Abstract
Introduction
The profiles of patients with COVID-19 have been widely studied, but little is known about differences in baseline characteristics and in outcomes between subjects with a ceiling of care assigned at hospital admission and subjects without a ceiling of care. The aim of this study is to compare, by ceiling of care, clinical features and outcomes of hospitalized subjects during four waves of COVID-19 in a metropolitan area in Catalonia.
Methods
Observational study conducted during the first (March–April 2020), second (October–November 2020), third (January–February 2021), and fourth wave (July–August 2021) of COVID-19 in five centers of Catalonia. All subjects were adults (> 18 years old) hospitalized with a proven SARS-CoV-2 infection and with therapeutic ceiling of care assessed by the attending physician at hospital admission.
Results
A total of 5813 subjects were analyzed. Subjects with a ceiling of care were mainly older (difference in median age of 20 years), with more comorbidities (Charlson index 3 points higher) and with fewer clinical signs at baseline than patients without a ceiling of care. Some features of their clinical profiles changed among waves. There were differences in treatments received during hospital admission across waves, but not between subjects with and without a ceiling of care. Subjects with a ceiling of care had a death incidence more than four times the death incidence of subjects a without a ceiling of care (risk ratio (RR) ranging from 3.5 in the first wave to almost 6 in the third and fourth). Incidence of severe pneumonia and complications for subjects with a ceiling of care was around 1.5 times the incidence in subjects without a ceiling of care.
Discussion
Analysis of hospitalized subjects with SARS-CoV-2 infection should be stratified according to therapeutic ceiling of care to avoid bias and outcome misestimation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00705-w.
Keywords: Therapeutic ceiling of care, COVID-19, Cohort studies, Real-world data
Key Summary Points
| Profiles of patients with COVID-19 have been widely studied, but little is known about differences in baseline characteristics and in outcomes between subjects with a ceiling of care assigned at hospital admission and subjects without a ceiling of care. |
| The aim of this study was to describe clinical characteristics and outcomes of patients with COVID-19 and to study the impact of their ceiling of care across waves. |
| Subjects with a ceiling of care were mainly older, with more comorbidities at baseline and some features of their clinical profiles change among waves. Subject with a ceiling of care in all waves had poorer outcomes than subjects without a ceiling of care. |
| As a result of differences at baseline and in outcomes between subjects with and without a ceiling of care, analysis should be stratified according to therapeutic ceiling of care to avoid bias. |
Introduction
Since the first confirmed case in Wuhan, China, in December 2019, COVID-19 rapidly spread throughout Europe, with the first outbreak in Italy in February 2020. Spain, like Italy, was one of the European countries worst hit by the COVID-19 pandemic in March 2020. Both presented the highest rates of excess mortality in western Europe [1–3].
The first imported case in Catalonia, Spain, was detected on February 25, 2020. More than 120,000 outpatient cases of COVID-19 were diagnosed in the so-called first wave from March to April 2020 [4]. The first wave of the pandemic in Catalonia had a severe impact on elderly people, and a national lockdown was required to avoid the collapse of the health system. From this point, seven successive waves of COVID-19 cases have been registered, and more than two and a half million cases have been detected [5].
Ceiling of care decisions concerning the life-prolonging treatments that a subject may receive are common practice when treating subjects with a critical prognosis. There is no full consensus on the criteria for the decision but in general decisions are based on clinical, ethical, and legal aspects [6]. In several COVID-19 peaks, as a result of the excess demand for critical care beds and the availability of clinical resources, decisions on potentially life-prolonging treatments had to be adapted to an emergency situation. Prioritization had to be done at hospital admission, or during hospitalization if a subject’s health status evolution demanded a more aggressive intervention such as admission to a critical care unit. Decisions were mainly based on each subject’s age, associated comorbidities, and the expected clinical benefit in relation to the availability of available resources [7]. Despite the importance of ceiling of care in allocating resources, the number of studies on COVID-19 with information about a ceiling of care is very limited.
We conducted an observational multicenter study in five hospitals, located in the south metropolitan area of Barcelona (Catalonia, Spain), to characterize all admitted subjects with COVID-19 in those hospitals during four waves from March 2020 to August 2021. The aim of this study was to describe each subject’s clinical profile and incidence of severe pneumonia, use of mechanical ventilation, clinical complications during hospitalization, and in-hospital death and to compare them by ceiling of care.
Methods
Study Design and Participants
The south metropolitan area of Barcelona [8] is a health administrative area with 1.2 million inhabitants in Catalonia (Spain). The coverage includes a mix of urban and rural areas, with a population of low and middle income. There are five centers located in this area, namely Hospital Universitari de Bellvitge, Consorci Sanitari Integral (Hospital Moisès Broggi, Hospital General de l’Hospitalet), Hospital de Viladecans, Hospital de Sant Boi de Llobregat, Consorci Sanitari de l’Alt Penedès i Garraf (Hospital Residència Sant Camil, Hospital Sant Antoni Abat, and Hospital Comarcal de l’Alt Penedès).
The MetroSud cohort is a prospective cohort of consecutive adult subjects (older than 18 years old) admitted to any of the five aforementioned centers. All subjects had a proven SARS-CoV-2 infection (with a positive PCR test or antigen test). The first wave included hospitalized subjects between March 1 and April 15, 2020; the second wave, from October 1 to November 31, 2020; the third, from January 1 to February 28, 2021; and the fourth, from July 1 to August 31, 2021. As a result of the burden of care, not all five centers included subjects in all waves (Table S1 in the supplementary material).
The study was approved by the ethics committee of all participating institutions in accordance with Spanish legislation and was performed in accordance with the Helsinki Declaration of 1964. The need for patient informed consent was waived by each ethics committee.
Study Variables
An electronic case report form in REDCap, a secure web-based software platform designed to build and manage online databases [9, 10], was designed ad hoc in March 2020 to collect study data. Demographic data (age, sex, race), comorbidities (obesity, smoking habit, Charlson score index [11], and other relevant findings on medical history), previous medications, clinical symptoms, epidemiologic profile (diagnostic date, acquisition, close contact, health worker, and recent travel abroad), vital signs (body temperature, FiO2, O2 saturation, blood pressure, pulse, and respiratory rate), laboratory results (D dimer, C-reactive protein, lactate dehydrogenase, leukocytes, and others), and respiratory auscultation (wheezing, rhoncus) were collected at baseline. Pneumonia severity index (PSI) and MuLBSTA score (score for viral pneumonia mortality) [12] (which predicts the 90-day mortality risk in subjects with viral pneumonia) were computed at admission.
