Abstract
Extracorporeal membrane oxygenation (ECMO) is a modality utilized for partially or completely supporting the cardiac and/or pulmonary function. There are multiple vascular access techniques depending upon the necessity and the mode of ECMO used. ECMO has evolved over the years as an integral part of the cardiac care discipline. Historically, this lifesaving modality began as an extension of cardiopulmonary bypass and was associated with adverse outcomes. Currently, ECMO has evolved as an accepted and viable solution to patients with severe cardiac/respiratory/cardiorespiratory failure that is refractory to conservative management. The outcomes of patients on ECMO are dependent on multiple factors originating from demographic and pathophysiological status of patients as well as the control of homeostasis during ECMO within the acceptable range. Various studies have been published by many practitioners over past decades since the dawn of ECMO era. A brief review of such experience is summated, and a conclusion is derived about the clinical course of the patients on ECMO, while adding the author’s experience about the same in a tertiary care large-volume center.
Keywords: ECMO, Lifesaving, Cardiac, Pulmonary, Vascular
Introduction
Extracorporeal membrane oxygenation (ECMO) is an advanced modality used to support the failing heart, lungs, or both. The support may be partial or full depending on the status of the cardiorespiratory function. Vascular access may be achieved centrally, peripherally, or as a hybrid technique. Over time, ECMO has evolved as an offshoot of cardiac surgery and has become an integral part of cardiac care where advanced and high-risk cardiac surgeries and interventional cardiology procedures are performed. Additionally, ECMO is an essential component of intensive care units where its use is indicated in severe respiratory failure that does not respond to conventional therapy. ECMO may be used as a bridging therapy (bridge to decision, bridge to recovery, and bridge to transplantation). Finally, ECMO may be used to facilitate resuscitation of the patients who are in refractory cardiac arrest; this is called extracorporeal cardiopulmonary resuscitation (ECPR).
History
Gibbon in 1953 had successfully utilized cardiopulmonary bypass (CPB) for the first time for the correction of atrial septal defect (ASD) using a screen oxygenator [1]. The first documented use of ECMO, a membrane oxygenator in a patient with severe respiratory failure following trauma, was performed by Hill and associates in 1972 [2]. In 1975, Dr Robert Bartlett successfully used ECMO to treat a new-born patient following respiratory failure secondary to meconium aspiration [3]. In 1989, the Extracorporeal Life Support Organization (ELSO) was founded in Michigan by Robert Bartlett and his associates. ECMO in India was first reported by K. V. Surendranath, D. P. Shetty, and team from BM Birla Heart Research Centre in Calcutta in December 1991 in a paper entitled “Long Term Cardiopulmonary Support in Children.”[4] The ELSO Society of India (ESOI) was established in India in 2010 for improving the awareness of ECMO in India. In 2013, the South and West Asia Chapter (SWAC) was incorporated by ELSO, and in 2015 Africa was added to SWAC making it the South and West Asia, Africa Chapter of ELSO (SWAAC) [5].
Landmark Trials
Zapol and associates published a trial in 1979 performed in 90 patients with acute respiratory failure (ARF) that demonstrated lack of long-term survival with the use of ECMO in patients with severe ARF. However, there were many limitations like the usage of the standard ventilation technique (high tidal volume and low PEEP), lack of guidelines on anticoagulation, patient selection, along with the use of veno-arterial (VA)-ECMO instead of veno-venous (VV)-ECMO [5]. The concept of extracorporeal removal of CO2 was put forth by Gattinoni and colleagues during 1980s in a series of publications. This technique was used with a combination of low-frequency, intermittent positive-pressure ventilation with a view to permit lung rest in patients with severe respiratory failure in conjunction [6, 7].
