Abstract
In this study, we fabricate a series of water-soluble anionic macrocyclic arenes, including pillar[5]arene (WP5), pillar[6]arene (WP6), leaning pillar[6]arene (WLT6), and biphenyl-extended pillar[6]arene (WBpP6), which show different separation capabilities toward low-molecular-weight organics, such as short chain haloalkanes, cyclic aliphatics, and aromatics, in water. The liquid–liquid distribution experiments are carried out at room temperature. The separation factor for low-molecular-weight organics is evaluated in the extraction of equimolar mixtures. WP6 demonstrates a high extraction efficiency of up to 89% in separating toluene/methylcyclohexane mixtures. These adsorbents also have the advantages of rapid adsorption, high separation efficiency, remarkable selectivity, and good recyclability. This work not only expands the application scope of macrocyclic chemistry, but also has practical research value for organics separation and water purification.
Keywords: low-molecular-weight organics, liquid–liquid extraction, host-guest chemistry, pillararene, separation
1. Introduction
The separation of high-value-added chemical feedstocks using feasible and environmentally friendly methods is always an essential topic in the chemical industry. As common precursors or solvents in the chemical industry, low-molecular-weight organics, such as short-chain haloalkanes, cyclic aliphatics, and aromatics, are widely used in synthetic fibers [1], surfactants [2], varnishes [3], pesticides [4,5], and so on. In particular, the separation of these organics based on their different boiling points is generally realized by conventional distillation. However, many aromatic isomers [6,7], short-chain alkane homologs [8], and cyclic aliphatics [9,10] have similar physical properties and close boiling points, making it difficult to guarantee chemical separation purity. In addition, the utilization of entrainers would cause additional energy and expense waste [11]. Therefore, it is necessary to develop cheap, convenient, and efficient separation methods to meet the requirements of industrial production. Meanwhile, the process of adsorptive separation is considered to be an effective, energy-efficient, and feasible method for separating liquid mixtures with close boiling points [12]. Adsorbents like activated carbon [13,14], zeolite [15], ordered mesoporous silica [16], and supramolecular macrocycles [17,18] have been extensively used to remove pollutants from water. Among them, supramolecular macrocycles, with their high selectivity and high recovery efficiency, emerge as one of the most effective receptors for these low-molecular-weight organics, and may eventually replace the current isolation techniques used to separate these mixtures.
Macrocycles like cyclodextrins [19], calixarenes [20,21], and pillararenes [22,23,24,25] are known for their unique cavity structures, which can accommodate specific guest molecules, as well as their diverse modifications and self-assembly behaviors. Their size-specific electron-rich hydrophobic cavities can selectively bind certain molecules through host–guest interactions [26,27,28]. The host–guest properties of macrocycles have been extensively applied in the fields of drug delivery [29,30,31], water purification [32], self-assembly [33], photochromism [34,35], and substance detection [36,37,38,39]. In particular, the development of pillararene-based macrocyclic arenes has increased rapidly since they were first reported on in 2008, and a variety of expanded versions of macrocyclic arenes, such as leaning pillar[6]arene [40], biphenyl-extended pillar[6]arene [41], geminiarenes [42], leggero pillar[5]arene [43], and hybrid [n] arenes [44], have been synthesized. They have also been widely employed in catalytic processes [45], molecular devices [46], substance detection [47], stimuli-responsive materials [48], medicine, and theranostics [49,50].
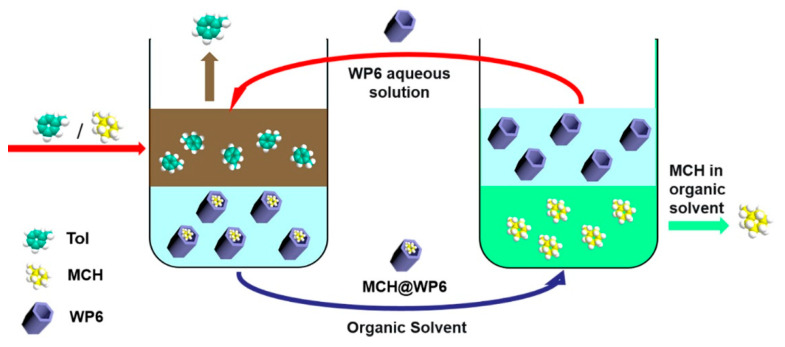
In this work, we successfully synthesized four carboxyl-modified anionic macrocyclic arenes and utilized their water solubility and host–guest properties to separate small organics in the water phase (Scheme 1). Control experiments confirmed that WP6 performed the best when extracting methylcyclohexane (MCH) from toluene (Tol) mixtures. This is the first report of a pillar[6]arene aqueous solution being applied in an extraction. Investigating the performance of water-soluble pillararenes as adsorptive separation materials for low-molecular-weight organics as we have done can provide a lot of valuable information.
