Abstract
Simple Summary
Hepatocellular cancer is the most common type of primary liver cancer. It is the third leading cause of cancer-related deaths worldwide and its incidence is increasing: >1 million new cases per year expected by 2025. Despite advances in treatment in recent years, diagnosis is associated with poor overall survival. Treatment of hepatocellular cancer depends on the patient’s general health and fitness, how well the liver is working, the number and size of tumors in the liver, and whether or not the tumor has spread to other neighboring or distant parts of the body. The combination of atezolizumab plus bevacizumab, two intravenously administered antibodies, is the preferred first-line treatment for patients with advanced hepatocellular cancer that has spread from the liver to other neighboring or distant parts of the body. This study investigated how long patients whose hepatocellular cancer continues to grow (progress) despite one or more prior tumor therapies live when they receive atezolizumab plus bevacizumab. These patients, treated with atezolizumab plus bevacizumab at various hospitals in Germany and Austria, lived about 16 months, which is about 5–8 months longer than patients receiving approved drugs. The safety profile was consistent with previous reports.
Abstract
Atezolizumab plus bevacizumab is the standard of care for first-line systemic therapy for advanced hepatocellular carcinoma (aHCC). Data on the efficacy and safety of atezolizumab plus bevacizumab in patients with aHCC who have received prior systemic therapy are not available. Methods: Patients with aHCC who received atezolizumab plus bevacizumab after at least one systemic treatment between December 2018 and March 2022 were retrospectively identified in 13 centers in Germany and Austria. Patient characteristics, tumor response rates, progression-free survival (PFS), overall survival (OS), and adverse events (AE) were analyzed. Results: A total of 50 patients were identified; 41 (82%) were male. The median age at initiation of treatment with atezolizumab plus bevacizumab was 65 years, 41 (82%) patients had cirrhosis, 30 (73%) Child A, 9 (22%) B, and 2 (5%) C. A total of 34 patients (68%) received atezolizumab plus bevacizumab in the second-line setting and 16 (32%) in later lines. The best radiologic tumor responses were complete remission (2%), partial remission (30%), stable disease (36%), and progressive disease (18%), resulting in an objective response rate of 32% and a disease control rate of 68%. Median OS was 16.0 months (95% confidence interval 5.6–26.4 months), and median PFS was 7.1 months (95% confidence interval 4.4–9.8 months). AE grades 3–4 were observed in seven (14%) and resulted in death in three patients (6%). There were five (10%) bleeding events with a grade ≥ 3, including one (2%) with a fatal outcome. Conclusions: Atezolizumab plus bevacizumab is effective in patients with aHCC who did not have access to this option as first-line therapy. The safety profile was consistent with previous reports.
Keywords: hepatocellular carcinoma, liver cancer, atezolizumab, bevacizumab, immune checkpoint inhibition, immunotherapy
1. Introduction
Hepatocellular carcinoma (HCC) accounts for roughly 80% of primary liver cancer, which is the third most leading cause of cancer-related deaths worldwide and affects more than 800,000 patients annually [1,2]. Patients with early stage HCC are eligible for treatments with curative intent, including local ablative procedures, surgical resection or liver transplantation, whereas systemic treatment is the therapy of choice for locally advanced or metastatic disease [3,4].
Since its approval in 2007, the tyrosine-kinase inhibitor (TKI) sorafenib has been the only available treatment for advanced-stage HCC (aHCC) for more than a decade [5,6]. However, since 2018, several new agents have been shown to be effective and have been approved for the treatment of aHCC. The TKI lenvatinib was noninferior to sorafenib in the phase III REFLECT trial, and represents an alternative first-line therapy [7], while three placebo-controlled phase III trials in eligible candidates with aHCC showed a survival benefit of approximately 3 months with either the TKI cabozantinib, or the TKI regorafenib for patients who had previously tolerated sorafenib, or the anti-VEGF2 monoclonal antibody (mAb) ramucirumab for patients with alpha-fetoprotein (AFP) ≥ 400 ng/ml [8,9,10].
Despite the immense breakthrough in cancer therapy with the discovery of monoclonal antibodies targeting immune checkpoints, no consistent survival benefit was achieved for patients with aHCC by monotherapy with the anti-programmed cell death 1 (PD-1) mAb nivolumab and pembrolizumab in the first- and second-line setting, respectively [11,12]. The intuition that combined inhibition of VEGF and PD-L1 may be the key to effective treatment of aHCC represents a paradigm shift in the treatment landscape of this tumor. In November 2019, the phase III IMbrave150 trial showed a survival benefit of about six months over sorafenib for aHCC patients treated with the anti-PD-L1 mAb atezolizumab in combination with the anti-VEGF mAb bevacizumab administered in first-line systemic treatment [13,14]. This trial led to the approval of atezolizumab plus bevacizumab for patients with aHCC who have not received prior systemic therapy [15,16]. Unfortunately, over the course of this revolution, patients with aHCC who had already received first-line therapy with sorafenib or lenvatinib were excluded from the most effective treatment available. Indeed, there are no reliable data on atezolizumab plus bevacizumab after first-line therapy.
