Abstract
Previous results have demonstrated that nonspecific protective immunity against lethal Francisella tularensis live vaccine strain (LVS) or Listeria monocytogenes infection can be stimulated either by sublethal infection with bacteria or by treatment with bacterial DNA given 3 days before lethal challenge. Here we characterize the ability of purified lipopolysaccharide (LPS) from F. tularensis LVS to stimulate similar early protective immunity. Treatment of mice with surprisingly small amounts of LVS LPS resulted in very strong and long-lived protection against lethal LVS challenge within 2 to 3 days. Despite this strong protective response, LPS purified from F. tularensis LVS did not activate murine B cells for proliferation or polyclonal immunoglobulin secretion, nor did it activate murine splenocytes for secretion of interleukin-4 (IL-4), IL-6, IL-12, or gamma interferon (IFN-γ). Immunization of mice with purified LVS LPS induced a weak specific anti-LPS immunoglobulin M (IgM) response and very little IgG; however, infection of mice with LVS bacteria resulted in vigorous IgM and IgG, particularly IgG2a, anti-LPS antibody responses. Studies using various immunodeficient mouse strains, including LPS-hyporesponsive C3H/HeJ mice, μMT− (B-cell-deficient) knockout mice, and IFN-γ-deficient mice, demonstrated that the mechanism of protection does not involve recognition through the Lpsn gene product; nonetheless, protection was dependent on B cells as well as IFN-γ.
Lipopolysaccharide (LPS), an integral component of the outer membrane of gram-negative bacteria, stimulates numerous immunobiological and pharmacological activities. During a bacterial infection, LPS may be recognized by host cells as a component of the bacterial surface, as well as following shedding of individual LPS molecules during bacterial growth or lysis. In mice, LPS purified from most pathogenic bacteria readily activates macrophages, B lymphocytes, neutrophils (32, 36), and T cells indirectly (41) for proliferation and/or production of a variety of cytokines and chemokines. Strains of inbred mice that are genetically hyporesponsive to LPS, such as C3H/HeJ, are paradoxically more susceptible to many gram-negative infections (38), indicating the importance of the molecule in influencing host-pathogen interactions. Overall, LPS recognition in mice has complex consequences and appears to be beneficial at lower doses of exposure but detrimental at higher doses.
Antibodies to LPS have been well studied both for diagnostic utility and for their contribution to protective immunity, particularly for extracellular bacteria such as Pseudomonas (7, 27). However, despite extensive study of immunobiological responses to LPS during infections such as those caused by salmonellae (31), the consequences of LPS recognition during infection with intracellular bacteria are less well understood. To determine the mechanisms of protective immunity operative against intracellular pathogens, we have characterized the murine protective immune response to the intracellular bacterium Francisella tularensis live vaccine strain (LVS). This small, gram-negative bacterium infects and replicates in macrophages and related cells (3, 17). LVS infections in mice are similar to human infections with fully virulent F. tularensis (39). Since survival of sublethal LVS infection leads to strong and easily measurable secondary protective immunity to LVS, we (8, 15, 17, 46) and others (2, 18, 40) have found the study of this infection in mice to be an informative in vivo model of immunity to intracellular pathogens. In contrast to the properties typically associated with LPS from many pathogens, LPS purified from LVS appears to lack many of the activities usually ascribed to this molecule. No traditional endotoxin has been associated with virulent F. tularensis (23). More recent reports indicated that purified LVS LPS was not endotoxic in d-galactosamine-sensitized mice (37) and failed to activate Limulus amoebocyte lysate (37). Further, LVS LPS also failed to stimulate human monocytes or peripheral blood lymphocytes to proliferate, produce tumor necrosis factor alpha (TNF-α), or produce interleukin-1 (IL-1) (37). Similarly, mouse peritoneal exudate macrophages treated with LVS LPS did not produce TNF-α or nitric oxide, and there was no increase in surface immunoglobulin expression by a mouse pre-B-cell line in response to LVS LPS (1). To date, the only reported biological activity of LVS LPS is activation of complement (21); no structural information is available.
On the other hand, in vivo experiments have suggested that LVS LPS contributes to the virulence of Francisella, in that LPS-defective Lpsd C3H/HeJ mice are reported to be more susceptible to LVS infection than Lpsn C3H/HeN (30). Francisella has apparently evolved the ability to undergo phase variation of LPS expression, such that F. tularensis normally expresses the “nontoxic” chemotype of LPS but occasionally switches to expression of a stimulatory chemotype of LPS that is characteristic of the closely related bacterium Francisella novicida (6); this indicates that regulated variation between LPSs of different biological properties confers a survival advantage on the bacterium. Further, detection of antibodies to Francisella LPS has been useful in diagnosis of human disease from natural infection (37, 42) as well as in demonstrating successful vaccination with LVS (44), indicating that Francisella LPS is immunogenic. Mice given repeated large doses of LVS LPS were protected against lethal LVS infection (19). The latter finding is particularly intriguing, since protection against intracellular pathogens has often been more difficult to achieve using killed bacteria or purified bacterial components than through infection with live attenuated organisms. Here, we report that treatment of mice with surprisingly small amounts of LVS LPS resulted in very strong and long-lived protection against lethal LVS challenge within 2 to 3 days. To understand the mechanism of protective immunity stimulated by LVS LPS against this intracellular infection, we performed a comprehensive characterization of the immunogenicity, polyclonal lymphocyte responses, and protective capacity of LVS LPS in mice.
MATERIALS AND METHODS
Animals.
Adult (6- to 12-week-old), specific-pathogen-free, male BALB/cByJ, C57BL/6J, and BALB/c.scid mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Male Igh6 (μMT− B-cell-deficient [25]) and male gamma interferon (IFN-γ) knockout (KO) mice (9) on a C57BL/6J background (>14 backcrosses) were purchased from the Induced Mutant Resource of The Jackson Laboratory. At least one mouse from each shipment of B-cell KO mice was sacrificed, and spleen cells were assessed by flow cytometry (see below), to confirm the phenotype of the mutation; no discrepancies were found. C3H/HeN, C3H/HeJ, and BALB/c.nu/nu mice were purchased from the Biological Resources Branch, Frederick Cancer Research and Development Center, National Cancer Institute (Frederick, Md.). The Lps phenotype of C3H/HeN and C3H/HeJ mice in each shipment was confirmed by testing LPS proliferative responses of spleen cells from randomly chosen mice in proliferation assays (see below; data not shown). All mice were housed in sterile micro-isolator cages in a barrier environment at the Center for Biologics Research and Evaluation (CBER), fed autoclaved food and water ad libitum, and routinely tested for common murine pathogens by a diagnostic service provided by the Division of Veterinary Services, CBER. The research described in this report was conducted in accordance with a protocol approved by the Animal Care and Use Committee of CBER.
Bacteria and growth conditions.
