Abstract
The meningococcal PorA protein is considered a promising vaccine candidate. Although much is understood regarding the structure of PorA proteins, little is known about the structure-function relationships of PorA antibodies. The aim of this study was to compare the functional and molecular characteristics of a human monoclonal antibody (MAb) and three murine MAbs specific for the PorA P1.7 serosubtype. Murine MAbs 207,B-4 (immunoglobulin G2a [IgG2a]) and MN14C11.6 (IgG2a) were both bactericidal and opsonophagocytic for P1.7-expressing meningococci, whereas human MAb SS269 (IgG3) and murine MAb 208,D-5 (IgA) initiated neither effector function. Epitope mapping with synthetic peptides revealed that MAbs 207,B-4 and 208,D-5 recognized the sequence ASGQ, which is the same specificity motif that a previous study had established for SS269 and MN14C11.6. Nucleotide and amino acid sequence analyses of the variable regions of the four MAbs showed that the SS269 VH region belonged to the VH3 family and was approximately 70% homologous to those of the murine MAbs which were all from the 7183 family, whereas the SS269 VL region belonged to the Vλ1-b family and was less than 40% homologous to those of the murine MAbs which were all members of the Vκ1 family. The Fab fragment of SS269 was cloned and expressed in Escherichia coli and was shown by enzyme-linked immunosorbent assay analyses to bind as well as intact SS269 MAb to P1.7,16 serosubtype group B strain 44/76. We conclude that distinct differences exist in the effector function activities and variable region gene sequences of human and murine P1.7-specific MAbs despite their recognition of similar epitopes.
Disseminated meningococcal infection is a fulminant disease with high morbidity and mortality (3). Immune defense against this illness depends on recognition of the bacterial surface by antibody and activation of complement (32). The current meningococcal vaccine is composed of the meningococcal capsular polysaccharides of serogroups A, C, Y, and W135 (7). Although the efficacy of the vaccine in adults and older children is quite high, the response is age dependent in younger children, and children younger than 2 years of age respond poorly (13, 25, 41). In addition, the vaccine does not include the serogroup B capsular polysaccharide, as it is poorly immunogenic (68), and therefore vaccination does not protect against disease due to serogroup B strains that are responsible for the majority of meningococcal infections in many countries (58). Thus, the development of a vaccine for protection against serogroup B infection has focused on outer membrane proteins as alternative targets (50).
The PorA class 1 outer membrane protein is a major outer membrane porin protein expressed by almost all meningococcal strains (23, 64, 65). Sequence comparisons of PorA proteins have shown strong homology among the proteins with the major variation confined to two discrete regions termed variable region 1 (VR1) and VR2 (42, 62). Based on a two-dimensional topology model, PorA has eight predicted surface-exposed loops, and VR1 and VR2 are located at the apices of the two longest loops: loops 1 and 4, respectively (62). Epitope mapping with murine monoclonal antibodies (MAbs) and synthetic peptides confirmed the surface exposure of loops 1 and 4 (62) and showed that most epitopes recognized by serosubtyping MAbs are localized to VR1 and VR2 (42, 43).
PorA is highly immunogenic in humans following infection or immunization (16, 26, 63), and the specific antibodies induced predominantly recognize epitopes within VR1 and VR2 as well as exhibit both bactericidal and opsonic functions (1, 39, 45, 52, 53). PorA-specific murine MAbs that bind to epitopes within VR1 and VR2 are bactericidal in vitro and protective in infant rats when administered passively (51, 55, 56). Thus, PorA protein is considered to be an important vaccine candidate either alone (54) or when administered as an outer membrane vesicle conjugated with the Haemophilus influenzae type b capsular polysaccharide (44).
Molecular epidemiological investigations have revealed that the P1.7 epitope within the VR1 of PorA is one of the most common serosubtype epitopes expressed by bacteria isolated from cases of meningococcal disease. The P1.7 epitope is expressed by epidemic strains of serogroup A meningococci isolated in West Africa and China (4, 64) and by serogroup B meningococci of the ET-5 complex, which have been commonly isolated in Europe, North America, and South America for decades (12). Recently, a hexavalent PorA meningococcal outer membrane vesicle vaccine was developed which covered more than 80% of the meningococcal PorA subtypes isolated in many countries (16). Vaccination of adult volunteers with the PorA vaccine induced bactericidal antibodies that were predominantly directed against P1.7 VR1 epitopes and to a lesser degree against P1.16 VR2 epitopes (52–54).
In a study of the human immune response to the P1.7 PorA protein, Delvig et al. reported the development of a human MAb, SS269, which was derived from the peripheral blood B lymphocytes of a volunteer immunized with an outer membrane vesicle meningococcal group B vaccine (19). A peptide analysis of the binding specificity of SS269 revealed that it was specific for an epitope present at the apex of the P1.7 VR1 loop which overlapped with the epitope recognized by murine P1.7-specific MAbs A'dam and MN14C11.6 (19). But despite the recognition of similar linear epitope sequences by the three MAbs and the fact that SS269 was an immunoglobulin G3 (IgG3) isotype known to fix complement (18), SS269 did not function in complement-dependent opsonophagocytosis or bactericidal effector assays as efficiently as the murine MAbs (19, 53). These findings suggest that the effector functions of P1.7-specific murine antibodies may not necessarily emulate those of human antibodies despite the recognition of similar epitopes.
In this study, we compared the effector function activities, peptide epitope specificities, and immunoglobulin variable region gene sequences of human MAb SS269 and three P1.7-specific murine MAbs to better understand the structure-function relationship of PorA antibodies. The data show that distinct differences exist in the functional activities and variable region gene sequences of human and murine P1.7-specific MAbs despite their recognition of similar epitopes. In addition, we describe the expression of the Fab fragment of MAb SS269 with binding function for a meningococcal strain expressing the P1.7 epitope.
MATERIALS AND METHODS
Neisseria meningitidis strains.
Strains 44/76 and 44/76-SL (B:15:P1.7,16:L3,7,9 ET-5), 188/87 (B:15:P1.7,16d ET-5), 29019 (A:4/21:P1.7,10, subgroup V), and 7973 (C:2:P1.2:L3,7) have been described previously (21, 27, 40, 64). Stock cultures were maintained in 10% skim milk at −70°C. Organisms were cultivated on gonococcal agar base (Difco, Detroit, Mich.) containing 2% IsoVitaleX (Becton Dickinson, Mountain View, Calif.) at 37°C in 5% CO2.
Hybridoma cell lines.
The human hybridoma SS269 (IgG3, λ light chain) (19) and three BALB/c murine hybridomas, 207,B-4 (IgG2a, κ light chain), 208,D-5 (IgA, κ light chain), and MN14C11.6 (IgG2a, κ light chain) (2), were selected for this study. The four hybridomas produce MAbs that recognize epitopes within the P1.7 loop of the PorA protein. Hybridomas 207,B-4 and 208,D-5 were prepared by immunizing mice with outer membrane vesicles from strain 188/87. The cell line MN14C11.6 was obtained from M. Maiden and I. Feavers of the National Biological Standards Board, Potters Bar, United Kingdom.
Bactericidal activity.
