Abstract
In this study, we have analyzed hematopoietic activity in the spleen, bone marrow, and blood of BALB/c and scid mice infected with Leishmania donovani. Our analysis demonstrates that infection induces a rapid but transient mobilization of progenitor cells into the circulation, associated with elevated levels of granulocyte/macrophage colony-stimulating factor (GM-CSF) and MIP-1α. From 14 to 28 days postinfection, when parasite expansion begins in the spleen and bone marrow, both the frequency and cell cycle activity of hematopoietic progenitors, particulary CFU-granulocyte, monocyte, are dramatically increased in these organs. This is associated with increased accumulation of mRNA for GM-CSF, M-CSF, and G-CSF, but not interleukin-3. Our data also illustrate that hematopoietic activity, as assessed by changes in the frequency of progenitor cell populations and their levels of cell cycle activity, can be regulated in both a T-cell-independent and T-cell-dependent, as well as in an organ-specific, manner. Collectively, these data add to our knowledge of the long-term changes which occur in organs in which L. donovani is able to persist.
The regulation of hematopoietic activity is an important homeostatic process of mammals. In the resting state, the bone marrow represents the main site of hematopoiesis in adult rodents, although a small percentage of myeloid precursors are present in the spleen (18, 54). Regulation of hematopoietic activity results from many extracellular matrix-cell and cell-cell interactions between a variety of stromal cell populations and hematopoietic stem cells and progenitor cells (7, 10, 12). This cooperation is mediated through transmembrane adhesion molecules, as well as the production of cytokines and chemokines with hematopoietic activity (2, 6, 44, 47). Alterations in the distribution of hematopoietic activity in the tissues may, however, occur as a result of increased hematopoietic stress. In addition, local changes in the balance of various cell lineages have also been attributed to recruitment from the bone marrow via the peripheral circulation. Increases in the hematopoietic activity of the spleen have been observed following experimental murine infection with Salmonella enterica serovar Typhimurium, Listeria monocytogenes, Plasmodium yoelii, and Leishmania major (33, 41, 54, 56, 57). However, there have been fewer comparative studies of infection-induced changes in hematopoietic activity in circumstances in which multiple tissues act as targets for infection (25–31).
In both clinical and experimental visceral leishmaniasis, Leishmania donovani and Leishmania infantum amastigotes replicate in mononuclear phagocytes of the liver, spleen, and bone marrow (1). Although the mechanisms of parasite control and acquisition of immunity in the liver of BALB/c mice has been extensively documented (15, 32, 55), recent interest has focused on the course of infection in the spleen. Unlike the liver, the spleen is persistently infected and suffers considerable pathological disruption to its microanatomy 46, 48; P. Gorak, unpublished data). In contrast, while the bone marrow has long been recognized as a site of infection in mice (3, 23), less is known about the relationship between parasite dynamics in this organ, changes in cytokine and chemokine expression, and local hematopoietic activity.
Therefore, we have conducted a comparative study of hematopoiesis in the spleen, bone marrow, and peripheral blood of BALB/c mice following infection with L. donovani. Our data indicate a marked temporal association between changes in myelopoiesis, probably driven by selected colony-stimulating factors (CSFs), and local parasite expansion. Furthermore, tissue-specific expression of chemokines and cytokines with hematopoietic activity is documented, and its implications for the regulation of organ-specific responses to L. donovani infection in mice are discussed.
MATERIALS AND METHODS
Animals and parasites.
Female specific-pathogen-free BALB/c mice were obtained from Tuck and Co. (Battlesbridge, United Kingdom). C.B-17 scid mice were obtained from a breeding colony maintained by Greg Bancroft at the London School of Hygiene and Tropical Medicine, derived from a parental stock provided by C. Hetherington (National Institute for Medical Research, London, United Kingdom). The mice were used at 8 to 10 weeks of age and housed under conventional conditions, with food and water provided ad libitum. No murine pathogens have been detected in our facility by routine sentinel screening. Parasites of the Ethiopian strain of L. donovani (LV9) were maintained by passage in Syrian hamsters as described elsewhere (48). The mice were infected with 2 × 107 L. donovani cells intravenously in 200 μl of RPMI or “sham” infected with an equivalent volume of naïve hamster spleen homogenate.
Determination of tissue parasite burden.
The mice were killed by cervical dislocation, and the livers, spleens, and femurs were removed. The parasite load was determined from impression smears following methanol fixation and Giemsa staining. For the spleen and liver, the parasite burden was expressed as Leishman-Donovan units (LDU), where LDU was equal to the number of parasites per 1,000 host nuclei times the organ weight in milligrams (49). For bone marrow, the parasite burden was determined both microscopically (as the number of parasites per 1,000 host nuclei in smears) and by limiting-dilution analysis on blood agar slopes (22). Briefly, nutrient agar (3 g; Difco, Scientific Laboratory Supplies, Ltd., Nottingham, United Kingdom) and NaCl (0.6 g; Sigma) were dissolved in 100 ml of double-distilled H2O and autoclaved for 60 min. Glucose (5 ml; 30% [wt/vol] (Sigma) in phosphate-buffered saline and 10 ml of citrated rabbit blood (Harlan Seralab Ltd., Crawley Down, United Kingdom) were then added, and the blood agar (50 μl) was dispensed into a flat-bottom 96-well plate (Nunc; Marathon Laboratory Supplies, London, United Kingdom). The plates were allowed to set at a 45° slant and were stored for up to 2 months in humidified boxes at 4°C before use. Three hours before the addition of tissue samples, the plates were placed at 26°C. Single-cell suspensions from bone marrow were prepared in Dulbecco modified Eagle medium F12 nutrient mix (Gibco) plus 5% (vol/vol) heat-inactivated fetal calf serum plus 100 μg of streptomycin/ml and 100 μl of penicillin/ml. Serial dilutions of cell suspensions were made, and 100 μl was added to each of 16 replicate wells. The plates were cultured in humidified, 5% (vol/vol) CO2 incubators at 25 to 26°C for 9 days. After that time, the number of wells negative for parasite growth were scored using an inverted microscope. The logarithm of the fraction of negative wells was plotted against the number of host cells plated at each serial dilution. The equation of the best-fit line was generated by a χ2 minimization method, using a computer program adapted from reference 50 and supplied by E. Prina, Institut Pasteur, Paris, France. The cell dilution yielding a fraction of 37% negative wells for parasite growth gives an estimate of the reciprocal parasite frequency.
Assays of colony-forming precursors and progenitors.
