Abstract
The benefits of physical exercise are well-known, but there are still many questions regarding COVID-19. Chow et al.’s 2022 study, titled Exerkines and Disease, showed that a special focus on exerkines can help to better understand the underlying mechanisms of physical exercise and disease. Exerkines are a group of promising molecules that may underlie the beneficial effects of physical exercise in diseases. The idea of exerkines is to understand the effects of physical exercise on diseases better. Exerkines have a high potential for the treatment of diseases and, considering that, there is still no study of the importance of exerkines on the most dangerous disease in the world in recent years, COVID-19. This raises the fundamental question of whether exerkines have the potential to manage COVID-19. Most of the studies focused on the general changes in physical exercise in patients with COVID-19, both during the illness and after discharge from the hospital, and did not investigate the basic differences. A unique look at the management of COVID-19 by exerkines, especially in obese and overweight women who experience high severity of COVID-19 and whose recovery period is long after discharge from the hospital, can help to understand the basic mechanisms. In this review, we explore the potential of exerkines in COVID-19 by practicing physical exercise to provide compelling practice recommendations with new insights.
Keywords: coronavirus disease 2019 (COVID-19), exerkines, females, women health, physical exercise, physical activity, SARS-CoV-2 virus, resistance training, aerobic exercise, Omicron
Key points
With the prevalence of COVID-19 in the world as a dangerous disease, many studies have given physical exercise interventions, but the understanding of the effective and basic mechanisms of physical exercise is still unclear.
The ACE2 receptor that the SARS-CoV-2 spike protein binds to is more expressed in fat tissue than in other organs, which is very high in obese and overweight women. Therefore, behavioral changes of adipokines in COVID-19 lead to high inflammation and, through crosstalk with other organs, such as muscle (myokines) or liver, hepatokines lead to a decrease in physical function and an increase in inflammation.
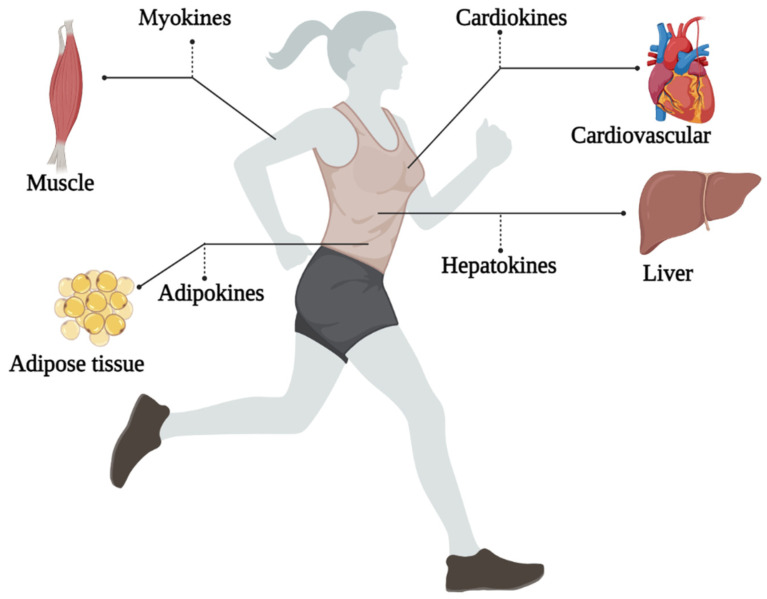
Exerkines include myokines (muscle), cardiokines (heart), hepatokines (liver), neurokines (nervous system), and baptokines (brown adipose tissue) and adipokines (adipose tissue).
Resistance training that includes weight training and exercise equipment can improve muscle strength and reduce inflammation by affecting myokines during COVID-19 disease. After discharge from the hospital, this physical exercise is suitable for returning to a quality of life. Moreover, aerobic exercise that includes moderate-intensity exercise (running on a treadmill or cycling) can have the greatest effect on the stimulation of exerkines.
Exerkines have a high potential for treating diseases, but investigating their high potential for COVID-19 and the underlying mechanisms remained unanswered.
The emerging research on exerkines opens up several promising avenues for future research where researchers can explore the potential importance of exerkines in elderly or coexisting COVID-19 patients.
Future studies on exerkines can be very effective in designing accurate physical exercise and more practical recommendations for patients, especially COVID-19 patients, during illness or after discharge from the hospital.
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the acute respiratory syndrome with coronavirus that primarily affects the lungs [1]. After three years of this disease, we understand who is more exposed to the symptoms of COVID-19 and who suffers from moderate and high severity of this disease [2]. The review of previous studies showed that most people infected with COVID-19 were obese or overweight [3,4], and disease severity and mortality were higher in obese subjects than in overweight subjects [5]. Moreover, during the illness, COVID-19 patients face a decrease in muscle strength (muscle atrophy), a decrease in muscle endurance, and other physiological factors, which can affect training programs in the long term after discharge from the hospital [6,7]. It has been determined that, in the long term, COVID-19 can lead to negative physiological changes and decrease the quality of life, reducing hope and motivation for physical activity, which worsens the course of the disease [8].
Gender is an essential determinant of mortality risk and immunological responses to COVID-19 [9,10,11]. Women face a higher risk of becoming infected during a pandemic because of their societal position, as the United Nations (UN) and the World Health Organization (WHO) reported [12,13,14]. Women are over-represented in health care professions [11,15,16,17]. On the other hand, obese and overweight women are more exposed to the complications of COVID-19 than normal women [18]. Being physically active or the lack thereof plays a vital role in obesity. Indubitably, obesity ensues from physical inactivity and vice versa [19,20,21]. Hormones can also affect sexual difference in viral infections such as COVID-19. The SARS-CoV-2 spike protein binds to the human angiotensin-converting enzyme 2 (ACE2) receptor. Studies have shown that estradiol, a primary female sex hormone, likely regulates ACE2 expression in airway epithelial cells, kidneys, heart, and adipose tissues [22,23]. In obese and overweight women, estradiol function [24] is disrupted and ACE2 expression increases [22,23].
Undeniable evidence has been supporting the vital role of physical exercise in improving immune system, body composition, and reducing the complications of COVID-19 [25,26]. Although the benefits of physical exercise in improving health and reducing the severity of COVID-19 are well known, the molecular mechanisms underlying these benefits associated with physical exercise are not yet defined and are being investigated [27,28]. Physical exercise generally includes aerobic, anaerobic, or resistance training, but physical activity includes occupational, sports, conditioning, household, or other activities [29,30,31]. Promoting physical activity seems to have a more significant effect on reducing the diseases severity [27,32,33,34]. In 2020, WHO reported that all adults should aim for 150 to 300 min of moderate-intensity physical activity per week or 75 to 150 min of vigorous-intensity physical activity per week, or an equivalent combination of moderate- and vigorous-intensity physical activity [35]. However, it is important to understand how physical activity can affect the acute or chronic immune response in obese women.
To make better recommendations for immunization in women, we need to understand the impact of physical exercise on all organs. Studying exerkines would be the key. The word exerkines has introduced in 2016 [36]. So far, there is no exact definition of exerkines, but the best explanation for seems to be: the release of myokines, cardiokines, hepatokines, and adipokines due to physical exercise [27]. That is, physical exercise can affect these “kines” and ultimately exerts its effects through endocrine, paracrine, and/or autocrine pathways (Figure 1) [35]. The idea of the present study originated from the study of Chow et al., 2022, titled Exerkine and Diseases in the journal Nature Reviews. The intensity, duration, frequency, and volume of training are all factors that can play an important role in these responses. Understanding the role of exerkines in the physiological and biological response to physical exercise is a vital step to provide training suggestions to reduce the severity of COVID-19 in new strains. Therefore, we will discuss how the training variables could affect exerkine response.
Figure 1.
An overview of the exerkines investigated in this study.
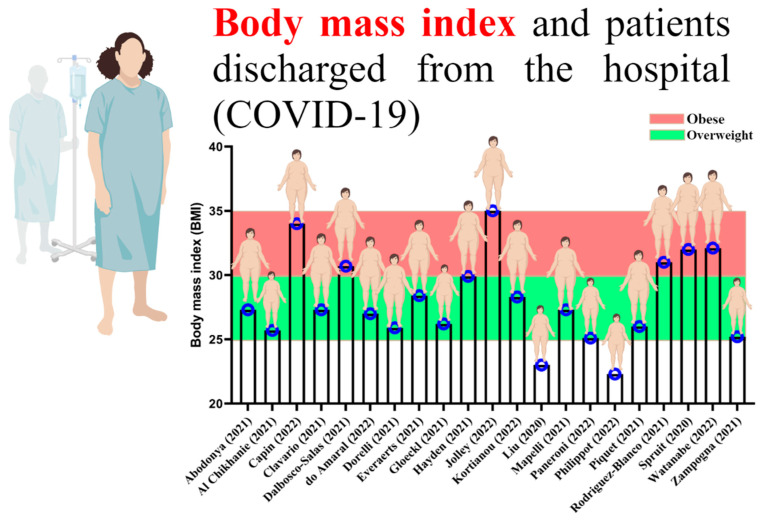
By reviewing past studies related to rehabilitation and physical exercise in the survivors of COVID-19, it can be well understood that most of those who were infected with COVID-19 were overweight and obese (Figure 2) [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. Obesity could affect the exerkine response to physical exercise, especially in women. Now that new strains of COVID-19 are on the way and obese women are experiencing a high severity of the disease, this study discusses the associations between gender, physical exercise, and exerkine secretion.
Figure 2.
Body mass index of patients with COVID-19 discharged from the hospital [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56].
2. The Potential Role of Exerkines Can Be Effective in COVID-19
It is well known that the most affected organs in COVID-19 include fat tissue, lungs, muscles, liver, and heart [57]. With the binding of SARS-CoV-2 spike protein to ACE2, physiological changes are created in the tissue, which can increase the disease process and influence the severity of the disease [58]. Moreover, the side effects of COVID-19 can stay with the patient long after discharge from the hospital and reduce the quality of life [59]. In this regard, most sports studies implementing “rehabilitation exercises” after COVID-19 had a general view of the effect of rehabilitation exercises on patients discharged from the hospital and did not investigate the fundamental mechanisms but highlighted the importance of physical exercise. Nevertheless, to provide a suitable physical exercise strategy for this disease, the mechanisms behind the curtain should be investigated. The idea of exerkines to take a more detailed look at diseases in 2022 by Chow et al. in the journal Nature Reviews Endocrinology gave rise to the idea that it might be possible to investigate the mechanisms behind the curtain and the effect of physical exercise in COVID-19 [27].
Exerkines look at the effect of physical exercise acutely or chronically on disease [27], and frequency, intensity, time, and type (FITT) can help to understand the effect of exerkines on disease better [27]. Exerkines are secreted in response to acute exercise (short-term physical exercise and less than 2 weeks), which is usually a part of aerobic or resistance training. Chronic exercise (long-term physical exercise and more than 2 weeks) is associated with changes in humoral factors, even at rest, suggesting that exerkine changes may reflect the effects of chronic exercise [60]. The acute exerkine response is influenced by the type of physical exercise, duration of physical exercise, background fitness, feeding–fasting status, and post-exercise sampling time [61]. Classical exerkines released during acute exercise, as found in human and animal models, include IL-6, IL-8, IL-1 receptor antagonist (IL-1ra), and IL-10. In a human study in which blood samples were collected before and after a marathon race (acute exercise), plasma levels of several cytokines (IL-6, IL-1ra, IL-10, and tumor necrosis factor (TNF)), when collected immediately, were higher than the base. Plasma levels measured after physical exercise were found to peak and remain high for up to 4 h after exercise [62]. Notably, the acute exerkine response does not necessarily parallel the chronic exerkine response [63].
