Abstract
In gram-negative bacteria, high-affinity iron uptake requires the TonB/ExbB/ExbD envelope complex to release iron chelates from their specific outer membrane receptors into the periplasm. Based on sequence similarities, the Bordetella pertussis tonB exbB exbD locus was identified on a cloned DNA fragment. The tight organization of the three genes suggests that they are cotranscribed. A putative Fur-binding sequence located upstream from tonB was detected in a Fur titration assay, indicating that the tonB exbB exbD operon may be Fur-repressed in high-iron growth conditions. Putative structural genes of the β-subunit of the histone-like protein HU and of a new two-component regulatory system were identified upstream from tonB and downstream from exbD, respectively. A B. pertussis ΔtonB exbB::Kmr mutant was constructed by allelic exchange and characterized. The mutant was impaired for growth in low-iron medium in vitro and could not use ferrichrome, desferal, or hemin as iron sources. Levels of production of the major bacterial toxins and adhesins were similar in the TonB+/TonB− pair. The ΔtonB exbB mutant was still responsive to chemical modulators of virulence; thus, the BvgA/BvgS two-component system is not TonB dependent. Nevertheless, in vivo in the mouse respiratory infection model, the colonization ability of the mutant was reduced compared to the parental strain.
Most bacteria require an iron concentration of 10−6 to 10−8 M for growth. In the host, iron is not readily available to microorganisms since Fe(III) is bound to transferrin (TF) in the serum and to lactoferrin (LF) in other secretions. The concentration of free iron in body fluids is estimated to be less than 10−18 M; thus, the ability of a pathogen to scavenge iron may represent an important virulence trait (65). Some bacteria, e.g., Neisseria spp. and Haemophilus influenzae, produce cell surface receptors for TF, LF, heme, or heme-containing proteins (10, 24, 37, 53). Others, e.g., Escherichia coli and Pseudomonas spp., secrete low-molecular-weight iron chelators termed siderophores which are able to remove Fe(III) from TF or LF (44). Iron-loaded siderophores can then bind to high-affinity receptors on the bacterial cell surface, and be internalized. In gram-negative bacteria the TonB/ExbB/ExbD complex, referred to as the Ton system, interacts with the outer membrane receptors involved in iron uptake and transduces the energy required for the transfer of Fe(III) from TF and LF or that of heme or ferrisiderophores into the periplasm. TonB is anchored in the inner membrane, where it is stabilized by the ExbB and ExbD proteins (for a review, see reference 43). Vitamin B12, group B colicins, and certain phages are also delivered into the cell via specific receptors and the Ton system in E. coli (13, 30, 50). Through a cycle of conformational changes TonB couples the cytoplasmic membrane protonmotive force to active transport across the outer membrane (35).
We were interested in deciphering the iron uptake systems and the potential influence of the iron regulatory network in virulence in bordetellae. Bordetella pertussis, the etiologic agent of whooping cough, Bordetella parapertussis, which infects humans and sheep, and Bordetella bronchiseptica, the causative agent of swine atrophic rhinitis and kennel cough, synthesize alcaligin, a hydroxamate-type siderophore. Bordetella avium, a poultry pathogen, does not seem to produce siderophore (17, 51). We and others independently identified and characterized alcR, the gene encoding an AraC-type activator of the alcaligin biosynthesis operon in B. pertussis, B. parapertussis, and B. bronchiseptica (6, 51). The expression of the recently identified alcaligin receptor gene is also AlcR regulated in B. bronchiseptica (6, 14). Surprisingly, the virulence of a B. pertussis alcR null mutant is not impaired in the mouse respiratory infection model (51). This observation suggests that B. pertussis possesses alcaligin-independent iron uptake systems which may contribute to efficient colonization of the host. An LF-binding protein has been detected in membrane fractions of bordetellae, but its role in iron uptake has not been established yet (40). In addition, several exogenous siderophore receptors have been identified in B. pertussis. These include BfeA, which binds enterobactin, and BfrB and BfrC, the receptors for unknown siderophores (3, 5). A B. bronchiseptica-specific receptor, BfrA, has also been characterized, but its ligand remains unidentified (4). B. pertussis is also able to use hemin as a sole iron source, suggesting that it produces an outer membrane heme receptor (4). Heme uptake is Ton dependent in several pathogens (29, 38, 60, 62), although in Neisseria gonorrhoeae and Haemophilus ducreyi heme uptake does not require the Ton system (8, 19). In order to evaluate the role of the Ton system in B. pertussis iron uptake and virulence, we first identified and characterized the tonB exbB exbD locus in this species. A B. pertussis ΔtonB exbB mutant was constructed and compared to the parental strain. The mutant presented reduced growth in iron-depleted medium and was deficient in exogenous siderophores, hemin and albomycin uptake. The mutant was also impaired in its ability to colonize the respiratory tract of infected mice, although the expression and in vitro regulation of Bvg-dependent virulence factors proved to be unaffected. Thus, our data suggest that TonB-dependent transport systems are important, yet Bvg-independent virulence traits in B. pertussis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this work are listed in Table 1. E. coli strains were grown at 37°C in Luria-Bertani (LB) medium (42) or on solid media obtained by addition of 1.5% (wt/vol) Bacto-Agar. In the Fur titration assay (58), the Lac phenotype of E. coli H1717 transformants was tested on MacConkey lactose agar plates containing 50 μM FeCl3. Bordetella strains were grown at 37°C on Bordet-Gengou (BG) agar base plates supplemented with 1% glycerol and 15% sheep blood. Liquid cultures were usually grown in modified Stainer-Scholte (SS) medium containing (per liter): 11.84 g of Na–l-glutamate · H2O, 0.24 g of l-proline, 2.5 g of NaCl, 0.5 g of KH2PO4, 0.2 g of KCl, 0.1 g of MgCl2 · 6H2O, 20 mg of CaCl2 · 2H2O, 1.5 g of Tris, 10 g of Casamino Acids, 1 g of dimethyl β-cyclodextrin (a gift from Teijin, Japan), 40 mg of l-cystein, 4 mg of nicotinic acid, 0.4 g of ascorbic acid, 0.15 g of glutathione, and 10 mg of FeSO4 · 7H2O. Low-iron medium was SS without addition of FeSO4 · 7H2O (SS-Fe). Some growth tests were performed in Casamino Acid-free SS medium or in SS supplemented with only 0.1% Casamino Acids. Modulation conditions were obtained by the addition of 50 mM MgSO4 or 15 mM nicotinic acid to SS. When necessary, antibiotics were included in the growth media at the following final concentrations: ampicillin (Ap), 150 mg/ml; chloramphenicol (Cm), 30 mg/ml; gentamicin (Gn), 10 μg/ml; kanamicin (Km), 30 μg/ml; nalidixic acid (Nal), 30 μg/ml; streptomicin (Sm), 100 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant featuresa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| RK5048 | metE70 tonB | 26 |
