Abstract
Background
The accessibility of health services is an important factor that affects the health outcomes of populations. A mobile clinic provides a wide range of services but in most countries the main focus is on health services for women and children. It is anticipated that improvement of the accessibility of health services via mobile clinics will improve women's and children's health.
Objectives
To evaluate the impact of mobile clinic services on women's and children's health.
Search methods
For related systematic reviews, we searched the Database of Abstracts of Reviews of Effectiveness (DARE), CRD; Health Technology Assessment Database (HTA), CRD; NHS Economic Evaluation Database (NHS EED), CRD (searched 20 February 2014).
For primary studies, we searched ISI Web of Science, for studies that have cited the included studies in this review (searched 18 January 2016); WHO ICTRP, and ClinicalTrials.gov (searched 23 May 2016); Cochrane Central Register of Controlled Trials (CENTRAL), part of The Cochrane Library.www.cochranelibrary.com (including the Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register) (searched 7 April 2015); MEDLINE, OvidSP (searched 7 April 2015); Embase, OvidSP (searched 7 April 2015); CINAHL, EbscoHost (searched 7 April 2015); Global Health, OvidSP (searched 8 April 2015); POPLINE, K4Health (searched 8 April 2015); Science Citation Index and Social Sciences Citation Index, ISI Web of Science (searched 8 April 2015); Global Health Library, WHO (searched 8 April 2015); PAHO, VHL (searched 8 April 2015); WHOLIS, WHO (searched 8 April 2015); LILACS, VHL (searched 9 April 2015).
Selection criteria
We included individual‐ and cluster‐randomised controlled trials (RCTs) and non‐RCTs. We included controlled before‐and‐after (CBA) studies provided they had at least two intervention sites and two control sites. Also, we included interrupted time series (ITS) studies if there was a clearly defined point in time when the intervention occurred and at least three data points before and three after the intervention. We defined the intervention of a mobile clinic as a clinic vehicle with a healthcare provider (with or without a nurse) and a driver that visited areas on a regular basis. The participants were women (18 years or older) and children (under the age of 18 years) in low‐, middle‐, and high‐income countries.
Data collection and analysis
Two review authors independently screened the titles and abstracts of studies identified by the search strategy, extracted data from the included studies using a specially‐designed data extraction form based on the Cochrane EPOC Group data collection checklist, and assessed full‐text articles for eligibility. All authors performed analyses, 'Risk of bias' assessments, and assessed the quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
Two cluster‐RCTs met the inclusion criteria of this review. Both studies were conducted in the USA.
One study tested whether offering onsite mobile mammography combined with health education was more effective at increasing breast cancer screening rates than offering health education only, including reminders to attend a static clinic for mammography. Women in the group offered mobile mammography and health education may be more likely to undergo mammography within three months of the intervention than those in the comparison group (55% versus 40%; odds ratio (OR) 1.83, 95% CI 1.22 to 2.74; low certainty evidence).
A cost‐effectiveness analysis of mammography at mobile versus static units found that the total cost per patient screened may be higher for mobile units than for static units. The incremental costs per patient screened for a mobile over a stationary unit were USD 61 and USD 45 for a mobile full digital unit and a mobile film unit respectively.
The second study compared asthma outcomes for children aged two to six years who received asthma care from a mobile asthma clinic and children who received standard asthma care from the usual (static) primary provider. Children who receive asthma care from a mobile asthma clinic may experience little or no difference in symptom‐free days, urgent care use and caregiver‐reported medication use compared to children who receive care from their usual primary care provider. All of the evidence was of low certainty.
Authors' conclusions
The paucity of evidence and the restricted range of contexts from which evidence is available make it difficult to draw conclusions on the impacts of mobile clinics on women's and children's health compared to static clinics. Further rigorous studies are needed in low‐, middle‐, and high‐income countries to evaluate the impacts of mobile clinics on women's and children's health.
Keywords: Aged; Aged, 80 and over; Child; Child, Preschool; Female; Humans; Middle Aged; ; Asthma; Asthma/therapy; Child Health Services; Child Health Services/economics; Child Health Services/statistics & numerical data; Cost-Benefit Analysis; Mammography; Mammography/statistics & numerical data; Maternal Health Services; Maternal Health Services/economics; Maternal Health Services/statistics & numerical data; Mobile Health Units; Mobile Health Units/economics; Mobile Health Units/statistics & numerical data; Randomized Controlled Trials as Topic; United States
Plain language summary
Mobile clinics for women's and children's health
What is the aim of this review?
The aim of this Cochrane Review was to assess the effects of mobile clinics on women’s and children’s health. Cochrane researchers searched for and analysed all relevant studies to answer this question.
Key messages
The review only included two studies. One included study showed that mobile clinics may increase the number of women who use mammography services, although the cost of mammography screening may be higher. The other study showed that mobile clinics may make little or no difference to children’s asthma symptoms, their use of medication and urgent care, or their caregivers’ quality of life. More studies are needed, including studies that measure the effect of mobile clinics on cost and on people’s access to healthcare, their satisfaction, health, and well‐being.
What was studied in the review?
In many settings, people have poor access to healthcare services because they live in remote or hard‐to‐reach areas. Women and children may find it very difficult to access health services because of financial or social circumstances.
One way to increase people’s access to healthcare services is by providing mobile clinics. A mobile clinic is a vehicle with a driver and clinical equipment, and is staffed by a healthcare provider, such as a doctor or nurse, that visits areas regularly to provide health services.
Mobile clinics are used in many countries and are often used to offer health services to women and children, such as antenatal care, childhood immunisation, family planning services, and breast cancer screening.
By taking health services to the community through mobile clinics, governments hope to increase the use of these services and improve people’s health. This Cochrane review aimed to explore the effect of mobile clinics on people’s access to and use of health care and on their satisfaction, health, and well‐being; as well as their cost and cost effectiveness, compared to permanent clinics.
What are the main results of the review?
The review authors included two studies, which were from the USA.
In the first study, women were either offered health education and mammography screening in mobile clinics or health education only including reminders to attend a permanent clinic that offered mammography screening. The study showed that:
· women offered mammography in mobile clinics may be more likely to undergo mammography (low certainty evidence);
· the cost of screening per woman may be higher for mobile clinics than for permanent clinics (low certainty evidence).
This study did not assess the effect of the mobile clinics on women’s health and well‐being, their access to services or their satisfaction with these services.
In the second study, children were offered asthma care either at mobile clinics or at their usual primary care provider. This study showed that mobile clinics:
· may make little or no difference to the children’s asthma symptom‐free days or their use of urgent care and medication (low certainty evidence);
· may make little or no difference to the quality of life of the children’s caregivers (low certainty evidence).
The study did not assess the effect of the mobile clinics on children’s access to services or their satisfaction with these services, or on the cost and cost‐effectiveness of using the mobile clinics.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to April 2015.
Summary of findings
Summary of findings for the main comparison. Mobile clinics versus static clinics for mammography screening.
| Immediate onsite mobile mammography with health education versus health education only (including reminders to attend a static clinic service for mammography) | |||||
| Participant or population: women aged 60 to 84 years at study entry. (Age of participants matches our selection criteria) Settings: High income country; USA (California) Intervention: mobile clinic ‐ women were offered immediate onsite mobile mammography with health education Comparison: static clinic ‐ women received health education only and were encouraged to have a mammogram at the usual static clinic | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Static clinic | Mobile clinic | ||||
| Health status and well‐being | The study did not assess this outcome | ||||
| Health behaviour: self‐reported mammography uptake (follow‐up: 3 months) | Study population | OR 1.83 (1.22 to 2.74) | 473 (1)1 | ⊕⊕⊖⊖ Low 2 |
|
| 382 per 1000 | 531 per 1000 | ||||
| Utilisation, coverage or access | The study did not assess this outcome | ||||
| Resource use: total cost per patient receiving mammography screening | The total cost per patient screened may be higher for mobile units than for stationary units (USD 41 for a stationary full digital screening unit; USD 102 for a mobile full digital screening unit; and USD 86 for mobile film screening unit). This evidence was of low certainty (⊕⊕⊖⊖)2 | ||||
| Satisfaction with care among healthcare recipients | The study did not assess this outcome | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; OR: odds ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation. | |||||
| GRADE Working Group grades of evidence High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different3 is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different3 is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different3 is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different3 is very high. | |||||
1Naeim 2009. 2We downgraded the quality of the evidence by 2 levels as we judged the study to be at moderate risk of bias and there was some imprecision around the estimate. 3Substantially different: a large enough difference that it might affect a decision
Summary of findings 2. Mobile clinics versus standard static services for childhood asthma care.
| Mobile clinics compared with standard 'static' services for childhood asthma care | |||
|
Paticipannt or population: children aged 2 to 6 years with persistent asthma and their caregivers. (Age of participants matches our selection criteria) Settings: High income country; USA (Baltimore) Intervention: mobile asthma clinic delivering screening, evaluation, and treatment services Comparison: static services comprising standard asthma care from the usual primary care provider | |||
| Outcomes | Impact | No of participants (studies) | Certainty of the evidence (GRADE) |
| Health status and well‐being (follow‐up: 12 months) |
Children who receive asthma care from a mobile asthma clinic may experience little or no difference in symptom‐free days and urgent care use, compared to children who receive standard asthma care. There may be little or no difference in quality of life among caregivers of children who receive asthma care from a mobile asthma clinic, compared to caregivers of children who receive standard asthma care. |
322 (1)1 |
Low 2 ⊕⊕⊖⊖ |
| Health behaviour (follow‐up: 12 months) |
There may be little or no difference in caregiver‐reported medication use among children who receive asthma care from a mobile asthma clinic compared to children who receive standard asthma care. | 322 (1)1 |
Low 2 ⊕⊕⊖⊖ |
| Utilisation, coverage or access | The study did not assess this outcome | ||
| Resource use | The study did not assess this outcome | ||
| Satisfaction with care among health care recipients | The study did not assess this outcome | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; OR: odds ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation. | |||
| GRADE Working Group grades of evidence High certainty: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different3 is low. Moderate certainty: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different3 is moderate. Low certainty: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different3 is high. Very low certainty: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different3 is very high. | |||
1Eakin 2012. 2We downgraded by two levels because we judged the study as at moderate risk of bias and due to imprecision. 3Substantially different: a large enough difference that it might affect a decision.
Background
Description of the condition
The accessibility of health services is an important factor that affects the health outcomes of populations. Several approaches have been employed to increase geographical healthcare coverage and utilisation, including building new facilities and use of outreach clinical services.
However, in many settings the building of new health centres and other facilities is not always feasible and does not necessarily result in more people receiving better services. For example, initiatives by governments, non‐governmental organisations (NGOs), and community self‐help schemes have often led to an increase in the building of health facilities. However, a study in Zimbabwe concluded that in some circumstances it was more economical to bring health staff to patients than vice versa, and that choosing geographically more optimal sites (i.e. further away from static clinics) would increase the cost‐effectiveness of outreach clinics (Vos 1990). Community‐based distribution programmes and mobile clinics are the most common outreach clinical services globally. Mobile clinics are used in a wide range of low‐ and middle‐income countries, e.g. in Nigeria (Onyia 1981), Thailand (Sriamporn 2006), Indonesia (Molyneaux 1988), and Egypt (El‐Zanaty 2001).
Description of the intervention
Mobile clinics have been widely used worldwide for many years. These clinics provide a wide range of services but their main focus in most countries is on health services for women and children. A mobile clinic is a clinic vehicle with a healthcare provider, such as a doctor or nurse, and a driver that visits areas on a regular basis (e.g. weekly, biweekly, or monthly) to provide health services.
Such services can enhance accessibility by providing services to underserved populations; they may therefore increase the potential to reach women and children who are underserved by static health services (ACCP 2004). For example, expanding access to contraceptives through mobile clinics can contribute to improving health by helping to reduce rates of unintended pregnancy and its associated morbidity and mortality (Welsh 2006).
In the USA, mobile clinics are used for screening for sexually transmitted diseases (STDs), human immunodeficiency virus (HIV) counselling (Ellen 2003), prenatal care (Edgerley 2007), and breast cancer screening (Skinner 1995). In Nigeria, mobile under‐fives clinics (clinics for children under five years old) provide health education, immunisation, malarial chemoprophylaxis, and regular weight monitoring (Onyia 1981). In Egypt, it was planned that mobile clinics should offer family planning services, antenatal care services, and counselling, examination, and investigation for some diseases, e.g. hepatitis B, HIV/autoimmune deficiency syndrome (AIDS), and health care for menopausal women. However, the main services offered by mobile clinics for the last 10 years have been restricted to family planning, antenatal care, and, occasionally, immunisation (El‐Zanaty 2001).
How the intervention might work
Mobile clinics could tackle some barriers of access proposed by various frameworks especially availability, geographic accessibility (distance between service location and households), and utilisation of health services (Jacobs 2012). It is anticipated that improvement of access would have a positive impact on the health of specific groups. For example, increased accessibility to family planning services for women may increase the use of effective contraceptive methods and reduce the number of unwanted pregnancies and illegal abortions. Similarly, increased accessibility to antenatal care may result in earlier diagnosis and referral of high‐risk pregnancies and better care for women with normal pregnancies, and so decrease the risk of having low birthweight babies (O'Connell 2010). Mobile clinics also could play a vital role in increasing utilisation of cervical cancer screening and raising awareness among women about importance of early detection of cervical cancer (Swaddiwudhipong 1995). For children, increased vaccination coverage and regular nutritional assessment may result in decreased infant and child morbidity and mortality in the long term.
Why it is important to do this review
Mobile clinics play an important role in offering services to vulnerable groups (i.e. women and children) living in remote or difficult‐to‐access areas where their access to health services is poor. Previous studies have suggested that mobile clinics may have the potential to increase access to antenatal care (Edgerley 2007; O'Connell 2010), immunisation, malaria chemoprophylaxis, and regular monitoring of weight (Onyia 1981), and may also have the potential to increase utilisation of breast cancer screening (Skinner 1995), cervical cancer screening (Swaddiwudhipong 1995), and screening for STDs and HIV counselling (Ellen 2003).
However, despite the wide use of mobile clinics across a range of settings, there is ongoing debate regarding their use compared to static clinics (Mercer 2005). For example, in Thailand mobile services were more heavily utilised than static services, which may be because these mobile services were more accessible and acceptable to women (ACCP 2004). In contrast, in rural areas of other countries, such as Bangladesh (Mercer 2005), and Egypt (El‐Gibaly 2008), static clinics were preferred by women to satellite clinics. In Bangladesh, women who were dissatisfied with the mobile clinics reported lack of medicines and unavailability of some services. In Egypt, static clinics were preferred because women perceived that the quality of family planning services offered by the static clinics is better than that offered by mobile clinics. In addition, continuity of care offered in the static clinics and availability of health provider on a daily basis were other causes for static clinic preferences (El‐Gibaly 2008). In Egypt, a national strategic plan has been adopted for the period 2007 to 2017 and includes provision of fee‐waiver family planning/reproductive health services using mobile clinics. The intention of this plan is to make these services accessible in areas not served by static clinics (El‐Zanaty 2001). Women and children are vulnerable and high risk groups. They may, in some low‐ and middle‐income countries, have limited mobility and therefore have difficulties accessing health services. Bringing the service to them may therefore have a greater impact on their health than on the health of other population groups, such as men, in the same countries.
In addition, the inclusion of economic evaluation has become an increasingly accepted component of health policy and planning. A number of country experiences have shown that information on the cost‐effectiveness of health interventions can be used alongside other types of information to inform different policy decisions (Hutubessy 2003). Also, information about cost‐effectiveness can guide policy makers to make the right judgements and address inequities based on the evidence presented from cost‐effectiveness studies about the pros and cons of the different policies and programmes (Oxman 2009). This is of particular importance in countries where health care demand exceeds supply and where resources for healthcare provision are very limited. For example, in Egypt the cost per client at a mobile clinic is lower than that at static clinics. However, reproductive health services offered by mobile clinics were used by only 6% of the target communities (women living in remote rural areas), which may reduce their cost‐effectiveness (El‐Gibaly 2008).
Objectives
To evaluate the impact of mobile clinic services on women's and children's health.
