Abstract
Culture supernatants of nontoxigenic nonepidemic clinical strains of Vibrio cholerae belonging to diverse serogroups were found to induce vacuolation of nonconfluent HeLa cells. The vacuoles became prominent 18 h after introduction of culture supernatant, and vacuolated cells survived for 48 h and then died. Only a fraction of the vacuolated cells took up neutral red dye, implying that there were differences in the vacuolar microenvironment. Further tests showed that the factor responsible for vacuolation was heat labile and proteinaceous. Vacuolating activity was completely neutralized by antibody to hemolysin of V. cholerae but not by antibody to vacuolating cytotoxin of Helicobacter pylori. Partial purification of the vacuolating factor led to elution of fractions, which showed both hemolytic and vacuolating activity. PCR amplification and cloning of the hemolysin structural gene (hlyA) into Escherichia coli DH5α led to isolation of clones producing cell vacuolating factor in a cell-associated form. Further, a null insertion mutation in the hlyA gene of a high-vacuolating-factor-producing strain led to complete abolition of both cell vacuolating and hemolytic activities. These analyses establish vacuolation as a potentially important but previously unrecognized property of V. cholerae El Tor hemolysin.
Much of the virulence of bacterial pathogens is initiated by secreted factors that induce specific biochemical changes in host tissue, culminating in pathology and disease. Cholera toxin (CT), the potent secretory toxin produced by toxigenic strains of Vibrio cholerae, is critically involved in key features of the disease cholera. Other putative factors, including zonula occludens toxin (Zot), accessory cholera enterotoxin (Ace), El Tor hemolysin, Shiga-like toxin, and heat-stable toxin, are reportedly produced by virulent strains of V. cholerae and are thought to contribute to the disease process (12). Despite attenuation of several virulence genes in recombinant candidate vaccine strains of V. cholerae, a safe and efficacious vaccine still eludes us. The residual diarrhea caused by genetically attenuated live oral vaccine strains of V. cholerae O1 prompted us to look for new factors secreted by V. cholerae.
One means of assessing the toxicity of the secreted products of bacterial pathogens is to study the effect of culture supernatants on eukaryotic cell lines (12). In this context, we have previously reported that cell-free culture supernatants of V. cholerae strains induced morphological changes, including elongation and rounding in eukaryotic cells (18, 21, 23). We initiated the present study to examine the mechanism as to how nontoxigenic nonepidemic V. cholerae strains are able to cause a disease that resembles cholera in absence of already known virulence determinants, especially CT, found in their toxigenic epidemic causing counterparts. While attempting this, we observed that some clinical strains of V. cholerae induce vacuolation on HeLa cells, which seemed reminiscent of that induced by VacA cytotoxin of H. pylori (6). Given that the vacuolating cytotoxin (VacA) of H. pylori is implicated in the etiology of peptic ulcer (6), it seemed that V. cholerae-induced vacuolation would also contribute to the disease it causes. In this study, we provide evidence that the El Tor hemolysin of V. cholerae induces cell vacuolation in HeLa cells.
MATERIALS AND METHODS
Bacterial strains and plasmids.
A collection of 110 V. cholerae strains belonging to different serogroups, each from different diarrheal patients from different parts of India, were used in this study. V. cholerae CO848b belonging to the O26 serogroup was used for purification of the cell vacuolating factor. The strains were biochemically characterized, and their serological identity was determined by using specific antisera; they were then further tested for the presence of virulence genes such as ctx, zot, and ace by PCR assays described previously (9, 18). Escherichia coli strains and plasmids used in this study are shown in Table 1. Strains were grown on Luria agar containing appropriate antibiotics when required. The antibiotics used were purchased from Sigma (St. Louis, Mo.) and were used in the following concentrations: streptomycin (1 mg/ml), kanamycin (50 μg/ml), tetracycline (12.5 μg/ml for E. coli and 2 μg/ml for V. cholerae), and ampicillin (100 μg/ml).
TABLE 1.