There has been a remarkable increase in the use of ECMO globally in the recent past: this is attributable to two important publications. Firstly, the Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial in 2009 supported the use of ECMO in specialized centers for patients with potentially reversible severe respiratory failure. The severity of respiratory failure was based on the derivation of the Murray Score that used four criteria, namely PaO2/FiO2 ratio, positive end-expiratory pressure (PEEP), dynamic lung compliance, and the number of quadrants infiltrated on the chest radiograph. A Murray Score greater than 3.0 was the main criterion institution of ECMO in addition to respiratory acidosis with a pH lower than 7.20. The CESAR trial showed an improvement in survival: 63% versus 43% by conventional treatment [8]. Its limitations were that there was no standard protocol for ventilatory management in the control group, whereas the ECMO group was managed with lung protective ventilation. Zapol and associates published a trial in 1979 performed in 90 patients with acute respiratory failure (ARF) that demonstrated lack of long-term survival with the use of ECMO in patients with severe ARF. However, there were many limitations like the usage of standard ventilation technique (high tidal volume and low PEEP), lack of guidelines on anticoagulation, patient selection, along with the use of veno-arterial (VA)-ECMO instead of veno-venous (VV)-ECMO [9]. The second publication that endorsed the use of ECMO in respiratory failure was the 2009 influenza A (H1N1) pandemic; the “ANZ-ECMO” was a case series published from Australia and New Zealand about the use of VV-ECMO in patients with ARDS, which showed a survival of 79% at 30 days [10]. This resulted in a dramatic increase in this use of VV-ECMO all over the world as a rescue therapy for patients with severe respiratory failure unresponsive to conventional therapy that included mechanical ventilation. However, the “ECMO to Rescue Lung Injury in severe ARDS” (the EOLIA) study demonstrated that in patients with very severe ARDS, 60-day mortality was not significantly lower with ECMO than with a strategy of conventional mechanical ventilation that included ECMO as rescue therapy [11]. In the EOLIA trial, indications for the use of VV-ECMO were patients with very severe ARDS, with by one of three criteria: (i) a ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) of less than 50 mmHg for more than 3 h; (ii) a PaO2:FiO2 of less than 80 mmHg for more than 6 h; or (iii) an arterial blood pH of less than 7.25 with a partial pressure of arterial carbon dioxide of equal to or greater than 60 mmHg for more than 6 h. VV-ECMO has been used for many years in treating ARDS and is now the widely used therapy for supporting patients awaiting lung transplantation for interstitial lung disease, cystic fibrosis, aspiration pneumonitis, and most recently for COVID-19 ARDS. Raleigh and associates have demonstrated from 10 studies that the use of ECMO in the pre-transplant period does not affect the post-transplant outcome compared to those who did not require ECMO [12]. However, Brodie et al. have highlighted that the role of extracorporeal life support in the management of adults with acute respiratory failure is being redefined by advances in technology and increasing evidence of its effectiveness. Future developments in the field will result from technological advances, an increased understanding of the physiology and biology of extracorporeal support, and increased knowledge of how it might benefit the treatment of a variety of clinical conditions [13].
Indications of VA-ECMO and VV-ECMO
VV-ECMO
VV-ECMO should be taken into consideration in patients with potentially reversible severe acute respiratory failure that is refractory to optimal conventional management. The indications for VV-ECMO may be conveniently categorized into (i) pathophysiologic criteria, (ii) criteria based on scoring systems, and (iii) specific clinical conditions. The pathophysiological criteria for initiation of ECMO are (i) hypoxemic respiratory failure (PaO2/FiO2 ratio < 80 mmHg despite optimal management including a trial of prone position (ii) hypercapnic respiratory failure with PaCO2 > 60 mmHg and pH < 7.25 despite optimal ventilatory management.
Specific indications for VV-ECMO other than respiratory failure in ARDS alluded above include severe air leak syndromes, for CO2 retention in mechanically ventilated patients despite a high (> 30 cm H2O) plateau pressure (Pplat), and for miscellaneous patient conditions such as airway support in a patient listed for lung transplantation and a patient with acute respiratory failure unresponsive to optimal care. The indications and contraindications of VV-ECMO and VA-ECMO are detailed in Tables 1 and 2.
Table 1.
Indications for ECMO
| VA-ECMO | VV-ECMO |
|---|---|
| 1. Refractory cardiogenic shock* | 1. Severe ARDS |
| 2. Combined cardiorespiratory failure | 2. Severe pneumonia/acute eosinophilic pneumonia |
| 3. Failure to wean from CPB | 3. Severe asthma |
| 4. Bridge to ventricular assist device and/or transplantation | 4. Thoracic trauma |
| 5. Unstable/refractory arrhythmias | 5. Aspiration pneumonitis |
| 6. Cardiac arrest without return of spontaneous circulation (ECPR) | 6. Inhalational injury |
| 7. Massive pulmonary embolism | 7. Air-leak syndromes |
|
8. Before and/or after lung transplant (peri-lung transplant) |
|
| 9. Bridge to recovery or transplantation | |
| 10. Diffuse alveolar/pulmonary hemorrhage |
* (based on SHOCK trial, 1999): (i) SBP < 90 mmHg or vasopressor support to maintain SBP > 90 mmHg; (ii) evidence of end-organ damage (urine output < 30 mL/h, cool extremities); (iii) hemodynamic criteria: CI < 2.2 L/min/m2, PCWP > 15 mmHg; VA veno-arterial, VV veno-venous, ARDS adult respiratory distress syndrome, CPB cardiopulmonary bypass, ECPR extracorporeal cardiopulmonary
Table 2.
Contraindications to ECMO
| Absolute | Relative |
|---|---|
| Acute intracranial hemorrhage | Advanced age |
| Massive stroke | Contraindications to anticoagulation |
| Active bleeding | Morbid obesity |
| Malignancy | End-stage liver disease |
|
Severe aortic insufficiency/aortic dissection (for VA-ECMO) immunosuppression |
Mechanical ventilation for more than 7 days with Pplat > 30 cm H2O and FiO2 > 90% |
Types and Techniques of ECMO
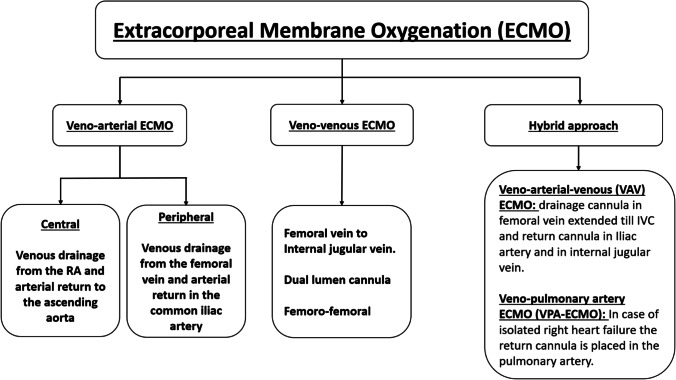
ECMO consists of two major types—veno-arterial (VA) ECMO and veno-venous (VV) ECMO (Fig. 1). There are further two subtypes in VA-ECMO—central and peripheral.
