Abstract
The pyrogenic response to supernatant fluids obtained from human peripheral blood mononuclear cells (PBMC) stimulated with staphylococcal enterotoxin A (SEA) was characteristic of a response to an endogenous pyrogen in that it was brief and monophasic and was destroyed by heating supernatant fluids at 70°C for 30 min. The febrile responses were in parallel with the levels of interleukin-1 (IL-1), tumor necrosis factor (TNF), interferon-γ (IFN-γ), IL-2, and IL-6 in supernatant fluids obtained from PBMC treated with SEA. Both the pyrogenicity and the levels of IL-1, TNF, IFN-γ, IL-2, and IL-6 in supernatant fluids started to rise at 6 to 18 h and reached their peak levels at 24 to 96 h after SEA incubation. Both the fever and the increased levels of IL-1, TNF, IFN-γ, IL-2, and IL-6 in supernatant fluids obtained from the SEA-stimulated PBMC were decreased by incubating SEA-PBMC with anisomycin (a protein synthesis inhibitor), aminoguanidine (an inhibitor of inducible nitric oxide synthase [NOS]), or dexamethasone (an inhibitor of NOS). The febrile response to supernatant fluids obtained from the SEA-stimulated PBMC was attenuated by adding either anti-IL-1β, anti-TNF-α, or anti-IFN-γ monoclonal antibody (MAb) to supernatant fluids. The antipyretic effects exerted by anti-IL-1β MAb were greater than those exerted by anti-TNF-α or anti-IFN-γ MAb. The data suggest that SEA acts through the NOS mechanisms in PBMC to stimulate synthesis of pyrogenic cytokines (in particular, the IL-1β).
The staphylococcal enterotoxins (SE) are secreted by a variance of Staphylococcus aureus and cause most common staphylococcal food poisoning and staphylococcus-associated toxic shock syndrome in humans and primates (1, 9, 15, 17, 19). The SE are classified into different toxin serotypes, such as SEA, SEB, SEC1, SEC2, and SEE (30). The SE, S. aureus toxic shock syndrome toxin 1, and group A streptococcal pyrogenic exotoxins are commonly considered superantigens because of their effects on the immune system (12, 14). The SE are 26- to 30-kDa proteins that bind with major histocompatibility class II molecules on antigen-presenting cells and stimulate T cells bearing Vβs on their receptor variable region (1, 5, 7). Intravenous administration of SEA is shown to produce fever, lethargy, shock, and death in cats, rabbits, and monkeys (3, 9, 17, 23, 26). In addition, our recent results demonstrate that the febrile responses are associated with increased levels of circulating interleukin-2 (IL-2), interferon (IFN), and tumor necrosis factor (TNF) in rabbits.
Other lines of evidence have shown that macrophages, neutrophils, endothelial cells, and hepatocytes are able to synthesize nitric oxide (NO) from l-arginine (24). Using arginine analogues such as NG-monomethyl-l-arginine and aminoguanidine (AG) to inhibit NO production, investigators have shown that NO mediates a variety of physiological events ranging from neurotransmission to the antimicrobial activity exhibited by mononuclear phagocytes in vitro (20). Our recent results have also demonstrated that febrile responses induced by intravenous injection of SEA are attenuated by pretreatment with AG (11). However, it was not known whether the NO pathway in peripheral blood mononuclear cells (PBMC) represent an important mechanism for modulation of SEA-induced synthesis or release of pyrogenic cytokines. In order to address the question properly, experiments were carried out to assess the pyrogenic responses in rabbits to intravenous injection of supernatant fluids obtained from PBMC treated with SEA alone or SEA plus inhibitors of NO synthase such as AG and dexamethasone (8, 27). At the same time, levels of IL-1, TNF, IFN-γ, IL-2, and IL-6 in the supernatant fluids were assessed in vitro. Furthermore, the effects of adding the anti-IL-1β, anti-TNF-α, and anti-IFN-γ monoclonal antibody (MAb) to the supernatant fluids from SEA-treated human PBMC on the pyrogenic responses to intravenous administration of the supernatant fluids were assessed in rabbits.
MATERIALS AND METHODS
Preparation of PBMC.
