Abstract
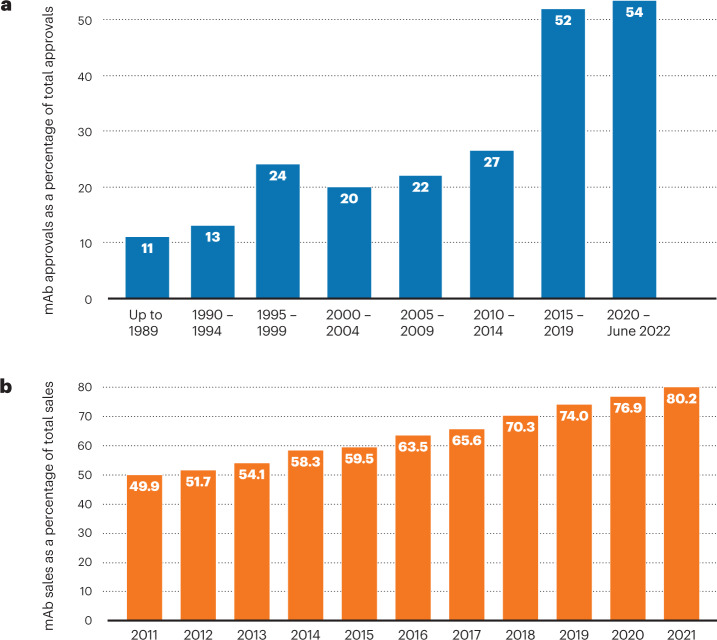
Monoclonal antibodies as a group continue to lead biopharmaceuticals in numbers of approvals and sales, although COVID-19 vaccines shot to the top of the list of highest-grossing individual products.
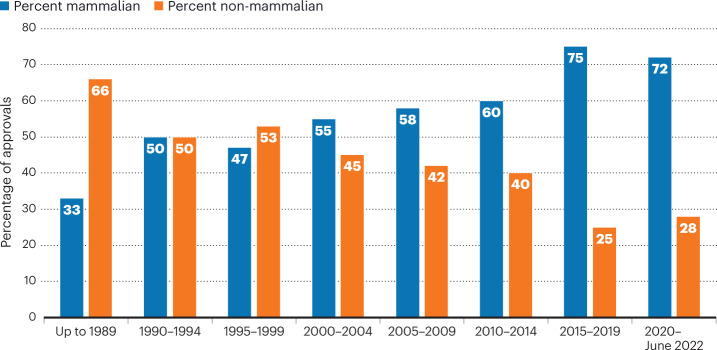
The past few years will forever be remembered as the years of a pandemic, the likes of which had not been seen for a century. And biopharmaceuticals took a starring role, with both COVID-19 vaccines and therapeutics dominating the news for the speed with which they were developed and their impact on global health. Nonetheless, regulatory agencies in both the United States and EU maintained the fast pace of prior years in moving products through their pipelines. This article is the latest survey of biopharmaceutical approvals, which we conduct every four years. The current survey period (January 2018–June 2022) witnessed the approval of 197 biopharmaceutical products (see Box 1 for definition) in the United States and/or EU, when counted by product trade name. Some products contain identical active ingredients or are sold under different trade names in the two regions; taking this into account, 180 distinct biopharmaceutical active ingredients entered the market.
Box 1 Biopharmaceuticals defined
Biopharmaceuticals (Table 1) are defined here as recombinant proteins, including recombinant antibodies, and nucleic acid- and genetically engineered cell-based products. They are listed in Table 1 consecutively from the most recent approval in each class, with registrations since 2018 indicated with boldface and withdrawals and discontinuations with italics. Eight categories are shown: recombinant clotting factors; recombinant thrombolytics, anticoagulants and other blood-related products; recombinant hormones; recombinant growth factors; recombinant interferons, interleukins and tumor necrosis factor; vaccines; monoclonal-antibody-based products; and other recombinant products. Where more than one drug in the same category was approved in a single year, they are listed alphabetically by trade name. In the case of several products that have been approved for multiple indications, only the first indication is listed here. Some product entries describe the product as being the same as another listed product. In such instances differences exist in terms of the approved indication range or the company holding the marketing authorizations, usually as a result of commercial agreements. Included are (COVID-19) therapeutics authorized under emergency procedures (Emergency Use Authorization in the United States and Conditional Marketing Authorisation in EU).
Table 1.
Biopharmaceuticals approved in the United States and European Union through end of June 2022
| Product | Company (location) | Therapeutic indication | Date approved |
|---|---|---|---|
| Recombinant clotting factors | |||
| Factor VIII | |||
| Esperoct (turoctocog alfa pegol), rh coagulation factor VIII, produced in a CHO cell line. PEGylated form of NovoEight (see later entry). |
Novo Nordisk (Bagsvaerd, Denmark) Novo Nordisk (Plainsboro, NJ, USA) |
Hemophilia A | 2019 (EU & US) |
| Adynovi (rurioctocog alfa pegol), extended-half-life PEGylated form of full-length r factor VIII product Advate (see below). Same product as Adynovate (see below). | Baxalta Innovations (Vienna) | Hemophilia A | 2018 (EU) |
| Jivi (damoctocog alfa pegol (EU), antihemophilic factor (recombinant), PEGylated-aucl (US)), PEGylated B-domain-deleted rh coagulation factor VIII, produced in BHK cells. |
Bayer (Leverkusen, Germany) Bayer HealthCare (Whippany, NJ, USA) |
Hemophilia A | 2018 (EU & US) |
| Afstyla (lonoctocog alfa), B-domain-truncated rh coagulation factor VIII, produced in CHO cells. | CSL Behring (Marburg, Germany, & Kankakee, IL, USA) | Hemophilia A | 2017 (EU) 2016 (US) |
| Vihuma (simoctocog alfa), rh B-domain-deleted factor VIII, produced in HEK cells. Same product as Nuwiq (see below). | Octapharma (Stockholm) | Hemophilia A | 2017 (EU) |
| Iblias (octocog alfa), rh coagulation factor VIII, produced in BHK cells using the same expression construct as Bayer’s Kogenate and Helixate. Same product as Kovaltry (see below). | Bayer Pharma (Berlin) | Hemophilia A |
2016 (EU) Withdrawn 2020 |
| Kovaltry (octocog alfa), rh coagulation factor VIII, produced in BHK cells using the same expression construct as Bayer’s Kogenate and Helixate. Same product as Iblias (see above). |
Bayer Pharma (Leverkusen, Germany) Bayer HealthCare (Whippany, NJ, USA) |
Hemophilia A | 2016 (EU & US) |
| Vonvendi (von Willebrand factor (recombinant)), produced in CHO cells. | Baxalta (Westlake Village, CA, USA) | von Willebrand disease | 2015 (US) |
| Nuwiq (simoctocog alfa), B-domain-deleted rh factor VIII, produced in HEK cells. Same product as Vihuma (see above). |
Octapharma USA (Hoboken, NJ, USA) Octapharma (Stockholm) |
Hemophilia A |
2015 (US) 2014 (EU) |
| Obizur (susoctocog alfa), r B-domain-deleted porcine factor VIII, produced in BHK cells. |
Baxalta Innovations (Vienna) Baxter Healthcare (Westlake Village, CA, USA) |
Acquired hemophilia due to development of autoantibodies against factor VIII |
2015 (EU) 2014 (US) |
| Adynovate (recombinant, PEGylated antihemophilic factor), extended-half-life PEGylated form of full-length r factor VIII product Advate (see below). Same product as Adynovi (see above). | Baxalta | Hemophilia A | 2015 (US) |
| Elocta (efmoroctocog alfa; EU), Eloctate (antihemophilic factor recombinant, Fc fusion protein; US), rh coagulation factor VIII–Fc fusion protein comprising B-domain-deleted human factor VIII covalently linked to the Fc domain of a human IgG, produced in HEK cells. |
Swedish Orphan Biovitrum (Stockholm) Biogen Idec (Cambridge, MA, USA) |
Hemophilia A |
2015 (EU) 2014 (US) |
| NovoEight (turoctocog alfa), rh factor VIII analog that, when activated, is structurally comparable to endogenous human factor VIIIa, produced in CHO cells. | Novo Nordisk (Bagsvaerd, Denmark, & Plainsboro, NJ, USA) | Hemophilia A | 2013 (EU & US) |
| Xyntha (antihemophilic factor), rh coagulation factor VIII, produced in CHO cells. | Pfizer/Wyeth (Philadelphia) | Hemophilia A | 2008 (US) |
| Advate (octocog alfa), rh factor VIII, produced in CHO cells. |
Takeda (Vienna) Baxter Healthcare (Westlake Village, CA, USA) |
Hemophilia A |
2004 (EU) 2003 (US) |
| Helixate NexGen (octocog alfa), rh factor VIII, produced in BHK cells. | Bayer (Berlin) | Hemophilia A |
2000 (EU) Withdrawn 2019 |
| ReFacto (moroctocog alfa), B-domain-deleted rh factor VIII, produced in CHO cells. |
Pfizer (Brussels) Genetics Institute (Cambridge, MA, USA) |
Hemophilia A |
2000 (US) 1999 (EU) |
| Kogenate, Helixate (antihemophilic factor), rh factor VIII, produced in BHK cells. Sold as Helixate by Aventis Behring through a license agreement. | Bayer (Leverkusen, Germany, & Berkeley, CA, USA) | Hemophilia A |
2000 (EU) 1993 (US) |
| Bioclate (antihemophilic factor), rh factor VIII, produced in CHO cells. | Aventis Behring (King of Prussia, PA, USA) | Hemophilia A | 1993 (US) |
| Recombinate (antihemophilic factor), rh factor VIII, produced in CHO cells. | Baxter Healthcare (Westlake Village, CA, USA) | Hemophilia A | 1992 (US) |
| Other blood factors | |||
| Sevenfact (coagulation factor VIIa (recombinant)-jncw; rh activated factor VII, produced in milk of transgenic rabbits. | HEMA Biologics (Louisville, KY, USA) | Hemophilia A or B | 2020 (US) |
| Ondexxya (andexanet alfa (EU), Andexxa (US)), engineered rh factor Xa lacking the coagulation activity of native FXa but retaining binding ability to FXa inhibitors, produced in CHO cells. |
AstraZeneca (Sodertalje, Sweden) Portola Pharmaceuticals (South San Francisco, CA, USA) Alexion Pharmaceuticals (Boston) |
Stopping life-threatening or uncontrolled bleeding in adults taking the anticoagulant medicines apixaban or rivaroxaban |
2019 (EU) 2018 (US) |
| Veyvondi (vonicog alfa), rh von Willebrand factor, produced in CHO cells. | Baxalta Innovations (Vienna) | von Willebrand disease | 2018 (EU) |
| Rebinyn (rh coagulation factor IX; US), Refixia (nonacog beta pegol; EU), rh coagulation factor IX, produced in CHO cells and PEGylated. | Novo Nordisk (Plainsboro, NJ, USA & Bagsvaerd, Denmark) | Hemophilia B | 2017 (EU & US) |
| Alprolix (eftrenonacog alfa), rh coagulation factor IX fused to a human IgG1 Fc domain, produced in HEK cells. |
Swedish Orphan Biovitrum (Stockholm) Bioverativ Therapeutics (Waltham, MA, USA) |
Hemophilia B |
2016 (EU) 2014 (US) |
| Idelvion (albutrepenonacog alfa), rh factor IX–albumin fusion protein, produced in CHO cells. | CSL Behring | Hemophilia B | 2016 (EU & US) |
| Ixinity (coagulation factor IX, recombinant)), rh coagulation factor IX, produced in CHO cells. | Aptevo BioTherapeutics (Berwyn, PA, USA) | Hemophilia B | 2015 (US) |
| Rixubis (nonacog gamma), rh factor IX, produced in CHO cells. |
Baxalta Innovations (Vienna) Baxter Healthcare (Lexington, MA, USA) |
Hemophilia B |
2014 (EU) 2013 (US) |
| Tretten (US), Novothirteen (EU) (catridecog), rh factor XIII A-subunit, produced in S. cerevisiae. | Novo Nordisk | Congenital factor XIII A-subunit deficiency |
2013 (US) 2012 (EU) |
| Recothrom (thrombin), rh factor Iia, produced in CHO cells. | Baxter Healthcare (Deerfield, IL, USA) | Control of minor bleeding during surgery | 2008 (US) |
| NovoSeven (eptacog alfa, activated), rh factor VIIa, produced in BHK cells. | Novo Nordisk | Some forms of hemophilia |
1996 (EU) 1999 (US) |
| Benefix (nonacog alfa), rh factor IX, produced in CHO cells. | Pfizer/Wyeth | Hemophilia B | 1997 (EU & US) |
| Recombinant thrombolytics, anticoagulants and other blood-related products | |||
| Tissue plasminogen activator (tPA) | |||
| Metalyse (tenecteplase), modified rh tPA, produced in CHO cells. | Boehringer Ingelheim (Ingelheim, Germany) | Myocardial infarction |
2001 (EU) Withdrawn 2005 |
| TNKase (tenecteplase), modified rh tPA, produced in CHO cells. | Roche/Genentech (South San Francisco, CA, USA) | Myocardial infarction | 2000 (US) |
| Ecokinase (reteplase), r tPA, produced in E. coli; differs from human tPA in the deletion of 3 of its 5 domains. | Roche (Welwyn Garden City, UK) | Acute myocardial infarction |
1996 (EU) Withdrawn 2000 |
| Rapilysin (reteplase), r tPA (see Ecokinase, above). | Actavis Group PTC (Hafnarfjordur, Iceland), Roche | Acute myocardial infarction | 1996 (EU) |
| Retavase (reteplase), r tPA (see Ecokinase, above). | Chiesi USA (Cary, NC, USA) | Acute myocardial infarction | 1996 (US) |
| Activase (alteplase), rh tPA, produced in CHO cells. | Roche/Genentech | Acute myocardial infarction | 1987 (US) |
| Hirudin | |||
| Refludan (lepirudin), r hirudin, produced in S. cerevisiae. | Bayer HealthCare (Leverkusen, Germany) | Anticoagulation therapy for heparin-associated thrombocytopenia |
1997 (EU) 1998 (US) Withdrawn 2012 |
| Revasc (desirudin), r hirudin, produced in S. cerevisiae. | Canyon Pharmaceuticals (London) | Prevention of venous thrombosis |
1997 (EU) Withdrawn 2014 |
| Other | |||
| Ruconest (conestat alfa), rh C1 esterase inhibitor, produced in the milk of transgenic rabbits. |
Pharming Healthcare (Warren, NJ, USA) Pharming Group (Leiden, the Netherlands) |
Acute angioedema |
2014 (US) 2010 (EU) |
| Jetrea (ocriplasmin), r truncated form of human plasmin, produced in Pichia pastoris. |
Inceptua (Bromma, Sweden) ThromboGenics (Iselin, NJ, USA) |
Symptomatic vitreomacular adhesion, vitreomacular traction |
2013 (EU) 2012 (US) |
| Atryn (rh antithrombin), produced in milk of transgenic goats. |
Laboratoire français du fractionnement et des biotechnologies (Les Ulis, France) rEVO Biologics (Framingham, MA, USA) |
Hereditary antithrombin deficiency |
2009 (US) 2006 (EU) Withdrawn 2018 (EU) |
| Kalbitor (ecallantide), plasma kallikrein inhibitor, produced in P. pastoris. | Dyax (Cambridge, MA, USA) | Hereditary angioedema | 2009 (US |
| Xigris (drotrecogin alfa), rh activated protein C, produced in a human cell line. | Eli Lilly (Houten, the Netherlands) | Severe sepsis |
2001 (US) 2002 (EU) Withdrawn 2012 |
| Recombinant hormones | |||
| Insulins | |||
| Inpremzia (rh insulin), produced in P. pastoris. Biosimilar to Actrapid. | Baxter Holding (Utrecht, the Netherlands) | Diabetes mellitus | 2022 (EU) |
| Truvelog Mix 30 (insulin aspart, produced in E. coli. Biosimilar to NovoMix. | Sanofi-Aventis (Paris) | Diabetes mellitus | 2022 (EU) |
| Kirsty (previously Kixelle) insulin aspart; fast-acting insulin analog, produced in P. pastoris. Biosimilar to NovoRapid. | Mylan Ireland (Dublin, Ireland) | Diabetes mellitus | 2021 (EU) |
| Rezvoglar (insulin glargine-aglr, long-acting human insulin analog, biosimilar to Lantus, produced in E. coli. | Eli Lilly | Diabetes mellitus | 2021 (US) |
| Semglee (insulin glargine (EU), insulin glargine-yfgn (US)); r insulin glargine, produced in P. pastoris. Biosimilar to Lantus. |
Mylan (Saint-Priest, France) Mylan Pharmaceuticals (Morgantown, WV, USA) |
Diabetes mellitus |
2021 (US) 2018 (EU) |
| Insulin aspart Sanofi (insulin aspart, r fast-acting insulin analog, produced in E. coli. Biosimilar to NovoRapid. | Sanofi-Aventis (Paris) | Diabetes mellitus | 2020 (EU) |
| Lyumjev (previously Liumjev; insulin lispro (EU), insulin lispro-aabc (US)); rh rapid-acting insulin analog, produced in E. coli. Same active ingredient as in Humalog, but new formulation. | Eli Lilly Nederland (Utrecht, the Netherlands) | Diabetes mellitus | 2020 (EU & US) |
| Myxredlin (rh insulin, produced in P. pastoris). | Baxter Healthcare (Deerfield, IL, USA) | Diabetes mellitus | 2019 (US) |
| Admelog (insulin lispro injection), rapid-acting human insulin analog, produced in E. coli. | Sanofi (Bridgewater, NJ, USA) | Diabetes mellitus | 2017 (US) |
| Fiasp (insulin aspart injection), rapid-acting insulin analog, produced in S. cerevisiae. | Novo Nordisk | Diabetes mellitus | 2017 (US & EU) |
| Insulin lispro Sanofi, produced in E. coli. Biosimilar to Humalog. | Sanofi-Aventis (Paris) | Diabetes mellitus | 2017 (EU) |
| Lusduna (insulin glargine), engineered insulin, produced in E. coli. Biosimilar to Lantus. | Merck Sharp & Dohme (Hoddesdon, UK) | Diabetes mellitus |
2017 (EU) Withdrawn 2018 (EU) 2017 (US, tentative) Withdrawn 2018 (US) |
| Suliqua (EU), Soliqua (US) (insulin glargine/lixisenatide), combination of long-acting insulin glargine, produced in E. coli, and a synthetically produced human GLP-1 analog. |
Sanofi-Aventis (Paris) Sanofi (Bridgewater, NJ, USA) |
Diabetes mellitus type 2 |
2017 (EU) 2016 (US) |
| Xultophy (insulin degludec/liraglutide), a combination of 2 previously approved products, Victoza and Tresiba. | Novo Nordisk | Diabetes mellitus type 2 |
2016 (US) 2014 (EU) |
| Abasaglar (previously Abasria; EU), Basaglar (US) (insulin glargine), produced in E. coli. Biosimilar (in EU) to Lantus. |
Eli Lilly (Utrecht, the Netherlands) Eli Lilly (Indianapolis, IN USA) |
Diabetes mellitus |
2015 (US) 2014 (EU) |
| Ryzodeg 70/30 (US), Ryzodeg (EU) (insulin degludec/insulin aspart), combination of two engineered insulins, produced in S. cerevisiae. | Novo Nordisk | Diabetes mellitus type 1 and 2 |
2015 (US) 2013 (EU) |
| Toujeo (insulin glargine, long-acting rh insulin analog), produced in E. coli (see also Lantus, below). Previously Optisulin in EU. |
Sanofi-Aventis Deutschland (Frankfurt) Sanofi (Bridgewater, NJ, USA) |
Diabetes mellitus |
2000 (EU) 2015 (US) |
| Tresiba (insulin degludec), engineered long-acting human insulin analog, produced in S. cerevisiae (see also Ryzodeg, above). | Novo Nordisk | Diabetes mellitus type 1 and 2 |
2015 (US) 2013 (EU) |
| Afrezza (rh insulin), produced in E. coli). | MannKind (Danbury, CT, USA) | Diabetes mellitus | 2014 (US) |
| Novolog mix (insulin aspart mix), 50:50 mixture of engineered rh insulins, produced in S. cerevisiae in soluble and protamine suspension forms. | Novo Nordisk | Diabetes mellitus | 2008 (US) |
| Insulin Human Winthrop (rh insulin), produced in E. coli. | Sanofi (Frankfurt) | Diabetes mellitus |
2007 (EU) Withdrawn 2018 |
| Exubera (inhalable rh insulin), produced in E. coli. | Pfizer (Sandwich, UK) | Diabetes mellitus |
2006 (EU & US) Withdrawn 2008 |
| Levemir (insulin detemir), long-acting rh insulin, produced in S. cerevisiae. | Novo Nordisk | Diabetes mellitus |
2005 (US) 2004 (EU) |
| Apidra (insulin glulisine), rapid-acting insulin analog, produced in E. coli. | Sanofi (Frankfurt) | Diabetes mellitus | 2004 (EU & US) |
| Actrapid, Velosulin, Monotard, Insulatard, Protaphane, Mixtard, Actraphane, Ultratard, rh insulin formulated as short-, intermediate- or long-acting products. | Novo Nordisk | Diabetes mellitus |
2002 (EU) Monotard and Ultratard withdrawn 2006 Velosulin withdrawn 2009 |
| Novolog (insulin aspart), short-acting rh insulin analog, produced in S. cerevisiae. | Novo Nordisk | Diabetes mellitus | 2001 (US) |
| Novolog mix 70/30 (contains insulin aspart, a short-acting rh insulin analog, as one ingredient). | Novo Nordisk | Diabetes mellitus | 2001 (US) |
| Novomix 30 (contains a mixture of insulin aspart, a short-acting rh insulin analog, produced in S. cerevisiae, in both soluble and crystalline forms). | Novo Nordisk | Diabetes mellitus | 2000 (EU) |
| Lantus (insulin glargine), long-acting rh insulin analog, produced in E. coli. | Sanofi (Frankfurt) | Diabetes mellitus | 2000 (EU & US) |
| NovoRapid (insulin aspart), rh insulin analog), produced in S. cerevisiae. | Novo Nordisk | Diabetes mellitus | 1999 (EU) |
| Liprolog (insulin lispro), insulin analog, produced in E. coli. | Eli Lilly (Houten, the Netherlands) | Diabetes mellitus |
1997 (EU) Withdrawn 2001 |
| Insuman (rh insulin), produced in E. coli. | Sanofi (Frankfurt) | Diabetes mellitus | 1997 (EU) |
| Humalog (insulin lispro), insulin analog, produced in E. coli. | Eli Lilly (Houten, the Netherlands) | Diabetes mellitus | 1996 (EU & US) |
| Novolin (rh insulin), produced in S. cerevisiae. | Novo Nordisk | Diabetes mellitus |
1991 (US) Withdrawn 2010 |
| Humulin (rh insulin), produced in E. coli. | Eli Lilly (Indianapolis, IN, USA) | Diabetes mellitus | 1982 (US) |
| Human growth hormone | |||
| Lonapegsomatropin Ascendis Pharma (lonapegsomatropin), rhGH, produced in E. coli and PEGylated. | Ascendis Pharma (Hellerup, Denmark) | Growth hormone deficiency | 2022 (EU) |
| Skytrofa (lonapegsomatropin-tcgd; r hGH, produced in E. coli and PEGylated. Same API as Lonapegsomatropin Ascendis Pharma. | Ascendis Pharma Endocrinology Division | Growth hormone deficiency | 2021 (US) |
| Sogroya (somapacitan (EU, somapacitan-beco (US)), long acting r hGH with L101C substitution and an albumin-binding C-16 fatty acid derivative attached, produced in E. coli. | Novo Nordisk | Growth hormone deficiency |
2021 (EU) 2020 (US) |
| Somatropin Biopartners (somatropin), r hGH, produced in S. cerevisiae. | Biopartners (Reutlingen, Germany) | Growth failure, growth hormone deficiency |
2013 (EU) Withdrawn 2017 |
| Accretropin (somatropin), r hGH, produced in E. coli. |
Emergent Biosolutions (Rockville, MD, USA) Cangene (Winnipeg, Canada) |
Growth failure or short stature associated with Turner syndrome in children | 2008 (US) |
| Valtropin (somatropin), r hGH, produced in S. cerevisiae. Biosimilar to Humatrope. | Biopartners, LG Life Sciences (Republic of Korea & Reutlingen, Germany) | Certain forms of growth disturbance in children and adults |
2007 (US) 2006 (EU) Withdrawn 2012 (EU), 2019 (US) |
| Omnitrope (somatropin), biosimilar to Genotropin (in EU) r hGH, produced in E. coli. |
Sandoz (Kundl, Austria) Sandoz (Princeton, NJ, USA) |
Certain forms of growth disturbance in children and adults | 2006 (EU & US) |
| Somavert (pegvisomant), r hGH analog (antagonist), produced in E. coli and PEGylated. | Pfizer | Acromegaly |
2003 (US) 2002 (EU) |
| Nutropin AQ (somatropin), r hGH, produced in E. coli. Different formulation of Nutropin (see below). | Ipsen Pharma (Boulogne-Billancourt, France) | Growth failure, Turner syndrome |
2001 (EU) 1994 (US) Withdrawn 2008 (EU) |
| Serostim (somatropin), r hGH, produced in mouse C127 cells. | EMD Serono (Rockland, MA, USA) | AIDS-associated catabolism and wasting | 1996 (US) |
