Abstract
In general there is a poor correlation between serum lipopolysaccharide (LPS; the biologically active constituent of endotoxin) levels and mortality in septic patients. The objective of this study was to determine if chemical, structural, or biological differences among LPS from different clinical isolates of gram-negative bacteria might explain this discrepancy. LPS preparations were made using the hot phenol-water extraction method from eight clinical isolates of gram-negative bacteria. As a percentage of the total weight of the LPS, the phosphate content ranged from 3.0 to 13.8% (average, 6.7 ± 3.6%), and the 2-keto-3-deoxyoctonate content ranged from 1.9 to 27.4% (average, 8.9 ± 8.5%). These values were not dissimilar to those obtained for a reference endotoxin. In a standard measure of LPS activity, the Limulus amoebocyte lysate assay, there was approximately a twofold difference between the least and most active preparations. The two preparations with the greatest difference in their ability to elicit the secretion of tumor necrosis factor alpha from a mouse peritoneal macrophage cell line were similar in lethality when administered to mice sensitized to the effects of LPS by d(+)-galactosamine. These relatively minor differences in LPS activity seem unlikely to explain the generally observed discrepancy between serum endotoxin levels and mortality in patients with gram-negative sepsis.
It is currently estimated that 500,000 cases of sepsis occur in the United States each year. Half of these are caused by gram-negative rods, and septic shock occurs in 50 to 60% of these cases (reviewed in reference 26). The mortality rate remains high despite the use of intensive care units and effective, broad-spectrum antibiotics. Bacterial lipopolysaccharide (LPS; the biologically active constituent of endotoxin) from gram-negative organisms is now well recognized to be a potent microbial toxin that has been postulated to play a critical role in the initiation of the proinflammatory events that contribute to the pathogenesis of this disease. The pathophysiologic mechanism(s) responsible for this illness are thought to result from the noncytotoxic interaction of LPS with host inflammatory mediator cells following its release from the bacterial outer cell membrane, resulting in the production of multiple proinflammatory cytokines, reactive oxygen and nitrogen intermediates, and bioactive lipids. The resultant systemic inflammatory response is thought to lead to multiorgan failure and often death (reviewed in references 14, 26, and 32).
A significant component of the experimental evidence supporting a relationship between endotoxin and sepsis derives from studies in which the administration of purified endotoxic LPS to experimental animals or human volunteers reproduces many of the clinical symptoms seen in patients with gram-negative sepsis. More recently, correlative data from in vitro, ex vivo, and in vivo studies using viable intact gram-negative microorganisms all provide strong indications that the production of cytokines depends, to at least some extent, on the degree to which various antibiotics induce the release of LPS from gram-negative bacteria (3, 5, 6, 15, 29). Furthermore, the amount of LPS released is now recognized to depend, at least in part, on the differential binding of various β-lactam antibiotics to selective penicillin-binding proteins (3, 15). Attempts to correlate circulating levels of LPS and/or cytokine levels with observed mortality in patients with gram-negative sepsis, however, have to date failed to provide an overall convincing case for cause and effect (4, 9, 13, 19, 20, 30). This apparent discrepancy may well be the result of an unappreciated variability in the biological activity and/or chemical structure of the LPS present in clinical bacterial isolates, as well as of differences in its release caused by different kinds of antibiotic treatment. Under such circumstances, it would be virtually impossible to differentiate the pathophysiologic potential of a given endotoxin level detected in the circulation with a projected outcome of a systemic inflammatory episode.
The studies presented here have been designed to test the hypothesis that LPS derived from clinical bacterial isolates is not uniform in its biological potential to induce a proinflammatory response in vitro. This hypothesis contrasts with the generally accepted underlying assumption made in most studies that LPS derived from different microbiological sources is of equal potency in biological systems. There exists at least some evidence to support a diversity of LPS biological activity, based upon observations that LPS activity can vary substantially depending upon both the type of organism and the method by which the LPS is extracted (23), the presence of outer membrane proteins in association with the LPS (22), the specific subunit composition of the LPS (i.e., presence of O-antigen subunits) (33), and the presence of O-antigen carbohydrates that are linked to the LPS (24). To date, these findings have not been extended to the full range of microorganisms usually encountered in clinical gram-negative bacterial sepsis.
An understanding of the range of biological diversity among the LPS of clinical isolates of gram-negative bacterial is clearly a necessary prerequisite to rational interpretation of the results of clinical endotoxin-cytokine assays in septic patients. To address these issues, we have undertaken a comprehensive series of studies in which LPS has been extracted and purified from a variety of clinical isolates of gram-negative bacteria. These various LPS have then been compared to one another in a variety of standard in vitro and in vivo assays that have traditionally been utilized to assess LPS biological activity. Qualitative structural analyses were carried out using silver staining after fractionation of LPS subunits using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Biological activity in vitro was assessed by the quantitative Limulus amoebocyte lysate assay and by the ability of the LPS preparations to initiate the secretion of tumor necrosis factor alpha (TNF-α) from a macrophage-like cell line. The in vivo potency of LPS was assessed using a sensitive mouse model of lethality.
