Abstract
In this multicenter retrospective study we aimed to evaluate the outcome of cardiac involvement in children affected by multisystem inflammatory syndrome (MIS-C), assessed through cardiac magnetic resonance (CMR). Children referring to three Italian tertiary pediatric centers between February 2020 and November 2021 with a diagnosis of MIS-C, who underwent CMR during a follow-up visit, were enrolled. Demographic, clinical, laboratory, treatment, and outcome data were collected. Twenty MIS-C patients (aged 9–17, median 12 years) were included in the study. Heart involvement at onset was testified by hypotension/shock (55%), laboratory evidence of myocardial involvement (100%), reduced LV ejection fraction (EF) on echocardiography (83%), and/or need for inotrope agents (40%); they all presented good clinical, laboratory, and echocardiographic response to treatment. CMR was performed after a median interval of 3 months from discharge. Pericardial effusion and myocardial edema were found in 5% of patients. Mild residual left ventricular (LV) dysfunction was found in 20% of patients, all showing normal echocardiographic LVEF at discharge. Minimal myocardial scars were found in 25% by late gadolinium enhancement (LGE). One patient was evaluated at two consecutive time points, showing partial resolution of a myocardial scar after 7 months from its first finding.
Conclusion: Despite the severity of heart involvement in the acute MIS-C phase, the mid-term cardiac outcome is good. Direct cardiac tissue viral invasion may be involved in MIS-C pathogenesis.
|
What is Known: • Heart involvement is common in MIS-C, but conflicting findings have been shown regarding cardiac outcome when assessed through cardiac MRI. | |
|
What is New: • Midterm cardiac MRI shows mild abnormalities in patients recovered from MIS-C with any grade of severity of cardiac involvement at presentation. |
Keywords: COVID-19, MIS-C, Cardiac MRI, Myocarditis, Outcome
Background
Since its appearance in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection showed a milder disease course in children when compared to adults [1]. However, children may develop a hyperinflammatory response to SARS-CoV-2 infection, called multisystem inflammatory syndrome (MIS-C) [2]. Heart involvement is common in MIS-C: myocarditis, ventricular dysfunction, valvular regurgitation, and pericardial effusion are more frequently reported than coronary artery abnormalities (CAA) [3]. Despite the severity of clinical manifestations at onset, an excellent cardiac outcome has been reported when evaluated through echocardiography at a 3-month follow-up [4]. However, conflicting findings concerning cardiac outcome have been shown when performing CMR in the acute phase of MIS-C [4–8]. Moreover, exhaustive data on cardiac recovery are lacking, so that cardiac follow-up recommendations for patients with MIS-C are still uncertain, being mostly derived from those applied for Kawasaki disease (KD) and viral myocarditis in the pre-COVID era [9, 10].
In this multicenter study, we aimed to evaluate the outcome of cardiac involvement in patients affected by MIS-C, assessed through CMR.
Methods
Study design and population
In this multicenter retrospective study, all MIS-C patients referring to three Italian tertiary pediatric centers (Institute for Maternal and Child Health, IRCCS “Burlo Garofolo”, Trieste; ASST Spedali Civili, Brescia; Meyer Children’s University Hospital, Florence) from February 1, 2020, to November 31, 2021 were enrolled. For the purpose of this study, only patients who underwent CMR, prescribed by the treating physician as standard of care, during follow-up visits were taken into account. Demographic, clinical, laboratory, treatment, and outcome data were collected in an anonymized database. Diagnosis of MIS-C was based on the WHO case definition [11].
CMR protocol and image analysis
Cardiac MRI was performed on 1.5-Tesla magnetic resonance scanners (Philips Intera®, Philips Ingenia®) with a cardiac phased-array receiver surface coil, ECG-gating, and breath-hold technique, using a dedicated cardiac software. The three centers presented similar protocols. Cine images in two-, three-, and four-chamber views and a stack of contiguous short-axis slices from the atrioventricular plane to the apex were acquired using balanced steady-state free precession (bSSFP) pulse sequence. T2-weighted short tau inversion recovery (STIR) or turbo spin echo (TSE) with fat suppression pulse sequences were acquired with the same slice coverage as cine images. Approximately 10 min after intravenous administration of 0.1 mmol/kg gadolinium-based contrast agent, late gadolinium enhancement (LGE) images were acquired using segmented T1-weighted inversion-recovery prepared gradient-echo or phase sensitive inversion recovery (PSIR) pulse sequences, individually adjusting inversion time to optimize nulling of apparently normal myocardium. Pre- and post-contrast T1-mapping was conducted with a MOLLI sequence in three short axis slices (basal, midventricular and apical). T2 mapping was conducted with a GRASE sequence in the same short axis slices. Studies were evaluated by a radiologist and/or a cardiologist with specific expertise in cardiac MRI at each center.