| Saizen (somatropin), r hGH, produced in mouse C127 cells. | EMD Serono (Rockland, MA, USA) | hGH deficiency in children | 1996 (US) |
| Genotropin (somatropin), r hGH, produced in E. coli. | Pfizer (New York) | hGH deficiency in children | 1995 (US) |
| Norditropin (somatropin), r hGH, produced in E. coli. | Novo Nordisk | Growth failure in children due to inadequate growth hormone secretion | 1995 (US) |
| Tev-Tropin, Bio-tropin (somatropin), r hGH, produced in E. coli. | Teva Pharmaceuticals (North Wales, PA, USA) | hGH deficiency in children | 1995 (US) |
| Nutropin (somatropin), r hGH, produced in E. coli. | Roche/Genentech | hGH deficiency in children | 1994 (US) |
| Humatrope (somatropin), r hGH, produced in E. coli. | Eli Lilly (Indianapolis) | hGH deficiency in children | 1987 (US) |
| Protropin (somatrem), r hGH differing from hGH by an extra N-terminal methionine, produced in E. coli. | Genentech (South San Francisco, CA, USA) | hGH deficiency in children |
1985 (US) Withdrawn 2004 |
| Follicle-stimulating hormone | |||
| Rekovelle (follitropin delta), rh FSH, produced in PER.C6 cells | Ferring Pharmaceuticals (Copenhagen) | Anovulation | 2016 (EU) |
| Bemfola (follitropin alfa), rh FSH, produced in CHO cells. Biosimilar to Gonal F. | Gedeon Richter (Budapest) | Anovulation (women), failure of spermatogenesis (men) | 2014 (EU) |
| Ovaleap (follitropin alfa), rh FSH, produced in CHO cells. Biosimilar to Gonal F. | Theramex Ireland (Dublin) | Infertility, subfertility | 2013 (EU) |
| Elonva (corifollitropin alfa), modified rh FSH with the C-terminal peptide of the β-subunit of hCG fused to the FSH β-chain, produced in CHO cells. | N.V. Organon (Oss, the Netherlands) | Controlled ovarian stimulation | 2010 (EU) |
| Fertavid (follitropin beta), rh FSH, produced in CHO cells. Active substance same as in Puregon (see below). | Merck Sharp & Dohme | Infertility |
2009 (EU) Withdrawn 2020 |
| Pergoveris (follitropin alfa/lutropin alfa) combination product containing rh FSH and rh luteinizing hormone, both produced in CHO cells. | Merck (Amsterdam) | Stimulation of follicular development in women with severe luteinizing hormone and FSH deficiency | 2007 (EU) |
| Follistim (follitropin beta), rh FSH, produced in CHO cells. | Merck (Whitehouse Station, NJ, USA) | Infertility | 1997 (US) |
| Puregon (follitropin beta), rh FSH, produced in CHO cells. | N.V. Organon | Anovulation and superovulation | 1996 (EU) |
| Gonal F (follitropin alfa), rh FSH, produced in CHO cells. | Merck Serono, EMD Serono (Rockland, MD, USA) | Anovulation and superovulation |
1997 (US) 1995 (EU) |
| Other hormones | |||
| Sondelbay (teriparatide), the active N-terminal fragment of human PTH, produced in E. coli. Biosimilar to Forsteo. | Accord Healthcare (Barcelona, Spain) | Osteoporosis | 2022 (EU) |
| Wegovy (semaglutide), r glucagon-like peptide-1 (GLP-1) analog with a linker and a fatty acid side chain, produced in Saccharomyces cerevisiae and then chemically modified. Same active substance as in Ozempic. | Novo Nordisk (Bagsvaerd, Denmark) | Weight loss and weight control | 2022 (EU) |
| Voxzogo (vosoritide), truncated (39-amino-acid) modified analog of the native human C-type natriuretic peptide, expressed in E. coli. |
BioMarin International (Cork, Ireland) BioMarin Pharmaceutical (Novato, CA, USA) |
Achondroplasia | 2021 (EU & US) |
| Livogiva (teriparatide; r 1-34 N-terminal fragment of endogenous human PTH, produced in Pseudomonas fluorescens. Biosimilar to Forsteo. | Theramex Ireland (Dublin) | Osteoporosis | 2020 (EU) |
| Qutavina (teriparatide; r 1-34 N-terminal fragment of endogenous human PTH, produced in P. fluorescens. Biosimilar to Forsteo. | EuroGenerics (Amsterdam, the Netherlands) | Osteoporosis |
2020 (EU) Withdrawn 2020 |
| Rybelsus (semaglutide; long-acting hGLP 1 analog (receptor agonist), produced in S. cerevisiae and chemically modified via fatty acid attachment (acylation). Same active substance as Ozempic, but developed for oral use. Oral bioavailability improved via inclusion of a novel absorption-enhancer excipient (salcaprozate sodium (SNAC), a fatty acid derivative). | Novo Nordisk | Type 2 diabetes |
2020 (EU) 2017 (US) |
| Myalepta (EU), Myalept (US) (metreleptin), rh leptin analog, produced in E. coli. |
Amryt Pharmaceuticals (Dublin) Aegerion Pharmaceuticals (Cambridge, MA, USA) |
Some forms of lipodystrophy |
2018 (EU) 2014 (US) |
| Ozempic (semaglutide), human GLP-1 receptor agonist, produced in S. cerevisiae and covalently modified by attachment of a C18 fatty acid. | Novo Nordisk | Diabetes mellitus type 2 |
2018 (EU) 2017 (US) |
| Movymia (teriparatide), rh PTH fragment, produced in E. coli. Biosimilar to Fortseo. Same product as Terrosa (see below). | Stada Arzneimittel (Bad Vilbel, Germany) | Osteoporosis | 2017 (EU) |
| Natpar (parathyroid hormone), rh PTH, full length, produced in E. coli. Same product as Preotact (see below). | Takeda Pharmaceuticals (Dublin) | Hypoparathyroidism | 2017 (EU) |
| Terrosa (teriparatide), rh PTH fragment, produced in E. coli. Biosimilar to Fortseo. Same product as Movymia (see above). | Gedeon Richter (Budapest) | Osteoporosis | 2017 (EU) |
| Natpara (parathyroid hormone), rh PTH, produced in E. coli. | Shire-NPS Pharmaceuticals (Lexington, MA, USA) | Hypocalcemia | 2015 (US) |
| Saxenda (liraglutide), human GLP-1 analog, produced in S. cerevisiae and covalently modified by palmitic acid. Active substance same as in Victoza (see below). | Novo Nordisk | Obesity | 2015 (EU) |
| Eperzan (EU), Tanzeum (US) (albiglutide), GLP-1 receptor agonist: two tandem copies of modified human GLP-1 fused to human albumin, produced in S. cerevisiae. |
GSK (Carrigaline, Ireland) GSK (Research Triangle Park, NC, USA) |
Diabetes mellitus type 2 |
2014 (EU & US) Withdrawn 2018 |
| Trulicity (dulaglutide), fusion protein consisting of a GLP-1 analog linked to a human IgG Fc domain, produced in a mammalian cell line. | Eli Lilly (Utrecht, the Netherlands, & Indianapolis) | Diabetes mellitus type 2 | 2014 (EU & US) |
| Gattex (US), Revestive (EU) (teduglutide), rh GLP-2 analog, produced in E. coli. |
Takeda (Dublin) Shire (Lexington, MA, USA) |
Short bowel syndrome | 2012 (EU & US) |
| Victoza (liraglutide), GLP-1 analog with attached fatty acid, produced in S. cerevisiae. | Novo Nordisk | Diabetes mellitus type 2 |
2010 (US) 2009 (EU) |
| Preotact, rh PTH, produced in E. coli. | NPS Pharma | Osteoporosis |
2006 (EU) Withdrawn 2014 |
| Fortical, r salmon calcitonin, produced in E. coli. |
Upsher-Smith Laboratories (Minneapolis, MN, USA) Unigene Laboratories (Fairfield, NJ, USA) |
Postmenopausal osteoporosis | 2005 (US) |
| Luveris (lutropin alfa), rh luteinizing hormone, produced in CHO cells. | Merck (Amsterdam) | Some forms of infertility |
2004 (US) 2000 (EU) Withdrawn 2007 (US) |
| Forsteo (EU), Forteo (US) (teriparatide), r shortened human PTH, produced in E. coli. |
Eli Lilly (Utrecht, the Netherlands) Lilly (Indianapolis) |
Established osteoporosis in some postmenopausal women |
2003 (EU) 1987 (US) |
| Natrecor (nesiritide), rh natriuretic peptide, produced in E. coli. | Johnson & Johnson/Scios (Titusville, NJ, USA) | Acutely decompensated congestive heart failure | 2001 (US) |
| Ovitrelle (EU), Ovidrel (US) (choriogonadotropin alfa) rh chorionic gonadotropin, produced in CHO cells. |
Merck (Amsterdam) EMD Serono (Rockville, MD, USA) |
Selected assisted reproductive techniques |
2001 (EU) 2000 (US) |
| Thyrogen (thyrotropin alfa), rh thyroid-stimulating hormone, produced in CHO cells. | Genzyme (Amsterdam & Cambridge, MA, USA) | Thyroid cancer (detection and treatment) |
1998 (US) 2000 (EU) |
| Forcaltonin, r salmon calcitonin, produced in E. coli. | Unigene UK (Bushey Heath, UK) | Paget disease |
1999 (EU) Withdrawn 2008 |
| Glucagen, rh glucagon, produced in S. cerevisiae. | Novo Nordisk | Hypoglycemia | 1998 (US) |
| Glucagon (glucagon, recombinant), rh glucagon, produced in E. coli. | Eli Lilly (Indianapolis) | Hypoglycemia | 1998 (US) |
| Bonsity. r PTH analog, expressed in P. fluorescens. | Pfenex (San Diego, CA, USA) | Osteoporosis | 1987 (US) |
| Recombinant growth factors | |||
| Erythropoietin | |||
| Retacrit (epoetin zeta (EU), epoetin alfa-epbx (US)), rh EPO, produced in CHO cells. Biosimilar to Eprex and Erypo. | Pfizer (Brussles, Belgium & Lake Forest, IL, USA) | Anemia |
2018 (US) 2007 (EU) |
| Biopoin (epoetin theta), rh EPO, produced in CHO cells. | Teva (Ulm, Germany) | Anemia | 2009 (EU) |
| Eporatio (epoetin theta), rh EPO, produced in CHO cells. | Ratiopharm (Ulm, Germany) | Anemia | 2009 (EU) |
| Abseamed (epoietin alfa), biosimilar to Eprex/Erypo, produced in CHO cells. Biosimilar to rh EPO. | Medice Arzneimittel Pütter (Iserlon, Germany) | Anemia associated with chronic renal failure | 2007 (EU) |
| Binocrit (epoetin alfa), biosimilar to Eprex/Erypo, produced in CHO cells. Biosimilar to rh EPO. | Sandoz (Kundl, Austria) | Anemia associated with chronic renal failure | 2007 (EU) |
| Epoetin alfa Hexal (epoietin alfa), biosimilar to Eprex/Erypo produced in CHO cells. Biosimilar to rh EPO. | Hexal (Holzkirchen, Germany) | Anemia associated with chronic renal failure | 2007 (EU) |
| Mircera (methoxy polyethylene glycol-epoetin beta), rh EPO, produced in CHO cells and PEGylated. | Roche (Grenzach-Wyhlen, Germany) | Anemia associated with chronic kidney disease | 2007 (EU & US) |
| Silapo (epoetin zeta), biosimilar toEprex/Erypo, produced in CHO cells. Biosimilar to rh EPO. | Stada (Bad Vilbel, Germany) | Anemia associated with chronic renal failure | 2007 (EU) |
| Dynepo (epoetin delta), rh EPO, produced in a human cell line. | Shire Pharmaceuticals (Hampshire, UK) | Anemia |
2002 (EU) Withdrawn 2009 |
| Aranesp (darbepoetin alfa), long-acting r EPO analog, produced in CHO cells (see Nespo, below) |
Amgen (Breda, the Netherlands) Amgen (Thousand Oaks, CA, USA) |
Anemia | 2001 (EU & US) |
| Nespo (darbepoetin alfa), long-acting r EPO analog, produced in CHO cells (see Aranesp above) | Dompé Biotec (Milan) | Anemia |
2001 (EU) Withdrawn 2008 |
| Neorecormon (epoietin beta), rh EPO, produced in CHO cells. | Roche | Anemia | 1997 (EU) |
| Procrit (epoietin alfa), rh EPO, produced in a mammalian cell line. | Janssen Biotech (Horsham, PA, USA) | Anemia | 1990 (US) |
| Epogen (epoietin alfa), rh EPO, produced in CHO cells. | Amgen | Anemia | 1989 (US) |
| Colony-stimulating factors | |||
| Fylnetra (pegfilgrastim-pbbk; rh G-CSF, produced in E. coli and PEGylated. Biosimilar to Neulasta. | Amneal Pharmaceuticals (Bridgewater, NJ, USA) | Neutropenia | 2022 (US) |
| Releuko (filgrastim-ayow; rh G-CSF, produced in E. coli. Biosimilar to Neupogen. | Kashiv BioSciences (Piscataway, NJ, USA) | Neutropenia | 2022 (US) |
| Stimufend (pegfilgrastim), rh G-CSFr produced in E. coli. Biosimilar to Neulasta. | Fresenius Kabi Deutschland (Höhe, Germany) | Neutropenia | 2022 (EU) |
| Nyvepria (pegfilgrastim (EU), pegfilgrastim-apgf (US)), rh G-CSF, expressed in E. coli and PEGylated. Biosimilar to Neulasta. |
Pfizer Europe MA EEIG (Brussels, Belgium) Pfizer (New York). |
Neutropenia | 2020 (EU & US) |
| Cegfila (previously Pegfilgrastim Mundipharma; pegfilgrastim), rh G-CSF, produced in E. coli and PEGylated Biosimilar to Neulasta. | Mundipharma (Dublin) | Neutropenia | 2019 (EU) |
| Grasustek (pegfilgrastim), rh-G-CSF, produced in E. coli and PEGylated. Biosimilar to Neulasta. | Juta Pharma (Flensburg, Germany, USA) | Neutropenia | 2019 (EU) |
| Ziextenzo (pegfilgrastim (EU), pegfilgrastim-bmez (US)), rh G-CSF, produced in E. coli and PEGylated. Biosimilar to Neulasta. | Sandoz (Kundl, Austria) | Neutropenia |
2019 (US) 2018 (EU) |
| Fulphila (pegfilgrastim-jmdb), rh G-CSF, produced in E. coli and PEGylated. Biosimilar to Neulasta. |
Mylan (Rockford, IL USA) Mylan (Saint-Priest, France) |
Neutropenia | 2018 (US and EU) |
| Nivestym (filgrastim-aafi; US), Nivestim (filgrastim; EU), rh G-CSF, produced in E. coli. Biosimilar to Neupogen. | Pfizer | Neutropenia |
2018 (US) 2010 (EU) |
| Pelgraz (pegfilgrastim), rh G-CSF, produced in E. coli and pEGylated. Biosimilar to Neulasta. | Accord Healthcare (Barcelona, Spain) | Neutropenia | 2018 (EU) |
| Pelmeg (pegfilgrastim), rh G-CSF, produced in E. coli and PEGylated. Biosimilar to Neulasta. | Cinfa Biotech (Olloki, Spain) | Neutropenia | 2018 (EU) |
| Udenyca (pegfilgrastim (EU), pegfilgrastim-cbqv (US)), rh G-CSF, produced in E. coli and PEGylated Biosimilar to Neulasta. |
ERA Consulting (Walsrode, Germany) Coherus BioSciences (Redwood City, CA, USA) |
Neutropenia |
2018 (EU & US) Withdrawn (EU) |
| Ristempa (pegfilgrastim), covalent conjugate of rh G-CSF, produced in E. coli and conjugated to 20-kDa PEG. | Amgen (Breda, the Netherlands) | Neutropenia |
2015 (EU) Withdrawn 2017 |
| Zarxio (US), Zarzio (EU) (filgrastim-sndz), rh G-CSF, produced in E. coli. | Sandoz (Princeton, NJ, USA, & Kundl, Austria) | Neutropenia |
2015 (US) 2009 (EU) |
| Accofil (filgrastim), G-CSF, produced in E. coli. Biosimilar to Neupogen. Same product as Grastofil (see below). | Accord Healthcare (Barcelona, Spain) | Neutropenia | 2014 (EU) |
| Grastofil (filgrastim), rh G-CSF, produced in E. coli. Biosimilar to Neupogen. Same product as Accofil (see above). | Accord Healthcare (Barcelona, Spain) | Neutropenia | 2013 (EU) |
| Lonquex (lipegfilgrastim), rh G-CSF, produced in E. coli and PEGylated. | Teva Pharmaceuticals (Utrecht, the Netherlands) | Neutropenia | 2013 (EU) |
| Granix (tbo-filgrastim), rh G-CSF, produced in E. coli. Same product as Tevagrastim (see below). | Teva Pharmaceuticals (North Wales, PA, USA) | Neutropenia | 2012 (US) |
| Filgrastim Hexal (filgrastim), biosimilar to Neupogen, produced in E. coli. Biosimilar rh G-CSF. | Hexal | Neutropenia | 2009 (EU) |
| Biograstim (filgrastim), biosimilar to Neupogen produced in E. coli. Biosimilar rh G-CSF. | ABZ-Pharma (Ulm, Germany) | Neutropenia |
2008 (EU) Withdrawn 2015 |
| Ratiograstim (filgrastim), biosimilar to Neupogen, produced in E. coli. Biosimilar rh G-CSF. | Ratiopharm (Ulm, Germany) | Neutropenia | 2008 (EU) |
| Tevagrastim (filgrastim), rh G-CS, produced in E. coli. Biosimilar to Neupogen. Same product as Granix (see above). | Teva (Radebeul, Germany) | Neutropenia | 2008 (EU) |
| Filgrastim Ratiopharm (filgrastim), produced in E. coli. Biosimilar to Filgrastim. | Ratiopharm | Neutropenia |
2008 (EU) Withdrawn 2011 |
| Neulasta (EU and US), Neupopeg (EU) (pegfilgrastim), PEGylated rh G-CSF. | Amgen (Breda, the Netherlands) | Chemotherapy-induced neutropenia |
2002 (EU & US) Neupopeg withdrawn 2008 (EU) |
| Leukine (sargramostim), rh GM-CSF differing from the native protein by an R23L substitution, produced in E. coli. | Partner Therapeutics (Lexington, MA, USA) | Autologous bone marrow transplantation |
1991 (US) Withdrawn 2008 and reformulated without EDTA 2008 |
| Neupogen (filgrastim), rh G-CSF differing from native protein by an extra N-terminal methionine, produced in E. coli. | Amgen (Thousand Oaks, CA, USA) | Chemotherapy-induced neutropenia | 1991 (US) |
| Other growth factors | |||
| Oxervate (cenegermin (EU), cenegermin-bkbj (US)), ophthalmic solution, rh nerve growth factor, produced in E. coli. |
Dompé Farmaceutici (Milan) Dompé U.S. (Boston) |
Neurotophic keratitis |
2018 (US) 2017 (EU) |
| Increlex (mecaserim), rh IGF-1, produced in E. coli. | Ipsen Pharma | Growth failure in children with IGF-1 deficiency or hGH gene deletion (long-term treatment) |
2007 (EU) 2005 (US) |
| iPlex (mecasermin rinfabate), a complex of rh IGF-1 and rh IGF binding protein-3, produced separately in E. coli. | Insmed (Glen Allen, VA, USA) | Growth failure in children with severe primary IGF-1 deficiency or hGH gene deletion (long-term treatment) |
2005 (US) Withdrawn 2007 for IGF-1 deficiency |
| Kepivance (palifermin), rh keratinocyte growth factor, produced in E. coli. | Swedish Orphan Biovitrum | Severe oral mucositis in selected patients with hematologic cancers |
2005 (EU) 2004 (US) Withdrawn 2016 (EU) |
| GEM 21S: Regranex (see below) and tricalcium phosphate; growth-factor-enhanced matrix. | BioMimetic Pharmaceuticals (Franklin, TN, USA) | Periodontally related defects | 2005 (US) |
| Regranex (becaplermin), rh platelet-derived growth factor receptor-BB, produced in S. cerevisiae. |
Janssen-Cilag International (Beerse, Belgium) Johnson & Johnson (Raritan, NJ, USA) |
Lower-extremity diabetic neuropathic ulcers |
1997 (US) 1999 (EU) Withdrawn 2012 (EU) |
| Recombinant interferons, interleukins and tumor necrosis factor | |||
| Interferon-α | |||
| Besremi (ropeginterferon alfa-2b (EU), ropeginterferon alfa-2b-njft (US); rh-interferon alfa-2b with an additional N-terminal proline conjugated to a 40-kDa two-arm PEG moiety, produced in E . coli. |
AOP Orphan Pharmaceuticals (Vienna) PharmaEssentia (Burlington, MA, USA) |
Polycythemia vera |
2021 (US) 2019 (EU) |
| PEG-Intron/Rebetol combo pack (peginterferon alfa-2b/ribavirin), rh IFN-α-2b, produced in E. coli and PEGylated, and ribavirin. | Schering Plough (Kenilworth, NJ, USA) | Chronic hepatitis C | 2008 (US) |
| Pegasys (peginterferon alfa-2a), IFN-α-2b, produced in E. coli and PEGylated. |
zr pharma (Vienna) Roche/Genentech |
Hepatitis C | 2002 (EU & US) |
| PEG-Intron (peginterferon alfa-2b), IFN-α-2b, produced in E. coli and PEGylated. | Merck Sharp & Dohme | Chronic hepatitis C |
2001 (US) 2000 (EU) Withdrawn 2021 (EU) |
| Viraferon (interferon alfa-2b), produced in E. coli. | Schering Plough (Brussels) | Chronic hepatitis B, C |
2000 (EU) Withdrawn 2008 |
| ViraferonPeg (peginterferon alfa-2b), IFN-α-2b, produced in E. coli and PEGylated. | Merck Sharp & Dohme | Chronic hepatitis C |
2000 (EU) Withdrawn 2021 |
| Intron A, Alfatronol (interferon alfa-2b), produced in E. coli. | Merck Sharp & Dohme | Cancer, genital warts, hepatitis B and C, HPV |
2000 (EU) 1986 (US) |
| Rebetron (ribavirin/interferon alfa-2b), produced in E. coli. | Schering Plough | Chronic hepatitis C | 1999 (US) |
| Infergen (interferon alficon-1), r IFN-α, synthetic type I, produced in E. coli. |
Astellas Pharma Europe (Leiderdorp, the Netherlands) Kadmon Pharmaceuticals (Warrendale, PA, USA) |
Chronic hepatitis C |
1999 (EU) 1997 (US) Withdrawn 2006 (EU), 2013 (US) |
| Roferon A (interferon alfa-2a), produced in E. coli. | Roche | Hairy cell leukemia |
1986 (US) Withdrawn 2007 |
| Interferon-β and interferon-γ | |||
| Plegridy (peginterferon beta-1a), rh IFN-β-1a, produced in CHO cells and PEGylated. | Biogen (Badhoevedorp, the Netherlands) | Multiple sclerosis | 2014 (EU & US) |
| Extavia (interferon beta-1b), rh IFN-β-1b, produced in E. coli. |
Novartis (Dublin) Novartis Pharmaceuticals (East Hanover, NJ USA) |
Multiple sclerosis |
2009 (US) 2008 (EU) |
| Rebif (interferon beta-1a), rh IFN-β-1a, produced in CHO cells. |
Merck (Amsterdam) EMD Serono (Rockland, MA, USA) |
Relapsing/remitting multiple sclerosis |
2002 (US) 1998 (EU) |
| Avonex (interferon beta-1a), rh IFN-β-1a, produced in CHO cells. | Biogen (Badhoevedorp, the Netherlands) | Relapsing multiple sclerosis |
1997 (EU) 1996 (US) |
| Betaferon (interferon beta-1b), r IFN-β-1b differing from native protein by C17S, produced in E. coli. | Bayer Pharma | Multiple sclerosis | 1995 (EU) |
| Betaseron (interferon β-1b), differing from human protein by C17S, produced in E. coli. |
Berlex Laboratories (Richmond, CA, USA) Chiron (Emeryville, CA, USA) |
Relapsing/remitting multiple sclerosis | 1993 (US) |
| Actimmune (interferon gamma-1b), produced in E. coli. | Vidara Therapeutics (Dublin) | Chronic granulomatous disease | 1990 (US) |
| Others | |||
| Kineret (anakinra), rh IL-1 receptor antagonist, produced in E. coli. | Swedish Orphan Biovitrum (Stockholm) | Rheumatoid arthritis | 2001 (US) |
| Beromun (tasonermin), rh TNF-α, produced in E. coli. | Belpharma (Luxembourg) | Adjunct to surgery for subsequent tumor removal, to prevent or delay amputation | 1999 (EU) |
| Neumega (oprelvekin), r IL-11 lacking N-terminal proline of native molecule, produced in E. coli. | Pfizer (Philadelphia), Genetics Institute | Prevention of chemotherapy-induced thrombocytopenia | 1997 (US) |