Scheme 1.
Schematic illustration of the separation process by WP6.
2. Results
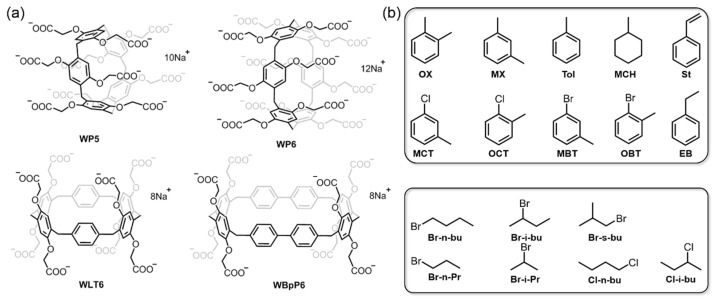
Four water-soluble anionic macrocyclic arenes, including WP5, WP6, WLT6, and WBpP6, bearing ten, twelve, eight, and eight carboxylate groups, respectively, were synthesized according to previously reported procedures (Figure 1a) [25,51,52,53]. All of the structures were fully characterized and confirmed using 1H NMR spectroscopy (Figures S1–S4), UV-vis absorption spectra (Figure S5), Fourier-transform infrared (FTIR) spectra (Figure S6), mass spectrometry (Figures S7–S10), and thermogravimetric analysis (Figure S11). To explore the liquid–liquid extraction performance of water-soluble pillararenes as adsorptive separation materials, we selected different types of low-molecular-weight organics with similar structures and close boiling points, such as short-chain haloalkanes, aromatics, and cyclic aliphatics, as adsorbates (Figure 1b). In addition, the effects of the pillararene concentration, proportion of mixtures, contacting time, and recycling time on separation performance were also carefully studied.
Figure 1.
Chemical structures of (a) four water-soluble anionic macrocyclic arenes and (b) selected small molecule organics acting as adsorbates.
We first used a liquid–liquid extraction method to investigate the separation capability of WP5. In a typical procedure, the equimolar binary organics mixture was added to the aqueous solution of WP5. After being stirred, the resulting mixture was left to undergo extraction for a few minutes. Thereafter, the aqueous phase was separated from the organic phase and a simple extraction was performed using deuterated chloroform to extract the organics adsorbed by WP5 in the aqueous phase. The composition of the organic mixture adsorbed by the aqueous phase was fully characterized by 1H NMR spectra (Figures S12–S15). The detailed results are given below.
According to the data in Table 1, multi-substituted aromatics and cyclic aliphatics had greater separation efficiencies than short-chain haloalkanes. WP5 performed best in separating different types of organics analogs, followed closely by WP6. This could be because water-soluble pillararenes have hydrophobic cavities that can form a stable inclusion complex with aromatics. In contrast, the experimental results of WLT6 and WBpP6 were far from impressive, which may be because their cavities are too large to combine well with guest molecules.
Table 1.
Composition of extracts after extraction by four water-soluble pillararenes according to the results of 1H NMR spectra.
| Mixtures | WP5 | WP6 | WLT6 | WBpP6 |
|---|---|---|---|---|
| OX | 0.15 | 0.44 | / | / |
| MX | 0.85 | 0.56 | ||
| MCT | 0.96 | 0.75 | 0.46 | 0.53 |
| OCT | 0.04 | 0.25 | 0.54 | 0.47 |
| MBT | 0.93 | 0.73 | 0.59 | 0.44 |
| OBT | 0.07 | 0.27 | 0.41 | 0.56 |
| St | 0.36 | 0.54 | 0.64 | / |
| EB | 0.64 | 0.46 | 0.36 | |
| Tol | 0.14 | 0.11 | / | / |
| MCH | 0.86 | 0.89 | ||
| Br-n-bu | 0.76 | 0.48 | 0.49 | 0.75 |
| Br-i-bu | 0.14 | 0.23 | 0.27 | / |
| Br-s-bu | 0.1 | 0.29 | 0.24 | 0.25 |
| Br-n-Pr | 0.71 | 0.43 | 0.52 | 0.55 |
| Br-i-Pr | 0.29 | 0.57 | 0.48 | 0.45 |
| Cl-n-bu | 0.60 | 0.42 | 0.48 | 0.47 |
| Cl-i-bu | 0.40 | 0.57 | 0.52 | 0.53 |
A lack of data means that there were no organics in the water phase to be extracted. Relevant results were obtained from the average of three independent extraction/release experiments.
