Abstract
Infection with the parasitic nematode Trichinella spiralis is initiated when the L1 larva invades host intestinal epithelial cells. Monoclonal antibodies specific for glycans on the larval surface and secreted glycoproteins protect the intestine against infection. Protective antibodies recognize tyvelose which caps the target glycan. In this study, we used an in vitro model of invasion to further examine the mechanism(s) by which tyvelose-specific antibodies protect epithelial cells against T. spiralis. Using cell lines that vary in susceptibility to invasion, we confirmed and clarified the results of our in vivo studies by documenting three modes of interference: exclusion of larvae from cells, encumbrance of larvae as they migrated within epithelial monolayers, and inhibition of parasite development. Excluded larvae bear cephalic caps (C. S. McVay et al., Infect. Immun. 66:1941–1945, 1998) of immune complexes that may physically block invasion or may interfere with sensory reception. Monovalent Fab fragments prepared from a tyvelose-specific antibody also excluded larvae from cells, demonstrating that antibody binding can inhibit the parasite in the absence of antigen aggregation and cap formation. In contrast, encumbered larvae caused extensive damage to the monolayer yet were not successful in establishing a niche, as reflected by their failure to molt. These results show that antibodies to tyvelose exhibit multiple modes of inhibitory activity, further implicating tyvelose-bearing glycoproteins as mediators of invasion and niche establishment by T. spiralis.
The parasitic nematode Trichinella spiralis has a wide host range which includes humans and over 100 other vertebrate species (10). T. spiralis infection is acquired by ingestion of muscle tissue containing L1 larvae. Enzymes in the acidic environment of the stomach free larvae from tissue, allowing them to initiate infection by invading columnar epithelial cells in the small intestine. Here, they rapidly undergo four molts, grow, and reproduce (10). Larval and adult stages localize to the crypt-villus junction, where they migrate in what appear to be epithelial syncytia (21, 22). Establishment of T. spiralis in this intestinal habitat is crucial for successful completion of the life cycle.
Although it has been known for many years that T. spiralis invades gut epithelium, the host-parasite relationship at this site is poorly understood. Our approach in investigating this relationship is based on the premise that the study of an effective host immune defense against a pathogen can reveal insights into the mechanisms of parasitism deployed by the agent. We have shown that niche establishment by T. spiralis is prevented in the rat by antibodies which are specific for L1 larval glycoproteins (1, 3). So-called rapid expulsion eliminates up to 100% of an oral dose of L1 larvae within hours of challenge (4, 9, 13, 15). Protective antibodies are specific for tyvelose (3,6-dideoxy-d-arabinohexose) which caps the antennae of tri- and tetra-antennary glycans shared by several secreted and surface glycoproteins (11, 20). Passive immunization of naive, suckling rats with immunoglobulin G (IgG) monoclonal antibodies (MAbs) specific for tyvelose appears to protect the intestine by (i) excluding larvae from intestinal epithelial cells by promoting their entrapment in mucus (7) and (ii) dislodging established larvae from their epithelial habitat (6). Antibodies also appear to immobilize larvae in the epithelium, although this effect is not always associated with expulsion (8, 19).
We have reported that monolayer cultures of several epithelial cell lines are invaded by T. spiralis L1 larvae (14) and have used this assay to examine more closely how antityvelose IgG interferes with the niche of the parasite. Tyvelose-specific antibodies exclude larvae from monolayers of otherwise susceptible Madin-Darby canine kidney (MDCK) cells (16). Excluded larvae bear cephalic caps of immune complexes formed by disgorged glycoproteins and tyvelose-specific antibodies (16, 18; J. Appleton and L. F. Gagliardo, unpublished observations). Antibody binding to surface glycoproteins contributes to efficient protection, perhaps by securing immune complexes to the body of the larva; however, the surface is not the primary target of protective immunity (16).
In this report we describe experiments aimed at extending our understanding of the mechanism(s) by which antibodies protect epithelial cells against T. spiralis. Using cell lines that vary in susceptibility to invasion, we found that tyvelose-specific antibodies excluded larvae from cells, encumbered larvae within cells, and also interfered with the development of the parasite.
MATERIALS AND METHODS
Tissue culture.
Strain I of the Madin-Darby canine kidney (MDCK) cell line was a gift from William Young (University of Kentucky) (17). The human colonic carcinoma cell line Caco-2 and the human intestinal epithelial cell line Henle 407 were obtained from the American Type Culture Collection (Manassas, Va.). Cells were maintained in minimal essential medium (Earle's salts) (MEM) with l-glutamine, nonessential amino acids (GIBCO Laboratories, Grand Island, N.Y.), and 10% fetal bovine serum (FBS). Cells were harvested by trypsinization (0.5% trypsin, 0.65 mM EDTA) and passaged no more than 15 times before use in experiments.
Parasite.
T. spiralis (pig strain) was maintained in irradiated AO rats. Infectious larvae were recovered by 1% pepsin-HCl digestion and activated as described by ManWarren et al. (14).