| Proleukin (aldesleukin) r IL-2, differs from native molecule in absence of N-terminal alanine and presence of C125S substitution, produced in E. coli. | Prometheus Laboratories (San Diego) | Renal cell carcinoma | 1992 (US) |
| Vaccines | |||
| Hepatitis B | |||
| PreHevbri (EU), Prehevbrio (US), r hepatitis B surface antigen produced in CHO cells genetically modified to produce the hepatitis B virus envelope proteins, which include the small (S), middle (pre-S2) and large (pre-S1) hepatitis B surface antigens (HBsAg), representing the active substance. |
VBI Vaccines (Amsterdam) VBI Vaccines (Cambridge, MA, USA) |
Hepatitis B vaccine |
2022 (EU) 2021 (US) |
| Heplisav B, r-hepatitis B surface antigen, produced in Hansenula polymorpha |
Dynavax (Dusseldorf, Germany) Dynavax Technologies (Berkeley, CA, USA) |
Hepatitis B vaccine |
2021 (EU) 2017 (US) |
| Vaxelis, multi-component vaccine containing r HBsAg, produced in S. cerevisiae as one component. |
MCM Vaccine (Swiftwater PA, USA) Merck (Whitehouse Station, NJ, USA) Sanofi Pasteur (Swiftwater PA, USA) |
Immunization against diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B and invasive disease due to Haemophilus influenzae type b | 2018 (US) |
| Hexacima (also sold as Hexyon), multi-component vaccine containing r HBsAg, produced in H. polymorpha as one component. | Sanofi Pasteur (Lyon, France) | Immunization against several pathogens and toxins | 2013 (EU) |
| Ambirix, combination vaccine containing r HBsAg, produced in S. cerevisiae as one component. | GSK (Rixensart, Belgium) | Immunization against hepatitis A and B | 2002 (EU) |
| Pediarix, combination vaccine containing r HBsAg, produced in S. cerevisiae as one component. | GSK | Immunization of children against various conditions inducing hepatitis B | 2002 (US) |
| HBVAXPRO (r HBsAg), produced in S. cerevisiae. | Merck Sharp & Dohme (Haarlem, the Netherlands) | Immunization of children and adolescents against hepatitis B | 2001 (EU) |
| Twinrix, combination vaccine containing r HBsAg, produced in S. cerevisiae as one component. | GSK | Immunization against hepatitis A and B |
2001 (US) 1997 (EU pediatric form) 1996 (EU adult form) |
| Infanrix-hexa, combination vaccine containing r HbsAg, produced in S. cerevisiae as one component. | GSK | Immunization against diphtheria, tetanus, pertussis, Haemophilus influenzae b, hepatitis B and polio | 2000 (EU) |
| Infanrix-penta, combination vaccine, containing r HbsAg, produced in S. cerevisiae as one component. | GSK | Immunization against diphtheria, tetanus, pertussis, polio, and hepatitis B |
2000 (EU) Withdrawn 2013 |
| Hepacare (r S, pre-S & pre-S2 HBsAg), produced in a murine cell line. | Evans Vaccines (Liverpool, UK) | Immunization against hepatitis B |
2000 (EU) Withdrawn 2002 |
| Hexavac, combination vaccine containing r HBsAg, produced in S. cerevisiae as one component. | Sanofi Pasteur | Immunization against diphtheria, tetanus, pertussis, hepatitis B, polio and H. influenzae b |
2000 (EU) Withdrawn 2012 |
| Procomvax, combination vaccine containing r HBsAg as one component. | Sanofi Pasteur | Immunization against H. influenzae b and hepatitis B |
1999 (EU) Withdrawn 2009 |
| Primavax, combination vaccine containing r HBsAg, produced in S. cerevisiae as one component. | Sanofi Pasteur | Immunization against diphtheria, tetanus and hepatitis B |
1998 (EU) Withdrawn 2000 |
| Engerix B, r HbsAg, produced in S. cerevisiae. | GSK | Immunization against hepatitis B | 1998 (US) |
| Infanrix Hep B, combination vaccine containing r HbsAg, produced in S. cerevisiae as one component. | GSK | Immunization against diphtheria, tetanus, pertussis and hepatitis B |
1997 (EU) Withdrawn 2005 |
| Comvax, combination vaccine containing HBsAg, produced in S. cerevisiae as one component. | Merck (Whitehouse Station, NJ, USA) | Immunization of infants against H. influenzae b and hepatitis B | 1996 (US) |
| Tritanrix-Hep B, combination vaccine containing r HBsAg, produced in S. cerevisiae as one component. | GSK | Immunization against hepatitis B, diphtheria, tetanus and pertussis |
1996 (EU) Withdrawn 2014 |
| Recombivax, r HBsAg, produced in S. cerevisiae. | Merck (Whitehouse Station, NJ, USA) | Immunization against hepatitis B | 1986 (US) |
| COVID-19 | |||
| Nuvaxovid/Novavax COVID-19, vaccine directed at SARS-CoV-2 r (full-length) spike protein, produced in Spodoptera frugiperda (Sf9) insect cells using r baculovirus system. | Novavax CZ (Jevany, Czechia) | Vaccine (COVID-19) |
2022 (US; Emergency Use Authorization) 2021 (EU; Conditional Marketing Authorisation) |
| Spikevax (previously COVID-19 Vaccine Moderna; elasomeran), ss mRNA produced by cell-free in vitro transcription from DNA templates encoding (full-length) viral spike (S) protein of SARS-CoV-2. |
Moderna Biotech Spain (Madrid) ModernaTX (Cambridge, MA, USA) |
Vaccine (COVID-19) |
2021 (EU; Conditional Marketing Authorization) 2020 (US; Emergency Use Authorisation) |
| Jcovden (COVID-19 vaccine Janssen), r replication-deficient (E1- and partially E3-gene-deleted) adenovirus type 26 encoding SARS-CoV-2 spike glycoprotein, propagated in a PER.C6 cell line (derived from human embryonal retina cells). | Janssen-Cilag International (Beerse, Belgium) | Vaccine (COVID-19) |
2021 (EU; Conditional Marketing Authorization) 2021 (US; Emergency Use Authorisation) |
| Vaxzevria, r replication-deficient (E1- and E3-deleted) chimpanzee adenovirus encoding SARS-CoV-2 spike protein combined with a tPA leader sequence, propagated in T-REx-293 cells (derivative of HEK293). | AstraZeneca (Sodertalje, Sweden) | Vaccine (COVID-19) | 2021 (EU; Conditional Marketing Authorisation) |
| Comirnaty (Pfizer BioNTech COVID-19 vaccine; tozinameran; COVID-19 mRNA vaccine, nucleoside modified), ss, 5′-capped mRNA produced by cell-free in vitro transcription from DNA templates, encoding SARS-CoV-2 spike (S) protein. |
BioNTech Manufacturing (Mainz, Germany) Pfizer (New York) |
Vaccine (COVID-19) |
2020 (US; Emergency Use Authorization) 2020 (EU; Conditional Marketing Authorisation) |
| Other | |||
| Vaxneuvance (Pneumococcal 15-valent Conjugate Vaccine), containing one r element, expressed in P. fluorescens. | Merck | Vaccine against Streptococcus pneumonia | 2021 (US) |
| Mvabea (ebolavirus vaccine MVA-BN-Filo), engineered vaccinia strain encoding proteins from different viral strains. | Janssen-Cilag International (Beerse, Belgium) | Ebolavirus vaccine | 2020 (EU) |
| Supemtek (Quadrivalent influenza vaccine), consisting of 4 r hemagglutinin (rHA) proteins, each expressed separately in Sf9 insect cells using a baculovirus protein expression vector. | Sanofi Pasteur (Lyon, France) | Influenza vaccine | 2020 (EU) |
| Vaxchora, live, attenuated Vibrio cholerae O1 strain Classical biotype genetically engineered via deletion of part of the enterotoxin catalytic subunit A gene and inclusion of a mercury resistance marker (allowing it to be distinguished from wild-type V. cholerae). | Emergent Netherlands (Amsterdam) | Cholera vaccine | 2020 (EU) |
| Zabdeno (Ebola vaccine; Ad26.ZEBOV-GP), r replication-incompetent, adenovirus type 26 (Ad26) encoding full-length glycoprotein (GP) of Ebola virus Zaire (ZEBOV) Mayinga strain. | Janssen-Cilag International (Beerse, Belgium) | Ebolavirus vaccine | 2020 (EU) |
| Dengvaxia, dengue tetravalent vaccine (live, attenuated) based upon r engineered yellow fever virus–dengue virus. |
Sanofi Pasteur (Lyon, France) Sanofi Pasteur (Swiftwater PA, USA) |
Dengue vaccine |
2019 (US) 2018 (EU) |
| Ervebo (Ebola Zaire Vaccine), live, r vesicular stomatitis virus with its envelope glycoprotein, replaced with ebolavirus Zaire surface glycoprotein, cultured in Vero cells. |
Merck Sharp & Dohme (Haarlem, the Netherlands) Merck (Whitehouse Station, NJ, USA) |
Ebolavirus vaccine | 2019 (EU & US) |
| Shingrix (zoster vaccine recombinant, adjuvanted), r Varicella zoster virus surface glycoprotein E antigen component, produced in CHO cells. |
GlaxoSmithKline Biologicals (Rixensart, Belgium) GlaxoSmithKline (Research Triangle Park, NC, USA) |
Herpes zoster (shingles) prevention |
2018 (EU) 2017 (US) |
| Trumenba (meningococcal group B vaccine), two r Neisseria meningitides serogroup B proteins independently expressed in E. coli. |
Pfizer (Brussels) Pfizer (Philadelphia) |
Vaccine against N. meningitides serogroup B |
2017 (EU) 2014 (US) |
| Pandemic influenza vaccine H5N1, vaccine derived from engineered viral strain containing gene segments from appropriate influenzavirus strains, produced in embryonated eggs. | MedImmune (Nijmegen, the Netherlands) | Influenza vaccine | 2016 (EU) |
| Bexsero (meningococcal group B vaccine), mixture of 3 N. meningitidis serogroup B proteins, produced in E. coli. |
GSK (Siena, Italy) GlaxoSmithKline (Research Triangle Park, NC, USA) |
Active immunization against N. meningitidis serogroup B |
2015 (US) 2013 (EU) |
| Gardasil 9, mixture of the major capsid protein (L1) of 9 strains of HPV, each produced in S. cerevisiae. |
MSD (Haarlem, the Netherlands) Merck (Whitehouse Station, NJ, USA) |
Active immunization for those above 9 years of age against HPV-caused cancers and genital warts |
2015 (EU) 2014 (US) |
| Flublok, r hemagglutinin proteins from 3 influenza viruses, produced in an insect cell line. | Protein Sciences (Meriden, CT, USA) | Immunization against influenza | 2013 (US) |
| Provenge (sipuleucel-T), autologous peripheral blood mononuclear cells in combination with r prostatic acid phosphatase linked to GM-CSF, produced in an insect cell line. | Dendreon (Seal Beach, CA, USA) | Prostate cancer |
2013 (EU) 2010 (US) Withdrawn 2015 (EU) |
| Cervarix, r C-terminally truncated major capsid L1 proteins from HPV types 16 and 18, produced in a baculovirus-based expression system | GSK | Prevention of cervical cancer |
2009 (US) 2007 (EU) |
| Gardasil (EU & US), Silgard (EU), r vaccine containing major capsid proteins from four HPV types, produced in S. cerevisiae. |
Merck Sharp & Dohme (Haarlem, the Netherlands) Merck (Whitehouse Station, NJ, USA) |
Vaccination against diseases caused by HPX | 2006 (EU & US) |
| Dukoral, Vibrio cholerae and r cholera toxin B subunit. | Valneva Sweden (Stockholm) | Immunization against disease caused by V. cholerae subunit O1 | 2004 (EU) |
| Lymerix (r OspA), Borrelia burgdorferi surface lipoprotein, produced in E. coli. | GSK | Immunization against Lyme disease |
1998 (US) Withdrawn 2002 |
| Triacelluvax, combination vaccine with r modified pertussis toxin as one component. | Chiron (Siena, Italy) | Immunization against diphtheria, tetanus and pertussis |
1999 (EU) Withdrawn 2002 |
| Monoclonal-antibody-based products | |||
| Alymsys (bevacizumab-maly (US), bevacizumab (EU)), humanized IgG1 targeting VEGF, produced in a CHO cell line. Biosimilar to avastin. Same product as Oyavas. |
Amneal Pharmaceuticals (Bridgewater, NJ, USA) Mabxience Research (Madrid) |
Colorectal & various other cancers |
2022 (US) 2021 (EU) |
| Bebtelovimab, rh IgG1 that binds an epitope of the SARS-CoV-2 spike protein RBD, expressed in CHO cell line. | Eli Lilly (Indianapolis) | COVID-19 | 2022 (US; Emergency Use Authorization) |
| Enjaym (sutimlimab-jome), humanized IgG4 targeting complement protein component 1s, produced in a CHO cell line. | Bioverativ USA (Waltham, MA, USA) | Cold agglutinin disease | 2022 (US) |
| Evusheld (tixagevimab & cilgavimab), combination of 2 human IgG1κ mAbs directed against 2 (non-overlapping) epitopes on the SARS-CoV-2 spike protein RBD, produced in CHO cell lines. | AstraZeneca (Sodertalje, Sweden) | COVID -19 prevention. |
2022 (EU) 2021 (US; Emergency Use Authorization) |
| Lunsumio (mosunetuzumab), humanized full-length anti-CD20/CD3 T-cell-engaging bispecific IgG1, produced in a CHO cell line. | Roche (Grenzach-Wyhlen, Germany) | Relapsed or refractory follicular lymphoma | 2022 (EU) |
| Opdualag (nivolumab and relatlimab-rmbw), combination of two IgG4κ mAbs targeting PD-1 and lymphocyte activation gene-3, both produced in CHO cells. | Bristol-Myers Squibb (Princeton, NJ, USA) | Unresectable or metastatic melanoma | 2022 (US) |
| Padcev (enfortumab vedotin (EU), enfortumab vedotin-ejfv (US)), antibody–drug conjugate (ADC) targeting nectin-4 (an adhesion protein highly expressed in urothelial cancer). Fully human IG1κ (produced in a CHO cell line) conjugated to monomethyl auristatin E (MMAE). |
Astellas Pharma Europe (Leiden, the Netherlands) Astellas Pharma US (Northbrook, Illinois, USA) |
Urothelial cancer |
2022 (EU) 2019 (US) |
| Saphnelo (anifrolumab (EU), anifrolumab-fnia (US)), human IgG1κ directed against type I IFN subunit 1 receptor, produced in mouse myeloma cells (NS0). |
AstraZeneca (Sodertalje, Sweden) AstraZeneca (Wilmington, DE, USA) |
Systemic lupus erythematosus |
2022 (EU) 2021 (US) |
| Uplizna (inebilizumab (EU), inebilizumab-cdon (US)), humanized, affinity-optimized, afucosylated (IgG1κ specific for the B-cell-specific surface antigen CD19, produced in fucosyltransferase-deficient CHO cells. |
Viela Bio (Schiphol, the Netherlands) Viela Bio (Gaithersburg, MD, USA) |
Neuromyelitis optica spectrum disorders |
2022 (EU) 2020 (US) |
| Vabysmo (faricimab-svoa), humanized bispecific IgG1, targeting VEGF-A and angiopoietin-2, produced in a CHO cell line. | Genentech | Neovascular (wet) age-related macular degeneration and diabetic macular edema | 2022 (US) |
| Vyepti (eptinezumab (EU), eptinezumab-jjmr (US)), humanized anti-CGRP IgG1 mAb, produced in P. pastoris. |
H. Lundbeck (Valby, Denmark) Lundbeck Seattle BioPharmaceuticals |
Migraine prevention |
2022 (EU) 2020 (US) |
| Abevmy (bevacizumab), r humanized, anti-VEGF-A IgG1κ, produced in a CHO cell line. Biosimilar to Avastin. | Mylan (Dublin) | Cancer (various) | 2021 (EU) |
| Adbry (tralokinumab-ldrm; US), Adtralza (tralokinumab; EU), anti-IL-13 human IgG4λ, manufactured in an NS0 murine cell line. |
LEO Pharma (Madison, NJ, USA) LEO Pharma (Ballerup, Denmark) |
Atopic dermatitis (eczema) | 2021 (US & EU) |
| Aduhelm (aducanumab-avwa), human IgG1 directed against aggregated soluble and insoluble forms of amyloid-β, produced in a CHO cell line. | Biogen (Cambridge, MA, USA) | Alzheimer’s disease | 2021 (US) |
| Bamlanivimab & eteseviman, rh IgG1 mAbs that bind distinct but overlapping epitopes in the SARS-CoV-2 spike protein RBD. | Eli Lilly (Indianapolis) | COVID 19 | 2021 (US; Emergency Use Authorization; Authorization paused in 2022 as product not sufficiently effective against Omicron variant) |
| Bimzelx (bimekizumab), humanized IgG1κ mAb targeting human IL-17A and 17F, produced in a CHO cell line. | UCB Pharma (Brussels) | Psoriasis | 2021 (EU) |
| Byooviz (ranibizumab-nuna (US), ranibizumab (EU)), r humanized IgG1κ Fab fragment targeting VEGF-A, produced in E. coli. Biosimilar to Lucentis. |
Samsung Bioepis NL (Delft, the Netherlands) Biogen (Cambridge, MA, USA) |
Age-related macular degeneration (wet) and some additional retinal conditions | 2021 (EU & US) |
| Enhertu (trastuzumab deruxtecan), ADC comprising humanized anti-HER2 IgG1κ (trastuzumab sequence), produced in CHO cells, conjugated to a topoisomerase I inhibitor derivative of exatecan. |
Daiichi Sankyo Europe (Munich, Germany) Daiichi Sankyo (Basking Ridge, NJ, USA) |
Metastatic breast cancer |
2021 (EU) 2019 (US) |
| Enspryng (satralizumab (EU), satralizumab-mwge (US)), r humanized IgG2 targeting soluble and membrane-bound IL-6 receptor, produced in a CHO cell line. |
Roche (Grenzach-Wyhlen, Germany) Genentech |
Neuromyelitis optica spectrum disorders |
2021(EU) 2020 (US) |
| Evkeeza (evinacumab-dgnb (US), evinacumab (EU)), rh-IgG4 mAb targeting angiopoietin-like 3, produced in a CHO cell line. |
Regeneron (Dublin) Regeneron (Tarrytown, NY, USA) |
Homozygous familial hypercholesterolemia | 2021 (EU & US) |
| Hukyndra (adalimumab), human IgG1κ targeting TNF, produced in a CHO cell line. Biosimilar to Humira. Same as Libmyris. | Stada Arzneimittel (Bad Vilbel, Germany) | Various inflammatory conditions | 2021 (EU) |
| Jemperli (dostarlimab-gxly (US), dostarlimab (EU)), humanized IgG4 mAb against programmed cell death protein 1 (PD-1), produced in CHO cells. |
GlaxoSmithKline (Dublin) GlaxoSmithKline (Research Triangle Park, NC, USA) |
Endometrial cancer | 2021 (EU & US) |
| Kesimpta (ofatumumab), rh IgG1 targeting B cell surface CD20, expressed in a mouse NS0 cell line. | Novartis (Dublin) | Multiple sclerosis | 2021 (EU) |
| Lextemy (bevacizumab), r humanized anti-VEGF-A IgG1κ, produced in a CHO cell line. Biosimilar to Avastin. Same product as Abevmy. | Mylan (Dublin) | Cancer (various) |
2021 (EU) Withdrawn 2021 |
| Libmyris (adalimumab), human IgG1κ targeting TNF, produced in a CHO cell line. Biosimilar to Humira. | Stada Arzneimittel (Bad Vilbel, Germany) | Various inflammatory conditions | 2021 (EU) |
| Minjuvi (EU), Monjivi (US) (tafasitamab (EU), tafasitamab-cxix (US)), humanized, Fc-engineered CD19-specific mAb, produced in a CHO cell line. |
Incyte Biosciences Distribution (Amsterdam) Morphosys US (Boston) |
Diffuse large B cell lymphoma |
2021(EU) 2020 (US) |
| Onbevzi (bevacizumab), humanized, anti-VEGF mAb, produced in CHO cells. Biosimilar to Avastin. | Samsung Bioepis (Delft, the Netherlands) | Cancers (various) | 2021 (EU) |
| Oyavas (bevacizumab), r humanized, anti-VEGF-A IgG1κ, produced in a CHO cell line. Biosimilar to Avastin. | Stada Arzneimittel (Hessen, Germany) | Cancer (various) | 2021 (EU) |
| Regkirona (regdanvimab), human IgG1 targeting the SARS-CoV-2 spike protein, produced in a CHO cell line. | Celltrion (Budapest) | COVID 19 (treatment) | 2021 (EU) |
| Ronapreve (EU), Regen-cov (US)) (casirivimab & imdevimab), combination of two human IgGs (IgG1κ and IgG1λ) targeting distinct epitopes of SARS-CoV-2 spike protein, both produced in CHO cell lines. |
Roche (Grenzach-Wyhlen, Germany) Regeneron (US) |
COVID-19 (prevention & treatment) |
2021 (EU) 2020 (US; Emergency Use Authorization; Authorization paused in 2022 as product not sufficiently effective against Omicron variant) |
| Rybrevant (amivantamab-vmjw (US), amivantamab (EU)), human low-fucose IgG1 bispecific antibody that binds the extracellular domains of EGF and MET receptors, produced in CHO cell lines. |
Janssen-Cilag (Beerse, Belgium) Janssen Biotech (Horsham, PA, USA) |
Advanced non-small-cell lung cancer | 2021 (EU & US) |
| Susvimo (ranibizumab), humanized IgG1κ antibody fragment specific for VEGF-A, produced in E. coli. | Genentech | Neovascular (wet) age-related macular degeneration | 2021 (US) |
| Tezspire (tezepelumab-ekko), human IgG2λ specific for thymic stromal lymphopoietin (TSLP), produced in a CHO cell line. | Amgen & AstraZeneca | Severe asthma | 2021 (US) |
| Tivdak (tisotumab vedotin-tftv), tissue factor (TF)-directed ADC comprising a human anti-TF IgG1κ antibody conjugated to monomethyl auristatin E (MMAE), produced in a CHO cell line. | Seagen (Bothell, WA, USA) | Cervical cancer | 2021 (US) |
| Trodelvy (sacituzumab govitecan (EU), sacituzumab govitecan-hziy (US)), ADC comprising an anti-Trop-2 humanized IgG1κ, produced in Sp2/0 cells, conjugated to camptothecin-derived topoisomerase I inhibitor SN-38. |
Gilead Sciences (Cork, Ireland) Immunomedics (Morris Plains, NJ, USA) |
Breast cancer (triple-negative) |
2021 (EU) 2020 (US) |
| Vyvgart (efgartigimod alfa-fcab), human IgG1-derived Fc fragment that binds the neonatal Fc receptor (FcRn), leading to reduced circulating IgG, produced in a CHO cell line. | Argenx (Boston) | Myasthenia gravis | 2021 (US) |
| Xevudy (sotrovimab in US), human IgG1 targeting the COVID-19 spike protein RBD, produced in a CHO cell line. |
GlaxoSmithKline (Dublin) GSK (Durham, NC, USA) |
Treating COVID-19 |
2021 (EU) 2021 (US; Emergency Use Authorization; Authorization paused in 2022 as product not sufficiently effective against Omicron variant) |
| Yuflyma (adalimumab), anti-TNF human IgG1, produced in a CHO cell line. Biosimilar to Humira. | Celltrion (Budapest) | Various inflammatory conditions | 2021 (EU) |
| Yusimry (adalimumab-aqvh), anti-TNF human IgG1, produced in a CHO cell line. Biosimilar to Humira. | Coherus BioSciences (Redwood City, CA, USA) | Various inflammatory conditions | 2021 (US) |
