Abstract
Background
Pressure ulcers affect approximately 10% of people in hospitals and older people are at highest risk. A correlation between inadequate nutritional intake and the development of pressure ulcers has been suggested by several studies, but the results have been inconsistent.
Objectives
To evaluate the effects of enteral and parenteral nutrition on the prevention and treatment of pressure ulcers.
Search methods
In March 2014, for this first update, we searched The Cochrane Wounds Group Specialised Trials Register, the Cochrane Central register of Controlled Trials (The Cochrane Library), the Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library), the Health Technology Assessment Database (HTA) (The Cochrane Library), the Cochrane Methodology Register (The Cochrane Library), NHS Economic Evaluation Database (The Cochrane Library), Ovid Medline, Ovid Embase and EBSCO CINAHL. No date, language or publication status limits were applied.
Selection criteria
Randomised controlled trials (RCTs) evaluating the effects of enteral or parenteral nutrition on the prevention and treatment of pressure ulcers, which measured the incidence of new ulcers, ulcer healing or changes in pressure ulcer severity. There were no restrictions on types of patient, setting, date, publication status or language.
Data collection and analysis
Two review authors independently screened for inclusion, and disagreement was resolved by discussion. Two review authors independently extracted data and assessed quality using the Cochrane Collaboration tool for assessing risk of bias.
Main results
We included 23 RCTs, many were small (between 9 and 4023 participants, median 88) and at high risk of bias.
Eleven trials compared a combination of nutritional supplements, consisting of a minimum of energy and protein in different dosages, for the prevention of pressure ulcers. A meta‐analysis of eight trials (6062 participants) that compared the effects of mixed nutritional supplements with standard hospital diet found no clear evidence of an effect of supplementation on pressure ulcer development (pooled RR 0.86; 95% CI 0.73 to 1.00; P value 0.05; I2 = 13%, random effects). This outcome is at unclear or high risk of bias.
Fourteen trials evaluated the effects of nutritional supplements on the healing of existing pressure ulcers: seven trials examined mixed nutritional supplements, three the effects of proteins, two trials examined zinc, and two studies examined ascorbic acid. The included trials were heterogeneous with regard to participants, interventions, comparisons and outcomes and meta‐analysis was not appropriate. There was no clear evidence of an improvement in pressure ulcer healing from the nutritional supplements evaluated in any of these individual studies.
Authors' conclusions
There is currently no clear evidence of a benefit associated with nutritional interventions for either the prevention or treatment of pressure ulcers. Further trials of high methodological quality are necessary.
Plain language summary
Dietary supplementation for preventing and treating pressure ulcers
Background Pressure ulcers (also called bed sores) are wounds caused by pressure at the weight‐bearing, bony points of immobilised people (such as hips, heels and elbows). Poor nutritional status, or dehydration, may weaken the skin and make people more vulnerable to developing pressure ulcers. Once a pressure ulcer has developed, it can become very large and difficult to heal.
Review Question We wanted to find out whether changing the diet (for example by giving supplements) could prevent the development of pressure ulcers. We also wanted to find out if dietary changes could help heal pressure ulcers that had already occurred.
The review of trials found that there is no clear evidence that nutritional interventions reduce the number of people who develop pressure ulcers or help the healing of existing pressure ulcers. More research is needed.
Background
A pressure ulcer ‐ also known as a pressure sore, decubitus ulcer or bedsore ‐ is defined as "localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear." Shear pressure occurs when layers of skin are forced to slide over one another or over deeper layers of tissue, for example, if a patient slides down the bed (EPUAP and NPUAP 2009a). Friction is also thought to contribute. Applied pressure affects cellular metabolism by decreasing or obliterating tissue circulation, resulting in insufficient blood flow to the skin and underlying tissues, and causing tissue ischaemia (deficient blood supply). Elderly patients with decreased mobility, limited mental status and increased skin friction and shear may have a higher risk of developing a pressure ulcer (Perneger 2002). Schoonhoven 2006 found that independent predictors of pressure ulcers were increased age, reduced (<54kg) or increased (>95kg) weight at admission, abnormal appearance of the skin, friction and shear, and surgery planned for the coming week (Schoonhoven 2006).
Pressure ulcer classification systems allow a consistent description for the severity and level of tissue injury of a pressure ulcer. The words "stage", "grade", and "category" may be used interchangeably to describe the levels of soft‐tissue injury (EPUAP and NPUAP 2009a). The classification includes Grades 1 through to 4. Grade 1 reflects persistent non‐blanching erythema (redness) of the skin, Grade 2 involves partial thickness skin loss (epidermis and dermis), Grade 3 reflects full thickness skin loss involving damage or necrosis of subcutaneous tissue whereas in Grade 4 the damage extends to the underlying bone, tendon or joint capsule (EPUAP and NPUAP 2009a).
Pressure ulcers affect a significant minority of people in hospitals and other care facilities. An economic analysis of the impact of pressure ulcer care in a 252‐bed elderly care unit in Glasgow reported that 41% of the patients suffered from some pressure damage. The incidence data were reported to show that 45% of these pressure ulcers were potentially preventable (Thomson 1999). The overall prevalence of pressure ulcers in all facilities in the United States was 12.3% in 2009, with 5% of the pressure ulcers considered to be facility‐associated. When Grade I ulcers were excluded, overall pressure ulcer prevalence was 9% (VanGilder 2009). A Swiss study showed an incidence of pressure ulcers (Grade 2 or more) of 10% in acute hospitals (Perneger 1998). Schoonhoven 2002 reported a weekly incidence of patients with Grade 2 pressure ulcers of 6.2% (95% confidence interval (CI) 5.2% to 7.2%) in two large Dutch hospitals. In 2001, a study of 3012 patients (mean age 65 years) from 165 wards in 11 hospitals in Germany estimated the prevalence of pressure ulcers as 24% to 39% (Dassen 2001). Between 2002 and 2008 the pressure ulcer prevalence rates in German long‐term care facilities decreased from 12.5% to 5.0%, while non‐blanchable erythema decreased from 6.6% to 3.5% (Lahmann 2010). The authors hypothesised that this decrease was due to more effective treatment strategies and better prevention. Another retrospective analysis of clinical records from 414 cancer patients admitted over six months for palliative care showed a prevalence of pressure ulcers of 22.9%, and an incidence of 6.7% (Hendrichova 2010).
The prevention of pressure ulcers involves a number of strategies designed to address both extrinsic factors, e.g. reducing the pressure duration or magnitude at the skin surface through repositioning or use of pressure relieving cushions or mattresses, and intrinsic factors, e.g. the ability of the patient's skin to remain intact and resist pressure damage by optimising hydration, circulation and nutrition. There is some evidence that the incidence and severity of pressure ulceration increases with poor nutrition (Bergstrom 1992;Berlowitz 1989). Decreased calorie intake, dehydration, and a drop in serum albumin levels may decrease the tolerance of skin and underlying tissue to pressure, friction, and shearing force, thus increasing the risk of skin breakdown and reducing wound healing ability (Mueller 2001). Serum albumin is commonly used as a measure of the amount of protein available in the blood for healing. The combination of low energy and low protein intake is often described as protein‐calorie or protein‐energy malnutrition.
A few studies have suggested a correlation between protein‐calorie malnutrition and pressure ulcers (Breslow 1991a; Finucane 1995; Strauss 1996). The effects of special diets in preventing and treating pressure ulcers has not yet been examined sufficiently, although many risk assessment tools include nutritional status (e.g. Braden 1994; Gosnell 1989). Nevertheless, there is a consensus that nutrition is an important factor, as shown by its incorporation in a variety of guidelines, e.g. the European Pressure Ulcer Advisory Panel (EPUAP) Pressure Ulcer Prevention Guidelines, which state, "Consider the impact of the following factors on an individual’s risk of pressure ulcer development: a) nutritional indicators . . ."; and "Screen and assess the nutritional status of every individual at risk of pressure ulcers in each health care setting", and urge care‐providers to, "Provide sufficient calories . . . provide adequate protein . . . provide and encourage adequate daily fluid intake for hydration . . . provide adequate vitamins and minerals" (EPUAP and NPUAP 2009a; EPUAP and NPUAP 2009b).
Enteral nutrition is nourishment such as a special diet, or supplements to normal eating or tube feeding, that are given via the mouth or by tube and absorbed through the digestive system. Parenteral nutrition is nourishment such as intravenous infusion or intramuscular injection given via the bloodstream. It is unclear if the route of administration (i.e. oral feeding, tube feeding or parenteral feeding) plays a role in pressure ulcer prevention and treatment
This update of the original systematic review was required to summarize the best research available and to enable evidence‐based guidance on the role of nutritional interventions in the prevention and treatment of pressure ulcers.
Objectives
To evaluate the effect of enteral and parenteral nutritional interventions (e.g. supplementation) on the prevention and treatment of pressure ulcers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of parallel or crossover design evaluating the effect of enteral and/or parenteral nutrition on the prevention and treatment of pressure ulcers by measuring the incidence of new ulcers, ulcer healing rates or changes in pressure ulcer severity.
Types of participants
People of any age and sex with or without existing pressure ulcers, in any care setting, irrespective of primary diagnosis. For the purpose of this review a pressure ulcer is defined as an area of localised damage to the skin and underlying tissue caused by pressure, shear, friction or a combination of these.
Types of interventions
Clearly described nutritional supplementation (enteral or parenteral nutrition) or special diets. Comparisons between supplementary nutrition plus standard diet versus standard diet alone and between different types of supplementary nutrition (e.g. enteral versus parenteral) were eligible.
Types of outcome measures
Primary outcomes
Proportion of participants developing new (incident) pressure ulcers (for prevention studies); and,
time to complete healing (for treatment studies).
Secondary outcomes
Acceptability of supplements;
side effects;
costs;
rate of complete healing;
rate in change of size of ulcer (absolute and relative);
health‐related quality of life.
Search methods for identification of studies
Electronic searches
The search methods section of the original published version of this review is shown in Appendix 1.
In March 2014, for this first update, we searched the following electronic databases to find reports of relevant RCTs:
The Cochrane Wounds Group Specialised Register (searched 25 March 2014);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 1);
The Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2014, Issue 1);
The Health Technology Assessment Database (HTA) (The Cochrane Library 2014, Issue 1);
The Cochrane Methodology Register (The Cochrane Library 2012, Issue 3);
NHS Economic Evaluation Database (The Cochrane Library 2014, Issue 1);
Ovid MEDLINE (1946 to March Week 2 2014);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, March 24, 2014);
Ovid EMBASE (1974 to 2014 March 24);
EBSCO CINAHL (1982 to 20 March 2014
The following strategy was used to search CENTRAL:
#1 MeSH descriptor: [Pressure Ulcer] explode all trees510 #2 pressure next (ulcer* or sore*) 1071 #3 decubitus next (ulcer* or sore*) 110 #4 (bed next sore*) or bedsore* 68 #5 #1 or #2 or #3 or #4 1150 #6 MeSH descriptor: [Nutritional Physiological Phenomena] explode all trees18062 #7 MeSH descriptor: [Nutrition Therapy] explode all trees6361 #8 MeSH descriptor: [Enteral Nutrition] explode all trees1407 #9 MeSH descriptor: [Parenteral Nutrition] explode all trees1466 #10 nutrition* 25947 #11 MeSH descriptor: [Diet] explode all trees10790 #12 diet* 35397 #13 tube next (fed or feed or feeding) 442 #14 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 52460 #15 #5 and #14 155
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format (Lefebvre 2011). The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2009). No date or language restrictions were applied. As the search strategy had been redesigned we screened all records regardless of publication year.
By updating the review we additionally searched several trial registers and searched in the first quarter of 2011:
Australian New Zealand Clinical Trials Registry;
CenterWatch Clinical Trials Listing Service;
Chinese Clinical Trial Register; ClinicalTrials.gov register;
Community Research & Development Information Service (of the European Union);
Current Controlled Trials metaRegister of Controlled Trials (mRCT) – active & archived registers;
German Trials Register;
Hong Kong Clinical Trials Register;
International Clinical Trials Registry Platform Search Portal;
International Standard Randomised Controlled Trial Number Register;
Netherlands Trial Register;
South African National Clinical Trial Register;
UK Clinical Trials Gateway;
UK National Research Register (NRR);
University Hospital Medical Information Network (UMIN);
Clinical Trials Registry (for Japan) – UMIN CTR.
Searching other resources
For the original review handsearching of conference proceedings and journals was performed, bibliographies of relevant articles were examined and experts in the field were contacted in order to find additional literature that might be relevant, however no further handsearching was undertaken for this update. The reference lists of all identified eligible studies and other published systematic reviews were searched in order to identify further eligible studies.
Data collection and analysis
Selection of studies
Results from the search were assessed for potential eligibility by two review authors independently, and disagreement was resolved by discussion with a third review author. Potentially relevant studies were retrieved in full and two review authors decided, independently, whether these studies met the inclusion criteria.
Data extraction and management
References identified from searches were entered into a bibliographic software package. Details of eligible studies were extracted and summarised using a data extraction sheet. Studies that had been published in duplicate were included only once except where multiple publications provided additional data. Data extraction was undertaken by two review authors simultaneously and independently. Any disagreements were resolved by discussion.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). Five domains of risk of bias were assessed according to the The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), namely: generation of randomisation sequence, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting )(see Appendix 5 for details of criteria on which the judgement will be based). We presented the assessment of risk of bias using a 'risk of bias summary figure', which shows all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
Assessment of heterogeneity
Where there was the potential to pool data from separate studies, we assessed between study heterogeneity with both the chi‐squared test and the I2. We regarded I2 greater than 60% as indicative of serious heterogeneity (Higgins 2003). Clinical heterogeneity was also considered.
Data synthesis
The method of synthesising the studies (i.e. random‐effects or fixed‐effect model) depended upon the heterogeneity of studies identified. In case of serious heterogeneity (i.e. where I2 > 60%) a random‐effects model was to be routinely applied when pooling was considered appropriate.
The following comparisons were planned:
Enteral compared with parenteral nutrition;
supplement/diet in addition to regular diet compared with regular diet alone;
comparisons between different types of supplement/diets.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were considered:
Characteristics of the setting (e.g. hospital in‐patients versus out‐patients);
method of feeding (e.g. enteral versus parenteral feeding);
characteristics of patients (e.g. people with pre‐existing malnutrition versus people without malnutrition).
Results
Description of studies
Results of the search
Our search strategy in 2003 identified 942 articles from online databases (MEDLINE (PubMed), CINAHL, and CENTRAL). A further 13 articles were retrieved by handsearching; 17 were referred to us by experts and manufacturers, and a further 23 were found by scanning bibliographies of relevant papers. In addition the Cochrane Wounds Group identified a further nine articles. After merging the results and removing duplicates, 912 citations were left and were reviewed independently. Two of the review authors had an initial overall agreement of 99% (904/912), and identified 16 studies related to potentially relevant trials, the text of which were retrieved in full. Disagreements were resolved by discussion and the rating of the third author. Eight trials met the inclusion criteria for the original version of this review.
Our search strategy in 2011 identified 175 articles from online databases (MEDLINE (PubMed), EMBASE, CINAHL, and CENTRAL), 19 by scanning bibliographies of relevant papers and seven by searching registration databases. In addition, the Cochrane Wounds Group identified a further six articles. After merging the results and removing duplicates, 197 citations were left and were reviewed independently. The two authors had an initial overall agreement of 98% (192/197), and identified 22 studies related to potentially relevant trials, full text copies of which were retrieved in full (see Figure 1). Disagreements were resolved by discussion. Fifteen trials met the inclusion criteria bringing the total number of included studies to 23 (27 citations).
1.
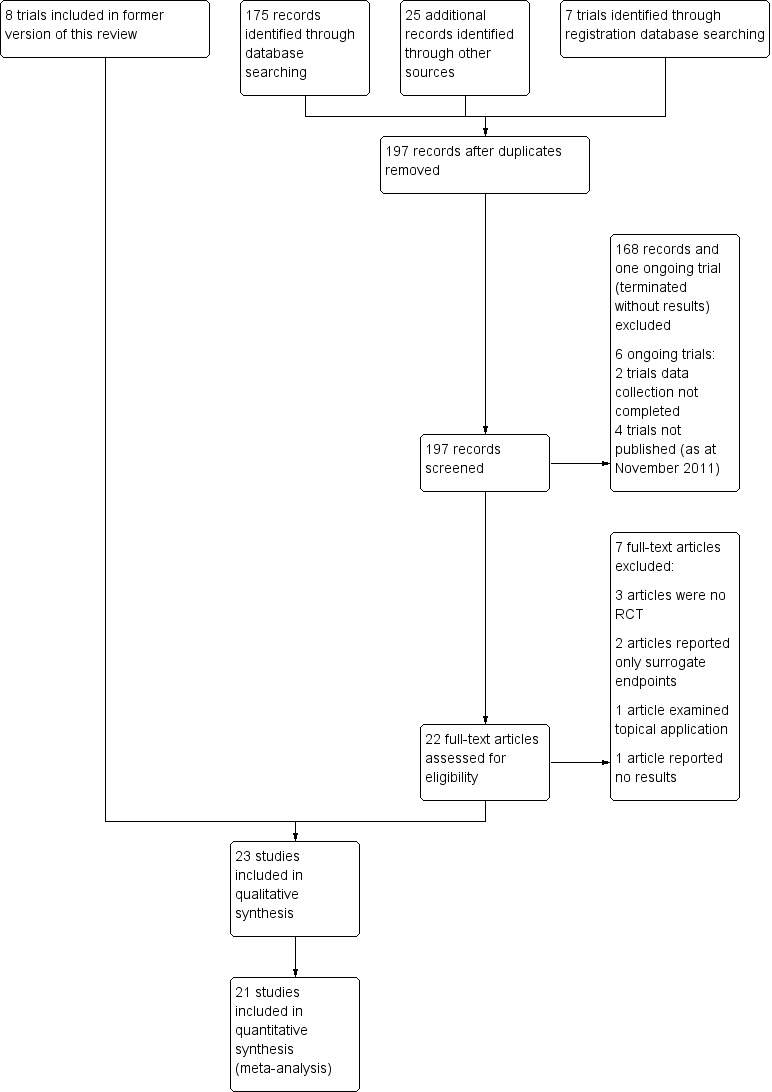
Study flow diagram.
Included studies
Twenty three RCTs are now included in the review (see Characteristics of included studies table); comprising 8 RCTs from the previous version of the review and 15 newly included RCTs.
Design
All included studies were RCTs. Six studies were multi‐centre trials (Bourdel‐M 2000; Dennis 2005; Meaume 2009; Ohura 2011; ter Riet 1995; van Anholt 2010).
Setting
Fifteen of the 23 trials were carried out in hospitals (Arias 2008; Benati 2001; Bourdel‐M 2000; Brewer 1967; Dennis 2005; Derossi 2009; Desneves 2005; Hartgrink 1998; Houwing 2003; Meaume 2009; Norris 1971; Ohura 2011; Olofsson 2007; Taylor 1974; Theilla 2007), and three in long‐term care facilities (Cereda 2009; Craig 1998; Lee 2006). One study was conducted in the long‐term care unit of a university hospital (Ek 1991). Two multi‐centre trials covered a range of settings, with long‐term care units and hospital wards (ter Riet 1995; van Anholt 2010). The Delmi 1990 trial was carried out in a orthopaedic ward, but some of the participants were transferred to a rehabilitation hospital. Chernoff 1990 did not mention the setting. Most studies were conducted in Europe (Benati 2001; Bourdel‐M 2000; Cereda 2009; Delmi 1990; Derossi 2009; Ek 1991; Hartgrink 1998; Houwing 2003; Olofsson 2007; Taylor 1974; ter Riet 1995), or the USA (Brewer 1967; Chernoff 1990; Craig 1998; Lee 2006; Norris 1971) with one each from Australia (Desneves 2005), Israel (Theilla 2007), Japan (Ohura 2011) and Urugay (Arias 2008). Three studies were international trials (Dennis 2005; Meaume 2009; van Anholt 2010).
Participants
Most of the studies included in the review were small. The median sample size was 88 participants, with a range from 12 (Chernoff 1990) to 4023 patients (Dennis 2005). Fourteen were conducted as treatment studies because the included participants already had pressure ulcers (Benati 2001; Brewer 1967; Cereda 2009; Chernoff 1990; Desneves 2005; Ek 1991; Lee 2006; Meaume 2009; Norris 1971; Ohura 2011; Taylor 1974; ter Riet 1995; Theilla 2007; van Anholt 2010). Five trials specifically recruited people with hip fractures (Delmi 1990; Derossi 2009; Hartgrink 1998; Houwing 2003; Olofsson 2007). Other patient populations, each with one trial, included stroke patients (Dennis 2005), and people with spinal cord injury (Brewer 1967). In several studies the mean age of the participants was over 80 years (Bourdel‐M 2000; Cereda 2009; Craig 1998; Delmi 1990; Hartgrink 1998; Houwing 2003; Meaume 2009; Olofsson 2007). The mean ages in the trials of Arias 2008 and Theilla 2007 were considerably lower at 60.2 years and 61.1 years respectively. Not all studies reported the mean age of the participants.