Fig. 1.
Types of extracorporeal membrane oxygenation (ECMO) techniques
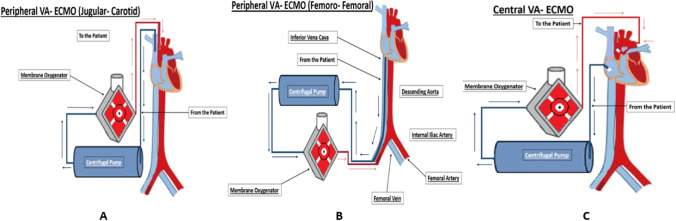
VA-ECMO provides rest to the heart by taking over the function of the heart perfusing the body. VV-ECMO provides rest to the lung by providing oxygenation and carbon dioxide removal. In VA-ECMO, the machine draws deoxygenated blood from the systemic venous circulation, oxygenates the blood, and supplies oxygenated blood to the systemic arterial circulation for perfusion to the whole body. This is achieved by placing cannula in the right atrium (RA) for the purpose of drawing the blood and placing another cannula in the ascending aorta for returning the blood. This is called central VA-ECMO. In peripheral VA-ECMO, one drainage cannula is inserted in the right femoral vein, the tip of which is positioned at the level of the right atrium, whereas the return cannula is inserted in the right femoral artery with the tip residing in the common iliac artery (please see Fig. 2).
Fig. 2.
Veno-arterial (VA) ECMO: (A) The venous drainage cannula inserted via the internal jugular vein into the right atrium; the arterial return cannula inserted via the carotid artery. (B) The venous drainage cannula inserted via the femoral vein into the mid RA and the arterial return cannula is inserted via the femoral artery. (C) Central VA ECMO: the venous drainage cannula inserted in the RA and the arterial return cannula inserted in the aorta
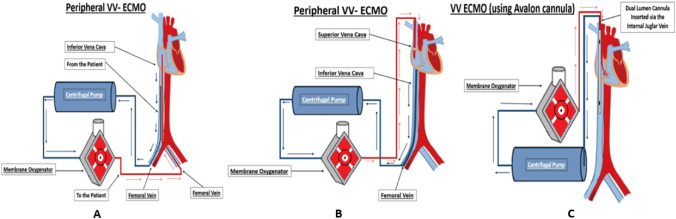
In VV-ECMO, three different configurations of cannulation are possible (please see Fig. 3): (i) femoro-jugular: the drainage cannula is inserted in the right femoral vein and advanced until the inferior vena cava-right atrium (IJV-RA) junction, and the return cannula is inserted in the right internal jugular vein (IJV) and advanced until the mid-RA; (ii) femoro-femoral: the drainage and return cannulae are inserted in femoral veins on either of the sides. The drainage cannula is advanced until the proximal IVC, while the tip of the other one, the return cannula, is advanced until the mid-RA. The return cannula may be in the same femoral vein as the drainage cannula, or it may be in the contralateral femoral vein. (iii) Dual lumen cannula: VV-ECMO may also be configured using a dual lumen cannula with one lumen draining blood from the inferior vena cava (IVC) and the superior vena cava (SVC). The oxygenated blood is returned to the RA through the other lumen with the opening of the return cannula facing towards the tricuspid valve. Transesophageal echocardiography (TEE) is useful in identifying the jet of blood directed towards the tricuspid valve. Hypoxemia during VV-ECMO may be due to one of the following reasons: (i) when patient’s cardiac output is greater than the ECMO flow, ECMO run may be associated with inadequate tissue oxygen delivery; in this instance, the ECMO flows can be increased in an attempt to increase/normalize oxygen delivery: consumption ratio of up to 5:1 but certainly above the critical threshold of 2:1; (ii) increased metabolic demand due to sepsis, fever, agitation, shivering, etc.; (iii) recirculation which refers to oxygenated blood from return cannula reaching the drainage cannula without entering the right ventricle due to the proximity of the cannulae in the RA. This leads to poor gas exchange. This may be identified by oxygenated bright red blood issuing out of the drainage cannula. This may be avoided by ensuring that the tips of the drainage and return cannula are at least 10 cm apart.
Fig. 3.