Human PBMC were obtained from freshly collected buffy coat fractions from healthy donors at the Tainan Blood Bank Center (Tainan City, Taiwan, Republic of China). They were isolated by centrifugation over a Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient at room temperature for 30 min in a Sorvall RT6000B (DuPont). The cells collected at the interface were washed three times with serum-free RPMI 1640 (GIBCO BRL, Grand Island, N.Y.) and subsequently resuspended in serum-free AIM-V medium (GIBCO BRL) containing 100 U of penicillin and 100 μg of streptomycin per ml. The PBMC at different concentrations were incubated with different concentrations of tested agents in a 37°C incubator. After different periods of incubation, the PBMC supernatant fluids were harvested by centrifugation (1,200 rpm) and stored at −80°C until experimentation.
Animals and pyrogen assay.
Adult male New Zealand White rabbits, weighing between 2.0 and 3.2 kg at the start of the study, were used. The pyrogen assay was carried out with unanesthetized animals restrained in rabbit stocks. Between experiments the animals were housed individually at an ambient temperature of 22 ± 1°C with a 12-h light-dark cycle, with the lights being switched on at 0600 h. Animal chow and water were allowed ad libitum. Experiments were conducted between 0900 and 2000 h, with each animal being used at an interval of not less than 13 days. Throughout the experiment, colonic temperatures were measured every minute with a copper constantan thermocouple connected to a thermometer (HR1300; Yokogawa, Tokyo, Japan). The colonic temperature of each animal was allowed to stabilize for at least 90 min before any injections. Only animals whose body temperatures were stable and in the range of 38.6 to 39.7°C were used to determine the effect of drug application.
Reagents.
All drug solutions were prepared in pyrogen-free glassware that was heated for 5 h before use. All solutions were passed through 0.22-μm-pore-size Millipore bacterial filters. Sterile SEA (Sigma Chemical Co., St. Louis, Mo.) was made up in 0.9% saline solution. Anisomycin (Sigma) was dissolved in 15% ethanol and then diluted to the required concentration with saline. Dexamethasone (Sigma), aminoguanidine (RBI, Natick, Mass.), and polymyxin B (Merck, Darmstadt, Germany) were dissolved in saline. All of the SEA solutions used in this study did not induce gelation in the Limulus amebocyte lysate test, so any contamination with endotoxin was below the level of 25 pg/ml. The experimental culture medium used was serum-free AIM-V medium (GIBCO BRL) containing 50 μg of gentamicin (Sigma) per ml. Monoclonal mouse anti-human (anti-h), interleukin-1β (anti-IL-1β), anti-h TNF-α (anti-TNF-α), and anti-h IFN-γ (anti-IFN-γ) were obtained from R&D (Minneapolis, Minn.), while an isotype-matched mouse immunoglobulin G1 (IgG1) control MAb was purchased from Chemicon International, Inc. (Temecula, Calif.).
IFN bioassay.
IFN activity in supernatant samples from drug-treated or vehicle-treated animals was tested by examining the vesicular stomatitis virus (Indiana strain) cytopathic effect on FL cells (10). IFN titers were expressed as units per milliliter and were defined as the reciprocal value of the dilution of sample that showed a 50% reduction in cytopathic effect. The reference IFN titer was determined, and the end point of the samples was adjusted. An internal laboratory standard human lymphoblastoid IFN (Wellcome Foundation, Ltd., London, England) was included in each assay for the present experiments. Reference human IFN (Ga23-902-530) obtained from The National Institute of Allergy and Infectious Diseases, National Institutes of Health, was used for calibration.
TNF bioassay.
TNF activity in supernatant samples was measured by an in vitro cytotoxicity assay with TNF-sensitive L.P3 cells (a kind gift from H. Fujiwara, Biomedical Research Center, Osaka University Medical School, Osaka, Japan) as previously described (10) with slight modifications. Briefly, 2.5 × 104 cells were plated in 96-well microplates (Nunc, Roskilde, Denmark) in RPMI 1640 (GIBCO BRL) containing 10% fetal bovine serum (FBS; GIBCO BRL) and incubated in a humidified atmosphere of 5% CO2 at 37°C for 4 h. After incubation, samples (100 μl) in a series of dilutions or recombinant human TNF-β (R&D), as an internal reference, were added to the wells, followed by the addition of 50 μl of actinomycin D (Sigma) at a final concentration of 1.6 μg/ml. After 24 h of incubation, the cells were washed with saline, stained with 0.05% crystal violet for 30 min, and then eluted with 50% ethanol in a 0.1% acetic acid solution. The microplates were read at 590 nm on a Multiskan photometer (MR5000; Dynatech, McLean, Va.). The sensitivity of the TNF bioassay was 0.3 U/ml.