| Zynlonta (loncastuximab tesirine-lpyl), CD19-directed humanized IgG1κ produced in a CHO cell line, conjugated to SG3199 (alkylating agent). | ADC Therapeutics (Murray Hill, NJ, USA) | Lymphoma | 2021 (US) |
| Adakveo (crizanlizumab (EU), crizanlizumab-tmca (US)), r humanized IgG2aκ mAb targeting human P-selectin, produced in a CHO cell line. |
Novartis (Dublin) Novartis (East Hanover, NJ, USA) |
Prevention of recurrent vaso-occlusive crises (VOCs) in sickle cell anemia |
2020 (EU) 2019 (US) |
| Amsparity (adalimumab), human IgG1 targeting TNF-α, produced in a CHO cell line. Biosimilar to Humira. | Pfizer (Brussels) | Various inflammatory conditions | 2020 (EU) |
| Aybintio (bevacizumab), humanized anti-VEGF mAb, produced in a CHO cell line. Biosimilar to Avastin. | Samsung Bioepis (Delft, the Netherlands) | Various cancers | 2020 (EU) |
| Beovu (brolucizumab (EU), brolucizumab-dbll (US)), humanized single-chain Fv (scFv) antibody fragment targeting vascular endothelial growth factor-A (VEGF-A), produced in E . coli. |
Novartis (Dublin) Novartis (East Hanover, NJ, USA) |
Wet age-related macular degeneration, macular oedema |
2020 (EU) 2019 (US) |
| Blenrep (belantamab mafodotin (EU), belantamab mafodotin-blmf (US)), ADC comprising monomethyl auristatin F conjugated to an afucosylated humanized IgG1κ, produced in a CHO cell line, targeting B cell maturation antigen (BCMA). |
GlaxoSmithKline (Dublin) GlaxoSmithKline (Research Triangle Park, NC, USA) |
Multiple myeloma | 2020 (EU & US) |
| Danyelza (naxitamab-gqgk), humanized IgG1 specific for glycolipid disialoganglioside (GD2), produced in a CHO cell line. | Y-mABs Therapeutics (New York, NY, USA) | Neuroblastoma | 2020 (US) |
| Darzalex Faspro (daratumumab and hyaluronidase-fihj), IgG1κ specific for the CD38 antigen in combination with rh hyaluronidase (which increases drug dispersion and absorption upon SC administration), both produced in CHO cell lines. | Janssen Biotech (Horsham, PA, USA) | Multiple myeloma | 2020 (US) |
| Ebanga (ansuvimab-zykl), Zaire ebolavirus glycoprotein (EBOV GP)-directed human IgG1, produced in a CHO cell line. | Ridgeback Biotherapeutics (Miami, FL, USA) | Infection by Zaire ebolavirus | 2020 (US) |
| Equidacent (bevacizumab), humanized IgG1 targeting VEGF, produced in a CHO cell line. Biosimilar to Avastin. | Centus Biotherapeutics (Dublin) | Cancers, various |
2020 (EU) Withdrawn 2020 |
| Hulio (adalimumab (EU), adalimumab-fkjp (US)), human IgG1 targeting TNF-α, expressed in CHO cells. Biosimilar to Humira. |
Mylan (Saint-Priest, France) Mylan Pharmaceuticals (Morgantown, WV, USA) |
Various inflammatory conditions mediated by TNF |
2020 (US) 2018 (EU) |
| Inmazeb (atoltivimab, maftivimab and odesivimab-ebgn), combination of Zaire ebolavirus glycoprotein-directed human IgG1s, produced in CHO cell lines. | Regeneron (Tarrytown, NY, USA) | Infection by Zaire ebolavirus | 2020 (US) |
| Margenza (margetuximab-cmkb), chimeric Fc-engineered IgG1κ specific for the extracellular domain of the human epidermal growth factor receptor 2 protein (HER2), produced in a CHO cell line. | MacroGenics (Rockville, MD, USA) | HER2positive breast cancer | 2020 (US) |
| Obiltoxaximab SFL (EU), Anthim (US) (obiltoxaximab), chimeric IgG1 targeting the B. anthracis protective antigen (PA), the cell-binding component of anthrax toxin. Produced in an NS0 cell line. |
SFL Pharmaceuticals Deutschland (Lörrach, Germany) Elusys Therapeutics (Pine Brook, NJ, USA) |
Inhalational anthrax |
2020 (EU) 2016 (US) |
| Phesgo (pertuzumab, trastuzumab), combination of 2 r humanized IgG1 mAbs targeting the human epidermal growth factor receptor 2 (HER2), both produced in CHO cells, along with rh hyaluronidase (vorhyaluronidase alfa) as an excipient. |
Roche (Grenzach-Wyhlen, Germany; EU) Genentech (South San Francisco, CA, USA; US) |
HER2-positive breast cancer | 2020 (EU & US) |
| Polivy (polatuzumab vedotin), ADC comprising a humanized IgG1, produced in CHO cells, targeting a component of the B cell receptor (CD79b) conjugated to monomethyl auristatin E (MMAE). |
Roche (Grenzach-Wyhlen, Germany) Genentech (South San Francisco, CA, USA) |
Diffuse large B cell lymphoma |
2020 (EU) 2019 (US) |
| Riabni (rituximab-arrx), chimeric IgG1κ directed against the CD20 antigen, produced in a CHO cell line. Biosimilar to Rituxan. | Amgen (Thousand Oaks, CA, USA) | Non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, Wegener’s granulomatosis and microscopic polyangiitis | 2020 (US) |
| Ruxience (rituximab (EU), rituximab-pvvr (US)), chimeric mouse/human IgG1 targeting B lymphocyte CD20, produced in a CHO cell line. Biosimilar to MabThera/Rituxan. |
Pfizer (Brussels) Pfizer (New York) |
Various cancers and inflammatory conditions |
2020 (EU) 2019 (US) |
| Sarclisa (isatuximab (EU), isatuximab-irfc (US)), chimeric anti-CD 38 IgG1, produced in a CHO cell line. |
Sanofi-Aventis (Paris) Sanofi-Aventis (Bridgewater, NJ, USA) |
Multiple myeloma | 2020 (EU & US) |
| Tepezza (teprotumumab-trbw), human IgG1 that binds IGF-1 receptor, produced in a CHO cell line. | Horizon Therapeutics (Lake Forest, IL, USA) | Thyroid eye disease | 2020 (US) |
| Zercepac (trastuzumab), humanized anti-HER2 mAb, produced in a CHO cell line. Biosimilar to Herceptin. | Accord Healthcare (Barcelona) | Breast and stomach cancers | 2020 (EU) |
| Abrilada (adalimumab-afzb), human IgG1 specific for TNF, produced in a CHO cell line. Biosimilar to Humira. | Pfizer (New York) | Various inflammatory conditions | 2019 (US) |
| Ajovy (fremanezumab (EU), fremanezumab-vfrm (US)), humanized IgG2 targeting both isoforms of CGRP, produced in a CHO cell line. |
Teva (Ulm, Germany) Teva Pharmaceuticals USA (North Wales, PA, USA) |
Migraine |
2019 (EU) 2018 (US) |
| Avsola (infliximab-axxq), anti-TNF chimeric IgG1κ, produced in a CHO cell line. Biosimilar to Remicade. | Amgen (Thousand Oaks, CA, USA) | Various inflammatory conditions | 2019 (US) |
| Cablivi (Caplacizumab (EU), caplacizumab-yhdp (US)), humanized bivalent nanobody comprising two identical building blocks joined by a tri-alanine linker and targeting the A1 domain of von Willebrand factor, produced in E. coli. |
Ablynx (Zwijnaarde, Belgium) Genzyme (Cambridge, MA, USA) |
Acquired thrombotic thrombocytopenic purpura |
2019 (US) 2018 (EU) |
| Evenity (romosozumab (EU), romosozumab-aqqg (US)), humanized IgG2 targeting sclerostin, produced in a CHO cell line. |
UCB Pharma (Brussels) Amgen (Thousand Oaks, CA, USA) |
Osteoporosis | 2019 (EU & US) |
| Hadlima (adalimumab-bwwd), anti-TNF human IgG1 produced in a CHO cell line. Biosimilar to Humira. Same product as Imraldi. | Merck (Whitehouse Station, NJ, USA) | Various inflammatory conditions | 2019 (US) |
| Herceptin Hylecta (trastuzumab and hyaluronidase-oysk). | Genentech | Breast cancer | 2019 (US) |
| Idacio (adalimumab), rh-IgG1 targeting TNF, produced in a CHO cell line. Biosimilar to Humira. Same as Kromeya (see below). | Fresenius Kabi Deutschland (Bad Homburg v.d. Höhe, Germany) | Various inflammatory conditions | 2019 (EU) |
| Kanjinti (trastuzumab (EU), trastuzumab-anns (US)), r humanized IgG1 against HER2, produced in CHO cells. Biosimilar to Herceptin. | Amgen Europe (Breda, the Netherlands) | Breast and gastric cancers |
2019 (US) 2018 (EU) |
| Kromeya (adalimumab, rh-IgG1 targeting TNF, produced in a CHO cell line. Biosimilar to Humira. Same as Idacio (see above). | Fresenius Kabi Deutschland (Bad Homburg v.d. Höhe, Germany) | Various inflammatory conditions |
2019 (EU) Withdrawn 2019 |
| Libtayo (cemiplimab-rwlc (US), cemiplimab (EU), human IgG4 specific for PD-1, produced in CHO cells. |
Regeneron Pharmaceuticals (Tarrytown, NY, USA) Regeneron Ireland (Dublin) |
Cutaneous squamous cell carcinoma |
2019 (EU) 2018 (US) |
| Ontruzant (trastuzumab (EU), trastuzminjmab-dttb USA), produced in CHO cells. Biosimilar to Herceptin. |
Samsung Bioepis (Delft, the Netherlands) Organon (Jersey City, NJ, USA) |
Breast and gastric cancers |
2019 (EU) 2017 (EU) |
| Skyrizi (risankizumab (EU), risankizumab-rzaa (US)), humanized IgG1 targeting IL-23, produced in a CHO cell line. |
AbbVie Deutschland (Rhein, Germany) AbbVie (North Chicago, IL, USA) |
Plaque psoriasis and psoriatic arthritis | 2019 (US & EU) |
| Trazimera (trastuzumab (EU), trastuzumab-qyyp, US)), humanized IgG, produced in a CHO cells. Biosimilar to Herceptin. |
Pfizer (Brussels) Pfizer (New York) |
Breast cancer, gastric or gastroesophageal junction adenocarcinoma |
2019 (US) 2018 (EU) |
| Trogarzo (ibalizumab (EU), ibalizumab-uiyk (US)), humanized IgG-4, targeting T-helper cells CD4 receptor, produced in an NS0 cell line. |
Theratechnologies Europe (Dublin) TaiMed Biologics (Irvine, CA, USA) & Theratechnologies (Montreal) |
Treatment of human immunodeficiency virus type 1 infection |
2019 (EU) 2018 (US) |
| Ultomiris (ravulizumab (EU), ravulizumab-cwvz (US)), r humanized IgG2/4 targeting complement component 5, produced in a CHO cell line. |
Alexion Europe (LevallPerret, France) Alexion Pharmaceuticals (Boston) |
Paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome |
2019 (EU) 2018 (US) |
| Zirabev (bevacizumab (EU), bevacizumab-bvzr (US)), humanized IgG1κ targeting VEGF, produced in CHO cell line. Biosimilar to Avastin. |
Pfizer Europe (Brussels) Pfizer (New York) |
Cancer (various) | 2019 (EU & US) |
| Aimovig (erenumab-aooe (US), erenumab (EU)), human IgG2 targeting the CGRP receptor, produced in CHO cells. |
Amgen (Thousand Oaks, CA, USA) Novartis (East Hanover, NJ, USA) Novartis Europharm (Dublin) |
Migraine | 2018 (EU & US) |
| Crysvita (burosumab (EU), burosumab-twza (US)), human IgG1 antibody to soluble fibroblast growth factor-23, produced in CHO cells. |
Kyowa Kirin (Hoofddorp, the Netherlands) Ultragenyx Pharmaceutical (Novato, CA, USA) |
X-linked hypophosphatemia | 2018 (EU & US) |
| Emgality (galcanezumab (EU), galcanezumab-gnlm (US)), humanized IgG4 that binds CGRP, produced in CHO cells. |
Eli Lilly Nederland (Utrecht, the Netherlands) Eli Lilly (Indianapolis) |
Migraine | 2018 (EU & US) |
| Fasenra (benralizumab), humanized, afucosylated IgG1 targeting the α subunit of the human IL-5 receptor, produced in CHO cells. | AstraZeneca (Södartälje, Sweden, & Wilmington, DE, USA) | Asthma |
2018 (EU) 2017 (US) |
| Gamifant (emapalumab-lzsg), r IgG1 targeting IFN-γ, produced in CHO cells. | Sobi (Waltham, MA, USA) | Primary hemophagocytic lymphohistiocytosis (HLH) | 2018 (US) |
| Halimatoz (adalimumab), anti-TNF IgG, produced in CHO cells. Biosimilar to Humira. Same product as Hefiya and Hyrimoz (see below). | Sandoz | Various inflammatory conditions mediated by TNF, including rheumatoid arthritis and plaque psoriasis |
2018 (EU) Withdrawn 2020 |
| Hefiya (adalimumab), anti-TNF IgG, produced in CHO cells. Biosimilar to Humira. Same product as Halimatoz and Hyrimoz (see above and below). | Sandoz | Various inflammatory conditions mediated by TNF, including polyarticular juvenile idiopathic arthritis and plaque psoriasis | 2018 (EU) |
| Hemlibra (emicizumab (EU), emicizumab-kxwh (US)), humanized, bispecific IgG4 capable of binding factors IXa and X, produced in CHO cells. |
Roche Registration (Welwyn Garden City, UK) Roche/Genentech (South San Francisco, CA, USA) |
Hemophilia A |
2018 (EU) 2017 (US) |
| Herzuma (trastuzumab (EU), trastuzumab-pkrb (US)), r humanized IgG1 against HER2, produced in CHO cells. Biosimilar to Herceptin. |
Celltrion Healthcare (Budapest), Celltrion (Incheon, Republic of Korea) Teva Pharmaceuticals USA (North Wales, PA, USA) |
Breast and gastric cancers (EU) Breast cancer (US) |
2018 (EU & US) |
| Hyrimoz (adalimumab (EU), adalimumab-adaz (US)), anti-TNF IgG, produced in CHO cells. Biosimilar to Humira. Same product as Halimatoz and Hefiya (see above). | Sandoz | Various inflammatory conditions mediated by TNF, including rheumatoid arthritis and plaque psoriasis | 2018 (EU & US) |
| Ilumya (tildrakizumab-asmn; US), Ilumetri (tildrakizumab; EU), humanized IgG1 that binds the p19 subunit of IL-23, produced in CHO cells. |
Merck (Whitehouse Station, NJ, USA) Almirall (Barcelona) |
Psoriasis | 2018 (US & EU) |
| Imfinzi (durvalumab), human IgG1 blocking the interaction of programmed cell death ligand-1 (PD-L1) with its receptor PD-1 and CD80, produced in CHO cells. |
AstraZeneca (Sodertalje, Sweden) AstraZeneca (Wilmington, DE, USA) |
Non-small-cell lung cancer Urothelial carcinoma |
2018 (EU) 2017 (US) |
| Mvasi (bevacizumab (EU), bevacizumab-awwb (US)), humanized IgG antibody to human VEGF-A1, produced in CHO cells. Biosimilar to Avastin. | Amgen Europe Amgen (Thousand Oaks, CA, USA) | Various cancers |
2018 (EU) 2017 (US) |
| Mylotarg (gemtuzumab ozogamicin), ADC targeting the CD33 surface antigen, consisting of a humanized IgG4 chemically conjugated to N-acetyl-γ-calicheamicin, produced in NS0 mouse myeloma cells. |
Pfizer (Brussels) Pfizer/Wyeth (Philadelphia) |
Acute myeloid leukemia |
2018 (EU) 2000 (US) Withdrawn 2010 (US) Reapproved 2017 (US) using modified dosage and regimen |
| Ocrevus (ocrelizumab), r humanized IgG1 targeting the CD20 surface antigen, produced in CHO cells. | Roche/Genentech (South San Francisco, CA, USA) | Multiple sclerosis |
2018 (EU) 2017 (US) |
| Ogivri (trastuzumab-dkst (US), trastuzumab (EU)), IgG produced in CHO cells. Biosimilar to Herceptin. |
Viatris (Dublin) Mylan (Morgantown, WV, USA) |
Breast and gastric cancers |
2018 (EU) 2017 (US) |
| Poteligeo (mogamulizumab (EU), mogamulizumab-kpkc (US)), defucosylated, humanized IgG1 that binds C-C chemokine receptor type 4 (CCR4), produced in CHO cells. |
Kyowa Kirin (Hoofddorp, the Netherlands) Kyowa Kirin (Bedminster, NJ, USA) |
Sezary syndrome, mycosis fungoides | 2018 (EU & US) |
| Takhzyro (lanadelumab (EU), lanadelumab-flyo (US)), human IgG1 targeting active plasma kallikrein, produced in CHO cells. |
Shire (Dublin) Dyax (Lexington, MA, USA) |
Hereditary angioedemas | 2018 (EU & US) |
| Truxima (rituximab (EU), rituximab-abbs (US)), chimeric IgG1 against cell surface antigen CD20, produced in CHO cells. Biosimilar to MabThera. Same product as Blitzima, Ritemvia (see above). |
Celltrion (Torony, Hungary) Teva Pharmaceuticals USA (North Wales, PA, USA) |
Selected cancers and autoimmune disorders (EU), non-Hodgkin’s lymphoma (US) |
2018 (US) 2017 (EU) |
| Zessly (infliximab), chimeric anti-TNF IgG1 produced in CHO cells. Biosimilar to Remicade. | Sandoz | Rheumatoid arthritis and selected additional inflammatory diseases | 2018 (EU) |
| Amgevita (adalimumab), anti-TNF human IgG1, produced in CHO cells. Biosimilar to Humira. Same product as Solymbic (see below). | Amgen Europe | Rheumatoid arthritis and selected additional inflammatory diseases |
2017 (EU) Withdrawn 2019 |
| Bavencio (avelumab), human IgG1 specific for programmed death ligand-1 (PD-L1), produced in CHO cells. |
Merck Europe (Amsterdam) Pfizer (New York) |
Metastatic Merkel cell carcinoma, urothelial carcinoma | 2017 (EU & US) |
| Besponsa (inotuzumab ozogamicin), ADC comprising a humanized IgG4 specific for human CD22, produced in CHO cells, covalently linked to the cytotoxic agent N-acetyl-γ-calicheamicin dimethylhydrazide. |
Pfizer (Brussels) Pfizer/Wyeth (Philadelphia) |
Acute lymphoblastic leukemia | 2017 (EU & US) |
| Blitzima (rituximab), chimeric IgG1 against cell surface antigen CD20, produced in CHO cells. Biosimilar to MabThera. Same product as Ritemvia, Truxima and Rituzena (see below). | Celltrion Healthcare (Budapest) | Non-Hodgkin’s lymphoma, CLL, granulomatosis | 2017 (EU) |
| Rixiamgelusd zarz ezo (adalimumab (EU), adalimumab-adbm (US)), rh IgG1 against human TNF, produced in CHO cells. Biosimilar to Humira. |
Boehringer Ingelheim (Rhein, Germany) Boehringer Ingelheim (Ridgefield, CT, USA) |
Range of inflammatory conditions, including psoriasis, rheumatoid arthritis and Crohn’s disease |
2017 (EU & US) Withdrawn 2019 (EU) |
| Dupixent (dupilumab), human IgG4 that binds the IL-4α receptor subunit, produced in CHO cells |
Sanofi-Aventis (Paris & Bridgewater, NJ, USA) Regeneron Pharmaceuticals (Tarrytown, NY, USA) |
Atopic dermatitis | 2017 (EU & US) |
| Imraldi (adalimumab), produced in CHO cells. Biosimilar to Humira. | Samsung Bioepis (Delft, the Netherlands) | Rheumatoid arthritis, selected additional inflammatory diseases | 2017 (EU) |
| Ixifi (infliximab-qbtx), produced in mammalian cells. Biosimilar to Remicade. | Pfizer (New York) | Various inflammatory conditions, including rheumatoid arthritis, Crohn’s disease, and psoriasis | 2017 (US) |
| Kevzara (sarilumab), human IgG1 that binds IL-6 receptors, produced in CHO cells. |
Sanofi-Aventis (Paris & Bridgewater, NJ, USA) Regeneron Pharmaceuticals (Tarrytown, NY, USA) |
Rheumatoid arthritis |
2017 (EU) 2017 (US) |
| Kyntheum (EU), Siliq (US) (brodalumab), human IgG2 against human IL-17 receptor A, produced in CHO cells. |
LEO Pharma (Ballerup, Denmark) Valeant Pharmaceuticals (Bridgewater, NJ, USA) |
Psoriasis |
2017 (EU) 2017 (US) |
| Qarziba (dinutuximab beta; previously dinutuximab beta EUSA and dinutuximab beta Apeiron), chimeric IgG1 against carbohydrate disialoganglioside GD2 that is overexpressed by cells of neuroectodermal origin such as neuroblastoma cells, produced in a CHO cell line. | EUSA Pharma (Schiphol-Rijk, the Netherlands) | Neuroblastoma | 2017 (EU) |
| Renflexis (infliximab-abda), chimeric IgG1 that binds TNF-α, produced in CHO cells. Biosimilar to Remicade. Same product as Flixabi (see below). | Merck (Kenilworth, NJ, USA) | Crohn’s disease and various other inflammatory conditions | 2017 (US) |
| Ritemvia (rituximab), produced in CHO cells. Biosimilar to MabThera. Same product as Blitzima, Rituzena and Truxima (see above and below). | Celltrion Healthcare (Budapest) | Non-Hodgkin’s lymphoma, granulomatosis with polyangiitis, microscopic polyangiitis |
2017 (EU) Withdrawn 2021 |
| Rituxan Hycela, rituximab and hyaluronidase human, both produced in CHO cells. | Biogen (Cambridge, MA, USA), Genentech | Follicular lymphoma, diffuse large B cell lymphoma, CLL | 2017 (US) |
| Rituzena (previously Tuxella) (rituximab), produced in CHO cells. Biosimilar to MabThera. Same product as Blitzima, Ritemvia and Truxima (see above and below). | Celltrion Healthcare (Budapest) | Non-Hodgkin’s lymphoma, CLL, granulomatosis with polyangiitis |
2017 (EU) Withdrawn 2019 |
| Rixathon (rituximab), chimeric IgG1 against cell surface antigen CD20, produced in CHO cells. Biosimilar to MabThera. Same product as Riximyo (see below). | Sandoz | Various conditions including non-Hodgkin’s lymphoma, CLL, rheumatoid arthritis | 2017 (EU) |
| Riximyo (rituximab), chimeric IgG1 against cell surface antigen CD20, produced in CHO cells. Biosimilar to MabThera. Same product as Rixathon (see above). | Sandoz | Various conditions including non-Hodgkin’s lymphoma and rheumatoid arthritis, but excluding CLL | 2017 (EU) |
| Solymbic (adalimumab), anti-TNF human IgG1 produced in CHO cells. Biosimilar to Humira. Same product as Amgevita and Amjevita (see above and below). | Amgen Europe | Rheumatoid arthritis and selected additional inflammatory diseases |
2017 (EU) Withdrawn 2018 |
| Tecentriq (atezolizumab), humanized IgG1 specific for programmed death ligand 1 (PD-L1), engineered to lack Fc glycosylation, produced in CHO cells. |
Roche Registration (Grenzach-Wyhlen, Germany) Genentech (South San Francisco, CA, USA) |
Urothelial carcinoma, non-small-cell lung cancer |
2017 (EU) 2016 (US) |
| Tremfya (guselkumab), human IgG1 that selectively binds the p19 subunit of IL-23, produced in CHO cells. |
Janssen-Cilag (Beerse, Belgium) Janssen Biotech (Horsham, PA, USA) |
Psoriasis | 2017 (EU & US) |
| Zinplava (bezlotoxumab), human IgG directed against Clostridium difficile toxin B, produced in CHO cells. |
Merck Sharp & Dohme Merck (Whitehouse Station, NJ, USA) |
C. difficile infection |
2017 (EU) 2016 (US) |
| Amjevita (adalimumab-atto), rh IgG1 specific for TNF, produced in CHO cells. Biosimilar to Humira. Same product as Solymbic and Amgevita (see above). | Amgen (Thousand Oaks, CA, USA) | Rheumatoid arthritis and selected additional inflammatory diseases | 2016 (US) |
| Cinqair (US), Cinqaero (EU) (reslizumab), humanized IgG4 against IL-5, produced in NS0 cells. |