MAbs.
Rat MAbs used in these experiments are described in Table 1. Antibodies were concentrated from ascites fluid (prepared in nude mice) or pooled normal rat sera by (NH4)2SO4 precipitation. We have described previously that this method yields MAbs of high purity from nude mouse ascites fluid (6). Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.).
TABLE 1.
MAbs used in experiments
Preparation of MAb 18H Fab fragments.
MAb 18H, concentrated from ascites fluid as described above, was digested with immobilized papain (Pierce, Rockford, Ill.). Fcγ fragments and any undigested antibodies were removed from digestion products by using an affinity column prepared with antibodies specific for rat Fcγ (Organon Teknika-Cappel, Malvern, Pa.). The unbound portion, containing the Fab fragments, was collected (Pharmacia Biotech, Piscataway, N.J.). Immunoblotting was used to verify the isolation and purity of Fab fragments. Blots from nonreduced, sodium dodecyl sulfate–10% polyacrylamide gels were probed with mouse anti-rat κ-chain MAb (MARK-1; kindly provided by H. Bazin, University of Louvain, Brussels, Belgium) (2) and developed with peroxidase-conjugated goat anti-mouse IgG to reveal the presence of Fab fragments. Duplicate blots were probed with peroxidase-conjugated goat anti-rat Fcγ (Organon Teknika-Cappel) to detect the presence of intact antibody or heavy chains. Blots were developed with a chemiluminescent substrate (Amersham Life Science, Inc., Cleveland, Ohio). Using these methods, Fab fragments were confirmed to be free of Fcγ, F(ab′)2 or whole IgG. Fragments were concentrated by filtration (Centriprep-10; Amicon Inc., Beverly, Mass.) and then centrifuged at 100,000 × g for 60 min to remove any protein aggregates.
Protection assay.
The invasion assay was performed as previously described (14), with modifications. Epithelial cells were grown to confluence on eight-well chamber slides (NUNC, Naperville, Ill.). Monolayers were overlaid with activated larvae suspended in MEM (without FBS) with 15 mM HEPES and 1.75% agarose containing the appropriate concentration of antibody. Following incubation for 1 to 2 h at 37°C in 5% CO2, chamber housings, gaskets, and media were removed from slides. Dead cells in monolayers were stained with 0.4% trypan blue in saline (Sigma, St. Louis, Mo.). Stained monolayers were rinsed in Dulbecco's phosphate-buffered saline (DPBS) (with MgCl2 and CaCl2) and fixed in 10% buffered formalin for 20 min. Cover slips were mounted on slides with Glycergel (DAKO Corp., Carpenteria, Calif.). A minimum of 25 microscope fields from each monolayer were captured using a 4× objective on a bright-field microscope (Labophot; Nikon) fitted with a black-and-white video camera (Cohu, Inc., San Diego, Calif.). A frame grabber captured the image, and the area of dead or damaged cells was determined with NIH Image 1.58 software.
The activity of larvae in Caco-2 monolayers was evaluated using two additional parameters: length measurements of the dead cell trails and number of worms retained in monolayers at the end of an experiment. Trail lengths were measured in trypan blue-stained monolayers, using the microscope, camera, and software described above. Trail length was also determined in monolayers stained with propidium iodide (14). These monolayers were examined with an inverted microscope (Diaphot; Nikon) equipped with epifluorescence (Opti-quip, Highland Mills, N.Y.) and a charge-coupled device camera (Hamamatsu Photonics K. K., Hamamatsu City, Japan). Images were analyzed using NIH Image 1.58. Because propidium iodide is more sensitive than trypan blue, values from monolayers stained with propidium iodide were much higher.
Molting assay.
We evaluated the influence of antityvelose IgG on larval development in culture. Caco-2 cells were grown in 12-well plates (Costar, Corning, N.Y.), inoculated, and cultured with larvae in the presence or absence of antibody for 24 h. We added 10% FBS to the agarose medium used in molting experiments in order to support cell viability. Larvae were recovered from wells and processed for fluorescent antibody staining using MAb 18H as previously described (14). Only L1 larvae display tyvelose and thus bind MAb 18H. The percentage of larvae that were not fluorescent (i.e., had molted) in each well was determined; this value was divided by the total larvae inoculated and expressed as the percent larvae molted.
Statistical analysis.
The mean area of dead cells per field, total trail length per monolayer, worm number per monolayer, or percentage of larvae that had molted (±1 standard deviation [SD]) was estimated for three or four monolayers per treatment. Mean values for the treatment groups were compared by analysis of variance, and significant differences were identified by Scheffé's mean separation test.
Images.
Images were captured using a charge-coupled device camera for fluorescence (Hamamatsu Photonics) or a black-and-white video camera for bright field (Cohu) and NIH Image 1.58. Files were imported into Adobe Photoshop 4.0 for labeling and formatting using a Power Macintosh 7200/120 computer and were printed by a dye diffusion printer (NP-1600; Codonics, Middleburg Heights, Ohio).
