecific color development in wells containing no added bacteria and bacteria bound to 3% BSA-coated wells were subtracted from test sample results before the number of bacteria bound specifically to NHBE cells was calculated.
For inhibition experiments, cytokeratins extracted from BEC (the CS fraction) or commercially obtained mixed epidermal cytokeratins (Sigma Biochemicals) were preincubated with bacteria for 1 h at 37°C, and bacterial binding to NHBE cells was carried out as usual. When the antibody to mixed epidermal cytokeratins was used as a potential inhibitor, the antibody was incubated with unfixed nonpermeabilized NHBE cells for 1 h at 37°C; the cells were then washed with PBS to remove nonbound antibody, incubated with 1:1,000-diluted antirabbit goat IgG (Jackson Immunoresearch, Inc., West Grove, Pa.) for 1 h to block the anti-mixed epidermal cytokeratin antibody, and then the bacterial binding assay was carried out as described above. Nonimmune serum instead of the antikeratin antibody was used in controls.
(iii) Bacterial binding to cells assayed by fluorescence microscopy.
NHBE cells were grown in Lab-Tek chamber slides (Nunc, Inc., Naperville, Ill.) to approximately 90% confluency. The spent medium was removed, and the cells were washed with PBS, blocked with BSA, and then incubated for 1 h with FITC-labeled B. cepacia (107 CFU/ml). The cells were washed five times with PBS to remove nonbound bacteria, fixed in 3% buffered formalin for 10 min at room temperature, and counterstained with Mayer's hematoxylin (Sigma Biochemicals). Fluorescent bacteria were visualized at 492 nm with a confocal microscope (TCS 4D equipped with an argon-krypton laser scanner; Leica, Heidelburg, Germany). Optical sectioning (ca. 0.3-μm thickness) of cells was carried out in the x-y plane to determine the location of bound bacteria.
To determine the effect of inhibitors, bacteria or cells were preincubated with potential inhibitors for 1 h at 37°C and washed three times to remove excess inhibitor, and the experiment was continued as described above. Potential inhibitors used in this study included the CS fraction isolated from BEC, mixed epidermal, and plantar stratum corneum cytokeratins (Sigma), the MAb to CK13, and the polyclonal antibody to mixed epidermal cytokeratins. To deplete the polyclonal antibody of anti-CK13 antibodies, it was absorbed with the insoluble CS fraction from BEC (107 BEC). After an overnight incubation at 4°C, the mixture was centrifuged and tested to ensure that the resulting supernatant showed no immunoreactivity with the 55-kDa CK13 protein of BEC. Nonimmune serum was also treated in a similar way to serve as a control in inhibition experiments. The anti-CK13 depleted antibody was then compared with untreated (intact) antibody preparations in bacterial binding inhibitor studies. Bacterial binding to cells was quantitated by counting the number of bacteria bound to cells in 10 representative fields, with each field containing 25 to 72 cells.
Detection of cytokeratins in blots and/or cells.
CM fractions (membrane and cytosolic proteins) and CS (cytoskeleton rich) fractions from epithelial cells were subjected to SDS-PAGE on 12% Novex gels, transferred to nitrocellulose, blocked with 3% BSA in Tris-buffered saline (TBS) for 2 h at room temperature, and incubated overnight at 4°C with MAbs to CK4 or CK13 or with the polyclonal antibody to mixed epidermal keratins. The blots were washed with TTBS and incubated with a secondary antibody (antimouse IgG or antirabbit IgG as appropriate) conjugated to alkaline phosphatase, and the immunoreactivity was detected after the addition of the substrate NBT-BCIP (Boehringer Mannheim, Burlington, Ontario, Canada). Semiquantitative comparisons were made by measuring the density of CK13 bands in various cell extracts.
Cellular CK13 was also detected by immunofluorescent localization. NHBE cells were grown on slides, blocked with 5% goat serum in TBS for 1 h at R.T., washed, and incubated for 1 h with the MAb to CK13 (0.8 μg of Fab fragments/ml). (In some experiments cells were first permeabilized by fixing in 100% methanol at −20°C for 5 min and then washed with PBS prior to blocking with goat serum). Nonbound antibody was removed by washing five times with 20 ml of TBS, and the cells were then incubated with antimouse IgG conjugated to the fluorophores CY3 or LRSC. The slides were washed again, counterstained with Mayer's hematoxylin, and examined by confocal microscopy. Control samples were treated identically, but normal mouse IgG was used instead of the antibody to CK13.
RESULTS
Biochemical identification of the BEC receptor for Cbl-positive B. cepacia.
The major B. cepacia isolate used in this study was BC7, which expresses cable pili and binds to the lower band of a 55-kDa doublet in BEC (37).
The lower band was subjected to amino acid analysis and N-terminal amino acid sequencing. The N terminus was blocked, but the amino acid profile was strikingly similar to that of type I cytokeratins, particularly CK13 (39), with enrichment in glutamic acid and glycine (>27 mol%), and approximately 35 mol% contributed by aspartic acid, serine, alanine, and leucine combined (data not presented). The band was subjected to in situ cyanogen bromide digestion and yielded nine peptides (<7.1 to 32 kDa), of which four (16 kDa, 10 kDa, and two others of <7.1 kDa) were in sufficient quantity to provide reliable N-terminal sequences (Fig. 1). Sequences 1 and 3 ranged from 70 to 100% similarity with all type I and type II cytokeratins, while sequences 2 and 4 were 93 and 90 similar, respectively, to CK13 of human squamous epithelia of the upper digestive tract (39). A part of peptide 2 sequence DAEEWFHA is specific to CK13 and is not found in any other type I cytokeratins. Amino acids that are dissimilar to those of the published CK13 sequence are shown in boldface.