During the successive COVID-19 waves, the daily clinical practice included the evaluation of the subjects’ ceiling of care, which was recorded in the subjects’ chart. Subjects’ ceiling of care was thus assessed by their attending physician at hospital admission. Therapeutic ceiling was defined as the maximum therapeutic effort to be offered to a subject on the basis of their age, their associated comorbidities, and the expected clinical benefit in relation to the availability of available resources. Criteria varied according to the burden of care and all decisions were based on the criteria of the attending physician and the availability of resources (intensive care unit (ICU) beds, number of non-invasive ventilators and high-flow nasal oxygen therapy devices). Those subjects assigned to no ceiling of care would have access to ICU and to receiving invasive mechanical ventilation (IMV). Otherwise, subjects assigned to ceiling of care will have limited access to ICU and if they require any respiratory support it will be a non-rebreather mask or a high-flow nasal cannula.
Treatments received during hospital stay (antiviral, antibiotic, statins, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists (ARA II), and corticosteroids), laboratory results, and medical management during ICU admission (type of ventilation, development of severe pneumonia) were also collected.
The evolution of COVID-19 knowledge and strategies over time resulted in changes in the variables collected in the electronic case report form (eCRF). For example, information about exposures or recent travels was deprecated rapidly after the first wave, as were treatments like hydroxychloroquine. We included information about the vaccination status of subjects in the third and fourth waves. Moreover, as a result of the burden of care, some data like laboratory results or vital signs during admission days were difficult to collect and finally discarded from the analysis because of an excess of missing data.
Clinical Outcomes
Cumulative incidence of intrahospital mortality was defined as the percentage of subjects who died during admission. Cumulative incidence of severe pneumonia was defined as the percentage of subjects who required a sustained (longer than 24 h) supply of oxygen therapy greater than FiO2 of 35% to maintain oxygen saturation above 95% at baseline or during hospital admission. Cumulative incidence of mechanical ventilation use was defined as the percentage of subjects who needed intubation during hospital admission. Main complications recorded during admission were cardiac events (heart failure, acute coronary event), respiratory complications (acute respiratory failure, venous thromboembolism, pneumonia), renal impairment, mental state alteration, and nosocomial infection. Cumulative incidences were defined as the percentage of subjects who presented each complication during hospital admission.
Statistical Methods
To define cohort characteristics, categorical variables were presented as the number of cases and percentages, while continuous variables were presented as the mean and standard deviation (SD) or median and interquartile range (IQR). To compare comorbidity prevalence in the four waves and by ceiling of care, radial charts were plotted. Log-binomial models (for death, severe pneumonia, and complications) were constructed to compare outcome incidence between ceiling of care levels in the three waves. Risk ratios (RR) and 95% confidence intervals were reported and represented on a forest plot. No formal sample size was computed and all consecutive subjects were included in the cohort. All analyses were performed with a two-sided significance level of 0.05 and conducted with the use of R software version 4.1.0 [13].
Results
Flowchart
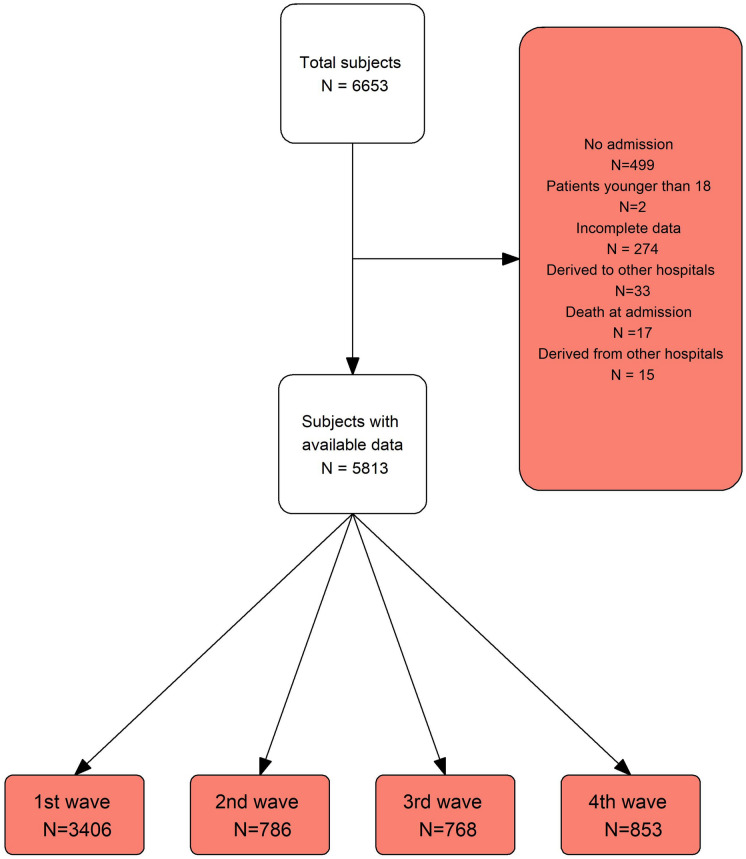
A total of 6653 subjects were included in the MetroSud cohort. Subjects who were admitted to hospital within less than 24 h (N = 499), subjects who died within the first 24 h (N = 17), subjects who had incomplete data in a pool of essential variables (age, sex, Charlson score, ceiling of care, and circumstances at discharge) (N = 274), or subjects admitted firstly in one hospital but transferred to another and treated in the latter (N = 48) were excluded from the analysis. All subjects were followed up until in-hospital death or hospital discharge (Fig. 1).
Fig. 1.
Flowchart of included subjects
Subject Baseline Characteristics by Ceiling of Care
Table 1 describes the demographic and clinical characteristics of included subjects by wave and stratified by ceiling of care. The percentage of subjects who were assigned a ceiling of care at hospital admission decreased across time from 39% in the first wave to percentages between 22.3% and 19.1%.