| H1717 | aroB fhuF::λ placMu; Kmr | 58 |
| XL1-Blue | High efficiency transformation; Tcr | Stratagene |
| SM10 | Mobilizing strain; Kmr | 57 |
| BL21(DE3)/pLysS | High-stringency expression host; Cmr | Novagen |
| B. avium 103004 | Smr | Institut Pasteur, Paris, France |
| B. bronchiseptica BB1015 | Smr but not rpsL | 51 |
| B. parapertussis PEP | Smr | 45 |
| B. pertussis | ||
| BPSM | Derivative of Tohama I rpsL; Smr Nalr | 41 |
| BPEP98 | Derivative of BPSM but ΔtonB exbB::ΩKmr | This study |
| BPLOW | Derivative of BPSM but ΔbvgAS | E. Willery, Lille, France |
| BPEP184 | Derivative of BPSM but alcR::Kmr | 51 |
| BPEP269 | Derivative of BPSM with pEP636 integration | This study |
| BPEP270 | Derivative of BPEP98 with pEP636 integration | This study |
| Plasmids | ||
| pSUTonBExbBD | pSU19 containing E. coli tonB, exbB, and exbD; Cmr | R. Kadner |
| pCG475 | pUC18 bearing BPSM tonB exbB exbD basR on a 3.1-kb EcoRI fragment; Apr | This study |
| pEP487 | pCG475 with a BamHI ΩKmr cassette inserted in the exbB BclI site; Apr Kmr | This study |
| pHP45Ω-Km | Source of ΩKmr cassette | 20 |
| pBCSK+ | High-copy-number vector; Cmr | Stratagene |
| pEP498 | pBSCK+ bearing BPSM tonB exbB exbD basR′ on a 3-kb EcoRI-XhoI fragment; Cmr | This study |
| pEP532 | pBSCK+ bearing the BB1015 tonB upstream region, (′metYpiuChupB) on a 2.4-kb EcoRI fragment; Cmr | This study |
| pJQ200mp18rpsL | Bordetella suicide vector, contains E. coli rpsL; Gnr | D. Raze, Little, France |
| pEP491 | pJQ200mp18rpsL bearing tonB exbB::ΩKmrexbB basR on an EcoRI fragment isolated from pEP487; Gnr Kmr | This study |
| pEP549 | Derivative of pEP491 through SalI deletion, bears ΩKmrexbD basR; Gnr Kmr | This study |
| pEP552 | Derivative of pEP549 through insertion of a 1.3-kb DNA region 5′ of tonB on the chromosome; Gnr Kmr | This study |
| pET24a+ | T7 promoter expression vector; Kmr | Novagen |
| pEP583 | pET24a+ derivative to produce Bp TonB; Kmr | This study |
| pJQ200mp18 | Bordetella suicide vctor; Gnr | 52 |
| pEP636 | pJQ200mp18 bearing ′piuC hupB tonB exbBD basR′ on a 4.5-kb XbaI-XhoI fragment; Gnr | This study |
Apr, Cmr, Gnr, Kmr, Nalr, Smr, and Tcr, resistance to ampicillin, chloramphenicol, gentamicin, kanamycin, nalidixic acid, streptomycin and tetracyclin, respectively.
DNA techniques.
Plasmid DNA was routinely isolated by the alkaline lysis method (55) or purified by using the Nucleobond AX kit (Macherey-Nagel, Hoerdt, France) for sequencing purposes. Restriction endonucleases and T4 DNA ligase were obtained from Roche (Meylan, France) and used according to standard procedures (55). DNA fragments were sequenced using an ABI PRISM Dye Terminator Cycle Sequencing kit and an ABI PRISM 377 sequencer (PE Applied Biosystems, Warrington, United Kingdom) and a combination of universal, reverse, and custom-synthesized primers. PCRs were carried out with VentR DNA polymerase (New England Biolabs, Inc., Beverly, Mass.).
Computer analysis of sequences.
The nucleotide and protein sequences were analyzed by using the DNA Strider 1.2 software (Service de Biochimie et de Génétique Moléculaire du CEA, Saclay, France). Sequence similarities were identified with the help of the BLASTN and BLASTP programs (2). Sequence alignments were performed with the Multalin 5.3.3 software (18). Oligonucleotides were designed using the Oligo 5.0 software (NBI, Plymouth, Minn.).
Construction of pCG475 and pEP491 and cloning of the B. bronchiseptica tonB upstream region.
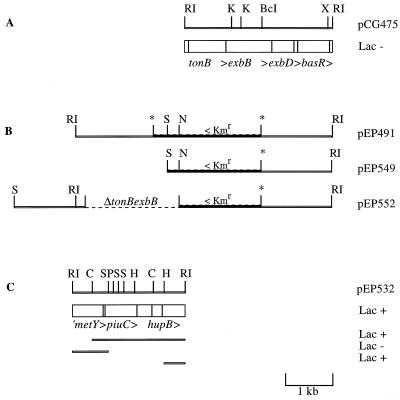
Plasmid pCG475 was isolated from a B. pertussis partial genomic DNA library we had previously constructed in pUC18. The BamHI ΩKmr cassette was isolated from pHP45Ω-Km and inserted into the unique BclI site in pCG475. The resulting plasmid, pEP487, was digested with EcoRI, and the 5.3-kb fragment bearing tonB exbB::ΩKmr exbD basR was cloned into the Bordetella suicide vector pJQ200mp18rpsL (Gnr) to obtain pEP491 (Fig. 1B). E. coli SM10 was transformed with pEP491 and used as a donor in conjugation with B. bronchiseptica BB1015. Genomic DNA of a Gnr Kmr BB1015 mutant bearing pEP491 inserted into the tonB locus was digested with NsiI, which does not cut pEP491, and ligated. The ligation mixture was used to transform E. coli XL1-Blue to gentamicin resistance. A recombinant plasmid resulting from the intramolecular ligation of a chromosomal NsiI fragment containing pEP491 was isolated. Restriction mapping of this plasmid permitted identification of the B. bronchiseptica tonB 5′ region. A 2.4-kb EcoRI DNA fragment localized immediately upstream from the tonB locus was subcloned into pBCSK+ to yield pEP532 (Fig. 1C).
FIG. 1.
Physical map of the Bordetella tonB exbB exbD chromosomal region and relevant constructions described in this study. The recombinant plasmid-associated Lac phenotypes in the Fur titration assay are indicated on the right. Blocks representing ORFs are drawn to scale; arrowheads indicate the direction of transcription. Certain restriction sites are indicated as follows: BclI (BcI), ClaI (C), EcoRI (RI), HincII (H), KpnI (K), NruI (N), PstI (P), SalI (S), and XhoI (X). (A) The B. pertussis tonB exbB exbD locus cloned as a 3.1-kb EcoRI fragment in pCG475. (B) Inserts in Bordetella suicide plasmids pEP491, pEP549, and pEP552. pEP552 was used for allelic exchange to construct the B. pertussis ΔtonB exbB::ΩKmR mutant BPEP98. (C) The B. bronchiseptica tonB upstream region subcloned as a 2.4-kb EcoRI fragment in pEP532. Derivatives tested in the Fur titration assay are indicated.
Construction of the B. pertussis ΔtonB exbB::ΩKmr mutant.
To delete tonB and exbB in pEP491, this plasmid was digested with SalI and religated to yield pEP549 (Fig. 1B). Oligonucleotides T13 (5′-GCCAGTTGTCCGAGCAGCAC-3′), which hybridizes between the first SalI site and the PstI site in piuC, and T14 (5′-CACGACGAAGCGGCATTCCTA-3′), which hybridizes 39 bp downstream from the EcoRI site preceding tonB, were used to amplify the tonB upstream region from B. pertussis BPSM genomic DNA. PCR conditions were 35 cycles of 1 min of denaturation at 96°C, 1 min of hybridization at 60°C, and 1.5 min of elongation at 72°C. The 1.6-kb PCR product containing the B. pertussis tonB upstream region (′piuChupB) was digested with SalI, and the resulting 1.3-kb fragment, which bore a SalI and a blunt-ended extremity, was inserted into pEP549 opened with SalI and NruI to generate pEP552 (Fig. 1B). The NruI site in pEP549 is located downstream of the Kmr gene; thus, pEP552 still confers resistance to Km. E. coli SM10 was transformed with pEP552 and used as a donor in conjugation with B. pertussis BPSM. Exconjugants were selected on BG-Nal-Km plates. All 27 isolated colonies tested showed a Smr Kmr Gns double recombination phenotype. Genomic DNA of two clones was prepared and subjected to Southern blot hybridization with the 1.9-kb KpnI-EcoRI fragment of pCG475 carrying exbB exbD. Correct allelic exchange was confirmed in both strains. One of these ΔtonB exbB::ΩKmr mutants, BPEP98, was chosen for further study.