The separation factor (α), which refers to the ratio of the relative content of the dominant extract before and after the extraction, was used to describe the extraction efficiency by different water-soluble pillararenes [54]. The relationship can be represented using the following equation, where xa and xb represent the mole quantity of components a and b in the mixed organic sources, and ya or yb is the mole quantity in the extracts:
The primary variables in this experiment were the composition of organic mixtures, concentration of macrocyclic arenes, contact time, and recycling time. Different combinations have been tested to determine the optimal separation conditions. To the best of our knowledge, the adsorptive separation of liquid organics by WP6 has not been reported thus far. Therefore, we used WP6 and WP5 as examples in the following explorations to investigate the extraction efficiency for separating Tol/MCH mixtures in different conditions. We specifically chose these pillararenes because they previously displayed the best separation performance.
2.1. Effect of Pillararene Concentration and Stirring Time
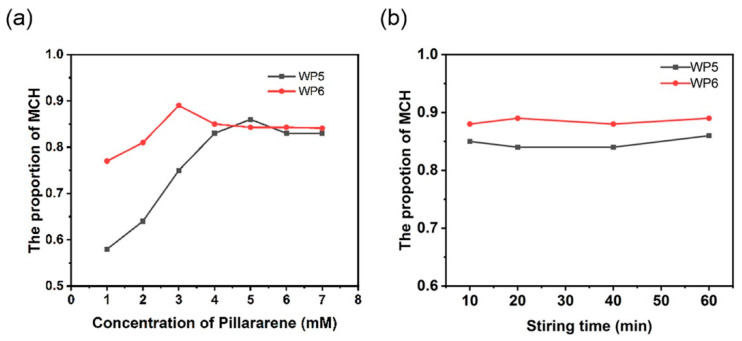
To gain more insight into the selectivity of pillararenes for mixtures separation, the effect of WP5 and WP6 concentrations on the proportion of MCH in the extraction phase was investigated. An equimolar Tol/MCH mixture (1:1) was prepared as the organic liquid source to be separated. It was found that the selectivity first increased, and then decreased slightly, as the pillararene concentration increased (Figure 2a). Due to the lower solubility of WP6 than WP5 in an aqueous solution, WP6 exhibited a sluggish selectivity for MCH at a low concentration range. When the separation efficiency reached the maximum value, the content of MCH in the aqueous phase underwent a slight decrease as the macrocycle concentration increased because the excess pillararenes could still adsorb a small amount of Tol. The best extraction efficiency (89%) was achieved when 3 mM WP6 was added in the extraction phase, and the optimal concentration of WP5 was 5 mM.
Figure 2.
The proportion of MCH in the extraction phase at different (a) pillararene concentrations and (b) stirring times.
The optimal pillararene concentrations (3 mM for WP6 and 5 mM for WP5) were used to explore the effect of stirring time on the separation efficiency. The experimental results showed that increasing the stirring time to somewhere between 10 and 60 min does not change the final content level of MCH in the extraction phase (Figure 2b). This means that the specific complexation between water-soluble pillararenes and MCH proceeds rapidly and is easily completed within 10 min under an uncomplicated condition. Thus, we can conclude that host–guest interactions exist between pillararenes and MCH molecules. As contact time is an important criterion used to evaluate the efficiency of a separation technique, the fact that water-soluble pillararenes have such a short stirring time means that they can be feasibly used in a wide range of practical operations.
2.2. Effect of Single Component Content
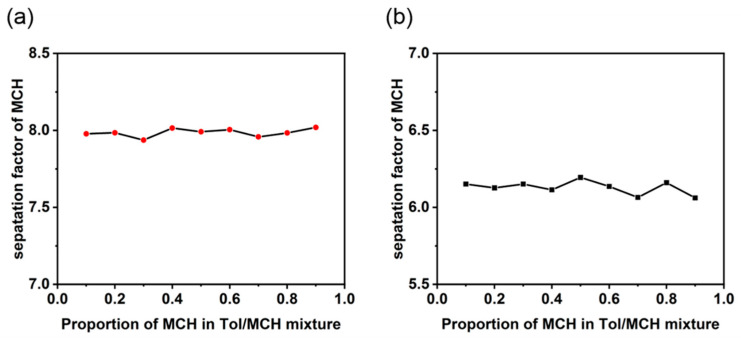
The extraction was subsequently performed using a different proportion of MCH in the Tol/MCH mixture. As the percentage of MCH was increased from 10% to 90%, there was no obvious change in the separation factor α of MCH after the organic phase was extracted by the arene aqueous solution (Figure 3). This result demonstrates that the pillararenes have excellent selectivity for MCH, and that the anionic macrocycles can be easily recycled from the mixed solution through the second round of liquid–liquid extraction by CDCl3. Meanwhile, MCH can be transferred into the organic phase of CDCl3. The average α value of WP6 is 8.08, which is slightly higher than that of WP5 (6.12), indicating that WP6 has better liquid–liquid phase extraction performance than MCH.
Figure 3.
The relationship between the separation factor of MCH and the proportion of MCH after an extraction treatment by (a) WP5 and (b) WP6.
