Abstract
Stereotactic brain biopsy is one of the most frequently performed brain surgeries. This review aimed to expose the latest cutting-edge and updated technologies and innovations available to neurosurgeons to safely perform stereotactic brain biopsy by minimizing the risks of complications and ensuring that the procedure is successful, leading to a histological diagnosis. We also examined methods for improving preoperative, intraoperative, and postoperative workflows. We performed a comprehensive state-of-the-art literature review. Intraoperative histology, fluorescence, and imaging techniques appear as smart tools to improve the diagnostic yield of biopsy. Constant innovations such as optical methods and augmented reality are also being made to increase patient safety. Robotics and integrated imaging techniques provide an enhanced intraoperative workflow. Patients’ management algorithms based on early discharge after biopsy optimize the patient’s personal experience and make the most efficient possible use of the available hospital resources. Many new trends are emerging, constantly improving patient care and safety, as well as surgical workflow. A parameter that must be considered is the cost-effectiveness of these devices and the possibility of using them on a daily basis. The decision to implement a new instrument in the surgical workflow should also be dependent on the number of procedures per year, the existing stereotactic equipment, and the experience of each center. Research on patients’ postbiopsy management is another mandatory approach to enhance the safety profile of stereotactic brain biopsy and patient satisfaction, as well as to reduce healthcare costs.
Keywords: Neurosurgery, Stereotactic brain biopsy, Robotics, Brain tumor, Brain surgery, Diagnostic yield, Safety
Introduction
Brain biopsy is one of the most frequently performed brain surgeries in neurosurgical centers that manage patients with brain tumors or non-neoplastic cryptogenic neurological diseases [11]. Various surgical methodologies can be employed to achieve a brain biopsy [32]. Among these, stereotactic brain biopsy is a minimally invasive neurosurgical technique used to acquire pathological brain tissue using a dedicated stereotactic needle. This procedure is indicated for multiple, deep-seated brain lesions and/or frail patients or those with poor prognosis. The goal of surgery is to obtain viable tissue representative of the lesion in order to provide a comprehensive histological analysis. The procedure uses imaging technologies, such as magnetic resonance imaging (MRI) or computed tomography (CT), to safely and precisely reach specific areas of the brain. Image-guided brain biopsy began with frame-based approaches incorporating CT scans for surgical planning [66]. For the past several years, frameless stereotactic systems using preoperative MRI have tended to supplant frame-based methods. The standard-of-care method for stereotactic brain needle biopsy involves the insertion of a 1.6- to 2-mm diameter needle cannula through a burr hole placed along a predetermined trajectory. The two cannulas had side windows that aligned when the target point was reached. The brain tissue was lodged into the cannula using suction and then cut by sliding the inner cannula up into the mandrel.
Complications following a brain biopsy are rare, but like all neurosurgical procedures, they carry some risks, such as seizures, brain edema, or infection [52]. The most specific and frequent complications related to this procedure are negative sampling, requiring a second biopsy procedure [8], and brain hemorrhage with potentially serious consequences [53]. In a mission to become safer and more effective, constant innovations are being made in this field of neurosurgery.
This literature review aims to expose the latest cutting-edge and updated technologies and innovations available to neurosurgeons to safely perform stereotactic brain biopsy by minimizing the risks of complications and ensuring that the procedure is successful, leading to a histological diagnosis. We also examined methods for improving preoperative, intraoperative, and postoperative workflows.
Innovations to increase biopsy diagnostic yield
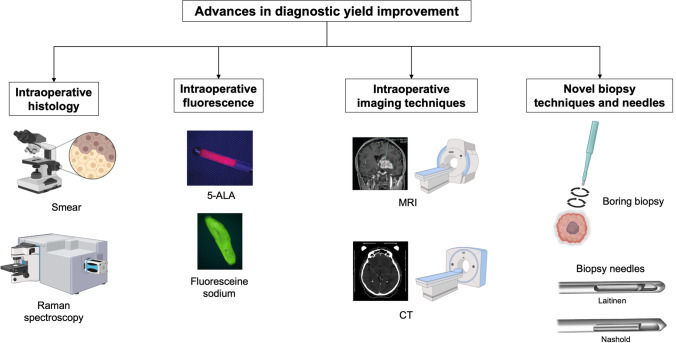
One of the main risks of stereotactic biopsy is to provide a sample that is non-contributory to a diagnosis. In a case series of patients with brain tumors, the rate of negative biopsies is close to 5%, exposing the patient to a second biopsy with potential morbidity and additional health care costs [8, 20, 53]. In patients biopsied for cryptogenic neurological disease, this rate reaches 30% [3, 36–38]. Innovations are partly driven by the causes of biopsy failures, from misguidance of the biopsy needle due to technical pitfalls and limitations [69] to uncertainties surrounding the pathological nature of the biopsy samples during the procedure (Fig. 1).
Fig. 1.
Advances and innovations in stereotactic brain biopsy diagnostic yield improvement. 5-ALA, 5-aminolevulinic acid; CT, computed tomography; MRI, magnetic resonance imaging. Figure created with BioRender.com
Intraoperative histopathological examination
Smear or frozen section
Intraoperative rapid smear or frozen-section histopathology is the oldest, but also the most reliable method to determine the presence of tumor or pathological tissue in biopsy specimens [10]. In 2019, Mathon et al. conducted a 2-year retrospective study on 145 patients for which a smear was performed during MRI-guided frame-based stereotactic biopsies [35]. They compared the negative biopsy rate between a historical cohort of 1638 patients brain-biopsied over a 10-year period (2007–2016) and the group of patients for which an intraoperative smear was performed. In the historical control group, the rate of negative biopsies was 2.6%, while there was no negative biopsy in the “smear group.” In five patients (3.4%), the first intraoperative smear was initially considered non-diagnostic; thus, further biopsies were performed deeper along the trajectory. The second smear resulted in a diagnosis in all patients. Another study reported that an intraoperative smear reduced the risk of negative biopsy from 11.1 to 3.7% [31].
Compared to frozen sections, the smear method has numerous advantages. It is faster and uses smaller amounts of tissue, allowing more tissue to be spared for definitive histopathological examination and molecular analysis. Moreover, contrary to frozen sections, the smear allows examination of the thin glial or neuronal cytoplasmic processes and identification of the glial or neuronal phenotype of tumor cells, or the presence of reactive astrocytes or a fibrillary background. Sharp nuclear details were also more visible in smears.
The intraoperative smear takes only a few minutes and does not unduly prolong the biopsy procedure [35]. However, the neuropathology laboratory must be located close to the operative room to receive the intraoperative sample without delay. The neuropathologist could also be in the operating room (OR) with a microscope because this gives the most reliable and instantaneous feedback to the operating surgeon [13]. Skilled and experienced neuropathologists should examine intraoperative smears to ensure a high level of reliability. This technique could thus be implemented in the surgical workflow to limit non-contributory biopsies and ultimately a second intervention.
