Abstract
Background: To examine the effect of the triglyceride-glucose (TyG) index on longitudinal cognitive decline in a healthy middle-aged-to-elderly population. Methods: We conducted a population-based longitudinal study. A total of 1774 participants without cognitive impairment were enrolled in the 4-year follow-up. They were divided into four groups according to the quartile of the TyG index. Multivariable-adjusted Cox proportional hazard models were performed to examine the association between the TyG index and cognitive decline. Discrimination tests were used to evaluate the incremental predictive value of the TyG index beyond conventional risk factors. Results: During the follow-up, compared with those in the bottom quartile group, participants in the top TyG quartile group presented a 51% increase in the risk of cognitive decline (OR 1.51 (95% CI: 1.06–2.14)). As shown by discrimination tests, adding the TyG index into the conventional model resulted in a slight improvement in predicting the risk of cognitive decline (NRI 16.00% (p = 0.004)). Conclusion: This study demonstrated that increasing values of the TyG index were positively associated with the risk of cognitive decline. Monitoring the TyG index may help in the early identification of individuals at high risk of cognitive deterioration.
Keywords: triglyceride-glucose index, cognitive decline, insulin resistance, risk factor, association
1. Introduction
With the aging of the global population, cognitive impairment is increasingly becoming a public health concern. Cognitive impairment, an aging-related disease, is clinically characterized by deterioration in memory, thinking, language, and executive function. Severe cognitive impairment may affect a person’s ability to perform daily activities and cause social and economic burdens [1,2]. The prevalence of dementia in China has increased by 5.6% in recent years, while the global prevalence has increased by 1.7% [3]. Many studies have shown that poor cognitive performance is associated with an increased risk of death, especially in middle-aged and elderly populations [4,5,6]. Research on risk factors for dementia has become a popular topic in recent years. Moreover, cognitive function changes over time. Therefore, it is necessary to identify and control the risk factors that may influence cognitive decline promptly.
Insulin resistance (IR) is defined as the reduced responsiveness of target tissue to insulin [7]. Previous studies have linked type 2 diabetes, caused by insulin resistance, to a higher risk of Alzheimer’s disease (AD) [8,9]. Other studies have found that increased serum insulin levels and insulin resistance are directly related to cognitive impairment [10,11,12]. The effect of this central IR on the occurrence and development of cognitive impairment may be related to changes in hippocampal synaptic plasticity [13]. Therefore, early detection and control of insulin resistance may be beneficial for the prevention of cognitive dysfunction. However, due to the complex testing process and high cost, the hyperinsulinemic-euglycemic clamp method is not commonly used in clinical settings to assess IR [14]. In addition, the degree of IR can be indexed by the homeostasis model assessment of insulin resistance (HOMA-IR) according to the homeostasis model assessment, which is calculated with the use of fasting glucose and insulin levels [15]. However, circulating insulin is not a routinely measured indicator, and this model has a high coefficient of variation due to the fluctuating pattern of insulin secretion, which makes HOMA-IR unsuitable for clinical diagnosis and large-scale studies [16].
The correlation between blood lipid levels and cognitive dysfunction has also received extensive amounts of attention. Previous studies have revealed that elevated blood lipid levels are linked to cognitive decline and the risk of AD [17,18,19,20,21]. Other studies have found that high lipid accumulation products are associated with cognitive performance and decline over four years, and this relationship may be influenced by sex and blood pressure [22,23]. Plasma triglycerides, simple lipids involved in energy storage and transportation, are thought to be closely associated with poor cognitive performance [24,25]. Increased plasma triglyceride levels may be involved in cognitive dysfunction through putative mechanisms such as blood–brain barrier disruption or an imbalance in amyloid metabolism [26,27,28,29]. Moreover, a previous study showed that elevated plasma triglyceride levels appear to be a risk factor for cognitive decline in elderly patients with diabetes [30]. Similarly, a cohort study also found that higher levels of triglycerides in midlife were associated with the decline in cognitive functions such as memory, attention, and executive function after 20 years of follow-up [20]. Another study explored whether triglyceride levels mediate cognitive decline in patients with major depressive disorder, and found that triglyceride levels are involved in the progression of depression and play an important role in memory decline [31].
Recently, the triglyceride-glucose (TyG) index calculated from fasting triglyceride and blood glucose has become easy to collect, cost-effective, and reliable, and has been suggested as a surrogate marker for evaluating insulin resistance [32,33]. Insulin resistance can be predicted better by the TyG index compared to the HOMA-IR index, according to researchers [34]. Previous studies have suggested that a high TyG index is associated with cardiovascular diseases, but population-based longitudinal studies on the association of the TyG index with cognitive impairment are lacking [35,36,37]. One study investigated the relationship between the TyG index and the burden of cerebral small vessel disease and cognitive impairment (CI) in elderly patients with diabetes and found a positive correlation between the increased TyG index and CI [38]. Another study revealed that the relationship between lung function and subsequent cognitive function in middle-aged and elderly people with a systemic low-grade inflammation state could be mediated by the TyG index [39]. Moreover, another study found that the TyG index was associated with a higher risk of dementia [40]. However, the relationship between the TyG index and cognitive decline remains poorly understood. Therefore, in this study, we aimed to characterize the association between the TyG index and longitudinal cognitive decline in healthy middle-aged and elderly populations.
2. Materials and Methods
2.1. Study Population
The Jidong Cognitive Impairment Cohort Study (CICS) is a community-based, long-term observational cohort study designed to investigate the potential prognostic factors and the risk of cognitive impairment [41]. The inclusion criteria for participants in this study include the following: (i) age of 40 years or older; (ii) no related diseases that may affect the cognitive function assessments, for instance, severe aphasia, hearing loss, visual impairment, psychosis or schizophrenia (documented in the questionnaire); and (iii) provision of signed informed consent. A total of 3617 participants aged 40 years or older were recruited into the CICS in 2015. Among these participants, the following were further excluded: 75 individuals without fasting blood glucose and triglyceride data in 2015, 29 individuals without an education record, 108 individuals with cognitive impairment in 2015, 932 individuals without cognitive examinations in 2015 and 2019, and 699 participants who were lost during follow-up. Eventually, a total of 1774 participants were ultimately enrolled in this study and were followed for 4 years until 2019.
The present study was performed in accordance with the guidelines described by the Helsinki Declaration and was approved by the Ethics Committees of Kailuan General Hospital of Tangshan City and the Medical Ethics Committee, the Staff Hospital of Jidong Oilfield Branch, China National Petroleum Corporation (No. 2013 YILUNZI 1). All the participants signed a written informed consent form prior to their inclusion in the study.
