Abstract
Bordetella pertussis, the agent of whooping cough, can invade and survive in several types of eukaryotic cell, including CHO, HeLa 229, and HEp-2 cells and macrophages. In this study, we analyzed bacterial invasiveness in nonrespiratory human HeLa epithelial cells and human HTE and HAE0 tracheal epithelial cells. Invasion assays and transmission electron microscopy analysis showed that B. pertussis strains invaded and survived, without multiplying, in HTE or HAE0 cells. This phenomenon was bvg regulated, but invasive properties differed between B. pertussis strains and isolates and the B. pertussis reference strain. Studies with B. pertussis mutant strains demonstrated that filamentous hemagglutinin, the major adhesin, was involved in the invasion of human tracheal epithelial cells by bacteria but not in that of HeLa cells. Fimbriae and pertussis toxin were not found to be involved. However, we found that the production of adenylate cyclase-hemolysin prevents the invasion of HeLa and HTE cells by B. pertussis because an adenylate cyclase-hemolysin-deficient mutant was found to be more invasive than the parental strain. The effect of adenylate cyclase-hemolysin was mediated by an increase in the cyclic AMP concentration in the cells. Pertactin (PRN), an adhesin, significantly inhibited the invasion of HTE cells by bacteria, probably via its interaction with adenylate cyclase-hemolysin. Isolates producing different PRNs were taken up similarly, indicating that the differences in the sequences of the PRNs produced by these isolates do not affect invasion. We concluded that filamentous hemagglutinin production favored invasion of human tracheal cells but that adenylate cyclase-hemolysin and PRN production significantly inhibited this process.
Bordetella pertussis, the etiological agent of whooping cough, is a strictly human respiratory pathogen. B. pertussis phase I strains synthesize and secrete many adhesins and toxins involved in the pathogenicity of the bacterium. The bacterium adheres to the respiratory epithelium via fimbrial-type adhesins such as fimbriae (FIM2 and FIM3), and non-fimbrial-type adhesins such as filamentous hemagglutinin (FHA) and pertactin (PRN), an outer membrane protein. Once the infection is established, toxins such as tracheal cytotoxin, a muramyl peptide; pertussis toxin (PT), an ADP-ribosylating toxin; and adenylate cyclase-hemolysin (AC-Hly), a protein belonging to the repeat-in-toxin family, participate in the toxic effects observed during the disease. AC-Hly is able to disrupt host cellular functions by penetrating the cells and by increasing, after activation by calmodulin, the intracellular cyclic AMP (cAMP) concentration (16). The synthesis of all of these virulence factors, except tracheal cytotoxin, is positively regulated by the Bordetella virulence gene (bvg) locus, a two-component signal transduction system (4). Mutations at the bvg locus result in phase IV variants which are unable to produce any of the known toxins and adhesins (48). The bvg locus also responds to environmental factors such as nicotinic acid, sulfate ions, and low temperature. If grown under these conditions, B. pertussis adopts a reversible phase IV phenotype (23). Intermediate-phase variants expressing some of the virulence genes have also been described (24). Such variants can be isolated by subculturing B. pertussis (12), but their in vivo significance and mechanisms of regulation are unknown.
It is widely accepted that B. pertussis adheres to various epithelial cells (11, 39, 40, 43–46). FHA is considered to be the principal adhesin produced by B. pertussis (8, 32, 39, 43, 46). However, FIM2 and FIM3 are known to be involved in adhesion to laryngeal Hep-2 cells (46), PT is involved in adhesion to ciliated and nonciliated epithelial cells (8, 43), and PRN is involved in adhesion to CHO epithelial cells (28).
Traditionally, B. pertussis has been thought of as an extracellular microorganism, producing adhesins and toxins on the surface of the respiratory epithelium. Many reports have demonstrated that the bacteria not only adhere to but may also invade epithelial cells. The first in vivo transmission electron microscopy (TEM) study of mice challenged intracerebrally with B. pertussis showed bacteria between the microvilli and within the cytoplasm of ependymal cells, ciliated cells with some phagocytic activity (17). In a rat model of lung infection by B. pertussis, bacteria were recovered immediately after infection but not on days 10 and 14. However, the bacterium reappeared on day 21. Electron microscopy of the lungs on day 14 showed that these organisms were residing within the cells, indicating that invasiveness properties of the bacteria might correlate with potential clinical effects in vivo (50).
In vitro, B. pertussis has been demonstrated to invade human epithelial cell lines of nonrespiratory origin, such as HeLa 229, CHO, and human laryngeal epithelial Hep-2 cells (7, 8, 25–27, 35, 40). The invasion of epithelial cells by B. pertussis was shown to be a bvg-dependent process, as bvg mutants were significantly less invasive (8, 10, 25, 40). However, the precise roles of bacterial adhesins and toxins in invasion are unknown because the results obtained are often contradictory. This may be because different epithelial cell lines and B. pertussis strains were used. With Tohama I-derived mutants, Ewanowich et al. (8), using human nonrespiratory HeLa 229 cells, and Roberts et al. (40), using human laryngeal epithelial Hep-2 cells, showed that FHA synthesis was involved in invasion. In contrast, with B. pertussis 18323-derived mutants, Lee et al. (26), using HeLa 229 cells, detected no defect in invasion in an FHA-deficient mutant. PRN has been shown to be involved in the uptake of B. pertussis by HeLa 229 cells (27) but not in that by Hep-2 cells (40). The role of FIM2 and FIM3 in the uptake of B. pertussis by epithelial cells has not been analyzed. Conflicting results have been obtained concerning the role of toxins in invasion. Ewanowich et al. (8) showed that PT stimulated invasion by B. pertussis whereas AC-Hly inhibited it, but Lee et al. found no role for these toxins in invasion (25). However, the mutants used in those two studies were constructed from different backgrounds.
We therefore tried to clarify the invasiveness properties of the B. pertussis Tohama I and 18323 parental strains and derived mutants using HeLa 229 cells and two human tracheal epithelial cell lines (HTE and HAE0) transformed by an origin-defective simian virus 40 (6, 13).
MATERIALS AND METHODS
B. pertussis strains and growth conditions.
The strains used in this study are listed in Table 1. Bacteria were grown on Bordet-Gengou agar (Difco) supplemented with 1% glycerol and 15% defibrinated sheep blood (BG) at 36°C for 72 h to detect hemolysis. Isolated colonies were then replated and incubated for 24 h before each experiment. Bacteria tested for invasion were never subcultured in vitro before the experiment.