2.3. Curve of Extraction Equilibrium
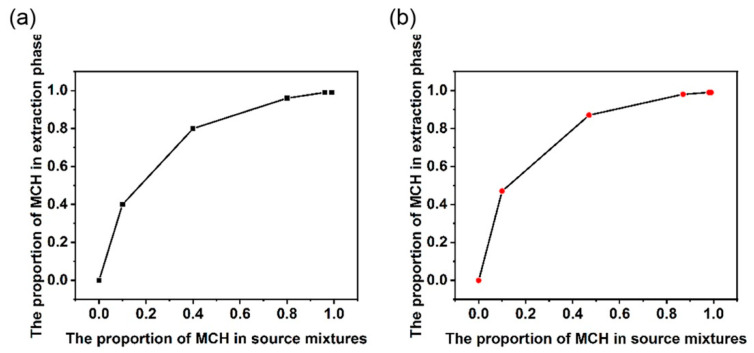
Like the gas–liquid equilibrium curve, the extraction equilibrium curve describes the relationship between the composition of the Tol/MCH mixture sources and the extraction efficiency. Therefore, the extraction equilibrium curve is beneficial in practical applications. For a given starting mixture containing different percentages of MCH, the curve provides the proportion of MCH that can be obtained in the extraction phase. Moreover, the α value can also be calculated using this curve.
The extraction equilibrium curve in Figure 4 was obtained from a starting organic phase source of Tol/MCH (v:v = 1:9). The resulting extraction phase and MCH content were used directly in the next extraction cycle to obtain consecutive sample points in Figure 4. It was found that after three extraction cycles, the proportion of MCH increased from the initial 10% to 87% by WP5 solution and to 95% in the case of WP6. After five extraction cycles, an MCH content level of 99% could be achieved for both cases. Thus, the extraction equilibrium curve allows us to determine how many extraction cycles will be required to reach a specific separation target from a given starting mixture. This facilitates the practical application of water-soluble pillararenes in chemical separation and water purification.
Figure 4.
Extraction equilibrium curve of (a) WP5 and (b) WP6.
2.4. Effect of Successive Cycles
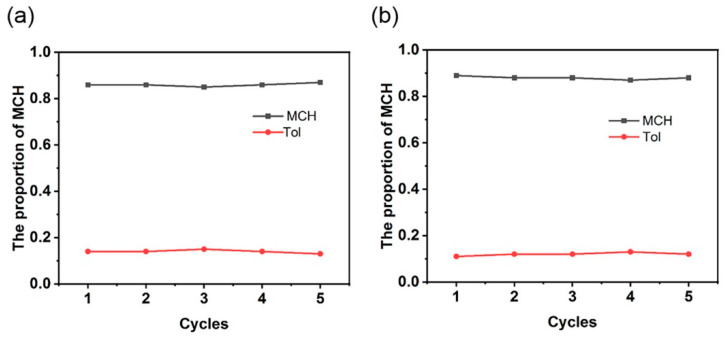
Reusability is a critical factor in wastewater treatment, thus we attempted to reactivate the adsorbents WP5 and WP6 through washing, re-precipitation, and filtration. The recovered water-soluble anionic macrocycles were first sonicated in deionized water for 30 min at room temperature, then washed with DCM/water (1:1, v/v) and deionized water. Next, the aqueous solution of anionic macrocyclic arenes was evaporated and the precipitated macrocyclic salts were filtered and then desiccated at 90 °C in vacuo for 12 h to make sure that the absorbed guest molecules were completely released from the cavities. The recovered anionic pillararenes were reused for five successive cycles in a D2O solution containing Tol/MCH mixtures (1:1, v/v). No obvious deactivation was found, clearly demonstrating the stability of water-soluble pillararenes as adsorptive separating materials (Figure 5).
Figure 5.
Relative uptakes of MCH and Tol by (a) WP5 and (b) WP6 for five cycles at 298 K.
3. Materials and Methods
3.1. General Information
Starting reagents and solvents were commercially available and used as received unless stated otherwise. Deionized water used in the relevant experiments was purified using an experimental water system (Lab-UV-20). All four macrocyclic arenes, including WP5, WP6, WLT6, and WBpP6, were synthesized according to the published procedures [25,51]. UV-vis spectra were recorded on a Shimadzu UV-2550 instrument. Fourier transform infrared spectroscopy (FT-IR) spectra were recorded on a Vertex 80 V spectrometer. Mass spectra were recorded on a Bruker Agilent1290-micrOTOF Q II High-resolution (HR) mass spectrometry instrument. Thermogravimetric analysis was performed by using a NETZSCH STA 449F3 QMS403D\Bruker V70 instrument, and each sample was heated to a temperature of 600 °C at a rate of 10 °C min−1 under a nitrogen atmosphere. 1H NMR spectra were recorded on a Bruker Avance III 400 MHz spectrometer at room temperature. Solution 1H NMR spectra were recorded using the deuterated solvent as the lock and the residual solvent as the internal reference.
