Abstract
Background
The Coronavirus disease-2019 (COVID-19) pandemic continues, and the death toll continues to surge. Ozone therapy has long been used in the treatment of a variety of infectious diseases, probably through its antioxidant properties and the supply of oxygen to hypoxic tissues. This systematic review and meta-analysis aimed to determine the efficacy of ozone on mortality in patients with COVID-19.
Methods
A systematic search was made of PubMed, Embase, Cochrane Library, and clinicaltrials.gov, without language restrictions. Prospective controlled trials on treatment of COVID-19 with ozone, compared with placebo or blank, were reviewed. Studies were pooled to risk ratios (RRs) and weighted mean differences (WMDs), with 95% confidence intervals (CIs).
Results
Eight trials (enrolling 371 participants) met the inclusion criteria. Ozone therapy showed significant effects on mortality (RR 0.38, 95% CI 0.17–0.85; P = 0.02), length of hospital stay (WMD −1.63 days, 95% CI −3.05 to −0.22 days; P = 0.02), and polymerase chain reaction (PCR) positivity (RR 0.07, 95% CI 0.01–0.34; P = 0.001).
Conclusions
Ozone therapy significantly reduced mortality, PCR positivity, and length of stay in hospitalized patients with COVID-19. Ozone therapy should be considered for COVID-19 patients.
Keywords: Mortality, Ozone, COVID-19, Meta-analysis
Graphical Abstract
1. Introduction
The Coronavirus disease-2019 (COVID-19) pandemic is the worst pandemic in more than 100 years, causing numerous infections and deaths worldwide.1 Despite the use of multiple drugs with different mechanisms, mortality from COVID-19 remains high, especially in older adults.1, 2 The mortality rate increases significantly with age, even up to 30% in patients aged 85 years or older.2 Therefore, there is an urgent need for treatments, pharmacological or otherwise, that can reduce mortality.
Medical ozone therapy is a complementary medical treatment primarily practiced in Europe and is believed to have immunomodulatory and antioxidant effects.3, 4 Ozone therapy has long been used in the treatment of a variety of infectious diseases,5 probably through its antioxidant properties and the supply of oxygen to hypoxic tissues.6, 7 In addition, ozone has shown an inhibitory effect on viral replication, creating great interest in the possibility of using this non-pharmacological adjunct in the treatment of COVID-19.4, 6, 8 Several clinical controlled trials have assessed the effects of ozone in hospitalized COVID-19 patients, and their results differed.
The aim of the present study therefore was to perform a systematic review and meta-analysis of controlled trials in order to determine the efficacy of ozone therapy on mortality in patients with COVID-19.
2. Methods
2.1. Data sources and search strategy
This systematic review and meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.9 The protocol was previously registered in March 2022 in the PROSPERO database (Review register: CRD42022320948). The PubMed, Embase, Cochrane Library, and clinicaltrials.gov were searched for studies up to March 28, 2022.
2.2. Study selection
To be eligible for inclusion in the meta-analysis studies had to meet the following criteria: (a) inclusion of hospitalized COVID-19 patients being 18 years or older; (b) polymerase chain reaction (PCR) positive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); and (c) use of a controlled design to make a comparison of ozone therapy with placebo or blank. The search strings used for the databases were (“COVID-19” OR “SARS-CoV-2” OR “SARS-CoV-19” OR “novel coronavirus 2019” OR “novel coronavirus pneumonia”) AND (“ozone” OR “autohemotherapy” OR “ozonization” OR “ozonized”). The reference lists of any relevant review articles were also screened to identify studies that might have been missed in this search. No language restrictions were applied to our study selection process. The full search strategies for all databases are provided in Table S1.
2.3. Data extraction and quality assessment
Two reviewers independently screened articles according to the inclusion criteria. The reviewers compared selected studies and differences were resolved by consensus. Data tables were used to collect all relevant data from texts, tables and figures of each included trial, including author, year of publication or last update posted, patient number and age, body mass index (BMI), types of ozone therapy, and outcomes such as mortality, length of hospital stay, PCR positivity, intensive care unit (ICU) admission, and tracheal intubation. Study quality was assessed using the Detsky Quality Assessment Scale.10, 11, 12, 13 This is a 20-point scale for studies with statistically significant results and a 21-point scale for studies without statistically significant results.