Raman spectroscopy
Recently, stimulated Raman scattering microscopy, a label-free optical imaging method, was developed to quickly generate in the OR digital hematoxylin-and-eosin-stained-like images for intraoperative near real-time histopathological tissue analysis. Using this new tool, Neidert et al. analyzed 429 stimulated Raman histology (SRH) samples from 108 patients and evaluated the use of this technology in the surgical workflow. This device can be used for intraoperative diagnosis, research, and quality control. During the first experiments, they had to improve process optimization regarding the tissue treatment; nevertheless, it was easily implemented in the surgical workflow in a “plug-and-play” manner [44]. In a second study, the same team quantified the neuropathological interpretability in a routine clinical setting without specialized training [61]. They tested the device on 117 samples of pathological tissue from 73 patients and a neuropathologist assessed image quality by scoring subjective tumor infiltration and stated a diagnosis based on the SRH images. The SRH imaging quality was high, and the detection of tumor cells classified as inconclusive was observed only in 4.2% of the cases. The diagnostic accuracy of SRH images was 87.7%.
In a multicenter prospective clinical trial including 278 patients, Hollon et al. combined SRH and artificial intelligence (i.e., deep convolutional neural networks trained on over 2.5 million SRH images) and showed that this diagnostic method was non-inferior to pathologist-based interpretation of conventional histological images (accuracy 94.6%) [21]. Using the same artificial intelligence process, Reinecke et al. demonstrated that SRH can reliably detect the microscopic presence of tumor and discriminate from non-neoplastic brain tissue in stereotactic biopsy specimens [49].
In conclusion, the interpretation of intraoperative histological images with SRH is rapid (within a few minutes) and independent of a traditional laboratory or an unevenly distributed pathology workforce. Hence, this new tool may pave the way for intraoperatively confirming the positivity of a biopsy sample.
Intraoperative fluorescence
In the neurosurgical field, tumor delimitation based on 5-aminolevulinic acid (5-ALA)-induced protoporphyrin IX (PpIX) or fluorescein sodium fluorescence is frequently used. Patients receive 5-ALA (20 mg/kg of body weight) approximately 4 h prior to the biopsy procedure. Administration of 5-ALA to the patient leads to the accumulation of red fluorescent PpIX in highly proliferating cells, such as tumor cells. As PpIX is only produced by vital cells, necrotic parts of the tumor do not show PpIX fluorescence. This is a useful tool for tumor delineation in resection surgery and for verifying tissue specimens during stereotactic biopsy sampling [67]. Millesi et al. compared the diagnostic yield from stereotactic brain tumor biopsies with the assistance of 5-ALA-induced fluorescence and those with the assistance of intraoperative histology [40]. The diagnostic rate was comparable between both strategies (98% vs. 100%, respectively). In addition, a positive predictive value of 100% was reported by the same team for all samples with strong or vague fluorescence-containing diagnostic lymphoma tissue according to histopathological examination [25].
Singh et al. studied the use of intravenous fluorescein sodium fluorescence to confirm pathological tissue samples in brain biopsies of gadolinium-enhancing tumors [57]. Their prospective observational study included 23 consecutive patients from whom 93 specimens were obtained and examined for the presence of fluorescence using a microscope with this visualization capability. They calculated the sensitivity and specificity of fluorescein detection based on histopathological confirmation. Overall, of the 93 specimens obtained, 58 were fluorescent samples, and all contained diagnostic tissue useful for tumor grading. Of the 35 non-fluorescent samples remaining, 12 (34.3%) did not contain any tumoral tissue, 11 (31.4%) contained minor hypercellularity or gliosis, and 12 (34.3%) contained a high proportion of necrotic tumor tissue. They concluded that the sensitivity and specificity of fluorescein fluorescence were 83% and 100%, respectively. This could be a useful and cost-effective tool to improve diagnostic accuracy by detecting pathological tissue in stereotactic brain biopsies and accelerating the procedure.
In a paper that was published in 2022, Xu and his team described their experience with 45 fluorescein-guided biopsies in 44 patients over a 5-year period and identified the distribution patterns in various histological diagnoses to create strategies to improve the effectiveness and precision of this procedure [68]. They carried out 25 frame-based and 20 frameless Varioguide (BrainLAB AG, Feldkirchen, Germany) image-guided biopsies. The intraoperative fluorescein uptake of 347 biopsy samples, with an average of eight samples per patient, was assessed, and the results were compared to the definitive histology. Sixty-three percent of the specimens obtained were fluorescein-positive. The specificity was 70%, and the sensitivity for high-grade gliomas was 85%. The specificity of fluorescein for contrast-enhancing lesions was 84%. Three samples were required to identify contrast-enhancing lesions, and five samples were required to provide a definitive histological diagnosis. Even though there was no indication of gadolinium enhancement, it is interesting to note that in the IDH-mutant WHO grade III group, astrocytomas showed fluorescein uptake. According to this patient series, fluorescein-guided stereotactic biopsy improves the chances of a successful neuropathological diagnosis and can reduce the number of samples required by half for contrast-enhancing lesions.
These two previous studies are the largest and most recent, but fluorescence has already been investigated by Thien et al. a few years ago on a smaller cohort with similar results [62]. Additionally and more recently, Thien et al. developed a low-cost and stand-alone device called Fluoropen to detect fluorescence in brain tumor tissue obtained by fluorescence-guided stereotactic needle biopsy [63]. The pen consisted of a light source fitted with color filters to create the required emission and visualization wavelengths. The proof-of-concept study consisted of four consecutive patients; a total of six samples were obtained, and each sample was examined for the presence of fluorescence using Fluoropen and compared with a microscope. Fluoropen was shown to have 100% concordance with the microscope and therefore could be a valid alternative to facilitate and expedite the procedure.
In conclusion, the literature supports the value of photodiagnosis and its high diagnostic yield, especially for high-grade tumors [50]. Although, its value seems more important in resection operations, fluorescence assistance during stereotactic biopsy of contrast-enhancing tumors may provide real-time confirmation of tumor tissue, increase the diagnostic yield, spare tissue samples, and reduce the time of intervention [7]. It represents a credible alternative for neurosurgical departments that have not undergone intraoperative histopathological examination by a neuropathologist.
Intraoperative imaging techniques
Both frame-based and frameless stereotactic biopsies are dependent on preoperative images and intraoperative anatomic registration to reach the target after careful planification and precalculated measurements in 3-dimensional space [66]. The limitation of these techniques is correlated to their relative inability to adapt to the shift of intracranial structures, to the anatomy, or to assure accuracy through real-time imaging and sampling on the target.
To overcome these limitations, Mohyeldin et al. developed a platform to integrate high Tesla intraoperative MRI (iMRI) with percutaneous frameless stereotactic biopsy [41]. Before that, the advantage of real-time feedback using iMRI technology in phantom experience [43, 55], non-human primate studies [51], and deep brain stimulation surgeries [30, 56, 59] has been investigated. The use of their platform was performed on five consecutive patients and showed that it would considerably lower the rate of misdiagnosis due to faulty targeting using real-time feedback, correction of the needle trajectory, confirmation of accurate position, and direct imaging of eventual complications [42]. This technology is best suited for small deep brain lesions found near important neurovascular structures. As the system works percutaneously, it can also be used for targets that may require an anterior starting point, such as the subfrontal approach to the hairline. The average operating time was approximately 2 h and tended to decrease with increasing experience [41].