Progenitor cell frequency in the bone marrow, spleen, and peripheral blood was determined at various times postinfection (p.i.) by analysis of their ability to produce colonies in semisolid methylcellulose culture (18, 51). Single-cell suspensions were prepared from spleens by passage through a 20-μm-pore-size nylon mesh, from bone marrow by flushing with cold RPMI, and from heparinized peripheral blood by Ficoll-Hypaque separation. The cells were washed in Iscove's modified Dulbecco's medium (Gibco) supplemented with 100 μg of penicillin/ml, 100 μg of streptomycin/ml, 20 mM sodium pyruvate, and 50 μM β-2-mercaptoethanol, and 70 μl (containing either 8 × 104 bone marrow cells, 1.2 × 106 spleen cells, or 4 × 106 peripheral blood mononuclear cells [PBMC]) was subsequently transferred to 4-ml Falcon tubes (Gibco). Stem cell factor (SCF) (final concentration, 25 μg/ml; R & D Systems) and hemin (bovine hemin chloride; final concentration, 100 μM; Sigma) were added to each tube to give a final volume of 100 μl. Using a 1-ml syringe fitted with a 16-gauge, blunt-ended needle (Stem Cell Technology), 900 μl of Methocult 3430 (0.1% [wt/vol] methylcellulose, 30% [vol/vol] fetal calf serum, 1% [wt/vol] bovine serum albumin, 100 μM 2-mercaptoethanol, 2 mM l-glutamine, 2% [vol/vol] pokeweed mitogen-stimulated murine spleen cell conditioned medium, and 3 U of recombinant human erythropoietin [Epo]/ml; Stem Cell Technology) was taken up and dispensed into each sample tube. The cells plus Methocult were mixed thoroughly and allowed to stand at room temperature for 5 min to allow air bubbles to rise to the top; 750 μl was carefully drawn into each syringe, and 250-μl triplicate samples were dispensed into the wells of a 24-well tissue culture plate (Falcon). The final number of cells plated per well was 2 × 104 bone marrow cells, 3 × 105 spleen cells, and 1 × 106 PBMC. In some experiments, cultures were also supplemented with 50 to 150 μg of sodium stibogluconate (Pentostam; Wellcome, Stevenage, United Kingdom)/ml or 0.1 to 1.0 μg of Fungizone (Squibb and Sons, Princeton, Ga.)/ml. The plates were incubated for 7 days at 37°C in 5% (vol/vol) O2–5% (vol/vol) CO2. At later time points in infection (days 28 and 56 p.i.), reduced numbers of spleen cells (1.5 × 105) and bone marrow cells (1.0 × 104) were plated per well to facilitate colony counting.
After 7 days of incubation at 37°C, the plates were examined microscopically for colony counting. Colonies (assessed by eye to contain greater than 50 cells) were scored as either CFU-granulocyte, erythrocyte, monocyte, megakaryocyte (GEMM; circular colonies with characteristic brown-pink coloring); CFU-granulocyte, monocyte (GM; spherical or dispersed clear or grey colonies); or burst-forming unit–erythrocyte (BFU-E); multicentered colonies with characteristic brown-pink coloring). To confirm identification, representative colonies were picked, cytospun onto glass microscope slides, and stained for hemoglobin content with a 5:1:1 mixture of 0.2% (wt/vol) O-dianisidine (Sigma) in methanol–3% (vol/vol) hydrogen peroxide solution (Sigma)–1% (wt/vol) sodium nitroferricyanide solution (Sigma) for 10 min at room temperature in the dark. The slides were rinsed under tap water and then counterstained with Giemsa stain. Erythroid cell types could be identified microscopically as those exhibiting a positive brown-yellow cytoplasmic reaction assessing the presence of hemoglobin, in contrast to myeloid cells showing a characteristic blue-grey cytoplasmic staining (51). Colony numbers are expressed as the number of each progenitor type within a fixed total number of cells, or within the whole tissue, based on the ratio of the number of cells assayed to whole-organ cell counts.
Progenitor [3H]thymidine suicide assay.
To determine the proliferative status of progenitor cells in the bone marrow and spleen, the proportion of cells in S phase of the cell cycle was determined (51). Single-cell suspensions were resuspended in IMDM at 106 bone marrow cells/ml or 107 spleen cells/ml. Replicate 1-ml samples were either untreated or pulsed with 50 μCi/ of l tritiated thymidine (specific activity, 25 Ci/mmol; ICN, Irvine, United Kingdom)/ml, and samples were incubated at 37°C for 20 min. The samples were then chased with excess cold thymidine (20% [wt/vol] in ice-cold IMDM; Sigma). The samples were then washed twice in IMDM and plated in colony assays, as described above. Colony formation was evaluated after 7 days, and the proportion of progenitor cells in S phase is expressed as the percentage reduction in CFU formation in samples treated with tritiated thymidine compared to controls.
Measurement of cytokine and chemokine mRNA accumulation.
mRNA was isolated and analyzed by a semiquantitative reverse transcription (RT)-PCR assay as previously described (16). All PCR primers and probes were as described, with the addition of granulocyte/macrophage colony-stimulating factor (GM-CSF) (39), G-CSF (41), M-CSF (41), MIP-1α (45), and SCF (41) primers and probes. The intensity of signals generated by mRNA encoding the housekeeping gene for hypoxanthine-guanine phosphoribosyl transferase (HPRT) was used to ensure approximately even loading of target cDNA into PCRs, and the results were calculated as the intensity of signals generated by cytokine products relative to signals generated by HPRT products for each sample tested. The data are presented as the relative fold increase in signal compared to control naïve mice analyzed at each time point and represent the mean ± standard error for three mice at each time point.
RESULTS
L. donovani accumulates in the bone marrow of BALB/c mice.
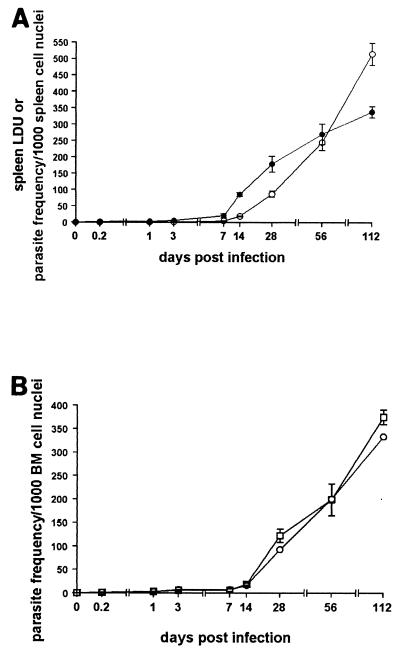
We first wished to determine the relative course of infection with L. donovani in the bone marrow and spleen of BALB/c mice. As previously shown (48), after an initial lag period during which parasite growth is not readily detectable, L. donovani amastigotes are detectable in increasing numbers in the spleen and are maintained in the tissue throughout the 112 days analyzed. The kinetics of parasite burden is similar irrespective of whether data are expressed as LDU (which introduces a correction for organ weight and thus represents total organ load) or as amastigotes per 1,000 host cells (Fig. 1A). In the bone marrow, the parasite burden was determined by two independent means. Firstly, amastigotes were enumerated from Giemsa-stained bone marrow tissue smears, in a fashion analogous to that used for the spleen. Secondly, pooled femur samples were serially diluted onto blood agar plates, allowing estimation of the parasite frequency by limiting-dilution analysis (23). As shown in Fig. 1B, parasite frequencies in the bone marrow, determined by either method, were identical. These data confirm that the bone marrow serves as a site of persistent infection and also indicate a striking similarity with the spleen in the time of onset of rapid amastigote accumulaton.
FIG. 1.
L. donovani causes a persistent infection in the bone marrow of BALB/c mice. Mice were infected with 2 × 107 L. donovani amastigotes, and the parasite burden was measured in the spleen (A) and bone marrow (B). In panel A, the parasite burden was calculated as LDU (○) or parasite frequency per 1,000 host cells (●), both determined from Giemsa-stained tissue smears. In panel B, the parasite frequency was determined from tissue smears (○) or by limiting-dilution analysis (□). The data represent the mean ± standard error of the mean for three mice for tissue smears (●) or triplicate cultures using cells pooled from three femurs in limiting-dilution assays. The data are representative of two independent experiments.
An increased frequency of hematopoietic progenitors is observed following L. donovani infection in BALB/c mice.
The data presented above show that parasites persist within both the bone marrow and spleen. To evaluate a possible association between parasite burden in these tissues and changes in hematopoietic activity, we assayed the frequency of progenitor cells capable of giving rise to mature colonies in semisolid methylcellulose culture. To support optimal in vitro colony growth, the cultures were supplemented with erythropoietin, a source of CSFs, and SCF (see Materials and Methods). Colony formation was not observed in the absence of these exogenous factors.
