Abstract
Simple Summary
Cholangitis/cholangiohepatitis is the most common inflammatory disease of the biliary system in cats. A tissue biopsy is crucial for a definitive diagnosis. Lymphocytic cholangitis is one type of cholangitis and is identified through histological findings. Treatment requires a long-term immunosuppressive drug. This article is the first report of multiple cyst-like hepatic lesions with histological findings of lymphocyte infiltration in a cat’s liver.
Abstract
A 5-year-old female neutered domestic short-haired cat presented with abdominal enlargement. An abdominal ultrasound revealed that large multiple hepatic cysts with irregular walls, hypoechoic fluid, and internal septations occupied most of the liver parenchyma. Serum liver enzymes, bilirubin, and bile acids concentrations were within normal limits. A fecal examination using simple floatation and formalin-ether sedimentation techniques was negative for liver fluke (Platynosomum fastosum), intestinal protozoa, and other helminth eggs. Praziquantel was prescribed for two distinct courses one month apart without obvious improvement of the hepatic cysts. An abdominal laparotomy and histopathological examination finally enabled diagnosis of cyst-like lymphocytic cholangiohepatitis of the liver tissue. Twelve weeks of oral prednisolone resulted in marked ultrasonographic improvement of the hepatic cysts. The liver parenchyma was heterogeneous and filled with multiple small anechoic cavities. Twenty-three months after ceasing the prednisolone, there was no recurrence of hepatic cysts.
Keywords: cat, cyst, cholangitis, cholangiohepatitis, liver
1. Introduction
Feline cholangitis/cholangiohepatitis is an inflammatory condition of two conjunctive organs: the gall bladder and the liver [1,2]. The prevalence of feline cholangitis ranges from 25% to 50% across the world [2,3,4,5,6]. According to the World Small Animal Veterinary Association Standardization Committee, there are four forms of feline cholangitis—neutrophilic cholangitis, lymphocytic cholangitis, destructive cholangitis, and chronic cholangitis—associated with liver fluke infestation [7]. Differentiation of the four distinct forms requires a tissue biopsy [7]. Lymphocytic cholangitis is characterized by the presence of an aggregation of lymphocytes around the bile ducts. Cholangiohepatitis occurs when the inflammation extends to the hepatic parenchyma [7]. Cats with lymphocytic cholangitis/cholangiohepatitis commonly present with weakness, anorexia, nausea, vomiting, weight loss, ascites, and jaundice [5,8]. They may have increased hepatic enzyme activities, total bilirubin, and hypergammaglobulinemia [9,10]. Some cats may develop mild nonregenerative anemia or poorly regenerative anemia [9,10]. Ultrasonographic assessment of a cat with lymphocytic cholangitis/cholangiohepatitis may detect normal hepatic parenchyma or diffuse heterogeneous liver parenchyma and biliary system abnormalities, such as bile duct dilation, presence of gall bladder sludge, and gall bladder wall or bile duct wall thickening [2,5,11,12]. Lymphocytic cholangitis/cholangiohepatitis may be associated with an immune-mediated condition [8,13]. An immunosuppressive dosage of prednisolone is recommended for effective treatment to minimize hepatocellular damage and increase appetite [10].
Although lymphocytic cholangitis/cholangiohepatitis is commonly found, cystic lesions of the hepatic parenchyma have not been described in cats with lymphocytic cholangitis/cholangiohepatitis. The present article represents the first report of clinical signs and characteristics of cyst-like lesions on the hepatic parenchyma with lymphocytic cholangitis/cholangiohepatitis in a cat.
2. Case Description
A 5-year-old female neutered domestic short-haired cat (5.3 kg) was brought to the Kasetsart University Veterinary Teaching Hospital with a 1-month history of progressive abdominal enlargement. Informed consent was obtained from the cat’s owner. The cat had indoor and outdoor access and was fed a commercial, complete, and balanced cat diet. The cat was alert and had hyporexia. A complete physical examination was performed and vital parameters were within normal limits. The clinical examination revealed that the cat had pale mucous membranes and moderate abdominal distension. Hematology and blood chemistry testing was performed using an automated hematology analyzer (Sysmex XN-1000TM Hematology Analyzer, Sysmex, Lincolnshire, IL, USA) and an automatic chemistry analyzer (IL Lab 650 chemistry system, Diamond Diagnostics, Holliston, MA, USA). A complete blood count indicated regenerative anemia with a hematocrit of 16.4% (reference interval (RI): 30–45%), an increased mean corpuscular volume of 60.07 fL (RI: 39–55 fL), absolute reticulocytosis of 225,000/µL (RI: 0–50,000/µL), and neutrophilia of 14,547 cells/µL (RI: 2500–12,500 cells/µL) (Table 1). A blood smear examination revealed anisocytosis, giant platelets, rouleaux formation, and toxic neutrophils. Serum creatinine, blood urea nitrogen, alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), bile acids, total protein, and albumin concentrations were within normal limits (Table 2). A high blood glucose concentration of 256 mg/dL (RI: 60–130 mg/dL) was found.
