Abstract
Introduction
Cutaneous lipohypertrophy (LH) is a thickened, “rubbery” lesion in the subcutaneous tissue following multiple injections performed at the same site, i.e., an incorrect injection technique. It is widespread, averaging 47% of insulin patients worldwide, and has severe direct and indirect consequences. Direct consequences consist mainly of poor metabolic control and frequent hypoglycemic events (HYPOs), and indirect ones of markedly increased healthcare costs related to hospital access due to acute events and long-term disease complications. This observation also holds for Italy, despite the National Health System organization expecting every patient with diabetes to undergo a series of visits by different care team members, each performing a specific treatment/education task. Indeed, the recent literature points to poor awareness of LH relevance and metabolic consequences among doctors from general and diabetic hospital wards, with educational deficiencies on correct injection practice in nurses too. The aim was to establish if, to what extent, and by whom they had received training on correct insulin injection techniques, and how many initially received notions had persisted over time.
Methods
We investigated the possible causes of such a failure from the point of view of 1160 insulin-requiring subjects with type 2 diabetes (T2DM), reporting for the first time to specialized diabetic structures through a validated questionnaire and, in the same patients, we searched for LH by inspection/palpation according to international guidelines, further confirmed by ultrasound scans. We then analyzed differences in education and injecting behavior between subjects classified as LH+ or LH− depending on the presence or absence of LH lesions.
Results
We documented significant educational gaps, with 50% of patients failing to refer to healthcare professionals and relying on their peers with diabetes, thought to be more experienced in 15% of the cases. Seventy-five percent of LH− patients received education from healthcare providers, while 90% of LH+ learned from another patient or could not remember how they knew, and 68% of LH+ versus 52% of LH− (p < 0.01) patients had failed to receive training on injection techniques by healthcare providers. All of this enabled the most disabling features of diabetes from the very beginning of the disease history.
Conclusions
This study documents, from the patients’ point of view, that educational gaps are significant and that, even in initially trained subjects, education on correct injection techniques has a fleeting effect if not regularly recalled. Therefore, to rehabilitate LH+ patients as soon as possible and prevent LH− patients from inadvertently slipping into the other group, there is an urgent need to educate doctors and nurses repeatedly on the importance of correctly injecting insulin to improve patients’ knowledge and skills.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-022-01341-w.
Keywords: Type 2 diabetes, Lipohypertrophy, Injection technique, Education, Education durability, Rehabilitation
Key Summary Points
| Lipohypertrophy is reported ubiquitously in insulin-treated people with type 2 diabetes, displaying an average frequency of 47%, up to a maximum of 75%, and subtly causing severe disabling consequences. |
| Its mere presence is proof of a lack of educational activities by the care team. |
| In doctors from general and diabetic hospital wards, awareness of the importance and metabolic consequences of lipohypertrophy appears to be poor. |
| Educational deficiencies on correct injection practice have also been documented among nurses. |
| This study documents, from the patients’ point of view, that educational gaps are significant. |
| Even in patients initially trained, education on correct injection techniques has a fleeting effect if not regularly recalled. |
| Therefore, for efficient preventative and rehabilitation purposes, it is urgent to educate both doctors and nurses repeatedly on the importance of correctly injecting insulin to improve patients’ knowledge and skills. |
Introduction
In Italy, assistance for people with type 2 diabetes (T2DM) relies on the primary care system and a network of about 650 diabetes care units (DCU). According to a validated protocol shared between the most representative scientific societies in the field, i.e., the Associazione dei Medici Diabetologi (AMD), Societa` Italiana di Diabetologia (SID), and Societa` Italiana di Medicina Generale (SIMG), the general practitioner (GP) has the responsibility for the diagnosis and suggests his patient for referral to the closest territorial or hospital diabetes unit (DCU) [1]. Unfortunately, this protocol is not widely and homogeneously implemented, and only a few Italian regions have issued new laws and funded health-related activities involving shared and fully integrated management plans. In real-life conditions, most GPs send their patients to DCUs only with persistent hyperglycemic levels or fast-progressing chronic complications [2].
Such a phenomenon causes a kind of dichotomy between (i) uncomplicated patients under treatment with oral hypoglycemic agents (OHAs) who consult their GPs and (ii) severely complicated, mostly insulin-treated patients with longstanding T2DM who rely on a DCU. Nonetheless, many GPs currently start insulin treatment more frequently than in the past by adding basal insulin to OHAs and fast-acting analogs at meal times, sometimes without completely stopping OHAs, before referring the patient to a DCU in case of failure to reach supposed glycemic targets or complications (unpublished personal observations).
Other patients access DCUs after being prescribed insulin in the hospital first and are sent to their GPs for follow-up or to a local DCU for the prescription of aids, education, or new generation insulins, accessible only through diabetologists authorized by the Italian Medicines Agency (AIFA). However, the patient can freely choose to refer to a DCU for treatment whenever needed.
Despite its apparent complexity, this process has been well understood and perfectly implemented for years. However, as documented by us a few years ago [2], owing to the different access pathways mentioned above, DCUs often meet people treated for diabetes, even those on insulin, for the first time between 6 months and 5 years from diagnosis. Each person with diabetes should then undergo a series of visits by members of the care team, each expected to perform a specific task. However, the high frequency of cutaneous lipohypertrophy (LH) due to incorrect injection techniques indicates that this does not occur.