Bacteria and plasmids used in the study
| Strain | Serogroup and/or description | Source and/or reference |
|---|---|---|
| V. choleraea | ||
| CO604 | O10 (4) | This work (clinical isolate) |
| CO848b | O26 Strs (32) | This work (clinical isolate) |
| AS16 | O144 (8) | This work (clinical isolate) |
| AS233 | O151 (16) | This work (clinical isolate) |
| DO40 | O37 (4) | This work (clinical isolate) |
| DO42 | O19 (4) | This work (clinical isolate) |
| DO45 | O8 (8) | This work (clinical isolate) |
| DO50 | O107 (16) | This work (clinical isolate) |
| DO56 | O145 (16) | This work (clinical isolate) |
| DO57 | O2 (8) | This work (clinical isolate) |
| DO62 | O186 (16) | This work (clinical isolate) |
| DO61 | O145 (4) | This work (clinical isolate) |
| DO69 | O145 (2) | This work (clinical isolate) |
| DO71 | O37 (2) | This work (clinical isolate) |
| DO76 | O125 (16) | This work (clinical isolate) |
| DO77 | O37 (16) | This work (clinical isolate) |
| CLO5 | O169 (16) | This work (clinical isolate) |
| CO853 | O139 (8) | This work (clinical isolate) |
| CO848b-str | O26 (32) | Spontaneous Strr mutant of CO848b |
| E. coli | ||
| SM10λpir | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu, Kmr (λpir) | 16 |
| S17-1λpir | Tpr SmrrecA thi pro hsdR−M+ RP4:2-Tc:Mu:Kmr Tn7 (λpir) | 7 |
| Plasmids | ||
| pBluescript | Ampr | 24 |
| pBR322 | Tetr | 4 |
| pKAS46 | Ampr Kmr | 25 |
| pRM | pBluescript hlyA | This work |
| pRM1 | pBluescript hlyA::tet | This work |
| pRM2 | pKAS46 hlyA::tet | This work |
All V. cholerae strains lacked ctxAB, zot, and ace but had the hlyA gene. Also, 92 other strains of various serogroups had no vacuolating titer. The value in parentheses is the reciprocal of the cell vacuolating activity of nonconcentrated culture supernatants.
Tissue culture assay.
AKI medium (1.5% Bacto-Peptone, 0.4% yeast extract, 0.5% NaCl, 0.3% filter-sterilized NaHCO3; pH 7.4) (10) was used to grow the V. cholerae strains at 37°C for 16 h in a rotary shaker at 100 rpm. The culture supernatant, obtained by centrifugation at 4°C at 10,000 × g was filter sterilized by using 0.22-μm-pore-size disposable filter units (Sigma), and the resultant cell-free culture filtrate (CFCF) was used for assay of vacuolating activity. HeLa cells were grown in Dulbecco modified Eagle medium (DMEM; Gibco Laboratories, Grand Island, N.Y.) supplemented with 10% horse serum and transferred from the growth flask to 96-well tissue culture plates (Nunc, Roskilde, Denmark), where the density of cells per well was maintained at 60 to 70% confluence. CFCF was serially diluted in DMEM containing 2% horse serum (Gibco) and added to cultured cells maintained in DMEM containing 2% horse serum. Cells were then incubated at 37°C in a humidified 5% CO2 atmosphere (Kendro Laboratory Product, Haraeus Instruments, Hanau, Germany) for 24 h. Morphological changes were observed by using an inverted microscope. The titer of the cell vacuolating activity in a sample was defined as the reciprocal of the highest dilution showing 50% vacuolation of HeLa cells.
Protease assay.
A freshly prepared concentrated culture supernatant of V. cholerae strain CO848b was incubated with 2.5 U of insoluble proteinase K-bead suspension at 37°C for 1 h. As a control, the supernatant was incubated under the same conditions without added protease. After incubation, the beads were eliminated by centrifugation at 3,000 × g for 1 min, and the supernatants were tested on nonconfluent HeLa cells after serial dilution as described earlier (17).
Purification of cell vacuolating factor.
Strain CO848b was grown in AKI medium for 16 h at 37°C with shaking (100 rpm) and then centrifuged at 4°C to obtain the culture supernatant. A cocktail of protease inhibitors (Sigma) was added to the supernatant to inhibit protease activity, and the supernatant was concentrated by ultrafiltration by using XM50 membrane (Millipore, Bedford, Mass.) and assayed for cell vacuolating activity. It was applied to a Sephacryl S-200 HR (Sigma) column (Pharmacia; 16 by 40 cm) preequilibrated with Tris-EDTA buffer (pH 7.4), and eluted fractions were assayed for cell vacuolating activity on nonconfluent HeLa cells.
Assay for hemolytic activity.