Using frameless stereotactic brain biopsy with intraoperative CT (iCT), Ikeda et al. studied the use of this adjuvant technique to assess the real target registration error and reported their preliminary experience on 10 patients [23]. During the procedure the iCT was conducted twice: once immediately before the biopsy with the 3-pin head holder and reference frame in place for self-registration of the navigation system and a second time for the precise confirmation of the localization of the inserted biopsy needle [23]. It provides a more reliable and accurate registration tool avoiding registration errors, especially in a prone or lateral position. One of the shortcomings of this technique is the double radiation exposure in one surgery, but it can be aided by the use of a low-dose-irradiated iCT. A similar surgical workflow using intraoperative verification with a mobile CT unit in combination with frameless neuronavigation-guided stereotaxy and preoperative MRI-based trajectory planning confirmed targeting accuracy with minimal radial trajectory deviation [5].
Novel biopsy techniques and needles
Recently, Ogiwara et al. reported a preliminary study on 26 patients of a novel biopsy method called “boring biopsy” [45]. The technique is based on a biopsy needle with a cylindrical tool able to gather columnar continuous specimens from the surface in the normal brain tissue to the tumor margin and within the lesion. They believe that continuous specimens are useful for improving the accuracy of histopathological diagnosis based on cellular changes and differentiation from normal tissues to the core of the lesion. Diagnosis was established in all cases, and no major complications were reported.
In an experimental on fresh swine brains, Trojanowski et al. compared the diagnostic histopathological quality obtained with different vacuum pressures (from 0 to 60 kPa), a novel needle rotation method, and using two needle types (Laitinen, Umea, Sweden, or Nashold, Radionics, USA) [64]. After analyzing 800 biopsy samples, they concluded that those are better for histopathological examination when obtained with higher vacuum pressure or with Laitinen needle.
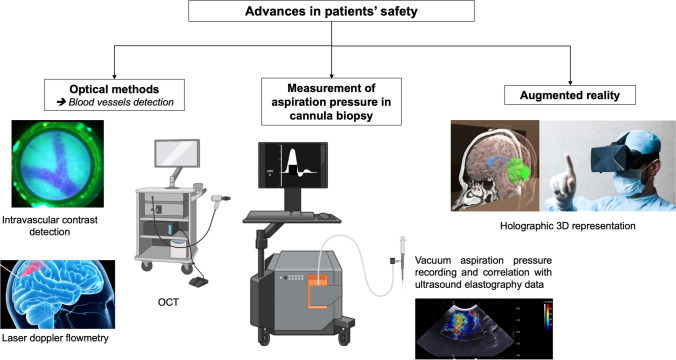
Innovations to improve patient’s safety
The most serious consequences of minimally invasive stereotactic biopsy are vessel damage and subsequent bleeding. The rate of postbiopsy symptomatic hemorrhage is reported to be between 0.9 and 8.6% [26, 53]. When considering all bleeding observed on systematic postoperative CT scans (symptomatic or asymptomatic), the range is much higher, up to 60% [27, 52].
The smooth and rounded tip of the biopsy needle protects the vessels by pushing them aside. Although the forward movement of the needle represents a risk of hemorrhage along the trajectory, the risk of vessel injury is higher with the suction and sliding movement of the biopsy needle during tissue acquisition. Therefore, more than a preoperative examination for navigation at an anatomical level, neurosurgeons need intraoperative feedback to improve the safety of biopsy sampling. Different techniques have been developed to enhance the safety of this critical step of the procedure (Fig. 2).
Fig. 2.
Advances and innovations in safety of patients undergoing stereotactic brain biopsy. OCT, optical coherence tomography. Figure created with BioRender.com and Freepik.com
Optical methods
Intravascular contrast detection
Göbel et al. conducted a clinical pilot trial on a minimally invasive endoscopic probe that can be inserted into the tissue through a regular biopsy needle [16]. The same fiber optics is used for both illumination and image detection enabling a clear demarcation of healthy tissue, tumorous tissue, and vasculature based on tissue autofluorescence, PpIX fluorescence, and indocyanine green (ICG) fluorescence. For vasculature, a dose of 200 mg/kg body weight ICG was administered intravenously immediately before starting the experiment, which allowed the surgeon to detect blood vessels at distances below 1 mm, which would be sufficient to change the propulsion path. ICG is frequently used in open vascular neurosurgery, but the detection of deep vessels might be altered due to the fast redistribution and possible leakage of blood that, if in contact with the probe, would result in false-positive measurements.
Similarly, in a study published in 2021, Richter et al. used a forward-looking probe to get direct feedback on PpIX fluorescence and blood flow detection on 20 stereotactic biopsies [54]. With this tool, they expect to shorten the time for the procedure, improve the diagnostic yield, and reduce the risk of bleeding complications.
Laser Doppler flowmetry
A 2.2-mm diameter forward-viewing probe that uses fluorescence detection in conjunction with laser Doppler flowmetry was described in two studies. The probe was designed to fit a Leksell stereotactic system. Laser Doppler flowmetry can assess brain perfusion and blood flow by measuring the frequency shift in the 780-nm backscattered laser light caused by cell movements in the capillaries [1].
Haj-Hosseini et al. developed a forward-looking fiber optic probe integrating real-time fluorescence spectral detection and laser Doppler spectroscopy with the goal of reducing the risk of artery injury and securely targeting tumor tissue during stereotactic brain biopsy procedures [19]. They described the use of the dual-mode probe in three stereotactic biopsy procedures. They measured PpIX fluorescence, autofluorescence, microvascular blood flow, and total light intensity along the trajectories in real-time in the OR. The signals were correlated with the radiology images and histopathology. The main goal of the studies was to establish whether there was a correlation between the measured blood flow and the anatomy along the trajectory, but it also showed that the system provided a clinically feasible method to increase the operational safety and efficiency.
These tools can aid in determining the optimal strategy for biopsy samples and patient safety by using real-time intraoperative information.
Optical coherence tomography
Optical coherence tomography (OCT) was initially presented by Kut et al. as a label-free technique for differentiating pathological and non-pathological human brain tissues at 1- to 1.5-mm penetration depth [28]. Later on, Ramakonar et al. demonstrated that OCT can accurately identify solid tissue and vessels in live patients with only minimal disruption to the current clinical workflow [48]. In 11 patients, they were able to intraoperatively detect blood vessels (diameter > 500 μm) with a sensitivity of 91.2% and a specificity of 97.7%. The only limitation of the device is that it is a side-viewing probe that performs imaging through the tissue-cutting window, so it does not prevent intracranial hemorrhage during needle penetration. Although the rate of hemorrhage is significantly reduced when there is no suction, indicating that bleeding occurs during tissue cutting, this does not completely eliminate the risk of hemorrhage.
Markwardt et al. conducted a study on ex vivo tissue using dual-wavelength remission spectrometry with a two-fiber probe to detect ≥ 100–500-μm diameter vessels at a maximal distance of 800 μm within the suction window [34]. Similarly, Pichette et al. conducted a sensitivity analysis to establish intrinsic vessel detection limits of interstitial optical tomography using brain tissue phantoms. They showed that they could detect vessels with diameters of > 300 μm located up to > 2 mm from the outer surface of the biopsy needle, corresponding to the volume of tissue aspirated during tissue extraction. The only limitation they pointed out was that when the surgeon had a high absorbance signal, they could not distinguish a single blood vessel from a dense cluster of small capillaries [46].
