Abstract
Objective
Advanced hybrid coronary revascularization is the integration of sternal-sparing multivessel coronary artery bypass grafting and percutaneous coronary intervention in patients with multivessel coronary artery disease. We sought to review our advanced hybrid coronary revascularization experience over an 8.5-year period using robotic totally endoscopic coronary artery bypass with bilateral internal thoracic artery grafts and percutaneous coronary intervention.
Methods
From August 2013 to February 2022, 664 patients underwent robotic totally endoscopic coronary artery bypass at our institution. Of the 293 patients who underwent totally endoscopic coronary artery bypass assigned to a hybrid revascularization strategy, 156 patients received bilateral internal thoracic artery grafts and are the subject of this review. Patients underwent percutaneous coronary intervention with drug-eluting stents before or after totally endoscopic coronary artery bypass. We reviewed early and midterm outcomes (up to 8 years) in this cohort of patients with intent-to-treat advanced hybrid coronary revascularization.
Results
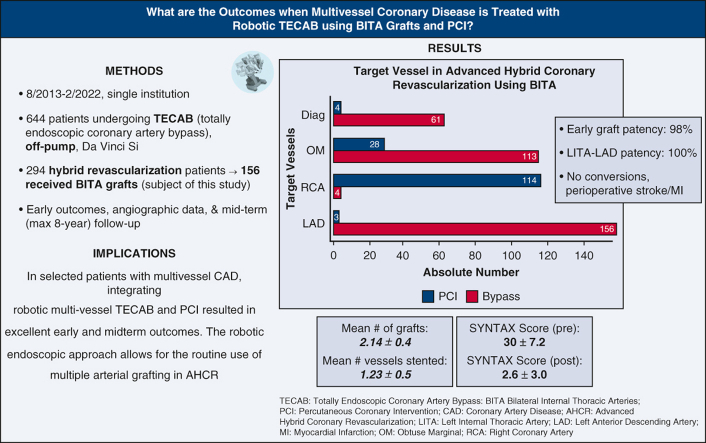
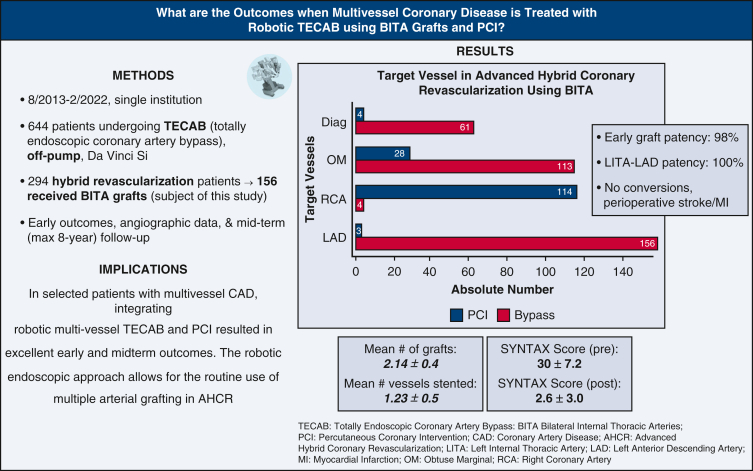
The mean age of patients was 65 ± 10 years. The mean Society of Thoracic Surgeons predicted risk of mortality was 1.26 ± 1.56. Triple-vessel disease occurred in 94% of patients, and 17% of patients had 70% or more left-main disease. The mean operative time was 311 ± 54 minutes, and the mean hospital length of stay was 2.7 ± 1.1 days. All patients had bilateral internal thoracic artery grafts; the total number of grafts was 334. Eight seven percentage of patients had totally endoscopic coronary artery bypass ×2, and 13% of patients had totally endoscopic coronary artery bypass ×3. One patient received totally endoscopic coronary artery bypass ×4. The mean number of grafts per patient was 2.14 ± 0.4, and the mean number of vessels stented was 1.23 ± 0.5. There were no conversions, perioperative stroke, or myocardial infarction. Early mortality occurred in 2 patients. Early graft patency was 98% (209/214 grafts); left internal thoracic artery to left anterior descending patency was 100% (66/66 grafts). At 8-year follow-up in 155 patients (mean 39 ± 26 months), all-cause and cardiac-related mortality were 11.6% and 3.9%, respectively. Freedom from major adverse cardiac/cerebrovascular events including repeat revascularization was 94%.
Conclusions
In patients with multivessel coronary artery disease, integrating robotic totally endoscopic coronary artery bypass with bilateral internal thoracic artery and percutaneous coronary intervention resulted in excellent early and midterm outcomes. Further studies are warranted.
Key Words: bilateral internal thoracic arteries, coronary artery bypass, hybrid revascularization, off-pump, percutaneous coronary intervention, robotic, TECAB
Abbreviations and Acronyms: AHCR, advanced hybrid coronary revascularization; BITA, bilateral internal thoracic artery; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; DAPT, dual-antiplatelet therapy; HCR, hybrid coronary revascularization; LAD, left anterior descending artery; LITA, left internal thoracic artery; LOS, length of stay; MACCE, major adverse cardiac/cerebrovascular events; MAG, multi-arterial grafting; MI, myocardial infarction; MIDCAB, minimally invasive direct coronary artery bypass; PCI, percutaneous coronary intervention; RCA, right coronary artery; RITA, right internal thoracic artery; SITA, single internal thoracic artery; TECAB, totally endoscopic coronary artery bypass
Graphical abstract
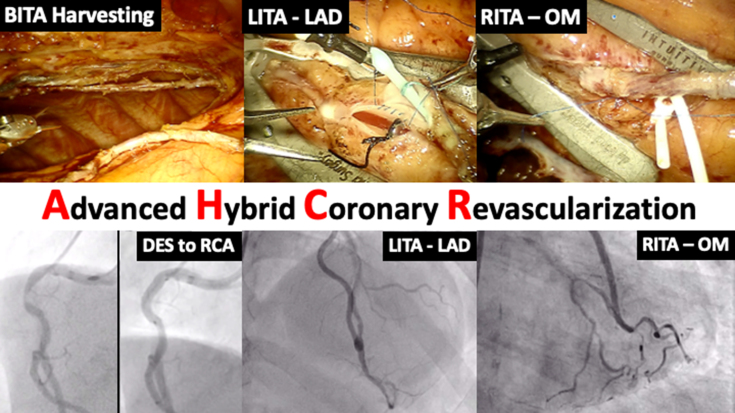
Outcomes of robotic TECAB with BITAs and PCI in patients with multivessel coronary disease.
