Abstract
Heart failure is defined as a clinical syndrome consisting of key symptoms and is due to a structural and/or functional alteration of the heart that results in increased intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise. One of the key mechanisms determining myocardial dysfunction in heart failure is oxidative stress. MicroRNAs (miRNAs, miRs) are short, endogenous, conserved, single-stranded non-coding RNAs of around 21–25 nucleotides in length that act as regulators of multiple processes. A systematic review following the PRISMA guidelines was performed on the evidence on the interplay between microRNA and oxidative stress in heart failure. A search of Pubmed, Embase, Scopus, and Scopus direct databases using the following search terms: ‘heart failure’ AND ‘oxidative stress’ AND ‘microRNA’ or ‘heart failure’ AND ‘oxidative stress’ AND ‘miRNA’ was conducted and resulted in 464 articles. Out of them, 15 full text articles were eligible for inclusion in the qualitative analysis. Multiple microRNAs are involved in the processes associated with oxidative stress leading to heart failure development including mitochondrial integrity and function, antioxidant defense, iron overload, ferroptosis, and survival pathways.
Keywords: oxidative stress, microRNA, heart failure, myocardial remodeling, myocardial hypertrophy, mitochondria, ROS
1. Introduction
1.1. microRNA
MicroRNAs (miRNAs, miRs) are short, endogenous, conserved, single-stranded non-coding RNAs of around 21–25 nucleotides in length [1]. miRNAs, when transcribed as precursors and processed into mature miRNA, can interact with target cellular messenger RNA (mRNA) transcripts [2]. In most cases, they induce target degradation. Through gene expression regulation, miRNAs have an important role in various cellular processes such as cell growth, proliferation, differentiation, apoptosis, and stress response [3,4,5]. The latest miRNBase (v22.1) contains microRNA sequences from 271 species: 38,589 precursors and 48,860 mature microRNAs. To date, the base includes 1917 precursors and 2654 mature sequences described in humans [6].
1.2. Oxidative Stress
Oxidative stress is recently widely described as an important factor in the pathophysiology of many human diseases [7,8,9]. It is caused by the overproduction of reactive oxygen species (ROS) which are the byproducts of normal cellular metabolism. ROS include both radicals and non-radicals, such as superoxide anions, hydrogen peroxide, hydroxyl ion, alkoxyl, singlet oxygen, ozone, and peroxyl radicals. Furthermore, because of their unique characteristics, excess ROS can not only trigger many molecular implications including inflammation and programmed cell death, but also target, terminally modify, and damage different molecules in the cell including proteins, lipids, and nucleic acids [10,11]. Oxidative stress has been widely described as a pathogenesis agent, diagnostic tool, and therapeutic target in medical conditions, including cardiovascular diseases [12].
1.2.1. ROS Origin, Formation, and Function
ROS are most often the result of the phenomenon described as free radical leak which is observed in mitochondria. In fact, mitochondria play a fundamental role in the cellular physiology by synthesizing adenosine triphosphate (ATP) in the process of Electron Transport Chain (ETC). The ETC contains four complexes (Complexes I–IV) embedded in the inner mitochondrial membrane (IMM), and two small electron carriers, ubiquinone (Q) and cytochrome (C), which carry electrons between the complexes until they are passed to the final electron acceptor—the oxygen. However, not all electrons within the ETC are transferred to the oxygen due to the free-radical leak. This results in the production of endogenous, mitochondrial ROS, mostly superoxide radical anion and H2O2 [11,12,13]. The accumulation of ROS inside mitochondria can provoke the process called mitochondrial permeability transition pore (mPTP) opening which results in the release of the ROS to the cytoplasm of the cell [14] and its activity there. Nonetheless, there are more mechanisms of mitochondrial ROS production described [15].
Non-mitochondrial ROS are produced with a key contribution of several enzymes including myeloperoxidase (MPO), xanthine oxidase (Xo), uncoupled nitric oxide synthase (NOS), lipoxygenase, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), which is considered as a significant source of ROS. The ROS-containing nitrogen are often distinguished as reactive nitrogen species (RNS). RNS in general are important in pathogenesis of cardiovascular events [12,16]. Finally, exogenous ROS can also be formed after the environmental factors exposure such as pollutants, cigarette smoke, ultraviolet (UV) radiation, and xenobiotics [17].
Despite the toxic effect and participation in pathogenesis of many diseases, it is worth mentioning that ROS generation is a natural part of aerobic life. Basal levels of ROS are essential for signaling function, defense against microorganisms, gene expression, growth, and death. Living organisms are equipped with mechanisms of ROS release control, including enzymatic e.g., superoxide dismutase (SOD), catalase (CAT), and non-enzymatic means e.g., ascorbate, beta-carotene.
1.2.2. microRNA and Oxidative Stress in Cardiovascular Diseases
Today, sophisticated methods of redox signaling pathways investigation allow the inquiry into the pathogenesis of many diseases, including widely discussed cardiovascular events and HF, to which ROS species may contribute [12]. There is evidence of interactions between cardiac miRNAs and ROS in different cardiovascular events including, e.g., atherosclerosis, myocardial infarction, diabetic cardiomyopathy, and chemotherapy-induced cardiomyopathy mainly in animal models [18]. In response to increased ROS concentration, the cardiovascular diseases can be initiated and developed by fibrosis, apoptosis, necrosis and proliferation or hypertrophy of both smooth and cardiac muscles, endothelial cells, and cardiac fibroblasts. It was proven that microRNAs are involved in these processes [19,20,21]. Moreover, some microRNAs have been identified as regulators of oxidative stress in cardiovascular system by interaction with ROS generators, antioxidant effectors, and signaling pathways [22].
This review presents current knowledge concerning the miRNA role involved in oxidative stress in the development of the heart failure. To our knowledge, no systematic reviews concerning miRNA and oxidative stress in heart failure have been published to date.