TABLE 1.
Bacterial strains and clinical isolates used in this study
| Strain or isolate | Adhesin or toxin production
|
Reference | |||||
|---|---|---|---|---|---|---|---|
| FIM2a | FIM3a | PRNb | FHAb | PTb | AC-Hlyb | ||
| 18323 | + | + | + | + | + | + | 37 |
| 18323H− | − | − | − | − | − | − | 22 |
| 18HS19 | + | + | + | + | + | − | 21 |
| SK16 | + | + | + | − | + | + | 25 |
| Tohama I | + | − | + | + | + | + | 49 |
| 359 | − | − | − | − | − | − | 48 |
| 348 | + | − | + | + | + | − | 49 |
| BPRA | + | + | + | + | − | + | 1 |
| BPMC | − | − | + | − | + | + | 29 |
| BBC42 | + | − | − | + | + | + | 40 |
| W28 | + | + | + | + | + | + | 47 |
| Fr287 | − | + | + | + | + | + | 5 |
| Hav | − | + | + | + | + | + | 14 |
Detected by agglutination.
Detected by Western blotting.
Detection of toxins and adhesins produced by B. pertussis parental strains and mutants.
AC-Hly, PT, PRN, and FHA were detected in bacterial suspensions by Western blotting with specific polyclonal sera as previously described (19). Fimbria production was detected by agglutination with anti-FIM2 and anti-FIM3 monoclonal antibodies (30).
Cells and growth conditions.
Two human tracheal epithelial cell lines (HTE and HAE0) derived from human primary tracheal epithelial cells by transfection by an origin-defective simian virus 40 T antigen (6, 13) and HeLa 229 cells (human epitheliumlike; ATCC CCL2.1) were used in invasion assays. For the cytotoxicity assay, the murine monocyte-macrophage-like cell line J774A.1 was used (22). Cells were plated in tissue culture trays (Corning Glass Works, Corning, N.Y.) coated with a collagen gel (Collagen G; Poly Labo, Strasbourg, France) for HTE and HAE0 cells or in noncoated trays for HeLa and J774A.1 cells. Cells were maintained in a 5% CO2 atmosphere at 37°C.
Invasion and persistence assays.
HTE cells were cultured in Dulbecco's modified Eagle medium (GIBCO Laboratories, Grand Island, N.Y.) supplemented with 2% Ultroser G (GIBCO Laboratories), streptomycin (100 μg/ml), and ampicillin (100 μg/ml) (Sigma).
HAE0 cells were cultured in minimum essential medium (GIBCO Laboratories) supplemented with 20% fetal calf serum (DAP, Vogelgrun, France), streptomycin (100 μg/ml), and ampicillin (100 μg/ml) (Sigma).
HeLa cells were cultured in Dulbecco's modified Eagle medium (GIBCO Laboratories) supplemented with 10% fetal calf serum (DAP), streptomycin (100 μg/ml), and ampicillin (100 μg/ml).
For invasion assays, epithelial cells were cultured to 70 or 80% confluence. Tissue culture trays (24 wells, coated) were seeded with approximately 7 × 104 epithelial cells per well 18 h before the assay.
B. pertussis cells grown for 24 h on BG were suspended in the appropriate complete medium without antibiotics to an optical density at 650 nm of 1.0. Approximately 7 × 106 CFU in 0.5 ml were added to each well (bacterium/cell ratio of 100:1) and incubated at 37°C in 5% CO2 for 5 h under static conditions. The bacterial inoculum was evaluated by plating dilutions of the bacterial suspension on BG and counting CFU after 3 days at 37°C. Monolayers were washed three times to remove nonadherent bacteria, and the medium was replaced with 0.5 ml of complete medium containing 100 μg of gentamicin (Sigma) per ml to inactivate the remaining extracellular organisms. After 2 h, the residual gentamicin was removed by extensive washing. Cells containing viable intracellular organisms were recovered from trypsin-treated and stripped monolayers. They were washed in complete medium and lysed in cold distilled water, and intracellular bacteria were counted by plating appropriate dilutions onto BG. Each strain was tested in triplicate. In separate experiments, B. pertussis strains suspended at a density equivalent to that used in invasion assays were treated by incubation with 100 μg of gentamicin per ml for 2 h in collagen-coated culture trays (for HAE0 and HTE cells). This resulted in a 99.998% decrease in CFU counts, indicating that gentamicin efficiently killed collagen-adherent bacteria.
We analyzed the viability of intracellular bacteria by carrying out a long-term survival experiment with HTE cells. Semiconfluent layers of cells were infected by incubation with the appropriate Bordetella strain (multiplicity of infection of 100) for 5 h. The epithelial cells were then washed extensively and incubated for 5 h with gentamicin (100 μg/ml). The concentration of gentamicin in the cell culture medium was then reduced to 10 μg/ml throughout the rest of the incubation period to prevent killing of the bacteria by penetration of antibiotic into the cells, as has been described previously (41). The number of viable intracellular B. pertussis cells was determined after 5, 24, and 48 h of incubation.
Cytotoxicity assay.
Cells and bacteria were cultured exactly as described for the invasion assay. Bacteria were added to HTE or J774A.1 (positive control) cells at a bacterium/cell ratio of 100:1 and counted by plating dilutions of the bacterial suspension on BG. Infected cells were incubated at 37°C in 5% CO2 for 5 h under static conditions. Cytotoxicity was determined using the CytoTox 96 assay (Promega), which measures lactate dehydrogenase activity released into the medium. The percentage of cytotoxicity was calculated in accordance with the manufacturer's instructions.
TEM.
HTE cell monolayers (approximately 3.5 × 105 cells) were grown for 24 h in 35-mm-diameter dishes coated with collagen. Approximately 3.5 × 107 B. pertussis Tohama I or AC-Hly-deficient mutant 348 bacteria from 24-h BG cultures were added to each monolayer and coincubated for 1, 3, or 5 h at 37°C under static conditions. Infected monolayers were fixed in tissue culture dishes with 1.6% glutaraldehyde (Merck) in 0.1 M phosphate buffer (pH 7.4), postfixed in 2% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4), dehydrated in an increasing series of ethanol concentrations, and embedded in epoxy resin. Ultrathin sections (70 to 80 nm) were cut with a diamond knife in a Reichert Ultracut S microtome. The sections were placed onto 200-mesh copper grids, stained with uranyl acetate and lead citrate, and examined with a JEOL 1010 transmission electron microscope operating at 80 kV.