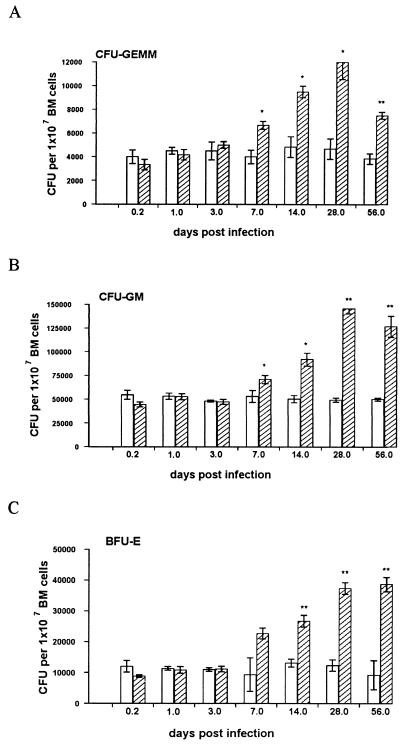
Colonies deriving from each progenitor cell type were readily observed in cultures of naïve bone marrow, though CFU-GM were more abundant than either BFU-E or CFU-GEMM (in a ratio of approximately 5:1:0.4, [Fig. 2]). Significant changes in progenitor frequency were seen over the course of L. donovani infection. Elevated levels of all three types of colony were observed by day 7 p.i., and they continued to rise until day 28 p.i., when they were present at approximately threefold frequency compared to those from naïve mice. However, the ratio of these three colony types remained similar (5:1.3:0.4).
FIG. 2.
L. donovani infection is associated with increased numbers of hematopoietic progenitors in the bone marrow of BALB/c mice. Bone marrow (BM) cells from L. donovani-infected (hatched bars) and age-matched naïve (open bars) mice were plated in hematopoietic methylcellulose colony assays (see Materials and Methods). After 7 days, mature colonies were scored as CFU-GEMM (A), CFU-GM (B), or BFU-E (C). The data represent the mean ± standard error of the mean for triplicate wells and are representative of two independent experiments. Significant differences between naïve and infected groups are indicated: ∗, P < 0.05; ∗∗, P < 0.005.
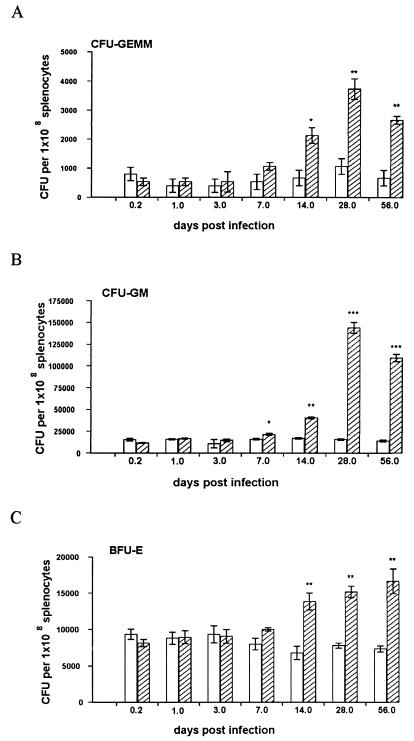
A lower frequency of progenitor cells was observed in the spleens of naïve mice compared to that in the bone marrow (Fig. 3). Furthermore, the ratio of colony types was different from that of bone marrow, with CFU-GM and BFU-E present in almost equal frequency (1.3:1:0.1 for CFU-GM, BFU-E, and CFU-GEMM, respectively). In the spleens of infected mice, significant changes in hematopoietic activity were first detected at day 14 p.i. Whereas the numbers of BFU-E stabilized at this level, the numbers of CFU-GM increased dramatically from day 14 to day 28 p.i. At day 28 p.i., CFU-GM were approximately tenfold more abundant on a per cell basis than BFU-E (Fig. 3). The spleen undergoes significant enlargement during the course of L. donovani infection (48). When adjusted for total cell number, the organ-specific capacity for myelopoiesis was increased 20- to 30-fold at later times in infection (Table 1). This analysis served to highlight both the extent to which progenitor cell frequency increases and the selective expansion of myeloid progenitors which occurs during infection.
FIG. 3.
L. donovani infection is associated with increased numbers of hematopoietic progenitors in the spleens of BALB/c mice. Spleen cells from L. donovani-infected (hatched bars) and age-matched naïve (open bars) mice were plated in hematopoietic methylcellulose colony assays. After 7 days, mature colonies were scored as CFU-GEMM (A), CFU-GM (B), or BFU-E (C). The data represent the mean ± standard error of the mean for triplicate wells and are representative of two independent experiments. Significant statistical differences between naïve and infected groups are indicated: ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005.
TABLE 1.
L. donovani infection increases hematopoietic progenitor cells in the spleen
| Days p.i. | Index of splenomegalya | Total fold increaseb
|
||
|---|---|---|---|---|
| CFU-GEMM | CFU-GM | BFU-E | ||
| 7 | 1.33 | 2.6 | 1.8 | 1.7 |
| 14 | 1.75 | 5.6 | 4.2 | 5.1 |
| 28 | 2.34 | 9.65 | 21.6 | 7.4 |
| 56 | 3.46 | 14.5 | 27.0 | 12.4 |
Index of splenomegaly = mean number of viable cells per infected spleen/mean number of viable cells per age-matched naïve spleen.
Splenic progenitor cell numbers (from the experiment shown in Fig. 3) were adjusted for splenomegaly that accompanies L. donovani infection.
Rapid and transient increases in the number of progenitor cells in the peripheral blood following L. donovani infection.
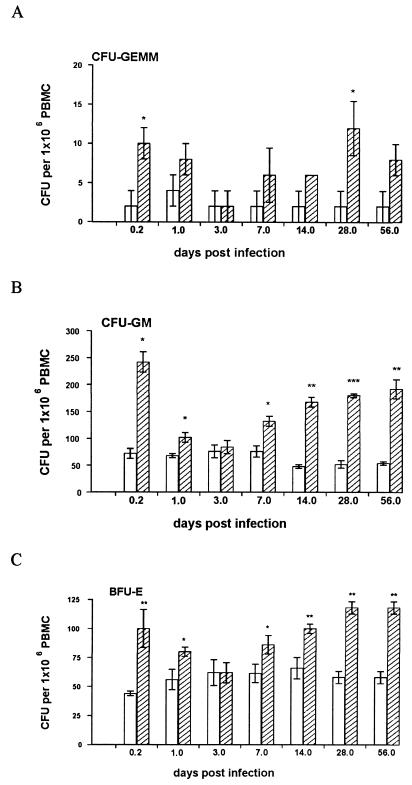
L. donovani infection fails to significantly alter the frequency of monocytes, neutrophils, and lymphocytes in peripheral blood. For example, comparing naïve mice to those at day 56 p.i., neutrophils, monocytes, and lymphocytes represent 8.1 ± 0.4, 26.7 ± 1.1, and 62.8 ± 0.1% versus 9.2 ± 0.8, 25.8 ± 1.1, and 63.4 ± 1.0% of the total, respectively. In contrast, progenitor cell activity was seen to vary during the course of infection (Fig. 4). A rapid, though transient, increase in the frequency of circulating CFU-GEMM, CFU-GM, and BFU-E was observed. This peaked at 5 h p.i. and declined to control levels by 72 h p.i. From day 7 p.i. onwards, there was a second rise in progenitor cell numbers in the peripheral blood, which was then maintained for the period of study. As seen in the spleen, the relative increase at these later time points was most dramatic for CFU-GM. Therefore, during the course of L. donovani infection, there is a biphasic mobilization of hematopoietic progenitor cells into the peripheral blood.