2.2. Data Collection
The information from all participants, including demographic characteristics and clinical examination information (age, sex, body mass index (BMI), smoking, alcohol consumption, educational level, and physical activity) and medical history (hypertension, dyslipidemia, diabetes mellitus), was collected through a series of comprehensive questionnaires. Regular physical activity was defined as four or more times per week of exercise. BMI was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as a self-reported history, any current use of antihypertensive medications, or a diagnosis of hypertension during healthcare examination (systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg). Diabetes mellitus was defined as a self-reported history, any current use of hypoglycemic medications, or fasting blood glucose levels ≥ 7.0 mmol/L. Hyperlipidemia was defined as a self-reported history, any current use of lipid-lowering therapy, or a diagnosis of hyperlipidemia during healthcare examination (serum triglyceride ≥ 1.7 mmol/L, total cholesterol ≥ 5.72 mmol/L, high-density lipoprotein ≤ 0.9 mmol/L). In addition, fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TGs), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels from blood samples were collected carefully from the antecubital vein in the morning under fasting conditions. Fasting blood glucose was measured using the hexokinase/glucose-6-phosphate dehydrogenase method, and triglyceride concentrations were determined by enzymatic methods (Mind Bioengineering Co., Ltd., Shanghai, China). All the blood samples were stored in serum-separated tubes and EDTA anticoagulant collection tubes at −80 °C to address potential sources of bias.
2.3. Assessment of the TyG Index
The TyG index was calculated as ln (fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2), as previously described [32,33].
2.4. Outcome Evaluation
All participants in this study completed the Chinese version of the Mini-Mental State Examination (MMSE) to evaluate cognitive function. The MMSE consists of 30 items that assess five cognitive domains: orientation (10 points), regulation (3 points), attention and calculation (5 points), recall (3 points) and language (10 points). Cognitive impairment was defined as the education-based cutoffs of MMSE scores: ≤17 for illiterate individuals, ≤20 for primary school graduates, and ≤24 for junior high school graduates or above. This cutoff point has been shown to have the best sensitivity and specificity in the Chinese population [42]. Generally, relatively high scores represent increased cognitive functioning.
2.5. Statistical Analysis
The data were tested for normality by using the Kolmogorov–Smirnov test. Continuous variables are presented as the means ± standard deviations for normally distributed data and were compared using ANOVA. Continuous variables that did not exhibit a normal distribution are presented as medians with interquartile ranges and were compared by the Kruskal–Wallis U test. Categorical variables were expressed as frequencies (proportions) and were compared using the χ2 test or Fisher’s exact test.
Participants in this study were divided into four categories according to the quartile of the TyG index, which was assessed at baseline. The association of the TyG index with cognitive decline was estimated by the use of the multivariate Cox proportional hazard regression model. Data reported as hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated after we adjusted for potential confounding factors. Model 1 was adjusted for age, sex and educational levels. Model 2 was additionally adjusted for smoking status, alcohol consumption, physical activity, body mass index, history of hypertension, and total cholesterol. To avoid collinearity, hypertension rather than systolic and diastolic blood pressure, BMI but not waist circumference, and total cholesterol but not HDL and LDL were used in the model fitting. In addition, restricted cubic spline analysis was used to address the dose–response relationship between the TyG index and cognitive decline. Furthermore, discrimination tests (C statistic, net reclassification improvement (NRI) and integrated discrimination improvement (IDI)) were used to evaluate the incremental predictive value of the TyG index beyond conventional risk factors. Additionally, subgroup analyses were conducted after stratification by age (<60 y or ≥60 y), sex, BMI (<25 or ≥25 kg/m2), hypertension, dyslipidemia and diabetes mellitus to test interactions and assess whether the effect of the TyG index on cognitive decline differed between different subgroups, which was adjusted by model 2.
In this study, p values < 0.05 were considered to be statistically significant and were two-sided.
All the statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Demographic and Clinical Characteristics
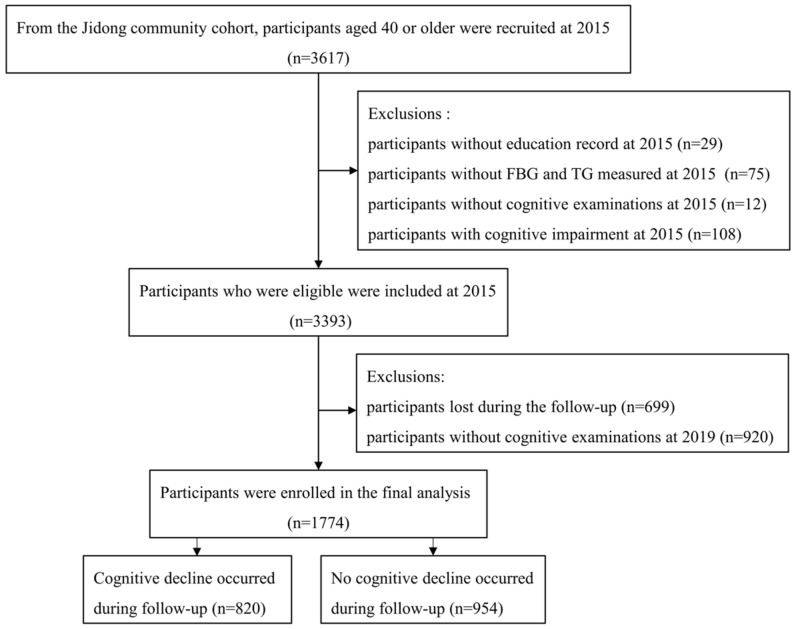
Among 3617 participants aged 40 or older in the CICS, 1774 participants who met the inclusion criteria were finally enrolled (Figure 1), and their baseline characteristics are shown in Table 1 and Table S1. Their mean age was 53.48 ± 8.47 years, 52.03% were women, and 90.98% had a junior high school educational level or higher. The participants were categorized into four groups according to the quartile of the TyG index at baseline (<7.87, 7.87–8.25, 8.25–8.68, ≥8.68). Compared with participants in the Q1 group, we found that patients with a higher TyG index were predominantly older, men, obese, more current smokers and drinkers, had a higher prevalence of hypertension, diabetes mellitus, and dyslipidemia, had a high FBG, TC, TG, LDL level and had a low HDL level.
Figure 1.
Flowchart of this study. Abbreviations: FBG, fasting blood glucose; TG, triglycerides.
Table 1.
