Abstract
The study of human cellular immune responses to parasite infection under field conditions is very complex. Often, the only practical site from which to sample the cellular responses is the peripheral blood. Sampling peripheral blood lymphocytes (PBL) relies on the assumption that these peripheral responses accurately reflect the immune responses acting locally at the site of infection. This is a particularly important point for the human intestinal helminth Trichuris trichiura, which solely inhabits the cecum and large intestine and so will stimulate a localized immune response. Using the well-defined model of T. trichiura, T. muris in the mouse, we have demonstrated that the dominant cytokine responses of the mesenteric lymph nodes (MLN) can be detected by sampling PBL. Resistant mice which mount a type 2 cytokine response in their MLN had PBL producing interleukin-4 (IL-4), IL-5, and IL-9, with negligible levels of gamma interferon (IFN-γ). Conversely, susceptible mice which mount a type 1 cytokine response in their MLN had PBL producing IFN-γ and negligible levels of type 2 cytokines. We have also shown that the PBL are capable of mounting a functional immune response against T. muris. PBL from immune mice were capable of transferring immunity to T. muris-infected severe combined immunodeficient (C.B-17 scid/scid) mice. Sampling PBL responses is therefore a viable option for monitoring human intestinal immune responses during T. trichiura infection in the field.
Studying human cellular immune responses during infection under field conditions poses many complications. This is especially true of parasites such as Trichuris trichiura, which reside specifically in the cecum and large intestine and hence stimulate a localized immune response. Studying the human cellular responses to this parasite either requires the taking of biopsies or the sampling of peripheral blood lymphocytes (PBL). Rectal biopsies from chronically infected children have shown increased numbers of mast cells, immunoglobulin E (IgE)-positive cells, tumor necrosis factor alpha (TNF-α+) cells, and evidence of increased numbers of monocytes (5, 14, 15). There was, however, no evidence of an effective cellular immune response against T. trichiura. No changes in the number of lamina propria CD3+ T cells were identified, and the number of intraepithelial CD3+ T cells decreased. A more effective method of investigating local T-cell responses would be to study the cellular responses occurring in the lymph nodes draining the mucosa, but this is largely impractical in human studies. The sampling of cellular responses of PBL provides an easier alternative, but it relies on the assumption that PBL responses accurately reflect those of the local intestinal responses. The reliance on this assumption is a very important issue, which to date has not been properly addressed.
Lymphocytes recirculate throughout the body from wherever they are initially activated; hence, parasite specific cells should be present within the blood for at least part of their life span. During mucosal immune responses, much of the initial antigen (Ag) presentation occurs in the Peyer's patches and mesenteric lymph nodes (MLN). Activated T and B cells then migrate to intestinal effector sites via the peripheral blood (1, 20). The recirculation of lymphocytes has been shown to be highly regulated, with different cell subsets following different migration patterns (4, 22). Naïve T cells tend to recirculate through the blood and lymph nodes, whereas effector/memory T cells migrate preferentially to tissue sites (16). CD4+ T cells, CD8+ T cells, and B cells have also been shown to follow different recirculation pathways (11, 23, 26, 27). The original hypotheses on the homing of lymphocytes revolved around the idea that lymphocytes were programmed to return to the site at which they were stimulated. Other workers have suggested that type 2 T cells tend to migrate preferentially to mucosa-associated tissue and that type 1 T cells home to peripheral organs and lymph nodes (19). Mucosa-associated sites are, however, capable of mounting a type 1 cytokine response, and type 2 cytokine responses are produced in the spleen (12, 24). Clearly, immune responses seen in the periphery may not necessarily represent those occurring at the mucosal sites, an assumption often made in the study of T-cell cytokine responses during human helminth infections.
The immune responses of mesenteric lymph node cells (MLNC) to T. muris have been well defined. T. muris occupies the same environmental niche as T. trichiura, is morphogenically similar, and is antigenically cross-reactive (21). Studies analyzing the Ag-specific MLNC cytokine responses to T. muris in different strains of mice have shown that the development of a type 2 CD4+ T-cell cytokine response is strongly associated with resistance (8, 10). Indeed T. muris infections in interleukin-4 (IL-4) and IL-13 knockout strains of mice have demonstrated the important roles these two cytokines play in expulsion (3). Conversely, development of a type 1 CD4+ T-cell cytokine response results in chronic infection, and depletion of gamma interferon (IFN-γ) will convert the phenotype of the host from susceptible to resistant. Such well-characterized local intestinal immune responses make this a good model for determining whether immune responses generated in peripheral sites give an accurate representation of the local mucosal immune responses. This in turn will reflect the relevance of monitoring the PBL response of humans infected with T. trichiura in the field.
