Abstract
Lung cancer is the second (11.4%) most commonly diagnosed cancer and the first (18%) to cause cancer-related deaths worldwide. The incidence of lung cancer varies significantly among men, women, and high and low-middle-income countries. Air pollution, inhalable agents, and tobacco smoking are a few of the critical factors that determine lung cancer incidence and mortality worldwide. Reactive oxygen species are known factors of lung carcinogenesis resulting from the xenobiotics and their mechanistic paths are under critical investigation. Reactive oxygen species exhibit dual roles in cells, as a tumorigenic and anti-proliferative factor, depending on spatiotemporal context. During the precancerous state, ROS promotes cancer origination through oxidative stress and base-pair substitution mutations in pro-oncogenes and tumor suppressor genes. At later stages of tumor progression, they help the cancer cells in invasion, and metastases by activating the NF-kB and MAPK pathways. However, at advanced stages, when ROS exceeds the threshold, it promotes cell cycle arrest and induces apoptosis in cancer cells. ROS activates extrinsic apoptosis through death receptors and intrinsic apoptosis through mitochondrial pathways. Moreover, ROS upregulates the expression of beclin-1 which is a critical component to initiate autophagy, another form of programmed cell death. ROS is additionally involved in an intermediatory step in necroptosis, which catalyzes and accelerates this form of cell death. Various therapeutic interventions have been attempted to exploit this cytotoxic potential of ROS to treat different cancers. Growing body of evidence suggests that ROS is also associated with chemoresistance and cancer cell immunity. Considering the multiple roles of ROS, this review highlights the exploitation of ROS for various therapeutic interventions. However, there are still gaps in the literature on the dual roles of ROS and the involvement of ROS in cancer cell immunity and therapy resistance.
Keywords: Reactive oxygen species, Oxidative stress, Cancer therapy, Lung cancer, Programmed cell death
Introduction
The inability of the cells to abide by contact inhibition paving its way toward uncontrolled proliferation causes cancer. Cancer cells can bypass all the checkpoints that are otherwise present in a normal mitotic division and thereby disrupt the functioning of the body. The cancer is named based on the site of origin; thus, lung cancer originates in the cells of the respiratory system. Lung cancer is among the most common ones with an estimation of 2.20 million new cases per year. About 27% of all cancer-related deaths are attributed to lung cancer making it responsible for being the leading cause of cancer-related deaths worldwide. The prognosis for lung cancer remains the poorest of all tumor types owing to the 5-year survival rate of an average of 10–20%. However, survival is particularly determined by the stage at which the diagnosis is made, the earliest stages have a 5-year survival of 92%, whereas the last stages have nil. There are two broad classifications in lung cancer namely—Non-Small Cell Lung Cancer (NSCLC) and Small Cell Lung Cancer (SCLC); the former accounts for 85% and the latter for about 15% of the total diagnoses [1, 2]. Lungs are also a primary metastatic site for cancer of other origins like the breast, skin, pancreas, and liver. 75% of lung cancer is endemic to smoking and the remaining is due to occupational exposure and air pollution [3].
Reactive oxygen species (ROS) are a regular ramification of cellular metabolism and the altered redox state of the cell due to these results in cancer. These are molecules with an extra unpaired electron, superoxide (O2·−) and hydroxyl (OH·), and as the name suggests it is unstable and highly reactive. Superoxides are processed by superoxide dismutase (SODs) to hydrogen peroxide (H2O2) and they further produce hydroxyls either via the Fenton reaction or the Haber–Weiss reaction. Hydrogen peroxide also gets converted to other damaging oxidants like hypochlorous acid and hypobromous acid. ROS can be generated exogenously and endogenously. The exogenous trigger is via exposure to environmental gases like NO2, SO2, CO, and particulate matter in the air like from cigarette smoke, while mitochondria and membrane-bound NADPH oxidases are the main intracellular sources of ROS involved in the signaling cascade. Cancer cells fine-tune the ROS signaling and its scavenging to their comfort. The expected scenario in the cell upon increased ROS production would be to induce oxidative stress and eventual cell death; however, in the cancer niche detoxification of ROS via scavengers like NRF2 (nuclear factor-erythroid 2-related factor 2), PRXs (peroxiredoxins), GPXs (glutathione peroxidases), SODs (superoxide dismutases), and CAT (catalase) help maintain the required ROS for pro-tumorigenic signaling.
Mitochondrial ROS (mROS) predominantly contributes to the cellular ROS with 1% of the total oxygen consumption being utilized in superoxide release. The cancer cells have an increased ROS production which elevates multiple signaling pathways ultimately leading to the transcription of cellular proliferation genes. The prime target of mROS is the inhibition of PTEN (phosphatase and tensin homolog), which leads to incessant activation of the PI3K/AKT pathway, finally resulting in enhanced proliferation and survival. The AKT activation further increases ROS levels promoting cancer cell proliferation and survival. ROS is also known to oxidize and inhibit other phosphatases like the MAPK phosphatases that result in growth factor receptor activation, inducing MAPK/ERK signaling, an established pro-proliferative signaling. MAPK/ERK pathway is specifically made conducive to lung cancer by mROS regulation of Kras-induced anchorage-independent growth of the cells [4]. ROS manipulates tumor cell survival by activating transcription factors like NF-κB and nuclear factor-erythroid 2-related factor (NRF2), which stimulate the expression of antioxidants to avoid ROS-mediated cell death. NRF-2 is negatively regulated by KEAP1 which is inhibited by ROS-mediated oxidation of cysteine residues on KEAP1 protein, thus stabilizing the former transcription factor to increase the transcription of GPXs to maintain the desired ROS balance in cancer cells. It is apparent that the annihilation of the mitochondrial respiratory chain reduces ROS production diminishing tumorigenesis and the knockout of NRF-2 promotes oxidative stress, thus killing the tumor. The cells in the cancer microenvironment thrive on hypoxia, hypoxia-induced angiogenesis, and glycolysis, wherein the role of mROS pitches in by activating and stabilizing HIF-1α an oxygen-sensitive subunit of HIF-1 protein via inhibition of PHD2 activity. In normoxia condition, PHD-2 degrades the HIF-1α, but under hypoxia, HIF-1α dimerizes with HIF-1β and localize to the nucleus to transcribe pro-angiogenic genes like VEGF1, and genes implicated in glycolysis, cell survival, and mobility. Hypoxia upregulates the Serine hydroxy methyltransferase 2 (SHMT2) enzyme, which increases the catabolism of mitochondrial serine to compensate for NADPH. Matrix metalloproteases that degrade the extracellular matrix are upregulated by mROS, and additionally, it also inhibits the activity of TIMP (tissue inhibitor of metalloproteinase). It is also known that mROS specifically targets Src Homology region 2 (SH2)-containing protein tyrosine Phosphatase 2 (SHP-2) and Focal adhesion kinase (FAK), enabling the cell to migrate and prevent cell death by anoikis, matrix detachment-induced apoptosis. Mitochondrial DNA is 5 times more susceptible to mutations via oxidative damage than nuclear DNA owing to its locality in the vicinity of Electron transport chain (ETC) and lack of histone proteins and DNA repair mechanisms. A cytochrome b mutation in mitochondrial complex II of ETC induces metastasis in cancer cells and mROS is comprehensive to all factors required for tumorigenesis from fabricating a tumor favorable microenvironment to metastasis [5, 6].
External factors regulating ROS in the lungs
Lungs are the main organ for gaseous exchange in the body, which makes them more vulnerable to ROS exposures. Factors like lifestyle, disease conditions, and medications are known to influence the ROS levels in the lungs. Lifestyle factors regulate ROS levels in different ways, for example, cigarette smoking and occupational hazards introduce exogenous ROS into the body, while healthy eating habits and supplements help scavenge ROS. Cigarette smoke enters the lungs in two phases: gaseous and tar, containing over 4000 chemical substances, including free radicals and other oxidants. The gaseous phase contains nitric oxide, an oxidant that readily reacts with superoxide (O2·−) to form peroxynitrite (ONOO−). The tar consists of more steady free radicals like the semiquinone radical, which readily reacts with oxygen to form O2·− and·H2O2. Individuals exposed to occupational and environmental hazards, including xenobiotics (pesticides and metals), physical factors (radiation, heat, noise), and biological agents, resulting in the production or exposure of ROS leading to formation of tumors, are depicted in Fig. 1. The occupational situations that are reported to be involved in oxidative stress-related toxicity are textile, metal, cement, pesticide industries, and radiology centers. The prolonged exposure to pollutants and allergens stimulates ROS generation and result in inflammation in the windpipe and asthma [6]. During infection, excessive amounts of ROS are generated in the respiratory tract to fight the invasion of virus and bacteria, but the downside of this defense mechanism is that these ROS needs to be scavenged adequately to avoid tissue damage. Indeed, recent reports suggest that COVID-19 infection and lung cancer are regulated through the ROS/NRF2-mediated mechanism. Medications used for various diseases may also result in ROS generation during the course of their metabolism, which may adversely affect the host cell physiology (Fig. 1). For example, the long-acting beta agonists asthma drug, indacaterol, enhances H2O2-induced ROS generation in THP1 cells. Doxorubicin (Dox) is an example of a drug generating ROS as an intermediate metabolite in the host system. Dox can be readily reduced to semiquinone by the mitochondrial enzymes, which in turn generate superoxide anion and H2O2. It is noteworthy that the ability of the drugs to induce ROS in host cells was exploited for anticancer and other treatment strategies. Another important lifestyle factor in modern-day life is physical exercise. Reports suggest that heavy physical workouts augment free radical (ROS) generation through the mitochondrial metabolism of skeletal muscles and other tissues, causing oxidative injury in the corresponding tissues. Certain diets and supplements can also operate the ROS levels in the human system. Vitamins E, C, and beta-carotene are considered major choices of antioxidants that are supplemented via diet in addition to the endogenous antioxidants. Antioxidants, in general, tend to diminish ROS and protect the host cells from oxidative injury and other adverse conditions [7].