Baseline characteristics in 2015 of these 1774 participants according to quartiles of the TyG index.
| Characteristic | Total | TyG Index | p Value | |||
|---|---|---|---|---|---|---|
| Q1 (<7.87) | Q2 (7.87–8.25) | Q3 (8.25–8.68) | Q4 (≥8.68) | |||
| N, (%) | 1774 | 437 | 450 | 444 | 443 | |
| Age, year, (mean ± SD) | 53.48 ± 8.47 | 51.91 ± 8.58 | 53.59 ± 8.57 | 54.48 ± 8.47 | 53.92 ± 8.09 | <0.001 |
| Sex, n (%) | <0.001 | |||||
| Male | 851(47.97) | 148(33.87) | 198(44.00) | 250(56.31) | 255(57.56) | |
| Female | 923(52.03) | 289(66.13) | 252(56.00) | 194(43.69) | 188(42.44) | |
| Educational level, n (%) | 0.601 | |||||
| Illiterate | 65(3.66) | 12(2.75) | 17(3.78) | 22(4.95) | 14(3.16) | |
| Primary | 95(5.36) | 21(4.81) | 28(6.22) | 22(4.95) | 24(5.42) | |
| Junior or above | 1614(90.98) | 404(92.45) | 405(90.00) | 400(90.09) | 405(91.42) | |
| Body mass index, kg/m2, (mean ± SD) | 25.02 ± 5.39 | 23.55 ± 9.22 | 24.52 ± 3.03 | 25.57 ± 2.99 | 26.43 ± 3.18 | <0.001 |
| Current smoking, n (%) | 394(22.21) | 66(15.10) | 81(18.00) | 114(25.68) | 133(30.02) | <0.001 |
| Current drinking, n (%) | 553(31.78) | 101(23.54) | 121(27.38) | 151(34.55) | 180(41.67) | <0.001 |
| Regular physical activity, n (%) | 883(60.90) | 210(57.69) | 222(61.16) | 239(66.02) | 212(58.73) | 0.489 |
| Medical history, n (%) | ||||||
| Hypertension | 632(35.63) | 87(19.91) | 135(30.00) | 179(40.32) | 231(52.14) | <0.001 |
| Diabetes mellitus | 226(12.74) | 10(2.29) | 25(5.56) | 56(12.61) | 135(30.47) | <0.001 |
| Dyslipidemia | 1012(57.05) | 60(13.73) | 132(29.33) | 382(86.04) | 438(98.87) | <0.001 |
| Laboratory test, (mean ± SD) | ||||||
| TyG index | 8.31 ± 0.62 | 7.60 ± 0.21 | 8.06 ± 0.11 | 8.45 ± 0.12 | 9.14 ± 0.44 | <0.001 |
| FBG, mg/dL | 6.21 ± 1.42 | 5.67 ± 0.49 | 5.90 ± 0.72 | 6.17 ± 0.96 | 7.08 ± 2.29 | <0.001 |
| LDL, mg/dL | 3.42 ± 0.82 | 3.01 ± 0.63 | 3.38 ± 0.79 | 3.61 ± 0.82 | 3.66 ± 0.86 | <0.001 |
| HDL, mg/dL | 1.27 ± 0.27 | 1.43 ± 0.27 | 1.32 ± 0.25 | 1.21 ± 0.24 | 1.12 ± 0.23 | <0.001 |
| TC, mg/dL | 5.15 ± 0.98 | 4.71 ± 0.78 | 5.09 ± 0.91 | 5.29 ± 0.95 | 5.51 ± 1.06 | <0.001 |
| TG, mg/dL | 2.00 ± 1.53 | 0.91 ± 0.17 | 1.36 ± 0.19 | 1.94 ± 0.31 | 3.79 ± 2.09 | <0.001 |
Abbreviations: Q, quartile; TyG, triglyceride-glucose; FBG, fasting blood glucose; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein levels.
3.2. Effect of the Tyg Index on Cognitive Decline
Table 2 shows the cognitive performance of participants with different TyG quartiles in 2015 and 2019. There was no significant difference in cognitive performance between these four groups in 2015 (28.51 ± 1.49 in Q1 vs. 28.46 ± 1.64 in Q4, p = 0.742). In 2019, participants in the highest TyG level group had worse cognitive performance (27.80 ± 2.79 in Q1 vs. 27.24 ± 3.12 in Q4, p = 0.002) and faster cognitive decline from 2015 to 2019 (0.70 ± 2.83 in Q1 vs. 1.21 ± 3.30 in Q4, p = 0.009) than those in the lowest TyG level group.
Table 2.
Cognitive function in 2015 and 2019 in participants according to the TyG quartiles.
| Overall | Q1 | Q2 | Q3 | Q4 | p Value | |
|---|---|---|---|---|---|---|
| MMSE in 2015 (mean ± SD) | 28.45 ± 1.58 | 28.51 ± 1.49 | 28.43 ± 1.62 | 28.39 ± 1.56 | 28.46 ± 1.64 | 0.742 |
| MMSE in 2019 (mean ± SD) | 27.38 ± 3.06 | 27.80 ± 2.79 | 27.48 ± 2.99 | 27.03 ± 3.30 | 27.24 ± 3.12 | 0.002 |
| MMSE decline from 2015 to 2019 (mean ± SD) | 1.06 ± 3.08 | 0.70 ± 2.83 | 0.95 ± 2.95 | 1.35 ± 3.18 | 1.21 ± 3.30 | 0.009 |
| Cognitive decline incidence N (%) | 820 (46.22) | 180 (41.19) | 198 (44.00) | 218 (49.10) | 224 (50.56) | 0.017 |
Abbreviations: TyG, triglyceride-glucose; MMSE, Mini-Mental State Examination.