FIG. 1.
N-terminal amino acid sequences of four CNBr fragments obtained from the 55-kDa B. cepacia receptor of BEC, and their alignment (underlined) with the published sequence of human CK13 (39). Dissimilar residues are shown in boldface.
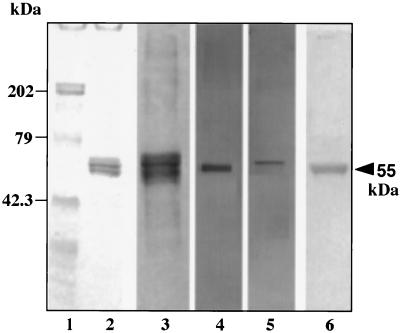
BEC were separated into a cytosol plus membrane-rich fraction (CM) and a cytoskeleton-rich fraction (CS). The CM fraction gave many Coomassie blue-stained bands after SDS-PAGE (not presented), while the CS fraction gave only a doublet centered at about 55 kDa (Fig. 2, lane 2). Western blot analyses with a polyclonal antibody to mixed epidermal cytokeratins were negative for the CM fraction (not presented) but reacted with components of the CS fraction in the range of 44 to 67 kDa (lane 3). A MAb specific for CK13 reacted with a band at approximately 55 kDa (lane 4), while an MAb specific for CK4 reacted with a more retarded band (lane 5). These two MAbs were chosen because CK4 and CK13 are known to be virtually the only cytokeratin pair present in the upper layers of oral epithelia (8), including buccal epithelial cells (27). Western blots of the CS and CM fractions were overlaid with 35S-labeled isolate BC7, and bacterial binding was observed only in the CS fraction, specifically to the 55-kDa band having the same mobility as CK13 (lane 6).
FIG. 2.
Immunoreactivity of the cytoskeleton-rich (CS) fraction of BEC and binding of B. cepacia. The CS fraction extracted from 104 BEC was separated by SDS-PAGE and Western blotted onto nitrocellulose. Lane 1, molecular mass standards in kilodaltons; lane 2, Coomassie blue staining; lane 3, reactivity with a polyclonal antibody (1:500 dilution) to mixed epidermal cytokeratins; lanes 4 and 5, reactivity with MAbs (0.04 μg of Fab fragments/ml) to CK13 and CK4, respectively; lane 6, overlay with 35S-labeled isolate BC7 (108 CFU/ml) for 1.5 h at 37°C and detection by autoradiography.
Inhibition of 35S-labeled B. cepacia binding to the BEC receptor.
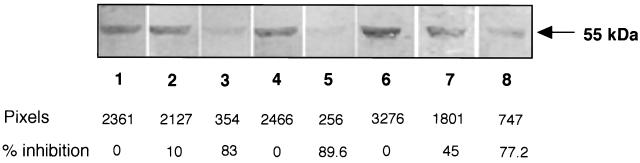
Inhibition studies were carried out using the same bacterial overlay procedure (Fig. 3). 35S-labeled BC7 (10 ml of 108 CFU/ml) were preincubated with 200 μg (by weight) of commercial mixed epidermal keratins solubilized in 40 μl of keratin solubilization buffer, and the mixture was then incubated with BEC blots of the CS fraction. Control incubations contained either no mixed epidermal keratins (lane 1) or only the keratin solubilization buffer (lane 2). As shown in lane 3, there was 83% inhibition of bacterial binding to CK13 by samples containing 200 μg of mixed epidermal cytokeratins. The CS fraction of BEC (containing 0.3 or 1.6 μg of CK13) was also inhibitory (45 and 77.2%, respectively) (lanes 7 and 8 relative to lane 6), as was a commercial polyclonal antibody to human epidermal cytokeratins (89.6%) (lane 5 versus lane 4). In separate Western blot overlay analyses, we confirmed that the polyclonal antibody to mixed epidermal cytokeratins did not react with B. cepacia proteins (not presented). This ruled out the possibility that the antibody blocked the adherence of B. cepacia to the BEC 55-kDa receptor by reacting with bacterial proteins rather than with the receptor protein. Plantar stratum corneum keratins, which are devoid of CK13 (41), did not cause inhibition of bacterial binding (data not presented).
FIG. 3.
Inhibition of isolate BC7 binding to the 55-kDa receptor of BEC. The cytoskeleton-rich (CS) fraction of BEC was separated by SDS-PAGE and blotted onto nitrocellulose. 35S-labeled isolate BC7 (10 ml of 108 CFU/ml) was added to the blots, and binding was detected by autoradiography. Lane 1, no inhibitor added; lanes 2 and 3, preincubation of bacteria with keratin solubilization buffer alone (lane 2) or with buffer plus mixed epidermal cytokeratins (200 μg) (lane 3); lanes 4 and 5, preincubation of blots with nonimmune serum (lane 4) or antiserum (1:40 dilution) to mixed epidermal cytokeratins (lane 5); lanes 6 to 8, preincubation of bacteria with keratin solubilization buffer (lane 6) or with buffer plus the BEC CS fraction containing 0.3 μg (lane 7) or 1.6 μg (lane 8) of CK13. Density (pixels) was measured on digitized images using NIH Image software. Inhibition of binding was calculated as a percentage of controls (set to 0% inhibition in each set of comparisons).
Specific immunoassays for CK13 indicated that CK13 comprised 1.02% by weight (2 μg/200 μg [total weight]) of the mixed epidermal cytokeratin suspension, and 8.35 μg/106 BEC (CS fraction), which amounts to 8.5% of the total protein in 106 BEC. Thus, the actual amounts of CK13 present in the inhibition mixtures for lanes 3, 7, and 8 of Fig. 3 were calculated to be 2.0 (36 nM), 0.3 (5.4 nM), and 1.6 μg (29 nM), respectively.