MATERIALS AND METHODS
Clinical isolates of gram-negative microorganisms.
Eight clinical isolates of gram-negative microorganisms representing four major genera commonly encountered in gram-negative infections (7, 8, 25) were obtained from the clinical pathology laboratory at the University of Kansas Medical Center. These included two blood isolates each of Citrobacter diversus, Escherichia coli, and Serratia marcescens and one sputum and one urine isolate of Proteus mirabilis. All cultures were grown in Trypticase soy broth to late log phase, harvested by centrifugation, and washed several times in sterile saline, and the LPS was extracted as described below. (Three isolates of Klebsiella sp. were also selected. LPS preparations from these isolates were analyzed by SDS-PAGE and silver staining as described below, but results of other analyses for the Klebsiella LPS preparations are not included here because of a concern of substantial contamination of the Klebsiella LPS preparations with capsular polysaccharide.)
Extraction and purification of LPS.
The standard hot phenol-water extraction method described by Westphal and Luderitz (34, 35) was used to extract LPS from approximately 5 to 10 g (wet weight) of bacteria. The late-log-phase bacteria were sedimented by centrifugation and then suspended in pyrogen-free distilled water to a concentration of approximately 1010 CFU/ml. Bacteria were extracted two times with 90% aqueous phenol at 68°C, and the combined aqueous extracts were then dialyzed extensively against double-distilled H2O at 4°C and lyophylized. Nucleic acids were removed by reconstitution of the LPS-enriched extracts to 10 mg/ml in 0.1 M acetate buffer with 0.02% MgSO4 and 0.4% chloroform and digestion with RNase (0.4 mg/ml; Sigma Chemical, St. Louis, Mo.) and DNase (20 μg/ml; Sigma) by incubation at 37°C overnight. Contaminating protein was then removed by the addition of proteinase K (20 μg/ml; Sigma) in 0.1 M Tris (pH 8.0), followed by heating at 60°C for 1 h and then incubation overnight at 37°C. Enzyme-digested LPS samples were then dialyzed extensively against multiple changes of deionized H2O. The resulting purified LPS was lyophilized, dried over P2O5 (Sigma), reconstituted in pyrogen-free distilled water at 1.0 mg/ml, and stored in aliquots at −70°C. LPS from E. coli O111:B4 prepared by phenol-water extraction was obtained from List Biologicals (Campbell, Calif.). “Rough” or R-chemotype LPS from Neisseria meningitidis 6275 was kindly provided by C. M. Tsai, Food and Drug Administration, Rockville, Md.
Analytical procedures.
Studies to characterize common chemical constituents of the core oligosaccharide-lipid A were carried out by using standard analytical procedures. Phosphate content was determined by the colorimetric method of Ames and Dubin (1); 2-keto-3-deoxyoctonate (KDO) was determined according to the thiobarbituric acid method of Karkhanis et al. (17). Protein contamination was estimated by using a Coomassie Protein Reagent Kit (Pierce, Rockford, Ill.).
SDS-PAGE and silver staining of LPS.
SDS-PAGE and LPS silver staining were performed as described by Tsai and Frasch (31). A modified Laemmli SDS-PAGE system was used with the Mini-Protean II gel apparatus (Bio-Rad, Hercules, Calif.) and 1.0-mm-thick gels.
LAL assays.
Limulus amoebocyte lysate (LAL) assays were carried out using a chromogenic assay (the QCL 1000 Kit purchased from BioWhittaker, Walkersville, Md.). The kit included vials of standard LPS (E. coli O111:B4) containing a defined number of endotoxin units (EU). The standard was reconstituted in pyrogen-free distilled H2O. A standard curve ranging from 0 to 1.0 EU/ml was constructed for each assay by plotting the optical density at 410 nm (OD410) versus EU per milliliter. Sample LPS preparations were assayed over a range of 0.01 to 0.1 ng/ml, and concentration was plotted against OD410. From the standard curve, the OD410 corresponding to 0.5 EU/ml was determined. This value was substituted into the formula for the line describing the sample LPS to determine the concentration (in nanograms per milliliter) of sample LPS corresponding to 0.5 EU/ml. The EU/nanogram values for each LPS were calculated from these data. Each LPS was assayed three times. Standards and samples were analyzed in duplicate, and absorption was measured at 410 nm by using a Dynatech 5000 microplate reader.
Secretion of TNF-α from LPS-stimulated mouse macrophages.
