Abstract
Macrophage class A scavenger receptors (SR-AI and SR-AII) contribute to host defense by binding polyanionic ligands such as lipopolysaccharide and lipoteichoic acid. SR-A knockout (SR-A−/−) mice are more susceptible to endotoxic shock and Listeria monocytogenes infection in vivo, possibly due to decreased clearance of lipopolysaccharide and microorganisms, respectively. We have used flow cytometry to analyze the role of SR-A and other scavenger-like receptors in phagocytosis of bacteria in vitro. Chinese hamster ovary cells stably transfected with human SR-A bound Escherichia coli and Staphylococcus aureus but ingested few organisms. Primary human monocyte-derived macrophages (Mφ) bound and ingested E. coli more efficiently, and this was partially but selectively blocked by the general SR inhibitor, poly(I). A specific and selective role for SR-A was shown, since bone marrow culture-derived Mφ from SR-A−/− mice ingested fewer E. coli organisms than did wild-type cells, while uptake of antibody-opsonized E. coli was unaffected. SR-A-dependent uptake of E. coli varied with the bacterial strain; ingestion of DH5α and K1 by SR-A−/− Mφ was reduced by 30 to 60% and 70 to 75%, respectively. Phagocytosis and endocytosis via SR-A were markedly down-modulated when Mφ were plated on serum-coated tissue culture plastic compared to bacteriologic plastic, where cell adhesion is mediated by SR-A and CR3, respectively. This paper demonstrates that SR-A can bind and ingest bacteria directly, consistent with a role in host defense in vivo, and highlights the importance of the source of the Mφ, bacterial strain, and culture conditions on receptor function in vitro.
Macrophages (Mφ) express several surface molecules to aid in the recognition of microorganisms: receptors for immunoglobulin (FcR) and complement (CR3) utilize opsonins for ingestion (1), while other pattern recognition molecules such as the mannose receptor are able to recognize conserved motifs on pathogen surfaces directly (13). Scavenger receptors (SR) were originally defined by their ability to recognize modified forms of low-density lipoprotein (LDL) (3, 29). Since family members such as the class A scavenger receptor (SR-A) are able to bind a broad range of polyanionic ligands, including lipopolysaccharide (LPS) (19) and lipoteichoic acid (LTA) (8), these receptors have been implicated in host defense against bacterial infections (28, 36).
SR-A are type II trimeric transmembrane glycoproteins and were initially cloned from bovine lung mRNA (26, 41). Three naturally occurring forms of SR-A are alternative splice variants of the same gene, whereas a distinct SR-A-like molecule, MARCO, is derived from a different gene (9, 10, 15). Each SR-A isoform expresses six domains: the N-terminal cytoplasmic, transmembrane, spacer, α-helical coiled-coil, collagenous, and C-terminal domains (2, 26, 41). SR-A type I (SR-AI) contains a C-terminal SR cysteine-rich domain of 110 amino acids. A similar protein motif is found on several other molecules on immune system cell surfaces including CD5 and CD6, although its function is still unclear (39, 40). Type II and type III SR-A (SR-AII and SR-AIII) express a short C terminus or truncated cysteine-rich domain, respectively. The ligand-binding region is in the positively charged collagenous domain of SR-AI and SR-AII; no difference in ligand binding has hitherto been detected between these isoforms (29). SR-AIII is trapped in the endoplasmic reticulum and has no known ligand-binding activity, although it can exert a dominant negative effect in cells which coexpress different SR-A isoforms (15).
SR-A is expressed by most tissue Mφ; however, its role in vivo is unclear since it is able to mediate disparate functions in vitro (17, 44). SR-A can endocytose modified low-density lipoproteins, which is important in foam cell formation and atherosclerosis (45), and Mφ from SR-A knockout (SR-A−/−) mice display a reduced capacity to phagocytose apoptotic thymocytes in vitro (38). SR-A has also been implicated in adhesion of murine Mφ in vitro. 2F8, a specific rat monoclonal antibody, inhibited the divalent cation-independent adhesion of murine Mφ-like cells to tissue culture plastic (TCP) coated with an unidentified ligand for SR-A present in bovine serum (12).
Several lines of evidence support a role for SR-A in phagocytic recognition of microorganisms. CHO cells transfected with bovine SR-A type I or type II specifically bound the lipid A moiety of LPS and its bioactive precursor, lipid IVA (19). In vitro competition binding studies with RAW264 Mφ-like cells demonstrated that SR-A could recognize and partially degrade LPS to a less active form without the concomitant release of proinflammatory cytokines. In vivo, SR-A−/− mice are more sensitive than control mice to LPS challenge after the animals have been primed with BCG (20). A septic-shock syndrome was associated with increased systemic production of tumor necrosis factor alpha, interleukin-6, and interleukin-1β by SR-A-deficient mice and could be partially prevented by anti-tumor necrosis factor alpha antibody treatment, consistent with a role for SR-A in endotoxin clearance and/or down-regulation of cytokine release.
In vitro binding studies showed that soluble forms of bovine SR-AI were able to bind LPS, as well as LTA and intact gram-positive bacteria (8). In vivo, SR-A−/− mice were more susceptible to Listeria monocytogenes infection than were wild-type (WT) control mice (45). L. monocytogenes was rapidly cleared from the circulation of both types of animal; 24 and 96 h after infection, there were more organisms in the livers and spleens of SR-A-deficient mice (45).
In none of these studies has phagocytic uptake by SR-A or other SR been demonstrated directly. Our preliminary in vitro studies have shown that BCG-activated peritoneal Mφ from SR-A−/− mice took up 40% fewer Escherichia coli organisms than did WT Mφ, but they did not define the role of SR-A in detail (37). In the present study, we have characterized the role of SR-A in bacterial phagocytosis in vitro. We used flow cytometry and microscopy to study the binding and ingestion of fluoresceinated E. coli and Staphylococcus aureus by various cell populations (primary cells, cell lines, and transfectants), using inhibitors and genetically deficient cells to establish a role for SR-A and its isoforms. We provide evidence that SR-A can mediate the binding and ingestion of a range of microorganisms and that its contribution varies markedly depending on the cell in which it is expressed, as well as the bacterial strain utilized and the in vitro conditions of cell culture.
MATERIALS AND METHODS
Reagents.
DiI (1,1′-dioctadecyl-1-3,3,3′,3′-tetramethylindocarbocyanine perchlorate)-labelled acetylated LDL (DiIAcLDL) and acetylated LDL (AcLDL) were obtained from Intracell (Rockville, Md.), and poly(I), poly(C), and Ficoll-Hypaque were obtained from Pharmacia Biotech (St. Albans, United Kingdom). X-VIVO 10 culture medium was obtained from Bio-Whittaker (Reading, United Kingdom), while all other culture media were from Gibco (Paisley, United Kingdom). Fluorescein isothiocyanate (FITC), rhodamine green X (RdGnX), FITC-labelled E. coli K-12, and FITC-labelled S. aureus were obtained from Molecular Probes (Eugene, Oreg.). Brewer thioglycolate medium, Luria broth, and E. coli LPS were obtained from Difco Laboratories (West Molesey, United Kingdom), and anti-E. coli rabbit polyclonal antiserum was obtained from Dako Ltd. (High Wycombe, United Kingdom). Unless stated otherwise, all other reagents were from Sigma (Poole, United Kingdom) and plastic products were from Becton Dickinson Labware (Oxford, United Kingdom). The E. coli K1 and the mouse anti-E. coli K1 capsule monoclonal antibodies were a kind gift from C. Tang (Paediatrics, Oxford University).
Animals.
Mice deficient in SR-AI and SR-AII (SR-A−/−) were produced as previously described (45). SR-A−/− and SR-A+/+ control 129/ICR mice of the same sex were used at 4 to 8 weeks of age and housed at the Sir William Dunn School of Pathology Services Building.
Cell isolation and culture.
Murine bone marrow-derived Mφ (BMMφ) were obtained and cultured by standard procedures. The cells were cultured in 15-cm bacteriologic plastic (BP) petri dishes containing RPMI 1640 supplemented with 50 IU of penicillin G per ml, 50 μg of streptomycin per ml, and 2 mM glutamine (PSG); 10% fetal calf serum (FCS); and 15% (vol/vol) L-cell conditioned medium (21). Before use, the Mφ were harvested with phosphate-buffered saline (PBS) containing 10 mM EDTA and 4 mg of Lidocaine-HCl per ml and plated in the appropriate culture medium at 106 Mφ per well of a six-well BP dish (Greiner, Gloucester, United Kingdom). Typically, the bone marrow from the femurs and tibias of one mouse gave 2 × 107 to 6 × 107 Mφ after 7 days in culture.