Interventions
The interventions in the included trials can be summarized as special nutrient supplementation or mixed nutritional supplements. Eleven studies considered mixed nutritional supplements as an intervention to prevent pressure ulcers (Arias 2008; Bourdel‐M 2000; Craig 1998; Delmi 1990; Dennis 2005; Derossi 2009; Ek 1991; Hartgrink 1998; Houwing 2003; Olofsson 2007; Theilla 2007). Mixed nutritional supplements included energy enriched supplements of protein alone and mixed supplements of protein, vitamins, carbohydrate, and lipids etc. All studies compared the nutritional intervention with a standard intervention, for example standard hospital diet, or standard hospital diet plus placebo.
Seven treatment studies considered special nutrients compared with placebo: two investigated the influence of ascorbic acid (Taylor 1974; ter Riet 1995); two the impact of zinc sulphate (Brewer 1967; Norris 1971); and three the impact of protein (Chernoff 1990; Lee 2006; Meaume 2009). Six studies considered mixed nutritional supplements as an intervention to treat pressure ulcers (Benati 2001; Cereda 2009; Desneves 2005; Ek 1991; Ohura 2011; van Anholt 2010). Ohura 2011 investigated the influence of increased energy in comparison with a standard amount of energy. The other studies compared the nutritional intervention with a standard intervention, for example standard hospital diet or standard hospital diet plus placebo.
Two studies considered the influence of mixed nutritional supplements on pressure ulcer healing as well as on the prevention of pressure ulcers (Ek 1991; Theilla 2007). In three studies the enteral nutrition or supplement was administered by nasogastric tube (Craig 1998; Hartgrink 1998; Ohura 2011).
Outcomes
Prevention
Five studies reported pressure ulcer incidence (Bourdel‐M 2000; Ek 1991; Hartgrink 1998; Houwing 2003; Theilla 2007). In six studies pressure ulcer incidence was considered as an in‐hospital complication (Arias 2008; Craig 1998; Delmi 1990; Dennis 2005; Derossi 2009; Olofsson 2007).
Treatment
The outcomes were heterogeneous. Two different validated scores were reported, namely the Pressure Ulcer Scale for Healing (PUSH, National Pressure Ulcer Advisory Panel; Thomas 1997; Günes 2009) and the Pressure Sore Status Tool (PSST; Bates‐Jensen 1992; Benati 2001). Five trials reported pressure ulcer healing with validated scores: four with PUSH (Cereda 2009; Desneves 2005; Lee 2006; van Anholt 2010), and one with PSST (Benati 2001). Other studies considered pressure ulcer size, pressure ulcer surface, or pressure ulcer volume (Cereda 2009; Chernoff 1990; Meaume 2009; Taylor 1974; ter Riet 1995). Five studies reported the number of people healed (Brewer 1967; Cereda 2009; Ohura 2011; Taylor 1974; ter Riet 1995), and one study noted adverse effects related to the supplements (Meaume 2009).
Excluded studies
See Characteristics of excluded studies table.
Fifteen studies were excluded from the review for the following reasons:six studies were not randomised (Barateau 1998; Bergstrom 1987; Bourdel‐M 1997; Breslow 1991; Langemo 2006; Neander 2004). Three trials used surrogate primary endpoints without specifically reporting pressure ulcers, or did not report any other outcomes predefined in this review (Eneroth 2006; Langkamp‐Henken 2000; Stotts 2009). One study used a topical application of vitamin A (Settel 1969). One study compared mixed nutritional supplements, but did not report any results (Schröder‐van den N 2004). One study appears to report on the same study and group of patients as the Ek 1991 trial, but does not report pressure ulcer outcomes (Larsson 1990). Breslow 1993 intended to conduct a RCT but switched to a CCT because groups were unbalanced and the trial had a high drop‐out rate; therefore the authors decided to exchange patients within the groups. One study (Myers 1990) did not explicitly describe the type of nutritional supplementation. Another trial we found in a registration database had been terminated without results because of a lack of patients (NCT00502372).
Ongoing studies
We identified six ongoing trials from different registration databases (NCT01107197; ACTRN12605000704695; NCT00228657; NCT01142570; ACTRN12610000526077; NCT01090076). In two trials the data collection was not complete (NCT01107197; NCT01142570), while another trial announced results for the third quarter of 2012 (ACTRN12605000704695). We contacted the principal investigators of the trials where all the data had been collected and asked for information about the outcomes in which we are interested (ACTRN12605000704695; NCT00228657; NCT01142570; ACTRN12610000526077; NCT01090076), but either they did not respond, or were not allowed to release any results prior to publication.
Risk of bias in included studies
All included studies were prospective RCTs. In general, most of the studies included in the review were small and had either an unclear, or high risk, of bias. Figure 2 and Figure 3 show judgments about the risk of bias for all the included studies. The descriptions of the risk of bias for each item and for each included trial are described in the Characteristics of included studies table.
2.
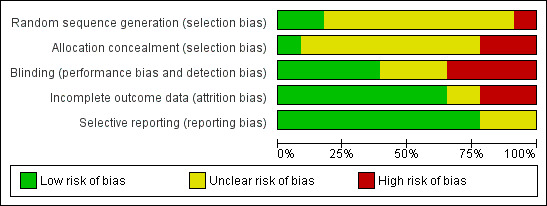
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
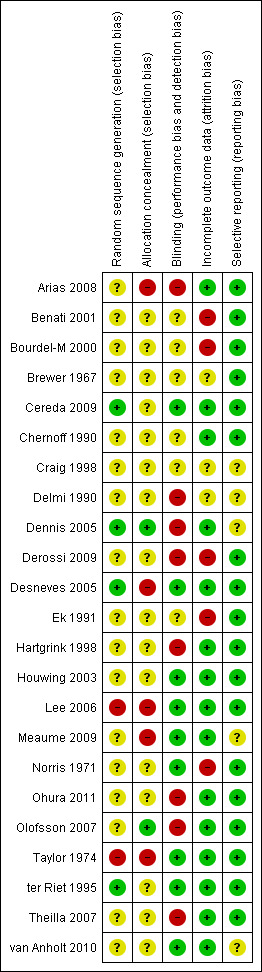
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation sequence generation
Only four out of 23 trials clearly reported adequate generation of randomisation sequence (Cereda 2009; Dennis 2005; Desneves 2005; ter Riet 1995).
Allocation concealment
Only one out of 23 trials clearly reported adequate allocation concealment (Dennis 2005).
Blinding
Seven trials tried to minimise performance bias by blinding the medical staff and patients to treatment allocation (Houwing 2003; Lee 2006; Meaume 2009; Norris 1971; Taylor 1974; ter Riet 1995; van Anholt 2010). Two trials blinded only the outcome assessor (Cereda 2009; Desneves 2005). Two other studies were described as blinded, but the methods were not reported (Brewer 1967; Craig 1998). Three trials were assumed not to have performed any blinding because blinding of participants or medical staff was not described, and so these were rated as being of unclear risk of bias (Bourdel‐M 2000; Chernoff 1990; Ek 1991). In eight trials, the intervention was apparent to patients and medical staff, and therefore blinding was deemed not to be possible (Arias 2008; Delmi 1990; Dennis 2005; Derossi 2009; Hartgrink 1998; Ohura 2011; Olofsson 2007; Theilla 2007).
Incomplete outcome data
Many of the trials reported the reasons for withdrawals and drop‐outs very accurately. Three trials minimised attrition bias by analysing data with an intention‐to‐treat approach (Dennis 2005; ter Riet 1995; van Anholt 2010). Two trials specified that there were no drop‐outs or withdrawals (Chernoff 1990; Houwing 2003). Six trials were judged to have a high risk of bias either because the reasons for losses‐to‐follow‐up were unclear (Benati 2001; Delmi 1990; Derossi 2009), or the drop‐out rate was unacceptably high (Hartgrink 1998; Norris 1971), or there was an imbalance in numbers of drop‐outs from different study groups (Hartgrink 1998). In the trial of Ek 1991 the drop‐outs were not comprehensibly described with regard to the numbers of participants in the intervention group and the control group; furthermore the number of patients evaluated was not clearly described.
Selective reporting
Most of the trials reported the outcome data analogous to the measurements described in the methods section. An unclear risk of selective reporting had to be assumed for five trials. Two of them did not describe all clinical outcomes, so it was not clear whether they were assessed (Craig 1998; Delmi 1990). Two trials did not report at least one pre‐defined outcome measure which was specified at the beginning of their study, but this is less relevant with regard to this review because outcomes relevant for this review have been reported (Dennis 2005; van Anholt 2010). One study stated for a subgroup that, "there have been no significant differences", but did not provide the results (Meaume 2009).
Risk of bias summary
All included trials had a certain risk of bias, with one or more quality domains judged as either unclear or at high risk of bias. Figure 2 shows judgements about each methodological quality item presented as percentages across all included trials. Interpretations and conclusion of the effects of the following interventions should be drawn against the background of these findings.
Effects of interventions
The included trials were heterogeneous with regard to patients (e.g. some surgical, some critically ill, some residents in nursing homes), and to nutritional interventions (e.g. type of nutritional intervention, form in which supplementation was applied, time of application, and dose and duration of nutritional supplementation).
PREVENTION STUDIES
The primary outcome in prevention studies was the proportion of participants who developed new pressure ulcers. The results are presented here as risk ratios (RR) with 95% confidence intervals (95% CI). The risk ratio is the proportion of people developing a new pressure ulcer in the intervention group divided by the proportion in the control group.
Mixed nutritional supplements compared with standard hospital diet (nine trials)
Delmi 1990: included 59 people recovering from hip fractures and presented data on the prevalence of pressure ulcers at three time points. There was no statistically significant difference between the two groups at the final follow‐up (six months).No pressure ulcers were present in the treatment group though there were two in the control group (RR 0.22, 95% CI 0.01 to 4.28). Ek 1991: included 501 patients who were expected to remain in hospital for more than three weeks, with follow‐up for up to 26 weeks. There was no statistically significant difference in pressure ulcer development between the two groups (RR 0.83, 95% CI 0.48 to 1.42; P value = 0.49). Hartgrink 1998: included 140 people recovering from hip fractures who were followed‐up for two weeks. After two weeks 25/48 (52%) of the patients remaining in the intervention group and 30/53 (56% )in the control group had pressure ulcers of Grade 2 or more (where Grade 2 was damage to at least the extent of blister formation).An intention‐to‐treat analysis indicated no difference in the incidence of sores of Grade 2 or above. Bourdel‐M 2000: included 672 people over 65 years of age who were in the acute phase of a critical illness; participants were followed‐up for 15 days or until discharged. At 15 days, the cumulative incidence of pressure ulcers (all grades) was 40% (118/295, calculated from relative numbers as absolute numbers have not been provided) in the nutritional intervention group compared with 48% (181/377, calculated from relative numbers as absolute numbers have not been provided) in the control group. There was a reduction in pressure ulcer development with supplementation (RR 0.83 (95% CI 0.70 to 0.99; P value = 0.04). The incidence of pressure ulcers was derived from the raw data and was not directly reported by the authors.The proportion of erythema was 90% for both groups and no significant differences in the development of erythema were detected between the two groups. Multivariate analysis, taking into account all diagnoses, potential risk factors and the intra‐ward correlation, indicated that the independent risk factors of developing a pressure ulcer were: serum albumin level at baseline, Kuntzman score at baseline, lower limb fracture, Norton score < 10, and belonging to the control group. Houwing 2003: included 103 hip fracture patients who were followed‐up for 28 days. There was no significant difference between the two groups; the incidence of pressure ulcers (grades I to 2) in the nutritional intervention group was 27/51 (55%) compared with 30/52 (59%) in the placebo group (RR 0.92, 95% CI 0.65 to 1.30; P value = 0.63). None of the participants developed a pressure ulcer of a grade higher than 2 but the incidence of pressure ulcers at Grade 2 was 18% in the nutritional intervention group versus 28% in the placebo group, which was not statistically significant (RR 0.66; 95% CI 0.31 to 1.38; P value = 0.27). Dennis 2005: included 4023 stroke patients who were able to swallow. There was no significant difference in pressure ulcer incidence between the two groups (15/2014 (0.75%) in the supplemented group compared with 26/2001 (1.30%) in the control group: RR 0.57, 95% CI 0.30 to 1.08; P value = 0.08). This trial only reported additional outcomes defined as secondary outcomes for this review. Quality‐of‐life (EUROQoL) information was available from 3086 (99%) patients, with no major differences between groups. Median utility (ranging from 0, i.e. death, to 1, i.e. perfect health) for all patients, including those who died, was 0.52 (interquartile range 0.03 to 0.74, P value = 0.96 for difference between groups) in both groups (difference between means = 0·001 (95%CI –0·023 to 0·025)). Olofsson 2007: included 199 patients with femoral neck fracture aged 70 years or older. There was no significant difference between the two groups (treatment group 7/83 (8.43%) and control group 14/74 (18.92%); RR 0.45, 95% CI 0.19 to 1.04; P value = 0.12). Arias 2008: randomised 667 patients with mild or serious malnutrition (Subjective Global Assessment B or C). There was no significant difference between the two groups (nutritional intervention group 33/264 (12.50%) compared with the control group 26/273 (9.52%); RR 1.31, 95% CI 0.81 to 2.13; P value = 0.27). Derossi 2009: included 107 hip‐fracture patients aged 65 and older, scheduled to undergo surgical treatment. Pressure ulcers were considered to be a complication. The authors noted that there were no differences between the intervention and control groups, but did not present any data, and did not respond to our request for clarification. This trial was therefore not included in the pooled analysis.
When the eight trials with available data were pooled using a fixed effect model there was a reduction in pressure ulcer incidence with supplementation (RR 0.84, 95% CI 0.74 to 0.96; I2 =13%). This difference was less clear when a random effects model was applied (RR = 0.86, 95% CI 0.73 to 1.00)(Analysis 1.1; Figure 4). Given the methodological differences between these studies (differences in interventions and duration of follow up), the random effects model is probably more appropriate, although some may argue that pooling at all is inappropriate.
1.1. Analysis.
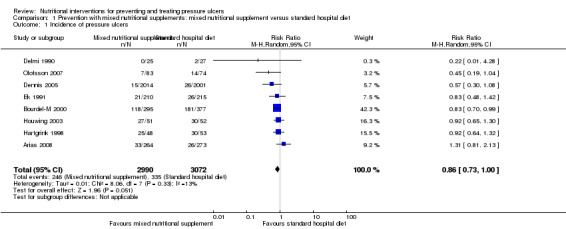
Comparison 1 Prevention with mixed nutritional supplements: mixed nutritional supplement versus standard hospital diet, Outcome 1 Incidence of pressure ulcers.
4.
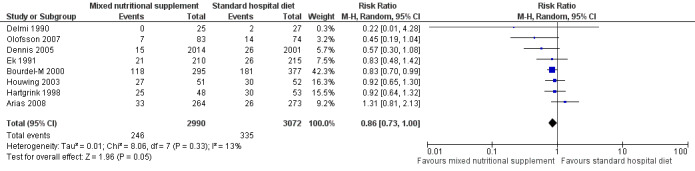
Forest plot of comparison: 1 Prevention with mixed nutritional supplements: mixed nutritional supplement versus standard hospital diet, outcome: 1.1 Incidence of pressure ulcers.
Mixed nutritional supplements compared with other nutritional interventions (two trials).
Craig 1998 included 34 people with a history of type 2 diabetes mellitus or documented hyperglycaemia and requiring total enteral nutrition support by nasogastric tube. Disease‐specific enteral nutritional formula was compared with standard high‐carbohydrate feeding. There was no difference in pressure ulcer development between the two groups (7/16 (43.75%) developed a pressure ulcer in the treatment group compared with 8/14 (57.14%) in the control group); RR 0.77 (95% CI 0.37 to 1.57; P value = 0.47; Analysis 2.1 ).
2.1. Analysis.
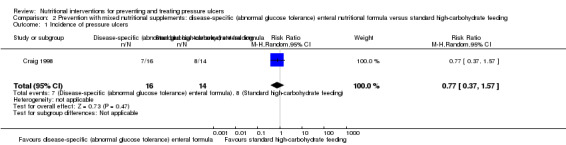
Comparison 2 Prevention with mixed nutritional supplements: disease‐specific (abnormal glucose tolerance) enteral nutritional formula versus standard high‐carbohydrate feeding, Outcome 1 Incidence of pressure ulcers.
Theilla 2007 included 100 intensive care patients suffering from acute lung injury and compared a high fat and low carbohydrate enteral formula which was enriched in lipids vitamins A, C, and E with a high‐fat and low‐carbohydrate enteral formula. There was no difference between the two groups in pressure ulcer development; there were eight new pressure ulcers in the supplemented group compared with 10 in the control group (RR 0.85, 95% CI 0.37 to 1.97; P value = 0.71; Analysis 3.1).
3.1. Analysis.

Comparison 3 Prevention with mixed nutritional supplements: high‐fat, low‐carbohydrate, enteral formula enriched in lipids (eicosapentanoicacid (EPA)), gamma‐linolenicacid (GLA), vitamins A, C and E versus high‐fat, low‐carbohydrate, enteral formula, Outcome 1 Incidence of pressure ulcers.
Summary of the effects of nutritional supplements on pressure ulcer development
Eleven studies investigated the effect of mixed nutritional supplements on pressure ulcer incidence (Arias 2008; Bourdel‐M 2000; Craig 1998; Delmi 1990; Dennis 2005; Derossi 2009; Ek 1991; Hartgrink 1998; Houwing 2003; Olofsson 2007; Theilla 2007). Overall the incidence of pressure ulcers was lower in the intervention group in all trials except for Arias 2008 however none of these differences was statistically significant with the exception of Bourdel‐M 2000. It was possible to pool eight of these trials in a meta‐analysis (Delmi 1990; Dennis 2005; Ek 1991; Arias 2008; Hartgrink 1998; Houwing 2003; Olofsson 2007). When a fixed effect model was applied, there was a reduction in the risk of pressure ulceration associated with nutritional supplementation (RR 0.84, 95% CI 0.74 to 0.96; P value = 0.01; I2 = 13%). This difference was not robust to analysis using a random effects model (RR 0.86, 95% CI 0.73 to 1.00; P value = 0.05) Analysis 1.1; Figure 4). There is clearly considerable uncertainty as to whether nutritional supplementation reduces pressure ulceration and heterogeneity in the studies that have explored this.
There was no evidence of a difference in pressure ulcer development when alternative nutritional supplements were compared with each other but there have only been two small trials.
Overall, these studies were at high or unclear risk of bias. Generally, studies were not at risk of selective reporting, but we were uncertain about the risk of bias in other key domains, for example, generation of allocation sequence and concealment of allocation. Blinding of participants, clinical staff and outcome assessors did not seem to have been performed adequately overall, with nearly all studies (with the exception of Houwing 2003) at high or unclear risk of performance bias.
TREATMENT STUDIES
Mixed nutritional supplements compared with other nutritional interventions (seven trials)
Seven studies investigated the effect of mixed nutritional supplements on the healing of existing pressure ulcers (Benati 2001; Cereda 2009; Ek 1991; Desneves 2005; Ohura 2011; Theilla 2007; van Anholt 2010).
Arginine‐enriched mixed nutritional supplement compared to standard hospital diet (four trials)
Four trials investigated the effect of an arginine‐enriched mixed nutritional supplement on pressure ulcers.
