Abstract
The use of extracellular vesicle (EV)-based vaccines is a strategically promising way to prevent cancer metastasis. The effective roles of immune cell-derived EVs have been well understood in the literature. In the present paper, we focus on cancer cell-derived EVs to enforce, more thoroughly, the use of EV-based vaccines against unexpected malignant cells that might appear in poor prognostic patients. As a model of such a cancer cell with high malignancy, Nanog-overexpressing melanoma cell lines were developed. As expected, Nanog overexpression enhanced the metastatic potential of melanomas. Against our expectations, a fantastic finding was obtained that determined that EVs derived from Nanog-overexpressing melanomas exhibited a metastasis-suppressive effect. This is considered to be a novel role for Nanog in regulating the property of cancer cell-derived EVs. Stimulated by this result, the review of Nanog’s roles in various cancer cells and their EVs has been updated once again. Although there was no other case presenting a similar contribution by Nanog, only one case suggested that NANOG and SOX might be better prognosis markers in head and neck squamous cell carcinomas. This review clarifies the varieties of Nanog-dependent phenomena and the relevant signaling factors. The information summarized in this study is, thus, suggestive enough to generate novel ideas for the construction of an EV-based versatile vaccine platform against cancer metastasis.
Keywords: Nanog, melanoma, extracellular vesicles (EVs), cancer cell, cancer metastasis, immune cells, extracellular vesicle (EV)-based vaccines
1. Introduction
The development of effective vaccines to prevent cancer metastasis is a socially important and urgent issue [1,2]. Although the quantity of target cancer cells or cancerous cells in prognostic patients might be very small, they can produce metastasis, as well as reactivate primary tumor sites, by the self-seeding of circulating tumor cells [3]. For such cases, the use of vaccines is well understood to be a strategically promising method. Immune cells can respond to malignant cells and activate protection systems that destroy or render them harmless. In fact, immune cells, such as dendritic cells, have been recognized as efficient resources for extracellular-vesicle (EV) vaccines. The only idea yet to be considered in the research, however, is whether immune cells can appropriately respond to cells with a high degree of malignancy. Cancer cells remaining in prognostic patients are likely to be cells with a high resistance to drugs and chemical stress [4,5]. Those malignant cells and EVs should, therefore, be crucial targets that might conversely undermine immune-protection systems. Therefore, a great expectation has arisen in the field for a novel idea to convert negative malignant factors to positive ones that support immune functions. The potential roles of cancer cell-derived EVs, as well as immune cell-derived EVs, should be considered for the construction of EV-based versatile vaccine platforms against cancer metastasis.
2. Why Nanog?
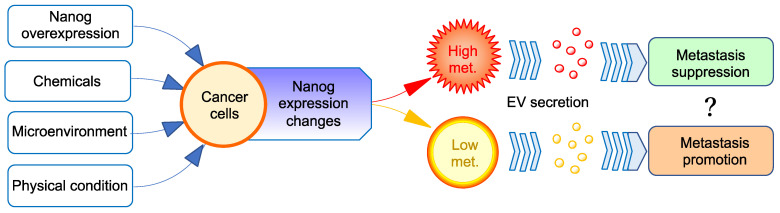
The first step of our experiment was to create a malignant cancer cell line with high metastatic potential. Mouse melanoma cell lines, B16-F10 and B16-BL6, were selected as the baseline for developing a novel cell library. These cell lines were genetically modified to create Nanog-overexpressing cell lines Nanog+F10 and Nanog+BL6. Nanog is a principal factor essential for the maintenance of the undifferentiated state (pluripotency, stemness) of embryonic stem cells. Nanog was thought to be able to increase the stemness of various other cells, and the stemness of cancer cells was suggested to be a crucial factor of malignancy. As expected, Nanog overexpression could enhance the metastatic potential of melanomas, indicating that a melanoma was made more malignant. EVs derived from B16-F10 cells exhibited a metastasis-promotion effect in the same way as those reported in other studies about other cancer cell-derived EVs. Unexpectedly, however, EVs derived from Nanog+F10 cells exhibited a metastasis-suppression effect (Figure 1). Such a Nanog-dependent effect of EVs was also observed for colon cancer. This result began with a simple idea of Nanog overexpression to increase stemness. However, it revealed an attractive phenomenon from the perspective of EV-based vaccines. Therefore, it would be important to investigate the detailed role of Nanog in this phenomenon.
Figure 1.
Paradoxical effects of EVs from Nanog-overexpressing cancer cells on their metastasis. Nanog expression level in cancer cells can be changed by genetic, chemical, microenvironmental, or physical factors. The higher the Nanog expression level, the higher the metastatic potential. The role of EVs in cancer metastasis has been thought to follow the metastatic potential of cancer cells, that is, EVs derived from metastatic cancer cells exhibit metastasis-promoting effects. However, in the case of cancer cells with a very high metastatic potential, contrary to our expectations, EVs may promote cancer metastasis.
3. Roles of Nanog in Cancer Cells
A high quantity of research papers have reported the potential roles of Nanog in various types of cancers. There were two or three recently published papers for each type of cancer selected, and they are summarized in Table 1. Short comments for respective papers are described, below, under Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5, Section 3.6, Section 3.7, Section 3.8, Section 3.9, Section 3.10, Section 3.11, Section 3.12 and Section 3.13.
Table 1.
Effects of the alteration of Nanog expression levels on cancer cell properties.
| Type of Cancer [Cell Line] |
NEL *1 Altering Factor [NEL] | Effects on Cancer Cell Functions | P or S *2 | Signaling Factors Downstream of Nanog [Role as a Marker of Cell Function] |
Ref. | |
|---|---|---|---|---|---|---|
| Breast cancer [MCF7] |
OE *3 [High] |
Cell adhesion ↑ | P | Itga9 ↑, Cldn11 ↑, Cldn10 ↑, Cldn6 ↑ | Array analysis | [6] |
| Ca signaling ↑ | Atp2a1 ↑, Cacna1h ↑, Gna14 ↑, Cacna1g ↑ | |||||
| Focal adhesion ↑ | Pdgfrα ↑, Pdgfrβ ↑, Itga11 ↑, Itgb5 ↑, Rasgrf1 ↑ | |||||
| p53 signaling (tumor suppressor gene) ↓ | Gadd45a ↓, Gadd45g ↓, Pten ↓, Bax ↓ | |||||
| Tumorigenesis by co-expression with Wnt-1 | ||||||
| Breast cancer [T47D, MCF7, MDA-MB-231] |
mTOR inhibitors, Chemotherapeutics [Up] |
Stem cell-like phenotype ↑ | P | NANOG ↑, SNAIL ↑, NODAL ↑ | [7] | |
| Cervical carcinoma [CaSki] |
OE [High] |
Resistance to complement-dependent cytotoxicity ↑ | P | CD59 ↑ through promoter occupancy, CD59 binds C8 and C9 and inhibits MAC (membrane attack complex) formation | [8] | |
| Cervical cancer [TC-1 (P0~P3)] |
Vaccination-induced cancer evolution (VICE) [Up] |
Resistance to cytotoxic T lymphocyte lysis ↑ Stem-like phenotype ↑ |
P | Stemness markers (CD133, CD44, ALDH) ↑ in TC-1(P3) | [9] | |
| Colon cancer [SW480, SW620, HT29, CT26] |
Furin [Down by furin repression] |
NANOG ↓, LGR5 ↓, Calcium transportation ↓ | S by NANOG ↓ | [10] | ||
| Colon/Colorectal cancer [HCT116, HT29] |
OE [High] |
Dormancy ↑, Chemoresistance ↑ |
P | Cell cycle regulating P21 ↑, P27 ↑ | [11] | |