3. Can Exerkines (Generated/Stimulated by Physical Activity/Physical Exercise) Help COVID-19 Patients?
Low-Intensity Physical Exercise
According to the American College of Sports Medicine, low-intensity training is recommended for people who are not fit or want to start exercising. Low-intensity exercise training may last 30 min or more [64]. Based on the study by Zinman et al., the desired intensity for this type of physical exercise is 20–40% of the maximum oxygen consumption (Vo2max), 35–45% of the maximum heart rate (HRmax), and Borg scale of 2–3/10 [64]. Considering that COVID-19 patients discharged from the hospital cannot perform physical activities for more than 30 min, they can spend less than 20 min to resume exercises [52].
A study found low-intensity interval resistance training can lead to beneficial changes in myokines [65]. In this study, subjects performed resistance training for 12 weeks, with 40% 1RM and 15 to 20 repetitions [65]. However, low-intensity physical exercise of 20–40 Vo2max or 35–45% of HRmax, such as running, cycling, or walking, for less than five weeks appears not to affect myokines [30,31,32,34]. The low intensity and duration of the training week are effective in these positive changes. This improvement in low-intensity exercises has also been noted in cardiokines (i.e., IL-33, IL-6, IL-18, IL-1β, and follistatin) [66,67,68]. However, low-intensity exercise did not seem to have much of an effect on hepatokines and adipokines (i.e., melanoprotein, fetuin-A, leptin, adiponectin, chemrin, resistin, visfatin, omentin, FGF21, ANGPTL4, and follistatin) [69,70,71,72,73,74,75,76,77,78]. Considering that most people infected with COVID-19 were overweight and obese, this obesity is related to COVID-19 [79]. In obese and overweight people, the severity of COVID-19 is very high, and it seems that, with the reduction in body fat percentage, despite improving body composition, improvement in adipokines is also created. Low-intensity training cannot effectively improve the behavioral changes of adipokines and reduce body fat [80,81,82,83,84,85,86,87,88].
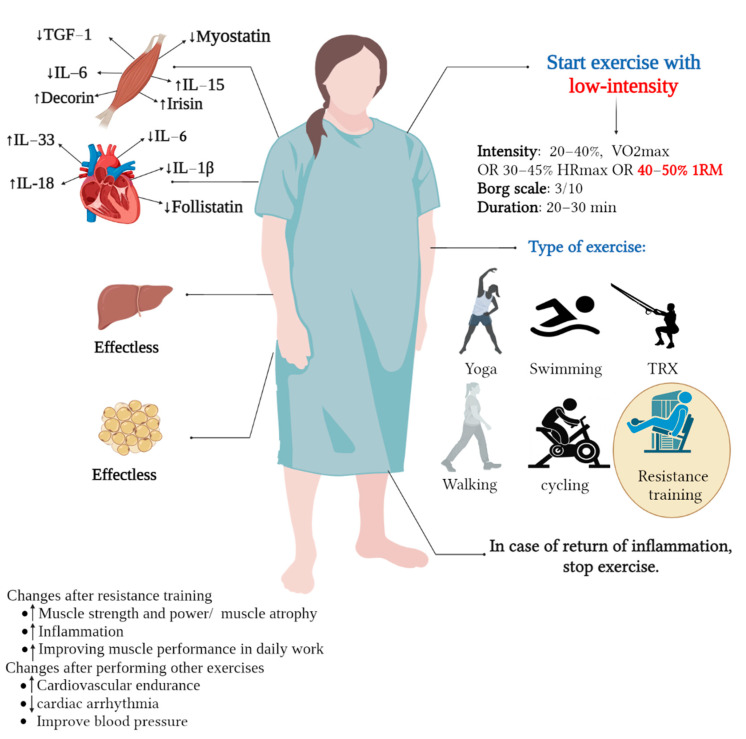
Thus, a low-intensity physical activity aimed at influencing exerkines appears appropriate for newly discharged or low-fit women. Of course, choosing the type of physical exercise is very important in influencing exerkines. Newly discharged women from the hospital (infected with COVID-19) need to restore strength [31]; so, it is well understood that low-intensity training can affect exerkines (myokines) more. Therefore, resistance training with a duration of 6 to 12 weeks and an intensity of 40% to 50% 1RM can be appropriate. Some studies have also suggested that exercises such as yoga, swimming, total body resistance exercise (TRX), walking, and cycling, as well as home exercises, such as body weight training, are also effective [89,90,91,92]. However, it seems that, in women who have just been discharged from the hospital, the duration should not be longer than 20 to 30 min to avoid re-inflammation. Therefore, in this short period of time, resistance training is suitable for women who have just been discharged from the hospital. Other means of physical exercise, such as cycling, running, or climbing, will be suitable for women with low physical fitness levels (see Figure 3 and Table 1).
Figure 3.
Effect of low-intensity exercise on exerkines in women with COVID-19 or discharged from hospital. TGF: transforming growth factor, IL: interleukin, RM: repetition maximum.
Table 1.
The effect of different types and intensities of physical exercise (low, moderate, and high) on the stimulation of exerkines.
| Physical Exercise Type (L/M/H) | Exerkines | |||||
|---|---|---|---|---|---|---|
| Myokines | Cardiokines | Adipokines | Hepatokines | Outcome | Reference | |
| Resistance training (Resistance weight training exercise with a resistance machine) |
(L/M) | (L/M) | (L/M) | (L/M) | Resistance training with low to moderate intensity can reduce inflammation and increase muscle strength by changing myokines. This can be effective in COVID-19 patients who suffer from decreased muscle strength and high inflammation during the illness. Moreover, resistance training with these intensities can benefit patients discharged from the hospital. However, high-intensity resistance training cannot positively affect myokines, and the results of articles have reported an increase in inflammation and a decrease in muscle strength. The effect of resistance training on cardiokines with low to moderate intensity can be suitable for improving high blood pressure and reducing inflammation. However, high-intensity resistance training may not be good for COVID-19 patients or those discharged from the hospital. Although studies on cardiokines are very limited, they can increase inflammation and possibly increase blood pressure. Low to moderate resistance training has a good effect on adipokines and hepatokines and can reduce inflammation, improve immune system function, improve insulin sensitivity, and increase fat tissue metabolism. In connection with high-intensity resistance training, the increase in inflammation is seen because of the behavioral changes in fat tissue and the decrease in the body function’s immune system. |
|
| ↓myostatin ↓TGF-β1 ↓IL-6 ↑IL-15 ↑decorin ↑irisin ↑BDNF ↑IL-15 ↑FGF ↔SPARC |
↓ANP ↔BNP ↑IL-33 ↓IL-6 ↑IL-18 ↓IL-1β ↓Follistatin ↑FGF ↔Sfrp ↑Neurotrophins ↓TNF-α ↓TGF-β |
↓Leptin ↑Adiponectin ↑Chemerin ↓Visfatin ↓Omentin ↑Vaspin ↔Progranulin ↔CTRP-4 |
↑Activin-E ↔ANGPTL3 ↔ANGPTL4 ↑ANGPTL6 ↑Fetuin-A ↑FGF21 ↓Follistatin ↑GDF15 ↓Hepassocin ↑IGF1 ↓LECT2 ↑Lipocalin 13 ↑Selenoprotein-P ↑Tsukushi |
[78,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110] | ||
| H | H | H | H | |||
| ↓myostatin ↓TGF-β1 ↑IL-6 ↑IL-15 ↑decorin ↑irisin ↑BDNF ↑IL-15 ↑FGF ↑SPARC |
↔ANP ↔BNP ↔IL-33 ↔IL-6 ↑IL-18 ↓IL-1β ↓Follistatin ↑FGF ↔Sfrp ↑Neurotrophins ↓TNF-α ↓TGF-β |
↔Leptin ↑↔Adiponectin ↑↓Chemerin ↓↔Visfatin ↓↑Omentin ↑↔Vaspin ↔↑Progranulin ↔↑CTRP-4 |
↓Activin-E ↑ANGPTL3 ↑ANGPTL4 ↓ANGPTL6 ↓Fetuin-A ↔FGF21 ↔Follistatin ↔GDF15 ↑Hepassocin ↔IGF1 ↔LECT2 ↓Lipocalin 13 ↔Selenoprotein-P ↔Tsukushi |
|||
| TRX | (L/M) | (L/M) | (L/M) | (L/M) | Low- to moderate-intensity TRX training can positively change some myokines, resulting in improved muscle strength and reduced inflammation. However, no studies were found in connection with high-intensity resistance training. Moreover, TRX training with low to moderate intensity can probably lead to a decrease in inflammation or an increase in metabolism and an improvement in the functioning of the immune system, with positive changes in adipokines; however, the results are limited and no study was found concerning high intensity. |
[111,112,113,114,115] |
| ↓myostatin ↔TGF-β1 ↔IL-6 ↔IL-15 ↔decorin ↑irisin ↔BDNF ↔IL-15 ↑FGF ↔SPARC |
N/A | ↓Leptin ↑Adiponectin ↑Chemerin ↓Visfatin ↓Omentin ↑Vaspin ↔Progranulin ↔CTRP-4 |
N/A | |||
| H | H | H | H | |||
| N/A | N/A | Leptin? Adiponectin? Chemerin? Visfatin? Omentin? Vaspin? Progranulin? CTRP-4? |
N/A | |||
| Yoga | (L/M) | (L/M) | (L/M) | (L/M) | No study focusing on exerkines was found. However, studies showed that practicing yoga can lead to a decrease in inflammatory cytokines and an increase in anti-inflammatory cytokines. However, it is impossible to conclude whether yoga focusing on exerkines is suitable. | [116,117,118,119,120] |
| N/A | N/A | N/A | N/A | |||
| H | H | H | H | |||
| N/A | N/A | N/A | N/A | |||
| Swimming | (L) | (L) | (L/M) | (L) | Low-intensity swimming training seems not to affect myokines, but moderate to intense training shows positive changes that can reduce inflammation and increase muscle strength. Concerning adipokines, low- to moderate-intensity swimming training can cause positive changes in adipokines and reduce inflammation and improve immune system function. Nevertheless, it seems like this concerns low intensity. No study was found in connection with other exerkines, but some studies have reported improved cardiovascular function and improved metabolism. |
[121,122,123,124,125,126,127,128,129,130] |
| ↔myostatin ↔TGF-β1 ↓IL-6 ↓IL-15 ↔decorin ↔irisin ↔BDNF ↔IL-15 ↔FGF ↔SPARC |
N/A | ↓Leptin ↑Adiponectin ↑Chemerin ↓Visfatin ↓Omentin ↑Vaspin ↔Progranulin ↔CTRP-4 |
N/A | |||
| (H/M) | (H/M) | (H) | (H/M) | |||
| ↑myostatin ↓TGF-β1 ↓IL-6 ↓IL-15 ↑decorin ↑irisin ↑BDNF ↑IL-15 ↑FGF ↔SPARC |
N/A | Leptin↑ Adiponectin↓Chemerin↑ Visfatin↑ Omentin↑ Vaspin↑ Progranulin↑ CTRP-4↑ |
N/A | |||