Rate of ulcer healing
Benati 2001 undertook a preliminary investigation but presented the results in graphical form only with no numerical data. The patients who received supplementation had a better pressure ulcer healing score (PSST). Three studies used the PUSH score as an outcome. Desneves 2005 compared the effect of two different kinds of supplement, i.e. an arginine, zinc, and vitamin C‐enriched supplement or a high‐protein, high‐energy supplement to standard hospital diet. The difference in mean PUSH scores was ‐4.40 (95% CI ‐7.57 to ‐1.23) in favour of the arginine, zinc and vitamin C‐enriched supplement. van Anholt 2010 found no difference when comparing a special supplement in non‐malnourished patients with placebo (difference in PUSH scores ‐0.70; 95% CI ‐13.16 to 11.76;); this paper includes a Repeated Measures Mixed Model. In the trial of Cereda 2009 the difference in the change in PUSH scores indicated better healing in the supplemented group (MD = ‐2.80; 95% CI ‐4.71 to ‐0.89; P value = 0.01). When these three studies were combined there was greater improvement in PUSH scores in people who received the arginine‐enriched supplement compared with those on the standard hospital diet (Mean Difference ‐3.18; 95% CI ‐4.80 to ‐1.56; P value = 0.0001; I2 = 0%) (Analysis 4.1; Figure 5). The validity of this result is undermined by the quality of reporting of the primary studies; this precluded our ability to accurately judge risk of bias.
4.1. Analysis.
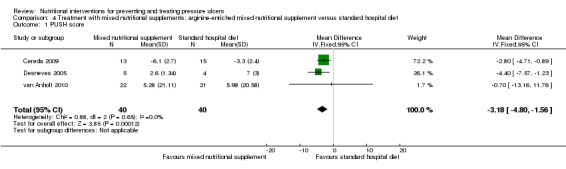
Comparison 4 Treatment with mixed nutritional supplements: arginine‐enriched mixed nutritional supplement versus standard hospital diet, Outcome 1 PUSH score.
5.
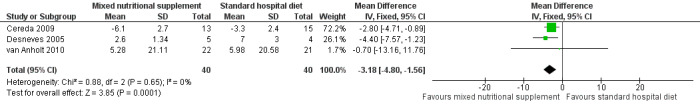
Forest plot of comparison: 4 Treatment with mixed nutritional supplements: arginine‐enriched mixed nutritional supplement versus standard hospital diet, outcome: 4.1 PUSH score.
Number of people healed
Cereda 2009 reported a complete healing of pressure ulcers in 1/13 patients in the nutritional intervention group versus 0/15 patients in the standard hospital diet group; clearly with only one healing event there is insufficient statistical power to detect a difference as statistically significant in this comparison (RR 0.29; 95% CI 0.01 to 6.60; P value = 0.44; Analysis 4.2).
4.2. Analysis.
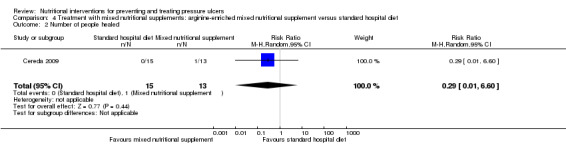
Comparison 4 Treatment with mixed nutritional supplements: arginine‐enriched mixed nutritional supplement versus standard hospital diet, Outcome 2 Number of people healed.
Ulcer size
The trials of Cereda 2009 and van Anholt 2010 both assessed difference in mean pressure ulcer size and were pooled (I2 = 0%); overall there was no clear evidence of a treatment effect of the arginine enriched supplement (compared with the standard hospital diet) though there is a lack of statistical power and a difference cannot be ruled out (MD ‐4.20 cm2 95% CI ‐ 9.80 to 1.40; P value = 0.14) (Analysis 4.3; Figure 6).
4.3. Analysis.
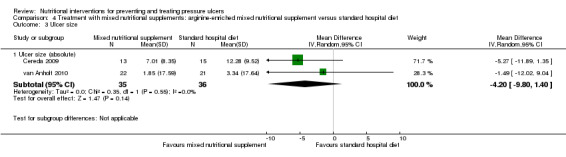
Comparison 4 Treatment with mixed nutritional supplements: arginine‐enriched mixed nutritional supplement versus standard hospital diet, Outcome 3 Ulcer size.
6.
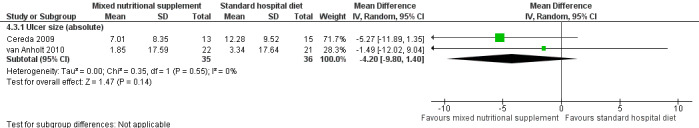
Forest plot of comparison: 4 Treatment with mixed nutritional supplements: arginine‐enriched mixed nutritional supplement versus standard hospital diet, outcome: 4.3 Ulcer size.
Mixed nutritional supplements compared with standard hospital diet (one trial and the third group of another trial)
Ulcer healing
Ek 1991: included 501 patients who were expected to remain in hospital for more than three weeks and were followed‐up for up to 26 weeks. The patients in the supplemented group had 67 pressure ulcers, those in the standard hospital diet group had 83 pressure ulcers. In the supplemented group 41.8% of the pressure ulcers healed and 51.3% improved. In the standard hospital diet group 30.3% of the pressure ulcers healed and 43.9% improved.No further analyses were undertaken as the number of patients evaluated in the groups was not clear from the trial report and there appears to have been inclusion of multiple pressure ulcers in individual patients.
Desneves 2005: compared the effect of a high‐energy, high‐protein supplement with a standard hospital diet in a study with 8 patients. The difference between mean PUSH scores was ‐1.00 (95% CI ‐4.76 to 2.76; P value = 0.60 Analysis 5.1) showing no difference between the groups however this comparison is grossly underpowered.
5.1. Analysis.
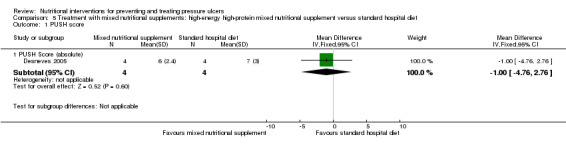
Comparison 5 Treatment with mixed nutritional supplements: high‐energy high‐protein mixed nutritional supplement versus standard hospital diet, Outcome 1 PUSH score.
High‐energy tube feeding versus standard‐energy tube feeding (one trial)
Ohura 2011: recruited 60 tube‐fed patients with pressure ulcers of NPUAP Grades 3 to 4 and compared high‐energy tube feeding with standard‐energy tube feeding. The number of people whose ulcers healed was 7/21 in the treatment group and 4/29 in the control group. There was therefore no clear evidence of a benefit of high energy tube feeding however this comparison is very underpowered (RR 0.41; 95 % CI 0.14 to 1.23; P value = 0.11, Analysis 6.1).
6.1. Analysis.
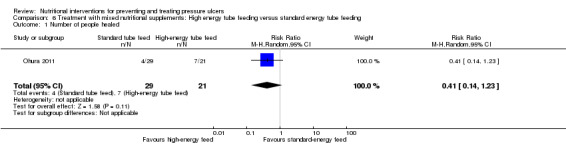
Comparison 6 Treatment with mixed nutritional supplements: High energy tube feeding versus standard energy tube feeding, Outcome 1 Number of people healed.
High‐fat, low‐carbohydrate, enteral formula enriched in lipids (eicosapentaenoic acid (EPA), gamma‐linolenic acid (GLA)), plus vitamins A, C and E versus high‐fat, low‐carbohydrate, enteral formula (one trial).
Theilla 2007 recruited 100 people in intensive‐care and suffering from acute lung injury. The study compared a high‐fat and low‐carbohydrate enteral formula enriched in lipids with vitamins A, C, and E with a high‐fat and low carbohydrate enteral formula and there was no clear evidence of a benefit with the enriched formula. In the group receiving the enriched high fat/low carbohydrate enteral formula only one participant out of 46 healed compared with 2/49 in the control group (RR 1.88 (95% CI 0.18 to 20.01) (Analysis 7.1) Again this is a grossly underpowered comparison.
7.1. Analysis.

Comparison 7 Treatment with mixed nutritional supplements: high‐fat, low‐carbohydrate, enteral formula enriched in lipids (eicosapentanoicacid (EPA)), gamma‐linolenicacid (GLA), vitamins A, C and E versus high‐fat, low‐carbohydrate, enteral formula, Outcome 1 Number of people healed.
Ascorbic acid (vitamin C) compared with placebo (two trials)
Two trials investigated the effect of ascorbic acid on pressure ulcer healing. In the trial of Taylor 1974, 20 people in surgical wards were followed‐up and data reported at one month. The study of ter Riet 1995 was intended to replicate the trial of Taylor with more participants (n = 88).
Ulcer healing
Healing rate
The mean healing rate in the trial of Taylor 1974 was 2.47 cm2/week in the intervention group compared with 1.45 cm2/week in the control group. The mean healing rate in the trial of ter Riet 1995 was 0.21 cm2/week in the intervention group (n = 43) and 0.27 cm2/week in the control group (n = 45) (difference ‐0.06 cm2/week); no standard deviations were reported and therefore pooling was not possible.
Number of ulcers healed
Taylor 1974 reported that 6/10 participants in the ascorbic acid group completely healed compared with 3/10 participants in the placebo group. This comparison is underpowered with only 9 events and therefore there is no clear evidence of a benefit associated with ascorbic acid supplementation on complete healing (RR 0.5 95% CI 0.17 to 1.46; P value = 0.21)(Analysis 8.1). ter Riet 1995 conducted an appropriate survival analysis to compare the overall risk of healing on ascorbic acid and placebo and found no difference (HR 0.78, 90%CI 0.44 to ‐1.39).
8.1. Analysis.
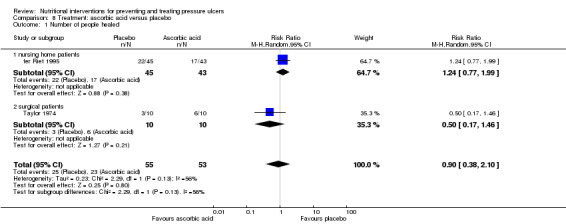
Comparison 8 Treatment: ascorbic acid versus placebo, Outcome 1 Number of people healed.
In order to allow comparison and meta‐analysis with the Taylor 1974 study, we extracted numbers healed from the survival curves of the ter Riet 1995 trial report. From the graphs, it appears that 17/43 in the ascorbic acid group were healed at 84 days compared with 22/45 in the placebo group (RR 1.24, 95% CI 0.77 to 1.99; P value = 0.38; calculated by review authors Analysis 8.1). The two trials were pooled using a random effects model (I2=56%) and overall there was no evidence of a benefit on pressure ulcer healing of ascorbic acid supplementation (RR 0.90, 95% CI 0.38 to 2.10; P value = 0.80; Analysis 8.1).
Ulcer size
Taylor 1974 reported a greater mean reduction in pressure ulcer area in the group treated with ascorbic acid (84% reduction (SD 24.04) after one month compared with a 42.7% in the placebo group (SD 23.43) . The overall difference in means was 41.30% in favour of the ascorbic acid (95% CI ‐62.11 to ‐20.49; P value=0.0001 Analysis 8.2).
8.2. Analysis.
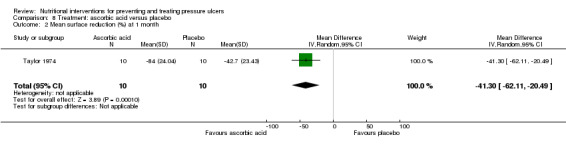
Comparison 8 Treatment: ascorbic acid versus placebo, Outcome 2 Mean surface reduction (%) at 1 month.
ter Riet 1995: the mean volume reduction was 0 ml/week in the intervention group and 0.20 ml/week in the control group (difference ‐0.20 ml/week). The mean "clinical change", where improvements (i.e. surface reduction, healing velocity, volume reduction) were scored on a scale from ‐100 to +100% was 17.89% per week in the intervention group and 26.08% per week in the control group (difference ‐8.19% per week).
In summary: the two studies that investigated the effect of ascorbic acid supplementation (compared with placebo) on existing pressure ulcers found no overall evidence of benefit.
Proteins or amino acids compared with other nutritional interventions (three trials)
Three trials investigated the effect of different proteins or amino acid products on pressure ulcer healing.
Very high protein (25% of calories) compared with high protein (16% of calories) (one trial)
At the start of the Chernoff 1990 study, pressure ulcers ranged in size from 1.0 cm2 to 46.4 cm2 in the very high protein (intervention) group and from 1.6 cm2 to 63.8 cm2 in the high protein (control) group. There was no difference in complete healing between treatment groups (RR 9.00 with 95% CI 0.59 to 137.65; P value = 0.11).
Concentrated, fortified, collagen protein hydrolysate supplement compared with placebo (one trial)
Lee 2006 examined the effect of a supplement of a concentrated, fortified, collagen protein hydrolysate supplement three times daily in 89 residents of long‐term care facilities with pressure ulcers of NPUAP grades 2, 3 and 4. There was a greater reduction in the difference in change in PUSH score in the supplemented group (decrease: ‐5.56 in the intervention group, ‐2.85 in the placebo group), however it should be noted that the PUSH Score was much higher in the intervention group at start of the trial. There was no statistically significant difference between the two groups in the study primary outcome of PUSH score at 8 weeks (MD = 0.33; 95% CI ‐1.74 to 2.40; P value = 0.76) (Analysis 9.1).
9.1. Analysis.
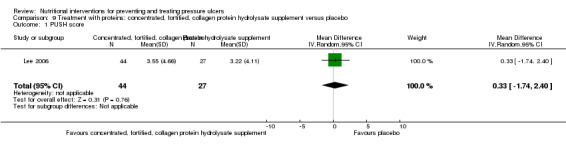
Comparison 9 Treatment with proteins: concentrated, fortified, collagen protein hydrolysate supplement versus placebo, Outcome 1 PUSH score.
Ornithine alpha‐ketoglutarate compared with placebo (one trial)
Meaume 2009 analysed the effect of 10 g ornithine alpha‐ketoglutarate daily on the healing of heel pressure ulcers NPUAP Grade 2 or 3 after accidental immobilization. Because of baseline imbalances in ulcer area in the two groups, the analysis was stratified by ulcer area. There was no evidence of a benefit of the ornithine supplement in people with baseline PU area ≤ 8cm2 (difference in mean change in area ‐0.6 cm2 (95% CI ‐1.90 to 0.70; P value = 0.36).
In the group with baseline PU area < 8 cm2 there was also no evidence of an effect of the ornithine supplement (difference in the mean change in PU area ‐5.50; 95% CI ‐34.04 to 23.04; P value = 0.71; Analysis 10.1). There were more adverse effects in the ornithine group (15/85 vs. 7/75) however this may have been a chance difference (RR 1.89, 95% CI 0.81 to 4.33; P value = 0.14; Analysis 10.2).
10.1. Analysis.
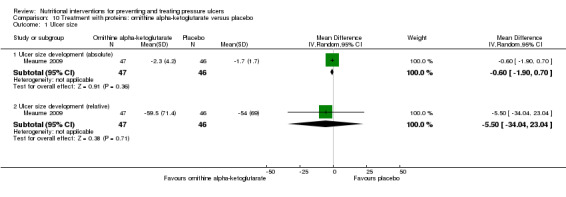
Comparison 10 Treatment with proteins: ornithine alpha‐ketoglutarate versus placebo, Outcome 1 Ulcer size.
10.2. Analysis.
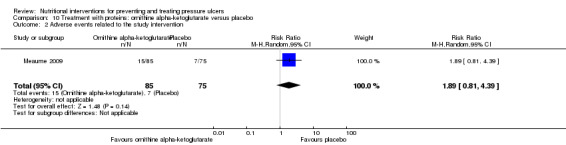
Comparison 10 Treatment with proteins: ornithine alpha‐ketoglutarate versus placebo, Outcome 2 Adverse events related to the study intervention.
There were no differences in wound area changes in the group with baseline PU area > 8 cm2
Zinc sulphate compared with placebo (two trials)
Two trials investigated the effect of zinc sulphate on the healing of pressure ulcers. In the Norris 1971 study, 20 people with pressure ulcers were treated with either zinc sulphate supplements or placebo. The zinc sulphate group showed a mean reduction in pressure ulcer volume of 10.1 ml (SD 9 ml) whilst those in the placebo group showed a mean reduction in pressure ulcer volume of 6.0 ml (SD 17.5 ml). There was no overall evidence of an effect of zinc sulphate on pressure ulcer volume (MD = ‐4.1 ml; 95% CI ‐16.30 to 8.10; P value = 0.51; Analysis 11.1).
11.1. Analysis.
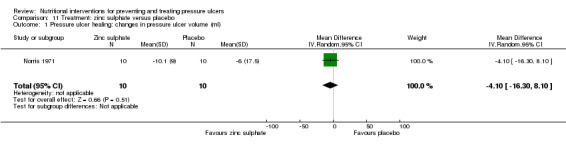
Comparison 11 Treatment: zinc sulphate versus placebo, Outcome 1 Pressure ulcer healing: changes in pressure ulcer volume (ml).
Brewer 1967 compared zinc sulphate with placebo in 14 patients with spinal cord injuries and poorly healing pressure ulcers. One person healed in the treatment group (n = 6) and one in the control group (n = 7) (RR 0.86;95% CI 0.07 to 10.96; P value = 0.91; Analysis 11.2).
11.2. Analysis.
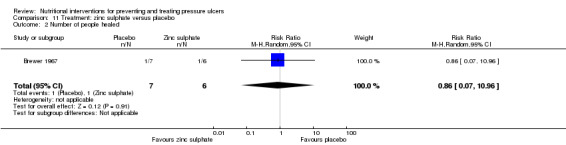
Comparison 11 Treatment: zinc sulphate versus placebo, Outcome 2 Number of people healed.
Discussion
The studies of nutritional supplementation vary in terms of interventions, outcome measurements and follow‐up; interpretation of these findings should be made with caution.
Summary of main results
Eleven studies compared a combination of nutritional supplements, consisting of a minimum of energy and protein in different dosages, for the prevention of pressure ulcers. A meta‐analysis was performed including eight trials comparing the effect of mixed nutritional supplements with standard hospital diet. The overall risk ratio (RR) was 0.86 (95% CI 0.73 to 1.00; P value = 0.05; see Analysis 1.1), Of the 6062 persons considered in this meta‐analysis 4015 were participants in the FOOD Trial (Dennis 2005). The other 2047 participants were allocated to seven trials which ranged in size from 52 to 672 participants. The trials examined clinically heterogenous interventions and were generally at high or unclear risk of bias. The clearest conclusion that can be drawn in the light of the volume and quality of this evidence is that it remains unclear whether nutritional supplementation reduces the risk of pressure ulcer development.
Fourteen studies evaluated the effects of nutritional supplements on the healing of existing pressure ulcers: seven trials examined mixed nutritional supplements, three the effect of protein supplements, two trials examined zinc, and two studies compared ascorbic acid with placebo. There is generally no clear evidence of improved pressure ulcer healing with nutritional supplements. There is some evidence of improved healing (as measured by a surrogate outcome measure, viz. PUSH scores) with an arginine enriched mixed nutritional supplement compared with standard hospital diet however there is no evidence of an effect on actual ulcer healing. Again most of the treatment studies were at unclear or high risk of bias.
Overall completeness and applicability of evidence
Many studies included few patients and some had a considerable drop‐out rate. Furthermore, the follow‐up time was often very short, making it unlikely that true effects of interventions would be detected. Some trials reported that laboratory markers of malnutrition improved during treatment, but the clinical effects of protein, calories, vitamin or zinc supplementation on the incidence of new ulcers or healing of existing ulcers was unclear.The validity of the PUSH score as a surrogate measure of pressure ulcer healing is unclear.
Quality of the evidence
All included trials were at risk of bias with one or more quality domains judged as unclear or at high risk of bias. Interpretations and conclusion of the effects of the interventions should be drawn against the background of these findings.
Authors' conclusions
Implications for practice.
Currently there is no clear evidence that nutritional interventions reduce the development of pressure ulcers or help them to heal. This conclusion should not be interpreted as nutritional interventions having no effect on pressure ulcers because the existing evidence base is of very low quality. People who are receiving health care and who are malnourished or at risk of malnutrition should receive expert nutritional assessment and intervention as per local practice.
Implications for research.
Further research with larger numbers of patients and sound methodology is required to procure evidence for the impact of nutrition on pressure ulcers. Consideration should be given to constituents of the supplement, and the method of application, as one study reported low tolerance of nasogastric tube feeding.
What's new
| Date | Event | Description |
|---|---|---|
| 25 March 2014 | New citation required and conclusions have changed | First update, new search, 15 additional trials included bringing the total to 23 trials. |
| 25 March 2014 | New search has been performed | Three review authors left the team and did not contribute to this update (G. Schloemer, O. Kuss, J. Behrens) |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 15 April 2008 | Amended | Converted to new review format. |
| 21 August 2003 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
The following people refereed the protocol and provided useful feedback: Mike Clark, Roberto Cassino, and Jane Kinniburgh. Special thanks go to Roberto Cassino, Mike Clark, Nicky Cullum, Andrew Jull, David Margolis, and Susan O'Meara for their helpful and constructive comments on this review. Special thanks go to Waltraud Mair for help with Spanish and Italian articles, Nadine Schumann for help with Italian articles, and Jens‐Olaf Hosenfeldt for help with Spanish articles. We are grateful for support and encouragement from Sally EM Bell‐Syer, E Andrea Nelson and Ruth Foxlee of the Cochrane Wounds Group. Thank you to Elizabeth Royle who copy edited the updated review. Special thanks go to Gabriele Schlömer and Johann Behrens for their contribution to the original version of the review.