Thioglycolate broth-elicited peritoneal Mφ (TPMφ) were prepared from mice that had been injected 4 days previously with 1 ml of Brewer's complete thioglycolate broth. The Mφ were harvested by peritoneal lavage with PBS, centrifuged, and plated at 106 Mφ per well in six-well BP dishes containing a defined, serum-free medium, Optimem, supplemented with PSG.
Human monocyte-derived Mφ (MDMφ) were isolated from buffy coats obtained through the National Blood Service Bristol Centre, Bristol, United Kingdom. Mononuclear cells were obtained by Ficoll-Hypaque density sedimentation and were washed four or five times with PBS to remove platelets. The cells were resuspended in RPMI 1640 supplemented with PSG and 5% heat-inactivated (56°C for 30 min) autologous human serum. Monocytes were separated from the lymphocytes by adherence for 90 min at 37°C to 75-cm polystyrene tissue culture plastic (TCP) flasks containing the above medium followed by six washes in warm RPMI 1640 to remove nonadherent cells. After 24 h, the monocytes were detached using PBS containing 4 mg of Lidocaine-HCl per ml and 10 mM EDTA, replated in six-well BP dishes at a density of 106 cells per well in X-VIVO 10 supplemented with 2% autologous heat-inactivated human serum, and incubated for 5 to 7 days before use.
THP-1 cells (human monocytic-like cell line) were cultured in RPMI 1640 medium supplemented with PSG and 10% FCS. Four days before use, the cells were harvested and plated in 24-well TCP dishes at 2 × 105 cells per well in culture medium containing 200 nM phorbol 12-myristate 13-acetate (PMA) to stimulate differentiation to mature Mφ-like cells.
CHO K1 cells (a nonphagocytic hamster ovary cell line) were routinely cultured in Ham's F12 medium supplemented with PSG and 10% FCS. CHO cells stably transfected with SR-AI or SR-AII (CHO hSR-AI and CHO hSR-AII, respectively) were produced as described previously (16). High-SR-A-expressing transfectants were maintained in MAC medium, i.e., Ham's F12 growth medium containing PSG, 3% lipoprotein-deficient FCS, 250 μmol of mevalonate per liter, 40 μmol of mevastatin per liter, and 3 μg of AcLDL per ml (14). Before use, CHO cells were harvested with PBS containing 10 mM EDTA and 0.1% (wt/vol) trypsin and plated in the appropriate culture medium at 2 × 105 cells per well of a 24-well TCP dish.
Bacterial culture and fluorescent labelling.
E. coli DH5α was inoculated in Luria broth with or without FITC (see below) and incubated overnight at 37°C on a shaker. Prior to use in uptake assays, the bacteria were washed three times with PBS and fixed with 4% paraformaldehyde. E. coli K1 was cultured as above in Luria broth containing 25 μg of nalidixic acid per ml.
FITC labelling of bacteria was performed as described previously (7). Briefly, bacteria were inoculated into Luria broth containing 4 mg of FITC per ml and incubated overnight at 37°C in a shaker. The bacteria were washed three times in PBS before use and fixed for 1 h at 4°C with 4% paraformaldehyde in PBS (pH 7). Before use, the fixed bacteria were washed twice in PBS to remove any residual fixative. RdGnX labelling of bacteria was performed as specified by the manufacturer (Molecular Probes).
E. coli was opsonized by resuspending FITC-labelled E. coli DH5α in rabbit anti-E. coli polyclonal antiserum and RdGnX-labelled E. coli K1 in mouse anti-E. coli K1 monoclonal antibody and incubating the mixture at 37°C for 30 min prior to the addition to cells.
Assay for bacterial association with eukaryotic cells.
The uptake assay was adapted from previous protocols (30, 35, 47). All cells, except THP-1 cells that were replated 72 h before use, were plated in the relevant culture medium in appropriate dishes 24 to 48 h before the assay. Unless stated otherwise, all Mφ populations were plated onto BP dishes. CHO cells were cultured in Ham's F12 medium supplemented with 3% lipoprotein-deficient serum; THP-1, MDMφ, and BMMφ were cultured in Optimem medium. To assay the uptake of bacteria, the cells were washed twice in PBS and then incubated with the relevant culture medium containing FITC-labelled E. coli K-12, FITC-labelled E. coli DH5α, or RdGnX-labelled E. coli K1 at doses specified in the figure legends. As required, the cells were preincubated for 30 min with inhibitor, which was retained throughout the assay. Unless otherwise stated, poly(I), an SR inhibitor, and its cognate nonligand, poly(C), were used at 50 μg/ml. As required, the cells were incubated with 2 μM cytochalasin D for 30 min and the inhibitor was retained throughout the assay. Each assay variable was examined in duplicate for the CHO cells and in triplicate for BMMφ. The endocytosis of 5 μg of DiIAcLDL per ml was examined as a functional control for SR-A in each assay. After incubation with ligand, the culture medium was removed and the cells were washed three times in PBS. Cells for flow cytometry were harvested from the culture dishes with 0.1% (wt/vol) trypsin and 10 mM EDTA in PBS for CHO cells or PBS containing 10 mM EDTA and 4 mg of Lidocaine-HCl per ml for BMMφ and fixed with 4% formaldehyde in PBS. Fluorescence was analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.) using the FL-1 or FL-2 photomultiplier where appropriate, and the results were analyzed with CellQuest software. The mean fluorescence of unloaded control cells was subtracted from the mean fluorescence of each assay condition, and the average was determined. Results are representative of at least three independent experiments. The statistical significance of results was determined using the paired Student t test, and significance was tested at the 95% confidence level.
The assay used above does not distinguish between binding and ingestion, which will collectively be referred to as uptake. Attempts to modify the flow cytometric assay in order to measure ingestion alone by trypan blue quenching (47) of extracellular fluorescence or protease treatment proved to be unsatisfactory (data not shown). Extensive washing of the cells proved the best method to remove most unbound bacteria.
Microscopy.
For fluorescence microscopy of CHO, MDMφ, and BMMφ, the culture and uptake assays were performed as described above, except that after incubation with bacteria, the cells were washed three times with PBS and fixed using 4% paraformaldehyde. Fluorescence microscopy was performed using a Zeiss Axiovert 25 CTL inverted microscope equipped with a 50-W mercury vapor lamp fitted with standard filter sets for viewing FITC and rhodamine fluorescence. CHO cells for confocal microscopy were plated in 24-well TCP dishes containing 13-mm-diameter glass coverslips, and the uptake assay was performed as above. The fixed cells were washed three times in PBS and permeabilized with 0.2% (vol/vol) Triton X-100 in PBS. The actin cytoskeleton was stained with tetramethylrhodamine-6-isothiocyanate (TRITC)-phalloidin at 100 μg/ml in Triton X-100 plus PBS for 45 min. Confocal microscopy was performed using a Bio-Rad MRC-1024 microscope mounted on a Nikon Diaphot 200 microscope equipped with a 60:1 Planapochromat NA 1.4 objective. A 15-mW air-cooled krypton-argon ion laser was used at 488, 568, and 647 nm. After selecting a focal plane, images were stored and analyzed using Lasersharp software (Bio-Rad, Hemel Hempstead, United Kingdom).
RESULTS
SR-A-mediated uptake of bacteria by CHO cells.
CHO cells are nonprofessional phagocytes, but transfection of appropriate receptors, like FcR, can induce these types of cells to ingest particles such as antibody-opsonized erythrocytes (5, 22, 25, 34). We wanted to investigate the role of SR-A in bacterial phagocytosis in the absence of other phagocytic receptors and to test human SR-A (hSR-A) since there has been no demonstration of its ability to bind and ingest gram-negative bacteria.
We therefore examined the ability of CHO cells, stably transfected with hSR-AI or hSR-AII, to associate with bacteria, compared with that of WT CHO K1 cells. CHO hSR-AI and CHO hSR-AII cells, which have been characterized previously (16), were cultivated in a selection medium, as described above, to retain high levels of receptor endocytic activity. We adapted a flow cytometry-based assay to obtain quantitative results with large populations of cells (30, 35, 47) and examined the binding of dead bacteria to SR-A to prevent possible bacterial invasion or evasion of ingestion.