Culture supernatants of test strains and fractions obtained after gel chromatography were assayed for hemolytic activity. Freshly prepared 1% rabbit erythrocytes were treated with an equal volume of serially diluted concentrated culture supernatant of strain CO848b grown in AKI medium, as were fractions showing vacuolating activity after gel chromatography. Purified El Tor hemolysin (a gift of K. Banerjee, National Institute of Cholera and Enteric Diseases, Calcutta, India) mixed with 1% rabbit erythrocytes was used as positive control. The mixtures were incubated at 37°C for 1 h and centrifuged at 2,000 × g for 3 min. The optical density at 540 nm (OD540) of the supernatant was read, and the hemolytic titer was calculated as the ratio of OD540 (test)/OD540 (control). A curve of the reciprocal titer versus the fraction number was plotted.
Neutralization assay.
Equal volumes of serial dilutions of 50-fold-concentrated culture supernatants were mixed with equal volumes of 1:2-diluted polyclonal anti-El Tor hemolysin antiserum (also a gift of K. Banerjee) or anti-H. pylori VacA neutralizing antiserum (20) (a gift of Hisao Kurazono, Department of Medical Technology, School of Health Sciences, Okayama University, Okayama, Japan) and were incubated for 1 h at 37°C. These were then added to nonconfluent HeLa cells and incubated overnight at 37°C in a humidified 5% CO2 atmosphere.
Neutral red assay.
A stock solution (10%) of purified grade neutral red (Sigma) was used for staining vacuolated cells. The staining solution was prepared before each experiment by diluting the stock solution 1:10 in Hanks balanced salt solution. After incubation with test samples for 21 h, the medium overlaying the HeLa cells was removed and 100 μl of staining solution per well was introduced for 4 min. The cells were then washed twice with 150 μl of 0.9% saline per well and observed under an inverted microscope to visualize the neutral red uptake by cells.
PCR amplification and cloning of hlyA gene from strain CO848b.
A phenol-chloroform method was used for DNA extraction (19). Amplification of hlyA gene from strain CO848b was done by using the following primers: hlyA (forward), CTG TCT AGA [XbaI] AGT GAG GTT TAT ATG CCA AAA CTC AAT CGT; hlyA (reverse), CTG CTC GAG [XhoI] TTA GTT CAA ATC AAA TTG AAC CCC TTT CAC CAA; and hlyB (reverse), GAT CCG ATT TTG CAC TTC GCC TAC CACT. These primers were designed from the El Tor hlyA sequence determined previously (3, 15). The composition of the 20 μl of PCR reaction mixture was as follows: 2.0 μl of 10× amplification buffer (500 mM KCl, 100 mM Tris HCl [pH 8.0]; 0.1% Triton X-100), 2 μl of 25 mM MgCl2, 2 μl of 2.5 mM concentrations of deoxynucleoside triphosphates, 1 μl (10 pmol/μl) each of the hlyA primers, 0.2 μl (5 U) of Taq polymerase (Promega), 1 μl of template DNA, and 10.8 μl of MilliQ water. The solution was overlaid with a drop of sterile mineral oil (Sigma), and PCR was performed in an automated thermal cycler (Perkin-Elmer, Norwalk, Conn.) for 30 cycles with the following cycling condition: denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 2 min. Amplified products were electrophoresed on 2% agarose gels and stained with ethidium bromide. A 1-kb molecular size ladder (New England Biolabs) was run in each gel.
The PCR product was purified by using a Qiagen PCR purification kit (Qiagen, Inc.), digested with the restriction enzymes XbaI and XhoI (cleavage sites in primers), and ligated with XbaI- and XhoI-cleaved pBluescript plasmid vector DNA to ensure transcription of the cloned hlyA DNA from the vector promoter. The ligated vector (pRM)-target DNA mix was transformed into competent E. coli DH5α cells following CaCl2 treatment (14). Transformants were selected on Luria agar containing ampicillin (100 μg/ml), isopropyl thiogalactosidase (40 μg/ml), and 5-bromo-4-chloro-3-indolyl-d-galactopyranoside (X-Gal) (40 μg/ml), and white colonies were tested for inserted DNA by PCR essentially as described above.
Generation of hlyA null insertion mutant in strain CO848b.