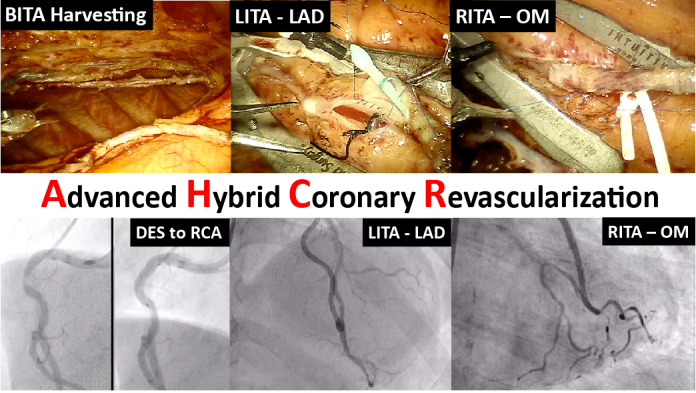
AHCR: Robotic beating-heart multivessel TECAB with BITA grafts. PCI and graft angiography. BITA, Bilateral internal thoracic artery; LITA, left internal thoracic artery; LAD, left anterior descending artery; RITA, right internal thoracic artery; OM, obtuse marginal artery; DES, Drug-eluting stent; RCA, right coronary artery.
Central Message.
In selected patients with multivessel CAD, robotic hybrid revascularization with BITAs and stents is safe and feasible.
Perspective.
Advanced hybrid coronary artery revascularization using robotic totally endoscopic coronary artery bypass with BITAs and stents is safe and feasible in selected patients with multivessel CAD.
Hybrid coronary revascularization (HCR) is designed to treat patients with multivessel coronary artery disease (CAD) by integrating sternal-sparing minimally invasive coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI). The safety and efficacy of the procedure have been reported to be similar compared with conventional CABG or multivessel PCI in randomized trials.1, 2, 3 The rational of HCR is to provide benefits of the long-term durability of surgical left internal thoracic artery (LITA) anastomosis to the left anterior descending (LAD) artery and less invasiveness of drug-eluting stents for the non-LAD lesions to achieve complete revascularization. Long-term observational studies have shown improved outcomes with a multi-arterial grafting (MAG) strategy, especially when bilateral internal thoracic artery (BITA) conduits are used.4 The term “advanced hybrid coronary revascularization” (AHCR) was coined to describe the concept of using 2 internal thoracic artery grafts as the surgical arm of HCR combined with PCI to achieve complete revascularization in patients with multivessel disease.5 Since 2013, we have performed robotic beating-heart totally endoscopic coronary artery bypass grafting (TECAB) as a standard surgical revascularization procedure for multivessel CAD.6,7 We have previously reported on the safety of AHCR using multivessel TECAB with PCI.8 Theoretically, this approach could be the most feasible option within other sternal-sparing approaches as the surgical arm of AHCR, because of the ease of harvesting BITA grafts and accessing left coronary targets on the posterolateral wall. In this study, we describe our AHCR strategy using robotic beating-heart TECAB with BITA grafts combined with PCI over an 8.5-year period for patients with multivessel CAD, and report on early and midterm clinical outcomes and angiographic patency.
Patients and Methods
Study Population
This is a single-center retrospective study. Between August 2013 and February 2022, 644 patients underwent robotic off-pump TECAB by a single experienced robotic team. A total of 293 patients were assigned to an intention-to-treat hybrid revascularization strategy, of whom 156 underwent AHCR with BITA grafting. We performed a retrospective review from our prospectively collected database with Institutional Review Board approval (#18-0742; date of approval 4/28/2020). Individual patient consent was waived given this was a de-identified retrospective study. Preoperative characteristics, intraoperative data, and postoperative outcomes were analyzed. Angiographic data were reviewed when available (in patients undergoing PCI after TECAB). Midterm clinical follow-up was completed by phone calls, email, or family/primary cardiologist contact, which we do for all TECAB patients on an annual basis. Major adverse cardiac/cerebrovascular events (MACCE) included cardiac-related death, myocardial infarction (MI), repeat cardiac surgery, and repeat revascularization.
Selection Criteria
Patients are referred to our robotic program or are self-referred seeking a sternal-sparing option for CABG and therefore are self-selected for this approach. Given we are a high-volume referral center for TECAB, all patients with multivessel CAD are initially considered for HCR. The AHCR approach is selected after extensive discussion among the heart team, consisting of the robotic cardiac surgery and interventional cardiology teams. Selection criteria are based on individual coronary anatomy and lesion complexity, notably those with left-sided coronary disease amenable to grafting by multivessel TECAB with BITA, and right coronary artery (RCA), or distal left circumflex artery (LCx) lesions amenable to PCI. The only absolute exclusion criterion for TECAB is fused pleural space from prior left thoracotomy or pulmonary resection. Chronic total occlusions of the RCA and distal LCx artery are not exclusion criteria and are discussed among the heart team as to the best option for revascularization. If PCI lesions are thought not to be suitable for atherectomy and or other advanced techniques, patients are considered for open CABG.
Hybrid Revascularization Approach
We have previously described our hybrid and advanced hybrid strategy8 in detail. PCI for hybrid revascularization is done before, simultaneous to, or after TECAB (most common). Duration of dual-antiplatelet therapy (DAPT) is at the discretion of the treating interventional cardiologist and in most cases informed by the type, caliber, number, and location of stents placed as well as the complexity of the percutaneous revascularization. In general, 1 year of DAPT was preferable for most patients and DAPT interruption was allowable after 3 months. Most patients who underwent PCI first received drug-eluting stents with DAPT interruption 3 to 6 months later. Graft patency is assessed using the Fitzgibbon classification when PCI is performed after TECAB.
Surgical Technique
We have previously described our surgical technique in detail.6,7,9 All procedures were performed using the da Vinci Si Surgical robot (Intuitive) on a beating heart. All procedures are planned to be off-pump. In this series, all patients received multivessel bypass with BITA grafting. The anastomotic technique early in this series was with the C-Port Flex-A distal coronary anastomotic stapler, until it was taken off the market in 2018 after which a robotic hand-sewn anastomosis technique was used. The LAD is most often bypassed using the LITA, and less commonly the right internal thoracic artery (RITA). Our primary aim is usually to preserve the LITA-LAD configuration and graft the in situ RITA to the remaining target (LCx branches, ramus, diagonal). In cases where the RITA cannot reach the second target, we perform RITA-LAD and use the LITA for the lateral wall target(s). If the in situ RITA can reach neither, we use it as a Y graft to the lateral wall. Transit time flowmetry is checked after anastomosis completion using the Medistim device (Medistim) Video 1.