(i) Characteristics of binding of E. coli by SR-A-transfected CHO cells.
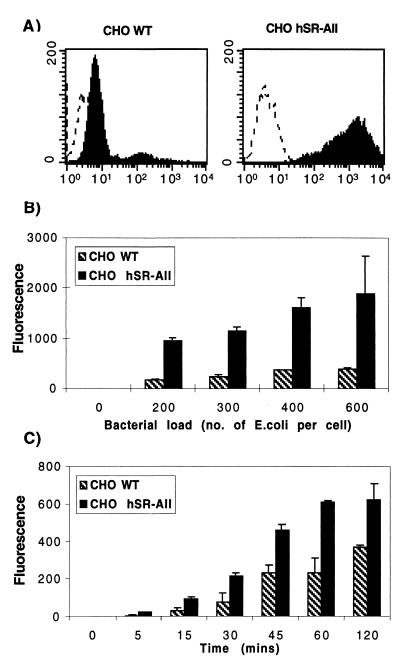
Since there are no reported differences in ligand binding by SR-AI and SR-AII (29), we initially compared CHO hSR-AII with WT cells to optimize the assay conditions. Bacterial association with SR-A-transfected CHO cells plated on TCP was investigated by incubating CHO WT and CHO hSR-AII cells with heat-killed FITC-labelled E. coli K-12 for 45 min (Fig. 1A). WT cells bound only a small number of bacteria; however, transfected cells bound more E. coli, with the mean fluorescence of CHO hSR-AII populations being five- to sixfold greater than that of controls. Figure 1B and C demonstrate the dose response and kinetics of association between SR-A and bacteria. CHO hSR-AII bound E. coli in a dose-dependent manner, with maximal association at approximately 400 E. coli organisms per cell; similarly, uptake by CHO hSR-AII and CHO WT depended on the length of incubation, reaching a plateau for transfectants after 60 min at a dose of 200 E. coli organisms per cell.
FIG. 1.
CHO hSR-AII cells associate with more E. coli K-12 than CHO WT cells. (A) CHO hSR-AII and CHO WT cells were incubated with heat-killed FITC-labelled E. coli K-12 (200 bacteria per cell) for 45 min at 37°C and analyzed by flow cytometry. The dotted line denotes control cells incubated with medium alone, and the solid line shows the increase in fluorescence after incubation with fluoresceinated bacteria. (B) CHO hSR-AII and CHO WT cells were incubated with increasing numbers of FITC-labelled E. coli K-12 organisms per cell for 45 min and analyzed as above. The mean fluorescence for each assay condition was averaged with the corresponding duplicate and plotted along with the standard deviation calculated for each assay condition. (C) FITC-labelled E. coli K-12 (200 bacteria per CHO cell) was incubated with transfected or control CHO cells for various times at 37°C and analyzed as above.
(ii) E. coli association with the SR-A transfectants is specific for SR-A.
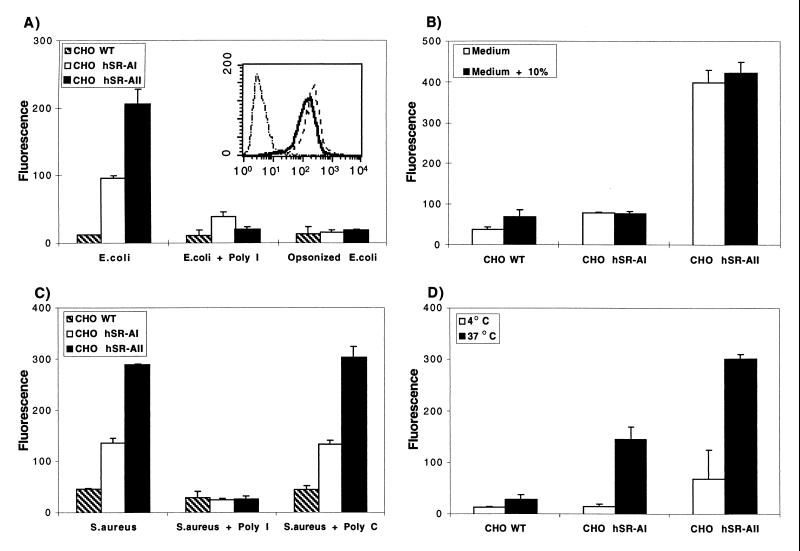
We examined uptake of FITC-labelled E. coli K-12 by the transfected CHO cells in the presence of inhibitors to confirm the specific association of hSR-A with bacteria (Fig. 2A). Poly(I) reduced the uptake of bacteria by hSR-AII by 90%. No difference in residual bacterial binding was detected between CHO hSR-AII and CHO WT cells in the presence of poly(I). Poly(C) had no effect on uptake (data not shown). Preopsonizing E. coli with anti-E. coli polyclonal antiserum at 37°C for 30 min abolished ingestion by these cells, which lack FcR. Poly(I) did not inhibit FITC-labelled E. coli K-12 uptake by WT cells.
FIG. 2.
In transfected CHO cells, association with bacteria is specific for SR-A, independent of the presence of serum, and sensitive to temperature. (A) CHO cells stably transfected with hSR-AI or hSR-AII were incubated with FITC-labelled E. coli K-12 or DiIAcLDL (inset) for 45 min. Binding to SR-A was blocked by poly(I) and SR-A ligands on the bacteria were blocked by preincubating the FITC-labelled E. coli K-12 with a rabbit-anti-E. coli K-12 polyclonal antiserum. The difference detected between CHO hSR-AI and CHO hSR-AII incubated with E. coli alone is significant (P < 0.01), but the difference after incubation with poly(I) is not (P > 0.05). The levels of DiIAcLDL endocytosis are shown in the inset. The fluorescence obtained for CHO WT (–·–·–); CHO hSR-AI (––––), and CHO hSR-AII (–) populations incubated with DiIAcLDL is shown. (B) CHO transfectants and control cells were incubated in the presence or absence of 10% heat-inactivated FCS and analyzed by flow cytometry. The difference in E. coli uptake by CHO WT in the presence and absence of serum is not significant (P > 0.1). (C) CHO cells stably transfected with SR-A were incubated with heat-killed FITC-labelled S. aureus (200 bacteria per CHO cell) for 45 min at 37°C in the presence or absence of poly(I) or its cognate non-SR-A ligand, poly(C). (D) CHO hSR-AI, CHO hSR-AII, and CHO WT cells were incubated with heat-killed FITC-labelled E. coli (100 bacteria per CHO cell) at 4 or 37°C and were analyzed as in Fig. 1. The difference detected between CHO WT cells incubated at 4 or 37°C is not significant (P > 0.15).
(iii) hSR-AI-transfected cells associate with fewer bacteria than do hSR-AII-transfected cells.
E. coli uptake by CHO hSR-AI was examined, and this transfectant consistently associated with fewer bacteria than did CHO hSR-AII (Fig. 2A), while the amounts of DiIAcLDL endocytosed by the two transfectants were similar (Fig. 2A, inset). hSR-AI always took up less E. coli than did hSR-AII, but E. coli association with CHO hSR-AI varied among experiments. CHO hSR-AI occasionally associated with levels of bacteria similar to those associated with CHO WT, but even low levels of E. coli binding to hSR-AI-transfected cells were judged to be specific by inhibitor analysis (Fig. 2A).
(iv) SR-A binds bacteria directly.
The inhibition of SR-A-mediated adhesion to TCP in vitro by 2F8 requires the presence of serum, which contains an unknown ligand for SR-A (12). We examined the role of serum in SR-A binding of bacteria to determine if SR-A could recognize bacteria directly or required precoating with opsonin. CHO cells were incubated with FITC-labelled E. coli K-12 in the presence or absence of 10% FCS. The association of bacteria with CHO WT, CHO hSR-AI, and CHO hSR-AII cells was unaltered in the presence of serum (Fig. 2B), indicating that SR-A could recognize the bacteria directly.
(v) SR-A transfected cells also recognize other strains of E. coli and gram-positive bacteria.
FITC-labelled E. coli K-12 organisms are heat killed and chemically modified by the fluorescent label; therefore, to check that SR-A association with bacteria was not mediated through modification of the bacterial cell surface, paraformaldehyde-fixed RdGnX- or FITC-labelled E. coli DH5α organisms were substituted for FITC-labelled E. coli K-12. CHO hSR-AI and CHO hSR-AII ingested both RdGnX-labelled and FITC-labelled E. coli DH5α, which was inhibited by poly(I) (data not shown). No difference in ingestion was detected between FITC-labelled E. coli K-12 and RdGnX-labelled E. coli DH5α, confirming that the method of killing the bacteria and the fluorescent label used did not alter SR-A recognition.