Methods
Criteria for considering studies for this review
Types of studies
We included the following study designs:
individual‐ and cluster‐randomised controlled trials (RCTs);
individual and cluster non‐randomised studies;
controlled before‐and‐after (CBA) studies that included at least two intervention sites and two control sites;
interrupted time series (ITS) studies with a clearly defined point in time; when the intervention occurred and at least three data points before and three after the intervention.
Types of participants
We included women (defined as aged 18 years or more) and children (from birth until the age of 18 years) in low‐, middle‐, and high‐income countries.
Types of interventions
A mobile clinic is a clinic vehicle (with a healthcare provider) that visits areas on a regular basis (e.g. weekly, every two weeks , or monthly) to provide health services. We assessed mobile clinics for their effectiveness in providing services to women and children. These services include promotive, preventive, and curative care for women and children, and might involve antenatal care, postnatal care, screening for sexually transmitted diseases (STDs), screening for breast and cervical cancer, screening for osteoporosis, human immunodeficiency virus (HIV) counselling, family planning, vaccination of children, and regular weight monitoring of children.
We compared:
mobile clinic services versus no services;
mobile clinic services versus static clinics;
combined mobile and static clinic services versus static clinics.
We excluded mobile clinics that provided services exclusively to men over the age of 18 years (since the interventions and outcomes of interest were related only to women and to children under the age of 18 years) and mobile clinics used in emergencies (such as in earthquakes). We didn't include outreach workers that used other means of transportation (e.g. bikes) to deliver e.g. family planning methods. This was because a mobile clinic is able to offer a different, and generally wider, range of services in a different way, compared to using a bike to reach a particular areas to deliver care. For example, the structure of a mobile clinic is often similar to that of a static clinic, and the mobile facility is usually staffed by a health professional trained to deliver a range of curative, promotive, and preventive services and with access in the mobile clinic to the equipment needed to undertake these activities (e.g. an ultrasound machine, instruments for examination, etc). In contrast, outreach workers who use other forms of transportation generally do not have access to this range of equipment and supplies. Also, we excluded a temporary clinic structure that was not mobile, but whose health workers only visited once a week (for example) to provide services to a particular community. Moreover, other types of outreach services are dealt with in other reviews, e.g. the Gruen 2003 review on specialist outreach clinics in primary care and rural hospital settings.
Types of outcome measures
Primary outcomes
-
Health outcomes: participant outcomes were:
health status and well‐being, including number of cases diagnosed, treated, or referred for treatment, including cases of STDs, high‐risk pregnancies, malnutrition among children and infectious diseases among children;
health behaviour: any measure of the extent to which service users adhered to recommended care plans. For example, for women, whether they attended for the recommended number of antenatal visits, and for children, whether they completed the vaccination schedule at the designated time;
-
utilisation, coverage, or access: we used coverage and service utilisation as proxies for the impact of health services on the health of women and children. Measures included:
contraceptive prevalence;
vaccination coverage among children;
use of other services provided by mobile clinics (e.g. osteoporosis screening, antenatal care, postnatal care, screening for STDs, screening for breast and cervical cancer, HIV counselling, regular weight monitoring of children etc.);
-
resource use, including:
cost‐effectiveness of services offered by the mobile clinics in comparison to static clinics.
Secondary outcomes
Satisfaction (assessed in any way) of healthcare recipients with care.
Search methods for identification of studies
Electronic searches
We searched the following databases for related systematic reviews:
Database of Abstracts of Reviews of Effectiveness (DARE), Centre for Reviews and Dissemination (CRD) (searched 20 February 2014)
Health Technology Assessment Database (HTA), Centre for Reviews and Dissemination (CRD) (searched 20 February 2014)
NHS Economic Evaluation Database (NHS EED), Centre for Reviews and Dissemination (CRD) (searched 20 February 2014)
We searched the following databases, with no language or date restrictions, for primary studies:
Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 3, part of The Cochrane Library.www.cochranelibrary.com (including the Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register) (searched 7 April 2015)
MEDLINE In‐Process & Other Non‐Indexed Citations, MEDLINE Daily, MEDLINE and OLDMEDLINE 1946 to Present, OvidSP (searched 7 April 2015)
Embase 1980 to 2015 Week 14, OvidSP (searched 7 April 2015)
CINAHL 1980 to present, EbscoHost (searched 7 April 2015)
Global Health 1973 to 2015 Week 13, OvidSP (searched 8 April 2015)
POPLINE, K4Health (searched 8 April 2015)
Science Citation Index and Social Sciences Citation Index 1975 to present, ISI Web of Science (searched 8 April 2015)
Global Health Library (GHL), Regional Indexes, Word Health Organization (WHO) (searched 8 April 2015)
Pan American Health Organization database (PAHO), Virtual Health Library (VHL) (searched 8 April 2015)
World Health Organization Library Information System (WHOLIS), WHO (searched 8 April 2015)
Latin American and Caribbean Health Sciences database (LILACS), Virtual Health Library (VHL) (searched 9 April 2015)
Searching other resources
Trial registries
International Clinical Trials Registry Platform (ICTRP), Word Health Organization (WHO): www.who.int/ictrp/en (searched 23 May 2016)
ClinicalTrials.gov, US National Institutes of Health (NIH): clinicaltrials.gov (searched 23 May 2016)
Other sources
We also:
reviewed the reference lists of relevant systematic reviews and studies
conducted a cited reference search for all included studies using Science Citation Index and Social Sciences Citation Index 1975 to present, and Emerging Sources Citation Index 2015 to present, ISI Web of Science (searched 18 January 2016)
See Appendix 1 for the search strategies we used.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts of studies identified by the search strategy to assess which studies met the inclusion criteria. We obtained the full‐text articles of potentially eligible studies. Two review authors (AF and GS) assessed the full‐text articles against the inclusion criteria of this review. Whenever there was uncertainty or disagreement, we reached consensus by discussion among the review authors (HAA). All full‐text articles that were excluded after full‐text assessment are listed in the 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors independently extracted details of study design, population, intervention, and comparison, and outcome data from included articles using a specially‐designed data extraction form based on the Cochrane EPOC Group data collection sheet.
The extracted data included:
participant characteristics (age, gender, education, ethnicity, health issues, etc.) and number of participants included in the study;
intervention characteristics (description of the mobile clinic including staffing, drugs, equipment, number of visits to the target area etc.) and comparison static clinic characteristics in the same terms. These descriptions also included the characteristics of the healthcare providers in both types of clinics (age, gender, profession, level of training, etc.) whenever reported in the included studies;
the setting (urban/rural) and description of other services provided in the served communities;
the country (low‐, middle‐, or high‐income);
description of the outcomes, timing of outcome assessment, and outcome data.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study using the nine standard Cochrane EPOC criteria and the seven standard criteria for ITS studies (EPOC 2015; Appendix 1). Judgements on the overall risk of bias took into account the likely magnitude and direction of the bias and whether we considered that the bias impacted on the findings. We assessed studies to be at high risk of bias if they scored 'high risk' in one or more of the following domains: sequence generation; allocation concealment; or selective outcome reporting (based on growing empirical evidence that these three factors are the most important in influencing risk of bias) (Higgins 2011). We judged the overall risk of bias as low if we assessed these key domains as at low risk of bias; unclear if one or more key domains were at unclear risk of bias; and high if one or more key domains were at high risk of bias.
Measures of treatment effect
For dichotomous outcomes, we had planned to use risk ratios (relative risk). For continuous outcomes measured in the same way, we had planned to use the mean difference. We had planned to use the standardised mean difference to combine the data from trials that measured the same outcome using different methods.
Unit of analysis issues
We used the odds ratio (OR) of the non‐randomised studies because it accounted for clustering in data. For example, in Reuben 2002, the primary analysis adjusted for correlation of observations within sites (cluster effect) using the Huber method (inflating the standard errors), which is a non‐parametric correction independent of observations when estimating the sample variance.
However, Eakin 2012 used a generalised estimating equation (GEE) to estimate the group population average for each outcome over time to control for the correlation among longitudinal measures within an individual, while adjusting for baseline level of each outcome.
In future updates, we may need to re‐analyse cluster‐RCTs if clusters were not taken into consideration during analysis. We will re‐analyse each study if it is possible to extract the following information from the trial report:
the number of clusters (or groups) randomised to each intervention group, or the average (mean) size of each cluster;
the outcome data ignoring the cluster design for the total number of individuals (e.g. number or proportion of individuals with events, or means and standard deviations (SDs));
an estimate of the intracluster (or intraclass) correlation coefficient (ICC).
We will present the point estimate of effect without any measure of uncertainty, and will note the presence of unit of analysis errors.
Dealing with missing data
We assumed that data were missing at random (as judged by the authors of each included study, i.e. missing due to refusal, or dropout). Therefore we analysed the available data only (and ignored the missing data).
In future updates, if we assume that data were not missing at random (as judged from the sociodemographic characters of the study participants compared to the outcome results in the same paper and the authors' justification for selecting a certain group versus another), whenever possible we will contact the original study investigator for missing data. In case of missing summary statistics (e.g. SDs), we will look for other statistics within each study to calculate the SD.
Assessment of heterogeneity
We were unable to assess heterogeneity as only two studies (three articles) met the inclusion criteria of this review. In future updates, we will assess clinical heterogeneity across the non‐randomised studies based on differences in populations, interventions, comparisons, and outcomes.
We will assess statistical heterogeneity using the T² value, the I² statistic, and the Chi² test and also using visual interpretation of forest plots. We will consider heterogeneity substantial where the T² value is greater than zero, and either the I² statistic is greater than 50% or there is a low P value (less than 0.10) in the Chi² test for heterogeneity. We will explore any heterogeneity (see the 'Subgroup analysis and investigation of heterogeneity' section below).
Assessment of reporting biases
We were unable to assess publication bias as only two studies (three articles) met the inclusion criteria. In future updates of this Cochrane review, we will assess publication bias using a funnel plot provided that there are 10 or more studies included in an analysis. We will judge publication bias to exist when we detect asymmetry in the funnel plot.
We assessed selective outcome reporting bias in each included study by comparing the outcomes reported in the results to those previously specified in the methodology section. Also we assessed this by checking whether the outcomes reported included those expected to have been measured in the study, based on the research question if the study protocol was unavailable.
In future updates, and provided that the study protocols are available, we will assess reporting bias by comparing if all of the prespecified (primary and secondary) outcomes list in the protocol are also reported in the published study. Where study protocols are unavailable, we will assess selective outcome reporting as above.
Data synthesis
Due to the small number of included studies and different outcomes reported, we were unable to perform data synthesis. In future updates of this review, we will group and compare studies according to similar outcome measures, e.g. non‐randomised studies that report contraceptive prevalence rate, vaccination coverage, percentage of breast cancer screened women, percentage of women with osteoporosis, etc. We will use a random‐effects model for meta‐analysis, based on the assumption that the true effects measured across non‐randomised studies are related but not the same due to differences in population, intervention, and setting. If studies are not sufficiently homogeneous to combine in a meta‐analysis, we will present the results of included studies in a forest plot but will not present the pooled estimate.
Subgroup analysis and investigation of heterogeneity
In future updates, we will investigate heterogeneity even if there is no statistically significant heterogeneity. We plan to perform the following subgroup analyses:
gender of the health care provider(s) that offers services to women included in the study, as the gender of the healthcare provider may affect the acceptability of the service and seeking of health care;
whether the study was conducted in a low‐, middle‐, or high‐income country as the socioeconomic level within the country may affect the quality and level of services offered by the mobile clinics;
the geographical setting of the study as living in a rural or urban area may affect the accessibility of the mobile clinic and may also affect quality.
Sensitivity analysis
In future updates of this review, we will conduct sensitivity analyses based upon the following to determine how robust and consistent the results are:
exclusion of all non‐RCTs;
exclusion of studies at high risk of bias (assessed using the Cochrane 'Risk of bias' tool described above);
exclusion of studies where we have obtained additional data from the study investigators;
variation of the ICC used to re‐analyse data from cluster‐RCTs
exclusion of data from studies that were re‐analysed.
'Summary of findings' tables
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence related to each of the key outcomes. We created 'Summary of findings' tables using the GRADEpro Guideline Development Tool (GDT) (available from www.gradepro.org). As we only had the complete information from one included study (Reuben 2002), we assessed the quality of the evidence for this study only and included it in the 'Summary of findings' table.
Results
Description of studies
Results of the search
The search retrieved 9516 records. There were 6910 after we removed duplicates. We screened 6910 records by title and abstract, and excluded 6820 articles. We assessed 90 full‐text articles for eligibility. We excluded 84 studies and three studies are awaiting classification while 3 articles (constitute 2 studies) were included. The reasons for exclusion: studies had an ineligible study design (n = 63); used an ineligible interventions (n = 16); were reviews rather than primary studies (n =5) (see Characteristics of excluded studies). We have presented this information in the study flow diagram in Figure 1.
1.

Study flow diagram.
Included studies
Two studies met the inclusion criteria of this review (Reuben 2002; Eakin 2012). Another published paper, Naeim 2009, was a cost‐effectiveness analysis and a secondary analysis of data collected in Reuben 2002. It focused on the cost‐effectiveness of the intervention.
Reuben 2002 is a cluster‐randomised controlled trial (RCT) that tested whether the offer of immediate, onsite, mobile mammography combined with health education was effective at increasing breast cancer screening rates versus health education alone, including encouragement to have a mammogram at the typical static clinic. The study randomised 60 community‐based meal sites, senior centres, and clubs in California, USA to either health education only or health education plus onsite mobile mammography over a two‐year period. There were 463 female participants (aged 60 to 84 years at study entry): 235 were offered health education and onsite mobile mammography and 228 received health education only.
The exclusion criteria of this study included recent mammography (within the past year), no telephone, inability to speak English or Spanish, and limited cognitive capacity to participate in the study (based on failure to be oriented to the date and day of the week, and inability to place numbers appropriately on an outline of a clock). The study assessed self‐reported mammography after three months through telephone interviews.
A secondary analysis, Naeim 2009, examined the cost‐effectiveness of mammography at mobile versus static (stationary) units.
The second included study was a cluster‐RCT that evaluated whether a 'Breathmobile' clinic would improve asthma outcomes compared to standard care, a static clinic (Eakin 2012). 'Head Start' programme sites in Baltimore, USA (66 sites in total) were the units of randomisation, while children were the unit of analysis. The Breathmobile is a mobile asthma clinic that delivers asthma screening, evaluation, and treatment services directly to inner city children at their schools or at the Head Start sites. A specially trained nurse practitioner, allergist, nurse, and driver/patient assistant provide care on the Breathmobile. The study included 322 children aged two to six years of age with persistent asthma. Eligibility criteria included caregiver reported physician diagnosed asthma or reactive airways disease and at least one of the following:
use of short acting beta agonist in the past four weeks;
asthma symptoms in the past four weeks; or
treated in the emergency department (ED) for asthma in past six months.
The study included both male and female children, and most participants were African‐American (more than 97%) in the two trial arms relevant to this review. Most children belonged to low‐income families.
Eakin 2012 included the following outcome measures:
symptom‐free days (SFD). This primary outcome was calculated by subtracting the number of days, or nights, or both, with asthma symptoms (i.e. cough, wheeze, shortness of breath) in the past 30 days, as reported by caregivers;
acute care and medication use. This measure included caregiver reports of ED visits, hospitalisations, prescribed asthma controller medication regimen (e.g. inhaled corticosteroid and leukotriene modifiers), and courses of oral corticosteroids in the previous six months;
caregiver quality of life. The study used the Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ), which is a 13‐item measure of activity limitations (four items) and emotional function (nine items) experienced by caregivers of asthmatic children. Responses on the PACQLQ are given on a 7‐point scale where 1 represents severe impairment and 7 represents non‐impairment. Higher scores indicate higher quality of life.
Excluded studies
We excluded 82 articles due to ineligible study design (n = 62); failure to meet our intervention definition (n = 16), or because they were not primary studies (n = 4) (see the 'Characteristics of excluded studies' table).
Risk of bias in included studies
We have presented a summary of the 'Risk of bias' assessments in Figure 2 and Figure 3, and provided the detailed assessments in the 'Characteristics of included studies' tables. Overall, we assessed the two included studies as at moderate risk of bias.
2.