| Embryonal carcinoma [NT2/D1] |
MIR630 [Down] |
Differentiation ↑ | S by NANOG ↓ | PKC ⇒ miR630 ↑ ⇒ NANOG 3′UTR ⇒ NANOG ↓ | [12] | |
| Somatic cancer [HeLa, HCT116] |
Nanog-C/CD2 as a Rad51 inhibitor [High] |
Inhibition of chemo-/radiotherapy-generated DNA damages of cancer cells mediated by Rad51 | S by Nanog-C/CD2 | Interaction of C/CD2 domain with Rad51 | [13] | |
| Hepatocellular carcinoma (HCC) [MHCC97H, -L, MHCCLM3, etc.] |
OE [High] |
EMT ↑, Invasion ↑ | P | Nodal ⇒ Smad3 | [14] | |
| Hepatocellular carcinoma [CD133+/CD49f+ TICs from HCC of alcoholic patients, HEK239T] |
Obesity, Alcohol, Virus [Up] |
Self-renewal ↑, Chemoresistance ↑ | P | Obesity, Alcohol ⇒ LPS ⇒ TLR4 ↑ ⇒ NANOG ↑ ⇒ FAO ↑, NANOG ⇒ OXPHOS ↓ |
[15] | |
| Hepatocellular carcinoma [MHCC97-L] |
Co-expression of Oct4 and Nanog [High] |
Stemness ↑, EMT ↑ | P | Stat3 ↑, Snail ↑ | [16] | |
| Melanoma (human) [A375] |
OE *3 [High] |
Amoeboid migration ↑ | P | ARHGAP22 ↑, DOCK10 ↑, EPHA2 ↑ | [17] | |
| Melanoma (mouse) [B16-F10] |
Hypoxia [Up] |
Spheroid formation ↑, Proliferation ↑ | P | Tgf-β1 ↑ | [18] | |
| Melanoma (mouse) [B16-BL6] |
OE [High] |
Proliferation ↑, Migration ↑, Invasion ↑ | P | Tgf-β1 ↓ | [19] | |
| Melanoma (mouse) [B16-F10] |
OE [High] |
Proliferation ↑, Migration ↑, Invasion ↑ | P | Tgf-β1 ↓ | [20] | |
| Ovarian [6 cell lines, Ascites of patients] |
HK2 [Up] |
Migration ↑, Invasion ↑, Metastasis ↑ | P | HK2 ⇒ FAK ↑ ⇒ ERK1/2 ↑ ⇒ MMP9 ↑ | [21] | |
| Stemness ↑ | P | HK2 ⇒ FAK ↑ ⇒ ERK1/2 ↑ ⇒ NANOG · SOX9 ↑ | ||||
| Pancreatic cancer stem cell (CSC) [AsPC-1, PANC-1, CSCs from primary tumors] |
α-Mangostin [Down] |
EMT ↓ | S by Nanog ↓ |
Gli signaling (Nanog, Oct4, c-Myc, Sox-2, KLF4) ↓ [human pancreatic CSCs marker: CD133+ /CD44+/CD24+/ ESA+] |
[22] | |
| Prostate cancer [DU145, PC3, 22Rv1] |
OE [High] |
Escape from NK cell attack ↑ | P | ICAM1 ↓ | [23] | |
| Prostate cancer [Xenograft models (LAPC4, LAPC9)] |
Endogenous NANOG [Varied] |
Castration resistance ↓ | P | NANOG co-occupies FOXA1 and androgen receptor loci ⇒ pro-differentiation genes ↓ | [24] | |
| Esophageal squamous carcinoma [Eca109] |
OE [High] |
Proliferation ↑, Invasion ↑, Stemness ↑ | P | IL6 ↑, STAT3 ↑, CCL5 ↑, VEGFA ↑, CCND1 ↑, Bcl-xL ↑ | [25] | |
| Head and neck squamous cell carcinomas (HNSC) [QLL1, SCC15, SCC25] |
CD44(+) [Higher than in CD44(-) cells] |
Migration ↑, Invasion ↑, Radiotherapy resistance ↑, EMT ↑ | P | NANOG and ERK1/2 synergistic effects | [26] | |
| HNSC | Patients [Varied] |
S | [NANOG and SOX2 ⇒ Better prognosis] | [27] | ||
| Nasopharyngeal Carcinoma | Patients [Varied] |
P | High frequency of Nanog, OCT4 at tumor invasive front, lymph node metastasis | [28] | ||
| Oral squamous cell carcinoma | Patients with/without lymph node metastasis [Varied] |
P | Positive-correlation [Nanog] vs [OCT4, NOTCH1, AGR2, KLF4] at mRNA [NANOG protein/mRNA:Poor prognosis marker?] |
[29] | ||
| Cancer stem cell (CSC) models [HNSC, non-small lung cancer, colon cancer, A549] |
NANOG overexpressed or varied [High] |
Migration ↑, Invasion ↑, EMT ↑ | P | BMI1 ⇒ E-Cadherin ↓ | [NANOG as a CSC marker (proposed)] | [30] |
| SNAIL2 ⇒ E-Cadherin ↓ | ||||||
| BMI1 ⇒ SNAIL1 ⇒ E-Cadherin ↓ | ||||||
| SNAIL2, BMI1 ⇒ SNAIL1 | ||||||
| Proliferation ↑, Self-renewal ↑, Chemoresistance ↑ | P | BMI1 | ||||
| Anti-apoptosis ↑, Chemoresistance ↑ | P | NANOG · STAT3 ⇒ miR21 ⇒ PDCD4(cancer resistance gene) ↓ | ||||
| Hedgehog signal (Hh), FAK [Varied] |
Stemness ↑ | P | Hh ⇒ PATCH1/2 ↓ ⇒ SMO ↑ ⇒ GLI1 ↑ ⇒ NANOG ↑ ⇒ GLI1 ↑ (Positive feedback) |
|||
| Anti-apoptosis ↑ | P |
FAK ⇒ NANOG ↑ ⇒ p53 ↓ ⇒ NANOG ↓ (Negative feedback) |
||||
| PD-1 therapy treated patients, Anti–PD-1 therapy model mice | Patients with 19 types of cancer [High] |
T-cell invasion ↓, Resistance to cytotoxic T-cell ↑ | P |
NANOG ⇒ HDAC1 ⇒ Cxcl10 ↓, MCL1 ↑ [Antitumor immunity marker] |
[31] | |
*1 NEL: Nanog expression level; *2 P or S: promotive or suppressive; *3 OE: overexpression; ↑: up-regulated; ↓: down-regulated.
3.1. Breast Cancer
The overexpression of NANOG increased cell adhesion. Additionally, p53, a tumor-suppressive gene, decreased. Concomitantly, the expression of downstream factors, such as Gadd45a, also decreased. The enhancement of Nanog expression promoted migration and invasion activities. Tumorigenesis was not induced by Nanog overexpression alone but by the co-expression with Wnt-1 [6].
Treatment with mTOR inhibitors and chemotherapeutic agents increased NANOG expression in a similar manner to hypoxia. Concurrently, the translation of a subset of SNAIL and NODAL mRNA isoforms was activated. The accumulation of these proteins enhanced the stem cell phenotype, increased drug resistance, and promoted metastasis [7].
3.2. Cervical Cancer
CD59 binds to C8 and C9 and, therefore, inhibits the formation of the membrane attack complex (MAC) that requires C9. Therefore, complement-dependent cytotoxicity (CDC) via MAC is inhibited by CD59. In a NANOG-overexpressing cell line (CaSki-NANOG), CD59 was up-regulated, and the resistance to CDC increased. NANOG directly bound to the CD59 promoter to enhance its expression activity [8].
According to the cancer immunoediting theory, heterologous tumor cells are continuously subject to host immune surveillance [32,33]. Cells vulnerable to immune surveillance are eliminated, while cells that evade detection and killing proliferate. Based on this idea, a method to create cancer cells with high immune-resistance levels, the vaccination-induced cancer evolution (VICE) method, was developed. TC-1(P3), which was obtained by repeating the subculturing process (removing the cancer cells that were inoculated into mice and then inoculating them into mice again) by this method three times, was the first cell line. Nanog expression was increased 10-fold compared to TC-1(P0). An increase in stemness markers (CD133, CD44, aldehyde dehydrogenase (ALDH)) was also observed [9]. It was shown that the higher the degree of malignancy of cancer cells, the higher the expression level of Nanog.
3.3. Colon Cancer/Colorectal Cancer
In colon cancer, LGR5 and NANOG are assumed to be stem cell markers. Therefore, the possibility of therapeutics targeting these markers was investigated in this study. As an example, furin, which belongs to the subtilisin-like proprotein convertase family, was investigated. Furin is involved in the activation of the functions, such as calcium transport, in colon cancer. Inhibitors of furin, such as PDX-1, Spn4A, and decanoyl-RVKR-chloromethylketone (CMK), were applied to investigate the effect of furin inhibition in vivo. As a result, it was understood that furin inhibition reduced the expression of stem cell markers and the malignancy of cancer cells [10].
In colorectal cancer, serum deprivation induced increased chemoresistance and enhanced dormancy through the increased expression of dormancy markers, and it also induced enhanced Nanog expression. The knockdown of Nanog abolished dormancy, whereas the overexpression of Nanog promoted dormancy through the transcription of P21 and P27. In the dormant state, cancer cells are malignant. Thus, enhanced Nanog expression is a factor in malignant transformation [11].