Teva Respiratory (Frazer, PA USA) Teva (Haarlem, the Netherlands) |
Asthma |
2016 (US) 2016 (EU) |
| Darzalex (daratumumab), human IgG1 against CD-38, produced in CHO cells. |
Janssen-Cilag Janssen Biotech |
Multiple myeloma |
2016 (EU) 2015 (US) |
| Empliciti (elotuzumab) humanized IgG1 against the cell surface receptor SLAMF7, produced in NS0 cells. | Bristol-Myers Squibb (Dublin & Princeton, NJ, USA) | Multiple myeloma (in combination with lenalidomide and dexamethasone) |
2016 (EU) 2015 (US) |
| Flixabi (infliximab), chimeric IgG1 against TNF-α, produced in CHO cells. Biosimilar to Remicade. Same product as Renflexis (see above). | Samsung Bioepis (Delft, the Netherlands) | Various forms of arthritis, psoriasis, colitis, Crohn’s disease, ankylosing spondylitis | 2016 (EU) |
| Inflectra (EU & US), Remsima (EU) (infliximab (EU), infliximab-dyyb (US)), chimeric IgG1 specific for TNF-α, produced in murine Sp2/0 cells. Biosimilar to Remicade. |
Inflectra: Hospira (Lake Forest, IL, USA) Pfizer (Brussels) Remsima: Celltrion (Budapest) |
Certain forms of arthritis and psoriasis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis |
2016 (US) 2013 (EU) |
| Lartruvo (olaratumab), rh IgG1 specific for human platelet-derived growth factor receptor-α, produced in NS0 cells. | Eli Lilly (Utrecht, the Netherlands, & Indianapolis) | Sarcoma |
2016 (EU & US) Withdrawn 2019 |
| Portrazza (necitumumab), human IgG1 against the ligand-binding site of human EGF receptor, produced in NS0 cells. | Eli Lilly (Utrecht, the Netherlands, & Indianapolis) | Non-small-cell lung cancer (in combination with gemcitabine and cisplatin) |
2016 (EU) 2015 (US) Withdrawn 2021 (EU) |
| Taltz (ixekizumab), humanized IgG4 against hIL-17A, produced in CHO cells. | Eli Lilly (Utrecht, the Netherlands, & Indianapolis) | Psoriasis | 2016 (EU & US) |
| Zinbryta (daclizumab), humanized IgG1 against IL-2Rα, produced in NS0 cells. |
Biogen (Cambridge, MA, US) Biogen Idec (Maidenhead, UK) |
Multiple sclerosis |
2016 (EU & US) Withdrawn 2018 |
| Blincyto (blinatumomab), bispecific T cell engager antibody construct (BiTE), produced in CHO cells. |
Amgen Europe Amgen (Thousand Oaks, CA, USA) |
Acute lymphoblastic leukemia |
2015 (EU) 2014 (US) |
| Cosentyx (secukinumab), human IgG1 selectively binding human IL-17a, produced in CHO cells. |
Novartis (Dublin) Novartis (East Hanover, NJ, USA) |
Moderate to severe plaque psoriasis in adults | 2015 (EU & US) |
| Keytruda (pembrolizumab), humanized IgG4 capable of binding to the receptor PD-1, produced in CHO cells. |
Merck Sharp & Dohme Merck (Whitehouse Station, NJ, USA) |
Advanced (unresectable or metastatic) melanoma in adults |
2015 (EU) 2014 (US) |
| Nivolumab BMS (nivolumab), human IgG4 against the receptor PD-1, produced in CHO cells. Same product as Opdivo (see below). | Bristol-Myers Squibb (Uxbridge, UK) | Locally advanced or metastatic squamous non-small-cell lung cancer after prior chemotherapy in adults |
July 2015 (EU) Withdrawn November 2015 |
| Nucala (mepolizumab), humanized IgG1 capable of binding human IL-5, produced in CHO cells. |
GlaxoSmithKline (Cork, Ireland) GSK (Research Triangle Park, NC, USA) |
Add-on treatment for severe refractory eosinophilic asthma in adult patients | 2015 (EU & US) |
| Opdivo (nivolumab), human IgG4 against the receptor PD-1, produced in CHO cells. Same product as nivolumab BMS (see above). | Bristol-Myers Squibb (Dublin, & Princeton, NJ, USA) | Melanoma (as monotherapy or in combination with ipilimumab), non-small-cell lung cancer, renal cell carcinoma |
2015 (EU) 2014 (US) |
| Praluent (alirocumab), human IgG1 targeting PCSK9, produced in CHO cells |
Sanofi-Aventis (Paris & Bridgewater, NJ, USA) Regeneron Pharmaceuticals (Tarrytown, NY, USA) |
Primary hypercholesterolemia or mixed dyslipidemia, as an adjunct to diet | 2015 (EU & US) |
| Praxbind (idarucizumab), humanized IgG1 Fab fragment capable of binding the anticoagulant drug dabigatran, produced in CHO cells. | Boehringer Ingelheim (Rhein, Germany, & Ridgefield, CT, USA) | Rapid reversal agent for the anticoagulant drug dabigatran | 2015 (EU & US) |
| Repatha (evolocumab), human IgG2 capable of binding human PCSK-9, produced in CHO cells. |
Amgen Europe Amgen (Thousand Oaks, CA, USA) |
Hypercholesterolemia and mixed dyslipidemia | 2015 (EU & US) |
| Unituxin (dinutuximab), chimeric IgG1 targeting human disialoganglioside (GD2), produced in Sp2/0 cells | United Therapeutics Chertsey (Surrey, UK, & Silver Spring, MD, USA) | Neuroblastoma (administered in combination with GM-CSF, IL-2 and isotretinoin) |
2015 (EU & US) Withdrawn 2017 (EU) |
| Cyramza (ramucirumab), human mAb that binds the VEGF-2 receptor, produced in NS0 cells. |
Eli Lilly Nederland (Utrecht, the Netherlands) Eli Lilly (Indianapolis) |
Gastric cancer | 2014 (EU & US) |
| Entyvio (vedolizumab), humanized IgG targeting the human α4β7 integrin, produced in CHO cells. |
Takeda Pharmaceuticals (Deerfield, IL, USA) Takeda Pharma (Taastrup, Denmark) |
Ulcerative colitis, Crohn’s disease | 2014 (EU & US) |
| Gazyva (US), Gazyvaro (EU) (obinutuzumab), humanized, glycoengineered mAb specific for B cell antigen CD20, produced in CHO cells. | Roche/Genentech | CLL |
2014 (EU) 2013 (US) |
| Sylvant (siltuximab), chimeric mAb that binds human IL-6, produced in CHO cells. | Janssen Biotech | Multicentric Castleman disease | 2014 (EU & US) |
| Kadcyla (trastuzumab emtansine), humanized mAb specific for HER2 antigen, produced in CHO cells and conjugated to the small-molecule cytotoxin DM1. | Roche | Breast cancer | 2013 (EU & US) |
| Simponi Aria (golimumab). Active substance same as that in Simponi (see below); different strength and mode of administration. | Janssen Biotech | Rheumatoid arthritis | 2013 (US) |
| Perjeta (pertuzumab), human mAb specific for HER2, produced in CHO cells. | Roche/Genentech | Breast cancer |
2013 (EU) 2012 (US) |
| Abthrax (raxibacumab), human IgG mAb against the protective antigen (PA) of B. anthracis, produced in NS0 cells. | GSK/Human Genome Sciences (Rockville, MD, USA) | Inhalational anthrax | 2012 (US) |
| Adcetris (brentuximab vedotin), chimeric mAb conjugate specific for human CD30 (expressed on the surface of lymphoma cells), produced in CHO cells. |
Takeda Pharma (Roskilde, Denmark) Seattle Genetics |
Lymphoma |
2012 (EU) 2011 (US) |
| Benlysta (belimumab), human mAb that targets human B-lymphocyte stimulator (BLyS), a B cell survival factor. Produced in NS0 cells. | Human Genome Sciences, GSK (Dublin) | Lupus | 2011 (EU & US) |
| Xgeva (denosumab) (see Prolia). | Amgen Europe | Bone loss associated with cancer |
2011 (EU) 2010 (US) |
| Yervoy (ipilimumab), human mAb binding to CTLA-4 (a negative regulator of T cell activation), thereby enhancing T cell activation and proliferation, produced in CHO cells. |
Bristol-Myers Squibb (Dublin) Bristol-Myers Squibb (Princeton, NJ, USA) |
Melanoma | 2011 (EU & US) |
| Actemra (US), RoActemra (EU) (tocilizumab), humanized mAb specific for IL-6, produced in a mammalian cell line. |
Roche (Grenzach-Wyhlen Germany) Genentech (South San Francisco, CA, USA) |
Rheumatoid arthritis |
2010 (US) 2009 (EU) |
| Arzerra (ofatumumab), human mAb specific for CD20, produced in NS0 hybridoma cells. | Novartis, Genmab (Greenford, UK) | CLL |
2010 (EU) 2009 (US) Withdrawn 2019 (EU) |
| Prolia (denosumab), human mAb specific for receptor activator of nuclear factor-κB ligand (RANKL), produced in CHO cells. | Amgen | Osteoporosis in postmenopausal women | 2010 (EU & US) |
| Scintimun (besilesomab), murine mAb against nonspecific cross-reacting antigen-95 (found on surface of granulocytes), produced in hybridoma cells. | CIS Bio International (Gif-sur-Yvette, France) | In vivo diagnosis or investigation of sites of inflammation or infection via scintigraphic imaging | 2010 (EU) |
| Cimzia (certolizumab pegol), anti-TNF-α humanized antibody Fab′ fragment, produced in E. coli and PEGylated. |
UCB Pharma (Brussels) UCB (Smyrna, GA, USA) |
Crohn’s disease, rheumatoid arthritis |
2009 (EU) 2008 (US) |
| Ilaris (canakinumab), human mAb specific for IL-1β, produced in Sp2/0 cells. |
Novartis Pharmaceuticals (East Hanover, New Jersey, USA) Novartis Europharm (Dublin) |
Cryopyrin-associated periodic syndromes (CAPS) | 2009 (EU & US) |
| Removab (catumaxomab), bispecific engineered antibody targeting the human epithelial cell adhesion molecule (EpCAM) and human CD3 expressed on T lymphocytes, respectively, produced in hybridoma cells. | Neovii Biotech (Gräfelfing, Germany) | Malignant ascites in patients with carcinomas expressing epithelial cell adhesion molecule |
2009 (EU) Withdrawn 2017 |
| Simponi (golimumab), human mAb specific for TNF-α, produced in Sp2/0 cells. |
Janssen Biologics (Leiden, the Netherlands) Janssen Biotech (Horsham, PA, USA) |
Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis | 2009 (EU & US) |
| Stelara (ustekinumab), human MAb specific for the p40 subunit of IL-12 and IL-23, produced in Sp2/0 cells. | Janssen-Cilag | Moderate to severe plaque psoriasis | 2009 (EU & US) |
| Lucentis (ranibizumab), humanized IgG fragment that binds and inactivates VEGF-A, produced in E. coli. |
Novartis (Dublin) Genentech |
Neovascular (wet) age-related macular degeneration |
2007 (EU) 2006 (US) |
| Soliris (eculizumab), humanized IgG that binds human C5 complement protein, produced in a murine myeloma cell line. | Alexion Pharmaceuticals (Cheshire, CT, USA, & Paris) | Paroxysmal nocturnal hemoglobinuria | 2007 (EU & US) |
| Vectibix (panitumumab), human mAb that binds human EGF receptor, produced in CHO cells. | Amgen | EGF-receptor-expressing colorectal carcinoma |
2007 (EU) 2006 (US) |
| Tysabri (natalizumab), humanized mAb against selected leukocyte integrins, produced in murine myeloma cells. |
Biogen (Cambridge, MA, USA) Biogen Netherlands (Badhoevedorp, the Netherlands) |
Relapsing forms of multiple sclerosis |
2006 (EU) 2004 (US) Suspended 2005 (US) Resumed 2006 (US) |
| Xolair (omalizumab), humanized mAb that binds IgE at the site of high-affinity IgE receptor binding, produced in CHO cells. | Roche/Genentech | Moderate to severe persistent asthma in adults and adolescents |
2005 (EU) 2003 (US) |
| Zevalin (ibritumomab tiuxetan), murine mAb against the CD20 antigen, produced in CHO cells. |
Ceft Biopharma (Prague) Spectrum Pharmaceuticals (Irvine, CA, USA) |
Non-Hodgkin’s lymphoma |
2004 (EU) 2002 (US) |
| Erbitux (cetuximab), chimeric mAb against human EGF receptor, produced in Sp2/0 cells. |
Merck (Amsterdam) Eli Lilly (Indianapolis) |
EGF-receptor-expressing metastatic colorectal cancer | 2004 (EU & US) |
| Raptiva (efalizumab), humanized mAb that binds LFA-1, which is expressed on all leukocytes; produced in CHO cells. |
Serono (London, UK) Genentech |
Chronic moderate to severe plaque psoriasis in adults |
2004 (EU) 2003 (US) Withdrawn 2009 |
| Avastin (bevacizumab), humanized mAb against VEGF, produced in CHO cells. | Roche/Genentech | Metastatic colorectal cancer, glioblastoma, metastatic renal carcinoma |
2005 (EU) 2004 (US) |
| NeutroSpec (fanolesomab), murine mAb against CD15, a surface antigen of selected leukocytes, produced in hybridoma cells. | Palatin Technologies (Cranbury, NJ, USA), Mallinckrodt Pharmaceuticals (Hazelwood, MO, USA) | Imaging of equivocal appendicitis |
2004 (US) Withdrawn 2005 |
| Humira (EU & US), Trudexa (EU) (adalimumab), anti-TNF human mAb, produced in CHO cells. | AbbVie (Maidenhead, UK) | Rheumatoid arthritis |
2003 (EU) 2002 (US) Trudexa withdrawn 2007 (EU) |
| Bexxar (tositumomab), radiolabeled mAb against CD20, produced in murine hybridoma cells. | GSK | CD20-positive follicular non-Hodgkin’s lymphoma |
2003 (US) Withdrawn 2014 |
| Mabcampath (EU), Campath (US) (alemtuzumab), humanized mAb against CD52, a surface antigen of B lymphocytes, produced in CHO cells. |
Genzyme (Naarden, the Netherlands) Millennium (Cambridge, MA, USA) |
CLL |
2001 (EU & US) Withdrawn 2012 (EU) |
| Mylotarg (gemtuzumab zogamicin), a humanized antibody–toxic antibiotic conjugate targeted against the CD33 antigen found on leukemic blast cells, produced in NS0 cells. | Wyeth (Madison, NJ, USA) | Acute myeloid leukemia |
2000 (US) Withdrawn 2010 Reintroduced in 2017 |
| Herceptin (trastuzumab), humanized mAb against HER2, produced in a murine cell line. | Roche (Grenzach-Wyhlen, Germany) | Treatment of metastatic breast cancer overexpressing HER2 protein |
2000 (EU) 1998 (US) |
| Remicade (infliximab), chimeric mAb against TNF-α, produced in Sp2/0 cells. | Janssen (Leiden, the Netherlands) | Crohn’s disease |
1999 (EU) 1998 (US) |
| Synagis (palivizumab) humanized mAb directed against an epitope on the surface of respiratory syncytial virus, produced in a murine myeloma cell line | AstraZeneca (Sodertalje, Sweden) | Prophylaxis of lower respiratory tract disease caused by syncytial virus in children |
1999 (EU) 1998 (US) |
| Zenapax (daclizumab), humanized mAb against the IL-2 receptor α-chain, produced in NS0 cells. |
Roche (Welwyn Garden City, UK) Biogen (Cambridge, MA, USA) |
Prevention of acute kidney transplant rejection |
1999 (EU) 1997 (US) Withdrawn 2009 (EU) |
| Humaspect (votumumab), human mAb against cytokeratin tumor-associated antigen, produced in a human lymphoblastoid cell line. | KS Biomedix (Farnham, UK) | Detection of carcinoma of the colon or rectum |
1998 (EU) Withdrawn 2004 |
| MabThera (EU), Rituxan (US) (rituximab), chimeric mAb against CD20 surface antigen of B lymphocytes, produced in CHO cells. | Genentech/Roche | Non-Hodgkin’s lymphoma |
1998 (EU) 1997 (US) |
| Simulect (basiliximab), chimeric mAb directed against the α-chain of the IL-2 receptor, produced in a murine myeloma cell line. | Novartis (Dublin) | Prophylaxis of acute organ rejection in allogeneic renal transplantation | 1998 (EU) |
| LeukoScan (sulesomab), murine mAb Fab fragment against granulocyte surface nonspecific cross-reacting antigen-90, produced in Sp2/0 cells. | Immunomedics (Darmstadt, Germany) | Diagnostic imaging for infection and inflammation in bone of patients with osteomyelitis |
1997 (EU) Withdrawn 2018 |
| Verluma (nofetumomab), murine mAb Fab fragment directed against carcinoma-associated antigen, produced in a murine cell line. | Boehringer Ingelheim, NeoRx (Seattle) | Detection of small-cell lung cancer |
1996 (US) Withdrawn 1999 |
| Tecnemab KI (anti-melanoma mAb fragments), murine mAb fragments (Fab/Fab2 mix) against HMW-MAA, produced in murine ascites culture. | Amersham Sorin (Milan) | Diagnosis of cutaneous melanoma lesions |
1996 (EU) Withdrawn 2000 |
| ProstaScint (capromab pentetate), murine mAb against the tumor surface antigen PSMA, produced in a murine cell line. | EUSA Pharma (Langhorne, PA, USA) | Detection, staging and follow-up of prostate adenocarcinoma |
1996 (US) Discontinued 2018 |
| MyoScint (imiciromab pentetate), murine mAb fragment directed against human cardiac myosin, produced in a murine cell line. | Centocor | Myocardial infarction imaging |
1996 (US) Withdrawn 1999 |
| CEA-scan (arcitumomab), murine mAb Fab fragment against human carcinoembryonic antigen (CEA), produced in mouse ascites. | Immunomedics | Detection of recurrent or metastatic colorectal cancer |
1996 (EU & US) Withdrawn 2005 (EU & US) |
| Indimacis 125 (igovomab), murine mAb Fab2 fragment against the tumor-associated antigen CA125, produced in a murine cell line. | CIS Bio (Gif-sur-Yvette, France) | Diagnosis of ovarian adenocarcinoma |
1996 (EU) Withdrawn 2009 |
| ReoPro (abciximab), Fab fragments derived from a chimeric mAb against the platelet surface receptor GPIIb/III, produced in a mammalian cell line. |
Janssen Biologics (Leiden, the Netherlands) Centocor |
Prevention of blood clots |
1994 (US) Withdrawn 2019 |
| OncoScint CR/OV (satumomab pendetide), murine mAb against the tumor-associated glycoprotein TAG-72, produced in a murine cell line. | Cytogen (Princeton, NJ, USA) | Detection, staging and follow-up of colorectal and ovarian cancers |
1992 (US) Withdrawn 2002 |
| Orthoclone OKT3 (muromomab CD3), murine mAb against the T-lymphocyte surface antigen CD3, produced in a murine cell line. | Centocor Ortho Biotech Products (Raritan, NJ, USA) | Reversal of acute kidney transplant rejection |
1986 (US) Withdrawn 2010 |
| Other recombinant products | |||
| Bone morphogenetic proteins | |||
| Opgenra (eptotermin alfa), rh BMP-7, produced in CHO cells | Olympus Biotech (Limerick, Ireland) | Posterolateral lumbar spinal fusion |
2009 (EU) Withdrawn 2016 |
| Infuse bone graft, containing dibotermin alfa, a rh BMP-2 produced in CHO cells, placed on an absorbable collagen sponge. Active substance same as that in Infuse (see below). | Wyeth (Madison, NJ, USA) | Acute open tibial shaft fracture | 2004 (US) |
| Inductos (dibotermin alfa), rh BMP-2, produced in CHO cells. | Medtronic BioPharma (Heerlen, the Netherlands) | Acute tibia fractures | 2002 (EU) |
| Infuse (rh BMP2), produced in CHO cells. | Medtronic Sofamor Danek (Memphis, TN, USA) | Promotes fusion of lower spine vertebrae | 2002 (US) |
| OP-1 implant (US), Osigraft (EU) (eptotermin alfa), rh BMP-7, produced in CHO cells. |
Olympus Biotech (Limerick, Ireland) Stryker Biotech (Hopkinton, MA, USA) |
Non-union of tibia |
2001 (EU & US) Withdrawn 2015 (EU) |
| Recombinant enzymes | |||
| Voraxaze (glucarpidase), Pseudomonas-derived exopeptidase enzyme capable of hydrolyzing the carboxy terminal glutamate residue from folic acid and its analogs, including methotrexate (MTX); produced recombinantly in E. coli. |
SERB SAS (Paris) BTG International (West Conshohocken, PA, USA) |
Reduce toxic plasma methotrexate concentrations |
2022 (EU) 2012 (US) |
| Nexviazyme (avalglucosidase alfa-ngpt), rh α-glucosidase produced in a CHO cell line, conjugated with multiple synthetic bis-mannose-6-phosphate (bis-M6P)-tetra-mannose glycans. | Genzyme (Cambridge, MA, USA) | Late-onset Pompe disease | 2021 (US) |
| Rylaze (asparaginase erwinia chrysanthemi (recombinant)-rywn), Erwinia chrystanthemi-derived asparaginase, produced recombinantly in P. fluorescens. | Jazz Pharmaceuticals (Palo Alto, CA, USA) | Acute lymphoblastic leukemia and lymphoblastic lymphoma | 2021 (US) |
| Idefirix (imlifidase; r Streptococcus pyogenes–derived protease that degrades IgG in the lower hinge region, produced in E. coli. | Hansa Biopharma (Lund, Sweden) | Preventing kidney transplant rejection | 2020 (EU) |
| Palynziq (pegvaliase (EU), pegvaliase-pqpz (US)), r phenylalanine ammonia lyase derived from the cyanobacterium Anabaena variabilis, produced in E. coli and PEGylated. |
BioMarin International (Cork, Ireland) BioMarin (Novato, CA, USA) |
Phenylketonuria |
2019 (EU) 2018 (US) |
| Lamzede (velmanase alfa), rh α-mannosidase, expressed in precursor form in CHO cells. | Chiesi Farmaceutici (Parma, Italy) | α-mannosidosis | 2018 (EU) |
| Mepsevii (vestronidase alfa-vjbk (US), vestronidase alfa (EU)), r human lysosomal β-glucuronidase, produced in CHO cells. |
Ultragenyx Germany (Berlin) Ultragenyx Pharmaceutical (Novato, CA, USA) |
Mucopolysaccharidosis VII |
2018 (EU) 2017 (US) |
| Revcovi (elapegademase-lvlr), PEG-conjugated r bovine adenosine deaminase, produced in E. coli. | Leadiant Biosciences (Gaithersburg, MD, USA) | Adenosine deaminase severe combined immune deficiency (ADA-SCID) | 2018 (US) |
| Brineura (cerliponase alfa), rh serine tripeptidyl peptidase-1, expressed in proenzyme form in CHO cells. |
BioMarin (Cork, Ireland) BioMarin |
CLN2 disease (tripeptidyl peptidase-1 deficiency) | 2017 (EU & US) |
| Oncaspar (pegaspargase), r asparaginase, produced in E. coli and conjugated to monomethoxypropylene glycol. | Les Laboratoires Servier (Suresnes, France) | Lymphoblastic leukemia, lymphoma | 2016 (EU) |
| Spectrila (asparaginase), r asparaginase, produced in E. coli. | Medac Gesellschaft für klinische Spezialpräparate (Wedel, Germany) | Lymphoblastic leukemia, lymphoma | 2016 (EU) |
| Kanuma (sebelipase alfa), rh lysosomal acid lipase, produced in the eggs of transgenic chickens.Alexion Europe (Rueil-Malmaison, France), Alexion Pharmaceuticals (Cheshire, CT, USA) | Enzyme replacement therapy in patients with lysosomal acid lipase deficiency | 2015 (EU & US) | |