IL-1 bioassay.
IL-1 was measured with the IL-1-dependent murine T-cell line D10N4M (a kind gift from C. C. Chao, Neuroimmunology and Host Defense Laboratory, Minneapolis Medical Research Foundation, Minneapolis, Minn.) as previously described (10, 28). Briefly, the D10N4M cells were maintained in RPMI 1640 (GIBCO BRL) with 10% FBS (GIBCO BRL), recombinant human IL-2 (20 ng/ml; R&D), recombinant human IL-1β (40 pg/ml; R&D), 50 μM 2-mercaptoethanol (Serva, Heidelberg, Germany), and concanavalin A (3 μg/ml; Sigma) and were fed every 3 days before being assayed. The serially diluted supernatant samples or recombinant human IL-1β (50 μl) as an internal reference was added to each microplate well (Nunc), followed by the addition of 50 μl of washed D10N4M cells (2 × 105/ml). After 72 h of incubation, the cells were pulsed with 0.5 μCi of [3H]thymidine (6.7 Ci/mmol; DuPont NEN, Boston, Mass.) per well for 4 h. The cells were harvested on glass fiber filters with an automatic cell harvester (Cambridge, Watertown, Mass.). The radioactivity incorporated was assayed in a liquid scintillation counter (LS 5000TA; Beckman, Fullerton, Calif.).
IL-2 bioassay.
IL-2 activity was measured with the IL-2-dependent CTLL-2 cell line (a kind gift from C. C. Chao) as previously described (2). Briefly, the CTLL-2 cells were maintained in RPMI 1640 (GIBCO BRL) with 10% FBS (GIBCO BRL), recombinant human IL-2 (20 U/ml; R&D), 50 μM 2-mercaptoethanol (Serva), and 50 μg of gentamicin (Sigma) per ml and were fed every 3 days before being assayed. The serially diluted supernatant samples or recombinant human IL-2 (50 μl; R&D) was incubated with 2 × 105 of CTLL-2 cells per ml (50 μl). After 24 h of incubation at 37°C, each well was pulsed with 0.5 μCi of [3H]thymidine (DuPont NEN) for 6 h. The cells were harvested on glass fiber filters with an automatic cell harvester (Cambridge). The radioactivity incorporated was assayed in a liquid scintillation counter (LS 5000TA; Beckman).
IL-6 bioassay.
The IL-6 activity was measured with the IL-6-dependent cell line 7TD1 as previously described (33). This cell line was kindly provided by J. Van Snick (Ludwig Institute for Cancer Research, Brussels, Belgium). Briefly, 7TD1 cells were cultured in RPMI 1640 (GIBCO BRL) containing 10% FBS, 2 ng of recombinant human IL-6 (R&D) per ml, and 50 μM 2-mercaptoethanol (Serva). Supernatant samples in serial dilutions or recombinant human IL-6 was added to each well of the microplates (Nunc), followed by the addition of washed 7TD1 cells (2 × 103 cells/well) in AIM-V medium (GIBCO BRL). The cells were incubated at 37°C in a CO2 incubator. After 3 days of incubation, each well was pulsed with 0.5 μCi of [3H]thymidine (DuPont NEN) for 6 h. The cells were harvested on glass fiber filters with an automatic cell harvester (Cambridge). The radioactivity incorporated was assayed in a liquid scintillation counter (LS 5000TA; Beckman).
Statistical analysis.
Animals were permitted at an ambient temperature of 24°C for at least 90 min to attain thermal balance before drugs were administered. The maximal elevation of colon temperature over the preinjection value (Δ°C) and the fever index (FI), the area under the curve produced in the 2-h period after the injection of drugs, in terms of degrees centigrade per 2 h were calculated (16). Results are expressed as the means ± the standard errors of the means (SEM) for n experiments. The results were compared by one-way analysis of variance (ANOVA), followed by Duncan's test when appropriate. A P value of <0.05 was considered significant.
RESULTS
Pyrogenic response to SEA-stimulated PBMC supernatant fluids.