The MAb for CK13 did not inhibit bacterial binding to CK13 (not presented), indicating that the antibody did not block the target epitope for the bacterial adhesin.
B. cepacia binding to epithelial cells correlates with cellular CK13 content.
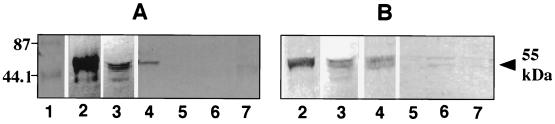
CK13 content and B. cepacia binding were measured in several different epithelial cells. The cytokeratin content of NHBE cells has not been reported. A-431 cells are from an epidermoid cell carcinoma of the vulva and have been reported to express CK13 (30). HEp-2 cells (epidermoid carcinoma of the larynx), A-549 (carcinoma of lung), and NCI-H520 (squamous carcinoma of lung) cells have been reported to express no or very low amounts of CK13 (1, 2, 26). Cells were cultured to 90 to 95% confluency, CM and CS fractions were prepared from each cell type and separated by SDS-PAGE, and proteins were transferred to nitrocellulose. As expected, none of the CM fractions showed CK13 immunoreactivity or served as a receptor for the bacteria (not presented). Among the CS fractions (Fig. 4), those from BEC, NHBE, and A-431 cells (lanes 2, 3, and 4 of panel A) contained immunoreactive CK13 bands at about 55 kDa, although the intensity of the CK13 signal in the CS fraction from 0.5 × 104 BEC (lane 2) was much greater than that from 1.1 × 105 NHBE cells (lane 3) or 5.6 × 104 A-431 cells (lane 4). Relative to the CK13 content of BEC (8.35 μg/106 cells), the CK13 content measured by immunoassay in NHBE cells was 0.48 μg/106 cells and in A-431 cells was 0.3 μg/106 cells. In NHBE cells, two or three immunoreactive bands were frequently observed (lane 3), but the major band was at 55 kDa. In bacterial overlays (panel B), we noted the highest binding of B. cepacia to the CK13 band of BEC (lane 2) and less binding to the CK13 bands of NHBE (lane 3) and A-431 (lane 4) cells. Very slight bacterial binding to the other cells was occasionally noted at the same position (lanes 5 to 7), in keeping with a low or undetectable CK13 content. Thus, a general correlation was noted between cellular CK13 content and binding of bacteria among the cells tested.
FIG. 4.
CS fractions from different cell types. Immunoreactivity with MAb to CK13 and BC7 binding. CS fractions from BEC and other epithelial cells were subjected to SDS-PAGE, and proteins were transferred to nitrocellulose membranes. One blot (A) was incubated with the MAb to CK13 (0.04 μg of Fab fragments/ml), and a separate identical blot (B) was incubated with 35S-BC7 (7 × 108 CFU/ml). CK13 was detected by using a second antibody conjugated to alkaline phosphatase. Bound bacteria were detected by autoradiography. Lane 1, prestained molecular mass standards (in kilodaltons); lanes 2 to 7 represent CS fractions from BEC (0.5 × 104), NHBE (1.1 × 105), A-431 (0.56 × 105), A-549 (5 × 105), NCI-H520 (5 × 105), and HEp-2 (5 × 105) cells, respectively.
Accessibility of CK13 in intact respiratory cells.
In an effort to establish that CK13 of intact airway cells can function as a surface receptor for cable-piliated B. cepacia, we carried out a number of experiments, including binding of B. cepacia to intact NHBE cells, inhibition of binding by cytokeratin-specific agents, and colocalization of CK13 and bound bacteria on NHBE cells.
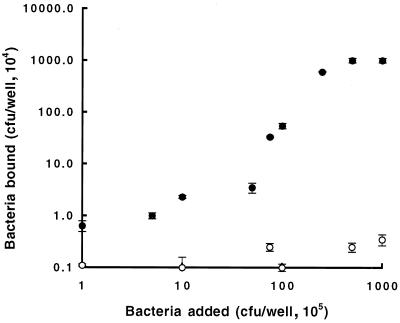
As shown in Fig. 5, isolate BC7 (Cbl positive) bound to NHBE cells in a concentration-dependent fashion, reaching a plateau at 2.5 × 106 CFU/well. Isolate BC45 (Cbl negative) showed negligible binding. These findings demonstrate the specificity for binding by the pilus-associated adhesin and indicate the saturability of the receptor on NHBE cells. Inhibition experiments were then carried out with BC7 used at a concentration that gives half-maximal binding (107 CFU/ml) (Table 1). Potential inhibitors were preincubated with BC7 or, in the case of the antibody to mixed epidermal cytokeratins, it was preincubated with NHBE cells, and then bacterial binding assays were carried out. Dose-dependent inhibition was observed for the CS fraction of BEC, mixed epidermal cytokeratins, and the anti-mixed epidermal cytokeratin antibody (Table 1). Plantar stratum corneum cytokeratins, which lack CK13 but contain cytokeratins 1, 2, and 9 (41), were noninhibitory. Higher concentrations of the inhibitors could not be tested for technical reasons, mainly because the increased volumes of keratin solubilization buffer needed to maintain solubility of the cytokeratins interfered in the assay. Unfortunately, purified CK13 is not available to allow more direct quantitation of its inhibitory activity. However, the results shown in Table 1 indicate that about 50 to 60% inhibition of binding was caused by mixtures containing approximately 0.2 to 0.84 μg of CK13 per ml, which is equivalent to 3.6 to 15 nM CK13. These findings are consistent with the interpretation that CK13 could be a receptor.
FIG. 5.