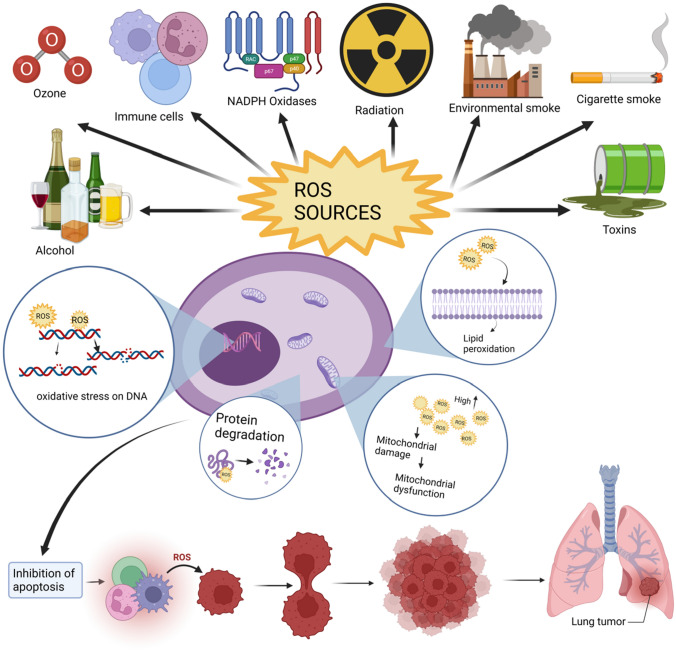
Fig. 1.
An overview of various sources and ill effects of ROS: Exogenous sources of ROS include radiation, environmental smoke, industrial toxins, alcohol, ozone, heavy metals, and cigarette smoke, as well as endogenous sources such as mitochondria, phagocytotic cells, lipids, protein, -oxidation of fatty acids in peroxisomes, cancer cells, and enzymes such as cytochrome p-450 and thiol oxidase in the endoplasmic reticulum [8]
ROS/oxidative stress leads to cancer, metastasis, and invasion
ROS can readily react with DNA bases to form DNA adducts, which leads to miscoding during replication. This may lead to several irreversible mutations in the genome, especially when they occur in oncogenes or tumor suppressor genes; it leads to cancerous state [9]. The oncogenes, K-Ras, Jun, and Myc and the tumor suppressor genes TP53, CDKN2A, and STK11 are the most commonly reported moieties to carry mutations in cancer patients. Moreover, lipid and protein peroxidation by the oxidants can also induce more free radicals and enhance the chances of carcinogenesis through DNA mutations. Chronic pulmonary obstructive disease is an inflammatory disease caused by free radicals released from tobacco smoking and has more possibilities for genotoxic changes and adds a 4.5-fold risk of developing lung cancer [1, 10]. When the cells have sufficient irreversible damage in their crucial genes, they attain a survival advantage state, which helps them to proliferate continuously, evading programmed cell death. These specific events are collectively known as cancer initiation and promotion [9, 11, 12]. ROS has been implicated in the regulation of integrins and other extracellular matrix proteins, which in turn regulate cellular migration and adhesion. This property of ROS has been investigated by several researchers with an interest in cancer cell migration and invasion. Although the exact mechanism of regulation remains to be elucidated, there is a growing body of evidence to suggest that integrin, an important cell anchoring protein, when interacting with antibodies or extracellular matrix proteins, produces cellular ROS through mitochondrial metabolism. Furthermore, it activates several oxidases, including NOX, 5-LOX, and COX-2; and Rac-1, a rho GTPase protein, which is involved in ROS-mediated actin cytoskeleton rearrangement required for cell migration. Moreover, ROS regulates stabilization and destabilization of VE-cadherin junctions, which in turn regulate cell–cell adhesion, leading to permeability change, migration, and proliferation. Breaking of extracellular matrix (ECM) by the cancer cells is termed as invasion, which is crucial for metastasis [13–15]. There are several proteases involved in degradation of ECM, of which matrix metalloproteinases, cathepsins, and urokinase plasminogen activator are relevant in cancer context. ROS has been associated with abnormal upregulation of these inhibitors through NF-kB and MAPK pathways. ROS can trigger SNAIL and promote epithelial-to-mesenchymal transition (EMT), and SNAIL has also been shown to increase ROS levels in prostate cancer cells [18, 20]. Interestingly, ROS and TGF-β signaling are interconnected during EMT. When TGF-β is stimulated, ROS increases the phosphorylation of Smad2 and p38, which upregulates α-SMA and fibronectin, respectively, and ERK1/2 activation leads to E-cadherin repression [16, 17]. Together, it is apparent that ROS regulates cancer cell metastasis and invasion through various signaling molecules.
Effect of ROS on cancer stem cells
Cancer stem cells (CSCs) can self-renew and differentiate into many lineages, like normal stem cells. This helps in accelerating tumor growth and heterogeneity. It is also possible for CSC to develop from differentiated cancer cells as a result of adaptation to the microenvironment and therapeutic pressures; such factors are responsible for their heterogeneity. Because of their high resistance, CSCs are resistant to conventional cancer treatments, which lead to metastasis and recurrence of cancer as well as the possibility of carcinogenesis due to EMT malfunction. This epithelial–mesenchymal transition (EMT) is important for embryonic development and the formation of various tissues or organs [18]. Based on their respective biological functions, EMTs can be divided into three distinct classes. Type 1 refers to Epithelial–Mesenchymal transition associated with embryo implantation, formation and development of the organ; type 2 corresponds to tissue regeneration, organ fibrosis and wound healing and type 3 tell how EMTs occur within tumor cells that have already undergone genetic and epigenetic changes. These types of EMT are essential for the progression of cancer and cancer stem cells.
The tumor microenvironment is typically rich in proteins like the growth factor TGF- β and cytokines that activate the pathway that affects the longevity of CSCs [19]. Based on this analysis, the researchers concluded that cytokines and growth factors increased cancer stem cells' flexibility and EMT properties. Apart from the role of the tumor niche, the researchers have also observed lower levels of ROS in cancer stem cells, this made the CSCs more sensitive to radiotherapy and minimized their ability to clone themselves. The excess level of ROS has increased oxidative stress that triggers cancer cell death and also damages protein, lipids, and DNA, which are regarded as oncogenic since they result in instability of the genome [20]. Regulation of ROS can be understood through the following example: in gastric cancer, a variant of CD44 (adhesion molecule) can protect CSCs in the membrane-proximal region by using insertion in alternative splicing; in the human liver, CD13 negatively regulates ROS that increases the ability of stem cells in it. And it has been observed that ROS has a specific effect on Cav-1 expression, motility of cells and also it helps in the migratory process with the help of Akt signaling in the non-small-cell lung cancer cell line [21].
Reports suggest that low concentration of ROS is necessary for CSCs, whereas a higher concentration of ROS promotes tumor growth. Research has shown that when transplanting CD24 and CD49 in mouse mammary CSCs, ROS levels were low and also, the CSCs were able to adhere and develop a novel tumor and it was demonstrated in head and neck tumors as well [22]. Together, for maintaining the prominent features of CSCs, ROS has a crucial role in redox control, cell signaling, also in therapeutic targets.
In human lung cancer A549 cells, Tan-IIA induces the ROS and decreases the mitochondrial membrane potential. Hence, it is mitochondrial dependent to increase apoptosis in Tan-IIA pathways in human A549. As TanIIA increases the production of ROS, it results in generation of cytochrome c that triggers apoptosis [22, 23]. In cancer cells, to increase the expression of CSCs and EMT, activation of the PI3K/AKT/mTOR pathway is needed as it triggers glycolysis to increase ROS levels. Since mTOR is needed for tumorigenic properties in ovarian CSCs. AKT also regulates ROS levels, Cellular longevity, and metastasis through signaling via the FOXO family of transcription factors. The production of manganese superoxide dismutase/superoxide dismutase-2 (Mn SOD/SOD2) also plays an important role for this process. Metastatic progression and invasion of markers are thought to catalase SOD2 in cancer such as colon, lung squamous, and prostate carcinoma. In addition, over expression of SOD2 induced mitochondrial ROS and HIF2 activity, as well as CSC formation, resulting in increased tumor invasiveness and poor prognosis for lung cancer patients. Activation and expression of Notch pathway components are related to insufficient outcomes and resistance to radiation or chemotherapy. The signaling cascade improves CSC’s drug resistance and proliferation by stimulating angiogenesis and EMT. Notch signaling also regulates CSCs by interfering with signaling protein as demonstrated by HER2 that promotes CSCs multiplication and proliferation in lung cancer. By upregulating AKT, Notch activates AKT in a manner that is independent of transcriptional regulation. Notch also increases ROS scavenging enzyme expression, resulting in low ROS levels [22, 24].