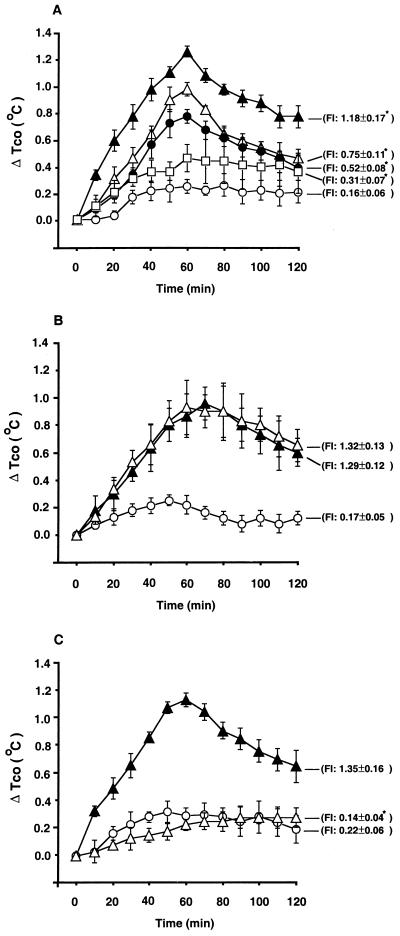
To ascertain whether SEA can act through PBMC to induce a pyrogenic response, supernatant fluids obtained from PBMC (107 cells/ml) treated for 72 h with SEA (1 ng/ml) were given intravenously (i.v.) to the rabbits. As shown in Fig. 1A, i.v. administration of supernatant fluids (0.125 to 2.0 ml/kg of body weight) produced dose-related fever. The colon temperature began to rise at 3 to 5 min after i.v. injection of supernatant fluids, peaked at 60 min, and returned to a preinjection level at 6 h. To exclude the possible contamination of endotoxin, the pyrogenic response to supernatant fluids from PBMC stimulated with SEA alone or SEA plus polymyxin B (50 μg/ml, an agent capable of blocking the activities of the lipid A portion of endotoxin) was assessed in rabbits. It was found that polymyxin B was unable to affect the pyrogenic response to supernatant fluids from SEA-stimulated PBMC (Fig. 1B). In addition, the pyrogenic response to supernatant fluids was completely abolished after heating the supernatant fluids at 70°C for 30 min (Fig. 1C). In the present study, 3 to 7 days elapsed between the times of exposure of PBMC to SEA and the times of exposure of rabbits to the fluids.
FIG. 1.
(A) Mean changes (±SEM) in the peak elevations of colonic temperature (ΔTco) and FI in rabbits treated with supernatant fluids obtained from PBMC (107 cells/ml) treated for 72 h with either AIM-V medium (1 ml/kg) or SEA (1 ng/ml) (○, n = 10). Either 0.125 ml (□, n = 5), 0.5 ml (●, n = 5), 1 ml (▵, n = 5), or 2 ml (▴, n = 5) of the supernatant fluids per kg was administered i.v. ∗, P < 0.05, significantly different from corresponding control values (AIM-V medium control group) (ANOVA). (B) Mean changes (±SEM) in peak elevations of colonic temperature (ΔTco) and FI in rabbits treated with supernatant fluids obtained from PBMC (107 cells/ml) treated for 72 h with AIM-V medium (○, n = 5), 1 ng of SEA (▴, n = 5) per ml, or 1 ng of SEA per ml plus 50 μg of polymyxin B per ml (▵, n = 5). The supernatant fluids (1 ml/kg) were administered i.v. into rabbits. (C) Mean changes (±SEM) in peak elevations of colonic temperature (ΔTco) and FI in rabbits treated with nonheated supernatant fluids obtained from PBMC (107 cells/ml) treated for 72 h with either AIM-V medium (○, n = 5) or SEA (1 ng/ml; ▴, n = 5), as well as with the heated supernatant fluids (70°C for 30 min) obtained from PBMC (107 cells/ml) treated for 72 h with SEA (1 ng/ml) (▵, n = 5). The supernatant fluids (1 ml/kg) were administered i.v. into rabbits. ∗, P < 0.05, significantly different from corresponding control values (nonheated SEA group, ▴) (ANOVA).
Cytokine production by SEA-stimulated PBMC.