Accordingly, apart from the ASCO guideline, which states that atezolizumab plus bevacizumab may be considered following first-line sorafenib or lenvatinib therapy “where patients did not have access to this option as first-line therapy,” [17] this recommendation is not supported by any other HCC guideline [3,18,19,20]. However, evidence from clinical practice and the peculiar mechanism of action of immune checkpoint inhibitors suggest that the use of atezolizumab plus bevacizumab is a rational option in patients with aHCC who progress after previous systemic therapies when other established therapeutic options are unavailable or contraindicated.
We present real-world data on the efficacy and safety of atezolizumab plus bevacizumab in patients with aHCC who have received at least one prior systemic therapy from 13 centers in Germany and Austria.
2. Material and Methods
2.1. Study Design and Selection of Patients
This is a post-approval retrospective study conducted to obtain information on the efficacy and safety of atezolizumab plus bevacizumab in patients with aHCC who have received at least one prior systemic therapy. Patients with histologically or radiologically confirmed aHCC who received at least one cycle of atezolizumab plus bevacizumab in the second- or further-lines between December 2018 and March 2022 were retrospectively identified at 13 centers in Germany and Austria. Consistent with a real-world dataset, no further inclusion and exclusion criteria were defined.
Demographic data, underlying liver disease, laboratory results, tumor-specific characteristics such as date of diagnosis, tumor stage at the time of diagnosis according to portal invasion, extrahepatic spread (EHS), and previous treatments, were retrospectively assessed. Liver function was classified using the Child–Pugh (CP) score, which is based on the presence of liver cirrhosis. Patients with HCC in cirrhosis were staged according to the Barcelona Clinical Liver Cancer (BCLC) classification. Patients were also assessed by the albumin–bilirubin (ALBI) score.
Adverse events (AEs) were graded according to the common terminology criteria for adverse events (CTCAE) version 5.0.
Response to treatment was assessed by the best radiological response under treatment by computed tomography or magnetic resonance imaging. Response was graded as complete and partial response (CR and PR), stable disease (SD), and progressive disease (PD) by local review according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Objective response rate (ORR) was defined as the proportion of patients achieving CR or PR. Disease control rate (DCR) was defined as the proportion of patients achieving a CR, PR, or SD as the best radiologic response. In addition to radiological response, AFP levels at baseline and during the treatment were documented. In addition to tumor progression, other reasons for treatment discontinuation such as worsening liver function and other AEs were analyzed. Patients were followed until death or data cut-off (31 March 2022). Patients whose last documented visit occurred more than 3 months before the data cut-off were considered lost to follow-up.
Atezolizumab in combination with bevacizumab has been approved by the European Medicines Agency (EMA), and the United States Food and Drug Administration (FDA) since 2020 for the treatment of patients with unresectable or advanced HCC who have not received prior systemic therapy. The recommended atezolizumab dose for HCC is 1200 mg i.v. followed by bevacizumab 15 mg/kg i.v. on the same day, every 3 weeks.
2.2. Statistical Analysis
Baseline characteristics were summarized using descriptive statistics with numbers, percentages, and median with ranges. Data are presented as medians and full ranges for continuous variables, and frequencies and percentages for categorical variables. Median duration of treatment was defined as time from the date of the first administration until the date of the last documented administration.
Overall survival (OS) was defined as the period of survival from the day treatment was initiated until the day death occurred. The date of last contact or data cutoff was used to censor patients who were still alive. Patients still receiving atezolizumab plus bevacizumab were censored at the time of data cut-off.
Progression-free survival (PFS) was defined as the time from the date of first administration of atezolizumab plus bevacizumab to radiologically confirmed disease progression or death, whichever occurred first. Patients who were alive and did not have radiologically confirmed progression were censored at the time of last contact or data cut-off. Patients who had at least one imaging follow-up were evaluable for radiologic response. Patients lost to follow-up without prior radiologic progression could not be evaluated.
OS and PFS were calculated using Kaplan–Meier survival analysis. Differences between groups were analyzed using the log rank test and expressed as median with the corresponding 95% confidence intervals (95% CI). Univariate and multivariate Cox regression models with stepwise likelihood ratio (forward selection) were used to analyze independent prognostic parameters. Sex, age, APF level, CP and BCLC stage, ALBI grade, presence of cirrhosis, EHS, portal vein thrombosis or infiltration, and prior ICI treatment were included in the model. Results were presented as hazard ratios (HR) with the corresponding 95% CI. Variables with statistical significance (p < 0.05) were included in the multivariate analysis. All tests were two-sided, and a p value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS (version 28.0, IBM, New York, NY, USA).
3. Results
3.1. Patients
A total of 50 patients from 13 centers (12 German centers and 1 Austrian center), were included. The main baseline characteristics are shown in Table 1. Individual patients had started treatment with atezolizumab plus bevacizumab in the time from 17 December 2018 to 26 June 2021. Data cut-off for the analysis was 31 March 2022. Forty-one patients (82%) were male and the median age at initiation of treatment with atezolizumab plus bevacizumab was 65 years with a range of 50–80 years. Most patients (n = 41, 82%) had cirrhosis, with CP stage A, B, and C in 30 (73%), 9 (22%), and 2 (5%), respectively. The majority of patients were staged as C according to BCLC criteria (23 of 41 patients with liver cirrhosis, 56%). The most common risk factors for HCC were alcohol consumption in 13 (25%), nonalcoholic fatty liver disease (NAFLD) in 12 (23%), hepatitis C virus (HCV) infection in 11 (21%), and hepatitis B virus (HBV) infection in 6 (12%) patients. When categorized by ALBI grade, 19 patients (38%) had grade A, 28 (56%) had B, and 3 (6%) had C.
