Abstract
Purpose of Review
Despite significant advances in detection and treatment for breast cancer, the breast cancer mortality rate for Black women remains 40% higher than that for White women. Timely work-up and treatment improve outcomes, yet no gold standard exists for which to guide providers.
Recent Findings
A large body of literature demonstrates disparities in time to treatment for breast cancer, and most studies show that Black women receive treatment later than their White counterparts. The COVID-19 pandemic has been projected to worsen these disparities, but the extent of this impact remains unknown.
Summary
In this review, we describe the available evidence on disparities in time to treatment, potential drivers, and possible mitigation strategies. Future research must address how the COVID-19 pandemic has impacted the timely treatment of breast cancer patients, particularly populations vulnerable to disparate outcomes. Improved access to multidisciplinary breast programs, patient navigation services, and establishment of standards for timely treatment are necessary.
Keywords: Disparities, Breast cancer, Cancer screening, Time to treatment
Introduction
Despite advances in screening/early detection and systemic therapy, breast cancer remains a leading cause of cancer death among women and is the most commonly diagnosed malignancy in women after skin cancer [1]. While overall mortality from breast cancer has improved in recent decades, disparities persist and mortality rates are 40% higher among African American compared to White American women [2]. Additionally, African American women are more likely to present at a younger age and with later stage disease; they are also more likely to have biologically aggressive tumors such as high-grade disease and estrogen-receptor-negative tumors [1]. Compounding these biological differences in breast cancer presentation are additional factors such as socioeconomic status and reduced access to high-quality, timely treatment among Black women. Prolonged time to treatment of newly diagnosed breast cancer has been associated with lower overall and disease-specific survival [3, 4•]. However, heterogeneity exists regarding the definition of a treatment delay, as there is no current standard for time to treatment. To date, several studies have characterized racial disparities in time to treatment and provide retrospective data on how this may explain discrepancies in survival [4•, 5–10]. In this review, we aim to summarize the current literature regarding disparities in time to treatment for breast cancer and the impact on survival, identify directions for future study considering the COVID-19 pandemic, and propose potential strategies to mitigate these time to treatment disparities.
Time to Treatment Impacts Survival
One of the difficulties in improving disparities in time to treatment is that there are currently no universally established benchmarks defining optimal intervals for initiating treatment following detection of a breast cancer. For example, time to treatment from the first diagnostic imaging study to core needle biopsy and time from the first positive needle biopsy to initial breast cancer surgery are included in quality measures developed by the National Consortium of Breast Centers [11]. Additionally, the Commission on Cancer (CoC) includes several measures regarding timely treatment for benchmark performance in CoC-accredited cancer programs [12] (Table 1). Evidence for these recommendations is driven by analyses of large national datasets that demonstrate that longer time to treatment is associated with worse outcomes, including reduced overall and disease-specific survival [3, 13–15]. Streamlining these definitions for providers would strengthen quality improvement efforts.
Table 1.
Commission on Cancer (CoC) quality measures in time to treatment for breast cancer
| Commission on Cancer (CoC) quality measures in time to treatment for breast cancer |
|---|
| ----Radiation therapy is administered within 1 year of diagnosis for women under age 70 receiving breast conservation surgery for breast cancer |
| ----Combination chemotherapy is recommended or administered within 4 months (120 days) of diagnosis for women under age 70 with AJCC T1cN0M or stage IB–III hormone receptor-negative breast cancer |
| ----Tamoxifen or third-generation aromatase inhibitor is recommended or administered within 1 year of diagnosis for women with AJCC T1cN0M0 or stage IB–III hormone receptor-positive breast cancer |
| ----Radiation therapy is recommended or administered following any mastectomy within 1 year of diagnosis for women with ≥ 4 positive regional lymph nodes |
A large population-based study of SEER-Medicare data comprised of more than 94,000 patients showed that overall survival diminished with progressively increasing intervals of treatment delay (≤ 30, 31–60, 61–90, 91–120, and 121–180 days) in time to surgery. In an analysis of the National Cancer Database (NCDB), the overall mortality hazard ratio was 1.10 for each increasing time interval [3]. For patients requiring neoadjuvant chemotherapy, a delay of more than 61 days from breast cancer diagnosis to initiation of neoadjuvant chemotherapy was associated with an increased risk of death [13]. Regarding adjuvant chemotherapy, multiple studies demonstrate adverse outcomes with initiation of therapy more than three months after diagnosis [14, 15].