Construction of BPEP269 and BPEP270.
Plasmid pEP498 was digested with PstI and EcoRI and ligated with the 1.5-kb PstI-EcoRI fragment from pEP532 to reconstitute the ′piuC hupB tonB exbBD basR′ locus in pBCSK+. Taking advantage of the XbaI site present in the multiple cloning site, the 4.5-kb XbaI-XhoI fragment was then cloned into pJQ200mp18 opened with XbaI and SalI to yield pEP636. This Gnr Bordetella suicide plasmid was introduced into BPSM and BPEP98 by conjugation with E. coli SM10(pEP636). Exconjugants were selected on BG-Sm-Gn. Two derivatives of BPSM and BPEP98 bearing pEP636 integrated on the chromosome, BPEP269 and BPEP270, respectively, were tested for iron source utilization and albomycin sensitivity.
Siderophore detection.
The Chrome Azurol S (CAS) assay (56) was used to assess alcaligin production by Bordetella cells grown to stationary phase in iron-limited (SS-Fe) medium. A 0.5-ml volume of culture supernatant was added to 0.5 ml of CAS solution, and the A630 of the CAS dye was measured after incubation for 4 h at room temperature.
Iron source utilization and albomycin sensitivity tests.
Fresh B. pertussis cells were scraped from BG plates and diluted into SS-Fe to an optical density at 600 nm (OD600) of 1. A 200-μl aliquot of this suspension was added to 20 ml of molten SS-Fe plus 0.1% Casamino Acids plus 10 μM EDDHA plus 0.8% agarose and poured into petri dishes. Agarose was used in plates because B. pertussis did not grow well on agar plates. Wells (4 mm in diameter) were punched in plates with a sterile plastic pipette and filled with 20 μl of 15 μM solutions of FeSO4, FeCl3, ferrichrome (Sigma Aldrich, St. Quentin Fallavier, France), desferal (a gift from Ciba-Geigy, Rueil Malmaison, France), or hemin (Sigma Aldrich) in SS-Fe or with SS-Fe alone as a control. Diameters of growth zones around wells were measured after 24 h of incubation at 37°C. To test albomycin sensitivity, 20 ml of molten SS-Fe plus 0.1% Casamino Acids plus 0.8% agarose were seeded with 200 μl of cell suspension and poured into petri dishes. Filter paper disks impregnated with 10 μl of albomycin (50 μg/ml in SS-Fe; a gift from K. Hantke and H.-P. Fiedler) or SS-Fe were applied to the surface. Growth inhibition was checked after 24 h of incubation at 37°C.
Mouse respiratory infection model.
After 24 h of growth on BG plates, BPSM or BPEP98 cells were resuspended in saline. Mice were intranasally infected with 50 μl of suspension containing 2 × 106 bacteria. Infected mice were sacrificed by cervical dislocation 2 h after exposure and at 5, 8, 12, and 16 and 22 days thereafter (two to four mice per time point). The lungs were removed and homogenized in saline with tissue grinders. Numeration of bacteria was performed on BG. To assess stability of the ΔtonB exbB::ΩKmr mutation, bacteria reisolated from the lungs of BPEP98-infected mice were tested for their resistance to Km and the absence of desferal, ferrichrome, or hemin utilization. All phenotypes had been retained.
B. pertussis TonB production in E. coli.
B. pertussis TonB was overexpressed using the T7 RNA polymerase-promoter system (Novagen, Madison, Wis.). The tonB open reading frame (ORF) was amplified from pCG475 with primers NdeI-TonB (5′-ATATCATATGCCTAGCCCCCAATCTGGT-3′) and TonB-EcoRI (5′-ATATGAATTCTGGCGGTGTTGATAGCGTTG-3′) by 35 PCR cycles of 1 min of denaturation at 96°C, 1 min of hybridization at 63°C, and 1 min of elongation at 72°C. The amplification product was digested with NdeI and EcoRI and cloned into pET24a+ to obtain pEP583. BL21(DE3)(pLysS) was transformed with either pET24a+ or pEP583 and grown in LB-Km-Cm at 37°C. At an OD600 of 0.6, cells were induced with 1 mM isopropyl-β-d-thiogalactoside (IPTG) and grown for another 1 to 3 h before proteins were precipitated with trichloroacetic acid (TCA).
Immunoblotting.
E. coli strains were grown in LB to an OD600 of 0.6, and then 1 ml of culture was precipitated at 4°C with one-third volume of 30% TCA, pelleted, washed with 50 mM Tris-HCl (pH 8.0), and then solubilized at 95°C for 5 min in 200 μl of Laemmli buffer (32). Bordetella strains were grown in SS to an OD600 of 3, and then a culture volume corresponding to 6 OD600 units was TCA precipitated (1 OD600 unit is equivalent to 1 ml of culture at an OD600 of 1), washed, and solubilized as described above for E. coli. Aliquots [15 μl for RK5048(pSUTonBExbBD), 5 μl for BL21(DE3)(pLysS) containing pET24a+ or pEP583, and 20 μl for Bordetella strains] were resolved by electrophoresis on sodium dodecyl sulfate (SDS)–11% polyacrylamide gels. Proteins were then electrotransfered to Immobilon-P membranes (Millipore, St-Quentin-en-Yvelines, France), probed with a 1:5,000 dilution of murine monoclonal antibody (MAb) 4H4 raised against E. coli TonB (a gift from K. Postle) (34), and developed by colorimetric detection with alkaline phosphatase-conjugated secondary antibodies.
Nucleotide sequence accession numbers.
Sequence data were submitted to the EMBL database under accession nos. AJ132741 and AJ132742.
RESULTS
Cloning and sequence analysis of the B. pertussis tonB exbB exbD locus.
In the course of cloning in connection with another research project 6 years ago, we had isolated pCG475, a recombinant plasmid bearing a B. pertussis 3.1-kb EcoRI DNA fragment. Sequence analysis of the insert revealed the presence of four ORFs located on the same DNA strand (Fig. 1A). Similarity searches with the deduced amino acid sequences suggested that the first three ORFs encode homologues of TonB, ExbB, and ExbD and that the fourth one codes for a transcriptional activator of a bacterial two-component regulatory system. This latter ORF was called basR. The putative tonB ATG start codon is located 80 bp downstream from the EcoRI site. It is not preceded by a sequence resembling the canonical E. coli ribosome binding site; this is often observed with B. pertussis genes. The putative exbB ATG initiation codon at position 882 is separated from the tonB TGA stop codon by 1 bp, and the putative exbD ATG start codon at position 1859 overlaps the exbB TAA termination codon. Such a tight organization suggests that the three genes are cotranscribed. Most tonB genes contain a Fur-binding sequence (FBS) in their promoter region, and their expression is repressed by Fur in iron-rich growth conditions (22, 46, 48, 49). No obvious promoter sequence was identified in the short region upstream from tonB in pCG475. In addition, a pBCSK+ derivative containing the 3-kb EcoRI-XhoI tonB exbB exbD basR′ fragment from pCG475 conferred a Lac− phenotype in the Fur titration assay, a genetic test for the presence of FBS using an E. coli indicator strain (58) (Fig. 1A). This suggested the absence of an FBS in the EcoRI-XhoI fragment and therefore that pCG475 does not contain the tonB promoter. A G+C-rich inverted sequence starting 27 bp downstream from the exbD stop codon (GCCCGCGCCGCGGGCGCGGGC) could constitute a termination signal for tonB exbB exbD transcription. Alternately, this sequence could play a role in basR expression, whose ORF starts 36 bp downstream from this hairpin structure with an ATG at position 2409. This ORF extends to position 3080, 60 bp upstream from the second EcoRI site (Fig. 1A).