We have compared the local MLNC cytokine responses to T. muris of resistant (BALB/K) and susceptible (AKR) H-2 haplotype-matched mice to the responses seen in peripheral sites, namely, the peripheral blood. This study demonstrates that the dominant cytokine responses produced in the MLN can also be detected by sampling PBL. In addition, we have transferred immunity to severe combined immunodeficient C.B-17 scid/scid (SCID) mice, using PBL from immune and naive BALB/c mice, showing that the parasite specific cells within the peripheral blood are immunologically functional.
MATERIALS AND METHODS
Animals.
Male AKR and BALB/K mice and male and female BALB/c mice were purchased from Harlan-Olac Ltd. (Bicestor, Oxon, United Kingdom) and were infected when 6 to 8 weeks old. C.B-17 scid/scid mice were bred and maintained in microisolator cages in the animal facility at the University of Manchester. The original breeding pairs were obtained from Charles River (Margate, Kent, United Kingdom). SCID mice were fed autoclaved food and water, and all manipulations were performed under laminar flow. Female mice were used when 4 to 12 weeks old.
Parasite and antigens.
T. muris life cycle was maintained within SCID mice. Experimental infections were performed with approximately 150 infective T. muris eggs, using oral gavage. The levels of infection were determined at each time point by counting the number of worms present in the large intestine and cecum. T. muris excretory/secretory (E/S) Ag were prepared as described by Artis et al. (2).
Preparation of MLNC and PBL.
MLN were removed from infected or uninfected mice and dissociated in Hanks' balanced salt solution (Gibco, Grand Island, N.Y.) supplemented with 2% fetal calf serum (Gibco), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (Gibco). MLNC were washed three times and resuspended at 5 × 106 cells/ml in RPMI 1640 medium (Gibco) containing 10% fetal calf serum, antibiotics as above, 2 mM l-glutamine (Gibco), and 7.5 × 10−5M monothioglycerol (Sigma-Aldrich, Poole, Dorset, United Kingdom). For the preparation of PBL, mice were exanguinated and the peripheral blood mixed 1:1 with phosphate-buffered saline supplemented with Dulbecco's A+B salts (Gibco) and heparin (20 IU/ml; CP Pharmaceuticals Ltd., Wrexham, United Kingdom). The PBL were isolated using density centrifugation; the blood was layered onto an equal volume of Histopaque 1083 (Sigma) and centrifuged at 500 × g for 20 min. The monocyte/lymphocyte layer was collected and treated as for the dissociated MLNC preparations.
In vitro restimulations and detection of cytokines.
Purified MLNC and PBL were dispensed into cluster plates (Nunclon, Roskilde, Denmark) with a maximum of 5 × 106 cells/well. T. muris E/S Ag were added to the relevant wells at a concentration of 50 μg/ml. Restimulation cultures were left at 37°C in 5% CO2 for approximately 28 h. Supernatants were harvested and standard sandwich enzyme-linked immunosorbent assay (ELISA) used to test for the presence of cytokines. IL-4, IL-5, and IFN-γ capture and biotinylated antibodies were obtained from Pharmingen (San Diego, Calif.). IL-9 capture antibody (229.4) was obtained from J. van Snick (Ludwig Institute for Cancer Research, Brussels, Belgium), and biotinylated IL-9 was bought from Pharmingen. The detection limit above background for each cytokine assay was calculated from 16 control wells incubated with culture medium alone as opposed to culture supernatants. Detection of a cytokine was considered significant only if its value lay above the average plus 3 standard deviations of the background level. Negligible levels of cytokines were seen when PBL and MLNC from each time point were cultured in the absence of Ag.
Adoptive transfer of immune and naive PBL.
T. muris-infected male or female BALB/c mice and uninfected littermates were sacrificed at day 20 or day 60 postinfection (p.i.). Worm burdens were counted to verify the resistant phenotype of the mice. The peripheral blood was pooled within groups, and the PBL were purified as above with the exception of being finally resuspended in Hanks' medium alone at a concentration of 108 cells/ml. SCID mice received 107 PBL intravenously or an equivalent volume of Hanks' medium and were infected with approximately 150 infective T. muris eggs 2 days later.