3.4. Embryonic Carcinoma
NANOG was shown to promote tumorigenesis in embryonic carcinomas. miRNAs that suppress NANOG expression were sought. The upstream factors of NANOG were surveyed. PKC was confirmed to be involved in the regulation of NANOG expression. A genome-wide analysis of miRNA expression was performed in the embryonal carcinoma cell line NT2/D1 in the presence of the PKC activator phorbol 12-myristate 13-acetate (PMA). As a result, an increased expression of MIR630 was confirmed. The transfection of MIR630 into embryonic carcinomas suppressed NANOG. The reactive site was NANOG 3′UTR [12].
3.5. Somatic Cancer
HeLa (cervical cancer) and HCT116 (human colon cancer) were used as somatic cancer cells. Rad51 is a protein involved in the homologous recombination (HR) repair of DNA damage. This protein prevents cancer cells that have been damaged by chemo or radiation therapies from dying. Therefore, Rad51 inhibitors were considered as effective for cancer treatment. Nanog was shown to be effective as a Rad51 inhibitor. Nanog interacted with Rad51 at the C or CD2 domain. Nanog-C/CD2 peptides were directly delivered to somatic cancer cells via nanoparticles or cell-membrane permeable peptides. The introduction of Nanog or moieties contributed to tumor suppression [13].
3.6. Hepatocellular Cancer
NANOG was activated by the TLR4-E2F1 pathway. NANOG suppressed mitochondrial oxidative phosphorylation genes (OXPHOS) and enhanced fatty acid oxidation (FAO) in tumor-initiating stem-like cells. FAO enhanced self-renewal and chemoresistance properties. On the other hand, restoring OXPHOS suppressed the self-renewal property [15].
3.7. Melanoma
The relationship between different motility modes and metastatic potential in human melanoma A375 was also investigated in this study. Mobility includes mesenchymal and amoeboid migrations [34]. A375 showed a mesenchymal motility mode, but the overexpression of NANOG or OCT4 increased amoeboid migration, resulting in an increased metastatic potential [17].
Nanog was up-regulated in mouse melanomas under hypoxia. This increased regulatory T cells (Treg) through the increased expression of Tgf-β1. Tregs are CD4+T cells that release the anti-inflammatory cytokine IL-10 and suppress immune responses. As a result, the proliferation and metastasis of cancer cells were promoted. The targeted inhibition of Nanog reduced Treg-like immunosuppressive cells and increased CD8+T cells (cytotoxic T cells), resulting in the suppression of cancer growth and metastasis [18].
3.8. Ovarian Cancer
Hexokinase 2 (HK2) is one of four isoenzymes. HK2 was overexpressed in ovarian cancer and showed significantly higher expression levels in ascites and metastases. Cell migration and invasion were enhanced by a NANOG-non-mediated pathway, HK2 ⇒ FAK ⇒ ERK1/2 ⇒ MMP9, and stem cell properties were enhanced by a NANOG-mediated pathway, HK2 ⇒ FAK ⇒ ERK1/2 ⇒ NANOG, SOX9 [21].
3.9. Pancreatic Cancer
In rare and highly malignant cancer stem cells, the hedgehog/glioma-associated oncogene homolog (HH/GLI)-signaling pathway regulates self-renewal, initiates and sustains tumor growth, and promotes drug resistance and metastasis [35]. The inhibitory effect of natural α-mangostin on this signaling pathway was examined. As a result, the expression of target genes (Nanog, Oct4, c-Myc, Sox-2, and KLF4) of this signal transduction system was inhibited, and an antitumor effect was observed. Conversely, the overexpression of Nanog abolished its inhibitory effect, suggesting that the effect of α-mangostin was mainly obtained by inhibiting Nanog expression. At the same time, it was concluded that the method targeting Nanog is preclinically effective for the prevention and treatment of pancreatic cancer [22].
3.10. Prostate Cancer
The relationship between cell–cell adhesion and the malignancy of prostate cancer cells DU145, PC3, and 22Rv1 was investigated. The overexpression of NANOG enhanced the ability to evade attacks from the NK cell MTA cell line (CD4 and CD56-positive T-cell line) [23]. NANOG suppressed the expression of ICAM1, a cell-adhesion molecule. Without ICAM1 on the cell surface, NK cells cannot recognize it, and cancer cells escape attack from NK cells.
3.11. Squamous Cell Carcinoma
In esophageal squamous cell carcinomas (ESCCs), the knockdown of NANOG clearly reduced cancer cell proliferation and the ability to resist drugs. It was presumed that IL-6/STAT3 was down-regulated [25].
In the case of head and neck squamous cell carcinoma (HNSCC) cells, a comparative analysis of CD44+ cells (indicator of stemness) and control CD44(−) cells revealed that Nanog or ERK1/2 was highly expressed in CD44+ cells. Thus, it was determined that they exhibited migration ↑, invasion ↑, radiotherapy resistance ↑, and EMT ↑ properties [26]. Nanog and ERK1/2 appeared to exhibit synergistic effects.
For HNSCC, 348 postoperative patients were also investigated [27]. As a result, NANOG protein was highly expressed in 72%, and SOX2 was highly expressed in 30%. The prognosis was better in NANOG’s and SOX2′s high expressions. In other words, NANOG and SOX2 can be used as good prognostic markers. Moreover, NANOG was also tumor site-specific and correlated with a favorable prognosis for pharyngeal tumors (rather than laryngeal) [27]. NANOG is probably uniquely considered a good prognostic marker. NANOG also serves as an independent prognostic factor in nasopharyngeal carcinomas [28].
The case of oral squamous cell carcinomas (OSCCs) was also investigated in 120 patient samples following surgery. As a result, the expressions of NANOG and OCT4 were higher in patients with lymph node metastases than in those without lymph node metastases, suggesting the possibility of NANOG as a malignant prognostic marker. However, protein and mRNA expression levels sometimes did not match. At the mRNA level, it was positively correlated with other cancer malignancy-associated factors: OCT4, SOX2, NOTCH1, AGR2, and KLF4 [29].
3.12. Cancer Stem Cells
The validity of NANOG as a cancer stem cell marker was discussed. It was proposed that NANOG might be considered as one of the markers, based on the following observations of multiple types of cancer cells, when NANOG is overexpressed. Following the enhancement of NANOG expression, there appeared increased expressions of BMI and SNAIL1/2, followed by the suppression of E-cadherin expression in various cancer cells (glioblastoma, non-small lung cancer, HNSC, colon cancer, and A549). In addition, an increased expression of NANOG ⇒ STAT3 ⇒ miR21 was followed by the down-regulation of programmed cell death 4 (PDCD4), resulting in the enhanced anti-apoptotic and chemoresistance properties of cancer cells. All of these are factors that increase the migratory ability, proliferative ability, and epithelial–mesenchymal transition (EMT), resulting in an increased malignancy as cancer cells [30].
On the other hand, the hedgehog signaling factor binds to PATCH1/2 (which is the receptor) and abolishes the inhibitory effect of PATCH1/2 on SMO. As a result, SMO ↑ ⇒ GLI1 ↑ ⇒ GLI1 nuclear translocation ⇒ NANOG promoter-activation ⇒ NANOG mRNA ↑ ⇒ NANOG protein ↑ ⇒ GLI1 ↑ (positive feedback). On the other hand, p53 (which induces apoptosis) represses the NANOG promoter ⇒ NANOG mRNA ↓ ⇒ NANOG protein ↓. Regardless of NANOG expression levels, NANOG represses p53, creating negative feedback. These positive and negative feedbacks suppress apoptosis, maintain cancer stemness, and contribute to cancer malignancy [30].
3.13. PD-1-Treated Patients and Their Model Mice
Programmed cell death protein 1 (PD-1) inhibitors and PD-L1 inhibitors are a group of checkpoint-blocking anticancer agents that block the activity of PD-1 and PDL1 immune-checkpoint proteins present on the cell’s surface. This immune-checkpoint inhibitor has emerged as a frontline therapy for several types of cancer. Using the transcriptional data obtained from cancer patients treated with PD-1 therapy and a newly established murine preclinical anti-PD-1 therapy-refractory model, NANOG was identified as a factor that enhanced patients’ resistance to immune-checkpoint inhibitors. NANOG regulated this immune checkpoint by suppressing T-cell infiltration and increasing resistance to killing by cytotoxic T lymphocytes (CTLs) through a histone deacetylase 1-dependent (HDAC1-dependent) regulation of CXCL10 and MCL1 [31].