F. tularensis LVS (ATCC 29684; American Type Culture Collection, Manassas, Va.) was cultured on modified Mueller-Hinton (MH) agar plates or in modified MH broth (Difco Laboratories, Detroit, Mich.) supplemented with ferric pyrophosphate and IsoVitaleX (Becton Dickinson, Cockeysville, Md.) as previously described (4, 18). Listeria monocytogenes strain EGD (ATCC 15313), a gift from William Schwan, was cultured in brain heart infusion broth or plates (Difco). One-milliliter aliquots of bacteria were frozen in broth alone at −70°C and periodically thawed for use; viable bacteria were quantified by plating serial dilutions on MH agar plates. The number of CFU after thawing varied less than 5% over a 6-month period.
Bacterial infections.
Mice were given 0.5 ml intraperitoneally (i.p.) or 0.1 ml intradermally (i.d.) of the indicated dilution of LPS or LVS; actual doses of bacteria inoculated were simultaneously determined by plate count. All materials, including bacteria, were diluted in phosphate-buffered saline (PBS; BioWhittaker, Walkersville, Md.) containing <0.01 ng of endotoxin per ml. Mean time to death was calculated by arithmetic mean ± standard deviation for all mice within a group that died; surviving mice were not included in this calculation.
LPS and DNA reagents.
LVS LPS was purified from whole F. tularensis LVS bacteria as previously described (6, 45). Briefly, a 1.5-liter culture of F. tularensis LVS was grown in 4-liter flasks at 37°C for 48 h in Trypticase soy broth with cysteine. The cultures were centrifuged at 3,000 × g for 15 min, washed three times in PBS, once in methanol, and once in acetone, and then lyophilized. The LPS was then extracted by the hot phenol method of Westphal and Luderitz (45). After the crude LPS was treated with DNase, RNase, and proteinase K, the pellet was harvested by centrifugation at 100,000 × g three times for 12 h. The purified LPS concentration was determined by measuring dry weight, followed by resuspension in sterile endotoxin-tested water (6). Contamination by DNA and protein in the final preparation was below limits of detection (6), and these preparations of LPS had no activity in a traditional chromogenic Limulus amoebocyte assay (BioWhittaker) at concentrations of up to 50 μg/ml (data not shown). Escherichia coli O111 LPS, E. coli O55 LPS, and concanavalin A were purchased from Sigma (St. Louis, Mo.). E. coli K235 LPS and Salmonella enterica serovar Typhimurium LPS were purchased from Ribi Immunochem (Hamilton, Mont.). All LPS preparations were reconstituted in endotoxin-free PBS and stored at 4°C. The sequence of the oligonucleotide used, synthesized by the CBER Core Facility, was TCT CCC AGC GTG CGC CAT (designated oligo 1 in reference 16).
Proliferation assay.
Single-cell suspensions were prepared from spleens from the indicated donor mice. Erythrocytes were lysed using ammonium chloride, and viable cells were enumerated by exclusion of trypan blue. For enrichment of B cells, spleen cells were resuspended to a concentration of 108 cells/ml and treated with a cocktail of (each at 10 μg/ml) anti-Thy1.2 (30-H12), anti-CD4 (RM4-5), anti-CD8 (53-6.7), and anti-γ/δ T-cell receptor (GL3) antibodies (all purchased from Pharmingen, San Diego, Calif., and determined to be optimal in separate experiments) for 30 min on ice, followed by treatment with 1:10 rabbit complement (Pel-Freeze, Brown Deer, Wis.) for 30 min at 37°C. Aliquots of both the starting spleen cell populations and the final B-cell-enriched populations were analyzed by flow cytometry using a FACScan. Cells were stained using a panel of monoclonal antibodies including fluorescein isothiocyanate-conjugated-anti-B220 phycoerythrin (PE)-anti-CD4, PE-anti-CD8, PE-anti-CD11b, and PE-anti-γ/δ T-cell receptor antibodies (all purchased from Pharmingen and chosen to recognize different epitopes from those used in cytotoxicity where available) in both one- and two-color staining protocols. Optimal concentrations for staining with each lot of each fluorochrome-labeled antibody were determined in separate experiments. Gates were set for viable lymphocytes and monocytes according to forward and side scatter profiles. Starting spleen cell populations used were about 50% B220+, 5 to 8% CD11b+, 30% CD4+, 10% CD8+, and 1 to 3% γ/δ+. B-cell-enriched populations used for transfer were >92% B220+, approximately 5 to 8% CD11b+, and <1% CD4+, CD8+, or γ/δ+. After preparation, spleen cells were cultured at 2 × 105 per well in triplicate groups in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), 10 mM HEPES, 2 mM glutamine, 1 mM sodium pyruvate, 0.075% sodium bicarbonate, and 5 × 10−5 M 2-mercaptoethanol (all purchased from GIBCO/Life Technologies, Gaithersburg, Md.). Cells were cultured in 96-well tissue culture plates (Costar, Boston, Mass.) at 37°C in a humidified atmosphere of 5% CO2 in air. For determination of proliferation, [3H]thymidine (0.5 μCi/well; specific activity, 6.0 Ci/mmol; New England Nuclear, Boston, Mass.) was added 16 h before harvesting and determination of incorporated radioactivity.
Antibody transfer protocol.
Normal mouse serum (NMS) was obtained by bleeding normal BALB/cByJ mice from the lateral tail vein and pooling the resulting serum. Normal BALB/cByJ mice were then immunized with 10 μg of LPS i.d., and immune mouse serum (IMS) was obtained 3 or 30 days later. The endpoint anti-LPS antibody titers of the pools used in these experiments were <1:10 immunoglobulin M (IgM) and IgG for NMS, 1:160 IgM and <1:10 IgG for day 3 IMS, and 1:320 IgM and 1:160 IgG, determined by enzyme-linked immunosorbent assay (ELISA) using LPS-coated plates (35; see below). BALB/cByJ mice were given 0.5 ml of a 1:4 dilution of these sera i.p. 1 day before challenge with 103 LVS bacteria i.p. (35).
Characterization of antibody response.
Blood was obtained via the lateral tail vein from the indicated groups of mice both before (prebleed) and after LPS immunization or LVS infection. Results used pooled sera are shown here; care was taken to pool approximately equal quantities of blood from individual mice within a group before preparation of sera. Titers of specific anti-LVS serum antibodies were determined as previously described (35). Briefly, Immulon 1 plates were coated overnight with 5 × 106 live LVS bacteria, washed, and blocked; samples were added in twofold serial dilutions and incubated for 2 h at 37°C. After washing, enzyme-labeled antibodies (goat anti-mouse immunoglobulin; anti-IgM; or anti-IgG that detects IgG1, IgG2a, IgG2b, and IgG3), directly conjugated to horseradish peroxidase (Southern Biotechnology Birmingham, Ala.), followed by ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] peroxidase substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.), were added for detection. Plates were read for optical density at 405 nm (OD405) with an OD600 reference. The endpoint titer of IMS was defined as the lowest dilution of immune serum that had a mean OD405 value greater than the mean OD value of the matched dilution of NMS plus 3 standard deviations and also greater than 0.050 (35). The starting dilution for most assays was 1:20, and thus 1:20 is the limit of detection for all assays unless otherwise stated. To detect antibodies directed against LVS LPS, the assay was modified such that Immulon 1 plates were coated with LPS rather than whole LVS bacteria. LVS LPS was diluted to 2 μg/ml in PBS (pH 7.2), 100 μl was added to each well, and plates were incubated for 2 h at 37°C and overnight at 4°C. The remainder of the assay was performed as described above. Plates coated in this manner readily reacted with a previously described anti-Francisella monoclonal antibody, designated Fran 4 (33), confirming that LPS successfully adhered to Immulon 1 plates and that this monoclonal antibody reacts with F. tularensis LVS LPS. The levels of binding of serum obtained from germ-free BALB/c mice and normal BALB/cByJ mice to LPS- or LVS (35)-coated ELISA plates were identical, indicating that these mice do not have natural antibodies either to LVS LPS or to LVS itself.