E. coli K1 is an encapsulated strain, which can cause genitourinary tract infections (23). To examine if the presence of a capsule alters SR-A recognition of E. coli, we examined RdGnX-labelled E. coli K1 association with the CHO transfectants. CHO hSR-AI and CHO hSR-AII, but not CHO WT, could take up E. coli K1. CHO hSR-AII cells bound more E. coli K1 than did CHO hSR-AI cells, and the association was similarly inhibited by poly(I) or by preopsonizing the E. coli K1 with anti-E. coli K1 capsule antibodies (data not shown), suggesting the presence of a ligand for SR-A on the capsular strain.
Dunne et al. (8) used competition binding studies with a soluble form of bovine SR-AI and SR-AII to show that they could bind gram-positive bacteria, including S. aureus, Streptococcus pyogenes, and L. monocytogenes. They also showed that the binding of these gram-positive bacteria to the Mφ-like cell line P388D1 was inhibited by the general SR inhibitor poly(G). However, since these cells can express other SR as well as SR-A, this was not a direct demonstration of cellular SR-A binding to these bacteria. We therefore investigated the uptake of gram-positive FITC-labelled S. aureus by the CHO transfectants. Both hSR-AI and hSR-AII took up more FITC-labelled S. aureus than did CHO WT cells (Fig. 2C), but the difference between the levels of association of the two isoforms with bacteria remained. Poly(C) had no effect on uptake, while poly(I) inhibited bacterial association with both CHO hSR-AI and CHO hSR-AII.
(vi) Regulation of SR-A-mediated ingestion by CHO transfectants.
Since the flow cytometry-based assay measures cell association, not ingestion, and since published quenching methods (47) to distinguish intra- from extracellular bacteria were unreliable in our hands, we investigated whether the CHO transfectants could ingest bound bacteria. One potential method to measure ingestion is to determine the temperature dependence of bacterial association, since at low temperatures cells are unable to ingest but can still bind bacteria. CHO WT, CHO hSR-AI, and CHO hSR-AII cells were incubated with FITC-labelled E. coli K-12 at 4 or 37°C. CHO hSR-AI and CHO hSR-AII cells associated with more FITC-labelled E. coli K-12 at 37 than at 4°C (Fig. 2D). Binding to CHO WT cells was unaffected by temperature. The association of FITC-labelled S. aureus was also dependent on temperature, with more bacteria associating with CHO hSR-AI and CHO hSR-AII at 37°C than at 4°C (data not shown). No difference in FITC-labelled S. aureus association was detected between bacteria incubated with the CHO transfectants at 37°C for the whole assay and bacteria bound to CHO cells at 4°C, washed to remove free bacteria, and then heated to 37°C (data not shown).
Cytochalasin D, an inhibitor of actin assembly, did not block the association of FITC-labelled E. coli K-12 with the CHO hSR-AI and hSR-AII transfectants (data not shown). The cytochalasin D was functional since it could inhibit bacterial uptake by primary Mφ (see below). These data indicated that the flow cytometric assay measured mostly binding of E. coli and S. aureus to the CHO cells.
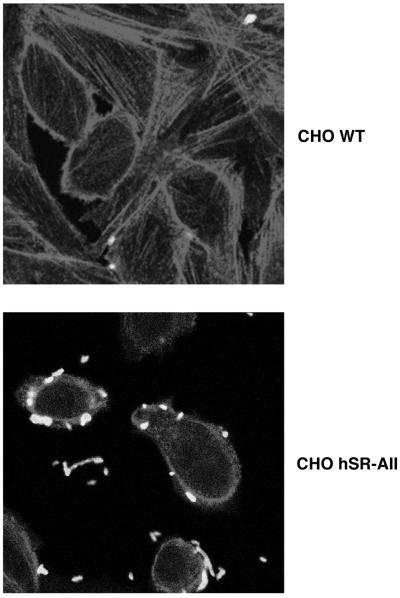
SR-A binding to ligand is reported to be temperature dependent, and since cytochalasin D did not block bacterial association in our assay, we used microscopy to visualize the interaction of E. coli with the CHO transfectants. Fluorescence microscopy (Fig. 3) demonstrated that CHO hSR-AI (results not shown) and CHO hSR-AII were not efficient at internalization since most bacteria remained bound to the cell surface and only a few were detected intracellularly. CHO WT cells bound few E. coli organisms, and no internalized FITC-labelled E. coli K-12 organisms were detected.
FIG. 3.
Most E. coli bacteria associated with CHO hSR-AII are bound to the extracellular surface of the transfectants. CHO hSR-AII and CHO WT cells were incubated with 400 E. coli DH5α bacteria per cell. The cells were permeabilized and actin stained with TRITC-phalloidin. The bacteria were stained with anti-E. coli polyclonal antibody. Cells were visualized using a confocal microscope. CHO hSR-AI incubated with E. coli DH5α gave similar results to CHO hSR-AII. Magnification, ×918.
Thus, we have shown that CHO cells transfected with hSR-A can associate specifically with gram-negative E. coli and gram-positive S. aureus. Binding was dependent on temperature, dose, and time but independent of the presence of serum. CHO hSR-AI and CHO hSR-AII bound bacteria efficiently, but ingestion was minimal. Since CHO cells may lack the appropriate expression of factors essential for efficient ingestion and since SR-A is a Mφ-specific receptor, we next examined the role of SR-A-mediated binding of bacteria in the context of other Mφ molecules.
Human Mφ phagocytose bacteria through SR.
We could not examine the specific role of hSR-A in bacterial phagocytosis by human Mφ, which express a range of SR including MARCO and SR-A, due to the lack of specific SR-A inhibitors. However, using general polyanionic inhibitors for SR, we investigated the proportion of total bacterial uptake mediated by the SR family in human Mφ.
(i) SR expressed by human MDMφ mediate E. coli uptake.
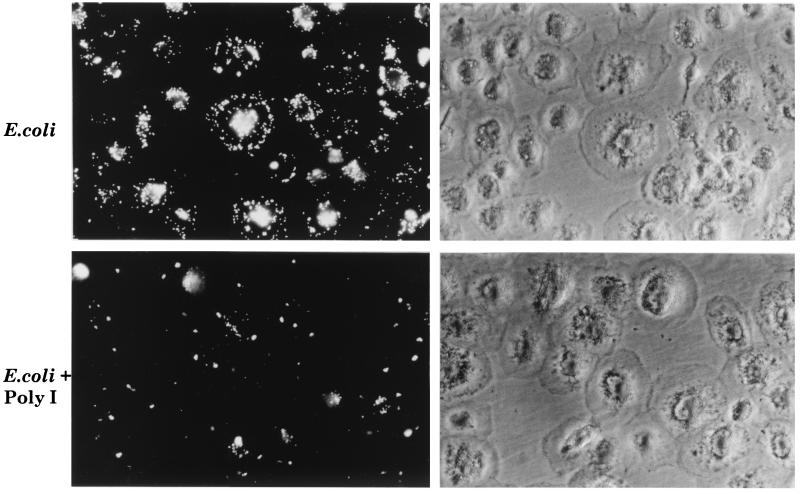
SR-A expression is upregulated during monocyte-to-Mφ differentiation; SR-A is not expressed on monocytes but appears on human MDMφ (16). Thus, monocytes were cultured for 5 to 7 days on BP (see below) before use and the resulting cells were judged morphologically and by flow cytometric analysis of DiIAcLDL uptake to have undergone maturation and to express SR. MDMφ were incubated with FITC-labelled E. coli K-12 in Optimem, a defined synthetic culture medium. Fluorescence microscopy showed that MDMφ associated with FITC-labelled E. coli K-12 and that this association could be partially inhibited by a saturating concentration of poly(I), a general inhibitor of all SR (Fig. 4). Flow cytometric analysis demonstrated that in the presence of poly(I), total FITC-labelled E. coli K-12 uptake was decreased by 70 to 75% compared with uptake by MDMφ incubated with bacteria alone (data not shown). MDMφ also associated with RdGnX-labelled E. coli K1 in a poly(I)-inhibitible manner, which reduced RdGnX-labelled E. coli K1 uptake by MDMφ by 50%. Cytochalasin D reduced the uptake of bacteria by MDMφ (data not shown).