The predicted sequences of B. pertussis TonB, ExbB, and ExbD were aligned with those of their respective closest homologues in the databases and with the corresponding E. coli sequences as a reference (Fig. 2). The B. pertussis TonB is a 266-amino-acid (aa) protein with a calculated molecular weight (MW) of 28,600. The highest degree of similarity was observed with Pseudomonas aeruginosa TonB (30% identity in a 275-aa overlap compared to 26% in a 240-aa overlap with E. coli TonB. Most TonB features are conserved: (i) a predicted N-terminal transmembrane segment (TM) containing a conserved His residue anchors TonB to the cytoplasmic membrane (Fig. 2A) and is involved in interactions of the E. coli TonB with ExbB (31, 35, 63); (ii) a Pro-rich domain, composed of 13 Glu-Pro and 9 Lys-Pro repeats in B. pertussis TonB, is proposed to span the periplasmic space in the case of E. coli TonB (36, 54). B. pertussis TonB, P. aeruginosa TonB, and E. coli TonB contain 20, 18, and 17% Pro, respectively; and (iii) a PXYP motif and highly conserved Val and Gly residues are present in the C-terminal third of the protein (Fig. 2A). In E. coli TonB this region has been proposed to interact with outer membrane receptors (33, 63).
FIG. 2.
Sequence alignments of the deduced B. pertussis (Bp) TonB (A), ExbB (B), and ExbD (C) proteins with their closest homologues and the corresponding E. coli (Ec) proteins. Pa, P. aeruginosa; Xc, X. campestris. Predicted transmembrane segments are highlighted in gray. Conserved residues are in boldface, and those important for function in E. coli and conserved in the other two proteins are underlined.
As a predicted 325-aa protein with a calculated MW of 33,800, B. pertussis ExbB is larger than most other ExbB proteins (220 to 240 aa). Its closest homologue is the Xanthomonas campestris ExbB (66), with 40% identity in a 253-aa overlap, compared to 25% identity with E. coli ExbB in a 257-aa overlap. In addition to the three TM domains typical of ExbB proteins, B. pertussis ExbB contains a putative fourth TM segment in its N-terminal extension (Fig. 2B). Such a characteristic is shared by the 329-aa Pseudomonas putida ExbB. Thus, in contrast to E. coli ExbB, the B. pertussis ExbB and P. putida ExbB N termini are predicted to be located in the cytoplasm. Apart from an Ala and Pro abundance, no striking sequence similarity could be detected between the N-terminal extensions of B. pertussis ExbB and P. putida ExbB. Most features of other ExbB proteins are conserved (59): (i) a VX3L/VX3LX3SX3W motif is present in the first common TM domain; (ii) the second conserved hydrophobic region is Gly-rich, while (iii) the last TM segment is Ala-rich and is followed by Asn and Arg residues present in most ExbB proteins (underlined in Fig. 2B).
B. pertussis ExbD is a predicted 155-aa protein with a calculated MW of 16,500. It is most similar to X. campestris ExbD1 (35% identity in a 140-aa overlap compared to 30% identity with E. coli ExbD in a 141-aa overlap). ExbD proteins are anchored in the inner membrane via a single N-terminal hydrophobic segment (Fig. 2C). An Asp residue required for E. coli ExbD activity is conserved in the B. pertussis ExbD TM domain (underlined in Fig. 2C). The remainder of E. coli ExbD extends into the periplasm where it interacts with TonB and ExbB. Leu132 in the C terminus of E. coli ExbD has been described as important for this activity (11). However, it is substituted by Phe in B. pertussis ExbD and X. campestris ExbD1 or by Ile in other ExbD proteins (21, 23, 28, 66).
While this manuscript was in preparation, Nicholson and Beall published an analysis of the B. bronchiseptica 19385 tonB exbBD locus. Their sequence data are very similar to ours except for 10 nucleotide changes and an 11-bp insertion in the B. pertussis BPSM hupB-tonB intergenic region. We identified 20 nucleotide changes between the BPSM and 19385 tonB genes, but only two generate amino acid substitutions in the deduced protein sequences: V139A and A151T. Only 5 of the 30 differences observed between BPSM and 19385 exbB lead to amino acid substitutions: D24G, A27T, A47T, I103V, and V120A. However, due to a 33-bp in-frame deletion, the 19385 ExbB contains only one of the two GTEAV/LNQAAQA motifs present in BPSM ExbB N-terminal extension. Comparing the 19385 and BPSM exbD genes, we noticed two changes generating residue substitutions E56Q and I65V and 14 silent ones.
Presence of the tonB locus in other Bordetella genomes.
B. parapertussis and B. bronchiseptica are closely related to B. pertussis, while B. avium is phylogenetically more distant. The presence of the tonB exbB exbD genes in these species was tested in Southern blot experiments. Chromosomal DNA from B. pertussis BPSM, B. bronchiseptica BB1015, B. parapertussis PEP, and B. avium 103004 was digested with EcoRI and probed with the 3-kb EcoRI-XhoI DNA fragment of pCG475 containing the whole tonB exbB exbD locus. A single hybridization product was detected in each genome: a 3.1-kb DNA fragment in B. pertussis and B. bronchiseptica and a larger DNA fragment, of about 10 and 15 kb, in B. parapertussis and B. avium, respectively (data not shown). Thus, the tonB exbB exbD locus is present in these four species. Furthermore, both EcoRI sites flanking this region appear to be conserved in B. pertussis and B. bronchiseptica. Since, unlike the other three species, B. avium does not seem to synthesize the siderophore alcaligin (17, 51), the presence of a ton locus in its genome suggests that iron uptake is mediated by other Ton-dependent systems in B. avium.
Cloning and sequencing of the tonB upstream region.
To localize the tonB promoter, we first isolated the tonB upstream region from B. bronchiseptica by using the strategy described in Materials and Methods. The 2.4-kb EcoRI fragment located immediately upstream from tonB was cloned into pBCSK+ to obtain pEP532 (Fig. 1C). Sequence analysis of this insert revealed the presence of three ORFs oriented in the same direction as the tonB gene (Fig. 1C). Databases were scanned for similarities to the deduced amino acid sequences. The first ORF translates into a product presenting 34% of identity in a 181-aa overlap with the C-terminal domain of MetY, an O-acetylhomoserine sulfhydrylase involved in methionine synthesis in Leptospira meyeri (7). The second ORF, starting 41 bp downstream from the metY termination codon, encodes a 226-aa protein homologous to PiuC, a putative P. aeruginosa iron uptake factor (65% identity in a 226-aa overlap) (47). No putative transcription terminator could be identified downstream from B. bronchiseptica piuC. A 322-bp noncoding region separates this ORF from the next one. The third ORF, hupB, codes for the 90-aa β-subunit of a putative histone-like protein HU (71% identity with HU-β from E. coli). A 12-bp inverted repeat, AGGCAAATCGGCGCTGCCGATTTGCCT, located 9 bp downstream from the hupB termination codon could form a transcriptional termination signal. Another inverted repeat GCGCGCTCGGCGCGTGCCGAGCGCGC is present 294 bp downstream from hupB. No similarity with any sequence in the databases could be detected in the 423-bp sequence downstream from hupB. Based on the B. bronchiseptica piuC and B. pertussis tonB sequences, we designed primers to PCR amplify the B. pertussis tonB upstream region. Sequence analysis of the PCR product indicated that this region is identical in both species.