Table 1.
Hematological profile at first visit, 4 weeks after doxycycline treatment, before prednisolone treatment, and 12 weeks after prednisolone treatment.
| Hematological Parameters | Reference | Unit | First Visit | 4 Weeks after Doxycycline Treatment | Before Prednisolone Treatment |
12 Weeks after Prednisolone Treatment |
|---|---|---|---|---|---|---|
| Hemoglobin | 10–15 | g/dL | 5.2 | 9.4 | 9.2 | 10.6 |
| Packed red cells | 30–45 | % | 16.4 | 27.5 | 29.1 | 31.1 |
| Red blood cells | 5–10 | ×106 cells/µL | 2.73 | 7.64 | 6.01 | 6.6 |
| Mean corpuscular volume | 39–55 | fL | 60.07 | 35.99 | 48.42 | 47.12 |
| Mean corpuscular hemoglobin concentration |
30–36 | g/dL | 31.71 | 34.18 | 31.62 | 34.08 |
| Total white blood cells | 5500–19,000 | cells/µL | 18,650 | 7410 | 8270 | 10,370 |
| Segmented neutrophils | 2500–12,500 | cells/µL | 14,547 | 5112.9 | 4854.5 | 6740.5 |
| Lymphocytes | 1500–7000 | cells/µL | 2797.5 | 1778.4 | 2299 | 3111 |
| Monocytes | 0–850 | cells/µL | 746 | 0 | 636.8 | 0 |
| Eosinophils | 0–750 | cells/µL | 559.5 | 518.7 | 479.7 | 518.5 |
| Basophils | Rare | cells/µL | 0 | 0 | 0 | 0 |
| Absolute reticulocytes | 0–50,000 | /µL | 225,000 | 9932 | N/A | N/A |
| Platelets | 300,000–800,000 | cells/µL | 471 | 146 | 52 | 229 |
| Protein (refract) | 6–7.5 | g/dL | 7.6 | 7.8 | 8 | 7.4 |
| Blood morphology | Anisocytosis, rouleaux formation, giant platelets | Microcytes, giant platelets | Anisocytosis, giant platelets | Rouleaux formation, giant platelets |
Table 2.
Blood chemistry profile at first visit, 4 weeks after doxycycline treatment, before prednisolone treatment, and 12 weeks after prednisolone treatment.
| Chemistry Parameters | Reference | Unit | First Visit | 4 Weeks after Doxycycline Treatment | Before Prednisolone Treatment |
12 Weeks after Prednisolone Treatment |
|---|---|---|---|---|---|---|
| Blood urea nitrogen | 15–34 | mg/dL | 30 | 28 | - | 22 |
| Creatinine | 1.1–2.2 | mg/dL | 1.56 | 1.36 | 1.69 | 1.64 |
| Glucose | 60–130 | mg/dL | 256 | 157 | - | 180 |
| Alanine aminotransferase | 28–76 | U/L | 46 | 63 | 49 | 61 |
| Alkaline phosphatase | 0–62 | U/L | 22 | - | - | 33 |
| Gamma-glutamyl transferase | 0–4 | U/L | 1 | - | - | 1 |
| Preprandial bile acids | 0–6.9 | µmol/L | 5 | - | - | - |
| Total bilirubin | 0.1–0.5 | mg/dL | 0.1 | - | - | 0.1 |
| Total protein | 5.8–7.8 | g/dL | 6.7 | 7.4 | 8.3 | 7.4 |
| Albumin | 2.6–4.2 | g/dL | 3.1 | 3.0 | 3.4 | 3.8 |
| Globulin | 2.9–7.7 | g/dL | 3.6 | 4.4 | 4.9 | 3.6 |
| Potassium | 3.0–4.8 | mEq/L | 4.38 | - | - | - |
The results of a combination enzyme-linked immunosorbent assay (ELISA) test for the feline leukemia (FeLV) antigen and the feline immunodeficiency virus (FIV) antibody, using a commercially available FeLV–FIV rapid immunochromatography test assay (Witness®, Lyon Cedex, France), was negative. Doxycycline (10 mg/kg/day for 28 days; Siadocin®, Siam Pharmaceutical, Bangkok, Thailand) was prescribed orally for suspected M. haemofelis because the cat had regenerative anemia and lived in an area where the tick-borne parasite is endemic, although the blood smear results were negative for hemoparasites.