3.14. Summary of NANOG Roles
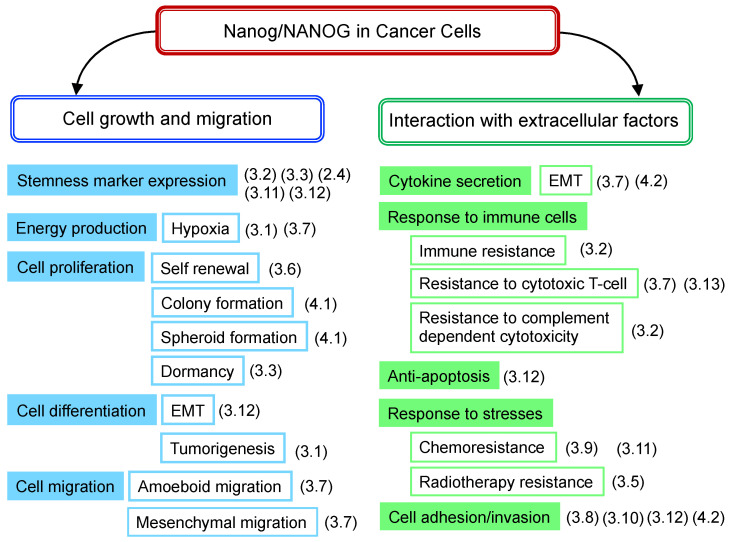
The role of Nanog, which has been clarified for various cancer cells, is related to the growth and migration of cancer cells themselves, as well as the interaction with various extracellular factors from the perspective of the effects on cancer cell functions (Figure 2). The following points summarize the contents of Table 1.
Figure 2.
Nanog-dependent phenomena observed in cancer cells. Contents listed in Table 1 are rearranged according to the phenomena of cancer cells. (3.1), (3.2), denote the number of sub-sections with a description of the phenomenon.
-
(a)
High levels of Nanog expression are associated with increased malignancy, which has been observed in many types of cancers. The only exception is the case of HNSC.
-
(b)
Nanog targeting, alone, does not necessarily lead to cancer cytocide.
-
(c)
The degree of malignancy of cancer cells is not solely governed by Nanog.
-
(d)
Cancer cells with high levels of Nanog expression have high metastatic potential. It shows potential as a marker of malignant prognosis. Indeed, Nanog has shown promise as a marker for predicting the efficacy of PD-1 therapy.
-
(e)
Molecular mechanisms, leading to malignant transformations, greatly differ depending on the type of cancer. There are almost no research reports about why NANOG signaling differs depending on cancer types. This point should be clarified for the use of Nanog as a therapeutic target.
-
(f)
From a therapeutic perspective, the enhancement of immune functions is essential and, therefore, novel ideas are required to combine Nanog-targeting therapy with immunotherapy.
4. Nanog Overexpression in Melanoma
4.1. Transcriptome Analysis
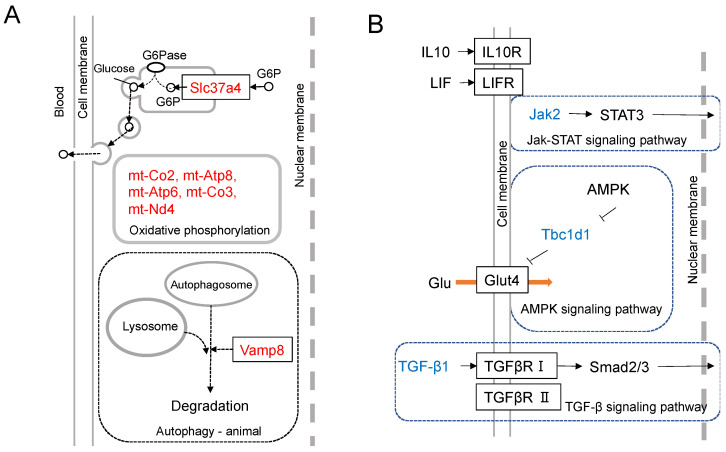
The transcriptome analysis of mouse melanoma cells was conducted to clarify the differential expression intensities between a cell line, B16-BL6, and its Nanog-overexpressing cell line Nanog+BL6. The up-regulated top-16 genes and down-regulated top15 genes were depicted [19]. The functional roles of up-regulated top-7 genes and down-regulated top-3 genes are illustrated in Figure 3A,B, respectively.
Figure 3.
Results of transcriptome analysis. (A) Differential expression of Nanog-dependent genes between Nanog+BL6 and B16-BL6 cell lines. Assumed-role diagrams of up-regulated top-7 genes (Slc37a4, mt-Atp6, mt-Nd3, mt-Co3, mt-Atp8, mt-Co2, and Vamp8). (B) Differential expression of Nanog-dependent genes between Nanog+BL6 and B16-BL6 cell lines. Assumed-role diagrams of down-regulated top-3 genes (Jak2, Tbc1d1, and TGF-β1).
Slc37a4 is a protein that transports glucose-6-phosphate (G6P) from the cytosol to the endoplasmic reticulum (ER). G6P is dephosphorylated in ER and released as glucose out to intercellular space or blood vessels. When cancer cells form colonies, glucose diffusion from the outer solution to the central cells takes a much longer time when compared to cells on the outer surface of the colony. In such a case, if a series of cells in contact with each other can relay glucose, glucose transport can be performed rapidly. The increased expression of Slc37a4 may contribute to the activation of such a glucose relay. The accelerated glucose supply throughout the colony will accelerate cell growth. The acceleration of energy production as ATP is facilitated by five genes (mt-Co2, mt-Atp8, mt-Atp6, mt Co3, and mt-Nd4) that may contribute to the acceleration of oxidative phosphorylation. Vesicle-associated membrane protein 8 (Vamp8) is involved in surviving the emergency of starvation. When cancer cells are placed in a state of starvation, they transport their own cytoplasm into autophagosomes, digest it, and use the nutrients.
The most down-regulated gene is Jak. Immunosuppression and malignant tumors are caused by the dysfunction of the Jak-STAT-signaling pathway. The down-regulation of Jak causes a similar condition and also produces more malignant melanomas. Glut4 facilitates glucose uptake. Tbc1d1 suppresses this uptake of glucose. Therefore, the suppression of Tbc1d1 stimulates glucose uptake activity and promotes cancer cell growth. Regarding Tgf-β1, however, it is necessary to consider its dual roles: tumor-suppressive in early stage tumors but tumor-promotive in advanced cancers [36,37,38]. Tumor-promotive roles are the promotion of angiogenesis, immunosuppression, and apoptosis induction. Transcriptome analysis indicated the down-regulation of Tgf-β1, although melanoma cells were made more malignant, which was supported by in vitro and in vivo tests.
4.2. Experimental Analyses
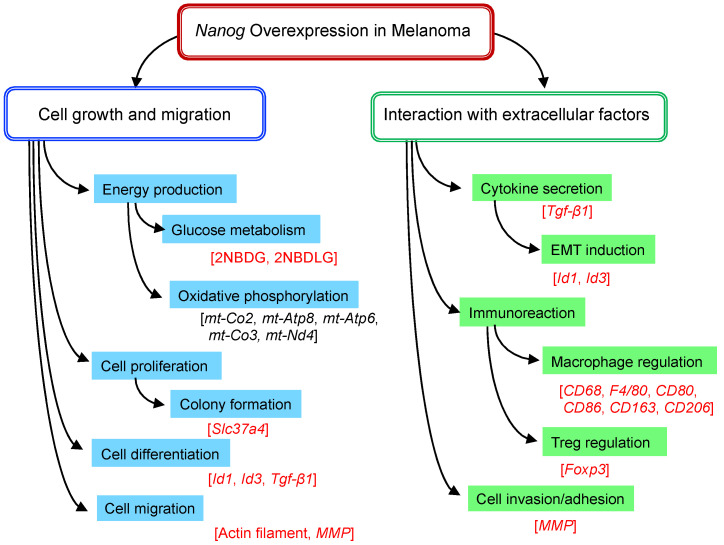
The in vitro and in vivo tests were conducted to investigate the effects of Nanog overexpression on the functional roles of melanoma cells. The cell lines were B16-F10, B16-BL6, Nanog+F10, and Nanog+BL6. The characteristic functions to be studied for a metastatic property evaluation are summarized in Figure 4.
Figure 4.
Nanog-dependent phenomena observed in melanoma. Relevant genes, proteins, or probes are described in brackets. Red letters in brackets indicate those analyzed by experiments; black letters in brackets indicate those analyzed only by transcriptome analysis.