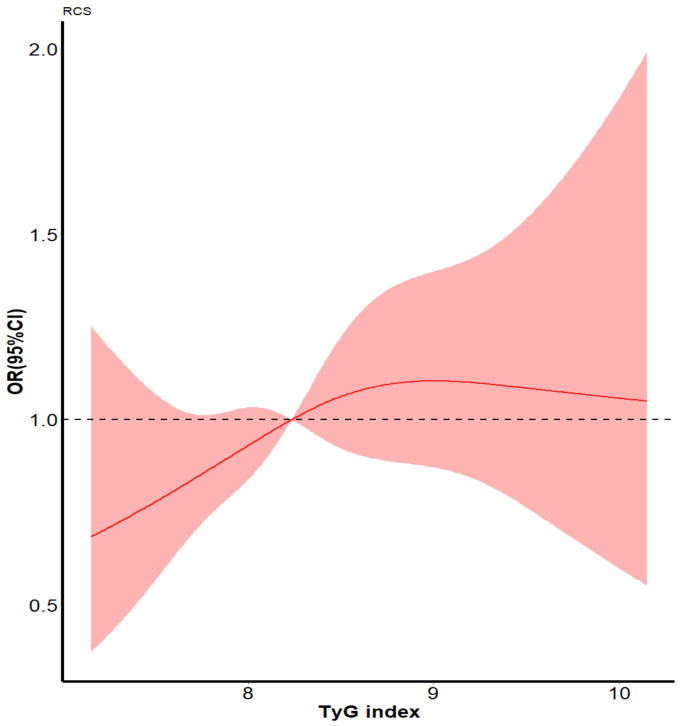
During the 4-year follow-up period from 2015 to 2019, among these 1774 participants, 820 (46.22%) participants presented cognitive decline. Associations of TyG levels with cognitive decline are presented in Table 3. After adjusting for the potential confounding factors mentioned above, the odds ratio for cognitive decline increased across TyG quartiles: 1.17 (95% CI: 0.85–1.62), 1.31 (95% CI: 0.93–1.83), and 1.51 (95% CI: 1.06–2.14) for the 2nd, 3rd, 4th quartiles, respectively, using the 1st quartile as the reference (p for trend = 0.020). Compared with those in the lowest TyG group, participants in the highest TyG group recorded a 51% increase in the risk of cognitive decline. Notably, the restricted cubic spline analysis also demonstrated that a higher TyG index represented a higher risk of cognitive decline (Figure 2).
Table 3.
ORs and 95% CIs for the association between the TyG index and the clinical outcome.
| Outcome | TyG Levels | p for Trend | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Cognitive decline from 2015 to 2019 | |||||
| Events, N(%) | 180 (41.19) | 198 (44.00) | 218 (49.10) | 224 (50.56) | |
| Unadjusted | 1 | 1.12 (0.86–1.46) | 1.38 (1.06–1.80) | 1.46 (1.12–1.91) | 0.002 |
| Model 1 | 1 | 1.07 (0.82–1.40) | 1.30 (0.99–1.70) | 1.40 (1.07–1.84) | 0.006 |
| Model 2 | 1 | 1.17 (0.85–1.62) | 1.31 (0.93–1.83) | 1.51 (1.06–2.14) | 0.020 |
Abbreviation: TyG, triglyceride-glucose. Odds ratios (ORs) with 95% confidence intervals (CIs) were expressed by using multivariate Cox regression models. The OR of the 1st quartile was set as the reference. Model 1: adjusted for age, sex, and educational levels. Model 2: adjusted for the factors in model 1 plus smoking status, alcohol consumption, physical activity, body mass index, history of hypertension, and total cholesterol.
Figure 2.
Spline models of the association between the TyG index and the risk of cognitive decline. The odds ratios from the multivariate Cox proportional hazard regression model were adjusted for the variables in model 2 in Table 3. The red line indicates the adjusted odds ratio, and the red area indicates the 95% confidence interval. Abbreviations: TyG, triglyceride-glucose.
After applying discrimination tests, we found that there was a slight improvement in predicting the risk of cognitive decline when adding the TyG index to the conventional model (including age, sex, educational levels, smoking status, alcohol consumption, physical activity, BMI, history of hypertension, total cholesterol) (NRI 16.00% (p = 0.004), IDI 0.40% (p = 0.030)) (Table 4).
Table 4.
Reclassification and disclination statistics for the clinical outcome when adding to the TyG index.
| Outcome | Model | C-Statistic | NRI | IDI | |||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p Value | Estimate (95% CI) | p Value | Estimate (95% CI) | p Value | ||
| Cognitive decline from 2015 to 2019 | Conventional model | 0.64 (0.61–0.67) | 0.414 | Ref. | 0.004 | Ref. | 0.030 |
| Conventional model +TyG | 0.65 (0.62–0.68) | 0.16 (0.05–0.27) | 0.004 (0.003–0.01) | ||||
Abbreviation: TyG, triglyceride-glucose; NRI, net reclassification improvement; IDI, integrated discrimination improvement; CI, confidence interval. Conventional model: added to factor-adjusted models, including age, sex, educational levels, smoking status, alcohol consumption, physical activity, body mass index, history of hypertension, and total cholesterol.
According to previous studies, several demographic and physiological factors might cause different effects of the TyG index on cognitive decline [40,41,42,43]. Thus, we further analyzed the interaction effects in this study. Regardless of the stratification of age, sex, BMI, hypertension, dyslipidemia and diabetes mellitus, participants in the highest quartile of the TyG index had a higher risk of cognitive decline than those in the lowest quartile. This association was significant in the female group (p value for interaction = 0.028). Moreover, the analysis showed no significant interaction effects between the TyG index and other stratified variables, including age, BMI, hypertension, dyslipidemia and diabetes mellitus (p value for interaction effects > 0.05) (Table S2).
4. Discussion
This longitudinal cohort study revealed a positive association between the TyG index and the risk of cognitive decline. Compared with those in the bottom of the fourth quartile, participants in the top of the fourth quartile showed a 51% increased risk of cognitive decline. The risk of this outcome increased with the increasing TyG quartile regardless of age, sex, educational levels, smoking status, alcohol consumption, physical activity, BMI, history of hypertension, and TC. Furthermore, adding the TyG index into the conventional model promoted the risk stratification ability. In addition, we found that the effect of the TyG index on the risk of cognitive decline remained consistent across the subgroup analyses, but this positive correlation was more significant among women.
In the past, the relationship between insulin resistance and dementia has been investigated; however, the HOMA-IR index has been mainly used in studies to determine the degree of IR; however, the HOMA-IR index values are cumbersome to collect and have low possibility of clinical application. Previous studies have reported that a higher risk of dementia was associated with increased insulin levels and HOMA-IR index [10,12,44,45,46,47]. In populations at high risk of cardiovascular disease, baseline HOMA-IR levels are associated with short-term cognitive deterioration [48]. Conversely, some researchers have found no association between dementia and insulin resistance [49]. Recently, a study found that elevated levels of the TyG index were associated with the risk of dementia and suggested that this index, as a marker of IR, might be a potential predictor of dementia development [40]; however, the evidence is still insufficient. The strength of this cohort study is that it further explored the effect of the TyG index, a surrogate marker of insulin resistance, on longitudinal cognitive function decline in a healthy middle-aged to elderly Chinese population.