Augmented reality
The use of augmented reality is expanding at an exponential rate each year, and it is most likely to be used in numerous fields of neurosurgery in the future. In a neurosurgical OR, the surgeon needs to have 3D vision and precise knowledge of the anatomy to go beyond anatomical borders through small surgical corridors to avoid injuring important neural and vascular structures. The use of a virtual access pathway, which can then be superimposed to guide surgery, might be a useful tool for avoiding potential problems before starting the procedure.
Very recently, Gibby et al. conducted a study to quantify the navigational accuracy of an advanced augmented reality device combining the VisAR system with Hololens 2 (Microsoft), a technology that converts DICOM data into holographic images [15]. With the help of this device, the surgeon has a virtual line of sight from which to design his route safely. More research is necessary for this new use of augmented reality, it could provide the surgeon with a significant advantage by eliminating the need to cognitively transform 2D data into a surgical field and enable safer target planning for stereotactic biopsies.
Measurement of aspiration pressure in cannula biopsy
Considering that excessive vacuum aspiration during stereotactic biopsy increases the risk of hemorrhage, Chan et al. assessed the optimal aspiration vacuum pressure for application in brain tumor biopsies and correlated this data with ultrasound elastography [9]. They recorded vacuum aspiration pressures using a T-connector pressure sensor during 11 biopsies. According to previous results provided by a preclinical study [64], they found that a vacuum pressure range of 40–66 kPa is safe and adequate for sampling various types of tumors with heterogeneous elastographic properties. Ultrasonographic elastography is an affordable tool that can indicate the minimum vacuum pressure required to achieve stereotactic brain biopsy in real time.
Innovations to improve perioperative workflow
Several tools have recently been developed to improve operative workflow. The purpose of these technologies is to shorten surgeries, simplify the flow of actions, and improve the comfort of both the patients and workers. Most of the technologies described below have been developed in recent years, and this sector is constantly growing to assist surgeons in achieving their objectives.
Imaging techniques
Various imaging systems integrated into the surgical workflow can increase the efficiency and comfort of the patient and surgeon. Preoperative preparation for planning a frame-based stereotactic brain biopsy is associated with important logistical effort and burden on the patient. Recently, Enders et al. developed and applied a new method for intraoperative acquisition in the planning dataset using a multiaxial robotic C-arc system called Artis Zeego ((AZ) Siemens) [12]. Fourteen patients with an indication-customized dose-reduced protocol underwent intraoperative imaging with AZ. The control group consisted of 10 patients who underwent conventional preoperative cranial computed tomography imaging. They compared the outcomes with regard to target deviation, diagnostic value of the biopsies, complications, and procedure time. A suitable intraoperative planning dataset was acquired using AZ. The total procedure time was significantly shorter, and biopsy was contributory for 12 patients (86%) in the AZ. Only 8 patients were diagnosed in the control group. There were no significant differences in target size, trajectory, or target deviation. They concluded that intraoperative imaging using AZ in frame-based stereotactic biopsy is an easy and feasible method with an accuracy comparable to that of conventional CT, with reduced radiation exposure. This system can significantly reduce the procedure length and undeniably improve the comfort of the patient and staff.
In contrast, in a retrospective study of 33 patients, Algin et al. investigated the safety and feasibility of CT and 3-T MR-guided freehand biopsy with 18/20-gauge coaxial needles in a single imaging unit [2]. The procedure was conducted under sedation and local anesthesia in their radiology department. They concluded that this technique was safe and feasible and that the biopsy workflow was simplified. This tool could be a valuable alternative for stereotaxic biopsies in centers that do not have stereotaxic equipment or experience.
In 2022, Sterk et al. reported the initial safety data and user experience of SmartFrame array (ClearPoint Neuro) on ten stereotactic procedures [60]. The SmartFrame array system is a MRI compatible frame placed on the skull with four 19-mm bone screws. After the placement of the frame, the next step of the procedure is optimization of the stereotactic trajectory based on real-time MRI. The goal of the frame was to support multitrajectory biopsies without additional adjustments. The accuracy of the frame is good because the radial error of the system is < 2 mm, and they achieved diagnostic tissue for all subcentimeter lesions biopsied. The safety profile revealed no procedural morbidity or mortality. This series suggested an average time of 80 min for a single-trajectory procedure. One advantage of real-time MRI biopsy is that there is no ionizing radiation and the same imaging technique is used for treatment planning and intra- and postbiopsy control.
Robotics
For nearly two decades, robotics has profoundly modified the neurosurgical landscape, particularly for stereotactic procedures. In recent years, major advances in robotics have been made in terms of steric hindrance and ease of use.
Mallereau et al. conducted a 12-year long, prospective, single center study to compare two frameless systems for brain biopsies: ROSA (Zimmer Biomet) Robotic-Assisted Stereotaxy and BrainLab Varioguide (BrainLab) image-guided stereotaxy [33]. They analyzed various parameters such as diagnosis, periprocedural complication, length of the procedure, and learning curve for each operator. They performed 526 biopsies on 516 consecutive patients, 314 with the ROSA robot, and 212 with the Varioguide. They found that a positive histological diagnosis was achieved in 97.4% of cases in the ROSA group versus 93.3% in the Varioguide group. However, no statistically significant differences were found in the percentage of postoperative complications and length of the procedure. For example, the hemorrhagic complication rate was 3.5% in the ROSA group and 4.7% in the Varioguide group. This study confirms that robotic surgery is safe, accurate, and reliable. A limitation of this study was that they compared two frameless robotic and image-guided surgery systems to prove the efficiency and safety of robotic surgery without a frame-based control group. In another study, Hu et al. compared the SINO (Sinovation Medical Technology Co., Ltd., Beijing, China) surgical robot-assisted frameless brain biopsy with standard frame-based stereotactic biopsy in terms of efficacy, accuracy, and complications [22]. Although there was no significant difference in diagnostic yield and postbiopsy complications between the frame-based group and the SINO robot-assisted group, the entry and target point errors were smaller in the robot-assisted group.
Based on the same idea as in the previous study, Spyrantis et al. proposed an experimental phantom study to compare the mechanical accuracy of the ROSA robot and the Leksell stereotactic frame [58]. Fifty trajectories were analyzed for each method. For both procedures, X-rays were used to precisely record the final cannula position; then, the coordinates were merged with the planning data, and the deviations were calculated. After analysis, similar to a previous study, they concluded that both methods proved to be very precise, but they recorded a higher degree of accuracy in robotic procedures.
The literature highlights the advantages of the robotized technique over the standard stereotactic technique. Robots have accurate, predefined, and reprogrammable trajectories. It minimizes the error that could be made by the surgeon in the various steps of manual settings or fixation and reduces inaccuracies due to the stereotactic frame or frameless surface-matching registration. It also allows repetition of the same trajectory numerous times with the same precision, without tremor or tiredness. However, despite numerous studies proving the higher accuracy of stereotactic robots compared to frame-based or frameless procedures, the positive clinical impact of robotics on diagnostic yield and safety appears negligible.