Appendices
Appendix 1. Search strategy for the original published version
The Cochrane Wounds Group Specialised Trials Register was searched for reports of trials evaluating nutritional interventions in the prevention and treatment of pressure ulcers in September 2002. The Trials Register has been developed and maintained by regular searches, using a maximally sensitive search strategy for retrieving randomised controlled trials, of 19 electronic databases, as well as handsearching of wound care journals and conference proceedings, and is regularly updated.
The Cochrane Central Register of Controlled Trials (CENTRAL) was also searched (Issue 3, 2002) using the following strategy: 1. (decubitus next ulcer*) 2. (bed and sore*) 3. (pressure and sore*) 4. (pressure and ulcer*) 5. DECUBITUS‐ULCER*:ME 6. ((((#1 or #2) or #3) or #4) or #5) 7. nutrition* 8. diet* 9. tube‐fe* 10. NUTRITION*:ME 11. DIET*:ME 12. DIET‐THERAPY*:ME 13. NUTRITIONAL‐SUPPORT*:ME 14. ENTERAL‐NUTRITION*:ME 15. PARENTERAL‐NUTRITION*:ME 16. ((((((((#7 or #8) or #9) or #10) or #11) or #12) or #13) or #14) or #15) 17. (#6 and #16)
MEDLINE was searched in June 2003 via PubMed using the following strategy: 1. (bed sore) OR bedsore OR (pressure sore) OR (decubitus ulcer) OR (pressure ulcer) OR (decubital ulcer) OR (ischaemic ulcer) 2. "Decubitus Ulcer"[MESH] 3. nutri* OR diet OR food 4. "nutrition"[MESH] OR "Diet"[MESH] OR "Food"[MESH] OR "Nutritional Support"[MESH] 5. enteral OR parenteral OR proteins OR vitamins OR minerals 6. "Amino Acids, Peptides, and Proteins"[MESH] OR "Dietary Supplements"[MESH] OR "Growth Substances, Pigments, and Vitamins"[MESH] OR "Enzymes, Coenzymes, and Enzyme Inhibitors"[MESH] OR "Lipids and Antilipaemic Agents"[MESH] OR "Minerals"[MESH] 7. therapy OR prophylaxis OR prevention 8. (randomized controlled trial[PTYP] OR drug therapy[SH] OR therapeutic use[SH:NOEXP] OR random*[WORD]) 9. systematic[sb] 10. (cohort studies[MESH] OR risk[MESH] OR (odds[WORD] AND ratio*[WORD]) OR (relative[WORD] AND risk[WORD]) OR (case control*[WORD] OR case‐control studies[MESH])) 11. (incidence[MESH] OR mortality[MESH] OR follow‐up studies[MESH] OR mortality[SH] OR prognos*[WORD] OR predict*[WORD] OR course[WORD]) 12. (#1 OR #2) AND (#3 OR #4 OR #5 OR #6) AND (#7 OR #8 OR #9 OR #10 OR #11)
CINAHL was searched via Ovid in June 2003 with the following query: 1. exp Pressure ulcer/nu, dh, pc, et, rf, th, me [Nursing, Diet Therapy, Prevention and Control, Etiology, Risk Factors, Therapy, Metabolism] 2. PARENTERAL NUTRITION SOLUTIONS/ or ENTERAL NUTRITION/ or TOTAL PARENTERAL NUTRITION/ or PERIPHERAL PARENTERAL NUTRITION/ or PARENTERAL NUTRITION/ or NUTRITION/ 3. 1 and 2
The listed databases were searched by the authors for eligible studies for the earliest entrance date possible until the latest search date. For this review there were no restrictions on date of publication, language of publication, or publication status (published or unpublished work). Experts in the field, such as scientific societies for wound healing and treatment, for nutrition and for nutritional medicine, were contacted and asked whether they had been involved in any further studies or were aware of recent or ongoing studies on the effect of nutrition in the prevention and treatment of pressure ulcers.
We handsearched the following conference proceedings to identify any research or relevant studies:
the Congress of the European Society of Parenteral and Enteral Nutrition (ESPEN) 1996 ‐2002
the Meetings of the European Pressure Ulcer Advisory Panel (EPUAP) 1997 ‐ 2000
Some additional journals to those stated in the protocol were considered suitable for handsearching. The following journals were searched by hand from 1996 to 2002:
Advances in Wound Care,
Advances in Food and Nutrition Research,
Clinical Nutrition,
European Journal of Clinical Nutrition,
European Journal of Nutrition,
Wundforum,
Zeitschrift fuer Wundbehandlung,
Zeitschrift fuer Wundheilung,
Zeitschrift fuer Gerontologie und Geriatrie,
Aktuelle Ernaehrungsmedizin,
Deutsches Wundjournal
Studies and articles cited in articles identified have also been checked for eligibility.
We tried to identify unpublished studies by contacting manufacturers of nutritional supplements (Fresenius, NutriScience, Pfrimmer, Braun, Ratiopharm, Aventis and Novartis), but this yielded no further studies.
Appendix 2. Ovid MEDLINE search strategy
1 exp Pressure ulcer/ (5595) 2 (pressure adj (ulcer$ or sore$)).ti,ab. (4721) 3 (decubitus adj (ulcer$ or sore$)).ti,ab. (618) 4 (bedsore$ or (bed adj sore$)).ti,ab. (258) 5 or/1‐4 (7036) 6 exp Nutrition Therapy/ (38354) 7 nutrition$.ti,ab. (98588) 8 diet$.ti,ab. (209761) 9 (tube adj (fed or feed or feeding)).ti,ab. (1628) 10 or/6‐9 (293406) 11 5 and 10 (546) 12 randomized controlled trial.pt. (261976) 13 controlled clinical trial.pt. (41370) 14 randomi?ed.ab. (257887) 15 placebo.ab. (97860) 16 clinical trials as topic.sh. (83790) 17 randomly.ab. (147290) 18 trial.ti. (80869) 19 or/12‐18 (605281) 20 exp animals/ not humans.sh. (1722788) 21 19 not 20 (550693) 22 11 and 21 (64)
Appendix 3. Ovid EMBASE search strategy
1 exp Decubitus/ (9677) 2 (pressure adj (ulcer$ or sore$)).ti,ab. (5976) 3 (decubitus adj (ulcer$ or sore$)).ti,ab. (821) 4 (bedsore$ or (bed adj sore$)).ti,ab. (427) 5 or/1‐4 (10912) 6 exp Nutrition/ (943162) 7 nutrition$.ti,ab. (148040) 8 diet$.ti,ab. (292491) 9 (tube adj (fed or feed or feeding)).ti,ab. (2443) 10 or/6‐9 (1054866) 11 5 and 10 (1274) 12 exp Clinical trial/ (814722) 13 Randomized controlled trial/ (296848) 14 Randomization/ (52199) 15 Single blind procedure/ (16283) 16 Double blind procedure/ (88664) 17 Crossover procedure/ (33182) 18 Placebo/ (173876) 19 Randomi?ed controlled trial$.tw. (85967) 20 RCT.tw. (11475) 21 Random allocation.tw. (961) 22 Randomly allocated.tw. (15027) 23 Allocated randomly.tw. (1254) 24 (allocated adj2 random).tw. (269) 25 Single blind$.tw. (10164) 26 Double blind$.tw. (94024) 27 ((treble or triple) adj blind$).tw. (253) 28 Placebo$.tw. (143416) 29 Prospective study/ (214584) 30 or/12‐29 (1132169) 31 Case study/ (17663) 32 Case report.tw. (175248) 33 Abstract report/ or letter/ (528846) 34 or/31‐33 (717245) 35 30 not 34 (1103264) 36 animal/ (752648) 37 human/ (9046339) 38 36 not 37 (504611) 39 35 not 38 (1079882) 40 11 and 39 (230)
Appendix 4. EBSCO CINAHL search strategy
S28 S15 and S27 S27 S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 S26 MH "Quantitative Studies" S25 TI placebo* or AB placebo* S24 MH "Placebos" S23 TI random* allocat* or AB random* allocat* S22 MH "Random Assignment" S21 TI randomi?ed control* trial* or AB randomi?ed control* trial* S20 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S19 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S18 TI clinic* N1 trial* or AB clinic* N1 trial* S17 PT Clinical trial S16 MH "Clinical Trials+" S15 S5 and S14 S14 S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 S13 TI ( tube fed or tube‐fed or tube feed* ) or AB ( tube fed or tube‐fed or tube feed* ) S12 TI diet* or AB diet* S11 (MH "Diet Therapy+") S10 (MH "Diet+") S9 TI nutrition* or AB nutrition* S8 (MH "Parenteral Nutrition+") S7 (MH "Enteral Nutrition") S6 (MH "Nutrition+") S5 S1 or S2 or S3 or S4 S4 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* ) S3 TI decubitus or AB decubitus S2 TI ( pressure ulcer* or pressure sore* ) or AB ( pressure ulcer* or pressure sore* ) S1 (MH "Pressure Ulcer")
Appendix 5. Risk of bias criteria
Was the allocation sequence adequately generated?
low risk of bias. The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random.
unclear; risk of bias. The trial is described as randomised but the method of sequence generation was not specified.
high risk of bias, the sequence generation method is not, or may not be, random. Quasi‐randomised studies, those using dates, names, or admittance numbers in order to allocate patients are considered inadequate, and will be excluded for the assessment of benefits, but not for harms.
Was allocation adequately concealed?
low risk of bias. Allocation was controlled by a central and independent randomisation unit, opaque and sealed envelopes or similar, so that intervention allocations could not have been foreseen in advance of, or during, enrolment.
unclear; risk of bias. The trial was described as randomised, but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
high risk of bias. If the allocation sequence was known to the investigators who assigned participants, or if the study was quasi‐randomised.
Was knowledge of the allocated interventions adequately prevented during the study?
low risk of bias. The trial was described as double blind and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial.
unclear; risk of bias. The trial was described as blinded, but the method of blinding was not described, so that knowledge of allocation was possible during the trial.
high risk of bias. The trial was not blinded, so that the allocation was known during the trial.
Were incomplete outcome data adequately addressed?
low risk of bias. Incomplete outcome data adequately addressed if the numbers and reasons for dropouts and withdrawals in all study groups were described, or if it was specified that there were no dropouts or withdrawals.
unclear; risk of bias. The report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
high risk of bias. Incomplete outcome data inadequately addressed if the number or reasons for dropouts and withdrawals were not described.
Are reports of the study free of suggestion of selective outcome reporting?
low risk of bias. Pre‐defined, or clinically relevant and reasonably expected outcomes are stated in the method section and are reported in the results section.
unclear; risk of bias. Not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported, or are not reported fully, or it is unclear whether data for these outcomes were recorded or not.
high risk of bias. One or more of the clinically relevant and reasonably expected outcomes were not reported; data on these outcomes were likely to have been recorded.
Data and analyses
Comparison 1. Prevention with mixed nutritional supplements: mixed nutritional supplement versus standard hospital diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of pressure ulcers | 8 | 6062 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.00] |
Comparison 2. Prevention with mixed nutritional supplements: disease‐specific (abnormal glucose tolerance) enteral nutritional formula versus standard high‐carbohydrate feeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of pressure ulcers | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.37, 1.57] |
Comparison 3. Prevention with mixed nutritional supplements: high‐fat, low‐carbohydrate, enteral formula enriched in lipids (eicosapentanoicacid (EPA)), gamma‐linolenicacid (GLA), vitamins A, C and E versus high‐fat, low‐carbohydrate, enteral formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of pressure ulcers | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.97] |
Comparison 4. Treatment with mixed nutritional supplements: arginine‐enriched mixed nutritional supplement versus standard hospital diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PUSH score | 3 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐3.18 [‐4.80, ‐1.56] |
| 2 Number of people healed | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.60] |
| 3 Ulcer size | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Ulcer size (absolute) | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐9.80, 1.40] |
Comparison 5. Treatment with mixed nutritional supplements: high‐energy high‐protein mixed nutritional supplement versus standard hospital diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PUSH score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 PUSH Score (absolute) | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.76, 2.76] |
Comparison 6. Treatment with mixed nutritional supplements: High energy tube feeding versus standard energy tube feeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people healed | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.23] |
Comparison 7. Treatment with mixed nutritional supplements: high‐fat, low‐carbohydrate, enteral formula enriched in lipids (eicosapentanoicacid (EPA)), gamma‐linolenicacid (GLA), vitamins A, C and E versus high‐fat, low‐carbohydrate, enteral formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people healed | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.18, 20.01] |
Comparison 8. Treatment: ascorbic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people healed | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.38, 2.10] |
| 1.1 nursing home patients | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.77, 1.99] |
| 1.2 surgical patients | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.17, 1.46] |
| 2 Mean surface reduction (%) at 1 month | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐41.3 [‐62.11, ‐20.49] |
Comparison 9. Treatment with proteins: concentrated, fortified, collagen protein hydrolysate supplement versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PUSH score | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐1.74, 2.40] |
Comparison 10. Treatment with proteins: ornithine alpha‐ketoglutarate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ulcer size | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Ulcer size development (absolute) | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.90, 0.70] |
| 1.2 Ulcer size development (relative) | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐34.04, 23.04] |
| 2 Adverse events related to the study intervention | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.81, 4.39] |
Comparison 11. Treatment: zinc sulphate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer healing: changes in pressure ulcer volume (ml) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐4.1 [‐16.30, 8.10] |
| 2 Number of people healed | 1 | 13 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.07, 10.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arias 2008.
| Methods | RCT, open‐label. Duration: 17 months, from May 2005‐September 2006. |
|
| Participants | Subjective Global Assessment (SGA) performed for 1700 hospital patients, 667 of whom had mild or serious malnutrition or were suspected of being malnourished (i.e. SGA status = B or C) and were included in the trial. Exclusion criteria: diabetes mellitus, decompensated liver disease with hepatic encephalopathy, impaired consciousness, impaired comprehension. Patients analysed (Drop out 130 patients): A) Intervention group (n = 264): 36.4% female, 63.6% male, mean age 62.0 years ± 18.8 (SD). B) Control group (n = 273): 63.1% female, 36.9% male, mean age 58.8 years ± 19.8 (SD). |
|
| Interventions | A) Nutritional intervention group (n = 264): standard hospital diet + oral supplement (with 1 kcal/ml; 14.0% protein; 31.5% fat; 54.5% carbohydrate) up to 700 ml/d. B) Control group (n = 273): standard hospital diet. |
|
| Outcomes | 1. Mortality. 2. Duration of hospital stay. 3. Complications. Outcomes 1‐3 were not considered in this review, except for incidence of pressure ulcers, which was considered to be a complication. |
|
| Notes | Location: Uruguay. Setting: hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | High risk | No allocation concealment intended. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 667 randomised, loss to follow‐up 130: 56 transferred to other institutions, 22 excluded because of gastrointestinal intolerance, 21 for insufficient compliance, 6 due to taste of the supplement, 25 for other described reasons. The reasons for missing outcome data in the groups were reported and balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Mortality, duration of hospital stay, and complications were described as the outcomes of interest and these were reported in the results section. |
Benati 2001.
| Methods | RCT. Duration: not stated; experimental treatment period 2 weeks. |
|
| Participants | Inpatients with severe cognitive impairment and pressure ulcers. Exclusion criteria: patients who were unlikely to benefit from nutritional supplementation. A) Treatment group A (n = 5): 20% female, 80% male. B) Treatment group B (n = 5): 40% female, 60% male. C) Control group (n = 6): 33.3% female, 66.6% male. Age range 72‐91 years. Activities of daily living scores ranged from 0‐3. |
|
| Interventions | A) Treatment group A: normal hospital diet plus 2 tetrapaks of a high‐protein supplement providing an additional 500 kcal, 37 g protein each day. B) Treatment group B: normal hospital diet plus 2 tetrapaks of a high‐protein supplement enriched with arginine, zinc and antioxidants providing an additional 500 kcal, 37 g protein (7.5 g arginine), 25 mg zinc, and antioxidants each day. C) Control group: normal hospital diet. |
|
| Outcomes | Pressure sore status tool (PSST) at days 0, 5, 10, and 15. | |
| Notes | Location: Italy. Setting: hospital, Department of Geriatric Medicine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36 participants were included. Results of 16 patients were presented without any explanation for the others. |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes were reported. |
Bourdel‐M 2000.
| Methods | Multicentred RCT, cluster‐randomised. Duration: not stated; experimental treatment period 15 days. |
|
| Participants | 672 patients without pressure ulcers < 65 years old in acute phase of a critical illness, unable to move themselves, unable to eat independently at admission. Exclusion criteria: existing pressure sore. A) Intervention group (n = 295): 67.5% female, 32.5% male, mean age 83.6 years ± 7.3 (SD). B) Control group (n = 377): 63.1% female, 36.9% male, mean age 83.6 years ± 7.1 (SD). |
|
| Interventions | A) Intervention group: standard diet (1800 kcal/d) and 2 oral supplements per day (each 200 ml; 200 kcal; 30% protein; 20% fat; 50% carbohydrate; minerals and vitamins, such as 1.8 mg zinc and 15 mg vitamin C). B) Control group: standard diet (1800 kcal/d). The nutritional intervention was implemented for up to 15 consecutive days or until discharge. Both groups underwent the same pressure ulcer prevention program (changing positions, special mattresses, cleaning care). |
|
| Outcomes | 1. Pressure ulcers were recorded each day using 4 grades:
I: erythematous skin;
2: superficial layers of broken or blistered skin;
3: subcutaneous tissue;
4: ulcer extends to muscle or bone. 2. Nutritional intake (energy and protein) at days 2, 5, 8, 12, and 15, or until death and discharge; which were not considered in this review. |
|
| Notes | Location: France. Setting: 19 wards of universal clinic or geriatric units with at least 40% of inpatients over 65 years. The intervention group included more patients with stroke, heart failure, and dyspnoea, and fewer with antecedent falls, delirium, lower limb fractures and digestive disease. Furthermore, the nutritional intervention group had a lower risk of developing pressure ulcers (Norton score) but was less dependent (Kuntzman score) and had a lower serum albumin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: “...9 wards were randomly selected for nutritional intervention...” (this is out of a total 19 wards included in the study). |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not explicitly reported. Patients and carers would not have been blinded to treatment assignment but "... nurses rated the volume ingested in each food category....the dietician using the Nutrisoft Bilnut program calculated intake. This information was not available to caregivers." Unclear if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 672 patients enrolled, 295 in the intervention and 377 in the control group. Evaluable at day 15: 107 in the intervention group and 244 in the control group. Death: 25 in intervention group, 22 in control group. Quote: “For subjects who died or were discharged without pressure ulcers before the day 15, the date of death or discharge were considered as censoring the data”. |
| Selective reporting (reporting bias) | Low risk | Nutritional intake and development of pressure ulcers were described as the outcomes of interest and these were both reported in the results section. |
Brewer 1967.
| Methods | RCT, double‐blind. Duration: 2‐3 months, date of baseline point: September 1967. |
|
| Participants | 14 spinal cord injured patients with poor healing decubitus ulcers of various sizes, types, locations, and duration (5 months‐ over 2 years). No further description available. | |
| Interventions | A) Intervention group (n = 7): 1 capsule of zinc sulphate daily (220 mg; 50 mg zinc). B) Control group (n = 7): 1 placebo capsule daily (lactose). |
|
| Outcomes | 1. Pressure ulcer healing. 2. Serum and urinary zinc increase after 2‐3 months; not considered in this review. |
|
| Notes | Setting and country not clearly described. Author was chief of the 'Spinal Cord Injury Service', Hines, Illinois, United States. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: “Selection of the type of capsule (zinc #1 or zinc #2) was made on random basis.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not clearly described but the description below would indicate that at least the participants were blinded. Quote: “It was conducted double blind utilizing two types of capsules prepared by the pharmacy”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Only one patient on zinc had to be discontinued because of upper gastrointestinal distress...”. Insufficient reporting. The outcomes for 1/7 subjects in the intervention group were missing. Results would be more convincing, if this case had been considered as a failure. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported (urinalyses, blood counts, blood chemistry). |
Cereda 2009.