3.2. Liquid-Liquid Extraction Experiments
WP6 (250 mg) was dissolved in 50 mL neat water to get a 3.0 mM WP6 aqueous solution. Then, 50 mL Tol/MCH mixtures (1:1, v/v) were added into the above solution. After stirring for 30 min, the water phase was separated from the benzene isomers phase. The emulsifying benzene isomers, which were liquid in the water phase, could be removed by centrifugation and filtration. The water phase was extracted by an organic solvent, CDCl3, for the 1H NMR experiment [55].
4. Conclusions
In conclusion, we demonstrated that water-soluble pillararenes could be used as rational separation adsorbents for low-molecular-weight organics. The carboxylate groups in the backbones of macrocycles provide them with good water solubility and the presence of hydrophobic intrinsic cavities allow them to selectively adsorb and separate specific organics. Although all of the pillararenes considered have a certain degree of adsorption capacity, the size of the cavity is the main factor that determines the separation performance. Although there have been previous reports of using pillar[6]arene to separate organic matters in the solid crystal state, the current work is the first time that water-soluble WP6 was used to separate and purify organics in the aqueous phase by liquid–liquid extraction. This not only expands the application scope of pillar[6]arene, but also provides a reference value for the application of other macrocyclic arenes in the future. In addition, the water-soluble anionic pillararenes not only have good separation properties, but also exhibit excellent stability and cycling performance. Therefore, this work creates more possibilities for the construction of high-performance adsorbents in organics separation and water purification, which is of great significance for energy conservation and environmental protection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238554/s1, Figures S1–S11: Characterization of four water-soluble anionic pillararenes; Figures S12–S15: 1H NMR spectra of the extraction phase.
Author Contributions
Conceptualization, W.W.; Methodology, W.W. and J.Y.; Validation, W.W., Z.L. and C.S.; Formal Analysis, W.W. and Z.L.; Supervision, Y.Y; Writing—original Draft Preparation, W.W. and Z.L.; Writing—review and Editing, Y.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
This research was funded by the National Natural Science Foundation of China (21871108).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zubris K.A.V., Richards B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005;138:201–211. doi: 10.1016/j.envpol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Garcia M.T., Campos E., Marsal A., Ribosa I. Fate and effects of amphoteric surfactants in the aquatic environment. Environ. Int. 2008;34:1001–1005. doi: 10.1016/j.envint.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Lochaiwatana Y., Poolthong S., Hirata I., Okazaki M., Swasdison S., Vongsavan N. The synthesis and characterization of a novel potassium chloride-fluoridated hydroxyapatite varnish for treating dentin hypersensitivity. Dent. Mater. J. 2015;34:31–40. doi: 10.4012/dmj.2014-102. [DOI] [PubMed] [Google Scholar]
- 4.Patel R.N. Biocatalysis: Synthesis of key intermediates for development of pharmaceuticals. ACS Catal. 2011;1:1056–1074. doi: 10.1021/cs200219b. [DOI] [Google Scholar]
- 5.Patel R.N. Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord. Chem. Rev. 2008;252:659–701. doi: 10.1016/j.ccr.2007.10.031. [DOI] [Google Scholar]
- 6.Jie K.-C., Liu M., Zhou Y.-J., Little M.A., Pulido A., Chong S.Y., Stephenson A., Hughes A.R., Sakakibara F., Ogoshi T., et al. Near-ideal xylene selectivity in adaptive molecular pillar[n]arene crystals. J. Am. Chem. Soc. 2018;140:6921–6930. doi: 10.1021/jacs.8b02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sajisha V.S., Maitra U. Remarkable isomer-selective gelation of aromatic solvents by a polymorph of a urea-linked bile acid-amino acid conjugate. RSC Adv. 2014;4:43167–43171. doi: 10.1039/C4RA08957J. [DOI] [Google Scholar]