| Walking OR running | (L/M) | (L/M) | (L/M) | (L/M) | Walking with low to moderate intensity (running) positively affects some myokines, reducing inflammation and, sometimes, increasing muscle hypertrophy. However, in intense running, negative changes in myokines can lead to increased inflammation. The association between cardiokines and low- to moderate-intensity walking suggests that changes in some cardiokines can lead to lower blood pressure and, in some cases, reduced inflammation. It also improves cardiovascular endurance. However, with high intensity, there are few results and, in some cases, an increase in inflammation is seen. In connection with walking with low to moderate intensity (running) and adipokines and hepatokines, it seems that the best results occur and significant changes are seen in adipokines and hepatokines, the results of which are reducing inflammation, improving the function of the immune system, increasing metabolism, and increasing insulin sensitivity. |
[78,131,132,133,134,135,136,137,138,139,140,141,142] |
| ↑myostatin ↓TGF-β1 ↓IL-6 ↓IL-15 ↔decorin ↔irisin ↑BDNF ↑IL-15 ↔FGF ↔SPARC |
↓ANP ↓BNP ↑IL-33 ↓IL-6 ↑IL-18 ↓IL-1β ↓Follistatin ↑FGF ↓Sfrp ↑Neurotrophins ↓TNF-α ↓TGF-β |
↓Leptin ↑Adiponectin ↑Chemerin ↓Visfatin ↓Omentin ↑Vaspin ↔Progranulin ↔CTRP-4 |
↑Activin-E ↔ANGPTL3 ↔ANGPTL4 ↑ANGPTL6 ↑Fetuin-A ↑FGF21 ↓Follistatin ↑GDF15 ↓Hepassocin ↑IGF1 ↓LECT2 ↑Lipocalin 13 ↑Selenoprotein-P ↑Tsukushi |
|||
| H | H | H | H | |||
| ↓myostatin ↑TGF-β1 ↑IL-6 ↑IL-15 ↓decorin ↓irisin ↔BDNF ↓IL-15 ↓FGF ↔SPARC |
↔? ANP ↔? BNP ↓IL-33 ↑IL-6 ↓IL-18 ↔↑IL-1β ↔Follistatin ↔FGF ↓Sfrp ↑Neurotrophins ↑TNF-α ↑TGF-β |
Leptin↑ Adiponectin↓ Chemerin↑ Visfatin↑ Omentin↑ Vaspin↑ Progranulin↑ CTRP-4↑ |
↓Activin-E ↑ANGPTL3 ↑ANGPTL4 ↓ANGPTL6 ↓Fetuin-A ↓FGF21 ↑Follistatin ↔GDF15 ↑Hepassocin ↓IGF1 ↓LECT2 ↓Lipocalin 13 ↔Selenoprotein-P ↔Tsukushi |
|||
| Cycling | (L/M) | (L/M) | (L/M) | (L/M) | Cycling with low to moderate intensity can increase the strength of lower body muscles and reduce inflammation by changing myokines, which cannot be seen at high intensity. Moreover, low- to moderate-intensity cycling can lead to increased muscular endurance, cardiovascular endurance, blood pressure, and cardiac output. These results were not seen at high intensity and increased inflammation occurs at high intensity. Low- to moderate-intensity cycling focusing on adipokines can reduce inflammation and improve immune function. No study was found in connection with high intensity and hepatokines changes. |
[99,135,143,144,145] |
| ↓myostatin ↔TGF-β1 ↓IL-6 ↓IL-15 ↑decorin ↑irisin ↑BDNF ↑IL-15 ↑FGF ↑SPARC |
↓ANP ↓BNP ↑IL-33 ↓IL-6 ↑IL-18 ↓IL-1β ↓Follistatin ↑FGF ↓Sfrp ↑Neurotrophins ↓TNF-α ↓TGF-β |
↓Leptin ↑Adiponectin ↑Chemerin ↓Visfatin ↓Omentin ↑Vaspin ↔Progranulin ↔CTRP-4 |
N/A | |||
| H | H | H | H | |||
| ↓myostatin ↑TGF-β1 ↑IL-6 ↑IL-15 ↓decorin ↓irisin ↔BDNF ↓IL-15 ↓FGF ↔SPARC |
↔? ANP ↔? BNP ↓IL-33 ↑IL-6 ↓IL-18 ↔↑IL-1β ↔Follistatin ↔FGF ↓Sfrp ↑Neurotrophins ↑TNF-α ↑TGF-β |
N/A | N/A | |||
L: low intensity, M: moderate intensity, H: high intensity, TGF: transforming growth factor, IL: interleukin, BDNF: brain-derived neurotrophic factor, FGF: fibroblast growth factor, SPARC: secreted protein and rich in cysteine, ANP: atrial natriuretic peptide, BNP: brain natriuretic peptide, Sfrp: secreted frizzled-related proteins, TNF: tumor necrosis factor, ANGPTL3-4-6: angiopoietin-like protein 3-4-6, GDF: growth differentiation factor, IGF: insulin-like growth factor, LECT: leukocyte cell-derived chemotaxin, CTRP: cancer therapeutics response portal, N/A: unreported or little irrelevant research, ↑: increase, ↓ decrease, ↔: unchanged or unknown, ?: not defined.
4. Can Exerkines (Generated/Stimulated by Physical Activity/Physical Exercise) Help Newly Discharged COVID-19 Patients?
4.1. Moderate-Intensity Physical Exercise
Moderate-intensity exercise is an exercise that may last more than 30 min, with an intensity of between 40% and 60% of the Vo2max, 55% and 70% of the HRmax, and Borg scale of 4–6/10 [64]. With the spread of COVID-19, many studies reported the importance of moderate-intensity exercise to reduce the severity of COVID-19 [34,146,147,148,149]. The common denominator of these studies was that their physical exercise recommendations for reducing the severity of COVID-19 seem to be the same or close to the same [34,146,147,148,149]. Moderate-intensity exercise has an intensity of between 40 and 65% Vo2max or 50 and 75% HRmax and Borg scale of 4–6/10 [34,64,146,147,148,149]. However, how moderate-intensity training affects exerkines can be interesting but controversial.
Regarding myokines, it seems that one of the best effects of moderate-intensity training is to increase apelin [150,151,152]. Apelin can stimulate muscle mitochondrial biogenesis and protein synthesis and enhance muscle stem cells to promote muscle regeneration [152]. An interesting study found that aerobic exercise can increase muscle hypertrophy in women [153]. It appears that moderate-intensity aerobic exercise can lead to an increase in follistatin and a decrease in myostatin activity [154,155]. Decreased myostatin function increases skeletal muscle growth and improves whole-body glycemic control [154,155]. However, the physical exercise that most affects muscle exerkines is resistance exercise [156]. It appears that eight weeks of moderate-intensity resistance training (40–60% 1RM) can lead to positive changes in myokines (increase IL-6, IL-8, follistatin, and IL-15 and decrease myostatin) [156]. The results of other studies also confirm that resistance training can have the best beneficial effect on myokines [157,158]. In another study, it was found that both aerobic exercise (running) and moderate-intensity resistance exercise can produce a positive change in myokines (increase IL-7 and IL-8 and decrease IL-6) [93]. However, the emphasis of studies for positive changes in myokines is resistance training. Moreover, 6 to 12 weeks seemed to be a good time for these positive changes [93,157,158].
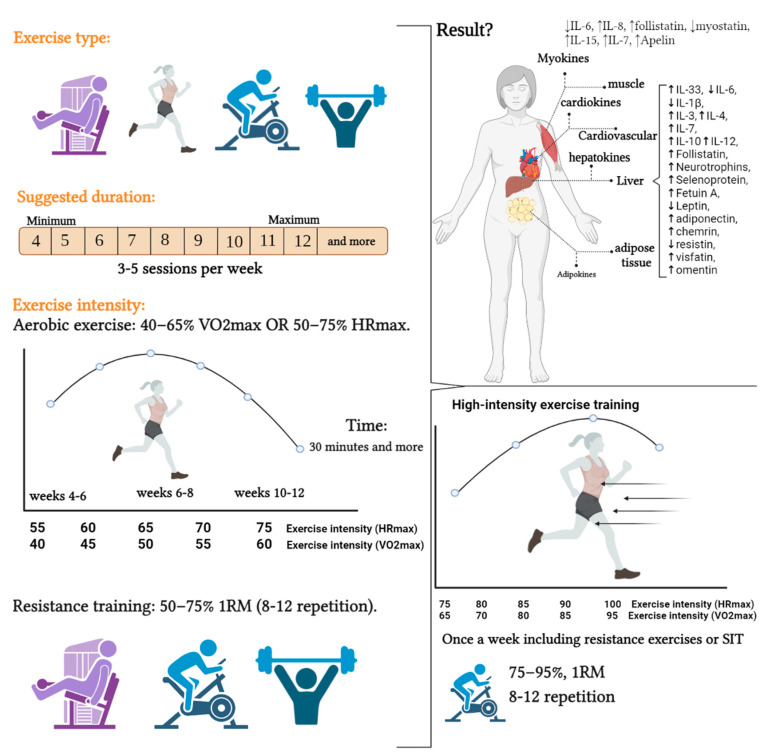
These positive changes due to moderate-intensity aerobic exercise do not affect other exerkines (cardiokines, hepatokines, and adipokines) and have significant positive effects (increase IL-33, IL-3, IL-4, IL-7, IL-10, IL-12, follistatin, neurotrophins, selenoprotein, fetuin-A, leptin, adiponectin, chemerin, resistin, visfatin, and omentin and decrease IL-6 and IL-1β) [63,68,131,159,160,161]. Therefore, both resistance training (weights or bodybuilding machines) and aerobic training (running on a treadmill and walking) will have a significant effect on exerkines. However, we can also mention combined exercises that can be very effective, with positive effects on exerkines to reduce the severity of COVID-19 [93,162,163]. Despite the fact that combined training (resistance and aerobic) can play a significant role in the positive impact of exerkines, current evidence shows that choosing the wrong type of movement and timing can lead to training interference and a negative effect on exerkines [164,165]. It is suggested to perform resistance exercises in one session and aerobic exercises in a separate session so that the phenomenon of training interference does not occur [164]. However, combined exercises that include aerobic exercises (40% and 60% of the Vo2max, 55% and 70% of the HRmax) and resistance exercises (40–60% 1RM with 10 to 15 repetitions) with a duration of 6 to 12 weeks can be effective in positively affecting exerkines. Thus, it is suggested that running on a treadmill, cycling, and resistance exercises are excellent choices for moderate-intensity exercise training [31,166].
Finally, moderate-intensity exercise may be the best approach to reduce disease severity in women with new strains of COVID-19. It is better to gradually enter moderate-intensity training after finishing the training period with low intensity. However, the type, duration, and intensity of physical exercise are very important. It is better to start with resistance exercises (exercises with weights and bodybuilding machines) with moderate intensity, and then use combined exercises in the following weeks. This can be especially important in women who have just been discharged from the hospital (see Figure 4 and Table 1).
Figure 4.
The effect of different type of exercise training on exerkines in women for immunization. SIT: sprint interval training, IL: interleukin, HRmax: heart rate maximum, Vo2max: maximum oxygen consumption.
4.2. High-Intensity Physical Exercise
It is well understood that, when high-intensity exercise occurs, these exercises can lead to an open-window phenomenon that ultimately suppresses the body’s immune system [167]. High-intensity exercise training leads to immune system dysfunction, inflammation, oxidative stress, and muscle damage and can endanger a person’s health. Markers of immune system function decrease from a few hours to a few days after a high-intensity exercise [165,168]. However, this response could vary among healthy, sick, trained, and untrained individuals [169]. High-intensity exercise does not appear appropriate for women recently discharged from the hospital due to COVID-19 or who are not physically fit. However, investigating the role of high-intensity training on exerkines can open a new process of training recommendations for women.