| Methods | RCT, single‐blinded. Duration: 5 months, from November 2007‐March 2008. |
|
| Participants | 30 elderly subjects with Grade 2, 3, and IV pressure ulcers (National Pressure Ulcer Advisory Panel staging system) of recent onset (history < 1‐month). Exclusion criteria: presence of acute illness (e.g. infection) or chronic disease possibly affecting the nutritional intervention and healing process (e.g. diabetes mellitus, peripheral vascular disease, autoimmune or neoplastic disorders), positive culture from pressure ulcer swab sampling, use of immunosuppressive therapies, development of the lesion > 1 month before evaluation, and lack of dietary adherence. A) Intervention group (n = 13): 69.2% female, 30.8% male, mean age 81.2 years ± 9.6 (SD), mean BMI 20.8 ± 3.2 (SD). B) Control group (n = 15): 60.0% female, 40.0% male, mean age 81.4 years ± 9.9 (SD), mean BMI 23.1 ± 5.0 (SD). Follow‐up in intervention group: 13/15 patients, 2 died. |
|
| Interventions | A) Intervention group: standard hospital diet plus supplement with at least 500 kcal, 34 g protein, 6 g arginine, 500 mg vitamin C, and 18 mg zinc (400 ml) . B) Control group: standard hospital diet (16% protein), at least 30 kcal/kg/d. 12‐week follow‐up. |
|
| Outcomes | 1. Pressure ulcer healing: Pressure Ulcer Scale for Healing, area measurement (mm2 and %) after 12 weeks. 2. Improvements in nutritional variables (weight, BMI, and biochemistry). 3. Infection occurrence. 4. Hospitalisation after 12 weeks. Outcomes 2‐3 not considered in this review. |
|
| Notes | Location: Italy, Province of Como. Setting: 4 long‐term care facilities. Supplements provided by Nutricia (Milan, Italy). No conflict of interest stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computer‐generated randomisation list”. |
| Allocation concealment (selection bias) | Unclear risk | Allocation not clearly described. Quote: “Allocation to the intervention groups was defined according to the computer‐generated randomisation list”. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patient and dietician unclear. Nurse providing wound care was blinded to the intervention. Operator who was blinded to the nutritional interventions performed PU assessments . |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 people in the treatment group died. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. Pressure ulcer healing (PUSH score) and lesion area (mm2 and %). Improvements in nutritional variables (weight, BMI, and biochemistry), infection occurrence and hospitalisation. |
Chernoff 1990.
| Methods | RCT. Duration: not stated; experimental treatment period 8 weeks. |
|
| Participants | 12 institutionalised tube‐fed patients with pressure ulcers. | |
| Interventions | A) Intervention group (n = 6): very high protein (25% of calories) dietary formula. B) Control group (n = 6): high protein (16% of calories). |
|
| Outcomes | Pressure ulcer healing, measured in % of decreasing surface after 8 weeks. | |
| Notes | Setting and country not clearly described. Authors' Institution was 'Medical Center and Division on Aging', University of Arkansas for Medical Science, Little Rock, Arkansas, United States. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: "Six patients each were randomised to a high protein (16% of calories) (HPD) or a very high protein (25% of calories)...". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not clearly reported, but did not report any missing data. |
| Selective reporting (reporting bias) | Low risk | Patients were monitored for 8 weeks to assess tolerance and pressure ulcer healing. Results for both of these outcomes were reported. |
Craig 1998.
| Methods | RCT, double‐blind. Duration: 10 months, from March 1995 to December 1995. |
|
| Participants | 34 patients ≥ 50 years old with a history of type 2 diabetes mellitus or documented hyperglycemias evidenced by either a plasma glucose random measurement of > 200 mg/dL or a fasting plasma glucose > 140 mg/dL on 2 occasions; required total enteral nutrition support by tube; were able to tolerate a volume of formula that maintained body weight. A) Intervention group (n = 18): mean age 80 years ± 2 (range 52‐100 years). B) Control group (n = 16): mean age 82 years ± 3 (range 52‐94 years). |
|
| Interventions | A) Intervention group: disease‐specific enteral nutritional formula: reduced‐carbohydrate, modified‐fat (Glucerna, Specialized Nutrition with Fiber for Patients with Abnormal Glucose Tolerance). Amounts per 1000 ml: 1000 kcal, 41.8 g protein (16.7% of energy; sodium and calcium caseinates), 93.7 g carbohydrate (33.3% of energy; maltodextrins, soy polysaccharide, fructose), 35.9 g fat (30% of energy; high‐oleic safflower oil, canola (rapeseed) oil). B) Control group: standard high‐carbohydrate feeding (Jevity Isotonic Liquid Nutrition with Fiber). Amounts per 1000 ml: 1060 kcal, 44.4 g protein (16.7% of energy; sodium and calcium caseinates), 141.7 g carbohydrate (53.3% of energy; Maltodextrin, soy polysaccharide), 35.9 g fat (30% of energy; high‐oleic safflower oil, canola oil, medium chain triglyceride (MCT) oil). 12‐week follow‐up. |
|
| Outcomes | 1. Subjects' metabolic response (especially glucose control and other routine serum chemistry). 2. Gastrointestinal tolerance. 3. Morbidity. 4. Medication usage. 5. Time spent by staff on resident care. 6. Status regarding clinical safety. Assessments 1‐6 were made daily, weekly or every 4 weeks. Clinical outcomes data collected daily: 7. Fevers. 8. Dehydration. 9. Pneumonia. 10. Urinary tract infections. 11. Skin infections. 12. Hypo‐ and hyperglycaemic events. 13. Vomiting. 14. Diarrhoea. 15. Number and severity of pressure ulcers. Outcomes 1‐14 were not considered in this review. |
|
| Notes | Location: USA, State of New York. Setting: 2 long‐term care facilities. Research supported by Ross Products Division, Abbott Laboratories, Columbus, Ohio. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: “Eligible subjects were randomised to receive, via enteral access device, either...”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not clearly described. Quote: “The study was conducted as a prospectively randomised, double‐blind, active treatment controlled, parallel group trial.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 subjects in the disease‐specific formula‐fed group and 2 subjects in the standard formula‐fed group died during the study period. One subject was removed from the standard formula‐fed group due to uncontrolled blood glucose levels. |
| Selective reporting (reporting bias) | Unclear risk | Not all clinical outcomes were reported: it was unclear whether dehydration, hypo‐ and hyperglycaemic events, vomiting, and diarrhoea occurred. |
Delmi 1990.
| Methods | RCT. Duration: 9 months, from March 1985‐November 1985. |
|
| Participants | 59 elderly patients (> 60 years old, mean age 82 years) with femoral neck fractures after accidental falls. Most patients had nutritional deficiencies on admission. Exclusion criteria: fractures from violent external trauma and pathological fractures (tumours, non‐osteoporotic osteopathies), patients with overt dementia or hepatic, renal or endocrine disease, gastrectomy or malabsorption, or treatment with phenytoin, steroids, barbiturates, fluoride or calcitonin. A) Intervention group (n = 27): 88.9% female, 11.1% male, mean age 80.4 years ± 8.5 (SD). B) Control group (n = 32): 90.6% female, 9.4% male, mean age 82.9 years ± 7.9 (SD). |
|
| Interventions | A) Intervention group: standard hospital diet with daily oral nutrition supplement (250 ml; 254 kcal; 20.4 g protein; 29.5 g carbohydrate; 5.8 g lipid; 525 mg calcium; 750 IU vitamin A; 25 IU vitamin D3, vitamins E, B1, B2, B6, B12, C, nicotinamide, folate, calcium pantothenate, biotin, minerals), started on admission, continued throughout 2nd (recovery) hospital (mean period 32 days); given at 8 pm (n = 27). B) Control group: standard hospital diet (n = 32). |
|
| Outcomes | Frequency of complications during the stay in orthopaedic ward and recovery hospital, and at 6 months: 1. Pressure ulcer. 2. Death. 3. Pneumonia. 4. Pyelonephritis. 5. Severe anaemia. 6. Deep vein thrombosis. 7. Acute renal insufficiency. 8. Pulmonary embolism. 9. Cardiac failure. Outcomes 2‐9 were not considered in this review. |
|
| Notes | Location: Switzerland, Geneva. Setting: orthopaedic unit of the University Hospital and 2nd (recovery) hospital. Baseline: no statistically significant difference between the 2 groups except for 25OHD plasma level (slightly lower in non‐supplemented patients). Supplements were provided by Sandoz‐Wander. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: "Patients were randomised into two groups." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported. Patients and care‐givers would not have been blinded to treatment assignment as the intervention group got additional supplements. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 59 patients (27:32) enrolled, 6‐month follow‐up in Table 3 showed 25:27 patients. Not clearly described. |
| Selective reporting (reporting bias) | Unclear risk | Rates of complications and mortality, with bedsores, severe anaemia, cardiac failure, infection and gastrointestinal ulcer were considered as major complications. All these results were reported in results section (Table 3). Minor complications were not reported, but, possibly, did not occur. |
Dennis 2005.
| Methods | Multicentred RCT, with ITT. Duration: 7.5 years from November 1996‐March 2004. |
|
| Participants | 4023 stroke patients who were able to swallow (maximum 7 days after stroke). Exclusion criterion: patients with subarachnoid haemorrhage. A) Intervention group (n = 2016): 46% female, 54% male, mean age 71 years ± 13 (SD). B) Control group (n = 2007): 47% female, 53% male, mean age 71 years ± 12 (SD). |
|
| Interventions | A) Intervention group: normal hospital diet plus oral protein energy supplements (equivalent to 360 ml at 6.27 kJ/ml and 62.5 g/L in protein every day) until discharge. B) Control group: normal hospital diet until discharge. Follow‐up at 6 months. |
|
| Outcomes | Primary outcomes: 1. Death. 2. Poor outcome (modified Rankin scale [MRS] grade 3‐5). 3. Overall survival. Secondary outcomes: 4. Patients’ vital status. 5. Place of residence. 6. Quality of life (EUROQoL). 7. Length of hospital stay. 8. Discharge destination. 10. Causes of death. 11. In‐hospital complications (including pressure ulcers). Outcomes 1‐10 not considered in this review; for outcome 11, only pressure ulcers incidence was considered. |
|
| Notes | Location: 15 countries. Setting: 125 hospitals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “After all baseline data had been entered and checked, the computer allocated the feeding regimen.” Quote: “A computer‐generated minimisation algorithm balanced treatment within each country, with age (> 75, < 75 years), sex, and predicted probability of poor outcome (< 35%, > 35%) as stratification variables”. |
| Allocation concealment (selection bias) | Low risk | Quote: “... the computer allocated the feeding regimen. Thus, baseline data were 100% complete and treatment allocation was concealed until it was given”. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No intention of blinding reported. Quote: “we could not mask this assessment to treatment allocation, and it was not feasible for local source data to be verified for the occurrence of these”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few drop outs, clearly described and balanced in numbers. Intention to treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Most outcomes (post stroke complications after randomisation and before discharge or in‐hospital death; patients’ vital status, functional ability, place of residence, and quality of life with the EUROQoL) were reported in the results section. Results for place of residence at follow‐up not reported. |
Derossi 2009.
| Methods | RCT. Duration: not stated. |
|
| Participants | 107 hip fracture patients, aged 65 and older, scheduled to undergo surgical treatment.
Exclusion criteria: dementia, dysphagia. A) Intervention group (n = 54): 83.3% female, 16.6% male, mean age 79.9 years ± 7.3 (SD). B) Control group (n = 53): 71.7% female, 28.3% male, mean age 80.4 years ± 6.8 (SD). |
|
| Interventions | A) Intervention group: oral nutrition supplement once daily containing: 345 mg L‐carnitine, 500 mg calcium, 250 mg magnesium, 5 μg vitamin D, 500 mg L‐leucine. B) Control group: normal hospital diet. |
|
| Outcomes | 1. Barthel index. 2. IADL index at admissions, at discharge and at follow‐up (40‐50 day after hospitalisation). 3. Length of hospital stay. 4. Changes in BMI. 5. Changes in upper arm circumferences during the study. 6. In‐hospital complications (pressure ulcers, infections, analgesic consumption). Otucomes 1‐5 not considered in this review; for outcome 6, only pressure ulcers were considered. |
|
| Notes | Location: Italy. Setting: hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 107 patients randomised, 53 in the intervention and 54 in the control group. At discharge: 49 participants in the intervention and 47 in the control group. The 11 subjects lost to follow‐up during the hospital stay were transferred to intensive care or could not be measured for unclearly described reasons. At follow‐up (40‐50 days after hospitalisation): 38 participants in the intervention and 41 in the control group. Reasons for loss to follow‐up were not clearly described. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported (nutritional status and the development and severity of pressure ulcers). |
Desneves 2005.
| Methods | RCT, single‐blinded. Duration: 7 months, date of baseline point: January 2004, 3‐week study period. |
|
| Participants | 16 in‐patients with pressure ulcer grades 2 to IV (according to the Australian Wound Management Assiciation Clinical Practice Guidelines). Exclusion criteria: patients with diabetes mellitus or a clinical suspicion or diagnosis of osteomyelitis. Individuals receiving more than 10 mg of steroids per day, nutrition support, or prescribed hydroxyurea. A) Intervention group I (n = 6): 33.3% female, 66.7% male, mean age 63.0 years ± 9.9 (SD). B) Intervention group II (n = 5): 40.0% female, 60.0% male, mean age 75.6 years ± 5.9 (SD). C) Control group (n = 5): 40.0% female, 60.0% male, mean age 83.2 years ± 1.1 (SD). |
|
| Interventions | A) Intervention group I: standard hospital diet plus 2 tetrapaks of a defined arginine‐containing supplement, supplying additional 2100 kJ (500 kcal), 21 g protein, 0 g fat, 500 mg vitamin C, 30 mg zinc and 9 g of arginine. B) Intervention group II: standard hospital diet plus 2 tetrapaks of a high‐protein, high‐energy supplement providing an additional 2100 kJ (500 kcal), 18 g protein, 0 g fat, 72 mg vitamin C and 7.5 mg zinc. C) Control group: standard hospital diet. All groups underwent the same pressure ulcer care according to standard ward practice. Other interventions occurred as per the Pressure Ulcer Prevention Strategies tool and equipment flow chart implemented in the hospital. |
|
| Outcomes | 1. Pressure ulcer healing (PUSH‐Score). 2. Blood chemistry. 3. Dietary intake. 4. Anthropometry (measurements of the body). Outcomes 2‐4 not considered in this review. |
|
| Notes | Location: Australia, Melbourne. Setting: Austin Health Hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence of dietary treatment allocation was determined before the beginning of the study by sorting a list of random numbers (generated using a computer program) in numerical order. |
| Allocation concealment (selection bias) | High risk | " over the 6‐month recruitment period, patients were allocated to a dietary treatment group in the order that they were recruited. The sequence of dietary treatment allocation was determined before the beginning of the study by sorting a list of random numbers (generated using a computer program) in numerical order. Before sorting the list of random numbers, each of the numbers was linked to a dietary intervention group." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “An independent person (Clinical Nurse Consultant), blinded to the dietary treatment, conducted the assessment of the pressure ulcers using the PUSH Tool”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Three of the 16 patients (one from each of the three dietary intervention groups) did not have assessments performed in the final week 3 of the study as one patient was discharged (from Diet B) and two died (from Diets A and C) after completion of their assessment at week 2.” |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported (PUSH‐Score, blood chemistry, dietary intake, anthropometry). |
Ek 1991.
| Methods | RCT. Duration: 19 months, from November 1985 ‐ May 1987. |
|
| Participants | Enrolled 501 patients who were expected to remain in hospital for more than 3 weeks. Data on nutritional state were obtained from only 482 patients, since 19 patients were not classified due to absence of data.The results relating the frequency of pressure sores were based on observations of 495 patients (307 women and 188 men) owing to missing information for 6 patients. 451 patients consented to enter the treatment study, 39 refused nutritional support and 9 in the control group were withdrawn because they developed clinical indications for nutritional support (numbers reported in the original publication). |
|
| Interventions | A) Intervention group: usual hospital diet (2200 kcal/day) plus standard supplement (400 kcal/day, 16% of energy protein, 36% of energy fat, 48% of energy carbohydrate). B) Control group: usual hospital diet (2200 kcal/day). |
|
| Outcomes | At admission, and on weeks 2, 4, 8 and 26. 1. Development of pressure ulcers. 2. Healing of pressure ulcers. 3. Nutritional state. Outcome 3 was not considered in this review. |
|
| Notes | Location: Sveden, Linkoping. Setting: Long‐term care clinic of an Universival Hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: "The patients were randomised to an experimental group who received extra nutritional support and to a control group." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not describe the number of patients evaluated clearly. |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes were reported. The authors performed comparisons between patients with or without pressure ulcers. |
Hartgrink 1998.
| Methods | RCT. Duration: 31 months, from May 1992 ‐ November 1995. |
|
| Participants | 140 patients with hip fracture and an increased pressure sore risk (8 points or more; scale according to the Dutch consensus meeting for the prevention of pressure ulcers in 1992).
Exclusion criteria: patients with pressure ulcers of Grade 2, or worse, at admission. A) Intervention group (n = 62): 72.2% female, 16.1% male, mean age 84.0 years ± 7.1 (SD). B) Control group (n = 67): 91.0% female, 9.0% male, mean age 83.3 years ± 8.1 (SD). |
|
| Interventions | A) Intervention group: standard hospital diet and additional nasogastric tube feeding with 1000 ml Nutrison Steriflo Energy‐plus (1500 kcal/L; 60 g/L protein) which was administered with a feeding pump between 9 pm and 5 am. B) Control group: standard hospital diet for 2 weeks. |
|
| Outcomes | 1. Development of pressure ulcers. 2. Severity of pressure ulcers: recorded each day using the following system (from the 1992 Dutch consensus meeting for the prevention of pressure ulcers): 0: normal skin; I: persistent erythema of the skin; 2: blister formation; 3: superficial (sub)cutaneous necrosis; 4: deep subcutaneous necrosis; 3. Nutritional intake. 4. Pressure ulcer risk score. 5. Haemoglobin concentration. 6. Total serum protein. 7. Total serum albumin. Outcomes 3‐7 were not considered in this review. |
|
| Notes | Location: Netherlands. Setting: hospital, department of surgery. 25/62 patients accepted nasogastric tube for more than 1 week, 16 patients for 2 weeks. Supplements and tubes were provided by Nutricia (Netherland). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: “... we randomised 140 patients...” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No intention of blinding reported. Quote: “Patients and physicians were not blinded with respect to treatment since it was judged unethical to discomfort the control group with a nasogastric tube”. Quote: “The presence and grade of pressure sores on the sacrum, trochanters, heels, and elsewhere were noted by two physicians independently. Any discrepancy between the two observers for any of the pressure‐sore grading was resolved by a third opinion, obtained from another physician, after which the three physicians reached consensus.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 140 randomised.
129 evaluable at admission (92%): 62/70 (89%) in the tube‐fed group and 67/70 (96%) in the tubeless group.
116 evaluable at week 1 (83%), 54 /70 (77%) in tube‐fed group and 62/70 (89%) in tubeless group. 101 evaluable at 2 weeks (72%), 48/70 (69%) in tube‐fed group and 53/70 (76%) in tubeless group. Explanations for exclusions after admission were provided, plus reasons for attrition at weeks 1 and 2. Losses were greater in the tube‐fed group. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported (nutritional status and the development and severity of pressure ulcers). |
Houwing 2003.
| Methods | RCT, double‐blind, placebo‐controlled. Duration: 21 months, from April1998‐December 1999. |
|
| Participants | 103 hip‐fracture patients at risk of developing pressure ulcers (8 points or more; scale according to the Dutch consensus meeting for the prevention of pressure ulcers in 1992).
