Abstract
The interaction of viridans streptococci with components of the extracellular matrix (ECM) plays an important role in the pathogenesis of infective endocarditis. We have identified a surface protein of Streptococcus mutans which binds the ECM constituent fibronectin (Fn). Initially, we found that S. mutans could adsorb soluble Fn in plasma, but with lower efficiency than Streptococcus pyogenes. In addition, S. mutans could bind immobilized Fn in a dose-dependent manner when tested using an enzyme-linked immunosorbent assay. Crude extracts of cell wall-associated proteins or extracellular proteins from S. mutans MT8148 specifically bound Fn through a protein with the molecular mass of ca. 130 kDa, as detected by far-Western immunoblotting. The candidate Fn binding protein (FBP-130) was purified to near homogeneity by using Fn coupled Sepharose 4B affinity column chromatography. A rabbit polyclonal antibody against FBP-130 reacted specifically with a protein of molecular mass of ca. 130 kDa in both cell wall and extracellular fractions, and the abundance of FBP was higher in the former than in the latter fractions. The purified FBP bound specifically to immobilized Fn, whereas the binding of soluble Fn to coated FBP could only be detected in the presence of high concentrations of Fn. The purified FBP, as well as anti-FBP immunoglobulin G, inhibited the adherence of S. mutans to immobilized Fn and endothelial cells (ECV304) in a dose-dependent manner. These results demonstrated that FBP-130 mediated the adherence of S. mutans specifically to Fn and endothelial cells in vitro. The characteristics of S. mutans and FBP-130 in binding Fn confirmed that viridans streptococci adopt different strategies in their interaction with ECM.
Viridans streptococci are a heterogeneous group of gram-positive bacteria that are commensal habitants of the human oral cavity. In addition to dental caries and dental related pyogenic infections, oral streptococci are also important agents of infective endocarditis (2, 35, 37). More than 20% of cases of viridans streptococci-induced endocarditis are caused by S. mutans, which is a primary pathogen of dental caries (14, 22). In Taiwan, Streptococcus oralis and Streptococcus sanguis are isolated most frequently from blood cultures in endocarditis, but S. mutans is responsible for the highest incidence of endocarditis in bacteremia-associated pyogenic infections (3). These findings suggest that S. mutans, when present in the circulation, has a predilection for colonizing damaged heart valve or tissues.
The mechanisms by which S. mutans and other viridans streptococci cause bacteremia and colonize heart valves are still not clear. It has been suggested that bacterial binding to components of the extracellular matrix (ECM), e.g., fibrin, platelets, and fibronectin (Fn), is crucial in the development of endocarditis (27). These components, which would not normally be exposed or deposited on healthy vascular tissues, may act as receptors for circulating bacteria. Fn is a dimeric glycoprotein found in a soluble form in plasma and in a fibrillar form in the ECM. Fn is composed of distinct domains that bind to a number of proteins, including integrins, collagens, fibrin, gelatin, and heparin (13). Binding to Fn has been shown to be an important virulence factor of streptococci and staphylococci causing endocarditis (18, 19, 29). Mutant strains of either S. sanguis or Staphylococcus aureus, defective in Fn binding, were constructed by transposon inactivation mutagenesis and were found to be less virulent than the parental strains in a rat model of endocarditis (18, 19). An interesting observation was that soluble Fn did not inhibit the binding of S. sanguis to immobilized Fn. It was proposed that S. sanguis binds to a conformationally specific domain on the immobilized Fn molecule that is not exposed on soluble Fn (20). However, the Fn binding receptor of S. sanguis was not identified in these experiments. Similar characteristics also were observed for another member of the sanguis group, Streptococcus gordonii. The preferential binding of S. gordonii to immobilized Fn was mediated by two surface proteins, CshA and CshB, with molecular masses of ca. 259 and 245 kDa, respectively (23). No information is available at present on the role of CshA or CshB in the pathogenesis of infective endocarditis or on the Fn binding characteristics of other viridans streptococci, such as S. mutans.
We have begun to study the mechanisms of S. mutans adherence by analyzing the plasma components adsorbed by this microorganism. Various strains of S. mutans were incubated with fresh plasma over various time intervals. The adsorbed components were analyzed by gel electrophoresis, and specific antiserum or monoclonal antibodies (MAbs) confirmed the proteins of interest. We present here data indicating that S. mutans can bind soluble and immobilized Fn in a manner distinct from S. sanguis. Fn enhances the binding of S. mutans to endothelial cells tested in vitro. We have also identified a cell wall-associated protein, FBP-130, as a receptor which binds Fn. The specific binding of S. mutans and FBP-130 to Fn was demonstrated by saturation binding and antibody inhibition studies.
MATERIALS AND METHODS
Bacteria.
All streptococcal strains were grown in Todd-Hewitt broth (Difco Laboratories, Inc., Detroit, Mich.) for 18 h at 37°C. Strains were stored at −80°C until needed. Tetracycline (Tc) and erythromycin (Em) were added to the media, as required, at concentrations of 10 and 5 μg/ml, respectively. S. mutans LN62DD, NHR1DD, NHS1, and NHS1DD, which are isogenic mutants expressing only GtfB (1), GtfC (11), GtfD (12), or no Gtf proteins, respectively, were provided by H. K. Kuramitsu (State University of New York, Buffalo). S. mutans XC strain was provided by T. Koga (Kyushu University). S. mutans MT8148R was provided by S. Hamada (Osaka University). S. sanguis ATCC 10549 and S. pyogenes ATCC 12345 were purchased from the American Type Culture Collection (ATCC).