J774A.1 cells, a mouse macrophage cell-like line (American Type Culture Collection, Rockville, Md.) were cultured in canted neck flasks in RPMI 1640 medium (Gibco, Grand Island, N.Y.) supplemented with 4 mM glutamine (Sigma), penicillin (100 U/ml; Sigma), and streptomycin (100 μg/ml; Sigma) and 10% heat-inactivated (56°C, 30 min) fetal bovine serum (endotoxin content of <0.06 ng/ml; Sigma) in a humidified incubator at 37°C with 5% CO2. Adherent cells were removed with a rubber policeman and used to make a suspension of 105 cells/ml in RPMI. Then, 0.5-ml aliquots were distributed to the wells of a 48-well tissue culture plate and allowed to adhere overnight. The cells were then stimulated with the purified LPS from the various clinical isolates or the standard E. coli O111:B4 LPS (range, 0.5 to 20 ng/ml) for 15 h. Mouse TNF-α was measured in the supernatants by enzyme immunoassay (Genzyme, Cambridge, Mass.).
Mouse lethality studies.
CF-1 outbred female mice, 8 to 10 weeks of age, were purchased from Sasco (Kingston, N.Y.) and were maintained in the University of Kansas Medical Center AALAC-accredited animal facility for 5 to 10 days before use. Mice were sensitized to the effects of LPS by the intraperitoneal injection of d-galactosamine (Sigma) (30 mg in 0.4 ml of pyrogen-free H2O per 25-g mouse) with or without LPS exactly according to the method of Galanos et al. (10, 11). Mortality was recorded 24 h after injection. The 50% lethal dose (LD50) was determined by the method of Reed and Muench (27).
RESULTS
Extraction and chemical characterization of LPS from clinical isolates of gram-negative organisms.
LPS was extracted from eight clinical isolates of gram-negative bacteria using the standard hot phenol method of Westphal et al. (34, 35). Yields of between 10 and 20 mg of LPS were obtained from about 5 g (wet weight) of bacterial pellets. Commercially prepared LPS from E. coli O111:B4 was included in these studies as a reference standard.
To assess the purity of the LPS preparations, experiments were carried out to detect contaminating nucleic acids and protein. Protein contamination was <2% for all but two of the LPS preparations (Table 1). A determination of the UV spectrum of each LPS preparation indicated the lack of a significant absorption peak at 260 nm, a finding consistent with the LPS preparations at 1.0 mg/ml having less than 1% nucleic acid by weight. Thus, we conclude that the LPS preparations were relatively pure.
TABLE 1.
Phosphate, KDO, and protein contents of various LPS preparationsa
| LPS prepn | % Phosphate | % KDO | % Protein |
|---|---|---|---|
| C. diversus | |||
| 1 | 10.4 (0.6) | 27.4 (1.7) | <2.1 |
| 2 | 8.4 (0.1) | 7.6 (2.4) | <2.1 |
| E. coli | |||
| O111 | 3.0 (1.4) | 19.0 (2.0) | <2.1 |
| 1 | 6.1 (2.7) | 4.5 (0.14) | <2.1 |
| 2 | 13.8 (0.7) | 5.6 (2.4) | <2.1 |
| P. mirabilis | |||
| 1 | 6.9 (0.18) | 4.6 (0.44) | 6.2 |
| 2 | 4.7 (0.78) | 4.2 (0.59) | 3.5 |
| S. marcescens | |||
| 1 | 3.2 (1.3) | 1.9 (0.6) | <2.1 |
| 2 | 4.0 (0.14) | 5.7 (0.4) | <2.1 |
Values in parentheses represent the standard deviations.
Our initial efforts were designed to determine the relative amounts of common chemical constituents usually found in the inner core and lipid A region of the LPS macromolecule. Phosphate is a constituent of lipid A (substituting the 1 and 4′ positions of the diglucosamine backbone). It is also associated with heptose and KDO, which link lipid A to the core oligosaccharide. Thus, phosphate serves as a measure of the relative amount of conserved LPS structures relative to the more diverse O antigen. Phosphate was quantitated by the ammonium molybdate assay, and KDO was quantitated by measuring thiobarbituric acid as described in Materials and Methods. The results of these assays are summarized in Table 1. As shown by these data, the phosphate content ranged, as a percentage of the total weight of the LPS, from 3.0 to 13.8% (average, 6.7 ± 3.6%), and the KDO content ranged from 1.9 to 27.4% (average, 8.9 ± 8.5%). These average values are not dissimilar from those obtained for a reference endotoxin (phosphate, 4.1%; KDO, 3.9%) (28).
Characterization of LPS subunit composition.