2.4. Risk of bias of included trials
Two reviewers independently assessed the risk of bias using the Cochrane collaboration risk of bias tool for RCTs,14 which considers allocation sequence generation, concealment of allocation, masking of participants and investigators, incomplete outcome reporting, selective outcome reporting, and other sources of bias. The Newcastle-Ottawa Scale15 was used to assess the risk of bias of observational studies, and full details are provided in Table S2. Disagreements were resolved through negotiation.
2.5. Outcomes
The primary outcome of this meta-analysis was mortality. Secondary outcomes were length of hospital stay, PCR positivity after treatment, ICU admission rate, and tracheal intubation rate.
2.6. Data synthesis and statistical analysis
Meta-analyses were conducted where applicable; otherwise, outcomes were presented in narrative form. Data were analyzed using the RevMan Version 5.4.1 (The Cochrane Collaboration). Next, risk ratios (RRs) for discontinuous outcomes, and weighted mean differences (WMDs) for continuous outcomes, with corresponding 95% confidence intervals (CIs) were computed for individual trials. Chi-squared and Higgins I2 tests were used to assess heterogeneity among included trials. If significant heterogeneity (p ≤ 0.10 for Chi-squared test results or I2 ≥ 50%) was obtained, we used a random-effects model, otherwise a fixed-effects model was used. And a P value < 0.05 was taken to indicate statistical significance. The P value of Egger’s linear regression test16, 17 (STATA version 12.0) was used to assess the presence of publication bias in included studies for each outcome.
2.7. Certainty of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) method was used to grade the quality or certainty of the outcomes and the strength of recommendations.18
3. Results
3.1. Study selection and characteristics
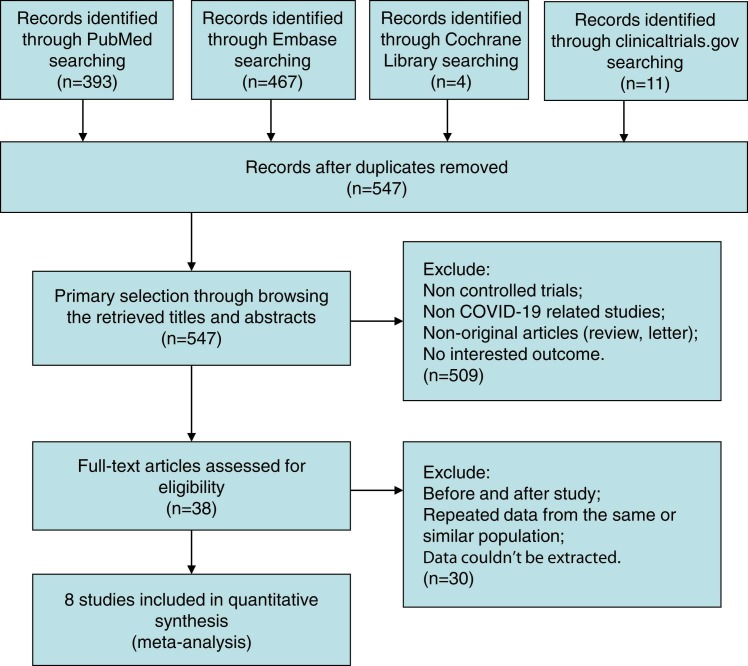
Of 875 trials recognized by the initial search, 38 were retrieved for more detailed assessment, and 8 trials5, 19, 20, 21, 22, 23, 24, 25 were included in this meta-analysis ( Fig. 1). Baseline characteristics of trials included in this meta-analysis are shown in Table 1. A total of 371 patients were included: 197 assigned to the ozone treatment groups and 174 to the control groups. The risk of bias results are summarized in Fig. S1 and Table S2.
Fig. 1.
Flow chart for selection of studies.
Table 1.