The bactericidal activities of the four MAbs were tested against strain 44/76-SL by using human serum as a complement source as previously described (1). Briefly, the assays were performed in microtiter plates with a bacterial inoculum of approximately 100 CFU per well. MAbs were tested against the test strain either as purified antibodies or as ascites fluid. When used as ascites fluid, the concentration of antibody was determined by using a capture enzyme-linked immunosorbent assay (ELISA) assay (45). Increasing concentrations of MAb were incubated with the bacteria prior to initiation of the bactericidal reaction with human serum at a final concentration of 25%. After 30 min at 37°C, brain heart infusion agar was added, and the plates were incubated overnight at 37°C in 5% CO2. Colonies were counted, and the percent kill was calculated by comparing test samples with controls containing MAb and heat-inactivated human serum. The results are presented as the lowest concentration of MAb resulting in greater than 50% killing of the strain.
Opsonophagocytic activity.
The opsonophagocytic activities of the different MAbs were tested against viable strain 44/76-SL by using human serum as a complement source and human peripheral blood polymorphonuclear leukocytes (PMN) as effector cells as previously described (1). The test was performed by using flow cytometry to measure respiratory burst (RB) by the PMN effector cells following phagocytosis of the test strain (1). Briefly, increasing concentrations of MAb determined as described above were incubated with log-phase-growth meningococci for 30 min at 37°C. Human serum at a final concentration of 10% was added as a complement source, and the mixture was further incubated for 10 min at 37°C. Finally, PMN from a donor heterozygous for the FcγRIILRHR allotype (66) which had been primed with dihydrorhodamine 123 (Molecular Probes, Eugene, Oreg.) were incubated with the opsonized bacteria at an effector/target ratio of 1:25 for 10 min at 37°C, after which RB was analyzed by flow cytometry. The results are presented as the lowest concentration of MAb resulting in RB in greater than 50% of the PMNs.
Epitope mapping.
The synthesis of N-terminally acetylated peptides on pins was performed as described previously (19). Briefly, the solid-phase synthesis of peptides on polyethylene rods was carried out using a commercial kit (Cambridge Research Biochemicals, Cambridge, United Kingdom). Synthetic peptides on pins composed of 4 to 10 amino acids from the PorA P1.7 loop of meningococcal strain H44/76 were synthesized to contain all possible sequences which spanned the NGGAS epitope of MAb SS269 and the ASGQ epitope of MAb MN14C11.6. The pins were screened by ELISA for reactivity to MAbs 207,B-4 and 208,D-5, and the assay was repeated twice for each MAb. Cell culture supernatants of each MAb were diluted 1:1,000 in phosphate-buffered saline (PBS) containing 1.0% bovine serum albumin (BSA) and incubated with the pins for 90 min. MAb binding to peptide sequences was detected by incubation of the pins for 90 min in a 1:1,500 dilution of affinity-purified horseradish peroxidase-conjugated goat anti-murine IgG (H+L; Bio-Rad, Richmond, Calif.) followed by azino-3-ethylbenzthiazodinsulfonate (Sigma, St. Louis, Mo.) substrate development. Control peptides reacting with a MAb supplied by the manufacturer were included to verify peptide coupling during synthesis.
PCR primers.
PCR primers for amplifying the Fab fragments of human immunoglobulin genes and mouse immunoglobulin genes were modified from those used by Chazenbalk et al. (14) and by Kettleborough et al. (34), respectively. Recognition sequences of restriction enzymes XhoI, SpeI, XbaI, or SacI were integrated into different primers to facilitate the cloning procedure outlined below. All primers that resulted in PCR products are listed in Table 1.
TABLE 1.
Oligonucleotide sequences of PCR primers
| Primer | Sequencea | Hybridoma(s) |
|---|---|---|
| Human VH3 | GAGGTGCAGCTCGAGGAGTCTGGG | SS269 |
| Human Cγ3 | TGTGTCACTAGTTGGGGTTTTGAGCTC | SS269 |
| Human Vλ | ACAGGBTCYBKSKCCGAGCTCRWRBTGACDCA | SS269 |
| Human Cλ | GCATTCTAGACTATTATGAACATTCTGTAGGGGC | SS269 |
| Mouse VH4 | GTGCAGCTCGAGGAGTCWGGRGGAG | 207,B-4, 208,D-5 |
| Mouse VH9 | AATTCTCGAGMASYTGSWGGWGWCTGGAGG | MN14C11.6 |
| Mouse Cγ2a | TCTTACTAGTGGGCMCTCTGGGCTC | 207,B-4 |
| Mouse JH | GAGACGGTGACTGCAGTGCCTTGGCCCCAGTA | MN14C11.6 |
| Mouse Cα | AGAGCAACTAGTATCCAATTCTTGGACGGG | 208,D-5 |
| Mouse Vκ3 | ATGTGAGCTCKTGATGACCCAAACTCCA | 207,B-4, 208,D-5, MN14C11.6 |
| Mouse Cκ | TCCTTCTAGATTACTAACACTCATTCCTGTTGAAGC | 207,B-4, 208,D-5, MN14C11.6 |
Recognition sequences for restriction enzymes are underlined. B = A or G; Y = C or T; K = G or T; R = A or G; W = A or T; D = A, G, or T; M = A or C; S = C or G.
Immunoglobulin gene cloning and in vitro expression.
Total RNA was prepared from approximately 106 hybridoma cells of SS269, 207,B-4, 208,D-5, and MN14C11.6 by using an acid guanidinium thiocyanate-phenol-chloroform method as described by the manufacturer (TriPure Reagent; Boehringer Mannheim, Indianapolis, Ind.). Five micrograms of total RNA was used for each first-strand cDNA synthesis reaction using the Superscript Preamplification System (Gibco BRL, Gaithersburg, Md.). The cDNAs coding for the heavy and light chains were then amplified by using the sets of primers listed in Table 1. Each PCR reaction was performed in a 50-μl volume containing 2.5 U of Extend Long Template DNA polymerase (Boehringer Mannheim) and AmpliWax PCR Gem 50 according to the Hot-Start protocol of the manufacturer (Perkin-Elmer, Norwalk, Conn.). PCR amplifications were done according to the following protocol: denaturation for 3 min at 94°C; 40 cycles of 40 s at 92°C, 40 s at 55°C, and 1.5 min at 72°C; and a final extension time of 5 min at 72°C.