Table 1.
Demographic characteristics and comorbidities through waves by ceiling of care
| Ceiling of care | No ceiling of care | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 1 | 2 | 3 | 4 | Total | 1 | 2 | 3 | 4 | |
| N = 1831 | N = 1330 | N = 175 | N = 163 | N = 163 | N = 3982 | N = 2076 | N = 611 | N = 605 | N = 690 | |
| Age | 80.0 [74.0; 86.0] | 79.0 [72.0; 85.0] | 83.0 [78.0; 88.0] | 83.0 [78.0; 87.0] | 85.0 [80.0; 89.0] | 59.0 [48.0; 69.0] | 59.0 [49.0; 69.0] | 62.0 [53.0; 71.0] | 63.0 [53.0; 72.0] | 49.0 [37.0; 63.0] |
| Sex | ||||||||||
| Women | 796 (43.5%) | 565 (42.5%) | 75 (42.9%) | 81 (49.7%) | 75 (46.0%) | 1567 (39.4%) | 855 (41.2%) | 222 (36.3%) | 248 (41.0%) | 242 (35.1%) |
| Long-term facility | 278 (15.2%) | 223 (16.8%) | 20 (11.4%) | 20 (12.3%) | 15 (9.20%) | 104 (2.61%) | 64 (3.08%) | 19 (3.11%) | 17 (2.81%) | 4 (0.58%) |
| Race | ||||||||||
| Caucasian | 1267 (96.3%) | 795 (96.1%) | 164 (94.8%) | 154 (97.5%) | 154 (98.1%) | 2470 (75.3%) | 1206 (78.0%) | 394 (68.8%) | 464 (86.4%) | 406 (65.1%) |
| Other | 48 (3.65%) | 32 (3.87%) | 9 (5.20%) | 4 (2.53%) | 3 (1.91%) | 811 (24.7%) | 341 (22.0%) | 179 (31.2%) | 73 (13.6%) | 218 (34.9%) |
| Smoker | ||||||||||
| Ex-smoker | 460 (25.1%) | 326 (24.5%) | 39 (22.3%) | 44 (27.0%) | 51 (31.3%) | 765 (19.2%) | 379 (18.3%) | 138 (22.6%) | 151 (25.0%) | 97 (14.1%) |
| No | 1294 (70.7%) | 958 (72.0%) | 125 (71.4%) | 108 (66.3%) | 103 (63.2%) | 2988 (75.0%) | 1593 (76.7%) | 448 (73.3%) | 415 (68.6%) | 532 (77.1%) |
| Yes | 77 (4.21%) | 46 (3.46%) | 11 (6.29%) | 11 (6.75%) | 9 (5.52%) | 229 (5.75%) | 104 (5.01%) | 25 (4.09%) | 39 (6.45%) | 61 (8.84%) |
| Alcohol consumption | 72 (3.93%) | 42 (3.16%) | 13 (7.43%) | 12 (7.36%) | 5 (3.07%) | 138 (3.47%) | 57 (2.75%) | 28 (4.58%) | 26 (4.30%) | 27 (3.91%) |
| Obesity (BMI > 30) | 451 (30.7%) | 285 (29.4%) | 51 (29.1%) | 65 (39.9%) | 50 (30.7%) | 1296 (36.2%) | 579 (34.6%) | 221 (36.2%) | 248 (41.0%) | 248 (35.9%) |
| Hypertension | 1274 (69.6%) | 881 (66.2%) | 137 (78.3%) | 124 (76.1%) | 132 (81.0%) | 1529 (38.4%) | 792 (38.2%) | 266 (43.5%) | 295 (48.8%) | 176 (25.5%) |
| Diabetes mellitus | 588 (32.1%) | 414 (31.1%) | 61 (34.9%) | 54 (33.1%) | 59 (36.2%) | 764 (19.2%) | 418 (20.1%) | 124 (20.3%) | 126 (20.8%) | 96 (13.9%) |
| COPD | 488 (26.7%) | 325 (24.4%) | 54 (30.9%) | 49 (30.1%) | 60 (36.8%) | 611 (15.3%) | 274 (13.2%) | 108 (17.7%) | 119 (19.7%) | 110 (15.9%) |
| Heart failure | 298 (16.3%) | 194 (14.6%) | 37 (21.1%) | 31 (19.0%) | 36 (22.1%) | 120 (3.01%) | 50 (2.41%) | 18 (2.95%) | 27 (4.46%) | 25 (3.62%) |
| Cerebrovascular disease | 241 (13.2%) | 164 (12.3%) | 24 (13.7%) | 29 (17.8%) | 24 (14.7%) | 125 (3.14%) | 66 (3.18%) | 22 (3.60%) | 23 (3.80%) | 14 (2.03%) |
| Dementia | 324 (17.7%) | 204 (15.3%) | 43 (24.6%) | 35 (21.5%) | 42 (25.8%) | 73 (1.83%) | 43 (2.07%) | 16 (2.62%) | 12 (1.98%) | 2 (0.29%) |