Baseline characteristics of trials included in meta-analysis.
| Study (Ref. #) |
Year | Quality Score | Randomization | Types of ozone therapy | n | Age, years (SD) | Male, % | BMI, kg/m2 (SD) |
|---|---|---|---|---|---|---|---|---|
| Araimo19 | 2021 | 17 | Randomized | Autohemotherapy Blank |
14 14 |
63.3 (12.1) 60.1 (14.4) |
64 50 |
28.2 (4.6) 28.9 (3.2) |
| Çolak5 | 2021 | 14 | Non-randomized | Autohemotherapy Blank |
37 18 |
58.0 (16.3) 64.7 (10.4) |
51.4 55.6 |
NR NR |
| Dengiz20 | 2022 | 16 | Randomized | Nebulization Blank |
15 15 |
51.6 (16.9) ^ | 60 53 |
NR NR |
| Fernández-Cuadros21 | 2021 | 14 | Non-randomized | Intra-rectal Blank |
14 14 |
84.4 (9.5) 83.0 (12.6) |
86 50 |
NR NR |
| Hernández22 | 2021 | 12 | Non-randomized | Autohemotherapy Blank |
9 9 |
64 (11) 71 (18) |
78 67 |
26.2 (4.5) 29.5 (7.1) |
| Shah23 | 2021 | 15 | Randomized | Intra-rectal + Autohemotherapy Blank |
30 30 |
44.0 (8.7) 43.6 (9.7) |
87 73 |
NR NR |
| Sozio24 | 2021 | 17 | Randomized | Autohemotherapy Blank |
48 44 |
63.5 (12.5) 64.2 (14.1) |
64.6 54.5 |
26.6 (4.5) 27.4 (6.0) |
| Tascini25 | 2021 | 12 | Non-randomized | Autohemotherapy Blank |
30 30 |
57 (12) 65 (13) |
77 60 |
NR NR |
Abbreviations: BMI, body mass index; NR, not reported; SD, standard deviation. ^ Average value of both groups.
3.2. Mortality
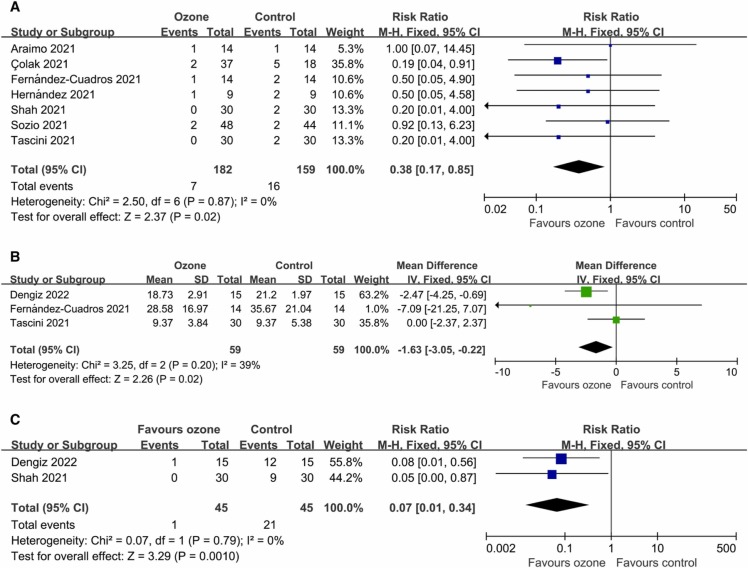
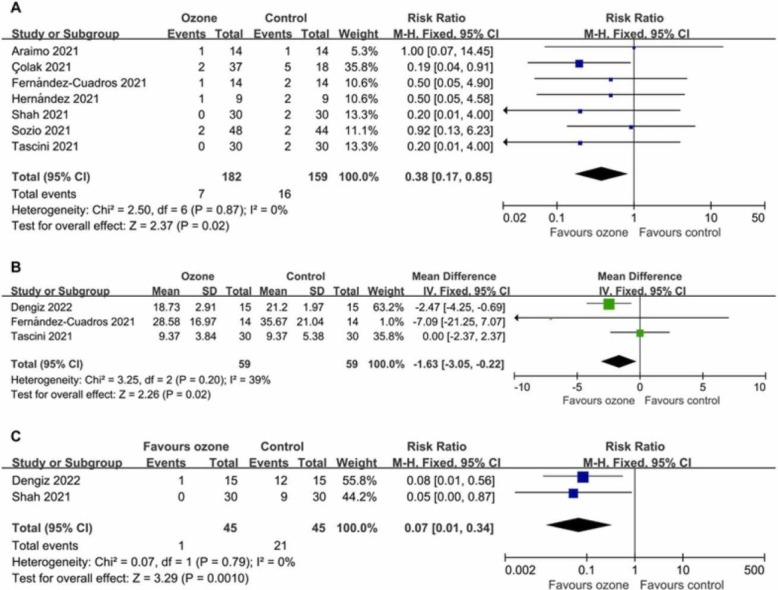
Data on mortality were available from seven controlled trials (341 patients). Compared with the control conditions, the mortality was significantly lower in the ozone therapy groups (RR 0.38, 95% CI 0.17–0.85; P = 0.02 [ Fig. 2A]), with a rate of 3.85% versus 10.06%. There was no significant heterogeneity (I2 = 0%; P = 0.87). Egger’s test (P = 0.736) did not show evidence of publication bias.