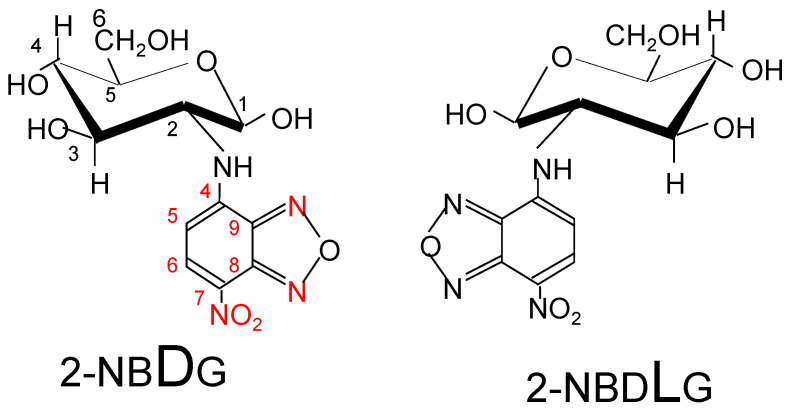
The glucose uptake activity is greater in cancer cells than in normal cells, and an analytical method used for visualizing glucose uptake activity has been introduced into cancer diagnostic methods. A pathological observation method that utilizes fluorescent glucose (Figure 5) was developed to distinguish between cancer cells, normal cells, and cells likely to become cancerous [39,40]. Normal cells only take up 2NBDG (D-type fluorophore), whereas cancer cells take up both 2NBDG and 2NBDLG (L-type fluorophore). In fact, it was confirmed that four melanoma cell lines tested could take in both 2NBDG and 2NBDLG. Furthermore, it was suggested that the total uptake of 2NBDG and 2NBDLG might be used as a marker of the degree of cancer cell malignancy.
Figure 5.
Fluorescent glucose analogs: 2NBDG: 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose, 2NBDLG: 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-L-glucose.
Cell–cell glucose relay was suggested to be one of Slc37a4′s roles. Accordingly, it suggested the promotion of glucose uptake as well. The knockdown of Slc37a4 caused a decrease in the uptake rate of 2NBDG, but there was no effect on the uptake rate of 2NBDLG [unpublished data]. Therefore, the up-regulation of Slc37a4 contributed to the increase in glucose uptake.
Since the expression level of Tgf-β1 was a controversial matter, it was analyzed at mRNA and protein levels. As a result, its expression was down-regulated at both levels [19]. Another study conducted elsewhere [18] demonstrated a conflicting result. Nanog expression in melanomas increased under a hypoxic condition, and the increase in Tgf-β1 expression followed. We suspected that this inconsistency might be caused by the difference in the expression level of Nanog. The Nanog expression level, up-regulated by a hypoxic condition, might be much lower than that up-regulated by genetic overexpression. The response of Tgf-β1 was concluded to be Nanog-expression level-dependent.
The expression of matrix metalloproteinase (MMP)s was studied since MMPs were believed to be relevant to invasion, although they were not included in the top 31 genes. They observed the increase in MMP9, a secretion-type MMP [19].
The interaction with immune cells was thought to principally occur via the EVs described below. The involvement of macrophage and Treg was investigated.
The results of the studies performed show that Nanog overexpression made melanoma cells more aggressive. In addition, melanoma cell lines with co-overexpressing Nanog, with Oct3/4 and/or Sox2, were created in order to further enhance stemness. Consequently, however, any combination could not create a cell line with a greater metastatic potential than the cell line with the overexpression of Nanog alone.
5. Properties of EVs Secreted from Cancer Cells
5.1. Tumor-Promoting Effect
The functional roles of dendritic cell-derived EVs and cancer cell-derived EVs have been well discussed in the literature [1,41,42]. Dendritic cell-derived EVs are regarded as promising materials for immunotherapy. In contrast, cancer cell-derived EVs are considered unsuitable. In fact, all of the 31 cases summarized in [42] showed that the effect of cancer cell-derived EVs on immune cells was the suppression or inactivation of immune activity. The active substances delivered by EVs were unknown in 10 out of 31 cases. Other cases, however, were six Fas cases, six TGF-β cases, and five miRNA cases. In the case of melanomas involving the Fas ligand, Jurkat and other lymphoid cells induced apoptosis associated with caspase activation [43]. Colon cancer also expressed the Fas ligand and TNF-α at the same time, resulting in the induction of T-cell apoptosis both in vivo and in vitro.
When cancer cell-derived EVs are taken up by other cancer cells of the same type, they change the properties of those cancer cells. There were eighteen types of cases summarized, and in all cases, EVs increased the proliferation, migration, invasion, EMT, and metastasis of cancer cells that took them in [4]. It also promoted the polarization of macrophages to the M2 type (tumor-promotive). In these examples, EV-delivered active substances included integrin αVβ6, apolipoprotein E, EGFR, Wnt4, IL-6, and TGF-β, as well as cell-specific miRNAs and long non-coding (lnc) RNAs. On the other hand, cancer cell-derived EVs are also transported to normal fibroblasts, and once taken up, the fibroblasts release EVs that have suppressive effects on immune cells [44].
Furthermore, cancer cells that have undergone anticancer drug treatment may be highly resistant to the drug. EVs released from such highly resistant cancer cells may change the non-resistant allogeneic cells to resistant cells. The effects of EVs derived from cancer cells that were resistant to anticancer agents, such as tamoxifen [45,46], cisplatin [47,48], and gemcitabine [49], were investigated. Cancer cells exposed to those EVs became more resistant to respective anticancer agents. EVs secreted from liver cancer stem cells induced Nanog in differentiated cancer cells, resulting in increased resistance to the anticancer drug regorafenib [50]. Small EVs secreted from gastric cancer cells enhanced the stemness of other gastric cancer cells and increased their resistance to oxaliplatin [51].
In another case, temozolomide (TMZ)-resistant and sensitive tumor cells were obtained from each of the TMZ-resistant (n = 36) and sensitive (n = 33) glioma patients. Circular RNA circ_0072083 expression was increased in resistant cells, and its knockdown reduced resistance, concomitantly reducing NANOG expression. EVs containing circ_0072083 released from resistant cells increased the resistance of sensitive cells to TMZ both in vitro and in xenograft models [5].
5.2. Metastasis-Inhibitory Effect
Cancer cells that have undergone chemotherapy, radiation therapy, and heat stimulation may increase resistance to each factor. This creates an increase in malignancy. EVs released from such malignant cancer cells enter other cancer cells and strengthen their resistance to the same factor as described above. However, EVs are also taken up by immune cells, such as dendritic cells. As a result, dendritic cells receive malignant cancer cell information and damage-related molecular patterns (DAMPs), such as DNA and RNA, which may enhance antitumor activity by activating intracellular virus-sensing pathways and producing inflammatory cytokines.
EVs released from breast cancer cells treated with the anticancer drug topotecan contained DNA that activated dendritic cells via a stimulator of interferon gene (STING) signaling [52]. In addition, when breast cancer model cells were irradiated with therapeutic radiation, the EVs released from these breast cancer cells were taken up by dendritic cells, and then, they activated the cyclic GMP-AMP synthase (cGAS) within the dendritic cells. In this case, dendritic cells were also activated via STING signals. In vivo, the EVs elicited a CD8+T-cell response and presented tumor-preventive effects [53].
The final case was the metastasis-suppressive effect of melanoma-derived EVs that initiated this review. There are still a few cases of metastasis inhibitory effects by cancer cell-derived EVs.
6. Suppression of Cancer Metastasis by Melanoma-Derived EV
6.1. Comparison of Metastatic Potential between B16-F10 and Nanog+F10
Metastatic colonies were analyzed two weeks after the introduction of mouse melanoma B16-F10 and Nanog+F10 from the mouse tail vein. Preliminary studies revealed that the highest number of metastatic colonies was generated on the liver. Therefore, liver was focused on as a predominant target organ, and the number and volume of metastatic colonies were quantitatively analyzed. As a result, those of Nanog+F10 increased 2.5 and 2.4 times, respectively, compared to B16-F10 [20]. At the same time, in vitro tests were conducted separately to investigate cell proliferation and migration. The results support the enhancement of the metastatic potential of Nanog+F10.
6.2. Comparison of the Effect of B16-F10-EV and Nanog+F10-EV on Melanoma Metastasis
EVs released from B16-F10 and Nanog+F10 were obtained, and their vaccine effects were investigated. EVs (5 μg/100 μL PBS) or PBS (100 μL as a control) were injected into the tail vein of 5–6-week-old mice 3 times per week for 3 weeks, and subsequently, melanoma cells (5 × 105 cells/250 μL PBS) were injected into the tail vein. Livers were separated and metastatic colonies were analyzed 2 weeks later. As a result, F10-EV increased metastasis, but Nanog+F10-EV suppressed metastasis [20].