| Mild renal insufficiency | 343 (18.7%) | 234 (17.6%) | 42 (24.0%) | 26 (16.0%) | 41 (25.2%) | 177 (4.44%) | 83 (4.00%) | 27 (4.42%) | 42 (6.94%) | 25 (3.62%) |
| Charlson index | 5.00 [4.00; 7.00] | 5.00 [4.00; 7.00] | 6.00 [5.00; 8.00] | 6.00 [5.00; 7.00] | 6.00 [5.00; 7.00] | 2.00 [1.00; 3.00] | 2.00 [1.00; 3.00] | 2.00 [1.00; 4.00] | 3.00 [1.00; 4.00] | 1.00 [0.00; 3.00] |
Data are presented as median [Q1; Q3] or N (%)
BMI body mass index
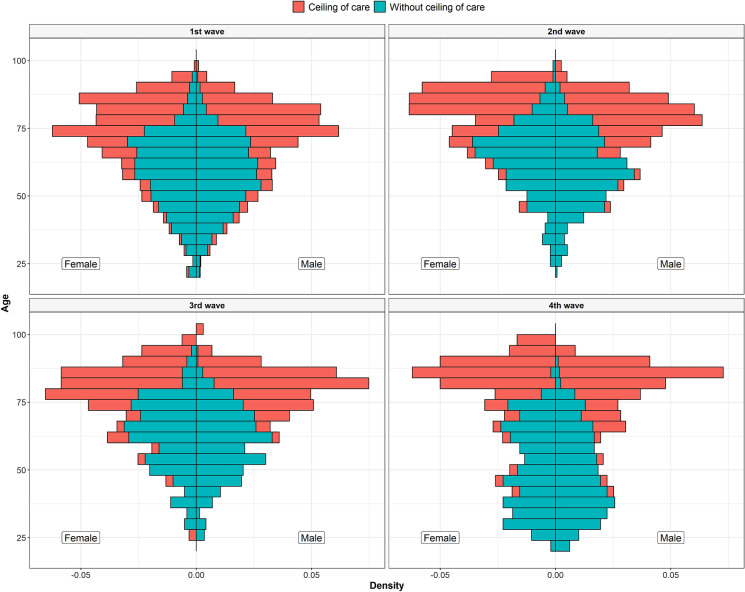
Figure 2 shows the distribution of sex and age according to ceiling of care in the four waves. The difference in median age between subjects with and without a ceiling of care was of 20 years in all waves except in the fourth (36 years difference). In relation to age, when stratifying by waves, there were no differences across the first three waves. However, subjects without a ceiling of care from the fourth wave were markedly younger than in the other three waves. The percentage of women was lower in subjects without a ceiling of care in all waves. Almost no subjects without a ceiling of care lived in a long-term facility whereas the percentage of subjects with a ceiling of care who lived in a long-term facility ranged from 17.8% in the first wave to 9.2% in the fourth. The most common race was Caucasian but with less predominance in subjects without a ceiling of care.
Fig. 2.
Histogram of age, by sex and ceiling of care for all waves
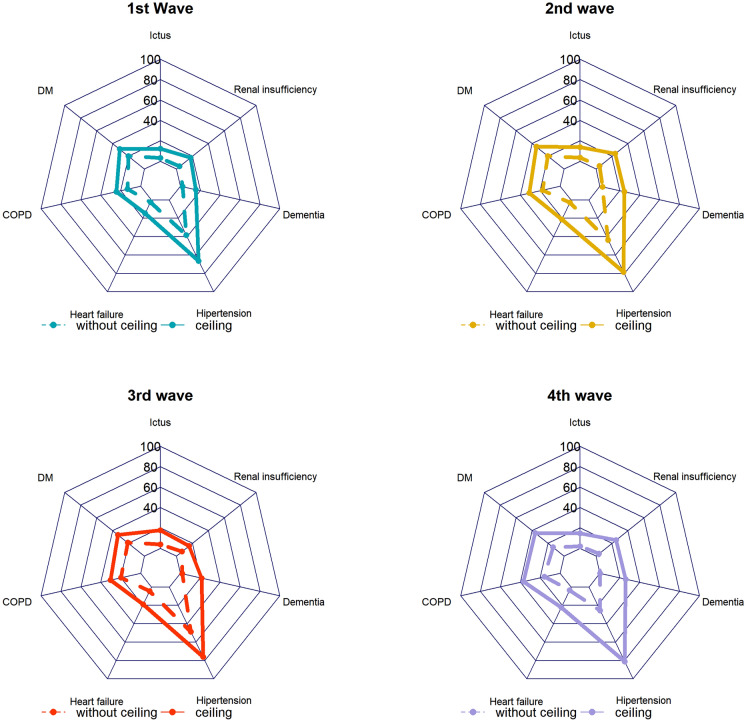
The most common comorbidities in all waves included hypertension, diabetes, obesity, and chronic obstructive pulmonary disease (COPD). Charlson index was 3 points lower in subjects without a ceiling of care. Radial charts in Fig. 3 show the prevalence of most common comorbidities for subjects with and without a ceiling of care in the four waves. Subjects with a ceiling of care had similar prevalence of the most common comorbidities across waves whereas subjects without a ceiling of care from the fourth wave had markedly fewer comorbidities than subjects without a ceiling of care from the other waves. In all waves, previous treatments (statins, corticosteroids, ACE inhibitors, ARA II, corticosteroids, and anticoagulants) were more common in subjects with a ceiling of care (Table S2 in the supplementary material).
Fig. 3.
Comorbidity comparison between waves and according to ceiling of care
Vaccination in Catalonia started in December 2020 in older and vulnerable people and in healthcare staff. Only 3.1% of subjects with a ceiling of care and only five subjects without a ceiling of care were vaccinated in the third wave. In the fourth wave, almost 85% of subjects with a ceiling of care and 35.2% of subjects without a ceiling of care had been vaccinated.
Table 2 describes the vital signs and severity scores at admission. Higher values of SatO2 and FiO2 were found in the fourth wave and were lower in the first one regardless of ceiling of care. Heart rate was higher in subjects without a ceiling of care in all waves.
Table 2.