LH occurs in patients with both T1DM and T2DM, and is characterized by a thickened, “rubbery” lesion in the subcutaneous tissue developing after multiple injections performed at the same site [3]. The identification and delimitation of LH-affected areas are not simple processes. Inspection and palpation are standard clinical practices used to identify LH [4] but can underestimate its rates without other additional maneuvers. The reliability of this method is potentially low, with high levels of interclinician variation [5]. Ultrasound scans have recently been shown to identify LH with significantly increased frequency compared with inspection or palpation. [6, 7]. LH is associated with increased glucose variability, poor metabolic control, and frequent hypoglycemic episodes [8–10]. Approximately one-third of physicians recognized the clinical harm related to LHs. Still, many ignore the social and economic costs of such lesions, including increasing healthcare costs, cosmetic effects, and severe psychological burden [11]. These adverse effects have additional impacts on long-term outcomes, including increased daily insulin doses and healthcare costs, which can dramatically worsen clinical, social, and economic results [8–15]. Even if LH causes are not yet fully understood, only some 40% of physicians recognized all known risk factors [8–11], including high body mass index (BMI) (still debated), frequent needle reuse, failure to rotate insulin injection sites, and insulin exposure duration [2, 5, 6, 10, 16, 17].
We were impressed by recently published literature concerning the attitude of doctors and nurses towards the identification of LHs from incorrect injection techniques. A recent survey from China revealed significant differences in awareness, knowledge, and behavior concerning LH across medical groups from various assistance levels with different hierarchical roles, seniority, and specialization so that, in less experienced doctors, the inadequacy was about 18.9% for LH identification and 54.7% for LH management [11]. These findings show that, despite some improvements in recent years, LH-related complications are still underestimated by many physicians and emphasize the need for comprehensive and continuous education on all aspects associated with LH, including physician awareness. China has the highest number of patients with diabetes in the world [17]. Many shortcomings in the insulin injection practice of nurses can be attributed to inadequate knowledge, suggesting the importance of being educated to improve compliance with injection guidelines [18, 19], as reported in a recent Chinese nationwide survey on knowledge, attitudes, and practices of 223,368 nurses within the field [19]. The study revealed deficiencies in all three items, especially in nurses working in endocrinology units, similar to results from previous studies conducted in other countries [20–26], albeit differing across regions. Approximately one-third of surveyed nurses had poor insulin injection knowledge scores, particularly concerning injection sites, needle disposal, and hypoglycemia management. Further, about one-quarter did not care about proper injection or repetitive use of insulin needles and were not entirely confident about teaching diabetic patients how to inject insulin correctly. Approximately two in three (67.28%) felt they needed insulin injection training. Indeed, nurse injection knowledge is expected to improve the performance of individual patients [5, 27, 28].
Based on previous analyses, we found it necessary to look for possible causes of such a failure, which might be related to factors other than mere health system dysfunction. To do so, we evaluated the point of view and needs of insulin-requiring patients with T2DM, crossing, for the first time, the threshold of specialized diabetic structures organized in multifunctional and autonomous teams.
The primary endpoint or our study was to establish if, to what extent, and by whom they had received training on correct insulin injection techniques and how many initially received notions had persisted over time. The secondary endpoint was to establish the relationship between the ability to inject insulin correctly and the presence or absence of LH and glycated hemoglobin (HbA1c) levels.
Methods
The present multicenter, observational study was conducted by eight outpatient diabetes units (DCUs) that share electronic record systems, diagnostic–therapeutic procedures, and operating standards, and participate in the continuous care improvement program of the National Clinical Diabetology Association (AMD) (https://www.aemmedi.it). All DCUs were part of the Nefrocenter Research Network affiliated with the National Health System, and with the University Hospital “Luigi Vancitelli” of Naples, whose Ethical-Scientific Committee approved the study protocol for all the DCUs (registration: protocol no. 227, 25 April 2021) under the original Declaration of Helsinki, and its subsequent amendments. All subjects were informed of the study purpose, requirements, and expectations and signed the informed consent form. At the end of the trial, all patients received the same structured education, tools, and devices as previously described [29, 30].
The specialists and nurses participating in the study had undergone full training on all procedures described.
The inclusion criteria were as follows: (i) 18–75 years of age; (ii) T2DM, (iii) being on insulin for at least 12 months and referring to the DCU for the first time; (iv) self-administering insulin through a pen for at least twice a day with or without other OHAs; (v) being free from handicaps, whether physical or mental (as evaluated by the Mini Mental Test), hindering protocol adherence; (vi) having no visual defects and being able to read a simple newspaper text; (vii) having received no education on LH within the preceding 6 months. The main exclusion criteria were (a) pregnancy or planned pregnancy within 3 months; (b) participation in other clinical trials within 3 months; (c) diagnosis of T1DM; (d) other significant medical diseases or unsuitability for the study as per the investigators’ judgment.
Each DCU enrolled the first 150 consecutive patients meeting the inclusion criteria, for a total of 1200 subjects with T2DM.
Data Collection and Endpoints
Baseline visits allowed demographic and clinical data collection and insulin injection behavior recording through a validated questionnaire [19, 31]. We further tested this questionnaire on a cohort of 85 insulin-treated people with T2DM after adding questions on if, by whom, where, and how they received the education on correct insulin injection techniques [32, 33]. For final validation of the new version, we performed a factorial analysis and an estimate of (i) comprehensibility based on the percentage of responses obtained to each question and scale completion; (ii) convergent validity to verify whether or not the score of an item correlated with the total of the pertaining scale; and (iii) internal consistency, i.e., the degree of agreement of the responses to the items relating to the same dimension in the responses to the items belonging to the same scale through the Cronbach alpha coefficient (values ≤ 0.70 are considered optimal). As part of the validation, the raw scores were transformed into a range from 0 to 100.
The questionnaires were independently self-completed by patients who could ask for help from the DCU healthcare staff whenever facing difficulties in question interpretation.