2. Materials and Methods
A computer search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) scheme by two independent observers in four major databases (Pubmed, Embase, Scopus, Scopus direct) using the following search terms: ‘heart failure’ AND ‘oxidative stress’ AND ‘microRNA’ or ‘heart failure’ AND ‘oxidative stress’ AND ‘miRNA’. The PRISMA statement consists of a 27-item checklist and a four-phase flow diagram whose aim is to help authors in the reporting of systematic reviews and meta-analyses [23].
After the database research, the obtained abstracts were screened by the authors and only the articles connected to the topic of microRNA, oxidative stress, and heart failure were included. Articles about cardiovascular diseases (CVD) in general, postpartum cardiomyopathy, diabetic cardiomyopathy, ischemia-reperfusion damage, and acute coronary syndromes were excluded. Finally, only 15 articles were outlined after the removal of 5 reviews on the topic. The methodology of the database research is summarized in Figure 1.
Figure 1.
Flowchart summarizing the literature search methodology.
3. Results
The final number of full-text original articles included in the analysis was n = 15 (Figure 1). The full list of relevant articles on this topic is listed in the Table 1. Below we explore the current evidence in detail.
Table 1.
A list of research studies evaluating out the role of microRNA that play a role in oxidative-stress mechanisms involved in heart failure according to the sections.
| No. | miRNA | Ref. | Type of Study 1 | Techniqe 2 | Main Conclusion |
|---|---|---|---|---|---|
| 3.1 Mitochondrial integrity and function | |||||
| 1 | miRNA-690 | Wang, X. et al. (2017) [24] | animal | RT qRT-PCR | microRNAs were enriched in mitochondria during heart failure, which establishes a link between microRNA and mitochondrion in heart failure. |
| miRNA-696 | |||||
| miRNA-532-5p | |||||
| miRNA-345-3p | |||||
| 2 | miRNA-122 | Shi, Y. et al. (2021) [25] | animal | qRT-PCR with the miRCute Enhanced Fluorescence Quantitative Assay Kit (Tiangen) | miR-122 causes cardiomyocyte apoptosis by inhibiting Hand2 transcription factor and consequently increasing mitochondrial fission. This mechanism likely contributes to heart failure and modulating this pathway could be therapeutically valuable against heart failure. |
| 3 | miRNA-15b | Roy, S. et al. (2013) [26] | animal/in vivo | qRT-PCR | Suppression of inducible miRNA-15b can prevent rapid loss of cardiac function in an animal adult heart and can be a key approach worthy of therapeutic consideration. |
| 3.2 Antioxidant defence | |||||
| 4 | miRNA-27a | Tian, C. et al. (2018) [27] | animal/in vivo | qRT-PCR | Increased expression of local microRNAs induced by myocardial infarction may contribute to oxidative stress by the inhibition of nuclear factor erythroid 2-related factor 2(NRF2) translation in chronic heart failure. |
| miRNA-28-3p | |||||
| miRNA-34a | |||||
| 5 | miRNA-27a * | Tian, C. et al. (2020) [28] | animal/in vivo | qRT-PCR | Cardiac fibroblast-secretion of miRNA27a *-enriched extracellular vesicles (EV) might act as a paracrine signaling mediator of cardiac hypertrophy and may have a potential to become novel therapeutic target. |
| 6 | miRNA-24-3p | Shimizu, T. et al. (2020) [29] | animal | RNA-sequencing, western blot, ELISA | Protein kinase R–like endoplasmic reticulum (ER) kinase (PERK)-mediated suppression of miRNAs by sildenafil improves cardiac dysfunction in heart failure. |
| 3.3 Iron overload and ferroptosis | |||||
| 7 | miRNA-224-5p | Zheng, H. et al. (2021) [30] | animal | qRT-PCR, Western blot analysis, luciferase reporter assay | Circulatory RNA (circRNA), microRNA(miRNA) and mRNA work in a regulatory network and reveal potential targets for the treatment of heart failure. |
| miRNA-296-3p | |||||
| miRNA-671-5p | |||||
| miRNA-1306-5p | |||||
| miRNA-3082-5p | |||||
| 3.4 Cardiac hypertrophy and remodelling | |||||
| 8 | miRNA-21-3p | Zhao, Y. et al. (2018) [31] | animal | miRNA microarray, bioinforma-tic analysis, qRT-PCR, Western blot | The injection of Yiqifumai (YQFM) has a potential effect which alleviates cardiac hypertrophy and apoptosis in chronic heart failure by miRNA expression regulation. |
| miRNA216-5p | |||||
| miRNA-219a-2-3p | |||||
| miRNA-381-3p | |||||
| miRNA-466c-5p | |||||
| miRNA-542-3p | |||||
| miRNA-702-5p | |||||
| 9 | miRNA-93 | Su, Q. et al. (2019) [32] | animal/in vivo | RT qRT-PCR, ELISA, Western blot | Upregulated miR-93 and downregulated LIM domain kinase 1 (LIMK1) reduce cardiac dysfunction and improve ventricular remodelling in rats with chronic heart failure. |
| 10 | miRNA-142-5p | Sharma, S. et al. (2012) [33] | animal/in vivo | qRT-PCR, Western blot | Downregulation of miR-142 is a critical element of adaptive cardiac muscle hypertrophy in response to hemodynamic stress. |
| miRNA-142-3p | |||||
| 11 | miRNA-195-5p | Shen, Y. et al. (2020) [34] | animal | RT qRT-PCR | Depleting miR-195-5p and up-regulating Chemokine receptor type 4 alleviates cardiac function injury in mice with heart failure and may be a potential candidate marker and therapeutic target for heart failure. |
| For Section 3.4 see also research studies no.: 4 [27] and 5 [28]. | |||||
| 3.5 Apoptosis | |||||
| 12 | miRNA-454 | Wang, Y. et al. (2021) [35] | animal | RT qRT-PCR, Western blot | The cardioprotective role of miR-454 is achieved through activation of the cyclic adenosine 3′5′-monophosphate(cAMP). |
| 3.6 Human studies | |||||
| 13 | miRNA-129-5p | Ramachandran, S. et al. (2017) [36] | human | isolation of mir129-5p from plasma microvesicles, qRT-PCR | miR129-5p was found to be a sensitive and specific biomarker for heart failure in univentricular heart disease in pediatric patients independent of ventricular morphology or stage of palliation. |
| 14 | miRNA-208a | Mohammadi et al. (2021) [37] | human | qRT-PCR | The results on different populations of cardiovascular patients (after myocardial infarction, with arrhythmia, heart failure etc.) showed increases in both oxidative stress, inflammation, apoptotic factors, and in the expression of miR-208a. |
| 3.7 Therapeutic potential | |||||
| 15 | miRNA-15b | Roy, S. et al. (2013) [26] | animal/in vivo | qRT-PCR | Suppression of inducible miRNA-15b can prevent rapid loss of cardiac function in an animal adult heart and can be a key approach worthy of therapeutic consideration. |
| For Section 3.5 see also research studies no.: 5 [28] and 11 [34]. | |||||
1 Types of study include: review, human—study based on human participants, animal—study based on animal models, in vivo—study based on research inside the organism. 2 qRT-PCR—quantitative Real-Time Polymerase Chain Reaction, RT qRT-PCR—Reverse Transcriptase quantitative Real-Time Polymerase Chain Reaction, *—passenger strand.