YAG lasers
A new tool has recently been described by Ha et al. using laser technology to make brain biopsy less invasive, faster, and safer [18]. In this “proof of principle” study, they used the yttrium aluminum garnet (YAG) laser as a high precision bone cutting technology that would perform a miniaturize necessary burr hole and also allow for a trajectory angulation much more tangential to the bone surface. The laser was used on a navigated multiaxial robotic arm and performs a “cold ablation” with a pulse energy of 650 mJ and pulse duration of 200 μs. Laser technology can be used to cut with high precision and low thermal strain to adjacent tissues when compared to mechanical bone drilling. The transmitted energy is nearly exclusively absorbed by the water molecules in the surrounding tissue. Potential future applications of this technology would be to miniaturize the hole in the skull bone to such an extent that the laser-created canal could serve as a guide for the biopsy needle with sufficient accuracy. In addition, the necessary skin incision can either be created by the laser itself or be much shorter when using a conventional scalpel. This technological advancement could provide neurosurgeons with the opportunity to sample biopsies of brain areas that are usually inaccessible or too hazardous.
Combined biopsy needle
Giannakou et al. studied phantoms using a new frameless MRI-guided robotic system that has the advantage that, with a single catheter insertion, the biopsy needle can perform both tissue suction and ablation of the lesion using high-intensity therapeutic ultrasound in cases of localized malignant tumors [14]. This new tool is useful for diagnosis, and if a tumor is proven malignant, its size allows it to ablate the lesion in a single surgery. It would reduce the surgical morbidity of multiple surgeries and possibly the hospitalization time.
Advances to improve postbiopsy workflow and patient’s management
We showed above that technological innovation is the cornerstone of stereotactic brain biopsy. Nevertheless, research on patients’ postbiopsy management is another fundamental way to optimize patient safety and personal experience and to make the most efficient possible use of the available hospital resources.
Early postbiopsy imaging
As little consensus exists on the postoperative care of patients undergoing stereotactic biopsy, several teams have sought to establish novel algorithms for their postoperative management and notably investigated the location of postbiopsy imaging. Recently, Riche et al. retrospectively examined 1500 consecutive cases to analyze the severity, timeline, risks factors, and management of complications after stereotactic brain biopsies [53]. The team proposed an algorithm for a better and safer postoperative management of stereotactic biopsy based on the results of the study and their experience. They pointed out that half of the symptomatic complications occurred within the first hour and three-quarters of complications occurred within the first 2 h following the biopsy. Consequently, they recommend close monitoring for 2 h in the recovery unit or the intensive care unit (ICU) and systematic CT scan after 2 h. The few existing studies on this topic also tended toward a short postbiopsy observation time to spot a complication or not in the patient [24, 29, 65]. ICU monitoring is continued for patients with a postbiopsy new neurological deficit. They also showed that more than 80% of delayed complications (i.e., after 2 h) occurred after 48 h and were mainly related to brain edema and seizures, which might have been prevented if cases were considered in the group of patients at risk using their algorithm. Indeed, the authors highlighted the strong value of a CT scan 2 h after the procedure. Thus, asymptomatic hemorrhage visible on CT scan was associated with delayed complications, and they recommended prescriptions of corticosteroids and antiepileptic medications to prevent brain edema and/or seizures.
Outpatient biopsy
The concept of outpatient management for stereotactic brain biopsies was introduced in the 2000s in North America [4, 24]. Early studies investigated the feasibility of outpatient care. This practice for patient management has become increasingly popular because it improves patient satisfaction and, ultimately, reduces costs and has been developed by several teams in the USA [6, 47], before reaching the British healthcare system [17].
More recently, another European team refined the outpatient stereotactic brain biopsy protocol by taking into account the location and findings of the systematic early postbiopsy CT scan. As discussed above [39], following the outpatient care management provided for 40 patients who underwent stereotactic brain biopsy, the authors indicated that all of the patients were discharged the same day and no patients had to be readmitted for complications in the month after the procedure. To safely perform ambulatory stereotactic brain biopsies, the latter suggested management recommendations and a prebiopsy checklist. The patient’s willingness, the distance between the hospital and the patient’s house, an overnight caregiver, and early morning surgery were some of the elements on the checklist. Other medical comorbidities, poor neurological state, uncontrolled seizures, and age > 80 years were excluded.
For selected cases, this is a promising adaptation to improve patient care; it can be easily implemented in other neurosurgical centers without lowering the level of care or endangering patient safety. A shorter hospital stay limits the risk of hospital-based complications, such as thromboembolic events and nosocomial infections, in addition to the well-known psychological benefits of the patient and family. This is particularly true during the COVID-19 pandemic, when patients are exposed to hospital clusters and, consequently, nosocomial contamination. However, the neurosurgical community should remember that early discharge should not be the primary aim, but rather the result of effective care and satisfying patient health status. Neurosurgeons must decide if outpatient brain biopsy is feasible in each individual case.
Conclusion
Many new trends are emerging, constantly improving patient care and safety, as well as surgical workflow. In particular, future advances in augmented reality as well as in artificial intelligence could enhance both preoperative planning of the biopsy trajectory and its intraoperative real-time viewing. A parameter that must be considered is the cost-effectiveness of these devices and the possibility of using them on a daily basis. Some of the technologies, such as 5-ALA to increase the diagnostic yield, ICG fluorescence to prevent vascular injury, or robotized stereotactic systems, are already used in other fields of neurosurgery, and an extension to the stereotactic biopsy procedures might be easily implemented. The decision to implement a new instrument in the surgical workflow should also be dependent on the number of procedures per year, the existing stereotactic equipment, and the experience of each center. Research on patients’ postbiopsy management is another mandatory approach to enhance the safety profile of stereotactic brain biopsy and patient satisfaction, as well as to reduce healthcare costs.
Author contribution
Conceptualization, B.M.; methodology, B.M.; validation, B.M. and A.B.; investigation, B.M. and A.B.; writing—original-draft preparation, A.B. and B.M.; writing—review and editing, A.B. and B.M.; supervision, B.M. All authors have read and agreed to the published version of the manuscript.