Vital signs and severity scores at hospital admission through waves by ceiling of care
| Ceiling of care | No ceiling of care | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 1 | 2 | 3 | 4 | Total | 1 | 2 | 3 | 4 | |
| N = 1828 | N = 1327 | N = 175 | N = 163 | N = 163 | N = 3980 | N = 2074 | N = 611 | N = 605 | N = 690 | |
| Temperature | 36.9 (0.99) | 37.0 (1.01) | 36.7 (0.93) | 36.7 (0.90) | 36.7 (0.86) | 37.0 (1.20) | 37.1 (1.42) | 37.0 (0.96) | 36.8 (0.90) | 36.9 (0.87) |
| FiO2 | 28.6 (18.0) | 27.6 (17.6) | 29.7 (18.4) | 32.5 (20.1) | 31.7 (18.3) | 29.4 (19.5) | 27.1 (17.4) | 30.7 (21.1) | 32.1 (22.0) | 32.4 (20.9) |
| SatO2 | 93.2 (6.15) | 92.8 (6.59) | 93.4 (5.06) | 94.1 (5.05) | 95.0 (3.54) | 94.5 (5.25) | 94.1 (6.19) | 94.5 (4.11) | 95.0 (4.45) | 95.4 (3.25) |
| SatO2/FiO2 | 386 (106) | 395 (102) | 375 (108) | 355 (118) | 356 (108) | 390 (111) | 405 (100.0) | 382 (116) | 373 (121) | 366 (120) |
| Systolic blood pressure | 131 (23.1) | 131 (22.7) | 134 (24.9) | 135 (22.6) | 132 (24.5) | 130 (20.0) | 130 (20.1) | 132 (20.6) | 133 (20.1) | 126 (18.5) |
| Diastolic blood pressure | 71.2 (14.2) | 71.4 (14.5) | 70.0 (12.4) | 73.0 (14.0) | 69.1 (13.4) | 75.4 (12.6) | 75.2 (13.1) | 75.1 (12.8) | 77.6 (12.4) | 74.5 (10.8) |
| Heart rate | 87.0 (18.3) | 88.4 (18.3) | 83.2 (17.6) | 84.5 (19.4) | 82.6 (16.6) | 89.5 (17.4) | 91.3 (17.5) | 88.8 (18.2) | 87.5 (15.6) | 86.4 (17.2) |
| Respiratory rate | 22.9 (6.81) | 23.3 (7.14) | 22.9 (6.19) | 23.1 (6.59) | 20.8 (5.12) | 21.5 (5.76) | 21.7 (6.24) | 21.8 (5.73) | 21.0 (5.06) | 21.0 (4.92) |
| ROX index | 18.2 (7.31) | 18.4 (7.30) | 17.9 (7.24) | 16.9 (7.53) | 18.2 (7.15) | 19.5 (7.49) | 20.2 (7.43) | 18.8 (7.60) | 18.9 (7.50) | 18.5 (7.38) |
| PSI group | ||||||||||
| 1 | 197 (12.4%) | 184 (16.9%) | 4 (2.30%) | 7 (4.40%) | 2 (1.24%) | 2208 (61.3%) | 1081 (63.5%) | 320 (52.9%) | 356 (59.1%) | 449 (65.4%) |
| 2 | 359 (22.6%) | 257 (23.6%) | 32 (18.4%) | 49 (30.8%) | 21 (13.0%) | 764 (21.2%) | 355 (20.9%) | 159 (26.3%) | 125 (20.8%) | 125 (18.2%) |
| 3 | 700 (44.2%) | 451 (41.3%) | 86 (49.4%) | 73 (45.9%) | 90 (55.9%) | 531 (14.8%) | 227 (13.3%) | 101 (16.7%) | 104 (17.3%) | 99 (14.4%) |
| 4 | 329 (20.8%) | 199 (18.2%) | 52 (29.9%) | 30 (18.9%) | 48 (29.8%) | 95 (2.64%) | 39 (2.29%) | 25 (4.13%) | 17 (2.82%) | 14 (2.04%) |
| MuLBSTA | 9.52 (3.39) | 9.40 (3.37) | 9.96 (3.29) | 10.3 (3.29) | 9.28 (3.57) | 7.84 (3.62) | 7.51 (3.70) | 8.38 (3.53) | 8.66 (3.60) | 7.61 (3.36) |
Data are presented as mean (SD) or N (%)
PSI pneumonia severity index
Regarding severity scores, subjects with a ceiling of care had PSI scores from 30 (first wave) to 50 (fourth wave) points higher than subjects without a ceiling of care. Subjects with a ceiling of care had a higher MuLBSTA score than subjects without a ceiling of care. Once stratified by ceiling of care, there were no differences in the MuLBSTA mortality score through waves.
Table S3 (supplementary material) shows clinical signs reported for subjects at hospital admission. All clinical signs were more frequent in subjects without a ceiling of care. The more reported were fever and cough (both higher in the first wave for both subjects with and without a ceiling of care) and shortness of breath (similar in the first wave for subjects with and without a ceiling of care and markedly lower than in the other waves).
Treatments Administered by Ceiling of Care
Treatments received during hospital admission varied between waves (Table S4 in the supplementary material) reflecting changes in scientific evidence. Treatments with unproven efficacy used during the first wave (98% hydroxychloroquine, 54.3% lopinavir/ritonavir, 49.6% azithromycin) were deprecated in the following waves. On the other hand, an increase in the use of remdesivir (from no use in the first wave to around 50–80% in the other three) and corticosteroids (lower than 45% in the first wave and higher than 85% in the other three) was observed. No differences in treatments administered by ceiling of care were found. Antibiotics were also used more frequently in the first wave (more than 84% of subjects for subjects with and without a ceiling) than in the other waves. The decreased in the percentage of subjects using antibiotics was higher in subjects without a ceiling of care. Tocilizumab was used in all waves, but more commonly for subjects without a ceiling of care and subjects from the fourth wave.
Outcomes by Ceiling of Care
Table 3 presents the main outcome incidences for each wave stratified by ceiling of care. In the first three waves, around 4 out of 10 subjects with a ceiling of care died in hospital whereas only 1 out of 10 subjects without a ceiling of care did. Percentages are lower for both groups in the fourth wave. Overall, subjects with a ceiling of care had a death incidence of more than four times the death incidence of subjects without a ceiling of care (RR ranging from 3.5 times more risk in the first wave to almost 6 in the third and fourth ones) (Fig. 4).
Table 3.