Percutaneous Coronary Intervention Strategy and Technique
Patients are discussed in a weekly multidisciplinary meeting to first determine the appropriateness for and sequence of AHCR. The last coronary angiogram before referral for TECAB is reviewed to determine the patient's baseline Synergy between Percutaneous coronary interventions with Taxus and Cardiac Surgery (SYNTAX) score and which lesions are unlikely to be revascularizable surgically. If TECAB-inaccessible lesions are deemed high risk, as in the case of lesions requiring atherectomy, intravascular lithotripsy, or chronic total occlusion interventions, the decision was often made to perform the PCI before TECAB. This approach was also used in the setting of critical RCA disease where septal collaterals from the LAD (“donor vessel”) could be jeopardized at the time of snaring the LAD for bypass. This sequence allowed for the option of grafting any failed PCI vessels, vis-à-vis modifying the TECAB surgical plan or converting to conventional (open) CABG. Patients with elevated residual SYNTAX score (>10) after TECAB were sometimes stratified to staged PCIs over 2 procedures to reduce radiation, time, and contrast use.
The PCI portion of the hybrid revascularization was performed using conventional radial access in the majority of cases and femoral approach in the minority. Calcium modification was performed in highly calcified or fibrocalcific lesions using orbital atherectomy (Diamondback 360, Cardiovascular Systems, Inc), rotational atherectomy (Rotablator, Boston Scientific), laser atherectomy (ELCA, Spectranetics), scoring balloon angioplasty (Angiosculpt, Spectranetics), and intravascular lithotripsy (Shockwave). Mechanical circulatory support via Impella (Abiomed) or intra-aortic balloon pump was provided for patients with poor systolic function and complex lesions.
Statistical Analysis
Continuous variables were tested for normality using the Shapiro–Wilk test. Those with normal distribution are expressed as mean ± standard deviation and those without as median (interquartile range). Categorical and sequential variables are expressed as the number and percentage of patients. Kaplan–Meier analysis was applied for midterm survival and freedom from MACCE. The statistical analyses were conducted using IBM SPSS 25.00 (IBM, Inc).
Results
Over the 8.5-year study period, 664 robotic beating-heart TECABs were performed. A total of 293 patients were intention-to-treat hybrid revascularization. Of these, 156 patients received BITA grafts during TECAB.
Demographics are shown in Table E1. Mean STS predicted mortality risk score was 1.26% ± 1.56%. Mean age was 65 years, and 14% were women. Comorbidities included 84% with hypertension, 38% with diabetes, 7% with peripheral vascular disease, 24% with prior MI, and 20% with chronic kidney disease. A total of 27 patients (17%) had ejection fraction less than 40%, 94% had triple-vessel disease, and 17% had significant left main disease.
The majority of patients (87%) underwent double-vessel TECAB, 13% underwent triple-vessel TECAB, and 1 patient underwent quadruple-vessel TECAB. Three patients required use of cardiopulmonary bypass for hemodynamic support due to difficult target exposure during the case (done using peripheral femoral cannulation); the remainder were performed off-pump. All cases were completed on a beating-heart. Intraoperative blood transfusion requirement was 6%. Mean operative time was 311 minutes. There were no conversions to sternotomy (Tables 1 and 2).
Table 1.
Intraoperative data
| Variables | All patients (n = 156) |
|---|---|
| TECAB 2 times, n (%) | 135 (86.5) |
| TECAB 3 times, n (%) | 20 (13) |
| TECAB 4 times, n (%) | 1 (0.5) |
| Operative time (min), mean ± SD | 311 ± 54 |
| BITA use, n (%) | 156 (100) |
| Concomitant procedure | 11 (7.1) |
| CPB use, n (%) | 3 (1.9) |
| Inotrope requirement, n (%) | 3 (1.9) |
| Intraoperative BTF use, n (%) | 10 (6.4) |
| Conversion, n (%) | 0 (0.0) |
| OR extubation, n (%) | 64 (41) |
TECAB, Totally endoscopic coronary artery bypass surgery; SD, standard deviation; BITA, bilateral internal thoracic artery; CPB, cardiopulmonary bypass; BTF, blood transfusion; OR, operating room.
Table 2.
Graft information
| Variables | Total grafts (n = 334) |
|---|---|
| Grafts per patient, mean ± SD | 2.14 ± 0.4 |
| Anastomosis technique | |
| Anastomotic device, n (%) | 178 (53) |
| Sutured, n (%) | 151 (45) |
| U-Clip constructed anastomosis, n (%) | 5 (2) |
| LITA flow, mean ± SD | 85 ± 45 |
| LITA PI, mean ± SD | 1.4 ± 0.5 |
| RITA flow, mean ± SD | 73 ± 37 |
| RITA PI, mean ± SD | 1.5 ± 0.6 |
| SYNTAX Scores | |
| Pre-AHCR | 30 ± 7.2 |
| Post-AHCR | 2.6 ± 3.0 |
SD, Standard deviation; LITA, left internal thoracic artery; RITA, right internal thoracic artery; PI, pulsatility index; SYNTAX, Synergy between Percutaneous coronary interventions with Taxus and Cardiac Surgery; AHCR, advanced hybrid coronary revascularization.
The mean number of grafts per patient was 2.14 ± 0.4, and mean number of target vessels stented was 1.23 ± 0.5 per patient. Tables E2 and E3 show the details on graft construction and stent types/location, respectively. Regarding the hybrid procedure, TECAB was planned first in 137 patients (87%), and PCI first in 19 patients (13%). In the TECAB first group, 6 patients had an unsuccessful attempt at PCI; 11 patients declined PCI after TECAB; 17 patients did not undergo PCI because of a change in management plan (because the target intended for PCI was grafted during TECAB or due to negative stress test postoperatively).
The mean hospital and ICU length of stay (LOS) were 2.7 ± 1.1 and 1.3 ± 0.7 days, respectively, after TECAB. Eleven percentage required postoperative blood transfusion, 10% had new-onset atrial fibrillation, 1% had pericarditis, and 3 patients required reintubation. There were no MIs or stroke. There was 1 return to the OR for bleeding. Mortality occurred in 2 patients with an observed/expected ratio of 1.02 (Table 3). Readmission within 30 days was 7%. Mean time to return to full activities and work was 15 and 19 days, respectively (Table E4).
Table 3.