Fig. 2.
Forest plot assessing the efficacy of ozone therapy on (A) mortality, (B) length of hospital stay, and (C) PCR positivity.
3.3. Length of hospital stay
Data on length of hospital stay were available from three trials (118 patients). The length of hospital stay was significantly shorter in the ozone groups (WMD −1.63 days, 95% CI −3.05 to −0.22 days; P = 0.02 [Fig. 2B]). There was no significant heterogeneity (I2 = 39%; P = 0.20). Egger’s test (P = 0.335) did not show evidence of publication bias.
3.4. PCR positivity
The number of patients with PCR positivity after treatment were extracted from two studies (90 patients). The PCR positive rate was significantly lower in the ozone therapy groups (RR 0.07, 95% CI 0.01–0.34; P = 0.001 [Fig. 2C]), with a rate of 2.22% versus 46.67%. There was no significant heterogeneity (I2 = 0%; P = 0.79).
3.5. ICU admission
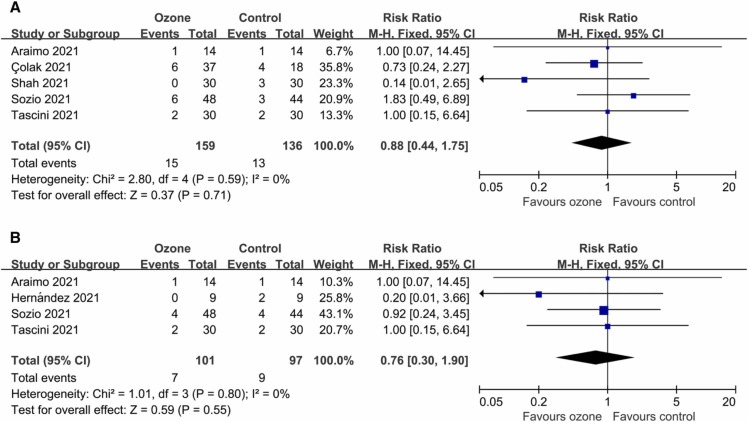
Five controlled trials reported data on ICU admission (295 patients). There was no statistically significant difference in ICU admission rates between the two groups (RR 0.88, 95% CI 0.44–1.75; P = 0.71 [ Fig. 3A]), with a proportion of 9.43% versus 9.56%. There was no significant heterogeneity (I2 = 0%; P = 0.59). Egger’s test (P = 0.453) did not show evidence of publication bias.
Fig. 3.
Forest plot assessing the efficacy of ozone therapy on (A) ICU admission rates, and (B) tracheal intubation rates.
3.6. Tracheal intubation
The number of patients with tracheal intubation were extracted from four studies (198 patients). There was no statistically significant difference in the rate of tracheal intubation between the two groups (RR 0.76, 95% CI 0.30–1.90; P = 0.55 [Fig. 3B]), with a proportion of 6.93% versus 9.28%. There was no significant heterogeneity (I2 = 0%; P = 0.80). Egger’s test (P = 0.411) did not show evidence of publication bias.