(A) Peripheral venous (VV) ECMO: The venous drainage cannula is inserted via the femoral vein into the IVC, tip positioned at the IVC-RA junction. The oxygenated blood is returned via the cannula inserted into the contralateral femoral vein and positioned in the mid RA. (B) Peripheral VV-ECMO: The venous drainage cannula inserted via the femoral vein and positioned at the IVC-RA junction and the oxygenated blood is returned through the cannula inserted via the internal jugular vein and positioned in the mid RA. (C) VV-ECMO by dual lumen cannula: The dual lumen cannula with multiple orifices is inserted via the internal jugular vein and positioned such that the orifices in the SVC and IVC. The oxygenated blood is returned through the same cannula via another lumen that opens and faces the tricuspid valve. The oxygenated blood is thus directed towards the tricuspid valve into the RV
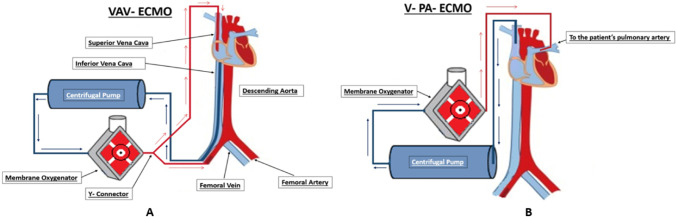
In cases of femoral VA-ECMO, two major problems relating to circuit/ECMO run may be encountered. (i) When the native heart recovers function with lungs yet to recover, the upper body may be perfused with deoxygenated blood emanating from the heart due to cardiac contraction. This leads to upper body being perfused with relatively deoxygenated blood whereas the lower parts of the body receive oxygenated blood. This is termed north/south or Harlequin syndrome. In such a case, an additional cannula is inserted in the right IJV that is connected to the arterial return cannula though a Y-connector. The oxygenated blood passes through the pulmonary circulation and returns to the left atrium and then to the left ventricle (LV), thus passing to the aorta; thus, the upper body gets perfused with adequately oxygenated blood. This configuration is called VAV-ECMO (Fig. 4). (ii) Left ventricular distension occurs in patients on VA-ECMO as a result of inability of the aortic valve to open in the setting of an increase in the afterload and decreased cardiac contractility, additionally myocardial blood flow from the Thebesian veins and bronchial blood return, and most importantly systemic venous return that is not captured by the ECMO venous cannula. This calls for strategies to unload the LV such as placement of LA vent, LV apical venting, atrial septostomy, or use of an intra-aortic balloon pump or Impella.
Fig. 4.
(A) VAV ECMO: The venous drainage cannula is inserted via the femoral vein and positioned at the IVC-RA junction and the arterial cannula inserted in the femoral artery. Additionally, a cannula supplying oxygenated blood is inserted using a Y-connector in the internal jugular vein to the RA; this is a treatment strategy for Harlequin syndrome. (B) V-PA ECMO: The venous drainage is via the internal jugular vein and the oxygenated blood is returned to the pulmonary artery; this strategy is used for patients with isolated right ventricular failure
In V-PA ECMO, the return cannula is placed in the main pulmonary artery using a graft, and a venous drainage cannula is either placed in right IJV or any one of the femoral veins. Alternatively, a dual lumen inflow cannula can be placed that drains simultaneously from the IVC and SVC. Yet another technique is where a cannula is placed via the right IJV with its inflow (venous drainage) ports in the RA and its tip with the outflow port resides in the main pulmonary artery.
The Seldinger technique is used for percutaneous cannulation using a guidewire and serial dilatations or a surgical cutdown is done followed by insertion of the cannulae. In the author’s institute, surgical cutdown is the preferred method and in non-emergency situations, a graft is placed over the femoral artery to attach the outflow cannula to avoid limb ischemia.
Outcomes of ECMO
Survival
ECMO is an invasive therapeutic modality for resuscitation of patients with severe cardiac, respiratory, or cardiorespiratory failure. Despite its increased utilization, ECMO may be associated with many adverse effects and complications. The ELSO registry showed decreased survival as the treatment duration increases [14]. Duration of VA-ECMO therapy amongst survivors and non-survivors was quoted to be similar in one study [15]. Smith et al. concluded that survival was poor in patients who were weaned early, and survival was also poor in patients with a long ECMO run. This suggests timing of weaning is crucial to have good outcome [16]. Survival decreases after ECMO therapy crosses 14 days due to the disease process and treatment complications adding a cumulative effect [17]. There is no effect on mortality with increased duration of VV-ECMO per se [18, 19].
Scoring
Survival after VA-ECMO can be predicted by means of survival after VA-ECMO (SAVE) score developed by ELSO in conjunction with the intensive care department of Alfred Hospital, Melbourne, in 2015 [20]. This score takes into consideration age, diagnosis, cardiac and respiratory vital parameters, renal function, and other organ functions like the central nervous system and liver function. It has a good predictive capability with area under the receiver operating characteristic curve of 0.68 (95% confidence interval: 0.64–0.71).
Complications of ECMO
Complications during ECMO can be broadly categorized into (i) circuit-related (technical) and (ii) patient-related.
Circuit-Related Complications
Circuit-related complications are (i) thrombosis: this is the most common complication that can have devastating effects: precise anticoagulation and constant vigilance are needed to avoid clotting in the ECMO circuits; (ii) circuit fracture may occur due to excessive pressure generation in the circuit; this will lead to sudden exsanguination of the patient and immediate replacement of the affected portion of the circuit or the entire apparatus may be necessary; (iii) gas embolism is an abrupt entrainment of air leading organ ischemia or a devastating air-lock leading to stoppage of circulation and is usually due to the creation of a significant negative pressure and air entrainment in the circuit. (iv) Cavitation, in which gas is forced out of the liquid medium by a circuit obstruction, can also lead to gas embolism.