Adsorption of Fn by streptococci.
Bacteria were harvested from overnight cultures, washed, and resuspended in phosphate-buffered saline (PBS) at 1010 cells/ml. Bacterial samples were mixed with 100 μl of human plasma, and the mixtures were incubated at room temperature for 5 to 30 min. After centrifugation, the pelleted bacteria were washed with 1.5 ml of PBSAT (PBS with 0.02% sodium azide and 0.05% Tween 20). Bound proteins and cell-wall-associated proteins were eluted with 8 M urea and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by silver staining or electrophoretic transfer to Hybond-P Super Membrane (Amersham, Buckinghamshire, United Kingdom) for Western blot analysis. Far-Western immunoblotting was used to detect the interaction between Fn-binding protein (FBP) and the cell surfaces of S. mutans and S. pyogenes. Briefly, cell-wall-associated proteins from these bacteria were extracted with 8 M urea. Proteins were separated by SDS-PAGE, transferred to Hybond-P membrane, and subsequently incubated with purified Fn (5 μg/ml). The Fn adsorbed by the candidate FBPs were reacted with anti-Fn MAb and detected by horseradish peroxidase (enhanced chemiluminescence [Amersham]).
Preparation and purification of FBP-130.
Human plasma was routinely collected from umbilical blood obtained from the Gynecology Department of National Taiwan University Hospital. Plasma Fn was purified by affinity chromatography as described previously (8, 9) or obtained commercially (Sigma). Gelatin-Sepharose columns were obtained commercially (Pharmacia, Uppsala, Sweden). Crude extracts of cell-wall-associated and extracellular proteins were prepared from 10-liter cultures of S. mutans MT-8148 as described previously (5, 6). Briefly, cells S. mutans MT8148 from the stationary phase were washed extensively with 10 mM sodium phosphate buffer and incubated with 8 M urea extraction buffer for 1 h at 25°C. The extract was then dialyzed against 10 mM sodium phosphate buffer (pH 6.5) to remove urea and subsequently concentrated by 60% (saturation) amonium sulfate precipitation, dialyzed against the same buffer containing 1 mM phenylmethylsulfonyl fluoride. Fn-binding protein (FBP-130) was purified from crude extracts of the cell-wall-associated fraction, essentially by affinity chromatography.
Briefly, purified Fn (5 mg) was coupled to CNBr-activated Sepharose 4B (Pharmacia), and 0.5-ml portions of crude extracts were applied to the column and passed through an additional three times. Unbound proteins were eluted with PBS, and FBP-130 was eluted with 8 M urea elution buffer. Fractions from major peaks were pooled, dialyzed exhaustively against PBS, frozen, and stored at −80°C until needed. Homogeneity of the purified FBP-130 was analyzed by SDS-PAGE, followed by silver staining. The bands were analyzed with an Electrophoresis Documentation and Analysis System 120 (Scientific Imaging Systems; Eastman Kodak Co., New York, N.Y.), and the purity of the FBP was found to be 98.5%. The protein concentrations were measured by using a modification of the method of Lowry et al. (21), with bicinchonic acid as the colorimetric detection reagent (BCA Protein Assay Reagent; Pierce Chemical Co., Rockford, Ill.).
Preparation of antisera and MAbs.
Antisera against FBP-130 were prepared by intracutaneous injection of New Zealand White rabbits in the back of the neck with an inoculum (3 ml) of purified FBP, 250 μg/ml in PBS, emulsified in Freund complete adjuvant. Two subsequent booster injections were given intravenously at 2-week intervals, and antisera were collected, assayed for antibody titer, and stored at 4°C until needed. Anti-Fn MAb was a gift from Wu-Nan Wen (Department of Biochemistry, College of Medicine, National Taiwan University). Anti-GtfB/C rabbit serum was prepared as described previously (6). Anti-FBP or anti-GtfB/C immunoglobulin G (IgG) was purified by affinity chromatography with an ImmunoPure (IgG) Purification Kit (Pierce).
Adherence and Fn-binding assays.
Adherence assays were modifications of an enzyme-linked immunosorbent assay (ELISA) described previously (20). Briefly, purified Fn (Sigma) was immobilized on microtiter plates (F16, high-binding, microwell module; Nunc, Roskilde, Denmark) by adding 50 μl of the protein solution (250 ng to 5 μg/ml in 0.05 M sodium carbonate buffer, pH 9.6) to each well and incubating the plates for 16 h at 4°C. Plates were washed three times with PBS before use (all subsequent washes were the same). Wild-type and mutant S. mutans GS-5 were grown at 37°C to late stationary phase and harvested by centrifugation for 10 min at 8,000 rpm in a Beckman-12 microcentrifuge (Beckman Instruments, Inc., Palo Alto, Calif.). Each bacterial pellet was washed and resuspended in 0.5 ml of PBS and adjusted to an absorbance of 0.1 at 550 nm on a Novaspect II spectrophotometer (Pharmacia). Streptococcal chains were disrupted by sonication, and the bacterial suspension was then applied to the Fn-coated assay plates (100 μl/well). Microtiter plates were incubated at room temperature for 30 min with orbital shaking. Nonadherent bacteria were removed with several washes of PBS. Adherent bacteria were fixed to the plates with a 15-min incubation at 60°C. After they cooled to room temperature, a 1:2,000 dilution of antiserum against S. mutans (4) was added to each well, followed by incubation for 1 h at 37°C. Plates were washed with PBS, and a 1:10,000 dilution (in PBS with 5% bovine serum albumin [BSA]) of alkaline phosphatase-labeled goat anti-rabbit IgG (Sigma) was applied and incubated for 1 h at 37°C. The substrate p-nitrophenylphosphate (Sigma) was used as a chromophore, and color was allowed to develop for 30 min, after which the A450 was measured by using a MicroELISA reader (Dynatech Corp., Alexandria, Va.). To confirm that the absorbance detected by ELISA reflected directly the number of adherent bacteria, bacteria were recovered, and the CFU were counted on brain heart infusion agar plates after anaerobic incubation for 48 h.