The presence of potential FBS in pEP532 was tested in the Fur titration assay (58). E. coli H1717(pEP532) presented a Lac+ phenotype, indicating the presence of at least one FBS on the EcoRI fragment (Fig. 1C). A derivative containing only the 423-bp HincII-EcoRI hupB downstream region also conferred a Lac+ phenotype in the assay. The EcoRI-SalI fragment containing ′metY and the piuC 5′-region was negative in the FBS assay (Fig. 1B), suggesting that, contrary to P. aeruginosa, the B. bronchiseptica piuC promoter does not contain any FBS. The HincII-EcoRI fragment was scanned for sequence similarity with the E. coli Fur-binding consensus sequence GATAATGATAATCATTATC. A putative GAGCTTGCGAATCATTCGC FBS (12 of 19 matches) was identified 36 bp upstream from the EcoRI site. Since FBSs usually overlap promoter sequences, this region most likely contains the tonB promoter.
A B. pertussis ΔtonB exbB mutant is affected in iron uptake.
To construct a B. pertussis tonB null mutant, the tonB upstream region was first spliced to the Kmr cassette of pEP491 as described in experimental procedures to yield pEP552 (Fig. 1B). This plasmid was then introduced into B. pertussis BPSM. Selection for Kmr Smr clones enabled us to isolate the ΔtonB exbB::ΩKmr BPEP98 mutant by allelic exchange. BPEP98 colonies were hemolytic on BG plates, indicating that the adenylate cyclase-hemolysin (AC-Hly) virulence factor was produced.
When BPSM and BPEP98 were grown to stationary phase in SS or SS-Fe medium, no difference in growth rate or in final yield could be detected between the two strains (data not shown). This suggests that traces of iron in SS-Fe medium were sufficient to feed BPEP98. The culture supernatants were tested for siderophore activity in the CAS assay (56). The levels of siderophore activity were similar for both strains (data not shown). No siderophore activity was detected in the iron-replete culture supernatants of either strain. Whole-cell lysates (WCLs) were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) analysis. No difference in the protein profiles of BPEP98 and BPSM was observed. Furthermore, both strains presented the same pattern of iron-repressed and iron-induced proteins (data not shown), indicating that the tonB mutant is still Fur regulated.
We next compared the growth of BPSM and BPEP98 at different concentrations of the Fe(III) chelator ethylenediamine di(o-hydroxyphenylacetic acid) (EDDHA) in SS-Fe media. For unknown reasons B. pertussis did not grow in liquid media even with very low concentrations of EDDHA. We therefore investigated their growth on solid SS media. BPSM and BPEP98 were first cultivated on BG plates and then restreaked onto SS-Fe–0.1% Casamino Acids agarose plates with or without addition of 10 μM FeSO4. The diameter of isolated colonies was measured after incubation for 6 days at 37°C. On SS plus Fe plates, both BPEP98 and BPSM formed ca. 1-mm-wide colonies. However, on SS-Fe plates, BPEP98 formed pinpoint colonies of about only 0.25 mm in diameter, compared to ca. 1 mm for the parental strain. Furthermore, in contrast to BPSM, the tonB mutant was not able to grow on SS-Fe plates containing 5 μM EDDHA (data not shown). These results suggest that BPEP98 is deficient in iron uptake.
The isogenic pair was tested for its ability to use different iron sources. SS-Fe plates containing 10 μM EDDHA were seeded with BPSM or BPEP98. Wells were punched in the agarose and filled with 15 μM solutions of FeSO4, FeCl3, or Fe(III)-loaded molecules such as hemin or desferal and ferrichrome siderophores. The plates were then incubated for 24 h at 37°C, after which the diameters of growth halos around the wells were measured. BPSM was able to grow using all five iron sources tested, whereas none of them promoted growth of the tonB mutant (Table 2). BPEP98 grew only around wells filled with 1 mM FeSO4 or FeCl3 (data not shown). Iron uptake of the avirulent B. pertussis ΔbvgAS mutant BPLOW was tested under the same conditions. Similar to BPSM, BPLOW could use all five iron sources (Table 2), indicating that the iron uptake systems involved are not Bvg dependent.
TABLE 2.
Iron sources utilization and albomycin sensitivity of B. pertussis BPSM, BPEP98, BPLOW, BPEP269, and BPEP270a
| Strain | Confluent growth halo diam (mm) with:
|
Albod | |||||
|---|---|---|---|---|---|---|---|
| SS-Fe | FeSO4 | FeCl3 | Hemin | DFb | FCc | ||
| BPSM | 0 | 9 | 9 | 21 | 27 | 27 | S |
| BPEP98 | 0 | 0 | 0 | 0 | 0 | 0 | R |
| BPLOW | 0 | 7 | 7 | 21 | 27 | 26 | S |
| BPEP269 | 0 | 6 | 11 | 20 | 27 | 27 | S |
| BPEP270 | 0 | 6 | 12 | 20 | 25 | 25 | S |
The diameters of confluent growth halos around wells filled with SS-Fe or the indicated iron source (15 μM solution in SS-Fe) were measured in millimeters after 24 h at 37°C. Results are the means for two separate experiments.
DF, desferal (ferrioxamin B).
FC, ferrichrome.
Sensitivity (S) or resistance (R) to albomycin.
In E. coli, the antibiotic albomycin is transported into the cell via the ferrichrome receptor and the Ton system; thus, tonB mutants are albomycin resistant. BPSM, BPEP98, and BPLOW were therefore tested for sensitivity to albomycin. As indicated in Table 2, BPSM and BPLOW were sensitive to albomycin, while the tonB mutant was totally resistant.
Iron uptake is restored by the integration of a tonB exbBD operon copy into the ΔtonB exbB mutant chromosome.
In order to complement the ΔtonB exbB mutation, we first cloned the B. pertussis tonB exbBD operon into a Bordetella multicopy plasmid. However, the introduction of this construct into BPEP98 or BPSM greatly reduced the viability of both strains, perhaps due to overproduction of the Ton system (data not shown). Thus, we reconstituted the ′piuC hupB tonB exbBD basR′ locus on a Bordetella suicide plasmid to obtain pEP636 as described in Materials and Methods. This plasmid was then introduced into BPSM and BPEP98 by conjugation. Selection for Gnr Smr clones enabled us to isolate BPEP269 and BPEP270 bearing pEP636 inserted on the chromosome in the tonB region. Both strains were able to grow on SS-Fe plates containing 5 μM EDDHA. Furthermore, as shown in Table 2, BPEP270 was able to utilize all iron sources tested and was albomycin sensitive. This phenotype indicated complementation of the ΔtonB exbB mutation.
The Ton system is required for efficient colonization in the mouse model.
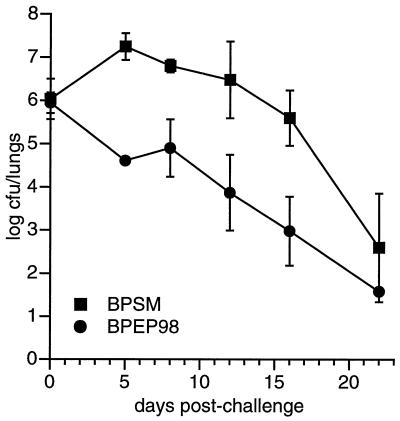
To test whether the Ton system is required for virulence, mice were infected with either BPSM or BPEP98, and bacteria in the lungs were numerated at different time intervals after infection. As shown in Fig. 3, the parental strain was able to adhere and multiply in the lungs. Then, 1 week after infection, the number of bacteria declined. The ΔtonB exbB mutant behavior was different; it was unable to multiply during the first phase of the infection, but it was cleared at a rate similar to that of the parental strain. Thus, BPEP98 is affected in its capacity to multiply in the respiratory tract of the mouse.