All patients underwent accurate evaluation at all their insulin injection sites by expert nurses or doctors certified as experts in the detection, grading, and measurement of LH, including visualization and palpation of the adipose tissue, as described by Gentile et al. [6, 28, 34]. Emphasis was placed on the need for oblique lighting to aid the visual detection of LH lesions, a warm environment, and a supine position with knees up to relax abdominal muscles. The location and size of LH were confirmed or identified by physical examination by two independent observers (one performed the physical examination and the other reviewed its results). LH presence was confirmed by ultrasound reevaluation as well. High-frequency B-mode ultrasound skin scans took advantage of linear 20 MHz probes (HD3; Philips NV, Amsterdam, the Netherlands) at all injection sites, as previously described [9].
Patients enrolled, and their caregivers were instructed how to identify severe (SeH) and symptomatic (SyH) hypoglycemic episodes in the 8 weeks after enrollment by checking associated blood-glucose meter readings. After that, all data were electronically recorded. HYPOs were defined according to American Diabetes Association (ADA) statements [35]. Briefly, a HYPO consisted of one or more symptoms (palpitations, tiredness, sweating, hunger, dizziness, and tremor) confirmed by a blood glucose (BG) reading ≤ 70 mg/dL. It was further classified as SeH (BG ≤ 50 mg/dL) and SyH (BG 50–70 mg/dL), as previously reported [36, 37]. Finally, a HYPO was defined as unexplained in the absence of any identified precipitating event, including changes in insulin dosage, diet composition, or amount of physical activity.
Statistical Analysis
In previous randomized controlled trials [9, 10, 19], HbA1c decreased by a mean of 0.58% (6.3 mmol/mol) during the study, and the standard deviation (SD) was 1.3%. We set the significance level at a = 0.05 (two-sided) and the power at 80%; therefore, the minimum sample size for each group was 120 participants. The sample size should have got to 160 participants per group to allow a dropout rate of 10–15%. Still, we chose 1200 as the final number of enrolled subjects, available in the Nefrocenter clinics, to increase the representativeness of the sample as much as possible. Using SAS version 9.4 (SAS Institute, Cary, NY, USA) for statistical analysis, we assessed intra- and intergroup differences among baseline, 3 months, and 6 months (values presented as mean ± SD, median, and interquartile range or percentage). We performed the Mann–Whitney U test and Pearson chi-square test to assess whether variables under investigation displayed any different mean levels/percentage distributions in patients with LH (LH+) from those without LH (LH−). Then, we entered all variables into an unadjusted odds ratio analysis according to the univariate binary logistic regression function and progressively removed significant ones from subsequent stepwise backward multivariate logistic regression analysis. After calibration and discrimination ability assessment by Hosmer–Lemeshow goodness of fit test (HLGOF) and receiver operating characteristic (ROC) analysis, we reported odds ratios as estimated in the refitted final model.
Results
The preliminary validation gave excellent results with the questionnaires, which were easy to understand and complete, and were well accepted by patients, thus reaching scores between 85% and 95% of the transformed values on a scale from 0 to 100, according to the established validation criteria. Of the 1200 enrolled subjects, 1160 completed the questionnaires correctly and were therefore included in the subsequent statistical analysis.
In greater detail, 59 out of the 1160 subjects completing the questionnaire asked staff for help to better understand questions without receiving any suggestions or directions. The other 40 (3.3%) subjects dropped out straight away with no answers to more than half of the questions.
The characteristics of the enrolled subjects are outlined in Table 1, which also shows the significant differences observed between subjects with and without LH (LH+: 487 subjects, 42%, and LH−). They proved similar in M/F ratio, age, BMI, diabetes duration, and time spent on insulin.
Table 1.
Clinical characteristics and injection habits of the patients at enrollment
| Overall | LH+ | LH− | p-Value | |
|---|---|---|---|---|
| Subjects N (%) | 1160 | 487 (42.0) | 673 (58.0) | n.s. |
| Sex (M/F) | 552/608 | 284 / 203 | 273/401 | – |
| Age (years) | 60.6 ± 9.0 | 61.9 ± 5.6 | 60.1 ± 3.6 | n.s. |
| BMI (kg/m2) | 32.4 ± 3.5 | 31.4 ± 7.2 | 32.7 ± 5.6 | n.s. |
| Diabetes duration (years) | 15.8 ± 7.6 | 14.5 ± 5.2 | 15.6 ± 7.5 | n.s. |
| Insulin treatment duration (years) | 7.6 ± 2.2 | 7.2 ± 3.1 | 7.7 ± 2.5 | n.s. |
| Two injections/day N (%) | 112 (9.6) | 8 (1.6) | 104 (15.5) | 0.001 |
| Three injections/day N (%) | 348 (21.3) | 99 (20.3) | 249 (36.9) | 0.001 |
| Four injections/day N (%) | 700 (60.4) | 380 (78.1) | 320 (47.6) | 0.001 |
| Total daily insulin dose (IU) | 61.6 ± 12.3 | 65.5 ± 10.6 | 53.6 ± 9.5 | 0.05 |
| HbA1c % (M ± SD) | 8.4 ± 1.1 | 8.6 ± 1.6 | 7.3 ± 1.0 | 0.01 |
| Fasting plasma glucose (mg/dL) (M ± SD) | 151.6 ± 26.6 | 165.7 ± 22.4 | 133.6 ± 18.7 | 0.05 |
| Needle reuse N (%) | 538 (46.4) | 480 (98.5) | 58 (8.6) | 0.0001 |
| Missing site rotation N (%) | 501 (43.2) | 485 (94.0) | 16 (2.3) | 0.0001 |
| Ice-cold insulin injection N (%) | 650 (56.0) | 478 (98.1) | 172 (25.5) | 0.0001 |
| Injection into LH nodules N (%) | 464 (40.0) | 464 (95.3) | – | – |
| HYPOs N and (%) of patients affected | 332 (46.2) | 289 (59.3) | 46 (6.8) | 0.0001 |
|
Overall HYPOS (N) Mean range |
506 0–1.2 |
371 0–2.7 |
135 0–0.9 |
|
| Severe HYPOs N (%) | 287 (56.7) | 262 (70.6) | 60 (44.4) | 0.001 |
| Symptomatic HYPOs N (%) | 219 (43.3) | 109 (29.4) | 75 (55.5) | 0.001 |
| Nocturnal HYPOs N (% of total severe) | 126 (43.9) | 112 (42.7) | 5 (20.0) | 0.001 |
Data are presented as mean ± standard deviation (M ± SD) or frequencies (%). Nocturnal hypoglycemic episodes were only severe
As depicted in Fig. 1, eight (1.6%) LH+ subjects and 104 (15.5%) LH− subjects were on two insulin injections/day (p < 0.001), 99 (20.3%) LH+ and 249 (36.9%) LH− were on three injections/day p < 0.001), and the remaining patients were on four injections/day [i.e., 380 (78.1%) LH+ and 320 (47.6%) LH−; p < 0.001] The total insulin dose was 65.5 ± 10.6 IU/day in LH+ and 53.6 ± 9.5 IU/day in LH− (p < 0.05) subjects. HbA1c values were significantly higher in LH+ than LH− (8.6 ± 1.6% versus 7.3 ± 1.0%, respectively; p < 0.01) (Fig. 2). Fasting plasma glucose was significantly higher in LH+ than LH− (165.7 ± 22.4 versus 133.6 ± 18.7 mg/dL, respectively; p < 0.05) (See Table 1).
