Abstract
Prostate cancer (PCa) is a leading cause of cancer death in men, worldwide. Mortality is highly related to metastasis and hormone resistance, but the molecular underlying mechanisms are poorly understood. We have studied the presence and role of cancer stem cells (CSCs) and the Epithelial–Mesenchymal transition (EMT) in PCa, using both in vitro and in vivo models, thereby providing evidence that the stemness–mesenchymal axis seems to be a critical process related to relapse, metastasis and resistance. These are complex and related processes that involve a cooperative action of different cancer cell subpopulations, in which CSCs and mesenchymal cancer cells (MCCs) would be responsible for invading, colonizing pre-metastatic niches, initiating metastasis and an evading treatments response. Manipulating the stemness–EMT axis genes on the androgen receptor (AR) may shed some light on the effect of this axis on metastasis and castration resistance in PCa. It is suggested that the EMT gene SNAI2/Slug up regulates the stemness gene Sox2, and vice versa, inducing AR expression, promoting metastasis and castration resistance. This approach will provide new sight about the role of the stemness–mesenchymal axis in the metastasis and resistance mechanisms in PCa and their potential control, contributing to develop new therapeutic strategies for patients with metastatic and castration-resistant PCa.
Keywords: prostate cancer, Epithelial–Mesenchymal transition, cancer stem cell, castration resistance, metastasis
1. Introduction
Prostate cancer (PCa) is a leading cause of oncologic death in men, worldwide [1]. Although new screening programs have increased the speed of early diagnosis and timely treatments with curative intentions [2], the high rate of relapse and metastasis remains as the major challenges [3,4]. At the beginning, PCa is sensitive to androgen action [5]. Testosterone, and its prostate metabolite dihydrotestosterone (DHT), induces cell proliferation, tumor growth, and probably, its dissemination [6]. For this reason, the treatments involving androgen deprivation therapy (ADT), when curative surgical prostate resection is not possible, have being developed [7,8]. Pharmacological castration using GnRH analogs, in order to block the hypothalamus–pituitary–testicular axis, provides the first-line therapy for disseminated PCa [7]. Nevertheless, at certain point of the treatment, the cells become androgen insensitive, resulting in a castration-resistant PCa (CRPC) with a poor prognosis [9]. The genetic and molecular mechanisms of androgen resistance are complex, and they are not completely understood [10]. The evidence suggests that, in some cases, the androgen receptor (AR) is involved in this resistance [8,11]. Gene amplification, mutations and other alterations in the AR gene have been reported [10]. In recent years, many articles have been published about AR-variant 7 (AR-7, a constitutively activated AR receptor), which is increased in CRPC, and it has been proposed as a prognostic biomarker, and it has been described as having an ligand-independent activated form [12,13,14]. In addition, alterations in androgen metabolism, and the local biosynthetic pathway within the prostate gland have been associated with androgen sensitivity [15,16]. Probably, CRPC is the result of a combination of these different mechanisms. On the other hand, recurrence and metastasis progression are also complex processes, involving a variety of mechanisms and genomic alterations of malignant cells [17,18,19]. It is well known that the epithelial–mesenchymal transition (EMT) is the main pathway by which the malignant epithelial cells (from carcinomas) switch their genetic program toward a mesenchymal phenotype, acquiring the characteristic hallmarks of cancer cells, such as invasive and metastatic behaviors, among others [18,20,21,22,23,24]. As a result, an epithelial cell loses its polarity, proliferation, differentiation and positioning controls, changing into a mesenchymal phenotype [25]. Interestingly, we have reported that ZEB1, a key EMT factor, is involved in the regulation of androgen synthesis in PCa cells [26]. However, increasing evidence indicates that tumors contain a heterogeneous cell population, [27] and probably, a cooperative action of these different types of malignant cells is needed to accomplish a successful metastatic process. In the last decade, a small subpopulation of malignant cells with stemness features have been identified and characterized in many cancers including PCa [4,28,29,30]. This population, called cancer stem cells (CSCs), has been proposed as responsible for relapse and metastasis [4]. In recent years, our group has contributed to this field in PCa [31,32,33].
2. Epithelial–Mesenchymal Transition and Cancer Stem Cells in Prostate Cancer
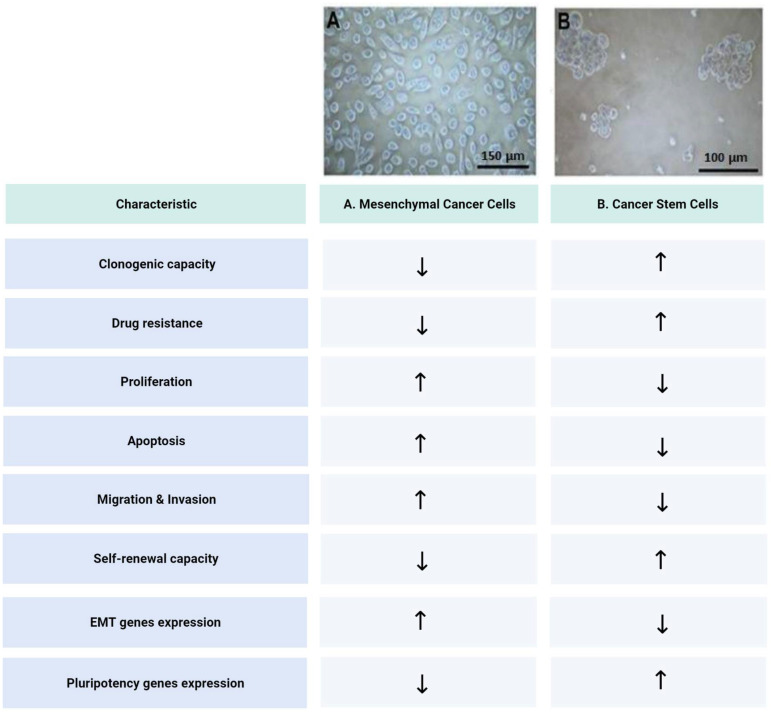
EMT occurs normally throughout embryonic development. A primary EMT occurs early in embryonic development (before implantation), and it continues after implantation during the mesoderm formation. Then, a secondary EMT takes place during mesodermal cells division after gastrulation. Finally, a tertiary EMT occurs during the organ formation stage [34]. During carcinogenesis (in carcinomas), a similar process takes place that transforms a malignant epithelial cell into a highly invasive mesenchymal-like cell. This process has been also called EMT [35,36,37]. The epithelial malignant cells progressively lose adhesion molecules such as E-cadherin, syndecans and tight junction molecules, while the gene expression factors such as SNAIL1, SNAI2/Slug and TWIST increase their expression together with the mesenchymal markers such as vimentin, N-cadherin and metalloproteinases, resulting in a migrating and invasive phenotype [38]. On the other hand, we have found that ZEB1 represses Syndecan 1 expression and promotes an aggressive phenotype in PCa cells. A detailed study of this process in PCa has been carried out in our laboratory [39,40,41,42,43]. There is evidence that this mesenchymal and invasive cell phenotype is involved in the metastatic process 19 [23]. Nevertheless, there is no direct proof that these mesenchymal cells have also a colonizing capacity. On the other hand, there is growing evidence suggesting that CSCs, which are present in most tumors as a small population of malignant cells, are finally responsible for relapse and metastasis [4,44,45]. In addition, we have identified and studied a CSCs population obtained from samples of human PCa. We have determined the molecular signature of stemness (CD133+/CD44+/ABCG2+/CD24-) [31] of this population, and we have evaluated its proliferation, migration, invasion and clonogenic capacity [32]. We were able to separate this CSCs population from the mesenchymal cancer cells (MCCs) by modifying the cultured conditions, which was followed by magnetic-associated cells sorting (MACS) [31]. In adherent conditions, most of the cells remain in mesenchymal-like state as they are evaluated by specific markers and functional assays. However, in non-adherent conditions, most of the mesenchymal adherent cells die by anoikis (anchorage-dependent apoptosis), and a few cells survive and rapidly form spheres that grow and remain for several weeks. After the MACS separation, the sphere-forming cells represent the enriched CSCs population. These CSCs were characterized by a functional assay, presenting a lower proliferation rate, an increased resistance to apoptosis and drugs treatments, a reduced invasive properties, and a high clonogenic capacity compared with the MCCs (Figure 1). In addition, these CSCs show no expression of GnRH-R or AR as well as many differentiation markers [32]. On the other hand, we have produced CSCs that were knocked down for several stemness genes such as Sox2, Klf4 and Myc. In these conditions, the cells reverse their phenotype toward a more mesenchymal form. Additionally, silencing Sox2 in the CSCs resulted in no metastatic progression in an orthotopic murine model (unpublished results). In addition, we isolated and characterized the miRNAs from CSCs exosomes and evaluated their possible association with metastasis [33].