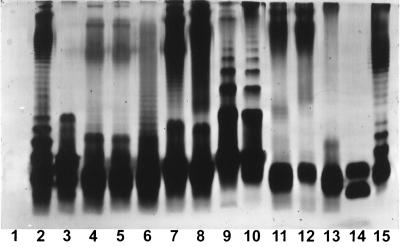
It is well recognized that purified LPS from most strains of gram-negative bacteria is heterogeneous with respect to the composition of individual LPS subunits that comprise the LPS macromolecule. Evidence for heterogeneity is readily detected by analysis by using SDS-PAGE, followed by silver staining to detect individual LPS subunits. The silver-staining profiles of the various LPS subunit patterns from the E. coli standard and the clinical isolates are shown in Fig. 1. Of interest, the majority of the LPS manifest the typical ladder-like pattern characteristic of S-LPS. However, two isolates of Klebsiella pneumoniae (lanes 12 and 13) have silver-staining patterns that strongly suggest an absence of significant amounts of O antigen-containing LPS subunits. Therefore, these would be designated as chemically incomplete or rough (R) forms of LPS. Even within species, it is clear that differences in the actual subunit patterns can be readily detected (e.g., compare lanes 2 and 3 and lanes 5 and 6). Thus, we conclude that, from a physical-chemical perspective, the LPS preparations, while similar, manifest unique differences from one another.
FIG. 1.
Silver stain of LPS fractionated over SDS-PAGE. Lane 1 is blank, and lanes 2 to 13 contain 3 μg of LPS. Lane 2, E. coli 1; lane 3, E. coli 2; lane 4, C. diversus 2; lane 5, C. diversus 2 prepared on a separate occasion; lane 6, C. diversus 1; lane 7, S. marcescens 2; lane 8, S. marcescens 1; lane 9, P. mirabilis 1; lane 10, P. mirabilis 2; lanes 11 to 13, LPS from three different isolates of K. pneumoniae; lane 14, 200 ng of “rough” (R) chemotype LPS from N. meningitidis from strain 6275; lane 15, 3 μg of LPS from E. coli O111:B4.
Activity of LPS preparations in the LAL assay.
The LAL assay is recognized as one of the most sensitive detection systems for determining the presence of LPS, and it is generally accepted as the method of choice for determining the presence of LPS in parenterally administered drugs and of endotoxin in body fluids. Since this assay has been used to establish correlations with outcome in cases of sepsis, it was felt to be important to use this measure of activity to assess the relative potency of the different LPS preparations.
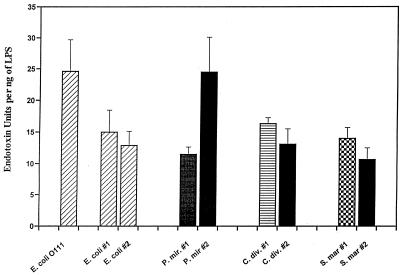
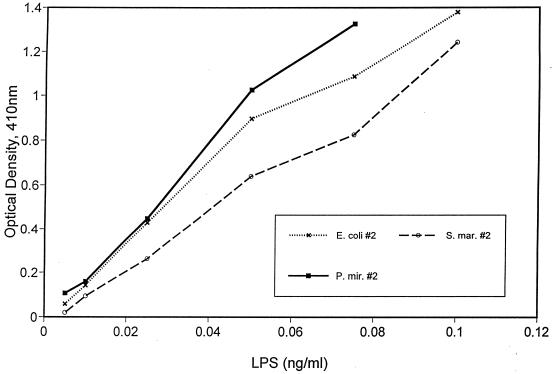
The LAL activity of the various sample LPS preparations was determined in chromogenic LAL assays in comparison to a known standard. The standard LPS was provided by the LAL supplier and consisted of vials of E. coli O111:B4 LPS containing a defined number of EU. Sample LPS preparations were compared to the standard curve obtained with LPS from E. coli O111:B4, and the data are expressed in EU per nanograms of sample LPS as explained in Materials and Methods. Investigators experienced with this assay recognize the considerable variation inherent to it. This was our experience as well. Although the technical performance of the assay was excellent, as suggested by the R-square values for the typical standard curves used in these assays (average R square value, 0.99; range, 0.96 to 1.0 [± 0.015]), there was considerable variation in the slope of the standard curve from one assay to the other (average slope, 1.0; range, 0.69 to 1.4 [± 0.32]). For a value of 0.5 OD units, the corresponding standard curve values of EU per milliliter would vary by as much as 1.7-fold. For each sample LPS, the average fold difference among the three determinations was 1.8 (range, 1.2 to 2.4), and this is reflected in the standard error (see below and Fig. 3). Thus, we conclude that there may be an approximately twofold variation in LAL activity for a given LPS preparation from one assay to another. Figure 2 shows typical dose-response curves obtained for three LPS preparations. These dose-activity profiles indicate that the assay is linear over the range of LPS concentrations studied. Figure 3 shows a summary of the results for each LPS preparation (three determinations per preparation). LPS from S. marcescens 2 had the lowest LAL activity (10.7 EU/ng [±1.9]), and E. coli O111:B4 had the highest (24.6 EU/ng [±5.1]). This represents a 2.3-fold difference between the least and most potent LPS preparations as determined in this assay. The average values by genus were: E. coli, 17.5 EU/ng (±6.2); P. mirabilis, 18.0 EU/ng (±9.2); C. diversus, 14.8 EU/ng (±2.3); and S. marcescens, 12.3 EU/ng (±2.4). Given the variability of the LAL assay as described above, these differences do not constitute a measurable degree of biologic variability.