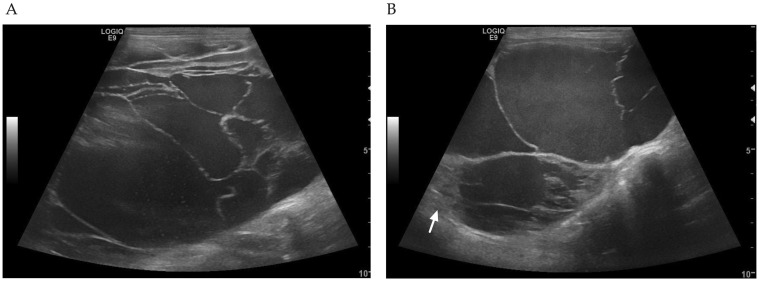
The abdominal ultrasound was performed by the Thai Board of Veterinary Surgeons, sub-specialty of veterinary diagnostic imaging, using a real-time scanner (LOGIQ E9, GE, Fairfield, CT, USA) with a 13 MHz broadband linear transducer. An abdominal ultrasound identified multiple large hepatic cysts occupying most of the liver (Figure 1). The cysts were characterized by thin and irregular walls with hypoechoic fluid and internal septations. There was only a small area of mild heterogeneous liver parenchyma. The other abdominal organs were unremarkable. Percutaneous ultrasound-guided needle aspiration of a hepatic cyst yielded a red and cloudy fluid. The fluid sample had a specific gravity of 1.035, a protein concentration of 5.7 g/dL, and a total nucleated cell count of 1910 cells/µL. Microscopic evaluation of the fluid sample revealed numerous erythrocytes, some macrophages, neutrophils, and lymphocytes. Fluid from the cysts was also submitted for a bacterial culture. No growth of bacteria was identified under aerobic or anaerobic conditions. Therefore, liver abscesses were ruled out. A fecal examination using simple floatation and formalin-ether sedimentation techniques was negative for liver fluke (Platynosomum fastosum), intestinal protozoa, and other helminth eggs. However, praziquantel (30 mg/kg/day; Opticide-FC®, Medicpharma Co., Ltd., Samut Sakhon, Thailand) was prescribed for 4 days.
Figure 1.
Ultrasonographic findings on the first visit. (A) Large hepatic cysts contained hypoechoic fluid and internal septations. (B) Hepatic cysts surrounded with irregular walls. There was a small area of mild heterogeneous liver parenchyma (arrow).
After 4 weeks of doxycycline administration, the cat’s weight was 5.5 kg. The cat was alert and had a normal appetite. Moderate abdominal distension was still found during the physical examination. Blood tests showed signs of improvement of the anemia, with a hematocrit of 27.5% (RI: 30–45%) and a decreased mean corpuscular volume of 35.99 fL (RI: 39–55 fL).
One month after the first dose of praziquantel administration, the cat received the second dose of praziquantel in a similar fashion to the initial dose. However, there was no noticeable improvement of the abdominal enlargement and hepatic cysts as determined by a clinical examination and an ultrasonographic evaluation. Parasitic cysts due to Platynosomum fastosum infection were unlikely. Therefore, an exploratory laparotomy was performed. Anesthesia was induced with a slow IV infusion of propofol (8 mg/kg; Troypofol, Troikaa Pharmaceuticals Ltd., India) and then maintained with isoflurane (Attane™, Piramal Critical Care, Inc., Bethlehem, PA, USA). Intramuscularis injection of morphine sulphate (0.2 mg/kg; M&H Manufacturing Co., Ltd., Samutprakarn, Thailand) was used for analgesia. Multiple large hepatic cystic lesions occupied the liver parenchyma and could not be removed. There was a small area of non-cystic liver parenchyma. Therefore, incisional biopsies of the liver and hepatic cyst walls were collected for histopathology. The cat’s anesthetic recovery went well. Intravenous fluid therapy using 0.9% NaCl (General Hospital Products Public Co., Ltd., Pathum Thani, Thailand) and antibiotic therapy using amoxicillin-clavulanate intravenously (20 mg/kg q8h; Cavumox®, Siam Pharmaceutical Co., Ltd., Bangkok, Thailand) was continued for postoperative care. Tramadol (3 mg/kg q12h, subcutaneous; Tramada-100®, L.B.S. Laboratory Ltd. Part., Bangkok, Thailand) was administered for analgesia. The cat was bright with a good appetite and was discharged 72 h postoperatively, with the sutures removed 7 days later.