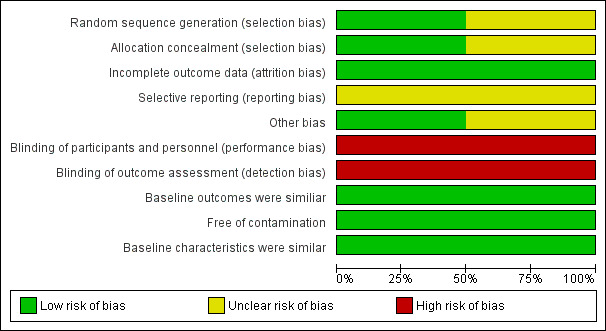
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
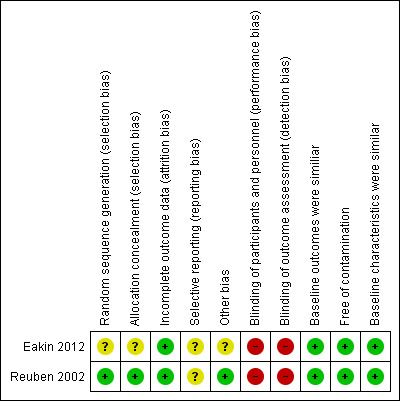
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
We assessed Reuben 2002 as at low risk of selection bias. Eakin 2012 was at unclear risk of bias as the study did not report adequately on how sequence generation was determined.
Blinding
In Reuben 2002 blinding of the participants and research associates was not possible (high risk of bias). The study reported that "on the day of the program, the presence or absence of the mobile mammography van revealed to participants which intervention group they had been assigned. For the same reason, the research associates administering the interventions also were aware of participants intervention status. While blinding was assured for outcome assessment since the outcomes assessor was unaware of the intervention group status of all participants”. The study used telephone interviews to ask women whether they had undergone mammography.
In Eakin 2012, blinding of the participants to the intervention could not be assured (high risk of bias); the study reports that the Breathmobile was present only at those Head Start sites assigned the Breathmobile. However, outcome assessment was blinded since the research assistants that conducted follow‐up telephone surveys were unaware of participants' group assignment.
Incomplete outcome data
We assessed the risk of attrition bias as low in both studies since the proportion of missing data was similar in the intervention and control groups.
Selective reporting
Selective reporting bias was unclear in both included studies. Although the studies reported all outcomes listed in the methods section in the results section, we were unable to assess the study protocols (unavailable).
Other potential sources of bias
For cluster‐RCTs, we assessed the following potential sources of bias.
Recruitment bias
Individuals were most likely recruited to the trial after the clusters had been randomised in both studies, but this was unclear for Eakin 2012.
Baseline characteristics
We assessed this as low risk as baseline characteristics were similar across intervention and control in the two included studies.
Loss of clusters
There was no evidence of loss of clusters in either study.
Incorrect analysis
We assessed Reuben 2002 as at low risk of bias as the study authors took clustering into consideration during the analysis using the Huber method (inflating standard errors). Eakin 2012 was unclear regarding this risk of bias item as the study authors used generalised estimating equations (GEE) but did not discuss adjustment for clustering explicitly.
Effects of interventions
Primary outcomes
1. Health outcomes
1.1 Health status and well‐being
One study, Eakin 2012, showed that children who receive asthma care from a mobile asthma clinic may experience little or no difference in symptom‐free days up to 12 months following the intervention versus children who receive standard asthma care from their primary care provider (low certainty evidence) (Table 2).
Eakin 2012 showed that children who receive asthma care from a mobile asthma clinic may experience little or no difference in urgent care use (including both emergency room use and hospitalisations) up to 12 months following the intervention versus children who receive standard asthma care from their primary care provider (low certainty evidence).
Eakin 2012 also assessed caregiver quality of life, and showed that there may be little or no difference in this measure at 12 months among caregivers of children who receive asthma care from a mobile asthma clinic compared to caregivers of children who receive standard asthma care from their primary care provider (low certainty evidence).
1.2 Health behaviour
One study, Reuben 2002, showed that women offered immediate, mobile onsite mammography and health education may be more likely than those offered health education only (including encouragement to have a mammogram at any usual static clinic) to report undergoing mammography within three months of the intervention (55% versus 40%; OR 1.83, 95% CI 1.22 to 2.74; low certainty evidence) (Analysis 1.1; unadjusted data only) (Table 1).
1.1. Analysis.
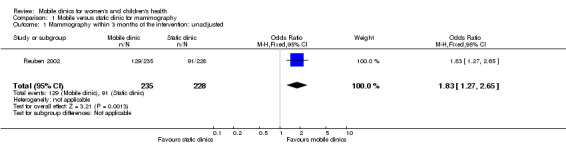
Comparison 1 Mobile versus static clinic for mammography, Outcome 1 Mammography within 3 months of the intervention: unadjusted.
The second included study, Eakin 2012, showed that there may be little or no difference in caregiver‐reported medication use at 12 months (including courses of oral steroids and prescriptions of inhaled corticosteroids) among children who receive asthma care from a mobile asthma clinic compared to children who receive standard asthma care from their primary care provider (low certainty evidence) (Table 2).
2. Utilisation, coverage, or access
None of the included studies assessed this outcome.
3. Resource use
Naeim 2009 reported a secondary cost‐effectiveness analysis of the data from Reuben 2002. This evaluation compared three types of screening program: stationary mammography units (with full digital film), mobile mammography with screen‐film, and mobile mammography with full digital film. The study included the following cost items: costs of equipment, mobile van, personnel, operating costs, processing and printing, and real estate costs. The study found that the total cost per patient screened was USD 41 for a stationary full digital unit, USD 102 for a mobile full digital unit, and USD 86 for mobile film unit. The study calculated the incremental cost per patient as the cost per mobile screening minus the cost per stationary screening. For mobile screening compared to screening at a stationary unit, the incremental cost per patient was USD 61 for a mobile full digital unit and USD 45 for a mobile film unit. For a mobile compared to a stationary unit, the incremental cost per screening was USD 264 for a mobile full digital unit and USD 207 for a mobile film unit. This evidence was of low certainty (Table 1).
Secondary outcomes
1. Satisfaction with care among healthcare recipients
None of the included studies assessed this outcome.
Discussion
Summary of main results
This Cochrane review aimed to assess the impact of mobile clinics that delivered services on any aspect of women's and children's health. However only two studies met the inclusion criteria. Reuben 2002 focused on mammography screening for women, and Eakin 2012 focused on care for children with asthma. Women who are offered mobile onsite mammography and health education may be more likely than those offered health education only to report undergoing mammography within three months of the intervention. However, the total cost per participant screened may be higher for mobile units than for stationary units. Children who receive asthma care from a mobile asthma clinic may experience little or no difference in symptom‐free days, urgent care use, and caregiver‐reported medication use versus children who receive standard asthma care from their usual primary care provider. In addition, there may be little or no difference in quality of life among caregivers of children who receive asthma care from a mobile asthma clinic compared to caregivers of children who receive standard asthma care. The certainty of the evidence was low for each of these outcomes.
Overall completeness and applicability of evidence
This Cochrane review aimed to evaluate the impact of mobile clinic services on women's and children's health. However, the available evidence was limited, and both included studies were conducted in a high‐income country among quite specific populations (children aged two to six years with persistent asthma and older women who might be eligible for mammography screening). This limits the applicability of the evidence to low‐ and middle‐income country settings, as well as to other populations. Mobile clinic utilisation and impact may be influenced by a various number of factors, including the reasons for using mobile services (e.g. improving geographical access to a package of health services or increasing coverage of targeted interventions to a targeted population) and the context in which these services operate.
For a number of review outcomes, including utilisation, coverage, or access and satisfaction with care among healthcare recipients, little or no data were available. For other outcomes, the data available were sparse. Further rigorous studies are needed that both cover a range of settings, populations, and health issues and that assess a wide range of outcomes relevant to understanding the effects of mobile clinics.
This review considered studies with a wide range of designs for inclusion and is based on comprehensive searches, without language or publication status restrictions. We searched for randomised controlled trials (RCTs), non‐randomised studies, controlled before‐and‐after (CBA) studies, and interrupted time series (ITS) studies. We included this range of designs as randomisation may not be feasible for some mobile clinic interventions. However, non‐randomised studies, CBA, and ITS studies are not as well indexed as RCTs in electronic databases and it is possible that we may have missed some studies.
Certainty of the evidence
The 'Summary of findings' tables for the main comparisons summarise the certainty of the evidence for the key outcomes (Table 1; Table 2).
Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, we assessed the certainty of the evidence as low. We judged both included studies as at moderate risk of bias overall and there was some imprecision around the estimate of effect (Reuben 2002; Eakin 2012). This suggests that the likelihood that the effect will be substantially different (i.e. the difference may be large enough to affect a decision) from that reported in the studies is high.
Potential biases in the review process
Identifying studies of mobile clinic interventions in electronic data base is challenging. However, our literature searches included many synonyms (mobile unit, mobile centres, mobile facilities, etc.) and we also searched a number of grey literature sources. Widening the scope of the eligible studies to include those carried out in high, middle or low income countries was an advantage to draw the attention to the absence of experimental studies evaluating the role of mobile clinics compared to the static clinics in low middle income countries.On the other hand, among the limitations which could be out of our control, is the possibility that we did not identify all eligible studies.The unpublished data were not obtained. Moreover, few outcomes were identified in the review compared to the proposed outcomes planned in the protocol. Many published papers evaluated the role of mobile clinic in offering maternal and child health services, however, because we limited our selection criteria to experimental designs only, none of the observational studies were included in this review. Another potential bias is that we excluded studies involving services offered to men which could have helped in better understanding the role played by the mobile clinics in improving health within communities and overcoming access barrier. However, we did exclude those studies because we were more interested in understanding mobile services offered to vulnerable populations (women and children) most.
Agreements and disagreements with other studies or reviews
Two other systematic reviews have explored the effects of mobile clinics. Mdege 2014 examined evidence on the use of mobile clinics to bring antiretroviral therapy closer to end users. The review did not identify any eligible studies on this topic. A second review, Vashishtha 2014, examined the use of mobile dental units. While Vashishtha 2014 identified a number of studies, none of these studies were eligible for inclusion in this Cochrane review as they did not meet our study design criteria.
We identified two other studies that evaluated the effects of mobile clinics for women's and child health but they did not use designs that were eligible for inclusion in this review (see the 'Characteristics of excluded studies' table). One cohort study, O'Connell 2010, evaluated the difference in prenatal care utilisation and birth outcomes among demographically similar women who used, or did not use, a mobile van (on a regular schedule and equipped to deliver services for women) for prenatal care in Florida, USA. This study suggested that adequate prenatal care was higher for mothers that utilised a mobile van compared to those who did not. One ITS study evaluated the use of mobile clinics for cervical cancer screening in rural Thailand (Swaddiwudhipong 1995), but did not meet our ITS study eligibility criteria. This study suggested that knowledge about cervical cancer and cervical cancer screening, as well as ever being screened for cervical cancer, increased after implementation of the mobile clinic intervention. The findings of both of these studies should be viewed with caution as these studies used designs that are at high risk of bias.
The inclusion of an economic perspective in the evaluation of health and health care has become an increasingly accepted component of health policy and planning. A number of country experiences have shown that cost‐effectiveness information can be used alongside other types of information to guide policy makers who address inequities and make evidence‐based decisions (Oxman 2009), and thus aid different policy decisions (Hutubessy 2003). In this Cochrane review, one study included a cost‐effectiveness analysis (Reuben 2002). Although mobile mammography was more effective, the costs per screen were much higher in the mobile units compared with stationary units. Incremental cost per patient for a mobile over a stationary unit was USD 61 for a mobile full digital unit and USD 45 for a mobile film unit. Other studies have suggested that mobile clinics might be most cost effective in rural and remote areas. For example, a cost‐effectiveness analysis of mobile clinics used for screening for cervical cancer in Japan showed that these had the highest benefit‐cost ratio (1.20) and were more suitable for rural areas compared to a detection centre and a private physician program (Takenaga 1985). A cross‐sectional study from Tunisia also showed that a mobile clinic service was cost‐effective in delivering family planing services to remote rural areas (Coeytaux 1989). This study highlights that clinic output was positively associated with literacy of the population, the number of centres served by a unit, and the frequency with which centres were served in a month. These factors may be important considerations for future evaluations.
Authors' conclusions
Implications for practice.
The limited evidence in this Cochrane review (only two small included studies) and the low certainty of the evidence makes it difficult to draw conclusions for practice in relation to mobile clinics for women's and children's health. Given these limitations, the results of this Cochrane review should be generalised with caution.
Implications for research.
We included only two studies in this Cochrane review, which were conducted in the USA. Therefore further well‐designed studies in low‐, middle‐, and high‐income countries are needed to evaluate the impacts of mobile clinics on women's and children's health. These studies should compare mobile clinic services with no services, mobile clinic services versus static clinics, and combined mobile and static clinic services versus static clinics. As far as possible, these studies should measure the full range of outcomes identified as important in this review and should address the impacts of mobile clinics on a wider range of health problems that affect women and children, including those living in both rural remote and urban settings. These studies should also assess resource use and cost effectiveness, given the importance of economic data in health policy and planning. Complementary studies that address the feasibility and acceptability of mobile clinic services in different settings would also be helpful.
Acknowledgements
We acknowledge the help and support of the Cochrane Effective Practice and Organisation of Care Group. The authors would also like to thank the following editors and peer referees who provided comments to improve the review: Simon Lewin (Editor), Jesse Uneke (Editor), Claire Glenton (PLS Editor), Lesley Bamford (Peer referee), Marit Johansen (Information Specialist).
The Norwegian Satellite of the Effective Practice and Organisation of Care (EPOC) Group receives funding from the Norwegian Agency for Development Cooperation (Norad), via the Norwegian Institute of Public Health to support review authors in the production of their reviews.