LV and RV volumes and function were measured using the standard volumetric technique from the cine short axis stack, with the endocardial border traced at end-diastole and end-systole for each slice and the epicardial border traced at end-diastole [12]. Volume and mass measures were indexed to body surface area. On T2-weighted images, edema was considered present when a signal hyperintensity was observed in two orthogonal planes and/or the ratio of signal intensity between the myocardium and the mean signal intensity of the skeletal muscle was ≥ 2 [13, 14]. Presence of LGE was defined by the presence of areas with increased signal intensity following administration of contrast medium in two orthogonal planes. Global T1 and T2 values were obtained by averaging all segmental T1 and T2 values, respectively.
Data analysis
Categorical variables were reported as absolute numbers and percentages. Continuous variables were expressed as means and standard deviations (SD) or as median and inter-quartile ranges (IQR), if not normally distributed.
Results
Population characteristics
Twenty children with a previous diagnosis of MIS-C had been evaluated with a cardiac MRI during their follow-up and were therefore included in the study. Data regarding their demographic characteristics, evidence of previous SARS-CoV-2 infection, and baseline symptoms at hospital admission for MIS-C, as well as laboratory markers, echocardiographic findings, treatment, and time of stay, are shown in Table 1.
Table 1.
Patient characteristics and clinical data
| ID | Age (years) | Sex | Ethnicity | Positive Serology for SARS-CoV-2 or previous PCR or exposure | Symptoms | Blood count abnormalities | CRP (mg/L) | hs-TnI or hs-TnT (× ULN) | NT-proBNP (× ULN) | Echocardiographic abnormalities | Treatment | PICU admission (days) | Time of hospitalization (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | M | Caucasian | PCR | Fever, oral mucositis | – | 75 | hs-TnT ×142 | – | Myopericarditis | NSAIDs, colchicine | – | 9 |
| 2 | 11 | M | Caucasian | IgG, PCR, exposure | Fever, rash, conjunctivitis, vomiting, diarrhea, hypotension | Anemia | 126 | hs-TnI ×4 | ×12 | LVEF 45–55%, mild mitral regurgitation | IVIG, MTP, inotropes | 3 | 19 |
| 3 | 12 | M | Caucasian | IgG, PCR | Fever, conjunctivitis, vomiting, diarrhea | Anemia, lymphopenia | 75 | Normal | ×5 | Normal | IVIG, MTP | – | 17 |
| 4 | 17 | M | Caucasian | IgG, PCR | Fever, vomiting, diarrhea, hypotension, hepatosplenomegaly | Lymphopenia | 154 | hs-TnI ×2 | – | LVEF 35–45%, CA aneurysm, pericardial effusion | IVIG, MTP, ASA | – | 21 |
| 5 | 9 | M | Asian | IgG, IgM | Fever, rash, conjunctivitis, dyspnea | Lymphopenia, thrombocytopenia | 300 | hs-TnT ×7 | ×12 | LVEF <35% | IVIG, MTP (pulses), anakinra, inotropes, heparin | 16 | 30 |
| 6 | 12 | M | Caucasian | IgG, exposure | Fever, rash, conjunctivitis, vomiting, diarrhea, hypotension, hepatomegaly | Anemia, thrombocytopenia | – | hs-TnI ×30 | ×11 | LVEF 50%, CA dilation | IVIG, MTP, anakinra, heparin | 5 | 46 |
| 7 | 11 | M | Caucasian | IgG, IgM | Fever, conjunctivitis, vomiting, diarrhea, dyspnea | – | 150 | hs-TnT ×6 | ×4 | Pericardial effusion | IVIG, MTP (pulses), heparin | 6 | 19 |
| 8 | 14 | F | Caucasian | IgG, IgM, exposure | Fever, vomiting, diarrhea, headache, dyspnea | Lymphopenia | 291 | hs-TnT ×16 | ×110 | – | IVIG, MTP (pulses), anakinra, inotropes, heparin | - | 26 |
| 9 | 14 | M | Caucasian | - | Fever, headache, lymphadenopathy, hypotension | Neutrophilia | 339 | hs-TnT ×23 | – | LVEF 35–45%, mild mitral regurgitation | IVIG, inotropes | 6 | 15 |
| 10 | 10 | F | Caucasian | IgG, IgM, PCR | Fever, rash, conjunctivitis, vomiting, diarrhea, hypotension | Anemia, lymphopenia, thrombocytopenia | 231 | hs-TnT ×2 | ×36 | LVEF 45–55% | IVIG, MTP, inotropes | 9 | 28 |
| 11 | 12 | M | African | IgM, PCR, exposure | Fever, vomiting, diarrhea, headache, dyspnea, cardiac arrest | Lymphopenia, neutrophilia | 31.5 | – | ×43 | LVEF <35% | IVIG, MTP, Anakinra, inotropes, heparin | 16 | 31 |