The binding activities of purified FBP-130 to soluble and immobilized Fn were measured by ELISA. Purified Fn (5 μg/ml) was immobilized on microtiter plates overnight at 4°C. After blocking with 1% BSA, purified FBP-130 or GtfC of various concentrations (0.1 to 8 μg/ml in PBS) was added, and the plates were incubated for 1 h at 37°C. Each concentration of the tested protein was tested in triplicate. After washing, the plates were processed as described above by using specific antibodies (anti-FBP or GtfC rabbit IgG). The procedure for detecting the binding of soluble Fn to FBP-130 was essentially the same, except that FBP-130 (5 μg/ml) was coated on the ELISA plates and the anti-Fn MAb was used. To determine the Fn-mediated adherence of S. mutans, an adherence assay was carried out using human endothelial cells ECV304 (ATCC) and bovine aortic endothelial cells (BAEC). Endothelial cell monolayers were maintained in 24-well tissue culture plates in RPMI 1640 medium supplemented with 10% fetal calf serum. To detect the indigenous and exogenous Fn bound to the endothelial cells, the monolayers were washed twice with serum-free AIM-V medium (GIBCO Laboratories, Grand Island, N.Y.) and incubated for 30 min at 37°C with anti-Fn MAb. After washing a further three times with serum-free AIM-V medium, monolayers were incubated at 37°C for 30 min with a 1:10,000 dilution of alkaline phosphatase-labeled goat anti-mouse IgG (Sigma). Finally, monolayers were washed three times with PBS, and Fn was detected by incubation with p-nitrophenylphosphate substrate (Sigma). For adherence assays, chain-disrupted bacteria were inoculated onto Fn-coated monolayers of endothelial cells, and plates were centrifuged gently (165 × g for 5 min) and then incubated for 30 min at 37°C in 5% CO2. The nonadherent organisms were removed by gently washing with serum-free M5 medium and PBS. The percentage of Fn-mediated adherence was calculated by dividing the number of adherent CFU per monolayer by the number of inoculated CFU.
Inhibition and competitive inhibition assay.
For inhibition assays, bacteria were preincubated 30 min with various concentrations of affinity column-purified anti-FBP, or control rabbit IgG, before binding to immobilized Fn on ELISA plates or to monolayers of endothelial cells (ECV304 or BAEC) coated with purified Fn. In the case of competitive inhibition, various concentrations of affinity-purified FBP-130 were preincubated for 30 min with the endothelial cell (ECV304 or BAEC) monolayers. After washing and removal of unbound FBP by centrifugation, bacteria were added to the wells, and the CFU of attached bacteria were determined. Inhibition was recorded as the percentage of bacteria attached (CFU), with IgG or in the presence of FBP, compared to the number of bacteria without IgG or in the absence of FBP.
RESULTS
With the long-term goal of identifying the adhesins of S. mutans involved in causing endocarditis, we began to analyze the plasma components adsorbed by this organism. Samples of plasma were supplemented with EDTA to prevent complement activation and adsorbed with S. mutans GS-5, a serotype c strain, for various time intervals. After adsorption, the bacterial cell surface proteins and the adsorbed plasma components were extracted and subjected to SDS-PAGE analysis. The S. mutans GS-5 could readily adsorb plasma proteins within 5 min, and the major components adsorbed were plasma proteins with molecular masses of ca. 255, 234, 71, 58, 50, and 42 kDa (Fig. 1A). The adsorption of plasma components appeared to be specific because some of the major components of the plasma fraction, such as albumin (67 kDa), were not bound to the bacteria and, therefore, were not detected on the gel (Fig. 1). Since the adsorption analysis could not distinguish between plasma components, bound proteins were eluted and subsequently analyzed by Western blotting with a specific antiserum that recognized plasma proteins. The adsorbed fractions, corresponding to the arrows 1 and 2 in Fig. 1A, were found to be Fn, which is present in human plasma in multiple forms, with molecular masses that range from 220 to 250 kDa due to differential splicing (13). The plasma Fn could readily be adsorbed by different strains of S. mutans, and adsorption could still be detected when the analysis was performed with purified Fn (Fig. 1B). When adsorption analysis was conducted with a fixed number of bacteria, S. mutans required a higher concentration of Fn for the bound Fn to be detected by Western blotting than did S. pyogenes (Fig. 1C). These results indicated that S. mutans could bind soluble Fn directly, rather than through interaction with other plasma components, and that its affinity for Fn was less than that of S. pyogenes, which has several Fn-binding proteins and can bind soluble Fn (7, 8, 10, 17, 26, 31, 32).