These data suggest that ensuring timely treatment for breast cancer patients is relevant to the goal of optimizing outcomes.
Disparities in Time to Treatment
Disparities in time to treatment for breast cancer have been well documented over the last two decades. Currently, a large body of data exists that is driven predominantly by retrospective studies, ranging from single institutional studies to investigations of large national datasets. Most find that regardless of the first treatment modality, racial and ethnic minorities, particularly Black women, experience prolonged treatment delays compared to White women (Table 2).
Table 2.
Recent studies evaluating disparities in time to treatment for breast cancer
| Study | Design | Setting and number of subjects | Main findings |
|---|---|---|---|
| Babatunde (2021) | Retrospective cohort | South Carolina Central Cancer Registry (SCCCR) and Office of Revenue and Fiscal Affairs (RFA), 2002–2010, n = 2155 |
• Black women received surgery, chemotherapy, and radiotherapy later than White counterparts • Unadjusted mean time to surgery from diagnosis was longer for Blacks |
| Benefield (2021) | Population-based study with in-home interview | Carolina Breast Cancer Study Phase III, 2008–2013, n = 437 | • After adjusting for stage, Black women with hormone receptor-positive/HER2- high-grade tumors were more likely to experience a treatment delay |
| Bleicher (2017) | Retrospective cohort | National Cancer Database, 2004–2015, n = 622,793 | • After adjusting for all other variables, factors that nearly or more than doubled odds of having > 90 days between diagnosis and surgery included Black race and Hispanic ethnicity |
| Bustami (2014) | Retrospective cohort study | NYU and Morristown Medical Center tumor registry, 2007–2011, n = 3071 | • Longest median time to surgery observed for African American patients and longer absolute difference between African American compared to Whites with difference less pronounced for Asian/other compared to White |
| Champion (2020) | Retrospective cohort | National Cancer Database, 2004–2015, n = 903,008 |
• After adjustment, Hispanic White women had longer time to surgery compared to non-Hispanic White women, regardless of treatment sequence • No significant racial differences in time to surgery among Hispanic patients |
| Doe (2020) | Retrospective cohort | Henry Ford Health System, 2015–2017, n = 541 |
• Mean time to treatment was significantly longer for Blacks than Whites both before and after implementation of multidisciplinary approach (MDC) • Before MDC, significantly more White patients were treated ≤ 60 days than Black and significantly more Black patients were treated > 60 days but this difference no longer appeared after MDC |
| Eaglehouse (2019) | Retrospective cohort | Department of Defense Central Cancer Registry and Military Health System Data Repository, 1998–2008, n = 4887 |
• In multivariable models, NHB women had longer time to surgery than NHW women • Regarding survival, addition of time to surgery to multivariable model did not substantially attenuate the HR estimates compared with adjusted model—NHB had higher risk for all-cause death compared to NHW women |
| Foy (2018) | Retrospective cohort | James Cancer Hospital, 2005–2014, n = 4593 |
• Mean number of days between diagnosis and treatment was significantly greater for Black women • Proportion of Black women with more than 90 days between diagnosis and treatment onset was significantly greater than White women |
| George (2015) | Retrospective cohort | NJ State Cancer Registry, 2005–2010, n = 575 |
• Median time to surgery was 29 days for White vs. 32 days for Black with 92% of White compared to 80% of Black patients receiving surgery within 2 months of diagnosis • Black patients more likely to experience surgical delay more than 3 months • In models adjusted for situational barriers, Black patients at increased risk for both diagnostic and surgical delay compared to Whites |
| Halpern (2016) | Retrospective cohort study | Medicaid data, 2006–2008, n = 7452 | • Black Medicaid beneficiaries were more likely to experience delays for breast-conserving surgery and outpatient and inpatient mastectomy |
| Hoppe (2019) | Retrospective cohort | National Cancer Database, 2004–2014, n = 546,351 |
• Black women had significantly longer times to first treatment, time to surgery, chemotherapy, radiation, and endocrine therapy than White women • Despite private insurance, Black women still had longer time to surgery than White patients |
| Jackson (2021) | Retrospective cohort | National Cancer Database, 2010–2016, n = 378,499 |
• Median time from diagnosis to first surgery was longer for Black women than White • 30.6% of Black women had surgery > 60 days from biopsy compared to 18.0% White • On multivariable logistic regression, Black race associated with increased odds of surgery > 60 days from diagnosis |