| 12 | 15 | M | Caucasian | Exposure | Fever, vomiting, diarrhea | Lymphopenia | 197 | – | ×2.5 | – | IVIG, MTP, heparin | – | 9 |
| 13 | 9 | F | Middle Eastern | IgG, IgM, exposure | Fever, rash, conjunctivitis, dyspnea, hypotension | Lymphopenia | 136 | – | ×7 | LVEF 45–55% | IVIG, MTP, heparin | 15 | 20 |
| 14 | 11 | M | Caucasian | IgG, PCR | Fever, cheilitis, conjunctivitis, vomiting, diarrhea, headache, hypotension | Lymphopenia, thrombocytopenia | 226 | TnI ×13 | ×6 | LVEF 50% | IVIG, MTP, ASA | – | 10 |
| 15 | 12 | M | Middle Eastern | IgG, exposure | Fever, rash, cheilitis, conjunctivitis, hypotension | – | 167 | TnT ×28 | – | LVEF 45–55%, mild mitral and tricuspid regurgitation | IVIG, MTP, ASA | – | 12 |
| 16 | 12 | M | Caucasian | IgG | Fever, rash, cheilitis, conjunctivitis, vomiting | Lymphopenia | 84 | TnI ×209 | ×35 | LVEF 45–55%, pericardial effusion, mild mitral regurgitation | IVIG, MTP, ASA | – | 8 |
| 17 | 11 | F | Caucasian | IgM, exposure | Fever, oral mucositis, lymphadenopathy, diarrhea, headache, dyspnea, hypotension, arthralgia | Lymphopenia | 114 | TnT ×24 | ×190 | LVEF <35%, CA aneurysms, moderate mitral and tricuspid regurgitation | IVIG, MTP, ASA, inotropes, heparin | 7 | 19 |
| 18 | 12 | F | Caucasian | PCR | Fever, rash, diarrhea, oliguria | Neutrophilia | 243 | TnI ×6 | ×115 | LVEF 48% | IVIG, MTP, ASA | – | 11 |
| 19 | 17 | M | Caucasian | IgG | Fever, rash, oral mucositis, conjunctivitis, lymphadenopathy | Thombocytopenia | 141 | TnI ×7 | ×3 | Normal | IVIG, MTP, ASA | – | 10 |
| 20 | 15 | M | Caucasian | IgG, IgM, exposure | Fever, diarrhea, headache, cough, myositis | Lymphopenia | 340 | TnT ×118 | Normal | LVEF 35–45% | IVIG, MTP, anakinra, remdesevir, inotropes, heparin | 8 | 29 |
PCR Polymerase Chain Reaction, CRP C-Reactive Protein, TnT Troponin T, hs-TnI High-Sensitivity Troponin I, ULN Upper Limit of Normal, NT-proBNP N-Terminal Prohormone of Brain Natriuretic Peptide, PICU Pediatric Intensive Care Unit, LVEF Left Ventricular Ejection Fraction, CA Coronary Artery, NSAIDs Non-Steroidal Anti-Inflammatory Drugs, IVIG Intravenous Immune Globulins, MTP Methylprednisolone, ASA Acetylsalicylic Acid
Most of the children were male (15/20, 75%) and Caucasian (16/20, 80%), with an age ranging from 9 to 17 years (median 12, IQR 11–14). Evidence of previous SARS-CoV-2 infection was based on detectable IgG and/or IgM antibodies in 16/20 children (80%), and a positive nasopharyngeal swab assessed by RT-PCR in 11/20 (55%); for two patients with negative serology and no reported positive RT-PCR, diagnosis of MIS-C was based on a recent contact with a subject with COVID-19 patient and on typical clinical presentation (with fever, myocarditis, shock, markedly elevated inflammatory markers, and no microbial cause) respectively.
All the patients presented with fever on admission. Gastrointestinal involvement (vomiting, diarrhea, abdominal pain) was present in 14/20 patients (70%); conjunctivitis, rash, and cheilitis/oral mucositis were present in 11/20 (55%), 9/20 (45%), and 6/20 (30%) patients respectively; dyspnea and/or cough in 7/20 (35%); and headache in 6/20 (30%). Remarkably, 11/20 patients (55%) presented with hypotension and/or shock. Six patients (30%) received oxygen support, with 2 of them requiring non-invasive ventilation (NIV); one patient needed mechanical ventilation (MV) and was the only one in our cohort to experience a cardiac arrest.
Markers of inflammation were markedly increased in all of our patients, with a median value of CRP of 152 mg/L (IQR 109–234). Laboratory evidence of myocardial involvement was found in all patients as well, either as elevated high-sensitivity troponin-T (hs-TnT, median 338 pg/mL, IQR 197–708), high-sensitivity troponin-I (hs-TnI, median 135 pg/mL, IQR 84–322), or NT-proBNP (median 5158 pg/mL, IQR 2110–28,941).