Early postoperative outcomes and adverse events
| Postoperative variables | All patients (n = 156) |
|---|---|
| Extubation within 6 h, n (%) | 129 (83) |
| Postoperative BTF use, n (%) | 17 (11) |
| Chest tube drainage total (mL), mean ± SD | 772 ± 584 |
| ICU LOS (d), mean ± SD | 1.30 ± 0.65 |
| Hospital LOS (d), mean ± SD | 2.73 ± 1.13 |
| Mortality, O/E | 1.02 |
| Postoperative adverse events | |
| Reintubation, n (%) | 3 (2.0) |
| Prolonged ventilation (>24 h), n (%) | 0 (0.0) |
| Wound infection, n (%) | 0 (0.0) |
| Acute kidney injury, n (%) | 5 (3.2) |
| Pericarditis, n (%) | 2 (1.3) |
| MI, n (%) | 0 (0.0) |
| New atrial fibrillation, n (%) | 16 (10) |
| Stroke or TIA, n (%) | 0 (0.0) |
| Reexploration for bleeding, n (%) | 1 (0.6) |
BTF, Blood transfusion; SD, standard deviation; ICU, intensive care unit; LOS, length of stay; O/E, observed/expected; MI, myocardial infarction; TIA, transient ischemic attack.
Mean preoperative SYNTAX score was 30 ± 7.2. Mean residual SYNTAX score after AHCR was 2.6 ± 3.0. Mean time to catheterization for patients undergoing PCI after TECAB was 1.78 months. Overall graft patency was 98% (209 patent grafts of 214 imaged—204 and 5 grafts Fitzgibbon A and B, respectively). RITA patency was 96% (100/104 grafts). LITA patency to all targets was 99% (109/110 grafts), with 100% LITA-LAD patency (Table 4).
Table 4.
Early angiographic graft patency
| Variables | |
|---|---|
| Time to catheterization (mo), mean ± SD | 1.78 ± 2.9 |
| Total ITA grafts imaged, n | 214 |
| Graft patency, n/total (%) | 209/214 (98) |
| LITA patency, n/110 total grafts (%) | 109 (99) |
| RITA patency, n/104 total grafts (%) | 100 (96) |
| LITA-LAD patency, n/66 total grafts (%) | 66 (100) |
SD, Standard deviation; ITA, internal thoracic artery; LITA, left internal thoracic artery; RITA, right internal thoracic artery; LAD, left anterior descending.
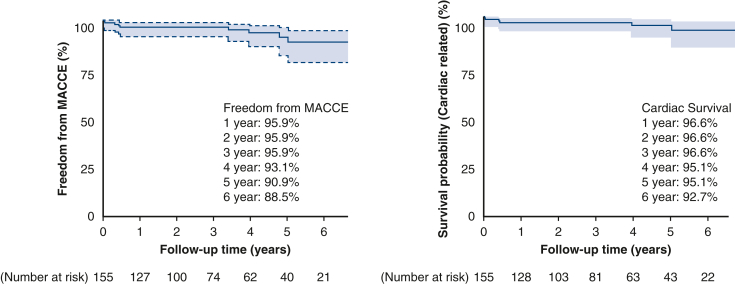
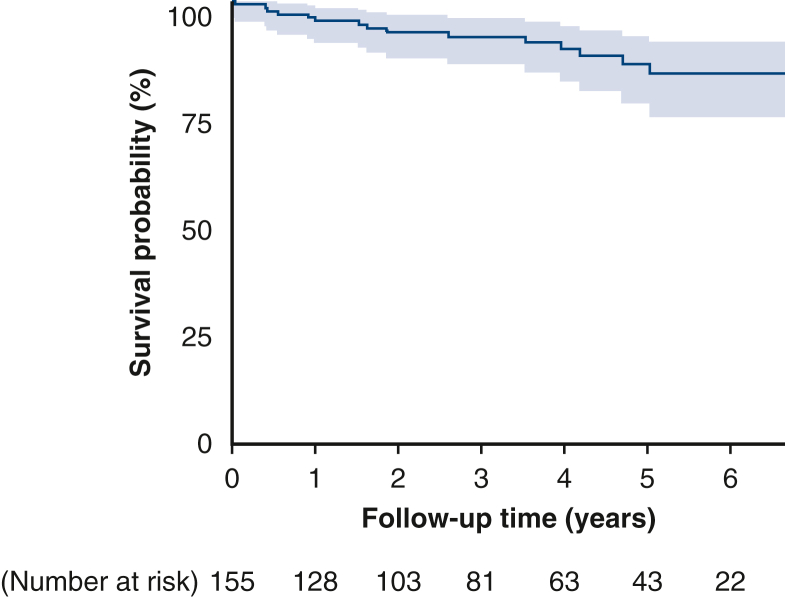
All patients were reached for midterm clinical follow-up (median of 37 months follow-up interquartile range, 18-60 months; longest 8 years). All-cause mortality was 11.6%, and cardiac-related mortality was 3.9% (Figures 1 and E1). Repeat cardiac surgery occurred in 1 patient who underwent redo CABG 3.5 years after TECAB despite having a patent LITA-LAD at the time of hybrid RCA stenting. Incidence of MI and unplanned PCI was 2.6% (4 patients) and 3.8% (6 patients), respectively. There were no surgical target vessel reinterventions. Freedom from angina was 96%, and freedom from MACCE was 94% (Table 5).
Figure 1.
Kaplan–Meier freedom from cardiac-related mortality and freedom from MACCE (95% confidence limit). MACCE, Major adverse cardiac and cerebrovascular events.
Figure E1.
Kaplan–Meier all-cause mortality curve (95% confidence limit).
Table 5.
Midterm follow-up
| Midterm variables | N = 155 |
|---|---|
| Average time to follow-up (mo), median [IQR] | 37 [18-60] |
| Longest follow-up, mo (y) | 94 (7.8) |
| Mortality, n (%) | 18 (11.6) |
| Cardiac-related mortality, n (%) | 6 (3.9) |
| Repeat cardiac surgery, n (%) | 1 (0.65) |
| MI, n (%) | 4 (2.6) |
| Repeat catheterization (unplanned), n (%) | 15 (10) |
| PCI, n (%) | 6 (3.8) |
| PCI in culprit (surgical) vessel | 0 (0.0) |
| Freedom from angina, n (%) | 149 (96) |
| Freedom from MACCE, n (%) | 146 (94) |
IQR, Interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; MACCE, major adverse cardiac and cerebrovascular events.