Heart failure is defined as a clinical syndrome consisting of key symptoms and is due to a structural and/or functional alteration of the heart that results in increased intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise [38]. One of the key mechanisms that determine the dysfunction of the heart muscle in heart failure is oxidative stress and mitochondrial dysfunction [39,40]. To date, several miRNAs have been identified and characterized in the heart failure basing on the animal models including: miRNA-27a, miRNA-28-3p, miRNA-34a, miR-122, miRNA-195-5p, miRNA-224-5p, miRNA-296-3p, miRNA-345-3p, miRNA-532, miRNA-671-5p, miRNA-690, miRNA696, miRNA-1306-5p, and miRNA-3082-5p (Table 1).
3.1. Mitochondrial Integrity and Function
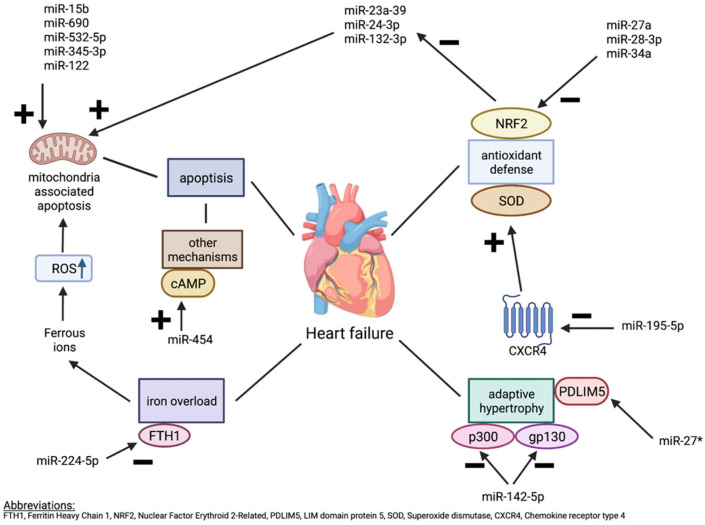
As described earlier, ROS formation strongly depends on the mitochondrial function. Wang, X. et al. (2017) [24] and Shi, Y. et al. (2021) [25] described association between microRNA and mitochondrial dysfunction contributing to cardiomyocyte apoptosis, and consequently heart failure. Wang et al. evaluated mitochondrial expression of microRNA in samples of mitochondrial RNA coming from animal model of HF (transverse aortic constriction (TAC) at early (4 weeks) and late (8 weeks) phase). The authors report on four microRNAs enriched in mitochondria of the failing heart (mmu-miR-690, mmu-miR-532-5p, mmu-miR-696, and mmu-miR-345-3p) (Figure 2). The pathway analysis revealed the association of this microRNAs with mitochondria biogenesis and fission, fatty acids metabolism, oxidative stress, and apoptosis [24]. Next, Hand2 transcription factor normally plays an essential role in cardiac morphogenesis and its disruption can cause impaired cardiac development, apoptosis of cardiomyocytes, and cardiac hypertrophy. miR-122 was found to inhibit Hand2 and consequently increase dynamin-related protein-1-mediated excessive mitochondrial fission which results in cardiomyocyte apoptosis.
Figure 2.
The balance between the apoptosis induced by mitochondrial oxidative stress and antioxidant defense mechanisms. (*—passenger strand).
The transgenic mice with excessive miR-122 expression not only showed macroscopically enlarged hearts with lowered contractile function but most importantly the Western blot quantification, immunofluorescence, and histochemical staining showed increased level of Dpr1 protein and mRNA in comparison to healthy controls. These results suggested that miR-122 overexpression leaded to cardiomyocyte apoptosis by mitochondrial fission, contributing to heart failure development in these transgenic mice [25] (Figure 2).
miRNA-15b is another microRNA species connected to the mitochondrial integrity and function in cardiomyocytes. In fact, suppression of dicer endonuclease, critical for maturation of miRNA results in rapid loss of cardiac function in mice model. MiRNA-15b was found to silence Pim-1 kinase, required for mitochondrial functioning and integrity of cardiac muscle (Figure 2). Authors analyzed mitochondrial structure by transmission electron microscope (TEM) and observed a loss of mitochondrial structural integrity in cardiac tissue represented by matrix swelling and unfolded cristae from Dicer−/− mice. Therefore, they concluded that targeting miRNA-15b could serve as a future therapeutic approach in the patients with HF [26].