To construct a hlyA null insertion mutant, a Tetr cartridge was cloned into a unique HpaI site in hlyA in the pBluescript hlyA clone (position 629 in the 2,223-bp open reading frame). The hlyA gene which was cloned into plasmid pRM was digested with HpaI enzyme to delete a 400-bp internal fragment (11) and then ligated to a blunt-ended 1.4-kb tetracycline fragment to generate the plasmid pRM1 (Table 1). The 1.4-kb tetracycline fragment was obtained after digestion of plasmid pBR322 with AvaI and EcoRI, followed by Klenow polymerase treatment. Plasmid pRM1, which has a backbone of the pBluescript plasmid, was then digested with KpnI and SacI to obtain a 3.2-kb fragment which consists of a truncated 1.8-kb hlyA gene disrupted by a 1.4-kb tetracycline fragment. This 3.2-kb gene fragment was ligated en bloc into suicide plasmid pKAS46 (25) to generate plasmid pRM2. This was transformed into E. coli S17-1λpir and then transferred by conjugation into E. coli SM10λ-pir, which is a good donor for conjugation with V. cholerae. E. coli SM10λpir harboring pRM2 was mated with V. cholerae CO848b-str and plated on Luria agar with streptomycin (1 mg/ml) and tetracycline (2 μg/ml). This process selected for V. cholerae that had acquired the mutated hlyA gene in plasmid pRM2. Exponential-phase cultures of the donor E. coli SM10λpir harboring the recombinant plasmid and recipient V. cholerae CO848b (Strr) strains were mixed in the ratio of 1:10, concentrated by centrifugation (300 × g, 5 min), and spread on cellulose acetate filters (Millipore) on nutrient agar plates. After incubation for 4 h at 37°C, the cells were resuspended in 10 ml of saline (0.9% [wt/vol] NaCl) and plated on Luria agar with tetracycline (2 gm/ml) and streptomycin (1 mg/ml). After homologous recombination, only those V. cholerae strains survived which had retained the hlyA tet insertion allele by allelic exchange. PCR with primers specific for the hlyA gene was performed to confirm that allelic replacement had occurred, and the isogenic strain thus generated was tested for cell vacuolating and hemolytic activities.
RESULTS
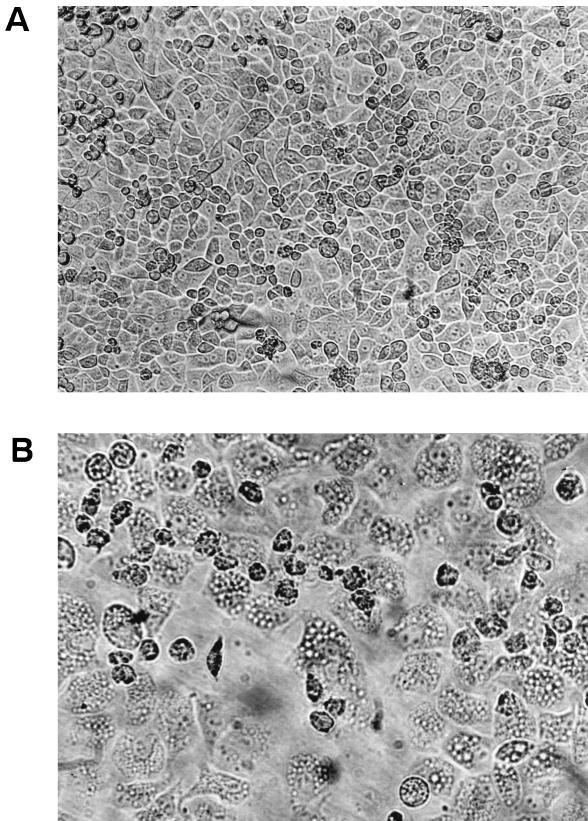
A total of 110 nonepidemic strains of V. cholerae belonging to different serogroups isolated from diarrheal patients from different parts of India were tested for their effects on a eukaryotic cell line. These strains lacked the ctx, zot, and ace genes that are characteristic of the epidemic strains. When grown in AKI medium at 37°C for 16 h, the culture supernatants of 18 of the 110 strains (Table 1), each belonging to a different serogroup, caused vacuolation in nonconfluent HeLa cells (Fig. 1). Generally, the vacuoles appeared 8 h after introduction of the culture supernatant and became prominent after 18 h of incubation. The vacuoles remained for 48 h, after which the cells died. Some vacuoles took up neutral red, while others did not (data not shown). Neutral red uptake indicates the nature of the vacuolar microenvironment; thus, some vacuoles were acidic, while others were basic.
FIG. 1.
(A) Morphology of normal confluent HeLa cells (magnification, ×20). (B) Morphology of vacuolated HeLa cells (magnification, ×40).