Two independent PCR products from each heavy and light chain derived from the four MAbs were selected for the subsequent cloning and sequencing procedures. Except for the heavy chain of MN14C11.6, the heavy- and light-chain PCR products were purified with QiaQuick PCR Purification Kit (Qiagen, Valencia, Calif.), double digested with either XhoI-SpeI or XbaI-SacI (Boehringer Mannheim), respectively, separated by agarose gel electrophoresis, and extracted from the gel with the QiaQuick Gel Extraction Kit (Qiagen). The products were then ligated into the corresponding site of the phagemid vector pComb3 (kindly provided by C. F. Barbas III, Scripps Research Institute, La Jolla, Calif.). Each ligation reaction was incubated overnight at 16°C in a 20-μl volume containing 1 U of T4 ligase (Boehringer Mannheim), and approximately 0.25 μg of PCR product and 0.25 μg of pComb3 vector. The ligated DNA molecules (2 μl each) were then transformed into E. coli XL-1 Blue MRF′ using an Electroporator II (Invitrogen, Carlsbad, Calif.). After an overnight incubation of the bacteria on Luria-Bertani agar plates supplemented with carbenicillin (20 μg/ml) and tetracycline (10 μl/ml), plasmids were prepared from single colonies with a Qiaprep Spin Miniprep Kit (Qiagen) and examined by agarose gel electrophoresis. The cloned inserts were then sequenced with primers pComb3-1 (GGTGGCGGCCGCAAATTCTATTTC) for the variable region of the heavy chain, pComb3-1013rev (GGCGACTAGCTAGTTTAGAATTCG) for the CH1 region of the heavy chain, pComb3-1013 (CGAATTCTAAACTAGCTAGTCGCC) for the variable region of the light chain, and pComb3-1166rev (TCACTATAGGGCGAATTGGGTACC) for the CL region of the light chain at the Biomolecular Research Center, University of California at San Francisco. The heavy-chain PCR product of MN14C11.6 was ligated into plasmid pCRII with a TA Cloning Kit (Invitrogen) and sequenced with universal primers of the Sp6 promoter and the T7 promoter.
Heavy-chain and light-chain gene fragments of the human hybridoma cell line SS269 were first cloned into separate pComb3 vectors and, after insertion of both gene fragments was confirmed by DNA sequencing, the heavy-chain fragment was excised and religated into the vector containing the light chain. This vector was then converted from a phage-display form to a soluble Fab producing form by removing the nucleotide sequence encoding the phage protein PIII from the vector followed by self-ligation. Soluble Fab was prepared from the cell pellet of a 10-ml overnight growth of E. coli XL-1 Blue MRF′ by repeatedly freezing (in a dry ice-ethanol bath) and thawing (in a 37°C water bath) the bacteria four times as previously described (8). Following centrifugation, the supernatant containing soluble Fab molecules was stored at −70°C prior to further characterization.
Whole-cell ELISA.
Bacteria grown overnight on plates were suspended to an optical density at 650 nm of 0.1 in PBS as described previously (31). Aliquots of 75 μl of the suspension were dispensed into wells of microtiter plates, and the plates were dried overnight. After a washing with PBS, nonspecific binding sites were blocked with PBS containing 0.1% BSA for 1 h. Wells were washed and incubated for 1 h with either a 1:10 dilution of the cell culture supernatant of MAb SS269 or a 1:10 dilution of the supernatant of an E. coli lysate containing the Fab fragment of MAb SS269. The binding of the MAb or its Fab fragment was detected with affinity-purified alkaline phosphatase-conjugated goat anti-human lambda chain antibody (Sigma) followed by p-nitrophenyl phosphate (Sigma) substrate development. Nonspecific absorbance was determined by reacting control wells with blocking solution in the absence of bacteria.
DNA and amino acid sequence analyses.
The DNA and protein sequence analysis software Lasergene (DNAStar, Madison, Wis.) was used for sequence editing and basic sequence analyses. The specific sequence domains of the immunoglobulin genes were defined and the domains of the different immunoglobulin genes were compared by using the computer program SAW (University of Alabama at Birmingham). Human immunoglobulin germ line gene comparisons were performed by using the V BASE database (http://www.mrc-cpe.cam.ac.uk/imt-doc/public/INTRO.html) and comparisons of murine germ line genes were performed by using DNAPLOT (http://www.genetik.uni-koeln.de/dnaplot). Similarities between germ line genes and the immunoglobulin gene sequences were calculated with MegAlign of Lasergene. Hydrophilicity structure predictions of MAb heavy chains were determined by using GPMAW protein analysis software (Lighthouse data, Odense, Denmark) based on the values of Kyte and Doolittle (37).
Nucleotide sequence accession numbers.
The nucleotide sequences of the MAb variable region genes have been deposited in GenBank under accession numbers AF191789 (207,B-4 VH), AF191790 (207,B-4 VL), AF191791 (208,D-5 VH), AF191792 (208,D-5 VL), AF191793 (MN14C11.6 VH), AF191794 (MN14C11.6 VL), AF191092 (SS269 VH), and AF191795 (SS269 VL).
RESULTS
Bactericidal activity of the P1.7 MAbs.
The bactericidal activities of human MAb SS269 and murine MAbs 207,B-4, 208,D-5, and MN14C11.6 were tested against strain 44/76-SL by using human serum as the complement source. As shown in Table 2, no bactericidal killing of strain 44/76-SL was detected for either human IgG3 SS269 or mouse IgA 208,D-5 at concentrations of the MAbs that exceeded 400 μg/ml. In contrast, both mouse IgG2a 207,B-4 and IgG2a MN14C11.6 showed high bactericidal activity. The lowest concentrations of the two murine MAbs resulting in greater than 50% killing of the test strain were found to be in the range of 0.5 to 1.0 μg/ml (Table 2).
TABLE 2.
Bactericidal and opsonophagocytic activities of the human and murine MAbs
| Hybridoma | Isotype (CH,CL) | Lowest concn giving bactericidal activity (μg/ml)a | Lowest concn giving opsonic activity (μg/ml)b |
|---|---|---|---|
| SS269 | γ3,λ | –c | >400 |
| 207,B-4 | γ2a,κ | 1 | 1.1 |
| 208,D-5 | α,κ | – | – |
| MN14C11.6 | γ2a,κ | 0.5 | 0.2 |
Lowest concentration giving >50% killing of strain 44/76-SL.
Lowest concentration giving RB in >50% of the human PMN effector cells when using opsonized strain 44/76-SL as the target.
–, Negative at the highest concentration tested.
Opsonophagocytic activity of the P1.7 MAbs.
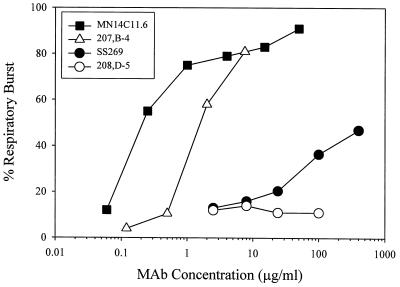
The opsonic function of the MAbs was measured with PMN as the effector cells and human serum as the complement source. The lowest concentrations of the four MAbs showing opsonic activity against strain 44/76-SL were determined as RB in more than 50% of PMN based on flow cytometric analyses. As shown in Table 2, the results paralleled those of the bactericidal assays in that SS269 and 208,D-5 showed little or no detectable opsonic activity, whereas 207,B-4 and MN14C11.6 were opsonic for the test strain at concentrations of 1.1 and 0.2 μg/ml, respectively. The dose-response curves of the opsonic activities showed that the curve of SS269 function was considerably less steep than those for 207,B-4 and MN14C11.6 and that SS269 did not induce RB in 50% of the PMNs at a concentration of 400 μg/ml (Fig. 1). In contrast, 207,B-4 and MN14C11.6 demonstrated high opsonophagocytic activity with >80% of PMNs exhibiting measurable RB at a concentration of the MAbs of <10 μg/ml. In addition, MAb 208,D-5, the IgA isotype, was negative for opsonic potential at a concentration of 100 μg/ml.
FIG. 1.