ROS in cancer tumor microenvironment
It is generally known that the tumor microenvironment (TME), which is made up of a wide variety of cell types, including numerous immune cells, cancer cells, and fibroblasts linked with cancer, produces a significant amount of ROS [11]. In lung tissues, benzo(a)pyrene (BaP), which is widely regarded as the substance that causes cancer at a higher rate than any other substance, triggers a cascade of chronic oxidative stress and inflammation and also increases the likelihood of mutation in certain genes, which may eventually result in the development of cancer. NF-κB, a transcriptional factor that is activated by oxidative stress, is implicated in triggering lung cancer through stimulation of the inflammatory cascade. It is reported that the inflammasome NOD-like receptor protein 3 (NLRP3), a type of intracellular multiprotein complex found in the microenvironment of a tumor, has been linked to the development of chronic inflammation in patients with lung cancer induced by benzo(a)pyrene (BaP) [25]. The formation of NLPR3 is responsible for the maturation and release of inflammatory cytokines in the microenvironment of a tumor. These cytokines, which promote cancer progression and are also responsible for immunological tolerance, are accountable for both of these processes [26]. One of the main factors contributing to the TME's resistance to immunotherapy, particularly immune checkpoint blockades, is the high levels of ROS [27, 28]. The CD4 + Foxp3 + regulatory T (Treg) cells are enrolled into the tumor microenvironment and act as potent immune suppressors, according to recent findings [29, 30]. Treg cells that have migrated into tumors experience apoptosis due to the elevated ROS levels in the TME. Notably, tumor-infiltrating apoptotic Treg cells reduce the ATP to adenosine, to suppress the immunity of programmed death ligand 1-blockade-mediated antitumor T-cells. These cells are extremely sensitive to ROS because of their weak NRF2 linked with the antioxidant defense system. It is significant to observe that dead Treg cells have a stronger immunosuppressive effect than living Treg cells [28]. This suppression of ROS can result in improved cancer immunotherapies. In fact, a new nano-scavenger that was anchored to the extracellular matrix prevented the apoptosis of suppressive immune cells by removing ROS [31].
Dual roles of ROS
ROS levels in noncancerous cells are closely regulated by balance between ROS production and scavenging, which is mainly caused by cellular respiration, and antioxidant levels, which are predominantly maintained by intracellular pools of glutathione and NADPH [32].
ROS act as signaling molecules/secondary messengers at low levels, regulating cellular and differentiation proliferation, inflammation associated with tissue maintenance, adaptive and innate immunity and aging, among other biological processes crucial for sustaining physiologic function (15) (Fig. 2). ROS have high reactivity and so play an essential function in a cell's redox balance. Increased amounts of ROS, on the other hand, can cause oxidative stress, resulting in damage to particular biomolecules like lipids, nucleic acids, and proteins and eventually death [33]. Apart from inducing cancer, ROS is also held responsible for the inhibition of cancer. This occurs due to extremely high levels of ROS accumulation above a cytotoxic threshold, which triggers the apoptotic pathways, ultimately leading to cancer cell senescence.
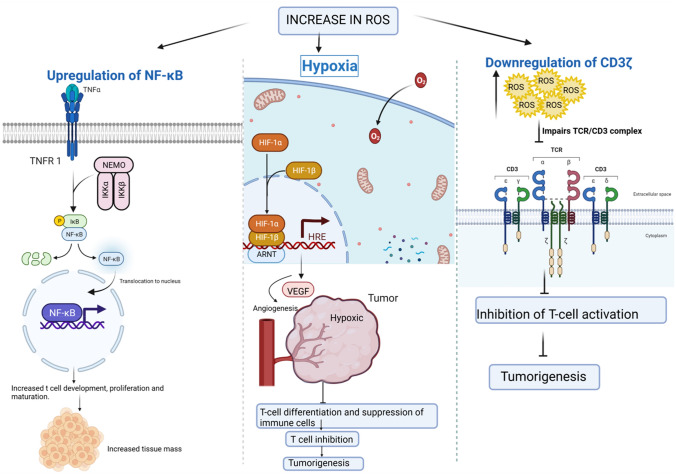
Fig. 2.
Increased ROS causes NF-κB transcription to be upregulated, resulting in increased tissue mass. As a result, tissues become hypoxic, triggering angiogenesis and the release of VEGF. However, excessive levels of VEGF lead to T-cell activation and tumor development [9, 13, 15]
As reported, overexpression of ROS causes normal cells to turn into cancerous cells, which activates oncogenes to promote tumorigenesis. ROS oxidizes various entities of the biomolecules, in this case, the bases of the nucleotide. This leads to the formation of various kinds of oxidation products which facilitate damage of the biomolecules via mutation and bond breaking. The ROS oxidizes guanine to form 7,8-dihydro-8-oxo-2-deoxyguanosine, causing G to mispair with T instead of C, while deoxyguanosine triphosphate is also oxidized to form 8-oxo-deoxyguanosine as a substrate, which causes A to mispair with C instead of T. When ROS oxidizes with adenine, there are two possible substrates: (i) 2-hydroxy-2-deoxyadenosine, which mispairs A with C, A with G, and A with T, and (ii) 7,8-dihydro-8-oxo-2-deoxyadenosine, which mispairs A with G, and A with C [34]. Exposure of purines and pyrimidines caused by hydrogen bond breakage, unfolding, double-, and single-strand breakage of DNA, facilitates ROS oxidation, thus resulting in DNA mutation and mispairing. Another way through which ROS damages DNA is by directly attacking and repressing the DNA repair system. This occurs when ROS attacks human 8-Oxoguanine DNA N-Glycosylase 1 (hOGG1) by oxidizing it. The hOGG1 is responsible for the elimination of 8-oxo-guanosine. So, in this case, the oxidation of hOGG1 makes 8-oxo-guanosine sticks around, which makes the turnover of epithelial cells faster. Colorectal carcinogenesis follows the very same mechanism [35].
T-cell death is induced due to high levels of ROS. This causes repression of T-cell differentiation, maturation, and activation. When ROS is increased, NF-κB is upregulated, causing T-cell development, maturation, and proliferation, resulting in more tissue mass. These new regions are in hypoxia conditions. This triggers HIF-1α induction to stimulate the release of vascular endothelial growth factor, hence resulting in angiogenesis [36]. When VEGF is overproduced, it blocks and represses T-cell development and migration, in turn causing immunodeficiency. An increase in the ROS downregulates the CD3 expression, thereby inactivating T-cells by suppressing immune responses. The immunosuppressive cells present in the tumor regions over express ROS to inhibit T-cell activation, thus promoting tumor metastasis. The myeloid-derived suppressor cells in the tumor region increase the count of CD8 + and CD4 + as a response to the defense mechanism of the above. This is again taken care of by ROS by releasing an apoptosis-inducing factor, ultimately leading to T-cell death.
Increased levels of ROS downregulate NK cell function by repressing CD16ζ expression, thereby abating the cytotoxicity of NK cells. The mechanism is similar, but with a slight difference, in various forms of carcinomas. In breast cancer, high amounts of ROS reduce the release of cytotoxic factors by NK cells by downregulating the expression of NK group 2 and its ligands, hence promoting cancer cell growth and metastasis. NK cells facilitate the elimination of malignant cells [37]. In melanoma, ROS spike causes decrease in the production of IFN-γ in the NK cells, which results in melanoma metastasis. ROS elevation also induces apoptosis leading to the death of NK cells. In chronic myelomonocytic leukemia, the cells themselves release ROS, which causes the death of NK cells, leading to the metastasis of chronic myelomonocytic leukemia. Apoptosis of NK cells in the liver occurs due to the high production of ROS in the mitochondria. This results in tumor growth leading to colorectal carcinoma.
Higher levels of ROS cause an increase in the production of NF-κB. This downregulates E-cadherins causing dissociation of the cell–cell junctions. This initiates endothelial–mesenchymal transition (EMT), which facilitates cancer cell invasion and metastasis (Fig. 3). Apart from NF-κB, ROS can also interact with TGF-β, PI3K/Akt, NRF2, HIF-1α signaling pathways to cause cell–cell disruption resulting in EMT [10].
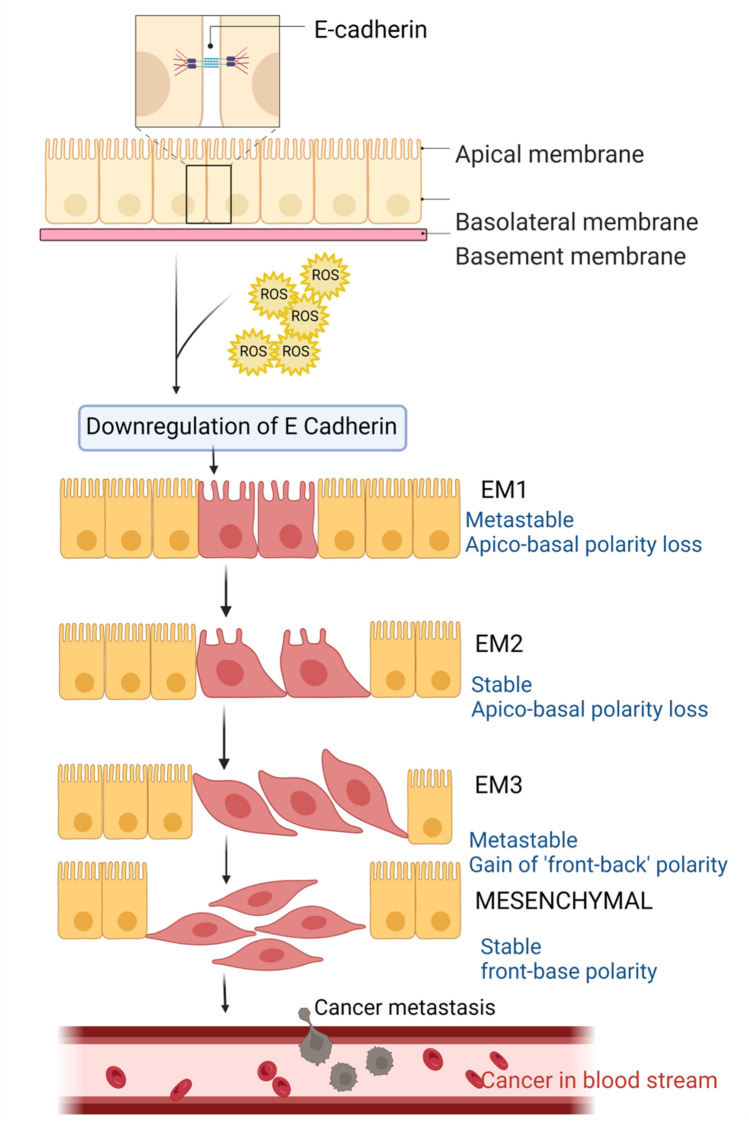
Fig. 3.