FIG. 3.
Colonization profiles of parental BPSM and BPEP98 ΔtonB exbB in the murine respiratory infection model.
The ΔtonB exbB mutant produces virulence factors and is sensitive to modulation signals.
The colonization of the mouse respiratory tract depends on the production of B. pertussis adhesins and toxins, such as filamentous hemagglutinin (FHA), pertactin (PRN), pertussis toxin (PTX), and AC-Hly (for a review, see reference 39). The production of these virulence factors is controlled by the two-component regulatory system BvgAS, which undergoes phenotypic modulation in response to MgSO4 or nicotinic acid. To investigate whether the tonB mutation affects the production of the Bvg-dependent virulence factors or modifies Bvg regulation, WCLs and culture supernatants of BPSM and BPEP98 grown in SS, SS plus MgSO4, or SS plus nicotinic acid were compared by SDS-PAGE and immunoblot analyses. Both strains were found to produce similar amounts of FHA, AC-Hly, PRN, and PTX (data not shown). Protein profiles of BPSM or BPEP98 grown in modulation conditions were identical (data not shown), indicating that BPEP98 is responsive to chemical modulators. These observations imply that TonB is not required for virulence factor production or for modulation.
TonB production is independent of the BvgA and AlcR activators.
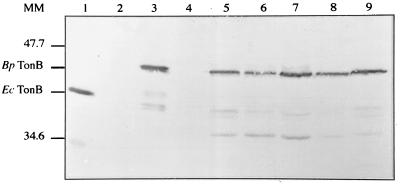
To determine whether tonB is Bvg or AlcR regulated, the presence of TonB was examined in B. pertussis bvgAS or alcR null mutants. WCLs of B. pertussis BPSM, BPEP98 (ΔtonB exbB), BPEP184 (alcR::Kmr), BPLOW (ΔbvgAS), and wild-type B. bronchiseptica and B. parapertussis strains were analyzed by immunoblotting using MAb 4H4 raised against E. coli TonB (34). As shown in Fig. 4, an immunoreactive protein was detected in all Bordetella extracts (lanes 5 to 9) except for BPEP98 (lane 4). This protein had an apparent MW of 42,000 compared to about 38,000 for E. coli TonB (lane 1) and presented the same migration profile as B. pertussis TonB overproduced in E. coli (lane 3). TonB proteins exhibit a retarded migration due to their high proline content. Some minor reactive polypeptides in lanes 5 to 9 correspond probably to B. pertussis TonB degradation products since they were not observed in the BPEP98 WCL (lane 4) but were detected in E. coli overproducing B. pertussis TonB (lane 1). These results show that (i) B. pertussis, B. bronchiseptica, and B. parapertussis synthesize a TonB homologue; that (ii) this protein is absent in B. pertussis ΔtonB exbB, and that (iii) B. pertussis TonB production does not require the BvgAS system or AlcR.
FIG. 4.
TonB immunoblots of WCLs. Lanes 1 to 3, E. coli strains RK5048(pSUTonBExbBD), BL21(DE3)/pLysS(pET24a+), and BL21(DE3)/pLysS(pEP583), respectively. Lanes 4 to 7, B. pertussis strains BPEP98 ΔtonBexbB, parental BPSM, BPEP184 alcR, and BPLOW ΔbvgAS, respectively. Lane 8, B. bronchiseptica BB1015. Lane 9, B. parapertussis PEP. The positions of B. pertussis TonB, E. coli TonB, and the molecular mass (MM) standards (in kilodaltons) are indicated on the left.
DISCUSSION
In this study we report the identification and functional characterization of the B. pertussis tonB exbB exbD locus. In light of their tight organization, tonB, exbB, and exbD are probably transcribed as a single operon. A similar gene arrangement has been documented for the Ton systems of Neisseria meningitidis (59), N. gonorrhoeae (8), X. campestris (66) and for the first set of Vibrio cholerae tonB genes (46). In other species, such as P. putida (9), H. influenzae (28), H. ducreyi (19), Pasteurella haemolytica (23), Helicobacter pylori (61), and in the second tonB locus of V. cholerae (46), genes are clustered in the order exbB exbD tonB. In Enterobacteriaceae, tonB is not linked to the exbB exbD genes on the chromosome (15, 16, 22, 25). The E. coli tonB promoter has been shown to be Fur repressed (49), and exbB exbD are cotranscribed from an iron-regulated promoter (1). We identified an FBS upstream from tonB, suggesting that expression of the B. pertussis tonB exbBD operon is also derepressed in low-iron growth conditions.
The deduced B. pertussis TonB sequence presents the highest degree of similarity with that of P. aeruginosa TonB (48), which is in agreement with the phylogenetic proximity of these two species. A second tonB gene was recently identified in P. aeruginosa through sequence analysis of the Pseudomonas Genome Project, but its physiological role has not been identified yet (67). This tonB2 gene precedes putative exbB and exbD genes on the P. aeruginosa chromosome, unlike tonB1, which is not linked to potential exb genes (E. Pradel, personal observation). Two sets of tonB exbB exbD genes have been identified in V. cholerae, and both of them are involved in iron uptake (46). We used the Bordetella BLAST server of the Sanger Centre to scan the available B. pertussis genomic DNA sequences for similarities with tonB, exbB, and exbD. A unique tonB exbBD locus was detected in the 543 assembled contigs which cover most of the B. pertussis genome. On a distinct contig, we identified two linked ORFs encoding products similar to B. pertussis ExbB and ExbD (27 and 35% conserved residues, respectively). However, the deduced proteins showed a higher degree of sequence similarity with P. aeruginosa TolQ and TolR (52 and 38% identity, respectively) (data not shown). No second ORF similar to tonB was detected. This analysis suggests that B. pertussis possesses a unique tonB exbBD operon and potential tolQR genes. The tolQR genes were not detected in our Southern hybridization experiments on B. pertussis chromosomal DNA, probably due to insufficient sequence conservation with the exbBD probe.
The B. pertussis TonB protein was overproduced in E. coli. Recombinant B. pertussis TonB was recognized by MAb 4H4 directed against E. coli TonB. This MAb has been shown to bind to the proline-rich region of E. coli TonB and to react with a unique protein in WCLs of a wide range of gram-negative species (34). However, 4H4 recognizes two putative TonB proteins in P. aeruginosa WCLs (34). A single protein presenting an electrophoretic migration similar to that of recombinant B. pertussis TonB was immunodetected in WCLs of B. pertussis, B. parapertussis, and B. bronchiseptica. This protein was absent from the WCL of a B. pertussis ΔtonB exbB::Kmr mutant. Together, these observations suggest that B. pertussis, B. bronchiseptica, and B. parapertussis produce only one TonB protein.
We showed that a B. pertussis ΔtonB exbB::Kmr mutant is more sensitive to iron deprivation than the parental strain, while it is still able to synthesize and secrete alcaligin in low-iron growth conditions. Brickman and Armstrong recently characterized FauA, the B. pertussis and B. bronchiseptica alcaligin receptor, and suggested that FauA is TonB dependent based on its primary structure (14). The reduced growth of the ΔtonB exbB strain in iron-restricted medium could result from its inability to transfer the ferrialcaligin complex to the periplasm in the absence of TonB. Furthermore, the mutant is unable to use exogenous siderophores or hemin as sole iron source and to internalize the antibiotic albomycin, a ferrichrome structural analogue. Three additional outer membrane siderophore receptors have been characterized in B. pertussis, and analysis of their primary structure suggested that these proteins are TonB dependent (3, 5). Although no B. pertussis receptor for ferrichrome, desferal (ferrioxamine B), or hemin has been identified yet, our data indicate that these iron uptake systems are also TonB dependent.