Appendices
Appendix 1. Search strategies
The Cochrane Central Register for Controlled Trials (CENTRAL)
CENTRAL, the Cochrane Library
| ID | Search | Hits |
| #1 | MeSH descriptor: [Mobile Health Units] this term only | 64 |
| #2 | (mobile or movable or moveable or traveling or travelling) near/3 (unit or units or clinic or clinics or center or centers or centre or centres or facility or facilities or hospital or hospitals):ti,ab,kw | 171 |
| #3 | (mobile or movable or moveable or traveling or travelling) near/3 (service* or health* or medicine or care):ti,ab,kw | 293 |
| #4 | (mobile or movable or moveable or traveling or travelling) near/3 (examination* or health next exam* or medical next exam* or diagnos* or treatment* or treating or therapy or therapies or surgery or operation* or prevent* or screen or screening or "x ray" or counsel* or laborator* or test or tests or testing or biopsy or biopsies or checkup* or check next up* or mammogra* or ultrasound or ultra next sound or vaccin* or immunisation or immunization):ti,ab,kw | 158 |
| #5 | (mobile or movable or moveable or traveling or travelling) near/3 (health* next personnel or health next care next personnel or medical next personnel or health* next professional* or health next care next professional* or medical next professional* or health* next provider* or health next care next provider* or medical next provider* or practitioner* or doctor or doctors or physician* or nurse or nurses or midwif* or midwiv*):ti,ab,kw | 22 |
| #6 | (medical or health* or mobile or clinic or clinical) next (bus or buses or van or vans or car or cars or truck or trucks or automobile* or trailer or trailers or wagon or wagons or wheel or wheels or vehicle or vehicles):ti,ab,kw | 17 |
| #7 | mobile near/3 (outreach or out next reach):ti,ab,kw | 7 |
| #8 | (#1 or #2 or #3 or #4 or #5 or #6 or #7) in Trials | 388 |
MEDLINE, OvidSP
| # | Searches | Results |
| 1 | Mobile Health Units/ | 3020 |
| 2 | ((mobile or movable or moveable or traveling or travelling) adj3 (unit? or clinic? or center? or centre? or facility or facilities or hospital?)).ti,ab. | 2732 |
| 3 | ((mobile or movable or moveable or traveling or travelling) adj3 (service? or health* or medicine or care)).ti,ab. | 2728 |
| 4 | ((mobile or movable or moveable or traveling or travelling) adj3 (examination? or health exam* or medical exam* or diagnos* or treatment? or treating or therapy or therapies or surgery or operation? or prevent* or screen or screening or x ray or counsel* or laborator* or test or tests or testing or biopsy or biopsies or checkup? or check up? or mammogra* or ultrasound or ultra sound or vaccin* or immunisation or immunization)).ti,ab. | 2116 |
| 5 | ((mobile or movable or moveable or traveling or travelling) adj3 (health* personnel or health care personnel or medical personnel or health* professional? or health care professional? or medical professional? or health* provider? or health care provider? or medical provider? or practitioner? or doctor? or physician? or nurse or nurses or midwif* or midwiv*)).ti,ab. | 351 |
| 6 | ((medical or health* or mobile or clinic or clinical) adj (bus or buses or van or vans or car or cars or truck? or automobile? or trailer? or wagon? or wheel? or vehicle?)).ti,ab. | 250 |
| 7 | (mobile adj3 (outreach or out reach)).ti,ab. | 85 |
| 8 | or/1‐7 | 8421 |
| 9 | randomized controlled trial.pt. | 388691 |
| 10 | pragmatic clinical trial.pt. | 132 |
| 11 | controlled clinical trial.pt. | 88951 |
| 12 | multicenter study.pt. | 182178 |
| 13 | Non‐Randomized Controlled Trials as Topic/ | 13 |
| 14 | Interrupted Time Series Analysis/ | 23 |
| 15 | Controlled Before‐After Studies/ | 27 |
| 16 | (randomis* or randomiz* or randomly allocat* or random allocat*).ti,ab. | 421108 |
| 17 | groups.ab. | 1431338 |
| 18 | (trial or multicenter or multi center or multicentre or multi centre).ti. | 159093 |
| 19 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 6819048 |
| 20 | or/9‐19 | 7591994 |
| 21 | exp Animals/ | 17805413 |
| 22 | Humans/ | 13794328 |
| 23 | 21 not (21 and 22) | 4011085 |
| 24 | review.pt. | 1954177 |
| 25 | meta analysis.pt. | 54332 |
| 26 | news.pt. | 167676 |
| 27 | comment.pt. | 618973 |
| 28 | editorial.pt. | 373544 |
| 29 | cochrane database of systematic reviews.jn. | 11153 |
| 30 | comment on.cm. | 618973 |
| 31 | (systematic review or literature review).ti. | 58811 |
| 32 | or/23‐31 | 6836573 |
| 33 | 20 not 32 | 5213356 |
| 34 | 8 and 33 | 2668 |
Embase, OvidSP
| # | Searches | Results |
| 1 | ((mobile or movable or moveable or traveling or travelling) adj3 (unit? or clinic? or center? or centre? or facility or facilities or hospital?)).ti,ab. | 3251 |
| 2 | ((mobile or movable or moveable or traveling or travelling) adj3 (service? or health* or medicine or care)).ti,ab. | 3153 |
| 3 | ((mobile or movable or moveable or traveling or travelling) adj3 (examination? or health exam* or medical exam* or diagnos* or treatment? or treating or therapy or therapies or surgery or operation? or prevent* or screen or screening or x ray or counsel* or laborator* or test or tests or testing or biopsy or biopsies or checkup? or check up? or mammogra* or ultrasound or ultra sound or vaccin* or immunisation or immunization)).ti,ab. | 2672 |
| 4 | ((mobile or movable or moveable or traveling or travelling) adj3 (health* personnel or health care personnel or medical personnel or health* professional? or health care professional? or medical professional? or health* provider? or health care provider? or medical provider? or practitioner? or doctor? or physician? or nurse or nurses or midwif* or midwiv*)).ti,ab. | 414 |
| 5 | ((medical or health* or mobile or clinic or clinical) adj (bus or buses or van or vans or car or cars or truck? or automobile? or trailer? or wagon? or wheel? or vehicle?)).ti,ab. | 320 |
| 6 | (mobile adj3 (outreach or out reach)).ti,ab. | 104 |
| 7 | or/1‐6 | 7990 |
| 8 | Randomized Controlled Trial/ | 365910 |
| 9 | Controlled Clinical Trial/ | 390179 |
| 10 | Quasi Experimental Study/ | 2330 |
| 11 | Pretest Posttest Control Group Design/ | 223 |
| 12 | Time Series Analysis/ | 15125 |
| 13 | Experimental Design/ | 10912 |
| 14 | Multicenter Study/ | 118438 |
| 15 | (randomis* or randomiz* or randomly or random allocat*).ti,ab. | 778278 |
| 16 | groups.ab. | 1805842 |
| 17 | (trial or multicentre or multicenter or multi centre or multi center).ti. | 207195 |
| 18 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 8141994 |
| 19 | or/8‐18 | 9100534 |
| 20 | (systematic review or literature review).ti. | 70713 |
| 21 | "cochrane database of systematic reviews".jn. | 3771 |
| 22 | (exp animal/ or nonhuman/) not exp human/ | 5224879 |
| 23 | or/20‐22 | 5298709 |
| 24 | 19 not 23 | 6862461 |
| 25 | 7 and 24 | 3356 |
| 26 | limit 25 to embase | 2524 |
CINAHL, EbscoHost
| # | Query | Results |
| S24 | S23 [Exclude MEDLINE records] | 302 |
| S23 | S8 and S22 | 1,142 |
| S22 | S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 | 1,219,859 |
| S21 | TI (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) OR AB (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 701,074 |
| S20 | TI ( randomis* or randomiz* or random* W0 allocat* ) OR AB ( randomis* or randomiz* or random* W0 allocat* ) | 81,751 |
| S19 | (MH "Health Services Research") | 7,044 |
| S18 | (MH "Multicenter Studies") | 9,168 |
| S17 | (MH "Quasi‐Experimental Studies+") | 7,903 |
| S16 | (MH "Pretest‐Posttest Design+") | 24,984 |
| S15 | (MH "Experimental Studies") | 14,117 |
| S14 | (MH "Nonrandomized Trials") | 157 |
| S13 | (MH "Intervention Trials") | 5,620 |
| S12 | (MH "Clinical Trials") | 81,626 |
| S11 | (MH "Randomized Controlled Trials") | 22,389 |
| S10 | PT research | 949,864 |
| S9 | PT clinical trial | 51,995 |
| S8 | S1 or S2 or S3 or S4 or S5 or S6 or S7 | 2,494 |
| S7 | TI ( mobile N3 (outreach or out W0 reach) ) OR AB ( mobile N3 (outreach or out W0 reach) ) | 47 |
| S6 | TI ( (medical or health* or mobile or clinic or clinical) W0 (bus or buses or van or vans or car or cars or truck or trucks or automobile* or trailer* or wagon* or wheel* or vehicle*) ) OR AB ( (medical or health* or mobile or clinic or clinical) W0 (bus or buses or van or vans or car or cars or truck or trucks or automobile* or trailer* or wagon* or wheel* or vehicle*) ) | 86 |
| S5 | TI ( (mobile or movable or moveable or traveling or travelling) N3 (health* W0 personnel or health W0 care W0 personnel or medical W0 personnel or health* W0 professional* or health W0 care W0 professional* or medical W0 professional* or health* W0 provider* or health W0 care W0 provider* or medical W0 provider* or practitioner* or doctor or doctors or physician* or nurse or nurses or midwif* or midwiv*) ) OR AB ( (mobile or movable or moveable or traveling or travelling) N3 (health* W0 personnel or health W0 care W0 personnel or medical W0 personnel or health* W0 professional* or health W0 care W0 professional* or medical W0 professional* or health* W0 provider* or health W0 care W0 provider* or medical W0 provider* or practitioner* or doctor or doctors or physician* or nurse or nurses or midwif* or midwiv*) ) | 212 |
| S4 | TI ( (mobile or movable or moveable or traveling or travelling) N3 (examination* or health W0 exam* or medical W0 exam* or diagnos* or treatment* or treating or therapy or therapies or surgery or operation* or prevent* or screen or screening or "x ray" or counsel* or laborator* or test or tests or testing or biopsy or biopsies or checkup* or check W0 up? or mammogra* or ultrasound or ultra W0 sound or vaccin* or immunisation or immunization) ) OR AB ( (mobile or movable or moveable or traveling or travelling) N3 (examination* or health W0 exam* or medical W0 exam* or diagnos* or treatment* or treating or therapy or therapies or surgery or operation* or prevent* or screen or screening or "x ray" or counsel* or laborator* or test or tests or testing or biopsy or biopsies or checkup* or check W0 up? or mammogra* or ultrasound or ultra W0 sound or vaccin* or immunisation or immunization) ) | 443 |
| S3 | TI ( (mobile or movable or moveable or traveling or travelling) N3 (service or services or health* or medicine or care) ) OR AB ( (mobile or movable or moveable or traveling or travelling) N3 (service or services or health* or medicine or care) ) | 917 |
| S2 | TI ( (mobile or movable or moveable or traveling or travelling) N3 (unit or units or clinic or clinics or center or centers or centre or centres or facility or facilities or hospital or hospitals) ) OR AB ( (mobile or movable or moveable or traveling or travelling) N3 (unit or units or clinic or clinics or center or centers or centre or centres or facility or facilities or hospital or hospitals) ) | 569 |
| S1 | (MH "Mobile Health Units") | 1,116 |
Global Health, OvidSP
| # | Searches | Results |
| 1 | ((mobile or movable or moveable or traveling or travelling) adj3 (unit? or clinic? or center? or centre? or facility or facilities or hospital?)).mp. | 553 |
| 2 | ((mobile or movable or moveable or traveling or travelling) adj3 (service? or health* or medicine or care)).mp. | 558 |
| 3 | ((mobile or movable or moveable or traveling or travelling) adj3 (examination? or health exam* or medical exam* or diagnos* or treatment? or treating or therapy or therapies or surgery or operation? or prevent* or screen or screening or x ray or counsel* or laborator* or test or tests or testing or biopsy or biopsies or checkup? or check up? or mammogra* or ultrasound or ultra sound or vaccin* or immunisation or immunization)).mp. | 496 |
| 4 | ((mobile or movable or moveable or traveling or travelling) adj3 (health* personnel or health care personnel or medical personnel or health* professional? or health care professional? or medical professional? or health* provider? or health care provider? or medical provider? or practitioner? or doctor? or physician? or nurse or nurses or midwif* or midwiv*)).mp. | 34 |
| 5 | ((medical or health* or mobile or clinic or clinical) adj (bus or buses or van or vans or car or cars or truck? or automobile? or trailer? or wagon? or wheel? or vehicle?)).mp. | 59 |
| 6 | (mobile adj3 (outreach or out reach)).mp. | 34 |
| 7 | or/1‐6 | 1343 |
| 8 | (randomis* or randomiz* or randomly allocat* or random allocat* or groups or trial or multicenter or multi center or multicentre or multi centre or intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).mp. | 1175972 |
| 9 | 7 and 8 | 755 |
POPLINE, K4Health
Two individual strategies.
1. Title: mobile AND Title: unit OR units OR clinic OR clinics OR mammography OR screening
2. All Fields: "mobile clinic" OR "mobile clinics" OR "mobile hospital" OR "mobile hospitals" OR "mobile health" OR "mobile healthcare" AND All Fields: randomised OR randomized OR "randomly allocated" OR "random allocation" OR "controlled trial" OR "control group" OR "control groups" OR quasiexperiment* OR "quasi experiment" OR "quasi experiments" OR "quasi experimental" OR "pseudo experiment" OR "pseudo experiments" OR "pseudo experimental" OR pseudoexperiment OR pseudoexperiments OR pseudoexperimental OR evaluat* OR "time series" OR "time point" OR "time points" OR "repeated measure" OR "repeated measures" OR "repeated measurement" OR "repeated measurements" OR "before and after" OR "pre and post" OR (("pretest" OR "pre test") AND ("posttest" OR "post test")) OR effect OR effects OR impact OR impacts OR intervention OR interventions OR trial OR multicenter OR "multi center" OR multicentre OR "multi centre"
Science Citation Index and Social Sciences Citation Index, ISI Web of Science
TOPIC: mobile NEAR/3 (clinic or clinics or hospital or hospitals or unit or units or mammography) AND TOPIC: woman* or women* or mother* or maternal* or child* AND TOPIC: randomised OR randomized or "randomly allocated" or "random allocation" or "controlled trial" or "control group" or "control groups" or quasiexperiment* or "quasi experiment" or "quasi experiments" or "quasi experimental" or pseudoexperiment* or "pseudo experiment" or "pseudo experiments" or "pseudo experimental" or evaluat* or "time series" or "time point" or "time points" or "repeated measure" or "repeated measures" or "repeated measurement" or "repeated measurements" or "before and after" or "pre and post" or (("pretest" or "pre test") AND ("posttest" or "post test")) or effect or effects or impact or impacts or intervention or interventions or trial or multicenter or "multi center" or multicentre or "multi centre"
Global Health Library, WHO
(Regional Indexes: AIM (AFRO), IMEMR (EMRO), IMSEAR (SEARO), WPRIM (WPRO), LILACS)
Searched in: Title
("mobile unit" or "mobile units" or "mobile clinic" or "mobile clinics" or "mobile facility" or "mobile facilities" or "mobile hospital" or "mobile hospitals" or "mobile health" or "mobile healthcare" or "mobile medical" or "mobile service" or "mobile services" or "mobile care" or "mobile surgery" or "mobile screening" or "mobile mammography" or "mobile vaccine" or "mobile vaccination" or "mobile immunisation" or "mobile immunization" or "mobile diagnostic" or "mobile diagnostics" or "mobile ultrasound" or "mobile ultra sound" or "mobile laboratory" or "mobile laboratories" or "mobile counseling" or "mobile counselling" or "mobile physician" or "mobile physicians" or "mobile doctor" or "mobile doctors" or "mobile nurse" or "mobile nurses" or "mobile nursing" or "mobile midwife" or "mobile midwives" or "mobile midwifery" or "mobile van" or "mobile vans" or "mobile vehicle" or "mobile vehicles" or "mobile truck" or "mobile trucks" or "medical van" or "medical vans" or "medical vehicle" or "medical vehicles" or "medical truck" or "medical trucks" or "clinic van" or "clinic vans" or "clinic vehicle" or "clinic vehicles" or "clinic truck" or "clinic trucks" or "clinical van" or "clinical vans" or "clinical vehicle" or "clinical vehicles" or "clinical truck" or "clinical trucks")
PAHO, VHL
"mobile health units" [Subject descriptor]
WHOLIS, VHL
"mobile health units" [Subject descriptor]
LILACS, VHL
Searched Advanced search in Title, abstract, subject
("Mobile Health Units" or "Unidades Móviles de Salud" or "Unidades Móveis de Saúde")
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)
Advanced Search in Title OR Condition OR Intervention AND Recruitment status is: All
(mobile clinic OR mobile clinics OR mobile health unit OR mobile health units OR mobile hospital OR mobile hospitals OR mobile health facility OR mobile health facilities)
ClinicalTrials.gov, NIH
Advanced Search – Searched in: Search Terms
("mobile clinic" OR "mobile clinics" OR "mobile health unit" OR "mobile health units" OR "mobile hospital" OR "mobile hospitals" OR "mobile health facility" OR "mobile health facilities")
DARE, NHSEED and HTA, CRD
(mobile NEAR/3 (unit or units or clinic or clinics or center or centers or centre or centres or facility or facilities or hospital or hospitals or service or services or health* or medicine or medical or examination or examinations or diagnos* or treatment or treatments or treating or therapy or therapies surgery or operation* or screen or screening or x ray or counsel* or laborator* or biopsy or biopsies or checkup* or check up or check ups or mammogra* or ultrasound or ultra sound or vaccin* or immunisation or immunization or personnel or practitioner* or doctor or doctors or physician* or nurse or nurses or midwif* or midwiv*))
Science Citation Index, Social Sciences Citation and Emerging Sources Citation Index, ISI Web of Science
Citation search for: Eakin 2012; Naeim 2009; Reuben 2002
Appendix 2. Cochrane Effective Practice and Organisation of Care (EPOC) suggested risk of bias criteria
Nine standard criteria are suggested for all RCTs, NRCTs and CBA studies:
-
Was the allocation sequence adequately generated?
Score “Low risk” if a random component in the sequence generation process is described (eg Referring to a random number table).
Score “High risk” when a nonrandom method is used (eg performed by date of admission). NRCTs and CBA studies should be scored “High risk”.
Score “Unclear risk” if not specified in the paper.
-
Was the allocation adequately concealed?