The results of this current study could be explained by the following possible mechanisms. Previous studies have shown that insulin metabolism and hemodynamic actions of insulin share common intracellular transduction pathways [50]. Insulin resistance might play an important role in arterial stiffness and the formation of atherosclerotic plaque by causing chronic inflammation, oxidative stress, impaired nitric oxide activity, and endothelial dysfunction, promoting foam cell formation, altering estrogen receptor expression and further leading to hemodynamic changes [51,52]. As a receptor expressed in blood vessels and adipose tissue, the mineralocorticoid receptors (MR) can regulate adipose tissue function and vascular tone. Studies have shown that the presence of metabolic abnormalities such as obesity, insulin resistance, and increased aldosterone levels, favors MR activation, which further promotes vascular endothelial dysfunction and accelerates arterial stiffness and atherosclerosis [53,54]. Arterial stiffness has gradually become a proxy of arterial aging, reflecting the state of vascular health. A negative correlation has been found between arterial stiffness and cognition over time, independently of other risk factors. The predictive value of arterial stiffness as a risk marker for cognitive deterioration and dementia is independent of age and traditional cardiovascular risk factors [55,56,57]. Moreover, long-term insulin resistance prevents insulin from crossing the blood–brain barrier, leading to decreased insulin levels in the brain, which in turn impairs neuronal function and synaptic building [58]. Moreover, peripheral insulin resistance can weaken the insulin signal in the brain, decrease the uptake of brain plasma insulin and reduce the clearance of brain Aβ [59]. Recent studies have shown that insulin resistance might enhance amyloid-β production or its neurotoxicity and might also be involved in tau phosphorylation and neurofibrillary tangle formation [60]. One study found that in cognitively normal individuals, increasing levels of HOMA-IR were associated with increases in CSF T-tau and P-tau [61]. Another study reported that higher insulin resistance might predict the degree of amyloid substance deposition in the temporal and frontal lobes of patients with Alzheimer’s disease [62]. Severe IR promotes amyloid Aβ production, increases AD-type amyloid plaque burden, and may cause hippocampal atrophy and impair memory task performance. Areas of the brain with high concentrations of insulin receptors, such as the medial temporal lobe and frontal lobe, were reported to be particularly sensitive to insulin signals and especially more vulnerable in AD patients [63]. Studies have found that higher IR levels were associated with a lower cerebral glucose metabolic rate in these areas in adults with or without AD [62,64]. Decreased glucose metabolism might affect cortical activity, resulting in impaired cognitive function. In other words, insulin resistance accelerates cognitive deterioration by substantially affecting hippocampal plasticity, changes in amyloid precursor protein metabolism, increased tau protein concentration, altered brain inflammation, and the involvement of the ApoEε4 allele [61,63,65,66].
Moreover, the TyG index is calculated from fasting triglycerides and blood glucose. Triglycerides may affect cognitive function by penetrating the blood–brain barrier and inducing central insulin receptor resistance [28,67]. In addition, elevated triglyceride levels and blood glucose levels may reflect increased neuroinflammation, which may also affect cognitive function [68]. Alternatively, during the development of insulin resistance, insulin cannot inhibit the production of glucose in the liver but paradoxically accelerates the synthesis of lipids, leading to an increase in triglycerides and blood glucose [69]. Moreover, as insulin resistance leads to reduced uptake of glucose by nerve cells, the energy supply of neurons decreases, leading to increased oxidative stress, neuroinflammation, and lipid dysregulation, all of which in turn accelerate neurodegeneration.
In this study, we also observed sex differences in the association of the TyG index and cognitive decline, suggesting that the TyG index might be a risk factor for cognitive decline in women. Similar results have been found in previous studies [70,71]. One study found that insulin resistance was more prevalent in women than in men [61]. Metabolic syndrome was associated with the risk of cognitive decline in women compared with men [72]. These findings might be due to changes in sex hormone levels in middle-aged and elderly women. Moreover, as women age, the decrease in estrogen production diminishes its neuroprotective effects, which may lead to pathological changes associated with cognitive impairment.
This study also had several limitations. First, due to the shortage of insulin concentration data, we could not compare the correlation between cognitive function decline and the TyG index and HOMA-IR index. Second, the effect size of the TyG index on cognitive decline was relatively small, possibly due to the influence of sample size and follow-up time, and this might have underestimated the association between the TyG index and cognitive function decline. Thus, for the interesting results of this study to be of greater breadth and relevance, longer follow-up should be considered. Third, owing to the limitations of this observational study, we could not establish a causal link between the TyG index and the risk of cognitive decline. Furthermore, this study collected only baseline data of the TyG index; nevertheless, as a marker that reflects metabolism, it may undergo dynamic changes. Thus, the effect of dynamic changes in the TyG index on cognitive deterioration needs to be further verified.
5. Conclusions
In conclusion, this study found that an increased TyG index was positively associated with the risk of cognitive decline; moreover, adding the TyG index to the conventional model might provide superior risk stratification. As a readily measurable, cost-effective, and reliable marker for evaluating insulin resistance, the TyG index has potential clinical predictive value in predicting longitudinal cognitive decline, but its use still needs to be further confirmed. This finding highlights the importance of more effectively monitoring the TyG index in healthy middle-aged and elderly populations to identify individuals who are at high risk for cognitive impairment. More medical and lifestyle interventions should be performed as early as possible to prevent the development of cognitive deterioration in people with a high TyG index.