Determination of intracellular cAMP.
Intracellular cAMP levels were determined in control and infected HTE cells as described previously (8). The cAMP levels were determined 5 h after the addition of forskolin (100 μM). 3-Isobutyl-1-methylxanthine (Sigma) was added to a concentration of 0.5 mM to inactivate cellular phosphodiesterases. HTE cells were scraped from wells with a rubber policeman, homogenized, and boiled for 3 min to denature the protein in the samples and to release cAMP. The cAMP concentration was then determined by enzyme immunoassay (Cayman Chemical) (38).
Statistical analysis.
Results are expressed as means ± standard error (SEM) for the indicated number of experiments. One-way analysis of variance was used to identify differences between the groups. If the analysis of variance result was significant, a nonparametric Mann-Whitney test was used to compare the means. P < 0.05 was considered significant.
RESULTS
Invasion of HeLa 229 cell monolayers by B. pertussis mutants.
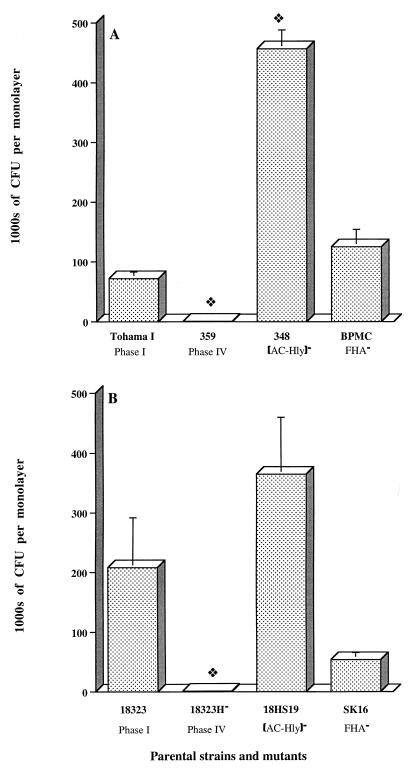
We used HeLa cells, two parental B. pertussis strains (Tohama I and 18323), and three sets of Tohama I- and 18323-derived mutants. We used a 5-h invasion period to achieve maximum adhesion and an additional 2 h for invasion so that our data could be compared with other results obtained with HeLa cells (8, 25). As shown in Fig. 1A and B, Tohama I and 18323 bacteria invaded HeLa cells similarly (Fig. 1A and B). Invasion of HeLa cells by B. pertussis required the expression of bvg-activated gene products (Fig. 1A and B) because both the phase IV Tohama I 359 and 18323H− variants, which produced no adhesins or toxins, were much less invasive than the parental strains (8, 25).
FIG. 1.
Invasion of HeLa 229 cell monolayers by B. pertussis parental strains and mutants. Each B. pertussis strain (7 × 106 CFU) was added to a separate well of a 24-well tissue culture plate, each of which contained 7 × 104 epithelial cells. The invasion of HeLa 229 cells by B. pertussis was assayed as described in Materials and Methods. The values shown are means ± SEM in thousands of CFU recovered from gentamicin-treated monolayers in three to six experiments. The diamond symbol indicates P < 0.05 versus the parental B. pertussis strain, Tohama I (A) or 18323 (B).
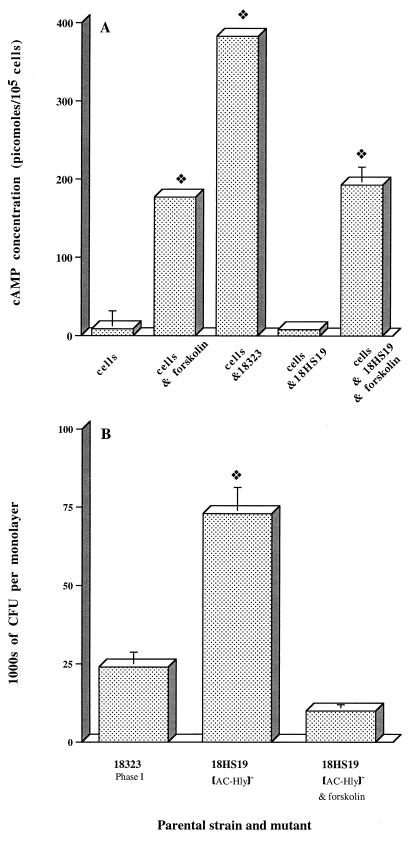
We also tested the invasiveness of two mutants deficient in AC-Hly production, one in the Tohama I background (348) and one in the 18323 background (18HS19). B. pertussis 348 was six times more invasive than parental strain Tohama I (Fig. 1A), but 18HS19 and parental strain 18323 were similar in invasiveness (Fig. 1B). Our results showed that AC-Hly synthesis inhibited cell invasion only for the strains with the Tohama I background.
We also studied the uptake of two FHA-deficient mutants, BPMC and SK16. In both backgrounds, FHA was not involved in the invasion process (Fig. 1A and B).
B. pertussis toxicity toward epithelial cells.
HeLa cells are a type of human epithelial cells of nonrespiratory origin. We therefore used HTE cells, a human tracheal epithelial cell line derived by transfection of human primary tracheal epithelial cells with an origin-defective simian virus 40 T antigen (13). We first tested whether B. pertussis was cytotoxic to HTE cells. The two parental strains and the phase IV variants were not cytotoxic to HTE cells after 5 h of contact (data not shown). B. pertussis 18323, used as a control, was highly toxic to J774A.1 cells (data not shown), consistent with previous data (22).
Invasion of HTE cells by B. pertussis and TEM examination of infected HTE cells.