FIG. 1.
Adsorption of plasma components by S. mutans and Western blot analysis. The elution analysis was carried out with samples containing a fixed number of bacteria, which were incubated with fixed amounts of plasma (A) or purified Fn (B and C) for 5 to 30 min. Bound proteins were eluted with 8 M urea, and the elutes were subjected to SDS-PAGE analysis under reducing conditions, followed by silver staining (A) or detection with anti-Fn MAb (B and C). (A) Adsorption analysis of plasma components by whole cells of S. mutans GS-5. Incubation times are indicated at the top of each lane. Major plasma components adsorbed by S. mutans are indicated by arrows. o/n, S. mutans from overnight culture; plasma, plasma control; BHI, S. mutans incubated without plasma for 30 min. (B) Western blot analysis of purified Fn adsorbed by whole cells of different laboratory strains (as indicated on top of each lane). −, without Fn; +, with Fn; MTR, MT8148R. (C) Western blot analysis of dose-dependent adsorption of purified Fn by S. mutans and S. pyogenes (as indicated). The Fn adsorbed (arrow) was reacted with anti-Fn MAb and subsequently was detected by horseradish peroxidase-labeled goat anti-mouse IgG (Sigma). M, prestained molecular mass marker (in kilodaltons).
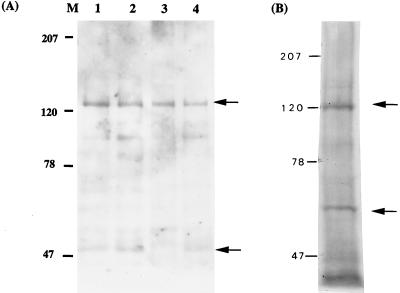
To confirm the binding of Fn by S. mutans and to identify the candidate adhesins responsible, cell surface proteins were extracted, and a far-Western immunoblot was carried out with purified Fn and an anti-Fn MAb. Membrane proteins of S. pyogenes served as a positive control in this experiment. Two proteins from S. pyogenes, of ca. 120 and 60 kDa, were detected clearly on the immunoblot (Fig. 2). Judging from the molecular masses, the former corresponds to the F1 or Sfb1 (10, 32) and the latter could be the FBP54 (7). In the case of S. mutans, two proteins, with molecular masses of ca. 130 and 55 kDa, were bound by Fn on the immunoblot (Fig. 2A). The binding ability of the 130-kDa protein appeared to be stronger than that of the 55-kDa protein, judging from the intensities on the blot. These two Fn-binding proteins also were identifiable in the cell-wall-associated fractions from another three isogenic mutants derived from S. mutans GS-5. All these mutants were defective in the expression of glucosyltransferases (GTFs), which are cell-wall-associated enzymes with molecular masses (after degradation) close to the protein bands detected (6). These results confirmed that S. mutans binds Fn specifically through the interaction of one or two cell surface protein components, and these are not GTFs or their degradation products.
FIG. 2.
Detection of Fn binding proteins of S. mutans (A) and S. pyogenes (B) by far-Western immunoblot. The cell-wall-associated proteins of S. mutans and S. pyogenes were transferred to polyvinylidene difluoride membrane and then incubated with purified Fn (0.2 μg) at room temperature for 18 h. After washing, the bound Fn was detected with anti-Fn MAb. (A) Lanes 1 to 4, GS-5, LN62DD, NHR1DD, and NHS1, respectively. Two positive bands at about 130 and 55 kDa were detected. (B) Two bands at around 120 and 60 kDa (arrows) were found in S. pyogenes.
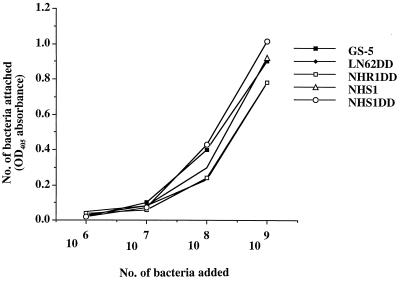
Adherence assays were carried out by ELISA to confirm the specificity of the interaction between Fn and surface components of S. mutans cells. The Fn was coated onto ELISA plates so that the binding ability of S. mutans to an immobilized form, rather than the soluble form, of Fn could be examined. Wild-type S. mutans GS-5 and the Gtf-defective isogenic mutants LN62DD, NHR1DD, NHS1, and NHS1DD bound immobilized Fn with equal efficiency in a dose-dependent manner and reached saturation at a cell density of 109 (Fig. 3). These results, in conjunction with the adsorption analysis, confirmed that S. mutans could bind both soluble and immobilized forms of human plasma Fn specifically, through cell surface protein components.
FIG. 3.
Dose-dependent adherence of S. mutans to immobilized Fn. Adherence assays were carried out in 96-well plates coated with purified Fn at 250 ng/well. Bacteria attached to Fn were detected with anti-S. mutans antiserum (■, GS-5; ⧫, LN62DD; □, NHR1DD; ▵, NHS1; ○, NHS1DD). Each point represents the mean value of triplicate experiments, and the standard deviation of individual sets of assays was within 10% of the average value. No significant difference was observed between the GS-5 and Gtf mutants in their abilities to adhere to immobilized Fn.