Patient-Related Complications
Neurologic Derangements
The literature has cited a vast myriad of neurological issues with the use of ECMO [21]. The causative factors for neurological derangements are microemboli, hypocapnia, cerebral hypoperfusion, and loss of cerebral autoregulation. In the neonatal age group, with VV-ECMO, the incidence of clinical seizures is 9% while that of intracranial hemorrhage is 7.4%. In the pediatric VV-ECMO group, the incidence of clinical seizures is 5.2% while that of intracranial hemorrhage is 6.2%. In adult patients, the data show a lower incidence of neurologic complications with VV-ECMO compared with VA ECMO. According to cumulative ELSO data, adult VV-ECMO patients have a 1.0% incidence of clinical seizures and a 3.8% incidence of intracranial hemorrhage. Adults on VA ECMO have a 1.7% incidence of seizures and a 2.4% incidence of intracranial hemorrhage.
Hemorrhage
Bleeding is the most common complication of ECMO particularly from cannulation sites due to systemic anticoagulation [22]. Bleeding is known to occur in the gastrointestinal tract, brain, and intrapulmonary and retroperitoneal space [23, 24]. It is thus, important to closely monitor the heparin dosage, activated clotting time (ACT), and activated partial thromboplastin time (aPTT) of patients on ECMO. Risk factors for hemorrhage in patients on ECMO are pre-ECMO cardiac arrest, sepsis, renal replacement therapy, influenza, hemolysis, and thrombocytopenia [25]. Arterial cannulation in VA-ECMO presents a higher bleeding risk compared with venous cannulations used in VV-ECMO.
Infection
The prevalence of hospital acquired infections in patients on ECMO is 10–12% with coagulase-negative Staphylococcus being the major culprit, followed by Candida, Pseudomonas aeruginosa, and Enterobacteriaceae [26]. The infection risk increases with duration of ECMO utilisation. Other factors like translocation of gut bacteria, ECMO-related impairment of immune system, colonisation of oxygenator, catheters, and cannulae are also other aetiologies [26]. Bizzaro et al. and Schmidt et al. have found that the rate of infection is directly related to the duration of ECMO therapy [27, 28].
Renal Dysfunction
Makdisi et al. have demarcated phases of renal performance of patients receiving ECMO support. They have stated that the first 24 to 48 h is the oliguric phase which occurs due to inflammatory response of the body. This is followed by a diuretic phase [29]. Continuous renal replacement therapy (CRRT) is utilized to maintain fluid balance on ECMO. Kielstein et al. have demonstrated that survival is reduced in patients requiring CRRT when on ECMO [30].
Extracorporeal Cardiopulmonary Resuscitation (ECPR)
ECPR, the adjunctive use of ECMO in routine CPR, is recognized by the American Heart Association (AHA) and the European Resuscitation Council (ERC) [31–33]. Zakhary et al. have found in their study on 75 patients with in-hospital and out-of-hospital cardiac arrest (OHCA) that 39% patients were successfully weaned from ECMO and 31% survived until hospital discharge amongst them [34]. Holmberg et al. in their review on ECPR included 25 observational studies including OHCA and in-hospital cardiac arrests. They found that, in spite of the increasing use of ECMO support over the past decade, the availability of ECPR is still lacking for OHCA. OHCA patients need to rely on rapid transportation to ECPR capable hospitals. According to them, there were no guidelines for the initiation of ECPR, confounders have not been adjusted, and vague accounting of past medical history of comorbidities like renal function or cardiac function of the patients exist [35]. With increasing utilisation of ECMO, for various indications there must exist clear guidelines about the initiation and termination of ECMO. It is required to define the inclusion and exclusion criteria for pediatric and adult patients who may need ECMO due to pulmonary or cardiac problems. The author has also commented that, there is a necessity of establishing a timing of the commencement of ECMO to avoid any doubt about occurrence of a delay in treating the patients and affecting their outcomes [36]. Table 3 shows the current criteria for ECPR.
Table 3.
Criteria for ECPR
| Witnessed arrest |
| Arrest to first CPR (“no-flow interval”) < 5 min |
| Initial cardiac rhythm of VF/pVT/PEA |
| Arrest to ECMO flow < 60 min “low flow interval* |
| Good-quality CPR with ETCO2 > 10 mmHg before cannulation for ECMO |
| Intermittent ROSC or recurrent VF |
| “Signs of life” during conventional CPR |
| The absence of severe comorbidities/malignancy |
| No known aortic valve regurgitation |
ECPR, ECMO cardiopulmonary resuscitation; CPR, cardiopulmonary resuscitation; VF, ventricular fibrillation; pVT, pulseless ventricular tachycardia; PEA pulseless electrical activity; ETCO2, end-tidal carbon dioxide; ROSC, return of spontaneous circulation
Maintenance on ECMO
Care of patients on ECMO need a multitude of health care professionals who understand the physiology of this form of therapy. At the bedside, a nurse and a perfusionist are available 24/7 to monitor the patients. In the author’s institute, lung protective ventilation is preferred for intubated patients on VV-ECMO. Those patients who are suspected to require prolonged ventilation may be tracheostomized. Patients are extubated if and when suitable. Arterial blood gas, flows, rotations per minute, sweep gas flow, and pre and post oxygenator membrane pressure are monitored periodically. The pre and post oxygenator partial pressure of oxygen is monitored to keep a track of oxygenator performance. Feeding is recommended that is monitored by a dietician. Renal replacement therapy is instituted as required.