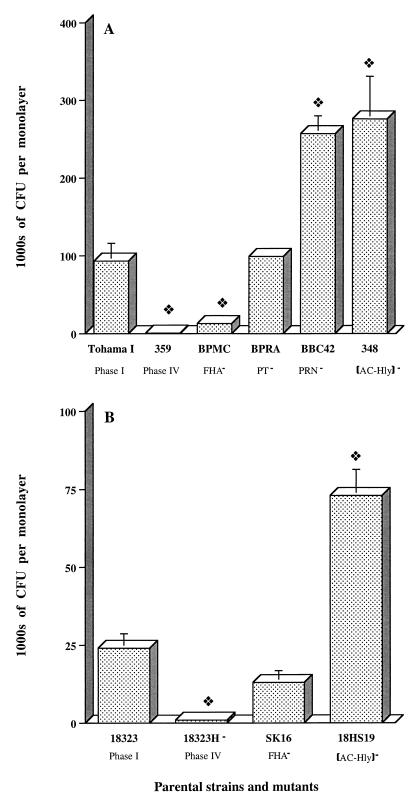
The capacity of the two parental strains and a number of mutants derived from these strains to invade the human tracheal epithelial cell lines HTE and HAE0 was assessed. Both parental strains Tohama I and 18323 invaded HTE cells, and Tohama I was significantly more invasive than 18323 (Fig. 2A and B). Invasion of HTE cells required vir-activated gene products because parental strains Tohama I and 18323 were 200 and 30 times, respectively, more invasive than phase IV variants 359 and 18323H−.
FIG. 2.
Invasion of HTE cell monolayers by B. pertussis mutants. Each B. pertussis strain (7 × 106 CFU) was added to an individual well of a 24-well tissue culture plate, each of which contained 7 × 104 epithelial cells. The invasion of HTE cells by B. pertussis was assayed as described in Materials and Methods. The values shown are means ± SEM in thousands of CFU recovered from gentamicin-treated monolayers in three to six experiments. The diamond symbol indicates P < 0.05 versus the parental B. pertussis strain, Tohama I (A) or 18323 (B).
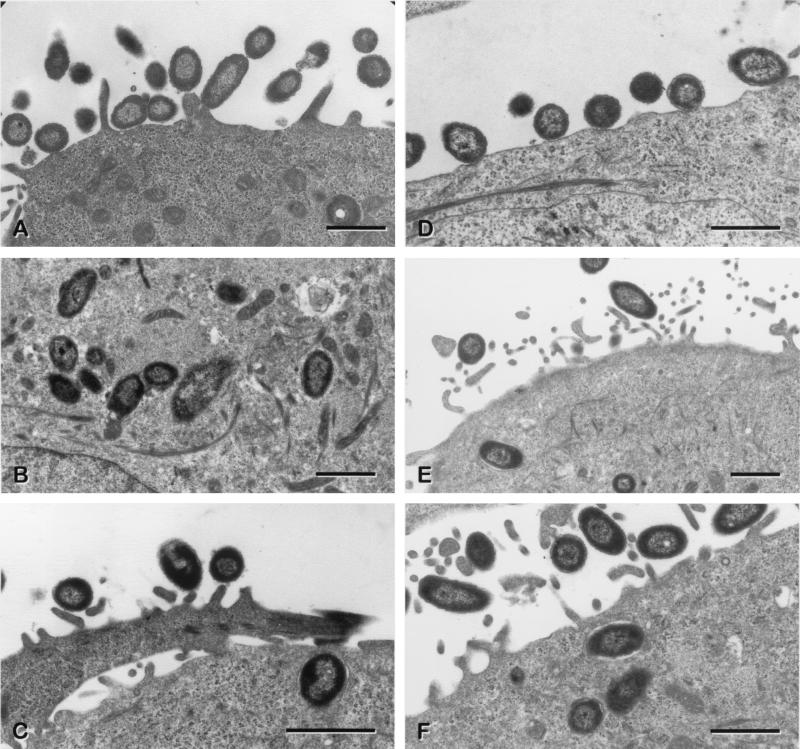
To confirm the presence of intracellular bacteria, sparsely seeded HTE monolayers were infected with approximately 3.5 × 107 Tohama I or AC-Hly-deficient mutant 348 bacteria for 1, 3, or 5 h and were examined by TEM. We also assessed whether the AC-Hly-deficient mutant 348, which is six times more invasive than the parental strain, caused any change in the morphology of the cells.
TEM showed that there were large numbers of adherent bacteria per cell 1 h after infection (Fig. 3A and D). Most intracellular bacteria were located singly within tight endocytic vacuoles (Fig. 3B, C, E and F). Very soon after the penetration of the bacteria into the cell, the vacuole was clearly observed to be in contact with the outer membrane of the bacteria (Fig. 3C and F). However, in some cases, the bacteria appeared to be free within the cytoplasm (Fig. 3B and F).
FIG. 3.
Transmission electron micrographs showing B. pertussis within HTE cells after 1 or 3 h of coincubation. Panels: A, adherent parental strain B. pertussis Tohama I; B and C, intracellular B. pertussis Tohama I; D, adherent AC-Hly-deficient mutant B. pertussis 348; E and F, intracellular AC-Hly-deficient mutant B. pertussis 348. The lack of AC-Hly expression did not appear to render these bacteria more susceptible to damage by lysosomal enzymes. In panels C and F, note the close proximity of the bacterial outer membrane to the membrane of the endocytic vacuole. For panels A and D, the coincubation period was 1 h. For panels B, C, E, and F, the coincubation period was 3 h.
No difference was observed between cells infected with the parental strain and those infected with the mutant. Similar results were obtained with HAE0 cells (data not shown).
Roles of FIM, FHA, PRN, and PT in the uptake of B. pertussis by HTE cells.
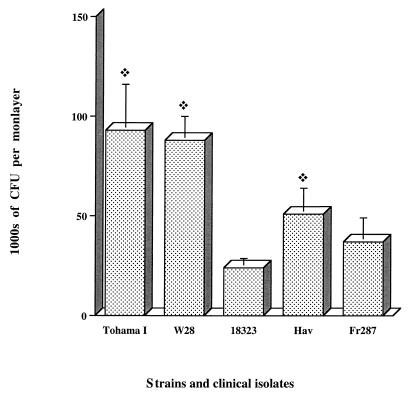
We investigated the role of FIM by comparing the Tohama I and W28 strains and a clinical isolate, Hav, producing FIM2, FIM2 and FIM3, or FIM3, respectively. The invasive capacity of the Tohama I strain was not significantly different from that of W28 or Hav (Fig. 4).
FIG. 4.
Invasion of HTE cell monolayers by B. pertussis isolates. Each B. pertussis strain or isolate (7 × 106 CFU) was added to a separate well of a 24-well tissue culture plate each of which contained 7 × 104 epithelial cells. The invasion of HTE cells by B. pertussis was assayed as described in Materials and Methods. The values shown are means ± SEM in thousands of CFU recovered from gentamicin-treated monolayers in three experiments. The diamond symbol indicates P < 0.05 compared to B. pertussis strain 18323.