RESULTS
High concentrations of antityvelose monoclonal IgG prevent invasion of MDCK cells.
We tested the ability of each MAb to prevent invasion of MDCK cells by L1 larvae. MAbs 9D, 9E, and 18H bind to tyvelose on secreted and surface glycoproteins and are protective in vivo (1, 3), while MAbs 18B and 16H have an alternate specificity for secreted and surface antigens and are not protective in vivo (1) (Table 1). Larvae cocultured with MAbs 18B and 16H or normal rat globulin (NRG) invaded and damaged MDCK monolayers. We observed no difference in the extracellular mobility or browsing behavior of the larvae in the presence of these antibodies compared to larvae inoculated in the absence of antibody. In contrast, larvae cultured in the presence of tyvelose MAbs were virtually excluded from the cells and did not damage monolayers (Table 2). As we have described previously, tyvelose antibodies induced the formation of cephalic caps on the larvae (16). Staining with fluorescein isothiocyanate-conjugated anti-rat IgG confirmed that the aggregates were comprised of immune complexes (not shown), as was originally described by Oliver-González (18). Caps form a barrier between the head of the larva and the cell. Furthermore, caps are positioned to interfere with sensory receptors in amphids and thus may prevent the larva from receiving sensory input from epithelial cells.
TABLE 2.
High concentrations of tyvelose-specific MAbs protect MDCK cells from invasion by T. spiralis larvae
| Treatment
|
Damage to monolayer (mm2, 103)a
|
||
|---|---|---|---|
| Antibody | Concn (mg/ml) | Uninfected control | Infected |
| NRG | 1.0 | 8 ± 2 | 26 ± 7b |
| 16H | 1.0 | 7 ± 1 | 29 ± 7b |
| 18B | 1.0 | 6 ± 1 | 25 ± 4b |
| 9D | 1.0 | 5 ± 3 | 8 ± 2 |
| 0.5 | ND | 9 ± 3 | |
| 9E | 1.0 | 8 ± 2 | 7 ± 2 |
| 0.5 | ND | 10 ± 1 | |
| 18H | 1.0 | 7 ± 3 | 5 ± 1 |
| 0.5 | ND | 11 ± 3 | |
Mean ±1 SD; n = 3 or 4 monolayers. ND, not determined.
Mean significantly different from that of treatment-matched, uninfected control (P ≤ 0.01).
Titration of tyvelose-specific antibodies in cultures of varying susceptibility to T. spiralis reveals two modes of protection.
To further investigate the protective modes of antibodies, we titrated antibodies at concentrations that were more physiologic. Furthermore, we compared human Caco-2 and Henle 407 cells with MDCK cells for susceptibility to invasion as well as protection against it.
(i) MDCK cells.
When grown in glass chamber slides, MDCK cells (strain I) formed a dense monolayer of spindle-shaped cells with an average density of 2.7 × 105 cells/cm2. Larvae browsed the monolayer for several minutes before invading cells. At the conclusion of experiments, larvae were readily dislodged from the monolayers during the process of staining and fixation. Larvae created plaques and short, serpentine trails of dead cells which were revealed by staining with trypan blue (14). These observations support the conclusion that MDCK cells were relatively resistant to infection in comparison with Caco-2 and Henle 407 cells (see below).
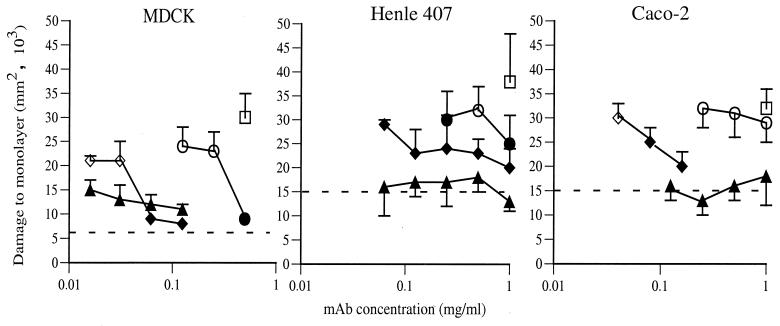
All three tyvelose-specific antibodies protected MDCK monolayers against larval damage, but antibody titrations revealed dramatic differences. As little as 0.016 mg of 18H or 0.061 mg of 9E per ml was protective, while MAb 9D failed to protect at concentrations below 0.50 mg/ml (Fig. 1). Each of the antibodies excluded larvae from the monolayers, and with 9D and 9E there were no intermediate levels of protection (i.e., partial reduction in damage to the monolayer).
FIG. 1.
Tyvelose-specific MAbs protect cultured epithelial cells from invasion by T. spiralis larvae. Confluent monolayers of MDCK, Henle 407, and Caco-2 cells were inoculated with L1 larvae in the presence of MAbs 9D (●), 9E (⧫), or 18H (▴) or of NRG or MAb 16H (as a negative control [□]) as described in Materials and Methods. Data points are means of areas of dead cells (±1 SD) in trypan blue-stained monolayers (n = 3 or 4). Open symbols indicate means that were significantly greater (P ≤ 0.05) than that of uninfected control cultures (represented by dashed lines).