Fig. 1.
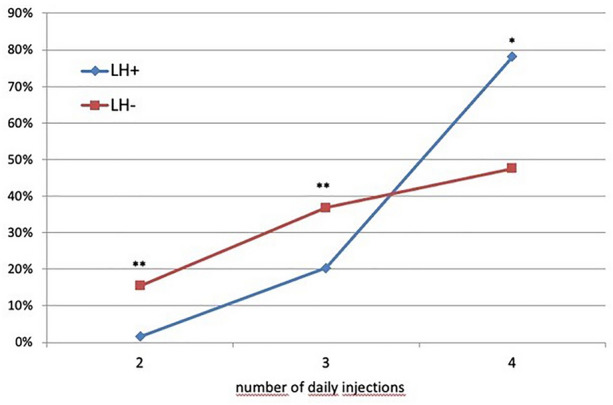
Frequency of LH in relation to the number of daily insulin injections (**p < 0.005; *p < 0.001)
Fig. 2.
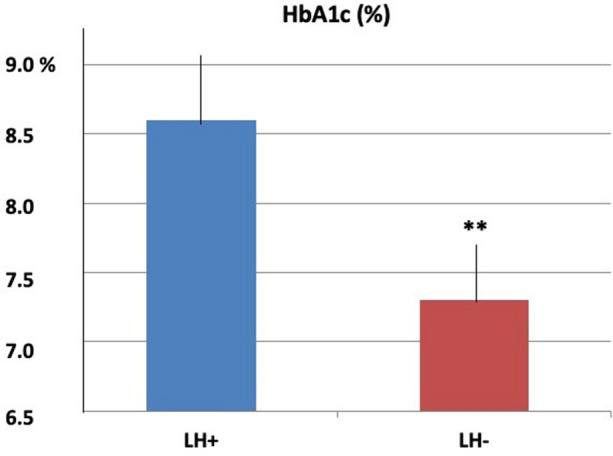
Mean HbA1c (%) values ± SD in subjects with (LH +) and without (LH−) lipohypertrophy, and significance of differences (**p < 0.01)
Regarding injection habits, needle reuse was significantly higher (up to > 5 times) in LH+ than in LH− [480 (98.5%) versus 58 (8.6%), respectively; p < 0.0001] (Table 1 and Fig. 3). Missing site rotation (94.0% versus 2.3%; p < 0.0001) and ice-cold insulin injection (98.1% versus 25.5%; p < 0.0001) were also greater in LH+ patients. Moreover, 464/487 LH+ subjects (95.3%) injected insulin into LH nodules and 289/487 (46.2%) LH+ subjects versus only 46/673 (6.8%) LH− (p < 0.0001) had one or more HYPOs.
Fig. 3.
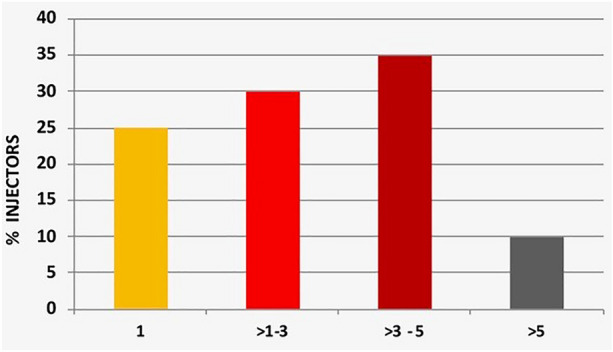
Frequency of needle reuse (%)
Out of a total of 371 Hypos, 262 SeHs (70.6%) occurred in LH+ versus 60/135 (44.4%) in LH− (p < 0.01), while SyHs were less frequent [i.e., 109/371 (29.4%) in LH+ versus 75/135 (55.5%) in LH−; p < 0.001]. Episodes of nocturnal hypoglycemia were all severe and significantly more frequent in LH+ than in LH− [112 (42.7%) versus 5 (20.0%), respectively; p < 0.001] (Table 1 and Fig. 4).
Fig. 4.