A Tohama I mutant deficient in FHA and FIM2 production (BPMC) was significantly less invasive than the parental strain (P < 0.05; Fig. 2A), suggesting a possible role for FHA in uptake. However, in the 18323 background, no significant difference was observed between the uptake of the parental strain and that of the FHA-deficient mutant, SK16 (Fig. 2B).
The role of two other adhesins, PRN and PT, was then analyzed in Tohama I-derived mutants. The PT-deficient mutant BPRA invaded HTE cells to an extent similar to that of parental strain Tohama I (Fig. 2A). However, we observed that PRN significantly inhibited the uptake of Tohama I (P < 0.05). Similar results were obtained with HAE0 cells (data not shown).
There are currently several variants of B. pertussis, synthesizing different PRNs, in circulation (5, 33, 34). We compared the invasive properties of the W28 and Tohama I strains which each produce a PRN similar to that of 18323 and two clinical B. pertussis isolates, Hav and Fr287, producing different PRNs (5). All of the strains and isolates tested had similar invasive capacities, and all were more invasive than 18323, except for isolate Fr287, which was intermediate (Fig. 4).
Role of AC-Hly in the uptake of B. pertussis by HTE cells.
We investigated the role of AC-Hly synthesis in the uptake of B. pertussis by HTE cells. AC-Hly-deficient mutants derived from Tohama I and 18323 were three times more invasive than the parental strains (Fig. 2A and B; P < 0.05).
We tested and confirmed that cAMP affected invasion by B. pertussis, as described previously for HeLa cells (8). cAMP levels in HTE monolayers were quantified after a 5-h coincubation in the presence of 0.5 mM 3-isobutyl-1-methylxanthine and 100 mM forskolin at 37°C. The concentrations of these molecules were kept constant throughout the experiment. As expected, HTE cells exposed to forskolin had significantly higher cAMP levels (Fig. 5A). The exposure to forskolin of HTE cells infected with the AC-Hly-deficient mutant 18HS19 increased intracellular cAMP levels (Fig. 5A) and decreased the extent of invasion by the mutant to a level similar to that of parental strain 18323 (Fig. 5B). These results demonstrate that high levels of cAMP inhibit the invasion by B. pertussis of respiratory epithelial cells. Similar results were obtained with HAE0 cells (data not shown).
FIG. 5.
Effect of cAMP on the invasion of HTE cells by B. pertussis. Each B. pertussis strain (7 × 106 CFU) was added to a separate well of a 24-well tissue culture plate each of which contained 7 × 104 epithelial cells. The invasion of HTE cells by B. pertussis was assayed as described in Materials and Methods. Forskolin was incubated with monolayers for 1 h before the addition of the AC-Hly-deficient mutant (B. pertussis 18HS19) to stimulate cAMP production. The effects of forskolin are reversible, and the concentration used (100 mM) was maintained throughout the experiment. (A) As a control, epithelial cells were incubated with (cells & forskolin) or without (control) forskolin. Results are expressed in picomoles of cAMP per 105 cells and are the means ± SEM of three independent determinations. The diamond symbol indicates P < 0.05 versus the control (cells). (B) Values are means ± SEM in thousands of CFU recovered from gentamicin-treated monolayers in three experiments. The diamond symbol indicates P < 0.05 versus B. pertussis 18323.
Intracellular survival of wild-type and mutant B. pertussis strains.
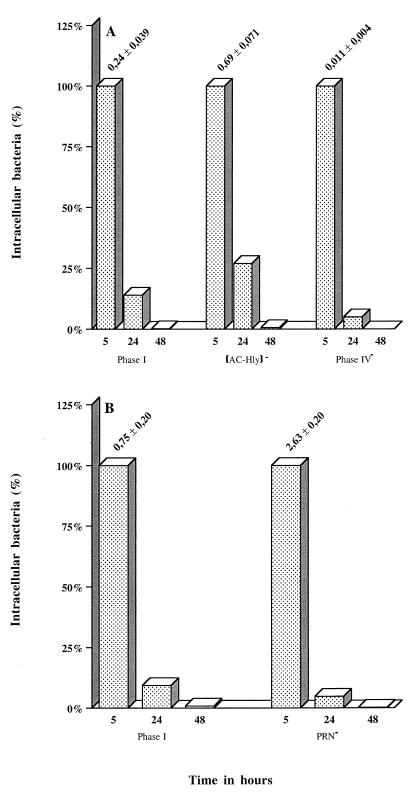
We assessed the relevance of the invasion process observed with B. pertussis by carrying out a 2-day survival experiment with HTE cells. Semiconfluent layers of cells were infected with parental strain 18323, with mutants derived from it, i.e., phase IV variant 18323H− and AC-Hly-deficient mutant 18HS19, with Tohama I, and with the PRN-deficient mutant derived from it, BBC42. For all of the strains and mutants tested, the number of viable B. pertussis cells decreased continually, until no viable cells were detected after 2 days (Fig. 6A and B).
FIG. 6.
Intracellular survival of B. pertussis parental and mutant strains in human respiratory epithelial HTE cells. Each B. pertussis strain (7 × 106 CFU) was added to a separate well of a 24-well tissue culture plate, each of which contained 7 × 104 epithelial cells. The invasion of HTE cells by B. pertussis was assayed as described in Materials and Methods. A value of 100% corresponds to the absolute number of viable intracellular bacteria of each strain in a coincubation period of 5 h. For the 24- and 48-h points, the percentages of surviving intracellular bacteria of each strain compared to the 5-h point, independently of the parental strain, are shown. The persistence experiment was performed twice with the parental strain B. pertussis Tohama I and HTE cells and only once with the parental strain B. pertussis 18323 and the mutants. The values above the histograms for the 5-h time point correspond to the means ± SEM of the absolute number of intracellular bacteria of each strain per cell in all of the four to six experiments performed for this time point.
DISCUSSION
It is now widely accepted that B. pertussis adheres to epithelial cells. FHA is the principal adhesin, but FIM, PT, and PRN are also involved in the adhesion process (8, 28, 40, 46). The question now is: can B. pertussis invade and survive in an epithelial cell? The answer to this question is of key importance for understanding the pathogenesis of whooping cough.