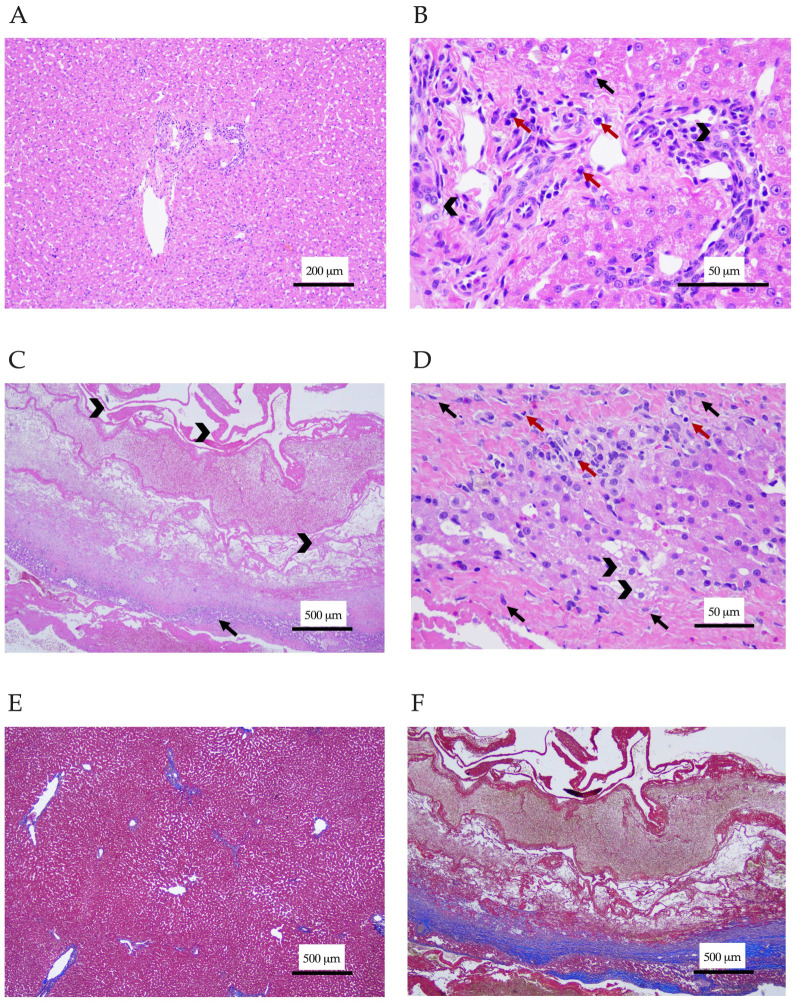
Three Thai board-certified pathologists analyzed the samples. Histopathology of the liver demonstrated lymphocytic cholangiohepatitis (Figure 2A,B). Mixed inflammatory cells including lymphocytes, plasma cells, macrophages, and a small number of neutrophils were seen in portal areas and had infiltrated the hepatic parenchyma. The hepatic cord was in a normal arrangement, but the hepatocytes exhibited cloudy swelling. Cyst wall sections revealed necrotizing hepatitis forming hepatic cyst-like lesions, which were composed of an abundance of eosinophilic fibrinous proteinaceous materials rearranging as a cyst-like wall without a lining of epithelial cells (Figure 2C,D). A small area of remaining hepatic cord showed the vacuolar degeneration of hepatocytes and infiltrated with mixed inflammatory cells, including neutrophils, lymphocytes, plasma cells, and macrophages. The necrotic lesion was surrounded by reactive fibroblastic cells, with thick fibrous connective tissue suggesting severe fibrosis. Tissue hemorrhage was also observed in the lesions. This finding confirmed the diagnosis of lymphocytic cholangiohepatitis. The Masson’s trichrome staining of liver section was performed to investigate the severity of fibrosis. The staining revealed normal collagen deposition around the portal areas and central veins, while the cyst-like wall section showed strongly positive staining, with the blue color indicating tissue fibrosis (Figure 2E,F). Prednisolone (1 mg/kg/day; Charoenbhaesaj Lab Co., Ltd., Bangkok, Thailand) was then prescribed. Two hepatoprotective agents, ursodeoxycholic acid (62.5 mg/d; Ursolin®, Berlin Pharmaceutical Industry Co., Ltd., Bangkok, Thailand) and silymarin (70 mg/day; Samarin®, Berlin Pharmaceutical Industry Co., Ltd., Bangkok, Thailand), were also administered.
Figure 2.
Histological section of the liver and cyst-like wall. (A,B) The liver section stained with hematoxylin-eosin showed infiltration of mononuclear inflammatory cells (red arrows) and neutrophils (black arrows) in periportal areas and the hepatic parenchyma. Bile duct epithelium cells are marked with arrowheads. Magnification ×100 (A) and ×400 (B). (C,D) The hepatic cyst-like wall section stained with hematoxylin-eosin. (C) Eosinophilic fibrinous proteinaceous materials rearranged as a cyst-like wall (arrowhead). The remaining hepatic cords (arrow) were infiltrated with a mix of inflammatory cells, including lymphocytes, plasma cells, macrophages, and some neutrophils. (D) Hepatic cords showed vacuolar degeneration of hepatocytes (arrowhead) and fibrosis (black arrow). Inflammatory cells (red arrow) were seen. Magnification ×40 (C) and ×400 (D). (E,F) The liver and cyst-like wall sections stained with Masson’s trichrome. (E) Normal collagen deposition around the portal areas and central veins presented in the liver section. (F) The cyst-like wall section had severe tissue fibrosis surrounding necrotic tissues. Magnification ×40 (E,F).