Discussion
In this study, we reviewed early and midterm outcomes of AHCR using robotic beating-heart TECAB with BITA grafts and PCI at our institution (Figure 2). The important findings of our study are as follows: Robotic beating-heart TECAB as a surgical arm of AHCR for multivessel CAD (1) was a safe and feasible procedure without risk of sternal wound infection; (2) resulted in short hospital LOS and early functional recovery; (3) was feasible in patients with a wide variety of risk factors, including high body mass index (BMI), prior cardiac surgery, low LV function, and left main disease; (4) showed similar long-term graft patency, survival and freedom from MACCE compared with traditional CABG data; and (5) treated a total of 3 coronary vessels (2.14 grafted and 1.23 stented) on average, which achieved complete revascularization as evidenced by low residual SYNTAX score (Figure 3).
Figure 2.
AHCR: Robotic beating-heart multivessel TECAB with BITA grafts + PCI and graft angiography. BITA, Bilateral internal thoracic artery; LITA, left internal thoracic artery; LAD, left anterior descending artery; RITA, right internal thoracic artery; OM, obtuse marginal artery; DES, drug-eluting stent; RCA, right coronary artery.
Figure 3.
Outcomes of robotic TECAB with BITA grafts and PCI in patients with multivessel coronary disease. TECAB, Totally endoscopic coronary artery bypass; BITA, bilateral internal thoracic artery; PCI, percutaneous coronary intervention; OM, obtuse marginal artery; LITA, left internal thoracic artery; LAD, left anterior descending artery; RCA, right coronary artery; MI, myocardial infarction; AHCR, advanced hybrid coronary revascularization; SYNTAX, Synergy between Percutaneous coronary interventions with Taxus and Cardiac Surgery.
Hybrid Coronary Revascularization and Advanced Hybrid Coronary Revascularization
The aim of HCR is to integrate the longevity of surgical LITA to LAD bypass using a sternal-sparing approach and the noninvasiveness of PCI to non-LAD targets to achieve complete revascularization. The safety and efficacy of the concept have been verified in several studies. In a recent Polish randomized trial, 5-year all-cause mortality of HCR was reported to be similar to conventional CABG.1 In the HREVS randomized trial comparing HCR, conventional CABG and multivessel PCI, Ganyukov and colleagues2 found no difference in residual myocardial ischemia and MACCE at 1 year. A recent meta-analysis showed that patients who underwent HCR had similar all-cause mortality and risk of MACCE, and shorter hospital LOS compared with conventional CABG.3 As a result of these and other positive data regarding early and midterm outcomes, this approach has become an acceptable alternative to conventional CABG or multivessel PCI for patients with multivessel disease. Although several studies have demonstrated superior early outcomes for HCR, long-term results compared with traditional CABG have not been fully validated. Patel and colleagues10 compared 207 HCR patients with 2-vessel disease propensity matched to patients undergoing off-pump CABG and found no difference in 8-year survival data obtained from the National Death Index. Giambruno and colleagues11 followed 147 HCR patients with 2-vessel disease for 96 months and compared them with a matched cohort of on-pump CABG patients with 2-vessel disease and found no difference in mortality or repeat revascularization. We believe these results can be expanded to patients with more than 2-vessel disease where HCR could provide superior short-term and comparable long-term outcomes to traditional CABG by using multivessel bypass, especially with BITA grafts. Based on this hypothesis, AHCR (which combines multivessel grafting with PCI) has become a major component of our hybrid revascularization practice, constituting more than 50% of all cases. The concept of AHCR is to gain further benefit from the surgical side of HCR by adding a second ITA graft to a second large left coronary target, thereby advancing the procedure from single arterial grafting to the LAD to MAG using BITA grafts. It is well known from observational studies that BITA grafting results in better long-term outcomes than single internal thoracic artery (SITA),4,12 despite the fact that a recent randomized trial (ART trial) did not validate the long-term benefits of BITA grafting.13 This may have been due to a large number of crossovers as well as other factors including the use of radial artery grafts in the SITA group. The ROMA trial, a prospective, unblinded, randomized, multicenter trial comparing outcomes of SITA bypass versus MAG using RITA or radial artery conduits, is in process to deliver further information.14 Hesitancy to use BITA grafts comes from the higher rate of mediastinitis in conventional CABG,15 which does not exist in AHCR because of the sternal-sparing nature of the procedure. Therefore, AHCR with BITA could theoretically improve long-term outcomes for patients with multivessel CAD without the risk of sternal wound infection.
Sternal-Sparing Coronary Artery Bypass Grafting in Advanced Hybrid Coronary Revascularization
Considering the sternal-sparing surgical arm of AHCR, minimally invasive direct coronary artery bypass grafting (MIDCAB) with or without robotic assistance or TECAB are both feasible options. MIDCAB has been mostly used for single LAD lesions; however, some institutions have published excellent outcomes of MIDCAB with BITA grafting.16 Nambiar and colleagues17 reviewed short-term outcomes of 819 patients who underwent beating-heart MIDCAB with LITA to RITA Y-composite grafts. Postoperative outcomes were excellent with a mean hospital LOS of 3.1 days and low mortality of 0.7%. Despite a general concern that minimally invasive CABG results in a lower number of grafts,18 this group reported 3.1 mean number of grafts comparable to conventional CABG results.19
In our view, robotic-assisted TECAB is the least invasive of the sternal-sparing options in either HCR or AHCR. Bonatti and colleagues5 reviewed outcomes of 88 HCR and 52 AHCR procedures using arrested-heart TECAB with a 5-year survival of 92.9%. We have reported outcomes of our initial series of 57 AHCR patients using robotic beating-heart TECAB in 2019.7 To the best of our knowledge, the present study reviews the largest number of patients, 156, who underwent AHCR with robotic TECAB. From this 8.5-year experience, we have found several advantages to this approach when compared with other sternal-sparing techniques.
First, the TECAB approach enables the use of BITA grafts more frequently and efficiently. Using BITA grafts for left coronary targets was reported to independently improve long-term survival in patients with stable CAD.20 We strongly believe in this concept and have used BITA grafts in 48% of our entire TECAB practice and in 89% of patients undergoing multivessel TECAB.7 Frequent use of BITA grafts in TECAB also enables us to increase the number of grafts, which is essential to achieve complete revascularization especially in patients with 3-vessel disease who constituted 94% of patients in the current series. Complete revascularization has been reported to be associated with improved long-term outcomes after CABG.21,22 In a previous study, we assessed the completeness of revascularization by calculating residual SYNTAX scores after AHCR with TECAB, using the postsurgical PCI angiogram. We showed that 86% of patients achieved complete or near-complete revascularization (residual SYNTAX score <8), which was associated with higher survival and freedom from MACCE.23 In that report, the mean preoperative SYNTAX score was 33.1 and mean residual SYNTAX score was 4.6, and in the present study we found a similar outcome with a mean preoperative SYNTAX score of 30 and residual SYNTAX score of 2.6.