Statistical analysis.
Statistical differences for cytokine data, and worm burdens were calculated using the Mann-Whitney U test.
RESULTS
Comparison of type 2 cytokine production by MLNC and PBL during T. muris infection.
To determine whether the Ag-specific cytokine responses produced locally by the MLNC during T. muris infection can also be detected in the periphery, resistant BALB/K and susceptible AKR mice were infected with T. muris. At various time points p.i., MLNC were sampled as a source of immune cells draining the site of infection, and PBL were sampled as a source of peripheral immune cells. The cells were restimulated in vitro with T. muris E/S Ag, and cytokine production was quantified. Three type 2 cytokines, IL-4, IL-5, and IL-9, are strongly associated with a protective response against T. muris and were used in this study to verify the presence of a protective type 2 cytokine response (3, 10). IFN-γ was chosen as a marker for the development of a type 1 cytokine response and susceptibility. The numbers of PBL retrieved from individual mice were low, precluding the study of multiple cytokines, and for this reason we decided to pool the PBL within each group. This allowed us to analyze a type 1 and multiple type 2 cytokines to give a clear reflection of the ongoing immune responses against T. muris infection. The results shown for the MLNC and PBL in Fig. 1 to 3 show one representative experiment of two.
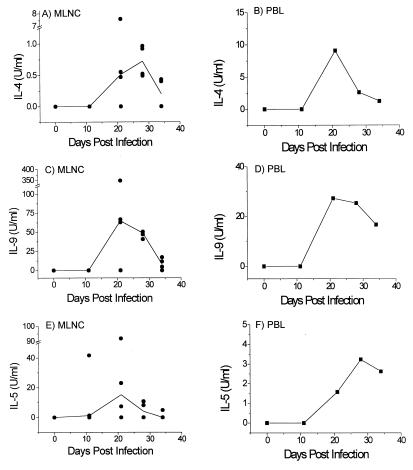
FIG. 1.
Type 2 cytokine production by BALB/K MLNC (A, C, and E) and PBL (B, D, and F) taken at various time points p.i. and restimulated in vitro with T. muris E/S Ag (n = 4 for each time point). Day 0 refers to uninfected control animals. In panels A, C, and E, solid lines show median values and circles represent individual animals. PBL at all time points, and MLNC at day 0, were pooled within groups.
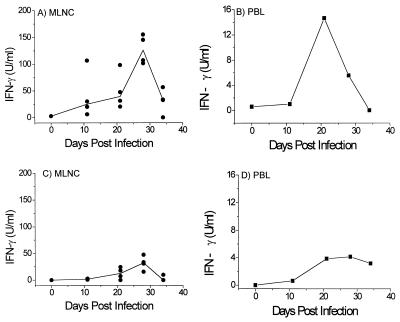
FIG. 3.
IFN-γ production by AKR MLNC and PBL (A and B, respectively) and BALB/K MLNC and PBL (C and D, respectively) taken at various time points p.i. and restimulated in vitro with T. muris E/S Ag. In panels A and C, solid lines show median values and circles refer to individual animals. PBL at all time points, and MLNC at day 0, were pooled within groups; n = 4 for BALB/K time points and for all AKR time points except for the uninfected controls at day 0, where n = 3.
In all experiments, the phenotypes of the susceptible AKR and resistant BALB/K mice were verified by counting the gut worm burdens. In agreement with previous work, AKR mice showed no reduction in worm burden and had fully developed adult worms after day 32 p.i. (10). BALB/K mice expelled the majority of worms by day 21 p.i. and had no worms after day 32 p.i. The AKR mice mounted a general type 1 response with high serum levels of parasite specific IgG2a and low specific IgG1. In contrast, the BALB/K mice had low levels of specific IgG2a and high specific IgG1 indicative of a type 2 response (results not shown).
The MLNC of the BALB/K mice produced type 2 cytokines characteristic of T. muris infection (8, 10) and shown typically for IL-4, IL-9, and IL-5 in Fig. 1A, C, and E. Levels of Ag-specific IL-4, IL-9, and IL-5 peaked at days 21 and 28 p.i. and then decreased as infection cleared. The level of IL-4 production in individual mice related to their production of IL-5 and IL-9, with the mice producing high IL-4 also producing high levels of IL-9 and IL-5, and vice versa.