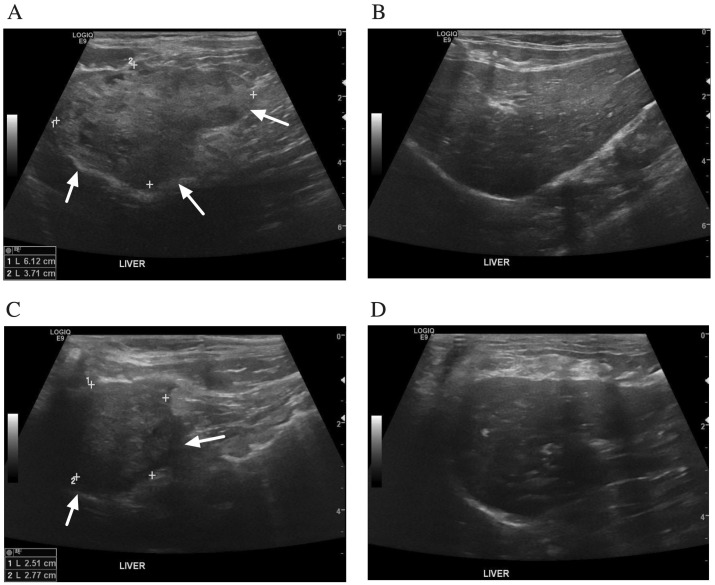
After 12 weeks of prednisolone administration, the abdominal distension had disappeared. In addition, improvement of the hepatic cysts was detected by abdominal ultrasonography. The abdominal ultrasound demonstrated a heterogeneous liver parenchyma filled with irregular, small anechoic cavities on the left lobe of the liver (Figure 3A,B). The right lobe of the liver showed a homogeneous parenchyma. Thickening of the gall bladder wall and common bile duct dilatation were demonstrated. There was no evidence of intrahepatic bile duct dilatation. Prednisolone was then tapered down 25% every 2 weeks until reaching a physiological dose of 0.25 mg/kg/day. After 1 week of a physiological dose of prednisolone, prednisolone administration was stopped.
Figure 3.
Ultrasonographic findings of the liver after treatment with prednisolone. (A,B) 12 weeks after prednisolone treatment. (A) The left lobe of liver showed a poorly defined mass (arrows) containing small irregular anechoic cavitations. (B) The right lobe of liver had a homogeneous parenchyma. There was no evidence of intrahepatic bile duct dilatation. (C,D) Twenty-three months after stopping prednisolone. (C) The left lobe of liver showed a heterogenous mass (arrows). (D) The right lobe of liver had a mild heterogenous parenchyma and round border. There was no evidence of common bile duct and intrahepatic bile duct dilatation.
Twenty-three months after stopping prednisolone, the cat was doing well, was active, and had a good appetite. An ultrasonographic examination showed no recurrence or progression of the hepatic cysts (Figure 3C,D). The left lobe of the liver showed a decrease in the size of liver mass. The right lobe of the liver showed mild heterogenous parenchyma and a round border. The gall bladder wall was thickening. No evidence of common bile duct and intrahepatic bile duct dilatation was identified. Liver enzymes and liver function tests were within the reference intervals.
3. Discussion
The hepatic cyst-like lesions in this present case were diagnosed as lymphocytic cholangiohepatitis. In general, biochemical parameter abnormalities of cats with lymphocytic cholangitis/cholangiohepatitis are often revealed by an increase in one or more liver enzyme activities [1,3]. Interestingly, the hepatic cyst-like lesions of this cat were widespread and occupied most of the liver parenchyma, but liver enzymes and liver function tests were within normal limits. This finding may be explained by the 70–80% of hepatic reserve capacity [14]. The high blood glucose at the first visit may be explained by the non-sedated restraint procedure and chronic illness stage [15]. The secretion of catecholamines, cortisol, glucagon, and cytokines during stress contribute to gluconeogenesis [16]. The serum fructosamine concentration should be measured to exclude diabetic mellitus from stress-related hyperglycemia [15]. Nonetheless, serum fructosamine was not analyzed in the present case. The blood glucose concentration decreased below the renal threshold after the symptomatic treatment.
True hepatic cysts are fluid-filled cavities lined with epithelium; however, the pathogenesis is not well defined [17]. The cysts are often thin, have a well-defined wall and no internal echoes or septations, and lack the extent of hepatic parenchymal involvement [18,19,20]. These hepatic cysts may be accompanied by polycystic kidney disease; therefore, the kidney should also be evaluated [21,22,23]. Polycystic kidney disease is an autosomal dominant genetic-related disease in Persian cats [21,22,23,24]. In the cat described in this case report, polycystic kidney disease could be ruled out because the ultrasonography results for the kidneys were normal and the cat’s breed was domestic short-haired [24].