| Khanna (2017) | Retrospective cohort | Boston Medical Center, 2004–2014, n = 1130 |
• Black women had longer time to treatment compared to all other race groups and significance primarily driven by comparison of Black vs. White • On multivariate model with race/ethnicity, marital status, stage, and first treatment delivered, race/ethnicity was the only independent predictor of time to treatment |
| Khorana (2019) | Retrospective cohort | National Cancer Database, 2004–2013, n = 1,368,024 |
• On multivariable analysis, race was one of the several predictors of delay • Black race was associated with increased time to initiation compared to White • Increased time to initiation was associated with worsened survival in stage I and II breast cancer |
| Lamb (2018) | Retrospective cohort | Methodist University Hospital, 2002–2012, n = 3072 | • Black women with stage 0, I, II, and III breast cancer all had significantly longer median time to surgery than White women |
| Polverini (2016) | Retrospective cohort | National Cancer Database, 2004–2012, n = 420,792 |
• As time to surgery increased, the percentage of Medicaid and uninsured patients and patients of Black or Hispanic race increased • Overall, only time to surgery > 12 weeks was associated with significantly shorter survival • When stratified by stage, stage I patients treated at 8 to < 12 weeks and > 12 weeks as well as stage II patients treated > 12 weeks had decreased overall survival compared with patients treated within 4 weeks |
| Reeder-Hayes (2019) | Retrospective cohort | Carolina Breast Cancer Study, unknown timeline, n = 2659 |
• Women with delayed treatment initiation were significantly more likely to be Black • Black women more frequently experience delayed treatment • Even in fully adjusted models, Black women had almost twice the frequency of delayed initiation compared to White women • After adjustment for age, receptor status, grade, and tumor size, a nonsignificant trend association with recurrence risk was suggested for patients with delayed initiation |
Examining time to treatment among early-stage breast cancer patients, Hoppe and colleagues analyzed over 540,000 patients within the National Cancer Database (NCDB). Black patients were found to have a significantly longer time to first treatment (35.5 days vs. 28.1 days) compared to White patients. This trend continued when stratified by treatment modality: surgery (36.6 days vs. 28.8 days), chemotherapy (88.1 days vs. 75.4 days), radiation (131.3 days vs. 99.1 days), and endocrine therapy (152.1 days vs. 126.5 days). Subset analysis among patients with private insurance found that the disparity remained, although it was reduced by 1.2 days [10].
In another NCDB study, time to surgery, defined as date of biopsy to first surgery, was examined among a cohort of 378,499 patients. Jackson et al. found that the odds of receiving surgery more than 60 days after diagnosis were higher among non-Hispanic Blacks (NHB) (OR 1.77, 95% CI 1.64–1.91) compared to non-Hispanic Whites. Specifically, 30.6% of NHB women had surgery more than 60 days after diagnosis compared to only 18% of White women [16•].
For patients undergoing primary surgical therapy for breast cancer, many factors may impact the interval from diagnosis to surgery such as genetic testing, consultation with a plastic surgeon for consideration of all surgical options such as contralateral prophylactic mastectomy (CPM) and reconstruction, and obtaining multiple oncology treatment opinions [3]. Recent work has found that racial and ethnic minorities are in fact less likely to undergo CPM and less likely to have genetic testing which would potentially reduce the odds of surgical delay for this patient population [17, 18]. However, most studies have nonetheless found that Black women tend to experience longer time to surgery than their White counterparts [5, 10, 16•, 19–23].
The role that other socioeconomic factors play in the treatment delays has been well studied, particularly the impact of insurance status. Analyzing patients from Phase 3 of the Carolina Breast Cancer Study, Reeder-Hayes et al. found that women with the longest treatment durations were more likely to be Black, younger, have a lower income, be uninsured or have Medicaid, be less educated, and have a higher stage at diagnosis [24]. Another study of 420,792 breast cancer patients from the NCDB undergoing primary surgical therapy found that as time to surgery increased, the percentage of Medicaid and uninsured patients also increased [8]. Importantly, other work has shown that higher Medicaid reimbursements for breast conserving surgery are associated with a decreased time from diagnosis to surgery [25]. These studies suggest that the etiology of these disparities is multifactorial and that insurance status clearly plays an important role.