Previously reported data showed that high-intensity interval training with a short duration (1 session to 1 week) has no effect on myokines (IL-6, IL-8, follistatin, myostatin, and IL-15) [170]. However, increasing the duration of training between 4 and 8 weeks led to a positive change in myokines [156,171]. Moreover, these positive changes are seen with high-intensity exercises in cardiokines, hepatokines, and adipokines [68,172,173,174]. However, maneuvering on high-intensity interval training and exerkines cannot be a suitable strategy to reduce the severity of the disease of COVID-19. Since most people who suffer from the severe COVID-19 are obese and overweight, high-intensity exercise is not a suitable strategy for them. Therefore, high-intensity interval training can be introduced once a week as a shock now (exercise with an intensity of 75% to 90% of HRmax and 65 to 95% of Vo2max) (see Figure 4 and Table 1).
5. Discussion
Although exercise’s health and disease benefits are well known, our understanding of the underlying mechanisms is still rudimentary. However, studies in the field of exerkines are expanding to give us a better experience of this pathway. Exerkines are increasingly recognized as critical mediators of exercise-related changes and health benefits, particularly in their role in interorganismic and systemic communication and co-ordination. However, many research gaps related to diseases can still be filled by examining exerkines. As COVID-19 spreads worldwide, many studies have been published, all of which report the effects of physical exercise alone on muscles and quality of life after hospital discharge [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. However, published studies have generally reported muscle strength after rehabilitation exercises and have not looked specifically at the underlying mechanisms affecting muscle or other organs [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. For this purpose, a review study was conducted to investigate the importance of exerkines in COVID-19 patients discharged from the hospital.
5.1. Findings on Exerkines and Women COVID-19 Patients
During the illness, COVID-19 patients experience decreased muscle strength, increased blood pressure, fatigue, and decreased performance. In more severe cases, it can lead to death. Women who are obese and overweight suffer from high severity of the disease. The mechanism of why obese people suffer from this disease more than thin people is unknown. Nevertheless, recent studies have shown that obese people express more ACE2 receptors on adipose tissue than lean people. This can result in more binding of SARS-CoV-2 spike protein to its receptor. Several studies have shown that COVID-19 in obese people can lead to significant behavioral changes in adipokines (increase in leptin, decrease in adiponectin, increase in resistin, and decrease in omentin). These behavioral changes can lead to increased inflammation in the body. Increased inflammation in adipose tissue can affect other organs, such as muscle, and lead to muscle atrophy or decreased strength.
The current review study investigated the importance of exerkines during illness (Figure 3 and Table 1). In this section, we examined various physical exercise and physical activity interventions on exerkines to determine the importance of exerkines in COVID-19 patients. Resistance exercises with low intensity seem to affect myokines’ positive changes (Figure 3 and Table 1). In this regard, it seems that resistance training with low intensity also affects cardiokines (Figure 3 and Table 1). However, low-intensity training did not significantly affect other exerkines, i.e., hepatokines and adipokines. In this section, we also reviewed other physical exercise interventions that do not seem effective for the COVID-19 patient, focusing on exerkines. The reason for not affecting these exerkines can be due to the low intensity or short duration of the physical exercise intervention (maybe yoga exercises are only helpful for reducing stress). In this section, we were looking for the best physical exercise recommendation that can be effective for COVID-19 patients, and it seems that resistance training can be a suitable recommendation. So, it seems that resistance training can be performed with training bands, light weights, or hand resistance. However, we found out in this section that training during illness should be less than 20 min so as not to cause fatigue. Using the Borg scale, which can be in the range of 3 out of 10, effectively controls the intensity of the exercise. Therefore, it is recommended that low-intensity resistance training can be effective as a functional strategy in COVID-19 patients (Figure 3 and Table 1).
5.2. Findings on Exerkines and Women COVID-19 Patients (after Discharge from the Hospital)
In the second part, we discussed the importance of exerkines in patients with COVID-19 discharged from the hospital. Which intensity and which type of training can shorten the rehabilitation period and increase the effectiveness is very important. Because the new strains of COVID-19 (Omicron) are increasing, which can reduce the severity of the disease in the case of new strains, most of the published studies have reported the importance of moderate- to low-intensity rehabilitation exercises that included resistance and aerobic exercises on muscle strength, sleep quality, blood pressure, walking, and overall quality of life. However, these results report a general view of these changes after exercise, which cannot be a precise physical exercise recommendation. The idea of exerkines in this study focused on the target of basic mechanisms after hospital discharge.
In this section, it was found that moderate-intensity aerobic and resistance exercises can have the most significant effects on adipokines and myokines, respectively (Figure 4 and Table 1), and there were limited studies related to hepatokines and cardiokines. It seems that acute exercises with fewer than four weeks cannot be a good idea to affect the exerkines, and the most significant effect was seen between 4 and 12 weeks (Figure 4 and Table 1). Resistance training using exercise machines provides more safety than free weights. After reviewing the studies, we found that, in the aerobic exercise section, running, cycling, and swimming can have the greatest effect on the exerkines (Table 1). Therefore, it seems that the best training advice can be resistance training with gym equipment and aerobic training (running). Not that we report that other physical exercise interventions are ineffective, but their significance in exerkines is still lacking. Of course, yoga or TRX exercises were also investigated, which do not seem to affect exerkines.
5.3. Conclusions
The review of studies showed that resistance training (weight training or resistance device) with low to moderate intensity could increase muscle strength and endurance and reduce inflammation by changing myokines. However, at high intensity, an increase in inflammation was seen. It can be suitable for COVID-19 patients discharged from the hospital (to return to a quality of life). This kind of resistance training with low to moderate intensity can reduce blood pressure, reduce inflammation, improve the function of the body’s immune system, and increase metabolism by changing cardiokines, adipokines, and hepatokines. TRX exercises performed with body weight appear to be effective for patients with low to moderate severity of COVID-19. However, in relation to yoga exercises, no study was found that reported stimulation of exerkines. A review of studies found that low- to moderate-intensity swimming can lead to stimulation of exerkines, which can be appropriate after hospital discharge. However, low-intensity walking and moderate-intensity running could have the best stimulation on exerkines, leading to increased immune system function, reduced inflammation, improved strength, increased muscular and cardiovascular endurance, and increased metabolism. Moreover, cycling, like walking and running, had the best effect on stimulating exerkines.
5.4. Limitations and Prospects for Future Research
Exerkines are a very promising idea for future research initiatives and, with their high potential as biomarkers to predict outcomes and facilitate personalized physical exercise programs to improve health and reduce disease, they can fill many scientific gaps. In this study, we examined four important exerkines that could effectively manage COVID-19, but other exerkines could be considered in prospective studies. For example, more than 600 myokines have been discovered so far, but we considered six of them (8). One of the limitations of this study was that we only could examine four important exerkines, and we could not examine the other two, hepatokine and neurokine. Unfortunately, technical issues have limited the investigation of the effects of exerkines on the human brain; on the other hand, baptokines have not been specifically looked at. This can be a good recommendation for future research to see how these two exerkines change with different physical exercise interventions. The current study focused on women, and it is suggested to investigate the importance of exerkines in men, older adults, or people with underlying diseases or focus on COVID-19. By examining the idea of exerkines in this study, it was found that studies using rehabilitation exercises reported a general view of changes. It is suggested that future studies address the importance of exerkines in rehabilitation exercises related to patients with COVID-19 discharged from the hospital. Considering that it has recently been determined that most ACE2 receptor is expressed on fat tissue and adipokines cause many behavioral changes, these behavioral changes in adipose tissue seem to affect other exerkines, such as myokine and hepatokine. It was also found that most of the patients with COVID-19 were obese and overweight, but the researchers did not examine the changes in fat tissue exerkine. It is suggested that researchers should investigate the importance of exerkines after discharge from the hospital or during illness in future studies. One of the points not seen in these studies was the examination of combined exercises. In future studies, researchers should focus on the importance of combined exercises and exerkines with a focus on COVID-19. It is also suggested that researchers investigate cytokine and interleukin responses during postexercise COVID-19 disease in future studies. As discussed, nutrition can influence the effect of exercise on exerkines by improving immune function [175]. It is suggested to investigate the importance of nutrition in combination with exerkines in future studies.
Author Contributions
Idea, Conceptualization, Project Administration and editing, K.S.; writing—original draft preparation, A.H.A.H. and K.K.; collected references and Investigation, S.J., E.A. and S.A. and Review & Editing, R.T.R. and A.K. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest upon submitting this article.
Funding Statement
This research has received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schett G., Manger B., Simon D., Caporali R. COVID-19 revisiting inflammatory pathways of arthritis. Nat. Rev. Rheumatol. 2020;16:465–470. doi: 10.1038/s41584-020-0451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W., Chua B.Y., Selva K.J., Kedzierski L., Ashhurst T.M., Haycroft E.R., Shoffner-Beck S.K., Hensen L., Boyd D.F., James F., et al. SARS-CoV-2 infection results in immune responses in the respiratory tract and peripheral blood that suggest mechanisms of disease severity. Nat. Commun. 2022;13:2774. doi: 10.1038/s41467-022-30088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu W., Rohli K.E., Yang S., Jia P. Impact of obesity on COVID-19 patients. J. Diabetes Complicat. 2021;35:107817. doi: 10.1016/j.jdiacomp.2020.107817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steenblock C., Hassanein M., Khan E.G., Yaman M., Kamel M., Barbir M., Lorke D.E., Everett D., Bejtullah S., Lohmann T. Obesity and COVID-19: What are the consequences? Horm. Metab. Res. 2022;54:496–502. doi: 10.1055/a-1878-9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q., He Q., Wang Z., Liu Y., Liu L. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 6.Soares M.N., Eggelbusch M., Naddaf E., Gerrits K.H., van der Schaaf M., van den Borst B., Wiersinga W.J., van Vugt M., Weijs P.J., Murray A.J. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J. Cachexia Sarcopenia Muscle. 2022;13:11–22. doi: 10.1002/jcsm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moro T., Paoli A. When COVID-19 affects muscle: Effects of quarantine in older adults. Eur. J. Transl. Myol. 2020;30:9069. doi: 10.4081/ejtm.2020.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Stefano V., Battaglia G., Giustino V., Gagliardo A., D’Aleo M., Giannini O., Palma A., Brighina F. Significant reduction of physical activity in patients with neuromuscular disease during COVID-19 pandemic: The long-term consequences of quarantine. J. Neurol. 2021;268:20–26. doi: 10.1007/s00415-020-10064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., Silva J., Mao T., Oh J.E., Tokuyama M. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi T., Iwasaki A. Sex differences in immune responses. Science. 2021;371:347–348. doi: 10.1126/science.abe7199. [DOI] [PubMed] [Google Scholar]
- 12.Burki T. The indirect impact of COVID-19 on women. Lancet Infect. Dis. 2020;20:904–905. doi: 10.1016/S1473-3099(20)30568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Gender and COVID-19: Advocacy Brief, 14 May 2020. World Health Organization; Geneva, Switzerland: 2020. [Google Scholar]
- 14.Boniol M., McIsaac M., Xu L., Wuliji T., Diallo K., Campbell J. Gender Equity in the Health Workforce: Analysis of 104 Countries. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 15.Connor J., Madhavan S., Mokashi M., Amanuel H., Johnson N.R., Pace L.E., Bartz D. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: A review. Soc. Sci. Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed S.B., Dumanski S.M. Sex, gender and COVID-19: A call to action. Can. J. Public Health. 2020;111:980–983. doi: 10.17269/s41997-020-00417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United Nations Policy Brief: The Impact of COVID-19 on Women—9 April 2020. 2020. [(accessed on 3 June 2022)]. Available online: https://unsdg.un.org/resources/policy-brief-impact-covid-19-women.