Figure 1.
Comparative summary of the main characteristics of mesenchymal cells and cancer stem cell from prostate cancer. (A) Mesenchymal cancer cells; (B) Cancer stem cells. ↑: Increased. ↓: Decreased.
3. Cancer Stem Cells and Epithelial–Mesenchymal Transition Control Prostate Cancer Progression
The CSCs represent less than 1% of a primary tumor even though have been suggested to drive tumor progression, relapse and metastasis [4,46,47]. The CSCs can respond to stressing conditions (hypoxia, oxidative damage, xenobiotics, etc.), modifying the gene expression program toward a migrating, invasive and resistant phenotype. It is believed that the CSCs may originate from transformed epithelial cells through EMT, leading to a stemness gene expression program [48]. On the other hand, to generate migrating and invading cells, the CSCs have to undergo EMT, and the EMT marker expression represents a PCa progression indicator. The EMT predictors include an increase in the N-cadherin and vimentin expression and a decrease in E-cadherin, EpCAM, and other epithelial markers [38]. When the migrating and invading cells come out from the primary tumor and reach the blood stream, they are usually called circulating tumor cells (CTCs). A small population of these CTCs survives in circulation, colonize distant tissues, proliferate and originate metastasis [49]. EpCAM is generally used to identify cancer cells in the circulation of PCa patients. Systems to detect the CTCs are based on EpCAM positivity, and several studies indicate that the number of CTCs-EpCAM+ increased with the PCa progression. However, if the stemness phenotype is responsible for metastasis, it is probably that neither EpCAM nor E-cadherin would be expressed on the CSCs [50,51]. When we were comparing normal men, localized PCa and metastatic patients, a higher number of CTCs-EpCAM+ was found in the last group [52]. On the other hand, in a transgenic mice model, only the cells undergoing EMT were capable of self-renewal compared with their epithelial and mesenchymal counterparts [4,53]. To add more complexity to the functional relationship between stemness and EMT, when E-cadherin was silenced in spheres that were obtained from a PC3 human PCa cell line, the EMT process was stimulated [54], while the E-cadherin expression induced stemness gene expression and sphere formation in DU 145 PCa cells [55,56]. These results indicated that a further investigation is needed to understand the stemness–EMT axis and its influence in metastasis and resistance. It is highly probably that both the CSCs and MCCs are also heterogeneous sub-populations. Among several other progression and metastasis markers in PCa, CD117 (c-Kit receptor) has been found to be highly expressed in PCa patients with high-grade tumors in comparison with those with low-grade tumors. CD133, one of the more well-known stemness markers, is increased in high-Gleason tumors, but it is not present in CTCs [57]. CD44, another stemness marker, was found to be expressed in invasive and self-renewing PCa cell lines together with other EMT and stemness markers [58,59]. In addition, the CD44 stemness marker was found to be expressed in patients who tested positive for chromogranin A [60], a neuroendocrine cell marker which is present in a very aggressive and resistance form of PCa. This suggests that PCa neuroendocrine cells (negative for AR and PSA) may be associated with CSCs, or they represent a subpopulation of them [61,62]. PCa neuroendocrine cells also express stemness markers, and identifying the relationship between these cell populations would shed light on the castration resistance progression of PCa, especially the neuroendocrine type. Recently, a cross-regulation of key EMT (SNAI2/Slug) and stemness (Sox2) genes have been reported [63,64,65], supporting the hypothesis that a stemness–EMT axis is operating and providing the necessary plasticity to guarantee the maintenance of tumor heterogeneity [66]. Recently, Zhao et al., have reported that Slug promotes hepatocellular cancer cell progression by increasing Sox2, but this study was performed in cell lines and xenografts in nude mice for a tumor growth evaluation [67]. Additionally, there is recent evidence that Sox2 can regulate AR and lineage plasticity in PCa cell lines and a xenograft model [68]. Moreover, there is increasing evidence, in several cancers, that this stemness–EMT axis is operating to promote cancer progression [69,70,71,72,73].
4. Role of Cancer Stem Cells in Metastatic Colonization and Progression
It has been calculated than more than 3 million cells per gram of tissue come out from the tumor to circulation every day in an average cancer patient. However, less than <0.01% of these cells can originate from a clinical metastasis [4,49,74]. This means that this lethal process is highly inefficient, even though it kills most of the cancer patients [75]. Once they are in the blood stream, the cancer cells from the tumor are called circulating tumor cells (CTCs). The subpopulation of CTCs that are able to colonize metastatic niche is often called metastasis-initiating cells (MICs), and when these cells grow in the colonized niche, they are named disseminated tumor cells (DTCs). Each cell kind is a sub-population of the predecessor. There is a consensus to call the CSCs to those cells that are able to colonize and develop micro-, and then, macro-metastases [76,77]. On the other hand, the capacity to leave the tumor, come into circulation, survive in circulation, colonize the selective tissue, survive in the metastatic niche, and finally, grow in this niche, requires EMT and the plasticity of the CSCs [66,75,78]. It is evident then, that not all CTCs, even not all DTCs, are able to develop metastasis. Many CTCs (rather, most of them) die in circulation. Even many DTCs can remain quiescent or die in the metastatic niche due to adverse microenvironment conditions. Therefore, only a small subpopulation (CSCs) has the ability to grow in the metastatic niche, and this recapitulates the primary tumor heterogeneity in a secondary site [76,79]. The stemness features of the CSCs, as asymmetric division and pluripotency, allows them to maintain the CSCs population as well as cell differentiation (EMT) to give rise all of the cell varieties of the original heterogeneous tumor [80]. In PCa, an increasing number of specific markers have been associated with tumor progression and therapeutic resistance. These markers have been found in CTCs and in bone metastasis DTCs. CXCR4 (SDF-1 chemokine receptor), EpCAM and EZH2 were found to be associated with relapse and distant metastasis, and clinical studies [81,82] suggest that these markers might drive metastasis. Given that some markers were restricted only in the prostate tumor suggests that they probably are not directly involved in the niche colonization and metastasis progression. However, CD117 and CXCR4 were expressed in bone metastatic foci at the levels shown in the primary tumor [4,83,84,85]. These findings suggest that CD117 and CXCR4 may be implicated in driving colonization, metastasis progression and dormancy escape. In addition, E-cadherin expression has been found to be associated with bone metastasis in clinical studies, giving more evidence that a mesenchymal–epithelial transition (an inverse EMT process) might be occurring [86]. Even when the specific molecular mechanisms for niche colonization survival, dormancy escape and further metastasis progression in the bones of PCa patients are still a challenge to be fully addressed, the evidence accumulated so far indicates that the CSCs play a crucial role.
