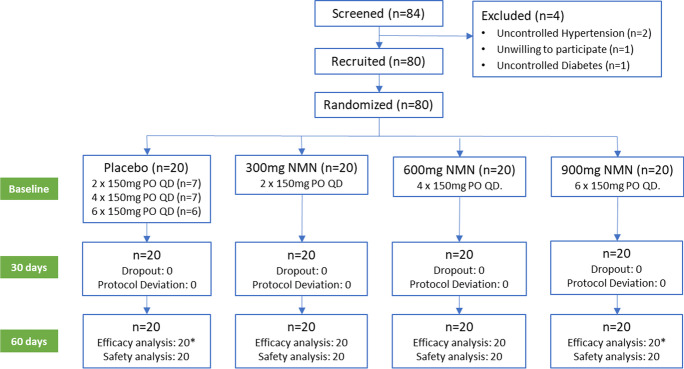
Fig. 1.
Trial flow for evaluating efficacy and safety of β-nicotinamide mononucleotide (AbinoNutra™NMN). Each recruited participant was instructed to take the assigned amount of either placebo or NMN orally once a day before breakfast with water of ambient temperature for 60 days. PO = orally, QD = once daily dosing. *Due to 1 participant each from placebo and 900 mg groups did not fast, Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) was based on 19 participants for these two groups