3.2. Antioxidant Defence
Nuclear factor erythroid 2-related factor 2 (Nrf2), an important antioxidant defense mechanism closely associated with oxidative stress-mediated cardiac remodeling in chronic heart failure (CHF), was also found to be inhibited by increased microRNA-27a and microRNA-28-3p. The microRNA-34a expression in the infarcted hearts contributed to decrease of Nrf2-targeted antioxidant enzymes (Figure 2). These microRNAs were preferentially incorporated into exosomes and secreted into the extracellular space in which microRNA-enriched exosomes mediated intercellular communication and Nrf2 dysregulation, which may play role in the setting of CHF [27]. On the other hand, the protective role of microRNA-27a has been reported as well. A study by Tian et al. evidenced that Kruppel-like factor 5 (KLF5), a pro-apoptotic member of the Kruppel-like transcription factor family, induces cell and tissue damage in myocardial infarction (MI) through downregulation of miR-27a and the subsequent activation of GFPT2/TGF-β/Smad2/3 axis (Figure 2) [31]. Some recently published data describe the role of miRNA-27a passenger strand (miRNA-27a*) especially as the hypertrophy mediator in post MI-hearts in a mechanism described below (see Section 3.4) [27,41]. Further on, Hua et al. described microRNA-34a role in cardiovascular fibrosis, and Smad4/TGF-β1 signaling. It was reported that the inhibition of miR-34 reduced cellular reactive oxygen species (ROS) through the miR-34a/SIRT1 signaling pathway, but also through alleviation of the Kruppel-like factor 4 (KLF4). Next, miRNA-34a eased oxidative stress (induced by H2O2) in protection of vascular endothelial cells by pointing SIRT1 (Figure 2) [42]. Based on the data above, it is clear that microRNA exert multidirectional effects, besides their effect on oxidative stress.
Oxidative/nitrosative stress has also been described as a trigger of heart dysfunction and remodeling. Activation of nitric oxide-cyclic guanosine monophosphate-protein kinase G signaling by sildenafil improves cardiac remodeling in a mouse model of pressure-overload-induced (PO-induced) heart failure. Sildenafil is a selective inhibitor of type 5 phosphodiesterase that provides positive effects on hemodynamics in pulmonary hypertension (PH). This drug has been approved for use in primary idiopathic PH, but it also is often utilized empirically in heart failure (HF), because it frequently results in PH [43]. Lewis et al. [44] found that sildenafil upregulated Nrf2, an important antioxidant defense player, by inhibiting the maturation of PO-induced miRNAs, such as miR-23a-3p, miR-24-3p, and miR-132-3p (Figure 2). These miRNAs govern the transition from compensated hypertrophy to decompensated hypertrophy and contribute to mitochondrial metabolism and redox homeostasis dysregulation. R–like endoplasmic reticulum (ER) kinase (PERK) was identified to be crucial for sildenafil-mediated improvement of mitochondrial dysfunction in failing hearts through the suppression of HF-induced miRNAs. All in, the suppression of the above miRNAs play a vital role in sildenafil-induced cardiac protection [44]. However, the data come from animal study and the results from human studies do provide ambiguous information on the effects of sildenafil treatment. Whereas sildenafil has been shown to improve hemodynamics in patients with HFrEF [45], potential adverse effects of sildenafil on mitochondrial function and endoplasmic reticulum stress in HFpEF patients have been reported [44,45]. Therefore, further studies in HFpEF and HFrEF patients are necessary to clarify the difference in its effects.
3.3. Iron Overload and Ferroptosis
Iron is an element essential for various enzymatic reactions and therefore maintaining normal cardiac function. However, excess iron in the cardiomyocytes leads to the increase of ROS formation, thus generating oxidative damage to large molecules such as DNA, membrane lipids and proteins [46]. Ferroptosis is an iron-dependent form of cellular death in which ferric iron (Fe3+) from Fenton reaction increases ROS concentration. In ferroptosis, ROS activate lipoxygenases which damage cellular membranes. It is generally a morphologically different process from apoptosis or necrosis while it manifests as dense mitochondria with loss of cristae and previously mentioned cellular membranes damage. Glutathione peroxidase 4 (GPX4) was described several times as an endogenous mechanism of ferroptosis alleviation [47] and may be an important factor regarding possible therapeutic modalities. Ferroptosis has been observed in the cell death as pathogenesis in many human diseases, also in heart diseases including ischemia/reperfusion damage in vitro studies [47].
Zheng et al. [30] report that Ferritin Heavy Chain 1 (FTH1) gene was markedly downregulated in mice with TAC, a method to induce acute heart failure, which in turn results in a release of a large amount of ferrous ions into the cytoplasm. In the above study, circular RNAs (circRNAs), mainly circSnx12, were the targeted molecules, but its correlation with miR-224-5p in heart failure mechanism was found. The authors identified a protein associated with iron metabolism (FTH1), predicted that miR-224-5p might bind to FTH1 3′UTR by TargetScan and RegRNA 2.0 database, and further determined that miR-224-5p could be a potential binding target for circSnx12 through starBase database, which was proven in their results. It was observed that high miR-224-5p expression can downregulate FTH1 expression, and therefore contribute to iron overload in myocardial cells, leading to cardiac cell death in the mechanism of ferroptosis [30]. The literature on miRNA and the role of ferroptosis in human heart failure is very limited, and therefore merits further exploration.