| Strensiq (asfotase alfa), dimeric fusion protein containing a soluble catalytic domain of human tissue-nonspecific alkaline phosphatase linked to an IgG Fc domain and a deca-aspartate peptide domain, produced in CHO cells. |
Alexion Europe (Rueil-Malmaison, France) Alexion (Cheshire, CT, USA) |
Enzyme replacement therapy in patients with pediatric-onset hypophosphatasia | 2015 (EU & US) |
| Vimizim (elosulfase alfa), rh N-acetlygalactosamine-6-sulfatase, produced in CHO cells. | BioMarin (Cork, Ireland) | Mucopolysaccharidosis IVA (Morquio A syndrome) | 2014 (EU & US) |
| Krystexxa (pegloticase), r urate oxidase, produced in E. coli and PEGylated. |
Savient Pharma (Dublin) Crealta Pharmaceuticals (Lake Forest, IL, USA) |
Gout |
2013 (EU) 2010 (US) Withdrawn 2016 (EU) |
| Elelyso (taliglucerase alfa), rh glucocerebrosidase, produced in engineered carrot root cell culture. | Pfizer (New York, NY, USA) | Gaucher disease | 2012 (US) |
| Lumizyme (alglucosidase alfa), rh acid-α-glucosidase, produced in CHO cells. | Sanofi Genzyme | Pompe disease (glycogen storage disease type II) | 2010 (US) |
| VPRIV (velaglucerase alfa), rh glucocerebrosidase, produced in a human fibroblast cell line. |
Shire (Dublin) Shire (Cambridge, MA USA) |
Gaucher disease | 2010 (EU & US) |
| Elaprase (idursulfase), rh iduronate-2-sulfatase, produced in a human cell line. | Shire | Mucopolysaccharidosis II (Hunter syndrome) |
2007 (EU) 2006 (US |
| Naglazyme (galsulfase), rh N-acetylgalactosamine-4-sulfatase, produced in CHO cells. | BioMarin (Cork, Ireland & Novato, CA, USA) | Long-term enzyme replacement therapy in mucopolysaccharidosis VI |
2006 (EU) 2005 (US) |
| Myozyme (algulcosidase alfa), rh acid glucosidase, produced in CHO cells. | Genzyme (Amsterdam) | Pompe disease |
2006 (EU & US) Discontinued 2014 (US) |
| Aldurazyme (laronidase), r α-l-iduronidase, produced in CHO cells. | Genzyme (Amsterdam) | Long-term replacement in mucopolysaccharidosis I | 2003 (EU & US) |
| Hylenex (hyaluronidase), rh hyaluronidase, produced in CHO cells. | Halozyme Therapeutics (San Diego) | Adjuvant to increase absorption and dispersion of other drugs | 2005 (US) |
| Fabrazyme (agalsidase beta), rh α-galactosidase, produced in CHO cells. | Genzyme | Fabry disease (α-galactosidase A deficiency) |
2003 (US) 2001 (EU) |
| Replagal (agalsidase alfa), rh α-galactosidase, produced in a human cell line. | Takeda (Dublin) | Fabry disease (α-galactosidase A deficiency) | 2001 (EU) |
| Fasturtec (EU), Elitek (US) (rasburicase), r urate oxidase, produced in S. cerevisiae. | Sanofi (Paris) | Hyperuricemia |
2002 (US) 2001 (EU) |
| Cerezyme (imiglucerase), rh β-glucocerebrosidase, produced in CHO cells. | Genzyme | Gaucher disease |
1997 (EU) 1994 (US) |
| Pulmozyme (dornase alpha), r DNase, produced in CHO cells. | Roche/Genentech | Cystic fibrosis | 1993 (US) |
| Fusion proteins | |||
| Kimmtrak (tebentafusp-tebn (US), tebentafusp (EU)); bispecific T cell engager fusion protein, produced in E. coli. |
Immunocore (Dublin; EU) Immunocore (Conshohocken, PA, USA; US) |
Uveal melanoma (type of eye cancer) | 2022 (EU & US) |
| Ngenla (somatrogon), r chimeric fusion protein of hGH with one copy of the C-terminal peptide (CTP) from the β-chain of human chorionic gonadotropin at the N-terminus and two copies of CTP at the C-terminus, produced in CHO cells. | Pfizer Europe MA EEIG (Brussels) | Growth disturbances due to growth hormone deficiency | 2022 (EU) |
| Elzonris (tagraxofusp-erzs (US), tagraxofusp (EU)), rh IL-3–truncated diphtheria toxin fusion protein, produced in E. coli. |
Stemline Therapeutics (Amsterdam) Stemline Therapeutics (New York) |
Blastic plasmacytoid dendritic cell neoplasm |
2021 (EU) 2018 (US) |
| Lumoxiti (moxetumomab pasudotox (EU), moxetumomab pasudotox-tdfk (US)), r-immunotoxin fusion protein consisting of an Ig light-chain variable domain (VL) and heavy-chain variable domain (VH) genetically fused to a truncated form of Pseudomonas exotoxin, targeting CD22 cell surface receptors, produced in E. coli. |
AstraZeneca (Sodertalje, Sweden) AstraZeneca Pharmaceuticals (Wilmington, DE, USA) |
Hairy cell leukemia |
2021 (EU) 2018 (US) Withdrawn 2021 (EU) |
| Nepexto (etanercept), r dimeric protein comprising 2 soluble p75 TNFR molecules fused to the human IgG1 Fc fragment, produced in a CHO cell line. Biosimilar to Enbrel. | Mylan (Dublin) | Various inflammatory conditions | 2020 (EU) |
| Reblozyl (luspatercept (EU), luspatercept-aamt (US)), r-fusion protein consisting of two modified extracellular domains of human activin receptor type IIB (ActRIIB) linked to the human IgG1 Fc domain. Binds selectively to transforming growth factor-beta (TGF-β) superfamily ligands; produced in CHO cell line. |
Bristol Myers Squibb Pharma EEIG (Dublin) Celgene (Summit, NJ, USA) & Acceleron Pharma, (Cambridge, MA, USA)) |
Certain (rare) forms of anemia |
2020 (EU) 2019 (US) |
| Benepali (etanercept, EU), Eticovo (etanercept-ykro, US)), rh TNF receptor–IgG Fc fusion protein, produced in CHO cells. Biosimilar to Enbrel. |
Samsung Bioepis (Delft, the Netherlands) Samsung Bioepis |
Arthritis, psoriasis, axial spondyloarthritis |
2019 (US) 2016 (EU) |
| Erelzi (etanercept (EU), etanercept-szzs (US)), r dimeric fusion protein consisting of TNF receptor extracellular domains linked to an IgG1 Fc region, produced in CHO cells. Biosimilar to Enbrel. | Sandoz (Kundl, Austria, & Princeton, NJ, USA) | Rheumatoid arthritis, selected other inflammatory diseases |
2017 (EU) 2016 (US) |
| Lifmior (etanercept), r dimeric fusion protein consisting of TNF receptor extracellular domains linked to an IgG1 Fc region, produced in CHO cells. Same product as Enbrel (see below). | Pfizer Europe MA EEIG (Brussels) | Rheumatoid arthritis, selected other inflammatory diseases |
2017 (EU) Withdrawn 2020 |
| Zaltrap (aflibercept), combination drug comprising binding domains of VEGF receptors 1 and 2 fused to an IgG Fc, produced in CHO cells. Same active substance as in Eylea (see below). |
Sanofi (Paris) Sanofi-Aventis US (Bridgewater, NJ, USA) |
Metastatic colorectal cancer |
2013 (EU) 2012 (US) |
| Eylea (aflibercept), fusion protein comprising extracellular ligand-binding domains of VEGF receptor fused to IgG Fc, produced in CHO cells. Same active substance as in Zaltrap (see above). |
Regeneron Pharmaceuticals (Tarrytown, NY, USA) Bayer (Berlin) |
Neovascular (wet) age-related macular degeneration |
2012 (EU) 2011 (US) |
| Nulojix (belatacept), fusion protein comprising extracellular domain of human CTLA4 fused to IgG Fc, which binds CD80 and CD86 on antigen-presenting cells, thereby inhibiting T cell activation; produced in CHO cells. | Bristol-Myers Squibb (Dublin) | Prophylaxis of organ rejection following kidney transplant | 2011 (EU & US) |
| Arcalyst (US), Rilonacept Regeneron (EU) (rilonacept), dimeric fusion protein with each monomer consisting of the ligand-binding domains of the human IL-1 receptor and IL-1 receptor accessory protein along with the Fc region of human IgG-1, produced in CHO cells. | Regeneron Pharmaceuticals | Cryopyrin-associated periodic syndromes (CAPS) |
2009 (EU) 2008 (US) Withdrawn 2012 (EU) |
| Nplate (romiplostim), dimeric fusion protein with each monomer consisting of two thrombopoietin RBDs and the Fc region of human IgG-1, produced in E. coli. | Amgen Europe | Thrombocytopenia |
2009 (EU) 2008 (US) |
| Orencia (abatacept), fusion protein that links the extracellular domain of human cytotoxic-T-lymphocyte-associated antigen-4 with modified Fc region of IgG1, produced in a mammalian cell line. | Bristol-Myers Squibb | Rheumatoid arthritis |
2007 (EU) 2005 (US) |
| Amevive (alefacept), dimeric fusion protein comprising the extracellular CD2-binding portion of human LFA-3 linked to the Fc region of human IgG1, produced in CHO cells. | Astellas Pharma (Deerfield, IL, USA) | Moderate to severe chronic plaque psoriasis in adults |
2003 (US) Withdrawn 2011 |
| Enbrel (etanercept), r TNF receptor–IgG fragment fusion protein, produced in CHO cells. Same product as Lifmior (see above). |
Amgen (Thousand Oaks, CA) Pfizer (Brussels) |
Rheumatoid arthritis |
2000 (EU) 1998 (US) |
| Ontak (denileukin diftitox), r IL-2–diphtheria toxin fusion protein that targets cells displaying a surface IL-2 receptor, produced in E. coli. |
Eisai (Tokyo) Ligand Pharmaceuticals (San Diego) |
Cutaneous T cell lymphoma |
1999 (US) Discontinued 2014 |
| Gene-therapy and nucleic acid-based products (nucleic acid-based vaccines are included in the ‘Vaccine’ section) | |||
| Amvuttra (vutrisiran), a transthyretin-directed siRNA. | Alnylam (Cambridge, MA, USA) | Polyneuropathy of hereditary transthyretin-mediated amyloidosis | 2022 (US) |
| Amondys 45 (casimersen), chemically synthesized PMO antisense oligonucleotide. | Sarepta Therapeutics (Cambridge, MA, USA) | Duchenne muscular dystrophy | 2021 (US) |
| Leqvio (inclisiran), chemically synthesized ds siRNA covalently linked to a triantennary GalNAc, facilitating hepatocyte update. |
Novartis (Dublin) Novartis (East Hanover, NJ, USA) |
Hypercholesterolemia, mixed dyslipidemia |
2021 (US) 2020 (EU) |
| Givlaari (givosiran), chemically synthesized, chemically modified ds siRNA conjugated to a triantennary GalNAc ligand to facilitate hepatic delivery. Silences aminolevulinate hepatic synthase 1 (ALAS1) mRNA. |
Alnylam Netherlands (Amsterdam) Alnylam (Cambridge, MA, USA) |
Acute hepatic porphyria |
2020 (EU) 2019 (US) |
| Oxlumo (Lumasiran), chemically synthesized ds siRNA covalently linked to a triantennary GalNAc, facilitating hepatocyte update. |
Alnylam (Amsterdam) Alnylam (Cambridge, MA, USA) |
Primary hyperoxaluria type 1 | 2020 (EU & US) |
| Viltepso (viltolarsen), chemically synthesized PMO antisense oligonucleotide. | NS Pharma (Paramus, NJ, USA) | Duchenne muscular dystrophy | 2020 (US) |
| Zolgensma (onasemnogene abeparvovec), nonreplicating adeno-associated vector housing the human survival motor neuron gene (SMN1). |
Novartis Europharm (Dublin) Novartis Gene Therapies (Bannockburn, IL, USA) |
Spinal muscular atrophy |
2020 (EU) 2019 (US) |
| Viondys 53 (golodirsen), chemically synthesized PMO antisense oligonucleotide. | Sarepta Therapeutics (Cambridge, MA, USA) | Duchenne muscular dystrophy | 2019 (US) |
| Waylivra (volanesorsen), synthetic antisense PTO oligonucleotide. | Akcea Therapeutics (Dublin) | Familial chylomicronemia syndrome | 2019 (EU) |
| Luxturna (voretigene neparvovec-rzyl (US), voretigene neparvovec (EU)), a live, nonreplicating adeno-associated virus genetically modified to express the human RPE65 gene. |
Novartis (Dublin) Spark Therapeutics (Philadelphia) |
Retinal dystrophy |
2018 (EU) 2017 (US) |
| Onpattro (patisiran), chemically synthesized, 21-nucleotide ds siRNA oligonucleotide, formulated as lipid nanoparticles. |
Alnylam (Amsterdam) Alnylam Pharmaceuticals (San Diego) |
Hereditary transthyretin amyloidosis | 2018 (EU & US) |
| Tegsedi (inotersen), a 20-nucleotide ss oligonucleotide manufactured by direct chemical synthesis. |
Akcea (Dublin) Ionis Pharmaceuticals (Carlsbad, CA, USA) |
Hereditary transthyretin amyloidosis | 2018 (EU & US) |
| Spinraza (nusinersen sodium), an 18-nucleotide antisense oligonucleotide manufactured by direct chemical synthesis. |
Biogen (Badhoevedorp, the Netherlands) Biogen (Cambridge, MA, USA) |
Spinal muscular atrophy |
2017 (EU) 2016 (US) |
| Exondys 51 (eteplirsen), a chemically synthesized antisense oligonucleotide. | Sarepta Therapeutics (Cambridge, MA, USA) | Duchenne muscular dystrophy | 2016 (US) |
| Imlygic (talimogene laherparepvec), an engineered herpes simplex virus type 1 capable of producing GM-CSF. | Amgen | Melanoma | 2015 (EU & US) |
| Kynamro (mipomersen sodium), a chemically synthesized antisense oligonucleotide. | Kastle Therapeutics (Chicago) | Familial hypercholesterolemia |
2013 (US) Discontinued 2022 |
| Glybera (alipogene tiparvovec), a human LPL gene housed in an engineered adeno-associated virus 1 vector. | uniQure (Amsterdam) | Lipoprotein lipase deficiency |
2012 (EU) Withdrawn 2017 |
| Macugen (pegaptanib sodium injection), a synthetic PEGylated oligonucleotide that specifically binds VEGF. |
Eyetech (Palm Beach Gardens, FL, USA) PharmaSwiss (Prague) |
Neovascular, age-related macular degeneration |
2006 (EU) 2004 (US) Withdrawn 2019 |
| Vitravene (fomivirsen), an antisense oligonucleotide. |
Isis Pharmaceuticals (Carlsbad, CA, USA) Novartis Ophthalmics Europe (Farnborough, UK) |
Cytomegalovirus retinitis in AIDS patients |
1999 (EU) 1998 (US) Withdrawn 2002 (EU), 2005 (US) |
| Engineered cell-based | |||
| Breyanzi (lisocabtagene maraleucel), autologous, purified CD8+ and CD4+ T cells, both engineered to encode an anti-CD19 CAR. |
Bristol-Myers Squibb (Dublin) Bristol-Myers Squibb (Bothell, WA, USA) |
B cell lymphoma |
2022 (EU) 2021 (US) |
| Carvykti (ciltacabtagene autoleucel), BCMA-directed genetically modified autologous T cell immunotherapy. | Janssen Biotech (Raritan, NJ, USA) | Multiple myeloma | 2022 (US) |
| Abecma (idecabtagene vicleucel), genetically modified autologous T cells transduced with an anti-BCMA CAR lentiviral vector. |
Bristol-Myers Squibb (Dublin) Celgene (Summit, NJ, USA) Bluebird Bio (Cambridge, MA, USA) |
Multiple myeloma | 2021 (EU & US) |
| Skysona (elivaldogene autotemcel), autologous CD34+-cell-enriched population containing hematopoietic stem cells transduced with a lentiviral vector (LVV) encoding ABCD1 cDNA for human adrenoleukodystrophy protein (ALDP). | Bluebird Bio (Utrecht, the Netherlands) | Early cerebral adrenoleukodystrophy |
2021 (EU) Withdrawn 2021 |
| Libmeldy (atidarsagene autotemcel), autologous CD34+ hematopoietic stem and progenitor cells (HSPCs), transduced with a lentiviral vector housing the arylsulfatase A gene. | Orchard Therapeutic (Amsterdam) | Metachromatic leukodystrophy | 2020 (EU) |
| Tecartus (brexucabtagene autoleucel), autologous peripheral blood T cells, CD4 and CD8 selected and CD3 and CD28 activated and transduced with retroviral vector expressing anti-CD19 CD28/CD3ζ chimeric antigen receptor. |
Kite Pharma (Amsterdam) Kite Pharma (Santa Monica, CA, USA) |
Mantle cell lymphoma | 2020 (EU & US) |
| Zynteglo (betibeglogene autotemcel), autologous CD34+-cell-enriched population containing hematopoietic stem cells transduced with a lentiglobin lentiviral vector encoding the β-A-T87Q-globin allele. | Bluebird Bio (Utrecht, the Netherlands) | β-thalassemia |
2019 (EU) Withdrawn 2022 |
| Kymriah (tisagenlecleucel), autologous T cells genetically modified to encode an anti-CD19 CAR comprising a murine single-chain antibody fragment (scFv) specific for CD19, followed by a CD8 hinge and transmembrane region fused to the intracellular signaling domains for 4-1BB (CD137) and CD3ζ. |
Novartis (Dublin) Novartis (East Hanover, NJ, USA) |
Acute lymphoblastic leukemia, large B cell lymphoma |
2018 (EU) 2017 (US) |
| Yescarta (axicabtagene ciloleucel), autologous T cells genetically modified to express a CAR comprising a murine anti-CD19 single-chain variable fragment (scFv) linked to CD28 and CD3ζ co-stimulatory domains. |
Kite Pharma (Amsterdam) Kite Pharma (Santa Monica, CA, USA) |
Large B cell lymphoma |
2018 (EU) 2017 (US) |
| Strimvelis, autologous CD34+ cells transduced with an engineered retroviral vector encoding the human adenosine deaminase sequence. | Orchard Therapeutics (Amsterdam) | Severe combined immunodeficiency | 2016 (EU) |
| Zalmoxis, allogeneic T cells genetically modified to express the herpes simplex thymidine kinase suicide gene and a truncated form of the human low-affinity nerve growth factor receptor gene. | MolMed (Milan) | Hematopoietic stem cell transplantation, graft-versus-host disease |
2016 (EU) Withdrawn 2019 |
Data were collected from several sources (http://www.fda.gov/, https://www.ema.europa.eu/en). Products are listed consecutively from most recent approval in each class, with registrations since 2018 in boldface and withdrawals and discontinuations in italics. r, recombinant; rh, recombinant human. ADC, antibody–drug conjugate; BHK, baby hamster kidney cell line; BMP, bone morphogenetic protein; CAR, chimeric antigen receptor; CGRP, calcitonin gene-related peptide; CHO, Chinese hamster ovary cell line; CLL, chronic lymphocytic leukemia; ds, double-stranded; EGF, epidermal growth factor; EPO, erythropoietin; FSH, follicle stimulating hormone; G-CSF, granulocyte colony-stimulating factor; GalNAc, N-acetylgalactosamine; GLP, glucagon-like peptide; GM-CSF, granulocyte-macrophage colony stimulating factor; HBsAg, hepatitis B surface antigen; HEK, human embryo kidney cell line; HER2, human epidermal growth factor receptor 2; hGH, human growth hormone; HPV, human papillomavirus; IFN, interferon; Ig, immunoglobulin; IGF, insulin-like growth factor; IL, interleukin; mAb, monoclonal antibody; PEG, polyethylene glycol; PTH, parathyroid hormone; RBD, receptor-binding domain; siRNA, small interfering RNA; ss, single-stranded; TNF, tumor necrosis factor; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor.
These new approvals bring the cumulative number of individual biopharmaceutical products (by trade name) licensed in these regions to 541, containing 435 distinct active biopharmaceutical ingredients. However, over the years, 98 products have been withdrawn from the market subsequent to approval in one or both regions, almost always for commercial reasons. Taking withdrawals into account, the number of individual biopharmaceutical products with current active licenses is estimated to be 443 (Table 1).
Annual approval numbers over the current survey period ranged from a low of 19 in Europe in 2019 to a high of 42, also in Europe, in 2018 (Fig. 1a). Annual approval rates were sustained or exceeded in both regions in 2020 and 2021, reflecting strong regulatory response, despite the unexpected burden on the agencies caused by the pandemic. Products approved over the current period include 97 monoclonal antibodies, 19 hormones, 16 nucleic acid/gene-therapy-based products and 16 vaccines (the latter two categories overlap as five of the (COVID-19) vaccines are nucleic acid based). Additional notable approval categories include colony-stimulating factors (CSFs; 12 products, all biosimilars), cell-based products (9), enzymes (8), fusion products (7) and clotting factors (6).
Fig. 1. Product approvals profile.
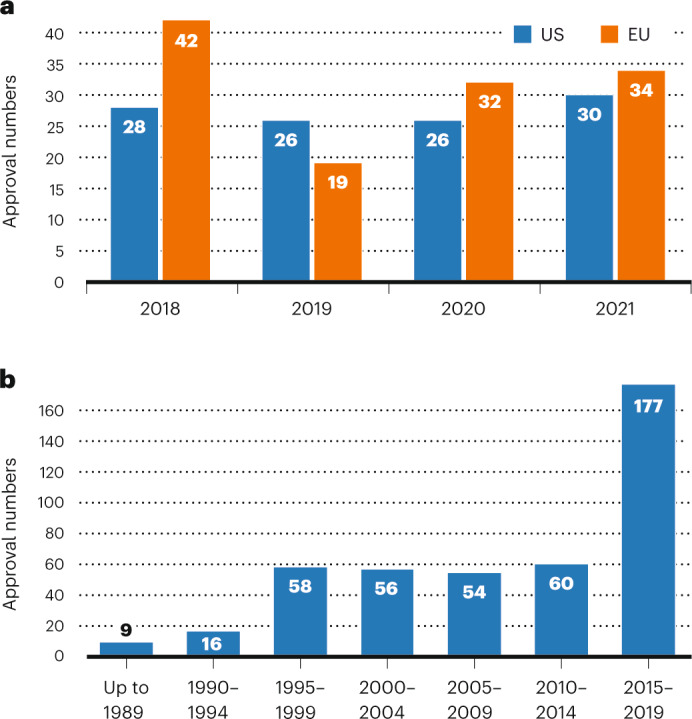
a, Annual product approval numbers (by product trade name) by individual region. b, Number of product approvals in one or both regions over the indicated periods.
Here we list all biopharmaceuticals approved from January 2018 to June 2022, examining what types of product reached the US and EU markets as well as the indication for which they were approved. As in previous articles1–4, we have not included tissue-engineering products, which the US Food and Drug Administration (FDA) classifies as medical devices.
In a snapshot
As in previous survey periods, new approvals followed predictable lines. Cancer was by far the most common indication (50 products). Other common indications included inflammation-related conditions (15 products), neutropenia (12 products), COVID-19 (11 products) and diabetes (10 products). Additional indications, less commonly targeted by biopharmaceuticals, included ebolavirus (the vaccines Mvabea, Zabdeno and Ervebo and the therapeutics Ebanga and Inmazeb), anthrax (the inhalation therapeutic Obiltoxaximab SFL), weight control and weight loss (Wegovy) and Alzheimer’s disease (Aduhelm).
Of the 197 biopharmaceutical products approved within the survey timeframe, 90 (46%) were genuinely new to the market, with the remainder representing biosimilars, me-too products and products previously approved elsewhere. Those 90 new products (by trade name) contained a total of 85 distinct active biopharmaceutical ingredients (Table 2). Looking at each region separately, 121 products were licensed in the United States, of which 70 (58%) were genuinely novel; 144 products gained marketing Authorization in the EU, of which 65 (45%) were genuinely novel.
Table 2.