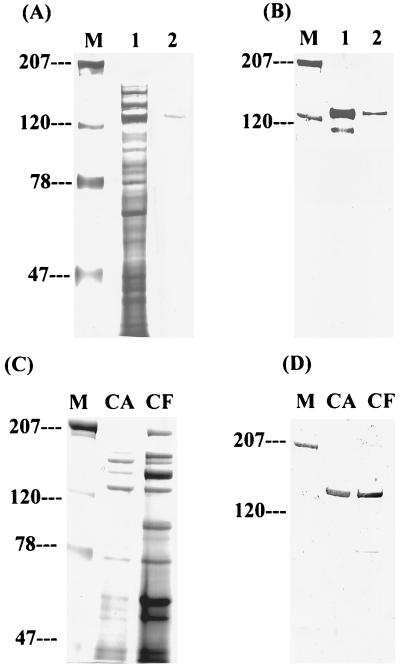
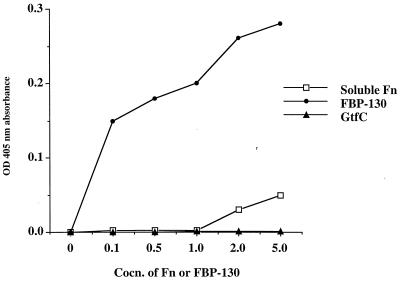
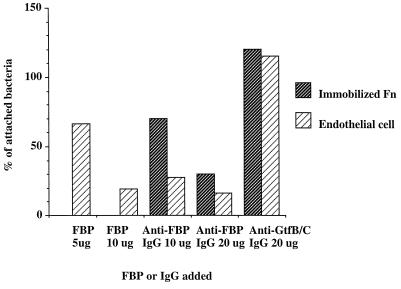
To purify candidate Fn-binding proteins, affinity column chromatography was established using CNBr-activated Sephorose coupled with Fn. Cell wall proteins were extracted and concentrated from a large batch of cultures (5, 6). A major band at around 130 kDa was obtained after eluting twice the Fn-treated affinity column (Fig. 4A), a result in agreement with the results obtained from far-Western blot analysis. The purified Fn-binding protein was named FBP-130 for its function and estimated molecular mass, based on the mobility on SDS-PAGE. Rabbit antiserum generated for FBP-130 specifically recognized the purified protein on a Western blot (Fig. 4B). The antiserum also reacted specifically with a protein of molecular mass ∼130 kDa in both cell wall and extracellular fractions, and the abundance of FBP was higher in the former than in the latter fraction (Fig. 4C and D). The localization of FBP-130 in the cell wall of S. mutans was further confirmed by examining different serotype c strains using fluorescent microscopy with purified rabbit polyclonal IgG against FBP-130. An ELISA was carried out to determine the specificity of binding of FBP-130 to Fn. The FBP-130 bound immobilized Fn specifically in a dose-dependent manner and reached saturation at a concentration of 5 μg/ml, whereas a control cell-wall-associated protein, purified GtfC, did not bind immobilized Fn (Fig. 5). Interestingly, Fn could only bind FBP-130 immobilized on an ELISA plate at a high concentration (Fig. 5). An inhibition ELISA was carried out by preincubation of bacteria with anti-FBP rabbit IgG to confirm the specificity of FBP-mediated adherence of S. mutans. Anti-FBP IgG, but not anti-GtfB/C IgG, specifically inhibited the binding of S. mutans to immobilized Fn in a dose-dependent manner, with up to 60% inhibition (Fig. 6). These results confirmed that FBP-130 of S. mutans binds Fn specifically and that the conformation of the Fn may affect the binding affinity.
FIG. 4.
Affinity purification and detection of FBP-130 from S. mutans. (A) Crude extracts of cell-wall-associated proteins (CA) were incubated with immobilized Fn. Unbound proteins were removed by extensive washing of the column, and bound protein was eluted with 8 M urea. The proteins were resolved on a 7.5% polyacrylamide gel stained with silver staining. Lane 1, 9 μg of crude extracts of CA proteins; lane 2, 0.75 μg of purified FBP-130. (B) Western blot analysis of FBP-130 in crude extracts (lane 1) and after purification (lane 2). The amount of protein loaded was the same as in panel A. The minor band detected in lane 1 may be a product of degradation of FBP-130. Anti-FBP rabbit serum at a dilution fold of 1:500 was used as primary antibody. (C) SDS-PAGE analysis of cell-wall-associated protein (CA) and extracellular (cell free [CF]) protein fractions, followed by Coomassie brilliant blue staining. The mass of total protein loaded for CA was 15 μg and for CF was 140 μg. (D) Detection of FBP-130 in cell-wall-associated fraction (CA) and extracellular fraction (CF) as determined by Western blot. The purified FBP migrated with a molecular size of approximately 130 kDa and was detectable predominantly in cell-wall-associated fractions. Anti-FBP rabbit serum at a dilution fold of 1:1,000 was used as primary antibody.
FIG. 5.
Specific and dose-dependent binding of FBP to soluble or immobilized Fn. Symbols: □, binding of soluble Fn to immobilized FBP; ●, binding of FBP to immobilized Fn; ▴, binding of GtfC to immobilized Fn. Each point represents the mean value of experiments done in triplicate, and the standard deviation of individual set of assays was within 5% of the average value. FBP specifically binds to immobilized Fn compared to purified GtfC. FBP at higher concentrations (6 and 8 μg/ml) did not give OD absorption values higher than that of 5 μg/ml; therefore, the saturation was achieved at a concentration of 5 μg/ml. Soluble Fn did not bind to FBP, unless a higher dose of soluble Fn (>5 μg) was added.