Echocardiography was performed in 18/20 patients (90%) on admission, resulting normal in two patients only (11%). Remarkably, reduced LVEF was found in 15/18 patients (83%), mostly with a mild (9/15; 60%) or moderate (3/15; 20%) depression; severely reduced LVEF was observed in the remaining 3 patients (20%, 15% of the entire cohort). Valvular regurgitation and pericardial effusion were found in 5/18 (28%) and 3/18 (17%) patients, respectively. Three patients (17%) experienced CA abnormalities, one with ectasia of the left anterior descending CA and two with aneurysms (left circumflex and left main CAs, respectively).
Apart from patient 1, who was treated with non-steroidal anti-inflammatory drugs (NSAIDs) and colchicine, all the other patients (19/20, 95%) received intravenous immune globulins (IVIG, 2 g/kg), combined with intravenous methylprednisolone in 18/20 (90%), either with a 1–2-mg/kg/day dosing regimen (10/18, 56%) or with 30-mg/kg pulses (8/18, 44%). Low-dose aspirin was started in 7/20 cases (35%). Eight (40%) patients required inotrope agents (such as adrenalin, dopamine, milrinone) and five (25%) biological therapy (anakinra) to control the disease. Ten patients (50%) were admitted to pediatric intensive care unit (PICU), either because requiring inotropes or non-invasive or mechanical ventilation, for a median time of 7.5 days (IQR 6–13.5), while the median total time of hospitalization was 19 days (IQR 11–26).
All the patients showed a good response to treatment and were discharged free of symptoms and with normal (9/20 patients, 45%) or markedly reduced (11/20 patients, 55%) levels of CRP, troponin, and NT-proBNP. Echocardiography was performed at discharge in most of our patients (19/20, 95%), with normal findings in most of them (15/19, 79%). Patients 15 and 16 (10%) showed residual mildly depressed LVEF, although improved compared to the ultrasound (US) at disease onset, and mild mitral regurgitation. Patient 16 showed residual pericardial effusion as well. Signs of residual myopericarditis were found in patients 1 and 9. No CA abnormalities were found.
Cardiac MRI
Apart from patient 1 who was studied 10 days after MIS-C onset, all of the patients received cardiac MRI with an interval of at least 1 month, with a median of 3 months (IQR 2–6) and a maximum of 9 months. Cardiac MRI was performed at two consecutive time points in patient 20, at 2 and 9 months respectively. Data regarding cardiac volumetric, functional and tissue characteristics are shown in Table 2.
Table 2.
Cardiac MRI results at follow-up
| ID | Time at follow up (months) | LV EF (%) | RV EF (%) | LV EDVI (ml/m2) | RV EDVI (ml/m2) | LV CO (L/min/m2) | RV CO (L/min/m2) | T1 mapping | T2 mapping | T2W | LGE | Pericardial effusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.3 | 48 | 48 | 81 | 72 | – | – | – | – | Normal | Lateral subepicardial, mid-wall inferoseptal | No |
| 2 | 1 | 50 | 53 | 105 | 115 | 4.1 | 4 | Normal | Normal | – | No | No |
| 3 | 1 | 58 | 53 | Normal | Normal | Normal | Normal | Normal | Normal | – | No | No |
| 4 | 1 | 66 | 47 | 98 | 103 | – | – | – | – | Normal | No | No |
| 5 | 2 | 69 | 67 | 56 | 63 | 4 | 4.4 | Normal | Normal | – | Inferoseptal and lateral mid-wall | No |
| 6 | 2 | 61 | 55 | 93 | 95 | 4.6 | 4.2 | Normal | Normal | – | Anteroseptal and inferolateral mid-wall | No |
| 7 | 2 | 62 | 60 | 74 | 81 | 3.7 | 3.9 | Normal | Normal | – | No | No |
| 8 | 2 | 57 | 54 | Normal | Normal | Normal | Normal | Normal | Prolonged | – | No | No |
| 9 | 2 | Normal | Normal | Normal | Normal | – | – | – | – | Normal | No | No |
| 10 | 3 | 54 | 62 | 68 | 60 | 2.8 | 2.9 | Normal | Normal | – | No | No |
| 11 | 3 | 50 | 43 | 88 | 104 | 3.5 | 3.5 | Normal | Normal | – | No | Thin layer |
| 12 | 3 | 61 | 56 | 74 | 80 | – | – | Normal | Normal | – | No | No |
| 13 | 4 | 43 | 47 | 112 | 96 | 4.1 | 3.8 | Prolonged | Normal | – | No | No |
| 14 | 5 | 65 | 58 | 86 | 97 | – | – | Prolonged | Normal | – | Inferolateral subepicardial | No |