3.7. Certainty of evidence
The GRADE assessment for the certainty of evidence for primary and secondary outcomes is summarized in Table S3.
4. Discussion
To our knowledge, this meta-analysis is the first designed specifically to evaluate the efficacy of ozone therapy in patients with COVID-19. Based on the present results, we observed that ozone significantly reduced mortality and PCR positivity, and shortened hospital stays.
COVID-19 is caused by SARS-CoV-2 and has caused a global pandemic. Although most patients have mild symptoms, further development of acute respiratory distress syndrome (ARDS) has been reported in approximately 20% of hospitalized patients with a more severe clinical presentation.26 The main causes of COVID-19-related deaths are respiratory failure, excessive inflammation, cytokine storm or multiple organ failure.5 Healthcare systems in most countries are overwhelmed as global COVID-19 surge continues. Therefore, there is an urgent need for safely, effective and accessible treatment to stop disease progression and shorten hospital stays, thereby preventing the collapse of the healthcare system.
Ozone therapy has been extensively researched and used for over a century,27 and its common types include autohemotherapy, nebulization, non-invasive rectal, mucosal, intramuscular/intraarticular/intradiscal/paravertebral injection23. Several studies6, 19 have explored possible anti-COVID-19 mechanisms of ozone therapy: (1) improving oxygen release in hypoxic tissues, (2) modulating antioxidant balance and inflammatory responses to prevent cytokine storm, and (3) inhibiting viral replication. Ozone therapy is very cheap and safe and does not develop resistance28, so it may play an important role in the treatment of COVID-19, especially for patients in low- and middle-income countries.21
The included studies used several different types of ozone therapy, and all studies lacked blinding which may have exaggerated treatment effects. Future large double-blind RCTs are needed to determine which type is more effective.
This study met most of the methodological criteria recommended for systematic reviews and meta-analyses.29 However, some limitations need to be considered when interpreting the results of this study. Firstly, some included trials had small sample sizes, which may have reduced the power of the results. Secondly, some included trials were non-randomized. Finally, this meta-analysis was not patient-level, so the results should be considered provisional.
5. Conclusions
Treatment with ozone reduced mortality, PCR positivity, and length of stay in hospitalized COVID-19 patients. Ozone therapy should be considered for patients with COVID-19.
Funding
No funding was received for this research.
Author contributions
All authors, led by D.H., were involved in the concept and protocol design of the meta-analysis. W.S. and G.W. screened the titles and abstracts and extracted data from the articles. Y.W. was primarily responsible for statistical analyses. D.H. was primarily involved in the interpretation of the quality data. All authors contributed to interpreting the results. G.W. and D.H accessed and verified the data. All authors contributed to the writing of the article and approved its submission. D.H. was responsible for the decision to submit the article.
Conflict of interest
None of the authors has a conflict of interest to declare.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ctim.2022.102907.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Qin J., Wang G., Han D. Benefits of plasma exchange on mortality in patients with COVID-19: a systematic review and meta-analysis. Int J Infect Dis IJID Publ Int Soc Infect Dis. 2022;122:332–336. doi: 10.1016/j.ijid.2022.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Rowen R.J. Ozone and oxidation therapies as a solution to the emerging crisis in infectious disease management: a review of current knowledge and experience. Med Gas Res. 2019;9:232–237. doi: 10.4103/2045-9912.273962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valdenassi L., Franzini M., Ricevuti G., Rinaldi L., Galoforo A.C., Tirelli U. Potential mechanisms by which the oxygen-ozone (O2-O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur Rev Med Pharmacol Sci. 2020;24 doi: 10.26355/eurrev_202004_20976. 4059-61. [DOI] [PubMed] [Google Scholar]