ELISPOT assay.
Numbers of cytokine-secreting spleen cells, after 8 h of in vitro stimulation with the indicated DNA preparations, were determined by enzyme-linked immunospot (ELISPOT) assay as previously described (26). Briefly, microtiter plates were coated with primary anticytokine antibodies and blocked, and serial dilutions of single spleen cell suspensions were incubated for 8 h at 37°C. Cytokine-secreting spots were detected by the addition of secondary biotinylated anticytokine antibodies, followed by avidin-conjugated alkaline phosphatase and 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium solution (Kirkegaard & Perry).
RESULTS
Protection against lethal F. tularensis LVS infection induced by LVS LPS.
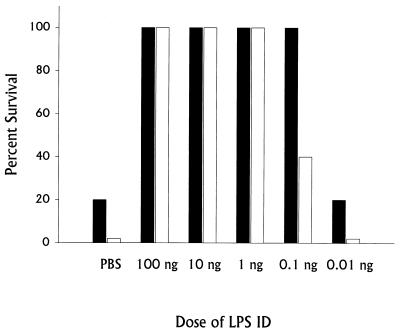
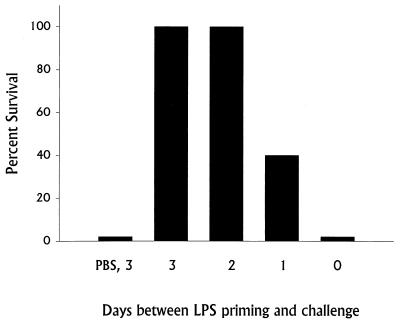
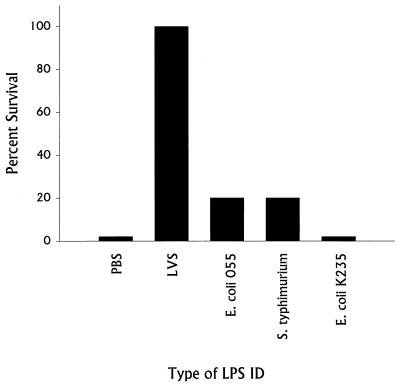
We have previously described a nonspecific, B-cell-dependent phase of innate immunity that leads to generation of strong protective immunity against lethal challenge very quickly after establishment of sublethal infection: normal mice (but not B-cell-deficient mice) given a sublethal dose of LVS i.d. on day 0 survive a subsequent lethal i.p. or intravenous challenge of over 106 50% lethal doses (LD50) given on day 3 (8, 13–15). To test whether LVS LPS has a role in this early protective immunity, BALB/cByJ mice were given various doses of LVS LPS i.d. on day 0 and challenged 3 days later with lethal doses of LVS, either 103 (1,000 LD50) or 104 (10,000 LD50) bacteria (Fig. 1). Very strong protection against lethal LVS infection was readily demonstrated. Remarkably, mice given doses as low as 0.1 ng of LVS LPS i.d. survived lethal LVS challenge, depending on the strength of the challenge given. In fact, mice given 100 ng of LVS LPS i.d. survived LVS challenge doses approaching 106 bacteria (1,000,000 LD50 [Fig. 2]). The time course of development of early protective immunity was identical to that previously observed using sublethal bacterial infection (13, 15), since all mice given LVS LPS 2 or 3 days before challenge with 104 LVS bacteria i.p. survived (Fig. 3). Very small amounts of anti-LPS antibodies, using either ELISA or Western blotting, were detected in serum from mice given 100 ng of LVS LPS i.d. 3 days earlier (see Materials and Methods), but anti-LPS antibodies could not be detected (titer of <1:10) by either method in serum from mice given 100 ng of LVS LPS i.d. 2 days earlier or at any time point in serum from mice given 1 ng of LVS LPS or less (data not shown). Further, in three experiments, 15 of 15 mice challenged with 104 LVS bacteria i.p. 3, 10, or 35 days after treatment with 100 ng of LVS LPS i.d. survived; however, in the same experiments none of 15 mice treated with 100 ng of LVS LPS i.d. and challenged 3, 10, or 35 days with 20 LD50 of L. monocytogenes survived. Early protection also could not be demonstrated using other types of LPS, in that mice given 100 ng of E. coli O55 LPS, Salmonella serovar Typhimurium LPS, or E. coli K235 LPS i.d. on day 0 and challenged with 103 LVS bacteria i.p. on day 3 did not survive (Fig. 4).
FIG. 1.
Dose response of protection against lethal LVS challenge stimulated by immunization with LVS LPS. Groups of five BALB/cByJ mice were given the indicated dose of LVS LPS (or PBS) i.d. Three days later all mice were challenged with either 103 LVS bacteria i.p. (black bars) or 104 LVS bacteria i.p. (open bars). Percent survival is shown for a single experiment. This experiment is representative of four experiments of similar design.
FIG. 2.
Strength of protection against lethal LVS challenge stimulated by immunization with LVS LPS. Groups of five BALB/cByJ mice were given 100 ng of LVS LPS (or PBS) i.d. Three days later all mice were challenged with the indicated number of LVS bacteria i.p. Percent survival is shown for a single experiment. This experiment is representative of three experiments of similar design.
FIG. 3.
Time course of development of protection against lethal LVS challenge stimulated by immunization with LVS LPS. Groups of five BALB/cByJ mice were given 100 ng of LVS LPS (or PBS) i.d. on the indicated day before all mice were challenged with 104 LVS bacteria i.p. Percent survival is shown for a single experiment. This experiment is representative of two experiments of similar design.
FIG. 4.
Ability of various types of LPS to stimulate protection against lethal LVS challenge. Groups of 5 BALB/cByJ mice were given 100 ng of the indicated LPS (or PBS) i.d.; three days later, all mice were challenged with 104 LVS bacteria i.p. Percent survival is shown for a single experiment. This experiment is representative of four experiments of similar design.
Determination of the cellular basis of protection against lethal infection stimulated by LVS LPS.