FIG. 4.
SR mediate bacterial uptake by MDMφ. MDMφ were incubated with heat-killed FITC-labelled E. coli K12 (20 bacteria per MDMφ [see below]) for 45 min. Representative fields detected by fluorescence and phase-contrast microscopy are shown. Magnification, ×366.
(ii) Mφ-like cell lines express SR that mediate bacteria uptake.
THP-1 cells are a human monocyte-like cell line that upon PMA treatment can differentiate into Mφ, which were previously shown to express SR-A (27, 31). Human SR-A was originally cloned from THP-1 cells (31), and to assess the role of SR in gram-negative bacterial uptake, we incubated PMA-differentiated THP-1 cells with FITC-labelled E. coli K-12 in the absence of serum and in the presence or absence of poly(I). THP-1 cells were plated on TCP associated with bacteria, and the uptake was inhibited by 30% in the presence of poly(I) (data not shown).
(iii) Mφ are more efficient at bacterial uptake than are transfected CHO cells.
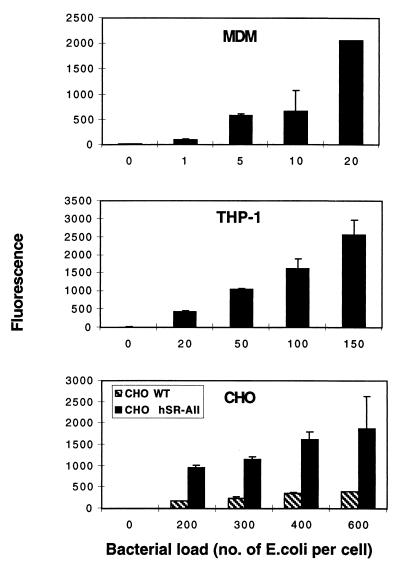
The optimal dose range of FITC-labelled E. coli K-12 required to produce substantial increases in population fluorescence was determined for MDMφ, PMA-treated THP-1 cells, and CHO transfectants. Comparison of this dose range emphasized the efficiency of uptake by MDMφ and, to a lesser extent, PMA-differentiated THP-1 cells compared with transfected CHO cells. Figure 5 shows that 400 E. coli organisms per CHO cell were required to produce similar increases in population fluorescence in the CHO transfectants compared with 150 and 20 bacteria for THP-1 and MDMφ, respectively. The requirement for different doses of bacteria by the various cells cannot be ascribed to differences in the labelling of E. coli K-12, since the same batch of organisms was used throughout.
FIG. 5.
Transfected CHO cells are not efficient at phagocytosis compared with phagocytes. MDMφ, PMA-treated THP-1, transfected CHO, and control cells were incubated with increasing doses of FITC-labelled E. coli K-12 for 45 min. The cells were analyzed by flow cytometry, and the mean fluorescence for each condition is plotted. Error bars indicate standard deviation.
Thus, undefined SR play a role in human Mφ binding and ingestion of E. coli. To define the role of SR-A in bacterial uptake by intact Mφ, we next studied selectively deficient SR-A−/− mouse Mφ.
SR-A is important in the uptake of E. coli by mouse BMMφ.
SR-A−/− mice were generated by the targeted disruption of exon 4 of the SR-A gene as described previously (45). These mice were used to demonstrate the role of SR-A in atherosclerosis and in phagocytosis of apoptotic cells in vitro (38, 45). We used BMMφ from SR-A−/− and 129/ICR control mice as a reproducible model system to characterize further the specific role of SR-A in bacterial phagocytosis and to extend these studies to immunologically nonactivated Mφ. The BMMφ were cultured in culture medium supplemented with L929 cell-conditioned medium, which contains macrophage colony-stimulating factor, that stimulates the maturation of the bone marrow progenitors and increases the expression of SR-A (6). We used Western blotting with 2F8, the anti-mouse SR-A antibody, to confirm that BMMφ from the SR-A−/− mice did not express SR-A (data not shown).
(i) SR-A−/− macrophages are deficient in phagocytosis of gram-negative bacteria.
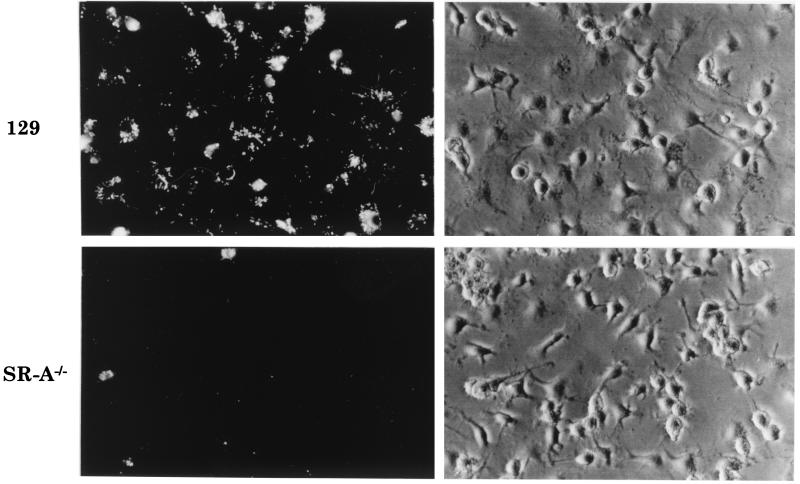
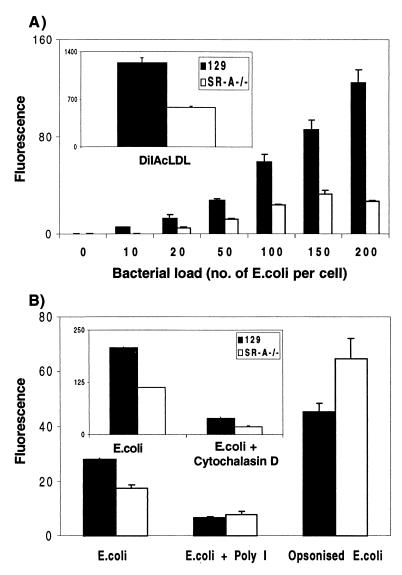
BMMφ from SR-A−/− and 129/ICR mice were incubated with increasing doses of FITC-labelled E. coli DH5α for 2 h. Fluorescence microscopy showed the difference in E. coli DH5α uptake between 129/ICR and SR-A−/− BMMφ (Fig. 6). Quantitation by flow cytometry (Fig. 7A) showed that SR-A−/− BMMφ consistently associated with 30 to 60% fewer FITC-labelled E. coli DH5α organisms than did 129/ICR BMMφ over a range of bacterial concentrations. SR-A−/− BMMφ also endocytosed 50% less DiIAcLDL than did 129/ICR Mφ (Fig. 7A, inset). The ingestion of E. coli DH5α fluorescently labelled with FITC or RdGnX was examined, and no difference in the levels of uptake by 129/ICR or SR-A−/− Mφ was detected, confirming that the fluorochromes were not affecting SR-A recognition of the bacteria (data not shown). Cytochalasin D reduced E. coli uptake by both SR-A−/− and 129/ICR Mφ to low levels (Fig. 7B, inset). In the presence of poly(I), uptake of bacteria was reduced by 50 to 75% in 129/ICR Mφ (Fig. 7B). Poly(I) only minimally inhibited the phagocytosis of bacteria by SR-A−/− Mφ; therefore, SR-A accounts for most of the SR-mediated ingestion by the 129/ICR Mφ in this system. The uptake of E. coli DH5α was dependent on the duration of incubation, with maximal uptake occurring at 2 h. The difference between the control and SR-A−/− Mφ was unaffected by time (data not shown).
FIG. 6.
BMMφ from SR-A−/− mice ingest fewer E. coli bacteria than do 129/ICR BMMφ. BMMφ from 129/ICR or SR-A−/− mice were incubated with paraformaldehyde-fixed RdGnX-labelled E. coli DH5α (60 E. coli bacteria per Mφ) for 2 h and analyzed by fluorescence and phase-contrast microscopy. Each field depicted here is representative of the whole population. Magnification, ×366.
FIG. 7.