Biopharmaceuticals approved in the United States and/or EU during the current survey period (January 2018–June 2022) by category
| Category | Products (by trade name) |
|---|---|
| Genuinely novel biopharmaceuticals (85 products) | Amvuttra, Lunsumio, Ondexxya, Veyvondi, Voxzogo, PreHevbri/Prehevbrio, Nuvaxovid/Novavax COVID-19 Vaccine, Spikevax, Jcovden, Vaxzevria, Comirnaty, Mvabea, Vaxchora, Zabdeno, Dengvaxia, Ervebo, Bebtelovimab, Enjaym, Evusheld, Opdualag, Padcev, Saphnelo, Uplizna, Vabysmo, Vyepti, Adbry, Aduhelm, Bamlanivimab & eteseviman, Enspryng, Evkeeza, Minjuvi/Monjivi, Regkirona, Ronapreve/Regen-cov, Rybrevant, Tezspire, Tivdak, Trodelvy, Vyvgart, Xevudy/Sotrovimab, Zynlonta, Adakveo, Blenrep, Danyelza, Ebanga, Inmazeb, Obiltoxaximab SFL, Polivy, Tepezza, Ajovy, Cablivi, Evenity, Trogarzo, Ultomiris, Aimovig, Crysvita, Emgality, Gamifant, Poteligeo, Takhzyro, Idefirix, Palynziq, Lamzede, Revcovi, Kimmtrak, Ngenla, Elzonris, Lumoxitib, Reblozyl, Amondys 45, Leqvio, Givlaari, Oxlumo, Viltepso, Zolgensma, Viondys 53, Waylivra, Onpattro, Tegsedi, Breyanzi, Carvykti, Abecma, Skysonab, Libmeldy, Tecartus, Zynteglo |
| Biosimilars (58 products) | Inpremzia, Truvelog Mix 30, Kirsty, Rezvoglar, Semglee, Insulin aspart Sanofi, Sondelbay, Livogiva, Qutavinab, Retacrita, Fylnetra, Releuko, Stimufend, Nyvepria, Cegfila, Grasustek, Ziextenzo, Fulphila, Nivestym/Nivestima, Pelgraz, Pelmeg, Udenycab, Alymsys, Abevmy, Byooviz, Hukyndra, Lextemyb, Libmyris, Onbevzi, Oyavas, Yuflyma, Yusimry, Amsparity, Aybintio, Equidacentb, Hulio, Riabni, Ruxience, Zercepac, Abrilada, Avsola, Hadlima, Idacio, Kanjinti, Kromeyab, Ontruzanta, Trazimera, Zirabev, Halimatozb, Hefiya, Herzuma, Hyrimoz, Mvasia, Ogivria, Truximaa, Zessly, Nepexto, Benepalia |
| ‘Me-too’ or incremental improvement on existing API: for example, reformulation, PEGylation, use in combination, different indication & related (31 products) | Esperoct, Adynovi, Jivi, Sevenfact, Lyumjev, Myxredlin, Lonapegsomatropin Ascendis Pharma, Skytrofa, Sogroya, Wegovy, Besremi, Heplisav B, Vaxelis, Vaxneuvance, Supemtek, Bimzelx, Enhertu, Jemperli, Kesimpta, Susvimo, Beovu, Margenza, Phesgo, Darzalex Faspro, Sarclisa, Herceptin Hylecta, Libtayo, Skyrizi, Ilumya/Ilumetri, Nexviazyme, Rylaze |
| Previously approved elsewhere1 (15 products) | Rybelsus, Myalepta/Myalept, Ozempic, Oxervate, Shingrix, Fasenra, Hemlibra, Imfinzi, Mylotarg, Ocrevus, Voraxaze, Mepsevii, Luxturna, Kymriah, Yescarta |
aBiosimilars approved in one region since 2018, but that were approved in the other region before 2018.
bProducts that were both approved and subsequently withdrawn from one or both regions within the survey timeframe.
In the same period, US regulators approved in total 244 non-biological pharmaceutical products containing novel molecular (chemical and biopharmaceutical) entities (NME); thus, 29% (70/244) of all genuinely novel drug approvals in the US were biopharmaceuticals. This compares to 40% for the previous survey period and 21–26% in survey periods before 2014. EU reporting formats for pharmaceuticals preclude calculation of an analogous figure for Europe.
Overall trends
Comparing approvals over the current survey period to those in earlier periods or to cumulative approvals confirms some interesting, if predictable, trends.
Since 2015, the rise in biopharmaceutical approval rates has been sustained. Between 2015 and 2019, 178 products gained approval, approximately three times the historical five-year approval average (Fig. 1b). Moreover, a further 117 products were approved in just the past year and a half, from January 2020 to June 2022. Contributing to this was a substantial increase in the numbers of both biosimilar and ‘me-too’-type products (Table 2) gaining approval in recent years. Although the absolute number of genuinely new biopharmaceutical entities also continued to increase (74 for the 2014–2018 survey, 85 for the current 2018–2022 period), as a proportion of total biopharmaceutical approvals the number of novel biopharmaceuticals has decreased over this period, to 45% (85/189) as compared to the previous period’s 56%.
Monoclonal antibodies stay strong
Monoclonal antibodies (mAbs) remain dominant in overall approvals, representing 53.5% of all approvals in the past four years (Fig. 2a) They also remain the most prominent category of genuinely new biopharmaceuticals coming on the market, constituting 51% of all genuinely new products approved in this survey period (Table 2) compared to 49% in the previous survey period (2014–2018).
Fig. 2. Monoclonal antibody statistics.
a, mAbs approved for the first time within the indicated periods, expressed as a percentage of total biopharmaceuticals approved for the first time within the same time period. b, Global annual mAb sales value, expressed as a percentage of total protein-based biopharmaceutical global sales for the indicated years. Financial data from LaMerie Business Intelligence.
The importance of mAbs to biopharmaceutical sales remains evident (Fig. 2b); the percentage of the total market value contributed by mAbs grew steadily during this survey, although COVID-19 vaccine sales affected this trend. However, with these vaccine revenues excluded, mAbs still represented 80% of total protein-based global biopharmaceutical sales last year. Also notable is an increase in approval rates for biosimilars, as well as for nucleic acid-based products and gene-engineered cells (Table 1).
Mammalian cell systems continue to be the most often used expression system during this time period. Of the 159 approved products made by recombinant means in cell-based systems, most (107, or 67%) are produced in mammalian cells. Nonetheless, this reverses a trend seen over many decades, as it is lower than the percentage of mammalian-cell-produced biopharmaceuticals approved during the last survey period (84%; Fig. 3). The expression systems used are invariably dictated by the post-translational modification (PTM) requirements of the products. A large proportion of, in particular, biosimilar and ‘me-too’-type products approved over the current survey period (Table 2) do not require glycosylation or other mammalian PTMs, enabling their production in nonmammalian and less expensive systems, most commonly Escherichia coli. Interestingly, when focusing solely on the genuinely novel active biopharmaceutical ingredients approved in this current period, a different story emerges, with 85% of these products made in mammalian systems.
Fig. 3. Expression systems.
Relative use of mammalian- versus non-mammalian-based production cell lines in the manufacture of biopharmaceuticals approved over the indicated periods. Each dataset is expressed as a percent of total biopharmaceutical product approvals for the period indicated.
As in previous surveys, the most often used mammalian cell culture system remains Chinese hamster ovary (CHO cells), which were used to produce 95 of those 107 individual products made in mammalian systems (89%). This reflects the well-known strengths of this production platform, including the ability to produce antibodies at titers of 3–8 g/liter at production scale5. Other mammalian systems used included NS0 mouse myeloma cells (7 products), as well as baby hamster kidney (BHK), human embryonic kidney (HEK), sp2/0 mouse myeloma cells and PER C6 immortalized primary human embryonic retinal cells (1 product each). Moreover, a single new product (Sevenfact) is produced via transgenic means, in the milk of transgenic rabbits—only the second recombinant protein produced in this transgenic system. (Two existing products are also made in transgenic systems: Atryn, approved in 2009, in transgenic goats' milk and Kanuma, approved in 2015, in transgenic chicken eggs.)
Of the nonmammalian production platforms, E. coli continues to dominate, used in the production of 36 products approved since 2018, with smaller numbers of products produced in Pichia pastoris (5) and Saccharomyces cerevisiae (4). Also notable is the bacterium Pseudomonas fluorescens, used to recombinantly produce the active ingredient (teriparatide) of the biosimilar Livogiva/Qutavina, as well as the active constituent of Rylaze (asparaginase) and one component of the multicomponent vaccine Vaxneuvance. Historically, P. fluorescens was used to produce a single biopharmaceutical, Bonsity, a recombinant parathyroid hormone (PTH) initially approved in 1987. The yeast Hansenula polymorpha is also used to produce one product approved in the current period (Heplisav B, a recombinant hepatitis B surface antigen). It was also used to produce the recombinant hepatitis B surface antigen active pharmaceutical ingredient (API) found in Hexacima/Hexyon, initially approved in 2013.
As with past surveys, most products approved during the current survey period are administered parenterally. A small number are administered directly to their intended site of action via nonparenteral means, such as the oral recombinant cholera vaccine Vaxchora (Table 1). Rybelsus (semaglutide), for type 2 diabetes, represents an interesting exception: this acylated, 39-amino-acid polypeptide is administered orally in tablet form, a first for the biopharma sector. The tablet also contains a novel excipient (salcaprozate sodium) as an absorption enhancer. This facilitates uptake of semaglutide across the epithelium of the gastrointestinal tract, and hence into the bloodstream. A bioavailability of 1% was recorded in humans during clinical studies.
Another interesting approval with challenging delivery is the Alzheimer’s product Aduhelm. This human IgG1, directed against aggregated soluble and insoluble forms of amyloid-β in the brain (a defining pathophysiological feature of Alzheimer’s), was approved in 2021 by the FDA under its accelerated approval process. Clinical studies confirmed that intravenous infusion of Aduhelm results in a reduction of amyloid-β plaques, although a clear and unambiguous link between this effect and appreciable clinical improvement remains to be established. This, along with some safety concerns, led the European Medicines Agency (EMA) to refuse to recommend approval in Europe. That an intravenous infusion of Aduhelm reduces amyloid-β plaques in the brain suggests that sufficient quantities of the antibody cross the blood–brain barrier to have a physiological effect. This finding may benefit other mAb-based therapies in development for diseases of the brain.
The impact of COVID-19
Clearly, COVID-19 represents the most significant and challenging new global threat to human health during the period of this survey. Since the first reported cases in November 2019, 636 million confirmed cases and 6.6 million deaths have been reported globally to the WHO (updated statistics available at https://COVID19.who.int).
Development and deployment of effective COVID-19 vaccines and therapeutics occurred with unprecedented speed, thanks to industry action and regulatory agility. Regulators shifted resources toward COVID-19-related activities and provided rapid scientific advice, compliance checks and accelerated assessment and evaluation procedures to product developers. Rolling reviews (regulatory assessment as data came in, rather than as part of a final marketing application) proved particularly effective. Such agility notwithstanding, FDA approvals of COVID-19 products were made through an existing framework for authorizing new drugs in emergency circumstances—the Emergency Use Authorization pathway (which is not strictly an approval)—whereas the EMA expedited approvals using their pre-existing Conditional Marketing Authorisation procedure. As a result, by September 2022, the FDA and EMA had, between them, approved or authorized 22 different COVID-19 medicines (6 vaccines and 16 therapeutics), of which 16 are biopharmaceuticals, mainly vaccines and mAbs. Updated product lists are available on the dedicated COVID-19 pages of both regulators’ websites.
Vaccination has had the greatest single impact on pandemic amelioration. As of November 2022, the World Health Organization estimates that a total of 13 billion vaccine doses have been administered globally. Data from the CDC show that, for those over 50 years of age, full vaccination decreases the risk of death by 12-fold). Approaches to vaccine API development and manufacture vary; approved vaccines include mRNA-based vaccines (Comirnaty and Spikevax), inactivated and adjuvanted SARS-CoV-2 virus (Valneva), engineered adenovirus encoding the SARS-CoV-2 spike protein (Vaxzevria and Jcovden) and recombinant spike protein (Nuvaxovid). From a technological perspective, mRNA-based vaccines have the greatest novelty and are likely to pave the way toward additional mRNA vaccines for COVID and non-COVID indications (Box 2).
Box 2 mRNA vaccines, COVID-19 and beyond.
The first two COVID-19 vaccines approved in the United States and EU were both mRNA-based, encoding the full-length SARS-CoV-2 spike protein. SARS-CoV-2 was identified as the causative agent of COVID-19 in December 2019. The first known cases of the disease in Europe and the United States were recorded on 12 and 16 January 2020, respectively. The full genome sequence of the original Wuhan strain (Wuhan-Hu-1) was published in GenBank on 13 January 2020, and the WHO declared COVID-19 to be a global pandemic on 11 March 2020. Pfizer-BioNTech’s mRNA COVID-19 Vaccine (Comirnaty) first gained Emergency Use Authorization in the United States on 11 December 2020 followed by conditional approval in the EU on 21 December 2020. Moderna’s mRNA COVID-19 Vaccine (Spikevax) gained Emergency Use Authorization status on 18 December 2020 in the United States and conditional approval in the EU on January 6th 2021.
The Authorization or approval of two vaccines within one year of pathogen identification is unparalleled. Historically, for example, it took almost 200 years from discovery of the infectious agent to develop a measles vaccine, and over 150 years in the case of polio. Luckily, several technical advancements were reported over the previous decade in the then nascent field of mRNA therapeutics, and several companies had already initiated vaccine developmental programs based on this technology, including BioNTech and Moderna.
Mimicking the native cellular transcription process, mRNA can be produced in vitro via incubation of (usually phage) RNA polymerase enzymes and ribonucleotide triphosphates (NTPs) with template DNA (usually linearized, plasmid DNA). Although protein synthesis following administration of in-vitro-transcribed mRNA to mice was reported in the 1990s, practical therapeutic application of the approach was beset by technical challenges, including mRNA instability, immunogenicity and inefficient in vivo delivery. More recently, many of these challenges have been largely overcome9. Optimization of mRNA sequences flanking the protein-coding region (the 5′ cap and 5′ untranslated region (UTR) at one end and the 3′ UTR and polyadenosine tail at the other) helps enhance both stability and the levels of expression. Incorporating chemically modified nucleosides (such as 1-methylpseudouridine) reduces the native immune response to naked mRNA, and the development of, in particular, lipid-based nanoparticles has substantially enhanced mRNA cellular delivery.
Usually administered intramuscularly, either the mRNA vaccines or some locally produced antigen are taken up by antigen-presenting cells, such as dendritic cells10,11. These cells then travel to lymph nodes, where they elicit adaptive immunity, incorporating both T cell and B cell immune responses.
Compared to conventional vaccines, mRNA-based vaccines have been considered to have several advantages, including a capacity for rapid development, relatively low cost, straightforward scale-up and manufacture, a potential for high level of efficacy and a strong safety profile (no risk of infection or insertional mutagenesis). The development, approval and deployment of Comirnaty and Spikevax validated such cited advantages and provides a sound platform for further mRNA-based approvals. The sequence-independent flexibility of the platform is further underscored by the recent introduction of bivalent versions of Spikevax and Comirnaty, each containing mRNA sequence combinations encoding the S proteins of both original and selected Omicron variants of SARS-CoV-2.
Several dozen such product, mainly targeting cancer and infectious disease, are at various stages of clinical development9–12. The ascendancy of mRNA-based vaccines is unlikely, however, to signal the demise of traditional vaccine modalities. Within a few months (and, in one case, within weeks) of the approval of Comirnaty and Spikevax, several additional SARS-CoV-19 vaccines based upon more traditional recombinant subunit and viral vector modalities gained approval. Moreover, mRNA vaccines still suffer from some drawbacks, including the requirement for cold chain storage and distribution (usually between –15 °C and –90 °C, depending on the product). mRNA-based platforms are thus likely to broaden as opposed to replace existing vaccine production modalities in the future.
Market value
In the current survey period, the market value of biopharmaceuticals has continued to rise. Consolidated data from various La Merie (http://www.lamerie.com) and Fierce Pharma (http://www.fiercepharma.com) financial reports indicate that total global sales for 2021 reached US $343 billion (Table 3). Indeed this figure is likely an under-representation, as revenues for biosimilars in some regions have not been publicly reported. Recombinant originator proteins, both mAb and non-mAb, collectively account for a lion’s share of this value ($271 billion), representing an increase of 44% over the $188 billion reported for this product category in our last survey, for 2017.
Table 3.
Total reported 2021 biopharmaceuticals global sales values
| Biopharmaceutical category | Reported sales value ($ billion) |
|---|---|
| Originatora recombinant proteins: mAbs | 217.3 |
| Originator recombinant proteins: non-mAbs | 53.6 |
| Covid vaccines (Comirnaty and Spikevax) | 54.5 |
| Biosimilars | 11.1 |
| Nucleic acid and engineered cell based | 6.8 |
| Total value | 343.3 |
aOriginator’ signifies non-biosimilar.
COVID-19 vaccines had the largest impact upon the biopharmaceutical landscape in commercial as well as technological and medicinal terms, with Comirnaty and Spikevax cumulatively generating revenues of $54.5 billion in 2021 (Table 3). Comirnaty ($36.8 billion) has displaced the long-time best-selling biopharmaceutical Humira ($21.2 billion) as the top-selling biopharmaceutical product, with Spikevax ($17.7 billion) ranking third in 2021. Indeed, Humira’s pre-eminence in the global biopharmaceutical market is likely over. In addition to the advent of COVID-19 vaccines, its ‘patent wall’ has all but ended, and a number of biosimilar rivals are likely to stream onto the US market in particular in the next year or two. Whereas Comirnaty is poised to retain the top spot globally this year, it is difficult to forecast the market for COVID-19 vaccines in future years. Much will depend on factors such as the course of the pandemic, the future need for booster programs, the severity of evolving viral strains and future approvals of additional COVID-19 medicines, both prophylactic and therapeutic. The unpredictability of the COVID-19 therapeutics markets is illustrated by Regeneron’s anti-spike-protein mAb-based product Regen-Cov (Ronapreve). After initially gaining an Emergency Use Authorization in November 2020, Regen-Cov generated $7.6 billion in global sales in 2021, but its lack of effectiveness against newer viral variants caused the FDA to effectively pause its use in January 2022.
From a commercial perspective, revenues generated by biosimilar, nucleic acid (excluding COVID-19 mRNA vaccines) and engineered cell-based products remain relative modest. Collectively they generated an estimated $17.9 billion in 2021, representing some 5% of the total biopharmaceutical market, and less than sales of Humira alone. Of the 73 biopharmaceuticals recording blockbuster status (sales above $1 billion) last year, two were biosimilars (Erelzi and Mvasi, recording sales of $1.5 and $1.1 billion, respectively) and two were (non-COVID) nucleic acid/gene-therapy-based products (Spinraza and Zolgensma, with sales of $1.9 and $1.3 billion, respectively). mAb-based products (including Fc fusion products) continue to represent the most lucrative single product class. Their total sales reached $217 billion last year, and they represented 15 of the top 20 products by sales generated (Table 4). In terms of target indications, the vast majority of such antibody-based products target inflammatory and autoimmune conditions (cumulative 2021 sales of $99.3 billion) and cancer (2021 cumulative sales of $68.4 billion).
Table 4.
The 20-top selling biopharmaceutical products in 2021
| Rank | Product | Sales, 2021 ($ billions)a | Year first approved | Company | Patent expiryb | Biosimilar version(s) approved |
|---|---|---|---|---|---|---|
| 1 | Comirnaty (COVID-19 Vaccine, mRNA) | 36.8 | 2020c | Pfizer & BioNTech | N/A | |
| 2 | Humira (adalimumab) | 21.2 | 2002 | AbbVie & Eisai |
2016 (US) 2018 (EU) |
Halimatoz/Hefiya/Hyrimoz, Amgevita/Amjevita/Solymbic, Cyltezo, Imraldi, Kromeya, Idacio, Hadlima, Abrilada, Hulio, Amsparity, Yusimry, Yuflyma, Libmyris, Hukyndra |
| 3 | Spikevax (COVID-19 Vaccine, mRNA) | 17.7 | 2020c | Moderna | N/A | |
| 4 | Keytruda (pembrolizumab) | 17.2 | 2014 | Merck |
2036 (US) 2028 (EU) |
|
| 5 | Stelara (ustekinumab) | 9.5 | 2009 | Janssen (Johnson & Johnson) |
2023 (US) 2024 (EU) |
|
| 6 | Eylea (aflibercept) | 9.4 | 2011 | Regeneron, Bayer | 2027 (EU & US) | |
| 7 | Opdivo (nivolumab) | 8.5 | 2014 | BMS, Ono |
2027 (US) 2026 (EU) |
|
| 8 | Ronapreve/Regen-Cov (casirivimab & imdevimab) | 7.6 | 2020 | Roche, Regeneron | N/A | |
| 9 | Trulicity (dulaglutide) | 6.7 | 2014 | Eli Lilly |
2026 (US) 2024 (EU) |
|
| 10 | Darzalex (daratumumab) | 6.0 | 2015 | Janssen |
2027 (US) 2026 (EU) |
|
| 11 | Dupixent (dupilumab) | 5.9 | 2017 | Sanofi-Aventis, Regeneron | N/A | |
| 12/13 | Prolia/Xgeva (denosumab) | 5.7 | 2010 | Amgen |
2025 (US) 2022 (EU) |
|
| 12/13 | Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant) | 5.7 | 2014 | Merck | 2028 (US & EU) | |
| 14 | Enbrel (etanercept) | 5.6 | 1998 | Amgen, Pfizer, Takeda Pharmaceuticals | 2015 (EU) 2028 (US) | Erelzi, Benepali/Eticovo, Nepexto |
| 15 | Ocrevus (ocrelizumab) | 5.5 | 2017 | Roche/Genentech |
2027 (EU) 2029 (US) |
|
| 16 | Cosentyx (secukinumab) | 4.7 | 2015 | Novartis |
2026 (US) N/A (EU) |
|
| 17 | Entyvio (vedolizumab) | 4.4 | 2014 | Takeda |
2026 (US) N/A (EU) |
|
| 18 | Perjeta (pertuzumab) | 4.3 | 2012 | Roche/Genentech |
2024 (US) 2023 (EU) |
|
| 19 | Soliris (eculizumab) | 4.2 | 2007 | Alexion |
2027 (US) 2020 (EU) |
|
| 20 | Lantus/Toujeo (insulin glargine) | 3.9 | 2000 | Sanofi | 2014 (EU & US) | Semglee, Lusduna, Abasaglar, Rezoglar |
aFinancial data from LaMerie Business Intelligence and Fierce Pharma.
bPatent data from various sources, predominantly http://gabi-journal.net/. Note that patent landscape for biologics can be particularly complex: issues such as follow-on patents subsequent to the main patent and patent litigation can delay biosimilar development and approval. cInitial date of Emergency Use Authorization or Conditional Marketing Authorisation.
N/A, data not available.
Although most classes of originator biopharmaceuticals continue to show strong year-on-year growth, a notable exception is that of originator ‘established therapeutic proteins’ (erythropoietins, interferons, CSFs, human growth hormone (hGH) and follicle-stimulating hormone (FSH)). Data from La Merie publishing shows that this product class generated total global revenues of $11.7 billion in 2021, down 14% compared with 2020 ($13.6 billion). This mirrors a longer-term trend, in which the sales value of this class of product has more than halved in the past decade (down from $26.6 billion in 2012) The underlying reasons for this decline include competition from biosimilars and the approval of additional therapeutics targeting the same indications.
Biosimilars hit the big time
The survey period witnessed a continued surge in biosimilar approvals, as this class of product gains global acceptance. When considered by product trade name, 94 biosimilars have gained approval in the EU and/or the United States since 2006, although 10 have been subsequently withdrawn for commercial reasons and not all are actively marketed as yet.
By product category, the 94 biosimilar approvals thus far include 2 hGHs, 5 erythropoietins (EPOs), 20 granulocyte CSFs (G-CSFs; filgrastim and PEGylated filgrastim), 2 FSHs, 9 engineered insulins, 51 antibody- or antibody-fusion-based products and 5 PTHs. The 94 licensed products are based on 72 distinct active ingredients (Table 5).
Table 5.