To determine the cellular basis of LVS LPS-stimulated protection, various immunocompromised and immunodeficient mice were immunized with 100 ng of LVS LPS i.d. and challenged with either 103 or 104 LVS bacteria i.p. on day 3 (Table 1). Both C3H/HeJ (Lpsn) and C3H/HeN (Lpsd) mice were fully protected by immunization with LVS LPS. Lymphocyte-deficient BALB/c.scid mice, B-cell KO mice, or IFN-γ KO mice treated with LVS LPS died within a week following lethal challenge, as did control (PBS-treated) mice. In contrast, T-cell-deficient BALB/c.nu/nu mice treated with LVS LPS survived lethal challenge for 2 to 3 weeks longer than control (PBS-treated) mice; these mice eventually succumbed to challenge after about 3 to 4 weeks (Table 1). Overall, 93% of B-cell KO mice (13 of 14 mice in three separate experiments) and 100% of IFN-γ KO mice (11 of 11 in three separate experiments) given 100 ng of LVS LPS i.d. died within a week following a 1,000- to 10,000-LD50 LVS challenge. On the other hand, only 20% of BALB/c.nu/nu mice given 100 ng of LVS LPS i.d. (3 of 15 mice in three separate experiments) died within a week following challenge with 1,000 to 10,000 LD50.
TABLE 1.
Stimulation of protection in LPS-defective and immunodeficient micea
| Mice | Amt of LPS given i.d. (ng) | No. of LVS bacteria given i.p. | No. of survivors/total | MTD |
|---|---|---|---|---|
| Expt 1 | ||||
| BALB/cByJ | None (PBS) | 103 | 0/5 | 6.2 ± 0.8 |
| BALB/cByJ | 100 | 103 | 5/5 | |
| C3H/HeJ | 100 | 103 | 4/4 | |
| C3H/HeN | 100 | 103 | 3/4 | 6 |
| Expt 2 | ||||
| BALB/cByJ | None (PBS) | 104 | 0/5 | 7.0 ± 0.0 |
| BALB/cByJ | 100 | 104 | 5/5 | |
| BALB/c.scid | 100 | 104 | 0/5 | 7.0 ± 0.0 |
| BALB/c.nu/nu | 100 | 104 | 0/5 | 26.2 ± 11.8 |
| C57BL/6J | 100 | 104 | 5/5 | |
| B-cell KO | 100 | 104 | 0/5 | 7.0 ± 0.0 |
| IFN-γ KO | 100 | 104 | 0/5 | 6.2 ± 0.5 |
The indicated mice (groups of five) were primed with PBS or LVS LPS i.d. on day 0 and challenged as indicated with 103 or 104 LVS bacteria i.p. on day 3. Actual priming and challenge doses were confirmed by plate count at the time of inoculation. Mice were observed for morbidity and mortality through day 60, at which time surviving mice were sacrificed to assess clearance of bacteria from spleens. MTD, mean time to death of those mice that died in relationship to the day of i.p. challenge. These experiments are representative of six total experiments of similar design.
Characterization of immune responses by B cells to F. tularensis LVS LPS.
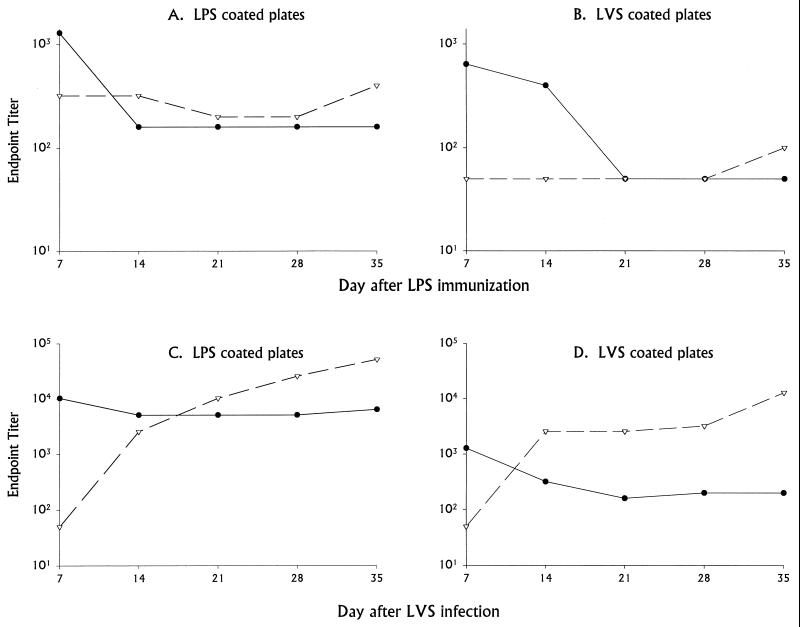
To begin to understand the basis of B-cell-dependent protection, we further characterized the immunobiological properties of LVS LPS, particularly those involving B-cell stimulation. Both specific and nonspecific responses to LVS LPS in mice were studied. First, the specific antibody response to LVS LPS was studied by immunizing BALB/cByJ mice with 100 ng of LPS i.d.; this dose was chosen following dose-response studies of protection (Fig. 1). Sequential serum samples were obtained at various time points after immunization with LPS, and serum titers of specific anti-LPS and anti-LVS bacterial antibodies were determined by ELISA. Binding of serum antibodies was assessed using both plates coated with LPS itself (the same preparation as that used for immunization; designated hereafter anti-LPS antibodies) and plates coated with whole LVS bacteria (designated hereafter anti-LVS antibodies). IgM anti-LPS antibodies were readily detected (using LPS-coated plates) by 7 days after immunization with LPS but declined thereafter (Fig. 5A). Small amounts of IgG anti-LPS antibodies were detected at all time points but did not increase noticeably with time. When the same serum samples were assayed on LVS-coated plates, IgM antibodies were detected at days 7 and 14, and IgG antibodies were detected only on day 35, all at low levels (Fig. 5B). Examination of the day 35 serum samples did not reveal a dominant subclass; when assayed on LPS-coated plates, levels of all were low: IgG1, 1:200; IgG2a, 1:100; IgG2b, 1:100; and IgG3, 1:200. The same samples assayed on LVS-coated plates had similar or lower titers (IgG1, <1:50; IgG2a, <1:50; IgG2b, 1:200; and IgG3, 1:200). There was no detectable anti-LPS antibody response in the serum of mice immunized with 1 or 0.1 ng of LVS LPS i.d. either 10 or 35 days after immunization; IgM and IgG titers of such sera, tested on either LVS LPS-coated plates or LVS-coated plates, were all <1:10.
FIG. 5.