Clinical manifestation of liver fluke infestation can be hepatic cysts. Detection of liver fluke eggs from fecal examination may be missed because of the intermittent egg shedding and low number of eggs in feces [25]. Cytology of bile aspirate could increase the sensitivity of parasitic egg detection [26]. The prevalence of liver fluke was reported as 2.7–8.9% in cats residing in Thailand [26,27,28]. Therefore, praziquantel should be prescribed to all cats with hepatobiliary diseases in Thailand. Echinococcosis is another possible cause of hepatic cysts in cats [29]. To the authors’ knowledge, echinococcosis in cats has never been reported in Thailand.
On occasions, hepatic cysts become more complex lesions, characterized by irregular walls, septations, internal debris, or varying degrees of echogenic content. In these cases, neoplasia, hematoma, abscess, necrosis, inflammation, parasites, or toxins must be included in the differential diagnosis [30,31]. In our case, the hepatic cysts had complex internal features and involved all lobes of the liver. Liver abscess and hematoma had been ruled out by percutaneous aspiration of cystic content for cytology and a bacterial culture. Parasitic cysts due to liver flukes were also unlikely because the lesions did not improve after two distinct courses of praziquantel treatment. Therefore, a liver histology was performed for a final diagnosis, which was lymphocytic cholangiohepatitis.
Lymphocytic cholangitis/cholangiohepatitis in cats is characterized as the infiltration of small lymphocytes in the portal areas, with varying degrees of fibrosis and biliary proliferation. The pathogenesis of lymphocytic cholangitis/cholangiohepatitis was proposed to be involved with an autoimmune mechanism and bacterial infection [7,32]. Ultrasonographic appearances of liver parenchyma in cats with lymphocytic cholangitis/cholangiohepatitis may be reported to be normal, hypoechoic, or hyperechoic [5,11,12]. Common sonographic findings of the biliary tract can be dilation of the bile ducts, gall bladder distension, increased gall bladder sludge, thickening of the gall bladder wall, thickening of the bile duct wall, or unremarkable findings [2,12].
To the authors’ knowledge, complex cyst-like lesions of the liver parenchyma of a cat with lymphocytic cholangitis/cholangiohepatitis have not been reported. The cyst-like lesions contained the areas of necrotizing hepatitis, tissue hemorrhage, and eosinophilic fibrinous proteinaceous materials. The cause of hepatic cyst-like lesions in this case remains unclear. The hepatic cyst might be the primary cause. The gradual distention of the cysts might induce biliary obstruction, which promotes bacterial infection followed by cholangitis [33,34,35,36]. On the other hand, lymphocytic cholangiohepatitis may contribute to severe necrosis of the liver and, in the end, form cyst-like structures. This hypothesis may support by the presence of thick fibrous connective tissues surrounding necrotic lesions, which was manifested by the Masson’s trichrome staining of cyst-like wall section.
Treatment with anti-inflammatory dosages of prednisolone clearly improved the hepatic cyst-like lesions in this study, possibly because the cat was diagnosed early and showed normal liver function. We suggest histological analysis should be performed in cats with complex hepatic cysts that involve large parts of the hepatic parenchyma.
4. Conclusions
We have demonstrated the first case of feline lymphocytic cholangiohepatitis that presented with cyst-like structures in the liver. Histopathological analysis is important for diagnosis and leads to successful treatment with prednisolone.
Acknowledgments
The authors are grateful for the support of the cat’s owner and the Veterinary Diagnostic Center, Faculty of Veterinary Medicine, Kasetsart University, for laboratory activities.
Author Contributions
Conceptualization, S.N.A. and P.S.; clinical case handling, S.N.A., P.C., A.K., and B.H.; data curation, S.N.A. and P.S.; writing—original draft preparation, S.N.A. and P.S.; formal analysis, S.N.A.; histopathological analyses, R.Y., P.M., and K.R.; writing—review and editing, S.N.A., R.Y., P.M., K.R., P.C., A.K., B.H., and P.S.; complementary examinations, P.C. and A.K.; surgical procedure, B.H.; supervision, P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All procedures performed on the cat were undertaken in the framework of routine clinical veterinary care. Thus, ethical review and approval were waived for this case report.