SR-A−/− BMMφ deficiency in bacterial phagocytosis is specific for SR-A. (A) BMMφ from SR-A−/− or control ICR/129 mice were incubated with increasing doses of paraformaldehyde-fixed FITC-labelled E. coli DH5α or DiIAcLDL (inset) for 2 h and analyzed by flow cytometry. (B) SR-A−/− or control 129/ICR Mφ were incubated with paraformaldehyde-fixed FITC-labelled E. coli DH5α (100 bacteria per Mφ) in the presence of an SR-A inhibitors, poly(I), or an inhibitor of phagocytosis, cytochalasin D (2 μM) (inset; the reduction in binding as well as ingestion may be because the major pool of SR-A is intracellular). To allow bacterial ingestion via the FcR, the FITC-labelled E. coli organisms were preincubated for 30 min with a rabbit anti-E. coli polyclonal antiserum in the absence of complement. The difference in mean fluorescence detected between 129/ICR and SR-A−/− Mφ incubated with E. coli is significant (P < 0.001). The greater uptake by SR-A−/− Mφ detected here was not reproducible and varied among experiments.
(ii) Phagocytosis by FcR is unaffected in SR-A−/− macrophages.
Uptake of bacteria by another receptor was studied to confirm that phagocytosis mediated by these molecules was unaffected in SR-A−/− Mφ. BMMφ from 129/ICR and SR-A−/− mice were incubated with unopsonized FITC-labelled E. coli DH5α or after opsonization with anti-E. coli antibodies for 30 min at 37°C (Fig. 7B). Opsonized FITC-labelled E. coli DH5α were ingested more efficiently by 129/ICR and SR-A−/− Mφ than were unopsonized E. coli bacteria, eliminating the previous deficiency of SR-A−/− Mφ. Opsonization of E. coli enhanced ingestion by 129/ICR and SR-A−/− to similar levels in different experiments, although this was variable.
(iii) The difference in bacterial uptake by SR-A−/− and 129/ICR Mφ varies with the E. coli strain.
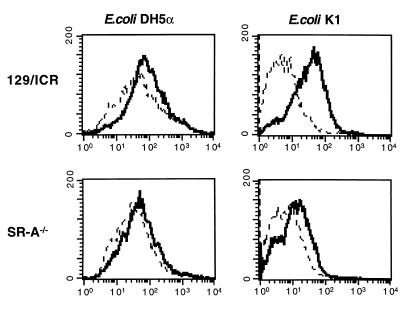
We compared the ingestion of encapsulated E. coli K1 by 129/ICR and SR-A−/− BMMφ with that of the E. coli DH5α strain. Flow cytometry showed that SR-A−/− Mφ barely internalized any E. coli K1 and that poly(I) reduced this ingestion to background levels (Fig. 8). SR-A−/− Mφ ingested 65 to 75% fewer E. coli K1 organisms than did 129/ICR Mφ. Poly(I) reduced E. coli K1 ingestion by 82% in 129/ICR Mφ. These data are in contrast to those obtained for E. coli DH5α and E. coli K-12 (not shown), where the differences in ingestion were smaller, i.e., 30 to 60% and 15% respectively, and poly(I) did not reduce uptake to background levels, indicating that binding was not entirely due to SR activity. These findings suggest that SR-A binds distinct ligands on different bacteria or to different extents and that SR-A is a more significant receptor for E. coli K1 than for E. coli DH5α.
FIG. 8.
The difference in E. coli ingestion between 129/ICR and SR-A−/− BMMφ is strain dependent. BMMφ from SR-A−/− or 129/ICR mice were incubated with paraformaldehyde-fixed RdGnX-labelled E. coli DH5α or K1 (60 bacteria per Mφ) in the presence or absence of poly(I). —, fluorescence obtained by flow cytometry for the Mφ populations incubated with E. coli alone; ––––, fluorescence for the Mφ populations incubated with E. coli in the presence of poly(I). Poly(I)-treated 129/ICR Mφ displayed 30% less mean fluorescence (significant at P = 0.01).
(iv) Heterogeneity of Mφ and activation by LPS do not eliminate the difference in E. coli uptake between 129/ICR and SR-A−/− Mφ.
Earlier preliminary studies on the role of SR-A in bacterial uptake used Mycobacterium bovis BCG-activated peritoneal Mφ (37). We therefore examined peritoneal Mφ elicited by another method to confirm that differences in E. coli uptake were not due only to BCG activation or bone marrow cultivation conditions. We examined uptake of E. coli by peritoneal Mφ elicited with thioglycolate broth from both SR-A−/− and 129/ICR mice and BMMφ activated by LPS. E. coli ingestion by TPMφ was reduced by 30% compared with that by 129/ICR control Mφ (data not shown). Poly(I) inhibited uptake by both 129/ICR and SR-A−/− Mφ, and the SR-mediated but SR-A-independent uptake of bacteria was enhanced, possibly due to increased expression of other SR. Activation of BMMφ with LPS 48 h prior to the addition of bacteria increased E. coli uptake by both 129/ICR and SR-A−/− Mφ 1.3- to 2.0-fold. However, the difference in uptake between SR-A−/− and 129/ICR Mφ remained the same (data not shown), suggesting that SR-independent rather than SR-A-dependent uptake mechanisms were affected by LPS. Poly(I) inhibited the activated Mφ more than it inhibited the unactivated control Mφ from the same mice. These data suggest that in this activation model, SR other than SR-A are up-regulated by the LPS and contribute to bacterial phagocytosis while SR-A contributes to E. coli uptake by a range of different Mφ populations. Table 1 summarizes the contribution of SR-A-dependent and -independent mechanisms to E. coli ingestion by different Mφ populations.
TABLE 1.
E. coli uptake by different Mφ populations via SR-A-dependent and -independent mechanisms
| E. coli strain | % Uptakea of E. coli by:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| BMMφb
|
TPMφb
|
MDMφc
|
THP-1c
|
|||||
| SR-A | Non-SR-A | SR-A | Non-SR-A | SR | Non-SR | SR | Non-SR-A | |
| K-12 | 15 | 85 | 60 | 40 | 30 | 70 | ||
| DH5α | 55 | 45 | 30 | 70 | ||||
| K1 | 70 | 30 | 50 | 50 | ||||
Percentages are derived from comparison of the levels of E. coli ingestion by 129/ICR and SR-A−/− Mφ.
The results are from single assays but are representative of at least three similar experiments.
Percentages are derived from comparison of the level of E. coli uptake in the presence or absence of the general SR inhibitor, poly(I).
(v) Adherence to TCP and SR-A ligands down-modulates SR-A in vitro.
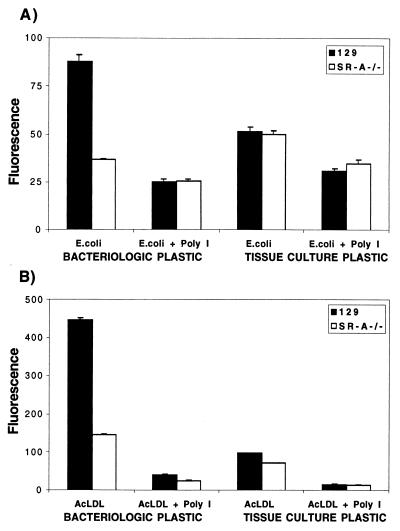
SR-A is a major receptor involved in the attachment of Mφ to TCP in the presence of serum (12), but adhesion to serum-coated BP is due to CR3 (42). Since the contribution of SR-A to bacterial binding is affected by the bacterial strain and Mφ heterogeneity, we examined the influence of adherence via SR-A on the role of SR-A in phagocytosis and endocytosis. We cultured BMMφ from 129/ICR and SR-A−/− mice on TCP or BP and compared the uptake of FITC-labelled E. coli DH5α and of DiIAcLDL (Fig. 9). On BP, SR-A−/− Mφ ingested 58% fewer FITC-labelled E. coli DH5α organisms and endocytosed 67% less DiIAcLDL than did 129/ICR Mφ. Uptake of FITC-labelled E. coli DH5α and DiIAcLDL by 129/ICR Mφ plated on TCP was reduced by 40 and 72%, respectively, compared with that by 129/ICR Mφ plated on BP. On TCP, no difference in the uptake of FITC-labelled E. coli DH5α or DiIAcLDL was detected between 129/ICR and SR-A−/− Mφ. Poly(I) inhibited the uptake of bacteria and DiIAcLDL by 71 and 78% respectively, when 129/ICR Mφ were plated on BP but had less effect on the residual activity of Mφ plated on TCP. Table 2 summarizes the effect of culture conditions on E. coli uptake by the different cell populations used in this study. We confirmed that the apparent receptor down-modulation on TCP was not due to damage to Mφ during cell detachment before analysis. Trypan blue exclusion on Mφ detached from both TCP and BP showed no loss of viability, and the Mφ could be replated and cultured. Therefore, the conditions of in vitro cultivation of Mφ have profound effects on the experimental analysis of SR-A functions in vitro.