Acknowledgments
We are grateful to all the research staff involved in the Jidong Cognitive Impairment Cohort Study (CICS). We appreciate the participants in this study for their contribution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11237153/s1, Table S1. Baseline characteristics of included vs. excluded participants in this study. Table S2. Subgroups analysis: Risk of cognitive decline based on the increasing the TyG quartiles with various clinical variables
Author Contributions
Y.Z. reviewed the manuscript, and takes responsibility for the integrity and accuracy of the data. S.L. contributed to the study concept and drafted the manuscript. X.D. analyzed the data and revised the manuscript for important statistical problems. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines described by the Helsinki Declaration, and was approved by the Ethics Committees of Kailuan General Hospital of Tangshan City and the Medical Ethics Committee, the Staff Hospital of Jidong Oilfield Branch, China National Petroleum Corporation (No. 2013 YILUNZI 1).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used and/or analyzed in the current study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 81972144).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jia L., Du Y., Chu L., Zhang Z., Li F., Lyu D., Li Y., Zhu M., Jiao H., Song Y., et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health. 2020;5:e661–e671. doi: 10.1016/S2468-2667(20)30185-7. [DOI] [PubMed] [Google Scholar]
- 2.Cong L., Ren Y., Wang Y., Hou T., Dong Y., Han X., Yin L., Zhang Q., Feng J., Wang L., et al. Mild cognitive impairment among rural-dwelling older adults in China: A community-based study. Alzheimer’s Dement. 2022;9:1–11. doi: 10.1002/alz.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators GBDD Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An R., Liu G.G. Cognitive impairment and mortality among the oldest-old Chinese. Int. J. Geriatr. Psychiatry. 2016;31:1345–1353. doi: 10.1002/gps.4442. [DOI] [PubMed] [Google Scholar]
- 5.Perna L., Wahl H.W., Mons U., Saum K.U., Holleczek B., Brenner H. Cognitive impairment, all-cause and cause-specific mortality among non-demented older adults. Age Ageing. 2015;44:445–451. doi: 10.1093/ageing/afu188. [DOI] [PubMed] [Google Scholar]
- 6.Batty G.D., Deary I.J., Zaninotto P. Association of cognitive function with causespecific mortality in middle and older age: Follow-up of participants in the English longitudinal study of ageing. Am. J. Epidemiol. 2016;183:183–190. doi: 10.1093/aje/kwv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. 2002;90:3G–10G. doi: 10.1016/S0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 8.Novak V., Milberg W., Hao Y., Munshi M., Novak P., Galica A., Manor B., Roberson P., Craft S., Abduljalil A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37:751–759. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S., Peters S.A., Woodward M., Mejia Arango S., Batty G.D., Beckett N., Beiser A., Borenstein A.R., Crane P.K., Haan M., et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39:300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooshmand B., Rusanen M., Ngandu T., Leiviskä J., Sindi S., von Arnim C.A., Falkai P., Soininen H., Tuomilehto J., Kivipelto M. Serum insulin and cognitive performance in older adults: A longitudinal study. Am. J. Med. 2019;132:367–373. doi: 10.1016/j.amjmed.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Neergaard J.S., Dragsbæk K., Christiansen C., Nielsen H.B., Brix S., Karsdal M.A., Henriksen K. Metabolic syndrome, insulin resistance, and cognitive dysfunction: Does your metabolic profile affect your brain? Diabetes. 2017;66:1957–1963. doi: 10.2337/db16-1444. [DOI] [PubMed] [Google Scholar]
- 12.Lutski M., Weinstein G., Goldbourt U., Tanne D. Insulin Resistance and Future Cognitive Performance and Cognitive Decline in Elderly Patients with Cardiovascular Disease. J. Alzheimer’s Dis. 2017;57:633–643. doi: 10.3233/JAD-161016. [DOI] [PubMed] [Google Scholar]
- 13.Barber T.M., Kyrou I., Randeva H.S., Weickert M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021;22:546. doi: 10.3390/ijms22020546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cersosimo E., Solis-Herrera C., Trautmann M., Malloy J., Triplitt C. Assessment of pancreaticβ-cell function: Review of methods and clinical applications. Curr. Diabetes Rev. 2014;10:2–42. doi: 10.2174/1573399810666140214093600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Q., Zhang T.Y., Cheng Y.J., Ma Y., Xu Y.K., Yang J.Q., Zhou Y.J. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: Results from an observational cohort study in China. Cardiovasc. Diabetol. 2020;19:108. doi: 10.1186/s12933-020-01086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S.E., Park C.Y., Sweeney G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit. Rev. Clin. Lab. Sci. 2015;52:180–190. doi: 10.3109/10408363.2015.1023429. [DOI] [PubMed] [Google Scholar]
- 17.Liu L., Zhang C., Lv X., Lai X., Xu L., Feng J., Song Y., Wang S., Zhan S. Sex-specific associations between lipids and cognitive decline in the middle-aged and elderly: A cohort study of Chinese adults. Alzheimer’s Res. Ther. 2020;12:164. doi: 10.1186/s13195-020-00731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Q., Wang F., Yang J., Peng H., Li Y., Li B., Wang S. Revealing a Novel Landscape of the Association Between Blood Lipid Levels and Alzheimer’s Disease: A Meta-Analysis of a Case-Control Study. Front. Aging Neurosci. 2020;11:370. doi: 10.3389/fnagi.2019.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C., Yin Z., Zhu P., Luo J., Shi X., Gao X. Blood cholesterol in late-life and cognitive decline: A longitudinal study of the Chinese elderly. Mol. Neurodegener. 2017;12:24. doi: 10.1186/s13024-017-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power M.C., Rawlings A., Sharrett A.R., Bandeen-Roche K., Coresh J., Ballantyne C.M., Pokharel Y., Michos E.D., Penman A., Alonso A., et al. Association of midlife lipids with 20-year cognitive change: A cohort study. Alzheimer’s Dement. 2018;14:167–177. doi: 10.1016/j.jalz.2017.07.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mielke M.M., Xue Q.L., Zhou J., Chaves P.H., Fried L.P., Carlson M.C. Baseline serum cholesterol is selectively associated with motor speed and not rates of cognitive decline: The Women’s Health and Aging Study II. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:619–624. doi: 10.1093/gerona/63.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Wei S., Zhou R., Shang S., Dang L., Gao L., Chen C., Huo K., Wang J., Wang J., et al. The Relationships Between Lipid Accumulation Product Levels and Cognitive Decline Over 4 Years in a Rural Area of Xi’an, China. Front. Aging Neurosci. 2021;13:761886. doi: 10.3389/fnagi.2021.761886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y., Li J., Yu G., Zhou X., Zhou W., Zhu L., Wang T., Huang X., Bao H., Cheng X. Association Between Lipid Accumulation Product and Cognitive Function in Hypertensive Patients With Normal Weight: Insight From the China H-type Hypertension Registry Study. Front. Neurol. 