Many studies have demonstrated that B. pertussis invades epithelial cells of nonrespiratory (8, 28) and respiratory (40, 41) origins. The cells generally used in previous studies were HeLa (8, 25), CHO (28), or Hep-2 (40) cells. However, the data obtained were often contradictory. We confirmed these variable results with nonrespiratory HeLa human epithelial cells first and then studied the invasiveness and survival properties of the bacterium with human epithelial cells of respiratory origin. In the present work, we performed only invasion assays in order to characterize the bacterial factors involved in the uptake of the bacteria into the epithelial cells. Each parental strain may differ in adherence to host cells; however, the uptake capacity comparison was always performed between the parental strain and mutants deficient in the expression of one factor.
We investigated interactions between B. pertussis and HeLa epithelial cells using two B. pertussis strains, well-known Japanese strain Tohama I and World Health Organization reference strain 18323. Both phase I strains invaded HeLa cells, whereas phase IV variants did not, indicating that invasion was dependent on bvg expression. We confirmed previous reports showing that production of AC-Hly inhibits the uptake by HeLa cells of B. pertussis strains of the Tohama I background but not those of the 18323 background. However, the observed difference in invasiveness between the parental strain (Tohama I) and the AC-Hly-deficient mutant (348) was larger than that reported in previous studies (8). FHA was not involved in the uptake of the 18323 strain by HeLa cells, confirming previous results (26). However, experiments with the FHA-deficient mutant BPMC did not confirm previous reports that FHA is involved in the uptake of the Tohama I strain (9). The different results generally obtained for Tohama I and 18323 may be attributed to the specific characteristics of the two strains. Differences are observed between the two strains when typing techniques, such as pulsed-field gel electrophoresis (14, 18, 42) or restriction fragment length polymorphism (47), are used to compare their DNAs. The proteins that they synthesize that are involved in pathogenicity, such as PT (2) and PRN (5), are different. In addition, 18323 is more virulent in a murine respiratory model (15).
We used human tracheal HTE and HAE0 epithelial cells. These cells were chosen because they have a respiratory origin, produce cytokeratins, and have microvilli and tight junctions (13). In addition, HAE0 cells are polar (6).
We checked that the two B. pertussis strains used were not toxic to HTE cells, indicating that the interactions between this bacterium and epithelial cells are different from that with phagocytic cells (22).
We found that Tohama I and 18323 invaded HTE cells and that adhesins and toxins were involved in this process. TEM examination demonstrated that the bacteria were present within the cells. Bacteria were located within the vacuoles, which were often observed in contact with the outer membranes of the bacteria. However, some bacteria were also observed free in the cytoplasm, an observation which was not made previously on human Caco-2 epithelial cells (41). No difference was observed between the parental strains and the mutants deficient in the synthesis of adhesins and toxins.
The Tohama I, W28, and Hav strains were similarly invasive despite differences in FIM production. Tohama I produces only FIM2, W28 produces FIM2 and FIM3, and Hav, a clinical isolate, produces only FIM3. This suggests that fimbriae are not involved in the uptake of B. pertussis strains. The 18323 strain, which produces FIM2 and FIM3 when not subcultured, was significantly less invasive than Tohama I, W28, and Hav. This suggests that a factor(s) involved in invasion differs between 18323 and the other B. pertussis strains. This factor may be FHA, because a Tohama I-derived FHA-FIM2 mutant was significantly less invasive than the parental strain. As FIM2 and FIM3 were not found to be involved in the uptake of B. pertussis strains, it is possible that FHA is involved in the invasion process. No difference was found between the uptake of 18323 and that of an FHA-deficient mutant derived from 18323. However, it should be noted that with the 18323 parental strain, as well as with the AC-Hly-deficient mutant derived from this strain, large standard errors were observed that could have masked FHA and AC-Hly effects. An alternative hypothesis could be that the FHA synthesized by 18323 is different from Tohama I FHA, as observed for PT and PRN. It was shown that 18323 is closer to isolates of B. bronchiseptica, the animal pathogen, than to other B. pertussis isolates (2). Since it is known that B. bronchiseptica FHA adheres less to human epithelial cells than does B. pertussis FHA (43), FHA may be involved in the uptake of B. pertussis into cells due to its role in adhesion to respiratory epithelial cells (43, 46). FHA has been shown to be involved in adhesion to human bronchial and laryngeal epithelial cells, whereas fimbriae are only involved in adhesion to laryngeal cells (46).
PT was not involved in the uptake of bacteria of the Tohama I background, but surprisingly, PRN significantly inhibited the uptake of bacteria into cells. Several variants of B. pertussis producing different PRNs are known to be in circulation, so we compared the uptake of various strains and isolates producing different PRNs and fimbriae. W28 and Tohama I produce similar types of PRN (5) and invaded cells similarly. The Hav and Fr287 isolates, which produce different PRNs (5) were also taken up similarly, indicating that the differences found in the PRNs produced by these isolates did not affect invasion.
AC-Hly inhibited the invasion of HTE cells by B. pertussis, regardless of the background used. The effect of AC-Hly was mediated by an increase in the cAMP concentration within the cells, as demonstrated by experiments performed in the presence of forskolin. However, how does PRN inhibit invasion? This protein might interact with AC-Hly, for example. We have shown that B. pertussis induces apoptosis in macrophages and that AC-Hly is responsible for this phenomenon (20, 22). However, we have also observed, as in this study, that the adenylate cyclase activity detected in bacterial suspensions of PRN-deficient mutants is lower than that detected in bacterial suspensions of the parental strain. These mutants are less able to cause DNA fragmentation in macrophages (20), and in this study, the PRN-deficient mutant behaved like the AC-Hly-deficient mutant. Thus, the effect of PRN could be indirect, via its interaction with AC-Hly. The interactions between AC-Hly and PRN might be direct, or PRN might have an effect on membrane protein structure important for AC-Hly export or conformation. Evidence in favor of this idea is provided by the difficulty encountered in efforts to separate PRN from AC-Hly during the purification of these proteins (36). We are currently trying to investigate the interactions between PRN and AC-Hly. If our assumption is correct, then PRN has a central role in B. pertussis pathogenicity, interacting with the major adhesin FHA (3) and with one of the major toxins, AC-Hly.