3.4. Cardiac Hypertrophy and Remodelling
One of the key pathophysiological components of the development and progression of the heart failure is the increased systemic pressure which increases the workload that the left ventricle must withstand. Therefore, the myocardium experiences structural and functional changes to comply with the increased demand resulting in the cardiac hypertrophy and/or remodeling [48]. The miRNAs that up to today have been correlated with the myocardial hypertrophy and remodeling in animal models include: miRNA-21-3p, miRNA-27a, miRNA-93miR216-5p, miRNA-142-3p, miRNA-142-5p, miRNA219a-2-3p, miRNA381-3p, miRNA466c-5p, miRNA542-3p, and miRNA-702-5p (Table 1).
The repression of miR-142-5p was found to be a crucial element of adaptive hypertrophy in response to hemodynamic and oxidative stress. As a result of physiologic repression of miR-142-5p transduced by mitogen-activated protein kinases (MAPK) and acetyltransferase p300, myocardial growth (mediated by p300) and activation of myocyte survival pathways (mediated by cytokine receptor gp130) are possible as both p300 and gp130 are direct targets of miR-142-5p (Figure 2). The cumulative effect is activation of cytokine-mediated survival signal pathway desirable for adaptive (advantageous) growth of myocardium and prevention of apoptosis triggered by acute hemodynamic stress [33], which leads to the myocardial hypertrophy and heart failure.
MicroRNA-27a passenger strand (miRNA-27a*) is upregulated in non-infarcted areas and in the border zone of the post MI heart as well as in extracellular vesicles (EV) secreted by cardiac fibroblasts and detected in plasma of CHF rats. One of the miRNA-27a* targets is LIM domain 5 (PDLIM5), a gene which has been shown to play a major role in cardiac structure and function (Figure 2) [27,41]. MiRNA-27* is therefore a potential novel therapeutic target in chronic heart as its inhibition could result in favorable PDLIM5 upregulation [27].
MiR-93 has been reported as a negative regulator of the immune response [49]. It also exhibits long-term protective roles via elevating angiogenesis atherosclerosis-associated cardiovascular disease such as myocardial infarction, stroke, and peripheral arterial disease [50]. It has been found that miR-93 is decreased and LIM domain kinase 1 (LIMK1), a serine/threonine kinase, is increased in CHF rats. Upregulation of miR-93 inhibited LIMK1, small GTPase RhoA (RhoA), and Rho kinase 1 (ROCK1) expression in CHF rats. Malondialdehyde (MDA) level was decreased while SOD and Total Antioxidant Capacity Colorimetric (T-AOC) levels were increased by up-regulation of miR-93 or downregulation of LIMK1 (Figure 2). MiR-93 mimics attenuated swelling, vacuolization, mitochondrial lysis and rupture, muscle fiber arrangement disorder, or even rupture of cells in CHF rats under TEM. MiRNA-93 was found to improve ventricular remodeling and reduce cardiac dysfunction, inflammatory markers, and indicators of apoptosis in rats with congestive heart failure [32].
Injection of anti-miR-195-5p in mice model of HF (TAC model of pressure overload) resulted in improved cardiac function and ameliorated inflammation, oxidative stress, and cardiomyocyte injury damage. The possible mechanisms behind that were upregulation of Chemokine receptor type 4 (CXCR4), the direct target of miR-195-5p, enhancement of superoxide dysmutase SOD, inhibited in TAC group, and subsequent inhibition of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway activation (Figure 2) [34].
In addition, a study was performed on induced oxidative stress in H9c2 myocardial cells to validate the anti-hypertrophy and anti-apoptosis effects of YQFM Yiqifumai (YQFM) injection, a traditional Chinese drug, derived from Shengmaifang, composed of Panax ginseng, Ophiopogon japonicas, and Schisandra chinensis [51]. In this study, a microarray-based approach was employed to investigate the miRNA expression profiles of YQFM in treating chronic heart failure and found out differentially reversed miRNAs regulated by YQFM: miR-21-3p, miR216-5p, miR219a-2-3p, miR381-3p, miR466c-5p, miR542-3p, and miR-702-5p [31]. It was hypothesized that these microRNAs may be responsible for counteracting anti-hypertrophic and anti-apoptotic effects of oxidative stress in heart muscle.
3.5. Apoptosis
Neural precursor cell expressed, developmentally downregulated 4-2 (NEDD4-2)/tropomyosin receptor kinase A (TrkA)/cyclic adenosine 3′,5′-monophosphate (cAMP) axis is described as playing role in degradation and apoptosis of cardiomyocytes. miR-454 activated the cAMP pathway via the NEDD4-2/TrkA/cAMP axis, which was found to be suppressed in HF, and alleviated myocardial damage in rats. miR-454 was aberrantly downregulated in the context of HF, while recent evidence suggests NEDD4-2 in cardiomyocytes. miR-454 exerted anti-apoptotic and protective effects on cardiomyocytes through inhibition of NEDD4-2, while NEDD4-2 stimulated ubiquitination and degradation of TrkA protein. Furthermore, miR-454 activated the cAMP pathway via the NEDD4-2/TrkA axis, which ultimately suppressed cardiomyocyte apoptosis and attenuated myocardial damage. Taken together, the key findings of the study highlight the cardioprotective role of miR-454, which is achieved through activation of the cAMP pathway by impairing NEDD4-2-induced TrkA ubiquitination [35].
3.6. Human Studies
To date, only one study regarding microRNA in heart failure was conducted on humans. Ramachandran, S. et al. (2017) [36] found that miR129-5p circulating in microvesicles is a sensitive and specific biomarker for heart failure in pediatric patients. The levels of miR129-5p were inversely related to the degree of clinical severity of heart failure assessed by Ross score in patients with univentricular heart congenital defect. At the same time, the authors describe that miR129-5p levels are downregulated in HL1 cells (cardiac muscle cell line) and human embryonic stem cell-derived cardiomyocytes exposed to oxidative stress. The authors demonstrated that bone morphogenetic protein receptor 2, which has been implicated in the development of pulmonary vascular disease, is a target of miR129-5p, and conversely regulated in response to oxidative stress in cell culture. Further studies are warranted in order to better understand the targets affected by miR129-5p and responsible for the development of heart failure in patients with univentricular physiology [36].