In both experiments, Ag-specific type 2 cytokines were produced at significant levels by the purified PBL, typically from day 21 onward (Fig. 1B, D, and F). IL-5 and IL-9 were produced at lower levels by the PBL than the MLNC at days 21 and 28 p.i., although by day 34 p.i. similar levels were produced by both cell populations. The PBL tended to produce higher levels of IL-4 than the MLNC particularly at day 21 p.i., although at this time point the MLNC from one mouse did produce IL-4 levels approaching that of the PBL. The production of relatively low levels of IL-4 by MLNC is often seen in this system, and higher levels of IL-4 are detected only if antibodies against the IL-4 receptor are added to the restimulation cultures preventing the responding cells from taking up the bioavailable IL-4 (3, 10). Although the PBL would be expected to produce lower levels of IL-4 than the MLNC due to a lower proportion of responding T cells in the PBL cultures, the lower number of cells responding to stimulation would also result in a lower demand for IL-4, possibly explaining the higher levels of bioavailable IL-4 detected in the PBL cultures.
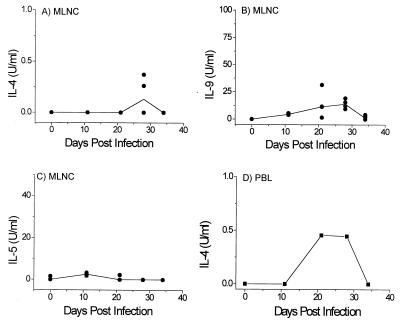
The MLNC of the susceptible AKR mice produced significantly lower IL-4 (Fig. 2A and 1A; P = 0.014 at days 21 and 28 p.i.), IL-9 (Fig. 2B and 1C; P = 0.014 at days 21 and 28 p.i.), and IL-5 (Fig. 2C and 1E; P = 0.014 at days 21 and 28) than the BALB/K mice. Early low levels of IL-5 and IL-9 were produced by the AKR MLNC at day 11 when the BALB/K MLNC were not producing detectable levels of cytokines (Fig. 2B and C). This early cytokine response failed to develop into a strong type 2 response, as thereafter IL-9 production remained low, IL-4 was produced only at day 28 p.i., and negligible levels of IL-5 were detected. The PBL failed to produce any detectable IL-9 or IL-5, but in one of two experiments they did produce low levels of IL-4 at days 21 and 28 p.i. (Fig. 2D).
FIG. 2.
Type 2 cytokine production by AKR MLNC (A to C) and IL-4 production by AKR PBL (D) taken at various time points p.i. and restimulated in vitro with T. muris E/S Ag (n = 4 for each time point except for uninfected controls, where n = 3). Day 0 refers to uninfected control animals. In panels A to C, solid lines show median values and circles represent individual animals. PBL at all time points, and MLNC at day 0, were pooled within groups.
Comparison of IFN-γ production by MLNC and PBL during T. muris infection.
Type 1 responses were also examined locally and peripherally via analysis of IFN-γ production. The MLNC of the susceptible AKR mice produced significantly higher levels of IFN-γ than those of the BALB/K mice (Fig. 3A and C; P = 0.029 at day 21 p.i.; P = 0.014 at day 28 p.i.). Peak levels of IFN-γ production by the MLNC from infected AKR mice occurred at days 21 and 28 p.i., and IFN-γ was produced by the PBL of the AKR mice, also peaking in both experiments at day 21 p.i. (Fig. 3B). Typically low or undetectable levels of IFN-γ were produced by the BALB/K PBL (Fig. 3D).
Transfer of immune PBL from resistant BALB/c mice into immunodeficient SCID mice results in protective immunity.
Having shown that the Ag-specific cytokine responses found in the periphery qualitatively reflect the local draining lymph node responses, we wanted to determine whether cells in the PBL compartment were capable of mounting a functional immune response. Thus, PBL were taken from infected and naive BALB/c mice that have a resistant phenotype and transferred into T- and B-cell-immunodeficient SCID mice which were subsequently infected with T. muris.