Epithelial–mesenchymal transition (EMT) in cancer metastasis: Down-regulation of E-cadherin results in loss of adherence. This results in a loss of apical-basal polarity and mesenchymal transition; thus, motility is increased and cancer metastasis is facilitated [18, 20, 22]
ROS inhibits the proliferation signaling pathway. An increase in the ROS production downregulates epithelial growth factor and its expression. This inhibits the phosphorylation of extracellular signal-regulated kinase, which leads to the decreased production of EGF and EGFR, ultimately inhibiting cancer cell growth. Ascorbic acid (sourced from vitamin C) and koumine are known to promote ROS production. They are also known to inhibit ERK phosphorylation, suppressing cancer cell carcinoma [38, 39]. Non-small cell lung cancer cells proliferation is inhibited when ROS blocks the PI3K/Akt/NF-κB signaling pathway [10, 40]. ROS buildup downregulates the production of cyclins and cyclin-dependent kinases and stops cancer cell proliferation, since CDKs and cyclins are responsible for the promotion of mitotic cell cycle of somatic cells. In multiple myeloma, ROS downregulates JK1 and JK2 to block cyclin D, B1, E, CDK2, and CDK4 to stop the cancer cell cycle and growth [9, 18]. If ROS is overexpressed in human non-small cancer cells, CDK1 is phosphorylated and cyclin B1 and CDK1 expression is suppressed. This causes arresting of G2/M phase in cell cycle [41]. Researchers found that Physalin A (PA) inhibits G2/M cell cycle progression in A549 cells by causing ROS to accumulate excessively in their cells. This effect was attributed to Physalin A's inhibition of the p38 MAPK/ROS pathway [25].
ROS induces tumor cell apoptosis. ROS promotes the release of Ca2 + ions from the lumen of the endoplasmic reticulum, which causes defects such as protein misfolding and/or unfolding. Overproduction of ROS causes phosphorylation of eIF2α. This activates transcription factor IV, inducing C/EBP homologous protein which leads to apoptosis. High levels of ROS accumulation cause it to oxidize cardiolipin (phospholipid), which causes cytochrome c (Cyt c) to detach from the outer surface of the mitochondrial membrane. The freely suspended Cyt c will now interact with the apoptotic protease activating factor 1 and forms an apoptosome. This in turn activates caspase-3 and caspase-9 to initiate apoptosis. In gastric cancer cells, α-heredin is known to trigger overproduction of ROS. In the A549 cell line, Atractylodin (ATR) which is known for its antitumor activities-induced apoptosis by upregulating ROS production by regulating STAT, MAPK, and NF-κB pathways and inhibits the G2/M phase of the cell cycle by mediating the AKT signaling pathway [42].
ROS enhances the transcription effects of P53 and then translocate it to the mitochondria. ROS accumulation can also damage cancer cell DNA leading to apoptosis. Mutations of P53 cause cancer. ROS accumulation can reverse and restore the normal functions of the mutated P53 in cancer cells. Occurrence of lung cancer is associated with various kinds of cell death, one of which is ferroptosis [43]. In ferroptosis, cell death occurs with the help of iron where the ROS is of lipid type. Ferroptosis significantly targets only cancerous cells due to its iron dependency. High amounts of ROS are accumulated due to faulty metabolism of iron along with the lack of natural antioxidants in the cells. This leads to lipid peroxidation followed by ferroptosis [44]. In a recent study, it was discovered that hemin promotes the growth of noncancerous lung cells while increasing the production of reactive oxygen species (ROS) that cause lipid peroxidation and ferroptosis in lung cancer cells [45].
ROS balance and homeostasis in cancer cells
Cancer cells have developed a reliable ROS detoxification system. As a result, cancer cells' reliance on antioxidant systems provides a unique vulnerability which must be targeted to cause targeted cell death. This is achieved by elevating oxidative stress level above the toxicity threshold, sparing the normal cells, which are distinguished by lower intracellular ROS levels. ROS serve as both 'good' and 'bad' molecules, regulating cellular physiology or causing cytotoxicity, relying on the duration, quantity, and location of their formation. The methods of ROS detoxification can be directly supported by malignant cells [46]. High doses of vitamin C (antioxidant) cause colon cancer cell death by increasing ROS levels [39]. Lung cancer progresses swifter when antioxidants like N-acetylcysteine are consumed and increasing Nrf2 gene expression causes accelerated lung tumor growth [47]. The methods of ROS detoxification can be directly supported by malignant cells.
In glycolysis, the pro-glycolytic shift caused by the activation of oncogenes and the loss of tumor suppressors gives tumors a selective advantage by giving them the building blocks they need to make the macromolecules that keep them growing and spreading. Since glycolytic intermediates can be transported into the metabolic pathways that directly or indirectly produce reducing equivalents, primarily PPP-derived NADPH or glutaminolysis-derived GSH, glucose metabolism is crucial in the regulation of redox homeostasis in malignancies. In order to reduce the load of ROS and avoid cell death by hydroperoxide, cancer cells enhance their glucose metabolism. By lowering ATP at intracellular levels and inhibiting the lactate dehydrogenase-A by the small drug FX11 prevents the growth of cancer cells.
In cancer cells, activation of pentose phosphate pathway constitutes a major feature, as this pathway synthesizes nucleotides, which is required during cell reproduction. PPP-dependent production of NADPH is enhanced by the regulation of glucose-6-phosphate dehydrogenase (G6PD). The availability of glucose may influence G6PD regulation: glucose funneling into the oxidative branch of PPP directly regulates the redox balance of human renal cell carcinoma cells (Fig. 4). The serine–glycine one-carbon metabolism (SGOC) is a network of biochemical reactions in which amino acids and their derivatives are converted into multiple outputs as carbon units that serve various cellular functions. These carbon units from glycine and serine depend on three different pathways: the folate cycle, the methionine cycle, and the trans-sulfuration pathway [20]. Recent evidence indicates that this pathway also plays a crucial role for redox balance [48].
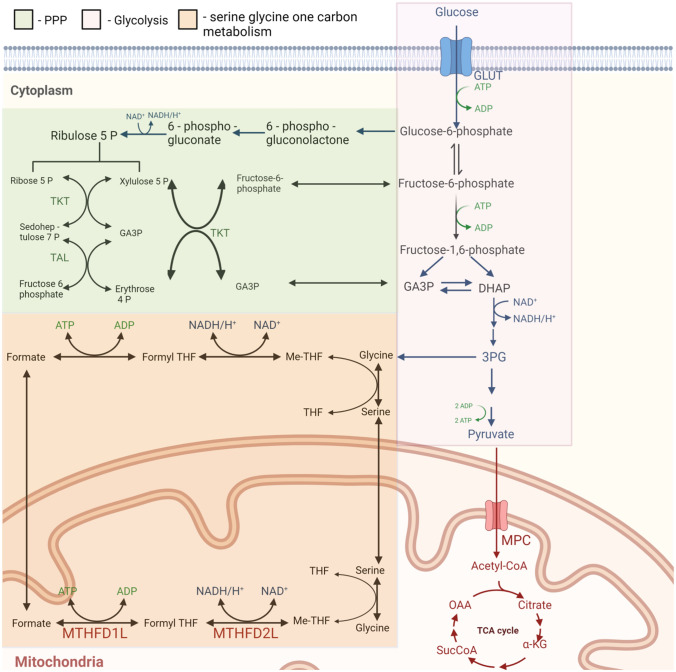
Fig. 4.
ROS Balance and Homeostasis. Metabolic pathways such as PPP, glycolysis, and serine-glycine one carbon involved in ROS balance and homeostasis in cancer cells [27, 48]
ROS-mediated cancer therapy
Roles of ROS in cancer chemotherapy
To treat cancer patients in a clinical context, chemotherapy has been widely employed. A majority of chemotherapy drugs generate ROS, and several of them can change the redox balance in cancer cells [12]. The main medications that enhance ROS in cancer cells are alkylating agents, camptothecins (irinotecan and topotecan), anthracyclines (daunorubicin, epirubicin, and doxorubicin), and platinum coordination complexes (cisplatin, oxaliplatin, and carboplatin) [49]. The two main causes of the increase in ROS in response to chemotherapeutic drugs are the production of ROS by mitochondria and the suppression of the cellular antioxidant system. For instance, cisplatin, one of the most popular and successful chemotherapy drugs for treating lung cancer, causes mitochondria-dependent ROS that cause nuclear DNA damage and ultimately lead to cell death. Because of the direct impact of cisplatin on mitochondrial DNA, there is an increase in ROS production that impairs the production of proteins for the electron transport chain.
ROS are crucial in the development of multidrug resistance. One of the main causes of chemotherapy's failure in the treatment of cancer is such resistance [50]. The plasma has P-glycoprotein (P-gp) and other transporter-based efflux pumps and these pumps are closely linked to drug resistance [15]. P-gp, a member of the large ATP-binding cassette protein family, is encoded by the MDR1/ABCB1 gene. P-gp plays a defensive role for the uptake of chemotherapeutic drugs [51]. NRF2 is turned on and overexpressed in cancer cells as a defense against too much ROS, as was previously mentioned. A NRF2 target gene called MDR1/ABCB1 is involved in the upregulation of P-gp and drug resistance in cancer cells [52]. In conclusion, elevated production of ROS in response to therapy is essential for destroying the cancer cells and this also plays an important role in drug resistance.
