Abstract
Objective
The purpose of the study is to examine presentation, injury patterns, and clinical course, for COVID-19-related peripheral nerve injury following mechanical ventilation.
Methods
A multicenter retrospective study of patients with COVID-19 complicated by acute respiratory distress syndrome (ARDS) that required mechanical ventilation was undertaken. Patient records were reviewed for intensive care unit and intubation characteristics, prone or lateral decubitus positioning, and the onset of neuropathy diagnosis.
Results
Between September 2020 and January 2022, 11 patients were diagnosed with peripheral neuropathy, including 9 with brachial plexopathy following COVID-19 infection. Each patient developed ARDS requiring mechanical ventilation for a median of 39 days. Six patients (54.5%) underwent prone positioning and 1 lateral decubitus. Neuropathies involved 5 brachial pan-plexopathies, 2 incomplete brachial plexopathies, 2 lower trunk plexopathies, 1 radial neuropathy, and 1 bilateral ulnar neuropathy. At a mean follow-up of 10.2 months, patients with brachial pan-plexopathies demonstrated signs of reinnervation proximally, and 1 resolved to a radial mononeuropathy; however, the majority have demonstrated minimal clinical improvements.
Conclusions
Our series demonstrates that peripheral neuropathies and especially brachial plexopathies have occurred following mechanical ventilation for ARDS-related COVID-19 infections. Contrary to prior COVID-19 studies, only 54.5% of these patients underwent prone positioning. Aside from a traumatic disturbance of prone positioning, the increased incidence of neuropathy may involve an atraumatic effect of COVID-19 via direct invasion of nerves, autoantibody targeting of nervous tissue, or hypercoagulation-induced microthrombotic angiopathy.
Key words: Acute respiratory distress syndrome, Brachial plexus, COVID-19, Prone positioning, Upper extremity neuropathy
Introduction
Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (Sars-CoV2) has presented unprecedented challenges to healthcare around the world. At the time of writing (April 24, 2022), there have been nearly 506 million confirmed cases of COVID-19, with 6.2 million deaths worldwide. Upon infection, the virus invades the respiratory mucosa, penetrating intracellularly and potentially inducing a cytokine storm.1 About a third of patients with COVID-19 progress to acute respiratory distress syndrome (ARDS), and 26% of COVID-19 patients require transfer to an intensive care unit (ICU).2, 3, 4 Experience with ARDS over the past 20 years has noted improved oxygenation and increased survival with prone positioning.3 , 5 These studies have further noted that a minimum of 16 hours in the prone position is ideal to obtain a survival benefit.5 , 6 From the initial randomized controlled trial of Prone Positioning in ARDS (ProSEVA), complications of prone positioning have been centered around pressure necrosis of the skin, followed by instances of unscheduled extubation, and endotracheal tube obstruction.5 However, over the course of the COVID-19 pandemic, patients with COVID-19 placed in the prone position have demonstrated acute peripheral neuropathy conditions with a higher-than-expected prevalence of upper extremity peripheral neuropathies.3 , 7 , 8
The increase in the presentation of COVID-19 ICU-related upper extremity neuropathy, especially pan-brachial plexopathies, could potentially be related to the sheer volume of ICU-ARDS admission necessitating prone positioning. Nonetheless, it remains unknown whether there is a direct or indirect effect of the Sars-CoV2 on peripheral nerves rendering them vulnerable to injury or if there is an inflammatory or vascular pathway that is associated with severe infection that predisposes peripheral nerves to injury. To offer additional insight into these previously uncommon complications, the purpose of this multicenter, retrospective study was to examine presentation, injury patterns, and clinical course for COVID-19-related peripheral nerve injury following mechanical ventilation.