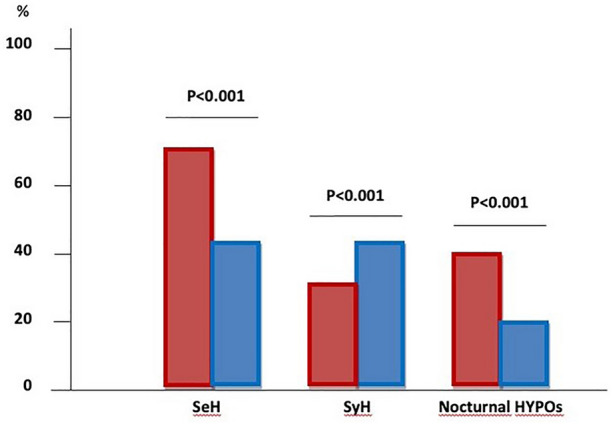
Frequency of severe (371) and symptomatic (135) total hypoglycemic episodes in LH+ (red columns) and LH− (blue columns) subjects. Nocturnal episodes were all severe
In Table 2, we summarized the results of the multivariate analysis of factors associated with LH, which confirmed what was already known from the literature, i.e., the significant association with diabetes duration (CI 95% 1.22–2.63; RR = 2.17), number of daily injections (CI 95% 1.86–2.91; RR = 2.38), high HbA1c levels (CI 5% 1.93–2–21; RR = 1.48), needle reuse (CI 95% 2.22–3.87; RR = 3.46), missing site rotation (CI 95% 2.89–4.90; RR = 3.67), ice-cold insulin injection (CI 95% 2.75–4.88; RR = 3.39), SeHs (CI 95% 2.48–4.56; RR = 3.49) and, notably, night-time HYPOs (CI 95%, 1.87–3.49; RR = 1.71).
Table 2.
Multivariate analysis of factors significantly associated with LH
| Factors associated with LH | ||
|---|---|---|
| (95% CI) | RR | |
| Age (years) | 1.00–1.19 | 0.88 |
| BMI (kg/m2) | 1.15–2.19 | 1.68 |
| Diabetes duration (years) | 0.78–1.08 | 0.79 |
| Insulin treatment duration (years) | 1.22–2.63 | 2.17 |
| N of daily injections | 1.86–2.91 | 2.38 |
| Total daily insulin dose (IU) | 1.93–2.21 | 1.48 |
| HbA1c % (M ± SD) | 1.55–2.88 | 2.04 |
| Fasting plasma glucose (mg/dL) (M ± SD) | 1.17–1 97 | 0.89 |
| Needle reuse (%) | 2.22–3.87 | 3.46 |
| Missing site rotation (%) | 2.89–4.90 | 3.67 |
| Ice-cold insulin injection (%) | 2.75–4.88 | 3.39 |
| Severe HYPOs (%) | 2.48–4.56 | 3.49 |
| Symptomatic HYPOs (%) | 0.98–1.88 | 0.91 |
| Nocturnal HYPOs (%) | 1.87–3.49 | 1.71 |
Figure 5 refers to the question: “who taught you how to correctly inject insulin?” It clearly shows that healthcare providers (35% diabetologists, 5% GPs, 2% pharmacists, and only 8% nurses) accounted for only 50% of the cases, with a surprising 35% of patients unable to remember educators, if any, and the remaining 15% stating to have got the necessary information from disease peers. Interestingly, 75% of LH− received education from healthcare providers, while 90% of LH+ learned from another patient or could not remember how.
Fig. 5.
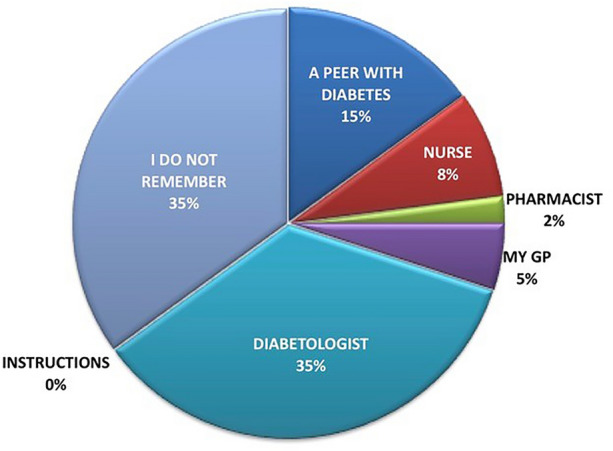
Answers to the question “who taught you how to correctly inject insulin?”
Finally, Fig. 6, referring to the question “Who taught you where within the body to inject insulin by rotating injection sites?” clarifies that only 48% claimed to have received education by a healthcare professional (35% diabetologists, 8% nurses, 3% GPs, and 2% pharmacists), 10% from another person with diabetes, and 32% by no one at all. In comparison, 10% could not even remember any details. Also, in this case, 68% of LH+ versus 52% of LH− (p < 0.01) patients had failed to receive training on injection techniques by healthcare providers, and only 12% of LH+ declared to have been trained by healthcare personnel.
Fig. 6.
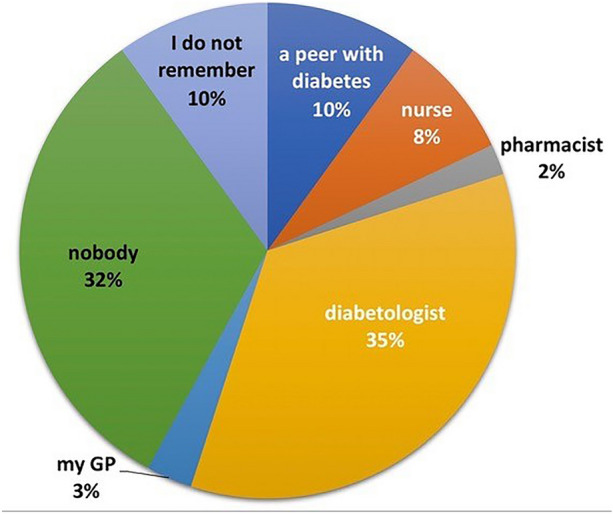
“Who taught you where within the body to inject insulin by rotating the injection sites?”