ROS-mediated programmed cell deaths pathways
As previously mentioned, cancer cells have higher ROS levels compared to non-malignant cells. Therefore, antioxidant enzyme activity increases in malignant cells to negate the detrimental effects of excess ROS production. Indeed, the disruption of this delicate balance has been of interest as potential anticancer interventions because either increasing ROS generation and/or decreasing antioxidant activity may result in activating of cell death pathways. Some of the key cell death pathways induced through the manipulation of ROS levels will be discussed below. Table 1 explains the anticancer compounds/drugs widely used for eliminating the cancer cell via modulating ROS generation.
Table 1.
Various compounds exhibiting anticancer potential through ROS-mediated programmed cell death pathways
| Cell death pathway induced | Stimulus | ROS type | Model system | References |
|---|---|---|---|---|
| Apoptosis | 3,3′-OH curcumin | H2O2 | HepG2 | [67] |
| Apoptosis | 6-gingerol | ROS | U937 & K562 | [68] |
| Apoptosis | Arctigenin | H2O2 & O2· − | MDA-MB-231 | [69] |
| Apoptosis | Arsenic trioxide | O2· − | HPF | [70] |
| Apoptosis | Artesunate | ROS | TE671 & RD18 | [5] |
| Apoptosis | Butein | ROS | HeLa | |
| Apoptosis | H2O2 & O2· − | HepG2 & Hep3B | [71] | |
| Apoptosis | Cannabidiol | ROS | Molt-4 &Jurkat | [72] |
| Apoptosis | Capsaicin | H2O2 | NB4 & Kasumi-1 | [73] |
| Apoptosis | ROS | HepG2 | ||
| Apoptosis | H2O2 & O2· − | BxPC-3 & AsPC-1 | ||
| Apoptosis | ROS | Jurkat | ||
| Apoptosis | Carnosic acid | ROS | IMR-32 | [74] |
| Apoptosis | ROS | Caski | ||
| Apoptosis | ROS | HCT116 | ||
| Apoptosis | Carnosol | ROS | HCT116 | [75] |
| Apoptosis | CAY10598 | ROS | HCT116 | [76] |
| Apoptosis | Celastrol | ROS | B16 | [77] |
| Apoptosis | Cepharanthine | ROS | H1299 & A549 | [1] |
| Apoptosis | Curcumin | H2O2 & O2· − | HuT-78 | [8] |
| Apoptosis | Curcumin | O2· − | HCT-116 | |
| Apoptosis | Curcumin | ROS | L929 | |
| Apoptosis | FTY720 | ROS | Jeko& Mino | [4] |
| Apoptosis | Gambogic acid | ROS | MDA-MB-231 | [78] |
| Apoptosis | Glucocorticoid | H2O2 | WEHI7.2 | |
| Apoptosis | Gossypol | ROS | COLO205 | [79] |
| Apoptosis | Isoliensinine | ROS | MDA-MB-231 | [80] |
| Apoptosis | LZ-106 | O2· − | H460 & A549 | [81] |
| Apoptosis | Methylglyoxal | O2· − | Jurkat | [82] |
| Apoptosis | Nimbolide | H2O2 | MG63 | [83] |
| Apoptosis | Patulin | O2· − | HCT116 & HEK293 | [28] |
| Apoptosis | Piperlongumine | H2O2 | EJ & U2OS | [84] |
| Apoptosis | Plumbagin | H2O2 & O2· − | A375.S2 | [85] |
| Apoptosis | Plumbagin | ROS | EL4 | |
| Apoptosis | Resveratrol | ROS | SGC7901 | [86] |
| Apoptosis | ROS | SUDHL4 & HBL-1 | ||
| Apoptosis | O2· − | U937 | ||
| Apoptosis | Rotenone | H2O2 | PC12 | [87] |
| Apoptosis | Salinomycin | ROS | PC3 | [88] |
| Apoptosis | Salvicine | H2O2 | HeLa | |
| Apoptosis | Sanguinarine | H2O2 | Jurkat& Molt-4 | |
| Apoptosis | Shikonin | O2· − | HL60 | [89] |
| Apoptosis | Sodium selenite | ROS | HepG2 | [16] |
| Apoptosis | Sodium selenite | ROS | HCT116 & SW480 | |
| Apoptosis | TRAIL | O2· − | Jurkat | |
| Apoptosis | Thymoquinone | ROS | MCF-7 | [90] |
| Apoptosis | H2O2 & O2· − | BC1 | ||
| Apoptosis | Wogonin | H2O2 | Jurkat | |
| Apoptosis | H2O2 | HepG2 | [91] | |
| Apoptosis | Zebularine | O2· − | Calu-6 | [3] |
| Apoptosis | Zerumbone | ROS | K562 | [92] |
| Autophagy/Apoptosis | Calyxin Y | H2O2 | NCI-H460 | [2] |
| Apoptosis/Autophagy | Cannabidiol | ROS | MDA-MB-231 | [93] |
| Autophagy/Apoptosis | Carnosol | ROS | MDA-MB-231 | [94] |
| Apoptosis/Autophagy | Celastrol | ROS | HOS & MG-63 | [95] |
| Apoptosis/Autophagy | Colistin | ROS | N2a | [96] |
| Autophagy/Apoptosis | Compound K | ROS | HCT-116 | [97] |
| Apoptosis/Autophagy | FTY720 | ROS | U266 | [98] |
| Apoptosis/Autophagy | Gambogic acid | ROS | T24 | [99] |
| Apoptosis/Autophagy | Isoorientin | ROS | HepG2 | [100] |
| Autophagy/Apoptosis | KIOM-C | ROS | HT1080 | [101] |
| Apoptosis/Autophagy | MomordinIc | H2O2 | HepG2 | [102] |
| Apoptosis/Autophagy | Plumbagin | ROS | PC-3 & DU145 | [103] |
| Apoptosis/Autophagy | Resveratrol | ROS | OVCAR-3 & Caov-3 | |
| Apoptosis/Autophagy | Sanguinarine | H2O2 | U87MG & U118MG | |
| Apoptosis/Autophagy | Sodium selenite | ROS | A549 | [17] |
| Autophagy | 2-methoxyestradiol | O2· − | U87MG & HEK293 | [60] |
| Autophagy | Bufalin | ROS | HT-29 & Caco-2 | [104] |
| Autophagy | Ciclopirox | ROS | Rh30 & RD | [105] |
| Autophagy | Cucurbitacin B | O2· − | HeLa | [106] |
| Autophagy | ROS | MCF-7 | ||
| Autophagy | Curcumin | ROS | ||
| Autophagy | Dichloroacetate | ROS | HT29 & HCT116 | [107] |
| Autophagy | Dihydroartemisinin | ROS | BxPC-3 & PANC-1 | |
| Autophagy | Rotenone | O2· − | U87MG & HEK293 | |
| Autophagy | Salinomycin | H2O2 | SW620 & RKO | [108] |
| Autophagy | Sodium selenite | O2· − | U87MG & T98G | [109] |
| Autophagy | Ursolic acid | ROS | U87MG | [110] |
| Apoptosis/Necrosis | Auranofin | O2· − | HeLa | |
| Necrosis | Salinomycin | ROS | U251MG | [111] |
| Necrosis | Sodium selenite | H2O2 & O2· − | Jurkat& J774.2 | |
| Necroptosis | Dimethylfumarate | ROS | CT26 | |
| Necroptosis | Lycorine | ROS | ARH-77 |
ROS and apoptosis
The most common and well characterized form of cell death is apoptosis, which is also known as type I programmed cell death. Caspases, a distinct family of aspartate-directed cysteine-dependent proteases, are responsible for both starting and finishing this process (Fig. 2). There are two major pathways for apoptosis, the extrinsic (death receptor-dependent) and the intrinsic (mitochondrial) mechanisms and ROS have been found to play a major role in both mechanisms [13].
Through the binding of death-inducing ligands like tumor necrosis factor-related apoptosis-inducing ligand (TRAIL-R1/2), Fas (FasL) and tumor necrosis factor receptor 1 to their corresponding receptors like TNF (TNFR), Fas (FasR) and death receptors (DR4 and DR5), ROS has been shown to initiate the extrinsic pathway of apoptosis. Adaptor proteins such as procaspases 8 and 10 and Fas-associated protein with death domain (FADD) are recruited to the cytoplasmic surface to form death-inducing signaling complexes when transmembrane death receptors are activated (DISCs). Following, caspases 8 and 10 are activated and this causes apoptosis by directly activating effector caspases [53] Cellular FLICE-inhibitory protein (c-FLIP) is one of the proteins that helps ROS initiates the extrinsic apoptosis. Pro-caspases and this protein compete with each other to attach to the adaptor protein, which prevents the creation of DISCs and induces apoptosis as a result. It has been demonstrated that ROS adversely control c-FLIP through two different ways, (i) the nitric oxide is negatively regulated by oxygen (O2), which stops S-nitrosation and subsequently enables ubiquitin-proteasomal destruction of c-FLIP [54], (ii) ROS sets c-FLIP up for ubiquitination and phosphorylation and this leads to the destruction of subsequent proteasomes.