Methods
This study was a multicenter retrospective review of patients following Institutional Review Board approval at Mayo Clinic in Minnesota (Rochester), Mayo Clinic in Florida (Jacksonville), Shirley Ryan AbilityLab, and Northwestern Memorial Hospital in Illinois (Chicago). Patients were identified by a review of each institution's peripheral nerve/brachial plexus clinic database. Inclusion criteria for analysis were patients older than 18 years, COVID-19 infection, and concern of brachial plexopathy on initial referral following treatment of severe COVID-19 infection requiring ICU level of care with mechanical ventilation. Exclusion criteria involved patients less than 18 years of age, COVID-19 infection that did not require ICU level of care, no electrodiagnostic studies, no previous peripheral nerve injury, and no evaluation by a peripheral nerve/brachial plexus specialist. For patients from the Shirley Ryan AbilityLab and Northwestern cohort, cases were reviewed after April 1, 2021, with some cases being excluded due to prior publication.9
Patient demographics, hand dominance, as well as comorbidities, including obesity, lung disease, diabetes, and tobacco use, were reviewed from the electronic medical record. Each patient was hospitalized for COVID-19 at institutions separate from the study centers. Inpatient records were obtained from these hospitals to confirm the timing of infection, progression to ARDS, length of intubation, institution of altered positioning such as prone or lateral decubitus, and timing to diagnosis of peripheral neuropathy or brachial plexopathy. When available from outside records, clinical findings, imaging, and electrodiagnostic studies were reviewed for initial analysis and working diagnosis.
Upon being seen at one of the study centers, each patient was evaluated by a peripheral nerve/brachial plexus specialist. Time from diagnosis to specialist evaluation, clinical exam findings, advanced imaging, electrodiagnostic findings, and diagnoses were reviewed. Treatment plans and, when available, follow-up encounters to understand clinical progression were reviewed.
Statistical Analysis
Continuous and categorical variables were reported with means, standard deviations, ranges, frequencies, and percentages. Descriptive statistics were performed by GraphPad Prism 9 (San Diego, California).
Results
Demographics and COVID-19 Management
Between September 2020 and January 2022, 11 patients were diagnosed with upper extremity neuropathy following severe COVID-19 infection. The mean age of these patients was 48.7 (±12.9, range 27–68), with 9 males (81.8%). The mean body mass index was 33.8 (±7.14), with 6 patients (54.5%) considered obese with a body mass index ≥30. Seven patients (63.6%) had dominant hand-sided injuries. All patients progressed to ARDS and required mechanical ventilation, with 6 (54.5%) undergoing prone positioning for ARDS and 1 with lateral decubitus positioning (Table 1 ). The median length of intubation was 39 days (range 18–60 days).
Table 1.
Patient Demographics and Intubation Characteristics
| Total Patients | 11 |
|---|---|
| Mean Age, SD, range (years) | 48.7, 12.9, 27–68 |
| Gender | |
| Male (%) | 9 (81.8) |
| Female | 2 (18.2) |
| BMI (mean, SD range) | 33.8, 7.14, 26.6–46.7 |
| Obesity (%) | 6 (54.5) |
| Diabetes (%) | 4 (36.3) |
| Dominant sided injury (%) | 7 (63.6) |
| ARDS (%) | 11 (100) |
| Intubation for COVID-19 | 11 (100) |
| Length of intubation, days (median range) | 39, 18–60 |
| Intubation positioning | |
| Supine | 4 (36.3) |
| Lateral decubitus (%) | 1 (9.09) |
| Prone positioning (%) | 6 (54.5) |
ARDS, acute respiratory distress syndrome; BMI, body mass index; COVID-19, Coronavirus Disease 2019.