FIG. 3.
Activity of different LPS in LAL assay by individual organism. Error bars indicate the standard error. P. mir., P. mirabilis; S. mar., S. marcescens.
FIG. 2.
Dose-response curves of four different LPS preparations in the Limulus assay. P. mir., P. mirabilis; S. mar., S. marcescens.
Secretion of TNF-α from LPS-stimulated mouse macrophages.
In the past decade, a large number of studies have strongly implicated proinflammatory cytokines, in particular, TNF-α, as a potentially important mediator of the septic response during gram-negative infections. We therefore carried out detailed quantitative assessments of LPS-induced TNF-α secretion. As an in vitro measure of their inflammatory potential, we evaluated the ability of selected LPS with different LAL bioactivities to stimulate the release of the inflammatory cytokine, TNF-α, from the mouse macrophage-like J774A.1 cell line, and we compared these results to their potency in the LAL assay (Table 2). As shown by the data in this table, there is clearly a large variability in the amount of TNF-α produced by the different LPS preparations. Of the five LPS preparations assayed for their ability to stimulate the release of TNF-α, E. coli 2 and S. marcescens 1 had the least activity in the LAL assays. However, E. coli 2 was the most effective at releasing TNF-α from J774 cells, and S. marcescens 1 was the least effective. Therefore, we conclude that the ability of an individual LPS preparation to induce the secretion of TNF-α from J774A.1 cells does not necessarily correlate to a precise 1:1 ratio with its potency in the LAL assay.
TABLE 2.
Summary of results for selected LPS preparations
| LPS prepn | EU/ng of LPS | pg of TNF-α/ng of LPSa | pg of TNF-α/EU | LD50b |
|---|---|---|---|---|
| E. coli 2 | 13 | 2.9 × 104 | 2.2 × 103 | 12 |
| S. marcescens 1 | 14 | 1.0 × 104 | 7.5 × 102 | 17 |
| C. diversus 2 | 15 | 1.5 × 104 | 1.0 × 103 | |
| C. diversus 1 | 16 | 1.5 × 104 | 9.2 × 102 | 10 |
| P. mirabilis 2 | 24 | 2.0 × 104 | 8.0 × 102 | 15 |
Values were calculated by dividing the amount of TNF-α produced in the supernatant (pg/ml) by the dose (ng/ml) of LPS used to stimulate J774A.1 cells. These values were taken from the linear portion of the dose-response curve for the individual LPS preparation.
LD50 values were determined by sensitizing mice with d-galactosamine as described in Materials and Methods. For each LPS, two control mice (no LPS) were included. Lethality was determined by using doses of 5, 10, 15, and 20 ng of LPS per 25-g mouse. Five mice were used at each dose. Thus, including controls, a total of 22 mice were used for each LPS preparation.
Determination of LD50 values.
In earlier published experiments to assess the lethal effects of LPS in mice, Galanos et al. (11) have shown that, in contrast to LPS alone, simultaneous intraperitoneal injection of d-galactosamine with LPS renders the mice up to 10,000-fold more sensitive to LPS. This effect is mediated by an increased sensitivity of the mouse to TNF-α elaborated by LPS-stimulated host inflammatory cells in vivo (10). Therefore, this is a useful model for examining the relationship between the in vitro results of LPS-induced TNF-α release from J774A.1 cells and the in vivo effects of LPS on mice. We have included in our analysis four of the five LPS preparations for which TNF-α inducing ability was assayed. As a standard of comparison, LPS from E. coli O111B:4 was determined to have an LD50 value of 11. E. coli 2, with the greatest TNF-α inducing ability, had an LD50 value of 12, while S. marcescens 1, with the least TNF-α inducing ability, had an LD50 value of 17. The LD50 values for LPS from E. coli 2 and P. mirabilis 2 (with, respectively, the least and greatest LAL activity of the five LPS preparations assayed for TNF-α inducing ability) were 12 and 15. These are considered minor differences for this assay. In this small sample, LPS preparations with differing activity in the TNF-α producing and LAL assays have virtually identical toxic effects in mice sensitized to LPS by d-galactosamine.
DISCUSSION
In order to investigate the possibility that differences in the inflammatory properties of LPS from gram-negative bacteria might account for the often observed discrepancy between detected levels of serum LPS in patients with sepsis and clinical outcome, we have tested the hypothesis that LPS from diverse clinical isolates of gram-negative bacteria differ significantly in several standard measures of LPS activity and LPS-induced inflammation. Our results show that there is little evidence to suggest that there are substantial differences among such LPS preparations when extracted from clinical isolates of bacteria using standard chemical methods.