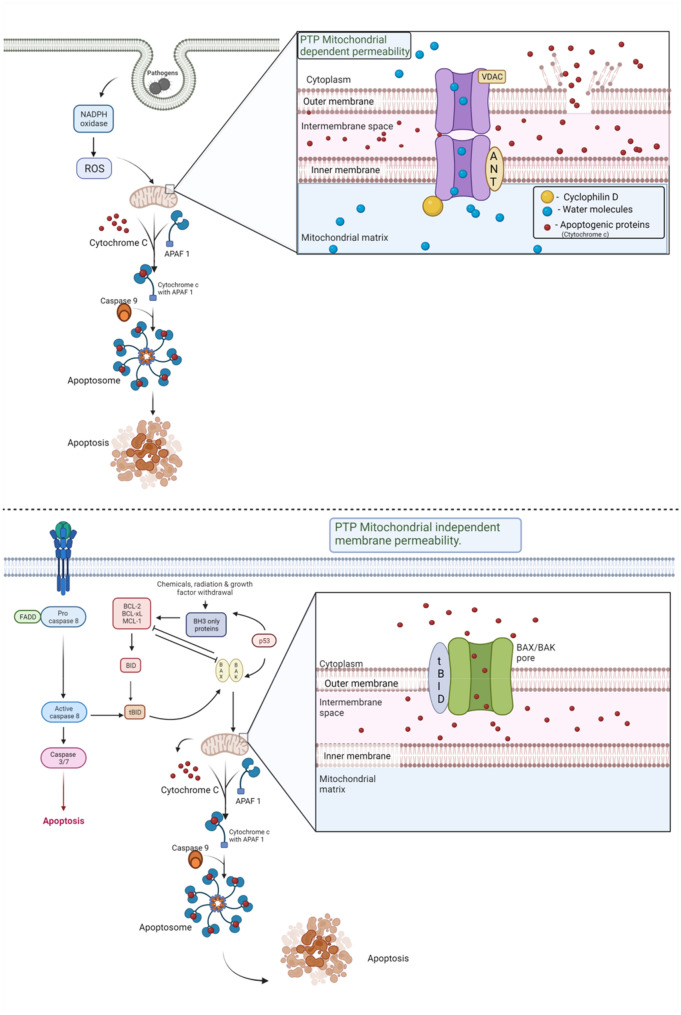
The intrinsic pathway of apoptosis is also referred to as the mitochondrial pathway. Indeed, the [54] mitochondria are a major source of ROS generation and mitochondria contribute a major function in initiating apoptosis. Due to this, mitochondria also often considered as a target of ROS. During apoptosis, the mitochondrial membrane becomes more permeable and release pro-apoptotic factors through the mitochondrial permeability transition pore (MPTP). Apoptosis-inducing factor (AIF), second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO), and cytochrome c (Cyt-c) are few of the substances released from the cytoplasm [9]. When cytochrome-c enters the cytosol, it forms a complex with apoptotic protease activating factor 1 (APAF1), which creates an apoptosome and activates caspase-9 [55]. A crucial component of intrinsic pathway, caspase-9 triggers the caspases 3, 6, and 7, which causes the breakdown of cellular proteins and induces apoptosis [56]. Additionally, in the mitochondria, Cardiolipin and Cyt-c form a combination that prevents Cardiolipin from being released into the cytosol. H2O2 inhibits this complex by oxidizing cardiolipin, which lowers its affinity for Cyt-c and permits its release into the cytosol [57]. Also, the ratio of pro-apoptotic Bcl-2 proteins (such as Bax, Bid, and Bad) to anti-apoptotic Bcl-2 proteins (such as Bcl-2 itself, Mcl-1 and Bcl-xL) controls intrinsic apoptosis [55]. (Fig. 5) Once again, ROS controls this equilibrium by directly oxidizing Bcl-2 at Cys229 and Cys158 by H2O2, which eliminates its anti-apoptotic activity, decreases the Bax and Bad ubiquitination and increases the Bcl-2 ubiquitination by O2 [12, 24].
Fig. 5.
An overview of apoptosis: The mitochondrial-mediated apoptosis can be permeability transition pore dependent or permeability transition pore independent [1, 56]
ROS and autophagy
Autophagy, also referred to as type II programmed cell death, is a process of self‐digestion which occurs in the lysosome and aims to recycle cytoplasmic components and damaged organelles when cells are exposed to stress [58]. In cancer cells, more recent literature has found that autophagy acts in a context-dependent manner and may function in tumor promotion and suppression [59]. Autophagy's redox regulation is, in reality, influenced by a number of variables, including the expression of ROS, time frame, and cell type. For instance, ROS affects cancer cells but does not cause autophagic cell death in untransformed cells [60]. Both the induction of autophagy by ROS generation and the decrease of ROS by autophagy show the close relationship between ROS and autophagy [61]. A key negative regulator of autophagy is mammalian target of rapamycin (mTOR), a serine/threonine kinase, and H2O2 inhibits mTOR activity resulting in autophagy [6, 62]. Autophagy is a multi-step process and is strategically controlled by autophagy-related genes (ATGs). ROS upregulates the expression of beclin-1 an important component for initiation of autophagy. Furthermore, nutrient starvation-induced H2O2 oxidizes ATG4 which promotes lipidation of LC-3 and increasing autophagosomes formation [63].
ROS and necroptosis
Necrosis was once thought to be an uncontrolled kind of cell death, but studies now suggest that there is another form of necrosis that is available and is controlled by a protein-mediated platform. This alternative form of necrosis is known as necroptosis. Necroptosis is started by death-inducing receptors like FasR and TNFR, once they have been stimulated by their corresponding ligands, just like the extrinsic apoptotic pathway. There are two critical regulators involved in necroptosis execution such as receptor-interacting protein kinases RIP1 and RIP3 [64]. In fact, RIP3 enhances the mitochondrial catalytic activity and increases the amount of ROS production in the mitochondria, interacting directly with metabolic enzymes [14, 65]. Furthermore, RIP3 phosphorylates PGAM5, which is a subunit of mitochondrial protein phosphatase. This role results in the activation and de-phosphorylation of the dynamin-related protein 1 (Drp1), which fragments the mitochondria, increases ROS production, and induces necroptosis as a result [66].
Future perspectives
The levels of reactive oxygen species (ROS) within cells are the target of many different chemotherapeutic approaches. The non-steroidal and anti-inflammatory drug sulindac is undergoing clinical trials for the treatment of tumors at the moment. It causes lung cancer cells to undergo induced apoptosis by increasing intracellular ROS. The propensity of mtDNA to mutate as a consequence of being subjected to reactive oxygen species (ROS) is something that could potentially be utilized in the treatment of various cancer treatments. Reactive oxygen species (ROS)-based nanoparticles such as gold nanoparticles and cerium-based nanoparticles induce cancer cell death by increasing the intracellular ROS.
Using 2-deoxyglucose as a glucose analog increases oxidative stress in the cell. This leads to cancer cell death due to the inhibition of glucose metabolism as cancerous cells are more vulnerable to glucose toxicity than non-cancerous cells. One way to activate ROS-based nanoparticles is through photodynamic therapy [44], also known as PDT. In this case, light energy is used to increase ROS activity inside the cell. With its photostability and low toxicity to non-cancerous cells, graphene has recently been used in PDT for cancer treatment purposes. Thus, activating intracellular ROS by suitable targeted therapy in cancer cells holds a great potential in anticancer treatment strategies.
Acknowledgements
The authors are grateful to the SRM Institute of Science and Technology for providing support for this work. KK is supported by the SRM IST scholarship.
Funding
The authors have not disclosed any funding.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
K. N. ArulJothi and K. Kumaran have contributed equally to this work.
Contributor Information
K. N. ArulJothi, Email: aruljotn@srmist.edu.in
Anand Krishnan, Email: Krishnana@ufs.ac.za.