Initial Diagnosis and Work-Up of Peripheral Neuropathy Prior to Referral
Initial diagnosis of peripheral nerve injury/brachial plexus injury was recognized when patients had the inability to utilize 1 or both upper extremities after being extubated. The mean time from infection to the diagnosis of neuropathy was 2.82 months (±2.04, range 1–7 months). Of the 11 patients, 5 demonstrated brachial pan-plexopathies, 2 demonstrated incomplete brachial plexopathies, 2 demonstrated lower trunk plexopathies, 1 had radial mononeuropathy, and 1 with bilateral ulnar neuropathies (Table 2 ). Of the 5 patients with brachial pan-plexopathy, 2 had deficits in both upper extremities. The patient who developed radial mononeuropathy was placed in lateral decubitus positioning (patient no. 10 in Table 3 ).
Table 2.
Peripheral Neuropathy Diagnosis at Time of COVID-19 Treatment
| Peripheral Nerve Complication | Number of Patients (and Percent of Cases) |
|---|---|
| Brachial pan-plexopathy | 5 (45.5) |
| Unilateral | 3 |
| Bilateral | 2 |
| Incomplete brachial plexopathy | 2 (18.2) |
| Unilateral | 1 |
| Bilateral | 1 |
| Lower trunk plexopathy (%) | 2 (18.2) |
| Radial mononeuropathy (%) | 1 (9.1) |
| Ulnar neuropathy (%) | 1 (9.1) |
| Neuropathic pain (%) | 9 (81.8) |
Time from Infection to Diagnosis of Nerve Injury:mean 2.82, SD 2.82, 2.04, range (mo) 1–7.
Table 3.
Outcomes of Patients with Peripheral Neuropathy Following COVID-19 Infection
| Patient No. | Age Gender |
Neurologic Injury | Positioning | Diagnosis to Specialist Evaluation (mo) | Follow-Up (mo) | Magnetic Resonance Imaging | Electrodiagnostic Studies | Clinical Course |
|---|---|---|---|---|---|---|---|---|
| 1 | 44F | Brachial pan-plexopathy | Prone | 12 | 3 | Diffuse brachial plexopathy with greatest involvement of lower trunk | ||
| 2 | 12 | Chronic patchy pan-brachial plexopathy, most affecting ulnar nerve innervated muscles | Improvement in shoulder and elbow function, poor hand function, continued dysesthesias to fingertips | |||||
| 2 | 57M | Brachial pan-plexopathy | Supine | 7 | 2 | Severe brachial pan-plexopathy | Minimal shoulder function | |
| 4 | 6 | Asymmetric decrease bulk of multiple muscles around left chest wall and shoulder in varying nerve distributions with chronic denervation changes. | Fibrillation potentials in a patchy distribution, severe left brachial pan-plexopathy | Shoulder abduction with deltoid 3/5 | ||||
| 5 | 12 | Severe diffuse brachial plexopathy, interval reinnervation of proximal muscles | Deltoid 4+, Biceps/Brachialis 1/5, Triceps 2/5 | |||||
| 3 | 37M | Brachial pan-plexopathy | Prone | 6 | 1 | Thickening and enhancements of roots and trunks consistent with brachial plexitis, no structural abnormality | ||
| 7 | 68M | Incomplete bilateral brachial plexopathy | Supine | 12 | 12 | Incomplete bilateral brachial pan-plexopathy affecting posterior cord and median and ulnar nerves | ||