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All study data are presented in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cullen J.M. Summary of the World Small Animal Veterinary Association standardization committee guide to classification of liver disease in dogs and cats. Vet. Clin. North. Am. Small. Anim. Pract. 2009;39:395–418. doi: 10.1016/j.cvsm.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callahan Clark J.E., Haddad J.L., Brown D.C., Morgan M.J., Van Winkle T.J., Rondeau M.P. Feline cholangitis: A necropsy study of 44 cats (1986–2008) J. Feline Med. Surg. 2011;13:570–576. doi: 10.1016/j.jfms.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagne J.M., Weiss D.J., Armstrong P.J. Histopathologic evaluation of feline inflammatory liver disease. Vet. Pathol. 1996;33:521–526. doi: 10.1177/030098589603300506. [DOI] [PubMed] [Google Scholar]
- 4.Hirose N., Uchida K., Kanemoto H., Ohno K., Chambers J.K., Nakayama H. A Retrospective Histopathological Survey on Canine and Feline Liver Diseases at the University of Tokyo between 2006 and 2012. J. Vet. Med. Sci. 2014;76:1015–1020. doi: 10.1292/jvms.14-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland L., Beatty J. Feline Cholangitis. Vet. Clin. North. Am. Small. Anim. Pract. 2017;47:703–724. doi: 10.1016/j.cvsm.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Bayton W.A., Westgarth C., Scase T., Price D.J., Bexfield N.H. Histopathological frequency of feline hepatobiliary disease in the UK. J. Small. Anim. Pract. 2018;59:404–410. doi: 10.1111/jsap.12810. [DOI] [PubMed] [Google Scholar]
- 7.van den Ingh T.S.G.A.M., Cullen J.M., Twedt D.C., Van Winkle T., Desmet V.J., Rothuizen J. Chapter 5—Morphological classification of biliary disorders of the canine and feline liver. In: Rothuizen J., Bunch S.E., Charles J.A., Cullen J.M., Desmet V.J., Szatmári V., Twedt D.C., van den Ingh T.S.G.A.M., Van Winkle T., Washabau R.J., editors. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. W.B. Saunders; Edinburgh, Scotland: 2006. pp. 61–76. [Google Scholar]
- 8.Day M.J. Immunohistochemical characterization of the lesions of feline progressive lymphocytic cholangitis/cholangiohepatitis. J. Comp. Pathol. 1998;119:135–147. doi: 10.1016/S0021-9975(98)80058-3. [DOI] [PubMed] [Google Scholar]
- 9.Prasse K.W., Mahaffey E.A., DeNovo R., Cornelius L. Chronic Lymphocytic Cholangitis in Three Cats. Vet. Pathol. 1982;19:99–108. doi: 10.1177/030098588201900201. [DOI] [PubMed] [Google Scholar]
- 10.Otte C.M., Penning L.C., Rothuizen J., Favier R.P. Retrospective comparison of prednisolone and ursodeoxycholic acid for the treatment of feline lymphocytic cholangitis. Vet. J. 2013;195:205–209. doi: 10.1016/j.tvjl.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Center S.A. Diseases of the gallbladder and biliary tree. Vet. Clin. North. Am. Small. Anim. Pract. 2009;39:543–598. doi: 10.1016/j.cvsm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Marolf A.J., Leach L., Gibbons D.S., Bachand A., Twedt D. Ultrasonographic findings of feline cholangitis. J. Am. Anim. Hosp. Assoc. 2012;48:36–42. doi: 10.5326/JAAHA-MS-5671. [DOI] [PubMed] [Google Scholar]
- 13.Warren A., Center S., McDonough S., Chiotti R., Goldstein R., Meseck E., Jacobsen M., Rowland P., Simpson K. Histopathologic features, immunophenotyping, clonality, and eubacterial fluorescence in situ hybridization in cats with lymphocytic cholangitis/cholangiohepatitis. Vet. Pathol. 2011;48:627–641. doi: 10.1177/0300985810384409. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence Y.A., Steiner J.M. Laboratory Evaluation of the Liver. Vet. Clin. North. Am. Small. Anim. Pract. 2017;47:539–553. doi: 10.1016/j.cvsm.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Frezoulis P.S., Oikonomidis I.L., Saridomichelakis M.N., Kasabalis D., Pappa A., Bouza-Rapti P., Chochlios T., Tsouloufi T.K., Kritsepi-Konstantinou M., Soubasis N. Prevalence, association with systemic inflammatory response syndrome and outcome of stress hyperglycaemia in sick cats. J. Small Anim. Pract. 2022;63:197–202. doi: 10.1111/jsap.13445. [DOI] [PubMed] [Google Scholar]
- 16.Dungan K.M., Braithwaite S.S., Preiser J.C. Stress hyperglycaemia. Lancet. 2009;373:1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafuente S., Fresno L., Anselmi C., Lloret A., Espada I., Santos L. Complete laparoscopic excision of a hepatic cyst and omentopexy in a Persian cat. JFMS Open Rep. 2018;4:2055116918817631. doi: 10.1177/2055116918817631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyland T.G., Larson M.M., Mattoon J.S. Liver. In: Mattoon J.S., Nyland T.G., editors. Small Animal Diagnostic Ultrasound. 3rd ed. Elsevier Saunders; St. Louis, MO, USA: 2015. pp. 332–399. [Google Scholar]