5. Different Malignant Cell Types Collaborate to Produce Distant Metastasis in Prostate Cancer
Revisiting the role of CSCs in metastasis, the fact that these cells with little invasive activity can leave the tumor and colonize the pre-metastatic niches strongly suggests that some kind of collaboration with highly invasive MCCs is occurring. Recently, Dr. Thomson’s group provided the first evidence about this potential cooperative action. Using commercial cell lines derived from PCa (PC3), they enriched a cell population with metastatic cells (TICs) using a strong epithelial program. In turn, they reduced the cell population with TICs using a mesenchymal program. The over-expression of mesenchymal genes in the first population decreased their TIC ability, whereas the knocking down of these genes enhanced the TIC capacity in the second population. Using immunocompromised NOD/SCID mice, they observed that when they were injected in combination, the mesenchymal cells increased the metastatic potential of the epithelial TIC-enriched population, suggesting a cooperative action between both of the cell types [87]. This hypothesis supports the idea that within a tumor, through EMT, the mesenchymal cells became the predominant population, giving the tumor a fully invasive capacity. However, it can be proposed that a small cell population that expresses a stemness cell program (CSCs) remains in the tumor, and this can escape passively with the bulk of the MCCs. Once they are in the metastatic niche, the CSCs can proliferate and produce progenitor cells that may further differentiate to an epithelial-like phenotype. This may explain our results (unpublished) and other observations, showing that in metastatic PCa samples, an increase in the epithelial markers and a decrease in the mesenchymal markers are observed, which has been called mesenchymal–epithelial transition (MET) [88,89]. Probably, the metastatic foci will generate the full heterogeneity of the original tumor, in which the epithelial-like cells will go through EMT again and probably keep a few CSCs. This is an interesting hypothesis that is worth proving using the CSCs and MCCs derived from patient’s tumors. In our laboratory, we have developed an orthotopic murine model for human PCa using NOD/SCID mice [90]. In this model, we have injected both CSCs and MCCs obtained from patient explants. Both of the types of cells produced metastasis. However, the prostate tumor from the CSCs was smaller than those from the MCCs. In addition, the metastasis from the CSCs was obtained more slowly that with the MCCs (unpublished). We interpret that the CSCs take longer to generate progenitors and all of the heterogeneity of the MCCs through EMT, and rather, the MCCs are already a mixed population including migrating and invading cells. These cells, through EMT, produce a population of CSCs that provide a metastatic capacity. This process seems to be faster than the other one. Taken together, these results and the others reported in different cancer types, such as hepatocellular carcinoma, strongly support the stemness–EMT axis, in which the driver genes for two processes are cross-regulated. This defines an epithelial-to-mesenchymal plasticity of the CSCs [66]. This axis may turn the focus of interest to identify new therapeutic targets.
6. Cancer Stems Cells and Epithelial–Mesenchymal Transition in Relapse and Castration Resistance
For the locally advanced PCa, radical prostatectomy and radiotherapy are the main treatments. When the PCa is disseminated, ADT and chemotherapy are the options [2,5,7]. Other treatments such as immunotherapy (i.e., dendritic cell-based vaccine Sipuleucel-T) have been developed recently [91,92]. For locally advanced PCa, the treatments have a curative intention. However, local recurrence and metastasis (and following castration resistance) are the main concerns after surgery or radiation (>30%). It is highly probably that the CSCs that remain in the surgical niche, in the irradiated tissue or in the circulation can promote the development of local relapse and/or distant metastasis. This is explained by the fact that the CSCs have been found to be resistant to most therapies, causing DNA damage in highly proliferative cells or directed to hormonal/signaling targets that affect mainly the bulk tumor cells but not the CSCs [93]. The CSCs markers such as CD117, EZH2, among others, which are expressed in the prostate primary tumor, resulted in being predictive for biochemical recurrence (rising PSA levels in the circulation) after radical prostatectomy [57,94]. It has been found that number of CTCs-CD117+ were elevated for more than 3 months after the radical prostatectomy in patients that later underwent biochemical recurrence [56], suggesting that circulating CSCs may be a useful predictor for early relapse [95]. Other clinical studies have shown that CRPC or the neuroendocrine PCa type have different CTCs (AR-low) [4,96]. In the murine models, CD166, a recently proposed stemness marker for colorectal cancer, was found to be up-regulated in PCa after castration [97]. In humans, EZH2 was increased in advanced PCa, and it was associated with poor survival [98]. All of this evidence points out that the CSCs have a major role in relapse and metastasis in PCa. Moreover, we and other groups have found that the CSCs show a significant resistant to chemotherapeutics and radiation [32,99]. In other studies, it was reported that the inhibition of CXRC4 significantly increased the PCa cell lines to docetaxel, which is one of the most common chemotherapeutic drugs used in PCa [100]. Additionally, silencing the EpCAM gene in PCa cell lines increases the patient’s sensitivity to radiation and chemotherapeutics in vivo [101]. Furthermore, other CSCs markers have been studied regarding therapeutic resistance. We have analyzed the influence of ATP-binding cassette (ABC) transporters, including ABCG2 (specific stemness marker), in PCa resistance, and we have found that several ABC transporters are over-expressed in the PCa cells. The pharmacological inhibition or knocking down of these ABC pumps resulted in a significant increase in drug sensitivity [102,103]. Our group and others have found that the ABCG2 transporter is highly expressed in the spheres from PCa which correlates with a level of high drug resistance of this CSCs population [32,104]. In several other studies, the CSCs have showed to have a level of high resistance to multiple drugs including taxanes, tyrosine kinase and topoisomerase inhibitors, among others [4,105,106,107]. ALDH1, which is another stemness marker, has been implicated in changes in the metabolism of chemotherapeutic agents, reducing radio-sensitivity in the PCa cell lines [105]. In patients treated with enzalutamide or abiraterone, the CTCs showed an increased expression of the AR-7 splicing variant and a decreased progression-free and overall survival [108]. In addition, AR-7 has been found to be expressed in the CSCs undergoing tumor progression during ADT [109,110]. Interestingly, the evidence indicates that AR expression can be induced in the CSCs during the progression to castrate resistance [111]. Additionally, the EMT marker SNAI2/slug is encoded by an androgen-regulated gene [112]. In addition, SNAI2/Slug increases the AR expression and binds to it, acting as co-activator and increasing the AR activity regardless of the androgen being absence. This has been proposed as a mechanism driving castration resistance [112]. Considering that SNAI2/Slug also cross-regulate with Sox2, and Sox2 can regulates AR [68], it is plausible that the stemness–EMT axis has a major role in castration resistance in PCa.