Discussion
Several times in the last few years, our group and other groups have outlined that educational deficiencies strongly affect the outcome of insulin treatment through the ability to identify LH and thus prevent their severe metabolic consequences [6, 9, 10, 34, 38]. Unlike many other countries, where the educator figure is officially there, in our NHS, an effective structured educational activity is on individual, spontaneous, nonremunerated grounds. Such a phenomenon is also dependent on substantial organization troubles affecting multidisciplinary teams, especially in the present COVID-19 pandemic requiring all possible human and structural resources to shift primarily toward acute problems. However, even without considering the current viral pandemic and the differences between the various organizational modalities, the high average rate of LHs is clear evidence of such a deficiency [6, 10, 34, 38].
In the current study, in addition to providing a further contribution to the LH field, we have gone deeper into what patients on insulin know or remember about correct injection techniques and metabolic consequences.
Four hundred eighty-seven subjects with LH (42%) and 673 without LH (58%) were identified in a cohort of 1160 type 2 diabetics on insulin, crossing, for the first time, the threshold of the DCUs participating in the study. These rates are very close to those reported in the literature [38]. Our analysis also confirmed that the main parameters associated with LH are long duration of insulin treatment, a high number of daily injections, repeated needle reuse, missing site rotation, and ice-cold insulin injection [6, 9]. The rate of hypoglycemia, especially severe or nocturnal, also closely correlated with the presence of LH, and significantly elevated HbA1c levels were present in patients with LH compared with those without, once again in agreement with literature data [5, 10, 11, 38, 39]. This is disabling in itself, and only the time and patience of healthcare personnel can enable progressive, yet slowly proceeding rehabilitation.
What surprised us much more, however, was the poor memory that patients claimed to have concerning professional figures involved in the education on insulin injections. Indeed, 50% of them had not referred to healthcare professionals for that. Moreover, they relied on their peers with diabetes, thought to be more experienced in 15% of the cases. In particular, when starting on insulin during a hospital stay for causes other than diabetes—which happened in 43 LH+ (8.9%) and 68 LH− (10.2%) patients—69% of the subjects reported no indications whatsoever concerning insulin injection procedures at discharge. Conversely, 70.7% of patients (n = 116) discharged on insulin from endocrinological wards [i.e., 58 LH+ (11.9%) and 58 LH− (8.6%)] were not only taught how to inject but also provided with the insulin pens used during the hospital stay. In patients starting in the hospital, the time interval between discharge and the time of enrollment ranged from 24 to 43 months. Our previous data on the durability of education concerning correct injection techniques did not exceed 6 months [29, 30, 40]. From this, it is clear that a therapeutic intervention, even if well performed, tends to be forgotten within a few months if not repeated and reinforced constantly. As expected, the LH percentage was significantly higher in subjects who had not received training from healthcare professionals. However, the equally alarming fact emerged that many patients do not remember any specific educational training upon hospital discharge.
These data coincide with a series of observations on the ability of doctors [12, 37] and nurses [20–26] to diagnose, treat, and prevent insulin technique errors in subjects with T2DM.
The limits of this study are those typical of all observational studies and, specifically, depending on the level of understanding of the questionnaire, albeit validated and administered, when needed, with the supervision of healthcare personnel supporting patients to understand questions and provide required answers without ever influencing the completer.
Conclusions
On the whole, physicians lack an adequate understanding of LH-related issues, especially in primary hospitals. Urgent needs in primary hospitals are as follows: increasing physician awareness of LH, establishing standardized diagnosis–treatment–care processes, and screening and educating patients regularly to rehabilitate patients otherwise left alone in their disabling disease. Furthermore, nurses have insufficient knowledge of appropriate insulin injection techniques despite having a good attitude and behavior towards injections. Learning can directly or indirectly affect the attitude toward insulin injection, indicating that hospitals should formulate unified norms and regularly organize training and assessment courses to improve nurses’ knowledge, philosophy, and behavior.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are indebted to all experimenters and components of the AMD-OSDI study group and of the Nefrocenter working group, all of whom are listed below. All DCUs were part of the Nefrocenter Research Network in Southern Italy, a private consortium supported by the National Health System in association with Naples University ‘‘Luigi Vanvitelli’’ for several clinical aspects, including the Ethics Committee’s autorization (trial registration no. 120—16.02.2019). Special thanks to Carolina La Rocca, national president of OSDI, to Marcello Grussu, National President of ANIAD, and to Dr. Paola Murano, General Manager of the Nefrocenter Research Network for the effective and continuous support offered as complimentary, for the realization of the study. We are also indebted with all patients who generously agreed to participate in the study.
Funding
No funding or sponsorship was received for this study or the publication of this article. No payment was requested for publication and online posting costs. None of the authors or coworkers received funding or another type of payment for this paper. No funding was required for the “Editorial and Other Assistance.”
Authorship
All named authors (Sandro Gentile, Giuseppina Guarino, Teresa Della Corte, Giampiero Marino, Ersilia Satta, Carmine Romano, Carmelo Alfarone, Maria Pasquarella, Laura Giordano, Fabrizio Loiacono, Maurizio Capece, Rossella Lamberti, and Felice Strollo) meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article taking responsibility for the integrity of the work as a whole, and gave their approval for this version to be published.
Authorship Contributions
Sandro Gentile and Felice Strollo designed the study and wrote the article. Ersilia Satta, Teresa Della-Corte, Giuseppina Guarino, Giampiero Marino, Carmine Romano, Carmelo Alfarone, Maria Pasquarella, Laura Giordano, Fabrizio Loiacono, Maurizio Capece, and Rossella Lamberti critically read and approved the paper. All authors contributed to data acquisition, critically assessed the results, and approved the final text. All collaborators critically read and approved the final text (see the complete list of collaborators in the Supplementary Material).