Targeting high mobility group box-1 (HMGB1) via transfection of miRNA-129-5p is yet another mechanism in which miRNA was found to protect heart muscle tissue. Upregulation of HMGB1 and downregulation of miRNA-129-5p was observed in patients with chronic heart failure. The mechanism was confirmed based on transfection of miRNA-129-5p into the rat models with reduced HMGB1 levels, which led to decrease in oxidative stress and inflammation in cardiac cells [52].
Increase in miRNA-208 expression and decrease in miR-1 expression along with significant decreases in the activity of antioxidant enzymes (CAT, SOD, and Gpx) have also been observed in heterogenic group of cardiovascular disease (CVD) patients. The study group consisted of four types of patients (acute coronary syndrome, myocardial infarction (MI), arrhythmia, and heart failure). The lowest concentration of GPX and SOD along with highest expression of miRNA-208 activity was observed in the group of heart failure. miRNA-1 was significantly downregulated in HF patients in comparison to other groups [37].
3.7. Therapeutic Potential
Both animal and in vivo studies detect key point mechanisms connected to miRNA and provide promising therapeutic targets for patients with heart failure. Multiple literature reports highlight the importance of miRNA further investigation which can reveal new potential therapeutic modalities [27,28,30,41,42].
The key miRNA with promising therapeutic potential based on the preclinical studies include miRNA-15b, miRNA-195, and miRNA-27a*.
3.7.1. miRNA-15b
As previously described, in murine hearts disturbed with Dicer depletion, miRNA-15b was found to be involved with heart failure. Elevated levels of miRNA-15b silence Pim-1 kinase and ADP-ribosylation factor-like 2 (Arl2), which both affect cellular ATP levels and show multipronged damaging effect it has on mitochondrial function. The study showed that injection of LNA (locked nucleic acid)-modified anti-miRNA-15b significantly decreased miRNA-15b levels in Dicer-deleted adult mice hearts. Moreover, anti-miRNA-15b treatment resulted in significant improvement in cardiac function as recorded by echocardiography and MRI. Furthermore, Hematoxylin/Eosin staining showed histopathological changes such as hypertrophied myofibers and myocyte disarray which were not observed in tissues of animals treated with anti-miRNA-15b. Altogether, these data point towards the hypothesis that strategies targeting the management of elevated miRNA-15b could be one of the potential therapeutic approaches in patients with HF [26].
3.7.2. miRNA-195
Another study provided substantial evidence that miR-195-5p, JAK2, and STAT1 expression were raised and CXCR4 expression was degraded in myocardial tissues of mice with HF (transverse aortic constriction, TAC model of pressure overload). The positive, alleviating impact of anti-miR-195-5p was found both in imaging modalities, biochemistry, and histopathology. The echocardiographic assessment proved decreased left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), interventricular septal thickness at systole (IVSS) and left ventricular posterior wall thickness in systole (LVPWS) as well as enhanced left ventricle ejection fraction (LVEF). The serum tests showed reduced lactate dehydrogenase (LDH), aspartate aminotransferase (AST), creatine kinase (CK) and creatine kinase myocardial band (CK-MB) levels, declined TNF-α, IL-1β, and Angiotensin II levels, and enhanced IL-10. Moreover, the apoptosis of cardiomyocytes was restrained. These multimodal, promising results in mice need to be further studied, and clinical research might be further extended for the treatment of HF in human patients [34].
3.7.3. miRNA-27a
miRNA-27a* was found to be upregulated in cardiac fibroblasts and packaged into EV resulting in a hypertrophic phenotype in mice with HF in a period of 3 weeks after MI. The researchers observed that systemic administration of miRNA-27a* inhibitor decreased cardiac miRNA-27a* and inhibited the expression of hypertrophic genes, partially improving cardiac function. The cardiac function was mainly assessed by echocardiography revealing that stroke volume was significantly improved, and the cardiac output trended to increase. However, cardiac fibroblasts may produce and secrete many different miRNAs, such as miRNA-21 and miRNA-27a, that contribute to cardiac hypertrophy by targeting the same gene. It will be difficult to determine which miRNA dominates in this pathological process and which inhibitors should be used to obtain the highest effectiveness. Moreover, the authors highlight that it is not clear at the present time what dose and route of administration are most effective. The authors predict that it may be more effective to develop a cardiac homing peptide-based EV delivery system for administration of miRNA-27a* inhibitor as therapy for HF [28].
4. Conclusions
Multiple microRNA species are involved in oxidative-stress-related processes within the failing heart. Current evidence provides detailed description of specific pathways and microRNAs involved, which could be used in the development of the diagnostic and therapeutic strategies for HF based on microRNA. To date, both microRNA-mimic and microRNA-inhibition in heart failure show potential in strengthening the antioxidant mechanisms and preventing unfavorable myocardial remodeling.