Potential Mitigation Strategies
Potential strategies to mitigate disparities in time to treatment for breast cancer include a multidisciplinary approach to breast cancer care and utilization of patient navigators.
Recent work by Doe et al. sought to examine whether a multidisciplinary approach to breast cancer care may improve disparities in time to treatment. In their analysis of 541 patients, mean time to treatment was significantly longer for Blacks than Whites among patients both before and after implementation of the multidisciplinary care program (MDC). Although the gap in time to treatment between races was shortened in the MDC group from 18.7 days to 8.5 days, the improvement did not achieve statistical significance [26]. These data suggest that a multidisciplinary approach to breast cancer treatment may be one potential method to reduce disparities but additional confirmatory studies are needed.
Other data have shown that with the use of patient navigation services, time from initial presentation to definitive treatment can be reduced. For example, in their analysis of patients at an urban safety net hospital, Haideri et al. found that 67% of women in the pre-navigation group received treatment within 60 days of presentation vs. 75% in the navigator group [27].
While minority patients have historically been underrepresented in National Cancer Institute (NCI)-designated cancer centers, one study elucidates how among certain communities comprised of predominantly minority patients, disparities may be reduced. For example, Parsons et al. retrospectively reviewed patients treated at an NCI-designated cancer center where 50% of the population identifies as Hispanic. On multivariate Cox proportional hazards modeling adjusting for age, cancer and treatment characteristics, and sociodemographic factors, no difference in time to treatment was found between Hispanic versus non-Hispanic White patients [28]. This finding underscores the importance of improving access to care at NCI-designated cancer centers particularly among communities where at least 50% of the population identifies as either Hispanic, Black, or other.
Disparities also appear to be less pronounced among patients treated within the U.S. military healthcare system. Outcomes of 6577 patients from the Department of Defense (DoD) central tumor registry demonstrate more equal access to treatment. Specifically, wait time from diagnosis to treatment was significantly shorter for Black women. Additionally, Black women have improved survival when treated at a DoD facility compared to within the general population. These findings are likely explained by the fact that military personnel have unique characteristics and similarities in education, lifestyle, and socioeconomic status that is more consistent among race groups. This includes equal wage and housing policies that may contribute to less inequity compared to that seen within the general population. This work is further evidence that disparities are driven by more than just race, but also social determinants of health [29]. Furthermore, U.S. military healthcare system may serve as a model for providing more equitable care.
Impact of the COVID-19 Pandemic on Time to Treatment
The COVID-19 pandemic has been the catalyst for a tremendous amount of research in understanding and addressing healthcare disparities in the United States. This has been largely driven by the disproportionate impact of the COVID-19 mortality on racial and ethnic minorities which mirrors disparities seen with cancer. For example, in recent data from the Kaiser Family Foundation, Black, Hispanic, and American Indian/Alaska Native people are at least twice as likely to die from COVID-19 as their White counterparts. These disparities are also seen with respect to hospitalization rates [30].
Beginning in the spring of 2020 in the Northeast, widespread shelter-in-place mandates were enacted to curb the spread of COVID-19. Simultaneously, large healthcare systems underwent a major reorganization to allocate resources toward pandemic management. These measures included diversion of resources and personnel and suspension of routine healthcare including cancer screenings. At this time, surgeons were faced with a necessary surgical pause, to conserve personal protective equipment, and in some institutions transform operating rooms toward caring for COVID-19 patients [31, 32].
In response to this, the American College of Surgeons developed a set of guidelines for the triage and prioritization of surgery relative to illness severity and time sensitivity [33]. Additionally, the American Society of Breast Surgeons, the National Accreditation Program for Breast Centers, the Commission on Cancer, and the National Comprehensive Cancer Network assembled a COVID-19 Pandemic Breast Cancer Consortium to establish guidelines for the treatment of breast cancer patients during this unprecedented time [34]. Treatment recommendations were based on various features including clinical stage, biologic and phenotype characteristics, and patient-related factors.