7. Orthotopic Model for the Study of Human Prostate Cancer Metastasis
As stated above, an NOD/SCID mouse has been widely used for the metastasis study of several human cancers [113]. A critical issue is the route by which the human cancer cells are injected. Many authors use subcutaneous, intravenous, or intra-cardiac administrations with different results. Lastly, orthotopic models has been developed (injection in the same mouse organ or tissue from which human cell derives). This model mimics, more accurately, the metastatic process. Lastly, a few reports on the orthotopic models for human PCa have been published [114,115]. We have developed a modification of an orthotopic model for PCa using a cell injection in one of the anterior lobes of the NOD/SCID mouse prostate [90]. This orthotopic injection resulted in a consistent and reproducible metastatic progression. Firstly, a fraction of tumor cells injected in the mouse prostate survived and generated a tumor derived from the injected cells (transduced with luciferase and red fluorescent protein genes). In a chronological sequence, the metastatic foci begin to appear in the liver, lungs and kidneys. The injection of the cell into the anterior lobe, instead the ventral prostate, has the advantage that it is possible to surgically remove the prostate tumor in order to evaluate the progression of the metastasis with or without the main “primary” prostate tumor. In this model, we have demonstrated the utility of prostatectomy during metastasis progression [116], and the effect of knocking down the stemness gene Sox2 on driving metastasis. In the current studies, we are establishing the progression toward a CRCP using surgical castration as an ADT. We consider that this orthotopic pre-clinical model represents a very suitable system to further study of relapse, resistance and metastasis of PCa.
8. Concluding Remarks
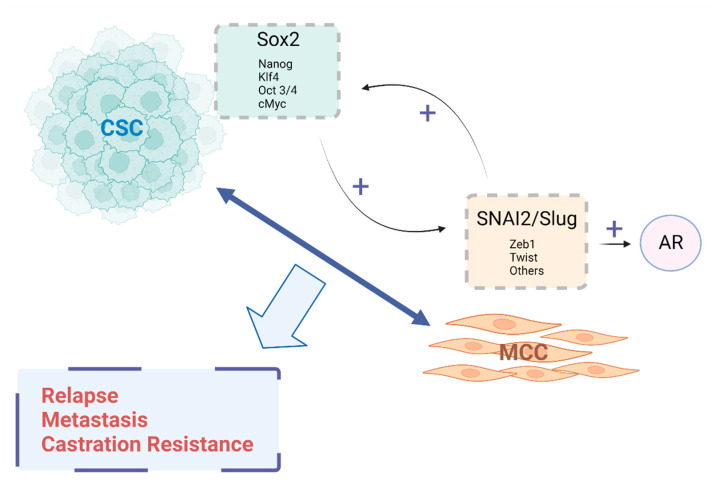
It may be suggested that the EMT gene SNAI2/Slug up regulates the stemness gene Sox2, and vice versa, inducing an androgen receptor expression, promoting metastasis and castration resistance in prostate cancer. This hypothesis is based on recent separate information about the influence on the CSCs and the EMT process in metastasis, relapse and treatment resistance in many cancers, including PCa. Recent evidence indicates that the generation of CSCs is dependent on EMT. It has been shown that several EMT factors increase the number of pluripotency genes. One of the best candidates is the SNAI2/Slug transcription factor. On the other hand, several stemness genes have been identified in PCa as being one of the most important, e.g., Sox2. This stemness transcription factor also regulates the EMT markers, establishing a stemness–EMT axis that allows it to generate the cell tumor heterogeneity. Additionally, Sox2 regulates several other differentiation genes such as the androgen receptor. The CSCs can originate from the cells undergoing EMT, and conversely, they are also capable of generating mesenchymal cells through differentiation. Both the stemness and EMT genes may inter-regulate their transcriptions. On the other hand, CRPC (the most lethal form of this cancer) has been also associated with the stemness–EMT axis. Takin the evidence together, it can be proposed that this stemness/EMT axis may promote androgen sensitivity changes, conducting to a castration resistance condition and metastasis (Figure 2).
Figure 2.
Proposed model for stemness–EMT axis in prostate cancer. CSC: cancer stem-like cell; MCC: Mesenchymal-like cancer cell; AR: Androgen Receptor.
Based on the background that is discussed above, it would be valuable to propose the study of the effects of manipulating the stemness–EMT axis genes SNAI2/Slug and Sox2, among others, on metastasis and castration resistance in PCa. The potential results of such a study would contribute to the understanding of the role of CSCs and EMT process upon the genetic, cellular and molecular mechanism of metastasis and hormone-resistance in PCa because these are still the major hints and challenges of the high mortality of this disease. Furthermore, new insight on these aspects obtained from pre-clinical models will have an impact on identifying new therapeutic targets for clinical use.