To determine the ability of SEA to stimulate the release of intermediate pyrogenic cytokines, cytokine production in supernatant fluids obtained from PBMC treated with SEA was assayed at different incubation times. As shown in Fig. 2, both the colonic temperature (Tco) and the levels of IL-1, TNF, IFN-γ, and IL-6 in supernatant fluids began to rise at 6 h, and they reached their peak levels at 72 to 96 h after the start of SEA-PBMC incubation. On the other hand, the level of IL-2 in supernatant fluids began to rise at 6 h, reached its peak level at 18 to 24 h, and almost returned to normal levels at 96 h after incubation. In addition, Table 1 shows that cytokine production by SEA-stimulated PBMC is PBMC number dependent. Over the cell number range of 104 to 107 cells/ml, both the cytokine production and FI are cell number dependent. Furthermore, over the dose range of 0.001 to 1 ng of SEA per ml, supernatant fluids from the SEA-stimulated PBMC (107 cells/ml) displayed dose-related fever and endogenous cytokine release (Table 2).
FIG. 2.
Changes in the release of pyrogenic cytokines from SEA-treated PBMC over time. Mean changes (±SEM) in the peak elevations of colonic temperature (ΔTco) in rabbits treated with supernatant fluids obtained from PBMC (107 cells/ml) treated for 6, 24, 48, 72, or 96 h with AIM-V medium or SEA (1 ng/ml). The supernatant fluids (1 ml/kg) were administered i.v. into rabbits as follows: medium control (○, n = 5), 6 h (▴, n = 5), 24 h (▵, n = 5), 48 h (●, n = 5), 72 h (□, n = 5), and 96 h (■, n = 5) (A), and the concentrations of IL-1 (B), TNF (C), IFN-γ (D), IL-2 (E), and IL-6 (F) in the supernatant fluids obtained from PBMC (107 cells/ml) treated for 1, 3, 6, 18, 24, 48, 72, 96, or 120 h with AIM-V medium (▵, n = 5) or SEA (1 ng/ml) (▴, n = 5) were as indicated.
TABLE 1.
Cytokine production in supernatant fluids obtained from PBMC (104 to 107 cells/ml) treated with AIM-V medium or SEA (1 ng/ml) and FI induced by the supernatant fluids (1 ml/kg, i.v.) in rabbitsa
| Treatment (cells/ml)b | Cytokine production (U/ml, mean ± SEM)
|
FI (°C/2 h) | ||||
|---|---|---|---|---|---|---|
| IL-1 | IL-2 | IL-6 | TNF | IFN | ||
| AIM-V medium | ||||||
| 104 | 17.0 ± 1.4 | 2.8 ± 0.4 | 0.3 ± 0.1 | 0.5 ± 0.1 | 2.5 ± 0.4 | 0.15 ± 0.06 |
| 105 | 17.2 ± 3.2 | 2.7 ± 0.5 | 0.6 ± 0.1 | 1.3 ± 0.3 | 1.5 ± 0.2 | 0.14 ± 0.05 |
| 106 | 20.2 ± 1.2 | 2.5 ± 0.3 | 0.6 ± 0.3 | 1.5 ± 0.4 | 2.0 ± 0.7 | 0.17 ± 0.07 |
| 107 | 24.2 ± 2.4 | 1.7 ± 0.6 | 0.5 ± 0.2 | 1.1 ± 0.5 | 3.5 ± 1.1 | 0.19 ± 0.07 |
| SEA (1 ng/ml) | ||||||
| 104 | 104.2 ± 26.0b | 3.4 ± 0.5 | 1.5 ± 0.2 | 1.0 ± 0.2 | 3.5 ± 0.4 | 0.16 ± 0.06 |
| 105 | 156.0 ± 15.0b | 4.4 ± 0.3 | 6.7 ± 0.4b | 32.7 ± 0.2b | 12.1 ± 2.0b | 0.62 ± 0.07b |
| 106 | 622.3 ± 52.1b | 11.0 ± 0.6b | 14.1 ± 0.7b | 96.0 ± 9.0b | 52.1 ± 6.2b | 1.04 ± 0.09b |
| 107 | 2,015.1 ± 59.2b | 29.2 ± 1.8b | 29.7 ± 1.5b | 183.0 ± 17.0b | 112.0 ± 1.1b | 1.22 ± 0.15b |
After 72 h of incubation, the supernatant fluids were collected for cytokine analysis and for pyrogen tests. Data represent the means (±SEM) of results of triplicate cultures and are representative of results in the three separate experiments. There were eight rabbits in each treatment group.
Significantly different from the corresponding control values (AIM-V medium group): P < 0.05 (ANOVA).