FIG. 4.
L. donovani infection induces biphasic mobilization of hematopoietic progenitors into the peripheral blood of BALB/c mice. Peripheral blood was removed from L. donovani-infected (hatched bars) and age-matched naïve (open bars) mice at various times p.i., and PBMC were plated in hematopoietic methylcellulose colony assays. After 7 days, mature colonies were scored as CFU-GEMM (A), CFU-GM (B), or BFU-E (C). The data represent the mean ± standard error of the mean for triplicate wells and are representative of two independent experiments. Significant statistical differences between naïve and infected groups are indicated: ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005.
Increases in splenic progenitor cell numbers are independent of the continual presence of parasites in colony assays.
The data shown above indicate that both parasite burden and the frequency of hematopoietic progenitor cells rise dramatically in the spleen from day 14 p.i. As our analysis of progenitor cells is based upon unfractionated cell populations, which contained variable numbers of infected macrophages, we wished to exclude the possibility that parasites per se influenced the in vitro colony-forming potential of progenitor cells. Therefore, we repeated these assays in the presence and absence of antileishmanial drugs. At the concentrations used, neither Pentostam nor Fungizone had an effect on spleen cell viability while they were able to kill 97% of L. donovani within 24 h (from 34 amastigotes/100 host cells to 1/100, as determined from cytospin preparations). As shown in Fig. 5, these drugs did not affect the number of colonies derived from either naïve or infected spleen cells. Thus, the presence of amastigotes or infected macrophages appears to have little influence on colony formation in vitro in the presence of optimal levels of growth factors.
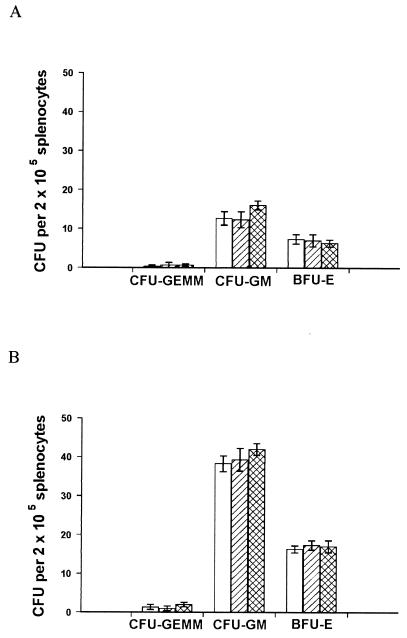
FIG. 5.
The presence of living parasites does not affect colony formation in methylcellulose cultures. Spleens were removed from age-matched naïve mice (A) and mice infected with L. donovani for 28 days (B). Replicate samples were plated in hematopoietic methylcellulose colony assays with no additions (open bars) or in the presence of 1 μg of Fungizone (hatched bars)/ml or 150 μg of Pentostam (cross-hatched bars)/ml. After 7 days, mature colonies were scored. The data represent the mean ± standard error of the mean for triplicate wells and are representative of two independent experiments.
Frequency of progenitor cells in the cell cycle is increased during L. donovani infection of BALB/c mice.
In vitro colony assays determine the frequency of progenitor cells capable of proliferating in response to hematopoietic growth or antiapoptotic factors. However, they do not directly evaluate the actual hematopoietic activity at the time of sacrifice. In order to address this issue, we performed suicide selection experiments with bone marrow and spleen cells from naïve and infected mice. Progenitor cells entering S phase during the pulse with radioactive thymidine subsequently die, and the percentage of progenitor cells in S phase of the cell cycle can be determined from the decrease in mature colony numbers after 7 days (51). As shown in Table 2, a fraction of each progenitor cell population was actively proliferating in the bone marrow of naïve BALB/c mice. In the spleens of naïve mice, the proportion of progenitor cells in cell cycle was much lower than that observed in the bone marrow, consistent with a majority of hematopoiesis occurring in the bone marrow in the resting state (18). Furthermore, the percentages of myeloid and erythroid colony-forming cells in S phase were comparable in the spleen, compared to the greater level of myeloid activity in the bone marrow. Following infection with L. donovani, the percentages of progenitor cells in S phase in both the spleen and bone marrow increased. In the bone marrow, the percentage of each progenitor population in S phase was increased approximately 1.5-fold as a result of infection. In contrast, the relative increase of cells in the cell cycle was considerably greater in the spleen (8-, 14-, and 8-fold increases in CFU-GEMM, CFU-GM, and BFU-E, respectively). The combination of selective expansion of CFU-GM progenitors (Fig. 3 and Table 1) and their active proliferation (Table 2) indicates that splenic myelopoiesis is specifically and extensively increased as a result of L. donovani infection.
TABLE 2.
L. donovani infection results in a differential increase in the proliferative status of progenitor cells in BALB/c and scid mice
| Cells | Percentage of cells in S phasea
|
|||||
|---|---|---|---|---|---|---|
| BALB/c
|
scid
|
|||||
| CFU-GEMM | CFU-GM | BFU-E | CFU-GEMM | CFU-GM | BFU-E | |
| Spleen | ||||||
| Naïve | 2.6 ± 2.1 | 3.7 ± 1.5 | 4.2 ± 1.6 | 8.0 ± 2.5 | 6.3 ± 1.4 | 6.7 ± 2.6 |
| d42 | 21.7 ± 13.3b | 53.0 ± 3.6 | 36.0 ± 3.6 | 13.7 ± 3.5 | 12.8 ± 2.9 | 11.4 ± 4.1 |
| BM | ||||||
| Naïve | 37.5 ± 2.6 | 42.1 ± 5.2 | 15.7 ± 1.8 | 26.3 ± 3.1 | 30.4 ± 3.2 | 16.9 ± 4.2 |
| d42 | 56.7 ± 2.3 | 65.0 ± 6.2 | 22.3 ± 2.0 | 37.0 ± 4.6 | 42.6 ± 0.9 | 26.3 ± 2.7 |
The percentage of spleen and bone marrow (BM) progenitor cells in S phase was determined by a [3H]thymidine kill assay, as described in Materials and Methods. The data are expressed as the percentage of decrease in colony formation following [3H]thymidine treatment compared to the mean number of colonies in control untreated samples and represent the mean ± standard error of the mean of triplicate cultures. d42, 42 days postinfection.
Data in bold face indicate statistically significant differences between naïve and infected mice (P < 0.05).
scid mice fail to increase hematopoietic activity in the spleen following L. donovani infection.
To examine the role of acquired immunity in the regulation of hematopoiesis during L. donovani infection, we also examined progenitor cell proliferation in scid mice (4, 16). Although the frequency of progenitors is increased in the bone marrow and spleen of naïve scid mice, absolute numbers per organ are comparable to those in coisogenic BALB/c mice (14) (data not shown). At day 42 p.i., when scid mice have a significantly higher parasite burden in the spleen than BALB/c mice (900 ± 36 versus 130 ± 9 amastigotes/1,000 spleen cells, respectively; P < 0.001), scid mice failed to upregulate the number of splenic CFU-GM as a result of L. donovani infection [(66.3 ± 2.3) × 103 versus (74.0 ± 2.7) × 103 CFU-GM/108 spleen cells in naïve and infected mice respectively]. Furthermore, no increase in the percentage of progenitor cells in the cell cycle was observed in the spleens of scid mice following infection with L. donovani (Table 2). In contrast, there was a significant increase in the number of progenitors in the cell cycle in the bone marrow of infected scid mice. This was of the same order of magnitude (approximately 1.5-fold) as that seen in BALB/c mice at this time. Hence, the spleen and bone marrow of scid mice differ in their ability to regulate hematopoiesis following L. donovani infection.