| 8 | 27F | Lower trunk plexopathy | Supine | 12 | 12 | Lower trunk plexopathy | ||
| 9 | 53M | Lower trunk plexopathy | Prone | 4 | 4 | Patchy lower trunk plexopathy affected median, posterior interosseous, and ulnar nerves | ||
| 10 | 44M | Radial mononeuropathy | Lateral Decubitus | 3 | 3 | Severe right radial mononeuropathy arising proximal to branch of triceps with some reinnervation to the triceps | No finger or wrist extension | |
| 11 | 59M | Bilateral ulnar neuropathy resolved on left side after 6 months, persistent on right | Supine | 28 | 28 | Normal caliber and signal intensity of the right ulnar nerve | Right severe ulnar neuropathy at or about the elbow | Dense numbness and wasting of right hand |
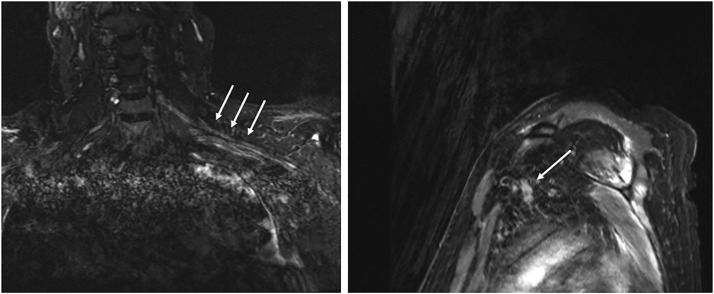
One patient (patient no. 3 in Table 3) with a brachial pan-plexopathy underwent magnetic resonance imaging (MRI) at an outside facility soon after demonstrating weakness, which found thickening and enhancements of roots and trunks of the brachial plexus consistent with brachial plexitis (Figure 1 . Another patient (patient No. 2 in Table 3) obtained an electrodiagnostic study shortly after diagnosis, demonstrating a severe brachial pan-plexopathy. It was further notable that patients with incomplete, lower trunk or pan-plexopathies endorsed severe neuropathic pain.
Figure 1.
MRI of 46 year old male with 9 month history of bilateral brachial pan-plexopathy following COVID-19 infection with protracted hospital course including management in prone positioning for ARDS demonstrating diffuse enhancement and thickening of the left brachial plexus (white arrows). MRI, magnetic resonance imaging; COVID-19, Coronavirus Disease 2019; ARDS, acute respiratory distress syndrome.
Follow-Up and Outcomes of Patients with Neuropathy Following COVID-19 Treatment
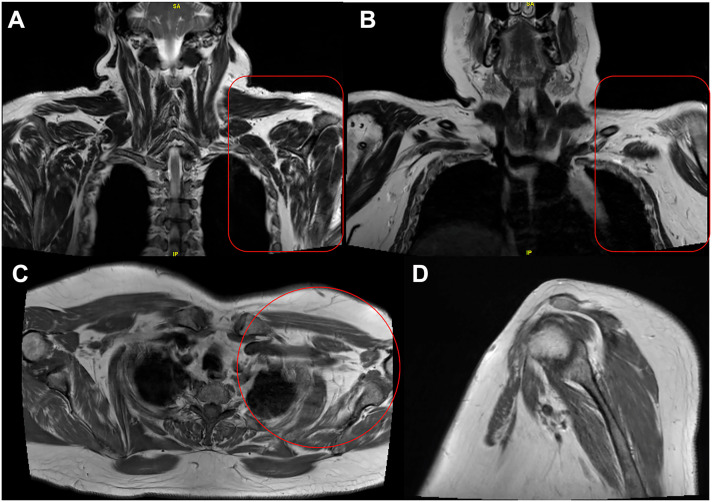
The mean time from injury to specialist evaluation was 9.5 months (±6.96 months, range 3–28 months). Patient No. 5 (Table 3), who developed bilateral brachial pan-plexopathy, had MRI on follow-up at 9 months that demonstrated diffuse enhancement and thickening of the left brachial plexus (Figure 1). Patient No. 2 and patient no. 3 both with brachial pan-plexopathy (Table 3) each received MRI evaluations at 6 months which noted asymmetric atrophy of the affected shoulder and chest wall muscles in varying nerve distributions (Figure 2 ). Concomitant electrodiagnostic studies from 6 months to 12 months noted a patchy distribution of pathology in patients with severe brachial pan-plexopathy as well as lower trunk plexopathy (Table 3, Patients 1, 2, 4, 5, 9). Patients with incomplete and lower trunk plexopathies demonstrated variable involvement of the plexus at the trunk, cord, and branch levels on electrodiagnostic studies (Table 3, Patients 6, 7, 8, 9).
Figure 2.