E. coli tonB mutants are relatively iron starved, and genes normally regulated by Fur are derepressed even in high-iron conditions (50). We observed no difference in the iron-regulated protein profiles of the B. pertussis ΔtonB exbB::Kmr and parental strains. Most likely, Fe(II) diffusion through porins is sufficient to maintain Fur repression in the mutant grown in iron-rich medium (36 μM FeSO4). Transport of periplasmic Fe(II) into the cell is TonB independent and may occur via a cytoplasmic membrane protein similar to the Feo system in E. coli (12). We also showed that the production of the major B. pertussis virulence factors, such as FHA, PRN, PTX, and AC-Hly, does not require TonB. In addition, phenotypic modulation in response to chemical stimuli occurs in the absence of the Ton system; thus, the BvgAS virulence regulatory system is TonB independent. Conversely, we established that the production of TonB in B. pertussis is BvgAS independent, which is consistent with our observation that ferrichrome, desferal, or hemin usage as iron sources is not affected in a ΔbvgAS mutant. Thus, tonB is not part of the bvg regulon. In addition, tonB is also not regulated by AlcR, the transcriptional activator of alcaligin biosynthesis and receptor genes (6, 51).
We had previously reported that a B. pertussis alcR mutant, while unable to produce alcaligin, is not impaired in a murine respiratory infection model (51). In the present study we demonstrate that the ΔtonB exbB mutant is affected in its capability to multiply in the mouse respiratory tract. This observation suggests that TonB-dependent iron uptake systems are required for efficient proliferation in vivo. Involvement of TonB in virulence expression in animal models in relation with iron uptake capability has been documented previously for H. influenzae (29), V. cholerae (27), and Salmonella typhimurium (64). We cannot discard the hypothesis that the B. pertussis TonB function may not be restricted to iron uptake. Recently, P. aeruginosa TonB has been shown to play a role in efflux-mediated multidrug resistance (67). We can therefore not exclude that in B. pertussis, the Ton system could be involved in the transport of other substrates or in the expression of yet-unidentified virulence factors in the host.
ACKNOWLEDGMENTS
We thank Kathleen Postle for her encouragement and the gift of anti-TonB MAbs, Robert Kadner and Dominique Raze for the gift of plasmids and strains, and Klaus Hantke and Hans-Peter Fiedler for that of albomycin. We are grateful to Eve Willery, Sabine Thiberge, and Claudie Gantiez for technical assistance; to Emmanuelle Fort for photographic work; and to Franck Biet and Alain Baulard for computer counseling. We acknowledge Teijin for the supply of dimethyl β-cyclodextrin, and Ciba-Geigy for that of desferal.
This work was supported by INSERM, the Institut Pasteur de Lille, the Région Nord-Pas-de-Calais, the Fondation de l'Institut Pasteur (Paris), and the Ministère de l'Education Nationale, de la Recherche, et de la Technologie.
REFERENCES
- 1.Ahmer B M, Thomas M G, Larsen R A, Postle K. Characterization of the exbBD operon of Escherichia coli and the role of ExbB and ExbD in TonB function and stability. J Bacteriol. 1995;177:4742–4747. doi: 10.1128/jb.177.16.4742-4747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beall B. Two iron-regulated putative ferric siderophore receptor genes in Bordetella bronchiseptica and Bordetella pertussis. Res Microbiol. 1998;149:189–201. doi: 10.1016/s0923-2508(98)80079-x. [DOI] [PubMed] [Google Scholar]
- 4.Beall B, Hoenes T. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology. 1997;143:135–145. doi: 10.1099/00221287-143-1-135. [DOI] [PubMed] [Google Scholar]
- 5.Beall B, Sanden G N. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology. 1995;141:3193–3205. doi: 10.1099/13500872-141-12-3193. [DOI] [PubMed] [Google Scholar]
- 6.Beaumont F C, Kang H Y, Brickman T J, Armstrong S K. Identification and characterization of alcR, a gene encoding an AraC-like regulator of alcaligin siderophore biosynthesis and transport in Bordetella pertussis and Bordetella bronchiseptica. J Bacteriol. 1998;180:862–870. doi: 10.1128/jb.180.4.862-870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belfaiza J, Martel A, Margarita D, Saint Girons I. Direct sulfhydrylation for methionine biosynthesis in Leptospira meyeri. J Bacteriol. 1998;180:250–255. doi: 10.1128/jb.180.2.250-255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas G D, Anderson J E, Sparling P F. Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB and exbD genes. Mol Microbiol. 1997;24:169–179. doi: 10.1046/j.1365-2958.1997.3421692.x. [DOI] [PubMed] [Google Scholar]
- 9.Bitter W, Tommassen J, Weisbeek P J. Identification and characterization of the exbB, exbD and tonB genes of Pseudomonas putida WCS358: their involvement in ferric-pseudobactin transport. Mol Microbiol. 1993;7:117–130. doi: 10.1111/j.1365-2958.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonnah R A, Yu R H, Wong H, Schryvers A B. Biochemical and immunological properties of lactoferrin binding proteins from Moraxella (Branhamella) catarrhalis. Microb Pathog. 1998;24:89–100. doi: 10.1006/mpat.1997.0173. [DOI] [PubMed] [Google Scholar]
- 11.Braun V, Gaisser S, Herrmann C, Kampfenkel K, Killmann H, Traub I. Energy-coupled transport across the outer membrane of Escherichia coli: ExbB binds ExbD and TonB in vitro, and leucine 132 in the periplasmic region and aspartate 25 in the transmembrane region are important for ExbD activity. J Bacteriol. 1996;178:2836–2845. doi: 10.1128/jb.178.10.2836-2845.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun V, Hantke K, Koster W. Bacterial iron transport: mechanisms, genetics, and regulation. Metal Ions Biol Syst. 1998;35:67–145. [PubMed] [Google Scholar]
- 13.Braun V, Pilsl H, Gross P. Colicins: structures, modes of action, transfer through membranes, and evolution. Arch Microbiol. 1994;161:199–206. doi: 10.1007/BF00248693. [DOI] [PubMed] [Google Scholar]
- 14.Brickman T J, Armstrong S K. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J Bacteriol. 1999;181:5958–66. doi: 10.1128/jb.181.19.5958-5966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruske A K, Anton M, Heller K J. Cloning and sequencing of the Klebsiella pneumoniae tonB gene and characterization of Escherichia coli-K. pneumoniae TonB hybrid proteins. Gene. 1993;131:9–16. doi: 10.1016/0378-1119(93)90663-n. [DOI] [PubMed] [Google Scholar]
- 16.Bruske A K, Heller K J. Molecular characterization of the Enterobacter aerogenes tonB gene: identification of a novel type of TonB box suppressor mutant. J Bacteriol. 1993;175:6158–6168. doi: 10.1128/jb.175.19.6158-6168.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connell T D, Dickenson A, Martone A J, Militello K T, Filiatraut M J, Hayman M L, Pitula J. Iron starvation of Bordetella avium stimulates expression of five outer membrane proteins and regulates a gene involved in acquiring iron from serum. Infect Immun. 1998;66:3597–3605. doi: 10.1128/iai.66.8.3597-3605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkins C, Totten P A, Olsen B, Thomas C E. Role of the Haemophilus ducreyi Ton system in internalization of heme from hemoglobin. Infect Immun. 1998;66:151–160. doi: 10.1128/iai.66.1.151-160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 21.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 22.Gaisser S, Braun V. The tonB gene of Serratia marcescens: sequence, activity and partial complementation of Escherichia coli tonB mutants. Mol Microbiol. 1991;5:2777–2787. doi: 10.1111/j.1365-2958.1991.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 23.Graham M R, Lo R Y. Cloning and characterization of the exbB-exbD-tonB locus of Pasteurella haemolytica A1. Gene. 1997;186:201–205. doi: 10.1016/s0378-1119(96)00703-2. [DOI] [PubMed] [Google Scholar]