Author Contributions
E.A.C.: Discussion of the literature and writing the review; S.I.: Selecting bibliography and discussion; H.R.C.: Analysis and discussion of references and data. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the studies cited in this review.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by “Fondo Nacional de Ciencia y Tecnología”, FONDECYT from the “Agencia Nacional de Investigación y Desarrollo”, ANID, grant number 1201704.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Litwin M.S., Tan H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA. 2017;317:2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 3.Luchini C., Fleischmann A., Boormans J.L., Fassan M., Nottegar A., Lucato P., Stubbs B., Solmi M., Porcaro A., Veronese N., et al. Extranodal extension of lymph node metastasis influences recurrence in prostate cancer: A systematic review and meta-analysis. Sci. Rep. 2017;7:2374. doi: 10.1038/s41598-017-02577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris K.S., Kerr B.A. Prostate Cancer Stem Cell Markers Drive Progression, Therapeutic Resistance, and Bone Metastasis. Stem. Cells Int. 2017;2017:8629234. doi: 10.1155/2017/8629234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizokami A., Kadono Y., Kitagawa Y., Izumi K., Konaka H. Therapies for castration-resistant prostate cancer in a new era: The indication of vintage hormonal therapy, chemotherapy and the new medicines. Int. J. Urol. 2017;24:566–572. doi: 10.1111/iju.13372. [DOI] [PubMed] [Google Scholar]
- 6.Ceder Y., Bjartell A., Culig Z., Rubin M.A., Tomlins S., Visakorpi T. The Molecular Evolution of Castration-resistant Prostate Cancer. Eur. Urol. Focus. 2016;2:506–513. doi: 10.1016/j.euf.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Hoda M.R., Kramer M.W., Merseburger A.S., Cronauer M.V. Androgen deprivation therapy with Leuprolide acetate for treatment of advanced prostate cancer. Expert Opin. Pharmacother. 2017;18:105–113. doi: 10.1080/14656566.2016.1258058. [DOI] [PubMed] [Google Scholar]
- 8.Einstein D.J., Arai S., Balk S.P. Targeting the androgen receptor and overcoming resistance in prostate cancer. Curr. Opin. Oncol. 2019;31:175–182. doi: 10.1097/CCO.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorente D., Fizazi K., Sweeney C., de Bono J.S. Optimal Treatment Sequence for Metastatic Castration-resistant Prostate Cancer. Eur. Urol. Focus. 2016;2:488–498. doi: 10.1016/j.euf.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Aurilio G., Cimadamore A., Mazzucchelli R., Lopez-Beltran A., Verri E., Scarpelli M., Massari F., Cheng L., Santoni M., Montironi R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells. 2020;9:2653. doi: 10.3390/cells9122653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galletti G., Leach B.I., Lam L., Tagawa S.T. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat. Rev. 2017;57:16–27. doi: 10.1016/j.ctrv.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Bastos D.A., Antonarakis E.S. CTC-derived AR-V7 detection as a prognostic and predictive biomarker in advanced prostate cancer. Expert Rev. Mol. Diagn. 2018;18:155–163. doi: 10.1080/14737159.2018.1427068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp A., Coleman I., Yuan W., Sprenger C., Dolling D., Rodrigues D.N., Russo J.W., Figueiredo I., Bertan C., Seed G., et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J. Clin. Investig. 2019;129:192–208. doi: 10.1172/JCI122819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sciarra A., Gentilucci A., Silvestri I., Salciccia S., Cattarino S., Scarpa S., Gatto A., Frantellizzi V., Von Heland M., Ricciuti G.P., et al. Androgen receptor variant 7 (AR-V7) in sequencing therapeutic agents for castratrion resistant prostate cancer: A critical review. Medicine. 2019;98:e15608. doi: 10.1097/MD.0000000000015608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez P., Zeigler-Johnson C.M., Spangler E., van der Merwe A., Jalloh M., Gueye S.M., Rebbeck T.R. Androgen Metabolism Gene Polymorphisms, Associations with Prostate Cancer Risk and Pathological Characteristics: A Comparative Analysis between South African and Senegalese Men. Prostate Cancer. 2012;2012:798634. doi: 10.1155/2012/798634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bader D.A., McGuire S.E. Tumour metabolism and its unique properties in prostate adenocarcinoma. Nat. Rev. Urol. 2020;17:214–231. doi: 10.1038/s41585-020-0288-x. [DOI] [PubMed] [Google Scholar]
- 17.Benko G., Spajić B., Krušlin B., Tomas D. Impact of the EpCAM expression on biochemical recurrence-free survival in clinically localized prostate cancer. Urol. Oncol. 2013;31:468–474. doi: 10.1016/j.urolonc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mollica V., Di Nunno V., Cimadamore A., Lopez-Beltran A., Cheng L., Santoni M., Scarpelli M., Montironi R., Massari F. Molecular Mechanisms Related to Hormone Inhibition Resistance in Prostate Cancer. Cells. 2019;8:43. doi: 10.3390/cells8010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dongre A., Weinberg R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari N., Tiwari V.K., Waldmeier L., Balwierz P.J., Arnold P., Pachkov M., Meyer-Schaller N., Schübeler D., van Nimwegen E., Christofori G. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–783. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Frisch S.M., Schaller M., Cieply B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J. Cell Sci. 2013;126:21–29. doi: 10.1242/jcs.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y.T., Wu K.J. Epigenetic regulation of epithelial-mesenchymal transition: Focusing on hypoxia and TGF-β signaling. J. Biomed. Sci. 2020;27:39. doi: 10.1186/s12929-020-00632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Brabletz S., Schuhwerk H., Brabletz T., Stemmler M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021;40:e108647. doi: 10.15252/embj.2021108647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera D., Orellana-Serradell O., Villar P., Torres M.J., Paciucci R., Contreras H.R. Silencing of the transcriptional factor ZEB1 alters the steroidogenic pathway, and increases the concentration of testosterone and DHT in DU145 cells. Oncol. Rep. 2019;41:1275–1283. doi: 10.3892/or.2018.6885. [DOI] [PubMed] [Google Scholar]
- 27.Haffner M.C., Zwart W., Roudier M.P., True L.D., Nelson W.G., Epstein J.I., De Marzo A.M., Nelson P.S., Yegnasubramanian S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021;18:79–92. doi: 10.1038/s41585-020-00400-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Najafi M., Farhood B., Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell Physiol. 2019;234:8381–8395. doi: 10.1002/jcp.27740. [DOI] [PubMed] [Google Scholar]
- 29.Lim J.R., Mouawad J., Gorton O.K., Bubb W.A., Kwan A.H. Cancer stem cell characteristics and their potential as therapeutic targets. Med. Oncol. 2021;38:76. doi: 10.1007/s12032-021-01524-8. [DOI] [PubMed] [Google Scholar]
- 30.Civenni G., Albino D., Shinde D., Vázquez R., Merulla J., Kokanovic A., Mapelli S.N., Carbone G.M., Catapano C.V. Transcriptional Reprogramming and Novel Therapeutic Approaches for Targeting Prostate Cancer Stem Cells. Front. Oncol. 2019;9:385. doi: 10.3389/fonc.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castellón E.A., Valenzuela R., Lillo J., Castillo V., Contreras H.R., Gallegos I., Mercado A., Huidobro C. Molecular signature of cancer stem cells isolated from prostate carcinoma and expression of stem markers in different Gleason grades and metastasis. Biol. Res. 2012;45:297–305. doi: 10.4067/S0716-97602012000300011. [DOI] [PubMed] [Google Scholar]