References
- 1.Hua P, Sun M, Zhang G, Zhang Y, Tian X, Li X, et al. Cepharanthine induces apoptosis through reactive oxygen species and mitochondrial dysfunction in human non-small-cell lung cancer cells. Biochem Biophys Res Commun. 2015;460:136–142. doi: 10.1016/j.bbrc.2015.02.131. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Yang L, Bing WX, Song WJ, di Geng Y, Shui YC, et al. Calyxin Y induces hydrogen peroxide-dependent autophagy and apoptosis via JNK activation in human non-small cell lung cancer NCI-H460 cells. Cancer Lett. 2013;340:51–62. doi: 10.1016/j.canlet.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 3.You BR, Park WH. Zebularine-induced apoptosis in Calu-6 lung cancer cells is influenced by ROS and GSH level changes. Tumor Biol. 2013;34:1145–1153. doi: 10.1007/s13277-013-0656-8. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Alinari L, Chen CS, Yan F, Dalton JT, Lapalombella R, et al. FTY720 shows promising in vitro and in vivo preclinical activity by downmodulating cyclin D1 and phospho-Akt in mantle cell lymphoma. Clin Cancer Res. 2010;16:3182–3192. doi: 10.1158/1078-0432.CCR-09-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beccafico S, Morozzi G, Marchetti MC, Riccardi C, Sidoni A, Donato R, et al. Artesunate induces ROS- and p38 MAPK-mediated apoptosis and counteracts tumor growth in vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis. 2015;36:1071–1083. doi: 10.1093/carcin/bgv098. [DOI] [PubMed] [Google Scholar]
- 6.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, et al. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shilo S, Tirosh O. Selenite activates caspase-independent necrotic cell death in Jurkat T cells and J774.2 macrophages by affecting mitochondrial oxidant generation. Antioxid Redox Signal. 2003;5:273–279. doi: 10.1089/152308603322110850. [DOI] [PubMed] [Google Scholar]
- 8.Watson JL, Hill R, Yaffe PB, Greenshields A, Walsh M, Lee PW, et al. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010;297:1–8. doi: 10.1016/j.canlet.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 10.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers (Basel) 2019;11:1191. doi: 10.3390/cancers11081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, et al. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res. 2018;37:266. doi: 10.1186/s13046-018-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta Mol Cell Res. 2016;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Gao Z, Liu X, Agarwal P, Zhao S, Conroy DW, et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat Commun. 2018;9:562. doi: 10.1038/s41467-018-02915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou Y, Niu P, Yang J, Yuan J, Wu T, Chen X. The JNK signaling pathway is involved in sodium-selenite-induced apoptosis mediated by reactive oxygen in HepG2 cells. Cancer Biol Ther. 2008;7:689–696. doi: 10.4161/cbt.7.5.5688. [DOI] [PubMed] [Google Scholar]
- 17.Park SH, Kim JH, Chi GY, Kim GY, Chang YC, Moon SK, et al. Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol Lett. 2012;212:252–261. doi: 10.1016/j.toxlet.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Yi BR, Kim NH, Choi KC. Role of the epithelial-mesenchymal transition and its effects on embryonic stem cells. Exp Mol Med. 2014;46:108. doi: 10.1038/emm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reczek CR, Chandel NS. The two faces of reactive oxygen species in cancer. Annu Rev Cancer Biol. 2017;1:79–98. doi: 10.1146/annurev-cancerbio-041916-065808. [DOI] [Google Scholar]
- 20.Wang SS, Jiang J, Liang XH, Tang YL. Links between cancer stem cells and epithelial– mesenchymal transition. Onco Targets Ther. 2015;8:2973–2980. doi: 10.2147/OTT.S91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luanpitpong S, Talbott SJ, Rojanasakul Y, Nimmannit U, Pongrakhananon V, Wang L, et al. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem. 2010;285:38832–38840. doi: 10.1074/jbc.M110.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci BioMed Central Ltd. 2018;25:20. doi: 10.1186/s12929-018-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu TL, Su CC. Tanshinone IIA induces apoptosis in human lung cancer A549 cells through the induction of reactive oxygen species and decreasing the mitochondrial membrane potential. Int J Mol Med. 2010;25:231–236. doi: 10.3892/ijmm_00000335. [DOI] [PubMed] [Google Scholar]
- 24.Luanpitpong S, Chanvorachote P, Stehlik C, Tse W, Callery PS, Wang L, et al. Regulation of apoptosis by Bcl-2 cysteine oxidation in human lung epithelial cells. Mol Biol Cell. 2013;24:858–869. doi: 10.1091/mbc.E12-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang N, Jian JF, Jie CS, Zhang Q, Wei MY, Yuan HuY, et al. Physalin A induces G2/M phase cell cycle arrest in human non-small cell lung cancer cells: involvement of the p38 MAPK/ROS pathway. Mol Cell Biochem. 2016;415:145–155. doi: 10.1007/s11010-016-2686-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Kong H, Zeng X, Liu W, Wang Z, Yan X, et al. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncol Rep Spandidos Publ. 2016;35:2053–2064. doi: 10.3892/or.2016.4569. [DOI] [PubMed] [Google Scholar]
- 27.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 31.Hu Z, Teng XL, Zhang T, Yu X, Ding R, Yi J, et al. SENP3 senses oxidative stress to facilitate STING-dependent dendritic cell antitumor function. Mol Cell. 2021;81:940–952.e5. doi: 10.1016/j.molcel.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Huang R, Chen H, Liang J, Li Y, Yang J, Luo C, et al. Dual role of reactive oxygen species and their application in cancer therapy. J Cancer Ivyspring Int Publ. 2021;12:5543–5561. doi: 10.7150/jca.54699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Li J, Zong L, Chen X, Chen K, Jiang Z, et al. Reactive oxygen species and targeted therapy for pancreatic cancer. Oxid Med Cell Longev. 2015;2016:1616781. doi: 10.1155/2016/1616781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, et al. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saikolappan S, Kumar B, Shishodia G, Koul S, Koul HK. Reactive oxygen species and cancer: a complex interaction. Cancer Lett. 2019;452:132–143. doi: 10.1016/j.canlet.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic Biol Med. 2017;104:144–164. doi: 10.1016/j.freeradbiomed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Shah MA, Rogoff HA. Implications of reactive oxygen species on cancer formation and its treatment. Semin Oncol. 2021;48:238–245. doi: 10.1053/j.seminoncol.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Yuan L, Yi C, Li W, et al. Koumine promotes ROS production to suppress hepatocellular carcinoma cell proliferation Via NF-κB and ERK/p38 MAPK signaling. Biomolecules. 2019;9:559. doi: 10.3390/biom9100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 1979;2015(350):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pei X, Xiao J, Wei G, Zhang Y, Lin F, Xiong Z, et al. Oenothein B inhibits human non-small cell lung cancer A549 cell proliferation by ROS-mediated PI3K/Akt/NF-κB signaling pathway. Chem Biol Interact. 2019;298:112–120. doi: 10.1016/j.cbi.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Kim C, Lee S-G, Yang WM, Arfuso F, Um J-Y, Kumar AP, et al. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018;431:123–141. doi: 10.1016/j.canlet.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 42.Zhang T, Li SM, Li YN, Cao JL, Xue H, Wang C, et al. Atractylodin induces apoptosis and inhibits the migration of A549 lung cancer cells by regulating ROS-mediated signaling pathways. Molecules. 2022;27:2946. doi: 10.3390/molecules27092946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen K, Zhang S, Jiao J, Zhao S. Ferroptosis and its potential role in lung cancer: updated evidence from pathogenesis to therapy. J Inflamm Res. 2021;14:7079–7090. doi: 10.2147/JIR.S347955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355:176–183. doi: 10.1016/j.canlet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Almahi WA, Yu KN, Mohammed F, Kong P, Han W. Hemin enhances radiosensitivity of lung cancer cells through ferroptosis. Exp Cell Res. 2022;410:112946. doi: 10.1016/j.yexcr.2021. [DOI] [PubMed] [Google Scholar]
- 46.le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7:308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 47.Kodama R, Kato M, Furuta S, Ueno S, Zhang Y, Matsuno K, et al. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18:32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 48.Panieri E, Santoro MM. Ros homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conklin KA. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 50.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 51.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 52.Jeddi F, Soozangar N, Sadeghi MR, Somi MH, Shirmohamadi M, Eftekhar-Sadat AT, et al. Nrf2 overexpression is associated with P-glycoprotein upregulation in gastric cancer. Biomed Pharmacother. 2018;97:286–292. doi: 10.1016/j.biopha.2017.10.129. [DOI] [PubMed] [Google Scholar]
- 53.Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Tumor suppressive functions of ceramide: evidence and mechanisms. Apoptosis. 2015;20:689–711. doi: 10.1007/s10495-015-1109-1. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Azad N, Kongkaneramit L, Chen F, Lu Y, Jiang B-H, et al. The fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J Immunol. 2008;180:3072–3080. doi: 10.4049/jimmunol.180.5.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 56.Ow YLP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 57.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, et al. Cytochrome C acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 58.Filomeni G, de Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. 2018;19:3466. doi: 10.3390/ijms19113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 2015;35:615–621. doi: 10.1007/s10571-015-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vandenabeele P, Galluzzi L, Berghe TV. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 65.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 1979;2009(325):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 67.Liu GY, Sun YZ, Zhou N, Du XM, Yang J, Guo SJ. 3,3′-OH curcumin causes apoptosis in HepG2 cells through ROS-mediated pathway. Eur J Med Chem. 2016;112:157–163. doi: 10.1016/j.ejmech.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 68.Rastogi N, Gara RK, Trivedi R, Singh A, Dixit P, Maurya R, et al. Gingerolinduced myeloid leukemia cell death is initiated by reactive oxygen species and activation of miR-27b expression. Free Radic Biol Med. 2014;68:288–301. doi: 10.1016/j.freeradbiomed.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 69.Hsieh CJ, Kuo PL, Hsu YC, Huang YF, Tsai EM, Hsu YL. Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic Biol Med. 2014;67:159–170. doi: 10.1016/j.freeradbiomed.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 70.You BR, Park WH. Arsenic trioxide induces human pulmonary fibroblast cell death via increasing ROS levels and GSH depletion. Oncol Rep. 2012;28:749–757. doi: 10.3892/or.2012.1852. [DOI] [PubMed] [Google Scholar]