Increases in progenitor cell proliferation are associated with increased hematopoietic growth factor mRNA accumulation.
The above-mentioned experiments indicate that hematopoiesis is enhanced to varying degrees in the bone marrow and spleen of L. donovani-infected BALB/c and scid mice. Given that hematopoiesis is stimulated and regulated by a number of CSFs, cytokines, and chemokines, the effect of L. donovani infection on the accumulation of mRNA for some of these factors was examined. The majority of factors analyzed were expressed constitutively in the bone marrow and spleen in both strains, consistent with active hematopoiesis occurring in these organs in the resting state. However, expression of γIP-10 was low or undetectable, and expression of interleukin 3 (IL-3) was also minimal. Tables 3 and 4 summarize the data from two independent experiments in which we measured the increases in mRNA accumulation observed in individual BALB/c and scid mice at various times p.i. L. donovani infection induced a rapid accumulation of mRNA for GM-CSF and MIP-1α in the bone marrow of both BALB/c and scid mice (Table 3), suggesting that this represents a direct T-cell-independent response to infection in this organ. In the spleen (Table 4), accumulation of mRNA at 5 h p.i. was only observed for MIP-1α and not GM-CSF, again in both strains of mice. Surprisingly, given previous studies of chemokine expression in the liver (9), neither MCP-1 nor γIP-10 mRNA accumulation was induced in either spleen or bone marrow immediately following infection. In the bone marrow of BALB/c mice, no significant changes in mRNA accumulation were subsequently observed until days 28 to 42 p.i. In the spleen significant levels of GM-CSF and M-CSF were only detectable at day 14 p.i. in one of two independent experiments. By days 28 to 42 p.i., significant increases in accumulation of mRNA for the growth factors G-CSF, M-CSF, and GM-CSF occurred in the bone marrow and spleens of BALB/c mice, correlating with late expansion of myeloid progenitor cell numbers and proliferative capacity (Fig. 2 and 3 and Table 2). In contrast, while later increases in the expression of CSFs were observed in the femurs of scid mice, accumulation of mRNA for myeloid growth factors was not apparent in the spleen. This restriction of CSF production to the bone marrow microenvironment may account for the selective inability of scid mice to increase splenic progenitor proliferation following L. donovani infection (Table 2). Together, these data indicate that the expression of cytokines and chemokines with hematopoietic activity is subject to both T-cell-dependent and T-cell-independent, as well as tissue-specific, regulation.
TABLE 3.
L. donovani infection of BALB/c and scid mice induces the accumulation of mRNA for hematopoietic growth factors in the bone marrow
| Cytokine or chemokine | Fold increase in mRNA accumulation over age-matched naïve control (p.i.)
|
|||||
|---|---|---|---|---|---|---|
| BALB/c
|
scid
|
|||||
| 5 h | 14 days | 42 daysb | 5 h | 14 days | 42 daysb | |
| Expt 1 | ||||||
| G-CSF | 1.04 ± 0.14c | 1.20 ± 0.37 | 1.65 ± 0.21d | 1.32 ± 0.21 | 1.10 ± 0.28 | 1.22 ± 0.21 |
| GM-CSF | 1.77 ± 0.11 | 1.30 ± 0.20 | 2.99 ± 0.18 | 1.67 ± 0.29 | 3.99 ± 1.38 | 1.69 ± 0.10 |
| IL-1β | 1.17 ± 0.49 | 0.95 ± 0.57 | 1.32 ± 0.29 | 1.56 ± 0.60 | 1.05 ± 0.18 | 1.09 ± 0.27 |
| IL-3 | 1.00 ± 0.02 | 1.01 ± 0.03 | 0.96 ± 0.41 | 0.67 ± 1.15 | 1.00 ± 0.06 | 1.23 ± 0.41 |
| M-CSF | 0.99 ± 0.16 | 1.45 ± 0.04 | 1.74 ± 0.21 | 1.38 ± 0.01 | 1.04 ± 0.29 | 1.42 ± 0.19 |
| SCF | 1.13 ± 0.22 | 0.98 ± 0.24 | 1.33 ± 0.62 | 1.76 ± 0.70 | 1.04 ± 0.31 | 1.77 ± 0.63 |
| MIP-1α | 1.83 ± 0.09 | 0.80 ± 0.30 | 1.23 ± 0.14 | 2.46 ± 0.70 | 0.85 ± 0.10 | 1.12 ± 0.44 |
| MCP-1 | 1.07 ± 1.59 | 0.96 ± 0.29 | 1.44 ± 0.30 | 0.91 ± 0.13 | 0.73 ± 0.40 | 1.33 ± 0.18 |
| γIP-10 | NDe | ND | ND | ND | ND | ND |
| Expt 2 | ||||||
| G-CSF | 1.23 ± 0.16 | 1.26 ± 0.31 | 1.76 ± 0.22 | 0.97 ± 0.22 | 1.08 ± 0.61 | 1.41 ± 0.12 |
| GM-CSF | 1.87 ± 0.26 | 1.54 ± 0.08 | 1.82 ± 0.3 | 1.48 ± 0.08 | 1.07 ± 0.28 | 1.62 ± 0.34 |
| M-CSF | 1.58 ± 0.46 | 1.79 ± 0.70 | 3.46 ± 0.48 | 0.79 ± 0.30 | 1.01 ± 0.70 | 1.32 ± 0.10 |
| MIP-1α | 1.31 ± 0.10 | 1.16 ± 0.12 | 1.00 ± 0.35 | 1.43 ± 0.15 | 1.66 ± 1.08 | 0.62 ± 0.29 |
mRNA for hematopoietic growth factors was measured by RT-PCR in bone marrow of BALB/c and scid mice infected with L. donovani. After normalizing against expression of HPRT, the fold increase in expression of each cytokine or chemokine was calculated for individual infected mice relative to the mean expression in age-matched naïve controls. In experiment 2, there were no significant changes in expression of IL-1β, IL-3, SCF, MCP-1, and γIP-10. These data are omitted for clarity.
Twenty-eight days for experiment 2.
Values represent the mean ± standard error of the mean for three animals analyzed individually at each time point.
Data in boldface represent significant statistical differences between naïve and infected mice (P < 0.05).
ND, undetectable levels of expression.
TABLE 4.
L. donovani infection of BALB/c and scid mice induces the accumulation of mRNA for hematopoietic growth factors in the spleen
| Cytokine or chemokine | Fold increase in mRNA accumulation over age-matched naïve control (p.i.)