- 32.Castillo V., Valenzuela R., Huidobro C., Contreras H.R., Castellon E.A. Functional characteristics of cancer stem cells and their role in drug resistance of prostate cancer. Int. J. Oncol. 2014;45:985–994. doi: 10.3892/ijo.2014.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez C.A., Andahur E.I., Valenzuela R., Castellón E.A., Fullá J.A., Ramos C.G., Triviño J.C. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7:3993–4008. doi: 10.18632/oncotarget.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D.H., Xing T., Yang Z., Dudek R., Lu Q., Chen Y.-H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017;7:1. doi: 10.3390/jcm7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Craene B., Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 36.Plygawko A.T., Kan S., Campbell K. Epithelial-mesenchymal plasticity: Emerging parallels between tissue morphogenesis and cancer metastasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020;375:20200087. doi: 10.1098/rstb.2020.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culig Z. Epithelial mesenchymal transition and resistance in endocrine-related cancers. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:1368–1375. doi: 10.1016/j.bbamcr.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Montanari M., Rossetti S., Cavaliere C., D’Aniello C., Malzone M.G., Vanacore D., Di Franco R., La Mantia E., Iovane G., Piscitelli R., et al. Epithelial-mesenchymal transition in prostate cancer: An overview. Oncotarget. 2017;8:35376–35389. doi: 10.18632/oncotarget.15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osorio L.A., Farfán N.M., Castellón E.A., Contreras H.R. SNAIL transcription factor increases the motility and invasive capacity of prostate cancer cells. Mol. Med. Rep. 2016;13:778–786. doi: 10.3892/mmr.2015.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poblete C.E., Fulla J., Gallardo M., Muñoz V., Castellón E.A., Gallegos I., Contreras H.R. Increased SNAIL expression and low syndecan levels are associated with high Gleason grade in prostate cancer. Int. J. Oncol. 2014;44:647–654. doi: 10.3892/ijo.2014.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farfán N., Ocarez N., Castellón E.A., Mejía N., de Herreros A.G., Contreras H.R. The transcriptional factor ZEB1 represses Syndecan 1 expression in prostate cancer. Sci. Rep. 2018;8:11467. doi: 10.1038/s41598-018-29829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orellana-Serradell O., Herrera D., Castellon E.A., Contreras H.R. The transcription factor ZEB1 promotes an aggressive phenotype in prostate cancer cell lines. Asian J. Androl. 2018;20:294–299. doi: 10.4103/aja.aja_61_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orellana-Serradell O., Herrera D., Castellón E.A., Contreras H.R. The transcription factor ZEB1 promotes chemoresistance in prostate cancer cell lines. Asian J. Androl. 2019;21:460–467. doi: 10.4103/aja.aja_1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babaei G., Aziz S.G., Jaghi N.Z.Z. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed. Pharmacother. 2021;133:110909. doi: 10.1016/j.biopha.2020.110909. [DOI] [PubMed] [Google Scholar]
- 45.Yin W., Wang J., Jiang L., James Kang Y. Cancer and stem cells. Exp. Biol. Med. 2021;246:1791–1801. doi: 10.1177/15353702211005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayob A.Z., Ramasamy T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018;25:20. doi: 10.1186/s12929-018-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen L.V., Vanner R., Dirks P., Eaves C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 48.Fang D., Kitamura H. Cancer stem cells and epithelial-mesenchymal transition in urothelial carcinoma: Possible pathways and potential therapeutic approaches. Int. J. Urol. 2018;25:7–17. doi: 10.1111/iju.13404. [DOI] [PubMed] [Google Scholar]
- 49.Rycaj K., Li H., Zhou J., Chen X., Tang D.G. Cellular determinants and microenvironmental regulation of prostate cancer metastasis. Semin. Cancer Biol. 2017;44:83–97. doi: 10.1016/j.semcancer.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schilling D., Todenhöfer T., Hennenlotter J., Schwentner C., Fehm T., Stenzl A. Isolated, disseminated and circulating tumour cells in prostate cancer. Nat. Rev. Urol. 2012;9:448–463. doi: 10.1038/nrurol.2012.136. [DOI] [PubMed] [Google Scholar]
- 51.Lowes L.E., Goodale D., Xia Y., Postenka C., Piaseczny M.M., Paczkowski F., Allan A.L. Epithelial-to-mesenchymal transition leads to disease-stage differences in circulating tumor cell detection and metastasis in pre-clinical models of prostate cancer. Oncotarget. 2016;7:76125–76139. doi: 10.18632/oncotarget.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theil G., Boehm C., Fischer K., Bialek J., Hoda R., Weber E., Schönburg S., Kawan F., Fornara P. In vivo isolation of circulating tumor cells in patients with different stages of prostate cancer. Oncol. Lett. 2021;21:357. doi: 10.3892/ol.2021.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruscetti M., Quach B., Dadashian E.L., Mulholland D.J., Wu H. Tracking and Functional Characterization of Epithelial-Mesenchymal Transition and Mesenchymal Tumor Cells during Prostate Cancer Metastasis. Cancer Res. 2015;75:2749–2759. doi: 10.1158/0008-5472.CAN-14-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deep G., Jain A.K., Ramteke A., Ting H., Vijendra K.C., Gangar S.C., Agarwal C., Agarwal R. SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Mol. Cancer. 2014;13:37. doi: 10.1186/1476-4598-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bae K.-M., Su Z., Frye C., McClellan S., Allan R.W., Andrejewski J.T., Kelley V., Jorgensen M., Steindler D.A., Vieweg J., et al. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. J. Urol. 2010;183:2045–2053. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bae K.M., Parker N.N., Dai Y., Vieweg J., Siemann D.W. E-cadherin plasticity in prostate cancer stem cell invasion. Am. J. Cancer Res. 2011;1:71–84. [PMC free article] [PubMed] [Google Scholar]
- 57.Kerr B.A., Miocinovic R., Smith A.K., West X.Z., Watts K.E., Alzayed A.W., Klink J.C., Mir M.C., Sturey T., Hansel D.E., et al. CD117⁺ cells in the circulation are predictive of advanced prostate cancer. Oncotarget. 2015;6:1889–1897. doi: 10.18632/oncotarget.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patrawala L., Calhoun T., Schneider-Broussard R., Li H., Bhatia B., Tang S., Reilly J.G., Chandra D., Zhou J., Claypool K., et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 59.Klarmann G.J., Hurt E.M., Mathews L.A., Zhang X., Duhagon M.A., Mistree T., Thomas S.B., Farrar W.L. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palapattu G.S., Wu C., Silvers C.R., Martin H.B., Williams K., Salamone L., Bushnell T., Huang L.-S., Yang Q., Huang J. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 2009;69:787–798. doi: 10.1002/pros.20928. [DOI] [PubMed] [Google Scholar]