Score “Low risk” if the unit of allocation was by institution, team or professional and allocation was performed on all units at the start of the study; or if the unit of allocation was by patient or episode of care and there was some form of centralised randomisation scheme, an on‐site computer system or sealed opaque envelopes were used. CBA studies should be scored “High risk”.
Score “Unclear risk” if not specified in the paper.
-
Were baseline outcome measurements similar?1,2
Score “Low risk” if performance or patient outcomes were measured prior to the intervention, and no important differences were present across study groups. In RCTs, score “Low risk” if imbalanced but appropriate adjusted analysis was performed (e.g. Analysis of covariance).
Score “High risk” if important differences were present and not adjusted for in analysis.
If RCTs have no baseline measure of outcome, score “Unclear risk”.
-
Were baseline characteristics similar?
Score “Low risk” if baseline characteristics of the study and control providers are reported and similar.
Score “Unclear risk” if it is not clear in the paper (e.g. characteristics are mentioned in text but no data were presented).
Score “High risk” if there is no report of characteristics in text or tables or if there are differences between control and intervention providers. Note that in some cases imbalance in patient characteristics may be due to recruitment bias whereby the provider was responsible for recruiting patients into the trial.
-
Were incomplete outcome data adequately addressed?1
Score “Low risk” if missing outcome measures were unlikely to bias the results (e.g. the proportion of missing data was similar in the intervention and control groups or the proportion of missing data was less than the effect size i.e. unlikely to overturn the study result).
Score “High risk” if missing outcome data was likely to bias the results.
Score “Unclear risk” if not specified in the paper (Do not assume 100% follow up unless stated explicitly).
-
Was knowledge of the allocated interventions adequately prevented during the study?1
Score “Low risk” if the authors state explicitly that the primary outcome variables were assessed blindly, or the outcomes are objective, e.g. length of hospital stay. Primary outcomes are those variables that correspond to the primary hypothesis or question as defined by the authors.
Score “High risk” if the outcomes were not assessed blindly.
Score “Unclear risk” if not specified in the paper.
-
Was the study adequately protected against contamination?
Score “Low risk” if allocation was by community, institution or practice and it is unlikely that the control group received the intervention.
Score “High risk” if it is likely that the control group received the intervention (e.g. if patients rather than professionals were randomised).
Score “Unclear risk” if professionals were allocated within a clinic or practice and it is possible that communication between intervention and control professionals could have occurred (e.g. physicians within practices were allocated to intervention or control)
-
Was the study free from selective outcome reporting?
Score “Low risk” if there is no evidence that outcomes were selectively reported (e.g. all relevant outcomes in the methods section are reported in the results section).
Score “High risk” if some important outcomes are subsequently omitted from the results.
Score “Unclear risk” if not specified in the paper.
-
Was the study free from other risks of bias?
Score “Low risk” if there is no evidence of other risk of biases
The seven standard criteria to be used for all interrupted time series (ITS) studies are as follows
Note: If the ITS study has ignored secular (trend) changes and performed a simple t‐test of the pre versus post intervention periods without further justification, the study should not be included in the review unless reanalysis is possible.
-
Was the intervention independent of other changes?
Score “Low risk” if there are compelling arguments that the intervention occurred independently of other changes over time and the outcome was not influenced by other confounding variables/historic events during study period. If Events/variables identified, note what they are.
Score “High risk” if reported that intervention was not independent of other changes in time.
-
Was the shape of the intervention effect pre‐specified?
Score “Low risk” if point of analysis is the point of intervention OR a rational explanation for the shape of intervention effect was given by the author(s). Where appropriate, this should include an explanation if the point of analysis is NOT the point of intervention.
Score “High risk” if it is clear that the condition above is not met.
-
Was the intervention unlikely to affect data collection?
Score “Low risk” if reported that intervention itself was unlikely to affect data collection (for example, sources and methods of data collection were the same before and after the intervention);
Score “High risk” if the intervention itself was likely to affect data collection (for example, any change in source or method of data collection reported).
-
Was knowledge of the allocated interventions adequately prevented during the study?3
Score “Low risk” if the authors state explicitly that the primary outcome variables were assessed blindly, or the outcomes are objective, e.g. length of hospital stay. Primary outcomes are those variables that correspond to the primary hypothesis or question as defined by the authors.
Score “High risk” if the outcomes were not assessed blindly.
Score “Unclear risk” if not specified in the paper.
-
Were incomplete outcome data adequately addressed?3
Score “Low risk” if missing outcome measures were unlikely to bias the results (e.g. the proportion of missing data was similar in the pre‐ and post‐intervention periods or the proportion of missing data was less than the effect size i.e. unlikely to overturn the study result).
Score “High risk” if missing outcome data was likely to bias the results.
Score “Unclear risk” if not specified in the paper (Do not assume 100% follow up unless stated explicitly).
-
Was the study free from selective outcome reporting?
Score “Low risk” if there is no evidence that outcomes were selectively reported (e.g. all relevant outcomes in the methods section are reported in the results section).
Score “High risk” if some important outcomes are subsequently omitted from the results.
Score “Unclear risk” if not specified in the paper.
-
Was the study free from other risks of bias?
Score “Low risk” if there is no evidence of other risk of biases. e.g. should consider if seasonality is an issue (i.e. if January to June comprises the preintervention period and July to December the post, could the “seasons’ have caused a spurious effect).
1 If some primary outcomes were imbalanced at baseline, assessed blindly or affected by missing data and others were not, each primary outcome can be scored separately.
2 If “Unclear risk” or “High risk”, but there is sufficient data in the paper to do an adjusted analysis (e.g. Baseline adjustment analysis or Intention to treat analysis) the criteria should be re scored as “Low risk”.
3 If some primary outcomes were assessed blindly or affected by missing data and others were not, each primary outcome can be scored separately.
Data and analyses
Comparison 1. Mobile versus static clinic for mammography.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mammography within 3 months of the intervention: unadjusted | 1 | 463 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.27, 2.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eakin 2012.
| Methods | Cluster‐randomised controlled trial (RCT). Unit of allocation: clusters. 66 Head Start sites were the units of randomisation for the Breathmobile intervention. Head Start is a programme of the United States Department of Health and Human Services that provides comprehensive early childhood education, health, nutrition and parent involvement services to low‐income children and their families. Setting: Baltimore, USA. |
|
| Participants | Participants: 322 children aged 2 to 6 years with persistent asthma. Inclusion criteria: criteria included caregiver‐reported physician‐diagnosed asthma or reactive airways disease, and at least one of the following:
The study included male and female children, and most participants were African‐American (more than 97%) in the 2 trial arms relevant to this review. Most participants belonged to low‐income families. |
|
| Interventions | The Breathmobile is a mobile asthma clinic that delivers asthma screening, evaluation, and treatment services directly to inner city children at their schools or Head Start sites. A specially trained nurse practitioner, allergist, nurse, and driver/patient assistant provided care on the Breathmobile. Head Start staff distributed a validated survey to all students to identify children with asthma or at risk for asthma. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not report the method used to ensure random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors used a block randomisation scheme by putting randomisation scheme into sealed envelopes, which were opened after families completed baseline surveys. However, it is unclear whether the envelopes were opaque to ensure allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was little difference in the percentage of missing data across the intervention (7%) and control groups (9%) for the first follow‐up compared to baseline, as well as at 12 months (12% for the intervention and 10% for the control group). Attrition of participants from baseline to 6 months and from 6 months to 12 months occurred as some children could not be contacted and a few refused to participate. The study did not mention the causes of attrition and reasons for drop‐out between groups. |
| Selective reporting (reporting bias) | Unclear risk | The study authors reported all outcomes that they stated in the objectives in the results, but we did not assess the study protocol as we couldn't obtain the protocol. |
| Other bias | Unclear risk | Recruitment bias: the time of recruitment relative to the time of randomisation was unclear as the study authors did not mention whether the study recruited individuals after the clusters had been randomised. Loss of clusters: there was no evidence of loss of clusters in the study. Incorrect analysis: the study authors did not mention adjustment for clustering explicitly. However, "generalized estimating equation (GEE) was used to estimate the group population average for each outcome overtime to control for the correlation among longitudinal measures within an individual, while adjusting for baseline level of each outcome". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study reported that the Breathmobile was present only at those sites assigned the Breathmobile and so staff and participants could not be blinded to assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The research assistants that conducted follow‐up telephone surveys were blinded to group assignment. However, caregivers reported all outcomes, and they were likely to have been aware of their group assignment. |
| Baseline outcomes were similiar All outcomes | Low risk | There were no differences in baseline outcomes by group. |
| Free of contamination All outcomes | Low risk | Allocation was by cluster and it is unlikely that the control group received the intervention. |
| Baseline characteristics were similar All outcomes | Low risk | There were no differences in baseline characteristics by group. |
Reuben 2002.
| Methods | Cluster‐RCT. Unit of allocation: clusters. 60 community‐based meal sites, senior centres, and clubs were randomised to offer either health education only or health education plus immediate, on‐site mobile mammography. Setting: California, USA. |
|
| Participants | Females aged 60 to 84 at study entry.
Ethnicity: White, Black, Asian American, Hispanic, American Indian. Exclusion criteria included recent mammography (within the past year), no telephone, inability to speak English or Spanish, and limited cognitive capacity to participate in the study (based on failure to be oriented to the date and day of the week, and inability to place numbers appropriately on an outline of a clock). |
|
| Interventions | Intervention: immediate, on‐site mobile mammography and health education. Participants were given the opportunity to have a mammogram directly after the health education session. Comparison: women who received health education only and were encouraged to have a mammogram at the typical static clinic. |
|
| Outcomes | Self reported mammography after 3 months. Total and incremental costs (reported in Naeim 2009). Some costs were estimated based on financial reports or data obtained from the Radiology Department while other costs (salaries of personnel) were obtained through online aggregated survey data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study authors determined sequence generation using a computer programme. |
| Allocation concealment (selection bias) | Low risk | The study authors ensured allocation concealment as they used a computer generator that randomly assigned the sites in each pair to one of the two study arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of missing data was similar in the intervention (8%) and control groups (7.6%), but the study authors didn't report the reasons for drop‐out among the intervention and the control groups. |
| Selective reporting (reporting bias) | Unclear risk | The study authors reported all outcomes in the methods section in the results section. However, we were unable to assess the study protocol because we couldn't obtain it |
| Other bias | Low risk | Recruitment bias: individuals were recruited to the trial after the clusters had been randomised. Loss of clusters: there was no evidence of loss of clusters in the study. Incorrect analysis: the study authors took clustering into consideration during the analysis using the Huber method (inflating standard errors). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants or research associates was not assured. The study reported that "on the day of the program, the presence or absence of the mobile mammography van revealed to participants which intervention group they had been assigned. For the same reason, the research associates administering the interventions also were aware of participants’ intervention status”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study used telephone interviews to collect information from women on whether they had undergone mammography. All outcomes were self‐reported by women who participated in the trial, and they are likely to have been aware of their group assignment. |
| Baseline outcomes were similiar All outcomes | Low risk | There were no differences in baseline outcomes by group. |
| Free of contamination All outcomes | Low risk | Allocation was by cluster and it is unlikely that the control group received the intervention. |
| Baseline characteristics were similar All outcomes | Low risk | There were only minor differences in baseline characteristics by group. |
Abbreviations:
ER: emergency department
GEE: generalized estimating equation
PACQLQ: Pediatric Asthma Caregiver's Quality of Life Questionnaire
RCT: randomised controlled trial.
SFD: symptom free days
USA: United States of America
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adelson 1992 | Ineligible study design: there was no comparison group. It is a (descriptive study). This study evaluated the the breast x‐ray programme in Sydney provided by a mobile clinic free of charge in order to determine the attendance rate, and therefore determine the best way of delivery of service in order to reduce breast cancer mortality in Australia. |
| Aneni 2013 | Ineligible study design. This was an interrupted time series (ITS) study but measured only at 2 points, not at 3 points. The study assessed children at the beginning of the study as baseline data, and then at the end of the intervention. |
| Babigumira 2009 | Ineligible study design (descriptive study). It is a cost‐effectiveness analysis, and the study authors used primary data from another study. |
| Bentham 1992 | Ineligible study design: it is a controlled before‐and‐after (CBA) study but with only 1 control site and 1 intervention site. The study used a caravan as a general mobile surgery unit and compared consultation rates of participants for 1 year before and after the operation of the mobile unit and a comparison between area served by the main surgery unit and a mobile surgery unit. |
| Bollinger 2010 | Ineligible study design: it is a retrospective non‐controlled study, it is a cost‐effectiveness analysis of a breath mobile programme. Computerised clinical data was revised for a breath mobile clinic (a free service for children with asthma in baltimore) and incremental cost effectiveness relations were determined. |
| Brooks 2013 | Ineligible study design: cross sectional study. Retrospective analysis of women undergoing mammography screening. The study objective was to evaluate the association of variables associated with outcomes for women undergoing breast cancer screening and clinical evaluation on a mobile unit. |
| Brun 1983 | Ineligible study design: descriptive study with no comparison group (non‐controlled study). The mobile clinic was operated for 3 years and its effectiveness was determined by a) the number of breast cancer cases detected compared to expected numbers derived from registers but no significant test or P values were presented; b) the proportion of stage 1 in the cancers detected; c) whether the service reached all socioeconomic levels; these measures were not compared to static clinics or to prior operation of mobile unit. |
| Busen 2008 | Ineligible study design: it is a descriptive study of group of homeless adolescents and street‐involved youth who utilised a mobile unit that provided medical and mental healthcare services. The study audited participants' records but there was no comparison group. |
| Casamassimo 1988 | Ineligible study design: it is a descriptive study. Ineligible comparisons: our comparisons include mobile versus none, mobile versus static, and combined mobile and static versus static. This study compared 2 mobile interventions (comparing 2 mobile treatment programmes (dental care) in Denver and Chicago in terms of types of services and fees). |
| Chilcote 1994 | Ineligible study design: descriptive study. The comparison in this study differs from that specified in the proposal (mobile clinic versus nothing, mobile clinic versus static, mobile clinic and static clinic versus static clinic). The comparisons in this study were between different organisations that used the same method of mobile screening. The study divided organisations into 4 groups: corporations, community groups, school systems, and government agencies. The organisations had different payment arrangements for the screening setting and then compared participation rates. |
| Chiomba 1983 | Ineligible study design: a descriptive study of maternal and child health (MCH) services in Malawi, and included 3 static and 1 under‐5 mobile clinics. The study based selection on easy accessibility. The study evaluated maternal and child health services and nutrition status of under‐5 in Mwanza district, Malawi, and the nutritional health status of children, attendance rate among the different 4 clinics. |
| Coeytaux 1989 | Ineligible study design (observational cross‐sectional study). This study compared the output of the mobile programme with that of the fixed (static) centres that provided family planning services in Tunisia. In addition, the study performed a cost‐effectiveness analysis for mobile teams and mobile clinics in 1985, but not for the fixed centres. The results showed that serving the remote rural areas was more costly than serving the nearby rich populations. However, mobile clinics remained the most cost‐effective tool to serve the people in the remote rural areas. |
