There can be significant interactions between intracellular pathogens infecting the same cells [1]. One possibility is that a chronic and/or latent first pathogen infection can cause conventional T-cell exhaustion for T-cells targeting itself, and also accelerate T-cell exhaustion for T-cells targeting a second pathogen [1]. This could involve creating a cytokine environment which facilitates T-cell exhaustion, inducing T-cell expressions of inhibitory receptors, such as PD-1; and inducing infected host cell expressions of inhibitory ligands, such as PD-L1, to bind to the T-cell inhibitory receptors [1]. A second distinct pathogen infection, particularly a virulent pathogen which creates high antigen titers, can benefit from the T-cell exhaustion facilitating cytokine environment, and/or expression of inhibitory ligands by the same infected cells, and accelerate a T-cell exhaustion for the second pathogen [1].
T-cell exhaustion has been repeatedly linked to COVID-19 cases having severe outcomes and/or mortality [2]. T-cell exhaustion can degrade the functions of both CD8+ and CD4+ T-cells. This also includes follicular helper CD4+ T-cells, located primarily in lymph node and spleen germinal centers, where these CD4+ T-cells have critical roles in enabling antibody affinity maturation, isotype switching, generation of memory B-cells and in B-cell differentiation into immunoglobulin (antibody) secreting plasma cells [3]. Accelerated T-cell exhaustion could have significant effects on first-time infections by the second pathogen, if germinal center follicular helper CD4+ T-cell functions are inhibited, and immunoglobulins (antibodies) from B-cells are numerically decreased or qualitatively inadequate in affinity selection/maturation from somatic hypermutation to suppress the second pathogen [1, 4].
Such inadequate B-cell populations have been documented in severe cases of COVID-19, where patients have relatively higher population fractions of the less-developed membrane-bround IgM immunoglobulins on B-cells, in contrast to relatively higher population fractions of the more developed membrane-bound IgG immunoglobulins on B-cells observed in control patients or patients with mild cases [5]. These observations of isotype differences in the immunoglobulin expression by B-cells suggest follicular helper CD4+ T-cell interactions with B-cells in germinal centers did not promote adequate somatic hypermutation and isotype switching and affinity maturation of immunoglobulins targeting the SARS-CoV-2 virus, which can be a result of CD4+ T-cell exhaustion in these severe cases [4].
In summary, accelerated T-cell exhaustion by its inhibition of follicular helper CD4+ T-cells critical for conventional B-cells, can cause impaired B-cells and their production of abnormally high proportions of less-developed antibodies.
One observed result of accelerated T-cell exhaustion may be elevated mortality rates for COVID-19 and other epidemics, in which the second pathogen infection quickly achieves T-cell exhaustion, and fatally overwhelms an individual's adaptive immune system [1].
However, dysfunctionality can have various degrees of expression. There may also be rarer instances of accelerated T-cell exhaustion which result in dyfunctional B-cells which release numerous quasi-dysfunctional immunoglobulins/antibodies sufficiently functional to avoid mortality and severe infections, but having alternative unfortunate consequences. In numerously documented cases, small percentages of mild cases of COVID-19 have resulted in extensive hyperinflammatory diseases, including multisystem inflammatory syndrome (MIS) and autoantibodies [6]. Hyperinflammatory diseases (MIS or other diseases similar to Kawasaki disease) could be consequences of abnormally high levels of antibodies secreted by B-cells and high titers of antigen–antibody immune complexes which could not be promptly phagocytized by a small percentage of individuals [6, 7].
Pathogenesis of hyperinflammatory diseases could be initiated by pathogens that evade/impair T-cell control and thus require antigen neutralization by a very large number of antibodies, creating very large numbers of antigen–antibody immune complexes which cannot be quickly phagocytized by certain individuals having transiently or permanently immuno-deficient immune systems [6]. The steps by which antigen–antibody immune complexes can initiate hyperinflammatory diseases through a Type III hypersensitivity immune reaction in immuno-deficient individuals having impaired phagocytosis have already been outlined [6]. There is also documented support from a 2020 study of intensive care cases of COVID-19, where it was observed that a TH1 (cell-mediated immunity) response evolved into a TH2 humoral immunity (antibody) response that then escalated into a Type III hypersensitivity immune reaction with deposition of antigen–antibody immune complexes in the walls of blood vessels to generate a severely inflammatory systemic vasculitis [8]. Additionally, ten puzzling aspects of hyperinflammatory diseases, including their prevalence in younger patients and mild cases and the tendency of hyperinflammatory diseases to occur only once, can be plausibly explained by the steps previously outlined [6].