To further characterize the vacuolating factor, we used V. cholerae strain CO848b, which had the highest titer of vacuolating activity CO848b (Table 1). First, concentrated culture supernatants of strain CO848b were incubated at various temperatures. Incubation at 60°C for 10 min completely abolished the cell vacuolating activity, whereas incubation at 50°C did not. The vacuolating activity also decayed slowly (fourfold decline after 1 week). Treatment of the supernatant with proteinase K-beads showed that the vacuolating factor was sensitive to proteases, thereby indicating that it was proteinaceous.
Culture supernatants of strain CO848b obtained after Amicon membrane concentration of >50 kDa showed vacuolating activity, which was then loaded onto a Sephacryl S-200 chromatographic column for further purification. Fractions eluting just after the void volume were found to have a high vacuolating titer, which also coincidentally showed high hemolytic activity. Coelution of fractions showing vacuolating and hemolytic activities thus prompted an antibody neutralization study with anti-El Tor hemolysin antiserum of V. cholerae and anti-VacA antiserum of H. pylori. The neutralization study showed that the cell vacuolating effect was completely neutralized by anti-El Tor hemolysin antibody but not by anti-VacA antibody (data not shown), which suggested that the vacuolating factor was related to the hemolysin of V. cholerae. We then PCR amplified the hlyA gene from the strain CO848b by using primers designed from the known El Tor sequence, cloned the gene into pBluescript, to generate plasmid pRM, and transformed this plasmid into E. coli DH5α. Culture supernatants of these E. coli transformants did not elicit vacuolation on cultured HeLa cells, whereas lysates of the same culture did, albeit at a lower titer (sixfold) compared to V. cholerae CO848b (Table 2).
TABLE 2.
Vacuolating and hemolytic titers of V. cholerae and E. coli strains with recombinant clonesa
| Strainb | Test sample | Reciprocal titer of:
|
|
|---|---|---|---|
| Cell vacuolating activity | Hemolytic activity | ||
| V. cholerae CO848b (wild type) | 50-Fold-concentrated culture supernatant | 1,024 | 512 |
| V. cholerae CO848b (Strr) | 50-Fold-concentrated culture supernatant | 1,024 | 512 |
| V. cholerae CO848b (hlyA::tet) | 50-Fold-concentrated culture supernatant | <2c | <2 |
| E. coli DH5α | 50-Fold-concentrated culture supernatant | <2 | <2 |
| E. coli DH5α | Cell sonicate | <2 | <2 |
| E. coli DH5α(pBSC-hlyA) | 50-Fold-concentrated culture supernatant | <2 | <2 |
| E. coli DH5α(pBSC-hlyA) | Cell sonicate | 16 | 32 |
V. cholerae and E. coli DH5α were grown in 50 ml of AKI medium, and the cell-free culture supernatant was concentrated 50-fold by ultrafiltration and then added to nonconfluent HeLa cells. The bacterial pellet obtained from recombinant E. coli DH5α(pBSC-hlyA) grown in 50 ml of Luria-Bertani medium was sonicated, and then the cell sonicate was added to HeLa cells.
Strr, Streptomycin resistant; hlyA::tet, hemolysin gene disrupted by tetracycline gene; pBSC-hlyA, plasmid pBluescript with cloned hemolysin (hlyA) gene.
A value of <2 depicts no vacuolating or hemolytic activities.
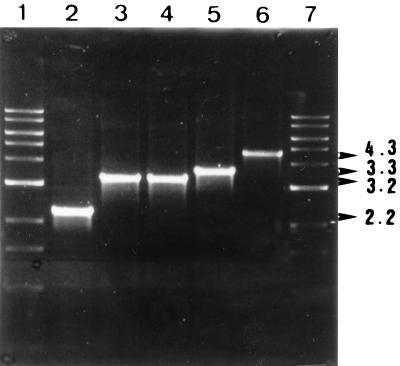
To test the idea that the Hly protein is responsible for the observed vacuolating activity, we made an hlyA-negative mutant derivative of V. cholerae. This entailed inserting a Tetr gene into the cloned hlyA gene in E. coli, moving the mutant allele to a shuttle suicide vector plasmid, and from there moving it to the V. cholerae chromosome by conjugation (E. coli donor strain harboring this plasmid with V. cholerae recipient) and selection for exconjugants that had received the hlyA insertion marker (Tetr) (the result of homologous recombination resulting in allelic replacement and loss of the suicide vector). To check for the positive clones, genomic DNA was isolated from the antibiotic-resistant colonies, and a PCR was done with two sets of primers. In the first set of PCRs with primer pair hlyA (forward) and hlyA (reverse), the wild-type colonies generated a 2.2-kb product, whereas plasmid pRM2 and the antibiotic-resistant colonies generated 3.2-kb product (lanes 2, 3, and 4, respectively; Fig. 2). The antibiotic-resistant colonies were thus V. cholerae colonies with the hlyA gene disrupted by tetracycline gene fragment. The second set of PCRs was carried with primer pair hlyA (forward) and hlyB (reverse), the latter being a gene adjacent to the hlyA gene (15). This reaction generated a 3.3-kb product for the wild-type colonies and 4.3-kb product for the antibiotic-resistant colonies (lanes 5 and 6, respectively; Fig. 2). These PCR results proved that the homologous recombination event took place, leading to the construction of an isogenic V. cholerae hlyA::tet strain. The cell-free culture supernatant of the hlyA mutant strain was devoid of both cell vacuolating and hemolytic activities (Table 2). This experiment established that cell vacuolation is caused by the El Tor hemolysin of V. cholerae.