(ii) Henle 407 cells.
When grown in glass chamber slides, Henle 407 cells formed a dense monolayer of cuboidal cells with an average density of 6.5 × 105 cells/cm2. Henle 407 cells appeared to be of intermediate susceptibility to invasion. Migrating larvae created serpentine trails of dead cells but were not retained in the monolayers during the process of fixation and staining.
Antibody 18H was highly protective of Henle 407 cells, preventing any significant damage at a concentration of 0.063 mg/ml (Fig. 1; endpoint was not determined). Titration curves for MAbs 9E and 9D were different for Henle 407 than for MDCK cultures (Fig. 1). Although MAb 9E afforded a significant level of protection at all concentrations tested, protection was never absolute (as seen with MAb 18H), even at concentrations as high as 1 mg/ml. MAb 9D was least protective, allowing larvae to invade and mediating a marginal, variable reduction in damage (Fig. 1).
(iii) Caco-2 cells.
When grown in glass chamber slides, Caco-2 cells were large and cuboidal, forming monolayers with an average density of 1.9 × 105 cells/cm2. Of the three cell lines tested, Caco-2 was the most susceptible to invasion by T. spiralis L1 larvae, according to the following criteria: (i) the period of larval browsing was reduced (larvae invaded monolayers within seconds of inoculation); (ii) larvae produced long, contiguous trails, suggesting that their occupation of the intracellular site was prolonged in comparison with the other cells; and (iii) larvae were retained in Caco-2 monolayers throughout the staining and fixing process, suggesting that they were more effectively sequestered in the monolayer. The latter characteristic allowed us to evaluate exclusion by counting the number of larvae retained in the monolayer at the end of the experiment.
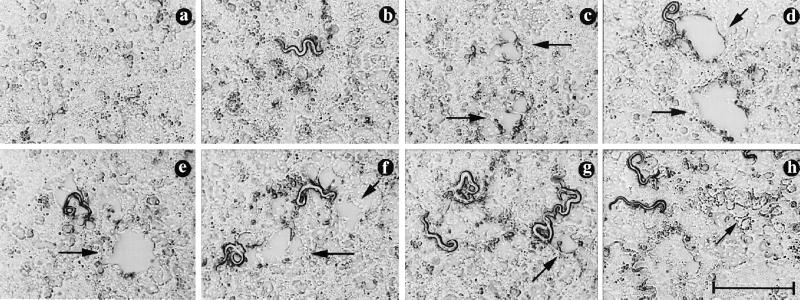
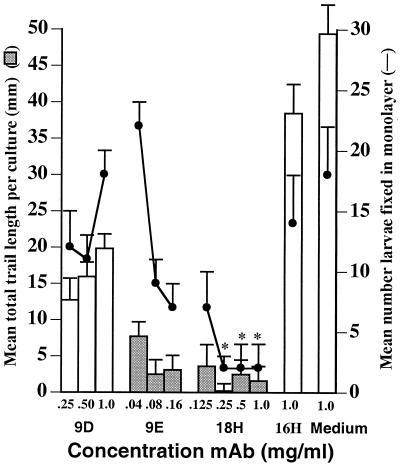
At concentrations of 0.25 mg/ml and greater, MAb 18H protected Caco-2 monolayers by excluding larvae from monolayers (Fig. 2b and c; Fig. 3). Larvae cultured with 0.125 mg of 18H per ml or less were less effectively excluded and larvae that entered cells became encumbered in them. Encumbered larvae eventually pulled up sections of the monolayer, creating plaques (Fig. 2d). Because plaques are essentially empty spaces in trypan blue-stained monolayers, image capture does not accurately record them. To better assess the effects of antibodies on larvae under these conditions, we measured the mean trail length for each culture, a parameter which reflects the combined outcomes of successful invasion and migration by larvae. Encumbrance was best demonstrated when larvae were cultured with 0.04 mg of MAb 9E per ml. Although large numbers of larvae invaded the monolayers, trail lengths were reduced dramatically (Fig. 3) and larvae created large plaques (Fig. 2e and f). Antibody 9D did not appear to prevent damage (Fig. 1), nor did it reduce the number of larvae in monolayers (Fig. 3). Nevertheless, larvae were inhibited in their migration by 9D, as reflected by reduced trail lengths (50 to 70% reduction), although the values were not statistically significantly different from controls (Fig. 3). The inhibitory effect of antibody 9D was demonstrated further by plaques and encumbered larvae that were evident in these cultures (Fig. 2g).
FIG. 2.