Furthermore, hyperinflammatory diseases including MIS typically occur two to four weeks after a SARS-CoV-2 infection [9]. This delay time is shorter than the time needed for conventional complete T-cell exhaustion [1], and this delay time is plausibly more consistent with a multistep process of accelerated T-cell exhaustion, inducing less-developed antibody production, resulting in extensive antigen–antibody immune complex titers in a phagocytosis impaired host, causing a Type III hypersensitivity immune reaction. This would then produce protease secretions and express or expose autoantigens which can result in autoantibodies, and this could ultimately achieve pathogenesis of a hyperinflammatory disease [1, 6–8].
The development of chronic autoimmune diseases is an alternative outcome from a high titer of antigen–antibody immune complexes, which cannot be quickly phagocytized in immuno-deficient individuals, which can cause a Type III hypersensitivity immune reaction with protease secretions which can express or expose autoantigens [7, 8, 10]. Therefore, it is plausible that accelerated T-cell exhaustion in immuno-deficient individuals can be an important factor in the pathogenesis of transient hyperinflammatory diseases, including MIS and the various types of Kawasaki diseases [6], and/or an important factor in the pathogenesis of chronic autoimmune diseases, such as diabetes, lupus or another autoimmune disease [7, 8, 10].
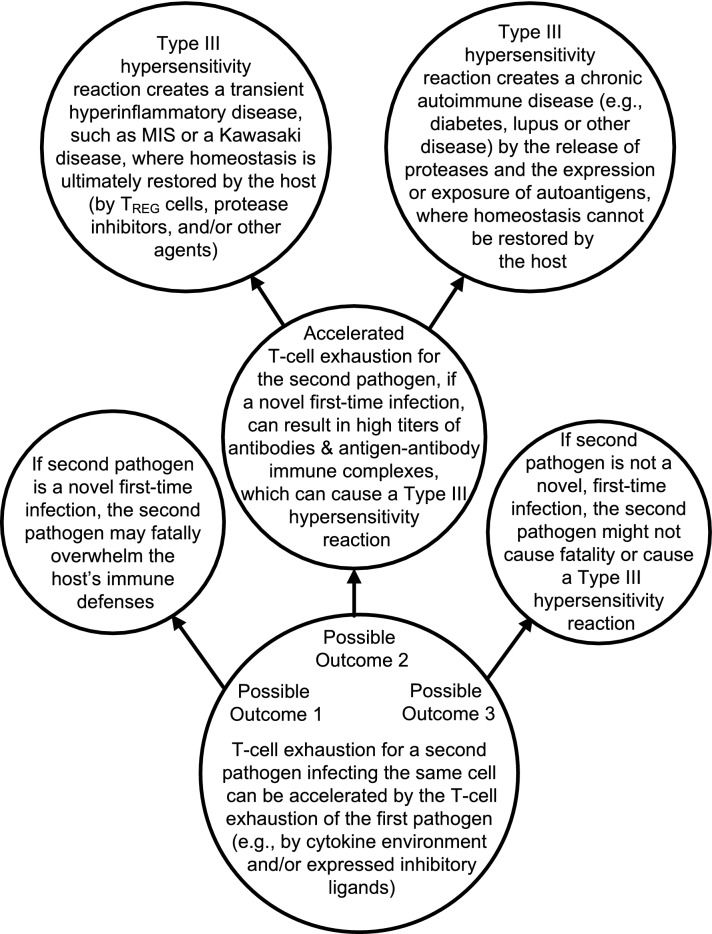
Figure 1 summarizes various outcomes possibly leading to transient hyperinflammatory diseases (e.g., MIS or one of the Kawasaki diseases) or chronic autoimmune diseases (e.g., diabetes, lupus, or another disease).