Table 1.
Baseline characteristics.
| Parameters | Patients, n = 50 (%) * |
|---|---|
| Median age, years (range) | 65 (50–80) |
| Gender, male/female | 41 (82)/9 (18) |
| ECOG, 0/1/2 | 27 (54)/17 (34)/6 (12) |
| Liver cirrhosis present | 41 (82) |
| Child-Pugh class, A/B/C | 30 (73)/9 (22)/2 (5) |
| BCLC stage, B/C/D | 7 (17)/23 (56)/11 (27) |
| ALBI score, grade 1/2/3 | 19 (38)/28 (56)/3 (6) |
| Risk factors for HCC: HCV/HBV/alcohol/NAFLD | 11 (21)/6 (12)/13 (25)/12 (23) |
| /other **/none | /5 (10)/5 (10) |
| AFP ≥ 400 ng/mL | 32 (64) |
| EHS | 27 (54) |
| Portal invasion | 17 (34) |
| EHS and/or portal invasion | 37 (74) |
| Prior surgical treatment | 20 (40) |
| Prior loco-regional treatment | 35 (70) |
| Prior loco-regional and/or surgical treatment | 40 (80) |
| Systemic treatment in first-line, lenvatinib/sorafenib/other *** | 26 (52)/16 (32)/11 (22) |
| Received atezolizumab plus bevacizumab in line, 2/3/4/5 | 34 (68)/9 (18)/6 (12)/1 (2) |
| Vital status at last follow-up, dead/alive/unknown | 23 (46), 22 (44), 5 (10) |
| Median observation period, months (range) | 10.1 (0.1–25.3) |
Abbreviations and notes: AFP, αlpha-fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinical Liver Cancer; ECOG, Eastern Cooperative Oncology Group; EHS, extrahepatic spread; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; * Due to rounding, the percentage may differ from the total. ** hemochromatosis, n = 3; Wilson’s disease, n = 1; beta-catenin adenoma, n = 1. The sum is higher than 50 as 2 patients had 2 risk factors. *** cabozantinib n = 2; nivolumab n = 2, pembrolizumab plus regorafenib n = 1; pembrolizumab plus envatinib n = 1; spartalizumab plus sorafenib n = 1; tislelizumab n = 1; regorafenib n = 1. The sum is higher than 50 as 3 patients received a combination of systemic therapy in first line.
A total of 34 patients (68%) received atezolizumab plus bevacizumab in the second-line setting and 16 (32%) in further lines. First-line treatment was a TKI in 42/50 patients. The median observation time was 10.1 months (range 0.1–25.3 months). At the last follow-up, 23 patients had died, 22 were alive, and 5 were no longer on follow-up. Additional baseline characteristics are shown in Table 1.
Reimbursement for off-label therapies was requested individually from the responsible health insurance company prior to the start of treatment and reimbursed on a case-by-case basis. Apart from the high remission rates reported in the Imbrave150 trial, off-label therapy with atezolizumab plus bevacizumab was often motivated by adverse events observed during prior TKI therapy, contraindications to TKIs (40%), or low AFP precluding therapy with ramucirumab (36%).
3.2. Treatment Response