Tyvelose-specific MAbs exclude and encumber L1 larvae in Caco-2 cultures. Digital images show fixed Caco-2 monolayers stained with trypan blue. (a) Uninfected control; (b to h) larvae cultured with 18H at 1.0 (b), 0.5 (c), and 0.125 (d) mg/ml, 9E at 0.16 (e) and 0.08 (f) mg/ml, 9D at 1.0 mg/ml (g), and nonprotective 16H at 1.0 mg/ml (h). Arrows indicate plaques (c to g) or trails of dead cells (h). Bar represents 1 mm. Images were captured as described in Materials and Methods. A Power Macintosh 7200/120 computer and Adobe Photoshop 4.0 were used to prepare the figure.
FIG. 3.
MAbs exclude or encumber L1 larvae in Caco-2 monolayers. L1 larvae were inoculated onto Caco-2 cells in the presence of medium alone or with MAb 16H (negative controls), 9D, 9E, or 18H as described in Materials and Methods. Bars represent means of summed trail lengths (±1 SD) per trypan blue-stained monolayer (n = 3 to 4 cultures); data points represent mean numbers of larvae fixed in monolayers (±1 SD) (n = 3 to 4). Shaded bars represent trail lengths that were significantly less than those of infected negative control monolayers (P < 0.005). Asterisks indicate mean larvae numbers that were significantly different from those of infected negative control cultures (P < 0.05).
In summary, two types of inhibitory activity were demonstrated by tyvelose-specific MAbs: antibodies caused larvae to be excluded from cells or to become encumbered in the monolayer. The relative protective efficacies of the MAbs were similar with all three cell lines tested: 18H > 9E > 9D.
Monovalent antibody binding to tyvelose prevents invasion of MDCK cells.
To clarify the requirement for antibody cross-linking in exclusion of larvae from epithelial cells or encumbrance in cells, we prepared Fab fragments of antibody 18H. The fragments were shown to be free of intact antibodies by Western blotting using highly sensitive, chemiluminescence-based detection.
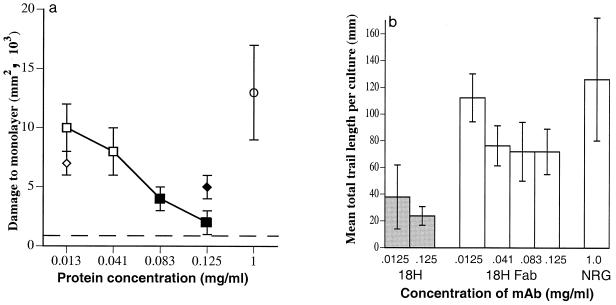
Fab fragments of 18H prevented T. spiralis larvae from invading MDCK cells, although higher molar concentrations of Fab (1.66 nM) than of intact 18H (0.1 nM) were required (Fig. 1 and 4a). This difference may be due, in large part, to the reduced avidity of the Fab fragments. Caps did not form on larvae cultured in the presence of 18H-Fab, supporting the conclusion that caps are immune complexes. Exclusion by 18H-Fab indicates that binding to tyvelose has an inhibitory effect on the larva which is distinct from cap formation.
FIG. 4.
(a) Fab fragments of MAb 18H block invasion of MDCK monolayers by T. spiralis larvae. L1 larvae were cocultured with intact MAb 18H (◊), 18H-Fab fragments (□), or NRG (○) (negative control). Damage was measured by image capture and analysis of trypan blue-stained monolayers as described in Materials and Methods (n = 3 to 4 monolayers). Open symbols represent values significantly greater than that of an uninoculated control (dashed line) (P ≤ 0.02). (b) Effect of Fab fragments of MAb 18H on migration of larvae in Caco-2 monolayers. The experiments were performed as for panel a except that monolayers were stained with propidium iodide, fixed, and analyzed as described in Materials and Methods. Bars represent mean summed length (±1 SD) of dead cell trails per monolayer (n = 3 to 4 monolayers). Shaded bars represent values significantly different from those for monolayers infected in the presence of NRG (P < 0.05).
When tested in Caco-2 cells, 18H-Fab inhibited migration by larvae by as much as 47%. However, the reduction was not statistically significant at any of the concentrations tested (Fig. 4b).
Antibodies inhibit molting of T. spiralis larvae in Caco-2 cells.
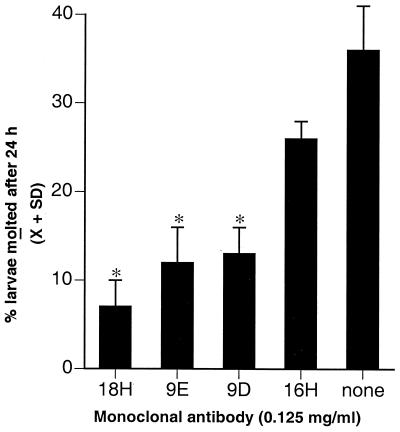
Preliminary experiments revealed that large numbers of L1 larvae will execute at least one molt when cultured in Caco-2 cells for 24 h (Fig. 5; L. F. Gagliardo, C. S. McVay, and J. Appleton, unpublished observations). In these cultures, both tyvelose-negative larvae (i.e., L2 or more mature stages) and tyvelose-positive, empty cuticles can be detected. Although FBS was present in molting experiments, we have found that FBS is not required for molting in vitro (Gagliardo and Appleton, unpublished observations). To determine whether the encumbrance of larvae in Caco-2 cells caused by tyvelose antibodies would interfere with larval development, we assessed molting in such cultures after 24 h. MAbs 18H and 9E consistently inhibited molting in these experiments when tested at concentrations of 0.125 mg/ml. Parallel cultures that were fixed after 2 h of incubation had large numbers of plaques and encumbered larvae (not shown) as observed in previous experiments. The presence of FBS in these experiments did not alter the activities of the MAbs. MAb 9D4 was highly variable in effect, although it sometimes inhibited molting (Fig. 5 and data not shown). These results indicate that the immediate encumbrance of larvae in cell layers had an additional, long-term inhibitory effect on parasite development.