PBL were first taken from male BALB/c mice at day 20 p.i. (PBL-20), as previous work showed that MLNC taken at this time point can transfer immunity (9, 25). SCID mice receiving immune PBL-20 had significantly reduced worm burdens at day 16 p.i. compared to infected nonreconstituted SCID mice (P = 0.028), whereas the SCID mice receiving naive PBL showed no reduction in worm numbers at day 16 p.i. (Table 1). To determine whether Ag-specific cells were still present in the periphery after infection had cleared, we took PBL from female BALB/c mice 60 days p.i. (PBL-60). Male and female BALB/c mice do not differ in their ability to expel T. muris, both being strongly resistant, and at this time point the mice will have been clear of worms for at least 30 days (7). The SCID mice receiving PBL-60 showed faster expulsion kinetics than the SCID mice receiving naive PBL (Table 2). At day 15 p.i., the SCID mice receiving PBL-60 had significantly reduced worm burdens (P = 0.028) whereas the SCID mice receiving naive PBL showed no reduction in worm numbers. Thus, immune PBL taken either during or after infection are capable of transferring immunity to immunodeficient SCID mice.
TABLE 1.
Worm burdens at day 16 p.i. of T. muris-infected SCID mice reconstituted with PBL from infected BALB/c mice taken 20 days p.i., from naïve uninfected BALB/c littermates, or from nonreconstituted controls
| Treatment | No. of worms recovered
|
|
|---|---|---|
| From individual mice | Median | |
| Infection alone | 108, 128, 143, 146 | 135.5 |
| Infection + immune PBL | 22, 23, 67 | 23a |
| Infection alone | 70, 100, 132, 134 | 116 |
| Infection + naive PBL | 108, 160, 170 | 160 |
Significantly lower worm burden (P < 0.05) than in the infected controls.
TABLE 2.
Worm burdens at day 15 p.i. of T. muris-infected SCID mice reconstituted with PBL from immune BALB/c mice taken 60 days p.i. from naive uninfected BALB/c littermates or nonreconstituted controls
| Treatment | No. of worms recovered
|
|
|---|---|---|
| From individual mice | Median | |
| Infection alone | 48, 145, 165, 230 | 155 |
| Infection + immune PBL | 10, 11, 23 | 11a |
| Infection + naive PBL | 105, 152 | 128.5 |
Significantly lower worm burden (P < 0.05) than in the infected controls.
DISCUSSION
The study of human immune responses to parasitic infections under field conditions is a very complex task both logistically and ethically. One problem in such studies is that often the infection resides in an organ or tissue that is inaccessible for sampling. This is particularly true for the intestinal nematode T. trichiura, which lives specifically in the cecum and large intestine and so will stimulate a localized immune response. Rectal biopsies have been performed on chronically infected children but have not provided any evidence of an effective T-cell response (5, 14, 15). As in many human studies, the only other practical site from which to sample cellular immune responses is the peripheral blood, and this has been done successfully for several human helminths (13, 17, 18). Sampling PBL, however, relies on the assumption that the immune responses of the peripheral blood reflect those acting locally at the site of infection. This assumption has not been adequately tested. Using the well-defined model of T. trichiura, T. muris in the mouse, we have demonstrated that the dominant cytokine responses produced locally by MLNC draining the site of infection can also be detected by sampling PBL. Hence, sampling PBL cytokine responses is a viable option for monitoring local gut immune responses.
In our experiments the MLNC from resistant BALB/K mice produced a dominant type 2 cytokine response consisting of IL-4, IL-5, and IL-9, whereas the susceptible AKR mice produced a dominant type 1 response, namely, IFN-γ. This polarization of MLNC cytokine production to T. muris by resistant and susceptible mouse strains has been well documented in the literature (8, 10). These cytokine responses were qualitatively reflected in the PBL compartment, with the BALB/K PBL producing IL-4, -5, and -9 and negligible levels of IFN-γ. Conversely the PBL from infected AKR mice produced IFN-γ with negligible levels of type 2 cytokines. The cytokine responses of both the MLNC and PBL were detected from day 21 p.i. onward, showing that there is no delay in the ability to detected the cytokine responses in the periphery.