| Crowe 2014 | Ineligible population (men who were educated and mostly were tested for prostate cancer) and ineligible study design. |
| Curpen 1995 | Ineligible intervention: the mobile clinic was just a tool for mammogram screening. The study mainly compared breast cancer prognostic factors among women aged 40 to 49 versus women aged 50 to 60 years. Study design: it is a retrospective study, the records of the mobile van were revised to determine the prognostic factors of breast cancer among the 2 age groups. |
| DeBoard 2011 | Ineligible intervention: this study described indicators of breast cancer utilisation with no reference to mobile clinics. |
| Dyer 1996 | Ineligible study design: descriptive retrospective study. This study compared costs of mobile clinics (which operate regularly) versus fixed clinics. The output measures included costs per minute consultation, but did not evaluate effectiveness and other output measures. |
| Edwards 2011 | Ineligible intervention: this study compared efficacy of different screening tests for cervical cancer. The mobile unit was only used as a method to transport women to the hospital to be screened. It is a descriptive study. |
| Ellen 2003 | Ineligible study design: it is a descriptive study that mainly compared the difference between characteristics of participants served by a human immunodeficiency virus (HIV) mobile clinic and a static clinic. |
| Ellen 2004 | Ineligible study design: a descriptive study of the postcounselling rates offered by a mobile clinic for HIV patients, with no comparison group (uncontrolled study). This study mainly compared the post‐test counselling (PTC) rates for HIV‐infected and uninfected individuals who received HIV counselling and testing on a mobilesexually transmitted disease (STD)/HIV screening clinic. |
| Foord 1995 | Ineligible study design: quasi‐experimental design. The intervention definition :improving service delivery (through training of traditional birth attendants (TBA) and nurses to offer better obstetric care) where each midwife would visit the village on her responsibility twice a month; accompanied by the TBA). The intervention also included organisation of the static clinic schedule. |
| Fox‐Rushby 1996 | Ineligible study design (quasi‐experimental design). This study evaluated the cost‐effectiveness of maternal services offered by new mobile outreach service in West Kiang versus maternal services offered by another facility in Upper Baddibu, Gambia. |
| Gatti 2013 | Ineligible intervention. The intervention was an interactive online programme where the participants enrolled in an online programme (1, 3, 6 months) and they were provided by an App where their weight was monitored by a company Coach to show the improvement in person wellness. |
| Greenberg 1987 | Ineligible study design. |
| Harmon 1989 | Ineligible intervention. This study defined the mobile service as: home visits for high risk pregnant patients by nurses to see the impact on hospital admissions. It is a descriptive study |
| Harrison 2011 | Ineligible intervention. This study defined the intervention as mobile phone that offered mental health information. |
| Held 1970 | This is a review article description of a university‐based mobile medical team service. This differs from the definition of mobile clinics in this Cochrane review. |
| Hensen 2014 | Ineligible study design (systematic review). Ineligible population (men). |
| Hill 2014 | Ineligible study design. This was a secondary analysis of Mobile Health Map project data. |
| Iredale 2011 | Ineligible study design: a descriptive study of participant's perception of mobile cancer support unit in South Wales.(uncontrolled study). It included collection of quantitative data from participants and a qualitative component to measure opinions and attitudes of participants. |
| Jin 2004 | Study design: descriptive study to assess patient satisfaction using self‐administered questionnaire and a cost effective analysis (cost per unit service delivered). There was no comparison group (uncontrolled study). Intervention: a nurse (offering care for diabetic patients) and an opthalmic clinician travelled and provided participants with main opthalmic services. During the first year, 25 clinics were held at 22 sites, each site was visited once, 3 sites were visited twice but not on a regular basis. Participants: male and female diabetic participants. |
| Kabbash 2010 | Ineligible study design: a cross sectional interview study of clients attending voluntary counselling and testing (VCT) centres throughout Egypt. We found no evidence for allocation of the participants. Moreover, the exposure (using the HIV counselling and testing services through mobile and static clinics) and the outcome (client satisfaction) were measured at the same time as clients were recruited after taking the service. "Clients were interviewed on days covering every working day of the week. Selection was performed using systematic sampling where every second client was chosen". The study evaluated HIV counselling and testing services offered by mobile versus static clinics by measuring client satisfaction. |
| Kahn 2003 | Ineligible study design: uncontrolled study (community‐based survey). The study used the mobile clinic for STD screening, and determined the feasibility to implement, the yield of screening, and the acceptability of the participants; although the mobile clinic seemed to be effective but there was no comparison group. |
| Kawichai 2012 | Ineligible intervention: no mobile clinic as defined in this Cochrane review. The study intervention was a mobile HIV voluntary counselling and testing (MVCT) that included trained nurses visiting rural areas and offering counselling services. |
| Khan 2013 | Ineligible intervention: mobile vaccination sites at which vaccination staff were based and people attended at fixed dates to these sites. There were no mobile clinics. |
| Khumalo‐Sakutukwa 2008 | Ineligible intervention: the study intervention included community mobilisation (community working groups‐outreach workers)‐community based VCT (provided in tents‐caravans and mobile outreach service, including a community worker that invited people to take the HIV test. The study intervention was multi‐component and not concurrent with the specified definition of a mobile clinic in this Cochrane review. |
| Kimmance 1970 | Ineligible study design. This study is an ITS but included only 2 data points. "The first after eighteen months of operation (May, 1967), the second a year later". This study evaluated the utilisation rates and immunisation rates of the medical services in the remote rural areas of Swaziland with special emphasis on the mobile clinics and how the distance from the clinic would affect the attendance. Utilisation rates of the mobile clinic: there was an increase in the number of attendants to the mobile clinics in 1968 than in 1967 (65.2% compared to 50.0% respectively). However, there was no baseline data prior to the implementation of the mobile clinics. |
| Kohli 1995 | Ineligible study design: this a descriptive study that specifically described the accessibility of a certain centre for women living in Lanarkshire in terms of costs and arrangements. |
| Lahuerta 2011 | Ineligible study design: it is a descriptive study. The study described the behavioural characteristics and HIV and syphilis prevalence between participants tested at a mobile van that offered voluntary counselling and testing compared to those tested at three sexually transmitted infection (STI) clinics in Guatemala. There was no evidence of randomisation of participants. |
| LeBaron 1998 | Ineligible intervention. The definition of mobile clinic differs from that specified in the protocol: the mobile clinic was just a tool to reach the children as a part of the residence based intervention (included door‐to‐door assessment and education campaigns followed by mobile van vaccination). The study used a mobile van as a temporarily onsite vaccination station, child care and transportation to providers and not on regular basis as specified in the protocol. This study evaluated the impact of interventions by a community‐based organisation on the vaccination coverage rates. Interventions included clinic‐based interventions through record review and community survey, residence‐based interventions through community saturation with vaccination messages and opportunities. The study used mobile clinics in this type of intervention. However, the effect of mobile clinic, as being available and accessible to the people, on the immunisation coverage rates was not obvious and could not be identified as a separate measure from the whole measured effect of the residence‐based intervention. |
| Massin‐Short 2010 | Ineligible study design: a descriptive study of a pilot mobile mammography screening project (non‐controlled study). Ineligible intervention: the mobile clinic in this study was assigned to work on only 5 specific dates throughout the study period; which is not a regular service as specified in the protocol. |
| Mauad 2010 | Ineligible study design: a descriptive longitudinal study performed between May 2003 and May 2004, a non‐controlled study. The study operated a mobile van to increase the accessibility for cervical cancer screening among sexually active women in Brazil; the study determined the yield of screening but not compared to either static clinic or to the screening rates before operation of the mobile unit. |
| Mauad 2011 | Ineligible study design: cross‐sectional study (non‐controlled study). The study aim was to evaluate the use of a mobile unit in the diagnosis and treatment of skin cancer in several poor regions of Brazil but the only available information was a description of case distribution of non‐melanoma and melanoma skin cancers in participants served by the mobile unit. |
| Mbopi‐Kéou 2007 | Ineligible study design: descriptive pilot study (no comparison) that described mobile HIV testing as evidence of a successful intervention that reached a needy population. |
| Meehan 2014 | This was not an intervention study; a comparative study of mobile to fixed clinic but ineligible for inclusion. |
| Merali 2014 | Ineligible study design. |
| Moharana 2014 | Ineligible study design. |
| Morano 2014 | Ineligible study design: a cross‐sectional study. |
| Mosleh 2015 | This is a prospective comparative cohort study. It compared hospital‐ and community‐based rehabilitation programmes. |
| Murphy 2012 | Ineligible intervention: the intervention was mobile crisis management teams. |
| Mushamiri 2015 | This is a mobile phone intervention and not a mobile clinic intervention. |
| O'Brien 1987 | Ineligible study design. |
| O'Connell 2010 | Ineligible study design: it is a cohort or cross‐sectional study (collected information on women who used the mobile clinic and others who didn't and then assessed the outcome). Participants: mothers who utilised the mobile van at least 1 time for their prenatal care and delivered between August 2007 through September 2008 were considered the mobile group (N = 182) and a comparison group of the same size who delivered within the same time period was randomly matched by sociodemographic characteristics. Intervention: the definition of the mobile clinic is concurrent with our proposal, it was on a regular schedule and was equipped to deliver services for women. The study evaluated if there was a difference in prenatal care utilisation and birth outcomes among demographically‐similar women who used or did not use a mobile van for prenatal care. |
| O'Malley 2002 | Ineligible study design: it is mainly a qualitative study with a quantitative component to calculate costs per screening tests. The qualitative study involved 21 interviews with key informants (providers, programme staff, administators) about accessibility and feasibility of mammography van. |
| Oriol 2009 | Ineligible study design: the study used published data to quantify the value of prevention practices and the value of preventing unnecessary use of emergency departments (EDs), and developed an empirical method to determine the value of a typical mobile health clinic. It is a methodology paper. |
| Padmadas 2014 | Ineligible study design: this is a cross‐sectional study. |
| Paone 2015 | This study used a mobile phone intervention and not a mobile clinic intervention. |
| Peek 2009 | Ineligible study design: it is a a retrospective cohort analysis and cross‐sectional survey to describe follow‐up patterns of women undergoing breast cancer screening on a mobile van. There was no comparison group (uncontrolled study). |
| Renck 2014 | Ineligible study design. |
| Riley 2014 | Ineligible study design: this is a cross‐sectional study |
| Schnippel 2015 | Ineligible study design: this is a cross‐sectional study that evaluated the costs of reproductive services offered by the mobile clinics. |
| Scholl 2011 | Ineligible study design: it is a review article on a mobile clinic programme in India. |
| Schwartze 2014 | Ineligible study design: this is a cross‐sectional study. |
| Schwitters 2015 | Ineligible study design: this is a qualitative study. |
| Silveira 2014 | Ineligible study design. This study compared screening and diagnostic tests for skin cancer where the participants were recruited from a mobile clinic. |
| Soclerstrom 1997 | Ineligible study design: a descriptive study of moves programme that included a mobile clinic that provided services for children (it describes the service rather than evaluating it). There was no comparison group. |
| Solon 1986 | It is a review article that discussed the family planning and health education in the Phillipines. The study referred to the mobile clinic as a nutri‐bus that offered nutrition services with no evaluation of the service (no information to evaluate). |
| Spagnolo 1995 | This is critical review for another cost‐effectiveness paper. |
| Spencer 1990 | Ineligible study design: a descriptive study. The intervention definition was a mobile team visited schools and communities; each consisted of a medical doctor, 2 laboratory, technicians, 2 clerks, 1 nurse, 2 laboratory workers, and 1 driver. The mobile team delivered chemotherapy to schools to reduce schistosomiasis prevalence. |
| Steward 2014 | Ineligible study design (cross sectional) |
| Swaddiwudhipong 1995 | Ineligible study design: an ITS but although the study defines clearly when the intervention took place in a certain point in time, the effects were measured at only 1 data point before the intervention (January 1991) and at 1 point after the intervention (1994). A mobile unit performed health education and Pap smears at 54 villages. The proportion of women who knew about Pap smear test and the proportion of women screened both increased after the intervention. |
| Swaddiwudhipong 1999 | Ineligible study design. It is an ITS but although the study defines clearly when the intervention took place in a certain point in time, the effects were measured at 1 data point before the intervention and at 1 point after the intervention (repeated study as Swaddiwudhipong 1995). |
| Swanson 2003 | Ineligible study design: study design is not mentioned in the study's methods section. “More detailed information can be found in an earlier publication”. However, we accessed the earlier publication through an internet search.. The study design is a cross‐sectional design, and compared homeless women satisfaction offered by the shelter outreach clinics/mobile clinics versus county/government clinic (mobile versus static). The mobile clinics were associated with higher satisfaction. |
| Takenaga 1985 | Ineligible study design: a cross‐sectional study (a cost‐benefit analysis and the study authors obtained the data from different sources). The study evaluated 3 early cervical cancer screening programmes (mobile, centre detection and private physician programme) according to cost benefit, efficiency, and accessibility. The mobile clinic programme was efficient in Japan according to the economic analysis undertaken in this study. |
| Tedrow 2012 | Ineligible study design: a qualitative study to explore community mobilisation mainly based on key informants. |
| Thandar 2015 | This was not an intervention study. but a cross‐sectional study. |
| Tianviwat 2009 | Ineligible study design: a cross‐sectional design. The study assessed whether the hospitals were using a mobile clinic or not as 1 of the baseline characteristics after the sampling strategy was done and a decision about the included schools was made. There was no allocation of the schools to hospital with or without an adjunct mobile clinic during the study design. Intervention: the study evaluated the secondary prevention measures (coverage, effectiveness, and protective effects) carried by hospital clinics with and without adjunct mobile clinics. So, the study authors compared the impact of static clinic versus the impact of combined static and mobile clinics. |
| Tonne 1979 | Ineligible study design, this is neither a controlled before‐and‐after study nor an ITS. We received no information on the participants’ demographics either at baseline or after. Intervention: school mobile dental service versus traditional dental service. Among 10 mobile clinics (school mobile dental services), 3 were chosen, and compared to 3 at a traditional pedodontic practice, comparing data from 7 years. The study authors chose an effectively analysis, but noted that they couldn't make a quantitative assessment of the intervention in both groups. |
| Treadway 1973 | It is a review article that summarises selected topics for a report that evaluated the family planning programme in India. The study reported that mobile units were 1 of the distribution methods that increased family planning methods acceptance, but there was no comparison between mobile and static clinics. There was no information to evaluate. |
| van der Pol 1998 | Ineligible study design: a descriptive study that included evaluation of mobile clinics through performance indicators. There was no baseline information concerning participants. |
| van Dijk 2014 | This is a cohort study. |
| Wang 2007 | Ineligible intervention. This study defined the intervention as movable acupuncture (no reference to mobile clinic as stated in this Cochrane review). |
| Williams 1989 | Ineligible intervention. The intervention did not include mobile clinics. The intervention was to receive a letter with an appointment compared to an open‐ended letter of invitation for breast cancer screening that was sometimes offered by a mobile clinic. Study design: randomised trial. |
| Yoon 2009 | Ineligible study design: a cross‐sectional study. A sample of 243,967 participants (screened from January to May 2007) was randomly chosen and then stratified according to the type of cancer (gastric, liver, colorectal, breast, or cervical) and the type of screening unit (general hospital, hospital, or mobile clinic). The study objectives were to evaluate participant satisfaction in the National Cancer Screening Program (NCSP) and to examine differences in satisfaction between mobile vans and static sites. |
| Zabos 2001 | Ineligible study design: a descriptive study (it describes the service rather than evaluates it) and includes a qualitative component to understand barriers of seeking care and degree of satisfaction of the service. The study used a mobile clinic (we care) programme to provide dental care for HIV patients. There was no baseline data concerning the participants and no statistical information. |
Abbreviations:
CBA: controlled before‐and‐after study
HIV: human immunodeficiency virus
ITS: Interrupted time series
MCH: maternal and child health
NCSP: National Cancer Screening Program
RCT: randomised controlled trial.