Monitoring
Monitoring during ECMO is crucial. Monitoring will encompass technical aspects of ECMO (pre and post pump pressures and deltaP) and patients’ physiological status. This entails invasive monitoring such as arterial blood pressure (BP), central venous pressure (CVP), pulmonary artery pressure (PAP) and pulmonary capillary wedge pressure (PCWP). The arterial pressure waveform provides information about mean arterial pressure and pulsatility. Invasive BP monitoring also provides an access for sampling for arterial blood gas analysis. Anticoagulation on ECMO is achieved by unfractionated heparin (UFH) and the options for monitoring are by means of activated clotting time (ACT), activated partial thromboplastin time (aPTT), and factor Xa assay. It is very important to monitor anticoagulation on ECMO, to prevent thrombotic complications, circuit clotting, and hemorrhage. The adverse effects of UFH are heparin-induced thrombocytopenia (HIT) of which there are 2 types—HIT 1 and HIT 2. It needs to be detected early, and the patient should be switched to other anticoagulants like argatroban or bivalirudin [37]. It is very important to monitor for infection when the patient is on ECMO using a protocolized approach on the basis of culture/sensitivity reports.
Weaning
Weaning from ECMO is a very complex task that requires multidisciplinary involvement. The approach to weaning will be dictated by echocardiographic parameters, lowering serum lactate levels, and adequate mixed venous oxygen saturation levels in VA-ECMO. In VV-ECMO, reduction of sweep gas to maintain acceptable gas exchange with radiological clearance will be a necessary prerequisite. Ventilation is set to provide adequate gas exchange along with escalation of inotropes. Once it is confirmed that the cardiorespiratory function is adequate, the ECMO is weaned off in OR/ICU followed by decannulation. Inability of the heart to exhibit adequate function warrants continuation of ECMO. This is considered as a failure to wean off ECMO. Alternatively, a technique termed as “AV-bridge” is utilized in ICU. Patients on VV-ECMO are weaned when the pulmonary physiology of the patient allows adequate oxygenation and carbon dioxide removal with minimal lung protective ventilation by reducing the sweep gas flow. Arterial blood gas monitoring is done hourly or half hourly during this process. Importance has been given to the presence of a cardiac anesthesiologist in an ECMO team for initiating and managing an ECMO inside and outside the hospital and transferring the patient on ECMO support [38]. European consensus is also in favor of a strong role of a cardiac anesthesiologist in an ECMO team [39]. Transesophageal echocardiography (TEE) helps in correct positioning of the ECMO cannulae and assessing the cardiac function [40].
Cost of ECMO
The cost of receiving ECMO support varies from region to region. In the USA, the mean charges range from USD 154,215 to USD 868,979 [41]. Tseng and associates, from Taiwan, have provided a breakdown of total hospital costs where 41% were spent on personnel, 26% on disposable items, 13% on medications, 10% on laboratory and radiological tests, 8% on blood products, and 2% on renal replacement therapy [41]. Dennis et al. have mean hospital costs in Australia for OHCA that were USD 42,658 and for IHCA they were USD 62,700 [42]. Jӓӓmaa-Holmberg et al. have stated in their study that the costs vary according to diagnoses for which the patients received ECMO therapy and they have provided the charges encountered according to the diagnosis [43]. The charges ranged from USD 63,271 to USD 126,605.
ECMO Experience at Narayana Institute of Cardiac Sciences
The details of experience from the author’s institution for the last 1000 cases of ECMO over the last 15 years are detailed in Table 4. Patients above the age of 18 were grouped under adult and there were 741 adult patients. There were 306 adult males: 337 male pediatric patients. The most frequent type of ECMO used was VA followed by VV. Commonest indication was failure to wean cardiopulmonary bypass in adults and cardiac arrest in pediatric population. Survival rate is 59.1% in adults and pediatric survival was 46.9%.
Table 4.