Exclusion criteria: terminal care, metastatic hip fracture, insulin‐dependent diabetes, renal disease, hepatic disease, morbid obesity, pregnancy or lactation. A) Intervention group (n = 51): 78.4% female, 21.6% male, mean age 81.5 years ± 0.9 (SE). B) Control group (n = 52): 84.6% female, 15.4% male, mean age 80.5 years ± 1.3 (SE). |
|
| Interventions | A) Intervention group: nutritional supplement (400 ml; 500 kcal; 40 g protein; 6 g L‐arginine; 20 mg zinc; 500 mg vitamin C; 200 mg vitamin E; 4 mg carotenoids) for a period of 4 weeks or until discharge. B) Control group: non‐caloric, water‐based placebo for a period of 4 weeks or until discharge. | |
| Outcomes | Assessed daily for 28 days, or until discharge, according to the four‐grade classification system defined in the treatment guidelines of the EPUAP. 1. Presence of pressure ulcers. 2. Grade of pressure ulcers. |
|
| Notes | Location: Netherlands. Setting: 3 centres treating patients with hip fractures. Power calculation: with 80% confidence and alpha of 5% to detect a 25% difference in pressure ulcer incidence: 350 patients per group. Sample size was not reached. Funded by Numico Research BV, Wageningen, the Netherlands. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: "... patients were randomised to receive the study or placebo supplement in addition to their regular diet." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and personnel: Quote: “Look and taste of both supplements were not exactly identical, but supplements were given in similar, blinded packages to mask the differences”. Nursing staff performed assessments but unclear if same ‘blinded’ personnel as those delivering the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients stayed in the study. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
Lee 2006.
| Methods | RCT, double‐blinded. Duration: 15 months, from October 2003‐December 2004. |
|
| Participants | 89 residents of long‐term care facilities with pressure ulcers grades 2, 3, or 4. Exclusion criteria: terminal diagnosis, hospice care, protein‐restricted diet due to renal insufficiency, active metabolic or gastrointestinal diseases that might interfere with nutrient absorption, distribution, metabolism, or excretion, food allergies, or use of corticosteroids or antibiotics for wound infection. No further description of participants available. |
|
| Interventions | A) Intervention group (n = 56): standard care and a concentrated, fortified, collagen protein hydrolysate supplement. Individual unit doses (each 15 g protein in a 45 ml unit dose) 3 times daily for 8 weeks. B) Control group (n = 33): standard care and placebo (noncaloric liquid indistinguishable from the study product in terms of colour, taste, and texture) 3 times daily for 8 weeks. |
|
| Outcomes | 1. Changes in PUSH tool scores at 8 weeks. 2. Supplement intake, which was not considered in this review. |
|
| Notes | Location: USA. Setting: 23 long‐term care facilities. The research was supported by Medical Nutrition USA, Inc, Englewood, NJ. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generation not adequate. Quote: "The first Patient in each building was randomised to the 'red' or 'white' group by the research assistant using the flip of a coin." Ensuring assignments were made by alternating between the 2 groups." |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate blinding. Subjects and staff were unaware of the group membership. Quote: “The placebo was a noncaloric liquid indistinguishable from the study product in terms of colour, taste, and texture. The placebo and the study product were each packaged in identical opaque white, uni‐dose bottled differentiated by a numeric code and a red dot or no dot on the label. Subjects and staff were unaware of the numeric code of the colours." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Eleven participants discontinued treatment because of adverse events that included hip fracture due to fall (n = 2), changes in renal laboratory values (n = 3), nausea or distention (n = 4) and death (n = 2). One person in each group died from causes unrelated to the study. For reasons unrelated to the study, 5 participants left their facilities before completion of the trial.” |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported (changes in PUSH tool score). |
Meaume 2009.
| Methods | Multicentred RCT, double‐blind, placebo‐controlled. Duration: not stated; experimental treatment period 6 weeks. |
|
| Participants | 160 patients > 60 years with a heel pressure ulcer, Grade 2 or 3 (NPUAP) after accidental immobilization, ulcer in the process of recovery with early signs of granulation tissue (at least 10% of red tissue on colour scale). Exclusion criteria: patients confined to bed 24 h/d before the episode triggering development of the pressure ulcer, pressure ulcers covered by necrosis or fibrin, infected pressure ulcers, poorly‐controlled type 1 or 2 diabetes, dialysis patients, active neoplastic disease, parenteral nutrition, serum albumin < 22 g/L, advanced peripheral arterial occlusive disease. A) Intervention group (n = 85): 65.9% female, 34.1% male, mean age 81.0 years ± 8.2 (SD). B) Control group (n = 75): 47.4% female, 52.6% male, mean age 80.5 years ± 9.6 (SD). Follow‐up 160/165, 5 patients did not take at least one dose of the study medication or have at least one post treatment evaluation. |
|
| Interventions | A) Intervention group: 10 g ornithine alpha‐ketoglutarate (OKG) daily for 6 weeks. B) Control group: placebo daily for 6 weeks. |
|
| Outcomes | Pressure ulcer development: 1. Area reduction. 2. Global closure rate. 3. Closure rate at each visit. 4. Cumulative closure rate. 5. Ulcers with 40 or 90% reduction. 6. Adverse effects related to the study medication. 7. Mini Nutritional Assessment (MNA). 8. Patient's appetite. 9. Body weight. 10. Serum albumin. 11. Serum pre‐albumin. 12. C reactive protein. Outcomes 3‐5 and 7‐12 were not considered in this review. |
|
| Notes | Location: 6 European countries. Setting: hospitals, 67 wards: geriatric, internal medicine, physical medicine and rehabilitation, trauma, plastic surgery, cardiology, neurology and dermatology. The study was sponsored by a grant from CHIESI France and Italy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization codes were generated by using CRITAL software” |
| Allocation concealment (selection bias) | High risk | A randomisation number was attributed according to the chronological order of entry of patients into the double‐blind period within each investigational site. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients: Quote: "... or one sachet of placebo (similar aspect and taste) was administered..." Investigators: Quote: "Ulcer tracing recorded by investigators were centrally and blindly measured by using AUTOCAD software. Two measurements were made by tracing (two independent evaluators) and the mean of the two values was used for analysis." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Efficacy analyses were performed on the intend to treat (ITT) population defined and categorized before code breaking. The Last Observation Carried Forward (LOCF) principle was applied to deal with missing efficiency time‐points." |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes reported. Outcomes: area reduction, global closure rate, closure rate at each visit, cumulative closure rate, ulcers with 40 or 90% reduction. Clinical/laboratory parameters reflecting nutritional and inflammatory status, OKG safety were mentioned in the result section. No details given for the group with sores > 8 cm2. No differences in these groups. Request to the author is done, but without response. |
Norris 1971.
| Methods | RCT, crossover design, double‐blind, placebo‐controlled. Duration: not stated; treatment period 24 weeks. |
|
| Participants | 14 patients with pressure ulcers: 35.7% female, 64.3% male. No further description available. Exclusion criteria: neoplastic disease, terminal phase of illness, superficial pressure sores, pressure sores where deep sinus tracts were involved. | |
| Interventions | A) 3 capsules of zinc sulphate (200 mg) (n = 7). B) 3 placebo capsules per day (n = 7). After 12 weeks the patients switched groups. | |
| Outcomes | Volume of pressure sore (crater) (Pories method). | |
| Notes | Location: USA, Baltimore. Setting: chronic disease hospital. Only 3/14 patients completed the study; ulcer volume measured at 4 week intervals. Supplements provided by CR Canfield and Company, Minneapolis, Minnesota. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Quote: "The patients were randomly divided into two groups." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant and personnel: Quote: "Identical‐appearing capsules of zinc sulphate (200 mg) and placebo were packaged in separate containers by the hospital pharmacy; they were labelled Zincate A and Zincate B. At the end of the study, when the code was broken, Zincate A provided to be zinc sulphate and Zincate B, placebo. Meanwhile, neither the physicians nor the nursing staff knew the exact contents of these capsules until completion of the study." Unclear who performed ulcer measurement, and, therefore, whether or not they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adequately addressed but unacceptable drop‐out rate. Only 3/14 patients completed the 24‐week crossover trial; 10/14 patients remained in the trial at 12 weeks; no ITT. |
| Selective reporting (reporting bias) | Low risk | Outcomes not pre‐specified; aim of trial described only, as objective criteria were used to measure the effects. |
Ohura 2011.
| Methods | Multicentred RCT. | |
| Participants | 60 tube‐fed patients with pressure ulcers grades 3‐4 (NPUAP), albumin 2.5‐3.5 g/dL, OH scale < 8.5 and Braden scale 9‐17. Exclusion criteria: severe liver or renal disorder, severe diabetes mellitus, arteriosclerosis obliterans, malignant tumour within past 5 years, "unmanageable severe general condition or unevaluable pressure ulcer wounds (necrotic tissue in 20% or more of the wound surface, wound before sharp debridement, 2 cm or more in depth of the undermining, multiple pressure ulcers and wound infection)". A) Intervention group (n = 21): 71.4% female, 28.6% male, mean age 81.40 years ± 8.9 (SD). B) Control group (n = 29): 65.5% female, 34.5% male, mean age 80.6 years ± 8.9 (SD). Follow‐up: 50/60 patients, reasons for drop‐out please see risk of bias table. |
|
| Interventions | All patients received: tube feeding of a special formula (protein 4.38 g, fat 2.23 g, and carbohydrate 15.62 g, copper 125 mg and zinc 0.64 mg per 100 ml of product. The ratio of ω‐3 to ω‐6 essential fatty acids was 1:3. A) Intervention group: number of calories calculated according to the range of Basal Energy Expenditure (BEE, calculated from the Harris–Benedict equation) x active factor 1.1, x stress factor 1.3–1.5. B) Control group: same number of calories as had before participating in the trial. |
|
| Outcomes | Pressure ulcers (measured every 2 weeks):
Nutritional state (measured every 6 weeks):
Adverse events. |
|
| Notes | Location: Japan. Setting: 35 hospitals. No adjustment for multiple testing. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Minimisation used; no information about sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study is described as an "open" trial. Observers could have been blinded. Some outcomes were objective (e.g. for nutritional state), but not all (e.g. for pressure ulcers). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 patients were randomised. 1 patient in the intervention group dropped out before administration of intervention. Further exclusions occurred due to study discontinuation within 4 weeks or for protocol violation: 1 patient in control group (reason not described) and 8 patients in intervention group (1 patient discontinued due to an adverse event for which a causal relationship with the study could not be ruled out, 3 patients discontinued due to an adverse event with no relationship to study intervention, 4 patients underwent prohibited treatment such as debridement or failure to consume the required calories). No ITT. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
Olofsson 2007.
| Methods | RCT. Duration: 3 years, from May 2000‐April 2003. |
|
| Participants | 199 patients with femoral neck fracture ≥ 70 years. 75.8% female, 24.2% male. No further description available. Exclusion criteria: severe rheumatoid arthritis, severe hip osteoarthritis, severe renal failure, metastatic fracture; bedridden before the injury. A) Intervention group (n = 83): 75% female, 25% male, mean age 81.1 years ± 6.8 (SD). B) Control group (n = 74): 77% female, 23% male, mean age 82.2 years ± 5.6 (SD). Follow‐up: 157/199 patients, reasons for drop‐out please see risk of bias table. |
|
| Interventions | A) Intervention group: postoperative special nutritional intervention programme in geriatric ward, i.e. a nutrition journal was kept for at least 4 days postoperatively to detect deficiencies in nutrition and record protein‐enriched meals . Furthermore, at least 2 nutritional and protein drinks were served each day during the whole hospitalisation.
(Supplementation daily: 600 kcal, 24 g protein from milk protein (16% of energy), 23.2 g fat from raps‐oil (rapeseed oil; 35% of energy), 73.6 g carbohydrate from maltodextrins and saccharose (sucrose; 49% of energy). B) Control group: conventional postoperative care routines in the orthopaedic department. |
|
| Outcomes | 4‐month follow‐up. 1. Mini Nutritional Assessment (MNA). 2. BMI. 3. S‐albumin. 4. B‐haemoglobin. 5. Length of hospital stay. 6. Delirium preoperatively. Postoperative complications during hospitalisation: 7. Delirium postoperatively. 8. Delirium during hospitalisation. 9. Nutrition difficulties. 10. Constipation. 11. Urinary tract infection. 12. Decubitus ulcers. Outcomes 1‐11 not considered in this review. |
|
| Notes | Location: Sweden, Umea. Setting: university hospital, orthopaedic department. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Quote: "Patients were randomised to postoperative care in a geriatric ward with a special intervention programme or to conventional care in the orthopaedic department." |
| Allocation concealment (selection bias) | Low risk | Quote: “... sealed, opaque envelopes stratified according to operation method. All participants received an envelope while in the emergency room, but it was not opened until immediately before surgery to ensure similar preoperative treatment. A nurse on duty at the orthopaedic department, who was not involved in the study, opened the envelope.” |
| Blinding (performance bias and detection bias) All outcomes | High risk | No intention of blinding described. Quote: "The staff on the intervention ward was aware of the nature of the study, and the staff working on the control ward was informed that a new care programme was being implemented and that it was being evaluated on the geriatric intervention ward.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 199 patients randomised. During hospitalisation: 6 patients died in the intervention group, and 7 in the control group. No MNA available: for 5 in the intervention group, and 8 in the control group. At 4‐month follow‐up: 3 patients in the intervention group, and 6 in the control group, died after discharge. No MNA available: for 4 in the intervention group, 1 in the control group. 1 patient in the intervention group declined to continue, and 1 patient in the control group moved to another city. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. Postoperative complications during hospitalisation, length of hospital stay, nutritional status (MNA, BMI, S‐albumin, B‐haemoglobin). |
Taylor 1974.
| Methods | RCT, double‐blind, placebo‐controlled. Duration: not stated; experimental treatment period 4 weeks. |
|
| Participants | 20 surgical patients with pressure ulcers. 60% female, 40% male, mean age 74.5 years (range 54‐88 years). No further description available. | |
| Interventions | A) Intervention group (n = 10): 500 mg ascorbic acid twice daily for 4 weeks.
B) Control group (n = 10): inert placebo twice daily for 4 weeks. All patients had standard hospital beds and mattresses, the same basic hospital diet, and similar local therapy for the pressure area. |
|
| Outcomes | 1. Changes in pressure ulcers after 1 month. 2. Mean rate of pressure ulcer healing per week. 3. Leucocyte ascorbic‐acids levels (not considered in this review). |
|
| Notes | Location: Great Britain, South Manchester. Setting: university hospital, division of surgery. Supplements provided by Merck Limited. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were allocated to the treatment groups A or B according to their year of birth.” Year of birth is non‐random sequence generation. |
| Allocation concealment (selection bias) | High risk | See above. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant and personnel: Quote: "Identical white tablets, A and B, were dispensed containing either 500 mg of ascorbic acid (E Merck Ltd) or an inert placebo.” Personnel and investigator: Quote: "The areas of the pressure sores were assessed weekly in three ways. .... The data were subsequently analysed by an independent observer”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information from 2 patients missing for 1 outcome that was not considered in this review. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
ter Riet 1995.
| Methods | RCT, double‐blind, ITT, per‐protocol, and sensitivity analysis. Duration: not stated; experimental treatment period 12 weeks. |
|
| Participants | 88 patients with pressure ulcers (partial thickness skin loss or worse). Patients with Grade 2 ulcers could participate only if de‐epithelialization had persisted for at least 7 days without interruption. No further description available. Exclusion criteria: difficulties with swallowing or frequent vomiting, osteomyelitis in the ulcer area, idiopathic haemochromatosis, thalassaemia major, sideroblastic anaemia, Cushing's syndrome or disease, pregnancy, radiotherapy in the ulcer area, use of antineoplastic agents or systematic glucocorticosteroids, terminally‐ill patients, surgical treatment of the ulcer (other than debridement) planned, or those already taking over 50 mg vitamin C per day. |
|
| Interventions | A) Intervention group (n = 43): 500 mg ascorbic acid twice daily B) Control group (n = 45): 10 mg ascorbic acid twice daily |
|
| Outcomes | 1. Wound survival time. 2. Wound closure probabilities per unit time. 3. Relative changes in surface area (% per week). 4. Mean healing velocity (cm2 per week). 5. Mean volume reduction. |
|
| Notes | Location: Netherlands. Setting: 11 nursing homes and 1 hospital. Most patients had nutritional deficiencies on admission. Supplements were provided by Hoffmann‐Laroche & Co Ltd, Basel. Water beds were provided by Lopital BV, Osterwijk. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was carried out in each stratum, using random permuted blocks (size = 4) prepared in advance with the help of a computer program. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients in the intervention group received effervescent tablets (Roche, Basel, Switzerland) containing 500 mg ascorbic acid twice daily (morning and early evening). The control group received indistinguishable tablets (with regard to colour, taste, smell, disintegration time, and friability) containing 10 mg of ascorbic acid. The investigators, nursing staff (and physiotherapist), and patients were blinded to treatment allocation. The main investigator and a research nurse performed most of the topical wound care themselves (weeks 1‐6 and then all even weeks to 12, except during weekends). Success of blinding was checked after 2 and 12 weeks. Two nursing home physicians and two senior staff nurses in nursing homes, who had an interest in pressure ulcers but were unaware of treatment allocation, scored all slides separately on 4 visual items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88 participants randomised. Intervention group: 3 died and 1 withdrew. Control group: 7 died and 2 withdrew. ITT analysis and per protocol conducted. 63 patients included in per protocol analysis. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
Theilla 2007.
| Methods | RCT. Duration: not stated; experimental treatment period 1 week. |
|
| Participants | 100 patients suffering from acute lung injury defined by a PaO2/FIO2 ratio below 250 were included. Exclusion criteria: head trauma, cerebral bleeding, coagulation disorders, receiving steroids in a dose > 0.25 mg/kg/day, taking methylprednisolone or non‐steroidal anti‐inflammatory agents, < 18 years, or pregnancy. Patients were also excluded if diarrhoea occurred more than 3 times. A) Intervention group (n = 46): 42.9% female, 57.1% male, mean age 62.3 years ± 17.2 (SD). B) Control group (n = 49): 37.0% female, 63.0% male, mean age 57.0 years ± 18.7 (SD). Follow‐up: 95/100 patients, reasons for drop‐out please see risk of bias table. |
|
| Interventions | A) Intervention group: high‐fat, low‐carbohydrate, enteral formula (Pulmocare, Ross Laboratories, Abbott, Chicago, USA) enriched in lipids (eicosapentaenoic acid (EPA), gamma‐linolenic acid (GLA)), and vitamins A, C and E. B) Control group: high‐fat, low‐carbohydrate, enteral formula (Pulmocare, Ross Laboratories, Abbott, Chicago, USA). |
|
| Outcomes | Days 4 and 7: 1. Pressure ulcer status. 2. Pressure ulcer incidence. 3. Pressure ulcer healing. Day 7: 4. BMI. 5. Nutritional intake. 6. Albumin. 7. Prealbumin. 8. Resting energy expenditure. 9. Harris‐Benedict equation. 10. Vitamin A. 11. Vitamin E. Days 7 and 14: 12. C‐reactive protein. 13. Procalcitonin. Outcomes 4‐13 were not considered in this review. |
|
| Notes | Location: Israel. Setting: intensive care unit. Supplements were provided by Abbott Laboratory Representatives (Promedico Company). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 people excluded because of diarrhoea or food intolerance. It was not clear whether exclusion happened before or after randomisation. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
van Anholt 2010.