Binding of B. cepacia to NHBE cells. NHBE cells were grown in 96-well plates until they were 80 to 90% confluent. The wells were blocked with 3% BSA for 1 h at 37°C, washed with PBS, and then incubated with 0.1 ml of B. cepacia (105 to 108 CFU/ml) for 1 h. The wells were washed five times with PBS to remove nonbound bacteria, and the bound bacteria were quantitated by enzyme-linked immunosorbent assay by using an antibody specific for B. cepacia. ●, Cbl-positive isolate BC7; ○, Cbl-negative isolate BC45. The mean ± the standard error of the mean of quadruplicate assays for each bacterial concentration is given.
TABLE 1.
Inhibition of BC7 binding to NHBE cells by BEC and epidermal cytokeratins and polyclonal antibody to epidermal cytokeratinsa
| Inhibitor(s) | CK13 content (μg/ml) | % Inhibition (range) |
|---|---|---|
| None (control) | 0.0 | |
| Mixed epidermal cytokeratins (μg/ml) | ||
| 2.5 | 0.025 | 5.2 (2.2–8.7) |
| 5.0 | 0.05 | 9.4 (6.3–13.1) |
| 10.0 | 0.1 | 32.3 (28–40) |
| 20.0 | 0.2 | 58.6 (51–67) |
| BEC CS fraction (cell number) | ||
| 104 | 0.08 | 37 (32–42) |
| 105 | 0.84 | 57 (49–63) |
| 106 | 8.4 | 73 (72–75) |
| Planar stratum corneum cytokeratins (20 μg/ml) | 0.0 | 0.0 |
| Polyclonal antibody to mixed epidermal cytokeratins (dilution) | ||
| 1:250 | 58 (54–61) | |
| 1:100 | 68 (65–69) | |
| 1:50 | 72 (69–73) |
NHBE cells were grown in 96-well plates until they were 80 to 90% confluent. Wells were blocked with 3% BSA for 1 h at 37°C, washed with PBS, and then incubated for 1 h at 37°C with BC7 (107 CFU/ml) preincubated with potential inhibitors (or PBS to provide background binding data). When the antibody to mixed epidermal cytokeratins was used as an inhibitor, NHBE cells were first incubated with the antibody for 1 h and then washed to remove excess antibody prior to the addition of bacteria. Wells were washed five times with PBS to remove nonbound bacteria and the bound bacteria quantitated by enzyme-linked immunosorbent assay by using an antibody specific to B. cepacia. Values represent the average of triplicate experiments, and the numbers in parentheses are the total ranges of values obtained.
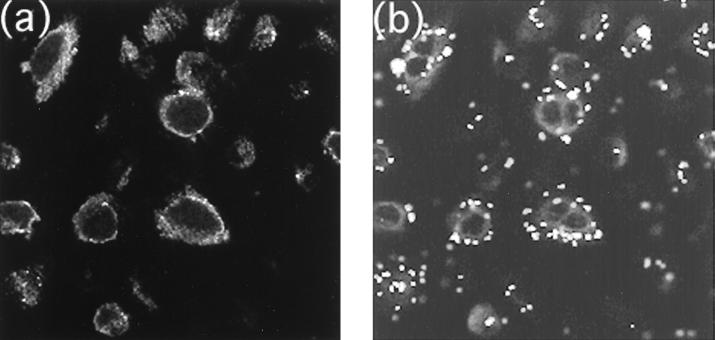
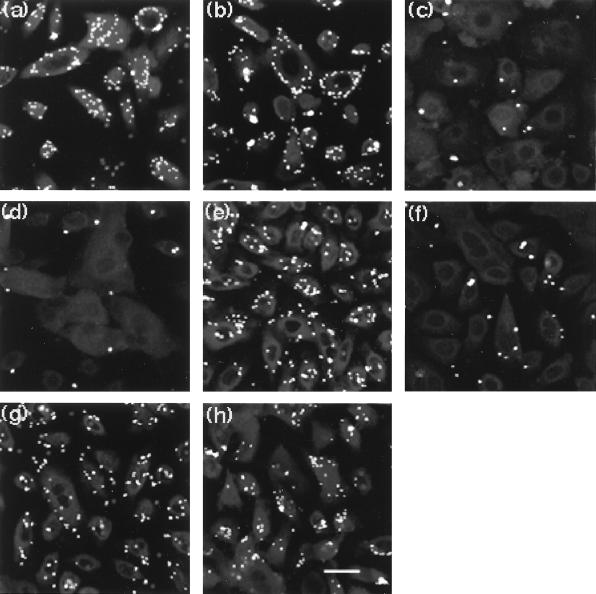
To assess surface distribution of CK13 and bound bacteria on intact cells, colocalization experiments were carried out on unfixed nonpermeabilized cells using confocal microscopy. Monolayers (80 to 90% confluent) of NHBE cells grown in Lab-Tek chamber slides were incubated with the MAb to CK13 for 30 min at 37°C, washed three times, and then incubated with FITC-labeled B. cepacia. After washing to remove nonbound bacteria, the slides were then incubated with anti-mouse IgG conjugated to the fluorophore LRSC to label bound CK13 antibody. We observed by light microscopy that some cells were lost during the various washing steps, which was expected because the cells were not fixed (to avoid permeabilization). The cells that remained were examined by confocal microscopy to determine the correspondence between CK13 distribution and bound bacteria (Fig. 6). Both CK13 (panel a) and bacteria (panel b) were noted around the periphery of cells in an almost identical distribution. Optical sectioning revealed no bacteria or CK13 signals in the interior of any of the cells (not presented). To ensure that the NHBE cells were really intact, they were incubated with an antibody to tubulin, a confirmed cytoplasmic protein (34). No staining was visible, but staining became prominent after the cells had been permeabilized by 100% cold methanol (not presented). CK13 also became much more prominent inside the cells after permeabilization (not presented). These experiments established that at least some CK13 produced by intact NHBE cells is present on cell surfaces. To confirm that surface CK13 was responsible for B. cepacia binding, fluorescence-labeled bound bacteria were visualized before and after added inhibitors. Binding was quantitated by counting the number of bacteria bound per cell in 10 representative microscopic fields. Each field contained from 25 to 72 NHBE cells. Mixed epidermal cytokeratins and CS fractions from BEC inhibited B. cepacia binding to NHBE cells by 96 to 97% (Fig. 7c and d and Table 2). The antiserum to mixed epidermal keratins was similarly inhibitory (95%) (panel f), an effect that was largely reversed by preabsorption of the antiserum against the BEC CS fraction to remove anti-CK13 antibodies (panel h). These findings help to support the interpretation that CK13 is available and can function as a surface receptor for Cbl-positive B. cepacia adherence to intact NHBE cells.