- 8.Abdallah D.J., Weiss R.G. n-alkanes gel n-alkanes (and many other organic liquids) Langmuir. 2000;16:352–355. doi: 10.1021/la990795r. [DOI] [Google Scholar]
- 9.Cai W.-Q., Li H.-Q., Yi Z. Selective oxidation of ethylhenzene to styrene with carbon dioxide. Prog. Chem. 2004;16:406–413. [Google Scholar]
- 10.Villaluenga J.P.G., Tabe-Mohammadi A. A review on the separation of benzene/cyclohexane mixtures by pervaporation processes. J. Membr. Sci. 2000;169:159–174. doi: 10.1016/S0376-7388(99)00337-3. [DOI] [Google Scholar]
- 11.Agrawal R., Gooty R.T. Misconceptions about efficiency and maturity of distillation. AlChE J. 2020;66:e16294. doi: 10.1002/aic.16294. [DOI] [Google Scholar]
- 12.Kawasaki J., Kosuge H., Habaki H., Morita Y. Separation of taxane compounds by liquid-liquid extraction. Chem. Eng. Commun. 2008;195:644–660. doi: 10.1080/00986440701555456. [DOI] [Google Scholar]
- 13.Pramanik B.K., Pramanik S.K., Suja F. A comparative study of coagulation, granular- and powdered-activated carbon for the removal of perfluorooctane sulfonate and perfluorooctanoate in drinking water treatment. Environ. Technol. 2015;36:2610–2617. doi: 10.1080/09593330.2015.1040079. [DOI] [PubMed] [Google Scholar]
- 14.Putra E.K., Pranowo R., Sunarso J., Indraswati N., Ismadji S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009;43:2419–2430. doi: 10.1016/j.watres.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Barlokova D., Ilavsky J. Natural zeolites with a surface MnO2 layer in water treatment. Chem. Listy. 2014;108:1153–1157. [Google Scholar]
- 16.Chew T.-L., Ahmad A.L., Bhatia S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of carbon dioxide (CO2) Adv. Colloid Interface Sci. 2010;153:43–57. doi: 10.1016/j.cis.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Chen T., Wang G., Hu W.-B., Liu Y.H.A., Yang H., Wen K. A pillar[5]arene conjugated polymer for removal of low-molecular-weight organic acids, amines, and alcohols from water. ACS Appl. Polym. Mater. 2020;2:5566–5573. doi: 10.1021/acsapm.0c00896. [DOI] [Google Scholar]
- 18.Xue M., Yang Y., Chi X.-D., Zhang Z.-B., Huang F.-H. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 2012;45:1294–1308. doi: 10.1021/ar2003418. [DOI] [PubMed] [Google Scholar]
- 19.Del Valle E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004;39:1033–1046. doi: 10.1016/S0032-9592(03)00258-9. [DOI] [Google Scholar]
- 20.Ikeda A., Shinkai S. Novel cavity design using calix[n]arene skeletons: Toward molecular recognition and metal binding. Chem. Rev. 1997;97:1713–1734. doi: 10.1021/cr960385x. [DOI] [PubMed] [Google Scholar]
- 21.Lou X.-Y., Song N., Yang Y.-W. Fluorescence resonance energy transfer systems in supramolecular macrocyclic chemistry. Molecules. 2017;22:1640. doi: 10.3390/molecules22101640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y.-W., Sun Y.-L., Song N. Switchable host-guest systems on surfaces. Acc. Chem. Res. 2014;47:1950–1960. doi: 10.1021/ar500022f. [DOI] [PubMed] [Google Scholar]
- 23.Cao D.-R., Kou Y.-H., Liang J.-Q., Chen Z.-Z., Wang L.-Y., Meier H. A facile and efficient preparation of pillararenes and a pillarquinone. Angew. Chem. Int. Ed. 2009;48:9721–9723. doi: 10.1002/anie.200904765. [DOI] [PubMed] [Google Scholar]
- 24.Qu D.-H., Wang Q.-C., Zhang Q.-W., Ma X., Tian H. Photoresponsive host-guest functional systems. Chem. Rev. 2015;115:7543–7588. doi: 10.1021/cr5006342. [DOI] [PubMed] [Google Scholar]
- 25.Dai D.-H., Yang J., Zou Y.-C., Wu J.-R., Tan L.-L., Wang Y., Li B., Lu T., Wang B., Yang Y.-W. Macrocyclic arenes-based conjugated macrocycle polymers for highly selective CO2 capture and iodine adsorption. Angew. Chem. Int. Ed. 2021;60:8967–8975. doi: 10.1002/anie.202015162. [DOI] [PubMed] [Google Scholar]