As a result, many breast cancer patients who would have been eligible for immediate surgery were now having their surgery postponed. While these guidelines were well intentioned, they may have inadvertently exacerbated disparities in time to treatment for surgery for Black patients [35•]. For example, previous studies have quantified the absolute difference in median time to surgery for Black versus White patients undergoing primary surgery. The majority demonstrate that Black patients consistently have surgery later than their White counterparts [5, 10, 16•, 19, 21–23].
Thus far, the impact of the COVID-19 surgical pause on outcomes for breast cancer patients remains unknown. Few studies have sought to quantify patient-reported delays in treatment and have identified patient factors that increase the odds of a delay. For example, one study of breast medical oncology patients from February 1, 2020, to April 30, 2020, who were scheduled for outpatient appointments found that 42.6% of patients had a COVID-19-related delay and/or change in treatment plan. The median COVID-19-related delay in systemic therapy was 24.5 days, and the median COVID-19-related delay for surgery and radiation was 47 and 55 days, respectively. On univariate analysis, Black/African American, Asian, and Other race groups were more likely to experience a COVID-19-related delay and/or change compared to White patients; however, on multivariate modeling, race and ethnicity were not associated with a delay [36•]. Another survey of breast cancer survivors in the United States found that race had no significant effect on patient-reported delays in care [37].
Future Directions
Future work is needed to characterize the impact of these widespread surgical delays, especially among vulnerable populations. Specifically, studies are needed that characterize time from diagnosis to surgery for breast cancer patients. Studies must be designed to account for social determinants of health such as financial stress, education, travel time to a healthcare facility, and geographical features specific to the pandemic, such as local lockdowns and suspensions of elective surgery.
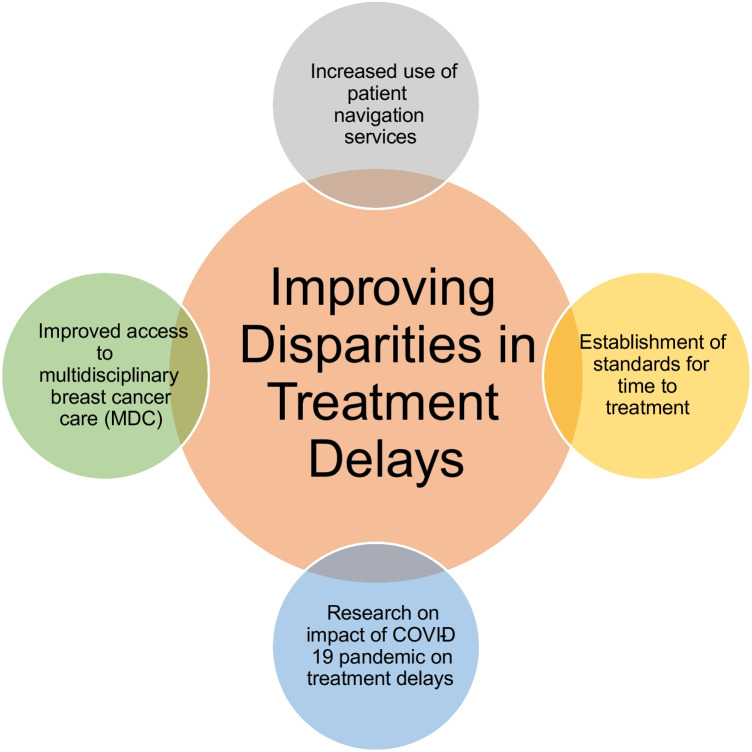
While a large body of data exists documenting disparities in time to treatment for breast cancer, a paucity of data exists for mitigation strategies. Designing targeted interventions for more equitable breast cancer care is complex and should include the following (Fig. 1):
- Increased implementation of multidisciplinary breast programs at hospitals with a large volume of breast cancer patients
-
oMultidisciplinary breast cancer programs should be designed to provide comprehensive but efficient breast cancer care. Enabling patients to be seen by multiple disciplines on a single day can help overcome barriers to timely treatment by minimizing the number of visits. Additionally, coordination between disciplines such as surgery, medical oncology, radiation oncology, plastic surgery, and genetic counseling at a single site can streamline care for patients and improve communication about individualized treatment plans.