- 18.Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324:1381–1383. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- 19.Cooper A.J., Gupta S.R., Moustafa A.F., Chao A.M. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr. Obes. Rep. 2021;10:458–466. doi: 10.1007/s13679-021-00453-x. [DOI] [PubMed] [Google Scholar]
- 20.Leblanc V., Hudon A.M., Royer M.M., Corneau L., Dodin S., Bégin C., Lemieux S. Differences between men and women in dietary intakes and metabolic profile in response to a 12-week nutritional intervention promoting the Mediterranean diet. J. Nutr. Sci. 2015;4:e13. doi: 10.1017/jns.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen H., Klein S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021;12:720952. doi: 10.3389/fimmu.2021.720952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelzig K.E., Canepa-Escaro F., Schiliro M., Berdnikovs S., Prakash Y., Chiarella S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020;318:L1280–L1281. doi: 10.1152/ajplung.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupte M., Thatcher S.E., Boustany-Kari C.M., Shoemaker R., Yiannikouris F., Zhang X., Karounos M., Cassis L.A. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fishman J., Boyar R.M., Hellman L. Influence of body weight on estradiol metabolism in young women. J. Clin. Endocrinol. Metab. 1975;41:989–991. doi: 10.1210/jcem-41-5-989. [DOI] [PubMed] [Google Scholar]
- 25.Khoramipour K., Basereh A., Hekmatikar A.A., Castell L., Ruhee R.T., Suzuki K. Physical activity and nutrition guidelines to help with the fight against COVID-19. J. Sports Sci. 2021;39:101–107. doi: 10.1080/02640414.2020.1807089. [DOI] [PubMed] [Google Scholar]
- 26.Shariat A., Cleland J.A., Hakakzadeh A. Home-based exercises during the COVID-19 quarantine situation for office workers: A commentary. Work. 2020;66:381–382. doi: 10.3233/WOR-203190. [DOI] [PubMed] [Google Scholar]
- 27.Chow L.S., Gerszten R.E., Taylor J.M., Pedersen B.K., van Praag H., Trappe S., Febbraio M.A., Galis Z.S., Gao Y., Haus J.M., et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022;18:273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. 2019;9:223. doi: 10.3390/biom9060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piercy K.L., Troiano R.P., Ballard R.M., Carlson S.A., Fulton J.E., Galuska D.A., George S.M., Olson R.D. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmadi Hekmatikar A.H., Ferreira Júnior J.B., Shahrbanian S., Suzuki K. Functional and Psychological Changes after Exercise Training in Post-COVID-19 Patients Discharged from the Hospital: A PRISMA-Compliant Systematic Review. Int. J. Environ. Res. Public Health. 2022;19:2290. doi: 10.3390/ijerph19042290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoramipour K., Hekmatikar A.A., Sotvan H. An overview of Fatmax and MFO in exercise. Razi J. Med. Sci. 2020;27:49–59. [Google Scholar]
- 33.Tayebi S.M., Hekmatikar A.A., Ghanbari-Niaki A., Fathi R. Ghrelin behavior in exercise and training. J Med. Sci. 2020;27:85–111. [Google Scholar]
- 34.Ahmadi Hekmatikar A., Haghshenas R., Mohammad Sadeghipor A. The effect of carbohydrate supplementation and pure water on interleukin 10, glucose and hematologicalindexes in male football players. Sport Physiol. Manag. Investig. 2019;11:135–145. [Google Scholar]
- 35.Bull F.C., Al-Ansari S.S., Biddle S., Borodulin K., Buman M.P., Cardon G., Carty C., Chaput J.-P., Chastin S., Chou R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safdar A., Saleem A., Tarnopolsky M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016;12:504–517. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- 37.Abodonya A.M., Abdelbasset W.K., Awad E.A., Elalfy I.E., Salem H.A., Elsayed S.H. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine. 2021;100:e25339. doi: 10.1097/MD.0000000000025339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Chikhanie Y., Veale D., Schoeffler M., Pépin J.L., Verges S., Hérengt F. Effectiveness of pulmonary rehabilitation in COVID-19 respiratory failure patients post-ICU. Respir. Physiol. Neurobiol. 2021;287:103639. doi: 10.1016/j.resp.2021.103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capin J.J., Jolley S.E., Morrow M., Connors M., Hare K., MaWhinney S., Nordon-Craft A., Rauzi M., Flynn S., Stevens-Lapsley J.E., et al. Safety, feasibility and initial efficacy of an app-facilitated telerehabilitation (AFTER) programme for COVID-19 survivors: A pilot randomised study. BMJ Open. 2022;12:e061285. doi: 10.1136/bmjopen-2022-061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clavario P., De Marzo V., Lotti R., Barbara C., Porcile A., Russo C., Beccaria F., Bonavia M., Bottaro L.C., Caltabellotta M. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int. J. Cardiol. 2021;340:113–118. doi: 10.1016/j.ijcard.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalbosco-Salas M., Torres-Castro R., Rojas Leyton A., Morales Zapata F., Henríquez Salazar E., Espinoza Bastías G., Beltrán Díaz M.E., Tapia Allers K., Mornhinweg Fonseca D., Vilaró J. Effectiveness of a Primary Care Telerehabilitation Program for Post-COVID-19 Patients: A Feasibility Study. J. Clin. Med. 2021;10:4428. doi: 10.3390/jcm10194428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.do Amaral V.T., Viana A.A., Heubel A.D., Linares S.N., Martinelli B., Witzler P.H.C., de Souza Zanini G., Mendes R., Ciolac E. Cardiovascular, Respiratory, and Functional Effects of Home-based Exercise Training after COVID-19 Hospitalization. Med. Sci. Sports Exerc. 2022;54:1795–1803. doi: 10.1249/MSS.0000000000002977. [DOI] [PubMed] [Google Scholar]
- 43.Dorelli G., Braggio M., Gabbiani D., Busti F., Caminati M., Senna G., Girelli D., Laveneziana P., Ferrari M., Sartori G., et al. Importance of Cardiopulmonary Exercise Testing amongst Subjects Recovering from COVID-19. Diagnostics. 2021;11:507. doi: 10.3390/diagnostics11030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everaerts S., Heyns A., Langer D., Beyens H., Hermans G., Troosters T., Gosselink R., Lorent N., Janssens W. COVID-19 recovery: Benefits of multidisciplinary respiratory rehabilitation. BMJ Open Respir. Res. 2021;8:e000837. doi: 10.1136/bmjresp-2020-000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gloeckl R., Leitl D., Jarosch I., Schneeberger T., Nell C., Stenzel N., Vogelmeier C.F., Kenn K., Koczulla A.R. Benefits of pulmonary rehabilitation in COVID-19: A prospective observational cohort study. ERJ Open Res. 2021;7:00108–02021. doi: 10.1183/23120541.00108-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayden M.C., Limbach M., Schuler M., Merkl S., Schwarzl G., Jakab K., Nowak D., Schultz K. Effectiveness of a three-week inpatient pulmonary rehabilitation program for patients after COVID-19: A prospective observational study. Int. J. Environ. Res. Public Health. 2021;18:9001. doi: 10.3390/ijerph18179001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kortianou E.A., Tsimouris D., Mavronasou A., Lekkas S., Kazatzis N., Apostolara Z.E., Isakoglou M., Dimakou G., Barmparessou Z., Tsikrika S. Application of a home-based exercise program combined with tele-rehabilitation in previously hospitalized patients with COVID-19: A feasibility, single-cohort interventional study. Pneumon. 2022;35:12. doi: 10.18332/pne/146521. [DOI] [Google Scholar]
- 48.Liu K., Zhang W., Yang Y., Zhang J., Li Y., Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020;39:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mapelli M., Vignati C., Gugliandolo P., Fumagalli D., Agostoni P. Feasibility of remote home monitoring with a T-shirt wearable device in post-recovery COVID-19 patients. J. Cardiovasc. Med. 2021;22:860–863. doi: 10.2459/JCM.0000000000001165. [DOI] [PubMed] [Google Scholar]
- 50.Paneroni M., Vitacca M., Bernocchi P., Bertacchini L., Scalvini S. Feasibility of tele-rehabilitation in survivors of COVID-19 pneumonia. Pulmonology. 2022;28:152. doi: 10.1016/j.pulmoe.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Philippot A., Moulin P., Charon M.-H., Balestra C., Dubois V., de Timary P., De Volder A., Bleyenheuft Y., Lambrechts K. Feasibility of Online High-Intensity Interval Training (HIIT) on Psychological Symptoms in Students in Lockdown During the COVID-19 Pandemic: A Randomized Controlled Trial. Front. Psychiatry. 2022;13:904283. doi: 10.3389/fpsyt.2022.904283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piquet V., Luczak C., Seiler F., Monaury J., Martini A., Ward A.B., Gracies J.M., Motavasseli D. Do Patients With COVID-19 Benefit from Rehabilitation? Functional Outcomes of the First 100 Patients in a COVID-19 Rehabilitation Unit. Arch. Phys. Med. Rehabil. 2021;102:1067–1074. doi: 10.1016/j.apmr.2021.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez-Blanco C., Gonzalez-Gerez J.J., Bernal-Utrera C., Anarte-Lazo E., Perez-Ale M., Saavedra-Hernandez M. Short-Term Effects of a Conditioning Telerehabilitation Program in Confined Patients Affected by COVID-19 in the Acute Phase. A Pilot Randomized Controlled Trial. Medicina. 2021;57:684. doi: 10.3390/medicina57070684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spruit M.A., Holland A.E., Singh S.J., Tonia T., Wilson K.C., Troosters T. COVID-19: Interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society-and American Thoracic Society-coordinated international task force. Eur. Respir. J. 2020;56:2002197. doi: 10.1183/13993003.02197-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe R., Kojima M., Yasuoka M., Kimura C., Kamiji K., Otani T., Tsujimura S., Fujita H., Nogimura A., Ozeki S. Home-Based Frailty Prevention Program for Older Women Participants of Kayoi-No-Ba during the COVID-19 Pandemic: A Feasibility Study. Int. J. Environ. Res. Public Health. 2022;19:6609. doi: 10.3390/ijerph19116609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zampogna E., Paneroni M., Belli S., Aliani M., Gandolfo A., Visca D., Bellanti M.T., Ambrosino N., Vitacca M. Pulmonary rehabilitation in patients recovering from COVID-19. Respiration. 2021;100:416–422. doi: 10.1159/000514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peiris S., Mesa H., Aysola A., Manivel J., Toledo J., Borges-Sa M., Aldighieri S., Reveiz L. Pathological findings in organs and tissues of patients with COVID-19: A systematic review. PLoS ONE. 2021;16:e0250708. doi: 10.1371/journal.pone.0250708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhalla V., Blish C.A., South A.M. A historical perspective on ACE2 in the COVID-19 era. J. Hum. Hypertens. 2021;35:935–939. doi: 10.1038/s41371-020-00459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zarei M., Bose D., Nouri-Vaskeh M., Tajiknia V., Zand R., Ghasemi M. Long-term side effects and lingering symptoms post COVID-19 recovery. Rev. Med. Virol. 2022;32:e2289. doi: 10.1002/rmv.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barisic A., Leatherdale S.T., Kreiger N. Importance of frequency, intensity, time and type (FITT) in physical activity assessment for epidemiological research. Can. J. Public Health. 2011;102:174–175. doi: 10.1007/BF03404889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leuchtmann A.B., Adak V., Dilbaz S., Handschin C. The role of the skeletal muscle secretome in mediating endurance and resistance training adaptations. Front. Physiol. 2021;12:1296. doi: 10.3389/fphys.2021.709807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wahren J., Felig P., Ahlborg G., Jorfeldt L. Glucose metabolism during leg exercise in man. J. Clin. Investig. 1971;50:2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magliulo L., Bondi D., Pini N., Marramiero L., Di Filippo E.S. The wonder exerkines—Novel insights: A critical state-of-the-art review. Mol. Cell. Biochem. 2021;447:105–113. doi: 10.1007/s11010-021-04264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zinman B., Ruderman N., Campaigne B.N., Devlin J.T., Schneider S.H., American Diabetes Association Physical activity/exercise and diabetes mellitus. Diabetes Care. 2003;26:s73–s77. doi: 10.2337/diacare.26.2007.s73. [DOI] [PubMed] [Google Scholar]