| 15 | 6 | 64 | 51 | 69 | 86 | – | – | – | – | Normal | No | No |
| 16 | 6.5 | 62 | 57 | 108 | 113 | - | - | Normal | Normal | – | No | No |
| 17 | 7 | Normal | Normal | Normal | Normal | – | – | – | – | Normal | No | No |
| 18 | 8 | 65 | 62 | 67 | 72 | – | – | Prolonged | Normal | – | No | No |
| 19 | 9 | 57 | 47 | 94 | 113 | – | – | Normal | Normal | – | No | No |
| 20 | 2 | 64 | 62 | 83 | 87 | 3.1 | 3.2 | Normal | Normal | – | Lateral subepicardial | No |
| 20 | 9 | 63 | 58 | 80 | 83 | 3.3 | 3.1 | Normal | Normal | – | Lateral subepicardial (reduced) | No |
LV Left Ventricle, RV Right Ventricle, EF Ejection Fraction, EDVI End-Diastolic Volume Index, CO Cardiac Output, LGE Late Gadolinium Enhancement
Sixteen patients (80%) had normal biventricular volumes and function. Mildly reduced LVEF was found in all of the remaining 4/20 (20%): patient 1, with the shortest follow up and signs of myopericarditis on discharge, and patients 2, 11, and 13 who all showed normal LVEF on echocardiography at discharge. Notably, patient 13 was also the only with increased indexed left ventricular size (LVEDVI 112 ml/m2). Prolonged T1 time as a sign of either persisting myocardial edema or fibrosis was found in 3/20 cases (15%), who were studied with an interval ranging from 4 (patient 13) to 8 months (patient 17). Prolonged T2 time as a more specific sign of myocardial edema was found in patient 8 only (1/20, 5%), who was studied with an interval of 2 months. A thin layer of pericardial effusion was revealed in patient 11 only (5%). Minimal late gadolinium enhancement (Fig. 1) was shown in 5/20 participants (25%), with patient 20 presenting it at a maximum 9-month interval after MIS-C onset, although reduced in entity compared to his previous MRI evaluation performed after 2 months; apart from patient 1, all of the remaining patients showing LGE (no. 5, 6, 14, and 20) had normal echocardiography at discharge.
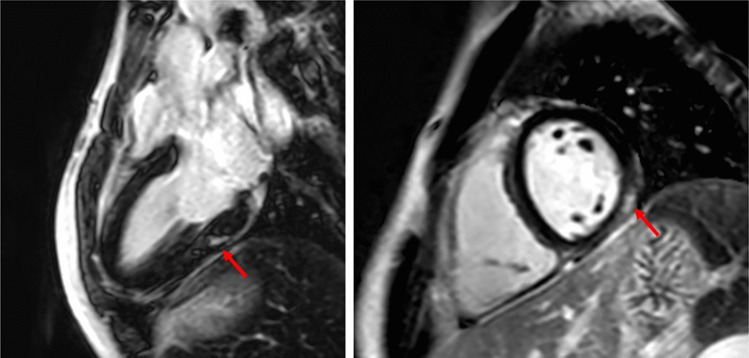
Fig. 1.
CMR imaging in a patient with previous MIS-C, showing subepicardial/intramyocardial LGE in the basal inferolateral wall of the left ventricle (red arrows). CMR, cardiac magnetic resonance; MIS-C, multisystem inflammatory syndrome in children; LGE, late gadolinium enhancement
Discussion
This is a multicenter study to evaluate cardiac involvement evolution of MIS-C patients through cardiac MRI.
Heart involvement is common in MIS-C, and the most frequent clinical manifestations are ventricular dysfunction, valvular regurgitation, arrhythmias, pericardial effusion, and CAA. In a study including 503 patients with MIS-C evaluated through echocardiography, depressed LVEF was found in 34%, being severely depressed in 22% of them, while CA aneurysm, mostly mild (93%), were found in 13% [3]. In another study including 286 MIS-C cases who underwent echocardiography, 42% had mitral regurgitation and 28% showed pericardial effusion [4]. Adequate supportive therapy and immune-modulatory treatment lead to complete resolution within days to weeks with low mortality rates [15]. Cardiac outcomes evaluated through echocardiography at a 3-month follow-up are excellent, with normalized LVEF and CA aneurysm resolution in nearly all patients [3, 4].
However, conflicting findings concerning cardiac outcome have been shown when performing CMR in the acute phase of MIS-C (i.e., within 1 month from onset). While a small case series [5] reported no myocardial damage, myocardial edema was demonstrated in all of the four patients from another case series [6]. In another study [7], myocardial edema was found in half of the 20 reported children, depressed LVEF in 35%, and a subendocardial scar in one patient. In another cohort including 17 children [8], 35% showed LGE, 12% pericardial effusion, and only one (6%) myocardial edema. Finally, in the large cohort studied by Valverde et al. [4], cardiac MRI was performed in 42 children showing myocardial edema in one-third, pericardial effusion in 24%, and LGE in 14%.