|
|||||
|---|---|---|---|---|---|---|
| BALB/c
|
scid
|
|||||
| 5 h | 14 days | 42 daysb | 5 h | 14 days | 42 daysb | |
| Expt 1 | ||||||
| G-CSF | 0.91 ± 0.55c | 1.26 ± 0.17 | 1.46 ± 0.23 | 0.85 ± 0.11 | 1.10 ± 0.23 | 1.44 ± 0.28 |
| GM-CSF | 1.10 ± 1.63 | 2.08 ± 0.48 | 3.21 ± 0.46 | 1.16 ± 0.11 | 1.30 ± 0.22 | 1.46 ± 0.29 |
| IL-1β | 1.30 ± 0.24 | 0.89 ± 0.18 | 1.22 ± 0.16 | 1.01 ± 0.21 | 0.66 ± 0.43 | 1.20 ± 0.31 |
| IL-3 | 0.93 ± 0.56 | 1.67 ± 2.90 | 1.03 ± 0.76 | 1.00 ± 0.02 | 1.33 ± 2.30 | 1.04 ± 0.11 |
| M-CSF | 0.68 ± 0.61 | 1.30 ± 0.08 | 1.49 ± 0.15 | 0.81 ± 0.19 | 1.16 ± 0.19 | 1.52 ± 0.36 |
| SCF | 1.27 ± 0.19 | 0.74 ± 0.34 | 1.22 ± 0.11 | 1.10 ± 0.24 | 1.05 ± 0.33 | 1.31 ± 0.21 |
| MIP-1α | 1.37 ± 0.09d | 0.74 ± 0.34 | 1.33 ± 0.19 | 1.42 ± 0.14 | 1.07 ± 0.17 | 1.25 ± 0.37 |
| MCP-1 | 0.85 ± 0.11 | 0.53 ± 0.45 | 1.44 ± 0.36 | 0.75 ± 0.18 | 1.08 ± 1.03 | 2.06 ± 1.23 |
| γIP-10 | 1.10 ± 0.65 | 1.32 ± 0.41 | 1.20 ± 0.34 | 0.98 ± 0.33 | 1.02 ± 0.16 | 1.36 ± 0.41 |
| 1.04 ± 0.13 | 1.31 ± 0.22 | 1.72 ± 0.23 | 1.22 ± 0.15 | 1.45 ± 0.38 | 1.29 ± 0.02 | |
| Expt 2 | ||||||
| G-CSF | 1.04 ± 0.13 | 1.31 ± 0.22 | 1.72 ± 0.23 | 1.22 ± 0.15 | 1.45 ± 0.38 | 1.29 ± 0.02 |
| GM-CSF | 1.26 ± 0.40 | 1.35 ± 0.53 | 2.78 ± 0.32 | 1.38 ± 0.10 | 1.76 ± 1.76 | 1.78 ± 0.34 |
| M-CSF | 1.11 ± 0.41 | 0.61 ± 0.29 | 2.50 ± 0.17 | 1.16 ± 0.52 | 1.53 ± 0.26 | 1.50 ± 0.41 |
| MIP-1α | 2.82 ± 0.51 | 1.86 ± 0.59 | 1.20 ± 0.43 | 2.61 ± 0.40 | 1.53 ± 0.36 | 0.56 ± 0.36 |
mRNA for hematopoietic growth factors was measured by RT-PCR in the spleens of BALB/c and scid mice infected with L. donovani. After normalizing against expression of HPRT, the fold increase in expression of each cytokine or chemokine was calculated for individual infected mice relative to the mean expression in age-matched naïve controls. In experiment 2, there were no significant changes in expression of IL-1β, IL-3, SCF, MCP-1, and γIP-10. These data are omitted for clarity.
Twenty-eight days in experiment 2.
Values represent the mean ± standard error of the mean for three animals analyzed individually at each time point.
Data in boldface represent significant statistical differences between naïve and infected mice (P < 0.05).
DISCUSSION
L. donovani infection of genetically susceptible mice results in a resolving parasite burden in the liver and a relatively persistent infection in the spleen and bone marrow (references 23 and 48 and this study). Here, we have characterized for the first time the local changes in hematopoietic activity in tissues which harbor persistent parasites and compared it with both parasite dynamics and the evolution of cytokine and chemokine responses in these organs. While the picture which emerges is undoubtedly one of extreme complexity, there are nevertheless a number of specific observations which warrant further comment.
First, L. donovani infection caused a rapid (5-h), yet transient, increase in progenitor cell frequency in the blood but not the spleen or bone marrow. Rapid mobilization of hematopoietic stem cells and progenitor cells from the bone marrow to the peripheral blood has also been described following the administration of lipopolysaccharide (18), as well as a variety of cytokines, including GM-CSF (42), G-CSF (34, 36), SCF (5), IL-8 (22), IL-12 (20), and MIP-1α (6). Although the mechanisms of cytokine-induced cellular mobilization have not been fully elucidated, the modification of adhesive interactions with stromal cells is thought to play a role (40, 53). However, the mechanisms of mobilization may differ among eliciting agents (43). Mobilization of progenitor cells immediately following L. donovani infection correlated with an increase in femoral mRNA accumulation for GM-CSF and MIP-1α at 5 h p.i., suggesting these two factors play a crucial role following L. donovani infection. Others have also described transient progenitor cell mobilization in response to GM-CSF (42). Although mobilization of stem cells and progenitors following cytokine or lipopolysaccharide administration is generally associated with a redistribution of hematopoietic precursors to the spleen (18, 34, 42), we were unable to detect any increases in spleen cell progenitor numbers within the first week of infection. However, since mRNA accumulation for MIP-1α was also induced in the spleens of infected mice, mobilization and recruitment of progenitor cells may balance each other in this organ. These data suggest a fundamental difference in tissue recruitment and activation of progenitor cells following L. donovani infection compared to that reported in other models.
Second, our data provide further evidence of tissue-specific regulation of cytokine and chemokine expression. We have previously demonstrated that L. donovani infection triggered a transient T-cell-independent chemokine response in the livers of infected mice. This response, largely mediated by Kupffer cells, involves early production of MIP-1α, MCP-1, and γIP-10 (9). It is apparent from the current study that while T-cell-independent responses also occur in other tissues, they are more restricted in nature. Thus, both bone marrow and spleen produce MIP-1α yet fail to accumulate significant levels of mRNA for MCP-1 or γIP-10. Furthermore, whereas there is T-cell-independent expression of GM-CSF in the bone marrow (Table 3), it is absent from the spleen (Table 4). The functional significance of these findings remains to be fully established. However, it is of interest that we recently postulated that γIP-10 is the key chemokine required to initiate effective host protective responses in the liver (9) and that in two organs in which parasites persist, we have failed to detect significant levels of this chemokine. Furthermore, we have recently demonstrated that L. donovani infection of a bone marrow-derived stromal macrophage line significantly affects its ability to regulate the hematopoietic activity of progenitor populations, under conditions where growth factors are limiting (8). Amastigote infection of these stromal macrophages selectively enhances CFU-GM production by a mechanism involving GM-CSF acting in concert with tumor necrosis factor alpha. Although MIP-1α is also produced by these cells, antibody neutralization experiments indicate that this chemokine plays no role in supporting colony growth in vitro. These data suggest that the major role of MIP-1α is, as discussed above, related to progenitor cell mobilization.
Third, as infection progressed the frequencies of CFU-GEMM, CFU-GM, and BFU-E increased in the bone marrow, spleen, and peripheral blood. This correlated with increased progenitor cell cycling in both the spleen and bone marrow and increased expression of mRNA for the growth factors GM-CSF, G-CSF, and M-CSF. These CSFs, together with IL-3, are usually described as the dominant molecules controlling the production and maturation of granulocytes (IL-3, GM-CSF, and G-CSF), and monocytes (IL-3, GM-CSF, and M-CSF) from more primitive, multipotential precursors (28, 52). Nevertheless, we have been unable to detect the accumulation of IL-3 mRNA in either the spleens or bone marrow of mice infected with L. donovani. Similarly, IL-3 protein has not been detected either in direct ex vivo cultures of spleen cells from long-term-infected mice (S. E. J. Cotterell, unpublished data) or in restimulation assays with leishmanial antigens (21). Hence, in contrast to the significant involvement of IL-3 in visceralizing stages of L. major infection (24), this cytokine plays a limited, if any, role in the alteration of hematopoietic activity caused by L. donovani infection. Interestingly, while the fold increase in expression of mRNA for each CSF was similar in the bone marrow and spleen, progenitor cell frequency and proliferative status was increased to a greater extent in the spleen than the bone marrow. Moreover, the myeloid progenitor number was selectively increased in the spleen, in comparison to similar increases in myeloid and erythroid hematopoiesis observed in the bone marrow. The significance of these observations is under further investigation.