Specifically, we were unable to detect large differences in the activity of the LPS preparations in chromogenic Limulus amoebocyte assays, a standard measure for detecting the presence of LPS in serum. Moreover, the differences determined in the ability of these preparations to elicit the release of TNF-α, thought to be a key mediator of inflammation, from macrophage-like cells did not correlate with the in vivo model of lethality in which mice are sensitized to TNF-α produced in response to LPS. Because the differences among the LPS preparations in the biological parameters studied in these experiments are small, they are not useful in understanding the apparent discrepancy between serum endotoxin levels and mortality.
The modified hot phenol-water extraction method of Luderitz and Westphal (34) worked well in purifying LPS from the clinical isolates as demonstrated by the low level of protein and nucleic acid contamination. In general, the phosphate and KDO contents of the LPS preparations were within the expected range, based on a comparison to other LPS. The silver-staining patterns of the LPS preparations demonstrated the typical ladder-like pattern characteristic of S-LPS. Thus, these preparations were suitable for the studies performed here. However, as a percentage of the total weight, two LPS preparations had KDO compositions that were substantially higher than the rest: C. diversus 1 and the standard LPS, E. coli O111:B4 (27.4 and 19.0% compared to a mean of 4.9% for the other six LPS preparations). The reasons for and significance of this finding are not clear. However, it is probable that other deoxy sugars present in the LPS were not adequately corrected for in the chemical analysis; nevertheless, since neither of the LPS preparations with a relatively high KDO content was characterized by especially high or low activity in any of the studies done here, we have not placed more significance upon this finding.
As pointed out above, it is generally accepted that the LAL assay is one of the most sensitive methods for detecting the presence of LPS, and it has therefore been the primary means for detection of LPS in plasma from patients with sepsis. However, it is not known if LPS activity in this assay varies among pathogenic strains of gram-negative bacteria isolated from clinical specimens or if activity in this assay correlates with the ability of LPS to induce inflammation or cause death. Within the limits of reproducibility of the assay, we were unable to detect a measurable difference in LAL activity among the eight LPS preparations studied.
TNF-α is considered a leading mediator of the septic response induced by gram-negative organisms during infection. Therefore, as a measure of their in vitro inflammatory potential, we chose to compare the ability of different LPS preparations to induce the release of TNF-α from a mouse macrophage-like cell line. We did not identify a strong positive 1:1 correlation between activity of individual LPS preparations in the LAL assay and the in vitro TNF-α induction studies. For example P. mirabilis 2 had twice the activity of E. coli 2 in the LAL assay but produced one-third less TNF-α. Subtle relationships between LAL activity and TNF-α inducing ability might be obscured by variations inherent to the LAL assay and TNF-α studies.
CF-1 mice sensitized to LPS with d-galactosamine according to the method of Galanos et al. (10, 11) were used to determine if differences existed in the toxic properties of the different LPS preparations. Of the five LPS preparations tested for TNF-α inducing capacity, those with the largest differences in LAL activity (E. coli 2 versus P. mirabilis 2) manifested only minor differences in LD50. Of these five LPS, the two preparations with the greatest difference in TNF-α inducing ability (E. coli 2 and S. marcescens 1) also demonstrated minor differences compared to each other. Therefore, we were unable to detect meaningful differences in lethality among the LPS preparations with the greatest disparity in both LAL activity and TNF-α inducing ability by using a murine model of LPS lethality. However, these findings are not inconsistent with the results recently published by Amura et al. (2), who reported a lack of correlation between in vivo lethality of LPS and in vitro ability to induce TNF-α production.
Although we were unable to define a difference in the bioactivity or toxicity among the LPS preparations studied in these experiments, it would nevertheless be premature to conclude that differences do not exist among LPS produced by gram-negative bacteria in clinical situations. This is because the LPS prepared for these studies was a chemically extracted and purified preparation virtually devoid of cell-associated membranes, proteins, and nucleic acids. Although this method of preparation has been a powerful tool in the study of LPS, the direct relevance of such preparations to the in vivo setting is not well known. The in vivo release of LPS may occur by a variety of mechanisms, including natural shedding of LPS from “blebs” formed on the surface of bacteria and through the process of bacterial lysis mediated either by humoral factors such as complement and antibody or by exogenous factors such as antibiotics. Indeed, antibiotics that inhibit penicillin-binding protein 3 result in disordered cell wall synthesis, elongation of bacteria into filamentous forms, a substantial increase in cell biomass, and subsequent release of large quantities of LPS from gram-negative bacteria (reviewed in reference 16). The precise composition and structure of antibiotic-released LPS is unknown, but it is clear that relevant questions should include whether other cellular components, such as proteins, lipids, or other cell wall and outer membrane structures might be associated with released LPS and also what role they might play in the inflammatory properties of such LPS preparations. For example, it is well established that isolation of LPS from gram-negative bacteria is often associated with coisolation of microbial outer membrane proteins, usually tightly bound to the LPS molecular complex (21). Further, the demonstrated biological activity of such proteins in stimulating cells of the immune-inflammatory system is well documented (12, 18, 22). Evidence from animal models of infection with gram-negative bacteria indicates that antibiotics which release greater quantities of LPS may result in higher mortality (5). Thus, there would appear to be a number of compelling reasons to explore in more detail the “natural state” of endotoxin generated under simulated situations likely to be operative in clinical sepsis.