Disclosures
Sandro Gentile, Giuseppina Guarino, Teresa Della Corte, Giampiero Marino, Ersilia Satta, Carmine Romano, Carmelo Alfarone, Maria Pasquarella, Laura Giordano, Fabrizio Loiacono, Maurizio Capece, Rossella Lamberti, and Felice Strollo have no financial interests to declare in relation to the present study.
Compliance with Ethics Guidelines
This study was carried out in compliance with good clinical practice standards and in accordance to the ethical guidelines of the 1964 Declaration of Helsinki and its subsequent amendments. The study protocol was approved by the Ethical and Scientific Committee of the reference center, University ‘‘Luigi Vanvitelli’’ Naples, Italy (Trial registration: Protocol n. 227, April 25, 2021) which served as the central reference ethical committee for all of the participating diabetes centers, with the latter an integral part of the same private consortium Nefrocenter Research Network, associated to the above-mentioned University. All of the subjects with T2DM participating in the study signed an informed consent form to be included in the present investigation.
Data Availability
The datasets analyzed during the present study are available from the corresponding author on reasonable request.
Footnotes
AMD = Associazione Medici Diabetologi (Italian Association of Diabetes Specialists)—OSDI = Operatori Sanitari di Diabetologia Italiani (Italian Diabetes Healthcare Professionals). ANIAD = Associazione Nazionale Italiana Atleti Diabetici (Italian National Association of Athletes with Diabetes).
References
- 1.Appropriatezza clinica, strutturale, tecnologica e operative per la prevenzione, diagnosi e terapia dell’obesita` e del diabete mellito. Quaderni del Ministero della salute n.10. Poligrafico dello Stato, Roma 2011. http://www.quadernidellasalute.it/archivio-quaderni/10-luglio-agosto-2011.php. Accessed 4 Mar 2022
- 2.ISSN 2038-5293. https://www.salute.gov.it/imgs/C_17_pubblicazioni_1707_allegato.pdf. Accessed 4 Aug 2021.
- 3.Strollo F, Guarino G, Marino G, Paolisso G, Gentile S. Different prevalence of metabolic control and chronic complication rate according to the time of referral to a diabetes care unit in the elderly. Acta Diabetol. 2014;51(3):447–453. doi: 10.1007/s00592-013-0537-z. [DOI] [PubMed] [Google Scholar]
- 4.Spollett G, Edelman SV, Mehner P, Walter C, Penfornis A. Improvement of insulin injection technique: examination of current issues and recommendations. Diabetes Educ. 2016;42:379–394. doi: 10.1177/0145721716648017. [DOI] [PubMed] [Google Scholar]
- 5.Frid AH, Kreugel G, Grassi G, Halimi S, Hicks D, Hirsch LJ, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91:1231–1255. doi: 10.1016/j.mayocp.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Gentile S, Guarino G, Giancaterini A, Guida P, Strollo F, AMD-OSDI Italian Injection Technique Study Group A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. Springerplus. 2016;5:563. doi: 10.1186/s40064-016-1978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertuzzi F, Meneghini E, Bruschi E, Luzi L, Nichelatti M, et al. Ultrasound characterization of insulin induced lipohypertrophy in type 1 diabetes mellitus. J Endocrinol Investig. 2017;40:1107–1113. doi: 10.1007/s40618-017-0675-1. [DOI] [PubMed] [Google Scholar]
- 8.Kapeluto JE, Paty BW, Chang SD, Meneilly GS. Ultrasound detection of insulin-induced lipohypertrophy in type 1 and type 2 diabetes. Diabet Med. 2018;35:1383–1390. doi: 10.1111/dme.13764. [DOI] [PubMed] [Google Scholar]
- 9.Gentile S, Guarino G, della Corte T, et al. Insulin-induced skin lipohypertrophy in type 2 diabetes: a multicenter regional survey in Southern Italy. Diabetes Ther. 2020;11(9):2001–2017. doi: 10.1007/s13300-020-00876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39:445–453. doi: 10.1016/j.diabet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Ji L, Sun Z, Li Q, et al. Lipohypertrophy in China: prevalence, risk factors, insulin consumption, and clinical impact. Diabetes Technol Ther. 2017;19(1):61–67. doi: 10.1089/dia.2016.0334. [DOI] [PubMed] [Google Scholar]
- 12.Shen M, Shi Y, Zheng S, Fan H, Xu J, Yang T. A systematic survey of physicians’ insights into lipohypertrophy. Front Public Health. 2021;9:738179. doi: 10.3389/fpubh.2021.738179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji L, Chandran A, Inocencio TJ, et al. The association between insurance coverage for insulin pen needles and healthcare resource utilization among insulin-dependent patients with diabetes in China. BMC Health Serv Res. 2018;18(1):300. doi: 10.1186/s12913-018-3095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile S, Strollo F, Nefrocenter Research Study Group Cost saving effects of a short-term educational intervention entailing lower hypoglycaemic event rates in people with type 1 diabetes and lipo-hypertrophy. Diabetes Res Clin Pract. 2018;143:320–321. doi: 10.1016/j.diabres.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Johansson UB, Amsberg S, Hannerz L, Wredling R, Adamson U, Arnqvist HJ, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28:2025–2027. doi: 10.2337/diacare.28.8.2025. [DOI] [PubMed] [Google Scholar]
- 16.Deeb A, Abdelrahman L, Tomy M, Suliman S, Akle M, Smith M, et al. Impact of insulin injection and infusion routines on lipohypertrophy and glycemic control in children and adults with diabetes. Diabetes Ther. 2019;10:259–267. doi: 10.1007/s13300-018-0561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta SS, Gupta KS, Gathe SS, Bamrah P, Gupta SS. Clinical implications of lipohypertrophy among people with type 1 diabetes in India. Diabetes Technol Ther. 2018;20:483–491. doi: 10.1089/dia.2018.0074. [DOI] [PubMed] [Google Scholar]
- 18.IDF Diabetes Atlas. 9th Edition. 2019. https://www.d-net.idf.org/upload/resources/material/20200302_133351IDFATLAS9e-final-web.pdf. Accessed 4 Aug 2021.