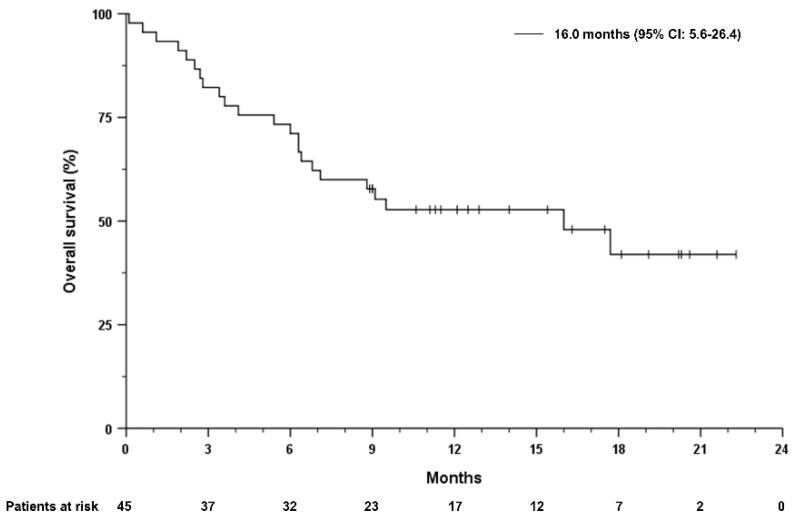
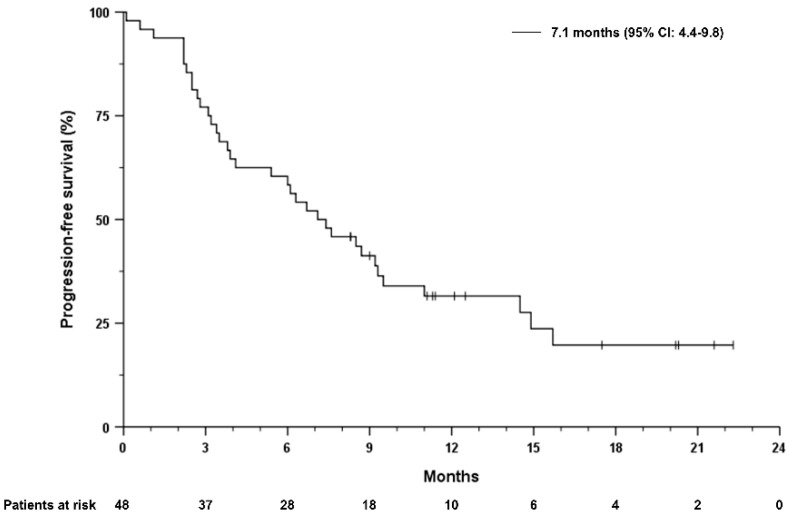
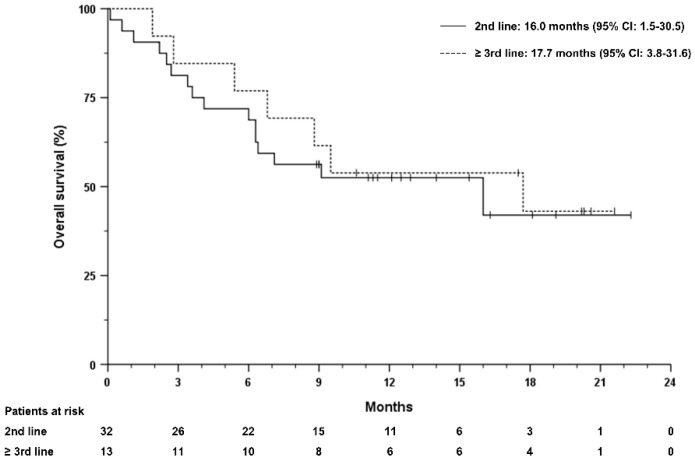
Median overall survival (mOS) from the start of first systemic treatment in the overall cohort was 31.6 months (95% CI 9.0–51.2). The median duration of treatment with atezolizumab plus bevacizumab was 7.0 months and ranged from 0.1 to 22.3 months. Median progression-free survival (mPFS) was 7.1 months (95% CI 4.4–9.8 months) and mOS after initiation of treatment with atezolizumab plus bevacizumab was 16.0 months (95% CI 5.6–26.4) months (Figure 1 and Figure 2). Patients receiving atezolizumab plus bevacizumab in the second-line had a mOS of 16 months (95% CI 1.5–30.5), compared to 17.7 months (95% CI 3.8–31.6) in patients receiving this combination in later lines (HR 0.85, 95% CI 0.35–2.1, p = 0.722, Figure 3). The mPFS was 6.3 months (95% CI 3.9–8.7 months) for patients who received atezolizumab plus bevacizumab in the second-line and 8.5 months (95% CI 6.5–10.5 months) in later lines (HR 0.69, 95% CI 0.32–1.45, p = 0.322).
Figure 1.
Overall survival of the overall study cohort, independent of the therapy line. Overall survival was defined by the date of treatment start to death from any cause. Tick marks indicate censored data.
Figure 2.
Progression-free survival of the overall study cohort, independent of the therapy line. Progression-free survival as the time from treatment start to radiographic progression or death from any cause. Tick marks indicate censored data.
Figure 3.
Overall survival stratified according to the number of previous therapy lines. Overall survival was defined by the date of treatment initiation until death from any cause. Tick marks indicate censored data.
Patients with CP-A achieved a mOS of 16.0 months (95% CI, 5.6–26.4), whereas it was 6.4 months (95% CI, 0.0–13.1) in patients with CP-B (p = 0.200). Patients with CP-A achieved a mPFS of 8.7 months (95% CI, 4.5–12.9), while it was 3.4 months (95% CI, 1.4–5.4) in patients with CP-B (p = 0.008).
The mOS was not reached in patients with ALBI grade 1, while it was 6.4 months (95% CI, 4.5–8.3) in patients with ALBI grade 2 and 6.3 months (95% CI, 0.2–12.4) in patients with ALBI grade 3.
Compared to patients with preserved liver function according to the ALBI grade (ALBI grade 1), survival of patients with impaired liver function was significantly shorter (ALBI 1 vs. ALBI 2: HR 3.4, 95% CI 1.14–10.30, p = 0.029; ALBI 1 vs. ALBI 2: HR 6.6, 95% CI: 1.46–29.89; p = 0.014). The mPFS for patients with ALBI grade 1, 2, and 3 was 7.6 months (95% CI, 5.3–9.9), 6.7 months (95% CI, 3.8–9.6), and 3.8 months (95% CI, 1.2–6.4), respectively, with no statistical difference among groups.