Demography and clinical details of the patients
| Demography and clinical details | Adult | Pediatric |
|---|---|---|
| Age (years); mean (SD) | 46.8 (15.3) | 8.5 (3.6) |
| Height (cm); mean (SD) | 158 (11.2) | 77.9 (32.2) |
| Weight (kg); mean (SD) | 62.5 (16.9) | 12.6 (9.8) |
| Male (n) | 306 | 337 |
| Female (n) | 435 | 209 |
| Mode of ECMO | ||
| VA | 331 | 531 |
| VV | 406 | 12 |
| VAV | 3 | 2 |
| VVA | 1 | 1 |
| Survival (%) | 59.1 | 46.8 |
| Indications | ||
| Hypoxemia | 93 | 41 |
| LV failure | 132 | 47 |
| Failure to wean | 193 | 54 |
| ARDS | 13 | 10 |
| RV failure | 97 | 25 |
| Supra-systemic PA pressure | 9 | 10 |
| Hypotension | 78 | 88 |
| Cardiac arrest | 58 | 200 |
| Bridge to transplant | 9 | 0 |
| Cardiogenic shock | 41 | 42 |
| Refractory arrhythmia | 0 | 9 |
| Cardiac tamponade | 0 | 2 |
| Pneumonia (COVID and others) | 4 | 0 |
| Meconium aspiration | 0 | 4 |
| Hyperkalemia | 0 | 2 |
| Hypoxic arrest | 0 | 9 |
| Chloroaniline inhalation | 1 | 0 |
| Bridge to recovery | 2 | 1 |
| ECPR | 11 | 2 |
Future
In the near future, there will be further advances in the technology of ECMO. Spherical chambers may replace the square-shaped membrane oxygenators to avoid formation of thrombi. Other advances may include paracorporeal membrane lung, automatic adjustment of sweep gas, and nitric oxide eluting plastic combined with anti-fibrin chemical of ECMO circuit to avoid anticoagulation [32].
ECMO is a modality which can transform patient outcomes when used judiciously. Timing all aspects of the ECMO run is crucial. Stringent monitoring, appropriate and timely trouble shooting strategies, and attention to detail along with teamwork are keys to a successful outcome. ECMO entails considerable investment in infrastructure, highly skilled and dedicated personnel, and substantial costs. The economics of ECMO vary from continent to continent. A center’s core team mix and experience are directly linked to outcomes.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirklin JW, Donald D, Harshbarger H, Hetzel P, Patrick R, Swan H, et al. Studies in extracorporeal circulation. I. Applicability of Gibbon-type pump-oxygenator to human intracardiac surgery: 40 cases. Ann Surg. 1956;144(1):2–8.1. doi: 10.1097/00000658-195607000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286(12):629–34. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett R. Esperanza: the first neonatal ECMO patient. ASAIO J. 2017;63:832–843. doi: 10.1097/MAT.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 4.Surendranath KV, Shetty DP, et al. Long term cardiopulmonary support for children. Indian J Extracorp Technol. 1991;2(3):120. [Google Scholar]
- 5.Pooboni S. ECMO in India, SWAAC ELSO: challenges and solutions. Indian J Thoracic Cardiovasc Surg. 2020;37(S2):344–350. doi: 10.1007/s12055-020-01031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L, Pesenti A, Rossi GP, Vesconi S, Fox U, Kolobow T, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. The Lancet. 1980;316:292–294. doi: 10.1016/S0140-6736(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F, et al. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256:881–886. doi: 10.1001/jama.1986.03380070087025. [DOI] [PubMed] [Google Scholar]
- 8.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 9.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA. 1979;242(20):2193–2196. doi: 10.1001/jama.1979.03300200023016. [DOI] [PubMed] [Google Scholar]
- 10.Davies A, Jones D, Beca J, Blackwell N, Forrest P, Gattas D, et al. The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators*. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 11.Combes A. Extracorporeal membrane oxygenation (ECMO) for severe acute respiratory distress syndrome (ARDS). The EOLIA (ECMO to rescue Lung Injury in severe ARDS) trial: a multicenter, international, randomized, controlled open trial [in French] Reanimation. 2011;20:49–61. doi: 10.1007/s13546-010-0002-8. [DOI] [Google Scholar]
- 12.Raleigh L, Ha R, Hill C. Extracorporeal membrane oxygenation applications in cardiac critical care. Semin Cardiothorac Vasc Anesth. 2015;19(4):342–352. doi: 10.1177/1089253215607065. [DOI] [PubMed] [Google Scholar]
- 13.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;13;322(6):557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 14.Merrill ED, Schoeneberg L, Sandesara P, Molitor-Kirsch E, O’Brien J, Dai H, et al. Outcomes after prolonged extracorporeal membrane oxygenation support in children with cardiac disease–Extracorporeal Life Support Organization registry study. J Thorac Cardiovasc Surg. 2014;148:582–588. doi: 10.1016/j.jtcvs.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care. 2013;17:R73. doi: 10.1186/cc12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith M, Vukomanovic A, Brodie D, Thiagarajan R, Rycus P, Buscher H. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: an analysis of the Extracorporeal Life Support Organization (ELSO) registry. Smith et al. Critical Care. 2017;21:45. doi: 10.1186/s13054-017-1633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta P, Robertson MJ, Beam B, Gossett JM, Schmitz ML, Carroll CL, et al. Relationship of ECMO duration with outcomes after pediatric cardiac surgery: a multi-institutional analysis. Minerva Anestesiol. 2015;81:619–627. [PubMed] [Google Scholar]