FIG. 6.
Inhibition and competitive inhibition by purified FBP or anti-FBP IgG of binding of S. mutans to immobilized Fn or monolayer endothelial cells (ECV304) preincubated with purified Fn (5 μg). Bacteria were preincubated with increased amounts of the FBP or IgG before binding to Fn (shaded bar) or endothelial cells (hatched bar). The number (CFU) of bacteria attached was quantified by direct counting of the colonies on mitis salivarius bacitracin (MSB) plates containing serially diluted mixtures of cells and bacteria trypsinized from the wells. Inhibition was expressed as the percentage of the number of bacteria treated compared to that of untreated bacteria, which was normalized to 100%. Each bar represents the mean value of experiments done in triplicate, and the standard deviation of each individual set of assays was within 8% of the average value.
Since the ability of bacteria to attach to endothelial cells is an important virulence factor in endocarditis (25), Fn-mediated adherence was examined using the endothelial cell line ECV304. Indigenous Fn could be detected on the surfaces of monolayers of ECV304, and S. mutans could bind ECV304 with an efficiency of 102 to 103 CFU per well. When exogenous Fn was added to a concentration of 5 μg/well, Fn was firmly attached to the ECV304 and enhanced about 100-fold (105 CFU/well) the attachment of S. mutans GS-5, as well as the GS-5-derived isogenic mutant, NHS1DD (no GTFs; data not shown). The inhibition and competitive inhibition experiments were conducted in the presence of exogenous Fn at a concentration of 5 μg/well to normalize the amount of Fn on ECV304. Preincubation with either FBP-130 or anti-FBP IgG inhibited the adherence of S. mutans to ECV304 in a dose-dependent manner (Fig. 6). Analogous results were obtained when different endothelial cells, BAEC, were analyzed. Therefore, the novel FBP, FBP-130, mediated specifically the adherence of S. mutans to Fn and endothelial cells in vitro.
DISCUSSION
Subacute bacterial endocarditis is an infectious disease associated with significant morbidity and mortality. Viridans streptococci, such as S. sanguis, S. mutans, S. gordonii, and S. oralis, are the major causative agents, accounting for 45 to 80% of cases (37). These streptococci can enter the blood through breaks in the oral microcirculation induced by trauma, including dental manipulations, oral hygiene procedures, mastication, and infections (24). These bacteremias may infect heart valves with underlying pathogenic changes, such as nonbacterial thrombotic vegetations (27). The adherent bacteria may also be embedded and protected in newly formed thrombi or platelet vegetations on the damaged heart valves. Consequently, initial adhesion and induction of thrombosis are considered to be important virulence traits of streptococci. Cumulative evidence has shown that binding to Fn, one of the major constituents of vegetations, is important in the development of infective endocarditis caused by various microorganisms. Fn-binding activity also is important for the pathogenesis of other bacterial infections, such as by S. aureus and S. pyogenes (8, 18). Therefore, it is interesting to investigate how these various pathogens bind to Fn. Unlike S. aureus and S. pyogenes, S. sanguis and S. gordonii preferentially bind immobilized, but not soluble, Fn (20, 23). In this investigation we identified a novel Fn-binding protein of S. mutans and confirmed that the binding of Fn by this member of the viridans streptococci is distinct from that of the sanguis group in several respects.
First, the plasma adsorption and far-Western analyses indicated that S. mutans could bind soluble Fn, even though the binding affinity was significantly lower than that of S. pyogenes. Similar experiments were conducted with strains of S. sanguis and S. gordonii: neither bound plasma or purified Fn in its soluble form and, therefore, no Fn signals were detected on immunoblots. These results also supported the concept that adsorption analysis alone could be used as a simple procedure for evaluating the binding ability of bacteria to soluble Fn or other serum components. A similar approach has been adopted recently to examine the binding of human complement inhibitor FHL-1 (factor H-like protein 1) by streptococcal M proteins (16). As shown in Fig. 1, at least four other plasma components were readily detectable on SDS-PAGE analysis, and further investigations are being carried out to identify them.
Other pathogenic microorganisms have been reported to interact differentially with soluble versus solid-phase Fn. Like the sanguis group of streptococci, group B streptococci and Yersinia sp. bind Fn adherent to a solid phase but do not bind soluble Fn (30, 33). In contrast, S. aureus binds Fn in both phases, which is similar to the situation with S. mutans. It was speculated that the surface domains presented by soluble and solid-phase Fn differ considerably (34). Since soluble Fn is ubiquitous in body fluids, it is logical that bacteria might evolve Fn-binding proteins which are not saturated by Fn in plasma, so that they may attach directly to tissue- or foreign body-associated Fn. Alternatively, microorganisms may bind to circulating Fn and become attached to tissues through Fn-fibrin, Fn-collagen, or Fn-Fn interactions (9, 38). In addition, binding of soluble Fn may facilitate the extravasation or migration from oral compartments to distant regions of the body, and coating of the microorganism with soluble Fn may facilitate escape from host immune surveillance.