- 19.Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide injection technique questionnaire study: population parameters and injection practices. Mayo Clin Proc. 2016;91(9):1212–1223. doi: 10.1016/j.mayocp.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Zhao F, Zhang M, Yuan L, Zheng Y, Huang J, Li Y, Li C. Insulin injection knowledge, attitudes, and practices of nurses in China: a cross-sectional nationwide study. Diabetes Ther. 2021;12(9):2451–2469. doi: 10.1007/s13300-021-01122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theofanidis D. In-hospital administration of insulin by nurses in northern Greece: an observational study. Diabetes Spectr. 2017;30(3):175–181. doi: 10.2337/ds16-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robb A, Reid B, Laird EA. Insulin knowledge and practice: a survey of district nurses in Northern Ireland. Br J Community Nurs. 2017;22(3):138–145. doi: 10.12968/bjcn.2017.22.3.138. [DOI] [PubMed] [Google Scholar]
- 23.Derr RL, Sivanandy MS, Bronich-Hall L, Rodriguez A. Insulin-related knowledge among health care professionals in internal medicine. Diabetes Spectr. 2007;20(3):177–185. doi: 10.2337/diaspect.20.3.177. [DOI] [Google Scholar]
- 24.Mushta AM. Study of insulin injection technique amongst the nursing staff. Pak J Med Sci. 2006;22(3):310–312. [Google Scholar]
- 25.Yacoub MI, Demeh WM, Darawad MW, Barr JL, Saleh AM, Saleh MY. An assessment of diabetes-related knowledge among registered nurses working in hospitals in Jordan. Int Nurs Rev. 2014;61(2):255–262. doi: 10.1111/inr.12090. [DOI] [PubMed] [Google Scholar]
- 26.Adhikari S, Poudel RS, Rajbanshi L, Shrestha S. Assessment of insulin injection practice of nurses working in a tertiary healthcare center of Nepal. Nurs Res Pract. 2018;2018:9375067. doi: 10.1155/2018/9375067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei SF, Xu P, Li YP, Wang P, Si DQ. Analysis of hidden dangers of non-endocrine insulin use and countermeasures. Nurs Pract Res. 2012;9(5):90–91. [Google Scholar]
- 28.Gentile S, Strollo F, Guarino G, Giancaterini A, Ames PRJ, Speese K, Guida P, Strauss K, on behalf of the AMD-OSDI Italian Injection Technique Study Group Factors hindering correct identification of unapparent lipohypertrophy. J Diab Metab Dis Contr. 2016;3:00065. [Google Scholar]
- 29.Gentile S, Guarino G, Della Corte T, et al. Role of structured education in reducing lypodistrophy and its metabolic complications in insulin-treated people with type 2 diabetes: a randomized multicenter case–control study. Diabetes Ther. 2021;12(5):1379–1398. doi: 10.1007/s13300-021-01006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gentile S, Guarino G, Della Corte T, et al. The durability of an intensive, structured education-based rehabilitation protocol for best insulin injection practice: the ISTERP-2 study. Diabetes Ther. 2021;12(9):2557–2569. doi: 10.1007/s13300-021-01108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Xing Q, Li J, Zhou J, Yuan Y, Wan Y, Pflug BK, Strauss KW, Hirsch LJ. Injection technique education in patients with diabetes injecting insulin into areas of lipohypertrophy: a randomized controlled trial. Diabetes Ther. 2021;12(3):813–826. doi: 10.1007/s13300-021-01013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards P. Questionnaires in clinical trials: guidelines for optimal design and administration. Trials. 2010;11(11):2. doi: 10.1186/1745-6215-11-2.PMID:20064225;PMCID:PMC2823735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vespasiani G, Nicoluci A, Erle G, Trento M, Miselli V. Validazione del questionario sulla conoscenza del diabete - GISED 2001. Giorn Ital Diabetol Metab 2002. https://www.gidm.it/validazione-del-questionario-sulla-conoscenza-del-diabete-gised-2001/. Accessed 4 Aug 2021.
- 34.Gentile S, Strollo F, Guarino G, et al. Why are so huge differences reported in the occurrence rate of skin lipohypertrophy? Does it depend on method defects or on lack of interest? Diabetes Metab Syndr. 2019;13(1):682–686. doi: 10.1016/j.dsx.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Supp 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giorda CB, Ozzello A, Gentile S, et al. Incidence and correlates of hypoglycemia in type 2 diabetes. The Hypos-1 study. J Diabetes Metab. 2014;5:344. [Google Scholar]
- 37.American Diabetes Association Clinical practice recommendations 2008. Hypoglycaemia and employment/licensure. Diabetes Care. 2008;31(Suppl 1):S94–123. doi: 10.2337/dc08-S094. [DOI] [PubMed] [Google Scholar]
- 38.Gentile S, Strollo F, Della Corte T, Marino G, Guarino G. Insulin related lipodystrophic lesions and hypoglycemia: double standards? Diabetes Metab Syndr. 2018;12(5):813–818. doi: 10.1016/j.dsx.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 39.Deng N, Zhang X, Zhao F, Wang Y, He H. Prevalence of lipohypertrophy in insulin-treated diabetes patients: a systematic review and meta-analysis. J Diabetes Investig. 2017;9:536–543. doi: 10.1111/jdi.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gentile S, Guarino G, Della Corte T, et al. The economic burden of insulin injection-induced lipohypertophy. role of education: the isterp-3 study. Adv Ther. 2022;39(5):2192–2207. doi: 10.1007/s12325-022-02105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the present study are available from the corresponding author on reasonable request.