- 19.Adler R., Wilson D.W. Biliary cystadenoma of cats. Vet. Pathol. 1995;32:415–418. doi: 10.1177/030098589503200411. [DOI] [PubMed] [Google Scholar]
- 20.d’Anjou M., Penninck D. Liver. In: Penninck D., d’Anjou M., editors. Atlas of Small Animal Ultrasonography. Wiley Blackwell; Oxford, UK: 2015. pp. 183–238. [Google Scholar]
- 21.Eaton K.A., Biller D.S., DiBartola S.P., Radin M.J., Wellman M.L. Autosomal dominant polycystic kidney disease in Persian and Persian-cross cats. Vet. Pathol. 1997;34:117–126. doi: 10.1177/030098589703400204. [DOI] [PubMed] [Google Scholar]
- 22.Bosje J.T., van den Ingh T.S., van der Linde-Sipman J.S. Polycystic kidney and liver disease in cats. Vet. Q. 1998;20:136–139. doi: 10.1080/01652176.1998.9694858. [DOI] [PubMed] [Google Scholar]
- 23.Schirrer L., Marín-García P.J., Llobat L. Feline Polycystic Kidney Disease: An Update. Vet. Sci. 2021;8:269. doi: 10.3390/vetsci8110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Supamanee N., Sattasathuchana P., Nilkumhang P., Teerapan W., Wajjawalku W., Thengchaisri N. A survey of feline polycystic kidney disease in 102 persian and persian-related Cats by ultrasonography. J. Kasetsart. Vet. 2010;20:77–84. [Google Scholar]
- 25.Stern J.k., Walden H.D.S., Marshall K., Sharkey L. What is your diagnosis? Bile from a cat. Vet Clin Path. 2020;49:354–355. doi: 10.1111/vcp.12840. [DOI] [PubMed] [Google Scholar]
- 26.Soe B.K., Adisakwattana P., Reamtong O., Anuracpreeda P., Sukhumavasi W. A first attempt at determining the antibody-specific pattern of Platynosomum fastosum crude antigen and identification of immunoreactive proteins for immunodiagnosis of feline platynosomiasis. Vet. World. 2022;15:2029–2038. doi: 10.14202/vetworld.2022.2029-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojekittikhun W., Chaisiri K., Mahittikorn A., Pubampen S., Sa-Nguankiat S., Ku-solsuk T., Maipanich W., Udonsom R., Mori H. Gastrointestinal parasites of dogs and cats in a refuge in Nakhon Nayok, Thailand. Southeast Asian J. Trop. Med. Public Health. 2014;45:31–39. [PubMed] [Google Scholar]
- 28.Jitsamai W., Khrutkham N., Hunprasit V., Chandrashekar R., Bowman D., Sukhu-mavasi W. Prevalence of endoparasitic and viral infections in client-owned cats in metropolitan Bangkok, Thailand, and the risk factors associated with feline hook-worm infections. Vet. Parasitol. Reg. Stud. Rep. 2021;25:100584. doi: 10.1016/j.vprsr.2021.100584. [DOI] [PubMed] [Google Scholar]
- 29.Da Silva A.M., Bastien M., Umhang G., Boué F., Bastid V., Boucher J.M., Cail-lot C., de Garam C.P., Renault C., Faisse M., et al. Oil contamination by Echinococcus multilocularis in rural and urban vegetable gardens in relation to fox, cat and dog faecal deposits. Parasite. 2021;28:74. doi: 10.1051/parasite/2021073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyland T.G., Koblik P.D., Tellyer S.E. Ultrasonographic evaluation of biliary cystadenomas in cats. Vet. Radiol. Ultrasound. 1999;40:300–306. doi: 10.1111/j.1740-8261.1999.tb00365.x. [DOI] [PubMed] [Google Scholar]
- 31.Braga R.R., Teixeira A.C., Oliveira J.A., Cavalcanti L.P. Prevalence of Platynosomum fastosum infection in free roaming cats in northeastern Brazil: Fluke burden and grading of lesions. Vet. Parasitol. 2016;227:20–25. doi: 10.1016/j.vetpar.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Otte C.M., Gutierrez O.P., Favier R.P., Rothuizen J., Penning L.C. Detection of bacterial DNA in bile of cats with lymphocytic cholangitis. Vet. Microbiol. 2012;156:217–221. doi: 10.1016/j.vetmic.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Best E.J., Bush D.J., Dye C. Suspected choledochal cyst in a domestic shorthair cat. J. Feline Med. Surg. 2010;12:814–817. doi: 10.1016/j.jfms.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grand J.G., Doucet M., Albaric O., Bureau S. Cyst of the common bile duct in a cat. Aust. Vet. J. 2010;88:268–271. doi: 10.1111/j.1751-0813.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- 35.Spain H.N., Penninck D.G., Webster C.R., Daure E., Jennings S.H. Ultrasonographic and clinicopathologic features of segmental dilatations of the common bile duct in four cats. JFMS Open Rep. 2017;3:2055116917716881. doi: 10.1177/2055116917716881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreeg M.E., Miller S.A., Cullen J.M. Choledochal cyst with secondary cholangitis, choledochitis, duodenal papillitis, and pancreatitis in a young domestic shorthair cat. J. Vet. Diagn. Invest. 2021;33:782–787. doi: 10.1177/10406387211017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are presented in the article.