-
o
- Increased funding for patient navigation services, particularly among hospitals treating a large population of medically underserved patients
-
oPatient navigation services have been shown to improve outcomes and reduce treatment intervals for patients requiring complex cancer care [38]. Since the expanded use of telehealth services since the COVID-19 pandemic, expansion of navigation services to include telehealth visits will be prudent to increase access [39]. However, considering the digital divide, this will require expanded resources to improve broadband internet services and education to familiarize patients with use of electronic communication methods. For example, orientation to the methods of communication available through the electronic medical record can empower patients to obtain enhanced follow-up and coordination of care.
-
o
- Establishment of standards for time to treatment to enhance awareness among providers
-
oMore visibility on how time to treatment is an essential element of cancer care that improves outcomes is needed, particularly among providers caring for a large proportion of racial minorities and Medicaid or underinsured patients. By establishing standards for acceptable treatment intervals from presentation to diagnosis, diagnosis to treatment, and delivery of adjuvant therapies, providers will have metrics to adhere to, similar to the monitoring of adverse events.
-
o
Fig. 1.
Strategies for improving disparities in treatment delays in the COVID-19 era
Conclusions
In conclusion, disparities in time to treatment for breast cancer have been well documented. While most studies have found significant differences in the interval from diagnosis to treatment for racial and ethnic minorities and Medicaid or uninsured patients, more work is needed to investigate the impact that the COVID-19 pandemic has had on the diagnosis and treatment of breast cancer patients, particularly among vulnerable populations. Mitigating differences in treatment delays is complex but should include more widespread availability of multidisciplinary breast programs and patient navigators, as recent data have indicated their potential to improve disparities.
Acknowledgements
We acknowledge the expertise and literature search assistance of Michelle R. Demetres of the Weill Cornell Medicine Samuel J. Wood Library.
Declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Breast Cancer Disparities.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, Törnberg S, Chen SL, Chiu SY, Fann JC, Ku MM, Wu WY, Hsu CY, Chen YC, Svane G, Azavedo E, Grundström H, Sundén P, Leifland K, Frodis E, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer. 2020;126:2971–2979. doi: 10.1002/cncr.32859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleicher RJ, Ruth K, Sigurdson ER, Beck JR, Ross E, Wong YN, Patel SA, Boraas M, Chang EI, Topham NS, Egleston BL. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.• Khorana AA, Tullio K, Elson P, Pennell NA, Grobmyer SR, Kalady MF, Raymond D, Abraham J, Klein EA, Walsh RM, Monteleone EE, Wei W, Hobbs B, Bolwell BJ. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS ONE. 2019;14:e0213209. 10.1371/journal.pone.0213209. (This study analysed the National Cancer Database to investigate the determinants of increased time to initiation of treatment for a new cancer diagnosis and the association with overall survival. Among patients with newly diagnosed breast cancer, increased time to initiation of treatment was associated with worsened survival for stages I and II breast cancer.).
- 5.Champion CD, Thomas SM, Plichta JK, Parrilla Castellar E, Rosenberger LH, Greenup RA, Hyslop T, Hwang ES, Fayanju OM. Disparities at the intersection of race and ethnicity: examining trends and outcomes in Hispanic women with breast cancer. JCO Oncol Pract. 2020;7:OP2000381. doi: 10.1200/OP.20.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaglehouse YL, Georg MW, Shriver CD, Zhu K. Racial differences in time to breast cancer surgery and overall survival in the US military health system. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foy KC, Fisher JL, Lustberg MB, Gray DM, DeGraffinreid CR, Paskett ED. Disparities in breast cancer tumor characteristics, treatment, time to treatment, and survival probability among African American and white women. NPJ Breast Cancer. 2018;20:4–7. doi: 10.1038/s41523-018-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polverini AC, Nelson RA, Marcinkowski E, Jones VC, Lai L, Mortimer JE, Taylor L, Vito C, Yim J, Kruper L. Time to treatment: measuring quality breast cancer care. Ann Surg Oncol. 2016;23:3392–3402. doi: 10.1245/s10434-016-5486-7. [DOI] [PubMed] [Google Scholar]
- 9.Khanna S, Kim KN, Qureshi MM, Agarwal A, Parikh D, Ko NY, Rand AE, Hirsch AE. Impact of patient demographics, tumor characteristics, and treatment type on treatment delay throughout breast cancer care at a diverse academic medical center. Int J Womens Health. 2017;9:887–896. doi: 10.2147/IJWH.S150064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoppe EJ, Hussain LR, Grannan KJ, Dunki-Jacobs EM, Lee DY, Wexelman BA. Racial disparities in breast cancer persist despite early detection: analysis of treatment of stage 1 breast cancer and effect of insurance status on disparities. Breast Cancer Res Treat. 2019;173:597–602. doi: 10.1007/s10549-018-5036-z. [DOI] [PubMed] [Google Scholar]
- 11.National quality measures for breast centers. https://www.nqmbc.org/about-us/what-is-nqmbc.cms; Accessed January 16, 2022.