Fig. 1.
summarizes accelerated T-cell exhaustion outcomes which can cause one of the transient hyperinflammatory diseases or one of the chronic autoimmune diseases or possibly a fatal outcome
In conclusion, interactions between two intracellular pathogens can have significant consequences, including accelerated T-cell exhaustion for a second pathogen. An immunologically novel second pathogen infection, especially a virulent pathogen that creates large antigen titers, could evade/impair T-cell defenses if there was accelerated exhaustion of T-cells targeting the second pathogen. Accelerated T-cell exhaustion can occur when the second pathogen can reuse the already expressed inhibitory ligands of the infected cells. It is logically plausible that accelerated T-cell exhaustion can be an important factor in causing several hyperinflammatory diseases and/or autoimmune diseases in phagocytosis impaired, immuno-deficient individuals, by inhibiting follicular helper CD4+ T-cell assistance to germinal center B-cell somatic hypermutation, affinity maturation and isotype switching of antibodies, eventually resulting in a Type III hypersensitivity immune reaction that leads to pathogenesis of a transient hyperinflammatory disease or a chronic autoimmune disease.
Acknowledgements
There are no acknowledgments.
Author contributions
No other author contributed to this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Not applicable.
Declarations
Conflict of interest
The author has no relevant financial or non-financial interests to disclose.
Ethical approval
No ethical approval was required as this is a review article with no original research data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roe K. Accelerated T-cell exhaustion: its pathogenesis and potentially severe outcomes. Hum Cell. 2022. 10.1007/s13577-022-00814-1. [DOI] [PMC free article] [PubMed]
- 2.Loretelli C, Abdelsalam A, D'Addio F, Ben Nasr M, Assi E, Usuelli V, Maestroni A, Seelam AJ, Ippolito E, Di Maggio S, Loreggian L, Radovanovic D, Vanetti C, Yang J, El Essawy B, Rossi A, Pastore I, Montefusco L, Lunati ME, Bolla AM, Biasin M, Antinori S, Santus P, Riva A, Zuccotti GV, Galli M, Rusconi S, Fiorina P. PD-1 blockade counteracts post-COVID-19 immune abnormalities and stimulates the anti-SARS-CoV-2 immune response. JCI Insight. 2021;6(24):e146701. doi: 10.1172/jci.insight.146701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yesillik S, Gupta S. Phenotypically defined subpopulations of circulating follicular helper T cells in common variable immunodeficiency. Immun Inflamm Dis. 2020;8(3):441–446. doi: 10.1002/iid3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavazzoni CB, Hanson BL, Podestà MA, Bechu ED, Clement RL, Zhang H, Daccache J, Reyes-Robles T, Hett EC, Vora KA, Fadeyi OO, Oslund RC, Hazuda DJ, Sage PT. Follicular T cells optimize the germinal center response to SARS-CoV-2 protein vaccination in mice. Cell Rep. 2022;38(8):110399. doi: 10.1016/j.celrep.2022.110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rendeiro AF, Casano J, Vorkas CK, Singh H, Morales A, DeSimone RA, Ellsworth GB, Soave R, Kapadia SN, Saito K, Brown CD, Hsu J, Kyriakides C, Chiu S, Cappelli LV, Cacciapuoti MT, Tam W, Galluzzi L, Simonson PD, Elemento O, Salvatore M, Inghirami G. Profiling of immune dysfunction in COVID-19 patients allows early prediction of disease progression. Life Sci Alliance. 2020;4(2):e202000955. doi: 10.26508/lsa.202000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roe K. Explanations for 10 of the most puzzling aspects of multisystem inflammatory syndrome and other Kawasaki-like diseases. J Clin Pharm Ther. 2022;47(4):539–543. doi: 10.1111/jcpt.13560. [DOI] [PubMed] [Google Scholar]
- 7.Roe K. An explanation of the pathogenesis of several autoimmune diseases in immuno-compromised individuals. Scand J Immunol. 2021;93(3):e12994. doi: 10.1111/sji.12994. [DOI] [PubMed] [Google Scholar]
- 8.Roncati L, Ligabue G, Fabbiani L, Malagoli C, Gallo G, Lusenti B, Nasillo V, Manenti A, Maiorana A. Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol. 2020;217:108487. doi: 10.1016/j.clim.2020.108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ailioaie LM, Ailioaie C, Litscher G. Implications of SARS-CoV-2 infection in systemic juvenile idiopathic arthritis. Int J Mol Sci. 2022;23(8):4268. doi: 10.3390/ijms23084268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessenbrock K, Fröhlich L, Sixt M, Lämmermann T, Pfister H, Bateman A, Belaaouaj A, Ring J, Ollert M, Fässler R, Jenne DE. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest. 2008;118(7):2438–2447. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.