FIG. 5.
Molting of L1 larvae in Caco-2 cells is inhibited by tyvelose-specific MAbs. Larvae were cultured without antibody (none) or with 0.125 mg of MAb 18H, 9E, 9D, or 16H per ml. The mean (±1 SD) of quadruplicate cultures is graphed for each treatment. Asterisks indicate means that were significantly different from those for monolayers infected in the presence of MAb 16H or no antibody (P < 0.001).
DISCUSSION
Suckling rat pups passively immunized with tyvelose-specific antibodies are protected against challenge infection with T. spiralis L1 larvae (1). The simplicity of this type of protective immunity has made it attractive for study, as it is free from complicating variables associated with immune and inflammatory responses that arise in animals that have been infected previously with the parasite. Evidence from passive immunization studies indicates that the specific interaction of protective antibodies with tyvelose moieties on secreted glycoproteins both prevents invasion and dislodges established larvae from host intestinal epithelium (6, 8, 19). The consequence of these activities, possibly together with undefined innate host defenses, is the rapid and sometimes complete expulsion of L1 larvae from the host small intestine.
Detailed investigation of expulsion is complicated by the problems attendant in evaluating the niche of T. spiralis in vivo. These challenges prompted us to apply an in vitro culture technique in which larvae and antibodies are inoculated onto the apical aspect of epithelial monolayers. We used human and canine cell lines combined with rat antibodies. Although there may be some unknown (but relevant) incompatibility between rat IgG and epithelial cells from other species, our choice of MDCK and Caco-2 cells was largely based on the fact that both are known to polarize, that is, to develop a differentiated epithelial cell phenotype in culture. We deemed this to be crucial to modeling the intestinal epithelium. Furthermore, both cell lines are susceptible to invasion by T. spiralis (14). There is no rat epithelial cell line available that shares these characteristics. The experimental system described here serves to model only the effects of luminal antityvelose IgG. Maternal IgG in suckling rats is not strictly luminal, as pups absorb antibodies from milk throughout the suckling period via an Fc receptor-driven transport across enterocytes (5). The distribution of IgG in the epithelium of the suckling rat differs from that of cultured epithelial cells, as Fc receptors have not been described in the cell lines used in this study. Nevertheless, the relevance of the in vitro model of luminal antibody is supported by earlier observations that antityvelose IgG F(ab′)2, which is confined to the lumen of the neonatal rat intestine, affords protection against infection with T. spiralis larvae (8). Obviously, there are other aspects of the neonatal intestine that are not reproduced in the in vitro system, for example, innate defenses such as complement and mucus. Although these potential cofactors were not evaluated in the experiments reported here, the model would accommodate their testing readily. The aim of the experiments described in this report was to identify direct inhibitory effects of luminal antibodies on T. spiralis larvae.
We compared three MAbs that differ in their protective capacities in vivo. Antibody 18H affords a weak level of protection (18 to 28%; statistically insignificant) to suckling rats, while antibodies 9D and 9E afford dramatic protection (1). All three antibodies protect adult rats under certain conditions (3). The antibodies differ in two respects that might influence their protective activities. First, they represent three different rat IgG subclasses (Table 1). Enzymatic removal of the Fc region of 18H to produce F(ab′)2 fragments does not improve its protective capacity in suckling rats, while similar treatment of 9E does not compromise its protective activity (8). Thus, the Fc region or isotype of these antibodies does not appear to play a role in protection. Second, detailed structural analysis of the glycan epitopes recognized by these antibodies has revealed that they vary in their fine specificities and affinities (12). The parasite glycans that are the binding targets for protective antibodies are large tri- and tetra-antennary, N-linked structures (20). The antennae of the tetra-antennary structures are of two types: three are fucosylated, branched structures; the fourth is unbranched (12). Antibody 18H shows a strong bias in binding to the nonfucosylated trisaccharide antenna [β-d-Tyvp (1-3)-β-d-GalNAcp(1-4)-β-d-GlcNAcp], 9E shows a strong bias for fucosylated tetrasaccharide antennae {β-d-Tyvp (1-3)-β-d-GalNAcp(1-4)[α-l-Fucp(1-3)]-β-d-GlcNAcp}, and 9D has the highest affinity for the tetrasaccharide of the three antibodies. These findings prompted us to attribute the highly protective nature of 9E and 9D to their binding to the more numerous, branched antennae (12); however, this has yet to be proven experimentally.