57 yoM with 6-month history of brachial pan-plexopathy following COVID-19 infection. MRI demonstrates asymmetric decreased bulk of multiple muscles around left chest wall and shoulder with varying nerve distributions consistent with chronic denervation changes and/or disuse atrophy. Views: (A) Mid Coronal T2 Dixon_in (B) Anterior Coronal T2 Dixon_in (C) Axial TSE T1 (D) Affected Left Sagittal TSE T1. COVID-19, Coronavirus Disease 2019; MRI, magnetic resonance imaging.
The mean follow-up was 10.2 months (±6.79 months). The resolution of plexopathies and neuropathies has been variable. Patients with brachial pan-plexopathy have shown gradual improvement in function, starting proximally with shoulder function followed by elbow flexion (Table 3, Patient No. 1, 2, 3). Patient No. 4 demonstrated resolution of initial bilateral brachial pan-plexopathy, which resolved to a right radial mononeuropathy at 3 months. Patient No. 11 with initial bilateral ulnar neuropathy resolved on the left side at 6 months, but continued on the right side, currently scheduled for cubital tunnel release. Treatment of these patients have involved nonoperative measures that include hand therapy, splinting, as well as continued follow-up. A discussion with each has ensued regarding the role of nerve transfers, tendon transfers, selected joint fusions, and other potential surgical interventions.
Discussion
Recognizing and understanding the sequelae of COVID-19 infection to assist with treatment modalities is imperative as the world continues to recover from this pandemic. This study presents 11 cases of peripheral neurologic complications, with 9 involving the brachial plexus that each occurred during the treatment of COVID-19 infection. Each patient in this series progressed to ARDS, requiring prolonged intubation and, for 54.5% of cases, prone position mechanical ventilation. Notably, patients with complete/partial brachial plexopathy were not all placed in the prone position. This finding gives credence to the hypothesis that there is a multifactorial compromise of the peripheral nerves secondary to a hyperinflammatory environment imposed by the viral infection, prolonged ICU courses, as well as patient comorbidities (e.g. diabetes, obesity, and age) that causes these nerves to be vulnerable to injury.
Prior to COVID-19, ICU-induced peripheral neuropathy, especially involving the brachial plexus, was not commonly reported. Of the few studies available, 1 by Goettler et al. from 2002, details brachial plexopathy in a case series of 2 patients, both associated with prone positioning in the ICU.10 Aside from the ICU, prone positioning during spine surgery has also been described as a risk factor for brachial plexopathy.11 , 12 However, within the short period of COVID-19 literature, there have been several reports of patients developing brachial plexopathy following prone positioning (Table 4 ). Douglas et al. studied 61 COVID-19 patients treated in the prone position for severe ARDS, finding 5 brachial plexopathies (8.2%), noting an unclear determination of whether this was due to the virus, prone positioning, or the critical illness itself.13 Miller et al. identified 15 COVID-19 patients and 30 limbs with peripheral nerve injuries following prone positioning in the ICU, finding the most common injury being to the ulnar nerve.14 Malik et al. reviewed 12 patients with 21 peripheral nerve injury sites, a majority being in the upper extremity.7 High rate of neurologic injury was found at the ulnar nerve in 6 cases and the brachial plexus in 2 cases.7 Authors of these studies do recognize the high rate of injury may be multifactorial rather than solely position-related as their cohort demonstrates high rates of diabetes, obesity, and advanced age, which are factors for both severe COVID-19 infection as well as peripheral neuropathy.3 , 17 , 18
Table 4.