Opsonophagocytic effector function activities of human MAb SS269 and murine MAbs 207,B-4, 208,D-5, and MN14C11.6. Phagocytic activity was tested against log-phase-growth strain 44/76-SL bacteria by using human serum as a complement source, human PMNs as effector cells, and increasing concentrations of each MAb as the opsonin. The test was performed by using flow cytometry to measure the respiratory burst induced in the effector cells following phagocytosis of the test strain.
Linear epitope mapping.
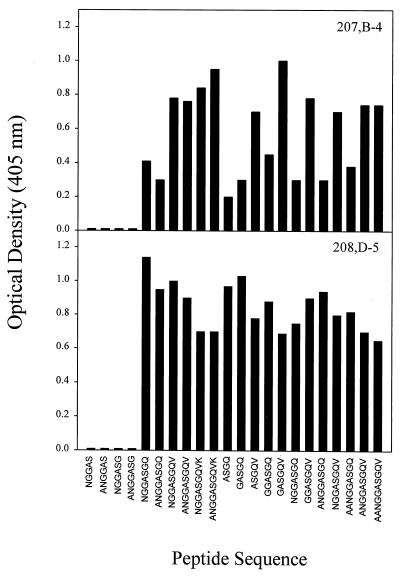
We next investigated the epitope specificities of the MAbs for the P1.7 VR1 region using synthetic peptide analyses. The epitope specificities of two of the MAbs, SS269 and MN14C11.6, had been determined previously (19). Synthetic peptides on pins composed of 4 to 10 amino acids from the P1.7 loop of meningococcal strain H44/76 were synthesized to contain all possible sequences which spanned the NGGAS epitope of MAb SS269 and the ASGQ epitope of MAb MN14C11.6 and were used to map the epitope specificities of murine MAbs 207,B-4 and 208,D-5. As shown in Fig. 2, MAb 207,B-4 bound most strongly to peptides containing the sequence ASGQV and bound less efficiently to peptides which contained only the sequence ASGQ (summarized as ASGQv). In contrast, MAb 208,D-5 bound strongly to ASGQ, and further addition of amino acids found in the P1.7 sequence did not influence its binding efficiency. Neither MAb bound to peptide sequences which lacked the glutamine (Q) residue. These results have been summarized in Table 3.
FIG. 2.
Epitope mapping of murine MAbs 207,B-4 and 208,D-5 by using synthetic peptides on pins. The 4- to 10-amino acid peptides were designed to cover the PorA P1.7 loop of meningococcal strain H44/76 which spans the NGGAS epitope of MAb SS269 and the ASGQ epitope of MAb MN14C11.6. The pins were screened by ELISA for reactivity to MAbs 207,B-4 and 208,D-5. Similar results were obtained in two independent experiments.
TABLE 3.
Human and murine MAb epitope specificities and variable region gene assignments
| Hybridoma | Class | Specificitya (reference) | VH family | VH germ line geneb | D gene | JH gene | VL family | VL germ line geneb | JL gene |
|---|---|---|---|---|---|---|---|---|---|
| SS269 | γ3,λ | aNGGASgq (19) | VH3a | V3-33.1 (L06618) | D3-10 | JH4 | Vλ1-b | VL-47.1 (Z73663) | JL3 |
| 207,B-4 | γ2a,κ | ASGQv | VH5 (7183)c | VHD6.96 (K02887) | D-FL16.1 | JH4 | Vκ1 | K1A5 (D00081) | JK1 |
| 208,D-5 | α,κ | ASGQ | VH5 (7183) | VHD6.96 (K02887) | D-FL16.1 | JH4 | Vκ1 | K1A5 (D00081) | JK1 |
| MN14C11.6 | γ2a,κ | ASGq (19) | VH5 (7183) | VHD6.96 (K02887) | D-SP2.1 | JH4 | Vκ1 | K5.1 (D00080) | JK1 |
Capital letters indicate essential amino acids of the linear epitopes, while the lowercase letters represent the amino acids that may increase binding efficiency.
GenBank accession numbers are given in parentheses.
VH5 is also named 7183.
Immunoglobulin variable region gene analyses.
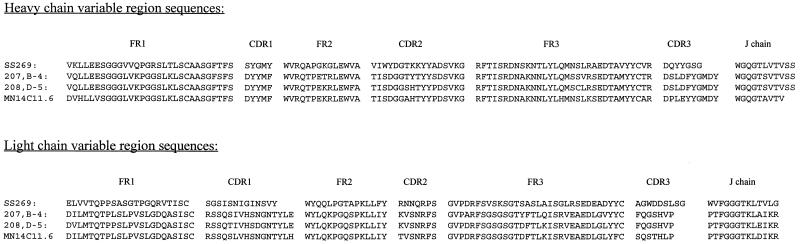
We next cloned the variable region genes for the VH and VL regions of the four MAbs and determined their nucleotide sequences. The deduced amino acid sequences were aligned and are shown in Fig. 3. All sequences were unique based on a search of the GenBank database. The results of the gene family assignments of the variable region gene sequences are summarized in Table 3.
FIG. 3.
Comparison of the amino acid sequences of the VH and VL genes of human MAb SS269 and murine MAbs 207,B-4, 208,D-5, and MN14C11.6 as deduced from nucleotide sequences. FR, framework regions; CDR, complementarity-determining regions.
The SS269 VH gene utilized a germ line gene of the VH3 family (Table 3). Within this family, the SS269 VH region shared 97.8% homology with the germ line gene V3-33.1 (without including the first 15 nucleotides which contained an artificial restriction enzyme insertion) (48). The VH genes of the three murine MAbs all belonged to the VH5 (also named VH7183) family (10); all three MAbs were most similar to the germ line gene VHD6.96 (69), with homologies of 97.4% for MN14C11.6 and 96.2% for both 207,B-4 and 208,D-5.
The SS269 Vλ gene was assigned to the Vλ1-b family and was most similar to germ line gene VL-47.1 (67), with a similarity of 97.9% (without including the first 12 nucleotides which contained an artificial restriction enzyme insertion). All three murine Vκ gene fragments belonged to the Vκ1 gene family but showed the highest sequence homology to different germ line genes (17, 46, 59). Both 207,B-4 and 208,D-5 shared high homology (98.6 and 97.6%, respectively) to K1A5, whereas the Vκ gene of MN14C11.6 showed 99% homology to the germ line gene K5.1. In addition, all three murine MAbs used the same J segment genes for both the heavy and light chains, whereas D gene utilization differed (Table 3).
A similarity comparison of the VH and VL amino acid sequences of the four MAbs revealed that the heavy chain of SS269 was approximately 70% homologous to those of the murine MAbs and that its light chain was less than 40% homologous. In contrast, high homology was observed among the heavy and light chains of the three murine MAbs: 95.2% between the VH of 207,B-4 and 208,D-5, 89.5% between the VH of 207,B-4 and MN14C11.6, 89% between the VH of 208,D-5 and MN14C11.6, 96.8% between the VL of 207,B-4 and 208,D-5, 94.4% between the VL of 207,B-4 and MN14C11.6, and 94.4% between the VL of 208,D-5 and MN14C11.6. A hydrophilicity structural prediction comparison of the human and murine MAb VH regions showed the greatest difference in the CDR3 region where the human MAb was considerably more hydrophilic than the three murine MAbs (results not shown).
Expression of the SS269 Fab fragment in E. coli.