Data availability
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Human and animal ethics
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Disclosure
All authors had access to the data and a role in writing the manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akshulakov SK, Kerimbayev TT, Biryuchkov MY, Urunbayev YA, Farhadi DS, Byvaltsev VA (2019) Current trends for improving safety of stereotactic brain biopsies: advanced optical methods for vessel avoidance and tumor detection. Front Oncol 9. 10.3389/fonc.2019.00947 [DOI] [PMC free article] [PubMed]
- 2.Algin O, Ayberk G. Feasibility of freehand CT and 3-T MR guided brain aspiration biopsies with 18/20-gauge coaxial needles. Jpn J Radiol. 2022 doi: 10.1007/s11604-022-01257-2. [DOI] [PubMed] [Google Scholar]
- 3.Bai HX, Zou Y, Lee AM, Lancaster E, Yang L. Diagnostic value and safety of brain biopsy in patients with cryptogenic neurological disease: a systematic review and meta-analysis of 831 cases. Neurosurgery. 2015;77:283–295. doi: 10.1227/NEU.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj RD, Bernstein M. Prospective feasibility study of outpatient stereotactic brain lesion biopsy. Neurosurgery. 2002;51:358–361. doi: 10.1097/00006123-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Bichsel O, Oertel MF, Stieglitz LH. Mobile intraoperative CT-assisted frameless stereotactic biopsies achieved single-millimeter trajectory accuracy for deep-seated brain lesions in a sample of 7 patients. BMC Neurol. 2021;21:285. doi: 10.1186/s12883-021-02322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulton M, Bernstein M. Outpatient brain tumor surgery: innovation in surgical neurooncology. J Neurosurg. 2008;108:649–654. doi: 10.3171/JNS/2008/108/4/0649. [DOI] [PubMed] [Google Scholar]
- 7.Catapano G, Sgulò FG, Seneca V, Iorio G, de Notaris M, di Nuzzo G. Fluorescein-assisted stereotactic needle biopsy of brain tumors: a single-center experience and systematic review. Neurosurg Rev. 2019;42:309–318. doi: 10.1007/s10143-018-0947-z. [DOI] [PubMed] [Google Scholar]
- 8.Chabaane M, Amelot A, Riche M, Bielle F, Mokhtari K, Carpentier A, Touat M, Mathon B. Efficacy of a second brain biopsy for intracranial lesions after initial negativity. J Clin Neurol. 2020;16:659–667. doi: 10.3988/jcn.2020.16.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DTM, Zheng L, Minxin Y, Philip CWY, Chi-Ping Ng S, Poon WS. Measurement of aspiration pressure in cannula brain tumour biopsy and its correlation with ultrasonographic elastography. J Clin Neurosci. 2022;103:9–13. doi: 10.1016/j.jocn.2022.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Colbassani HJ, Nishio S, Sweeney KM, Bakay RA, Takei Y. CT-assisted stereotactic brain biopsy: value of intraoperative frozen section diagnosis. J Neurol Neurosurg Psychiatry. 1988;51:332–341. doi: 10.1136/jnnp.51.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do TH, Howard MA, Palzer EF, Huling JD, Alvi MA, Cramer SW, Zhu P, Johnson RA, Jean J, Lu J, Jonason AB, Hanson J, Sabal L, Sun KW, McGovern RA, Chen CC. Readmission risk of malignant brain tumor patients undergoing laser interstitial thermal therapy (LITT) and stereotactic needle biopsy (SNB): a covariate balancing weights analysis of the National Readmissions Database (NRD) J Neurooncol. 2022 doi: 10.1007/s11060-022-04093-6. [DOI] [PubMed] [Google Scholar]
- 12.Enders F, Rothfuss A, Brehmer S, Stallkamp J, Schulte DM, Hänggi D. Optimized intraoperative imaging for stereotactic planning with a multiaxial robotic C-arm system: technical note and case series. J Neurol Surg A Cent Eur Neurosurg. 2021 doi: 10.1055/s-0041-1731754. [DOI] [PubMed] [Google Scholar]
- 13.Furtak J, Śledzińska P, Bebyn MG, Szylberg T, Krajewski S, Birski M, Harat M. Infratentorial stereotactic biopsy of brainstem and cerebellar lesions. Brain Sci. 2021;11:1432. doi: 10.3390/brainsci11111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannakou M, Yiallouras C, Menikou G, Ioannides C, Damianou C. MRI-guided frameless biopsy robotic system with the inclusion of unfocused ultrasound transducer for brain cancer ablation. Int J Med Robot. 2019;15:e1951. doi: 10.1002/rcs.1951. [DOI] [PubMed] [Google Scholar]
- 15.Gibby W, Cvetko S, Gibby A, Gibby C, Sorensen K, Andrews EG, Maroon J, Parr R (2021) The application of augmented reality-based navigation for accurate target acquisition of deep brain sites: advances in neurosurgical guidance. J Neurosurg 1–7. 10.3171/2021.9.JNS21510 [DOI] [PubMed]
- 16.Göbel W, Brucker D, Kienast Y, Johansson A, Kniebühler G, Rühm A, Eigenbrod S, Fischer S, Goetz M, Kreth F-W, Ehrhardt A, Stepp H, Irion K-M, Herms J. Optical needle endoscope for safe and precise stereotactically guided biopsy sampling in neurosurgery. Opt Express. 2012;20:26117–26126. doi: 10.1364/OE.20.026117. [DOI] [PubMed] [Google Scholar]
- 17.Grundy PL, Weidmann C, Bernstein M. Day-case neurosurgery for brain tumours: the early United Kingdom experience. Br J Neurosurg. 2008;22:360–367. doi: 10.1080/02688690801961858. [DOI] [PubMed] [Google Scholar]
- 18.Ha TT, Thieringer FM, Bammerlin M, Cordier D. High precision bone cutting by Er: YAG lasers might minimize the invasiveness of navigated brain biopsies. Front Oncol. 2021;11:690374. doi: 10.3389/fonc.2021.690374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haj-Hosseini N, Richter JCO, Milos P, Hallbeck M, Wårdell K. 5-ALA fluorescence and laser Doppler flowmetry for guidance in a stereotactic brain tumor biopsy. Biomed Opt Express. 2018;9:2284–2296. doi: 10.1364/BOE.9.002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall WA. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82:1749–1755. doi: 10.1002/(SICI)1097-0142(19980501)82:9<1756::AID-CNCR23>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Hollon TC, Pandian B, Adapa AR, Urias E, Save AV, Khalsa SSS, Eichberg DG, D’Amico RS, Farooq ZU, Lewis S, Petridis PD, Marie T, Shah AH, Garton HJL, Maher CO, Heth JA, McKean EL, Sullivan SE, Hervey-Jumper SL, Patil PG, Thompson BG, Sagher O, McKhann GM, Komotar RJ, Ivan ME, Snuderl M, Otten ML, Johnson TD, Sisti MB, Bruce JN, Muraszko KM, Trautman J, Freudiger CW, Canoll P, Lee H, Camelo-Piragua S, Orringer DA. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat Med. 2020;26:52–58. doi: 10.1038/s41591-019-0715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Cai P, Zhang H, Adilijiang A, Peng J, Li Y, Che S, Lan F, Liu C. A comparation between frame-based and robot-assisted in stereotactic biopsy. Front Neurol. 2022;13:928070. doi: 10.3389/fneur.2022.928070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda N, Katayama Y, Kawabata S, Furuse M, Tsuji Y, Nonoguchi N, Yagi R, Kameda M, Takami T, Kuroiwa T, Wanibuchi M. Frameless stereotactic biopsy with intraoperative computed tomography “assessment of efficacy and real target registration error”. Neurol Med Chir (Tokyo) 2022;62:195–202. doi: 10.2176/jns-nmc.2021-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaakaji W, Barnett GH, Bernhard D, Warbel A, Valaitis K, Stamp S. Clinical and economic consequences of early discharge of patients following supratentorial stereotactic brain biopsy. J Neurosurg. 2001;94:892–898. doi: 10.3171/jns.2001.94.6.0892. [DOI] [PubMed] [Google Scholar]