Currently, there are few studies comparing the activity of LPS released from bacteria following interaction with antibiotics and LPS chemically extracted using the modified hot phenol-water method. The findings reported here, therefore, demonstrating that there is little variation between purified LPS of clinical isolates from different species, provide a solid foundation upon which such questions can be addressed experimentally. We are actively investigating this question by using the clinical isolates of gram-negative bacteria used for the experiments described here. By incubating these bacteria with different β-lactam antibiotics and isolating the LPS-associated subcellular components released into the growth medium by filtration and centrifugation, we will be able to compare the activity of antibiotic-released LPS with the LPS prepared by hot phenol-water extraction for the experiments reported here.
In summary, we have prepared LPS from clinical isolates of gram-negative bacteria by using the modified hot phenol-water method and characterized them chemically, structurally, and physiologically. There were only small differences among these LPS preparations with respect to their activity in the LAL assay, their ability to elicit the secretions of TNF-α from a macrophage-like cell line, or their toxicity in mice sensitized to the effects of LPS by d-galactosamine. These differences are insufficient to explain the lack of correlation between serum endotoxin levels and mortality in sepsis.
ACKNOWLEDGMENTS
We would like to acknowledge the assistance of S. N. Vogel (Uniformed Services, University of the Health Sciences), A. S. Cross (University of Maryland), and S. M. Opal (Brown University) for guidance in these experiments. C. M. Tsai (Food and Drug Administration, Rockville, Md.) generously allowed us to use his laboratory for the SDS-PAGE and silver-staining procedures. Technical assistance was provided by Paula Worley, Rebecca Hegarty, and Eugene Gregory.
This work was supported by grants from the National Institutes of Health (AI23447, DCM, and an Institutional Development Award; Principal Investigator S. W. Russell, University of Kansas Medical Center) and in part through an unrestricted medical grant from Merck and Co. Inc., West Point, Pa.
REFERENCES
- 1.Ames B N, Dubin D T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- 2.Amura C R, Silverstein R, Morrison D C. TI: mechanisms involved in the pathogenesis of sepsis are not necessarily reflected by in vitro cell activation studies. Infect Immun. 1998;66:5372–5378. doi: 10.1128/iai.66.11.5372-5378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arditi M, Kabat W, Yogev R. Antibiotic-induced bacterial killing stimulates tumor necrosis factor-alpha release in whole blood. J Infect Dis. 1993;167:240–244. doi: 10.1093/infdis/167.1.240. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Mollnes T E, Kierulf P. Complement activation and endotoxin levels in systemic meningococcal disease. J Infect Dis. 1989;160:58–65. doi: 10.1093/infdis/160.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Bucklin S E, Morrison D C. Differences in therapeutic efficacy among cell wall-active antibiotics in a mouse model of gram-negative sepsis. J Infect Dis. 1995;172:1519–1527. doi: 10.1093/infdis/172.6.1519. [DOI] [PubMed] [Google Scholar]
- 6.Dofferhoff A S, Esselink M T, de Vries Hospers H G, van Zanten A, Bom V J, Weits J, Vellenga E. The release of endotoxin from antibiotic-treated Escherichia coli and the production of tumour necrosis factor by human monocytes. J Antimicrob Chemother. 1993;31:373–384. doi: 10.1093/jac/31.3.373. [DOI] [PubMed] [Google Scholar]
- 7.Doran T I. The role of citrobacter in clinical disease of children: review. Clin Infect Dis. 1999;28:384–394. doi: 10.1086/515106. [DOI] [PubMed] [Google Scholar]
- 8.Edmond M B, Wallace S E, McClish D K, Pfaller M A, Jones R N, Wenzel R P. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 9.Elin R J, Robinson R A, Levine A S, Wolff S M. Lack of clinical usefulness of the limulus test in the diagnosis of endotoxemia. N Engl J Med. 1975;293:521–524. doi: 10.1056/NEJM197509112931102. [DOI] [PubMed] [Google Scholar]
- 10.Galanos C, Freudenberg M A, Katschinski T, Salomao R, Mossmann H, Kumazawa Y. Tumor necrosis factor and host response to endotoxin. In: Ryan J L, Morrison D C, editors. Bacterial endotoxic lipopolysaccharides: immunopharmacology and pathophysiology. Ann Arbor, Mich: CRC; 1992. pp. 75–104. [Google Scholar]
- 11.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman G W, Sultzer B M. Endotoxin protein is a mitogen and polyclonal activator of human B lymphocytes. J Exp Med. 1979;149:713–723. doi: 10.1084/jem.149.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurley J C. Antibiotic-induced release of endotoxin: a reappraisal. Clin Infect Dis. 1992;15:840–854. doi: 10.1093/clind/15.5.840. [DOI] [PubMed] [Google Scholar]