FIG. 2.
Agarose gel electrophoresis profile of PCR product generated by using different sets of primers during the construction of hemolysin null mutant. Lanes 1 and 7 show the 1-kb DNA ladder (New England Biolabs). Lanes 2, 3, and 4 show the PCR product generated from wild-type CO848b, plasmid pRM, and CO848b hlyA::tet, respectively, with primers hlyA (forward) and hlyA (reverse). Lanes 5 and 6 show the PCR product generated from CO848b (wild type) and CO848 (hlyA::tet), respectively, with primers hlyA (forward) and hlyB (reverse).
DISCUSSION
The demonstration that the V. cholerae hemolysin exhibits vacuolating activity on nucleated mammalian cells is interesting for several reasons. First, only a few other bacterial toxins with vacuolating activity have been identified to date. Second, by demonstrating that hemolysin induces vacuolation in nucleated mammalian cells, the current study raises the possibility that this toxin might contribute to gastrointestinal symptoms associated with some V. cholerae infections, particularly infections involving V. cholerae strains that do not express well-established enterotoxins (e.g., CT). Third, if V. cholerae hemolysin can affect intestinal cells, this effect might help explain the mild gastrointestinal effects noted when human volunteers are given V. cholerae vaccine strains that have been attenuated for expression of established enterotoxins.
Unlike H. pylori VacA-induced vacuoles, only a fraction of the V. cholerae vacuoles took up neutral red dye. However, it must be mentioned that there is no amino acid sequence homology between V. cholerae El Tor hemolysin and VacA of H. pylori. This diversity in V. cholerae relative to the uniformity for H. pylori neutral red dye uptake indicates a difference in the vacuolar microenvironments induced by these two gastrointestinal pathogens. While characterizing the cell vacuolating activity in the Indian V. cholerae strains, a separate analysis of a probably similar vacuolating activity of Mexican nonepidemic V. cholerae strains on Vero cells had implicated hlyA (P. Figueroa and D. E. Berg, unpublished data). These preliminary data then prompted us to test whether the hlyA was the only determinant responsible for the vacuolating activity. The hlyA structural gene from the test strain CO848b was PCR amplified and cloned in E. coli DH5 cells. The cloned gene product in E. coli DH5 induced cell vacuolation in a cell-associated form, which may be due to the lack of an effective transport machinery to export the HlyA protein. We then generated an hlyA null insertion mutant derivative of strain CO848b, which was unable to induce cell vacuolating effect on HeLa cell and did not show the hemolytic effect in rabbit red blood cells. This conclusively established the role played by hemolysin in cell vacuolation. However, only 15% of the 110 strains of V. cholerae exhibited the vacuolating activity, even though all of them had the hlyA gene. Possibly, the vacuolating factor of the remaining strains was produced in amounts that could not be detected by our HeLa cell assay. On the other hand, the protein may have had a different host cell specificity since HeLa is not an intestinal cell line. An earlier report had linked cell vacuolation to a heat-labile protease-sensitive vacuolating cytotoxin in stool samples of Italian children with diarrhea (13). However, no organism was isolated from the stool samples, and thus the agent responsible for this activity was not determined.