- 65.Ataeinosrat A., Saeidi A., Abednatanzi H., Rahmani H., Daloii A.A., Pashaei Z., Hojati V., Basati G., Mossayebi A., Laher I. Intensity Dependent Effects of Interval Resistance Training on Myokines and Cardiovascular Risk Factors in Males with Obesity. Front. Endocrinol. 2022;13:895512. doi: 10.3389/fendo.2022.895512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belardinelli R., Georgiou D., Scocco V., Barstow T.J., Purcaro A. Low intensity exercise training in patients with chronic heart failure. J. Am. Coll. Cardiol. 1995;26:975–982. doi: 10.1016/0735-1097(95)00267-1. [DOI] [PubMed] [Google Scholar]
- 67.Yaman B., Akpınar O., Kemal H.S., Cerit L., Sezenöz B., Açıkgöz E., Duygu H. The beneficial effect of low-intensity exercise on cardiac performance assessed by two-dimensional speckle tracking echocardiography. Echocardiography. 2020;37:1989–1999. doi: 10.1111/echo.14891. [DOI] [PubMed] [Google Scholar]
- 68.Hanani M., Gaeini A., Nuri R., Hemati Nafar M. The effect of intensity of interval training on expression of cordial myostatin and follistatin proteins in rats with myocardial infarction. Razi J. Med. Sci. 2020;27:239–252. [Google Scholar]
- 69.Kon M., Ebi Y., Nakagaki K. Effects of acute sprint interval exercise on follistatin-like 1 and apelin secretions. Arch. Physiol. Biochem. 2021;127:223–227. doi: 10.1080/13813455.2019.1628067. [DOI] [PubMed] [Google Scholar]
- 70.Willis S.A., Sargeant J.A., Thackray A.E., Yates T., Stensel D.J., Aithal G.P., King J.A. Effect of exercise intensity on circulating hepatokine concentrations in healthy men. Appl. Physiol. Nutr. Metab. 2019;44:1065–1072. doi: 10.1139/apnm-2018-0818. [DOI] [PubMed] [Google Scholar]
- 71.Görgens S.W., Eckardt K., Jensen J., Drevon C.A., Eckel J. Exercise and Regulation of Adipokine and Myokine Production. Prog. Mol. Biol. Transl. Sci. 2015;135:313–336. doi: 10.1016/bs.pmbts.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Ingerslev B., Hansen J.S., Hoffmann C., Clemmesen J.O., Secher N.H., Scheler M., Hrabĕ de Angelis M., Häring H.U., Pedersen B.K., Weigert C., et al. Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and cAMP. Mol. Metab. 2017;6:1286–1295. doi: 10.1016/j.molmet.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim K.H., Kim S.H., Min Y.-K., Yang H.-M., Lee J.-B., Lee M.-S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS ONE. 2013;8:e63517. doi: 10.1371/journal.pone.0063517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sargeant J.A., Aithal G.P., Takamura T., Misu H., Takayama H., Douglas J.A., Turner M.C., Stensel D.J., Nimmo M.A., Webb D.R., et al. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and overweight/obese men. Appl. Physiol. Nutr. Metab. 2018;43:482–490. doi: 10.1139/apnm-2017-0639. [DOI] [PubMed] [Google Scholar]
- 75.Misu H., Takayama H., Saito Y., Mita Y., Kikuchi A., Ishii K.A., Chikamoto K., Kanamori T., Tajima N., Lan F., et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 2017;23:508–516. doi: 10.1038/nm.4295. [DOI] [PubMed] [Google Scholar]
- 76.Golbidi S., Laher I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014;2014:726861. doi: 10.1155/2014/726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park K.M., Park S.C., Kang S. Effects of resistance exercise on adipokine factors and body composition in pre- and postmenopausal women. J. Exerc. Rehabil. 2019;15:676–682. doi: 10.12965/jer.1938368.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonzalez-Gil A.M., Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: A review. Nutrients. 2020;12:1899. doi: 10.3390/nu12061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Méry G., Epaulard O., Borel A.-L., Toussaint B., Le Gouellec A. COVID-19: Underlying adipokine storm and angiotensin 1-7 umbrella. Front. Immunol. 2020;11:1714. doi: 10.3389/fimmu.2020.01714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mosaffa N., Abedi B. The Effect of Eight Week Interval training on The Serum VCAM-1 and PAI-1 in Obesity Women. J. Sport Biosci. 2018;10:193–206. [Google Scholar]
- 81.Voss S., Nikolovski Z., Bourdon P., Alsayrafi M., Schumacher Y. The effect of cumulative endurance exercise on leptin and adiponectin and their role as markers to monitor training load. Biol. Sport. 2016;33:23–28. doi: 10.5604/20831862.1180173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jamurtas A.Z., Theocharis V., Koukoulis G., Stakias N., Fatouros I., Kouretas D., Koutedakis Y. The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur. J. Appl. Physiol. 2006;97:122–126. doi: 10.1007/s00421-006-0169-x. [DOI] [PubMed] [Google Scholar]
- 83.Kraemer R.R., Aboudehen K.S., Carruth A.K., Durand R.J., Acevedo E.O., Hebert E.P., Johnson L.G., Castracane V.D. Adiponectin responses to continuous and progressively intense intermittent exercise. Med. Sci. Sport. Exerc. 2003;35:1320–1325. doi: 10.1249/01.MSS.0000079072.23998.F3. [DOI] [PubMed] [Google Scholar]
- 84.Jürimäe J., Hofmann P., Jürimäe T., Mäestu J., Purge P., Wonisch M., Pokan R., Von Duvillard S. Plasma adiponectin response to sculling exercise at individual anaerobic threshold in college level male rowers. Int. J. Sports Med. 2006;27:272–277. doi: 10.1055/s-2005-865661. [DOI] [PubMed] [Google Scholar]
- 85.Racil G., Ben Ounis O., Hammouda O., Kallel A., Zouhal H., Chamari K., Amri M. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur. J. Appl. Physiol. 2013;113:2531–2540. doi: 10.1007/s00421-013-2689-5. [DOI] [PubMed] [Google Scholar]
- 86.Ferguson M.A., White L.J., McCoy S., Kim H.-W., Petty T., Wilsey J. Plasma adiponectin response to acute exercise in healthy subjects. Eur. J. Appl. Physiol. 2004;91:324–329. doi: 10.1007/s00421-003-0985-1. [DOI] [PubMed] [Google Scholar]
- 87.Vardar S.A., Karaca A., Güldiken S., Palabıyık O., Süt N., Demir A.M. High-intensity interval training acutely alters plasma adipokine levels in young overweight/obese women. Arch. Physiol. Biochem. 2018;124:149–155. doi: 10.1080/13813455.2017.1369998. [DOI] [PubMed] [Google Scholar]
- 88.De Souza D.C., Matos V.A., Dos Santos V.O., Medeiros I.F., Marinho C.S., Nascimento P.R., Dorneles G.P., Peres A., Müller C.H., Krause M., et al. Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front. Physiol. 2018;9:567. doi: 10.3389/fphys.2018.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagendra H. Yoga for COVID-19. Int. J. Yoga. 2020;13:87. doi: 10.4103/ijoy.IJOY_27_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yaacoub S., Khabsa J., El-Khoury R., El-Harakeh A., Lotfi T., Saad Z., Itani Z., Khamis A.M., El Mikati I., Cuello-Garcia C.A., et al. COVID-19 transmission during swimming-related activities: A rapid systematic review. BMC Infect. Dis. 2021;21:1112. doi: 10.1186/s12879-021-06802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vitale J.A., Bonato M., Borghi S., Messina C., Albano D., Corbetta S., Sconfienza L.M., Banfi G. Home-based resistance training for older subjects during the COVID-19 outbreak in Italy: Preliminary results of a six-months RCT. Int. J. Environ. Res. Public Health. 2020;17:9533. doi: 10.3390/ijerph17249533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Budi D.R., Widyaningsih R., Nur L., Agustan B., Dwi D., Qohhar W., Asnaldi A. Cycling during COVID-19 pandemic: Sports or lifestyle. Int. J. Hum. Mov. Sports Sci. 2021;9:765–771. doi: 10.13189/saj.2021.090422. [DOI] [Google Scholar]
- 93.Ahn N., Kim K. Effects of Aerobic and Resistance Exercise on Myokines in High Fat Diet-Induced Middle-Aged Obese Rats. Int. J. Environ. Res. Public Health. 2020;17:2685. doi: 10.3390/ijerph17082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Urzi F., Marusic U., Ličen S., Buzan E. Effects of elastic resistance training on functional performance and Myokines in older women—A randomized controlled trial. J. Am. Med. Dir. Assoc. 2019;20:830–834.e2. doi: 10.1016/j.jamda.2019.01.151. [DOI] [PubMed] [Google Scholar]
- 95.Zunner B.E., Wachsmuth N.B., Eckstein M.L., Scherl L., Schierbauer J.R., Haupt S., Stumpf C., Reusch L., Moser O. Myokines and Resistance Training: A Narrative Review. Int. J. Mol. Sci. 2022;23:3501. doi: 10.3390/ijms23073501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eckardt K., Görgens S.W., Raschke S., Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia. 2014;57:1087–1099. doi: 10.1007/s00125-014-3224-x. [DOI] [PubMed] [Google Scholar]
- 97.Cornish S.M., Bugera E.M., Duhamel T.A., Peeler J.D., Anderson J.E. A focused review of myokines as a potential contributor to muscle hypertrophy from resistance-based exercise. Eur. J. Appl. Physiol. 2020;120:941–959. doi: 10.1007/s00421-020-04337-1. [DOI] [PubMed] [Google Scholar]
- 98.Kang S., Park I., Lim S.-T. Changing Levels of Myokines after Aerobic Training and Resistance Training in Post-Menopausal Obese Females: A Randomized Controlled Trial. Sustainability. 2020;12:8413. doi: 10.3390/su12208413. [DOI] [Google Scholar]
- 99.Catoire M., Mensink M., Kalkhoven E., Schrauwen P., Kersten S. Identification of human exercise-induced myokines using secretome analysis. Physiol. Genom. 2014;46:256–267. doi: 10.1152/physiolgenomics.00174.2013. [DOI] [PubMed] [Google Scholar]