2022;12:732757. doi: 10.3389/fneur.2021.732757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Q., Li Q., Zhao J., Wu T., Ji L., Huang G., Ma F. Relationship between plasma lipids and mild cognitive impairment in the elderly Chinese: A case-control study. Lipids Health Dis. 2016;15:146. doi: 10.1186/s12944-016-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parthasarathy V., Frazier D.T., Bettcher B.M., Jastrzab L., Chao L., Reed B., Mungas D., Weiner M., DeCarli C., Chui H., et al. Triglycerides are negatively correlated with cognitive function in nondemented aging adults. Neuropsychology. 2017;31:682–688. doi: 10.1037/neu0000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimache A.M., Șalaru D.L., Sascău R., Stătescu C. The Role of High Triglycerides Level in Predicting Cognitive Impairment: A Review of Current Evidence. Nutrients. 2021;13:2118. doi: 10.3390/nu13062118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nägga K., Gustavsson A.M., Stomrud E., Lindqvist D., van Westen D., Blennow K., Zetterberg H., Melander O., Hansson O. Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology. 2018;90:e73–e81. doi: 10.1212/WNL.0000000000004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banks W.A., Farr S.A., Salameh T.S., Niehoff M.L., Rhea E.M., Morley J.E., Hanson A.J., Hansen K.M., Craft S. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int. J. Obes. 2018;42:391–397. doi: 10.1038/ijo.2017.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peloso G.M., Beiser A.S., Destefano A.L., Seshadri S. Genetic Interaction with Plasma Lipids on Alzheimer’s Disease in the Framingham Heart Study. J. Alzheimer’s Dis. 2018;66:1275–1282. doi: 10.3233/JAD-180751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimering M.B., Knight J., Ge L., Bahn G., VADT Investigators Predictors of Cognitive Decline in Older Adult Type 2 Diabetes from the Veterans Affairs Diabetes Trial. Front. Endocrinol. 2016;7:123. doi: 10.3389/fendo.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao T.N., Yin G.Z., Yin X.L., Wu J.Q., Du X.D., Zhu H.L., Liu J.H., Wang X.Q., Xu D.W., Tang W.J., et al. Elevated triglyceride levels are associated with cognitive impairments among patients with major depressive disorder. Compr. Psychiatry. 2017;75:103–109. doi: 10.1016/j.comppsych.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Khan S., Sobia F., Niazi N., Manzoor S., Fazal N., Ahmad F. Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 2018;10:74. doi: 10.1186/s13098-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazidi M., Kengne A., Katsiki N., Mikhailidis D., Banach M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J. Diabetes Complicat. 2018;32:266–270. doi: 10.1016/j.jdiacomp.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Vasques A.C.J., Novaes F.S., de Oliveira M.D.S., Souza J.R.M., Yamanaka A., Pareja J.C., Tambascia M.A., Saad M.J.A., Geloneze B. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Barzegar N., Tohidi M., Hasheminia M., Azizi F., Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc. Diabetol. 2020;19:155. doi: 10.1186/s12933-020-01121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong S., Han K., Park C. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: A population-based study. BMC Med. 2020;18:361. doi: 10.1186/s12916-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su W., Chen S., Huang Y., Huang J., Wu P., Hsu W., Lee M. Comparison of the effects of fasting glucose, hemoglobin A, and triglyceride-glucose index on cardiovascular events in type 2 diabetes mellitus. Nutrients. 2019;11:2838. doi: 10.3390/nu11112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng Z., Feng J., Dong Y., Xu J., Jiang X., Chen H., Qi Q., Li R., Chen W., Lv P. Triglyceride glucose index is associated with cerebral small vessel disease burden and cognitive impairment in elderly patients with type 2 diabetes mellitus. Front. Endocrinol. 2022;13:970122. doi: 10.3389/fendo.2022.970122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C., Lu Z., Wang X., Zhang J., Zhang D., Li S. The chain mediating role of C-reactive protein and triglyceride-glucose index between lung function and cognitive function in a systemic low-grade inflammation state. J. Psychiatr. Res. 2022;155:380–386. doi: 10.1016/j.jpsychires.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Hong S., Han K., Park C.Y. The insulin resistance by triglyceride glucose index and risk for dementia: Population-based study. Alzheimer’s Res. Ther. 2021;13:9. doi: 10.1186/s13195-020-00758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song D.Y., Wang X.W., Wang S., Ge S.Q., Ding G.Y., Chen X.Y., Chen Y.R., Liu H.M., Xie X.M., Xing W.J., et al. Jidong cognitive impairment cohort study: Objectives, design, and baseline screening. Neural Regen. Res. 2020;15:1111–1119. doi: 10.4103/1673-5374.266070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H., Jia J., Yang Z. Mini-Mental State Examination in Elderly Chinese: A Population-Based Normative Study. J. Alzheimer’s Dis. 2016;53:487–496. doi: 10.3233/JAD-160119. [DOI] [PubMed] [Google Scholar]
- 43.Lv X., Li W., Ma Y., Chen H., Zeng Y., Yu X., Hofman A., Wang H. Cognitive decline and mortality among community-dwelling Chinese older people. BMC Med. 2019;17:63. doi: 10.1186/s12916-019-1295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He R., Zheng R., Li J., Cao Q., Hou T., Zhao Z., Xu M., Chen Y., Lu J., Wang T., et al. Individual and Combined Associations of Glucose Metabolic Components With Cognitive Function Modified by Obesity. Front. Endocrinol. 2021;12:769120. doi: 10.3389/fendo.2021.769120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pekkala T., Hall A., Mangialasche F., Kemppainen N., Mecocci P., Ngandu T., Rinne J.O., Soininen H., Tuomilehto J., Kivipelto M., et al. Association of Peripheral Insulin Resistance and Other Markers of Type 2 Diabetes Mellitus with Brain Amyloid Deposition in Healthy Individuals at Risk of Dementia. J. Alzheimer’s Dis. 2020;76:1243–1248. doi: 10.3233/JAD-200145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cezaretto A., de Almeida-Pititto B., Alencar G.P., Suemoto C.K., Bensenor I., Lotufo P.A., Ferreira S.R. Utility of combined inflammatory biomarkers for the identification of cognitive dysfunction in non-diabetic participants of the ELSA-Brasil. Psychoneuroendocrinology. 2019;103:61–66. doi: 10.1016/j.psyneuen.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Teixeira M.M., de Azeredo Passos V.M., Barreto S.M., Schmidt M.I., Duncan B.B., Beleigoli A.M., Fonseca M.D.J.M., Vidigal P.G., Figueiredo R.C., Colosimo E., et al. Markers of adiposity, insulin resistance, prediabetes and cognitive function at baseline of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Diabetes Res. Clin. Pract. 2020;170:108499. doi: 10.1016/j.diabres.2020.108499. [DOI] [PubMed] [Google Scholar]
- 48.Gómez-Martínez C., Babio N., Júlvez J., Becerra-Tomás N., Martínez-González M.Á., Corella D., Castañer O., Romaguera D., Vioque J., Alonso-Gómez Á.M., et al. Glycemic Dysregulations Are Associated with Worsening Cognitive Function in Older Participants at High Risk of Cardiovascular Disease: Two-Year Follow-up in the PREDIMED-Plus Study. Front. Endocrinol. 2021;12:754347. doi: 10.3389/fendo.2021.754347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rönnemaa E., Zethelius B., Sundelöf J., Sundström J., Degerman-Gunnarsson M., Lannfelt L., Berne C., Kilander L. Glucose metabolism and the risk of Alzheimer’s disease and dementia: A population-based 12 year follow-up study in 71-year-old men. Diabetologia. 2009;52:1504–1510. doi: 10.1007/s00125-009-1393-9. [DOI] [PubMed] [Google Scholar]