In the early stages of this study, we repeatedly obtained two PCR products from SS269 VH gene cDNA, one that was 348 bp in length and a second that was 360 bp. The DNA sequences of each product indicated that both sequences were in frame and belonged to the human VH3 family but that only the 348-bp product had been spliced to the CH1 γ3 domain.
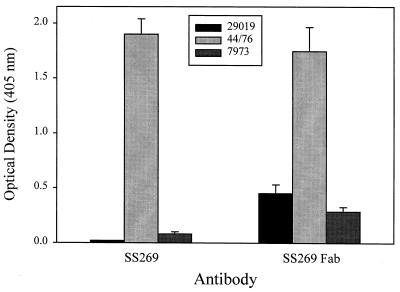
To confirm that the 348-bp product was a functional transcript, we cloned the VL chain gene and the VH Fd gene fragment of SS269 and expressed the Fab fragment in E. coli. After we excised the DNA sequence encoding phage protein PIII from the phagemid vector pComb3, soluble Fab was expressed in E. coli. Figure 4 shows that the SS269 Fab had the same binding reactivity as intact SS269 MAb against strain 44/76 expressing a P1.7,16 PorA protein. When tested against strains expressing P1.7,10 and P1.2 PorA proteins, the Fab of SS269, unlike intact SS269, showed reactivity toward both strains, albeit at a level well below that observed for strain 44/76. The results were consistent with a previous study which reported that SS269 MAb reacted with P1.7,16 but not with P1.7,10 PorA proteins (19).
FIG. 4.
Whole-bacterium ELISA of the binding of MAb SS269 and SS269 Fab to three strains of N. meningitidis. ELISA plate wells were coated overnight with bacteria and then probed with either MAb SS269 or SS269 Fab. Strains 44/76, 29019, and 7973 express PorA serosubtypes P1.7,16, P1.7,10, and P1.2, respectively.
DISCUSSION
The meningococcal PorA outer membrane protein is considered a promising vaccine candidate because it is expressed by nearly all meningococcal strains (23), it is highly immunogenic in humans (16, 26), and bactericidal antibodies directed against PorA protect against meningococcal infection in an animal model (56). Although much is understood regarding the structure of PorA proteins (42, 62), little is known about the structure-function relationships of PorA antibodies. To this end, we took advantage of the fact that the PorA protein is one of only a few meningococcal proteins against which human MAbs have been reported (19, 22). We compared the functional and molecular characteristics of a human MAb directed against the P1.7 epitope of the PorA protein to those of three murine P1.7-specific MAbs and found that distinct differences exist in their opsonophagocytic and bactericidal effector function activities and variable region gene sequences despite their recognition of similar epitopes.
Murine MAbs 207,B-4 and MN14C11.6 were both bactericidal and opsonophagocytic for P1.7-expressing meningococci, whereas human MAb SS269 and murine MAb 208,D-5 initiated neither effector function. The ability of 207,B-4 and MN14C11.6 to initiate complement-dependent effector mechanisms is consistent with their IgG2a isotype which is known to activate complement efficiently (38), and the observed bactericidal activity of MN14C11.6 is in agreement with previous reports (53, 62). In contrast, SS269, an IgG3 isotype known to fix complement (18) did not mediate bactericidal or opsonic functions. This was not due to low affinity of SS269 for P1.7 since the affinity of SS269 is similar to that of murine MAb A'dam which is bactericidal and opsonic for P1.7 meningococci (19), suggesting that a structural characteristic of SS269 other than isotype or affinity influences its functional activity. Interestingly, Pollard et al. concluded that the bactericidal responses to a P1.7 strain in children following meningococcal infection derived from qualitative rather than quantitative differences in the complement-dependent functional activity of human anti-meningococcal IgG antibody (49). The finding that 208,D-5 did not initiate complement-dependent effector functions is presumably due to its IgA isotype constant region, which is a poor activator of complement compared with those of IgG and IgM (33). This result is consistent with the reported lack of opsonophagocytic activity exhibited by human PorA-specific IgA antibodies induced during meningococcal disease (39).
The fact that SS269 was not bactericidal is in accordance with the study by Delvig et al. which reported no bactericidal killing of a P1.7 strain by SS269 (19). However, in contrast to the findings presented here, they also found that SS269 was efficient in the opsonophagocytosis of strain 44/76. This difference is most likely due to the fact that their opsonophagocytosis experiments were performed with ethanol-fixed bacteria (19), whereas viable bacteria in the log phase of growth were used as targets for opsonophagocytosis in the present report. Our data showed only low levels of opsonophagocytic activity by SS269 against viable bacteria which did not reach 50% RB of the neutrophils for the maximum concentration tested. This contrasted with MAbs 207,B-4 and MN14C11.6, which showed strong opsonic activity. Apparently, the binding of SS269 to its epitope renders the MAb nonfunctional for bactericidal and opsonic activities despite it being an IgG3 subclass antibody which normally is highly efficient for both effector functions (11). Further investigation is required to determine the degree to which SS269 is representative of the human immune response to P1.7 epitopes.
Peptide epitope mapping revealed that MAbs 207,B-4 and 208,D-5 recognized amino acid sequences in the presumed apex of the P1.7 loop (62), which is the same site of binding established previously for MAbs SS269 and MN14C11.6 (19). The four MAbs shared recognition of the ASGQ motif in which alanine and serine were found to be necessary for epitope binding by all of the MAbs. It is likely that these two amino acids form key hydrogen bonds with contact residues of the Fab antigen combining sites as proposed for the recognition of the PorA P1.16 epitope by a murine MAb (61). For the three murine MAbs, glycine was also required for binding. For 207,B-4 and 208,D-5, all four amino acids in the ASGQ motif were required for binding although the binding of 207,B-4 was improved by the addition of a C-terminal valine residue (ASGQv). In contrast, glycine and glutamine were not necessary for SS269 epitope recognition but rather for increased binding efficiency and, also unlike the murine MAbs, SS269 required NGG for its minimal epitope (19). It may be that SS269 recognizes a conformational epitope only partially mimicked by short peptides as postulated previously (19). A possible explanation for the poor functional activities of SS269 is that it may not recognize its epitope through a peptide-induced tight conformational fit as described for the interaction of a bactericidal murine MAb directed against the P1.16 epitope in loop 4 of PorA (60). This in turn would increase the off-rate of the SS269-P1.7 complex resulting in poor induction of complement-mediated bactericidal and phagocytic activities. This suggestion is supported by the reported low avidity of SS269 for a P1.7 strain (19).
Nucleotide sequence analyses revealed that the VH region of SS269 belonged to the VH3 gene family and that all three murine VH regions were encoded by genes belonging to the VH7183 family. Based on a phylogenetic association of progenitor VH gene segments, these two families are classified as members of VH subgroup III (57). It is interesting to note that both of these families are more frequently utilized by self-reactive antibodies than other families (5, 20). We determined that the amino acid sequence of the VH region of SS269 was approximately 70% similar to those of the murine MAbs, which is in agreement with the finding that nearly all human VH3 family members are at least 70% homologous to mouse subgroup III gene families, including the 7183 family (28). Molecular modeling studies have demonstrated that such VH subgroup and family identity predicts similarity in the solvent-exposed β loop subdomains of FR1 and FR3 both within and across species (35). This is especially important for FR3, since residues within FR3 can influence the conformation of CDR2 (15), suggesting that conservation of FR3 residues across species may provide initial family-associated constraints to antibody affinity for specific antigen epitopes. Thus, our data indicate that the immunoglobulin response of humans and mice to P1.7 epitopes may be biased toward the expression of related subgroup III family VH genes, perhaps as the result of evolutionary pressure to generate related antigen binding site conformations reactive toward P1.7 epitopes. Alternatively, the subgroup III relatedness of the four MAbs may simply reflect the reported overexpression of VH3 and VH7183 gene families in humans and mice, respectively (36, 69).