- 100.Hosseini M., Bambaeichi E., Sarir H., Kargarfard M. Effect of training with or without Ziziphus jujuba extract on cardiokines in heart tissue of myocardial infarcted rats. Int. J. Prev. Med. 2019;10:103. doi: 10.4103/ijpvm.IJPVM_367_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Piano A., de Mello M.T., Sanches P.d.L., da Silva P.L., Campos R.M., Carnier J., Corgosinho F., Foschini D., Masquio D.L., Tock L., et al. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur. J. Gastroenterol. Hepatol. 2012;24:1313–1324. doi: 10.1097/MEG.0b013e32835793ac. [DOI] [PubMed] [Google Scholar]
- 102.Ward L.J., Nilsson S., Hammar M., Lindh-Åstrand L., Berin E., Lindblom H., Spetz Holm A.-C., Rubér M., Li W. Resistance training decreases plasma levels of adipokines in postmenopausal women. Sci. Rep. 2020;10:19837. doi: 10.1038/s41598-020-76901-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Negaresh R., Motl R.W., Mokhtarzade M., Dalgas U., Patel D., Shamsi M.M., Majdinasab N., Ranjbar R., Zimmer P., Baker J.S. Effects of exercise training on cytokines and adipokines in multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2018;24:91–100. doi: 10.1016/j.msard.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 104.Akbarpour M., Ozgoli G., Aryamanesh Z., Mojab F., Majd H.A., Karami M., Amirmozaffari N., Dody M., Bazzazi N., Akbarzadeh S. The Effect of Resistance Training on Serum Levels of Adipokine and Inflammatory Markers of Cardiovascular Disease in Obese Men. Qom Univ. Med. Sci. J. 2013;7:1–10. [Google Scholar]
- 105.Banitalebi E., Mardanpour Shahrekordi Z., Kazemi A.R., Bagheri L., Amani Shalamzari S., Faramarzi M. Comparing the effects of eight weeks of combined training (Endurance and Resistance) in different orders on inflammatory factors and adipokines among elderly females. Women’s Health Bull. 2016;3:1–10. doi: 10.17795/whb-30990. [DOI] [Google Scholar]
- 106.Jensen-Cody S.O., Potthoff M.J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metab. 2021;44:101138. doi: 10.1016/j.molmet.2020.101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keihanian A., Arazi H., Kargarfard M. Effects of aerobic versus resistance training on serum fetuin-A, fetuin-B, and fibroblast growth factor-21 levels in male diabetic patients. Physiol. Int. 2019;106:70–80. doi: 10.1556/2060.106.2019.01. [DOI] [PubMed] [Google Scholar]
- 108.Hashemi Jokar S.E., Peeri M., Azarbayjani M.A. The effect of high, low and moderate intensity circuit resistance training on the levels of hepatocyte-derived fibrinogen-related protein 1 (HFREP1) and lipid profile in obese postmenopausal women. J. Basic Res. Med. Sci. 2022;9:61–70. [Google Scholar]
- 109.Keihaniyan A., Arazi H., Kargarfard M. The Effect of Eight-Week Resistance and Aerobic Training on Lipid Profile and Serum Levels of Hepatokine HFREP1 in Obese Men with Type 2 Diabetes. Sport Physiol. 2018;10:85–98. [Google Scholar]
- 110.Ghorbanian B., Saberi Y. The Response of Some Hepatokines and Insulin Resistance to High Intensity Interval Training in Women with Non-Alcoholic Fatty Liver. J. Appl. Exerc. Physiol. 2020;16:57–71. [Google Scholar]
- 111.Manabe Y., Takagi M., Nakamura-Yamada M., Goto-Inoue N., Taoka M., Isobe T., Fujii N.L. Redox proteins are constitutively secreted by skeletal muscle. J. Physiol. Sci. 2014;64:401–409. doi: 10.1007/s12576-014-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rahimi M., Nazarali P., Alizadeh R. Pilates and TRX training methods can improve insulin resistance in overweight women by increasing an exercise-hormone, Irisin. J. Diabetes Metab. Disord. 2021;20:1455–1460. doi: 10.1007/s40200-021-00887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Timmons J.A., Baar K., Davidsen P.K., Atherton P.J. Is irisin a human exercise gene? Nature. 2012;488:E9–E10. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 114.Olarescu N.C., Ueland T., Godang K., Lindberg-Larsen R., Jørgensen J., Bollerslev J. Inflammatory adipokines contribute to insulin resistance in active acromegaly and respond differently to different treatment modalities. Eur. J. Endocrinol. 2014;170:39–48. doi: 10.1530/EJE-13-0523. [DOI] [PubMed] [Google Scholar]
- 115.Rahmatollahi M., Valizade-Orang A., Bahram M.E., Jafarnezhad A. Effect of 12 Weeks TRX Training on Irisin and Chemerin Profile in Male Older Adults. Sci. J. Rehabil. Med. 2021 doi: 10.22037/jrm.2021.115599.2765. [DOI] [Google Scholar]
- 116.Chaliki K., Delgado D., Dong D., Moreno M., Lee J., McCulloch P., Harris J., Lambert B. Acute Effects of a Single Session of Hot Yoga on Caloric Expenditure, Range of Motion, and Metabolism. Proc. Int. J. Exerc. Sci. Conf. Proc. 2019;2:61. [Google Scholar]
- 117.Cahn B.R., Goodman M.S., Peterson C.T., Maturi R., Mills P.J. Yoga, meditation and mind-body health: Increased BDNF, cortisol awakening response, and altered inflammatory marker expression after a 3-month yoga and meditation retreat. Front. Hum. Neurosci. 2017;11:315. doi: 10.3389/fnhum.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yadav R., Yadav R.K., Khadgawat R., Pandey R.M. Comparative efficacy of a 12 week yoga-based lifestyle intervention and dietary intervention on adipokines, inflammation, and oxidative stress in adults with metabolic syndrome: A randomized controlled trial. Transl. Behav. Med. 2019;9:594–604. doi: 10.1093/tbm/iby060. [DOI] [PubMed] [Google Scholar]
- 119.Supriya R., Yu A.P., Lee P.H., Lai C.W., Cheng K.K., Yau S.Y., Chan L.W., Yung B.Y., Siu P.M. Yoga training modulates adipokines in adults with high-normal blood pressure and metabolic syndrome. Scand. J. Med. Sci. Sports. 2018;28:1130–1138. doi: 10.1111/sms.13029. [DOI] [PubMed] [Google Scholar]
- 120.Yadav R., Yadav R.K., Khadgawat R., Mehta N. OS 28-06 beneficial effects of a 12-week yoga-based lifestyle intervention on cardio-metabolic risk factors and adipokines in subjects with pre-hypertension or hypertension. J. Hypertens. 2016;34:e252. doi: 10.1097/01.hjh.0000500572.10167.f4. [DOI] [Google Scholar]
- 121.Kapilevich L.V., Kironenko T., Zakharova A., Kabachkova A., Orlov S. Level of interleukins IL-6 and IL-15 in blood plasma of mice after forced swimming test. Bull. Exp. Biol. Med. 2017;163:10–13. doi: 10.1007/s10517-017-3725-y. [DOI] [PubMed] [Google Scholar]
- 122.Peng C.-C., Chen K.-C., Hsieh C.-L., Peng R.Y. Swimming exercise prevents fibrogenesis in chronic kidney disease by inhibiting the myofibroblast transdifferentiation. PLoS ONE. 2012;7:e37388. doi: 10.1371/journal.pone.0037388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakamura M., Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 124.Mohr M., Nordsborg N.B., Lindenskov A., Steinholm H., Nielsen H.P., Mortensen J., Weihe P., Krustrup P. High-intensity intermittent swimming improves cardiovascular health status for women with mild hypertension. Biomed. Res. Int. 2014;2014:728289. doi: 10.1155/2014/728289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zahraei H., Mogharnasi M., Afzalpour M.E., Fanaei H. The effect of 8 weeks of continuous and high intensity interval swimming on chemerin levels in liver and visceral fat tissues and insulin resistance in male rats with metabolic syndrome. J. Sport Exerc. Physiol. 2022;15:33–44. doi: 10.52547/joeppa.15.1.33. [DOI] [Google Scholar]
- 126.Cabrera A., Pineda W., del Pilar Correa Valencia N., Gutierrez M. Body mass conversion and improved insulin response in Colombian Paso horses subjected to a swimming training program. Comp. Exerc. Physiol. 2022;18:211–218. doi: 10.3920/CEP210024. [DOI] [Google Scholar]
- 127.Hansen J., Brandt C., Nielsen A.R., Hojman P., Whitham M., Febbraio M.A., Pedersen B.K., Plomgaard P. Exercise induces a marked increase in plasma follistatin: Evidence that follistatin is a contraction-induced hepatokine. Endocrinology. 2011;152:164–171. doi: 10.1210/en.2010-0868. [DOI] [PubMed] [Google Scholar]
- 128.Dobashi S., Nakamura A., Saito K., Ando D., Koyama K. Acute swimming exercise, but not exposure to moderate hypoxic conditions reduces circulating selenoprotein P levels in short-term, high-fat diet-fed rats. J. Phys. Fit. Sports Med. 2019;8:181–184. doi: 10.7600/jpfsm.8.181. [DOI] [Google Scholar]
- 129.Nazari M., Kordi M., Minasian V., Saffar Kohneh Quchan A.H. Ameliorating effect of 6-week swimming exercise on mice with experimental autoimmune encephalomyelitis (EAE) by reducing fetuin-A and increasing AMPK & NAD⁺ levels in liver tissue. Iran. J. Basic Med. Sci. 2022;25:1016–1020. doi: 10.22038/IJBMS.2022.65117.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Silva R., Bueno P., Avó L., Nonaka K., Selistre-Araújo H., Leal A. Effect of physical training on liver expression of activin A and follistatin in a nonalcoholic fatty liver disease model in rats. Braz. J. Med. Biol. Res. 2014;47:746–752. doi: 10.1590/1414-431X20143869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arabzadeh E., Samadian Z., Tofighi A., Tolouei Azar J. Alteration of follistatin-like 1, neuron-derived neurotrophic factor, and vascular endothelial growth factor in diabetic cardiac muscle after moderate-intensity aerobic exercise with insulin. Sport Sci. Health. 2020;16:491–499. doi: 10.1007/s11332-020-00631-9. [DOI] [Google Scholar]
- 132.Hutchinson K.A., Mohammad S., Garneau L., McInnis K., Aguer C., Adamo K.B. Examination of the myokine response in pregnant and non-pregnant women following an acute bout of moderate-intensity walking. Front. Physiol. 2019;10:1188. doi: 10.3389/fphys.2019.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Da Silva Lage V.K., de Paula F.A., Lima L.P., Santos J.N.V., Dos Santos J.M., Viegas Â.A., da Silva G.P., de Almeida H.C., Teixeira A.L.d.S.N., Leopoldino A.A.O. Plasma levels of myokines and inflammatory markers are related with functional and respiratory performance in older adults with COPD and sarcopenia. Exp. Gerontol. 2022;164:111834. doi: 10.1016/j.exger.2022.111834. [DOI] [PubMed] [Google Scholar]
- 134.Gmiat A., Mieszkowski J., Prusik K., Kortas J., Kochanowicz A., Radulska A., Lipiński M., Tomczyk M., Jaworska J., Antosiewicz J., et al. Changes in pro-inflammatory markers and leucine concentrations in response to Nordic Walking training combined with vitamin D supplementation in elderly women. Biogerontology. 2017;18:535–548. doi: 10.1007/s10522-017-9694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wahl P., Hein M., Achtzehn S., Bloch W., Mester J. Acute effects of superimposed electromyostimulation during cycling on myokines and markers of muscle damage. J. Musculoskelet. Neuronal. Interact. 2015;15:53. [PMC free article] [PubMed] [Google Scholar]