Literature Review of COVID-19-Related Peripheral Neuropathy Following Prone Positioning
| Study | Design | Findings |
|---|---|---|
| Douglas et al.13 | Single-center retrospective study | 61 patients with COVID-19 treated with prone position ventilation, 5 (8.2%) brachial plexopathies |
| Miller et al.14 | Single-center retrospective study | 114 patients with treated with prone positioning, 15 (13.2%) patients and 30 peripheral neuropathies, most common was ulnar nerve (12/30) followed by cords of brachial plexus (10/30) |
| Malik et al.7 | Single-center retrospective study | 12 patients treated with prone position, 6 (50%) with ulnar neuropathy and 2 (16.7%) with brachial plexopathy |
| Diprose et al.15 | Case report | 55F, BMI 42.6, bilateral upper limb neuropathies with comprised of individual nerve lesions affecting axillary and suprascapular nerves, sparing brachioradialis and biceps. |
| Sayegh et al.16 | Case series | Single center case series noting ulnar neuropathy in 3 patients following prone positioning |
COVID-19, Coronavirus Disease 2019; BMI, body mass index.
Consistent with our series of brachial plexopathy patients, the evidence against prone positioning alone was reported by Michaelson et al., where only 1 of 4 cases of brachial plexopathy was positioned prone.19 Patients with brachial plexopathy in their series were also noted to have an incomplete plexopathy picture with sparing of axillary and suprascapular nerves. Han et al. also report on a patient with neuropathy who contracted COVID-19, requiring intubation, but did not undergo prone positioning.20 Their patient was noted to be in a hypercoagulable state with elevated D-dimers and, on day 24, developed a rash that was consistent with thrombotic microvascular injury. Four days later, he was noted to have severe weakness of the left upper extremity, with sparing of the deltoid, infraspinatus, and pectoral muscles.20 Similar to our patients with complete and partial plexopathies, electrodiagnostic studies from Han et al. noted a patchy pattern of axonotmesis with sparing of some fascicles and severe denervation of the other fascicles that run through the same trunks and cords.20 With these findings, Michaelson et al. and Han et al. postulated that an uncontrolled systemic inflammation resulting in hypercoagulation may have led to the development of microthombotic angiopathy of the vasa nervorum about the brachial plexus leading to paucifascicular infarctions and subsequent plexopathy.19 Needham et al. have also described 11 patients with a mononeuritis multiplex with pathology most similar to vasculitic neuropathies given multifocal sites alongside their associated electrodiagnostic evidence.21 Similarly, in our series of patients, several had incomplete pan-brachial plexopathy with severe involvement of nerves at the trunk, cord, and branch level alongside both diffuse and patchy electrodiagnostic findings.
Another pathophysiologic consideration may be in relation to neuralgic amyotrophy during which, in addition to mechanical vulnerability in the intubated ICU environment, an auto-immune trigger from the viral infection, leads to neuronal injury.22 Impact of peripheral nerves may also occur directly through the angiotensin-converting enzyme 2 receptor, which has been identified as the functional receptor for Sars-CoV2, with the expression of ACE2 on neurons.23, 24, 25, 26 Others have reported that COVID-19 has been associated with acute inflammatory demyelinating polyneuropathy, increasing the vulnerability of peripheral nerves to injury.27 In addition, multifactorial considerations such as obesity, age, diabetes, hypovolemia, or ICU interventions through the use of muscle paralytic agents, long periods of sedation, and mechanical ventilation may all be involved in such peripheral neuropathy complications associated with COVID-19.11 , 28, 29, 30, 31 Taken together, a double crush phenomenon of prone positioning in addition to a systemic, metabolic, or direct viral insult to the peripheral nerve secondary to the viral infection may be more plausible as opposed to prone positioning alone in the etiology and rise of brachial plexus injuries.20 , 32, 33, 34
We recognize the limitations of this study and those inherent to retrospective analyses. The patient cohort is small, with only 11 patients identified in 3 institutions. As these patients were referred for tertiary care, documentation of their prior critical care course or details on positioning protocols were limited, with most only detailing prone positioning and no other forms of positioning (i.e., lateral decubitus, etc.). In addition, accessory information on instances of hypoxic respiratory failure, hypotension, hypovolemia, or agitation and use of restraints that all may influence the integrity of the peripheral nerves was limited. The time from recognition of the upper extremity neuropathy to evaluation by a specialist was prolonged (9.5 months) coinciding with a delay in electrodiagnostic evaluation and may have represented the difficulty of obtaining specialty care during the pandemic. We recommend specialist evaluation at the first evidence of peripheral nerve or brachial plexopathy recognition with a detailed exam and electrodiagnostic studies. These limitations notwithstanding, this multicenter series provides additional information regarding the development of neuropathies following severe COVID-19 infection.