- 25.Kiesel B, Millesi M, Woehrer A, Furtner J, Bavand A, Roetzer T, Mischkulnig M, Wolfsberger S, Preusser M, Knosp E, Widhalm G. 5-ALA-induced fluorescence as a marker for diagnostic tissue in stereotactic biopsies of intracranial lymphomas: experience in 41 patients. Neurosurg Focus. 2018;44:E7. doi: 10.3171/2018.3.FOCUS1859. [DOI] [PubMed] [Google Scholar]
- 26.Kreth FW, Muacevic A, Medele R, Bise K, Meyer T, Reulen HJ. The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brain tumours–a prospective study. Acta Neurochir (Wien) 2001;143:539–545. doi: 10.1007/s007010170058. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni AV, Guha A, Lozano A, Bernstein M. Incidence of silent hemorrhage and delayed deterioration after stereotactic brain biopsy. J Neurosurg. 1998;89:31–35. doi: 10.3171/jns.1998.89.1.0031. [DOI] [PubMed] [Google Scholar]
- 28.Kut C, Chaichana KL, Xi J, Raza SM, Ye X, McVeigh ER, Rodriguez FJ, Quiñones-Hinojosa A, Li X. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med. 2015;7:292ra100. doi: 10.1126/scitranslmed.3010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lara-Almunia M, Hernandez-Vicente J. Symptomatic intracranial hemorrhages and frame-based stereotactic brain biopsy. Surg Neurol Int. 2020;11:218. doi: 10.25259/SNI_102_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson PS, Starr PA, Bates G, Tansey L, Richardson RM, Martin AJ. An optimized system for interventional magnetic resonance imaging-guided stereotactic surgery: preliminary evaluation of targeting accuracy. Neurosurgery. 2012;70:95–103. doi: 10.1227/NEU.0b013e31822f4a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livermore LJ, Ma R, Bojanic S, Pereira EAC. Yield and complications of frame-based and frameless stereotactic brain biopsy–the value of intra-operative histological analysis. Br J Neurosurg. 2014;28:637–644. doi: 10.3109/02688697.2014.887657. [DOI] [PubMed] [Google Scholar]
- 32.Malaizé H, Laigle-Donadey F, Riche M, Marijon P, Mokhtari K, Bielle F, Tran S, Nichelli L, Beccaria K, Idbaih A, Hoang-Xuan K, Touat M, Carpentier A, Mathon B, PSL BRAIN-BIOPSY STUDY GROUP Roles and outcomes of stereotactic biopsy for adult patients with brainstem lesion. J Neurooncol. 2022 doi: 10.1007/s11060-022-04129-x. [DOI] [PubMed] [Google Scholar]
- 33.Mallereau C-H, Chibbaro S, Ganau M, Benmekhbi M, Cebula H, Dannhoff G, Santin M-N, Ollivier I, Chaussemy D, Hugo Coca A, Proust F, Todeschi J. Pushing the boundaries of accuracy and reliability during stereotactic procedures: a prospective study on 526 biopsies comparing the frameless robotic and image-guided surgery systems. J Clin Neurosci. 2022;95:203–212. doi: 10.1016/j.jocn.2021.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Markwardt NA, Stepp H, Franz G, Sroka R, Goetz M, Zelenkov P, Rühm A. Remission spectrometry for blood vessel detection during stereotactic biopsy of brain tumors. J Biophotonics. 2017;10:1080–1094. doi: 10.1002/jbio.201600193. [DOI] [PubMed] [Google Scholar]
- 35.Mathon B, Amelot A, Mokhtari K, Bielle F. Increasing the diagnostic yield of stereotactic brain biopsy using intraoperative histological smear. Clin Neurol Neurosurg. 2019;186:105544. doi: 10.1016/j.clineuro.2019.105544. [DOI] [PubMed] [Google Scholar]
- 36.Mathon B, de Chambrun MP, Le Joncour A, Amelot A. Letter to the Editor. Brain biopsy in children and adults with neurological diseases of unknown etiology: two sides of the same coin? J Neurosurg Pediatr. 2020;27:120–122. doi: 10.3171/2020.7.PEDS20619. [DOI] [PubMed] [Google Scholar]
- 37.Mathon B, Favreau M, Degos V, Amelot A, Le Joncour A, Weiss N, Rohaut B, Le Guennec L, Boch A-L, Carpentier A, Bielle F, Mokhtari K, Idbaih A, Touat M, Combes A, Demoule A, Shotar E, Navarro V, Raux M, Demeret S, Pineton De Chambrun M, PSL BRAIN-BIOPSY STUDY GROUP Brain biopsy for neurological diseases of unknown etiology in critically ill patients: feasibility, safety, and diagnostic yield. Crit Care Med. 2022;50:e516–e525. doi: 10.1097/CCM.0000000000005439. [DOI] [PubMed] [Google Scholar]
- 38.Mathon B, Le Joncour A, Bielle F, Mokhtari K, Boch A-L, Peyre M, AmouraCacoub ZP, Younan N, Demeret S, Shotar E, Burrel S, Fekkar A, Robert J, Amelot A, Pineton de Chambrun M, PSL BRAIN-BIOPSY STUDY GROUP Neurological diseases of unknown etiology: brain-biopsy diagnostic yields and safety. Eur J Intern Med. 2020;80:78–85. doi: 10.1016/j.ejim.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 39.Mathon B, Marijon P, Riche M, Degos V, Carpentier A, PSL BRAIN-BIOPSY STUDY GROUP Outpatient stereotactic brain biopsies. Neurosurg Rev. 2022;45:661–671. doi: 10.1007/s10143-021-01593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millesi M, Kiesel B, Wöhrer A, Mercea PA, Bissolo M, Roetzer T, Wolfsberger S, Furtner J, Knosp E, Widhalm G. Is intraoperative pathology needed if 5-aminolevulinic-acid-induced tissue fluorescence is found in stereotactic brain tumor biopsy? Neurosurgery. 2020;86:366–373. doi: 10.1093/neuros/nyz086. [DOI] [PubMed] [Google Scholar]
- 41.Mohyeldin A, Elder JB. Stereotactic biopsy platforms with intraoperative imaging guidance. Neurosurg Clin N Am. 2017;28:465–475. doi: 10.1016/j.nec.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Mohyeldin A, Lonser RR, Bradley Elder J. Real-time magnetic resonance imaging-guided frameless stereotactic brain biopsy: technical note. J Neurosurg. 2016;124:1039–1046. doi: 10.3171/2015.5.JNS1589. [DOI] [PubMed] [Google Scholar]
- 43.Moriarty TM, Quinones-Hinojosa A, Larson PS, Alexander E, Gleason PL, Schwartz RB, Jolesz FA, Black PM. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: stereotactic brain biopsy. Neurosurgery. 2000;47:1138–1145. doi: 10.1097/00006123-200011000-00023. [DOI] [PubMed] [Google Scholar]