- 24.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannavy K, Barr G C, Dorman C J, Adamson J, Mazengera L R, Gallagher M P, Evans J S, Levine B A, Trayer I P, Higgins C F. TonB protein of Salmonella typhimurium. A model for signal transduction between membranes. J Mol Biol. 1990;216:897–910. doi: 10.1016/S0022-2836(99)80009-6. [DOI] [PubMed] [Google Scholar]
- 26.Heller K J, Kadner R J, Gunther K. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene. 1988;64:147–153. doi: 10.1016/0378-1119(88)90488-x. [DOI] [PubMed] [Google Scholar]
- 27.Henderson D P, Payne S M. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect Immun. 1994;62:5120–5125. doi: 10.1128/iai.62.11.5120-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarosik G P, Hansen E J. Cloning and sequencing of the Haemophilus influenzae exbB and exbD genes. Gene. 1995;152:89–92. doi: 10.1016/0378-1119(94)00675-i. [DOI] [PubMed] [Google Scholar]
- 29.Jarosik G P, Sanders J D, Cope L D, Muller-Eberhard U, Hansen E J. A functional tonB gene is required for both utilization of heme and virulence expression by Haemophilus influenzae type b. Infect Immun. 1994;62:2470–2477. doi: 10.1128/iai.62.6.2470-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadner R J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990;4:2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson M, Hannavy K, Higgins C F. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol Microbiol. 1993;8:379–388. doi: 10.1111/j.1365-2958.1993.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Larsen R A, Foster-Hartnett D, McIntosh M A, Postle K. Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J Bacteriol. 1997;179:3213–3221. doi: 10.1128/jb.179.10.3213-3221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen R A, Myers P S, Skare J T, Seachord C L, Darveau R P, Postle K. Identification of TonB homologs in the family Enterobacteriaceae and evidence for conservation of TonB-dependent energy transduction complexes. J Bacteriol. 1996;178:1363–1373. doi: 10.1128/jb.178.5.1363-1373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen R A, Thomas M G, Postle K. Protonmotive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol Microbiol. 1999;31:1809–1824. doi: 10.1046/j.1365-2958.1999.01317.x. [DOI] [PubMed] [Google Scholar]
- 36.Larsen R A, Wood G E, Postle K. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol Microbiol. 1993;10:943–953. doi: 10.1111/j.1365-2958.1993.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee B C. Quelling the red menace: haem capture by bacteria. Mol Microbiol. 1995;18:383–390. doi: 10.1111/j.1365-2958.1995.mmi_18030383.x. [DOI] [PubMed] [Google Scholar]
- 38.Litwin C M, Byrne B L. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect Immun. 1998;66:3134–3141. doi: 10.1128/iai.66.7.3134-3141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locht C. Molecular aspects of Bordetella pertussis pathogenesis. Int Microbiol. 1999;2:137–144. [PubMed] [Google Scholar]
- 40.Menozzi F D, Gantiez C, Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991;59:3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J H, Leininger E, Brennan M J, Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller J H. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 43.Moeck G S, Coulton J W. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol Microbiol. 1998;28:675–681. doi: 10.1046/j.1365-2958.1998.00817.x. [DOI] [PubMed] [Google Scholar]
- 44.Neilands J B, Nakamura K. Detection, determination, isolation, characterization and regulation of microbial iron chelates. In: Winkelmann G, editor. Handbook of microbial iron chelates. Boca Raton, Fla: CRC Press; 1991. pp. 1–15. [Google Scholar]
- 45.Nordmann P, François B, Menozzi F D, Commare M C, Barois A. Whooping cough associated with Bordetella parapertussis in a human immunodeficiency virus-infected child. Pediatr Infect Dis J. 1992;11:248. [PubMed] [Google Scholar]
- 46.Occhino D A, Wyckoff E E, Henderson D P, Wrona T J, Payne S M. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol Microbiol. 1998;29:1493–1507. doi: 10.1046/j.1365-2958.1998.01034.x. [DOI] [PubMed] [Google Scholar]
- 47.Ochsner U A, Vasil M L. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc Natl Acad Sci USA. 1996;93:4409–4414. doi: 10.1073/pnas.93.9.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poole K, Zhao Q, Neshat S, Heinrichs D E, Dean C R. The Pseudomonas aeruginosa tonB gene encodes a novel TonB protein. Microbiology. 1996;142:1449–1458. doi: 10.1099/13500872-142-6-1449. [DOI] [PubMed] [Google Scholar]
- 49.Postle K. Aerobic regulation of the Escherichia coli tonB gene by changes in iron availability and the fur locus. J Bacteriol. 1990;172:2287–2293. doi: 10.1128/jb.172.5.2287-2293.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 51.Pradel E, Guiso N, Locht C. Identification of AlcR, an AraC-type regulator of alcaligin siderophore synthesis in Bordetella bronchiseptica and Bordetella pertussis. J Bacteriol. 1998;180:871–880. doi: 10.1128/jb.180.4.871-880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 53.Ren Z, Jin H, Morton D J, Stull T L. hgpB, a gene encoding a second Haemophilus influenzae hemoglobin- and hemoglobin-haptoglobin-binding protein. Infect Immun. 1998;66:4733–4741. doi: 10.1128/iai.66.10.4733-4741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roof S K, Allard J D, Bertrand K P, Postle K. Analysis of Escherichia coli TonB membrane topology by use of PhoA fusions. J Bacteriol. 1991;173:5554–5557. doi: 10.1128/jb.173.17.5554-5557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 56.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 57.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–790. [Google Scholar]
- 58.Stojiljkovic I, Baumler A J, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 59.Stojiljkovic I, Srinivasan N. Neisseria meningitidis tonB, exbB, and exbD genes: Ton-dependent utilization of protein-bound iron in Neisseriae. J Bacteriol. 1997;179:805–812. doi: 10.1128/jb.179.3.805-812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas C E, Olsen B, Elkins C. Cloning and characterization of tdhA, a locus encoding a TonB-dependent heme receptor from Haemophilus ducreyi. Infect Immun. 1998;66:4254–4262. doi: 10.1128/iai.66.9.4254-4262.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 62.Torres A G, Payne S M. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1997;23:825–833. doi: 10.1046/j.1365-2958.1997.2641628.x. [DOI] [PubMed] [Google Scholar]
- 63.Traub I, Gaisser S, Braun V. Activity domains of the TonB protein. Mol Microbiol. 1993;8:409–423. doi: 10.1111/j.1365-2958.1993.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 64.Tsolis R M, Baumler A J, Heffron F, Stojiljkovic I. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect Immun. 1996;64:4549–4556. doi: 10.1128/iai.64.11.4549-4556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberg E D. Acquisition of iron and other nutrients in vivo. In: Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. Washington, DC.: American Society for Microbiology; 1995. pp. 81–95. [Google Scholar]
- 66.Wiggerich H G, Klauke B, Koplin R, Priefer U B, Puhler A. Unusual structure of the tonB-exb DNA region of Xanthomonas campestris pv. campestris: tonB, exbB, and exbD1 are essential for ferric iron uptake, but exbD2 is not. J Bacteriol. 1997;179:7103–7110. doi: 10.1128/jb.179.22.7103-7110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Q, Li X Z, Mistry A, Srikumar R, Zhang L, Lomovskaya O, Poole K. Influence of the TonB energy-coupling protein on efflux-mediated multidrug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:2225–2231. doi: 10.1128/aac.42.9.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]