- 50.Tesauro M., Canale M.P., Rodia G., Di Daniele N., Lauro D., Scuteri A., Cardillo C. Metabolic syndrome, chronic kidney, and cardiovascular diseases: Role of adipokines. Cardiol. Res. Pract. 2011;2011:653182. doi: 10.4061/2011/653182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janus A., Szahidewicz-Krupska E., Mazur G., Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediat. Inflamm. 2016;2016:3634948. doi: 10.1155/2016/3634948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoscheidt S.M., Kellawan J.M., Berman S.E., Rivera-Rivera L.A., Krause R.A., Oh J.M., Beeri M.S., Rowley H.A., Wieben O., Carlsson C.M., et al. Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J. Cereb. Blood Flow Metab. 2017;37:2249–2261. doi: 10.1177/0271678X16663214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feraco A., Marzolla V., Scuteri A., Armani A., Caprio M. Mineralocorticoid Receptors in Metabolic Syndrome: From Physiology to Disease. Trends Endocrinol. Metab. 2020;31:205–217. doi: 10.1016/j.tem.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Gorini S., Marzolla V., Mammi C., Armani A., Caprio M. Mineralocorticoid Receptor and Aldosterone-Related Biomarkers of End-Organ Damage in Cardiometabolic Disease. Biomolecules. 2018;8:96. doi: 10.3390/biom8030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scuteri A., Benetos A., Sierra C., Coca A., Chicherio C., Frisoni G.B., Gasecki D., Hering D., Lovic D., Manios E., et al. Routine assessment of cognitive function in older patients with hypertension seen by primary care physicians: Why and how-a decision-making support from the working group on ‘hypertension and the brain’ of the European Society of Hypertension and from the European Geriatric Medicine Society. J. Hypertens. 2021;39:90–100. doi: 10.1097/HJH.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 56.Nilsson P.M., Laurent S., Cunha P.G., Olsen M.H., Rietzschel E., Franco O.H., Ryliškytė L., Strazhesko I., Vlachopoulos C., Chen C.H., et al. Characteristics of healthy vascular ageing in pooled population-based cohort studies: The global Metabolic syndrome and Artery Research Consortium. J. Hypertens. 2018;36:2340–2349. doi: 10.1097/HJH.0000000000001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamballais S., Sajjad A., Leening M.J.G., Gaillard R., Franco O.H., Mattace-Raso F.U.S., Jaddoe V.W.V., Roza S.J., Tiemeier H., Ikram M.A. Association of Blood Pressure and Arterial Stiffness with Cognition in 2 Population-Based Child and Adult Cohorts. J. Am. Heart Assoc. 2018;7:e009847. doi: 10.1161/JAHA.118.009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arnold S.E., Arvanitakis Z., Macauley-Rambach S.L., Koenig A.M., Wang H.Y., Ahima R.S., Craft S., Gandy S., Buettner C., Stoeckel L.E., et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grillo C.A., Woodruff J.L., Macht V.A., Reagan L.P. Insulin resistance and hippocampal dysfunction: Disentangling peripheral and brain causes from consequences. Exp. Neurol. 2019;318:71–77. doi: 10.1016/j.expneurol.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Ekblad L.L., Johansson J., Helin S., Viitanen M., Laine H., Puukka P., Jula A., Rinne J.O. Midlife insulin resistance, APOE genotype, and late-life brain amyloid accumulation. Neurology. 2018;90:e1150–e1157. doi: 10.1212/WNL.0000000000005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laws S.M., Gaskin S., Woodfield A., Srikanth V., Bruce D., Fraser P.E., Porter T., Newsholme P., Wijesekara N., Burnham S., et al. Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci. Rep. 2017;7:9766. doi: 10.1038/s41598-017-09577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willette A.A., Bendlin B.B., Starks E.J., Birdsill A.C., Johnson S.C., Christian B.T., Okonkwo O.C., La Rue A., Hermann B.P., Koscik R.L., et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer’s disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoscheidt S.M., Starks E.J., Oh J.M., Zetterberg H., Blennow K., Krause R.A., Gleason C.E., Puglielli L., Atwood C.S., Carlsson C.M., et al. Insulin Resistance is Associated with Increased Levels of Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease and Reduced Memory Function in At-Risk Healthy Middle-Aged Adults. J. Alzheimer’s Dis. 2016;52:1373–1383. doi: 10.3233/JAD-160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willette A.A., Xu G., Johnson S.C., Birdsill A.C., Jonaitis E.M., Sager M.A., Hermann B.P., La Rue A., Asthana S., Bendlin B.B. Insulin Resistance, Brain Atrophy, and Cognitive Performance in Late Middle–Aged Adults. Diabetes Care. 2013;36:443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tyagi A., Pugazhenthi S. Targeting Insulin Resistance to Treat Cognitive Dysfunction. Mol. Neurobiol. 2021;58:2672–2691. doi: 10.1007/s12035-021-02283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spinelli M., Fusco S., Grassi C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front. Neurosci. 2019;13:788. doi: 10.3389/fnins.2019.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez-Vilaret K.M., Cantero J.L., Fernandez-Alvarez M., Calero M., Calero O., Lindín M., Zurrón M., Díaz F., Atienza M. Impaired glucose metabolism reduces the neuroprotective action of adipocytokines in cognitively normal older adults with insulin resistance. Aging. 2021;13:23936–23952. doi: 10.18632/aging.203668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rönnbäck C., Hansson E. The Importance and Control of Low-Grade Inflammation Due to Damage of Cellular Barrier Systems That May Lead to Systemic Inflammation. Front. Neurol. 2019;10:533. doi: 10.3389/fneur.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santoleri D., Titchenell P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell. Mol. Gastroenterol. Hepatol. 2019;7:447–456. doi: 10.1016/j.jcmgh.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ekblad L.L., Rinne J.O., Puukka P.J., Laine H.K., Ahtiluoto S.E., Sulkava R.O., Viitanen M.H., Jula A.M. Insulin resistance is associated with poorer verbal fluency performance in women. Diabetologia. 2015;58:2545–2553. doi: 10.1007/s00125-015-3715-4. [DOI] [PubMed] [Google Scholar]
- 71.Schuur M., Henneman P., van Swieten J.C., Zillikens M.C., de Koning I., Janssens A.C., Witteman J.C., Aulchenko Y.S., Frants R.R., Oostra B.A., et al. Insulin-resistance and metabolic syndrome are related to executive function in women in a large family-based study. Eur. J. Epidemiol. 2010;25:561–568. doi: 10.1007/s10654-010-9476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McEvoy L.K., Laughlin G.A., Barrett-Connor E., Bergstrom J., Kritz-Silverstein D., Der-Martirosian C., von Mühlen D. Metabolic syndrome and 16-year cognitive decline in community-dwelling older adults. Ann. Epidemiol. 2012;22:310–317. doi: 10.1016/j.annepidem.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used and/or analyzed in the current study are available on reasonable request from the corresponding author.