| Methods | Multicentred RCT, double‐blind, placebo‐controlled. Duration: 17 months, from August 2007‐December 2008. |
|
| Participants | 47 patients with pressure ulcers grade 3 or 4 EPUAP; receiving standard care and a standard (institutional) diet without nutritional supplements for at least 2 weeks before the study. Aged 18‐90 years. Exclusion criteria: malnutrition (BMI < 18.5 if 18‐70 years old, or weighing < 21 kg if over 70 years); severe medical conditions, non pressure‐related ulcers (e.g. diabetic ulcers), life expectancy < 6 months, receiving palliative care, use of corticosteroids, and/or dietary restrictions, i.e. a protein‐restricted diet. A) Intervention group (n = 22): 63.6% female, 36.4% male, mean age 76.2 years ± 3.2 (SE). B) Control group (n = 21): 47.6% female, 52.4% male, mean age 73.0 years ± 3.3 (SE). Follow‐up: 43/47 patients, reasons for drop‐out please see risk of bias table. |
|
| Interventions | A) Intervention group: high‐energy supplement enriched with arginine, antioxidants, and other micronutrient oral supplements (Cubitan). 3 x 200 ml/d: provides per serving: 250 kcal, 28.4 g carbohydrate (45%), 20 g protein (30%; including 3 g arginine), 7 g fat (25%), 238 mg vitamin A, 250 mg vitamin C, 38 mg vitamin E (a‐tocopherol‐equivalents), 1.5 mg carotenoids, 9 mg zinc, 64 mg selenium, 1.35 mg copper and 200 mg folic acid. B) Control group: non‐caloric, flavoured placebo (similar in taste and appearance). |
|
| Outcomes | Primary outcome: 1. Pressure ulcer healing (changes in pressure ulcer surface over 8 weeks). Secondary outcomes: 2. Change in the Pressure Ulcer Scale for Healing (PUSH). Other parameters: 3. BMI. 4. Malnutrition Universal Screening Tool scores. 5. Blood parameters (vitamin C, zinc, alanine aminotransferase, g‐glutamyl transpeptidase, creatinine, blood cell and platelet counts, haemoglobin, troponin I, transthyretin, and C‐reactive protein. 6. Number of dressings applied during the preceding week. 7. Mobility and activity levels. Outcomes 3‐7 were not considered in this review. |
|
| Notes | Location: 4 countries. Setting: 8 healthcare centres, hospitals, and long‐term care facilities. The study was sponsored by Nutricia Advanced Medical Nutrition. 2 authors were employees of Nutricia Advanced Medical Nutrition, which is part of Danone Research. Another author had received several (unrestricted) research grants from Nutricia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Quote: “Patients were randomly allocated to receive a ...”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “All participants were prescribed three coded servings (three times 200 mL) per day between meals to be consumed preferably within 1 h.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47 patients were randomised, 43 in ITT analysis. Quote: “Four patients were not included in the ITT analysis due to death, hospitalisation, exceeding the inclusion criteria for BMI or withdrawal of informed consent before consuming a single serving of the (control) product.” Other dropouts reasonable and balanced in numbers. |
| Selective reporting (reporting bias) | Unclear risk | The most important prespecified outcomes reported in the result section (primary and secondary endpoints). Not all other parameters are described in Table 3. |
Abbreviations
< = less than > = greater than ≥ = greater than or equal to ω = omega d = day(s) BMI = Body Mass Index EPUAP = European Pressure Ulcer Advisory Panel IADL index = Instrumental Activities of Daily Living ITT = intention‐to‐treat (analysis) IU = International Units NPUAP = National Pressure Ulcer Advisory Panel OH scale = Japanese patient intrinsic risk factor scale; self‐sustainable ability to move unassisted, morbid bony prominence, edema, and articular contracture PaO2/FIO2 ratio = ratio of partial pressure of oxygen in arterial blood by percentage of oxygen in a gas mixture PUSH = Pressure Ulcer Scale for Healing RCT = randomised clinical trial SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barateau 1998 | Not an RCT. Prospective, multicentered, randomised survey to study the Influence of nutrition in prevention of bedsores among elderly patients, in hospital, suffering from acute infections. |
| Bergstrom 1987 | 129 institutionalised elderly who were at risk but did not have pressure ulcers at admission were studied to determine wether dietary and serum zinc and copper differ between those who develop pressure ulcers and those who did not develop pressure ulcers. Not an RCT or CCT. |
| Bourdel‐M 1997 | Retrospective case‐control study with 108 patients to discover early and late tolerance of long‐term feeding with PEG for older and frail patients. Not an RCT or CCT. |
| Breslow 1991 | Comparison of nutritional status and dietary intake of 14 tube fed nursing home patients with pressure ulcers to 12 tube fed patients without pressure ulcers. Not an RCT or CCT. |
| Breslow 1993 | 28 malnourished patients with pressure ulcers received 24% protein or 14% protein supplements for a period of 8 weeks. First RCT, then CCT justified by unbalanced groups and high drop‐out rate; effects of bed type on results are unclear and pressure ulcers were treated differently. |
| Eneroth 2006 | Pressure ulcers, and other outcomes we predefined for this review, were not measured. 40 elderly patients after hip fracture received 1000 kcal daily intravenous supplement for 3 days, followed by a 400 kcal oral nutritional supplement for 7 days to decrease fracture‐related complications. 40 patients in the control group were given standard hospital food and beverages. |
| Langemo 2006 | Not an RCT. |
| Langkamp‐Henken 2000 | 32 nursing home residents with pressure ulcers received 0g, 8.5 g or 17 g arginine for 4 weeks. Not pressure ulcers but only immune functions were measured. |
| Larsson 1990 | 501 geriatric patients received standard hospital diet or additional nutritional supplements for 26 weeks. Pressure ulcers not measured. |
| Myers 1990 | 80 patients with pressure ulcers were treated with wound care, with nutritional support, with both or with standard hospital treatment for 7 days. Nutritional supplementation was not clearly described. |
| NCT00502372 | Terminated without results because of a lack of patients. |
| Neander 2004 | Not an RCT. 193 elderly people living in sheltered housing, who had shown a decline in serum protein and intracellular zinc, were studied to determine whether a supplement containing 20 g protein, 3 g arginine, 250 mg vitamin C and 9 mg zinc reduced the incidence of pressure ulcers. |
| Schröder‐van den N 2004 | No results reported. The effects of 2 different types of sip feeds on pressure ulcer healing were compared in a high‐risk population in a rehabilitation centre. |
| Settel 1969 | No nutritional intervention. The effects of a submicroscopic emulsion containing Allantoin and vitamin A on the prevention and treatment of pressure ulcers was examined. |
| Stotts 2009 | Pressure ulcers, and other outcomes we predefined for this review, were not measured. The effects of increased fluid intake on collagen deposition, estimated total body water, and subcutaneous tissue oxygenation were estimated in 64 nursing‐home residents who were at risk for pressure ulcers. |
Abbreviation
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Candela‐Zamora 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Caulfield 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Chen 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Leigh 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Starke 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Sugihara 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Theilla 2012a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Theilla 2012b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12605000704695.
| Trial name or title | |
| Methods | RCT, blinded. |
| Participants | Setting: intensive care units, Australia. Participants: 1470 male and female patients not currently receiving oral, enteral or parenteral nutritional support, and for whom the treating physician does not expect to begin oral, enteral or parenteral nutritional support within the next 24 h. Exclusion criteria: patients who have been on ICU for > 24 h, or whom the treating physician expects to discharge from ICU within the next 24 h; inability to start parenteral nutrition within 24 h of ICU admission; contraindication for enteral nutrition where patient would normally be supported with parenteral nutrition; admission to ICU for treatment of thermal injury (burns) or palliative care; patient is moribund and not expected to survive 24 h, or is brain dead or suspected of being brain dead; patients admitted to the study ICU directly from another ICU; or where the treating physician believes there is an absolute contraindication to treatment received in either study arm. |
| Interventions | A) Early parenteral nutrition initiated within 24 h of ICU admission at a rate to achieve approximately 25‐30 kcal/kg/d. Early parenteral nutrition will be maintained until enteral nutrition is initiated. B) Standard care. |
| Outcomes | Primary outcome: 60 day all‐cause landmark mortality. Secondary outcome: ICU and hospital length of stay (measured at ICU and hospital discharge) and SF1 (measured at day 60). Further secondary outcomes measured during ICU stay: degree and days of organ dysfunction, days of mechanical ventilation, days of renal replacement therapy, days of inotrope requirement, daily blood glucose levels, daily insulin dose, duration and severity of pressure ulcers, antibiotic usage and nutritional status. |
| Starting date | |
| Contact information | |
| Notes | Location: Australia. Registration number: ACTRN12605000704695aa. Funding: Government funding body e.g. Australian Research Council. Results announced for the 3rd quarter 2012. Author contacted and advised us to wait for publication. |
ACTRN12610000526077.
| Trial name or title | |
| Methods | RCT, single‐blind. |
| Participants | Setting: emergency department, Australia. Participants: estimated enrolment of 130 patients aged 60 years or above, both genders. Inclusion criteria: malnourished patients aged 60 years or above, admitted to emergency department (malnutrition assessed with the "Malnutrition Screening Tool"). Exclusion criteria: patients unable to give informed consent; with triage category I (highest priority); already receiving dietetic care, or admitted from a health care facility (including nursing‐homes). |
| Interventions | A) Individualised nutritional care from a dietitian, beginning on day of presentation to Emergency Department, with monthly follow‐up over the phone for a 3‐month period. B) Usual care as per Community Hospital Interface Program (CHIP) i.e. nursing staff and community support. |
| Outcomes | Primary outcomes: weight assessed using bioelectrical impedance analysis (BIA) scales, nutritional status assessed via Subjective Global Assessment (SGA) and Patient Generated Subjective Global Assessment (PGSGA), protein and energy intake assessed using 24 h recall entered into the Food Works (Registered Trademark) software program. All primary outcomes will be assessed at baseline and again after 3 months. Secondary outcomes: quality of life assessed with EuroQol 5 dimension questionnaire, geriatric depression scale (GDS), alcohol use disorders identification test (AUDIT). These outcomes will be assessed at baseline and again after 3 months. Further secondary outcomes include: hospital and emergency department admissions, length of stay, incidence of pressure ulcers, and death, which will be assessed through Hospital Information Management Services over 3 months from date of randomisation. The Incidence of falls and fractures resulting from falls, as reported by patients, will be assessed for 6 months immediately prior to baseline, and from baseline for 3 months. |
| Starting date | |
| Contact information | |
| Notes | Location: Australia. Registration number: ACTRN12610000526077aa. Funding: Government funding body e.g. Australian Research Council. Further details required. Study suspended. |
NCT00228657.
| Trial name or title | The formation and severity of pressure ulcers associated with 4% albumin versus 0.9% sodium chloride administration (sub study of SAFE Protocol 153711). |
| Methods | RCT, double‐blind. |
| Participants | Setting: ICU, Australia. Participants: 1100 patients from the SAFE study, without existing pressure ulcers, 18 years or over, both genders. Inclusion criteria: patients in whom fluid resuscitation required for intravascular fluid depletion in addition to iv fluid required for nutrition, or to replace ongoing insensible losses, urinary losses, or ongoing losses from other sites (e.g. fistula losses from the gastrointestinal tract, urinary losses from diabetes insipidus, cerebral salt wasting syndrome, or the polyuric phase of acute renal failure), or to restore normonatraemia (normal sodium levels in the blood); ICU clinician considers that both interventions (4% human albumin solution or 0.9% sodium chloride) are equally appropriate and there is no specific indication, or contraindication, for either. Requirement for fluid resuscitation must be supported by at least one out of the seven defined clinical signs. Exclusion criteria: a known previous adverse reaction to human albumin solution; any known religious objection to the administration of human blood products; a requirement to receive plasmapheresis during this ICU admission; an admission to ICU following cardiac surgery, liver transplantation surgery, or for treatment of burns; brain death present or likely to be diagnosed within next 24 h of fluid resuscitation being required; moribund patients expected to die within next 24 h; patients previously enrolled and completed follow‐up in the SAFE study; if the patient has previously received fluid resuscitation that was prescribed within the study ICU and during this current ICU admission; if the patient has been transferred to the study ICU from a non‐study ICU and received a fluid bolus or fluid resuscitation for the treatment of volume depletion in that non‐study ICU. |
| Interventions | A) Intervention: intravascular fluid resuscitation with 4% albumin. B) Control: intravascular fluid resuscitation with 0.9% sodium chloride. |
| Outcomes | Incidence and severity of pressure injuries. |
| Starting date | July 2002. Anticipated end date: June 2003. |
| Contact information | Shena M Graham, The Alfred Hospital, Prahran, Melbourne, Australia. |
| Notes | Location: Australia. Registration number: NCT00228657. Sponsors and Collaborators: Bayside Health. Further details required. Awaiting email reply from author. |
NCT01090076.
| Trial name or title | |
| Methods | RCT, single‐blind. |
| Participants | Setting: Hospital, Singapore. Participants: estimated enrolment of 40 patients aged 21 years or more, both genders. Inclusion criteria: patients with non‐healing pressure ulcers grades 2, 3 or 4, admitted to Changi General Hospital for > 2 weeks, able to attend outpatient follow‐up appointments for dietary and wound review. Exclusion criteria: patients with poorly controlled diabetes mellitus (HbA1c >7.0%); with lower extremity ulcers with untreated peripheral vascular disease; deep tissue infection and/or requiring debridement of necrotic or sloughy tissue, or with severe sepsis; patients in Metabolic Intensive Care Unit (MICU) or Surgical Intensive Care Unit (SICU); those who are medically unstable, or receiving palliative care; patients on total parenteral nutrition, or who receive any other wound healing supplements (e.g. zinc, vitamin A and vitamin C); patients who require restriction of protein or fluid (< 1 L/d), or who cannot tolerate oral intake > 70% EER and/or fluid intake 30 ml/kg BW; patients unable to attend outpatient follow‐up appointments or unable to give consent (absence of next‐of‐kin). |
| Interventions | A) Dietary supplement of a specialised amino acid mixture, 2 sachets/d (each sachet provides additional 79 kcal, 7 g L‐arginine, 7 g L‐glutamine and 1.2 g beta‐hydroxy‐beta methyl butyrate). B) 2 sachets of placebo/d. |
| Outcomes | Primary outcome: wound size (percentage change in wound size, length, depth, area) and viable wound tissue (percentage change in proportion of viable wound tissue) assessed weekly up to week 4. |
| Starting date | |
| Contact information | |
| Notes | Location: Singapore. Registration number: NCT01090076a. Sponsors and collaborators: Changi General Hospital; Abbott. Further details required. Awaiting full‐text retrieval.. |
NCT01107197.
| Trial name or title | Nutritional support in malnourished pressure ulcer patients: the Oligoelement Sore Trial (OEST). |
| Methods | RCT, double‐blind. |
| Participants | Setting: home‐care or long‐term care. Participants: estimated enrolment of 310 patients, male and female, aged 18‐90 years. Inclusion: malnourished patients in home‐care or long‐term care, with Grade 2, 3 or 4 pressure ulcers, with reduced food intake, but able to drink supplement. Exclusion criteria: any organ failure, decompensated diabetes (HbA1C > 7%), current or previous neoplastic disease (< 1 year since last treatment CT or RT), connective tissue disease, infected wounds, anaemia (haemoglobin < 10 g/dL), respiratory insufficiency (COPD), cellulitis, sepsis or osteomyelitis, obesity, immunosuppressive therapy, use of steroids, poor tolerance to sip feeding. |
| Interventions | A) Intervention group: dietary supplement: oral formula enriched in arginine, zinc and antioxidant vitamins (Cubitan; NUTRICIA Italia). B) Control group: dietary supplement: control formula isonitrogenous isocaloric oral formula. |
| Outcomes | Primary outcome: rate of healing (8‐week follow‐up). Healing defined as complete healing, or reduction in ulcer area as continuous and categorical variable (> = 40%). Secondary outcomes: incidence of infections and reduction in costs of care (8‐week follow‐up), cost analyses are based on antibiotic therapy (days, dose and type of medication), number and type of dressing throughout the study, number of additional visits by healthcare professionals (outside those scheduled in the protocol). |
| Starting date | February 2010. End date: December 2012. |
| Contact information | Federico D'Andrea, MD; Azienda Ospedaliero Universitaria Maggiore della Carita. DIETOLOGIA@MAGGIOREOSP.NOVARA.IT. |
| Notes | Location: Italy. Registration number: NCT01107197. Sponsors and collaborators: Azienda Ospedaliero Universitaria Maggiore della Carita; NUTRICIA Italia. Further details required. Awaiting email reply from author. |
NCT01142570.
| Trial name or title | Effect of enteral nutrition enriched in protein and based on indirect calorimetry measurement in chronically critically ill patients. |
| Methods | RCT, double‐blind. |
| Participants | Setting: private home‐care or institutionalised long term care. Participants: estimated enrolment of 60 patients, aged 65‐90 years, both genders. Inclusion criteria: patients requiring mechanical ventilation (more than 21 days) by tracheostomy . Exclusion criteria: blood pH < 7.3 due to metabolic causes; patients with blood albumin level less than 2.2 g/dL. |
| Interventions | Part one: patients will receive caloric support as dictated by Hariss‐Benedict Formula (group 1), or by indirect calorimetry (group 2). Part 2: for patients not been weaned from ventilator 7 days after hospitalisation: 1a) Dietary supplement with a protein dose of 1.1‐1.5 g/kg body weight (calculated by the HARISS BENEDICT equation). 2a) Dietary supplement with a protein dose of 1.1 g/kg body weight (assessed with indirect calorimetry). 2b) Dietary supplement with a protein dose of 1.5 g/kg body weight (assessed with indirect calorimetry). |
| Outcomes | Primary outcomes: successful weaning from ventilation (spontaneous breathing), length of hospital stay in days, re‐admission to ICU, mortality, length of mechanical ventilation in h/day. Secondary outcomes: incidence of infectious diseases, development and progression of pressure ulcers, checked intake of daily insulin among different groups of patients (glucose control). |
| Starting date | September 2010. Anticipated end date: November 2012. |
| Contact information | Gregory Papirov, MD; Beit‐ Rivka Hospital, Petah‐Tikva, Israel. |
| Notes | Location: Israel. Registration number: NCT01142570. Sponsors and collaborators: Rabin Medical Center; Beit Rivka Hospital. Further details required. Awaiting email reply from author. |
Abbreviations
> = more than < = less than BW = body weight CT = chemotherapy COPD = chronic obstructive pulmonary disease d = day(s) EER = Existence energy rate h = hour(s) ICU = intensive care unit iv = intravascular RT = radiotherapy
Differences between protocol and review
None.
Contributions of authors
Gero Langer wrote the protocol, searched databases, conducted the handsearch, contacted experts and manufacturers, examined bibliographies, assessed the studies, entered the data, wrote the review, and worked on the final version of the update. Astrid Fink assessed the studies, entered the data and prepared a draft version of this update.
Contributions of editorial base:
Nicky Cullum: edited the review, advised on methodology, interpretation and review content. Approved the final review and review update prior to submission. Sally Bell‐Syer: coordinated the editorial process. Advised on methodology, interpretation and content. Edited and copy edited the review and the updated review. Ruth Foxlee: designed the search strategy, ran the searches and edited the search methods section for the update. Rachel Richardson: edited the update of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR/Department of Health (England). (Cochrane Wounds Group), UK.