Radiologic response was assessed in 43 patients (86%) according to RECIST criteria v1.1. Of these patients, 1 (2%) achieved a complete response (CR), 15 (30%) achieved a partial response (PR), and 18 (36%) had a stable disease (SD), while 9 (18%) had progressive disease (PD). The overall response rate (ORR) was 32%, and the disease control rate (DCR) was 68% (Table 2).
Table 2.
Response rates.
| Best Documented Response | Patients, n = 50 (%) | 2nd Line, n = 34 (%) | ≥3rd Line, n = 16 (%) |
|---|---|---|---|
| Complete response (CR) | 1 (2) | 1 (3) | 0 (0) |
| Partial response (PR) | 15 (30) | 10 (29) | 5 (31) |
| Stable disease (SD) | 18 (36) | 12 (35) | 6 (38) |
| Progressive disease | 9 (18) | 5 (15) | 4 (25) |
| Not evaluable | 7 (14) | 6 (18) | 1 (6) |
| Objective response rate (ORR) | 16 (32) | 11 (32) | 5 (31) |
| Disease control rate (DCR) | 34 (68) | 23 (68) | 11 (69) |
Notes: Radiological response was available for 43 patients (86% of the efficacy population). The percentages may differ from the total due to rounding.
Response rates were similar in patients who received atezolizumab plus bevacizumab after one, two, or more prior lines of systemic treatment (Table 2).
OS was not significantly different between patients with AFP levels ≥ 400 ng/mL compared to patients with low AFP levels (p = 0.744). The median OS was 17.7 months (95% CI 2.0–33.4 months) in patients with AFP levels > 400 ng/mL; compared to patients with lower AFP levels, mOS was 16.0 months (95% CI 3.6–28.4 months). Patients who had previously received treatment with a checkpoint inhibitor had similar mOS compared to patients without ICI therapy prior to the initiation of treatment with atezolizumab plus bevacizumab (p = 0.81).
3.3. Safety
All patients included in the present analysis received at least one dose of atezolizumab plus bevacizumab and were monitored for the development of treatment-related adverse events (TRAE). At the time of data cut-off, 38 patients (76%) had discontinued treatment with atezolizumab plus bevacizumab or had died. In four cases (8%), the bevacizumab medication was paused due to TRAE or planned surgery, in three cases (6%), bevacizumab was not continued. In 25 patients (50%) at least one TRAE was reported, whereas the total number of observed TRAEs was 36. Events of grade 3–4 were observed in seven (14%) cases and led to the death of three patients (6%). The most common TRAEs were bleeding events in seven (14%), rash/exanthem in six (12%), and fatigue in four (8%) patients. There were five (10%) bleeding events of grade ≥ 3, one of them (2%) with fatal outcome was reported. The incidence of each TRAE is shown in Table 3.
Table 3.
Treatment-related adverse events (TRAE) during the treatment in the safety population.
| TRAE | Any Grade, n (%) |
Grade 1–2, n (%) |
Grade 3–4, n (%) |
Death, n (%) |
|---|---|---|---|---|
| Rash/exanthema | 6 (12) | 6 (12) | 0 | 0 |
| Esophageal variceal bleeding | 4 (8) | 0 | 3 (6) | 1 (2) |
| Fatigue | 4 (8) | 4 (8) | 0 | 0 |
| Thyroid toxicity | 3 (6) | 3 (6) | 0 | 0 |
| Hepatotoxicity/hepatitis | 2 (4) | 0 | 1 (2) | 1 (2) |
| Epistaxis | 2 (4) | 2 (4) | 0 | 0 |
| Hyponatremia | 2 (4) | 2 (4) | 0 | 0 |
| Hypertension | 2 (4) | 2 (4) | 0 | 0 |
| Pruritus | 2 (4) | 2 (4) | 0 | 0 |
| Hypoglycemia | 1 (2) | 0 | 0 | 1 (2) |
| Retroperitoneal bleeding | 1 (2) | 0 | 1 (2) | 0 |
| Hyperglycemia | 1 (2) | 0 | 1 (2) | 0 |
| Worsening asthma | 1 (2) | 0 | 1 (2) | 0 |
| Hepatic encephalopathy | 1 (2) | 1 (2) | 0 | 0 |
| Dyspnea | 1 (2) | 1 (2) | 0 | 0 |
| Dysphonia | 1 (2) | 1 (2) | 0 | 0 |
| Infusion reaction | 1 (2) | 1 (2) | 0 | 0 |
| Appetite loss | 1 (2) | 1 (2) | 0 | 0 |
3.4. Prognostic Markers Associated with Survival
Apart from a significant correlation of OS with the ALBI score (HR 3.42, 95% CI 1.14–10.30 for ALBI grade 1 vs. 2 and HR 6.6, 95% CI 1.46–29.89 for ALBI grade 1 vs. 3), no other prognostic markers were identified. In the multivariate Cox regression model, ALBI score was independently associated with OS (HR 0.05, 95% CI 0.004–0.52 for ALBI grade 1 vs. 2 and HR 0.13, 95% CI 0.013–1.23 for ALBI grade 1 vs. 3, respectively).