STD: sexually transmitted disease
TBA: traditional birth attendant
VCT: voluntary counselling and testing
Characteristics of studies awaiting assessment [ordered by study ID]
Coronado 2014.
| Methods | Clinical randomised controlled trial (RCT) |
| Participants | 600 Latino women aged 42 to 74 who were non‐compliant with breast cancer screening guidelines |
| Interventions | A theory‐based counselling programme that consisted of a ‘promotora’ or community health worker‐led home‐based intervention to encourage breast cancer screening. At the clinic‐level, two clinics offered additional mammography services provided by a mobile mammography unit operated by the Seattle Cancer Care Alliance. |
| Outcomes | The rate of mammography uptake over the 1‐year follow‐up period. |
| Notes | We need the full‐text paper as the abstract is non‐informative about the results and intended measured outcome. |
Kotschan 2014.
| Methods | Not known |
| Participants | Women |
| Interventions | Mobile educational units and mobile clinics offering breast cancer screening in 2 study sites in South Africa: Two educational cars, one educational truck & one mobile Women's Health Cancer Unit,servicing 103 clinics and 15 hospitals in Gauteng, Limpopo, Mpumulanga, Kwa‐Zulu Natal, Orange Free State and North West Province‐on a rotational basis. Another educational car and mammography unit servicing, Western Cape, Eastern Cape and Northern Cape offering services to two hospitals and theirs referring clinics, on a weekly basis. |
| Outcomes | Reduce the incidence of breast cancer in South Africa and ultimately reduce the mortality by encouraging early detection of breast lumps and prompt referral |
| Notes | We need the full‐text paper as even the abstract is unavailable. |
Labhardt 2014.
| Methods | Cluster‐RCT |
| Participants | All people resident within the study area and not known to be HIV‐positive were eligible except children less than 18 years coming without an accompanying caregiver. |
| Interventions | The 6 clusters in the home based HIV testing and counselling (HB‐HTC) group received 30 1‐d multi‐disease campaigns (5 villages per cluster) that delivered services by going door‐to‐door, whereas the six clusters in mobile clinic HIV testing and counselling (MC‐HTC) group received campaigns involving community gatherings in the 30 villages with subsequent service provision in mobile clinics. |
| Outcomes | At the individual level Primary outcomes: number of participants that take up HIV testing and counselling (HTC), the proportion of participants newly tested HIV‐positive among participants who took up HTC, linkage to care within 28 days after a positive HIV test among participants who tested HIV‐positive during the campaign. Secondary outcomes: the age group distribution of persons accessing the campaigns (less than 12, 12 to 24, and ≥ 25 years), the proportion of first‐time testers among people who took up HTC, male participation, the clinical World Health Organization (WHO) stage of participants newly diagnosed HIV‐positive, as assessed by the study nurse during the campaign, the CD4 cell count among participants newly diagnosed HIV‐positive. At the cluster level: the number of persons who tested HIV‐positive through routine facility‐based HTC during the 28 d following the campaign in the specific cluster. |
| Notes | We have not yet assessed this study. |
Abbreviations:
HB‐HTC: home based HIV testing and counselling
HTC:HIV testing and counselling
MC‐HTC: mobile clinic HIV testing and counselling
RCT: randomised controlled trial
Differences between protocol and review
There was no difference between the protocol and review as we followed the same proposed methodology mentioned in the protocol. However, the subgroup analysis and the sensitivity analysis were not carried out because of the scarcity of the eligible studies.
Contributions of authors
HAA conceived the protocol and is the guarantor of this Cochrane review. OE participated in the protocol design and reviewed the draft of the protocol. AF and GA extracted data and prepared the review draft under the guidance of HAA. All authors approved the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
Norwegian Public Health Institute, Norway.
Declarations of interest
HAA has no known conflicts of interest. AF has no known conflicts of interest. GA has no known conflicts of interest. OE has no known conflicts of interest.
New
References
References to studies included in this review
Eakin 2012 {published data only}
- Eakin MN, Rand CS, Bilderback A, Bollinger ME, Butz A, Kandasamy V, et al. Asthma In Head Start children: effects of the Breathmobile program and family communication on asthma outcomes. The Journal of Allergy and Clinical Immunology 2012;129(3):664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reuben 2002 {published data only}
- Naeim A, Keeler E, Bassett LW, Parikh J, Bastani R, Reuben DB. Cost‐effectiveness of increasing access to mammography through mobile mammography for older women. Journal of the American Geriatrics Society 2009;57(2):285‐90. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Bassett LW, Hirsch SH, Jackson CA, Bastani R. A randomized clinical trial to assess the benefit of offering on‐site mobile mammography in addition to health education for older women. American Journal of Roentgenology 2002;176(6):1509‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adelson 1992 {published data only}
- Adelson P, Irwig L, Turnbull D. Evaluating the impact of a promotional campaign for screening mammography: who attends?. Australian Journal of Public Health 1992;16(1):66‐71. [DOI] [PubMed] [Google Scholar]
Aneni 2013 {published data only}
- Aneni E, Beer IH, Hanson L, Rijnen B, Brenan AT, Feeley FG. Mobile primary healthcare services and health outcomes of children in rural Namibia. Rural and Remote Health 2013;13(3):2380. [PubMed] [Google Scholar]
Babigumira 2009 {published data only}
- Babigumira JB, Sethi AK, Smyth KA, Singer ME. Cost effectiveness of facility‐based care, home‐based care and mobile clinics for provision of antiretroviral therapy in Uganda. Pharmacoeconomics 2009;27(11):963‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bentham 1992 {published data only}
- Bentham G, Haynes R. Evaluation of a mobile branch surgery in a rural area. Social Science & Medicine 1992;34(1):97‐102. [DOI] [PubMed] [Google Scholar]
Bollinger 2010 {published data only}
- Bollinger ME, Morphew T, Mullins. Symptom free day improvement for underserved children in the breathmobile program: is it worth the cost?. The Journal of Allergy and Clinical Immunology 2010;125(2):AB185. [Google Scholar]
Brooks 2013 {published data only}
- Brooks SE, Hembree TM, Shelton BJ, Beache SC, Aschbacher G, Schervish PH, et al. Mobile mammography in underserved populations: analysis of outcomes of 3,923 women. Journal of Community Health 2013;38(5):900‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brun 1983 {published data only}
- Brun C, Grime LP. Effectiveness of a mobile breast screening service in the Blackburn Health District ‐ analysis of the first 3 years' results. Public Health 1983;97(2):102‐8. [DOI] [PubMed] [Google Scholar]
Busen 2008 {published data only}
- Busen NH, Engebretson JC. Facilitating risk reduction among homeless and street‐involved youth. Journal of the American Academy of Nurse Practitioners 2008;20(11):567‐75. [DOI] [PubMed] [Google Scholar]
Casamassimo 1988 {published data only}
- Casamassimo PS, Coffee LM, Leviton FJ. A comparison of two mobile treatment programs for the homebound and nursing home patient. Special Care in Dentistry 1988;8(2):77‐81. [DOI] [PubMed] [Google Scholar]
Chilcote 1994 {published data only}
- Chilcote WA, Barry M, Paushter DM, Desberg A, Churchill E, Jeric R. Patient‐initiated breast cancer screening: results of a comprehensive community and workplace sponsored program. Breast Disease 1994;7(2):151‐6. [Google Scholar]
Chiomba 1983 {published data only}
- Chiomba WM. Evaluation of maternal and child health services and nutrition status of under fives in Mwanza district, Malawi. East African Medical Journal 1983;60(6):380‐5. [PubMed] [Google Scholar]
Coeytaux 1989 {published data only}
- Coeytaux F, Donaldson D, Aloui T, Kilani T, Fourati H. An evaluation of the cost‐effectiveness of mobile family planning services in Tunisia. Studies in Family Planning 1989;20(3):158‐69. [PubMed] [Google Scholar]
Crowe 2014 {published data only}
- Crowe H, Howard N, Bugeja P, Murphy D, Wootten A, Challacombe B, et al. Introduction of a novel, mobile, nurse‐led prostate cancer education and testing service. Australian Journal of Advanced Nursing 2014;31(4):14‐23. [Google Scholar]
Curpen 1995 {published data only}
- Curpen BN, Sickles EA, Sollitto RA, Ominsky SH, Galvin HB, Frankel SD. The comparative value of mammographic screening for women 40‐49 years old versus women 50‐64 years old. American Journal of Roentgenology 1995;164(5):1099‐103. [DOI] [PubMed] [Google Scholar]
DeBoard 2011 {published data only}
- DeBoard RA. Indicators facilitating health service use in diagnosis and treatment of breast cancer. Communicating Nursing Research 2011;44:576. [Google Scholar]
Dyer 1996 {published data only}
- Dyer JJ. Comparative costs of mobile and fixed‐clinic primary health care services . Suid‐Afrikaanse Tydskrif Vir Geneeskunde [South African Medical Journal] 1996;86(5):528‐30. [PubMed] [Google Scholar]
Edwards 2011 {published data only}
- Edwards C, Sirotin N, Alene IAN, Riggs E, Taylor K . Effectiveness of a mobile cervical cancer screening program in Andhra Pradesh, India. Journal of General Internal Medicine 2011;26:S337. [Google Scholar]
Ellen 2003 {published data only}
- Ellen JM, Bonu S, Arruda JS, Ward MA, Vogel R . Comparison of clients of a mobile health van and a traditional STD clinic. Journal of Acquired Immune Deficiency Syndromes 2003;32(4):388‐93. [DOI] [PubMed] [Google Scholar]
Ellen 2004 {published data only}
- Ellen JM, Liang TS, Jacob CA, Erbelding E, Christmyer C. Post‐HIV test counselling of clients of a mobile STD/HIV clinic. International Journal of STD & AIDS 2004;15(11):728‐31. [DOI] [PubMed] [Google Scholar]
Foord 1995 {published data only}
- Foord F. Gambia: evaluation of the mobile health care service in West Kiang district. World Health Statistics Quarterly. Rapport Trimestriel de Statistiques Sanitaires Mondiales 1995;48(1):18‐22. [PubMed] [Google Scholar]
Fox‐Rushby 1996 {published data only}
- Fox‐Rushby JA, Foord F. Costs, effects and cost‐effectiveness analysis of a mobile maternal health care service in West Kiang, The Gambia. Health Policy 1996;35(2):123‐43. [DOI] [PubMed] [Google Scholar]
Gatti 2013 {published data only}
- Gatti F, Brivio E, Galimberti C. Evaluation of a personal mobile coaching service for health tracking. Studies in Health Technology & Informatics 2013;191:154‐7. [PubMed] [Google Scholar]
Greenberg 1987 {published data only}
- Greenberg J, Naschak C, Sachs R. Family planning on wheels: costs and benefits of a mobile delivery system compared to the traditional clinic.. 115th Annual Meeting of the American Public Health Association (APHA), New Orleans, Louisiana. Data on file. 18 ‐ 22 October 1987.
Harmon 1989 {published data only}
- Harmon JS, Barry M. Antenatal testing, mobile outpatient monitoring service. Journal of Obstetric, Gynecologic & Neonatal Nursing 1989;18(1):21‐4. [DOI] [PubMed] [Google Scholar]
Harrison 2011 {published data only}
- Harrison V, Proudfoot J, Wee P, Parker G, Pavlovic D, Manicavasagar V. Mobile mental health: review of the emerging field and proof of concept study. Journal of Mental Health 2011;20(6):509‐24. [DOI] [PubMed] [Google Scholar]
Held 1970 {published data only}
- Held B. Family planning in rural areas. 2. Florida: a university‐based mobile medical team. Family Planning Perspectives 1970;2(3):32‐4. [PubMed] [Google Scholar]
Hensen 2014 {published data only}
- Hensen B, Taoka S, Lewis JJ, Weiss HA, Hargreaves J. Systematic review of strategies to increase men's HIV‐testing in sub‐Saharan Africa. Aids 2014;28(14):2133‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 2014 {published data only}
- Hill CF, Powers BW, Jain SH, Bennet J, Vavasis A, Oriol NE. Mobile health clinics in the era of reform. The American Journal of Managed Care 2014;20(3):261‐4. [PubMed] [Google Scholar]
Iredale 2011 {published data only}
- Iredale R, Hilgart J, Hayward J. Patient perceptions of a mobile cancer support unit in South Wales. European Journal of Cancer Care 2011;20(4):555‐60. [DOI] [PubMed] [Google Scholar]
Jin 2004 {published data only}
- Jin AJ, Martin D, Maberley D, Dawson KG, Seccombe DW, Beattie J. Evaluation of a mobile diabetes care telemedicine clinic serving Aboriginal communities in Northern British Columbia, Canada. International Journal of Circumpolar Health 2004;63(Suppl 2):124‐8. [DOI] [PubMed] [Google Scholar]
Kabbash 2010 {published data only}
- Kabbash IA, Hassan NM, Al‐Nawawy AN, Attalla AA, Mekheimer SI. Evaluation of HIV voluntary counselling and testing services in Egypt. Part 1: client satisfaction. Eastern Mediterranean Health Journal 2010;16(5):481‐90. [PubMed] [Google Scholar]
Kahn 2003 {published data only}
- Kahn RH, Moseley KE, Thilges JN, Johnson G, Farley TA. Community‐based screening and treatment for STDs: results from a mobile clinic initiative. Sexually Transmitted Diseases 2003;30(8):654‐8. [DOI] [PubMed] [Google Scholar]
Kawichai 2012 {published data only}
- Kawichai S, Celentano D, Srithanaviboonchai K, Wichajarn M, Pancharoen K, Chariyalertsak C, et al. NIMH Project Accept (HPTN 043) HIV/AIDS community mobilization (CM) to promote mobile HIV voluntary counseling and testing (MVCT) in rural communities in Northern Thailand: modifications by experience. AIDS and Behavior 2012;16(5):1227‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2013 {published data only}
- Khan IA, Saha A, Chowdhury F, Khan AI, Uddin MJ, Begum YA, et al. Coverage and cost of a large oral cholera vaccination program in a high‐risk cholera endemic urban population in Dhaka, Bangladesh. Vaccine 2013;31(51):6058‐64. [DOI] [PubMed] [Google Scholar]
Khumalo‐Sakutukwa 2008 {published data only}
- Khumalo‐Sakutukwa G, Morin SF, Fritz K, Charlebois ED, Rooyen H, Chingono A, et al. Project Accept (HPTN 043): a community‐based intervention to reduce HIV incidence in populations at risk for HIV in sub‐Saharan Africa and Thailand. Journal of Acquired Immune Deficiency Syndromes 2008;49(4):422‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimmance 1970 {published data only}
- Kimmance KJ. Evaluation of the work of a mobile outpatient unit in Swaziland. Journal of Tropical Pediatrics 1970;16(2):62‐7. [DOI] [PubMed] [Google Scholar]
Kohli 1995 {published data only}
- Kohli HS, Teo PYK, Howie FMC, Dobson HM. How accessible is the Breast Screening Assessment Centre for Lanarkshire women?. Health Bulletin 1995;53(3):153‐8. [PubMed] [Google Scholar]
Lahuerta 2011 {published data only}
- Lahuerta M, Sabidó M, Giardina F, Hernández G, Palacios JF, Ortiz R, et al. Comparison of users of an HIV/syphilis screening community‐based mobile van and traditional voluntary counselling and testing sites in Guatemala. Sexually Transmitted Infections 2011;87(2):136‐40. [DOI] [PubMed] [Google Scholar]
LeBaron 1998 {published data only}
- LeBaron CW, Starnes D, Dini EF, Chambliss JW, Chaney M. The impact of interventions by a community‐based organization on inner‐city vaccination coverage: Fulton County, Georgia, 1992‐1993. Archives of Pediatrics & Adolescent Medicine 1998;152(4):327‐32. [DOI] [PubMed] [Google Scholar]
Massin‐Short 2010 {published data only}
- Massin‐Short SB, Grullón MA, Judge CM, Ruderman KR, Grullón M, Lora V. A mobile mammography pilot project to increase screening among Latina women of low socioeconomic status. Public Health Reports 2010;125(5):765‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mauad 2010 {published data only}
- Mauad EC, Nicolau SM, Gomes UA, Costa Vieira RA, Castro Mattos JS, Longatto‐Filho A, et al . Can mobile units improve the strategies for cervical cancer prevention?. Diagnostic Cytopathology 2010;38(10):727‐30. [DOI] [PubMed] [Google Scholar]
Mauad 2011 {published data only}
- Mauad EC, Silva TB, Latorre MRDO, Vieira RAC, Haikel Jr RL, Vazquez VL, et al. Opportunistic screening for skin cancer using a mobile unit in Brazil. BMC Dermatology 2011;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mbopi‐Kéou 2007 {published data only}
- Mbopi‐Kéou FX, Ongolo‐Zogo P, Angwafo F 3rd, Ndumbe PM, Bélec L. High impact of mobile units for mass HIV testing in Africa. AIDS 2007;21(14):1994‐6. [DOI] [PubMed] [Google Scholar]
Meehan 2014 {published data only}
- Meehan SA, Naidoo P, Claassens MM, Lombard C, Beyers N. Characteristics of clients who access mobile compared to clinic HIV counselling and testing services: a matched study from Cape Town, South Africa. BMC Health Services Research 2014;14:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merali 2014 {published data only}
- Merali HS, Morgan JF, Uk S, Phlan S, Wang LT, Korng S. The Lake Clinic – providing primary care to isolated floating villages on the Tonle Sap Lake, Cambodia. Rural and Remote Health 2014;14:2612. [PubMed] [Google Scholar]
Moharana 2014 {published data only}
- Moharana PR, Kumari N, Trehan S, Sahani NC. Mobile family planning unit: an innovation for expanding accessibility to family planning services in Bihar. Indian Journal of Public Health 2014;58(4):289‐90. [DOI] [PubMed] [Google Scholar]
Morano 2014 {published data only}
- Morano JP, Zelenev A, Walton MR, Bruce RD, Altice FL. Latent tuberculosis infection screening in foreign‐born populations: a successful mobile clinic outreach model. American Journal of Public Health 2014;104(8):1508‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosleh 2015 {published data only}
- Mosleh SM, Bond CM, Lee AJ, Kiger A, Campbell NC. Effects of community based cardiac rehabilitation: Comparison with a hospital‐based programme. European Journal of Cardiovascular Nursing 2015;14(2):108‐16. [DOI] [PubMed] [Google Scholar]
Murphy 2012 {published data only}
- Murphy K. Crisis intervention teams and mobile crisis management. North Carolina Medical Journal 2012;73(3):200. [PubMed] [Google Scholar]
Mushamiri 2015 {published data only}
- Mushamiri I, Luo C, Iiams‐Hauser C, Ben Amor Y. Evaluation of the impact of a mobile health system on adherence to antenatal and postnatal care and prevention of mother‐to‐child transmission of HIV programs in Kenya. BMC Public Health 2015;15:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 1987 {published data only}
- O'Brien A, Anderson C. Evaluation of the effectiveness of a community‐based prenatal health education program. Family & Community Health 1987;10(2):30‐8. [DOI] [PubMed] [Google Scholar]
O'Connell 2010 {published data only}
- O'Connell E, Zhang G, Leguen F, Prince J. Impact of a mobile van on prenatal care utilization and birth outcomes in Miami‐Dade County. Maternal and Child Health Journal 2010;14(4):528‐34. [DOI] [PubMed] [Google Scholar]
O'Malley 2002 {published data only}
- O'Malley AS, Lawrence W, Liang W, Yabroff R, Lynn J, Kerner J, et al. Feasibility of mobile cancer screening and prevention. Journal of Health Care for the Poor and Underserved 2002;13(3):298‐319. [PubMed] [Google Scholar]
Oriol 2009 {published data only}
- Oriol NE, Cote PJ, Vavasis AP, Bennet J, DeLorenzo D, Blanc P, et al. Calculating the return on investment of mobile healthcare. BMC Medicine 2009;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Padmadas 2014 {published data only}
- Padmadas SS, Amoako Johnson F, Leone T, Dahal GP. Do mobile family planning clinics facilitate vasectomy use in Nepal?. Contraception 2014;89(6):557‐63. [DOI] [PubMed] [Google Scholar]
Paone 2015 {published data only}
- Paone M, Whitehouse SR, Evans D. 51. JUST TRAC it!: assessing a Mobile‐Health (mhealth) intervention for youth with chronic health conditions and/or disabilities (CHC/Ds) to encourage engagement and readiness behaviors for transition. Journal of Adolescent Health 2015;56(2 Suppl 1):S27‐8. [Google Scholar]
Peek 2009 {published data only}
- Peek ME, Han JH. Compliance and self‐reported barriers to follow‐up of abnormal screening mammograms among women utilizing a county mobile mammography van. Health Care for Women International 2009;30(10):857‐70. [DOI] [PubMed] [Google Scholar]
Renck 2014 {published data only}
- Renck DV, Barros F, Domingues MR, Gonzalez MC, Sclowitz ML, Caputo EL, et al. Equity in access to breast cancer screening in a mobile mammography program in southern Rio Grande do Sul State, Brazil [Equidade no acesso ao rastreamento mamográfico do câncer de mama com intervenção de mamógrafo móvel no sul do Rio Grande do Sul, Brasil]. Cadernos de Saúde Pública 2014;30(1):88‐96. [DOI] [PubMed] [Google Scholar]
Riley 2014 {published data only}
- Riley EC, Roland L, Barkley L, Pan J, Rai S, Mizuguchi S. Impact of location to repeat mobile mammography utilization trends: 10‐year analysis of a comprehensive urban cancer center. Journal of Clinical Oncology 2014;32(5S):Abstract 6577. [Google Scholar]
Schnippel 2015 {published data only}
- Schnippel K, Lince‐Deroche N, Handel T, Molefi S, Bruce S, Firnhaber C. Cost evaluation of reproductive and primary health care mobile service delivery for women in two rural districts in South Africa. PLoS One 2015;10(3):e0119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scholl 2011 {published data only}
- Scholl E, Peterson J. Mobile clinics in India take to the road. Bringing HIV testing and counseling and sexually transmitted infection services to those most at risk. https://aidsfree.usaid.gov/sites/default/files/mobile_clinics_in_india_take_to_the_road.pdf. Arlington, Virginia, John Snow[JSI].