Another advantage of TECAB in AHCR is its low complication rate and early functional recovery. No risk of mediastinal infection exists in TECAB, even in high-risk patients with diabetes or morbid obesity.24 In a recent systematic review paper investigating 2397 TECAB cases, the mean hospital LOS was 5.8 days.25 Our robotic TECAB approach has been conducted without cardiopulmonary bypass (conversion rate 1.9%), which might contribute to the shorter LOS of 2.73 days in the present study. The duration of opioid use after discharge was only 2.9 days, which was also contributed to by avoiding larger incisions or rib spreading.
A final benefit of TECAB is the ability to perform the procedure using the same endoscopic access on patients with a wide range of risk factors. Davierwala and colleagues16 mentioned in their study of MIDCAB with BITA grafts that good clinical outcomes were partly due to favorable patient selection, excluding patients with lung disease because of lack of tolerance of single-lung ventilation. In our experience, intolerance to single-lung ventilation is not a contraindication because of controlled double-lung ventilation by adjusting the carbon dioxide insufflation in the left pleural space, as we have previously described.26 High BMI is also an important risk factor in MIDCAB with BITA, and it was mentioned that morbid obesity should be considered a contraindication.16 Morbid obesity has not been a contraindication to TECAB with BITA in our practice,27 and 42% of the patients in the present study had BMI greater than 30. In addition, patients with prior cardiac surgery28,29 or low ejection fraction30 are no longer considered to be a contraindication in our TECAB practice.
PCI using current generation drug-eluting stents has been shown to have repeat revascularization rates as low as 3.2% at 3 years follow-up in one recent publication,31 making them suitable adjuncts in non-LAD targets for patients with multivessel disease. One of the benefits of the HCR procedure when PCI is performed after the surgery is the ability to assess graft patency. Early angiographic patency in this study was 98% overall and 100% for LITA-LAD grafts. Our midterm outcomes were comparable to the results of traditional CABG and to the series of simple HCR in patients with 2-vessel disease discussed,10,11 despite the fact that the overwhelming majority of patients in this series had 3-vessel disease with high preoperative SYNTAX scores. The risk of incomplete revascularization should not be underestimated using this approach. In our series, 17 patients declined or had unsuccessful PCI after TECAB. This has been reported in other HCR series and should be taken into account when choosing which approach to take for a specific patient. It is important to note that even in these patients with incomplete revascularization, having 2 ITA grafts on the 2 most important targets of the left coronary system can be advantageous for long-term survival.20
With good early and long-term outcomes achieved using the least-invasive approach to MAG applicable in a wide range of patients, robotic beating-heart TECAB with BITA grafts should be a sound surgical option when considering AHCR. Experienced surgical and interventional teams in a fully integrated heart-team environment are crucial to the success of this strategy. Advanced PCI techniques are necessary to handle complex lesions not amenable to endoscopic grafting. New developments in robotic technology and adjunctive robotic instruments will be necessary to facilitate broader adoption of this approach. Further long-term follow-up and randomized trials are necessary to verify this strategy compared with conventional approaches.
Study Limitations
This is a retrospective single-institution series with all the inherent potential biases therein. Because we are a referral center for robotic cardiac surgery, selection bias cannot be ruled out. Widespread adoption of this approach will be limited to experienced teams. Although the midterm follow-up was complete (based on our system of contacting all patients in our database on a yearly basis), the angiographic patency data were early and long-term angiographic follow-up was not obtained.
Conclusions
In selected patients with multivessel CAD, integrating robotic TECAB with BITA grafts and PCI to achieve AHCR resulted in excellent early and midterm outcomes. The TECAB approach facilitates easy harvesting and grafting of 2 ITA conduits to 2 left coronary targets using a totally endoscopic approach. Further studies are warranted.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/1347.

Conflict of Interest Statement
H.H.B. discloses he is a proctor for Intuitive Surgical, maker of the da Vinci robot. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
Table E1.
Preoperative characteristics
| Variables | All patients (n = 156) |
|---|---|
| Age (y), mean [range] | 65 [31-91] |
| Female gender, n (%) | 55 (35) |
| STS score, mean [range] | 1.26 [0.22-10.5] |
| BMI >30, n (%) | 65 (42) |
| Hypertension, n (%) | 131 (84) |
| Dyslipidemia, n (%) | 121 (78) |
| Diabetes mellitus, n (%) | 60 (38) |
| Peripheral vascular disease, n (%) | 11 (7.1) |
| Chronic renal failure, n (%) | 31 (20) |
| Renal failure on dialysis, n (%) | 5 (3.3) |
| COPD, n (%) | 8 (5.1) |
| Congestive heart failure, n (%) | 22 (14) |
| Ejection fraction <40%, n (%) | 27 (17) |
| Atrial fibrillation, n (%) | 13 (8.3) |
| Prior cerebrovascular accident, n (%) | 12 (7.7) |
| Prior MI, n (%) | 38 (23) |
| Prior PCI, n (%) | 47 (30) |
| Previous cardiac surgery, n (%) | 0 (0.0) |
| Left main disease, n (%) | 27 (17) |
| 3-vessel disease, n (%) | 147 (94) |
| 2-vessel disease, n (%) | 9 (6.0) |
STS, Society of Thoracic Surgeons; BMI, body mass index; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Table E2.
Conduits and targets
| TECAB × 2, n (%) | N = 135 |
|---|---|
| LITA-LAD, RITA-Diag/Ramus/Cx/OM | 84 (62) |
| RITA-LAD, LITA-Diag/Ramus/Cx/OM | 43 (32) |
| LITA-LAD, RITA-Diag/OM (Y) | 2 (1.6) |
| LITA-OM, RITA-Diag | 1 (0.7) |
| LITA-LAD (distal), RITA-LAD (proximal) | 1 (0.7) |
| RITA-LAD, LITA-PLB | 4 (3) |
| TECAB × 3, n (%) | N = 20 |
|---|---|
| LITA-LAD, LITA-Diag (sequential or Y), RITA-Cx/OM | 12 (60) |
| LITA-LAD, RITA-Diag/OM, RITA-Diag/Ramus/Cx/OM (sequential or Y) | 1 (5.0) |
| RITA-LAD, LITA-Diag/Cx, LITA-OM (sequential or Y) | 6 (30) |
| RITA-LAD, RITA-Diag/Cx, LITA-OM (sequential or Y) | 1 (5.0) |
| TECAB × 4, n (%) | N = 1 |
|---|---|
| LITA-LAD, LITA-Diag, LITA-OM, RITA-OM | 1 (100) |
TECAB, Totally endoscopic coronary artery bypass; LITA, left internal thoracic artery; LAD, left anterior descending artery; RITA, right internal thoracic artery; Cx, circumflex artery; OM, obtuse marginal artery; PLB, posterolateral branch.