Outcome incidence through waves by ceiling of care
| Ceiling of care | No ceiling of care | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 1 | 2 | 3 | 4 | Total | 1 | 2 | 3 | 4 | |
| N = 1828 | N = 1327 | N = 175 | N = 163 | N = 163 | N = 3981 | N = 2075 | N = 611 | N = 605 | N = 690 | |
| Death | 682 (37.3%) | 490 (36.9%) | 70 (40.0%) | 73 (44.8%) | 49 (30.1%) | 362 (9.09%) | 218 (10.5%) | 62 (10.1%) | 46 (7.60%) | 36 (5.22%) |
| Severe pneumonia | 830 (45.5%) | 543 (41.0%) | 100 (57.1%) | 103 (63.2%) | 84 (51.5%) | 1485 (37.3%) | 670 (32.3%) | 254 (41.8%) | 266 (44.0%) | 295 (42.8%) |
| Mechanical ventilation | 18 (0.99%) | 14 (1.06%) | 2 (1.14%) | 2 (1.23%) | 0 (0.00%) | 484 (12.2%) | 269 (13.0%) | 79 (13.0%) | 67 (11.1%) | 69 (10.0%) |
| Complications | 853 (46.7%) | 600 (45.2%) | 98 (56.0%) | 83 (50.9%) | 72 (44.2%) | 1405 (35.3%) | 778 (37.5%) | 217 (35.6%) | 206 (34.0%) | 204 (29.6%) |
| Cardiac complications | 165 (9.03%) | 100 (7.54%) | 29 (16.6%) | 23 (14.1%) | 13 (7.98%) | 190 (4.77%) | 116 (5.59%) | 26 (4.26%) | 26 (4.30%) | 22 (3.19%) |
| Respiratory complications | 83 (4.54%) | 56 (4.22%) | 11 (6.29%) | 5 (3.07%) | 11 (6.75%) | 396 (9.95%) | 210 (10.1%) | 80 (13.1%) | 39 (6.45%) | 67 (9.71%) |
| Renal impairment | 299 (16.4%) | 226 (17.0%) | 29 (16.6%) | 26 (16.0%) | 18 (11.0%) | 318 (7.99%) | 195 (9.40%) | 44 (7.21%) | 44 (7.27%) | 35 (5.07%) |
| Mental state alteration | 256 (14.0%) | 185 (13.9%) | 30 (17.1%) | 25 (15.3%) | 16 (9.82%) | 151 (3.79%) | 89 (4.29%) | 24 (3.93%) | 17 (2.81%) | 21 (3.04%) |
Data are presented as N (%)
Fig. 4.
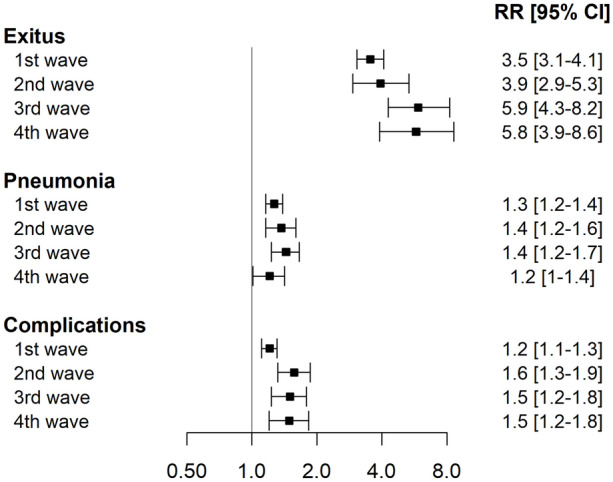
Forest plot of risk ratios for death, severe pneumonia, and complications for subjects with a ceiling of care versus no ceiling of care in each wave
Severe pneumonia was less frequent in the first wave for both subjects with and without a ceiling of care. In the second, third, and fourth wave, more than 40% of subjects developed or presented severe pneumonia (regardless of ceiling of care) at hospital admission. In all waves, subjects with a ceiling of care had a higher incidence of severe pneumonia than subjects without a ceiling of care (RR ranging from 1.2 to 1.4).
Regarding invasive mechanical ventilation, in subjects with no ceiling of care there were no differences in the percentage of subjects with invasive mechanical ventilation across waves (percentage ranging from 13% in the first to 10% in the fourth).
Incidence of complications in subjects with a ceiling of care was around 1.5 times the incidence of complications in subjects without a ceiling of care (lower in the first wave, RR = 1.2). In subjects without a ceiling of care, the percentage of complications was lower in the fourth wave. Subjects with a ceiling of care in the second and third waves had more complications than subjects with a ceiling of care in the first and fourth waves.
Regarding the type of complications, no relevant differences were found in the incidence of cardiac complications between subjects with and without a ceiling of care in the first wave while in the other waves, subjects with a ceiling of care had around three times more cardiac complications than subjects without a ceiling of care. On the contrary, respiratory complications were more likely to occur in subjects without a ceiling of care in all waves. Incidence of renal impairment in subjects with a ceiling of care was twice the incidence in subjects without a therapeutic ceiling in all waves. Subjects without a ceiling of care had almost no mental state alterations. For subjects with a ceiling of care, mental state alteration was less common in the fourth wave.
Discussion
To our knowledge, this is the first large cohort study with information on multiple waves that describes the baseline characteristics and outcomes of subjects according to ceiling of care assigned at hospital admission. Comparison of in-hospital 30-day mortality incidence in subjects with and without a ceiling of care showed that estimation may be biased if ceiling of care is not considered. Same circumstances apply to severe pneumonia, use of mechanical ventilation, and clinical complications. The impact differs depending on the wave studied.
Regarding subjects’ characteristics, subjects with no ceiling of care were younger, had fewer comorbidities, and had fewer previous treatments at hospital admission than subjects who were assigned a ceiling of care in all waves. The most common comorbidities in all waves included hypertension, diabetes, and COPD (all more common in the second and third waves), in line with findings in the Catalonia National Survey of Health in 2020 [14]. Regarding treatments administered during hospital admission, treatments with no proven efficacy [15] were abandoned after the first wave. On the other hand, following scientific evidence [16, 17], the use of remdesivir and corticosteroids increased in the other three waves. In this study, subjects with a ceiling of care more frequently received antibiotic treatment during all waves than subjects without a ceiling of care. Antibiotics use decreased throughout waves according to World Health Organization guidelines [18]. As previously authors have shown [19], the prevalence of bacterial infection in COVID-19 is approximately 8.6%; however, more than 60% of subjects received antibiotics. Furthermore, international guidelines recommend the involvement of antimicrobial stewardship programs in antibiotic decisions in palliative care subjects [20].