- 14.Ingalls R R, Heine H, Lien E, Yoshimura A, Golenbock D. Lipopolysaccharide recognition, CD14, and lipopolysaccharide receptors. Infect Dis Clin North Am. 1999;13:341–353. doi: 10.1016/s0891-5520(05)70078-7. [DOI] [PubMed] [Google Scholar]
- 15.Jackson J J, Kropp H. Beta-lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J Infect Dis. 1992;165:1033–1041. doi: 10.1093/infdis/165.6.1033. [DOI] [PubMed] [Google Scholar]
- 16.Jackson J J, Kropp H. Differences in mode of action of β-lactam antibiotics influence morphology, LPS release and in vivo antibiotic efficacy. J Endotoxin Res. 1996;3:201–218. [Google Scholar]
- 17.Karkhanis Y D, Zeltner J Y, Jackson J J, Carlo D J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 18.Killion J W, Morrison D C. Determinants of immunity to murine salmonellosis: studies involving immunization with lipopolysaccharide-lipid A-associated protein complexes in C3H/HeJ mice. FEMS Microbiol Immunol. 1988;1:41–53. doi: 10.1111/j.1574-6968.1988.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 19.Levin J, Poore T E, Young N S, Margolis S, Zauber N P, Townes A S, Bell W R. Gram-negative sepsis: detection of endotoxemia with the limulus test, with studies of associated changes in blood coagulation, serum lipids, and complement. Ann Intern Med. 1972;76:1–7. doi: 10.7326/0003-4819-76-1-1. [DOI] [PubMed] [Google Scholar]
- 20.Levin J, Poore T E, Zauber N P, Oser R S. Detection of endotoxin in the blood of patients with sepsis due to gram-negative bacteria. N Engl J Med. 1970;283:1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- 21.Manthey C L, Vogel S N. Elimination of trace endotoxin protein from rough chemotype LPS. J Endotoxin Res. 1994;1:84–89. [Google Scholar]
- 22.Morrison D C, Betz S J, Jacobs D M. Isolation of a lipid A bound polypeptide responsible for “LPS-initiated” mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976;144:840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison D C, Leive L. Fractions of lipopolysaccharide from Escherichia coli O111B:4 prepared by two extraction procedures. J Biol Chem. 1975;250:2911–2919. [PubMed] [Google Scholar]
- 24.Morrison D C, Vukajlovich S W, Ryan J L, Levin J. Structural requirements for gelation of the Limulus amebocyte lysate by endotoxin. Prog Clin Biol Res. 1987;231:55–73. [PubMed] [Google Scholar]
- 25.Pfaller M A, Jones R N, Marshall S A, Coffman S L, Hollis R J, Edmond M B, Wenzel R P. Inducible ampC beta-lactamase producing gram-negative bacilli from blood stream infections: frequency, antimicrobial susceptibility, and molecular epidemiology in a national surveillance program (SCOPE) Diagn Microbiol Infect Dis. 1997;28:211–219. doi: 10.1016/s0732-8893(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 26.Rangel-Frausto M S. The epidemiology of bacterial sepsis. Infect Dis Clin North Am. 1999;13:299–312. doi: 10.1016/s0891-5520(05)70076-3. [DOI] [PubMed] [Google Scholar]
- 27.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 28.Rudbach J A, Akiya F I, Elin R J, Hochstein H D, Luoma M K, Milner E C, Milner K C, Thomas K R. Preparation and properties of a national reference endotoxin. J Clin Microbiol. 1976;3:21–25. doi: 10.1128/jcm.3.1.21-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon D M, Koenig G, Trenholme G M. Differences in release of tumor necrosis factor from THP-1 cells stimulated by filtrates of antibiotic-killed Escherichia coli. J Infect Dis. 1991;164:800–802. doi: 10.1093/infdis/164.4.800. [DOI] [PubMed] [Google Scholar]
- 30.Stumacher R J, Kovnat M J, McCabe W R. Limitations of the usefulness of the Limulus assay for endotoxin. N Engl J Med. 1973;288:1261–1264. doi: 10.1056/NEJM197306142882402. [DOI] [PubMed] [Google Scholar]
- 31.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 32.van der, P. T, van Deventer S J. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin North Am. 1999;13:413–426. doi: 10.1016/s0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 33.Vukajlovich S W, Morrison D C. Activation of murine spleen cells by lipid A: negative modulation of lipid A mitogenic activity by O-antigen polysaccharide. J Immunol. 1985;135:2546–2550. [PubMed] [Google Scholar]
- 34.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. In: Whistler R L, editor. Methods in carbohydrate chemistry. New York, N.Y: Academic Press, Inc.; 1965. pp. 83–91. [Google Scholar]
- 35.Westphal O, Luderitz O, Bister F. Über die Extraktion von Bakterien mit Phenol/Wasser. Z Naturforschung B. 1952;7B:148–155. [Google Scholar]