B. pertussis invaded but did not multiply in epithelial cells, as previously reported for the Tohama I parental strain on human Caco-2 epithelial cells (41). This may be because B. pertussis uses epithelial cells to hide from the host immune system or to reach other cells. No difference in persistence was observed between the parental strain and PRN- or AC-Hly-deficient mutants, even though the mutants were more invasive. These data indicate that FHA production is required for invasion but that AC-Hly and PRN production significantly inhibits this process. So, what is the biological significance of these results? Phase I B. pertussis may be mostly extracellular, secreting adhesins and toxins at the cell surface, but during infection, modulation may occur and an intermediate-phase variant, not producing AC-Hly, may play a key role in invasion. Such variants are more invasive than the phase I strain and may escape the immune response. It has already been observed that B. pertussis invades macrophages and that AC-Hly production stops after uptake (31). An alternative hypothesis could be that B. pertussis has evolved anti-invasive mechanisms such as the expression of AC-Hly to avoid destruction within tracheal epithelial cells. Further experiments are required to investigate these issues further.
In the present work, our data indicate that human tracheal cell invasion is ultimately followed by killing of B. pertussis. It could be important to analyze whether other invasive bacteria are also killed by these cell lines.
In conclusion, this study confirmed (i) that there are major differences between B. pertussis strains or isolates and the 18323 World Health Organization reference strain which account for previous conflicting results and (ii) that B. pertussis invades human respiratory epithelial cells and showed the opposite effects of FHA and of AC-Hly and PRN on this invasive potential of B. pertussis.
ACKNOWLEDGMENTS
The first and second authors contributed equally to this work.
We thank D. C. Gruenert for the gift of the human respiratory epithelial cell lines HTE and HAE0. We thank A. Ullmann, H. Sakamoto, C. Locht R. Rappuoli, and G. Dougan for providing strains and B. Meade for providing anti-FIM2 and anti-FIM3 monoclonal antibodies. We thank B. Chavinier for expert technical assistance with the electron microscope and N. Khelef for critical reading of the manuscript.
L. Bassinet was supported by a grant from the Fondation pour la Recherche Médicale. This work was supported by the Institut Pasteur Fondation.
REFERENCES
- 1.Antoine R, Locht C. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect Immun. 1990;58:1518–1526. doi: 10.1128/iai.58.6.1518-1526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arico B, Gross R, Smida J, Rappuoli R. Evolutionary relationships in the genus Bordetella. Mol Microbiol. 1987;1:301–308. doi: 10.1111/j.1365-2958.1987.tb01936.x. [DOI] [PubMed] [Google Scholar]
- 3.Arico B, Nuti S, Scarlato V, Rappuoli R. Adhesion of Bordetella pertussis to eukaryotic cells requires a time-dependent export and maturation of filamentous hemagglutinin. Proc Natl Acad Sci USA. 1993;90:9204–9208. doi: 10.1073/pnas.90.19.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arico B, Scarlato V, Monack D M, Falkow S, Rappuoli R. Structural and genetic analysis of the bvg locus in Bordetella species. Mol Microbiol. 1991;5:2481–2491. doi: 10.1111/j.1365-2958.1991.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 5.Boursaux-Eude C, Thiberge S, Carletti G, Guiso N. Intranasal murine model of Bordetella pertussis infection. II. Sequence variation and protection induced by a tricomponent acellular vaccine. Vaccine. 1999;17:2651–2660. doi: 10.1016/s0264-410x(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 6.Cozens A L, Yezzi M J, Yamaha M, Steiger D, Wagner J A, Garber S S, Chin L, Simon E M, Cutting G R, Gardner P, Friend D S, Basbaum C B, Gruenert D C. A transformed human epithelial cell line that retains tight junctions post crisis. In Vitro Cell Dev Biol. 1992;28:735–744. doi: 10.1007/BF02631062. [DOI] [PubMed] [Google Scholar]
- 7.Everest P, Li J, Douce G, Charles I, De Azavedo J, Chatfield S, Dougan G, Roberts M. Role of the Bordetella pertussis P.69/pertactin protein and the P.69/pertactin RGD motif in the adherence to and invasion of mammalian cells. Microbiology. 1996;142:3261–3268. doi: 10.1099/13500872-142-11-3261. [DOI] [PubMed] [Google Scholar]
- 8.Ewanowich C A, Melton A R, Weiss A A, Sherburne R K, Peppler M S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989;57:2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewanowich C A, Sherburne R K, Man S F P, Peppler M S. Bordetella parapertussis invasion of HeLa 229 cells and human respiratory epithelial cells in primary culture. Infect Immun. 1989;57:1240–1247. doi: 10.1128/iai.57.4.1240-1247.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman R L, Nordensson K, Wilson L, Akporiaye E T, Yocum D E. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992;60:4578–4585. doi: 10.1128/iai.60.11.4578-4585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funnel S G P, Robinson A. A novel adherence assay for Bordetella pertussis using tracheal organ cultures. FEMS Microbiol Lett. 1993;110:197–204. doi: 10.1111/j.1574-6968.1993.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldman S, Hanski E, Fish F. Spontaneous phase variation in Bordetella pertussis in a multistep non-random process. EMBO J. 1984;3:1353–1356. doi: 10.1002/j.1460-2075.1984.tb01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruenert D C, Basbaum C B, Welsh M J, Li M, Finkbeiner W E, Nadel J A. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci USA. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiso N. Isolation, identification and characterization of Bordetella pertussis. Dev Biol Stand. 1997;89:233–238. [PubMed] [Google Scholar]
- 15.Guiso N, Rocancourt M, Szatanik M, Alonso J M. Bordetella adenylate cyclase is a virulence associated factor and an immunoprotective antigen. Microb Pathog. 1989;7:373–380. doi: 10.1016/0882-4010(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 16.Hewlett E L. Pertussis: current concepts of pathogenesis and prevention. Pediatr Infect Dis J. 1997;16:S78–S84. doi: 10.1097/00006454-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 17.Hopewell J W, Holt L B, Desombre T R. An electron-microscope study of intracerebral infection of mice with low-virulence Bordetella pertussis. J Med Microbiol. 1972;5:154–157. doi: 10.1099/00222615-5-1-154. [DOI] [PubMed] [Google Scholar]
- 18.Khattak M N, Matthews R C. A comparison of the DNA fragment patterns of the mouse-virulent challenge strains and clinical isolates of Bordetella pertussis. J Infect. 1993;27:119–124. doi: 10.1016/0163-4453(93)94566-t. [DOI] [PubMed] [Google Scholar]
- 19.Khelef N, Danve B, Quentin-Millet M J, Guiso N. Bordetella pertussis and Bordetella parapertussis: two immunologically distinct species. Infect Immun. 1993;61:486–490. doi: 10.1128/iai.61.2.486-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khelef N, Guiso N. Induction of macrophage apoptosis by Bordetella pertussis adenylate cyclase-hemolysin. FEMS Microbiol Lett. 1995;134:27–32. doi: 10.1111/j.1574-6968.1995.tb07909.x. [DOI] [PubMed] [Google Scholar]
- 21.Khelef N, Sakamoto H, Guiso N. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb Pathog. 1992;12:227–235. doi: 10.1016/0882-4010(92)90057-u. [DOI] [PubMed] [Google Scholar]
- 22.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knapp S, Mekalanos J J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988;170:5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacey B. Antigenic modulation of Bordetella pertussis. J Hyg. 1960;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C K, Roberts A L, Finn A M, Knapp S, Mekalanos J J. A new assay for invasion of HeLa 229 cells by Bordetella pertussis: effects of inhibitors, phenotypic modulation, and genetic alterations. Infect Immun. 1990;58:2516–2522. doi: 10.1128/iai.58.8.2516-2522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, C. K., A. L. Roberts, T. M. Finn, and J. J. Mekalanos. 1990. Invasion of HeLa 229, Chinese hamster ovary, and U937 cells by Bordetella pertussis, p. 115–123. In C. R. Manclark, DHHS publication no. (FDA) 90-1164 (ed.), Proceedings of the Sixth International Symposium on Pertussis. Department of Health and Services, U.S. Public Health Service, Bethesda, Md.