Although this study shows the potential for sampling the cytokine responses of PBL as an indication of the intestinal immune responses, the complexities of studying human infections still need to be considered. Individuals are often infected with multiple parasitic infections, posing problems with responses against potentially cross-reactive Ag and the possibility of one parasite modulating the cytokine response of another. Work studying Schistosoma mansoni and T. muris coinfections have shown that infecting a susceptible strain of mouse with T. muris during a concurrent S. mansoni infection results in the expulsion of T. muris (6). This is associated with the down-regulation of the type 1 response against T. muris, presumably due to the ongoing type 2 response induced against S. mansoni eggs. Thus, the ability of one infection to modulate the response against a second infection is a real issue. These arguments, however, apply to any human study and could also be raised if it were possible to directly sample MLNC of humans. A different consideration is also raised by intestinal parasites which do not just reside in the intestine but migrate to different internal organs and tissues within the body. In this case, sampling the PBL response will sample a component of the immune response raised at each tissue or organ site. These questions are a problem in human studies, but the careful selection of Ag for restimulation, knowledge of the parasites life cycle, and verification of the infection status of the subject will help to minimize these problems.
This study also demonstrated that the peripheral cellular response did not just phenotypically reflect the local MLN response but was immunologically functional. PBL taken from naturally resistant male and female BALB/c mice infected with T. muris were capable of transferring immunity to SCID mice lacking T and B cells. SCID mice receiving immune PBL expelled a large proportion of their worm burden by day 15 or 16 p.i., whereas no expulsion at these time points was seen in SCID mice receiving naive PBL. Resistance was transferred when BALB/c PBL were sampled during the memory phase of the response against T. muris (day 60 p.i.), as well as during the effector stage (day 20 p.i.). This demonstrates that functional parasite-specific memory cells were still recirculating in the peripheral blood after the parasite had been expelled.
Overall, this study shows that sampling PBL immune responses is a viable method of monitoring the local intestinal immune responses. The major cytokine responses of the MLNC were produced by PBL, and both effector-stage and memory-stage PBL from immune BALB/c mice were capable of transferring resistance to SCID mice recipients. Parasite-specific cells therefore do not remain localized at the intestinal site of the infection and can be detected in the periphery as they recirculate through the peripheral blood.
ACKNOWLEDGMENTS
We thank Richard Grencis for discussion and paper critique; we also thank the staff of the BSU at the University of Manchester for animal maintenance.
This work was supported by the Wellcome Trust (grants 44494/Z/95/Z and 047366/Z/96/Z.
REFERENCES
- 1.Abreu-Martin M T, Targan S R. Regulation of immune responses of the intestinal mucosa. Crit Rev Immunol. 1996;16:277–309. doi: 10.1615/critrevimmunol.v16.i3.30. [DOI] [PubMed] [Google Scholar]
- 2.Artis D, Potten C S, Else K J, Finkelman F D, Grencis R K. Trichuris muris: host intestinal epithelial cell hyperproliferation during chronic infection is regulated by Interferon-γ. Exp Parasitol. 1999;92:144–153. doi: 10.1006/expr.1999.4407. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft A, McKenzie A N, Grencis R K. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 4.Bradley L M, Watson S R. Lymphocyte migration into tissue: the paradigm derived from CD4 subsets. Curr Opin Immunol. 1996;8:312–320. doi: 10.1016/s0952-7915(96)80118-x. [DOI] [PubMed] [Google Scholar]
- 5.Cooper E S, Spencer J, Whyte-Alleng C A, Cromwell O, Whitney P, Venugopal S, Bundy D A P, Haynes B, MacDonald T T. Immediate hypersensitivity in colon of children with chronic Trichuris trichiura dysentery. Lancet. 1991;338:1104–1107. doi: 10.1016/0140-6736(91)91964-v. [DOI] [PubMed] [Google Scholar]