Results from in vitro studies reported here would appear to contradict the findings in suckling rats. Specifically, 18H affected larval behavior in all three cell lines, 9E was intermediate in effect, while 9D consistently was only marginally active. Comparison of data collected in vitro and in vivo indicates that the cell culture system most effectively models early events in protective immunity. For example, oral administration of 18H or 18H-F(ab′)2 fragments cause larvae to become entrapped in mucus in the intestinal lumen during the first hour following infection (8). Although the molecular events involved in mucus entrapment are not known, mucus-entrapped larvae are coated with antibody (6). Because F(ab′)2 fragments are restricted to the intestinal lumen, mucus entrapment by 18H-F(ab′)2 fragments shows that entrapment occurs when larvae bind luminal antibodies. Thus, 18H is capable of interacting with larvae in the lumen of the gut, and this interaction temporarily compromises the ability of the larva to invade the epithelium. We have reproduced this effect in vitro by showing that one inhibitory effect of apical (or luminal) 18H was exclusion of larvae from epithelial monolayers.
Antibody 9E was less efficient in excluding larvae from MDCK cells although it was quite effective at inhibiting larval migration in the Caco-2 monolayers. Previous reports described the encumbrance of larvae in intestinal tissue in passively immunized rats (8, 19). In normal rats, larvae are recoverable from intestinal tissue by incubating intestines (luminal contents are first removed) in warm saline for 5 h. Passive immunization with 9E causes large numbers of larvae to be inhibited from migrating into saline; these larvae can be recovered only by enzymatic digestion of the intestine. This immobility occurred in weaned rats that were passively immunized intraperitoneally with 9E and also in suckling rats immunized by the same route with F(ab′)2 fragments of 9E. The basis for the immobility was difficult to evaluate further. In the present study we found that tyvelose-specific antibodies caused larvae to become entangled in the cells they invaded. Extensive damage to monolayers was caused by encumbered larvae as they thrashed and pulled cells off the glass. This type of encumbrance was the predominant effect of MAbs 9E and 9D in Caco-2 cell cultures. Whether or not it is the same effect described as immobility in vivo is not certain. Regardless, encumbrance clearly interfered with the niche of the larva. Further evidence for niche disruption was found when antibodies that encumbered larvae also prevented molting. Molting by T. spiralis in vitro has never before been described, and the specific host factors required for larval development are unknown.
To test the hypothesis that immune complex formation was required for encumbrance or to block entry of larvae into cells, we prepared monovalent Fab fragments of MAb 18H. The fragments had limited (statistically insignificant) effects in Caco-2 cultures, suggesting that encumbrance may be dependent on cross-linking by antibodies. This conclusion requires more testing. In contrast, 18H-Fab excluded larvae from MDCK cells, indicating that simple blocking of tyvelose by Fab fragments interferes with invasion. Thus, although caps of immune complexes may form a physical barrier and/or block sensory receptors and thereby interfere with invasion, these are not the only means of preventing larvae from entering cells. These observations implicate tyvelose-bearing molecules as active participants in the process of invasion and enhance interest in their function in parasitism. To date there has been no convincing demonstration of function for any of these glycoproteins.
In summary, we have described three inhibitory activities of antibodies specific for tyvelose-bearing glycoproteins of T. spiralis L1 larvae: exclusion of larvae from epithelial cells, encumbrance of larvae as they migrated within epithelial monolayers, and interference with molting. Exclusion occurred not only when whole antibodies formed caps of immune complexes around the larval stoma but also when Fab fragments were used and there were no caps evident. The latter result further implicates parasite glycoproteins as mediators of invasion. The phenomena observed correlate with certain antibody-mediated effects that we have described in passively immunized suckling rats. It is reasonable to conclude that in the presence of a polyclonal antibody response the different effects work in combination, possibly in cooperation with innate host defenses, to cause nematode expulsion from the intestine.
ACKNOWLEDGMENTS
This research was supported by USPHS grant AI 14490 from the National Institute of Allergy and Infectious Diseases. P.B. was supported by a summer research fellowship from the U.S. Department of Agriculture (grant 95-38411-2476).
We thank B. Butcher for helpful discussions and F. Romaris for constructive criticism of the manuscript.