- 136.Llanos A.A., Krok J.L., Peng J., Pennell M.L., Vitolins M.Z., Degraffinreid C.R., Paskett E.D. Effects of a walking intervention using mobile technology and interactive voice response on serum adipokines among postmenopausal women at increased breast cancer risk. Horm. Cancer. 2014;5:98–103. doi: 10.1007/s12672-013-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neumayr G., Engler C., Lunger L., Lechleitner P. Effects of a One-week Vacation with Various Activity Programs on Metabolism and Adipokines. Int. J. Sports Med. 2021;42:703–707. doi: 10.1055/a-1297-4669. [DOI] [PubMed] [Google Scholar]
- 138.Tota Ł., Pilch W., Piotrowska A., Pałka T., Pilch P. The effect of 12-week-long nordic walking exercise on body composition, changes in lipid and carbohydrate metabolism indices, concentration of selected adipokines and calcidiol in healthy middle-aged women. Cent. Eur. J. Sport Sci. Med. 2017;20:69–80. doi: 10.18276/cej.2017.4-08. [DOI] [Google Scholar]
- 139.Seo D.-I., So W.-Y., Sung D.J. Changes in insulin resistance and adipokines in obese women following a 12-week programme of combined exercise training. S. Afr. J. Res. Sport Phys. Educ. Recreat. 2016;38:39–147. [Google Scholar]
- 140.Stefanyk L.E., Dyck D.J. The interaction between adipokines, diet and exercise on muscle insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:255–259. doi: 10.1097/MCO.0b013e328338236e. [DOI] [PubMed] [Google Scholar]
- 141.Babaei P., Hosseini R. Exercise training modulates adipokines dysregulations in metabolic syndrome. Sports Med. Health Sci. 2022;4:18–28. doi: 10.1016/j.smhs.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sargeant J.A. Ph.D. Thesis. Loughborough University; Loughborough, UK: 2018. Exercise and Insulin Sensitivity: Interaction with Intrahepatic Triglyceride and Hepatokines. [Google Scholar]
- 143.Garneau L., Parsons S.A., Smith S.R., Mulvihill E.E., Sparks L.M., Aguer C. Plasma myokine concentrations after acute exercise in non-obese and obese sedentary women. Front. Physiol. 2020;11:18. doi: 10.3389/fphys.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chikamoto K., Misu H., Takayama H., Kikuchi A., Ishii K.-A., Lan F., Takata N., Tajima-Shirasaki N., Takeshita Y., Tsugane H., et al. Rapid response of the steatosis-sensing hepatokine LECT2 during diet-induced weight cycling in mice. Biochem. Biophys. Res. Commun. 2016;478:1310–1316. doi: 10.1016/j.bbrc.2016.08.117. [DOI] [PubMed] [Google Scholar]
- 145.Takata N., Ishii K.-A., Takayama H., Nagashimada M., Kamoshita K., Tanaka T., Kikuchi A., Takeshita Y., Matsumoto Y., Ota T., et al. LECT2 as a hepatokine links liver steatosis to inflammation via activating tissue macrophages in NASH. Sci. Rep. 2021;11:555. doi: 10.1038/s41598-020-80689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vancini R.L., Andrade M.S., Viana R.B., Nikolaidis P.T., Knechtle B., Campanharo C.R., de Almeida A.A., Gentil P., de Lira C.A. Physical exercise and COVID-19 pandemic in PubMed: Two months of dynamics and one year of original scientific production. Sport. Med. Health Sci. 2021;3:80–92. doi: 10.1016/j.smhs.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scartoni F.R., Sant’Ana L.d.O., Murillo-Rodriguez E., Yamamoto T., Imperatori C., Budde H., Vianna J.M., Machado S. Physical exercise and immune system in the elderly: Implications and importance in COVID-19 pandemic period. Front. Psychol. 2020;11:593903. doi: 10.3389/fpsyg.2020.593903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dixit S. Can moderate intensity aerobic exercise be an effective and valuable therapy in preventing and controlling the pandemic of COVID-19? Med. Hypotheses. 2020;143:109854. doi: 10.1016/j.mehy.2020.109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin J., Guo T., Becker B., Yu Q., Chen S.-T., Brendon S., Hossain M.M., Cunha P.M., Soares F.C., Veronese N., et al. Depression is associated with moderate-intensity physical activity among college students during the COVID-19 pandemic: Differs by activity level, gender and gender role. Psychol. Res. Behav. Manag. 2020;13:1123. doi: 10.2147/PRBM.S277435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Besse-Patin A., Montastier E., Vinel C., Castan-Laurell I., Louche K., Dray C., Daviaud D., Mir L., Marques M., Thalamas C., et al. Effect of endurance training on skeletal muscle myokine expression in obese men: Identification of apelin as a novel myokine. Int. J. Obes. 2014;38:707–713. doi: 10.1038/ijo.2013.158. [DOI] [PubMed] [Google Scholar]
- 151.Kwak S.E., Cho S.C., Bae J.H., Lee J., Shin H.E., Di Zhang D., Lee Y.-I., Song W. Effects of exercise-induced apelin on muscle function and cognitive function in aged mice. Exp. Gerontol. 2019;127:110710. doi: 10.1016/j.exger.2019.110710. [DOI] [PubMed] [Google Scholar]
- 152.Vinel C., Lukjanenko L., Batut A., Deleruyelle S., Pradere J.-P., Le Gonidec S., Dortignac A., Geoffre N., Pereira O., Karaz S., et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018;24:1360–1371. doi: 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- 153.Chapman M.A., Arif M., Emanuelsson E.B., Reitzner S.M., Lindholm M.E., Mardinoglu A., Sundberg C.J. Skeletal muscle transcriptomic comparison between long-term trained and untrained men and women. Cell Rep. 2020;31:107808. doi: 10.1016/j.celrep.2020.107808. [DOI] [PubMed] [Google Scholar]
- 154.McPherron A.C., Lawler A.M., Lee S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 155.Domin R., Dadej D., Pytka M., Zybek-Kocik A., Ruchała M., Guzik P. Effect of various exercise regimens on selected exercise-induced cytokines in healthy people. Int. J. Environ. Res. Public Health. 2021;18:1261. doi: 10.3390/ijerph18031261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yeo N.H., Woo J., Shin K.O., Park J.Y., Kang S. The effects of different exercise intensity on myokine and angiogenesis factors. J. Sports Med. Phys. Fit. 2012;52:448–454. [PubMed] [Google Scholar]
- 157.Banitalebi E., Kazemi A., Faramarzi M., Nasiri S., Haghighi M.M. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. 2019;217:101–109. doi: 10.1016/j.lfs.2018.11.062. [DOI] [PubMed] [Google Scholar]
- 158.Bugera E.M., Duhamel T.A., Peeler J.D., Cornish S.M. The systemic myokine response of decorin, interleukin-6 (IL-6) and interleukin-15 (IL-15) to an acute bout of blood flow restricted exercise. Eur. J. Appl. Physiol. 2018;118:2679–2686. doi: 10.1007/s00421-018-3995-8. [DOI] [PubMed] [Google Scholar]
- 159.Vints W.A., Levin O., Fujiyama H., Verbunt J., Masiulis N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front. Neuroendocrinol. 2022;66:100993. doi: 10.1016/j.yfrne.2022.100993. [DOI] [PubMed] [Google Scholar]
- 160.Türkel İ., Özerkliğ B., Atakan M.M., Aktitiz S., Koşar Ş.N., Yazgan B. Exercise and Metabolic Health: The Emerging Roles of Novel Exerkines. Curr. Protein Pept. Sci. 2022;23:437–455. doi: 10.2174/1389203723666220629163524. [DOI] [PubMed] [Google Scholar]
- 161.Correia J.C., Ruas J.L. Exercised cytokines promote endurance. Science. 2020;368:470–471. doi: 10.1126/science.abb4116. [DOI] [PubMed] [Google Scholar]
- 162.Shokri E., Heidarianpour A., Razavi Z. Positive effect of combined exercise on adipokines levels and pubertal signs in overweight and obese girls with central precocious puberty. Lipids Health Dis. 2021;20:152. doi: 10.1186/s12944-021-01588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.You T., Nicklas B.J. Effects of exercise on adipokines and the metabolic syndrome. Curr. Diabetes Rep. 2008;8:7–11. doi: 10.1007/s11892-008-0003-4. [DOI] [PubMed] [Google Scholar]
- 164.Fyfe J.J., Bishop D.J., Stepto N.K. Interference between concurrent resistance and endurance exercise: Molecular bases and the role of individual training variables. Sports Med. 2014;44:743–762. doi: 10.1007/s40279-014-0162-1. [DOI] [PubMed] [Google Scholar]
- 165.Suzuki K., Totsuka M., Nakaji S., Yamada M., Kudoh S., Liu Q., Sugawara K., Yamaya K., Sato K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999;87:1360–1367. doi: 10.1152/jappl.1999.87.4.1360. [DOI] [PubMed] [Google Scholar]
- 166.Jiménez-Pavón D., Carbonell-Baeza A., Lavie C.J. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: Special focus in older people. Prog. Cardiovasc. Dis. 2020;63:386. doi: 10.1016/j.pcad.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kakanis M., Peake J., Hooper S., Gray B., Marshall-Gradisnik S. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. J. Sci. Med. Sport. 2010;13:e85–e86. doi: 10.1016/j.jsams.2010.10.642. [DOI] [PubMed] [Google Scholar]
- 168.Campbell J.P., Turner J.E. Debunking the myth of exercise-induced immune suppression: Redefining the impact of exercise on immunological health across the lifespan. Front. Immunol. 2018;9:648. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Molanouri Shamsi M., Hassan Z.M., Quinn L.S., Gharakhanlou R., Baghersad L., Mahdavi M. Time course of IL-15 expression after acute resistance exercise in trained rats: Effect of diabetes and skeletal muscle phenotype. Endocrine. 2015;49:396–403. doi: 10.1007/s12020-014-0501-x. [DOI] [PubMed] [Google Scholar]
- 170.He Z., Tian Y., Valenzuela P.L., Huang C., Zhao J., Hong P., He Z., Yin S., Lucia A. Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach. Front. Physiol. 2018;9:1735. doi: 10.3389/fphys.2018.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Farhani F., Shahrbanian S., Auais M., Hekmatikar A.H.A., Suzuki K. Effects of Aerobic Training on Brain Plasticity in Patients with Mild Cognitive Impairment: A Systematic Review of Randomized Controlled Trials. Brain Sci. 2022;12:732. doi: 10.3390/brainsci12060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dun Y., Smith J.R., Liu S., Olson T.P. High-Intensity Interval Training in Cardiac Rehabilitation. Clin. Geriatr. Med. 2019;35:469–487. doi: 10.1016/j.cger.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang C., Xing J., Zhao B., Wang Y., Zhang L., Wang Y., Zheng M., Liu G. The Effects of High-Intensity Interval Training on Exercise Capacity and Prognosis in Heart Failure and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Cardiovasc. Ther. 2022;2022:4273809. doi: 10.1155/2022/4273809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seo D.Y., Park S.H., Marquez J., Kwak H.B., Kim T.N., Bae J.H., Koh J.H., Han J. Hepatokines as a Molecular Transducer of Exercise. J. Clin. Med. 2021;10:385. doi: 10.3390/jcm10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Suzuki K. Recent Progress in Applicability of Exercise Immunology and Inflammation Research to Sports Nutrition. Nutrients. 2021;13:4299. doi: 10.3390/nu13124299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.