Conclusion
This series provides insight that both traumatic and atraumatic causes of peripheral neuropathy may be associated with COVID-19 infections. Furthermore, there may be an interplay of traumatic and atraumatic etiologies whereby the traumatic disturbance of the plexus from prone positioning with shoulder abduction align with the physiologic vulnerability of the peripheral nerves during infection. This physiologic vulnerability stems from either a hypercoagulation state and the development of microthrombotic angiopathy with vascular compromise, a direct viral invasion, or an autoantibody targeting of the nervous tissue.35 Ongoing treatments have been nonoperative in nature, with some patients demonstrating signs of reinnervation. As we proceed further in the recovery process for patients infected with COVID-19, recognizing long-term complications and optimal treatments for these pathologies become necessary. Ongoing follow-up with the development of larger observational and longitudinal studies is needed to understand the course and recovery of COVID-19 related neuropathies.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranucci M., Ballotta A., di Dedda U., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sánchez-Soblechero A., García C.A., Sáez Ansotegui A., et al. Upper trunk brachial plexopathy as a consequence of prone positioning due to SARS-CoV-2 acute respiratory distress syndrome. Muscle Nerve. 2020;62:E76–E78. doi: 10.1002/mus.27055. [DOI] [PubMed] [Google Scholar]
- 4.Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattinoni L., Tognoni G., Pesenti A., et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 6.Guérin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 7.Malik G.R., Wolfe A.R., Soriano R., et al. Injury-prone: peripheral nerve injuries associated with prone positioning for COVID-19-related acute respiratory distress syndrome. Br J Anaesth. 2020;125:e478–e480. doi: 10.1016/j.bja.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugliera L., Filippi M., del Carro U., et al. Nerve Compression injuries after prolonged prone position ventilation in patients with SARS-CoV-2: a case series. Arch Phys Med Rehabil. 2021;102:359–362. doi: 10.1016/j.apmr.2020.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franz C.K., Murthy N.K., Malik G.R., et al. The distribution of acquired peripheral nerve injuries associated with severe COVID-19 implicate a mechanism of entrapment neuropathy: a multicenter case series and clinical feasibility study of a wearable, wireless pressure sensor. J Neuroeng Rehabil. 2022;19:108. doi: 10.1186/s12984-022-01089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goettler C.E., Pryor J.P., Reilly P.M. Brachial plexopathy after prone positioning. Crit Care. 2002;6:540–542. doi: 10.1186/cc1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uribe J.S., Kolla J., Omar H., et al. Brachial plexus injury following spinal surgery. J Neurosurg Spine. 2010;13:552–558. doi: 10.3171/2010.4.SPINE09682. [DOI] [PubMed] [Google Scholar]
- 12.DePasse J.M., Palumbo M.A., Haque M., Eberson C.P., Daniels A.H. Complications associated with prone positioning in elective spinal surgery. World J Orthop. 2015;6:351–359. doi: 10.5312/wjo.v6.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas I.S., Rosenthal C.A., Swanson D.D., et al. Safety and outcomes of prolonged usual care prone position mechanical ventilation to treat acute coronavirus disease 2019 hypoxemic respiratory failure. Crit Care Med. 2021;49:490–502. doi: 10.1097/CCM.0000000000004818. [DOI] [PubMed] [Google Scholar]