Finally, this study reveals a striking correlation between increased hematopoietic activity and parasite growth in both the spleen and bone marrow. The “safe-target” hypothesis, where increased numbers of immature myeloid cells provide a site for rapid multiplication of amastigotes, has been frequently applied to infections with L. major (13, 33). In this model, both susceptibility to infection and myelopoiesis are regulated by IL-3 and GM-CSF (17, 19, 24), and the parasite has a tropism for immature myeloid cells (11, 33). In contrast, L. donovani infection occurs most readily in mature macrophage populations (11), IL-3 is limiting during infection (reference 21 and this report), and GM-CSF is required for resistance in the liver (35). Therefore, the increased level of myelopoiesis, if biologically relevant, may not serve the same function in these two models. In addition, it should be noted that parasite growth can be significant even in the absence of increases in local hematopoietic activity, as shown here in the spleens of scid mice. Investigation into the precise relationship between hematopoietic activity and parasite growth is hampered by the difficulties associated with long-term cytokine neutralization in vivo and the redundancy of hematopoietic growth factors in gene-targeted mice (37, 38). Nevertheless, the future development of conditional or cell-type-specific mutants should aid in addressing this question.
ACKNOWLEDGMENTS
This work was supported by grants from the Wellcome Trust and the British Medical Research Council. S.E.J.C. is a recipient of a Wellcome Trust Prize Studentship, and C.R.E. is the recipient of a Wellcome Trust Career Development Award.
REFERENCES
- 1.Baker R, Chiodini P, Kaye P M. Leishmaniasis. In: Geraint James D, Zumla A, editors. The granulomatous disorders. Cambridge, United Kingdom: Cambridge University Press; 1999. pp. 212–234. [Google Scholar]
- 2.Behringer D, Kresin V, Henschler R, Mertelsmann R, Lindemann A. Cytokine and chemokine production by CD34+ haemopoietic progenitor cells: detection in single cells. Br J Haematol. 1997;97:9–14. doi: 10.1046/j.1365-2141.1997.d01-2143.x. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell J M, Hale C, Roberts M B, Ulczak O M, Liew F Y, Howard J G. An H-11-linked gene has a parallel effect on Leishmania major and L. donovani infections in mice. Immunogenetics . 1985;21:385–395. doi: 10.1007/BF00430803. [DOI] [PubMed] [Google Scholar]
- 4.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 5.Briddell R A, Hartley C A, Smith K A, McNiece I K. Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential. Blood. 1993;82:1720–1723. [PubMed] [Google Scholar]
- 6.Broxmeyer H E, Mantel C R, Aronica S M. Biology and mechanisms of action of synergistically stimulated myeloid progenitor cell proliferation and suppression by chemokines. Stem Cells. 1997;15:69–77. doi: 10.1002/stem.5530150811. [DOI] [PubMed] [Google Scholar]
- 7.Clark B R, Gallagher J T, Dexter T M. Cell adhesion in the stromal regulation of haemopoiesis. Baillieres Clin Haematol. 1992;5:619–652. doi: 10.1016/s0950-3536(11)80010-7. [DOI] [PubMed] [Google Scholar]
- 8.Cotterell, S., C. Engwerda, and P. Kaye. Leishmania donovani infection of bone marrow stromal marcrophages selectively enhances myelopoiesis, by a mechanism involving GM-CSF and TNF-alpha. Blood, in press. [PubMed]
- 9.Cotterell S E, Engwerda C R, Kaye P M. Leishmania donovani infection initiates T cell-independent chemokine responses, which are subsequently amplified in a T cell-independent manner. Eur J Immunol. 1999;29:203–214. doi: 10.1002/(SICI)1521-4141(199901)29:01<203::AID-IMMU203>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Crocker P R, Gordon S. Isolation and characterization of resident stromal macrophages and hematopoietic cell clusters from mouse bone marrow. J Exp Med. 1985;162:993–1014. doi: 10.1084/jem.162.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies E V, Singleton A M, Blackwell J M. Differences in Lsh gene control over systemic Leishmania major and Leishmania donovani or Leishmania mexicana mexicana infections are caused by differential targeting to infiltrating and resident liver macrophage populations. Infect Immun. 1988;56:1128–1134. doi: 10.1128/iai.56.5.1128-1134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dexter T M, Allen T D, Lajtha L G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 13.Djoko-Tamnou J, Leclerc C, Modabber F, Chedid L. Studies on visceral Leishmania tropica infection in BALB/c mice. I. Clinical features and cellular changes. Clin Exp Immunol. 1981;46:493–498. [PMC free article] [PubMed] [Google Scholar]
- 14.Dorshkind K, Keller G M, Phillips R A, Miller R G, Bosma G C, O'Toole M, Bosma M J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984;132:1804–1808. [PubMed] [Google Scholar]
- 15.Engwerda C R, Murphy M L, Cotterell S E, Smelt S C, Kaye P M. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol. 1998;28:669–680. doi: 10.1002/(SICI)1521-4141(199802)28:02<669::AID-IMMU669>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Engwerda C R, Smelt S C, Kaye P M. An in vivo analysis of cytokine production during Leishmania donovani infection in scid mice. Exp Parasitol. 1996;84:195–202. doi: 10.1006/expr.1996.0105. [DOI] [PubMed] [Google Scholar]
- 17.Feng Z Y, Louis J, Kindler V, Pedrazzini T, Eliason J F, Behin R, Vassalli P. Aggravation of experimental cutaneous leishmaniasis in mice by administration of interleukin 3. Eur J Immunol. 1988;18:1245–1251. doi: 10.1002/eji.1830180815. [DOI] [PubMed] [Google Scholar]
- 18.Gao J L, Wynn T A, Chang Y, Lee E J, Broxmeyer H E, Cooper S, Tiffany H L, Westphal H, Kwon-Chung J, Murphy P M. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greil J, Bodendorfer B, Rollinghoff M, Solbach W. Application of recombinant granulocyte-macrophage colony-stimulating factor has a detrimental effect in experimental murine leishmaniasis. Eur J Immunol. 1988;18:1527–1533. doi: 10.1002/eji.1830181009. [DOI] [PubMed] [Google Scholar]
- 20.Jackson J D, Yan Y, Brunda M J, Kelsey L S, Talmadge J E. Interleukin-12 enhances peripheral hematopoiesis in vivo. Blood. 1995;85:2371–2376. [PubMed] [Google Scholar]
- 21.Kaye P M, Curry A J, Blackwell J M. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991;146:2763–2770. [PubMed] [Google Scholar]
- 22.Laterveer L, Lindley I J, Hamilton M S, Willemze R, Fibbe W E. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85:2269–2275. [PubMed] [Google Scholar]
- 23.Leclercq V, Lebastard M, Belkaid Y, Louis J, Milon G. The outcome of the parasitic process initiated by Leishmania infantum in laboratory mice: a tissue-dependent pattern controlled by the Lsh and MHC loci. J Immunol. 1996;157:4537–4545. [PubMed] [Google Scholar]
- 24.Lelchuk R, Graveley R, Liew F Y. Susceptibility to murine cutaneous leishmaniasis correlates with the capacity to generate interleukin 3 in response to leishmania antigen in vitro. Cell Immunol. 1988;111:66–76. doi: 10.1016/0008-8749(88)90051-2. [DOI] [PubMed] [Google Scholar]