Declarations of interest
None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Arias 2008 {published and unpublished data}
- Arias S, Bruzzone I, Blanco V, Inchausti M, Garcia F, Casavieja G, et al. Identification and early nutritional support in hospitalized malnourished patients [Reconomimiento y soporte nutricional precoz en pacientes hospitalizados desnutridos]. Nutrición Hospitalaria 2008;23(4):348‐53. [PubMed] [Google Scholar]
Benati 2001 {published data only}
- Benati G, Delvecchio S, Cilla D, Pedone V. Impact on pressure ulcer healing of an arginine‐enriched nutritional solution in patients with severe cognitive impairment. Archives of Gerontology and Geriatrics 2001 Jan;33(Suppl 1):43‐7. [DOI] [PubMed] [Google Scholar]
Bourdel‐M 2000 {published data only}
- Bourdel‐Marchasson I, Barateau M, Rondeau V, Dequae‐Merchadou L, Salles‐Montaudon N, Emeriau JP, et al. A multi‐center trial of the effects of oral nutritional supplementation in critically ill older inpatients. GAGE Group. Groupe Aquitain Geriatrique d'Evaluation. Nutrition 2000 Jan;16(1):1‐5. [DOI] [PubMed] [Google Scholar]
Brewer 1967 {published data only}
- Brewer RD, Mihaldzic N, Dietz A. The effect of oral zinc sulfate on the healing of decubitus ulcers in spinal cord injured patients. Proceedings of the Annual Clinical‐Spinal Cord Injury Conference; September 27‐29, 1967. 1967:70‐2. [PubMed]
Cereda 2009 {published data only}
- Cereda E, Gini A, Pedrolli C, Vanotti A. Disease‐specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: a randomized controlled trial. Journal of the American Geriatrics Society 2009;57(8):1395‐1402. [DOI] [PubMed] [Google Scholar]
- Cereda E, Gini A, Pusani C, Borghi B, Pedrolli C, Vanotti A. Disease‐specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: a randomized controlled trial. Clinical Nutrition 2008;3(Suppl. 1):19. [DOI] [PubMed] [Google Scholar]
Chernoff 1990 {published data only}
- Chernoff RS, Milton KY, Lipschitz DA. The effect of a very high‐protein liquid formula on decubitus ulcers healing in long‐term tube‐fed institutionalized patients. Journal of the American Dietetic Association 1990;90:A‐130. [Google Scholar]
Craig 1998 {published data only}
- Craig LD, Nicholson S, Silverstone FA, Kennedy RD. Use of a reduced‐carbohydrate, modified‐fat enteral formula for improving metabolic control and clinical outcomes in long‐term care residents with type 2 diabetes: results of a pilot trial. Nutrition 1998;14(6):529‐34. [DOI] [PubMed] [Google Scholar]
Delmi 1990 {published data only}
- Delmi M, Rapin CH, Bengoa JM, Delmas PD, Vasey H, Bonjour JP. Dietary supplementation in elderly patients with fractured neck of the femur. Lancet 1990 Apr 28;335(8696):1013‐6. [DOI] [PubMed] [Google Scholar]
Dennis 2005 {published data only}
- Dennis MS, Lewis SC, Warlow C. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): a multicentre randomised controlled trial. Lancet 2005;365(9461):755‐63. [DOI] [PubMed] [Google Scholar]
Derossi 2009 {published data only}
- Derossi D, Bo A, Bergonzi R, Scivoletto G. Six‐week administration of a mixture of ergogenic and osteotrophic ingredients (RestorfastTM) improve the clinical course of elderly patients after hip fracture surgery [La somministrazione per sei settimane di un composto a base die sostanze osteotrofiche ed ergogene (RestorfastTM) migliora il decorso clinio in anziani sottopasti a chirugia del femore]. Trends in Medicine 2009;9:235‐42. [Google Scholar]
Desneves 2005 {published data only}
- Desneves KJ, Todorovic BE, Cassar A, Crowe TC. Treatment with supplementary arginine, vitamin C and zinc in patients with pressure ulcers: a randomised controlled trial. Clinical Nutrition 2005;24(6):979‐87. [DOI] [PubMed] [Google Scholar]
Ek 1991 {published data only}
- Ek AC, Unosson M, Larsson J, Schenck H, Bjurulf P. The development and healing of pressure sores related to the nutritional state. Clinical Nutrition 1991;10(5):245‐50. [DOI] [PubMed] [Google Scholar]
- Larsson J, Unosson M, Ek AC, Nilsson L. Effect of dietary supplement on nutritional status and clinical outcome in 501 geriatric patients: a randomized study. Clinical Nutrition 1990;9(4):179‐84. [DOI] [PubMed] [Google Scholar]
Hartgrink 1998 {published data only}
- Hartgrink HH, Wille J, Konig P, Hermans J, Breslau PJ. Pressure sores and tube feeding in patients with a fracture of the hip: a randomized clinical trial. Clinical Nutrition 1998 Dec;17(6):287‐92. [DOI] [PubMed] [Google Scholar]
Houwing 2003 {published data only}
- Houwing R, Rozendaal M, Wouters‐Wesseling W, Beulens J, Buskens E, Haalboom J. The effect of nutritional supplementation on the prevention of pressure ulcers (PU) in hip‐fracture patients. Clinical Nutrition 2002;21(Suppl. 1):84. [DOI] [PubMed] [Google Scholar]
- Houwing R, Rozendaal M, Wouters‐Wesseling W, Beulens JWJ, Buskens E, Haalboom J. A randomised, double‐blind assessment of the effect of nutritional supplementation on the prevention of pressure ulcers in hip‐fracture patients. Clinical Nutrition 2003;22(4):401‐5. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee SK, Posthauer ME, Dorner B, Redovian V, Maloney MJ. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: a randomized controlled trial. Advances in Skin and Wound Care 2006;19(2):92‐6. [DOI] [PubMed] [Google Scholar]
Meaume 2009 {published data only}
- Meaume S, Kerihuel JC, Constans T, Teot L, Lerebours E, Kern J, et al. Efficacy and safety of ornithine alpha‐ketoglutarate in heel pressure ulcers in elderly patients: results of a randomized controlled trial. Journal of Nutrition, Health and Aging 2009;13(7):623‐30. [DOI] [PubMed] [Google Scholar]
Norris 1971 {published data only}
- Norris JR, Reynolds RE. The effect of oral zinc sulfate therapy on decubitus ulcers. Journal of the American Geriatric Society 1971;19:793‐7. [Google Scholar]
Ohura 2011 {published data only}
- Ohura T, Nakajo T, Okada S, Omura K, Adachi K. Evaluation of effects of nutrition intervention on healing of pressure ulcers and nutritional states (randomized controlled trial). Wound Repair and Regeneration 2011;19(3):330‐6. [DOI] [PubMed] [Google Scholar]
Olofsson 2007 {published and unpublished data}
- Olofsson B, Stenvall M, Lundstrom M, Svensson O, Gustafson Y. Malnutrition in hip fracture patients: an intervention study. Journal of Clinical Nursing 2007;16(11):2027‐38. [DOI] [PubMed] [Google Scholar]
Taylor 1974 {published data only}
- Taylor TV, Rimmer S, Day B, Butcher J, Dymock IW. Ascorbic acid supplementation in the treatment of pressure‐sores. Lancet 1974 Sep 7;2(7880):544‐6. [DOI] [PubMed] [Google Scholar]
ter Riet 1995 {published data only}
- ter Riet G, Kessels AG, Knipschild PG. Randomized clinical trial of ascorbic acid in the treatment of pressure ulcers. Journal of Clinical Epidemiology 1995 Dec;48(12):1453‐60. [DOI] [PubMed] [Google Scholar]
Theilla 2007 {published data only}
- Theilla M, Singer P, Cohen J, DeKeyser F. A diet enriched in eicosapentanoic acid, gamma‐linolenic acid and antioxidants in the prevention of new pressure ulcer formation in critically ill patients with acute lung injury: A randomized, prospective, controlled study. Clinical Nutrition 2007;26(6):752‐7. [DOI] [PubMed] [Google Scholar]
van Anholt 2010 {published and unpublished data}
- Schols JMGA. Specific nutritional support for pressure ulcer healing in non‐malnourished patients; a value based intervention. Results of the cube study. EWMA Journal 2011;11(suppl 2):79. [Google Scholar]
- Anholt RD, Sobotka L, Meijer EP, Heyman H, Groen HW, Topinková E, et al. Specific nutritional support accelerates pressure ulcer healing and reduces wound care intensity in non‐malnourished patients. Nutrition 2010;26(9):867‐72. [DOI] [PubMed] [Google Scholar]
- Anholt RD, Sobotka L, Meijer EP, Schols JM. An arginine‐ and micronutrient‐enriched nutritional supplement accelerates pressure ulcer healing and reduces wound care in non‐malnourished patients. EWMA Journal. 2010; Vol. 10:abstract 29.
References to studies excluded from this review
Barateau 1998 {published data only}
- Barateau M, Corompt A, Soulan J, Bourdel‐Marchasson I. Multicenter nursing study on the importance of nutritional support for the prevention of bedsores in the elderly at risk. Recherche en Soins Infirmiers 1998;Dec(55):42‐9. [PubMed] [Google Scholar]
Bergstrom 1987 {published data only}
- Bergstrom N, Braden B, Milne D. Are dietary and serum zinc and copper factors in the development of pressure sores in institutionalized elderly. Federation Proceedings 1987;46:902. [Google Scholar]
Bourdel‐M 1997 {published data only}
- Bourdel‐Marchasson I, Dumas F, Pinganaud G, Emeriau JP, Decamps A. of percutaneous endoscopic gastrostomy in long‐term enteral feeding in a nursing home. International Journal for Quality in Health Care 1997;9(4):297‐302. [DOI] [PubMed] [Google Scholar]
Breslow 1991 {published data only}
- Breslow RA, Hallfrisch J, Goldberg AP. Malnutrition in tubefed nursing home patients with pressure sores. Journal of Parenteral and Enteral Nutrition 1991 Nov‐Dec;15(6):663‐8. [DOI] [PubMed] [Google Scholar]
Breslow 1993 {published data only}
- Breslow RA, Hallfrisch J, Guy DG, Crawley B, Goldberg AP. The importance of dietary protein in healing pressure ulcers. Journal of the American Geriatrics Society 1993;41(4):357‐62. [DOI] [PubMed] [Google Scholar]
Eneroth 2006 {published data only}
- Eneroth M, Olsson UB, Thorngren KG. Nutritional supplementation decreases hip fracture‐related complications. Clinical Orthopaedics and related Research 2006;451:212‐7. [DOI] [PubMed] [Google Scholar]
Langemo 2006 {published data only}
- Langemo D, Anderson J, Hanson D, Hunter S, Thompson P, Posthauer ME. Nutritional considerations in wound care. Advances in Skin and Wound Care 2006;19(6):297‐8, 300, 303. [DOI] [PubMed] [Google Scholar]
Langkamp‐Henken 2000 {published data only}
- Langkamp‐Henken B, Herrlinger‐Garcia KA, Stechmiller JK, Nickerson‐Troy JA, Lewis B, Moffatt L. Arginine supplementation is well tolerated but does not enhance mitogen‐induced lymphocyte proliferation in elderly nursing home residents with pressure ulcers. Journal of Parenteral and Enteral Nutrition 2000 Sep‐Oct;24(5):280‐7. [DOI] [PubMed] [Google Scholar]
Larsson 1990 {published data only}
- Larsson J, Unosson M, Ek AC, Nilsson L. Effect of dietary supplement on nutritional status and clinical outcome in 501 geriatric patients: a randomized study. Clinical Nutrition 1990;9(4):179‐84. [DOI] [PubMed] [Google Scholar]
Myers 1990 {published data only}
- Myers SA, Takiguchi S, Slavish S, Rose CL. Consistent wound care and nutritional support in treatment. Decubitus 1990 Aug;3(3):16‐28. [PubMed] [Google Scholar]
NCT00502372 {published and unpublished data}
- NCT00502372. Effect of an oral supplement enriched in amino acids and the leucine metabolite B‐hydroxyB‐methylbutyrate (HMB). http://clinicaltrials.gov/show/NCT00502372 (accessed 14 November 2013).
Neander 2004 {published data only}
- Neander K, Hesse F, Wagenaar L, Wouters‐Wesseling W. A specific nutritional supplement reduces the incidence of pressure ulcers in elderly people. Journal of Parenteral and Enteral Nutrition 2004;28(1):S31‐2. [Google Scholar]
Schröder‐van den N 2004 {published data only}
- Schröder‐van den Nieuwendijk DL, Jager‐van den Ende MA. The effect of a pressure ulcer specific ‐ versus a protein enriched ‐ sip feed on pressure ulcer healing. 2nd World Union of Wound Healing Societies Meeting; 2004 ,8‐13 July; Paris. 2004:159.
Settel 1969 {published data only}
- Settel E. Decubitus ulcer. A double blind study of an old problem and a new treatment. Medical Times 1969;97(4):220‐30. [PubMed] [Google Scholar]
Stotts 2009 {published data only}
- Stotts NA, Hopf HW, Kayser‐Jones J, Chertow GM, Cooper BA, Wu H‐S. Increased fluid intake does not augment capacity to lay down new collagen in nursing home residents at risk for pressure ulcers: A randomized, controlled clinical trial. Wound Repair and Regeneration 2009;17(6):780‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Candela‐Zamora 2010 {published data only}
- Candela‐Zamora MD, Martín‐Gómez MA, Solas‐Gómez B, Fernández‐Pérez C, Martín‐González M, Manzanedo‐Basilio L, et al. Comparative study of the effectiveness two of hyperoxygenated fatty acids in the treatment of grade I ulcers in geriatric hospitalized patients [Estudio comparativo de efectividad de dos ácidos grasos hiperoxigenados en el tratamiento de úlceras de grado I en pacientes geriátricos hospitalizados]. Enfermería Clínica 2010;20(1):10‐6. [DOI] [PubMed] [Google Scholar]
Caulfield 2012 {published data only}
- Caulfield C. Examining the benefits of an oral nutritional supplement (ONS) with regard to its safety, tolerance and clinical efficacy in improving nutritional status of undernourished, hospitalised patients. Clinical Nutrition 2012;7(1):280. [Google Scholar]
Chen 2004 {published data only}
- Chen Q. Effect of egg albumen combined with infrared ray irradiation to treat patients with bedsore induced by fecal incontinence. Chinese Nursing Research 2004;17(9a):1550‐1. [Google Scholar]
Leigh 2012 {published data only}
- Leigh B, Desneves K, Rafferty J, Pearce L, King S, Woodward MC, et al. The effect of different doses of an arginine‐containing supplement on the healing of pressure ulcers. Journal of Wound Care 2012;21(3):150‐6. [DOI] [PubMed] [Google Scholar]
Starke 2011 {published data only}
- Starke J, Schneider H, Alteheld B, Stehle P, Meier R. Short‐term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clinical Nutrition 2011;30(2):194‐201. [DOI] [PubMed] [Google Scholar]
Sugihara 2012 {published data only}
- Sugihara F, Koizumi S, Inoue N. A randomized controlled trial to determine the effectiveness, safety and tolerability of collagen hydrolysate as add‐on nutritional supplement in management of subjects with pressure ulcer. 4th Congress of the World Union of Wound Healing Societies; September 2‐6; Yokohama, Japan. Yokohama, Japan, 2012:256.
Theilla 2012a {published data only}
- Theilla M, Schwartz B, Cohen J, Shapiro H, Anbar R, Singer P. Impact of a Nutritional Formula Enriched in Fish Oil and Micronutrients on Pressure Ulcers in Critical Care Patients. American Journal of Critical Care 2012;21(4):e102‐9. [DOI] [PubMed] [Google Scholar]
Theilla 2012b {published data only}
- Theilla M, Schwartz B, Zimra Y, Shapiro H, Anbar R, Rabizadeh E, et al. Enteral n‐3 fatty acids and micronutrients enhance percentage of positive neutrophil and lymphocyte adhesion molecules: a potential mediator of pressure ulcer healing in critically ill patients. British Journal of Nutrition 2012;107(7):1056‐61. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12605000704695 {published data only}
- ACTRN12605000704695. The effects of early parenteral nutrition compared to standard care on 60 day landmark mortality in the critically ill patient: A level I randomised controlled trial. http://www.anzctr.org.au/trial_view.aspx?ID=863 (accessed 4 May 2012).
ACTRN12610000526077 {published data only}
- ACTRN12610000526077. In older patients presenting to the Emergency Department, do those who receive a nutrition intervention achieve a better nutritional status than those who receive standard care only? A comparison of two service models. http://www.anzctr.org.au/trial_view.aspx?id=335631 (accessed 4 May 2012).
NCT00228657 {published data only}
- NCT00228657. The formation and severity of pressure ulcers associated with 4% albumin vs. 0.9% sodium chloride administration (substudy of SAFE protocol 153711). http://clinicaltrials.gov/ct2/show/NCT00228657 (accessed 10 Dec 2013).
NCT01090076 {published data only}
- NCT01090076. The use of specialised amino acid mixture in pressure ulcer wound healing rates ‐ A placebo controlled trial. http://clinicaltrials.gov/ct2/show/NCT01090076 (accessed 4 May 2012).
NCT01107197 {published data only}
- NCT01107197. Nutritional support in malnourished pressure ulcer patients: the oligoelement sore trial (OEST). http://clinicaltrials.gov/ct2/show/NCT01107197 (accessed 10 Dec 2013).
NCT01142570 {published data only}
- NCT01142570. Effect of enteral nutrition enriched in protein and based on indirect calorimetry measurement in chronically critically ill patients. http://clinicaltrials.gov/ct2/show/NCT01142570 (accessed 10 Dec 2013).
Additional references
Bates‐Jensen 1992
- Bates‐Jensen BM, Vredevoe DL, Brecht ML. Validity and reliability of the Pressure Sore Status Tool. Decubitus 1992;5(6):20‐8. [PubMed] [Google Scholar]
Bergstrom 1992
- Bergstrom N, Braden MJ, Laguzza A, Holman V. A prospective study of pressure sore risk among institutionalized elderly. Journal of the American Geriatrics Society 1992;40(8):747‐58. [DOI] [PubMed] [Google Scholar]
Berlowitz 1989
- Berlowitz DR, Wilking SV. Risk factors for pressure sores. A comparison of cross‐sectional and cohort‐derived data. Journal of the American Geriatrics Society 1989;37(11):1043‐50. [DOI] [PubMed] [Google Scholar]
Braden 1994
- Braden BJ, Bergstrom N. Predictive validity of the Braden scale for pressure sore risk in a nursing home population. Research Nursing Health 1994;17(6):459‐70. [DOI] [PubMed] [Google Scholar]
Breslow 1991a
- Breslow R. Nutritional status and dietary intake of patients with pressure ulcers: review of research literature 1943 to 1989. Decubitus 1991;4(1):16‐21. [PubMed] [Google Scholar]
Dassen 2001
- Dassen T. Dekubitus Sturzereignisse Pflegeabhängigkeit ‐ Prävalenzerhebung 2001. Berlin: Zentrum für Human‐ und Gesundheitswissenschaften der Berliner Hochschulmedizin, Institut für Medizin‐/Pflegepädagogik und Pflegewissenschaft, 2001. [Google Scholar]
EPUAP and NPUAP 2009a
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: quick reference guide. http://www.epuap.org/guidelines/Final_Quick_Prevention.pdf. Washington DC: National Pressure Ulcer Advisory Panel, 2009.
EPUAP and NPUAP 2009b
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Treatment of pressure ulcers: Quick Reference Guide. http://www.epuap.org/guidelines/Final_Quick_Treatment.pdf. Washington DC: National Pressure Ulcer Advisory Panel, 2009.
Finucane 1995
- Finucane TE. Malnutrition, tube feeding and pressure sores: data are incomplete. Journal of the American Geriatrics Society 1995;43(4):447‐51. [DOI] [PubMed] [Google Scholar]
Gosnell 1989
- Gosnell DJ. Gosnell pressure sore risk assessment instrument revision. Journal of Enterostomal Therapy 1989;16(6):272. [DOI] [PubMed] [Google Scholar]
Günes 2009
- Günes UY. A prospective study evaluating the Pressure Ulcer Scale for Healing (PUSH Tool) to assess stage II, stage III, and stage IV pressure ulcers. Ostomy/Wound Management 2009;55(5):48‐52. [PubMed] [Google Scholar]
Hendrichova 2010
- Hendrichova I, Castelli M, Mastroianni C, Mirabella F, Surdo L, Marinis MG, et al. Pressure ulcers in cancer palliative care patients. Palliative Medicine 2010;24(7):669‐73. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lahmann 2010
- Lahmann NA, Dassen T, Poehler A, Kottner J. Pressure ulcer prevalence rates from 2002 to 2008 in German long‐term care facilities. Aging Clinical and Experimental Research 2010;22(2):152‐6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mueller 2001
- Mueller SD, Hoerist K, Bahnsen B. Prophylaxe und Therapie des Dekubitalleidens ‐ Bedeutung der Ernaehrungsmedizin. http://www.diet‐aachen.de 2001.
Perneger 1998
- Perneger TV, Heliot C, Rae AC, Borst F, Gaspoz JM. Hospital‐acquired pressure ulcers: risk factors and use of preventive devices. Archives of Internal Medicine 1998;158(17):1940‐5. [DOI] [PubMed] [Google Scholar]
Perneger 2002
- Perneger TV, Rae AC, Gaspoz JM, Borst F, Vitek O, Heliot C. Screening for pressure ulcer risk in an acute care hospital: development of a brief bedside scale. Journal of Clinical Epidemiology 2002;55(5):498‐505. [DOI] [PubMed] [Google Scholar]
Schoonhoven 2002
- Schoonhoven l, Haalboom JRE, Bousema MT, Algra A, Grobbee DE, Grypdonck MH, et al. Prospective cohort study of routine use of risk assessment scales for prediction of pressure ulcers. BMJ 2002;325:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoonhoven 2006
- Schoonhoven L, Grobbee DE, Donders ART, Algra A, Grypdonck HM, Bousema MT, et al. Prediction of pressure ulcer development in hospitalized patients: a tool for risk assessment. Quality and Safety in Health Care 2006;15(1):65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2009
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 28 July 2009).
Strauss 1996
- Strauss EA, Margolis DJ. Malnutrition in patients with pressure ulcers: morbidity, mortality, and clinically practical assessments. Advances in Wound Care 1996;9(5):37‐40. [PubMed] [Google Scholar]
Thomas 1997
- Thomas DR, Rodeheaver GT, Bartolucci AA, Franz RA, Sussman C, Ferrell BA, et al. Pressure ulcer scale for healing: derivation and validation of the PUSH tool. The PUSH Task Force. Advances in Wound Care 1997;10(5):96‐101. [PubMed] [Google Scholar]
Thomson 1999
- Thomson JS, Brooks RG. The economics of preventing and treating pressure ulcers: a pilot study. Journal of Wound Care 1999;8(6):312‐6. [DOI] [PubMed] [Google Scholar]
VanGilder 2009
- VanGilder A, Amlung S, Harrison P, Meyer S. Results of the 2008–2009 International Pressure Ulcer Prevalence Survey and a 3‐year, acute care, unit‐specific analysis. Ostomy/Wound Management 2009;55(11):39‐45. [PubMed] [Google Scholar]
References to other published versions of this review
Langer 2003
- Langer G, Schloemer G, Knerr A, Kuss O, Behrens J. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003216] [DOI] [PubMed] [Google Scholar]