It was of interest to determine whether differences exist in the affinity of S. mutans for various forms of Fn and whether different mechanisms of binding are involved. The plasma adsorption assay confirmed that S. mutans binds soluble Fn with an affinity lower than that of the S. pyogenes, which possesses multiple Fn-binding proteins, including protein F (10), SfbI (32), serum opacity factor/SfBII (17), FBP54 (7), and glyceraldehyde-3-phosphate dehydrogenase (26). A signal at ca. 55 kDa, in addition to the signal of FBP-130, was detected by far-Western blotting. Western blot analysis of the cell-wall-associated and extracellular protein fractions confirmed that anti-FBP rabbit IgG did not react with the 55-kDa entity and, therefore, this 55-kDa protein might not be a degradation product of FBP-130. This result suggested that at least two Fn-binding proteins might exist on the cell surface of S. mutans. The existence of other Fn-binding elements may also account for the remaining binding activity detectable in the inhibition assay with purified FBP-130 and competitive inhibition experiments on endothelial cells (Fig. 6). Nevertheless, the results of these experiments and the fact that FBP-130 is the predominant eluent from a Fn-activated affinity column confirmed that FBP-130 is the major Fn-binding protein of S. mutans. Specific interactions between S. mutans or FBP-130 and Fn were demonstrated by saturation binding and specific antibody inhibition studies. In addition, an ELISA adherence test of purified FBP-130 suggested that the FBP-130 binds immobilized Fn more readily than soluble Fn, since a higher concentration (>5 μg/ml) of soluble than immobilized Fn was required for the binding to be detectable by ELISA. The binding domains in soluble and immobilized Fn are currently being investigated.
Adherence to endothelial cells is considered to be important for the pathogenesis of bacterial endocarditis (28, 38). Endothelial cells are capable of producing Fn, which in turn is present on the cell surfaces (15). It has been shown that microbial adhesion to Fn-coated surfaces parallels adhesion to endothelial cell surfaces for various strains of bacteria (25). Therefore, Fn is critical for bacterial adherence to vascular surfaces. We have found that Fn is readily detectable on monolayers of ECV304, and S. mutans could bind to this endothelial cell line through interaction with Fn. The specificity of Fn mediated adherence to ECV304 was confirmed by inhibition and competitive inhibition studies. It has been shown that surface-localized GTF mediated adhesion of S. gordonii to human umbilical vein endothelial cells in vitro (36). S. mutans possesses two surface-localized GTFs, GtfB and GtfC (11, 12), which synthesize insoluble glucan polymers from sucrose and are important for bacterial colonization of tooth surfaces. Distinct from the findings with S. gordonii, we found that a GtfB/C-deleted strain, NHS1, and a GtfB/C/D-inactivated strain, NHS1DD, attached equally as well to ECV 304 cells as did the wild-type strain. In parallel with this finding was the finding that the Fn-binding protein FBP-130 was found to have equal binding capacity in these GTF-defective isogenic strains as with the parental GS-5 (Fig. 2 and 3). Therefore, GTFs affected neither the expression of surface FBP-130 nor the adherence of S. mutans to endothelial cells in vitro. Although S. mutans may be found in the same compartment (the oral cavity) as the sanguis group of streptococci, as well as sharing other characteristics, it has evolved different mechanisms of interaction with components of the ECM such as Fn. Such differences might affect the pathogenic process and the severity of infective endocarditis.
ACKNOWLEDGMENTS
We thank H. K. Kuramitsu, T. Koga, and S. Hamada for bacterial strains. We thank Tim J. Harrison, Reader in Molecular Virology, Royal Free and University College Medical School, for his kind review and help in the preparation of this manuscript.
This work was supported in part by the National Science Council (grants NSC-852331-B002-024, 862314, and 89-2314-B-002-184) and the National Health Research Institute (grant DOH88-HR-814).
REFERENCES
- 1.Aoki H, Shiroza T, Hayakawa M, Sato S, Kuramitsu H K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986;53:587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour L M. Twelve-year review of recurrent native valve infectious endocarditis: a disease of the modern antibiotic era. Rev Infect Dis. 1988;10:1163–1170. doi: 10.1093/clinids/10.6.1163. [DOI] [PubMed] [Google Scholar]
- 3.Chang S C, Luh K T, Deng L J, Hsieh W C. Bacteriology of viridans streptococcal bacteremia. Chin J Microbiol Immunol. 1987;20:311–318. [PubMed] [Google Scholar]
- 4.Chia J S, Hsu T Y, Teng L J, Chen J Y, Hahn L J, Yang C S. Glucosyltransferase gene polymorphism among Streptococcus mutans strains. Infect Immun. 1991;59:1656–1660. doi: 10.1128/iai.59.5.1656-1660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia J S, Lin R H, Lin S W, Chen J Y, Yang C S. Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect Immun. 1993;61:4689–4695. doi: 10.1128/iai.61.11.4689-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chia J S, Lin S W, Hsu T Y, Chen J Y, Kwan H W, Yang C S. Analysis of a DNA polymorphic region in the gtfB and gtfC genes of Streptococcus mutans strains. Infect Immun. 1993;61:1563–1566. doi: 10.1128/iai.61.4.1563-1566.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtney H S, Ofek I, Simpson W A, Hasty D L, Beachey E H. Binding of Streptococcus pyogenes to soluble and insoluble fibronectin. Infect Immun. 