- 25.Marchal G, Milon G. Control of hemopoiesis in mice by sensitized L3T4+ Lyt2-lymphocytes during infection with bacillus Calmette-Guerin. Proc Natl Acad Sci USA. 1986;83:3977–3981. doi: 10.1073/pnas.83.11.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchal G, Milon G. Decreased erythropoiesis: the origin of the BCG induced anaemia in mice. Br J Haematol. 1981;48:551–560. doi: 10.1111/j.1365-2141.1981.tb02752.x. [DOI] [PubMed] [Google Scholar]
- 27.Marchal G, Milon G. Numeration of DTH-mediating T lymphocytes in mice under optimal titration conditions. Ann Immunol (Paris) 1984;135C:353–364. doi: 10.1016/s0769-2625(84)80965-4. [DOI] [PubMed] [Google Scholar]
- 28.Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science. 1991;254:529–533. doi: 10.1126/science.1948028. [DOI] [PubMed] [Google Scholar]
- 29.Milon G, Marchal G, Lebastard M. BDG infection in mice: a model for in vivo analysis of T lymphocytes as regulators of haemopoiesis. Ann Immunol. 1984;135C:291–294. [Google Scholar]
- 30.Milon G, Goossens P, Marchal G. Role of T lymphocytes during murine BCG infection. In: van Furth R, editor. Mononuclear phagocytes—characteristics, physiology and function. Boston, Mass: Martinus Nijhoff Publishers; 1985. pp. 553–559. [Google Scholar]
- 31.Milon G, Gheorghiu M, Lagranderie M, Lebastard M, Marchal G. BCG-induced anaemia in mice: no direct effect of the growth of bacilli. Ann Immunol (Paris) 1984;135C:195–204. doi: 10.1016/s0769-2625(84)81153-8. [DOI] [PubMed] [Google Scholar]
- 32.Miralles G D, Stoeckle M Y, McDermott D F, Finkelman F D, Murray H W. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect Immun. 1994;62:1058–1063. doi: 10.1128/iai.62.3.1058-1063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirkovich A M, Galelli A, Allison A C, Modabber F Z. Increased myelopoiesis during Leishmania major infection in mice: generation of ‘safe targets,’ a possible way to evade the effector immune mechanism. Clin Exp Immunol. 1986;64:1–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Molineux G, Pojda Z, Dexter T M. A comparison of hematopoiesis in normal and splenectomized mice treated with granulocyte colony-stimulating factor. Blood. 1990;75:563–569. [PubMed] [Google Scholar]
- 35.Murray H W, Cervia J S, Hariprashad J, Taylor A P, Stoeckle M Y, Hockman H. Effect of granulocyte-macrophage colony-stimulating factor in experimental visceral leishmaniasis. J Clin Investig. 1995;95:1183–1192. doi: 10.1172/JCI117767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neben S, Marcus K, Mauch P. Mobilization of hematopoietic stem and progenitor cell subpopulations from the marrow to the blood of mice following cyclophosphamide and/or granulocyte colony-stimulating factor. Blood. 1993;81:1960–1967. [PubMed] [Google Scholar]
- 37.Nilsson S K, Lieschke G J, Garcia-Wijnen C C, Williams B, Tzelepis D, Hodgson G, Grail D, Dunn A R, Bertoncello I. Granulocyte-macrophage colony-stimulating factor is not responsible for the correction of hematopoietic deficiencies in the maturing op/op mouse. Blood. 1995;86:66–72. [PubMed] [Google Scholar]
- 38.Nishinakamura R, Miyajima A, Mee P J, Tybulewicz V L, Murray R. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood. 1996;88:2458–2464. [PubMed] [Google Scholar]
- 39.O'Garra A, Stapleton G, Dhar V, Pearce M, Schumacher J, Rugo H, Barbis D, Stall A, Cupp J, Moore K, et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2:821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- 40.Papayannopoulou T, Craddock C, Nakamoto B, Priestley G V, Wolf N S. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson V M, Adamovicz J J, Elliott T B, Moore M M, Madonna G S, Jackson W E, III, Ledney G D, Gause W C. Gene expression of hematoregulatory cytokines is elevated endogenously after sublethal gamma irradiation and is differentially enhanced by therapeutic administration of biologic response modifiers. J Immunol. 1994;153:2321–2330. [PubMed] [Google Scholar]
- 42.Pojda Z, Molineux G, Dexter T M. Effects of long-term in vivo treatment of mice with purified murine recombinant GM-CSF. Exp Hematol. 1989;17:1100–1104. [PubMed] [Google Scholar]
- 43.Pruijt J F, van Kooyk Y, Figdor C G, Lindley I J, Willemze R, Fibbe W E. Anti-LFA-1 blocking antibodies prevent mobilization of hematopoietic progenitor cells induced by interleukin-8. Blood. 1998;91:4099–4105. [PubMed] [Google Scholar]
- 44.Quesenberry P, McElrath E, Buxton E, Kittler E, Crittendon R, Temeles D. Constitutive production of multiple hemopoietic growth factors by murine Dexter stroma. Blood. 1990;76:161a. [Google Scholar]
- 45.Rhoades E R, Cooper A M, Orme I M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63:3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saha B, Nanda-Roy H, Pakrashi A, Chakrabarti R N, Roy S. Immunobiological studies on experimental visceral leishmaniasis. I. Changes in lymphoid organs and their possible role in pathogenesis. Eur J Immunol. 1991;21:577–581. doi: 10.1002/eji.1830210307. [DOI] [PubMed] [Google Scholar]
- 47.Simmons P J, Levesque J P, Zannettino A C. Adhesion molecules in haemopoiesis. Baillieres Clin Haematol. 1997;10:485–505. doi: 10.1016/s0950-3536(97)80022-4. [DOI] [PubMed] [Google Scholar]
- 48.Smelt S C, Engwerda C R, McCrossen M, Kaye P M. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158:3813–3821. [PubMed] [Google Scholar]
- 49.Stauber L A. Host resistance to the Khartoum strain of Leishmania donovani. Rice Institute Pamphlet. 1958;45:80–86. [Google Scholar]
- 50.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 51.Testa N J, Dexter T M. The regulation of haematopoietic cell production. In: Hoffbrand L V A, Lewis S M, Tuddenham E G D, editors. Postgraduate hematology. Oxford, United Kingdom: Butterworth/Heinemann; 1999. pp. 1–12. [Google Scholar]
- 52.Valledor A F, Borras F E, Cullell-Young M, Celada A. Transcription factors that regulate monocyte/macrophage differentiation. J Leukoc Biol. 1998;63:405–417. doi: 10.1002/jlb.63.4.405. [DOI] [PubMed] [Google Scholar]
- 53.Vermeulen M, Le Pesteur F, Gagnerault M C, Mary J Y, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 54.Weiss L. Mechanisms of splenic control of murine malaria: cellular reactions of the spleen in lethal (strain 17XL) Plasmodium yoelii malaria in BALB/c mice, and the consequences of pre-infective splenectomy. Am J Trop Med Hyg. 1989;41:144–160. doi: 10.4269/ajtmh.1989.41.144. [DOI] [PubMed] [Google Scholar]
- 55.Wilson M E, Sandor M, Blum A M, Young B M, Metwali A, Elliott D, Lynch R G, Weinstock J V. Local suppression of IFN-gamma in hepatic granulomas correlates with tissue-specific replication of Leishmania chagasi. J Immunol. 1996;156:2231–2239. [PubMed] [Google Scholar]
- 56.Young A M, Cheers C. Colony-forming cells and colony-stimulating activity during listeriosis in genetically resistant or susceptible mice. Cell Immunol. 1986;97:227–237. doi: 10.1016/0008-8749(86)90393-x. [DOI] [PubMed] [Google Scholar]
- 57.Zhan Y, Lieschke G J, Grail D, Dunn A R, Cheers C. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood. 1998;91:863–869. [PubMed] [Google Scholar]