- 61.Chang Y.-T., Lin T.-P., Campbell M., Pan C.-C., Lee S.-H., Lee H.-C., Yang M.-H., Kung H.-J., Chang P.-C. REST is a crucial regulator for acquiring EMT-like and stemness phenotypes in hormone-refractory prostate cancer. Sci. Rep. 2017;7:42795. doi: 10.1038/srep42795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies A.H., Beltran H., Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 2018;15:271–286. doi: 10.1038/nrurol.2018.22. [DOI] [PubMed] [Google Scholar]
- 63.Russo M.V., Esposito S., Tupone M.G., Manzoli L., Airoldi I., Pompa P., Cindolo L., Schips L., Sorrentino C., Di Carlo E. SOX2 boosts major tumor progression genes in prostate cancer and is a functional biomarker of lymph node metastasis. Oncotarget. 2016;7:12372–12385. doi: 10.18632/oncotarget.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esposito S., Russo M.V., Airoldi I., Tupone M.G., Sorrentino C., Barbarito G., Di Meo S., Di Carlo E. SNAI2/Slug gene is silenced in prostate cancer and regulates neuroendocrine differentiation, metastasis-suppressor and pluripotency gene expression. Oncotarget. 2015;6:17121–17134. doi: 10.18632/oncotarget.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soundararajan R., Paranjape A.N., Maity S., Aparicio A., Mani S.A. EMT, stemness and tumor plasticity in aggressive variant neuroendocrine prostate cancers. Biochim. Biophys. Acta Rev. Cancer. 2018;1870:229–238. doi: 10.1016/j.bbcan.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jayachandran A., Dhungel B., Steel J.C. Epithelial-to-mesenchymal plasticity of cancer stem cells: Therapeutic targets in hepatocellular carcinoma. J. Hematol. Oncol. 2016;9:74. doi: 10.1186/s13045-016-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X., Sun B., Sun D., Liu T., Che N., Gu Q., Dong X., Li R., Liu Y., Li J. Slug promotes hepatocellular cancer cell progression by increasing sox2 and nanog expression. Oncol. Rep. 2015;33:149–156. doi: 10.3892/or.2014.3562. [DOI] [PubMed] [Google Scholar]
- 68.Mu P., Zhang Z., Benelli M., Karthaus W.R., Hoover E., Chen C.-C., Wongvipat J., Ku S.-Y., Gao D., Cao Z., et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsumoto T., Yokoi A., Hashimura M., Oguri Y., Akiya M., Saegusa M. TGF-β-mediated LEFTY/Akt/GSK-3β/Snail axis modulates epithelial-mesenchymal transition and cancer stem cell properties in ovarian clear cell carcinomas. Mol. Carcinog. 2018;57:957–967. doi: 10.1002/mc.22816. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., Weinberg R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018;12:361–373. doi: 10.1007/s11684-018-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bocci F., Jolly M.K., George J.T., Levine H., Onuchic J.N. A mechanism-based computational model to capture the interconnections among epithelial-mesenchymal transition, cancer stem cells and Notch-Jagged signaling. Oncotarget. 2018;9:29906–29920. doi: 10.18632/oncotarget.25692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang K., Ji W., Yu Y., Li Z., Niu X., Xia W., Lu S. FGFR1-ERK1/2-SOX2 axis promotes cell proliferation, epithelial-mesenchymal transition, and metastasis in FGFR1-amplified lung cancer. Oncogene. 2018;37:5340–5354. doi: 10.1038/s41388-018-0311-3. Erratum in Oncogene 2020, 39, 6619–6620. [DOI] [PubMed] [Google Scholar]
- 73.Pradella D., Naro C., Sette C., Ghigna C. EMT and stemness: Flexible processes tuned by alternative splicing in development and cancer progression. Mol. Cancer. 2017;16:8. doi: 10.1186/s12943-016-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butler T.P., Gullino P.M. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35:512–516. [PubMed] [Google Scholar]
- 75.Celià-Terrassa T., Kang Y. Metastatic niche functions and therapeutic opportunities. Nat. Cell Biol. 2018;20:868–877. doi: 10.1038/s41556-018-0145-9. [DOI] [PubMed] [Google Scholar]
- 76.Ganesh K., Massagué J. Targeting metastatic cancer. Nat. Med. 2021;27:34–44. doi: 10.1038/s41591-020-01195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cackowski F.C., Heath E.I. Prostate cancer dormancy and recurrence. Cancer Lett. 2022;524:103–108. doi: 10.1016/j.canlet.2021.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rycaj K., Tang D.G. Metastasis and Metastatic Cells: A Historical Perspective and Current Analysis. In: Liu H., Lathias D.J., editors. Cancer Stem Cells. 1st ed. Elsevier; Cambridge, MA, USA: 2016. pp. 317–340. [Google Scholar]
- 79.Mei W., Lin X., Kapoor A., Gu Y., Zhao K., Tang D. The Contributions of Prostate Cancer Stem Cells in Prostate Cancer Initiation and Metastasis. Cancers. 2019;11:434. doi: 10.3390/cancers11040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsika A., Srinivasan B., Day C., Mader S.A., Kiernan D.M., Broomfield A., Fu J., Hooper J.D., Kench J.G., Samaratunga H. Cancer stem cell markers in prostate cancer: An immunohistochemical study of ALDH1, SOX2 and EZH2. Pathology. 2015;47:622–628. doi: 10.1097/PAT.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 81.Conley-LaComb M.K., Huang W., Wang S., Shi D., Jung Y.S., Najy A., Fridman R., Bonfil R.D., Cher M.L., Chen Y.Q., et al. PTEN regulates PDGF ligand switch for β-PDGFR signaling in prostate cancer. Am. J. Pathol. 2012;180:1017–1027. doi: 10.1016/j.ajpath.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massoner P., Thomm T., Mack B., Untergasser G., Martowicz A.A., Bobowski K., Klocker H., Gires O.O., Puhr M. EpCAM is overexpressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200c/205. Br. J. Cancer. 2014;111:955–964. doi: 10.1038/bjc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mainetti L.E., Zhe X., Diedrich J., Saliganan A.D., Cho W.J., Cher M.L., Heath E., Fridman R., Kim H.-R.C., Bonfil R.D. Bone-induced c-kit expression in prostate cancer: A driver of intraosseous tumor growth. Int. J. Cancer. 2015;136:11–20. doi: 10.1002/ijc.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harris K.S., Shi L., Foster B.M., Mobley M.E., Elliott P.L., Song C.J., Watabe K., Langefeld C.D., Kerr B.A. CD117/c-kit defines a prostate CSC-like subpopulation driving progression and TKI resistance. Sci. Rep. 2021;11:1465. doi: 10.1038/s41598-021-81126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Domanska U.M., Timmer-Bosscha H., Nagengast W.B., Munnink T.H.O., Kruizinga R.C., Ananias H.J., Kliphuis N.M., Huls G., De Vries E.G., de Jong I.J., et al. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia. 2012;14:709–718. doi: 10.1593/neo.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Putzke A.P., Ventura A.P., Bailey A.M., Akture C., Opoku-Ansah J., Çeliktaş M., Hwang M.S., Darling D.S., Coleman I.M., Nelson P.S., et al. Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am. J. Pathol. 2011;179:400–410. doi: 10.1016/j.ajpath.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Celià-Terrassa T., Meca-Cortés O., Mateo F., de Paz A.M., Rubio N., Arnal-Estapé A., Ell B.J., Bermudo R., Díaz A., Guerra-Rebollo M., et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J. Clin. Investig. 2012;122:1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakir B., Chiarella A.M., Pitarresi J.R., Rustgi A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020;30:764–776. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bullock M.D., Sayan A.E., Packham G.K., Mirnezami A.H. MicroRNAs: Critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol. Cell. 2012;104:3–12. doi: 10.1111/boc.201100115. [DOI] [PubMed] [Google Scholar]