Time course of specific antibody responses in BALB/cByJ mice immunized with LVS LPS compared to infection with LVS. Serum samples were obtained from five normal BALB/cByJ mice (prebleeds), and approximately equal amounts of blood from each mouse were pooled. Mice were then given 100 ng of LPS i.d. (A and B) or 104 LVS bacteria i.d. (C and D); actual bacterial doses were confirmed by plate count at the time of inoculation. On the indicated days after immunization or infection, pooled serum samples were obtained and tested by ELISA using plates coated with either purified LPS (A and C) or whole LVS bacteria (B and D). Antibodies were detected by the use of either horseradish peroxidase-labeled goat anti-mouse IgM (●) or anti-mouse IgG (▿) antibodies. Endpoint titers from a single representative experiment using pooled serum samples are shown; titers are defined in relationship to the binding of matched prebleed normal mouse serum, which was uniformly low and similar to that of germfree mouse serum (see Materials and Methods). This experiment is representative of three experiments of similar design.
For comparison, other mice were infected with 104 LVS bacteria i.d. at the same time as LPS immunization, and serum samples were assayed simultaneously on both LVS-coated plates (anti-LVS antibodies) and LPS-coated plates (anti-LPS antibodies). As previously described (35), IgM anti-LVS antibodies were readily detected using LVS-coated plates by day 7 in serum from mice given this sublethal infection, and levels declined slightly but remained detectable thereafter; IgG antibacterial antibodies were detected by 14 days after infection and increased thereafter (Fig. 5D). When the same serum samples were assayed on LPS-coated plates, very high amounts of IgM anti-LPS antibodies were detected throughout the course of the study, as were IgG anti-LPS antibodies from day 14 and beyond (Fig. 5C). When day 35 serum samples from LVS-infected mice were studied for isotype of IgG antibodies, the endpoint anti-LVS antibody titers in this experiment were quite high: IgG1, 1:800; IgG2a, 1:12,800; IgG2b, 1:6,400; and IgG3, 1:800. The same samples assayed on LPS-coated plates were similarly very high (IgG1, 1:6,400; IgG2a, 1:25,600; IgG2b, 1:800; and IgG3, 1:6,400).
To determine whether the antibody response to LVS LPS was thymus independent, a single experiment compared serum antibody titers produced after immunization of BALB/cByJ or athymic BALB/c.nu/nu mice with 100 ng of LPS i.d. Serum IgM titers were quite similar in both groups of mice at all time points, and IgG titers were low or undetectable (data not shown).
To confirm the specificity of the antibodies generated following immunization with LPS, day 3 and day 30 serum samples from both LPS-immunized mice and LVS-infected mice were analyzed by Western blotting using either LVS LPS, E. coli LPS, or whole, heat-killed bacteria as antigens. This analysis demonstrated that, as expected, day 30 serum from mice immunized with LVS LPS reacted with both LVS LPS and heat-killed LVS, but not E. coli LPS, in a characteristic LPS ladder-like pattern. Day 30 serum from mice infected with LVS bacteria recognized a number of individual protein bands as well as a ladder-like pattern of bands when reacted with heat-killed bacteria but only a ladder-like pattern of bands when reacted with LVS LPS. Only very faint reactivity was detected using either day 3 IMS to react with either LVS LPS or heat-killed LVS bacteria as antigens (data not shown).
To determine whether anti-LPS antibodies were able to transfer protection against lethal challenge to naive recipient mice, BALB/cByJ mice were given 0.5 ml i.p. each of day 3 or day 30 anti-LPS IMS and challenged with 102 LVS bacteria i.p. immediately after transfer (35). Alternatively, mice were immunized with 100 ng of LVS LPS i.d. 3 days earlier and challenged at the same time as mice that received IMS. In two experiments of similar design, all of 10 mice survived lethal LVS challenge following LPS immunization, but none of 10 mice survived after receiving day 3 anti-LPS IMS, and none of 10 mice survived after receiving day 30 anti-LPS IMS. Thus, anti-LVS LPS antibodies, even when produced by immunization with much higher amounts of LVS LPS (10 μg) than were effective in protection, were unable to transfer protection against lethal challenge.
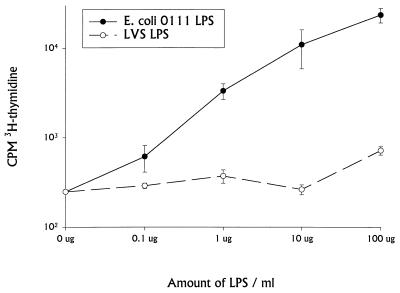
To examine the nonspecific lymphocyte stimulation activities of LVS LPS, lymphocyte proliferation assays were performed. Spleen cells from naive BALB/cByJ mice were cultured with various doses of either E. coli O111 LPS or LVS LPS. [3H]thymidine was then added overnight 1, 2, or 3 days after initiation of cultures. Data from day 2 are shown in Fig. 6, but trends were comparable on all days. E. coli O111 LPS (Fig. 6) and concanavalin A (see the legend to Fig. 6) readily induced proliferation of murine spleen cells. However, LVS LPS did not stimulate proliferation at any dose tested up to 100 μg/ml. Similar results were obtained using enriched B cells (data not shown). Culture supernatants from spleen cells stimulated with E. coli O111 LPS contained large amounts of secreted murine IgM, but no such increase in polyclonal immunoglobulin was observed in supernatants collected from LVS LPS stimulated cultures at any time point (data not shown).
FIG. 6.
Induction of proliferation of BALB/cByJ spleen cells by LVS LPS. Spleen cells obtained from normal BALB/cByJ mice were cultured at 2 × 105/well in complete medium with the indicated amounts of E. coli O111 or LVS LPS. Proliferation was assessed by adding [3H]thymidine overnight on day 2 before harvesting and counting. Mean uptake of 3H per well for triplicate wells is shown; error bars indicate standard deviation of the mean. The response to 1 μg of concanavalin A per ml was 138,244 ± 16,481 in this experiment. This experiment is representative of four experiments of similar design.
The ability of LVS LPS to stimulate cytokine secretion from spleen cells was also studied (Table 2). Mice were treated with either PBS, LVS LPS, or oligonucleotide DNA (as a positive control [16]). Three days later, spleens were removed and studied for ex vivo cytokine production by ELISPOT assay; results shown here included LPS or DNA in the medium during the 8-h incubation period of the assay (Table 2), but numbers of ELISPOTs per 106 cells were very similar in parallel cultures that did not contain LPS or DNA in the medium (data not shown). Only DNA treatment, not LVS LPS treatment, 3 days before assay noticeably increased the numbers of cells secreting IFN-γ, IL-12, and IL-6 (but not IL-4). To determine whether LPS primed lymphocytes for an increase in cytokine production that was revealed only upon infection, mice were treated with PBS or 100 ng of LPS i.d. and infected with 103 LVS bacteria 3 days later; spleens were tested by ELISPOT assay 3 days after infection. Large increases in numbers of ELISPOTs for IFN-γ were observed after LVS infection alone, but there was no increase or decrease in numbers of ELISPOTs for IFN-γ with LPS priming, nor was there an increase in numbers of ELISPOTs for IL-12, IL-6, or IL-4 following either infection alone or LPS priming and infection (data not shown). Thus, LVS LPS, unlike LPS derived from traditional enteric bacteria, did not stimulate murine B cells or other lymphocytes directly for polyclonal lymphocyte proliferation or immunoglobulin secretion, nor did it induce cytokine secretion from murine splenocytes (for the cytokines tested).