- 14.Miller C., O’Sullivan J., Jeffrey J., Power D. Brachial plexus neuropathies during the COVID-19 pandemic: a retrospective case series of 15 patients in critical care. Phys Ther. 2021;101:pzaa191. doi: 10.1093/ptj/pzaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diprose W.K., Bainbridge L., Frith R.W., Anderson N.E. Bilateral upper limb neuropathies after prone ventilation for COVID-19 pneumonia. Neurol Clin Pract. 2021;11:e211–e213. doi: 10.1212/CPJ.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayegh M.J., Larsen C.G., Pinpin C., Intravia J.M., Nellans K.W. Ulnar neuropathy after intermittent prone positioning for COVID-19 infection: a preliminary report of 3 cases. JBJS Case Connect. 2021;11 doi: 10.2106/JBJS.CC.20.00729. [DOI] [PubMed] [Google Scholar]
- 17.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxen M.A. Literature review for office-based anesthesia. Anesth Prog. 2020;67:60–62. doi: 10.2344/0003-3006-67.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaelson N.M., Malhotra A., Wang Z., et al. Peripheral neurological complications during COVID-19: a single center experience. J Neurol Sci. 2022;434:120118. doi: 10.1016/j.jns.2021.120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han C.Y., Tarr A.M., Gewirtz A.N., et al. Brachial plexopathy as a complication of COVID-19. BMJ Case Rep. 2021;14:e237459. doi: 10.1136/bcr-2020-237459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Needham E., Newcombe V., Michell A., et al. Mononeuritis multiplex: an unexpectedly frequent feature of severe COVID-19. J Neurol. 2021;268:2685–2689. doi: 10.1007/s00415-020-10321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Alfen N. Clinical and pathophysiological concepts of neuralgic amyotrophy. Nat Rev Neurol. 2011;7:315–322. doi: 10.1038/nrneurol.2011.62. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez A., Amirianfar E., Mason M.C., Huang L., Jose J., Tiu T. Extended neuralgic amyotrophy syndrome in a confirmed COVID-19 patient after intensive care unit and inpatient rehabilitation stay. Am J Phys Med Rehabil. 2021;100:733–736. doi: 10.1097/PHM.0000000000001795. [DOI] [PubMed] [Google Scholar]
- 24.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 25.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell Mol Neurobiol. 2022;42:305–309. doi: 10.1007/s10571-020-00915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koralnik I.J., Tyler K.L. COVID-19: a global threat to the nervous system. Ann Neurol. 2020;88:1–11. doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jone H.D. Ulnar nerve damage following general anaesthetic. Anaesthesia. 1967;22:471–475. doi: 10.1111/j.1365-2044.1967.tb02768.x. [DOI] [PubMed] [Google Scholar]
- 29.Chung I., Glow J.A., Dimopoulos V., et al. Upper-limb somatosensory evoked potential monitoring in lumbosacral spine surgery: a prognostic marker for position-related ulnar nerve injury. Spine J. 2009;9:287–295. doi: 10.1016/j.spinee.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Brunette K.E.J., Hutchinson D.O., Ismail H. Bilateral brachial plexopathy following laparoscopic bariatric surgery. Anaesth Intensive Care. 2005;33:812–815. doi: 10.1177/0310057X0503300619. [DOI] [PubMed] [Google Scholar]
- 31.Zhou C., Wu L., Ni F., Ji W., Wu J., Zhang H. Critical illness polyneuropathy and myopathy: a systematic review. Neural Regen Res. 2014;9:101–110. doi: 10.4103/1673-5374.125337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toscano G., Palmerini F., Ravaglia S., et al. Guillain–barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Eijk J.J.J., Groothuis J.T., van Alfen N. Neuralgic amyotrophy: an update on diagnosis, pathophysiology, and treatment. Muscle Nerve. 2016;53:337–350. doi: 10.1002/mus.25008. [DOI] [PubMed] [Google Scholar]