FIG. 9.
Cultivation on TCP down-regulates SR-A phagocytic and endocytic function. BMMφ from SR-A−/− or control ICR/129 mice were incubated with paraformaldehyde-fixed FITC-labelled E. coli DH5α (A) or DiIAcLDL (B) in the presence of SR-A inhibitors and analyzed as before in Fig. 7. The uptake of E. coli on TCP by 129/ICR and SR-A−/− BMMφ represents SR-A-independent uptake alone. The difference in DiIAcLDL endocytosis between 129/ICR and SR-A−/− BMMφ is significant (P < 0.001).
TABLE 2.
Effect of culture conditions on E. coli uptake by different cell populationsa via SR-A-dependent and -independent mechanisms
| Culture condition | % Uptake of E. coli by:
|
||||||
|---|---|---|---|---|---|---|---|
| BMMφb
|
MDMc
|
CHOd
|
|||||
| SR-A | Non-SR-A | SR | Non-SR | WT | hSR-AI | hSR-AII | |
| Adhesion to TCP | 0 | 100 | 60 | 40 | 10 | 40 | 100 |
| Adhesion to BPe | 55 | 45 | 70 | 30 | |||
Cells were cultivated on the appropriate substratum in the presence of FCS. The results shown are from single assays but are representative of at least three similar experiments.
Percentages are derived from comparison of the levels of E. coli ingestion by 129/ICR and SR-A−/− Mφ.
Percentages are derived from comparison of the level of E. coli uptake in the presence or absence of the general SR inhibitor, poly(I).
Percentages were derived from comparison of the level of E. coli uptake by CHO hSR-AII.
BMMφ plated on BP and activated by LPS had no effect on SR-A-mediated uptake but increased SR-A-independent ingestion of E. coli.
DISCUSSION
We have shown that the role of SR-A in bacterial phagocytosis depends on both the host cell and the microorganism. In primary Mφ incubated with selected E. coli and S. aureus in vitro, SR-A is necessary for efficient binding and ingestion in the absence of opsonins such as specific antibody. The extent of involvement of SR-A, other SR, and different types of receptors varies with the cell source, activation state, and culture conditions, especially adhesion to a serum-coated substratum. In transfected CHO cells, the SR-A is sufficient to bind microbial and other ligand-bearing particles but ingestion is poor, indicating that additional Mφ components are required for efficient uptake. Further studies are required to extend these studies to live microorganisms in vitro and in vivo.
We utilized SR-A-deficient murine Mφ and CHO human transfectants to examine the roles of SR-AI and SR-AII together and independently. A general SR inhibitor, poly(I) versus poly(C), was used to assess the contribution of other receptors in human and murine cells. Table 1 summarizes the contribution of SR-A-dependent and -independent mechanisms in various cells challenged with different strains of E. coli. SR-A contributed 15 to 70% of the nonopsonic uptake in a single system of BMMφ, depending on the strain used. Although LPS is a candidate ligand, it is not the only one, since encapsulated bacteria in which the LPS may be masked were also recognized. Only a limited range of organisms was examined, but S. aureus was also recognized by SR-A, possibly via LTA. We predict that bacteria will vary widely in the expression of potential ligands for SR-A.
The relative contribution of other SR also varies, depending on the cell source. With human cells, it was not possible to distinguish SR-A from other polyanion-sensitive receptors, but results suggest that regulation of SR-A overall can be independent of that of other SR. Candidate alternative receptors include MARCO, which is up-regulated by LPS, and polyanion-independent receptors such as CD14 (18, 46). The total contributions of SR-dependent and non-SR-dependent recognition also varied in different cells.
Table 2 summarizes the effect of culture conditions on SR-A-dependent and -independent uptake of E. coli, as well as the role of SR-AI and SR-AII isoforms in bacterial binding by CHO transfectants. Key variables included cell adhesion to a serum-coated substratum; the addition of macrophage colony-stimulating factor, a potent inducer of SR-A in the bone marrow culture system (6); and the addition of LPS, which enhanced SR-A-independent uptake. Down-regulation of SR-A endocytosis and phagocytosis results from decreased expression of SR-A on the free surfaces of cells cultivated on undefined ligands present in serum, which bind selectively to TCP (12) but not to BP, where adhesion is mediated mainly by CR3 (42). Down-regulation of specific phagocytic receptors when Mφ adhere to substrata coated with ligands such as antibody is well known (33) and has been previously demonstrated for SR-A (38). Our present study took this work into account, where possible, by cultivating cells on BP. The cell lines we used (CHO and THP-1) did not adhere to BP, and the results shown when CHO transfectants were cultivated on TCP may underestimate the SR-A contribution to bacterial recognition. Adhesion of Mφ such as Kupffer cells can be expected to influence phagocytic clearance in vivo depending on the expression of extracellular matrix ligands for SR-A and other receptors. It should be noted that fibronectin and poorly defined ligands present in the extracellular matrix can also up-regulate ingestion via different phagocytic receptors such as CR3 (24, 48) and CD36 (32, 49).
One of our unexpected findings was the increased efficiency of the type II SR-A with respect to the type I SR-A in binding of bacteria. Most primary Mφ express both isoforms, and CHO transfectants were matched as far as possible to express similar levels of DiIAcLDL endocytic activity. Results with SR-AII were more consistent than with SR-AI. There have been no clear indications previously about the possible functional significance of the additional SR cysteine-rich domain. Further studies are needed to compare the plasma membrane and intracellular levels and surface distribution of each isoform; an intriguing possibility is that the SR cysteine-rich domain plays a distinct role in adhesion and/or phagocytosis, in contrast to endocytosis.
The CHO transfectants were less efficient in binding E. coli than were “professional” phagocytes and Mφ-like cell lines (“semiprofessional” phagocytes) and were particularly poor at ingestion. This could be due to lower surface levels, clustering or recycling of SR-A, absence of other Mφ-restricted phagocytic receptors, or limiting levels of intracellular molecules involved in signalling and actin assembly. Although several other phagocytic receptors, for example, FcR (5, 22, 25, 34) and mannose receptors (11), are able to mediate ingestion in non-Mφ transfectants (CHO and COS cells), their efficiency may also vary considerably compared with their phagocytic activity in Mφ. It will be interesting to identify the molecules and mechanisms which regulate ingestion via SR-A and other SR. It has been shown previously that cotransfection of the insulin receptor can enhance the endocytosis of SR-A ligands by transfected cells (43) and that ingestion via FcR and CR3 in transfected cells was enhanced by cotransfection of the small GTPases: Rho, Rac, and CdC42 (4).
It will be important to extend the present studies to living organisms and to examine the effect of SR-A-mediated binding and uptake on the fate of the microbe and on the cellular and host responses to ingestion. Pathogenic organisms, including virulent strains of E. coli, may be able to vary the expression and accessibility of potential ligands for SR-A, modulating their capture, clearance, and killing by the Mφ. In turn, Mφ are able to regulate their ability to release different cytokines and activate acquired immune responses. SR-A could be more important in initial bacterial dissemination than when amplified phagocytosis via antibody and complement come into operation. However, the innate response may have long-lasting effects on the efficiency and nature of the adaptive immune response, and SR-A-dependent recognition of intact organisms and microbial constituents could play a crucial role in limiting the pathologic sequelae of infection such as septic shock.
ACKNOWLEDGMENTS
We thank R. Moxon and C. Tang for helpful discussion.
L.P. is supported by Goodger and Harry Crossley Scholarships, and P.J.G. held a Goodger Scholarship. Work in the laboratory of S.G. is supported by a grant from the Medical Research Council. Facilities for imaging and confocal microscopy were provided by a grant from the Wellcome Trust.