TABLE 2.
Stimulation of cytokine production by LVS LPSa
| Treatment | No. of ELISPOTs/106 spleen cells
|
|||
|---|---|---|---|---|
| IFN-γ | IL-12 | IL-6 | IL-4 | |
| PBS | 116 | 94 | 163 | 344 |
| LPS | 92 | 105 | 173 | 261 |
| DNA | 476 | 1,280 | 576 | 297 |
Three days before assay, groups of four BALB/cByJ mice were treated with either PBS (100 μl i.d.), oligonucleotide DNA (20 μg i.p.), or LVS LPS (100 ng i.d.). All mice were sacrificed on the same day; spleens were removed and assayed for ELISPOT formation for the indicated cytokines, including LVS LPS (50 ng/ml) in the medium (for LPS-treated mice) or oligonucleotide DNA (1 μg/ml) (for DNA treated mice), during 8 h of in vitro culture. Errors are not shown for clarity of presentation but were always less than 25% of the mean. This experiment is representative of three experiments of similar design.
DISCUSSION
The data presented here demonstrate that LPS purified from F. tularensis LVS lacks the ability to nonspecifically activate murine B cells for proliferation or polyclonal immunoglobulin secretion and does not stimulate murine splenocytes to secrete IL-12, IL-6, IL-4, or IFN-γ (Fig. 6 and Table 2). Similarly, previous results indicate that LVS LPS is unable to activate murine (1) or human (37) macrophages. LVS LPS does stimulate specific antibody production and in purified form is a weak immunogen that induces primarily an IgM response (Fig. 5). When recognized by the murine immune system as a component of the bacterium, however, the resulting specific antibody response is vigorous and is characterized by the production of large amounts of IgG, particularly IgG2a (Fig. 5 and Results). Despite the apparent absence of nonspecific immunostimulatory activity and minimal specific antibody production, however, treatment of mice with surprisingly small amounts of LVS LPS stimulates very strong B-cell-dependent protection against lethal LVS challenge within 2 to 3 days (Table 1; Fig. 1 to 3). The mechanism of protection is fundamentally different from activation by other types of enteric LPS and does not involve recognition through the Lpsn gene product (Fig. 4; Table 1). Thus, LVS LPS is a highly unusual LPS, and induction of protection involving B cells is likely effected through an indirect means rather than through traditional direct activation of B cells.
The protection provided by purified LVS LPS is remarkable for the ability of this purified component, rather than live bacteria as is usually necessary, to provide protection against this intracellular pathogen. Previous studies clearly indicated that immunization of mice with heat-killed LVS, for instance, did not provide any measurable protection against challenge with either LVS or fully virulent F. tularensis strain Schu 4 (5, 11, 20, 22). Of note in this regard is the fact that another study using repeated large doses of LVS LPS as a vaccine demonstrated protection against lethal LVS challenge but not Schu 4 (19, 20) challenge. These and other early studies have led to the proposal that Francisella subunit vaccines and various extracts of bacteria may provide some weak protection against challenge with LVS or less virulent strains of F. tularensis but not against more virulent strains such as Schu 4. The studies performed here do not address this point directly, nor do they provide information on the whether LPS from Francisella is a realistic vaccine candidate. They nonetheless demonstrate that under some circumstances a purified bacterial component can provide substantial protection against a challenge with an intracellular bacterium that is fully virulent for mice.
Although the structure of LVS LPS has not been determined, these results clearly imply that the chemical composition of the lipid A moiety of LVS LPS is quite different from that of enteric LPS and that LVS LPS lacks a functional lipid A. This apparently confers a survival advantage on the bacterium, since its LPS does not participate in induction of nitric oxide production that might limit its intracellular growth (6). Further, since other data indicate that LVS LPS is unable to block macrophage stimulation by functional LPS (1), the structure must be distinct enough to not permit recognition as an antagonist for traditional LPSs. The ability of LVS LPS to stimulate protection in C3H/HeJ mice, which are defective in the ability to recognize and respond to enteric LPS at least in part due to a point mutation in Toll-like receptor 4 (24, 34, 43), suggests that LVS LPS is recognized through receptors other than Tlr4.
Most other purified LPS chemotypes that stimulate polyclonal activation also stimulate strong primary IgM antibody responses (32); the lack of lipid A-related activity presumably explains why LVS LPS is such a weak immunogen. LVS LPS has more similarities to other nonmitogenic carbohydrate antigens such as capsular polysaccharides, which typically stimulate weak primary IgM and IgG3 responses as well. Our previous results also demonstrated that athymic nu/nu mice also produce only small amounts of anti-LVS IgM when immunized with live bacteria (15), as did nu/nu mice immunized with purified LVS LPS (see Results), and thus it is reasonable to conclude that LVS LPS should be considered a thymus-independent antigen.
The failure to detect binding of IgG anti-LPS antibodies to LVS-coated plates (Fig. 5) may imply that the LPS epitopes recognized by these antibodies are not readily accessible on the surface of LVS bacteria, at least when bacteria are prepared and allowed to adhere to ELISA plates in this manner. In contrast, infection with bacteria stimulates IgG anti-LPS antibodies that are readily detected on LVS- and LPS-coated plates (Fig. 5), and thus the epitope(s) recognized by these antibodies appears to be readily accessible on the bacterial surface. Alternatively, the results may indicate that anti-LPS antibodies, particularly the small amounts of IgG antibodies produced after immunization with purified LPS, are of very low affinity compared to those produced after immunization with live bacteria; thus, their binding to bacteria coating plates may be easily disrupted in the course of the assay. The results of studying anti-LPS antibodies produced after immunization with live bacteria also indicate that a large portion of the serum anti-LVS response is comprised of anti-LPS antibodies (Fig. 5); this is entirely consistent with similar observations in human serological studies of tularemia, in which serum from people either vaccinated with LVS (44) or recovering from natural infection (28, 42) exhibit high titers of anti-Francisella LPS antibodies. The predominance of the IgG2a isotype likely reflects the large induction of IFN-γ following LVS infection.