4. Discussion
To our knowledge, this is the largest cohort in the literature of patients with aHCC receiving atezolizumab plus bevacizumab after prior systemic therapy. We have shown that atezolizumab plus bevacizumab resulted in higher ORR, PFS, and OS than expected with standard treatment [8,9,10] in patients who had disease progression after at least one systemic treatment for aHCC, regardless of serum AFP level. In addition, both the efficacy and safety profiles of atezolizumab plus bevacizumab in our cohort were similar to those in patients treated in the IMbrave150 trial, which did not allow prior systemic treatment. Thus, the results of this multicenter retrospective study support the clinical use of atezolizumab plus bevacizumab in patients who did not have access to this option as a first-line therapy in the pre-approval period, who had contraindications to established therapies after first-line TKI-based therapy, or for whom no other approved treatment was available.
Because 32% of patients received atezolizumab plus bevacizumab as a third-line treatment or later, the favorable survival of these patients may be due in part to less aggressive tumor biology rather than treatment effect. However, an objective response was seen in both this subset of patients who had received at least two prior systemic therapies and in the larger proportion of patients (68%) who received atezolizumab plus bevacizumab as a second-line therapy, strongly supporting the evidence for the intrinsic efficacy of atezolizumab plus bevacizumab.
Approximately 20% of patients in our cohort had impaired liver function (CP B). However, the efficacy and safety of atezolizumab plus bevacizumab was independent of the CP stage in our cohort. This is consistent with recently published data on the use of atezolizumab plus bevacizumab [21] in first-line treatment. Nevertheless, multivariate Cox regression identified liver function (ALBI score) as the strongest prognostic marker in our cohort, underscoring the known effect of liver function in determining prognosis in these patients [22,23].
Obvious limitations of our study are its retrospective nature and the lack of central radiological assessment at predefined intervals. On the other hand, a positive effect of atezolizumab plus bevacizumab was observed, although these patients had unfavorable baseline characteristics that would have prevented their inclusion in clinical trials: these include the fact that many patients who received atezolizumab plus bevacizumab had contraindications to TKI-based treatment, poor performance status (12% of patients had a ECOG PS of 2) and/or poorer liver function (27% had CP B or C). Despite these limitations, atezolizumab plus bevacizumab proved safe and demonstrated clinical efficacy after one or more prior lines of treatment, exceeding the ORR, PFS, and OS reported in contemporary studies.
A number of different immune checkpoint inhibition (ICI)-based treatment strategies have been evaluated in global phase III clinical trials. The ICI monotherapy with durvalumab was shown to be non-inferior to the TKI sorafenib with improved safety profile [24]. However, among combination therapies, only the ICI combination with durvalumab and tremelimumab provided a survival benefit over sorafenib [24], whereas both the COSMIC-312 and LEAP-002 trials, which investigated the superiority of a combination TKI and ICI compared with TKI monotherapy, provided negative results with respect to mOS [25,26]. Of note, in the COSMIC-312 trial, the combination of the TKI cabozantinib with the ICI atezolizumab was superior to a TKI monotherapy in terms of PFS, whereas the significantly higher percentage of patients who received subsequent therapy with VEGF(R)-targeted antibody or ICI in the standard arm may have contributed to preventing a mOS benefit in the experimental arm. These results are consistent with our retrospective analysis and indicate that the bar has been raised for future first-line trials in aHCC. While future studies on the optimal treatment sequence for patients with advanced HCC are still pending, our results support current ASCO recommendations and should be considered in other international guidelines.
Acknowledgments
We would like to thank the health insurance providers that supported our applications and bore the costs for the off-label therapy with atezolizumab plus bevacizumab. This work contains substantial parts of the doctoral thesis of F.S.
Abbreviations
AE, adverse events; AFP, alpha-fetoprotein; aHCC, advanced stage hepatocellular carcinoma; ALBI, albumin-bilirubin; ASCO, American Society of Clinical Oncology; BCLC, Barcelona clinic liver cancer; CI, confidence interval; CP, Child-Pugh; CR, complete response; CT, computed tomography; CTCAE, common terminology criteria for adverse events; DCR, disease control rate; ECOG-PS, Eastern Cooperative Oncology Group performance status; EHS, extrahepatic spread; EMA, European Medical Agency; FDA, Food and Drug Administration; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibition; mAb, monoclonal antibody; MRI, magnetic resonance imaging; NAFLD, nonalcoholic fatty liver disease; ORR, objective response rate; OS, overall survival; PD, progressive disease; PD-L1, programmed cell death-ligand 1; PFS, progression free survival; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; TKI, tyrosine-kinase inhibitor; TRAE, treatment-related adverse events; VEGF, vascular endothelial growth factor.
Author Contributions
F.S. and M.V. designed the study, analyzed, and interpreted the data and drafted the manuscript. F.S., M.P., B.S., T.J.E., N.S., M.A.G.-C., O.W., F.F., V.H., E.N.D.T., N.B.K., R.M., T.W.F., F.K., F.v.B., S.L., S.K., D.B., M.S., L.S.J., J.B., C.M., V.K. and M.V. collected the data and revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript and agreed t to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the 1975 Declaration of Helsinki and approved by the ethics committee of the medical faculty and university hospital of the Otto-von-Guericke University Magdeburg in 06/2022 with the protocol code 70/22.