6.3. Role of Tgf-β1 in the Anti-Metastasis Effect
As a mechanism of the metastasis-suppressive effect, (i) the effect of Tgf-β1 in EV and (ii) the tumor suppressive effect by immune cells (macrophages, Tregs) were presumed. Regarding (i), B16-F10, Nanog+F10, F10-EV, Nanog+F10-EV, and Tgf-β1 knockdown cell lines and EVs obtained from them (Tgf-β1(−)-F10, Tgf-β1(−)-F10-EV) were used. Tgf-β1 gene expression level and protein concentration (pg/μg EVs) in those cell lines—and EVs therefrom—were analyzed. The concentration of Tgf-β1 protein in EVs and the effect on metastasis were summarized as F10-EV (3.9 pg/μg EVs, promotive), Nanog+F10-EV (1.6 pg/μg EVs, suppressive), and Tgf-β1(−)-F10-EV (0.5 pg /μg EVs, suppressive) [20]. At high concentrations of Tgf-β1, the role of Tgf-β1 was the promotion of metastasis. In contrast, it turned into the suppression of metastasis at lower levels than a threshold of 1.6–3.9 pg/μg EVs.
Although there are few papers that report the quantitative studies conducted on the role of TGF-β1 in EVs, we obtained a couple of papers that may support the validity of such a threshold level. Exosomes derived from melanoma A375 cells contained 10–15 pg/μg TGF-β and inactivated T cells, suggesting a metastasis-promotive role [54]. In contrast, EVs derived from murine colon carcinoma cells that had been genetically modified with an overexpression of shRNA for Tgf-β1 could induce tumor growth inhibition [55]. This suggests a metastasis-suppressive role at a sufficiently low level of Tgf-β1.
Tgf-β1 is involved in the regulation of EMT, suppressing EMT in the early stages of tumors but conversely promoting EMT in the late stages of tumors, but its concentration dependence is unclear [37,56,57]. Considering that Tgf-β1 is associated with various factors, it is conceivable that the concentration dependence of Tgf-β1 is not simple. Although the concentration dependence of Tgf-β1 revealed in this study is a phenomenon in a limited concentration range, it is highly suggestive in considering the multifaceted role of Tgf-β1.
6.4. Role of Immune Cells in Preventing Metastasis
Regarding (ii), we first examined the involvement of macrophages according to the test schedule. As a result of examining the expressions of six types of macrophage markers (pan-macrophage [CD68, F4/80], M1-type [CD80, CD86], and M2-type [CD163, CD206] macrophage markers), it was revealed that only the expression of the tumor-promotive M2-type marker CD163 was significantly reduced [20].
Tumor-associated macrophages that exhibit tumor-promotive effects are M2-type macrophages, the majority of which are CD163-positive macrophages [58]. In addition, a positive correlation between the infiltration of CD163-positive macrophages into cancer and PD-L1 expression in cancer has been reported from observing tissues of various cancer patients [59,60,61,62]. PD-L1 is an immunosuppressive receptor that suppresses T-cell proliferation and cytokine secretion [62]. Therefore, it is possible that the reduction in CD163 by Nanog+F10-EVs reduced the suppressive effect on T cells, resulting in increased anti-tumor immunity activity.
Regarding (ii), we examined the effects of Nanog+F10-EV on Foxp3, which was a specific marker of Treg activation in the spleen, and observed that the expression of Foxa3 was significantly suppressed. Treg inhibits cytotoxic T cells and macrophages by secreting cytokines, such as IL-10 and IL-35, and the cytotoxic T-lymphocyte antigen 4 (CTLA-4) ligand. Treg also inhibits acquired immunity by suppressing dendritic cells [63]. An artificial Treg inhibitor introduced into mice increased the tumor infiltration of cytotoxic T cells and suppressed subcutaneous melanoma cell tumors [64]. Therefore, it was inferred that the suppression of Foxp3 in the spleen contributed to the metastasis-suppression effect of Nanog+F10-EV.
6.5. Quantitative Analysis of the Effects of EVs Taken Up by Macrophages
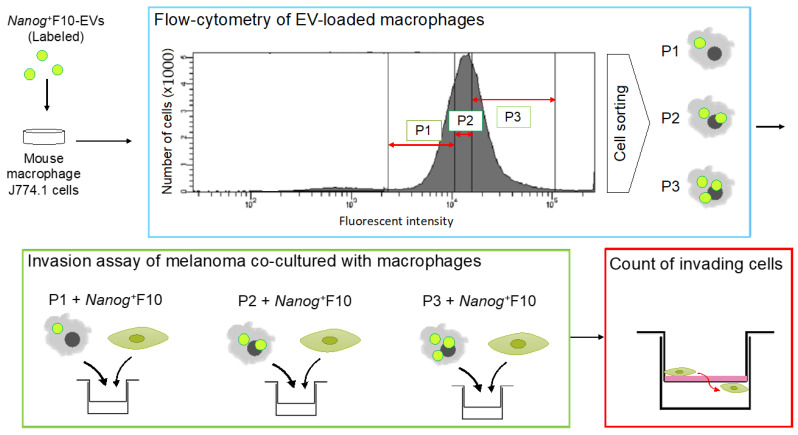
The involvement of CD163 was investigated by in vitro experiments using a macrophage cell line J774.1. In a similar manner to the in vivo test described above, Nanog+F10-EV caused a suppression effect on CD163 expression in J774.1.
Subsequently, this suppressive effect of Nanog+F10-EV is further analyzed quantitatively. J774.1 cells are fractionated with a cell sorter according to the differences in EV uptake (Figure 6). Then, each fraction was tested for its invasion ability with Transwell test kits. The number of filtrated cells is counted and compared to the control. Higher uptake of Nanog+F10-EV will result in higher infiltration.
Figure 6.
Experimental protocol to analyze the effects of EV-uptake quantity on the invasion ability of macrophages. EVs are labeled with a fluorescent probe. J774.1 cells are fractionated, with a cell sorter, into P1, P2, and P3 fractions, respectively, according to the intensities of fluorescence of EVs. Each fraction of J774.1 cells is co-cultured with Nanog+F10 cells and tested for invasion ability with Transwell® invasion assay kits. The number of melanoma cells that invade the Transwell membrane are counted.
6.6. A Mechanism of Metastasis-Suppressive Effects by Nanog+F10-EV
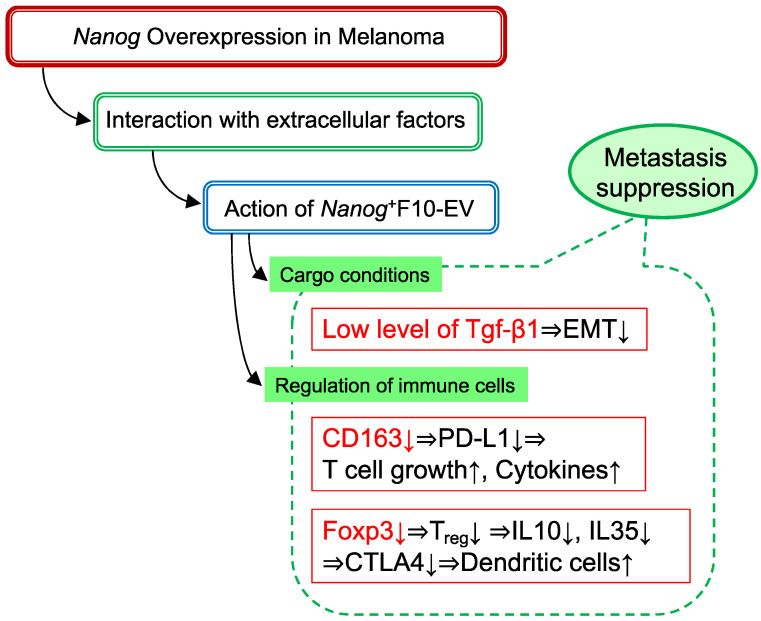
Figure 7 summarizes a mechanism in which Tgf-β1, CD163, and Foxp3 are involved. This is specific to melanomas.
Figure 7.
A postulated mechanism of metastasis-suppressive effects by Nanog+F10-EV.
7. Prospects for EV Cancer Vaccines
There are many studies on cancer cell-derived EVs. However, there are only a few papers [20,52,53] reporting metastasis-suppression effects. Among them, only one paper [20] addresses the Nanog-dependent phenomenon. Therefore, metastasis suppression by cancer cell-derived EVs is, to date, an extremely rare phenomenon. Recently, however, a similar phenomenon was observed for colon cancer-derived EVs. We expect that similar anti-metastasis effects will be observed for EVs derived from Nanog-overexpressing cells of other cancers in the near future.
To elucidate the molecular mechanism of the metastasis-suppression phenomenon, much effort must be focused on the quantitative analyses of various cargos of EVs. In the case of melanomas, Tgf-β1 was selected as a predominant factor, and an idea for its quantity threshold in EVs could be proposed. However, various other components coexist in EVs. It is necessary to analyze them to evaluate their possible involvement. Based on these analyses, we will be able to discuss whether metastasis is suppressed or promoted as a total effect.