Similarly to our findings, another European study [21] also found that subjects assigned a ceiling of care below full intensive treatment were of advanced age and had more previous comorbidities than subjects without a ceiling of care. Supporting our results, it also found that although subjects without a ceiling of care presented worse symptoms at hospital admission, outcomes were more favorable for them than for subjects with a ceiling of care. Our study adds that the observed differences between those subjects with and without a ceiling effect remained throughout the analyzed waves.
A systematic review of 33 studies from the first wave [22] showed an overall 17.1% mortality rate (95% CI 12.7; 22.7) for patients with COVID-19 admitted to hospital. But when only looking into studies with non-critically ill subjects, the overall mortality rate decreased to 11.5% (95% CI 7.7; 16.9), consistent with our mortality incidences in subjects without a ceiling of care. In the same way, overall mortality in studies with only critically ill subjects was 40.5% (95% CI 31.2; 50.6), again very similar to our figures for the four waves in subjects with a ceiling of care. However, critically ill subjects from these studies are subjects admitted to ICU and our subjects without a ceiling of care were potential candidates for ICU. Studies with non-critically ill subjects that reported higher mortality incidences analyzed a mix of subjects with and without a ceiling of care [23] or included only subjects who had severe pneumonia [24, 25]. Another study [26] based on subjects admitted with COVID-19 in eight university hospitals in Catalonia between February 2020 and February 2021 presented mortality incidences among waves from 8.3% to 16.6%. This study did not stratify by ceiling of care, so these figures lay between the higher incidence we observed in mortality rates in subjects with a ceiling of care and the lower incidence rates in subjects without a ceiling of care.
A living systematic review by the COVID-PRECISE (Precise Risk Estimation to optimize covid-19 Care for Infected or Suspected patients in diverse sEttings) group analyzed more than 200 COVID-19 prediction models [27] on diagnostic, mortality, progression to severe disease, intensive care unit admission, ventilation, intubation, or length of hospital stay. Of the analyzed models, 107 were prognostic models. The authors concluded that the proposed models were poorly reported, at high risk of bias, with probably an optimistic reported performance, and they do not recommend any of the reviewed models for use in current practice. Importantly, the most frequently identified bias was the inclusion or exclusion criteria of participants, in part related to mixing participants with and without a ceiling of care and not accounting for it. This reinforces our idea that analyzing all together subjects with and without a ceiling of care may overestimate the incidence of mortality and other outcomes for subjects without a ceiling of care. This applies especially for waves in which the indication of ceiling of care was conditioned by the demand on critical care beds and the availability of clinical resources.
The number of subjects with a ceiling of care decreased through waves and its profile changed across them. Our hypothesis is that this could be related to the change in the available resources to allocate subjects, knowledge on COVID-19 through time, vaccination impact, and differences between virus strains. During the outbreak, each attending physician made decisions based on clinical characteristics and the expected clinical benefit considering the availability of available resources. Under a scenario without harmonized guidelines, decisions may appear arbitrary or resemble medical practice variation [28]. The Spanish Society of Intensive Care suggested [29] performing triage at hospital admission to give priority to subjects with a greater life expectancy for all diseases. Nevertheless, guidelines have not been validated in COVID-19 [30], but some recommendations have been made to reach a fair allocation of resources [31].
This study has some limitations. To the extent that we assumed that the missing data were random, we restricted the analysis to subjects with complete data on a group of variables that we identified as essential. Selection bias was unavoidable since the cohort contains only patients with COVID-19 that were able to be hospitalized. Subjects who did suffer COVID-19 but were not admitted to a hospital because of the excess of assistance demand in each wave might bias some results. Moreover, this is a study with data from a metropolitan area of Catalonia (Spain). Thus, the generalizability of our findings may be limited as a result of sociodemographic differences between countries and hospital resources available [32]. In addition, ceiling of care does not have a consensus definition and depends on the attending physician. On the other hand, the strengths of this study are the large number of subjects included from different hospitals and from four different waves and the availability of information about the ceiling of care.
In summary, here we report the clinical characteristics and outcomes of a large cohort of subjects with SARS-2-CoV infection consecutively admitted to five hospitals in a metropolitan area in Spain during four waves of the pandemic according to ceiling of care assigned by clinicians at hospital admission. Our findings provide information about the evolution of characteristics and outcomes of COVID-19 and show the importance of analyzing separately subjects with and without a ceiling of care so as not to overestimate the incidence of mortality and other outcomes related to COVID-19. Further research should consider ceiling of care information to correctly report outcomes and characteristics of patients with COVID-19.
Conclusions
Subjects with a ceiling of care in all waves had poorer outcomes than subjects without a ceiling of care. Analysis should be stratified according to therapeutic ceiling of care to avoid overestimation of incidence of outcomes in subjects without a ceiling of care.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Funding
This study was partially funded by Secretaria d’Universitats i Recerca del Departament d’Empresa i Coneixement de la Generalitat de Catalunya (2020PANDE00148). The funding was used to fund the Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Conceptualization was performed by AR-M, JC, SV, CT, GG, and NP. Data collection was performed by GA, AR, IO, AFS, AR-M, EI, and VD-B. Statistical analysis was performed by CT and NP. The first draft of the manuscript was written by NP and revised by SV, JC, and CT. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
List of Investigators
MetroSud Study group: Carlota Gudiol, Judit Aranda-Lobo, Marta Arroyo, Carlos Pérez-López, Montserrat Sanmartí, Encarna Moreno, Maria C Alvarez Mª, Ana Faura, Martha Gónzalez, Paula Cruz, Mireia Colom, Andrea Perez, Laura Serrano. Divine Study group: Mireia Besalú, Erik Cobo, Jordi Cortés, Daniel Fernández, Leire Garmendia, Pilar Hereu, Klaus Langohr, Núria Pérez-Álvarez, Xavier Piulachs.
Disclosures
Cristian Tebé has received fees for speaker lectures and talks from Amgen, Boehringer Ingelheim, and Gedeon Richter, outside the submitted work. Natàlia Pallarès, Gabriela Abelenda-Alonso, Alexander Rombauts, Isabel Oriol, Antonella F Simonetti, Alejandro Rodríguez-Molinero, Elisenda Izquierdo, Vicens Díaz-Brito, Gemma Molist, Guadalupe Gómez Melis, Jordi Carratalà and Sebastián Videla have nothing to disclose. Alexander Rombauts changed his affiliation to Infectious Diseases Unit, Hospital de la Santa Creu i Sant Pau, Barcelona. Antonella F Simonetti changed her affiliation to Infectious Diseases Unit, Hospital de la Santa Creu i Sant Pau, Barcelona.