Informed Consent Statement
Due to the retrospective character of the study, consent to participate was waived.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
F.S. reports no conflicts of interest. M.P. is an investigator for Bayer, BMS, Eisai, Ipsen, Lilly, and Roche; he received speaker honoraria from Bayer, BMS, Eisai, Lilly, MSD, and Roche; he is a consultant for Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; he received travel support from Bayer, BMS, and Roche. B.S. received travel support from Abbvie, Ipsen and Gilead and is supported by a scientific scholarship granted by the University of Piemonte Orientale, Novara, Italy. T.J.E. reports no conflicts of interest. N.S. reports no conflicts of interest. M.A.G.-C. received Honoraria for speaker, consultancy, and advisory role from Roche, BMS, MSD, EISAI, Lilly, AstraZeneca and Amgen. O.W. received Honoraria for speaker, consultancy, and advisory role from Amgen, AstraZeneca, Bayer, BMS, Celgene, Eisai, Incyte, Ipsen, Merck Serono, MSD, Novartis, Roche, and Servier. F.F. has received travel support from Ipsen, and speaker’s fees from AbbVie, MSD, Ipsen, Esai and Fresenius. K. V.H. reports no conflicts of interest. E.N.D.T. has served as a paid consultant for AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, Pfizer, IPSEN, and Roche. He has received reimbursement of meeting attendance fees and travel expenses from Arqule, Astrazeneca, BMS, Bayer, Celsion and Roche, and lecture honoraria from BMS and Falk. He has received third-party funding for scientific research from Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, and Roche. N.B.K. has received reimbursement of meeting attendance fees and travel expenses from EISAI and lecture honorarium from Falk. R.M. reports no conflicts of interest. T.W.F. reports no conflicts of interest. F.K. received Honoraria for speaker and/or advisory role from Eisai, Sirtex, Servier, Janssen, and Bayer. F.v.B. received payment or honoraria for lectures, presentations, speakers’ bureaus, advisory board or educational events from Roche, Eisai, Ipsen, MSD, Astra Zeneca, Gilead Sciences, Sirtex, and Gilead Sciences. S.L. reports no conflicts of interest. S.K. received Honoraria for speaker, consultancy, and advisory role from BMS, MSD, Bayer, AstraZeneca, Ipsen, Amgen, AAA, Servier and Advanz PharmaDB. D.B. has served as a paid consultant for Bayer Healthcare, Boston Scientific, and Shionogi. Lectures: Falk Foundation, W.L. Gore & Associates. M.S. served as a consult for Bayer Healthcare, and Falk Foundation. L.S.J. has served as a paid consultant for AstraZeneca, EISAI and BS and has received lecture honoraria from Falk and BS. J.B. served as Consultant for BTGPLC, BMS, MSD, Roche, and Eisai. He served as Speaker for BTGPLC, Eisai, and Novartis. He received travel support from Ipsen, BTGPLC, and BMS. He is an investigator for MSD, BMS, and Lilly. C.M. reports no conflicts of interest. V.K. received honoraria for speakers’ corner from AbbVie, Gilead, Falk, Albireo, and CSL Behring, and participated in an advisory board for AstraZeneca. M.V. received honoraria for speaker, consultancy, and advisory role from Roche, BMS, MSD, EISAI, Bayer, Lilly, AstraZeneca, Merck Serono, Sirtex, Ipsen, Nordic Pharma, and Amgen.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim E., Viatour P. Hepatocellular Carcinoma: Old Friends and New Tricks. Exp. Mol. Med. 2020;52:1898–1907. doi: 10.1038/s12276-020-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Siegel R.L., Rosenberg P.S., Jemal A. Emerging Cancer Trends among Young Adults in the USA: Analysis of a Population-Based Cancer Registry. Lancet Public Health. 2019;4:e137–e147. doi: 10.1016/S2468-2667(18)30267-6. [DOI] [PubMed] [Google Scholar]
- 3.Voesch A.S., Bitzer M., Blödt S., Follmann M., Freudenberger P., Langer T., Lorenz P., Jansen P.L., Steubesand N., Galle P., et al. S3-Leitlinie: Diagnostik Und Therapie Des Hepatozellulären Karzinoms Und Biliärer Karzinome. Z. Gastroenterol. 2022;60:131–185. doi: 10.1055/a-1589-7585. [DOI] [PubMed] [Google Scholar]
- 4.Galle P.R., Forner A., Llovet J.M., Mazzaferro V., Piscaglia F., Raoul J.L., Schirmacher P., Vilgrain V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.-F., de Oliveira A.C., Santoro A., Raoul J.-L., Forner A., et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.S., et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet. Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., Cicin I., Merle P., Chen Y.H., Park J.W., et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhu A.X., Kang Y.-K., Yen C.-J., Finn R.S., Galle P.R., Llovet J.M., Assenat E., Brandi G., Lim H.Y., Pracht M., et al. REACH-2: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study of Ramucirumab versus Placebo as Second-Line Treatment in Patients with Advanced Hepatocellular Carcinoma (HCC) and Elevated Baseline Alpha-Fetoprotein (AFP) Following First-Line Sorafe. J. Clin. Oncol. 2018;36:4003. doi: 10.1200/JCO.2018.36.15_suppl.4003. [DOI] [Google Scholar]
- 11.Yau T., Park J.W., Finn R.S., Cheng A.-L., Mathurin P., Edeline J., Kudo M., Han K.-H., Harding J.J., Merle P., et al. CheckMate 459: A Randomized, Multi-Center Phase III Study of Nivolumab (NIVO) vs Sorafenib (SOR) as First-Line (1L) Treatment in Patients (Pts) with Advanced Hepatocellular Carcinoma (AHCC) Ann. Oncol. 2019;30:v874–v875. doi: 10.1093/annonc/mdz394.029. [DOI] [Google Scholar]
- 12.Finn R.S., Chan S.L., Zhu A.X., Knox J.J., Cheng A.-L., Siegel A.B., Bautista O., Watson P., Kudo M. KEYNOTE-240: Randomized Phase III Study of Pembrolizumab versus Best Supportive Care for Second-Line Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2017;35:TPS503. doi: 10.1200/JCO.2017.35.4_suppl.TPS503. [DOI] [Google Scholar]
- 13.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 14.Cheng A.L., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Lim H.Y., Kudo M., Breder V., Merle P., et al. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J. Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency Tecentriq. [(accessed on 7 May 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tecentriq.