Our previous studies have demonstrated that normal mice, but not lymphocyte-deficient scid mice or B-cell KO mice, given a sublethal infection with LVS survive a strong lethal challenge with LVS given only 2 to 3 days later (8, 13). This early protective immunity, which was also demonstrable in L. monocytogenes infection in mice (14), is nonspecific and requires IFN-γ and lymphocytes, particularly B cells. Importantly, lack of specificity was indicated by the ability of sublethal infection with LVS to generate protection against lethal challenge with L. monocytogenes (14), although LVS-mediated protection against lethal Salmonella serovar Typhimurium challenge could not be demonstrated (13). We have recently demonstrated that genomic or oligonucleotide bacterial DNA containing unmethylated CpG motifs was able to stimulate protection against lethal LVS or Listeria challenge within 2 to 3 days after administration. As for early protection stimulated by bacterial infection, the protective mechanism was also dependent on lymphocytes, particularly B cells and IFN-γ (16). Here, we wished to determine whether LVS LPS is similarly a candidate bacterial component involved in the stimulation of early protective immunity. Like results of using live bacteria as an immunogen (8, 13, 15), protection stimulated by LVS LPS required low doses, was effective against very large doses of lethal LVS challenge, developed within 2 to 3 days, and was dependent on B cells and IFN-γ (Fig. 1 to 3; Table 1). Protection is clearly not due to contaminating bacterial DNA, which is undetectable in these LPS preparations. Further, unlike results using live bacteria or DNA (14, 16), LVS LPS did not stimulate protection against Listeria (see Results). There are several possible interpretations of this apparent discrepancy. First, early protective immunity may not be a function of LVS LPS but of other bacterial components such as DNA. This would be consistent with other studies demonstrating that sublethal infection with F. novicida, which has a different chemotype of LPS on its surface, provides protection against lethal challenge with F. tularensis LVS (T. Kieffer and K. L. Elkins, unpublished results); such results suggest that LVS LPS may be sufficient for early protection under some circumstances but is not necessary. A second possibility is that LVS LPS may be unable to stimulate the full range of immune mediators necessary for protection against Listeria when introduced as a purified molecule in isolation from the rest of the bacterium but can do so for LVS in conjunction with infection itself (e.g., when the challenge is introduced); specificity in this case would be only apparent, rather than due to clonal recognition of LPS by B- or T-cell receptors that is the hallmark of specific immunity. This concept would also be consistent with the observation that IFN-γ is required for LPS-stimulated protection (Table 1), despite the inability of LPS to stimulate IFN-γ production by murine splenocytes (Table 2); as demonstrated by ELISPOT assay, IFN-γ production by splenocytes is stimulated by LVS infection alone (see Results).
A third possibility is that protection stimulated by LVS LPS against LVS is indeed specific and due to the production of anti-LPS antibodies that are unavailable in B-cell-deficient mice. Our previous results have demonstrated that serum (10, 35) or monoclonal anti-LVS antibodies (33) transfer only very weak protection against lethal LVS challenge under limited experiment circumstances. We believe that it is highly unlikely that LPS-stimulated protection is mediated by specific antibodies for a number of reasons. First, in other circumstances such as the stimulation of early protection by whole bacteria, the defect in B-cell-deficient mice is readily reconstituted by transfer of naive B cells but not immune mouse serum (8, 12). Second, the antibody response to 100 ng of LVS LPS is quite weak at its peak on day 7 after immunization and barely detectable by very sensitive techniques on day 3 when lethal challenge is introduced (Fig. 5 and Results); in addition, immune serum from mice immunized 3 days earlier with LVS LPS was unable to transfer protection against a relatively weak lethal LVS challenge (Results). Further, there is no detectable anti-LPS antibody response in the serum of mice given 1 ng (or even 0.1 ng) of LVS LPS i.d. at any time point (Results), yet this dose of LPS is able to elicit strong protection (Fig. 1). Third, large doses of day 3 immune serum from LVS LPS-immunized mice are unable to transfer protection against even a relatively small lethal challenge of 102 LVS bacteria i.p. (Results).
Instead, we propose that LVS LPS stimulates potent protection against lethal LVS challenge via cytokines and chemokines whose production or expression of function is directly or indirectly dependent on B cells. Resolution of LVS infection has previously been shown to be dependent on TNF-α (29). Since LVS LPS also does not stimulate production of TNF-α (1, 37), another bacterial component must be responsible for elicitation of this cytokine during survival of LVS infection. Similarly, LVS LPS-stimulated protection was dependent on IFN-γ (Table 1), but LVS LPS did not stimulate production of IFN-γ from spleen cells (Table 2) or peritoneal cells (data not shown) in vitro. Because natural killer (NK) cells are an important source of IFN-γ in innate immune responses, future studies will determine the ability of LVS LPS to stimulate NK cells (which are very few in spleen cell populations) for IFN-γ production and cytotoxicity. However, since scid mice, which contain abundant functional NK cells, were not protected against lethal LVS challenge upon immunization with LVS LPS (Table 1), any stimulation of NK cells by LVS LPS could at best be necessary but not sufficient for protection.
Further understanding of the functional capabilities of LVS LPS is important to determining whether the molecule may be useful as a vaccine candidate or as an adjuvant for other antigens. Thus, future studies will continue to elucidate the structure-function relationships of this unusual LPS.
ACKNOWLEDGMENTS
We thank Tonya Rhinehart-Jones for excellent technical assistance and our CBER colleagues Catharine M. Bosio, Dorothy Scott, and Kathryn Stein for critical readings of the manuscript.
Footnotes
This article is dedicated to the memory of Roberta D. Shahin, our friend and colleague, whose insight, encouragement, and companionship were instrumental throughout the progression of these and many other studies.