Compliance with Ethics Guidelines
The study was approved by the ethics committee of all participating institutions in accordance with Spanish legislation and was performed in accordance with the Helsinki Declaration of 1964. The patient informed consent was waived by each ethics committee.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jordi Carratalà and Sebastián Videla are joint senior authors.
Contributor Information
Cristian Tebé, Email: ctebe@idibell.cat.
the MetroSud and Divine study groups:
Carlota Gudiol, Judit Aranda-Lobo, Marta Arroyo, Carlos Pérez-López, Montserrat Sanmartí, Encarna Moreno, Maria C. Mª Alvarez, Ana Faura, Martha Gónzalez, Paula Cruz, Mireia Colom, Andrea Perez, Laura Serrano, Mireia Besalú, Erik Cobo, Jordi Cortés, Daniel Fernández, Leire Garmendia, Pilar Hereu, Klaus Langohr, Núria Pérez-Álvarez, and Xavier Piulachs
References
- 1.COVID-19 Situation Dashboard. European Centre for Disease Prevention and Control. https://qap.ecdc.europa.eu/public/extensions/COVID-19/COVID-19.html#eu-eea-daily-tab. Accessed 12 Jul 2022.
- 2.Spain: Coronavirus Pandemic Country Profile. Our World in Data. https://ourworldindata.org/coronavirus/country/spain. Accessed 12 Jul 2022.
- 3.Spain: WHO Coronavirus Disease (COVID-19) dashboard with vaccination data. WHO. https://covid19.who.int/region/euro/country/es. Accessed 12 Jul 2022.
- 4.Burn E, Tebé C, Fernandez-Bertolin S, et al. The natural history of symptomatic COVID-19 during the first wave in Catalonia. Nat Commun. 2021;12(1):777. doi: 10.1038/s41467-021-21100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19-Diagnòstics AP. SIVIC. https://sivic.salut.gencat.cat/covid. Accessed 12 Jul 2022.
- 6.Norwegian Directorate of Health. Decision-making processes in the limitation of lifeprolonging treatment[Internet]; 2013[cited 2022 Nov 28]. Available from: https://www.helsedirektoratet.no/veiledere/beslutningsprosesser-ved-begrensning-av-livsforlengende-behandling/engelsk-versjon.
- 7.Segrelles-Calvo G, de Granda-Orive JI, López-Padilla D, Zamora GE. Therapeutic limitation in elderly patients: reflections regarding COVID19. Arch Bronconeumol Engl Ed. 2020;56(10):677–679. doi: 10.1016/j.arbres.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regió Sanitària Barcelona. CatSalut. Servei Català de la Salut. https://catsalut.gencat.cat/ca/coneix-catsalut/catsalut-territori/barcelona/. Accessed 12 Jul 2022.
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 12 Jul 2022.
- 14.Direcció General de Planificació en Salut. L’estat de salut, els comportaments relacionats amb la salut i l’ús de serveis sanitaris a Catalunya, 2020[Internet]; 2020 [cited 2022 Nov 28]. Available from: https://salutweb.gencat.cat/esca.
- 15.Mitjà O, Corbacho-Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild coronavirus disease 2019: a randomized, controlled trial. Clin Infect Dis. 2021;73(11):E4073–E4081. doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. JAMA. 2020;324(13):1330. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical management of COVID-19-Interim Guidance (May 2020)-World. ReliefWeb. https://reliefweb.int/report/world/clinical-management-covid-19-interim-guidance-may-2020. Accessed 12 Sep 2022.
- 19.Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27(4):520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung KC, Lee LW, Liew YX, et al. Antibiotic stewardship program (ASP) in palliative care: antibiotics, to give or not to give. Eur J Clin Microbiol Infect Dis. 2022;41(1):29–36. doi: 10.1007/s10096-021-04325-z. [DOI] [PubMed] [Google Scholar]
- 21.Straw S, McGinlay M, Drozd M, et al. Advanced care planning during the COVID-19 pandemic: ceiling of care decisions and their implications for observational data. BMC Palliat Care. 2021;20(1):10. doi: 10.1186/s12904-021-00711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macedo A, Gonçalves N, Febra C. COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis. Ann Epidemiol. 2021;57:14–21. doi: 10.1016/j.annepidem.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X, Du RH, Wang R, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roso-Llorach A, Serra-Picamal X, Cos FX, et al. Evolving mortality and clinical outcomes of hospitalized subjects during successive COVID-19 waves in Catalonia, Spain. Glob Epidemiol. 2022;4:100071. doi: 10.1016/j.gloepi.2022.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wennberg JE. Dealing with medical practice variations: a proposal for action. Health Aff (Millwood) 1984;3(2):6–32. doi: 10.1377/hlthaff.3.2.6. [DOI] [PubMed] [Google Scholar]
- 29.Rubio O, Estella A, Cabre L, Saralegui-Reta I, Martin MC, Zapata L, Esquerda M, Ferrer R, Castellanos A, Trenado J, Amblas J. Recomendaciones éticas para la toma de decisiones difíciles en las unidades de cuidados intensivos ante la situación excepcional de crisis por la pandemia por COVID-19: revisión rápida y consenso de expertos [Ethical recommendations for a difficult decision-making in intensive care units due to the exceptional situation of crisis by the COVID-19 pandemia: A rapid review & consensus of experts]. Med Intensiva (Engl Ed). 2020;44(7):439–45. [DOI] [PMC free article] [PubMed]
- 30.Herreros B, Gella P, Real de Asua D. Triage during the COVID-19 epidemic in Spain: better and worse ethical arguments. J Med Ethics. 2020;46(7):455–458. doi: 10.1136/medethics-2020-106352. [DOI] [PubMed] [Google Scholar]
- 31.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 32.Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38(10):1647–1653. doi: 10.1007/s00134-012-2627-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.