Author Contributions
Conceptualization, D.K.-T.; methodology, D.K.-T.; validation, D.K.-T., J.H.-S., M.K. and L.P.; formal analysis, D.K.-T. and J.H.-S.; investigation, D.K.-T. and J.H.-S., resources, D.K.-T. and J.H.-S.; data curation, D.K.-T. and J.H.-S.; writing—original draft preparation, D.K.-T. and J.H.-S.; writing—review and editing, D.K.-T., J.H.-S., M.K. and L.P.; visualization, D.K.-T. and J.H.-S., supervision, D.K.-T.; project administration, D.K.-T.; funding acquisition, D.K.-T., J.H.-S., M.K. and L.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Donde M.J., Rochussen A.M., Kapoor S., Taylor A.I. Targeting non-coding RNA family members with artificial endonuclease XNAzymes. Commun. Biol. 2022;5:1010. doi: 10.1038/s42003-022-03987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chipman L.B., Pasquinelli A.E. miRNA Targeting: Growing beyond the Seed. Trends Genet. 2019;35:215–222. doi: 10.1016/j.tig.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou S.-S., Jin J.-P., Wang J.-Q., Zhang Z.-G., Freedman J.H., Zheng Y., Cai L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018;39:1073–1084. doi: 10.1038/aps.2018.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Çakmak H.A., Demir M. MicroRNA and Cardiovascular Diseases. Balkan. Med. J. 2020;37:60–71. doi: 10.4274/balkanmedj.galenos.2020.2020.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozomara A., Birgaoanu A., Griffiths-Jones S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019;47:55–162. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klisic A., Kavaric N., Ninic A., Kotur-Stevuljevic J. Oxidative stress and cardiometabolic biomarkers in patients with non-alcoholic fatty liver disease. Sci. Rep. 2021;11:18455. doi: 10.1038/s41598-021-97686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee I., Dhar R., Singh S., Sharma J.B., Nag T.C., Mridha A.R., Jaiswal P., Biswas S., Karmakar S. Oxidative stress-induced impairment of trophoblast function causes preeclampsia through the unfolded protein response pathway. Sci. Rep. 2021;11:18415. doi: 10.1038/s41598-021-97799-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahmy R., Almutairi N.M., Al-Muammar M.N., Bhat R.S., Moubayed N., El-Ansary A. Controlled diabetes amends oxidative stress as mechanism related to severity of diabetic retinopathy. Sci. Rep. 2021;11:17670. doi: 10.1038/s41598-021-96891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh A., Shcherbik N. Effects of Oxidative Stress on Protein Translation: Implications for Cardiovascular Diseases. Int. J. Mol. Sci. 2020;21:2661. doi: 10.3390/ijms21082661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lennicke C., Cochemé H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell. 2021;16:3691–3707. doi: 10.1016/j.molcel.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Senoner T., Dichtl W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients. 2019;11:2090. doi: 10.3390/nu11092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Kang P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants. 2020;17:1292. doi: 10.3390/antiox9121292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rottenberg H., Hoek J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell. 2017;16:943–955. doi: 10.1111/acel.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tejero J., Shiva S., Gladwin M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019;99:311–379. doi: 10.1152/physrev.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandes R.P., Weissmann N., Schröder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Lei X.G., Zhu J.-H., Cheng W.-H., Bao Y., Ho Y.-S., Reddi A.R., Holmgren A., Arnér E.S.J. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2016;96:307–364. doi: 10.1152/physrev.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Climent M., Viggiani G., Chen Y.W., Coulis G., Castaldi A. MicroRNA and ROS Crosstalk in Cardiac and Pulmonary Diseases. Int. J. Mol. Sci. 2020;21:4370. doi: 10.3390/ijms21124370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Duan L., Li Y., Liu B. Long noncoding RNA/circular noncoding RNA–miRNA–mRNA axes in cardiovascular diseases. Life Sci. 2019;233:116440. doi: 10.1016/j.lfs.2019.04.066. [DOI] [PubMed] [Google Scholar]
- 20.Colpaert R.M.W., Calore M. MicroRNAs in Cardiac Diseases. Cells. 2019;8:737. doi: 10.3390/cells8070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng J., Zhong Q. Advanced research on the microRNA mechanism in heart failure. Int. J. Cardiol. 2016;220:61–64. doi: 10.1016/j.ijcard.2016.06.185. [DOI] [PubMed] [Google Scholar]
- 22.Gong Y.Y., Luo J.Y., Wang L., Huang Y. MicroRNAs Regulating Reactive Oxygen Species in Cardiovascular Diseases. Antioxid. Redox Signal. 2018;29:1092–1107. doi: 10.1089/ars.2017.7328. [DOI] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;21:7. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Song C., Zhou X., Han X., Li J., Wang Z., Shang H., Liu Y., Cao H. Mitochondria Associated MicroRNA Expression Profiling of Heart Failure. Biomed. Res. Int. 2017;2017:4042509. doi: 10.1155/2017/4042509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y., Zhang Z., Yin Q., Fu C., Barszczyk A., Zhang X., Wang J., Yang D. Cardiac-specific overexpression of miR-122 induces mitochondria-dependent cardiomyocyte apoptosis and promotes heart failure by inhibiting Hand2. J. Cell Mol. Med. 2021;25:5326–5334. doi: 10.1111/jcmm.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy S., Banerjee J., Gnyawali S.C., Khanna S., He G., Pfeiffer D., Zweier J.L., Sen C.K. Suppression of Induced microRNA-15b Prevents Rapid Loss of Cardiac Function in a Dicer Depleted Model of Cardiac Dysfunction. PLoS ONE. 2013;8:e66789. doi: 10.1371/journal.pone.0066789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian C., Gao L., Zimmerman M.C., Zucker I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018;314:928–939. doi: 10.1152/ajpheart.00602.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian C., Hu G., Gao L., Hackfort B.T., Zucker I.H. Extracellular vesicular MicroRNA-27a* contributes to cardiac hypertrophy in chronic heart failure. J. Mol. Cell Cardiol. 2020;143:120–131. doi: 10.1016/j.yjmcc.