- 12.Commission on Cancer (CoC). CoC quality measures. https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/quality-measures.ashx; Accessed January 16, 2022.
- 13.de Melo GD, Lei X, Giordano SH, Valero V, Barcenas CH, Hortobagyi GN, Chavez-MacGregor M. Impact of delayed neoadjuvant systemic chemotherapy on overall survival among patients with breast cancer. Oncologist. 2020;25:749–757. doi: 10.1634/theoncologist.2019-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99:313–321. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 15.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2:322–329. doi: 10.1001/jamaoncol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.• Jackson DK, Li Y, Eskander MF, Tsung A, Oppong BA, Bhattacharyya O, Paskett ED, Obeng-Gyasi S. Racial disparities in low-value surgical care and time to surgery in high-volume hospitals. J Surg Oncol. 2021;123:676–86. 10.1002/jso.26320 (This study examines racial differences in time to surgery among women receiving treatment at high-volume hospitals utilizing stage I-III breast cancer patients from the National Cancer Database. Black patients treated at high-volume hospitals had higher rates of surgical delay and were less likely to undergo low-value surgical procedures.). [DOI] [PubMed]
- 17.Kim Y, McCarthy AM, Bristol M, Armstrong K. Disparities in contralateral prophylactic mastectomy use among women with early-stage breast cancer. NPJ Breast Cancer. 2017;3. 10.1038/s41523-017-0004-z. [DOI] [PMC free article] [PubMed]
- 18.McCarthy AM, Bristol M, Domchek SM, Groeneveld PW, Kim Y, Motanya UN, Shea JA, Armstrong K. Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. J Clin Oncol. 2016;34:2610–2618. doi: 10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babatunde OA, Eberth JM, Felder TM, Moran R, Hughes-Halbert C, Truman S, Hebert JR, Heiney S, Adams SA. Racial disparities and diagnosis-to-treatment time among patients diagnosed with breast cancer in South Carolina. J Racial Ethn Health Disparities. 2021:10.1007/s40615-020-00935-z. [DOI] [PMC free article] [PubMed]
- 20.Benefield HC, Reeder-Hayes KE, Nichols HB, Calhoun BC, Love MI, Kirk EL, Geradts J, Hoadley KA, Cole SR, Earp HS, Olshan AF, Carey LA, Perou CM, Troester MA. Outcomes of hormone-receptor positive, HER2-negative breast cancers by race and tumor biological features. JNCI Cancer Spectr. 2020;5:pkaa072. doi: 10.1093/jncics/pkaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bustami RT, Shulkin DB, O'Donnell N, Whitman ED. Variations in time to receiving first surgical treatment for breast cancer as a function of racial/ethnic background: a cohort study. JRSM Open. 2014;5:2042533313515863. doi: 10.1177/2042533313515863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George P, Chandwani S, Gabel M, Ambrosone CB, Rhoads G, Bandera EV, Demissie K. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt) 2015;24:209–217. doi: 10.1089/jwh.2014.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamb EP, Pritchard FE, Nouer SS, Tolley EA, Boyd BS, Davidson JT, Munene G, Fleming MD. Understanding disparities in breast cancer care in Memphis. Tennessee Am Surg. 2018;84:620–627. [PubMed] [Google Scholar]
- 24.Reeder-Hayes KE, Mayer SE, Olshan AF, Wheeler SB, Carey LA, Tse CK, Bell ME, Troester MA. Race and delays in breast cancer treatment across the care continuum in the Carolina Breast Cancer Study. Cancer. 2019;125:3985–3992. doi: 10.1002/cncr.32378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halpern MT, Schrag D. Effects of state-level medicaid policies and patient characteristics on time to breast cancer surgery among medicaid beneficiaries. Breast Cancer Res Treat. 2016;158:573–581. doi: 10.1007/s10549-016-3879-8. [DOI] [PubMed] [Google Scholar]