Biosimilar products that had gained marketing Authorization within the EU and/or the US by June 2022
| Product type | Trade name(s) | Year (and region) approved | Reference product | Manufacturer(s) of the biologically active substance |
|---|---|---|---|---|
| Somatropin-based | ||||
| hGH-based | Omnitrope | 2006 (EU) | Genotropin | Sandoz (Kundl, Austria) |
| Valtropin |
2006 (EU) Withdrawn 2012 |
Humatrope | LG Life Sciences (Jeonbuk-do, Republic of Korea) | |
| Epoetin-based | ||||
| EPO-based | Retacrit |
2018 (US) 2007 (EU) |
Eprex/Erypo (EU) Epogen/Procrit (US) |
Norbitec (Uetersen, Germany; EU) |
| Binocrit | 2007 (EU) | Eprex/Erypo | Rentschler (Laupheim, Germany) & Lek (Menges, Slovenia) | |
| Epoetin alfa hexal | 2007 (EU) | Eprex/Erypo | ||
| Abseamed | 2007 (EU) | Eprex/Erypo | ||
| Silapo | 2007 (EU) | Eprex/Erypo | Norbitec | |
| Filgrastim-based | ||||
| G-CSF-based | Releuko | 2022 (US) | Neupogen | Kashiv Biosciences (Chicago) |
| Nivestim (EU)/Nivestym (US) |
2010 (EU) 2018 (US) |
Neupogen | Hospira (Zagreb, Croatia) | |
| Ratiograstim | 2008 (EU) | Neupogen | Sicor (Vilnius, Lithuania) | |
| Filgrastim Ratiopharm |
2008 (EU) Withdrawn 2011 |
Neupogen | ||
| Biograstim |
2008 (EU) Withdrawn 2015 |
Neupogen | ||
| Tevagrastim | 2008 (EU) | Neupogen | ||
| Zarzio (EU)/Zarxio (US) |
2009 (EU) 2015 (US) |
Neupogen | Sandoz (Kundl, Austria) | |
| Filgrastim hexal | 2009 (EU) | Neupogen | ||
| Grastofil | 2013 (EU) | Neupogen | Intas Biopharmaceuticals (Gujarat, India) | |
| Accofil | 2014 (EU) | Neupogen | ||
| Pegfilgrastim | Fylnetra | 2022 (US) | Neulasta | Kashiv Biosciences (Chicago) |
| Stimufend | 2022 (EU) | Neulasta | Fujifilm Diosynth Biotechnologies (Billingham, UK) | |
| Nyvepria | 2020 (EU & US) | Neulasta | Hospira | |
| Cegfila (previously pegfilgrastim Mundipharma) | 2019 (EU) | Neulasta | 3P Biopharmaceuticals, (Noain, Spain) | |
| Pelmeg | 2018 (EU) | Neulasta | ||
| Grasustek | 2019 (EU) | Neulasta | USV Private (Navi Mumbai, India) | |
| Ziextenzo |
2019 (US) 2018 (EU) |
Neulasta | Lek & Sandoz (Kundl, Austria) | |
| Pelgraz | 2018 (EU) | Neulasta | Intas Pharmaceuticals (Ahmedabad, India) | |
| Udenyca |
2018 (EU & US) Withdrawn (EU) |
Neulasta | KBI Biopharma (Boulder, CO, USA) | |
| Fulphila | 2018 (US & EU) | Neulasta | Biocon Biologics (Bangalore, India) | |
| FSH-based | ||||
| FSH-based | Ovaleap | 2013 (EU) | Gonal F | Merckle Biotech (Ulm, Germany) |
| Bemfola | 2014 (EU) | Gonal F | Polymun Scientific Immunbiologische Forschung (Klosterneuburg, Austria) | |
| Insulin-based | ||||
| Insulin-based | Inpremzia | 2022 (EU) | Actrapid | Biocon (Bangalore, India) |
| Insulin glargine-based | Rezvoglar | 2021 (US) | Lantus | Eli Lilly (Indianapolis, IN, USA), Lilly del Caribe (Carolina, Puerto Rico, USA) & Lilly France (Fegersheim, France) |
| Semglee |
2021 (US) 2018 (EU) |
Lantus | Biocon (Johor, Malaysia) | |
| Abasaglar | 2014 (EU) | Lantus | Lilly del Caribe Eli Lilly | |
| Lusduna |
2017 (EU) Withdrawn 2018 2017 (tentative, US) Withdrawn 2018 |
Lantus | Merck Sharp & Dohme (Elkton, VA, USA) | |
| Insulin lispro-based | Insulin lispro Sanofi | 2017 (EU) | Humalog | Sanofi-Aventis (Frankfurt) |
| Insulin aspart-based | Truvelog Mix 30 | 2022 (EU) | NovoMix | Sanofi-Aventis |
| Kirsty | 2021 (EU) | NovoRapid | Biocon | |
| Insulin aspart Sanofi | 2020 (EU) | NovoRapid | Sanofi-Aventis | |
| Mab-based and related | ||||
| Infliximab-based | Avsola | 2019 (US) | Remicade | R* |
| Inflectra |
2013 (EU) 2016 (US) |
Remicade | Celltrion (Incheon, Republic of Korea) | |
| Remsima | 2014 (EU) | Remicade | ||
| Flixabi | 2016 (EU) | Remicade | Biogen (Hillerod, Denmark) & Samsung Bioepis (Incheon, Republic of Korea) | |
| Renflexis | 2017 (US) | Remicade | ||
| Ixifi | 2017 (US) | Remicade | Pfizer | |
| Zessly | 2018 (EU) | Remicade | Boehringer Ingelheim (Biberach an der Riss, Germany) | |
| Adalimumab-based | Hukyndra | 2021 (EU) | Humira | Alvotech (Reykjavik) |
| Libmyris | 2021 (EU) | Humira | ||
| Yuflyma | 2021 (EU) | Humira | Celltrion | |
| Yusimry | 2021 (US) | Humira | R* | |
| Amsparity | 2020 (EU) | Humira | Wyeth BioPharma (Andover, MA, USA) | |
| Hulio |
2020 (US) 2018 (EU) |
Humira | Kyowa Kirin, Takasaki (Gunma, Japan) | |
| Abrilada | 2019 (US) | Humira | R* | |
| Hadlima | 2019 (US) | Humira | Samsung Bioepis | |
| Imraldi | 2017 (EU) | Humira | ||
| Idacio | 2019 (EU) | Humira | Merck Serono (Corsier-sur-Vevey, Switzerland) | |
| Kromeya |
2019 (EU) Withdrawn 2019 |
Humira | ||
| Amgevita (EU)/Amjevita (US) |
2016 (US) 2017 (EU) |
Humira | Amgen (Thousand Oaks, CA, USA) | |
| Solymbic | 2017 (EU) | Humira | ||
| Cyltezo | 2017 (EU & US) | Humira | Boehringer Ingelheim (Fremont, CA, USA) | |
| Halimatoz |
2018 (EU) Withdrawn 2020 |
Humira | Catalent Biologics (Bloomington, IN, USA) & Sandoz (Langkampfen, Austria) | |
| Hefiya | 2018 (EU) | Humira | ||
| Hyrimoz | 2018 (EU & US) | Humira | ||
| Rituximab-based | Riabni | 2020 (US) | Rituxan | Immunex (West Greenwich, RI, USA) |
| Ruxience |
2020 (EU) 2019 (US) |
MabThera | Boehringer Ingelheim (Biberach an der Riss, Germany) | |
| Truxima |
2018 (US) 2017 (EU) |
MabThera | Celltrion | |
| Blitzima | 2017 (EU) | MabThera | ||
| Ritemvia | 2017 (EU) | MabThera | ||
| Rituzena | 2017 (EU) | MabThera | ||
| Rixathon | 2017 (EU) | MabThera | Sandoz (Langkampfen, Austria) | |
| Riximyo | 2017 (EU) | MabThera | ||
| Trastuzumab-based | Zercepac | 2020 (EU) | Herceptin | Shanghai Henlius Biopharmaceutical (Shanghai, China) & WuXi Biologics (WuXi, China) |
| Kanjinti |
2019 (US) 2018 (EU) |
Herceptin | Patheon Biologics (Groningen, the Netherlands) & Immunex | |
| Ontruzant |
2019 (US) 2017 (EU) |
Herceptin | Fujifilm Diosynth (Hillerød, Denmark) & Samsung Biologics (Incheon, Republic of Korea) | |
| Trazimera |
2019 (US) 2018 (EU) |
Herceptin | Boehringer Ingelheim (Biberach an der Riss, Germany) | |
| Herzuma | 2018 (EU & US) | Herceptin | Celltrion | |
| Ogivri |
2018 (EU) 2017 (US) |
Herceptin | Biocon (Bangalore, India) | |
| Bevacizumab-based | Alymsys | 2022 (US) | Avastin | GH GENHELIX (Armunia, Spain) |
| Oyavas | 2021 (EU) | Avastin | ||
| Abevmy | 2021 (EU) | Avastin | Biocon Biologics (Bangalore, India) | |
| Lextemy |
2021 (EU) Withdrawn 2021 |
Avastin | ||
| Onbevzi | 2021 (EU) | Avastin | Biogen (Hillerød, Denmark) | |
| Aybintio | 2020 (EU) | Avastin | Fujifilm Diosynth Biotechnologies (Hillerød, Denmark) | |
| Equidacent |
2020 (EU) Withdrawn 2020 |
Avastin | Kyowa Kirin (Takasaki, Japan) | |
| Zirabev | 2019 (EU & US) | Avastin | Wyeth (Andover, MA, USA) | |
| Mvasi |
2018 (EU) 2017 (US) |
Avastin | Amgen | |
| Ranibizumab-based | Byooviz | 2021 (EU & US) | Lucentis | Wacker Biotech (Jena, Germany) |
| Etanercept-based | Nepexto | 2020 (EU) | Enbrel | Lupin (Taluka Mulshi, India) |
| Benepali/Eticovo |
2019 (US) 2016 (EU) |
Enbrel | Fujifilm Diosynth & Samsung (Incheon, Republic of Korea) | |
| Erelzi |
2016 (US) 2017 (EU) |
Enbrel | Sandoz (Langkampfen, Austria) & Novartis (Singapore) | |
| Teriparatide | ||||
| Teriparatide-based | Sondelbay | 2022 (EU) | Forsteo | Intas Pharmaceuticals (Ahmedabad, India) |
| Livogiva | 2020 (EU) | Forsteo | Cytovance Biologics (Oklahoma City, OK, USA) | |
| Qutavina |
2020 (EU) Withdrawn 2020 |
Forsteo | Cytovance Biologics | |
| Movymia | 2017 (EU) | Forsteo | Richter-Helm BioLogics (Bovenau, Germany) | |
| Terrosa | 2017 (EU) | Forsteo | ||
R*, manufacturing site details of biological active substance redacted in FDA documentation.
By region, 83 biosimilar products have received marketing Authorization in the EU, with 44 of these (53%) having gained Authorization within the current survey period. In the United States, a total of 37 products have thus far been licensed, of which 27 (73%) were approved within this survey period. The acceleration in biosimilar approval rates seen in our last survey is thus maintained in this one. Notable approval trends since 2018 include the approval of a raft of polyethylene glycol (PEG)-filgrastims (biosimilars to Neulasta; 2021 global sales of $2 billion), a number of engineered insulins and mAbs with biosimilarity to adalimumab (Humira; 2021 sales of $21 billion), trastuzumab (Herceptin; 2021 sales of $2.9 billion and bevacizumab (Avastin; 2021 sales of $3.3 billion).
Despite the large number of recent biosimilar approvals, both the revenues generated and the overall savings accrued to patients and healthcare systems remain relatively modest in both the EU and United States. A recent report by IQVIA6 prepared for the European Commission, estimates the total European biosimilars market to have reached €8.8 billion in 2021, amounting to savings of €5.7 billion (savings calculated as actual spend versus the pre-biosimilar cost of the originator, reference product).
Moreover, the report finds that biosimilars recently launched in Europe achieved 50% penetration of the originator market in less than a year, whereas earlier biosimilars typically took over two years to reach an equivalent position. Biosimilars approved in the EU are considered automatically interchangeable from a medical viewpoint, although decisions regarding actual substitution (dispensing one medicine instead of another medicine without consulting the prescriber) are made at individual EU member state level.
According to FDA data, generic drugs account for 90% of all prescriptions in the United States and provided savings of more than $1 trillion to the US health care system over a decade. The statistics for biosimilars are, predictably, more modest. The Pharmaceutical Research and Manufacturers of America (PhRMA; www.phrma.org) reported that annualized savings from biosimilars reached $6.5 billion in 2020, with average biosimilar sales prices being as much as 45% less than the branded biologics price at the time of first biosimilar launch.
US biosimilar approval and market penetration is influenced by regulatory, legal and developmental cost considerations. For example, biosimilar status in the United States does not automatically equate to interchangeability (and hence substitution for the reference product without the involvement of the prescriber). Interchangeable biosimilar products must meet additional regulatory requirements, as outlined by the Biologics Price Competition and Innovation Act. The US patent litigation landscape in this space can also slow or stop putative biosimilar products reaching the market. Additionally, a recent study found that most comparative efficacy trials supporting FDA biosimilar approvals were larger (median 504 patients), longer (median of 52 weeks) and more costly (estimated median cost of $20.8 million) than pivotal trials for new molecular entities7. As witnessed in Europe, however, more recent US biosimilars are achieving faster market uptake, with bevacizumab, trastuzumab and rituximab biosimilars achieving 42%, 38% and 20% uptake within their first year on the market, trending toward 60% by the end of year two.
mAb approvals
Monoclonal antibodies continue to dominate both in approval numbers (in the case of both originator and biosimilar categories) and commercial value. All newly approved antibodies were engineered in some way: they are either humanized or fully human, and most were additionally engineered to enhance or stabilize specific functional and/or structural characteristics. Jemperli and Evkeeza, both IgG4 mAbs, reportedly tend to form half-antibodies. To prevent this, each heavy chain contains a serine-to-proline substitution in the hinge region of the Fc domain, which stabilizes disulfide bonds between the two heavy chains.
Skyrizi, a humanized IgG1, represents another example of engineering. Used to treat plaque psoriasis and psoriatic arthritis, it acts by selectively binding the p19 subunit of IL-23, thereby inhibiting the latter from binding to its receptor. The framework of the antibody was engineered with two mutations in the Fc region, Leu234Ala and Leu235Ala, to reduce its potential effector function, which does not contribute to the product’s mode of action. The C-terminal lysine of the heavy chain was also deleted to reduce potential charge heterogeneity.
The period also witnessed the approval of five glycoengineered (afucosylated or low-fucose) products: Uplizna, Rybrevant, Blenrep, Fasenra and Poteligeo. Removal of the fucose residue in the antibody’s Fc glycocomponent can increase antibody-dependent cellular cytotoxicity (ADCC), potentially boosting the potency of mAbs whose mode of action depends on this antibody effector function.
Additional engineered formats that came on stream include three bispecific full-size mAbs: Vabysmo, the above-mentioned Rybrevant and the bispecific T cell engager (BiTE) product Lunsumio. Cabilivi, a bivalent nanobody, also gained approval, the first approval of a domain fragment. Three antigen-binding fragments also entered the market: Byooviz, Susvimo and Beovu. All three target macular degeneration and are administered by intravitreal injection. The smaller size of antibody fragments enables delivery of a high molar dose to the limited volume of the eye’s vitreous body, which may enhance tissue penetration at the retina and prolong the therapeutic effect.
Six new antibody–drug conjugates (ADCs) were approved during the survey period (Padcef, Enhertu, Tivdak, Trodelvy, Zynlonta and Blenrep), joining five previously approved ADC products. ADCs consist of an antibody chemically conjugated to a cytotoxic payload. Antibody-mediated binding to the target cell is followed by internalization, with subsequent intracellular cytotoxin release and action. Advances in cytotoxin discovery and chemical linker design have fueled increasing numbers of ADCs coming on stream. Cumulatively, ADCs generated $5.4 billion in 2021, with two such products achieving blockbuster status (Kadcyla and Adcetris).
The migraine therapy Vyepti, a humanized anti-calcitonin-gene-related peptide (CGRP) IgG1 antibody, is the first antibody to be produced in P. pastoris. Following intravenous administration, it binds CGRP, preventing its binding to its receptors, which influence the initiation, frequency and severity of migraine attacks. The mAb’s heavy chain N-glycosylation site has been removed via protein engineering, which eliminates any potential immunogenicity issues in humans due to a yeast-derived glycocomponent. Although the lack of a glycocomponent prevents ADCC and complement-dependent cytotoxicity (CDC) effector functions, the product’s mode of action does not rely on such functionality.
The current period also witnessed the conditional approval and emergency Authorization of several mAb-based products to treat COVID-19. The efficacy of mAb-based preparations aimed at SARS-CoV-2 may be compromised by mutations affecting the viral spike protein, as illustrated by products such as bamlanivimab and eteseviman (which are administered together) and REGEN-COV. This year, the FDA restricted the use of both products due to the emergence of the Omicron variant.
Nucleic acid-based approvals
Two of the most technically innovative, medically impactful and commercially successful products coming on stream in this survey period fall into this category—the COVID-19 mRNA vaccines Spikevax and Comirnaty. An additional 12 nucleic acid-based products were approved, adding substantially to the seven such products previously approved (Table 1). The new approvals include five small interfering RNA (siRNA)-based products (Amvuttra, Leqvio, Givlaari, Oxlumo and Onpattro), five antisense-based products (Amondys 45, Viltepso, Viondys 53, Waylivra and Tegsedi) and two gene therapy products (Zolgensma and Luxturna), which deliver therapeutic genes in adeno-associated viral vectors. Although 12 approvals signal progress in this field, almost all are orphan products and undergoing additional monitoring.
siRNA, antisense RNA and gene therapies therefore have as yet to make a broad impact on the mainstream biopharma market, particularly in regard to sales value. These modalities may benefit from lessons learned during the development of COVID-19 mRNA vaccines, but they face steeper technical challenges. mRNA vaccines capitalize on the massive amplification provided by the immune system—small doses administered intramuscularly and taken up by local antigen-presenting cells lead to a system-wide adaptive immune response (Box 2). In contrast, most non-vaccine nucleic acid products require substantially higher dosages, systemic administration, delivery to a specific tissue, prolonged therapeutic action and, in many cases, a non-immunogenic profile that allows chronic administration. These additional technical hurdles remain largely unresolved.
Engineered cell-based approvals
New cell-based therapies flooded into the market during this survey period, with nine such products gaining approval in the EU and/or United States. Previously there were but two. The majority of the new approvals (six products) are based on CAR-T cells, indicated for the treatment of blood-borne malignancies (multiple myeloma, leukemia and, in particular, lymphoma).
Many tumors manage to evade immune surveillance by downregulating the expression of major histocompatibility antigen class I (MHC-I) molecules. This prevents MHC-I-mediated presentation of tumor-specific peptides on the cancer cell surface and recognition of the MHC-I–peptide complex by cytolytic T lymphocytes via their T cell receptors (TCRs), which triggers cancer cell destruction by activated T cells.
CAR-T cells harness cancer-killing T cells independent of the MHC–TCR pathway. The CAR-T cell approach is now arguably the leading technology in this regard, surpassing alternatives such as T-cell-directed bispecific antibodies. CAR-T cells are genetically engineered to express on their surface a chimeric antigen receptor (CAR) that fuses an extracellular antibody fragment (usually a single-chain variable fragment, or scFv) specific for the target tumor surface antigen to intracellular T-cell-activating domains. CAR-T cell therapies are autologous, requiring isolation of a patient’s T lymphocytes via leukapheresis and ex vivo engineering of the cells to express the CAR. The engineered T cells are expanded in cell culture and cryopreserved until infused back into the patient.
CAR-T cell therapies have proven most effective against B cell cancers, whereas their extension to solid tumors remains challenging. Initial efficacy can be followed by cancer recurrence arising from tumor evolution. The approach can also present safety concerns, particularly cytokine release syndrome. The autologous nature of CAR-T cell therapy is inherently costly, with treatment list prices typically in the region of $400,000–$500,000.
Genetically engineered cell-based products have also been developed for non-cancer indications. One example is Zynteglo, a hematopoietic-stem-cell-based gene therapy approved in the EU in 2019 as an orphan product for the treatment of transfusion-dependent β-thalassemia. Zynteglo consists of autologous hematopoietic stem cells transduced with a functional β-globin gene. After infusion, the engineered cells repopulate the hematopoietic compartment, with clinical studies reporting ongoing expression of the β-globin gene 36 months after treatment. However, for commercial reasons, the sponsor company, Bluebird Bio, informed the EMA earlier this year of its intention to withdraw the product from the market. The treatment cost was in the region of €1.5 million per patient. In a further twist, the FDA approved Zynteglo in August of this year, reportedly with an associated price tag of $2.8 million per patient.
Traditional biotech product approvals
The current survey period also witnessed the approval of 37 traditional biotech products classified as new by regulatory authorities in terms of active substance—nine fewer than in our previous survey. Traditional products refer to those produced naturally or via nonrecombinant means in or by a biological source. The profile of approvals (Supplementary Table 1) largely mirrors product types approved in previous surveys, and include a range of blood-derived products and natural extracts, as well as traditional (nonrecombinant) vaccines and un-engineered cells.
Future directions
Although estimates vary, data published by PhRMA indicate that there are more than 7,800 biopharmaceutical products in clinical development globally, of which over 1,000 have reached phase 3 trials. Cancer remains by far the single most common indication, with other common target indications including genetic disorders, cardiovascular disease, as well as neurological, eye and blood disorders—all leading causes of mortality or morbidity, particularly in the West. Almost a third of products in clinical development (2,533) are mAb based, maintaining these as the single largest experimental product class. Smaller but still notable numbers of gene-modified cell therapies (348) and nucleic acid- and gene-based therapies (546) are currently being assessed in the clinic. On the whole, therefore, the industry retains a strong experimental product pipeline. Data from Evaluate Pharma indicates that total global biotech products sales continue to steadily increase as a percentage of overall global pharmaceutical sales, growing from 18% in 2010 to over 30% currently.
There are more than 100 (non-COVID-19) mAb-based products currently in late-stage clinical development. Antibody approvals over the next several years will likely mirror the profile of this antibody cohort. Some 60 of these experimental mAbs target cancer. Although 18 target liquid malignancies, the majority target a range of solid tumor types including ovarian, prostate, melanoma, breast, small-cell lung cancer and renal cancer. Almost a third (18 products) are bispecific, and one-fifth (12 products) are antibody conjugates. The remaining 54 non-cancer mAbs target a wide range of conditions; almost all are human or humanized monospecific products. In addition to this cohort of experimental products, over a dozen anti-COVID-19 mAbs remain in clinical studies, although the impact of these products will ultimately depend upon their efficacy against current and future SARS-CoV-2 variants.
Earlier stages in the mAb developmental pipeline display a greater diversity of antibody formats (ADCs, bispecific and fragments) and a larger proportion of products targeting solid as opposed to hematological malignancies. For example, 80% of ADCs in oncology clinical trials target solid tumor types8. Solid tumors were the most common global causes of cancer death in 2020 according to the WHO, including lung (1.80 million deaths), colon and rectum (916,000 deaths), liver (830,000 deaths), stomach (769,000 deaths) and breast (685.000 deaths).
Nucleic acid and engineered cell-based therapies continue to represent a vibrant and growing sector of experimental as well as approved biopharmaceuticals. Recent validation by COVID-19 is providing particular impetus to the field of mRNA vaccines, and advances in CAR-T-cell-based therapeutic approaches will continue to drive the developmental pipeline in this field, particularly against solid tumors. Although only two gene therapy products based upon viral delivery were approved in the current survey period (Zolgensma and Luxturna), several such products are showing success in clinical trials. Indeed, one additional such product (BioMarin’s hemophilia A product Roctavian) has recently gained approval in Europe, and a biological license application (BLA) is currently being considered by the FDA. Roctavian’s active substance, valoctocogene roxaparvovec, comprises a nonreplicating recombinant adeno-associated viral vector housing a functional human factor VIII cDNA under the control of a liver-specific promoter. Clinical studies show that increased factor VIII expression was sustained for (so far) at least two years, with the need for additional factor VIII replacement treatment dropping by 97.5%. Reports from industry sources indicate that Biomarin anticipates Roctavian’s list price in Europe to be on the order of €1.5 million euros, net of all discounts.
Biosimilars will also continue to feature with increasing prominence on the biopharmaceutical landscape. Various US-facing reports indicate that almost 100 biosimilars targeting the American market are in clinical development and that cumulative sales of biosimilars over the next five years could total $80 billion. A recent report by Allied Market Research forecasts the global biosimilars market to reach as much as $143 billion by 2031, fuelled by sales of biosimilar mAbs, CSFs, EPO, insulins and hGH. Forecasts can vary, however. A report from Research & Markets predicts that global biosimilar sales will reach $88 billion by 2030, whereas Global Market Insights put the value at $100 billion.
COVID-19 is likely to feature on the biopharmaceutical landscape over the foreseeable future. mRNA and other vaccines are expected to require updating to match novel SARS-CoV-2 variants. Tracking data maintained by the WHO estimates there are currently 172 Covid vaccines in clinical development globally, of which 55 (32%) are protein subunit based, 40 (23%) are RNA based, and 23 (13%) are (nonreplicating) viral vector based.
The biopharmaceutical sector’s impressive response to the global COVID-19 pandemic is likely to inform and accelerate broader innovation in the sector, particularly within the vaccine space. Finally, regulatory experience accrued in the last survey period should accelerate the speed of the drug development and approval processes for future medicines.
Supplementary information
Supplementary Table 1
Contributor Information
Gary Walsh, Email: gary.walsh@ul.ie.
Eithne Walsh, Email: eithne.walsh14@gmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41587-022-01582-x.
References
- 1.Walsh G. Nat. Biotechnol. 2018;36:1136–1145. doi: 10.1038/nbt.4305. [DOI] [PubMed] [Google Scholar]
- 2.Walsh G. Nat. Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 3.Walsh G. Nat. Biotechnol. 2010;28:917–924. doi: 10.1038/nbt0910-917. [DOI] [PubMed] [Google Scholar]
- 4.Walsh G. Nat. Biotechnol. 2006;24:769–776. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- 5.Kelley, B., Kiss, R. & Laird, M. (2018). in New Bioprocessing Strategies: Development and Manufacturing of Recombinant Antibodies and Proteins (eds Kiss, B., Gottschalk, U. & Pohlscheidt, M.) 443–462 (Springer, 2018). [DOI] [PubMed]
- 6.Troein, P. et al. The impact of biosimilar competition in Europe. IQVIA (2021); https://www.iqvia.com/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2021
- 7.Moore TJ, Mouslim MC, Blunt JL, Alexander GC, Shermock KM. JAMA Intern. Med. 2021;181:52–60. doi: 10.1001/jamainternmed.2020.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean AQ, Luo S, Twomey JD, Zhang B. MAbs. 2021;13:1951427. doi: 10.1080/19420862.2021.1951427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardi N, Hogan MJ, Porter FW, Weissman D. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettini E, Locci M. Vaccines (Basel) 2021;9:147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cagigi A, Loré K. Vaccines (Basel) 2021;9:61. doi: 10.3390/vaccines9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, et al. Mol. Cancer. 2021;20:33. doi: 10.1186/s12943-021-01311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1