REFERENCES
- 1.Appleton J A, Schain L R, McGregor D D. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988;65:487–492. [PMC free article] [PubMed] [Google Scholar]
- 2.Bazin H, Cormont F, De Clercq L. Rat monoclonal antibodies. II. A rapid and efficient method of purification from ascitic fluid or serum. J Immunol Methods. 1984;71:9–16. doi: 10.1016/0022-1759(84)90200-x. [DOI] [PubMed] [Google Scholar]
- 3.Bell R G, Appleton J A, Negrao-Correa D A, Adams L S. Rapid expulsion of Trichinella spiralis in adult rats mediated by monoclonal antibodies of distinct IgG isotypes. Immunology. 1992;75:520–527. [PMC free article] [PubMed] [Google Scholar]
- 4.Bell R G, McGregor D D, Despommier D D. Trichinella spiralis: mediation of the intestinal component of protective immunity in the rat by multiple, phase-specific, antiparasitic responses. Exp Parasitol. 1979;47:140–157. doi: 10.1016/0014-4894(79)90068-7. [DOI] [PubMed] [Google Scholar]
- 5.Borthistle B K, Kubo R T, Brown W R, Grey H M. Studies on receptors for IgG on epithelial cells of the rat intestine. J Immunol. 1977;119:471–476. [PubMed] [Google Scholar]
- 6.Carlisle M S, McGregor D D, Appleton J A. The role of mucus in antibody-mediated rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1990;70:126–132. [PMC free article] [PubMed] [Google Scholar]
- 7.Carlisle M S, McGregor D D, Appleton J A. Intestinal mucus entrapment of Trichinella spiralis larvae induced by specific antibodies. Immunology. 1991;74:546–554. [PMC free article] [PubMed] [Google Scholar]
- 8.Carlisle M S, McGregor D D, Appleton J A. The role of the antibody Fc region in rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1991;74:552–558. [PMC free article] [PubMed] [Google Scholar]
- 9.Castro G A, Badial-Aceves F, Adams P R, Copeland E M, Dudvick S J. Response of immunized, parenterally nourished rats to challenge infection with the nematode, Trichinella spiralis. J Nutr. 1976;106:1484–1491. doi: 10.1093/jn/106.10.1484. [DOI] [PubMed] [Google Scholar]
- 10.Despommier D D. Biology. In: Campbell W C, editor. Trichinella and trichinosis. New York, N.Y: Plenum Press; 1983. pp. 75–151. [Google Scholar]
- 11.Ellis L A, Reason A J, Morris H R, Dell A, Iglesias R, Ubeira F M, Appleton J A. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology. 1994;4:585–592. doi: 10.1093/glycob/4.5.585. [DOI] [PubMed] [Google Scholar]
- 12.Ellis L A, McVay C S, Probert M A, Zhang J, Bundle D R, Appleton J A. Terminal β-linked tyvelose creates unique epitopes in Trichinella spiralis glycan antigens. Glycobiology. 1997;7:383–390. doi: 10.1093/glycob/7.3.383. [DOI] [PubMed] [Google Scholar]
- 13.Love R J, Ogilvie B M, McLaren D J. The immune mechanism which expels the intestinal stage of Trichinella spiralis from rats. Immunology. 1976;30:7–15. [PMC free article] [PubMed] [Google Scholar]
- 14.ManWarren T, Gagliardo L, Geyer J, McVay C, Pearce-Kelling S, Appleton J. Invasion of intestinal epithelia in vitro by the parasitic nematode Trichinella spiralis. Infect Immun. 1997;11:4806–4812. doi: 10.1128/iai.65.11.4806-4812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy O R. Rapid loss of Trichinella spiralis larvae fed to immune rats and its bearing on the mechanisms of immunity. Am J Hyg. 1940;32:105–116. [Google Scholar]
- 16.McVay C S, Tsung A, Appleton J. Participation of parasite surface glycoproteins in antibody-mediated protection of epithelia against Trichinella spiralis. Infect Immun. 1998;66:1941–1945. doi: 10.1128/iai.66.5.1941-1945.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols G E, Lovejoy J C, Borgnam C A, Sanders J M, Young W J. Isolation and characterization of two types of MDCK epithelial cell clones based on glycosphingolipid pattern. Biochim Biophys Acta. 1986;887:1–12. doi: 10.1016/0167-4889(86)90115-1. [DOI] [PubMed] [Google Scholar]
- 18.Oliver-González J. The in vitro action of immune serum on the larvae and adults of Trichinella spiralis. J Infect Dis. 1940;67:292–300. [Google Scholar]
- 19.Otubu O E, Carlisle-Nowak M S, McGregor D D, Jacobson R H, Appleton J A. Trichinella spiralis: the effect of specific antibody on muscle larvae in the small intestines of weaned rats. Exp Parasitol. 1993;76:394–400. doi: 10.1006/expr.1993.1048. [DOI] [PubMed] [Google Scholar]
- 20.Reason A J, Ellis L A, Appleton J A, Wisnewski N, Grieve R B, McNeil M, Wassom D L, Morris H R, Dell A. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology. 1994;4:593–603. doi: 10.1093/glycob/4.5.593. [DOI] [PubMed] [Google Scholar]
- 21.Wright K A. Trichinella spiralis: an intracellular parasite in the intestinal phase. J Parasitol. 1979;65:441–445. [PubMed] [Google Scholar]
- 22.Wright K A, Weidman E, Hong H. The distribution of cells killed by Trichinella spiralis in the mucosal epithelium of two strains of mice. J Parasitol. 1987;73:935–939. [PubMed] [Google Scholar]