1986;53:454–459. doi: 10.1128/iai.53.3.454-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engvall E, Ruoslahti E. Binding of soluble from of fibroblast surface protein, fibronectin, to collagen. J Cancer Res. 1977;10:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 10.Hanski E, Caparon M G. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus—Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988;56:1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989;57:2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiroshi M. Interaction of fibronectin with integrin receptors: evidence by use of synthetic peptides. Peptides. 1997;18:899–907. doi: 10.1016/s0196-9781(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 14.Horaud T, Delbos F. Viridans streptococci in infective endocarditis:species distribution and susceptibility to antibiotics. Eur Heart J. 1984;5(Suppl. C):39–44. doi: 10.1093/eurheartj/5.suppl_c.39. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe E, Mosher D F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978;147:1779–1783. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnsson E, Berggard K, Kotarsky H, Hellwage J, Zipfel P F, Sjobring U, Lindahl G. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J Immunol. 1998;161:4894–4901. [PubMed] [Google Scholar]
- 17.Kreikemeyer B, Talay S R, Chhatwal G S. Characterizations of a novel fibronectin-binding surface protein in group A streptococcus. Mol Microbiol. 1995;17:137–145. doi: 10.1111/j.1365-2958.1995.mmi_17010137.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuypers J M, Proctor R A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989;57:2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowrance J H, Baddour L M, Simpson W A. The role of fibronectin binding in the rat model of experimental endocarditis caused by Streptococcus sanguis. J Clin Investig. 1990;86:7–13. doi: 10.1172/JCI114717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowrance J H, Hasty D L, Simpson W A. Adherence of Streptococcus sanguis to comformationally specific determinants in fibronectin. Infect Immun. 1988;56:2279–2285. doi: 10.1128/iai.56.9.2279-2285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNab R, Holmes A R, Clarke J M, Tannock G W, Jenkinson H F. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect Immun. 1996;64:4204–4210. doi: 10.1128/iai.64.10.4204-4210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ness P M, Perkins H A. Transient bacteremia after dental procedures and other minor manipulations. Transfusion. 1980;20:82–85. doi: 10.1046/j.1537-2995.1980.20180125046.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa S K, Yurberg E R, Hatcher V B, Levitt M A, Lowy F D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985;50:218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pancholi V, Fischetti V. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheld W M. Pathogenesis and pathophysiology of infective endocarditis. In: Sande M A, Kaye D, Root R K, editors. Endocarditis. New York, N.Y: Churchill Livingstone, Ltd.; 1984. pp. 1–32. [Google Scholar]
- 28.Scheld W M, Strunk R W, Balian G, Calderone R A. Microbial adhesion to fibronectin in vitro correlates with the production of endocarditis in rabbits. Proc Soc Exp Biol Med. 1985;180:474–482. doi: 10.3181/00379727-180-42205. [DOI] [PubMed] [Google Scholar]
- 29.Schennings T, Heimdahl A, Coster K, Flock J I. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb Pathog. 1993;15:227–236. doi: 10.1006/mpat.1993.1073. [DOI] [PubMed] [Google Scholar]
- 30.Schulze-Koops H, Burghardt H, Heesemann J, Kirsch T, Swoboda B, Bull C, Goodman S, Emmrich F. Outer membrane protein YadA of enteropathogenic yersiniae mediates specific binding to cellular but not plasma fibronectin. Infect Immun. 1992;61:2513–2519. doi: 10.1128/iai.61.6.2513-2519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sela S, Aviv A, Tovi A, Burstein I, Caparon M G, Hanski E. Protein F: an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol Microbiol. 1993;10:1049–1055. doi: 10.1111/j.1365-2958.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 32.Talay S R, Valentin-Weigand P, Jerlstrom P G, Timmis K N, Chhatwal G S. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;60:3873–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura G S, Rubens C E. Group B streptococci (GBS) adhere to a variant of fibronectin attached to a solid phase. Mol Microbiol. 1994;15:581–589. doi: 10.1111/j.1365-2958.1995.tb02271.x. [DOI] [PubMed] [Google Scholar]
- 34.Underwood P A, Steele J G, Dalton B A. Effects of polystyrene surface chemistry on the biological activity of solid phase fibronectin and vitronectin analyzed with monoclonal antibodies. J Cell Sci. 1993;104:793–803. doi: 10.1242/jcs.104.3.793. [DOI] [PubMed] [Google Scholar]
- 35.Van Reyn F C. Infective endocarditis: an analysis based on strict case definition. Ann Intern Med. 1981;94:505–518. doi: 10.7326/0003-4819-94-4-505. [DOI] [PubMed] [Google Scholar]
- 36.Vacca-Smith A M, Jones C A, Levine M J, Stinson M W. Glucosyltransferase mediates adhesion of streptococcus gordonii to human endothelial cell in vitro. Infect Immun. 1994;62:2187–2194. doi: 10.1128/iai.62.6.2187-2194.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van de Rijn I, George M, Bouvet A, Roberts R B. Enzyme-linked immunosorbent assay for the detection of antibodies to nutritionally variant streptococci in patients with endocarditis. J Infect Dis. 1986;153:116–121. doi: 10.1093/infdis/153.1.116. [DOI] [PubMed] [Google Scholar]
- 38.Vercellotti G M, Lussenhop D, Peterson P K, Furcht L T, McCarthy J B, Jacob H S, Moldow C F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984;103:34–43. [PubMed] [Google Scholar]