- 6.Curry A J, Else K J, Jones F, Bancroft A, Grencis R K, Dunne D W. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J Exp Med. 1995;181:769–774. doi: 10.1084/jem.181.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Else K, Wakelin D. Genetic variation in the humoral immune responses of mice to the nematode Trichuris muris. Parasite Immunol. 1989;11:77–90. doi: 10.1111/j.1365-3024.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 8.Else K J, Finkelman F D, Maliszewski C R, Grencis R K. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Else K J, Grencis R K. Antibody-independent effector mechanisms in resistance to the intestinal nematode parasite Trichuris muris. Infect Immun. 1996;64:2950–2954. doi: 10.1128/iai.64.8.2950-2954.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Else K J, Hültner L, Grencis R K. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of Th-cell subsets in resistant versus susceptible mice. Immunology. 1992;75:232–237. [PMC free article] [PubMed] [Google Scholar]
- 11.Freitas A A, Rose M, Rocha B. Random recirculation of small T lymphocytes from thoracic duct lymph in the mouse. Cell Immunol. 1980;56:29–39. doi: 10.1016/0008-8749(80)90078-7. [DOI] [PubMed] [Google Scholar]
- 12.James S P, Kwan W C, Sneller M C. T cells in inductive and effector compartments of the intestinal mucosal immune system of nonhuman primates differ in lymphokine mRNA expression, lymphokine utilization, and regulatory function. J Immunol. 1990;144:1251–1256. [PubMed] [Google Scholar]
- 13.King C L, Mahanty S, Kumaraswami V, Abrams J S, Regunathan J, Jayaraman K, Ottesen E A, Nutman T B. Cytokine control of parasite-specific anergy in human lymphatic filariasis. J Clin Investig. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald T T, Choy M Y, Spencer J, Richman P I, Diss T, Hanchard B, Venugopal S, Bundy D A P, Cooper E S. Histopathology and immunohistochemistry of the caecum in children with the Trichuris dysentery syndrome. J Clin Pathol. 1991;44:194–199. doi: 10.1136/jcp.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald T T, Spencer J, Murch S H, Choy M Y, Venugopal S, Bundy D A P, Cooper E S. Immunoepidemiology of intestinal helminthic infections. 3. Mucosal macrophages and cytokine production in the colon of children with Trichuris trichiura dysentery. Trans R Soc Trop Med Hyg. 1994;88:265–268. doi: 10.1016/0035-9203(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 16.Mackay C R, Marston W L, Dudler L, Spertini O, Teddler T F, Hein W R. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 17.Mahanty S, King C L, Kumaraswami V, Regunathan J, Maya A, Jayaraman K, Abrams J S, Ottesen E A, Nutman T B. IL-4- and IL-5-secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151:3704–3711. [PubMed] [Google Scholar]
- 18.Malaquias L C C, Falcão P L, Silveira A M S, Gazzinelli G, Prata A, Coffman R L, Pizziolo V, Souza C P, Colley D G, Correa-Oliveira R. Cytokine regulation of human immune responses to Schistosoma mansoni: analysis of the role of IL-4, IL-5 and IL-10 on peripheral blood mononuclear cell responses. Scand J Immunol. 1997;46:393–398. doi: 10.1046/j.1365-3083.1997.d01-136.x. [DOI] [PubMed] [Google Scholar]
- 19.Meeusen E N T, Premier R R, Brandon M R. Tissue-specific migration of lymphocytes: a key role for Th1 and Th2 cells? Immunol Today. 1996;17:421–424. doi: 10.1016/0167-5699(96)10055-4. [DOI] [PubMed] [Google Scholar]
- 20.Mowat A M, Viney J L. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 21.Roach T I, Wakelin D, Else K J, Bundy D A P. Antigenic cross-reactivity between the human whipworm, Trichuris trichiura, and the mouse trichuroids Trichuris muris and Trichinella spiralis. Parasite Immunol. 1988;10:279–291. doi: 10.1111/j.1365-3024.1988.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 23.Stevens S K, Weissman I L, Butcher E C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J Immunol. 1982;128:844–851. [PubMed] [Google Scholar]
- 24.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissue. Frequencies of CD4+ and CD8+ T cells that secrete IFN-γ and IL-5. J Immunol. 1990;145:68–77. [PubMed] [Google Scholar]
- 25.Wakelin D. Immune expulsion of Trichuris muris from mice during a primary infection: analysis of the components involved. Parasitology. 1975;70:397–405. doi: 10.1017/s0031182000052173. [DOI] [PubMed] [Google Scholar]
- 26.Washington E A, Kimpton W G, Cahill R N P. CD4+ lymphocytes are extracted from blood by peripheral lymph nodes at different rates from other T cell subsets and B cells. Eur J Immunol. 1988;18:2093–2096. doi: 10.1002/eji.1830181235. [DOI] [PubMed] [Google Scholar]
- 27.Witherden D A, Kimpton W G, Washington E A, Cahill R N P. Non-random migration of CD4+, CD8+ and γδ+T19+ lymphocytes through peripheral lymph nodes. Immunology. 1990;70:235–240. [PMC free article] [PubMed] [Google Scholar]