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu T., Taguchi A., Higashijima Y., Takubo N., Kanki Y., Urade Y., Wada Y. PERK-mediated suppression of microRNAs by sildenafil improves mitochondrial dysfunction in heart failure. iScience. 2020;23:101410. doi: 10.1016/j.isci.2020.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H., Shi L., Tong C., Liu Y., Hou M. circSnx12 Is Involved in Ferroptosis During Heart Failure by Targeting miR-224-5p. Front. Cardiovasc. Med. 2021;8:656093. doi: 10.3389/fcvm.2021.656093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y., Li Y., Tong L., Liang X., Zhang H., Li L., Fan G., Wang Y. Analysis of microRNA Expression Profiles Induced by Yiqifumai Injection in Rats with Chronic Heart Failure. Front. Physiol. 2018;9:48. doi: 10.3389/fphys.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su Q., Zhang P., Yu D., Wu Z., Li D., Shen F., Liao P., Yin G. Upregulation of miR-93 and inhibition of LIMK1 improve ventricular remodeling and alleviate cardiac dysfunction in rats with chronic heart failure by inhibiting RhoA/ROCK signaling pathway activation. Aging. 2019;11:7570–7586. doi: 10.18632/aging.102272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma S., Liu J., Wei J., Yuan H., Zhang T., Bishopric N.H. Repression of miR-142 by p300 and MAPK is required for survival signalling via gp130 during adaptive hypertrophy. EMBO Mol. Med. 2012;4:617–632. doi: 10.1002/emmm.201200234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Y., Zhang W., Lee L., Hong M., Lee M., Chou G., Yu L., Sui Y., Chou B. Down-regulated microRNA-195-5p and up-regulated CXCR4 attenuates the heart function injury of heart failure mice via inactivating JAK/STAT pathway. Int. Immunopharmacol. 2020;82:106225. doi: 10.1016/j.intimp.2020.106225. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Pan W., Bai X., Wang X., Wang Y., Yin Y. microRNA-454-mediated NEDD4-2/TrkA/cAMP axis in heart failure: Mechanisms and cardioprotective implications. J. Cell Mol. Med. 2021;25:5082–5098. doi: 10.1111/jcmm.16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramachandran S., Lowenthal A., Ritner C., Lowenthal S., Bernstein H.S. Plasma microvesicle analysis identifies microRNA 129-5p as a biomarker of heart failure in univentricular heart disease. PLoS ONE. 2017;12:e0183624. doi: 10.1371/journal.pone.0183624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadi A., Karami A.R.B., Mashtani V.D., Sahraei T., Tarashoki Z.B., Khattavian E., Mobarak S., Kazerouni H.M., Radmanesh E. Evaluation of Oxidative Stress, Apoptosis, and Expression of MicroRNA-208a and MicroRNA-1 in Cardiovascular Patients. Rep. Biochem. Mol. Biol. 2021;10:183–196. doi: 10.52547/rbmb.10.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. ESC Scientific Document Group, 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab670. [DOI] [PubMed] [Google Scholar]
- 39.van der Pol A., van Gilst W.H., Voors A.A., van der Meer P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagan L.U., Gomes M.J., Martinez P.F., Okoshi M.P. Oxidative Stress and Heart Failure: Mechanisms, Signalling Pathways, and Therapeutics. Oxid. Med. Cell Longev. 2022;2022:9829505. doi: 10.1155/2022/9829505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Z., Zhang Y., Lyu X. Promoting roles of KLF5 in myocardial infarction in mice involving microRNA-27a suppression and the following GFPT2/TGF-β/Smad2/3 axis activation. Cell Cycle. 2021;20:874–893. doi: 10.1080/15384101.2021.1907512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua C.C., Liu X.M., Liang L.R., Wang L.F., Zhong J.C. Targeting the microRNA-34a as a Novel Therapeutic Strategy for Cardiovascular Diseases. Front. Cardiovasc. Med. 2022;8:784044. doi: 10.3389/fcvm.2021.784044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guglin M., Rajagopalan N., Anaya P., Charnigo R. Sildenafil in heart failure with reactive pulmonary hypertension (Sildenafil HF) clinical trial (rationale and design) Pulm. Circ. 2016;6:161–167. doi: 10.1086/685548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis G.D., Lachmann J., Camuso J., Lepore J.J., Shin J., Martinovic M.E., Systrom D.M., Bloch K.D., Semigran M.J. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 45.Wang H., Anstrom K., Ilkayeva O., Muehlbauer M.J., Bain J.R., McNulty S., Newgard C.B., Kraus W.E., Hernandez A., Felker G.M., et al. Sildenafil Treatment in Heart Failure With Preserved Ejection Fraction: Targeted Metabolomic Profiling in the RELAX Trial. JAMA Cardiol. 2017;2:896–901. doi: 10.1001/jamacardio.2017.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson E.R., Shah Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013;3:315–330. doi: 10.1002/cphy.c120016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Re D.P., Amgalan D., Linkermann A., Liu Q., Kitsis R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019;99:1765–1817. doi: 10.1152/physrev.00022.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee C.S., Auld J. Heart Failure: A Primer. Crit. Care Nurs. Clin. N. Am. 2015;27:413–425. doi: 10.1016/j.cnc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Yang W.H., Tsai C.H., Fong Y.C., Huang Y.L., Wang S.J., Chang Y.S., Tang C.H. Leptin induces oncostatin M production in osteoblasts by downregulating miR-93 through the Akt signaling pathway. Int. J. Mol. Sci. 2014;15:15778–15790. doi: 10.3390/ijms150915778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li K., Lin T., Chen L., Wang N. MicroRNA-93 elevation after myocardial infarction is cardiac protective. Med. Hypotheses. 2017;106:23–25. doi: 10.1016/j.mehy.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Li F., Tan Y.S., Chen H.L., Yan Y., Zhai K.F., Li D.P., Kou J.P., Yu B.Y. Identification of schisandrin as a vascular endothelium protective component in YiQiFuMai Powder Injection using HUVECs binding and HPLC-DAD-Q-TOF-MS/MS analysis. J. Pharmacol. Sci. 2015;129:1–8. doi: 10.1016/j.jphs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Xiao N., Zhang J., Chen C., Wan Y., Wang N., Yang J. miR-129-5p improves cardiac function in rats with chronic heart failure through targeting HMGB1. Mamm. Genome. 2019;30:276–288. doi: 10.1007/s00335-019-09817-0. [DOI] [PubMed] [Google Scholar]