- 26.Li M.-H., Lou X.-Y., Yang Y.-W. Pillararene-based molecular-scale porous materials. Chem. Commun. 2021;57:13429–13447. doi: 10.1039/D1CC06105D. [DOI] [PubMed] [Google Scholar]
- 27.Kursunlu A.N., Acikbas Y., Ozmen M., Erdogan M., Capan R. Haloalkanes and aromatic hydrocarbons sensing using langmuir-blodgett thin film of pillar[5]arene-biphenylcarboxylic acid. Colloid Surf. A. 2019;565:108–117. doi: 10.1016/j.colsurfa.2018.12.050. [DOI] [Google Scholar]
- 28.Bastug E., Kursunlu A.N., Guler E. A fluorescent clever macrocycle: Deca-bodipy bearing a pillar[5]arene and its selective binding of asparagine in half -aqueous medium. J. Lumin. 2020;225:117343–117348. doi: 10.1016/j.jlumin.2020.117343. [DOI] [Google Scholar]
- 29.Wu M.-X., Gao J., Wang F., Yang J., Song N., Jin X.-Y., Mi P., Tian J., Luo J.-Y., Liang F., et al. Multistimuli responsive core-shell nanoplatform constructed from Fe3O4@MOF equipped with Pillar[6]arene nanovalves. Small. 2018;14:1704440–1704446. doi: 10.1002/smll.201704440. [DOI] [PubMed] [Google Scholar]
- 30.Chi X.-D., Ji X.-F., Xia D.-Y., Huang F.-H. A dual-responsive supra-amphiphilic polypseudorotaxane constructed from a water-soluble pillar[7]arene and an azobenzene-containing random copolymer. J. Am. Chem. Soc. 2015;137:1440–1443. doi: 10.1021/ja512978n. [DOI] [PubMed] [Google Scholar]
- 31.Chai Y., Chen L.-M., Zhang Y.-H., Zhao L., Meng Z., Chen J.-Y., Li C.-J., Meng Q.-B. Reversing neuromuscular blocking agent decamethonium by carboxylatopillar[6]arene based on host-guest encapsulation. Chin. Chem. Lett. 2022;33:3003–3006. doi: 10.1016/j.cclet.2021.11.087. [DOI] [Google Scholar]
- 32.Zhang H., Wu J.-R., Wang X., Li X.-S., Wu M.-X., Liang F., Yang Y.-W. One-pot solvothermal synthesis of carboxylatopillar[5]arene-modified Fe3O4 magnetic nanoparticles for ultrafast separation of cationic dyes. Dyes Pigment. 2019;162:512–516. doi: 10.1016/j.dyepig.2018.10.061. [DOI] [Google Scholar]
- 33.Cai Y., Zhang Z.-C., Ding Y., Hu L.-P., Wang J., Chen T.-T., Yao Y. Recent development of pillar[n]arene-based amphiphiles. Chin. Chem. Lett. 2021;32:1267–1279. doi: 10.1016/j.cclet.2020.10.036. [DOI] [Google Scholar]
- 34.Liu S.-Y., Yan T.-S., Wu Q.-X., Xu Z., Han J. Accelerating the thermal fading rate of photochromic naphthopyrans by pillar[5]arene-based conjugated macrocycle polymer. Chin. Chem. Lett. 2022;33:239–242. doi: 10.1016/j.cclet.2021.07.023. [DOI] [Google Scholar]
- 35.Wang D.-H., Wang J., Wang Y., Yang Y.-W. A fluorescent linear conjugated polymer constructed from pillararene and anthracene. Molecules. 2022;27:3162. doi: 10.3390/molecules27103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y.-F., Lou X.-Y., Wang C.-Y., Wang Y., Jia Y., Lin Q., Yang Y.-W. Synthesis of stimuli-responsive pillararene-based supramolecular polymer materials for the detection and separation of metal ions. Chin. Chem. Lett. 2022:107877–107894. doi: 10.1016/j.cclet.2022.107877. [DOI] [Google Scholar]
- 37.Zhang H., Huang K.-T., Ding L., Yang J., Yang Y.-W., Liang F. Electrochemical determination of paraquat using a glassy carbon electrode decorated with pillararene-coated nitrogen-doped carbon dots. Chin. Chem. Lett. 2022;33:1537–1540. doi: 10.1016/j.cclet.2021.09.002. [DOI] [Google Scholar]
- 38.Wang W.-M., Dai D., Wu J.-R., Wang C., Wang Y., Yang Y.-W. Renewable supramolecular assembly-induced emission enhancement system for efficient detection and removal of silver(I) Dye. Pigment. 2022;207:110712. doi: 10.1016/j.dyepig.2022.110712. [DOI] [Google Scholar]
- 39.Li Z., Yang Z., Zhang Y., Yang B., Yang Y.-W. An acidochromic and nitroaromatic responsive hydrazone-linked pillararene framework using a macrocycle-to-framework strategy. Angew. Chem. Int. Ed. 2022;61:e202206144. doi: 10.1002/anie.202206144. [DOI] [PubMed] [Google Scholar]
- 40.Wu J.-R., Mu A.U., Li B., Wang C.-Y., Fang L., Yang Y.-W. Desymmetrized leaning pillar[6]arene. Angew. Chem. Int. Ed. 2018;57:9853–9858. doi: 10.1002/anie.201805980. [DOI] [PubMed] [Google Scholar]