Table E3.
Stent information
| Variables | |
|---|---|
| Targets vessels stented, mean ± SD | 1.23 ± 0.5 |
| Stent location, % | |
| RCA | 77 |
| LCx/OM | 19 |
| Ramus/diagonal | 2 |
| Left main | 2 |
| Stent type, % | |
| Resolute | 63 |
| Orsiro | 9 |
| Xience | 7.5 |
| Promus | 7.5 |
| Bare mental stent | 7 |
| PTCA | 1.5 |
| Unknown | 4.5 |
SD, Standard deviation; RCA, right coronary artery; LCx, left coronary circumflex artery; OM, obtuse marginal artery; PTCA, percutaneous transluminal coronary angioplasty.
Table E4.
Return to work/activities and postoperative opioid use
| Postoperative variable | N = 156 patients |
|---|---|
| Readmission within 30 d, n (%) | 7 (4.5) |
| Last day of opioid medication after discharge (d), mean ± SD | 2.9 ± 4.6 |
| Patients taking no opioids postoperatively, % | 53% |
| Patients off opioids within 1 wk, % | 87% |
| Time to return to full normal activities (d), mean ± SD | 15 ± 10 |
| Return to full activities within 2 wk, % | 64% |
| Time to return to work (d), mean ± SD (n = 42) | 19 ± 13 |
| Return to work within 2 wk, % | 20% |
SD, Standard deviation.
Supplementary Data
Representative AHCR case demonstrating robotic totally endoscopic multivessel arterial grafting with BITA grafts in a patient with triple-vessel CAD, followed by PCI with a drug-eluting stent to the RCA and postoperative graft angiography showing patent grafts. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00451-5/fulltext.
References
- 1.Tajstra M., Hrapkowicz T., Hawranek M., Filipiak K., Gierlotka M., Zembala M., et al. Hybrid coronary revascularization in selected patients with multivessel disease: 5-year clinical outcomes of the prospective randomized pilot study. JACC Cardiovasc Interv. 2018;11:847–852. doi: 10.1016/j.jcin.2018.01.271. [DOI] [PubMed] [Google Scholar]
- 2.Ganyukov V., Kochergin N., Shilov A., Tarasov R., Skupien J., Szot W., et al. Randomized clinical trial of surgical vs. percutaneous vs. hybrid revascularization in multivessel coronary artery disease: residual myocardial ischemia and clinical outcomes at one year-hybrid coronary REvascularization versus stenting or surgery (HREVS) J Interv Cardiol. 2020;2020:5458064. doi: 10.1155/2020/5458064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardar P., Kundu A., Bischoff M., Chatterjee S., Owan T., Nairooz R., et al. Hybrid coronary revascularization versus coronary artery bypass grafting in patients with multivessel coronary artery disease: a meta-analysis. Catheter Cardiovasc Interv. 2018;91:203–212. doi: 10.1002/ccd.27098. [DOI] [PubMed] [Google Scholar]
- 4.Dorman M.J., Kurlansky P.A., Traad E.A., Galbut D.L., Zucker M., Ebra G. Bilateral internal mammary artery grafting enhances survival in diabetic patients: a 30-year follow-up of propensity score-matched cohorts. Circulation. 2012;18:2935–2942. doi: 10.1161/CIRCULATIONAHA.112.117606. [DOI] [PubMed] [Google Scholar]
- 5.Bonatti J.O., Zimrin D., Lehr E.J., Vesely M., Kon Z.N., Wehman B., et al. Hybrid coronary revascularization using robotic totally endoscopic surgery: perioperative outcomes and 5-year results. Ann Thorac Surg. 2012;94:1920–1926. doi: 10.1016/j.athoracsur.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Balkhy H.H., Nisivaco S., Kitahara H., Torregrossa G., Patel B., Grady K., et al. Robotic off-pump totally endoscopic coronary artery bypass in the current era: report of 544 patients. Eur J Cardiothorac Surg. 2022;61:439–446. doi: 10.1093/ejcts/ezab378. [DOI] [PubMed] [Google Scholar]
- 7.Balkhy H.H., Nisivaco S.M., Hashimoto M., Torregrossa G., Grady K. Robotic total endoscopic coronary bypass in 570 patients: impact of anastomotic technique in two eras. Ann Thorac Surg. 2022;114:476–482. doi: 10.1016/j.athoracsur.2021.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Kitahara H., Hirai T., McCrorey M., Patel B., Nisivaco S., Nathan S., et al. Hybrid coronary revascularization: mid-term outcomes of robotic multivessel bypass and percutaneous interventions. J Thorac Cardiovasc Surg. 2019;157:1829–1836.e1. doi: 10.1016/j.jtcvs.2018.08.126. [DOI] [PubMed] [Google Scholar]
- 9.Balkhy H.H., Nathan S., Arnsdorf S.E., Krienbring D.J. Right internal mammary artery use in 140 robotic totally endoscopic coronary bypass cases: toward multiarterial grafting. Innovations (Phila) 2017;12:9–14. doi: 10.1097/IMI.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 10.Patel N.C., Hemli J.M., Kim M.C., Seetharam K., Pirelli L., Brinster D.R., et al. Short- and intermediate-term outcomes of hybrid coronary revascularization for double-vessel disease. J Thorac Cardiovasc Surg. 2018;156:1799–1807.e3. doi: 10.1016/j.jtcvs.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 11.Giambruno V., Jones P., Khaliel F., Chu M.W., Teefy P., Sridhar K., et al. Hybrid coronary revascularization versus on-pump coronary artery bypass grafting. Ann Thorac Surg. 2018;105:1330–1335. doi: 10.1016/j.athoracsur.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Yi G., Shine B., Rehman S.M., Altman D.G., Taggard D.P. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation. 2014;130:539–545. doi: 10.1161/CIRCULATIONAHA.113.004255. [DOI] [PubMed] [Google Scholar]
- 13.Taggart D.P., Benedetto U., Gerry S., Altman D.G., Gray A.M., Lees B., et al. Bilateral versus single internal-thoracic-artery grafts at 10 years. N Engl J Med. 2019;380:437–446. doi: 10.1056/NEJMoa1808783. [DOI] [PubMed] [Google Scholar]