- 26.Doe S, Petersen S, Buekers T, Swain M. Does a multidisciplinary approach to invasive breast cancer care improve time to treatment and patient compliance? J Natl Med Assoc. 2020;112:268–274. doi: 10.1016/j.jnma.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haideri NA, Moormeier JA. Impact of patient navigation from diagnosis to treatment in an urban safety net breast cancer population. J Cancer. 2011;2:467–473. doi: 10.7150/jca.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons HM, Lathrop KI, Schmidt S, Mazo-Canola M, Trevino-Jones J, Speck H, Karnad AB. Breast cancer treatment delays in a majority minority community: is there a difference? J Oncol Pract. 2015;11:e144–e153. doi: 10.1200/JOP.2014.000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojcik BE, Spinks MK, Optenberg SA. Breast carcinoma survival analysis for African American and white women in an equal-access health care system. Cancer. 1998;82:1310–1318. doi: 10.1002/(sici)1097-0142(19980401)82:7<1310::aid-cncr14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser Family Foundation. COVID-19 cases and deaths by race/ethnicity: current data and changes over time. https://www.kff.org/racial-equity-and-health-policy/issue-brief/covid-19-cases-and-deaths-by-race-ethnicity-current-data-and-changes-over-time/; Accessed January 16, 2022.
- 31.Bartlett DL, Howe JR, Chang G, Crago A, Hogg M, Karakousis G, Levine E, Maker A, Mamounas E, McGuire K, Merchant N, Shibata D, Sohn V, Solorzano C, Turaga K, White R, Yang A, Yoon S, Society of Surgical Oncology Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol. 2020;27:1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382:e52. doi: 10.1056/NEJMc2010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American College of Surgeons. COVID-19: recommendations for management of elective surgical procedures. https://www.facs.org/covid-19/clinical-guidance/elective-surgery; Accessed January 16, 2022.
- 34.Dietz JR, Moran MS, Isakoff SJ, Kurtzman SH, Willey SC, Burstein HJ, Bleicher RJ, Lyons JA, Sarantou T, Baron PL, Stevens RE, Boolbol SK, Anderson BO, Shulman LN, Gradishar WJ, Monticciolo DL, Plecha DM, Nelson H, Yao KA. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181:487–497. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.• Obeng-Gyasi S, Oppong B, Paskett ED, Lustberg M. Purposeful surgical delay and the coronavirus pandemic: how will black breast cancer patients fare? Breast Cancer Res Treat. 2020;182:527–30. 10.1007/s10549-020-05740-0. An important editorial that examines the possible implications of the pandemic-related surgical delays on black breast cancer patients and directions for future study to further elucidate the impact.). [DOI] [PMC free article] [PubMed]
- 36.• Satish T, Raghunathan R, Prigoff JG, Wright JD, Hillyer GA, Trivedi MS, Kalinsky K, Crew KD, Hershman DL, Accordino MK. Care delivery impact of the COVID-19 pandemic on breast cancer care. JCO Oncol Pract. 2021;17:e1215–24. 10.1200/OP.20.01062. (As one of the first studies to examine how breast cancer care in the United States was affected by the COVID-19 pandemic, this study found that among breast oncology patients, patients who identified as Black/African American, Asian, or Other races were more likely to experience a delay or change in their treatment compared to Whites; additionally, they found that Medicaid compared with commercial insurance was associated with increased odds of a delay and/or change.). [DOI] [PubMed]
- 37.Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184:249–254. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muñoz R, Farshidpour L, Chaudhary UB, Fathi AH. Multidisciplinary cancer care model: a positive association between oncology nurse navigation and improved outcomes for patients with cancer. Clin J Oncol Nurs. 2018;22:E141–E145. doi: 10.1188/18.CJON.E141-E145. [DOI] [PubMed] [Google Scholar]
- 39.Jaffe DH, Lee L, Huynh S, Haskell TP. Health inequalities in the use of telehealth in the United States in the lens of COVID-19. Popul Health Manag. 2020;23:368–377. doi: 10.1089/pop.2020.0186. [DOI] [PubMed] [Google Scholar]