Since neither the isotype nor the affinity for P1.7 accounted for the poor bactericidal and phagocytic effector functions of SS269, it may be that structural differences between the VH regions of SS269 and those of 207,B-4 and MN14C11.6 gave rise to effector function differences. Although the VH regions of the three MAbs shared structural characteristics, differences in key portions of these regions were apparent, which is consistent with the report that differences exist between humans and mice in the structural repertoire of the VH germ line gene segments (6). Certainly, the species restriction of natural meningococcal infection to humans supports the concept of a species-specific antibody response to P1.7 resulting in divergent levels of immunological protection. This explanation for the observed effector function differences is supported by two studies which demonstrated that differences mainly in the CDR3 portion of the VH region between antibodies with identical VL and constant regions can affect complement activation by IgG (29, 30). Importantly, the CDR3 region of SS269 was three amino acids shorter in length with a higher degree of hydrophilicity than those of 207,B-4 and MN14C11.6.
We cloned and expressed the Fab fragment of SS269 and found that it bound as well as the intact SS269 MAb to strain 44/76 expressing a P1.7,16 PorA protein. When tested against strains expressing P1.7,10 and P1.2 PorA proteins, the Fab of SS269, unlike intact SS269, showed reactivity toward both strains, albeit well below that observed for strain 44/76. Since PorA proteins are known to express silent P1.7 epitopes which are physically masked in the native protein (9, 43), it may be that the SS269 Fab due to its smaller size reacts with P1.7 epitopes not otherwise accessible to the intact SS269 MAb. Considering that P1.7-expressing strains are frequently isolated as causative agents of serogroup B infections in the continuing absence of a group B vaccine (12, 24), the successful cloning and expression of a functional SS269 Fab provides the means by which anti-P1.7 human antibodies can be generated for the passive immunization of high-risk individuals (22). Such clinically relevant antibodies could be constructed with bactericidal and phagocytic effector functions by combining human constant regions with either human or murine variable regions specific for P1.7 epitopes as described by Norderhaug et al. (47).
In conclusion, the data presented here contribute to our understanding of the human immune response to the meningococcal PorA protein. Given the importance of the PorA protein as a vaccine candidate, further studies of the immunochemistry of PorA antibodies are warranted.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grant AI32944 (G.A.J.) and the Department of Veterans Affairs (J.M.G.).
We gratefully acknowledge receipt of hybridoma cell lines and MAbs from Jan Kolberg, Department of Vaccinology, National Institute of Public Health, Oslo, Norway, and the measurement of bactericidal activity performed by Arne Høiby, Department of Bacteriology, National Institute of Public Health, Oslo, Norway. We also gratefully acknowledge the receipt of PCR primers for the MN14C11.6 VH gene from Martin Maiden and Ian Feavers, National Biological Standards Board, N.I.B.S.C., Potters Bar, United Kingdom.
Footnotes
Report no. 92 from The Center for Immunochemistry.
REFERENCES
- 1.Aase A, Høiby E A, Michaelsen T E. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand J Immunol. 1998;47:388–396. doi: 10.1046/j.1365-3083.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 2.Abdillahi H, Poolman J T. Definition of meningococcal class 1 OMP subtyping antigens by monoclonal antibodies. FEMS Microbiol Immunol. 1988;1:139–144. doi: 10.1111/j.1574-6968.1988.tb02366.x. [DOI] [PubMed] [Google Scholar]
- 3.Achtman M. Global epidemiology of meningococcal disease. In: Cartwright K, editor. Meningococcal disease. Chichester, England: John Wiley & Sons; 1995. pp. 159–175. [Google Scholar]
- 4.Achtman M, Kusecek B, Morelli G, Eickmann K, Wang J F, Crowe B, Wall R A, Hassan-King M, Moore P S, Zollinger W. A comparison of the variable antigens expressed by clone IV-1 and subgroup III of Neisseria meningitidis serogroup A. J Infect Dis. 1992;165:53–68. doi: 10.1093/infdis/165.1.53. [DOI] [PubMed] [Google Scholar]
- 5.Adib-Conquy M, Gilbert M, Christodoulou C, Avrameas S. Reactivity and structure of a mouse anti-F(ab′)2 IgM. Comparison of its variable region sequences with those of a structurally close polyreactive natural IgM. Mol Immunol. 1994;31:555–562. doi: 10.1016/0161-5890(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 6.Almagro J C, Hernandez I, Ramirez M D C, Vargas-Madrazo E. The differences between the structural repertoires of VH germ-line gene segments of mice and humans: implication for the molecular mechanism of the immune response. Mol Immunol. 1997;34:1199–1214. doi: 10.1016/s0161-5890(97)00118-1. [DOI] [PubMed] [Google Scholar]
- 7.Armand J, Arminjon F, Mynard M C, Lafaix C. Tetravalent meningococcal polysaccharide vaccine groups A, C, Y, W 135: clinical and serological evaluation. J Biol Stand. 1982;10:335–339. doi: 10.1016/s0092-1157(82)80010-3. [DOI] [PubMed] [Google Scholar]
- 8.Barbas C F, Lerner R A. Combinatorial immunoglobulin libraries on the surface of phage (Phabs): rapid selection of antigen-specific Fabs. Methods Companion Methods Enzymol. 1991;2:119–124. [Google Scholar]
- 9.Bart A, Dankert J, van der Ende A. Antigenic variation of the class I outer membrane protein in hyperendemic Neisseria meningitidis strains in The Netherlands. Infect Immun. 1999;67:3842–3846. doi: 10.1128/iai.67.8.3842-3846.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodeur P H, Osman G E, Mackle J J, Lalor T M. The organization of the mouse Igh-V locus. Dispersion, interspersion, and the evolution of VH gene family clusters. J Exp Med. 1988;168:2261–2278. doi: 10.1084/jem.168.6.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton D R, Woof J M. Human antibody effector function. Adv Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- 12.Caugant D A, Frøholm L O, Bøvre K, Holten E, Frasch C E, Mocca L F, Zollinger W D, Selander R K. Intercontinental spread of Neisseria meningitidis clones of the ET-5 complex. Antonie Leeuwenhoek. 1987;53:389–394. doi: 10.1007/BF00415492. [DOI] [PubMed] [Google Scholar]
- 13.Ceesay S J, Allen S J, Menon A, Todd J E, Cham K, Carlone G M, Turner S H, Gheesling L L, DeWitt W, Plikaytis B D. Decline in meningococcal antibody levels in African children 5 years after vaccination and the lack of an effect of booster immunization. J Infect Dis. 1993;167:1212–1216. doi: 10.1093/infdis/167.5.1212. [DOI] [PubMed] [Google Scholar]