- 90.Cifuentes F.F., Valenzuela R.H., Contreras H.R., Castellón E.A. Development of an orthotopic model of human metastatic prostate cancer in the NOD-SCIDγ mouse (Mus musculus) anterior prostate. Oncol. Lett. 2015;10:2142–2148. doi: 10.3892/ol.2015.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rizzo A., Mollica V., Cimadamore A., Santoni M., Scarpelli M., Giunchi F., Cheng L., Lopez-Beltran A., Fiorentino M., Montironi R., et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells. 2020;9:2051. doi: 10.3390/cells9092051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sutherland S.I.M., Ju X., Horvath L.G., Clark G.J. Moving on From Sipuleucel-T: New Dendritic Cell Vaccine Strategies for Prostate Cancer. Front. Immunol. 2021;12:641307. doi: 10.3389/fimmu.2021.641307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan C.H., Rosen J.M. The mechanisms of therapy resistance in cancer stem cells. In: Liu H., Lathias D.J., editors. Cancer Stem Cells. 1st ed. Elsevier; Cambridge, MA, USA: 2016. pp. 395–410. [Google Scholar]
- 94.Hoogland A.M., Verhoef E.I., Roobol M.J., Schröder F.H., Wildhagen M.F., van der Kwast T.H., Jenster G., van Leenders G.J. Validation of stem cell markers in clinical prostate cancer: α6-integrin is predictive for non-aggressive disease. Prostate. 2014;74:488–496. doi: 10.1002/pros.22768. [DOI] [PubMed] [Google Scholar]
- 95.Yang M., Zhang X., Guo L., Liu X., Wu J., Zhu H. Research Progress for the Clinical Application of Circulating Tumor Cells in Prostate Cancer Diagnosis and Treatment. Biomed. Res. Int. 2021;2021:6230826. doi: 10.1155/2021/6230826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conteduca V., Ku S.-Y., Fernandez L., Dago-Rodriquez A., Lee J., Jendrisak A., Slade M., Gilbertson C., Manohar J., Sigouros M., et al. Circulating tumor cell heterogeneity in neuroendocrine prostate cancer by single cell copy number analysis. NPJ Precis Oncol. 2021;5:76. doi: 10.1038/s41698-021-00211-1. Erratum in NPJ Precis Oncol. 2021, 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiao J., Hindoyan A., Wang S., Tran L.M., Goldstein A.S., Lawson D., Chen D., Li Y., Guo C., Zhang B., et al. Identification of CD166 as a surface marker for enriching prostate stem/progenitor and cancer initiating cells. PLoS ONE. 2012;7:e42564. doi: 10.1371/journal.pone.0042564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park S.H., Fong K.W., Mong E., Martin M.C., Schiltz G.E., Yu J. Going beyond Polycomb: EZH2 functions in prostate cancer. Oncogene. 2021;40:5788–5798. doi: 10.1038/s41388-021-01982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsao T., Beretov J., Ni J., Bai X., Bucci J., Graham P., Li Y. Cancer stem cells in prostate cancer radioresistance. Cancer Lett. 2019;465:94–104. doi: 10.1016/j.canlet.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 100.Dubrovska A., Elliott J., Salamone R.J., Telegeev G.D., Stakhovsky A.E., Schepotin I.B., Yan F., Wang Y., Bouchez L.C., Kularatne S.A., et al. CXCR4 expression in prostate cancer progenitor cells. PLoS ONE. 2012;7:e31226. doi: 10.1371/journal.pone.0031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ni J., Cozzi P., Beretov J., Duan W., Bucci J., Graham P., Li Y. Epithelial cell adhesion molecule (EpCAM) is involved in prostate cancer chemotherapy/radiotherapy response in vivo. BMC Cancer. 2018;18:1092. doi: 10.1186/s12885-018-5010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sánchez C., Mendoza P., Contreras H.R., Vergara J., McCubrey J.A., Huidobro C., Castellón E.A. Expression of multidrug resistance proteins in prostate cancer is related with cell sensitivity to chemotherapeutic drugs. Prostate. 2009;69:1448–1459. doi: 10.1002/pros.20991. [DOI] [PubMed] [Google Scholar]
- 103.Sánchez C., Mercado A., Contreras H.R., Mendoza P., Cabezas J., Acevedo C., Huidobro C., Castellón E.A. Chemotherapy sensitivity recovery of prostate cancer cells by functional inhibition and knock down of multidrug resistance proteins. Prostate. 2011;71:1810–1817. doi: 10.1002/pros.21398. [DOI] [PubMed] [Google Scholar]
- 104.Patrawala L., Calhoun T., Schneider-Broussard R., Zhou J., Claypool K., Tang D.G. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 105.Cojoc M., Mäbert K., Muders M.H., Dubrovska A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin. Cancer Biol. 2015;31:16–27. doi: 10.1016/j.semcancer.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 106.Garcia-Mayea Y., Mir C., Masson F., Paciucci R., LLeonart M.E. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020;60:166–180. doi: 10.1016/j.semcancer.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 107.Shu H., Bin Yuan B., Huang Y., Wang L., He B., Sun Q., Sun L. High expression of ABCG2 is associated with chemotherapy resistance of osteosarcoma. J. Orthop. Surg. Res. 2021;16:85. doi: 10.1186/s13018-021-02204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Antonarakis E.S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J.C., Chen Y., Mohammad T.A., Chen Y., Fedor H.L., et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scher H.I., Lu D., Schreiber N.A., Louw J., Graf R.P., Vargas H.A., Johnson A., Jendrisak A., Bambury R., Danila D., et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016;2:1441–1449. doi: 10.1001/jamaoncol.2016.1828. Erratum in JAMA Oncol. 2016, 2, 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y., Chan S.C., Brand L.J., Hwang T.H., Silverstein K.A., Dehm S.M. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deng Q., Tang D.G. Androgen receptor and prostate cancer stem cells: Biological mechanisms and clinical implications. Endocr. Relat. Cancer. 2015;22:T209–T220. doi: 10.1530/ERC-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu K., Gore C., Yang L., Fazli L., Gleave M., Pong R.-C., Xiao G., Zhang L., Yun E.-J., Tseng S.-F., et al. Slug, a unique androgen-regulated transcription factor, coordinates androgen receptor to facilitate castration resistance in prostate cancer. Mol. Endocrinol. 2012;26:1496–1507. doi: 10.1210/me.2011-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abdolahi S., Ghazvinian Z., Muhammadnejad S., Saleh M., Asadzadeh Aghdaei H., Baghaei K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J. Transl. Med. 2022;20:206. doi: 10.1186/s12967-022-03405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y., Xue H., Cutz J.-C., Bayani J., Mawji N.R., Chen W.G., Goetz L.J., Hayward S.W., Sadar M.D., Gilks C.B., et al. An orthotopic metastatic prostate cancer model in SCID mice via grafting of a transplantable human prostate tumor line. Lab. Investig. 2005;85:1392–1404. doi: 10.1038/labinvest.3700335. [DOI] [PubMed] [Google Scholar]
- 115.Tumati V., Mathur S., Song K., Hsieh J.-T., Zhao D., Takahashi M., Dobin T., Gandee L., Solberg T.D., Habib A.A., et al. Development of a locally advanced orthotopic prostate tumor model in rats for assessment of combined modality therapy. Int. J. Oncol. 2013;42:1613–1619. doi: 10.3892/ijo.2013.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cifuentes F.F., Valenzuela R.H., Contreras H.R., Castellón E.A. Surgical cytoreduction of the primary tumor reduces metastatic progression in a mouse model of prostate cancer. Oncol. Rep. 2015;34:2837–2844. doi: 10.3892/or.2015.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.