Remarkably, in our cohort, all the patients presented a severe acute disease and suffered from acute cardiac involvement, with an 83% prevalence of depressed LVEF (being severely reduced in 20%) which is at least two times higher than reported frequency in non-selected MIS-C patients [3]. CA abnormalities, valvular regurgitation, and pericardial effusion frequencies in our cohort were instead in line with existing literature [3, 4]. More than half of them presented with hypotension and/or shock, similarly to larger studied cohorts [4]; a nonfatal cardiac arrest was observed as well. LV systolic dysfunction was still present in 10% at discharge, comparably to other reports [16]. In fact, our data, although limited to a few cases, are to consider representative of children with any grade of severity of cardiac involvement from MIS-C.
The interval occurred between MIS-C onset, and cardiac MRI in our cohort was variable, as a result of the multicenter design and the retrospective protocol of our study, with patient 1, in particular, being evaluated in the acute phase of the MIS-C episode. The findings in this patient of a mildly depressed LVEF and of LGE are not therefore surprising when considering other cohorts evaluated in the acute phase [4–8], with a considerable prevalence of myocardial edema, depressed LVEF, pericardial effusion, and LGE as an expression of a recent cardiac involvement, as mentioned above.
A few other studies have evaluated MIS-C cardiac involvement evolution through cardiac MRI at a midterm timepoint. In the case series by Tannoury et al. [17], cardiac MRI was performed in two MIS-C patients 3.5 and 1.5 months after recovery respectively, showing mild myocardial scarring as evident by LGE in the latter. In contrast, in the retrospective study by Bartoszek et al. [18], 19 patients with evidence of previous MIS-C cardiac involvement were studied through cardiac MRI 3 months after recovery, showing no signs of LGE nor depressed LVEF, but pericardial effusion in 16% and myocardial edema as assessed through T2 signal intensity ratio (though with normal T1 and T2 mapping values) in only 1 patient (5%). In their prospective controlled study, Webster et al. [19] did not find depressed LVEF or myocardial edema in any of 6 MIS-C patients at a median of 2 months from the acute phase, although elevated troponin had been found in only two of their MIS-C participants and only two had showed reduced LVEF on echocardiography during initial admission. Moreover, they did not evaluate LGE as no gadolinium contrast agent was administered. Finally, Barris et al. [20] reported of 9 MIS-C patients with cardiac involvement in the acute phase, as testified by either elevated troponin or reduced LVEF, who underwent cardiac MRI after a median 9.4-month interval, with no evidence of depressed LVEF or LGE but a considerable prevalence (44%) of myocardial edema. Of note, the latter was assessed through T2 signal intensity ratio and not through T1 and T2 mapping, which can overcome some of its limitations [21].
In contrast to all of these studies, we found a 20% prevalence of mildly reduced LVEF, at a maximum interval of 4 months after MIS-C onset. Persisting mild LV dysfunction at a 4–9-month interval, although infrequent, has been described in a pediatric MIS-C cohort evaluated through echocardiography [22]. However, the clinical relevance of this finding remains unclear considering that all of our patients with reduced LVEF when assessed through cardiac MRI had normal echocardiography at discharge. Notably, the two techniques are not directly comparable when assessing cardiac volumes and function, since cardiac MRI allows a three-dimensional measurement while echocardiography relies on a summation-of-disks approach (Simpson’s biplane rule). It would therefore be interesting to compare cardiac MRI and echocardiography data obtained at the same time for each patient, data that unfortunately were not available in our cohort. Such a comparison would be of great help in MIS-C patients when questioning if the differences we observed between the two techniques actually rely on an MRI overinterpretation, an ongoing pathological process leading to scarring and fibrosis (although all but one of the patients presenting LGE in our cohort had normal LVEF on cardiac MRI), or a more precise measurement through cardiac MRI as observed in patients affected by other disorders [23].
The MRI protocol in our study included T2 mapping, through which we found a 5% prevalence of myocardial edema, as a sign of ongoing myocarditis persisting up to 2 months after the acute phase. Moreover, we found a 5% prevalence of persisting minimal pericardial effusion at 3 months after the onset. These results confirm the findings by Bartoszek et al. [18].
Finally, in contrast to all of the previous observations, we observed LGE as an expression of persisting scars in 25% of our patients, although minimal in the entity. MIS-C is considered the result of an abnormal immune response to SARS-CoV-2 where cardiac involvement could recognize its pathophysiology in postinfectious myocarditis, similarly to Kawasaki-related myocarditis [24], which usually occurs without residual myocardial fibrosis [25]. Nevertheless, myocardial scars are typical findings in either viral or immune-mediated myocarditis [26]. In particular, in a prospective study including 18 adolescents with acute myocarditis in the “pre-COVID era,” cardiac MRI performed after 6–9 months revealed a 44% prevalence of persisting scars [27]. Given the post-mortem findings showing virus particles in the cardiac tissue of patients died for MIS-C [28], a direct viral invasion could be hypothesized as the cause of damage in MIS-C. Remarkably, the long-term risk of a major adverse cardiac event such as ventricular arrhythmias or sudden cardiac death in patients with myocarditis is more than doubled in presence of persistent scars, even though their extent should be considered when assessing this risk [29]. We are not able to assess such a risk in our cohort, and, more importantly, to predict the evolution of myocardial scars in MIS-C patients. Of note, the one patient (no. 20) in our cohort receiving two consecutive cardiac MRI evaluations showed a reduction of LGE entity after 7 months from its first finding.