- 14.Chazenbalk G D, Portolano S, Russo D, Hutchison J S, Rapoport B, McLachlan S. Human organ-specific autoimmune disease. Molecular cloning and expression of an autoantibody gene repertoire for a major autoantigen reveals an antigenic immunodominant region and restricted immunoglobulin gene usage in the target organ. J Clin Investig. 1993;92:62–74. doi: 10.1172/JCI116600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chothia C, Lesk A M, Tramontano A, Levitt M, Smith-Gill S J, Air G, Sheriff S, Padlan E A, Davies D, Tulip W R. Conformations of immunoglobulin hypervariable regions. Nature. 1989;342:877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- 16.Claassen I, Meylis J, Van Der Ley P, Peeters C, Brons H, Robert J, Borsboom D, van der Ark A, van Straaten I, Roholl P, Kuipers B, Poolman J. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–1008. doi: 10.1016/0264-410x(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 17.Corbet S, Milili M, Fougereau M, Schiff C. Two Vκ germ-line genes related to the GAT idiotypic network Ab1 and Ab3/Ab1′ account for the major subfamilies of the mouse Vκ-1 variability subgroup. J Immunol. 1987;138:932–939. [PubMed] [Google Scholar]
- 18.Dangl J L, Wensel T G, Morrison S L, Stryer L, Herzenberg L A, Oi V T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988;7:1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delvig A, Jahn S, Kusecek B, Heckels J E, Rosenqvist E, Høiby E A, Michaelsen T E, Achtman M. A comparison of human and murine monoclonal IgGs specific for the P1.7 PorA protein of Neisseria meningitidis. Mol Immunol. 1994;31:1257–1267. doi: 10.1016/0161-5890(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 20.Dersimonian H, Schwartz R S, Barrett K J, Stollar B D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987;139:2496–2501. [PubMed] [Google Scholar]
- 21.Estabrook M M, Griffiss J M, Jarvis G A. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65:4436–4444. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández de Cossío M E, Ohlin M, Llano M, Selander B, Cruz S, del Valle J, Borrebaeck C A. Human monoclonal antibodies against an epitope on the class 5c outer membrane protein common to many pathogenic strains of Neisseria meningitidis. J Infect Dis. 1992;166:1322–1328. doi: 10.1093/infdis/166.6.1322. [DOI] [PubMed] [Google Scholar]
- 23.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 24.Fredriksen J H, Rosenqvist E, Wedege E, Bryn K, Bjune G, Frøholm L O, Lindbak A K, Møgster B, Namork E, Rye U. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–79. [PubMed] [Google Scholar]
- 25.Gold R, Lepow M L, Goldschneider I, Draper T F, Gotshlich E C. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J Infect Dis. 1979;140:690–697. doi: 10.1093/infdis/140.5.690. [DOI] [PubMed] [Google Scholar]
- 26.Guttormsen H K, Wetzler L M, Solberg C O. Humoral immune response to class 1 outer membrane protein during the course of meningococcal disease. Infect Immun. 1994;62:1437–1443. doi: 10.1128/iai.62.4.1437-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol. 1979;9:186–188. doi: 10.1128/jcm.9.2.186-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honjo T, Matsuda F. Immunoglobulin heavy chain loci of mouse and human. In: Honjo T, Alt F W, editors. Immunoglobulin genes. San Diego, Calif: Academic Press; 1995. pp. 145–171. [Google Scholar]
- 29.Horgan C, Brown K, Pincus S H. Alteration in H chain V region affects complement activation by chimeric antibodies. J Immunol. 1990;145:2527–2532. [PubMed] [Google Scholar]
- 30.Horgan C, Brown K, Pincus S H. Effect of H chain V region on complement activation by immobilized immune complexes. J Immunol. 1992;149:127–135. [PubMed] [Google Scholar]
- 31.Jarvis G A. Analysis of C3 deposition and degradation on Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1994;62:1755–1760. doi: 10.1128/iai.62.5.1755-1760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarvis G A. Recognition and control of neisserial infection by antibody and complement. Trends Microbiol. 1995;3:198–201. doi: 10.1016/s0966-842x(00)88921-0. [DOI] [PubMed] [Google Scholar]
- 33.Kerr M A. The structure and function of human IgA. Biochem J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kettleborough C A, Saldanha J, Ansell K H, Bendig M M. Optimization of primers for cloning libraries of mouse immunoglobulin genes using the polymerase chain reaction. Eur J Immunol. 1993;23:206–211. doi: 10.1002/eji.1830230132. [DOI] [PubMed] [Google Scholar]
- 35.Kirkham P M, Mortari F, Newton J A, Schroeder H J. Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 1992;11:603–609. doi: 10.1002/j.1460-2075.1992.tb05092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraj P, Rao S P, Glas A M, Hardy R R, Milner E C, Silberstein L E. The human heavy chain Ig V region gene repertoire is biased at all stages of B cell ontogeny, including early pre-B cells. J Immunol. 1997;158:5824–5832. [PubMed] [Google Scholar]
- 37.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 38.Leatherbarrow R J, Rademacher T W, Dwek R A, Woof J M, Clark A, Burton D R, Richardson N, Feinstein A. Effector functions of a monoclonal aglycosylated mouse IgG2a: binding and activation of complement component C1 and interaction with human monocyte Fc receptor. Mol Immunol. 1985;22:407–415. doi: 10.1016/0161-5890(85)90125-7. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann A K, Halstensen A, Aaberge I S, Holst J, Michaelsen T E, Sørnes S, Wetzler L M, Guttormsen H. Human opsonins induced during meningococcal disease recognize outer membrane proteins PorA and PorB. Infect Immun. 1999;67:2552–2560. doi: 10.1128/iai.67.5.2552-2560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malorny B, Morelli G, Kusecek B, Kolberg J, Achtman M. Sequence diversity, predicted two-dimensional protein structure, and epitope mapping of neisserial Opa proteins. J Bacteriol. 1998;180:1323–1330. doi: 10.1128/jb.180.5.1323-1330.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maslanka S E, Tappero J W, Plikaytis B D, Brumberg R S, Dykes J K, Gheesling L L, Donaldson K B, Schuchat A, Pullman J, Jones M, Bushmaker J, Carlone G M. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect Immun. 1998;66:2453–2459. doi: 10.1128/iai.66.6.2453-2459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGuinness B, Barlow A K, Clarke I N, Farley J E, Anilionis A, Poolman J T, Heckels J E. Deduced amino acid sequences of class 1 protein PorA from three strains of Neisseria meningitidis. Synthetic peptides define the epitopes responsible for serosubtype specificity. J Exp Med. 1990;171:1871–1882. doi: 10.1084/jem.171.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuinness B T, Lambden P R, Heckels J E. Class 1 outer membrane protein of Neisseria meningitidis: epitope analysis of the antigenic diversity between strains, implications for subtype definition and molecular epidemiology. Mol Microbiol. 1993;7:505–514. doi: 10.1111/j.1365-2958.1993.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 44.Mulholland E K, Ahonkhai V I, Greenwood A M, Jonas L C, Lukacs L J, Mink C M, Staub J M, Todd J, Vella P P, Greenwood B M. Safety and immunogenicity of Haemophilus influenzae type B-Neisseria meningitidis group B outer membrane protein complex conjugate vaccine mixed in the syringe with diphtheria-tetanus-pertussis vaccine in young Gambian infants. Pediatr Infect Dis J. 1993;12:632–637. doi: 10.1097/00006454-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Næss L M, Aarvak T, Aase A, Oftung F, Høiby E A, Sandin R, Michaelsen T E. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine. 1999;17:754–764. doi: 10.1016/s0264-410x(98)00259-x. [DOI] [PubMed] [Google Scholar]