- 14.Gaudino M., Alexander J.H., Bakaeen F.G., Ballman K., Barili F., Calafiore A.M., et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial-rationale and study protocol. Eur J Cardiothorac Surg. 2017;52:1031–1040. doi: 10.1093/ejcts/ezx358. [DOI] [PubMed] [Google Scholar]
- 15.Schwann T.A., Habib R.H., Wallace A., Shahian D.M., O'Brien S., Jacobs J.P., et al. Operative outcomes of multiple-arterial versus single-arterial coronary bypass grafting. Ann Thorac Surg. 2018;105:1109–1119. doi: 10.1016/j.athoracsur.2017.10.058. [DOI] [PubMed] [Google Scholar]
- 16.Davierwala P.M., Verevkin A., Sgouropoulou S., Hasheminejad E., von Aspern K., Misfeld M., et al. Minimally invasive coronary bypass surgery with bilateral internal thoracic arteries: early outcomes and angiographic patency. J Thorac Cardiovasc Surg. 2021;162:1109–1119.e4. doi: 10.1016/j.jtcvs.2019.12.136. [DOI] [PubMed] [Google Scholar]
- 17.Nambiar P., Kumar S., Mittal C.M., Saksena K. Minimally invasive coronary artery bypass grafting with bilateral internal thoracic arteries: will this be the future? J Thorac Cardiovasc Surg. 2018;155:190–197. doi: 10.1016/j.jtcvs.2017.07.088. [DOI] [PubMed] [Google Scholar]
- 18.Benedetto U., Caputo M., Patel N.N., Fiorentino F., Bryan A., Angelini G.D. Longterm survival after off-pump versus on-pump coronary artery bypass graft surgery. Does completeness of revascularization play a role? Int J Cardiol. 2017;246:32–36. doi: 10.1016/j.ijcard.2017.04.087. [DOI] [PubMed] [Google Scholar]
- 19.Khan N.E., De Souza A., Mister R., Flather M., Clague J., Davies S., et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21–28. doi: 10.1056/NEJMoa031282. [DOI] [PubMed] [Google Scholar]
- 20.Bakaeen F.G., Ravichandren K., Blackstone E.H., Houghtaling P.L., Soltesz E.G., Johnston D.R., et al. Coronary artery target selection and survival after bilateral internal thoracic artery grafting. J Am Coll Cardiol. 2020;75:258–268. doi: 10.1016/j.jacc.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Omer S., Cornwell L.D., Rosengart T.K., Kelly R.F., Ward H.B., Holman W.L., et al. Completeness of coronary revascularization and survival: impact of age and off-pump surgery. J Thorac Cardiovasc Surg. 2014;148:1307–1315.e1. doi: 10.1016/j.jtcvs.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Melina G., Angeloni E., Refice S., Benegiamo C., Lechiancole A., Matteucci M., et al. Residual SYNTAX score following coronary artery bypass grafting. Eur J Cardiothorac Surg. 2017;51:547–553. doi: 10.1093/ejcts/ezw356. [DOI] [PubMed] [Google Scholar]
- 23.Balkhy H.H., Kitahara H., Hirai T., Matsukage H., Nathan S. Residual SYNTAX score after advanced hybrid robotic totally endoscopic coronary revascularization. Ann Thorac Surg. 2020;109:1826–1832. doi: 10.1016/j.athoracsur.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Kitahara H., Patel B., McCrorey M., Nisivaco S., Balkhy H.H. Morbid obesity does not increase morbidity or mortality in robotic cardiac surgery. Innovations (Phila) 2017;12:434–439. doi: 10.1097/IMI.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 25.Göbölös L., Ramahi J., Obeso A., Bartel T., Hogan M., Traina M., et al. Robotic totally endoscopic coronary artery bypass grafting: systematic review of clinical outcomes from the past two decades. Innovations (Phila) 2019;14:5–16. doi: 10.1177/1556984519827703. [DOI] [PubMed] [Google Scholar]
- 26.Balkhy H.H., Nisivaco S., Tung A., Torregrossa G., Mehta S. Does intolerance of single-lung ventilation preclude robotic off-pump totally endoscopic coronary bypass surgery? Innovations (Phila) 2020;15:456–462. doi: 10.1177/1556984520940462. [DOI] [PubMed] [Google Scholar]
- 27.Kitahara H., Patel B., McCrorey M., Nisivaco S., Balkhy H.H. Is robotic beating heart totally endoscopic coronary artery bypass feasible for BMI > 35 morbidly obese patients? Int J Med Robot. 2018;14:e1911. doi: 10.1002/rcs.1911. [DOI] [PubMed] [Google Scholar]
- 28.Nisivaco S., McCrorey M., Krienbring D., Patel B., Srivastava S., Balkhy H.H. Redo robotic endoscopic beating heart coronary bypass (TECAB) after previous TECAB. Ann Thorac Surg. 2017;104:e417–e419. doi: 10.1016/j.athoracsur.2017.06.067. [DOI] [PubMed] [Google Scholar]
- 29.Kitahara H., Wehman B., Balkhy H.H. Can robotic-assisted surgery overcome the risk of mortality in cardiac reoperation? Innovations (Phila) 2018;13:438–444. doi: 10.1097/IMI.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 30.Peev M.P., Nisivaco S., Torregrossa G., Arastu A., Shahul S., Balkhy H.H. Robotic off-pump totally endoscopic coronary artery bypass in patients with low ejection fraction. Innovations (Phila) 2022;17:50–55. doi: 10.1177/15569845211073929. [DOI] [PubMed] [Google Scholar]
- 31.Van Hemert N.D., Voskuil M., Rosemeijer R., Stein M., Frambach P., Periera B., et al. 3-Year clinical outcomes after implantation of permanent-polymer vs polymer-free stents: ReCre8 Landmark Analysis. JACC Cardiovasc Interv. 2021;14:2477–2486. doi: 10.1016/j.jcin.2021.08.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative AHCR case demonstrating robotic totally endoscopic multivessel arterial grafting with BITA grafts in a patient with triple-vessel CAD, followed by PCI with a drug-eluting stent to the RCA and postoperative graft angiography showing patent grafts. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00451-5/fulltext.