In conclusion, our data suggest that despite the severity of heart involvement in the acute MIS-C phase, the cardiac outcome is good. Larger longitudinal studies are needed on MIS-C patients in order to confirm this positive evolution and to establish the effective need and duration for physical activity restrictions or specific cardioprotective treatment [30].
Our study has four main limitations. Firstly is the small sample size, which carries a risk of selection bias, although attenuated by the multicenter design of our study; secondly, the variable time interval between acute MIS-C and cardiac MRI scan; thirdly, the heterogeneity of the radiologists who analyzed cardiac MRI results, without any double or triple check to evaluate inter-reader variability; and finally, the lack of a control group, which could help to avoid MRI findings overinterpretation [31].
Abbreviations
- bSSFP
Balanced steady-state free precession
- CA
Coronary artery
- CMR
Cardiac magnetic resonance
- COVID-19
Coronavirus disease 2019
- CRP
C-reactive protein
- ECG
Electrocardiography
- EF
Ejection fraction
- hs-TnI
High-sensitivity troponin I
- hs-TnT
High-sensitivity troponin T
- IVIG
Intravenous immune globulins
- KD
Kawasaki disease
- LGE
Late gadolinium enhancement
- LV
Left ventricle
- MIS-C
Multisystem inflammatory syndrome in children
- MV
Mechanical ventilation
- NIV
Non-invasive ventilation
- NSAIDs
Non-steroidal anti-inflammatory drugs
- NT-proBNP
N-terminal fragment brain natriuretic peptide
- PICU
Pediatric intensive care unit
- PSIR
Phase-sensitive inversion recovery
- RT-PCR
Real-time polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- STIR
Short Tau inversion recovery
- TSE
Turbo spin echo
- US
Ultrasound
- WHO
World Health Organization
Authors’ contributions
All the authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Simone Benvenuto, Sara Della Paolera, Sara Abu Rumeileh, Thomas Caiffa, Marco Cattalini, and Andrea Taddio. The first draft of the manuscript was written by Simone Benvenuto, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Funding
Funded by Italian Ministry of Health, RC 12/20.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to individual privacy reasons.
Declarations
Ethics approval
Obtained by the Institutional Review Board of the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy (RC 12/20).
Consent to participate and consent for publication
Retrospective study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 2.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, Soma VL, Maddux AB, Mourani PM, Bowens C, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, Kuciñska B, Mannarino S, Tamariz-Martel A, Gutierrez-Larraya F, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143(1):21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 5.Prieto LM, Toral B, LLorente A, Coca D, Blázquez-Gamero D. Cardiovascular magnetic resonance imaging in children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 and heart dysfunction. Clinical microbiology and infection: the official publication of the Euro Soc Clin Microbiol Infect Dis. 2021;27(4):648–650. doi: 10.1016/j.cmi.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, Schnuriger A, Lorrot M, Guedj R, Ducou le Pointe H. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297(3):E283–E288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, Cleary A, Stewart K, Adhvaryu K, Savis A. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2021;22(8):896–903. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirico D, Basso A, Reffo E, Cavaliere A, Castaldi B, Sabatino J, Meneghel A, Martini G, Da Dalt L, Zulian F, Di Salvo G. Early echocardiographic and cardiac MRI findings in multisystem inflammatory syndrome in children. J Clin Med. 2021;10(15):3360. doi: 10.3390/jcm10153360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperotto F, Friedman KG, Son M, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180(2):307–322. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen SE, Friedrich MG, Leiner T, Elias MD, Ferreira VM, Fenski M, Flamm SD, Fogel M, Garg R, Halushka MK. Cardiovascular magnetic resonance for patients with COVID-19. JACC Cardiovasc Imaging. 2022;15(4):685–699. doi: 10.1016/j.jcmg.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (2020) Multisystem inflammatory syndrome in children and adolescents with COVID-19
- 12.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson: Official J Soc Cardiovasc Magn Reson. 2006;8(3):417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 15.Bowen A, Miller AD, Zambrano LD, Wu MJ, Oster ME, Godfred-Cato S, Belay ED, Campbell AP (2021) Demographic and clinical factors associated with death among persons <21 years old with multisystem inflammatory syndrome in children-United States. Open Forum Infect Dis 8(8):ofab388. 10.1093/ofid/ofab388 [DOI] [PMC free article] [PubMed]