- 46.Ng K H, Lavigueur A, Richard L, Boivrette M, Maclean S, Cloutier D, Gibson D M. Characterization of allelic Vκ-1 region genes in inbred strains of mice. J Immunol. 1989;143:638–648. [PubMed] [Google Scholar]
- 47.Norderhaug L, Olafsen T, Michaelsen T E, Sandlie I. Versatile vectors for transient and stable expression of recombinant antibody molecules in mammalian cells. J Immunol Methods. 1997;204:77–87. doi: 10.1016/s0022-1759(97)00034-3. [DOI] [PubMed] [Google Scholar]
- 48.Olee T, Yang P M, Siminovitch K A, Olsen N J, Hillson J, Wu J, Kozin F, Carson D A, Chen P P. Molecular basis of an autoantibody-associated restriction fragment length polymorphism that confers susceptibility to autoimmune diseases. J Clin Investig. 1991;88:193–203. doi: 10.1172/JCI115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollard A J, Galassini R, van der Voort E M, Booy R, Langford P, Nadel S, Ison C, Kroll J S, Poolman J, Levin M. Humoral immune responses to Neisseria meningitidis in children. Infect Immun. 1999;67:2441–2451. doi: 10.1128/iai.67.5.2441-2451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poolman J T. Development of a meningococcal vaccine. Infect Agents Dis. 1995;4:13–28. [PubMed] [Google Scholar]
- 51.Poolman J T, Timmermans H A, Hopman C T, Teerlink T, Van Vught P A, Witvliet M H, Beuvery E C. Comparison of meningococcal outer membrane protein vaccines solubilized with detergent or C polysaccharide. Antonie Leeuwenhoek. 1987;53:413–419. doi: 10.1007/BF00415495. [DOI] [PubMed] [Google Scholar]
- 52.Rouppe van der Voort E M, Kuipers B, Brugghe H F, van Unen L M, Timmermans H A, Hoogerhout P, Poolman J T. Epitope specificity of murine and human bactericidal antibodies against PorA P1.7,16 induced with experimental meningococcal group B vaccines. FEMS Immunol Med Microbiol. 1997;17:139–148. doi: 10.1111/j.1574-695X.1997.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 53.Rouppe van der Voort E M, van der Ley P, van der Biezen J, George S, Tunnela O, van Dijken H, Kuipers B, Poolman J. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect Immun. 1996;64:2745–2751. doi: 10.1128/iai.64.7.2745-2751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rouppe van der Voort E M, van Dijken H, Kuipers B, van der Biezen J, van der Ley P, Meylis J, Claassen I, Poolman J. Human B- and T-cell responses after immunization with a hexavalent PorA meningococcal outer membrane vesicle vaccine. Infect Immun. 1997;65:5184–5190. doi: 10.1128/iai.65.12.5184-5190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saukkonen K, Abdillahi H, Poolman J T, Leinonen M. Protective efficacy of monoclonal antibodies to class 1 and class 3 outer membrane proteins of Neisseria meningitidis B:15:P1.16 in infant rat infection model: new prospects for vaccine development. Microb Pathog. 1987;3:261–267. doi: 10.1016/0882-4010(87)90059-3. [DOI] [PubMed] [Google Scholar]
- 56.Saukkonen K, Leinonen M, Abdillahi H, Poolman J T. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine. 1989;7:325–328. doi: 10.1016/0264-410x(89)90194-1. [DOI] [PubMed] [Google Scholar]
- 57.Schroeder H J, Hillson J L, Perlmutter R M. Structure and evolution of mammalian VH families. Int Immunol. 1990;2:41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz B, Moore P S, Broome C V. Global epidemiology of meningococcal disease. Clin Microbiol Rev. 1989;2:S118–S124. doi: 10.1128/cmr.2.suppl.s118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strohal R, Helmberg A, Kroemer G, Kofler R. Mouse Vκ gene classification by nucleic acid sequence similarity. Immunogenetics. 1989;30:475–493. doi: 10.1007/BF02421180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Elsen J, Vandeputte-Rutten L, Kroon J, Gros P. Bactericidal antibody recognition of meningococcal PorA by induced fit. Comparison of liganded and unliganded Fab structures. J Biol Chem. 1999;274:1495–1501. doi: 10.1074/jbc.274.3.1495. [DOI] [PubMed] [Google Scholar]
- 61.van den Elsen J M H, Herron J N, Hoogerhout P, Poolman J T, Boel E, Logtenberg T, Wilting J, Crommelin D J, Kroon J, Gros P. Bactericidal antibody recognition of a PorA epitope of Neisseria meningitidis: crystal structure of a Fab fragment in complex with a fluorescein-conjugated peptide. Proteins. 1997;29:113–125. doi: 10.1002/(sici)1097-0134(199709)29:1<113::aid-prot9>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 62.van der Ley P, Heckels J E, Virji M, Hoogerhout P, Poolman J T. Topology of outer membrane porins in pathogenic Neisseria spp. Infect Immun. 1991;59:2963–2971. doi: 10.1128/iai.59.9.2963-2971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Ley P, Poolman J T. Construction of a multivalent meningococcal vaccine strain based on the class 1 outer membrane protein. Infect Immun. 1992;60:3156–3161. doi: 10.1128/iai.60.8.3156-3161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J F, Caugant D A, Li X, Hu X, Poolman J T, Crowe B A, Achtman M. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People's Republic of China. Infect Immun. 1992;60:5267–5282. doi: 10.1128/iai.60.12.5267-5282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J F, Caugant D A, Morelli G, Koumarae B, Achtman M. Antigenic and epidemiologic properties of the ET-37 complex of Neisseria meningitidis. J Infect Dis. 1993;167:1320–1329. doi: 10.1093/infdis/167.6.1320. [DOI] [PubMed] [Google Scholar]
- 66.Warmerdam P A, van de Winkel J G, Vlug A, Westerdaal N A, Capel P J. A single amino acid in the second Ig-like domain of the human Fcγ receptor II is critical for human IgG2 binding. J Immunol. 1991;147:1338–1343. [PubMed] [Google Scholar]
- 67.Williams S C, Frippiat J P, Tomlinson I M, Ignatovich O, Lefranc M P, Winter G. Sequence and evolution of the human germline V lambda repertoire. J Mol Biol. 1996;264:220–232. doi: 10.1006/jmbi.1996.0636. [DOI] [PubMed] [Google Scholar]
- 68.Wyle F A, Artenstein M S, Brandt B L, Tramont E C, Kasper D L, Altieri P L, Berman S L, Lowenthal J P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 69.Yancopoulos G D, Desiderio S V, Paskind M, Kearney J F, Baltimore D, Alt F W. Preferential utilization of the most JH-proximal VH gene segments in the pre-B-cell lines. Nature. 1984;311:727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]