Nanog+F10-EV and F10-EV are a suitable pair for the differential analysis of EV cargo components. Our plan is to analyze those components, such as miRNAs and cytokines. Although the analytical results only concentrate on melanoma-relevant matter, they are sure to contribute to the construction of an EV-based versatile vaccine platform against cancer metastasis.
Acknowledgments
We thank Hideaki Matsuoka of Tokyo University of Agriculture and Technology for his valuable advice on cell analysis.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fernández-Delgado I., Calzada-Fraile D., Sánchez-Madrid S. Immune regulation by dendritic cell extracellular vesicles in cancer immunotherapy and vaccines. Cancers. 2020;12:3558. doi: 10.3390/cancers12123558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tay B.Q., Wright Q., Ladwa R., Perry C., Leggatt G., Simpson F., Wells J.W., Panizza B.J., Frazer I.H., Cruz J.L.G. Evolution of cancer vaccines—Challenges, achievements, and future directions. Vaccines. 2021;9:535. doi: 10.3390/vaccines9050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M.Y., Oskarsson T., Acharyya S., Nguyen D.X., Zhang X.H., Norton L., Massagué J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha D., Roy S., Saha P., Chatterjee N., Bishayee A. Trends in research on exosomes in cancer progression and anticancer therapy. Cancers. 2021;13:326. doi: 10.3390/cancers13020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding C., Yi X., Chen X., Wu Z., You H., Chen X., Zhang G., Sun Y., Bu X., Wu X., et al. Warburg effect-promoted exosomal circ_0072083 releasing up-regulates NANGO expression through multiple pathways and enhances temozolomide resistance in glioma. J. Exp. Clin. Cancer Res. 2021;40:164. doi: 10.1186/s13046-021-01942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X., Mazur S.J., Lin T., Appella E., Xu Y. The pluripotency factor Nanog promotes breast cancer tumorigenesis and metastasis. Oncogene. 2014;33:2655–2664. doi: 10.1038/onc.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewer M., Lee L., Leibovitch M., Zhang G., Liu J., Findlay S.D., Vincent K.M., Tandoc K., Dieters-Castator D., Quail D.F., et al. Translational control of breast cancer plasticity. Nat. Commun. 2020;11:2498. doi: 10.1038/s41467-020-16352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son S.W., Cho E., Cho H., Woo S.R., Lee H.J., Oh S.J., Kim S., Kim J.H., Chung E.J., Chung J.Y., et al. NANOG confers resistance to complement-dependent cytotoxicity in immune-edited tumor cells through up-regulating CD59. Sci. Rep. 2022;12:8652. doi: 10.1038/s41598-022-12692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noh K.H., Lee Y.H., Jeon J.H., Kang T.H., Mao C.P., Wu T.C., Kim T.W. Cancer vaccination drives Nanog-dependent evolution of tumor cells towards an immune-resistant and stem-like phenotype. Cancer Res. 2012;72:1717–1727. doi: 10.1158/0008-5472.CAN-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descarpentrie J., Araúzo-Bravo M.J., He Z., François A., González Á., Garcia-Gallastegi P., Badiola I., Evrard S., Pernot S., Creemers J.W.M., et al. Role of furin in colon cancer stem cells malignant phenotype and expression of LGR5 and NANOG in KRAS and BRAF-mutated colon tumors. Cancers. 2022;14:1195. doi: 10.3390/cancers14051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M., Peng R., Wang H., Yang Z., Zhang H., Zhang Y., Wang M., Wang H., Lin J., Zhao Q., et al. Nanog mediated by FAO/ACLY signaling induces cellular dormancy in colorectal cancer cells. Cell Death Dis. 2022;13:159. doi: 10.1038/s41419-022-04606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu W.K., Hung L.M., Hou C.W., Chen J.K. MicroRNA 630 represses NANOG expression through transcriptional and post-transcriptional regulation in human embryonal carcinoma cells. Int. J. Mol. Sci. 2021;21:46. doi: 10.3390/ijms23010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin Y., Wang J., Wu Y., Li Q., Dong M., Liu C., He Q., Wang R., Wang D., Jiang S., et al. Identification of Nanog as a novel inhibitor of Rad51. Cell Death Dis. 2022;13:193. doi: 10.1038/s41419-022-04644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun C., Sun L., Jiang K., Gao D.M., Kang X.N., Wang C., Zhang S., Huang S., Qin X., Li Y., et al. NANOG promotes liver cancer cell invasion by inducing epithelial–mesenchymal transition through NODAL/SMAD3 signaling pathway. Int. J. Biochem. Cell Biol. 2013;45:1099–1108. doi: 10.1016/j.biocel.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Chen C.L., Kumar D.B.U., Punj V., Xu J., Sher L., Tahara S.M., Hess S., Machida K. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016;23:206–219. doi: 10.1016/j.cmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin X., Zhang B.H., Zheng S.S., Gao D.M., Qiu S.J., Wu W.Z., Ren Z.G. Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling. J. Hematol. Oncol. 2015;8:23. doi: 10.1186/s13045-015-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrull A., Ghislin S., Deshayes F., Lauriol J., Alcaide-Loridan C., Middendorp S. Nanog and Oct4 overexpression increases motility and transmigration of melanoma cells. J. Cancer Res. Clin. Oncol. 2012;138:1145–1154. doi: 10.1007/s00432-012-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasmim M., Noman M.Z., Messai Y., Bordereaux D., Gros G., Baud V., Chouaib S. Cutting edge: Hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-β1. J. Immunol. 2013;191:5802–5806. doi: 10.4049/jimmunol.1302140. [DOI] [PubMed] [Google Scholar]
- 19.Saito M., Kishi R., Sasai T., Hatakenaka T., Matsuki N., Minagawa S. Effect of Nanog overexpression on the metastatic potential of a mouse melanoma cell line B16-BL6. Mol. Cell. Biochem. 2021;476:2651–2661. doi: 10.1007/s11010-021-04110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatakenaka T., Matsuki N., Minagawa S., Khoo C.S.M., Saito M. Anti-metastatic function of extracellular vesicles derived from Nanog-overexpressing melanoma. Curr. Oncol. 2022;29:1029–1046. doi: 10.3390/curroncol29020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu M.K.Y., Jiang Y.X., Wang J.J., Leung T.H., Han C.Y., Tsang B.K., Cheung A.N., Ngan H.Y.S., Chan K.K.L. Hexokinase 2 regulates ovarian cancer cell migration, invasion and stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 signaling cascades. Cancers. 2019;11:813. doi: 10.3390/cancers11060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y., Yu W., Shrivastava A., Srivastava R.K., Shankar S. Inhibition of pancreatic cancer stem cell characteristics by α-Mangostin: Molecular mechanisms involving Sonic hedgehog and Nanog. J. Cell. Mol. Med. 2019;23:2719–2730. doi: 10.1111/jcmm.14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saga K., Park J., Nimura K., Kawamura N., Ishibashi A., Nonomura N., Kaneda Y. NANOG helps cancer cells escape NK cell attack by downregulating ICAM1 during tumorigenesis. J. Exp. Clin. Cancer Res. 2019;38:416. doi: 10.1186/s13046-019-1429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeter C.R., Liu B., Lu Y., Chao H.P., Zhang D., Liu X., Chen X., Li Q., Rycaj K., Calhoun-Davis T., et al. NANOG reprograms prostate cancer cells to castration resistance via dynamically repressing and engaging the AR/FOXA1 signaling axis. Cell Discov. 2016;2:16041. doi: 10.1038/celldisc.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L., Zhang X., Xiang X., Xiong R., Xiao D., Chen Z., Liu K., Feng G. NANOG promotes cell proliferation, invasion, and stemness via IL-6/STAT3 signaling in esophageal squamous carcinoma. Technol. Cancer Res. Treat. 2021;20:15330338211038492. doi: 10.1177/15330338211038492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C., Yoon C., Zhou W.H., Zhou Y.C., Zhou W.W., Liu H., Yang X., Lu J., Lee S.Y., Huang K. ERK1/2-Nanog signaling pathway enhances CD44(+) cancer stem-like cell phenotypes and epithelial-to-mesenchymal transition in head and neck squamous cell carcinomas. Cell Death Dis. 2020;11:266. doi: 10.1038/s41419-020-2448-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Pedregal-Mallo D., Hermida-Prado F., Granda-Díaz R., Montoro-Jiménez I., Allonca E., Pozo-Agundo E., Álvarez-Fernández M., Álvarez-Marcos C., García-Pedrero J.M., Rodrigo J.P. Prognostic significance of the pluripotency factors NANOG, SOX2, and OCT4 in head and neck squamous cell carcinomas. Cancers. 2020;12:1794. doi: 10.3390/cancers12071794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo W., Li S., Peng B., Ye Y., Deng X., Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS ONE. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grubelnik G., Boštjančič E., Grošelj A., Zidar N. Expression of NANOG and its regulation in oral squamous cell carcinoma. Biomed Res. Int. 2020;2020:8573793. doi: 10.1155/2020/8573793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gawlik-Rzemieniewska N., Bednarek I. The role of NANOG transcriptional factor in the development of malignant phenotype of cancer cells. Cancer Biol. Ther. 2016;17:1–10. doi: 10.1080/15384047.2015.1121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh S.J., Lee H.J., Song K.H., Kim S., Cho E., Lee J., Bosenberg M.W., Kim T.W. Targeting the NANOG/HDAC1 axis reverses resistance to PD-1 blockade by reinvigorating the antitumor immunity cycle. J. Clin. Investig. 2022;15:132. doi: 10.1172/JCI147908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 34.Bear J.E., Haugh J.M. Directed migration of mesenchymal cells: Where signaling and the cytoskeleton meet. Curr. Opin. Cell Biol. 2014;30:74–82. doi: 10.1016/j.ceb.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peer E., Tesanovic S., Aberger F. Next-generation Hedgehog/GLI pathway inhibitors for cancer therapy. Cancers. 2019;11:538. doi: 10.3390/cancers11040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seoane J., Gomis R. TGF-β family signaling in tumor suppression and cancer progression. Cold Spring Harbor Perspect. Biol. 2017;9:a022277. doi: 10.1101/cshperspect.a022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabregat I., Caballero-Díaz D. Transforming growth factor-β-induced cell plasticity in liver fibrosis and hepatocarcinogenesis. Front. Oncol. 2018;8:357. doi: 10.3389/fonc.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suriyamurthy S., Baker D., Dijke P.T., Iyengar P.V. Epigenetic reprogramming of TGF-β signaling in breast cancer. Cancers. 2019;11:726. doi: 10.3390/cancers11050726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada K., Saito M., Matsuoka H., Inagaki N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nat. Protoc. 2007;2:753–762. doi: 10.1038/nprot.2007.76. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto T., Nishiuchi Y., Teshima T., Matsuoka H., Yamada K. Synthesis of 2-NBDLG, a fluorescent derivative of L-glucosamine; the antipode of D-glucose tracer 2-NBDG. Tetrahedron Lett. 2008;49:6876–6878. doi: 10.1016/j.tetlet.2008.09.089. [DOI] [Google Scholar]
- 41.Gehrmann U., Näslund T.I., Hiltbrunner S., Larssen P., Gabrislsson S. Harnessing the exosome-induced immune response for cancer immunotherapy. Semin. Cancer Biol. 2014;28:58–67. doi: 10.1016/j.semcancer.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Czernek L., Düchler M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch. Immunol. Ther. Exp. 2017;65:311–323. doi: 10.1007/s00005-016-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andreola G., Rivoltini L., Castelli C., Huber V., Perego P., Deho P., Squarcina P., Accornero P., Lozupone F., Lugini L. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurywchak P., Tavormina J., Kalluri R. The emerging roles of exosomes in the modulation of immune responses in cancer. Genome Med. 2018;10:23. doi: 10.1186/s13073-018-0535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Y., Lai X., Yu S., Chen S., Ma Y., Zhang Y., Li H., Zhu X., Yao L., Zhang J. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res. Treat. 2014;147:423–431. doi: 10.1007/s10549-014-3037-0. [DOI] [PubMed] [Google Scholar]
- 46.Xu C.G., Yang M.F., Ren Y.Q., Wu C.H., Wang L.Q. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016;20:4362–4368. [PubMed] [Google Scholar]
- 47.Qin X., Yu S., Zhou L., Shi M., Hu Y., Xu X., Shen B., Liu S., Yan D., Feng J. Cisplatin-resistant lung cancer cell–derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100–5p-dependent manner. Int. J. Nanomed. 2017;12:3721–3733. doi: 10.2147/IJN.S131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J., Lv B., Su Y., Wang X., Bu J., Yao L. Exosome-mediated transfer of lncRNA HOTTIP promotes cisplatin resistance in gastric cancer cells by regulating HMGA1/miR-218 axis. OncoTargets Ther. 2019;12:11325–11338. doi: 10.2147/OTT.S231846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards K.E., Zeleniak A.E., Fishel M.L., Wu J., Littlepage L.E., Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H., Hou J., Liu K., Liu Q., Shen L., Liu B., Lu Q., Zhang N., Che L., Li J., et al. RAB27A-dependent release of exosomes by liver cancer stem cells induces Nanog expression in their differentiated progenies and confers regorafenib resistance. J. Gastroenterol. Hepatol. 2021;36:3429–3437. doi: 10.1111/jgh.15619. [DOI] [PubMed] [Google Scholar]
- 51.Zhao L.-J., Li Y.-Y., Zhang Y.-T., Fan Q.-Q., Ren H.-M., Zhang C., Mardinoglu A., Chen W.-C., Pang J.-R., Shen D.-D., et al. Lysine demethylase LSD1 delivered via small extracellular vesicles promotes gastric cancer cell stemness. EMBO Rep. 2021;22:e50922. doi: 10.15252/embr.202050922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitai Y., Kawasaki T., Sueyoshi T., Kobiyama K., Ishii K.J., Zou J., Akira S., Matsuda T., Kawai T. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J. Immunol. 2017;198:1649–1659. doi: 10.4049/jimmunol.1601694. [DOI] [PubMed] [Google Scholar]
- 53.Diamond J.M., Vanpouille-Box C., Spada S., Rudqvist N.P., Chapman J.R., Ueberheide B.M., Pilones K.A., Sarfraz Y., Formenti S.C., Demaria S. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol. Res. 2018;6:910–920. doi: 10.1158/2326-6066.CIR-17-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Düchler M., Czernek L., Peczek L., Cypryk W., Sztiller-Sikorska M., Czyz M. Melanoma-derived extracellular vesicles bear the potential for the induction of antigen-specific tolerance. Cells. 2019;8:665. doi: 10.3390/cells8070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossowska J., Anger N., Wegierek K., Szczygieł A., Mierzejewska J., Milczarek M., Szermer-Olearnik B., Pajtasz-Piasecka E. Antitumor potential of extracellular vesicles released by genetically modified murine colon carcinoma cells with overexpression of interleukin-12 and shRNA for TGF-β1. Front. Immunol. 2019;10:211. doi: 10.3389/fimmu.2019.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.David C.J., Massagué J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018;19:419–435. doi: 10.1038/s41580-018-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh H.W., Lee S.S., Chang C.Y., Lang Y.D., Jou Y.S. A new switch for TGFβ in cancer. Cancer Res. 2019;79:3797–3805. doi: 10.1158/0008-5472.CAN-18-2019. [DOI] [PubMed] [Google Scholar]
- 58.Fujimura T., Aiba S. Significance of immunosuppressive cells as a target for immunotherapies in melanoma and non-melanoma skin cancers. Biomolecules. 2020;10:1087. doi: 10.3390/biom10081087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo F., Feng Y.C., Zhao G., Zhang R., Cheng Z.Z., Kong W.N., Wu H.L., Xu B., Lv X., Ma X.M. Tumor-associated CD163+ M2 macrophage infiltration is highly associated with PD-L1 expression in cervical cancer. Cancer Manag. Res. 2020;12:5831–5843. doi: 10.2147/CMAR.S257692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubota K., Moriyama M., Furukawa S., Rafiul H.A.S.M., Maruse Y., Jinno T., Tanaka A., Ohta M., Ishiguro N., Yamauchi M., et al. CD163+ CD204+ tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci. Rep. 2017;7:1755. doi: 10.1038/s41598-017-01661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harada K., Dong X., Estrella J.S., Correa A.M., Xu Y., Hofstetter W.L., Sudo K., Onodera H., Suzuki K., Suzuki A., et al. Tumor-associated macrophage infiltration is highly associated with PD-L1 expression in gastric adenocarcinoma. Gastric Cancer. 2018;21:31–40. doi: 10.1007/s10120-017-0760-3. [DOI] [PubMed] [Google Scholar]
- 62.Obeid J.M., Erdag G., Smolkin M.E., Deacon D.H., Patterson J.W., Chen L., Bullock T.N., Slingluff C.L. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology. 2016;5:e1235107. doi: 10.1080/2162402X.2016.1235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeuchi Y., Nishikawa H. Roles of regulatory T cells in cancer immunity. Int. Immunol. 2016;28:401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimizu J., Yamazaki S., Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: A common basis between tumor immunity and autoimmunity. J. Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.