REFERENCES
- 1.Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenas J, Penman M, Vasile E, Acton S, Freeman M, Krieger M. Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J Lipid Res. 1993;34:983–1000. [PubMed] [Google Scholar]
- 3.Brown M S, Goldstein J L. Lipoprotein metabolism in the macrophage: implications for cholestrol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 4.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 5.Davis W, Harrison P T, Hutchinson M J, Allen J M. Two distinct regions of FC gamma RI initiate separate signalling pathways involved in endocytosis and phagocytosis. EMBO J. 1995;14:432–441. doi: 10.1002/j.1460-2075.1995.tb07019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Villiers W J, Fraser I P, Hughes D A, Doyle A G, Gordon S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J Exp Med. 1994;180:705–709. doi: 10.1084/jem.180.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drevets D A, Elliott A M. Fluorescence labeling of bacteria for studies of intracellular pathogenesis. J Immunol Methods. 1995;187:69–79. doi: 10.1016/0022-1759(95)00168-a. [DOI] [PubMed] [Google Scholar]
- 8.Dunne D W, Resnick D, Greenberg J, Krieger M, Joiner K A. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA. 1994;91:1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 10.Emi M, Asaoka H, Matsumoto A, Itakura H, Kurihara Y, Wada Y, Kanamori H, Yazaki Y, Takahashi E, Lepert M, et al. Structure, organization, and chromosomal mapping of the human macrophage scavenger receptor gene. J Biol Chem. 1993;268:2120–2125. [PubMed] [Google Scholar]
- 11.Ezekowitz R A, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364:343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- 13.Fraser I P, Ezekowitz R A B. Mannose receptor and phagocytosis. In: Gordon S, editor. Phagocytosis and pathogens. Vol. 5. Greenwich, Conn: JAI Press; 1999. pp. 85–99. [Google Scholar]
- 14.Freeman M, Ekkel Y, Rohrer L, Penman M, Freedman N J, Chisholm G M, Krieger M. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1991;88:4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gough P J, Greaves D R, Gordon S. A naturally occurring isoform of the human macrophage scavenger receptor (SR-A) gene generated by alternative splicing blocks modified LDL uptake. J Lipid Res. 1998;39:531–543. [PubMed] [Google Scholar]
- 16.Gough P J, Greaves D R, Suzuki H, Hakkinen T, Hiltunen M O, Turunen M, Herttuala S Y, Kodama T, Gordon S. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:461–471. doi: 10.1161/01.atv.19.3.461. [DOI] [PubMed] [Google Scholar]
- 17.Greaves D R, Gough P J, Gordon S. Recent progress in defining the role of scavenger receptors in lipid transport, atherosclerosis and host defence. Curr Opin Lipidol. 1998;9:425–432. doi: 10.1097/00041433-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Grunwald U, Fan X, Jack R S, Workalemahu G, Kallies A, Stelter F, Schutt C. Monocytes can phagocytose Gram-negative bacteria by a CD14-dependent mechanism. J Immunol. 1996;157:4119–4125. [PubMed] [Google Scholar]
- 19.Hampton R Y, Golenbock D T, Penman M, Krieger M, Raetz C R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 20.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hume D A, Gordon S. Optimal conditions for proliferation of bone marrow-derived mouse macrophages in culture: the roles of CSF-1, serum, Ca2+, and adherence. J Cell Physiol. 1983;117:189–194. doi: 10.1002/jcp.1041170209. [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson M J, Harrison P T, Allen J M. Transmembrane association with gamma-chain is required for Fc gamma RI-mediated phagocytosis in transfected COS cells. Biochem Soc Trans. 1995;23:121S. doi: 10.1042/bst023121s. [DOI] [PubMed] [Google Scholar]
- 23.Ikaheimo R, Siitonen A, Karkkainen U, Kuosmanen P, Makela P H. Characteristics of Escherichia coli in acute community-acquired cystitis of adult women. Scand J Infect Dis. 1993;25:705–712. doi: 10.3109/00365549309008567. [DOI] [PubMed] [Google Scholar]
- 24.Johnson E, Larsen T, Hetland G. Phagocytosis of agarose beads by receptors for C3b (CR1) and iC3b (CR3) on human alveolar macrophages cultured on fibronectin in vitro. A scanning electron microscopic study. Scand J Immunol. 1986;24:653–660. doi: 10.1111/j.1365-3083.1986.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 25.Joiner K A, Fuhrman S A, Miettinen H M, Kasper L H, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- 26.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 27.Kodama T, Reddy P, Kishimoto C, Krieger M. Purification and characterisation of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci USA. 1988;85:9238–9242. doi: 10.1073/pnas.85.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 30.Matsson P, Fossum C, Larsson B. Evaluation of flow cytometry and fluorescence microscopy for the estimation of bovine mononuclear phagocytes. J Immunol Methods. 1985;78:13–24. doi: 10.1016/0022-1759(85)90325-4. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto A, Naito M, Itakura H, Ikemoto S, Asaoka H, Hayekawa I, Kanamori H, Aburatani H, Takaku F, Suzuki H, et al. Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci USA. 1990;87:9133–9137. doi: 10.1073/pnas.87.23.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxeiner H, Husemann J, Thomas C A, Loike J D, El Khoury J, Silverstein S C. Complementary roles for scavenger receptor A and CD36 of human monocyte-derived macrophages in adhesion to surfaces coated with oxidized low-density lipoproteins and in secretion of H202. J Exp Med. 1998;188:2257–2265. doi: 10.1084/jem.188.12.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michl J, Pieczonka M M, Unkeless J C, Silverstein S C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979;150:607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagarajan S, Chesla S, Cobern L, Anderson P, Zhu C, Selvaraj P. Ligand binding and phagocytosis by CD16 (Fc gamma receptor III) isoforms. Phagocytic signaling by associated zeta and gamma subunits in Chinese hamster ovary cells. J Biol Chem. 1995;270:25762–25770. doi: 10.1074/jbc.270.43.25762. [DOI] [PubMed] [Google Scholar]
- 35.Oda T, Maeda H. A new simple fluorometric assay for phagocytosis. J Immunol Methods. 1986;88:175–183. doi: 10.1016/0022-1759(86)90004-9. [DOI] [PubMed] [Google Scholar]
- 36.Pearson A M. Scavenger receptors in innate immunity. Curr Opin Immunol. 1996;8:20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
- 37.Platt N, Haworth R, da Silva R P, Gordon S. Scavenger receptors and phagocytosis of bacteria and apoptotic cells. In: Gordon S, editor. Phagocytosis: the host. Vol. 5. Greenwich, Conn: JAI Press; 1999. pp. 69–83. [Google Scholar]
- 38.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Resnick D, Chatterton J E, Schwartz K, Slayter H, Krieger M. Structures of class A macrophage scavenger receptors. Electron microscopic study of flexible, multidomain, fibrous proteins and determination of the disulfide bond pattern of the scavenger receptor cysteine-rich domain. J Biol Chem. 1996;271:26924–26930. doi: 10.1074/jbc.271.43.26924. [DOI] [PubMed] [Google Scholar]
- 40.Resnick D, Pearson A, Krieger M. The SRCR superfamily: a family reminiscent of the Ig superfamily. Trends Biochem Sci. 1994;19:5–8. doi: 10.1016/0968-0004(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 41.Rohrer L, Freeman M, Kodama T, Perman M, Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990;343:570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- 42.Rosen H, Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med. 1987;166:1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sano H, Higashi T, Matsumoto K, Melkko J, Jinnouchi Y, Ikeda K, Ghira Y, Makino H, Smedsrod B, Horiuchi S. Insulin enhances macrophage scavenger receptor-mediated endocytic uptake of advanced glycation end products. J Biol Chem. 1998;273:8630–8637. doi: 10.1074/jbc.273.15.8630. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Sakayuchi H, Kruijt J K, Higashi T, Suzuki T, van Berkel T J, Horiuchi S, Takahashi K, Yazaki Y, Kodama T. The multiple roles of macrophage scavenger receptors (MSR) in vivo: resistance to atherosclerosis and susceptibility to infection in MSR knockout mice. J Atheroscler Thromb. 1997;4:1–11. doi: 10.5551/jat1994.4.1. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 46.van der Laan L J, Dopp E A, Haworth R, Pikkarainen T, Kargas M, Elomaa O, Dijkstra C D, Gordon S, Tryggvason K, Kraal G. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J Immunol. 1999;162:939–947. [PubMed] [Google Scholar]
- 47.Wan C P, Park C S, Lau B H. A rapid and simple microfluorometric phagocytosis assay. J Immunol Methods. 1993;162:1–7. doi: 10.1016/0022-1759(93)90400-2. [DOI] [PubMed] [Google Scholar]
- 48.Wright S D, Craigmyle L S, Silverstein S C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983;158:1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yesner L M, Huh H Y, Pearce S F, Silverstein R L. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol. 1996;16:1019–1025. doi: 10.1161/01.atv.16.8.1019. [DOI] [PubMed] [Google Scholar]