FIG. 6.
Colocalization of bound B. cepacia and CK13 on NHBE cells. NHBE cells were incubated with the mouse MAb to CK13 and FITC-labeled B. cepacia BC7 as described in Materials and Methods, washed, and then incubated with antimouse antibody conjugated with the fluorophore LRSC. Cells were counterstained with Mayer's hematoxylin. Panels a and b represent CK13 localization and B. cepacia binding, respectively. Magnification bar, 10 μm.
FIG. 7.
Inhibition of BC7 binding to NHBE cells by BEC CS fraction, mixed epidermal cytokeratins, and polyclonal antibody to mixed epidermal cytokeratins. FITC-labeled BC7 (107 CFU/ml) were incubated with NHBE cells for 1 h and washed, and bound bacteria were detected by confocal microscopy. (a) No inhibitor added. (b to d) Preincubation of bacteria with keratin solubilization buffer (control) (b), buffer plus mixed epidermal cytokeratins (20 μg/ml) (c), and buffer plus BEC CS fraction (containing approximately 8.3 μg of CK13 per ml) (d). (e to h) Preincubation of cells with nonimmune serum control (e), antiserum to mixed epidermal cytokeratins (f), nonimmune serum preadsorbed on BEC CS fraction (g), and antibody to mixed epidermal cytokeratins preabsorbed on BEC CS fraction (h). Magnification bar, 10 μm.
TABLE 2.
Inhibition of BC7 binding to NHBE cellsa
| Inhibitor(s) | Binding (bacteria/10 cells) |
|---|---|
| None (control) | 54 ± 5.1 |
| Mixed epidermal cytokeratins (20 μg/ml) | 3.3 ± 0.53 |
| BEC CS fraction (8.3 μg/ml) | 4 ± 0.43 |
| Nonimmune serum (1:50 dilution) | 50 ± 4.5 |
| Antibody to mixed epidermal cytokeratins (1:50 dilution) | 5.3 ± 0.48 |
| Nonimmune serum absorbed on BEC CS fraction | 53 ± 2.9 |
| Antibody to mixed epidermal cytokeratins absorbed on BEC CS fraction | 16 ± 2.31 |
FITC-labeled isolate BC7 (107 CFU/ml) was incubated with NHBE cells grown in Lab-Tek chamber slides for 1 h in the presence or absence of potential inhibitors. The cells were washed and counterstained with hematoxylin, and bacteria were detected by fluorescence microscopy. Bound bacteria per cell were counted for all cells in 10 representative microscopic fields (each field containing at least 25 NHBE cells) and then averaged. Values represent the mean number of bacteria bound ± the standard error of the mean.
DISCUSSION
The present study indicates that cable-piliated B. cepacia, of which isolate BC7 is a typical example, bind to a 55-kDa epithelial protein having the size, internal peptide sequences, and immunoreactive properties characteristic of CK13. No other binding receptors for Cbl-positive B. cepacia were found in the combined membrane and cytoplasmic fractions of epithelial cells. Bacterial binding to the isolated CK13 and to CK13-containing epithelial cells (BEC, A431, and NHBE cells) was inhibited by the cytokeratin-enriched cytoskeleton (CS) fraction of BEC, by mixed epidermal cytokeratins (which contain small amounts of CK13), and by a polyclonal antibody to mixed epidermal cytokeratins. Preabsorption of the antibody with the CS fraction of BEC (which contains mainly CK13 and CK4) reversed the inhibitory effect of the antibody. Mixtures containing as little as 3.6 to 15 nM CK13 caused 50 to 60% inhibition of Cbl-positive B. cepacia binding to intact NHBE cells. This is a sufficiently low range to be appropriate for a receptor molecule. Binding was not inhibited by cytokeratins of plantar stratum corneum, which are devoid of CK13 (41). Although the MAb to CK13 did not inhibit bacterial binding, this result cannot be taken as a lack of evidence for CK13 as the bacterial target receptor. MAbs generally recognize only a single epitope (10), and there is no compelling reason to expect that this epitope should also be the recognition site for Cbl-positive B. cepacia.