Schwartze 2014 {published data only}
- Schwartze J, Wolf KH, Bargen T, Rochon M, Wagner M, Bannenberg U, et al. Rollende arztpraxis ‐ first results of an ambulant mobile care model for rural areas. Studies in Health Technology and Informatics 2014;202:295‐8. [PubMed] [Google Scholar]
Schwitters 2015 {published data only}
- Schwitters A, Lederer P, Zilversmit L, Gudo PS, Ramiro I, Cumba L, et al. Barriers to health care in rural Mozambique: a rapid ethnographic assessment of planned mobile health clinics for ART. Global Health: Science and Practice 2015;3(1):109‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silveira 2014 {published data only}
- Silveira CEG, Silva TB, Fregnani JHGT, Costa Vieira RA, Haikel RL Jr, Syrjänen K, et al. Digital photography in skin cancer screening by mobile units in remote areas of Brazil. BMC Dermatology 2014;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soclerstrom 1997 {published data only}
- Soclerstrom EK, Long TM, Sherman J. MoVES: incorporating developmental services on a pediatric mobile health care clinic. Infants & Young Children 1997;9(3):78‐86. [Google Scholar]
Solon 1986 {published data only}
- Solon F. Family welfare through improved health care. JOICFP Review 1986;12:19‐21. [PubMed] [Google Scholar]
Spagnolo 1995 {published data only}
- Spagnolo G, Zappa M, Paci E, Giorgi D, Rosselli del Turco M. [Evaluation of the costs of mammographic screening programme in the city of Florence]. Epidemiologia e Prevenzione 1995;19(65):330‐7. [PubMed] [Google Scholar]
Spencer 1990 {published data only}
- Spencer HC, Ruiz‐Tibén E, Mansour NS, Cline BL . Evaluation of UNICEF/Arab Republic of Egypt/WHO schistosomiasis Control Project in Beheira Governorate. American Journal of Tropical Medicine & Hygiene 1990;42(5):441‐8. [DOI] [PubMed] [Google Scholar]
Steward 2014 {published data only}
- Steward LT, Kraenzle S, Drake BF, Lyons S, Goodman MS. An evaluation of mobile mammography outreach in urban and rural communities.. Journal of Clinical Oncology 2014;32(15 Supplement):e17524. [Google Scholar]
Swaddiwudhipong 1995 {published data only}
- Swaddiwudhipong W, Chaovakiratipong C, Nguntra P, Mahasakpan P, Lerdlukanavonge P, Koonchote S. Effect of a mobile unit on changes in knowledge and use of cervical cancer screening among rural Thai women. International Journal of Epidemiology 1995;24(3):493‐8. [DOI] [PubMed] [Google Scholar]
Swaddiwudhipong 1999 {published data only}
- Swaddiwudhipong W, Chaovakiratipong C, Nguntra P, Mahasakpan P, Tatip Y, Boonmak C . A mobile unit: an effective service for cervical cancer screening among rural Thai women. International Journal of Epidemiology 1999;28(1):35‐9. [DOI] [PubMed] [Google Scholar]
Swanson 2003 {published data only}
- Swanson KA, Andersen R, Gelberg L. Patient satisfaction for homeless women. Journal of Women's Health 2003;12(7):675‐86. [DOI] [PubMed] [Google Scholar]
Takenaga 1985 {published data only}
- Takenaga N, Kai I, Ohi G. Evaluation of three cervical cancer detection programs in Japan with special reference to cost‐benefit analysis. Cancer 1985;55(10):2514‐9. [DOI] [PubMed] [Google Scholar]
Tedrow 2012 {published data only}
- Tedrow VA, Zelaya CE, Kennedy CE, Morin SF, Khumalo‐Sakutukwa G, Sweat MD, et al. No "magic bullet": exploring community mobilization strategies used in a multi‐site community based randomized controlled trial: Project Accept (HPTN 043). AIDS and Behavior 2012;16(5):1217‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thandar 2015 {published data only}
- Thandar MM, Kyaw MP, Jimba M, Yasuoka J. Caregivers' treatment‐seeking behaviour for children under age five in malaria‐endemic areas of rural Myanmar: a cross‐sectional study . Malaria Journal 2015;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tianviwat 2009 {published data only}
- Tianviwat S, Birch S, Chongsuvivatwong V. Comparison of the effects of secondary prevention in schoolchildren between hospitals with and without mobile dental services in Southern Thailand. Journal of Oral Science 2009;51(1):97‐102. [DOI] [PubMed] [Google Scholar]
Tonne 1979 {published data only}
- Tonne R. The efficiency of school clinics. Stomatologie der DDR 1979;29(9):657‐61. [PubMed] [Google Scholar]
Treadway 1973 {published data only}
- Treadway RC, Forrest JE. Family planning program in India: an evaluation. Studies in Family Planning 1973;4(6):149‐56. [PubMed] [Google Scholar]
van der Pol 1998 {published data only}
- Pol M, Varwijk W. Feasibility of mobile provision of health services: a study of child monitoring centres in The Netherlands. The International Journal of Health Planning & Management 1998;13(3):244‐54. [DOI] [PubMed] [Google Scholar]
van Dijk 2014 {published data only}
- Dijk JH, Moss WJ, Hamangaba F, Munsanje B, Sutcliffe CG. Scaling‐up access to antiretroviral therapy for children: a cohort study evaluating care and treatment at mobile and hospital‐affiliated HIV clinics in rural Zambia. PLoS One 2014;9(8):e104884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang QF, Wang GY. Observations on the efficacy of acupuncture and moxibustion plus blood‐letting puncture and movable cupping in treating acne. Shanghai Journal of Acupuncture and Moxibustion [Shang Hai Zhen Jiu Za Zhi] 2007;26(12):20‐1. [Google Scholar]
Williams 1989 {published data only}
- Williams EM, Vessey MP. Randomised trial of two strategies offering women mobile screening for beast cancer. BMJ 1989;299(6692):158‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2009 {published data only}
- Yoon NH, Lee HY, Kwak MS, Choi KS, Jun JK, Kim MK, et al. Comparison of satisfaction with cancer screening at mobile van and static sites: National Cancer Screening Program in Korea. Japanese Journal of Clinical Oncology 2009;39(3):169‐74. [DOI] [PubMed] [Google Scholar]
Zabos 2001 {published data only}
- Zabos GP, Trinh C. Bringing the mountain to Mohammed: a mobile dental team serves a community‐based program for people with HIV/AIDS. American Journal of Public Health 2001;91(8):1187‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Coronado 2014 {published data only}
- Coronado GD, Jimenez R, Martinez‐Gutierrez J, McLerran D, Ornelas I, Patrick D, et al. Multi‐level Intervention to increase participation in mammography screening: ¡Fortaleza Latina! study design . Contemporary Clinical Trials 2014;38(2):350‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotschan 2014 {published data only}
- Kotschan N. Early detection saves lives through mobile screening units . Asia‐Pacific Journal of Clinical Oncology 2014;10:30. [Google Scholar]
Labhardt 2014 {published data only}
- Labhardt ND, Motlomelo M, Cerutti B, Pfeiffer K, Kamele M, Hobbins MA, et al. Home‐based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster‐randomized trial . PLoS Medicine 2014;11(12):e1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACCP 2004
- Alliance for Cervical Cancer Prevention. Chapter 6: Delivering clinical services and strengthening linkages at planning and implementing cervical cancer prevention and control programs: a manual for managers. In: Planning and Implementing Cervical Cancer Prevention and Control Programs: A Manual for Managers. 2004. http://www.searo.who.int/nepal/mediacentre/2004_planning_and_implementing_cervical_cancer_prevention_n_control_programs.pdf?ua=1 (accessed 28 June 2007).
Edgerley 2007
- Edgerley LP, El‐Sayed YY, Druzin ML, Kiernan M, Daniels KI. Use of a community mobile health van to increase early access to prenatal care . Maternal and Child Health Journal 2007;11(3):235‐9. [DOI] [PubMed] [Google Scholar]
El‐Gibaly 2008
- El‐Gibaly OMH, Moftah FM, Salah M, Al‐Attar GST. Have mobile clinics increased access to family planning methods in Assiut Governorate? . Assiut Medical Journal 2008;32:49‐57. [Google Scholar]
El‐Zanaty 2001
- El‐Zanaty F, Hamed R. Sustainability of mobile clinics in reproductive health and family planning service delivery. El‐Zanaty and associates, Research Management Unit, National Population Council 2001:1‐48.
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services 2015:Available at: http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
Gruen 2003
- Gruen RL, Weeramanthri TS, Knight SE, Bailie RS. Specialist outreach clinics in primary care and rural hospital settings. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003798.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutubessy 2003
- Hutubessy R, Chisholm D, Tan‐Torres Edejer T, WHO‐CHOICE. Generalized cost‐effectiveness analysis for national‐level priority‐setting in the health sector. Cost Effectiveness and Resource Allocation 2003;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2012
- Jacobs B, Ir P, Bigdeli M, Annear PL, Damme W. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in low‐income Asian countries. Health Policy and Planning 2012;27(4):288–300. [DOI] [PubMed] [Google Scholar]
Mdege 2014
- Mdege ND, Chindove S. Bringing antiretroviral therapy (ART) closer to the end‐user through mobile clinics and home‐based ART: systematic review shows more evidence on the effectiveness and cost effectiveness is needed. The International Journal of Health Planning and Management 2014;29(1):e31‐47. [DOI] [PubMed] [Google Scholar]
Mercer 2005
- Mercer A, Ashraf A, Huq NL, Haseen F, Uddin AH, Reza M. Use of family planning services in the transition to a static clinic system in Bangladesh: 1998‐2002. International Family Planning Perspectives 2005;31(3):115‐23. [DOI] [PubMed] [Google Scholar]
Molyneaux 1988
- Molyneaux JW, Alimoeso S, Lerman C, Moeljodihardjo S. Program impacts on contraceptive distribution and method mix in the Indonesian family planning program: causal modelling with pooled cross‐sectional and time series data from East Java. Indonesian Journal of Demography [Majalah Demografi Indonesia] 1988;15(30):37‐67. [PubMed] [Google Scholar]
Onyia 1981
- Onyia DN, Sanda O. Mobile under‐fives clinic in Ekpoma, Nigeria. Tropical Doctor 1981;11(3):128‐31. [DOI] [PubMed] [Google Scholar]
Oxman 2009
- Oxman AD, Fretheim A, Lavis JN, Lewin S. SUPPORT Tools for evidence‐informed health Policymaking (STP) 12: Finding and using research evidence about resource use and costs. Health Research Policy and Systems 2009;7(Suppl 1):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 1995
- Skinner CS, Zerr AD, Damson RL. Incorporating mobile mammography units into primary care: focus group interviews among inner‐city health centre patients. Health Education Research 1995;10(2):179‐89. [DOI] [PubMed] [Google Scholar]
Sriamporn 2006
- Sriamporn S, Khuhaprema T, Parkin M. Cervical cancer screening in Thailand: an overview. Journal of Medical Screening 2006;13(Suppl 1):S39‐43. [PubMed] [Google Scholar]
Vashishtha 2014
- Vashishtha V, Kote S, Basavaraj P, Singla A, Pandita V, Malhi RK. Reach the unreached ‐ a systematic review on mobile dental units. Journal of Clinical Diagnostic Research 2014;8(8):ZE05‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 1990
- Vos J, Borgdorff MW, Kachidza EG. Cost and output of mobile clinics in a commercial farming area in Zimbabwe. Social Science and Medicine 1990;31(11):1207‐11. [DOI] [PubMed] [Google Scholar]
Welsh 2006
- Welsh MJ, Stanback J, Shelton J. Access to modern contraception. Best Practice & Research. Clinical Obstetrics & Gynaecology 2006;20(3):323‐38. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abdel‐Aleem 2012
- Abdel‐Aleem H, El‐Gibaly OMH, EL‐Gazzar AFES, Al‐Attar GST. Mobile clinics for women’s and children’s health. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009677] [DOI] [PMC free article] [PubMed] [Google Scholar]