- 44.Neidert N, Straehle J, Erny D, Sacalean V, El Rahal A, Steybe D, Schmelzeisen R, Vlachos A, Reinacher PC, Coenen VA, Mizaikoff B, Heiland DH, Prinz M, Beck J, Schnell O. Stimulated Raman histology in the neurosurgical workflow of a major European neurosurgical center - part A. Neurosurg Rev. 2022;45:1731–1739. doi: 10.1007/s10143-021-01712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogiwara T, Nitta J, Fujii Y, Watanabe G, Kuwabara H, Agata M, Kobayashi H, Miyaoka Y, Kitamura S, Hanaoka Y, Goto T, Iwaya M, Hongo K, Horiuchi T. A preliminary study of the diagnostic efficacy and safety of the novel boring biopsy for brain lesions. Sci Rep. 2022;12:4387. doi: 10.1038/s41598-022-08366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pichette J, Goyette A, Picot F, Tremblay M-A, Soulez G, Wilson BC, Leblond F. Sensitivity analysis aimed at blood vessels detection using interstitial optical tomography during brain needle biopsy procedures. Biomed Opt Express. 2015;6:4238–4254. doi: 10.1364/BOE.6.004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purzner T, Purzner J, Massicotte EM, Bernstein M. Outpatient brain tumor surgery and spinal decompression: a prospective study of 1003 patients. Neurosurgery. 2011;69:119–126. doi: 10.1227/NEU.0b013e318215a270. [DOI] [PubMed] [Google Scholar]
- 48.Ramakonar H, Quirk BC, Kirk RW, Li J, Jacques A, Lind CRP, McLaughlin RA. Intraoperative detection of blood vessels with an imaging needle during neurosurgery in humans. Sci Adv. 2018;4:eaav4992. doi: 10.1126/sciadv.aav4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reinecke D, von Spreckelsen N, Mawrin C, Ion-Margineanu A, Fürtjes G, Jünger ST, Khalid F, Freudiger CW, Timmer M, Ruge MI, Goldbrunner R, Neuschmelting V. Novel rapid intraoperative qualitative tumor detection by a residual convolutional neural network using label-free stimulated Raman scattering microscopy. Acta Neuropathol Commun. 2022;10:109. doi: 10.1186/s40478-022-01411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rey-Dios R, Hattab EM, Cohen-Gadol AA. Use of intraoperative fluorescein sodium fluorescence to improve the accuracy of tissue diagnosis during stereotactic needle biopsy of high-grade gliomas. Acta Neurochir (Wien) 2014;156:1071–1075. doi: 10.1007/s00701-014-2097-6. [DOI] [PubMed] [Google Scholar]
- 51.Richardson RM, Kells AP, Martin AJ, Larson PS, Starr PA, Piferi PG, Bates G, Tansey L, Rosenbluth KH, Bringas JR, Berger MS, Bankiewicz KS. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotact Funct Neurosurg. 2011;89:141–151. doi: 10.1159/000323544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riche M, Amelot A, Peyre M, Capelle L, Carpentier A, Mathon B. Complications after frame-based stereotactic brain biopsy: a systematic review. Neurosurg Rev. 2021;44:301–307. doi: 10.1007/s10143-019-01234-w. [DOI] [PubMed] [Google Scholar]
- 53.Riche M, Marijon P, Amelot A, Bielle F, Mokhtari K, de Chambrun MP, Joncour AL, Idbaih A, Touat M, Do C-H, Deme M, Pasqualotto R, Jacquens A, Degos V, Shotar E, Chougar L, Carpentier A, Mathon B. Severity, timeline, and management of complications after stereotactic brain biopsy. J Neurosurg. 2022;136:867–876. doi: 10.3171/2021.3.JNS21134. [DOI] [PubMed] [Google Scholar]
- 54.Richter J, Haj-Hosseini N, Milos P, Hallbeck M, Wårdell K. Optical Brain Biopsy with a Fluorescence and Vessel Tracing Probe. Oper Neurosurg (Hagerstown) 2021;21:217–224. doi: 10.1093/ons/opab216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salas S, Brimacombe M, Schulder M. Stereotactic accuracy of a compact intraoperative MRI system. Stereotact Funct Neurosurg. 2007;85:69–74. doi: 10.1159/000097921. [DOI] [PubMed] [Google Scholar]
- 56.Sillay KA, Rusy D, Buyan-Dent L, Ninman NL, Vigen KK. Wide-bore 1.5 T MRI-guided deep brain stimulation surgery: initial experience and technique comparison. Clin Neurol Neurosurg. 2014;127:79–85. doi: 10.1016/j.clineuro.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Singh DK, Khan KA, Singh AK, Kaif M, Yadav K, Kumar Singh R, Ahmad F (2021) Fluorescein sodium fluorescence: role in stereotactic brain biopsy. Br J Neurosurg 1–4. 10.1080/02688697.2021.2016615 [DOI] [PubMed]
- 58.Spyrantis A, Woebbecke T, Rueß D, Constantinescu A, Gierich A, Luyken K, Visser-Vandewalle V, Herrmann E, Gessler F, Czabanka M, Treuer H, Ruge M, Freiman TM. Accuracy of robotic and frame-based stereotactic neurosurgery in a phantom model. Front Neurorobot. 2022;16:762317. doi: 10.3389/fnbot.2022.762317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg. 2010;112:479–490. doi: 10.3171/2009.6.JNS081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sterk B, Taha B, Osswald C, Bell R, Chen L, Chen CC (2022) Initial clinical experience with clearpoint smartframe array-aided stereotactic procedures. World Neurosurg S1878–8750(22)00242-X. 10.1016/j.wneu.2022.02.095 [DOI] [PubMed]
- 61.Straehle J, Erny D, Neidert N, Heiland DH, El Rahal A, Sacalean V, Steybe D, Schmelzeisen R, Vlachos A, Mizaikoff B, Reinacher PC, Coenen VA, Prinz M, Beck J, Schnell O. Neuropathological interpretation of stimulated Raman histology images of brain and spine tumors: part B. Neurosurg Rev. 2022;45:1721–1729. doi: 10.1007/s10143-021-01711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thien A, Han JX, Kumar K, Ng YP, Rao JP, Ng WH, King NKK. Investigation of the usefulness of fluorescein sodium fluorescence in stereotactic brain biopsy. Acta Neurochir (Wien) 2018;160:317–324. doi: 10.1007/s00701-017-3429-0. [DOI] [PubMed] [Google Scholar]
- 63.Thien A, Rao JP, Ng WH, King NKK. The Fluoropen: a simple low-cost device to detect intraoperative fluorescein fluorescence in stereotactic needle biopsy of brain tumors. Acta Neurochir (Wien) 2017;159:371–375. doi: 10.1007/s00701-016-3041-8. [DOI] [PubMed] [Google Scholar]
- 64.Trojanowski P, Jarosz B, Szczepanek D. The diagnostic quality of needle brain biopsy specimens obtained with different sampling methods - experimental study. Sci Rep. 2019;9:8077. doi: 10.1038/s41598-019-44622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warnick RE, Longmore LM, Paul CA, Bode LA. Postoperative management of patients after stereotactic biopsy: results of a survey of the AANS/CNS section on tumors and a single institution study. J Neurooncol. 2003;62:289–296. doi: 10.1023/a:1023315206736. [DOI] [PubMed] [Google Scholar]
- 66.Wen DY, Hall WA, Miller DA, Seljeskog EL, Maxwell RE. Targeted brain biopsy: a comparison of freehand computed tomography-guided and stereotactic techniques. Neurosurgery. 1993;32:407–412. doi: 10.1227/00006123-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 67.Widhalm G, Minchev G, Woehrer A, Preusser M, Kiesel B, Furtner J, Mert A, Di Ieva A, Tomanek B, Prayer D, Marosi C, Hainfellner JA, Knosp E, Wolfsberger S. Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg Rev. 2012;35:381–391. doi: 10.1007/s10143-012-0374-5. [DOI] [PubMed] [Google Scholar]
- 68.Xu R, Rösler J, Teich W, Radke J, Früh A, Scherschinski L, Onken J, Vajkoczy P, Misch M, Faust K. Correlation of tumor pathology with fluorescein uptake and MRI contrast-enhancement in stereotactic biopsies. J Clin Med. 2022;11:3330. doi: 10.3390/jcm11123330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zrinzo L. Pitfalls in precision stereotactic surgery. Surg Neurol Int. 2012;3:S53–61. doi: 10.4103/2152-7806.91612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.