Production of the El Tor hemolysin was the basis of differentiation between classical and El Tor biotypes of V. cholerae O1 when the El Tor strains appeared in 1961, but over the years this test has lost its significance because recent isolates of the El Tor biotype and also the O139 Bengal strains do not produce El Tor hemolysin, although the gene is present in all El Tor O1 and O139 strains of V. cholerae. It is not certain why the expression of this gene has become silent over a period of three decades. There is already a wealth of information on the biological relevance of the El Tor hemolysin, some of which is controversial. Previous studies have shown that the classical isolates of V. cholerae possess an 11-bp deletion in the structural gene for the El Tor hemolysin leading to the production of a 27-kDa nonhemolytic but enterotoxic truncated product HlyA∗ compared to the 82-kDa hemolysin HlyA produced by El Tor strains of V. cholerae (2). To test the hypothesis that the El Tor hemolysin was responsible for the residual diarrhea seen with Δctx strains of V. cholerae, Kaper et al. (12) constructed derivatives of such strains, which were mutated in the hlyA gene by deletion of an internal 400-bp HpaI fragment. When tested in volunteers, the ΔhlyA strains CVD104 and CVD105 still caused diarrhea in 33% of the subjects, indicating that the hemolysin is probably not the cause of diarrhea seen in recipients of Δctx V. cholerae strains. However, according to Alm et al. (2) removal of the 400-bp HpaI fragment would only decrease HlyA∗ by 2.5 kDa, and thus they felt that this may not satisfactorily inactivate the HlyA product. According to these investigators, experiments with the CT-less vaccine candidate JBK70 and its HlyA::Kmr mutant suggest that HlyA∗ may be responsible for the residual diarrhea observed in CT-less vaccine strains.
Although, we have been studying the morphological alterations in cell lines induced by culture supernatants of V. cholerae strains, we noticed that only 15% of strains induced a vacuolating effect. The presence of CT in toxigenic V. cholerae strains may obliterate the cell vacuolating effect, which may have led to the conclusion that toxigenic V. cholerae strains do not produce cell vacuolating factor but, interestingly, CO853, a nontoxigenic O139 strain, induced cell vacuolation. V. cholerae clinical isolates have also been reported to produce a cytotoxin (cell-rounding factor) which induces rounding of cultured HeLa cell line (17, 22). The exact mechanism of how the El Tor or hemolysin induces vacuolation was not studied here, but it can be inferred that intoxication of cells with mature hemolysin after the action of cell protease on the prohemolysin moiety leads to the dramatic vacuolation in the cell cytoplasm.
Like V. cholerae, Aeromonas hydrophila also secretes a pore-forming cytotoxin known as aerolysin which causes vacuolation in BHK cells (1). A phenotype similar to that of aerolysin-induced vacuolation has also been observed with Serratia marcescens hemolysin (ShlA) (8). Both hemolysin and aerolysin form pores in the lipid bilayer, and most studies have been focused on the mechanism leading to membrane insertion and pore formation by using artificial membranes as well as erythrocytes (5, 22). The mechanism by which nontoxigenic (CT−) nonepidemic strains of V. cholerae (often associated with sporadic diarrhea) cause secretory diarrhea is unknown and remains a great mystery. It is interesting to speculate that the vacuolation seen here is critically important to this process by ways not clearly discerned thus far.
ACKNOWLEDGMENTS
We thank Ronald Taylor for the allelic replacement system.
This work was supported by grants AI38166, DK48029, and HG00820 from The National Institutes of Health. R.M. was the recipient of the 1998 UNESCO-ASM travel award to work in the lab of D.E.B. This research was also supported in part by grants from the Japan International Cooperation Agency (JICA/NICED project no. 054-1061-E-0) to The National Institute of Cholera and Enteric Diseases.