To date, few cellular receptors for any bacteria have been characterized, and cytokeratins as a potential class of molecules have been largely overlooked. This is not surprising, given that cytokeratins have been assumed to be exclusively intracellular in location. They form a complex intracellular scaffold of intermediate filaments connecting cell organelles to the nucleus and plasma membrane, and in epithelial cells they extend into desmosomes by interacting with desmosomal components (18). CK13 is commonly used as a marker of nonkeratinized squamous epithelium (45), where it is found in superficial, intermediate, and suprabasal cells. It is known to be prominent in the oral mucosa and esophagus (13, 26, 27, 45) and is also present in the nonkeratinized stratified squamous epithelia of the nose and trachea (26, 29). BEC as a model of respiratory epithelial cells are useful but have the limitation that they are “damaged,” since they are permeable to trypan blue and are unable to replicate in culture (28). Thus, internally located CK13 in BEC may be sufficiently “exposed” for B. cepacia to adhere. This may not be the case for intact epithelial cells in more distal airways, a consideration which led us to test for the distribution of the cytokeratin and its action to bind B. cepacia in primary cultures of normal human bronchial epithelial (NHBE) cells. Our findings suggest that a small amount of CK13 is indeed exposed at the surface of nonpermeabilized NHBE cells and can function to bind B. cepacia. It is likely that CK13 availability at cell surfaces is normally low but is increased by the chronic inflammation typical of CF lungs, since it is known that an increase in CK13 expression occurs in transitional respiratory epithelium undergoing squamous metaplasia and/or repair following epithelial injury (21, 32, 42). Previous infection with P. aeruginosa may serve to upregulate CK13 and make CF patients particularly vulnerable to infection by Cbl-positive B. cepacia.
Our findings are not the first to suggest that cytokeratins are accessible at epithelial surfaces. Godfroid et al. (9), Diaz et al. (5), and Hembrough et al. (14) showed that CK8 and CK18 are present on the surface of cultured human and mouse keratinocytes. Cytokeratins (type not specified) were also identified on injured corneal epithelial cells, where they functioned as receptors for pili isolated from P. aeruginosa PAK strain (49). It is possible therefore that cytokeratins are more common at cell surfaces than is usually assumed and may have hitherto undiscovered roles in recognition processes.
Isolate BC45 is identical to isolate BC7, as judged by DNA-based typing methods (43), but it does not bind to CK13. In an earlier study (38) electron microscopy revealed that 14 of 15 genetically identical Toronto B. cepacia isolates expressed cable pili on the bacterial surface. The single exception was isolate BC45, which appears to be a naturally occurring “isogenic” mutant. This isolate binds preferentially to galactolipids rather than to CK13 of BEC (37, 44). It is possible that other Cbl-negative pathogenic isolates of the B. cepacia complex also use galactolipid receptors for initial attachment to CF host cells, although rigorous testing with other genomovars has not been carried out. B. cepacia strains are also known to infect patients with chronic granulomatous disease (CGD) (19). One CGD isolate, designated JTC, was available to us from the B. cepacia research panel held at the University of British Columbia. JTC belongs to the Cbl-negative genomovar II group (46), and we would therefore not expect CK13 to serve as a target receptor in host cells.
Recently, a case report (20) documented B. cepacia lung infection in a previously healthy non-CF mother of two CF children. The infecting strain was typed as genomovar III, cblA positive. This raises the possibility that Cbl-positive B. cepacia could pose a threat to healthy family members who are repeatedly exposed to this strain. Given the propensity of BC7 to bind to CK13, it is also possible that normal host BEC and other upper airway transitional or squamous cells can serve as a reservoir for B. cepacia, with more distal spread during repeat exposure to the organism, perhaps as a consequence of episodes of mild airway infections by viruses or other pathogens. Fortunately, no other similar cases have been reported, and we assume that infection of healthy individuals by Cbl-positive B. cepacia will continue to be a very rare occurrence.
The cellular consequences, if any, of cytokeratin-bacterial interactions are unknown, but are worthy of future investigation, since the extensive cytokeratin network within cells makes it possible that surface perturbation could initiate intracellular changes. A few examples of this have been observed, including the deleterious effects of adenovirus and human papillomavirus binding to vimentin and cytokeratins in HeLa cells and human keratinocytes, respectively (6, 47). Both cause rearrangements and disruption of intermediate filaments. Vimentin is also disrupted by the binding of P. aeruginosa exotoxin A to CVB-1 cells (40). If similar disruptions occur with Cbl-positive B. cepacia binding to epithelial CK13, the net effect may be to impair cell function and facilitate translocation of bacteria across the epithelial barrier. Investigations to explore some of these possibilities are ongoing.