- 18.Camboni D, Philipp A, Lubnow M, Bein T, Haneya A, Diez C, et al. Support time-dependent outcome analysis for veno-venous extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 2011;40:1341–1347. doi: 10.1016/j.ejcts.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 19.Staudacher DL, Bode C, Wengenmayer T. Duration of extracorporeal membrane oxygenation is a poor predictor of hospital survival. J Crit Care. 2016;32:207–208. doi: 10.1016/j.jcrc.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36(33):2246–2256. doi: 10.1093/eurheartj/ehv194. [DOI] [PubMed] [Google Scholar]
- 21.Xie A, Phan K, Tsai Y-C, Yan TD, Forrest P. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: a metaanalysis. J Cardiothorac Vasc Anesth. 2015;29:637–645. doi: 10.1053/j.jvca.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Akihiko Inoue MD, Toru Hifumi MD, Tetsuya Sakamoto MD, Yasuhiro Kuroda MD. Extracorporeal cardiopulmonary resuscitation for out- of- hospital cardiac arrest in adult patients. J Am Heart Assoc. 2020;9(7):e015291. doi: 10.1161/JAHA.119.015291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swol J, Belohlavek J, Brodie D, Bellezzo J, Weingart SD, Shinar Z, Schober A, Bachetta M, Haft JW, Ichiba S, et al. Extracorporeal life support in the emergency department: a narrative review for the emergency physician. Resuscitation. 2018;133:108–117. doi: 10.1016/j.resuscitation.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Otani T, Sawano H, Natsukawa T, Matsuoka R, Nakashima T, Takahagi M, Hayashi Y. D- Dimer predicts bleeding complication in out- of- hospital cardiac arrest resuscitated with ECMO. Am J Emerg Med. 2018;36:1003–1008. doi: 10.1016/j.ajem.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Alexandros Cavayas Y, et al. Intracranial hemorrhage in adults on ECMO. Perfusion. 2018;33:42. doi: 10.1177/0267659118766435. [DOI] [PubMed] [Google Scholar]
- 26.Biffi S, Di Bella S, Scaravilli V, Peri AM, Grasselli G, Alagna L, Pesenti A, Gori A. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017;50(1):9–16. doi: 10.1016/j.ijantimicag.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P, Extracorporeal Life Support Organization Task Force on Infections. Extracorporeal Membrane Oxygenation Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;112:277. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt M, Bréchot N, Hariri S, Guiguet M, Luyt CE, Makri R, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55:1633–1641. doi: 10.1093/cid/cis783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makdisi G, Wang IW. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166–E176. doi: 10.3978/j.issn.2072-1439.2015.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kielstein JT, Heiden AM, Beutel G, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 2013;28:86–90. doi: 10.1093/ndt/gfs398. [DOI] [PubMed] [Google Scholar]
- 31.Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: Adult advanced cardiovascular life support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S444–S464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 32.de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, et al. Part 12: Pediatric advanced life support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S526–S542. doi: 10.1161/CIR.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soar J, Nolan JP, Böttiger BW, Perkins GD, Lott C, Carli P, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3 Adult advanced life support. Resuscitation. 2015;95:10047. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Zakhary B, Nanjayya VB, Sheldrake J, Collins K, Ihle JF, Pellegrino V. Predictors of mortality after extracorporeal cardiopulmonary resuscitation. Crit Care Resusc. 2018;20(3):223–230. [PubMed] [Google Scholar]
- 35.Holmberg MJ, Geri G, Wiberg S, Guerguerian A-M, Donnino MW, Nolan JP, Deakin CD, Andersen LW, International Liaison Committee on Resuscitation’s (ILCOR) Advanced Life Support and Pediatric Task Forces Extracorporeal cardiopulmonary resuscitation for cardiac arrest: A systematic review. Resuscitation. 2018;131:91–100. doi: 10.1016/j.resuscitation.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakravarthy M. ECMO - the way to go. Ann Card Anaesth. 2011;14:1–2. doi: 10.4103/0971-9784.74391. [DOI] [PubMed] [Google Scholar]
- 37.Barlett RH. ECMO: the next 10 years. Egyp J Critical Care Med. 2016;4:7–10. doi: 10.1016/j.ejccm.2016.01.003. [DOI] [Google Scholar]
- 38.Dalia AA, et al. Extracorporeal membrane oxygenation is a team sport: institutional survival benefits of a formalized ECMO team. J Cardiothoracic Vasc Anesth. 2018;85:902. doi: 10.1053/j.jvca.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Nwozuzu A, Fontes ML, Schonberger RB. Mobile extracorporeal membrane oxygenation teams: the North American versus the European experience. J Cardiothoracic Vasc Anesth. 2016;30(6):1441–1448.86. doi: 10.1053/j.jvca.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Combes A, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 41.Tseng YH, Wu MY, Tsai FC, Chen HJ, Lin PJ. Costs associated with extracorporeal life support used in adults: a single-center study. Acta Cardiol Sin. 2011;27(4):221–228. [Google Scholar]
- 42.Dennis M, Zmudzki F, Burns B, Scott S, Gattas D, Reynolds C, Sydney ECMO Research Interest Group et al. Cost effectiveness and quality of life analysis of extracorporeal cardiopulmonary resuscitation (ECPR) for refractory cardiac arrest. Resuscitation. 2019;139:49–56. doi: 10.1016/j.resuscitation.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Jäämaa-Holmberg S, Salmela B, Suojaranta R, Lemstrom KB, Lommi J. Cost-utility of venoarterial extracorporeal membrane oxygenation in cardiogenic shock and cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2020;9(4):333–341. doi: 10.1177/2048872619900090. [DOI] [PubMed] [Google Scholar]