REFERENCES
- 1.Ancuta P, Pedron R, Girard R, Sandström G, Chaby R. Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect Immun. 1996;64:2041–2046. doi: 10.1128/iai.64.6.2041-2046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony L S D, Kongshavn P A L. Experimental murine tularemia caused by Francisella tularensis, live vaccine strain: a model of acquired cellular resistance. Microb Pathog. 1987;2:3–14. doi: 10.1016/0882-4010(87)90110-0. [DOI] [PubMed] [Google Scholar]
- 3.Anthony L S D, Morrissey P J, Nano F E. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from l-arginine metabolism. J Immunol. 1992;148:1829–1834. [PubMed] [Google Scholar]
- 4.Baker C N, Hollis D G, Thornsberry C. Anti-microbial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J Clin Microbiol. 1985;22:212–215. doi: 10.1128/jcm.22.2.212-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claflin J L, Larson C L. Infection-immunity in tularemia: specificity of cellular immunity. Infect Immun. 1972;5:311–318. doi: 10.1128/iai.5.3.311-318.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowley S, Myltseva S, Nano F E. Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity, and nitric oxide production. Mol Microbiol. 1996;20:867–874. doi: 10.1111/j.1365-2958.1996.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 7.Cryz S J, Furer E, Sadoff J C, Fredeking T, Que J U, Cross A S. Production and characterization of a human hyperimmune intravenous immunoglobulin against Pseudomonas aeruginosa and Klebsiella species. J Infect Dis. 1991;163:1055–1061. doi: 10.1093/infdis/163.5.1055. [DOI] [PubMed] [Google Scholar]
- 8.Culkin S J, Rhinehart-Jones T, Elkins K L. A novel role for B cells in early protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1997;158:3277–3284. [PubMed] [Google Scholar]
- 9.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 10.Drabick J J, Narayanan R B, Williams J C, Leduc J W, Nacy C A. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308:83–87. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Eigelsbach H T, Downs C M. Prophylactic effectiveness of live and killed tularemia vaccines. J Immunol. 1961;87:415–425. [PubMed] [Google Scholar]
- 12.Elkins K L, Bosio C M, Rhinehart-Jones T R. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the model intracellular bacterium, Francisella tularensis live vaccine strain. Infect Immun. 1999;67:6002–6007. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkins K L, Leiby D A, Winegar R K, Nacy C A, Fortier A H. Rapid generation of specific protective immunity to Francisella tularensis. Infect Immun. 1992;60:4571–4577. doi: 10.1128/iai.60.11.4571-4577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins K L, MacIntyre A T, Rhinehart-Jones T R. Nonspecific early protective immunity in Francisella and Listeria infections can be dependent on lymphocytes. Infect Immun. 1998;66:3467–3469. doi: 10.1128/iai.66.7.3467-3469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkins K L, Rhinehart-Jones T, Nacy C A, Winegar R K, Fortier A H. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect Immun. 1993;61:823–829. doi: 10.1128/iai.61.3.823-829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkins K L, Rhinehart-Jones T R, Stibitz C, Conover J S, Klinman D M. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162:2291–2298. [PubMed] [Google Scholar]
- 17.Fortier A H, Green S J, Polsinelli T, Jones T R, Crawford R M, Leiby D A, Elkins K L, Meltzer M S, Nacy C A. Life and death of an intracellular pathogen: Francisella tularensis and the macrophage. Immunol Ser. 1994;60:349–361. [PubMed] [Google Scholar]
- 18.Fortier A H, Slayter M V, Ziemba R, Meltzer M S, Nacy C A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulop M, Manchee R, Titball R. Role of lipopolysaccharide and a major outer membrane protein from Francisella tularensis in the induction of immunity against tularemia. Vaccine. 1995;13:1220–1225. doi: 10.1016/0264-410x(95)00062-6. [DOI] [PubMed] [Google Scholar]
- 20.Fulop M, Manchee R, Titball R. Role of two outer membrane antigens in the induction of protective immunity against Francisella tularensis in strains of different virulence. FEMS Immunol Med Microbiol. 1996;13:245–247. doi: 10.1111/j.1574-695X.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 21.Fulop M, Webber T, Manchee R. Activation of the complement system by Francisella tularensis lipopolysaccharide. Microbiologica. 1993;16:141–148. [PubMed] [Google Scholar]
- 22.Hambleton P, Evans C G T, Hood A M, Strange R E. Vaccine potencies of the live vaccine strain of Francisella tularensis and isolated bacterial components. Br J Exp Pathol. 1974;55:363–373. [PMC free article] [PubMed] [Google Scholar]
- 23.Hood A M. Virulence factors of Francisella tularensis. J Hyg. 1977;79:47–65. doi: 10.1017/s0022172400052840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 25.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 26.Klinman D M, Nutman T B. ELISPOT assay to detect cytokine-secreting murine and human cells. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Brooklyn, N.Y: Greene and Associates; 1994. pp. 6.19.1–6.19.8. [Google Scholar]
- 27.Kohzuki T, Egucki Y, Kato M, Irie K, Ohtsuka H, Higuchi A, Noguchi H. Protective activity of anti-exotoxin monoclonal antibody against mice infected with toxin-producing Pseudomonas aeruginosa. J Infect Dis. 1993;167:119–125. doi: 10.1093/infdis/167.1.113. [DOI] [PubMed] [Google Scholar]
- 28.Koskela P, Salminen A. Humoral immunity against Francisella tularensis after natural infection. J Clin Microbiol. 1985;22:973–979. doi: 10.1128/jcm.22.6.973-979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leiby D A, Fortier A H, Crawford R M, Schreiber R D, Nacy C A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macela A, Stulik J, Hernychova L, Kroca M, Krocova Z, Kovarova H. The immune response against Francisella tularensis live vaccine strain in Lpsn and Lpsd mice. FEMS Immunol Med Microbiol. 1996;13:235–238. doi: 10.1111/j.1574-695X.1996.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 31.Mastroeni P, Villarreal-Ramos B, Hormaeche C E. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison D C, Ryan J L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 33.Narayanan R B, Drabick J J, Williams J C, Fortier A H, Meltzer M S, Sadoff J C, Bolt C R, Nacy C A. Immunotherapy of tularemia: characterization of a monoclonal antibody reactive with Francisella tularensis. J Leukoc Biol. 1993;53:112–116. doi: 10.1002/jlb.53.1.112. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhinehart-Jones T R, Fortier A H, Elkins K L. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rietschel R T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zähringer U, Seydel U, Di Padova F, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 37.Sandström G, Sjöstedt A, Johansson T, Kuoppa K, Williams J C. Immunogenicity and toxicity of lipopolysaccharide from Francisella tularensis LVS. FEMS Microbiol Immunol. 1992;105:201–210. doi: 10.1111/j.1574-6968.1992.tb05902.x. [DOI] [PubMed] [Google Scholar]
- 38.Sultzer B M, Castagna R, Bandekar J, Wong P. Lipolysaccharide nonresponder cells: the C3H/HeJ defect. Immunobiology. 1993;187:257–271. doi: 10.1016/S0171-2985(11)80343-8. [DOI] [PubMed] [Google Scholar]
- 39.Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 40.Tärnvik A, Eriksson M, Sandström G, Sjöstedt A. Francisella tularensis—a model for studies of the immune response to intracellular bacteria in man. Immunology. 1992;76:349–354. [PMC free article] [PubMed] [Google Scholar]
- 41.Tough D F, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J Exp Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viljanen M K, Nurmi T, Salminen A. Enzyme-linked immunosorbent assay (ELISA) with bacterial sonicate antigen for IgM, IgA, and IgG antibodies to Francisella tularensis: comparison with bacterial agglutination test and ELISA with lipopolysaccharide antigen. J Infect Dis. 1983;148:715–720. doi: 10.1093/infdis/148.4.715. [DOI] [PubMed] [Google Scholar]
- 43.Vogel S N, Johnson D, Perera P Y, Medvedev A, Lariviere L, Qureshi S T, Malo D. Cutting edge: functional characterization of the effect of the C3H/HeJ defect in mice that lack an Lpsn gene: in vivo evidence for a dominant negative mutation. J Immunol. 1999;162:5666–5670. [PubMed] [Google Scholar]
- 44.Waag D M, McKee K T, Jr, Sandstrom G, Pratt L L K, Bolt C R, England M J, Nelson G O, Williams J C. Cell-mediated and humoral immune responses after vaccination of human volunteers with the live vaccine strain of Francisella tularensis. Clin Diagn Lab Immunol. 1995;2:143–148. doi: 10.1128/cdli.2.2.143-148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westphal O, Luderitz O. Chemische Erforschung von Lipopolysacchariden gramnegativer Bacterien. Angew Chem. 1954;66:407–417. [Google Scholar]
- 46.Yee D, Rhinehart-Jones T R, Elkins K L. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1996;157:5042–5048. [PubMed] [Google Scholar]