REFERENCES
- 1.Blobel G A, Moll R, Franke W W, Vogt-Moykopf I. Cytokeratins in normal lung and lung carcinomas. Virchows Arch B Cell Pathol. 1984;45:407–429. doi: 10.1007/BF02889883. [DOI] [PubMed] [Google Scholar]
- 2.Broers J L, Carney D N, Klein Rot M, Schaart G, Lane E B, Vooijs G P, Ramaekers F C. Intermediate filament proteins in classic and variant types of small cell lung carcinoma cell lines: a biochemical and immunochemical analysis using a panel of monoclonal and polyclonal antibodies. J Cell Sci. 1986;83:37–60. doi: 10.1242/jcs.83.1.37. [DOI] [PubMed] [Google Scholar]
- 3.Burns J L, Wardsworth C D, Barry J J, Goodall C P. Nucleotide sequence analysis of a gene from Burkholderia cepacia encoding an outer membrane lipoprotein involved in multiple antibiotic resistance. Antimicrob Agents Chemother. 1996;40:307–313. doi: 10.1128/aac.40.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darling P, Chan M, Cox A D, Sokol P A. Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect Immun. 1998;66:874–877. doi: 10.1128/iai.66.2.874-877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz L A, Sampaio S A P, Martins C R, Rivitti E A, Macca M L, Roscoe J T, Takahashi Y, Labib R S, Patel H P, Mutasim D F, Kugan E M, Anhalt G J. An autoantibody in pemphigus serum, specific for the 59 KD keratin, selectively binds the surface of keratinocytes: evidence for an extracellular keratin domain. J Investig Dermatol. 1987;89:287–295. doi: 10.1111/1523-1747.ep12471451. [DOI] [PubMed] [Google Scholar]
- 6.Doorbar J, Ely S, Sterling J, McLean C, Crawford L. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of epithelial cell intermediate filament network. Nature. 1991;352:824–827. doi: 10.1038/352824a0. [DOI] [PubMed] [Google Scholar]
- 7.Falk P, Roth K A, Boren T, Westblom T U, Gordon J I. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc Natl Acad Sci USA. 1993;90:2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke W W, Schiller D L, Moll R, Winter S, Schmid E, Engelbrecht I, Denk H, Krepler R, Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981;153:933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- 9.Godfroid E, Geuskens M, Dupressoir T, Parent I, Szpirer C. Cytokeratins are exposed on the outer surface of established human mammary carcinoma cells. J Cell Sci. 1991;99:565–607. doi: 10.1242/jcs.99.3.595. [DOI] [PubMed] [Google Scholar]
- 10.Goding J W, editor. Monoclonal antibodies: principles and practice. 2nd ed. New York, N.Y: Academic Press, Inc.; 1986. [Google Scholar]
- 11.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govan J R W, Hughes J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 13.Grace M P, Kim K H, True L D, Fuchs E. Keratin expression in normal esophageal epithelium and squamous cell carcinoma of the esophagus. Cancer Res. 1985;45:841–846. [PubMed] [Google Scholar]
- 14.Hembrough T A, Vasudevan J, Allietta M M, Glass II W F, Gonias S L. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J Cell Sci. 1995;108:1071–1082. doi: 10.1242/jcs.108.3.1071. [DOI] [PubMed] [Google Scholar]
- 15.Hughes J E, Stewart J, Barclay G R, Govan J R. Priming of neutrophil respiratory burst activity by lipopolysaccharide from Burkholderia cepacia. Infect Immun. 1997;65:4281–4287. doi: 10.1128/iai.65.10.4281-4287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchison M L, Poxton I R, Govan J R. Burkholderia cepacia produces a hemolysin that is capable of inducing apoptosis and degranulation of mammalian phagocytes. Infect Immun. 1998;66:2033–2039. doi: 10.1128/iai.66.5.2033-2039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen H K, Kovesi T A, Koch C, Corey M, Hoiby N, Levison H. Pseudomonas aeruginosa and Burkholderia cepacia infection in cystic fibrosis patients treated in Toronto and Copenhagen. Pediatr Pulmonol. 1998;26:89–96. doi: 10.1002/(sici)1099-0496(199808)26:2<89::aid-ppul3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Jones J C, Green K J. Intermediate filament-plasma membrane interactions. Curr Opin Cell Biol. 1991;3:127–132. doi: 10.1016/0955-0674(91)90175-x. [DOI] [PubMed] [Google Scholar]
- 19.Lacy D, Spencer D, Goldstein A, Weller P, Darbyshire P. Chronic granulomatous disease presenting in childhood with Pseudomonas cepacia septicaemia. J Infect. 1993;27:301–304. doi: 10.1016/0163-4453(93)92271-w. [DOI] [PubMed] [Google Scholar]
- 20.Ledson M J, Gallagher M J, Corkill J E, Hart C A, Walshaw M J. Cross infection between cystic fibrosis patients colonised with Burkholderia cepacia. Thorax. 1998;53:432–436. doi: 10.1136/thx.53.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leube R E, Rustad T J. Squamous cell metaplasia in the human lung: molecular characteristics of epithelial stratification. Virchows Arch B Cell Pathol. 1991;61:227–253. doi: 10.1007/BF02890425. [DOI] [PubMed] [Google Scholar]
- 22.LiPuma J J. Burkholderia cepacia. Management issues and new insights. Clin Chest Med. 1998;19:473–86. doi: 10.1016/s0272-5231(05)70094-0. [DOI] [PubMed] [Google Scholar]
- 23.Lonon M K, Woods D E, Straus D C. Production of lipase by clinical isolates of Pseudomonas cepacia. J Clin Microbiol. 1988;26:976–984. doi: 10.1128/jcm.26.5.979-984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahenthiralingam E, Campbell M E, Henry D A, Speert D P. Epidemiology of Burkholderia cepacia infection in patients with cystic fibrosis: analysis by randomly amplified polymorphic DNA fingerprinting. J Clin Microbiol. 1996;34:2914–2920. doi: 10.1128/jcm.34.12.2914-2920.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKevitt A I, Bajaksouzian S, Klinger J D, Woods D E. Purification and characterization of an extracellular protease from Pseudomonas cepacia. Infect Immun. 1989;57:771–778. doi: 10.1128/iai.57.3.771-778.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moll R, Franke W W, Schiller D L, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 27.Morgan P R, Leigh I M, Purkis P E, Gardner I D, van Muijen G N P, Lane E B. Site variation in keratin expression in human oral epithelia—an immunocytochemical study of individual keratins. Epithelia. 1987;1:31–43. [Google Scholar]