- 16.Capone CA, Misra N, Ganigara M, Epstein S, Rajan S, Acharya SS, Hayes DA, Kearney MB, Romano A, Friedman RA, et al. Six month follow-up of patients with multi-system inflammatory syndrome in children. Pediatrics. 2021;148(4):e2021050973. doi: 10.1542/peds.2021-050973. [DOI] [PubMed] [Google Scholar]
- 17.Tannoury TE, Bulbul ZR, Bitar FF. Cardiac manifestations and short-term outcomes of multisystem inflammatory syndrome in Middle Eastern children during the COVID-19 pandemic: a case series. Cardiol Young. 2022;32(1):165–168. doi: 10.1017/S1047951121002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartoszek M, Małek ŁA, Barczuk-Falęcka M, Brzewski M. Cardiac magnetic resonance follow-up of children after pediatric inflammatory multisystem syndrome temporALLY associated with SARS-CoV-2 with initial cardiac involvement. J Magn Reson Imaging : JMRI. 2022;55(3):883–891. doi: 10.1002/jmri.27870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster G, Patel AB, Carr MR, Rigsby CK, Rychlik K, Rowley AH, Robinson JD. Cardiovascular magnetic resonance imaging in children after recovery from symptomatic COVID-19 or MIS-C: a prospective study. J Cardiovasc Magn Reson : J Soc Cardiovasc Magn Reson. 2021;23(1):86. doi: 10.1186/s12968-021-00786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barris DM, Keelan J, Ahluwalia N, Jhaveri S, Cohen J, Stern K, Seiden HS, Glass L. Midterm outcomes and cardiac magnetic resonance imaging following multisystem inflammatory syndrome in children. J Pediatr. 2022;241:237–241.e1. doi: 10.1016/j.jpeds.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Pan JA, Lee YJ, Salerno M. Diagnostic performance of extracellular volume, native T1, and T2 mapping versus Lake Louise Criteria by cardiac magnetic resonance for detection of acute myocarditis: a meta-analysis. Circ Cardiovasc Imaging. 2018;11(7):e007598. doi: 10.1161/CIRCIMAGING.118.007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooqi KM, Chan A, Weller RJ, Mi J, Jiang P, Abrahams E, Ferris A, Krishnan US, Pasumarti N, Suh S, et al. Longitudinal outcomes for multisystem inflammatory syndrome in children. Pediatrics. 2021;148(2):e2021051155. doi: 10.1542/peds.2021-051155. [DOI] [PubMed] [Google Scholar]
- 23.Brunklaus A, Parish E, Muntoni F, Scuplak S, Tucker SK, Fenton M, Hughes ML, Manzur AY. The value of cardiac MRI versus echocardiography in the pre-operative assessment of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2015;19(4):395–401. doi: 10.1016/j.ejpn.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20(8):453–454. doi: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratis K, Hachmann P, Child N, Krasemann T, Hussain T, Mavrogeni S, Botnar R, Razavi R, Greil G. Cardiac magnetic resonance feature tracking in Kawasaki disease convalescence. Ann Pediatr Cardiol. 2017;10(1):18–25. doi: 10.4103/0974-2069.197046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebai F, Brun S, Petermann A, Ribes D, Prévot G, Cariou E, Lavie-Badie Y, Faguer S, Galinier M, Carrié D, et al. Cardiac magnetic resonance imaging with late gadolinium enhancement in acute myocarditis: towards differentiation between immune-mediated and viral-related aetiologies. Arch Cardiovasc Dis. 2019;112(10):559–566. doi: 10.1016/j.acvd.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Małek ŁA, Kamińska H, Barczuk-Falęcka M, Ferreira VM, Wójcicka J, Brzewski M, Werner B. Children with acute myocarditis often have persistent subclinical changes as revealed by cardiac magnetic resonance. J Magn Reson Imaging : JMRI. 2020;52(2):488–496. doi: 10.1002/jmri.27036. [DOI] [PubMed] [Google Scholar]
- 28.Dolhnikoff M, Ferranti JF, de Almeida Monteiro RA, Duarte-Neto AN, Gomes-Gouvêa MS, Degaspare NV, Delgado AF, Fiorita CM, Leal GN, Rodrigues RM, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4(10):790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, Jerosch-Herold M, Kwong RY. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70(16):1964–1976. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.https://www.acc.org/latest-in-cardiology/articles/2020/07/13/13/37/returning-to-play-after-coronavirus-infection. Accessed 17 Dec 2021
- 31.Maleszewski JJ, Young PM, Ackerman MJ, Halushka MK. Urgent need for studies of the late effects of SARS-CoV-2 on the cardiovascular system. Circulation. 2021;143(13):1271–1273. doi: 10.1161/CIRCULATIONAHA.120.051362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to individual privacy reasons.