- 41.Dai D.-H., Li Z., Yang J., Wang C.-Y., Wu J.-R., Wang Y., Zhang D.-M., Yang Y.-W. Supramolecular assembly-induced emission enhancement for efficient mercury(II) detection and removal. J. Am. Chem. Soc. 2019;141:4756–4763. doi: 10.1021/jacs.9b01546. [DOI] [PubMed] [Google Scholar]
- 42.Wu J.-R., Wu G.-X., Yang Y.-W. Pillararene-inspired macrocycles: From extended pillar[n]arenes to geminiarenes. Acc. Chem. Res. 2022;55:3191–3204. doi: 10.1021/acs.accounts.2c00555. [DOI] [PubMed] [Google Scholar]
- 43.Wu J.-R., Wu G.-X., Cai Z., Li D.-X., Li M.-H., Wang Y., Yang Y.-W. A water-soluble leggero pillar[5]arene. Molecules. 2022;27:6259. doi: 10.3390/molecules27196259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boinski T., Cieszkowski A., Rosa B., Szumna A. Hybrid[n]arenes through thermodynamically driven macrocyclization reactions. J. Org. Chem. 2015;80:3488–3495. doi: 10.1021/acs.joc.5b00099. [DOI] [PubMed] [Google Scholar]
- 45.Wang K.-Y., Jordan J.-H., Velmurugan K., Tian X.-Q., Zuo M.-Z., Hu X.-Y., Wang L.-Y. Role of functionalized pillararene architectures in supramolecular catalysis. Angew. Chem. Int. Ed. 2021;60:9205–9214. doi: 10.1002/anie.202010150. [DOI] [PubMed] [Google Scholar]
- 46.Kato K., Fa S., Ohtani S., Shi T.-H., Brouwer A.M., Ogoshi T. Noncovalently bound and mechanically interlocked systems using pillar[n]arenes. Chem. Soc. Rev. 2022;51:3648–3687. doi: 10.1039/D2CS00169A. [DOI] [PubMed] [Google Scholar]
- 47.Tian M.-M., Chen D.-X., Sun Y.-L., Yang Y.-W., Jia Q. Pillararene-functionalized Fe3O4 nanoparticles as magnetic solid-phase extraction adsorbent for pesticide residue analysis in beverage samples. RSC Adv. 2013;3:22111–22119. doi: 10.1039/c3ra43752c. [DOI] [Google Scholar]
- 48.Liu Z.-J., Wu J.-R., Wang C.-Y., Yang J., Wang Y., Yang Y.-W. Stimuli-responsive fluorescent supramolecular polymer network based on a monofunctionalized leaning tower[6]arene. Chin. Chem. Lett. 2019;30:2299–2303. doi: 10.1016/j.cclet.2019.10.023. [DOI] [Google Scholar]
- 49.Zhu H.-T.-Z., Li Q., Khalil-Cruz L.E., Khashab N.M., Yu G.C., Huang F.-H. Pillararene-based supramolecular systems for theranostics and bioapplications. Sci. China Chem. 2021;64:688–700. doi: 10.1007/s11426-020-9932-9. [DOI] [Google Scholar]
- 50.Yang J., Dai D.-H., Ma L.-J., Yang Y.-W. Molecular-scale drug delivery systems loaded with oxaliplatin for supramolecular chemotherapy. Chin. Chem. Lett. 2021;32:729–734. doi: 10.1016/j.cclet.2020.08.035. [DOI] [Google Scholar]
- 51.Wang X., Ji K.Y., Rockenbauer A., Liu Y.-P., Song Y.-G. Host-guest interaction of nitroxide radicals with water-soluble pillar[6]arenes. Org. Biomol. Chem. 2020;18:2321–2325. doi: 10.1039/D0OB00341G. [DOI] [PubMed] [Google Scholar]
- 52.Ogoshi T., Hashizume M., Yamagishi T.A., Nakamoto Y. Synthesis, conformational and host-guest properties of water-soluble pillar[5]arene. Chem. Commun. 2010;46:3708–3710. doi: 10.1039/c0cc00348d. [DOI] [PubMed] [Google Scholar]
- 53.Yu G.-C., Xue M., Zhang Z.-B., Li J.-Y., Han C.-Y., Huang F.-H. A water-soluble pillar[6]arene: Synthesis, host-guest chemistry, and its application in dispersion of multiwalled carbon nanotubes in water. J. Am. Chem. Soc. 2012;134:13248–13251. doi: 10.1021/ja306399f. [DOI] [PubMed] [Google Scholar]
- 54.Uemasu I., Kushiyama S. Selective separation of benzene from hydrocarbon mixtures via liquid-liquid extraction method using aqueous solutions of substituted cyclodextrins. Fuel Process. Technol. 2004;85:1519–1526. doi: 10.1016/j.fuproc.2003.10.003. [DOI] [Google Scholar]
- 55.Zhang G.-W., Moosa B., Chen A.-P., Khashab N.-M. Separation and detection of meta-andortho-substituted benzene isomers by using a water-soluble pillar[5]arene. Chempluschem. 2020;85:1244–1248. doi: 10.1002/cplu.202000275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.