- 28.Moss-Salentijn L, Hendricks-Klyvert M, editors. Dental and oral tissues: an introduction. 3rd ed. Philadelphia, Pa: Lea & Febiger; 1990. [Google Scholar]
- 29.Nagle R B, Moll R, Weidauer H, Nemetschek H, Franke W W. Different patterns of cytokeratin expression in the normal epithelia of the upper respiratory tract. Differentiation. 1982;30:130–140. doi: 10.1111/j.1432-0436.1985.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 30.Nelson W G, Sun T-T. The 50- and 58-kilodalton keratin classes as molecular markers for stratified squamous epithelia: cell culture studies. J Cell Biol. 1983;97:244–251. doi: 10.1083/jcb.97.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitt T L, Kaufmann M E, Patel P S, Benge L C, Gaskin S, Livermore D M. Type characterization and antibiotic susceptibility of Burkholderia cepacia isolates from patients with cystic fibrosis in the United Kingdom and the Republic of Ireland. J Med Microbiol. 1996;44:203–210. doi: 10.1099/00222615-44-3-203. [DOI] [PubMed] [Google Scholar]
- 32.Roger P, Puchelle E, Bajolet-Laudinat O, Tournier J M, Debordeaux C, Plotkowski M C, Cohen J H, Sheppard D, de Bentzmann S. Fibronectin and α5β1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur Respir J. 1999;13:1301–1309. [PubMed] [Google Scholar]
- 33.Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol. 1997;145:794–803. doi: 10.1093/oxfordjournals.aje.a009172. [DOI] [PubMed] [Google Scholar]
- 34.Safiejko-Mroczka B, Bell P B. Bifunctional protein cross-linking reagents improve labeling of cytoskeletal proteins for qualitative and quantitative fluorescence microscopy. J Histochem Cytochem. 1996;44:641–656. doi: 10.1177/44.6.8666749. [DOI] [PubMed] [Google Scholar]
- 35.Sajjan U S, Corey M, Karmali M, Forstner J F. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Investig. 1991;89:648–656. doi: 10.1172/JCI115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sajjan U S, Forstner J F. Identification of the mucin-binding adhesin of isolated Pseudomonas cepacia from patients with cystic fibrosis. Infect Immun. 1992;60:1434–1440. doi: 10.1128/iai.60.4.1434-1440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sajjan U S, Forstner J F. Role of a 22-kilodalton pilin protein in binding of Pseudomonas cepacia to buccal epithelial cells. Infect Immun. 1993;61:3157–3163. doi: 10.1128/iai.61.8.3157-3163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sajjan U S, Sun L, Goldstein R, Forstner J F. Cable (cbl) type II pili of cystic fibrosis-associated Burkholderia (Pseudomonas) cepacia: nucleotide sequence of the cblA major subunit pilin gene and novel morphology of the assembled appendage fibers. J Bacteriol. 1995;177:1030–1038. doi: 10.1128/jb.177.4.1030-1038.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz P, Wachter E, Hochstrasser K, Wild A G, Mischke D. Sequence of a human keratin 13 specific cDNA encompassing coil 1B through the 3′ end. Biochem Biophys Res Commun. 1989;162:1522–1527. doi: 10.1016/0006-291x(89)90847-4. [DOI] [PubMed] [Google Scholar]
- 40.Sharpe A H, Chen L B, Murphy J R, Fields B N. Specific disruption of vimentin filament organization in monkey kidney CV-1 cells by diphtheria toxin, exotoxin A, and cycloheximide. Proc Natl Acad Sci USA. 1980;77:7267–7271. doi: 10.1073/pnas.77.12.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smack D P, Korge B P, James W D. Keratin and keratinization. J Am Acad Dermatol. 1994;30:85–102. doi: 10.1016/s0190-9622(94)70012-5. [DOI] [PubMed] [Google Scholar]
- 42.Stosiek P, Kasper M, Moll R. Changes in cytokeratin expression accompany squamous metaplasia of the human respiratory epithelium. Virchows Arch A Pathol Anat. 1992;421:133–141. doi: 10.1007/BF01607046. [DOI] [PubMed] [Google Scholar]
- 43.Sun L, Jiang R-Z, Steinbach S, Holmes A, Campanelli C, Forstner J, Sajjan U, Tan Y, Riley M, Goldstein R. The emergence of a highly transmissible lineage of cbl+ Pseudomonas (Burkholderia) cepacia causing epidemics in North America and Britain. Nat Med. 1995;1:661–666. doi: 10.1038/nm0795-661. [DOI] [PubMed] [Google Scholar]
- 44.Sylvester F A, Sajjan U S, Forstner J F. Burkholderia (basonym Pseudomonas) cepacia binding to lipid receptors. Infect Immun. 1996;64:1420–1425. doi: 10.1128/iai.64.4.1420-1425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Muijen G N P, Ruiter D J, Franke W W, Achtstatter T, Haasnoot W H B, Ponec M, Warnaar S O. Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res. 1986;162:97–113. doi: 10.1016/0014-4827(86)90429-5. [DOI] [PubMed] [Google Scholar]
- 46.Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, Revets H, Lauwers S, Gillis M, Kersters K, Govan J R W. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- 47.White E, Cipriani R. Role of adenovirus E1B proteins in transformation: altered organization of intermediate filaments in transformed cells that express the 19-kilodalton protein. Mol Cell Biol. 1990;10:120–130. doi: 10.1128/mcb.10.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whiteford M L, Wilkinson J D, McColl J H, Conlon F M, Michie J R, Evans T J, Paton J Y. Outcome of Burkholderia (Pseudomonas) cepacia colonisation in children with cystic fibrosis following a hospital outbreak. Thorax. 1995;50:1194–1198. doi: 10.1136/thx.50.11.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Kurpakus M, Hazlett L D. Some P. aeruginosa pilus-binding proteins of human corneal epithelium are cytokeratins. Curr Eye Res. 1996;15:782–791. doi: 10.3109/02713689609003463. [DOI] [PubMed] [Google Scholar]
- 50.Zughaier S M, Ryley H C, Jackson S K. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenostrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect Immun. 1999;67:1505–1507. doi: 10.1128/iai.67.3.1505-1507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zughaier S M, Ryley H C, Jackson S K. A melanin pigment purified from an epidemic strain of Burkholderia cepacia attenuates monocyte respiratory burst activity by scavenging superoxide anion. Infect Immun. 1999;67:908–913. doi: 10.1128/iai.67.2.908-913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]