- 16.FDA FDA Approves Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma. [(accessed on 7 May 2022)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma.
- 17.Gordan J.D., Kennedy E.B., Abou-Alfa G.K., Beg M.S., Brower S.T., Gade T.P., Goff L., Gupta S., Guy J., Harris W.P., et al. Systemic Therapy for Advanced Hepatocellula Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020;38:4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 18.Pentheroudakis G. Reply: Recent EUpdates to the ESMO Clinical Practice Guidelines on Hepatocellular Carcinoma, Cancer of the Pancreas, Soft Tissue and Visceral Sarcomas, Cancer of the Prostate and Gastric Cancer. Ann. Oncol. 2019;30:1396–1397. doi: 10.1093/annonc/mdz180. [DOI] [PubMed] [Google Scholar]
- 19.Su G.L., Altayar O., O’Shea R., Shah R., Estfan B., Wenzell C., Sultan S., Falck-Ytter Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology. 2022;162:920–934. doi: 10.1053/j.gastro.2021.12.276. [DOI] [PubMed] [Google Scholar]
- 20.Yau T., Tai D., Chan S.L., Huang Y.-H., Choo S.P., Hsu C., Cheung T.T., Lin S.-M., Yong W.P., Lee J., et al. Systemic Treatment of Advanced Unresectable Hepatocellular Carcinoma After First-Line Therapy: Expert Recommendations from Hong Kong, Singapore and Taiwan. Liver Cancer. 2022;11:426–439. doi: 10.1159/000525582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessio A., Fulgenzi C.A.M., Nishida N., Schönlein M., von Felden J., Schulze K., Wege H., Gaillard V.E., Saeed A., Wietharn B., et al. Preliminary Evidence of Safety and Tolerability of Atezolizumab plus Bevacizumab in Patients with Hepatocellular Carcinoma and Child-Pugh A and B Cirrhosis: A Real-World Study. Hepatology. 2022;76:1000–1012. doi: 10.1002/hep.32468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welland S., Leyh C., Finkelmeier F., Jefremow A., Shmanko K., Gonzalez-Carmona M.A., Kandulski A., Jeliazkova P., Best J., Fründt T.W., et al. Real-World Data for Lenvatinib in Hepatocellular Carcinoma (ELEVATOR): A Retrospective Multicenter Study. Liver Cancer. 2022;11:219–232. doi: 10.1159/000521746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himmelsbach V., Pinter M., Scheiner B., Venerito M., Sinner F., Zimpel C., Marquardt J.U., Trojan J., Waidmann O., Finkelmeier F. Efficacy and Safety of Atezolizumab and Bevacizumab in the Real-World Treatment of Advanced Hepatocellular Carcinoma: Experience from Four Tertiary Centers. Cancers. 2022;14:1722. doi: 10.3390/cancers14071722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou-Alfa G.K., Lau G., Kudo M., Chan S.L., Kelley R.K., Furuse J., Sukeepaisarnjaroen W., Kang Y.-K., Van Dao T., De Toni E.N., et al. Tremelimumab Plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 25.Kelley R.K., Rimassa L., Cheng A., Kaseb A., Qin S., Zhu A.X., Chan S.L., Melkadze T. Articles Cabozantinib Plus Atezolizumab versus Sorafenib for Advanced Hepatocellular Carcinoma (COSMIC-312): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2022;23:995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 26.Merck & Co. Merck and Eisai Provide Update on Phase 3 LEAP-002 Trial Evaluating KEYTRUDA® (Pembrolizumab) Plus LENVIMA® (Lenvatinib) versus LENVIMA Monotherapy in Patients With Unresectable Hepatocellular Carcinoma. [(accessed on 7 August 2022)]. Available online: https://www.merck.com/news/merck-and-eisai-provide-update-on-phase-3-leap-002-trial-evaluating-keytruda-pembrolizumab-plus-lenvima-lenvatinib-versus-lenvima-monotherapy-in-patients-with-unresectable-hepatocellul/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.