- 71.Moon DO, Kim MO, Choi YH, Hyun JW, Chang WY, Kim GY. Butein induces G2/M phase arrest and apoptosis in human hepatoma cancer cells through ROS generation. Cancer Lett. 2010;288:204–213. doi: 10.1016/j.canlet.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 72.McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- 73.Macho A, Calzado MA, Muñoz-Blanco J, Gómez-Díaz C, Gajate C, Mollinedo F, et al. Selective induction of apoptosis by capsaicin in transformed cells: the role of reactive oxygen species and calcium. Cell Death Differ. 1999;6:155–165. doi: 10.1038/sj.cdd.4400465. [DOI] [PubMed] [Google Scholar]
- 74.Kim DH, Park KW, Chae IG, Kundu J, Kim EH, Kundu JK, et al. Carnosic acid inhibits STAT3 signaling and induces apoptosis through generation of ROS in human colon cancer HCT116 cells. Mol Carcinog. 2016;55:1096–1110. doi: 10.1002/mc.22353. [DOI] [PubMed] [Google Scholar]
- 75.Park KW, Kundu J, Chae IG, Kim DH, Yu MH, Kundu JK, et al. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int J Oncol. 2014;44:1309–1315. doi: 10.3892/ijo.2014.2281. [DOI] [PubMed] [Google Scholar]
- 76.Chae IG, Kim DH, Kundu J, Jeong CH, Kundu JK, Chun KS. Generation of ROS by CAY10598 leads to inactivation of STAT3 signaling and induction of apoptosis in human colon cancer HCT116 cells. Free Radic Res. 2014;48:1311–1321. doi: 10.3109/10715762.2014.951838. [DOI] [PubMed] [Google Scholar]
- 77.Lee JH, Won YS, Park KH, Lee MK, Tachibana H, Yamada K, et al. Celastrol inhibits growth and induces apoptotic cell death in melanoma cells via the activation ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT signaling. Apoptosis. 2012;17:1275–1286. doi: 10.1007/s10495-012-0767-5. [DOI] [PubMed] [Google Scholar]
- 78.Li C, Qi Q, Lu N, Dai Q, Li F, Wang X, et al. Gambogic acid promotes apoptosis and resistance to metastatic potential in MDA-MB-231 human breast carcinoma cells. Biochem Cell Biol. 2012;90:718–730. doi: 10.1139/o2012-030. [DOI] [PubMed] [Google Scholar]
- 79.Ko CH, Shen SC, Yang LY, Lin CW, Chen YC. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int J Cancer. 2007;121:1670–1679. doi: 10.1002/ijc.22910. [DOI] [PubMed] [Google Scholar]
- 80.Zhang X, Wang X, Wu T, Li B, Liu T, Wang R, et al. Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci Rep. 2015;5:12579. doi: 10.1038/srep12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang L, Yuan Y, Fu C, Xu X, Zhou J, Wang S, et al. LZ-106, a novel analog of enoxacin, inducing apoptosis via activation of ROS-dependent DNA damage response in NSCLCs. Free Radic Biol Med. 2016;95:155–168. doi: 10.1016/j.freeradbiomed.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Du J, Suzuki H, Nagase F, Akhand AA, Ma XY, Yokoyama T, et al. Superoxide-mediated early oxidation and activation of ASK1 are important for initiating methylglyoxal-induced apoptosis process. Free Radic Biol Med. 2001;31:469–478. doi: 10.1016/s0891-5849(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 83.Liu JF, Hou CH, Lin FL, Tsao YT, Hou SM. Nimbolide induces ROS-regulated apoptosis and inhibits cell migration in osteosarcoma. Int J Mol Sci. 2015;16:23405–23424. doi: 10.3390/ijms161023405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Wang CCC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008;259:82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 86.Guha P, Dey A, Sen R, Chatterjee M, Chattopadhyay S, Bandyopadhyay SK. Intracellular GSH depletion triggered mitochondrial bax translocation to accomplish resveratrol-induced apoptosis in the U937 cell line. J Pharmacol Exp Ther. 2011;336:206–214. doi: 10.1124/jpet.110.171983. [DOI] [PubMed] [Google Scholar]
- 87.Liu C, Ye Y, Zhou Q, Zhang R, Zhang H, Liu W, et al. Crosstalk between Ca2+ signaling and mitochondrial H2O2 is required for rotenone inhibition of mTOR signaling pathway leading to neuronal apoptosis. Oncotarget. 2016;7:7534–7549. doi: 10.18632/oncotarget.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim KY, Yu SN, Lee SY, Chun SS, Choi YL, Park YM, et al. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun. 2011;413:80–86. doi: 10.1016/j.bbrc.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 89.Duan D, Zhang B, Yao J, Liu Y, Fang J. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic Biol Med. 2014;70:182–193. doi: 10.1016/j.freeradbiomed.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 90.Woo CC, Hsu A, Kumar AP, Sethi G, Tan KHB. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS ONE. 2013;8:e75356. doi: 10.1371/journal.pone.0075356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei L, Lu N, Dai Q, Rong J, Chen Y, Li Z, et al. Different apoptotic effects of wogonin via induction of H2O 2 generation and Ca2+ overload in malignant hepatoma and normal hepatic cells. J Cell Biochem. 2010;111:1629–16241. doi: 10.1002/jcb.22898. [DOI] [PubMed] [Google Scholar]
- 92.Rajan I, Jayasree PR, Kumar PRM. Zerumbone induces mitochondria-mediated apoptosis via increased calcium, generation of reactive oxygen species and upregulation of soluble histone H2AX in K562 chronic myelogenous leukemia cells. Tumor Biol. 2015;36:8479–8489. doi: 10.1007/s13277-015-3583-z. [DOI] [PubMed] [Google Scholar]
- 93.Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10:1161–1172. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- 94.al Dhaheri Y, Attoub S, Ramadan G, Arafat K, Bajbouj K, Karuvantevida N, et al. Carnosol induces ROS-mediated beclin1-independent autophagy and apoptosis in triple negative breast cancer. PLoS One. 2014;9:e109630. doi: 10.1371/journal.pone.0109630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie T, et al. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: an in vitro and in vivo study. Cell Death Dis. 2015;6:e1604. doi: 10.1038/cddis.2014.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dai C, Tang S, Velkov T, Xiao X. Colistin-induced apoptosis of neuroblastoma-2a cells involves the generation of reactive oxygen species, mitochondrial dysfunction, and autophagy. Mol Neurobiol. 2016;53:4685–4700. doi: 10.1007/s12035-015-9396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim AD, Kang KA, Kim HS, Kim DH, Choi YH, Lee SJ, et al. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liao A, Hu R, Zhao Q, Li J, Li Y, Yao K, et al. Autophagy induced by FTY720 promotes apoptosis in U266 cells. Eur J Pharm Sci. 2012;45:600–605. doi: 10.1016/j.ejps.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 99.Ishaq M, Khan MA, Sharma K, Sharma G, Dutta RK, Majumdar S. Gambogic acid induced oxidative stress dependent caspase activation regulates both apoptosis and autophagy by targeting various key molecules (NF-κB, Beclin-1, p62 and NBR1) in human bladder cancer cells. Biochim Biophys Acta Gen Subj. 2014;1840:3374–3384. doi: 10.1016/j.bbagen.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 100.Yuan L, Wei S, Wang J, Liu X. Isoorientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-Related p53, PI3K/Akt, JNK, and p38 signaling pathways in HepG2 cancer cells. J Agric Food Chem. 2014;62:5390–5400. doi: 10.1021/jf500903g. [DOI] [PubMed] [Google Scholar]
- 101.Kim A, Im M, Yim NH, Kim T, Ma JY. A novel herbal medicine, KIOM-C, induces autophagic and apoptotic cell death mediated by activation of JNK and reactive oxygen species in HT1080 human fibrosarcoma cells. PLoS ONE. 2014;9:e98703. doi: 10.1371/journal.pone.0098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mi Y, Xiao C, Du Q, Wu W, Qi G, Liu X. Momordin Ic couples apoptosis with autophagy in human hepatoblastoma cancer cells by reactive oxygen species (ROS)-mediated PI3K/Akt and MAPK signaling pathways. Free Radic Biol Med. 2016;90:230–242. doi: 10.1016/j.freeradbiomed.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 103.Checker R, Gambhir L, Sharma D, Kumar M, Sandur SK. Plumbagin induces apoptosis in lymphoma cells via oxidative stress mediated glutathionylation and inhibition of mitogen-activated protein kinase phosphatases (MKP1/2) Cancer Lett. 2015;357:265–278. doi: 10.1016/j.canlet.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 104.Xie CM, Chan WY, Yu S, Zhao J, Cheng CHK. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic Biol Med. 2011;51:1365–1375. doi: 10.1016/j.freeradbiomed.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 105.Zhang T, Li Y, Park KA, Byun HS, Won M, Jeon J, et al. Cucurbitacin induces autophagy through mitochondrial ROS production which counteracts to limit caspase-dependent apoptosis. Autophagy. 2012;8:559–576. doi: 10.4161/auto.18867. [DOI] [PubMed] [Google Scholar]
- 106.Ren G, Sha T, Guo J, Li W, Lu J, Chen X. Cucurbitacin B induces DNA damage and autophagy mediated by reactive oxygen species (ROS) in MCF-7 breast cancer cells. J Nat Med. 2015;69:522–530. doi: 10.1007/s11418-015-0918-4. [DOI] [PubMed] [Google Scholar]
- 107.Lin G, Hill DK, Andrejeva G, Boult JKR, Troy H, Fong ACLFWT, et al. Dichloroacetate induces autophagy in colorectal cancer cells and tumours. Br J Cancer. 2014;111:375–385. doi: 10.1038/bjc.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Verdoodt B, Vogt M, Schmitz I, Liffers ST, Tannapfel A, Mirmohammadsadegh A. Salinomycin induces autophagy in colon and breast cancer cells with concomitant generation of reactive oxygen species. PLoS ONE. 2012;7:e44132. doi: 10.1371/journal.pone.0044132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim EH, Sohn S, Kwon HJ, Kim SU, Kim MJ, Lee SJ, et al. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007;67:6314–6324. doi: 10.1158/0008-5472.CAN-06-4217. [DOI] [PubMed] [Google Scholar]
- 110.Shen S, Zhang Y, Zhang R, Tu X, Gong X. Ursolic acid induces autophagy in U87MG cells via ROS-dependent endoplasmic reticulum stress. Chem Biol Interact. 2014;218:28–41. doi: 10.1016/j.cbi.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 111.Luo Y, Roy M, Xiao X, Sun S, Liang L, Chen H, et al. Lycorine induces programmed necrosis in the multiple myeloma cell line ARH-77. Tumor Biol. 2015;36:2937–2945. doi: 10.1007/s13277-014-2924-7. [DOI] [PubMed] [Google Scholar]