- 5.Çolak Ş., Genç Yavuz B., Yavuz M., et al. Effectiveness of ozone therapy in addition to conventional treatment on mortality in patients with COVID-19. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez-Sánchez G., Schwartz A., Donna V.D. Potential cytoprotective activity of ozone therapy in SARS-CoV-2/COVID-19. Antioxidants. 2020:9. doi: 10.3390/antiox9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavo B., Pérez J.L., López L., et al. Effect of ozone therapy on muscle oxygenation. J Altern Complement Med. 2003;9:251–256. doi: 10.1089/10755530360623365. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z., Dong M., Hu K. A preliminary evaluation on the efficacy of ozone therapy in the treatment of COVID-19. J Med Virol. 2020;92 doi: 10.1002/jmv.26040. 2348-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(264–9):W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Detsky A.S., Naylor C.D., O'Rourke K., McGeer A.J., L'Abbé K.A. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;45:255–265. doi: 10.1016/0895-4356(92)90085-2. [DOI] [PubMed] [Google Scholar]
- 11.Shang W., Wang G., Wang Y., Han D. The safety of long-term use of inhaled corticosteroids in patients with asthma: a systematic review and meta-analysis. Clin Immunol. 2022;236 doi: 10.1016/j.clim.2022.108960. [DOI] [PubMed] [Google Scholar]
- 12.Qin J., Wang G., Han D. Benefits of LAMA in patients with asthma-COPD overlap: a systematic review and meta-analysis. Clin Immunol. 2022;237 doi: 10.1016/j.clim.2022.108986. [DOI] [PubMed] [Google Scholar]
- 13.Shang W., Zhang Y., Liu L., Chen F., Wang G., Han D. Benefits of continuous positive airway pressure on blood pressure in patients with hypertension and obstructive sleep apnea: a meta-analysis. Hypertens Res Off J Jpn Soc Hypertens. 2022 doi: 10.1038/s41440-022-00954-9. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ Clin Res Ed. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Shang W., Zhang Y., Han D. Benefits of tolvaptan on early dyspnea relief in patients with acute heart failure: a meta-analysis. Clin Cardiol. 2022 doi: 10.1002/clc.23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin J., Wang G., Han D. Selexipag in patients with pulmonary hypertension: a systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol. 2022 doi: 10.1016/j.cpcardiol.2022.101466. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt G., Oxman A.D., Akl E.A., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Araimo F., Imperiale C., Tordiglione P., et al. Ozone as adjuvant support in the treatment of COVID-19: a preliminary report of probiozovid trial. J Med Virol. 2021;93 doi: 10.1002/jmv.26636. 2210-20. [DOI] [PubMed] [Google Scholar]
- 20.Dengiz E., Özcan Ç., Güven Y., et al. Ozone gas applied through nebulization as adjuvant treatment for lung respiratory diseases due to COVID-19 infections: a prospective randomized trial. Med Gas Res. 2022;12:55–59. doi: 10.4103/2045-9912.326001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Cuadros M.E., Albaladejo-Florín M.J., Álava-Rabasa S., et al. Compassionate use of rectal ozone (O(3)) in severe COVID-19 pneumonia: a case-control study. SN Compr Clin Med. 2021:1–15. doi: 10.1007/s42399-021-00849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández A., Viñals M., Pablos A., et al. Ozone therapy for patients with COVID-19 pneumonia: preliminary report of a prospective case-control study. Int Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah M., Captain J., Vaidya V., et al. Safety and efficacy of ozone therapy in mild to moderate COVID-19 patients: a phase 1/11 randomized control trial (SEOT study) Int Immunopharmacol. 2021;91 doi: 10.1016/j.intimp.2020.107301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sozio E., De Monte A., Sermann G., et al. CORonavirus-19 mild to moderate pneumonia management with blood ozonization in patients with respiratory failure (CORMOR) multicentric prospective randomized clinical trial. Int Immunopharmacol. 2021;98 doi: 10.1016/j.intimp.2021.107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tascini C., Sermann G., Pagotto A., et al. Blood ozonization in patients with mild to moderate COVID-19 pneumonia: a single centre experience. Intern Emerg Med. 2021;16 doi: 10.1007/s11739-020-02542-6. 669-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30211-7. 507-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elvis A.M., Ekta J.S. Ozone therapy: a clinical review. J Nat Sci Biol Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cattel F., Giordano S., Bertiond C., et al. Ozone therapy in COVID-19: A narrative review. Virus Res. 2021;291 doi: 10.1016/j.virusres.2020.198207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material