REFERENCES
- 1.Abrami L, Fivaz M, Glanser P-E, Parton R G, Vander Goot F G. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1988;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R A, Mayrhofer G, Kotlarski I, Manning P A. Amino-terminal domain of the El Tor haemolysin of Vibrio cholerae O1 is expressed in classical strains and is cytotoxic. Vaccine. 1991;9:588–594. doi: 10.1016/0264-410x(91)90247-4. [DOI] [PubMed] [Google Scholar]
- 3.Alm R A, Stroeher U H, Manning P A. Extracellular proteins of Vibrio cholerae: nucleotide sequence of the structural gene (hlyA) for the haemolysin of the haemolytic El Tor strain O17 and characterization of the hly A mutation in the non-haemolytic classical 569B. Mol Microbiol. 1988;2:481–488. doi: 10.1111/j.1365-2958.1988.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 4.Boliver F, Rodriguez R L, Geene P J, Betlach M C, Heyneker H L, Boyer H W, Crosa J H, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95. [PubMed] [Google Scholar]
- 5.Buckley J T. Purification of cloned proaerolysin mutant of Aeromonas salmonicida. Biochem Cell Biol. 1990;68:221–224. doi: 10.1139/o90-029. [DOI] [PubMed] [Google Scholar]
- 6.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 7.De Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in gram-negative with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 8.Hertle R, Hilger M, Kocher S W, Walev I. Cytotoxic action of Serratia marcesceus hemolysin on human epithelial cells. Infect Immun. 1999;67:817–825. doi: 10.1128/iai.67.2.817-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshino K, Yamasaki S, Mukhopadhyay A K, Chakraborty S, Basu A, Bhattacharya S K, Nair G B, Shimada T, Takeda Y. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Med Biol Immunol. 1998;20:201–207. doi: 10.1111/j.1574-695X.1998.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 10.Iwanaga M, Kuyyakanond T. New medium for the production of cholera toxin by Vibrio cholerae O1 biotype ElTor. J Clin Microbiol. 1985;22:405–408. doi: 10.1128/jcm.22.3.405-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaper J B, Lockman H, Baldine M M, Levine M M. Recombinant live oral cholera vaccine. Bio/Technology. 1984;2:345–349. [Google Scholar]
- 12.Kaper J B, Morris J G, Jr, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luzzi I, Covacci A, Sensini S, Pezzella C, Crotti D, Rappuoli R, Caprioli A. Detection of a vacuolating cytotoxin in stools from children with diarrhoea. Clin Infect Dis. 1996;23:101–106. doi: 10.1093/clinids/23.1.101. [DOI] [PubMed] [Google Scholar]
- 14.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 15.Manning P A, Brown M H, Heuzenroeder M W. Cloning of the structural gene (hly) for the haemolysin of Vibrio cholerae El Tor strain O17. Gene. 1984;31:225–231. doi: 10.1016/0378-1119(84)90213-0. [DOI] [PubMed] [Google Scholar]
- 16.Miller V L, Mekalonos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outermembrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitra R, Saha P K, Basu I, Venkataraman A, Ramakrishna B S, Albert M J, Takeda Y, Nair G B. Characterization of non-membrane damaging cytotoxin of non-toxigenic Vibrio cholerae O1 and its relevance to disease. FEMS Microbiol Lett. 1998;169:331–339. doi: 10.1111/j.1574-6968.1998.tb13337.x. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay A K, Garg S, Mitra R, Basu A, Dutta D, Bhattacharya S K, Shimada T, Takeda T, Takeda Y, Nair G B. Temporal shifts in traits of Vibrio cholerae strains isolated from hospitalized patients in Calcutta: a 3-year (1993–1995) analysis. J Clin Microbiol. 1996;34:2537–2543. doi: 10.1128/jcm.34.10.2537-2543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray M G, Thompson W F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata H, Wada A, Kurazono H, Yahiro K, Shirasaka D, Ikemura T, Aoyama N, Wang A P, Makiyama K, Kohno S, Hirayama T. Application of Bead-ELISA method to detect Helicobacter pylori VacA. Microb Pathog. 1999;26:103–110. doi: 10.1006/mpat.1998.0256. [DOI] [PubMed] [Google Scholar]
- 21.Ramamurthy T, Bag P K, Pal A, Bhattacharya S K, Bhattacharya M K, Sen D, Shimada T, Takeda T, Takeda Y, Nair G B. Virulence patterns of Vibrio cholerae non-O1 strains isolated from hospitalized patients with acute diarrhoea in Calcutta, India. J Med Microbiol. 1993;39:310–317. doi: 10.1099/00222615-39-4-310. [DOI] [PubMed] [Google Scholar]
- 22.Saha N, Banerjee K. Carbohydrate-mediated regulation of interaction of Vibrio cholerae hemolysin with erythrocyte and phospholipid vesicle. J Biol Chem. 1995;272:162–167. doi: 10.1074/jbc.272.1.162. [DOI] [PubMed] [Google Scholar]
- 23.Saha P K, Koley H, Nair G B. Purification and characterization of an extracellular secretogenic non-membrane damaging cytotoxin produced by clinical strains of Vibrio cholerae non-O1. Infect Immun. 1996;64:3101–3108. doi: 10.1128/iai.64.8.3101-3108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Short J M, Fernandez J M, Sorge J A, Huse W D. ZAP: a bacteriophage expression vector with in vivo excision properties. Nucleic Acids Res. 1988;16:7583. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skorupski K, Taylor R K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]