- 27.Leininger E, Ewanowich C A, Bhargava A, Peppler M S, Kenimer J G, Brennan M J. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992;60:2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leininger E, Roberts M, Kenimer J G, Charles I G, Fairweather N, Novotny P, Brennan M J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Locht C, Geoffroy M C, Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992;11:3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manclark C R, Meade B D, Burstyn D G. Serological response to Bordetella pertussis. In: Rose N R, Friedman H, Fahey J L, editors. Manual of clinical laboratory immunology. 3rd ed. Washington, D.C.: American Society for Microbiology; 1986. pp. 388–394. [Google Scholar]
- 31.Masure H R. Modulation of adenylate cyclase toxin production as Bordetella pertussis enters human macrophages. Proc Natl Acad Sci USA. 1992;89:6521–6525. doi: 10.1073/pnas.89.14.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J H, Leininger E, Brennan M J, Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mooi F R, He Q S, van Oirschot H, Mertsola J. Variation in the Bordetella pertussis virulence factors pertussis toxin and pertactin in vaccine strains and clinical isolates in Finland. Infect Immun. 1999;67:3133–3134. doi: 10.1128/iai.67.6.3133-3134.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mooi F R, van Oirschot H, Heuvelman K, van der Heide H G, Gaastra W, Willems R J. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect Immun. 1998;66:670–675. doi: 10.1128/iai.66.2.670-675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mouallem M, Farfel Z, Hanski E. Bordetella pertussis adenylate cyclase toxin: intoxication of host cells by bacterial invasion. Infect Immun. 1990;58:3759–3764. doi: 10.1128/iai.58.11.3759-3764.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novotny P, Chubb A P, Cownley K, Montaraz J A. Adenylate cyclase activity of a 68,000-molecular-weight protein isolated from the outer membrane of Bordetella bronchiseptica. Infect Immun. 1985;50:199–206. doi: 10.1128/iai.50.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittman M. Genus Bordetella. In: Krieg N R, Holt J G, editors. Bergey's manual of systemic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 388–393. [Google Scholar]
- 38.Pradelles P, Grassi J, Chabardes D, Guiso N. Enzyme immunoassays of adenosine cyclic 3′,5′-monophosphate and guanosine cyclic 3′,5′-monophosphate using acetylcholinesterase. Anal Chem. 1989;61:447–453. doi: 10.1021/ac00180a014. [DOI] [PubMed] [Google Scholar]
- 39.Relman D A, Domenighini M, Tuomanen E, Rappuoli R. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci USA. 1989;86:2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts M, Fairweather N F, Leininger E, Pickard D, Hewlett E L, Robinson A, Hayward C, Dougan G, Charles I G. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol. 1991;5:1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 41.Schipper H, Krohne G F, Gross R. Epithelial cell invasion and survival of Bordetella bronchiseptica. Infect Immun. 1994;62:3008–3011. doi: 10.1128/iai.62.7.3008-3011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stibitz S, Yang M S. Genomic fluidity of Bordetella pertussis assessed by a new method for chromosomal mapping. J Bacteriol. 1997;179:5820–5826. doi: 10.1128/jb.179.18.5820-5826.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuomanen E, Weiss A A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985;152:118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- 44.Tuomanen E I, Hendley J O. Adherence of Bordetella pertussis to human respiratory epithelial cells. J Infect Dis. 1983;148:125–130. doi: 10.1093/infdis/148.1.125. [DOI] [PubMed] [Google Scholar]
- 45.Urisu A, Cowell J L, Manclark C R. Involvement of filamentous hemagglutinin in the adherence of Bordetella pertussis to human WiDr cell cultures. Dev Biol Stand. 1985;61:205–214. [PubMed] [Google Scholar]
- 46.Van den Berg B, Beekhuizen H, Willems R J L, Mooi F R, Van Furth R. Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infect Immun. 1999;67:1056–1062. doi: 10.1128/iai.67.3.1056-1062.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van der Zee A, Vernooij S, Peeters M, Van Embden J, Mooi F R. Dynamics of the population structure of Bordetella pertussis as measured by IS1002-associated RFLP: comparison of pre- and post-vaccination strains and global distribution. Microbiology. 1996;142:3479–3485. doi: 10.1099/13500872-142-12-3479. [DOI] [PubMed] [Google Scholar]
- 48.Weiss A A, Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984;43:263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woods D E, Franklin R, Cryz S J, Ganss M, Peppler M, Ewanowich C. Development of a rat model for respiratory infection with Bordetella pertussis. Infect Immun. 1989;57:1018–1024. doi: 10.1128/iai.57.4.1018-1024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]