TABLE 2.
Cytokine production in the supernatant fluids obtained from PBMC (107 cells/ml) treated with AIM-V medium or various concentrations of SEA and the FI induced by the supernatant fluids (1 ml/kg, i.v.) in rabbitsa
| Treatment (ng/ml) | Cytokine production (U/ml, mean ± SEM)
|
FI (°C/2 h) (n) | ||||
|---|---|---|---|---|---|---|
| IL-1 | IL-2 | IL-6 | TNF | IFN | ||
| AIM-V medium | 4 ± 1.2 | 1 ± 0.2 | 0.9 ± 0.2 | 1.2 ± 0.3 | 2 ± 0.2 | 0.16 ± 0.08 (8) |
| SEA (1.0) | 2,432 ± 131.2b | 20 ± 2.3b | 20 ± 3.4b | 189 ± 26.6b | 111 ± 11.2b | 1.36 ± 0.13b (6) |
| SEA (0.1) | 2,339 ± 294.0b | 18 ± 1.6b | 10 ± 0.4b | 158 ± 19.0b | 123 ± 21.0b | 1.00 ± 0.12b (6) |
| SEA (0.01) | 2,007 ± 95.3b | 17 ± 0.8b | 8 ± 0.9b | 138 ± 20.3b | 95 ± 5.9b | 0.96 ± 0.08b (6) |
| SEA (0.001) | 850 ± 90.6b | 9 ± 0.6b | 7 ± 0.7b | 111 ± 3.8b | 81 ± 7.2b | 0.65 ± 0.08b (6) |
After 72 h of incubation, the supernatant fluids were collected for cytokine analysis and for pyrogen tests. Data represent the means (±SEM) of results of triplicate cultures and are representative of results in the three separate experiments. n, number of rabbits tested.
Significantly different from the corresponding control values (AIM-V medium group): P < 0.05 (ANOVA).
Effects of cytokine-specific MAbs on the pyrogenic response to supernatant fluids from SEA-stimulated PBMC.
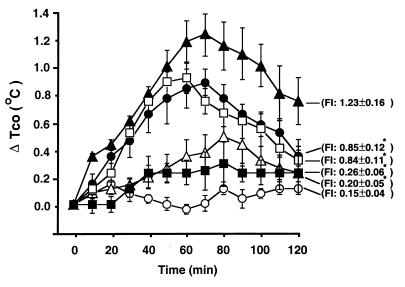
To determine whether the pyrogenic response is mediated by a specific cytokine in supernatant fluids from SEA-stimulated PBMC, we added several cytokine-specific MAbs to supernatant fluids at 37°C for 30 min before they were administered i.v. into rabbits. As shown in Fig. 3, MAbs to TNF-α or IFN-γ had a very weak neutralizing or antipyretic effect. However, anti-IL-1β MAb alone or a combination of anti-IL-1β, anti-TNF-α, and anti-IFN-γ MAbs abrogated almost completely the pyrogenic response to supernatant fluids. The antipyretic effect exerted by anti-IL-1β MAb was greater than that exerted by the anti-TNF-α or anti-IFN-γ MAb (P <0.05; ANOVA). IgG1 control MAb did not affect the pyrogenic response to supernatant fluids from the SEA-stimulated PBMC.
FIG. 3.
Mean changes (±SEM) in the peak elevations of colonic temperature (ΔTco) and FI in rabbits treated with supernatant fluids obtained from PBMC (107 cells/ml) treated at 37°C for 30 min with AIM-V medium (○, n = 5), SEA (1 ng/ml) incubated with control antibody (▴, n = 5), SEA (1 ng/ml) incubated with anti-IL-1β MAb (50 μg/ml) (▵, n = 5), SEA (1 ng/ml) incubated with anti-TNF-α MAb (50 μg/ml) (●, n = 5), SEA (1 ng/ml) incubated with anti-IFN-γ MAb (50 μg/ml) (□, n = 5), or SEA (1 ng/ml) incubated with anti-IL-1β MAb (50 μg/ml) plus anti-TNF-α MAb (50 μg/ml) plus anti-IFN-γ MAb (50 μg/ml) (■, n = 5). The supernatant fluids (1.2 ml/kg) were administered i.v. into rabbits. ∗, Significantly different from corresponding control values (SEA-treated PBMC incubated control antibody group) (P < 0.05; ANOVA).
Effect of anisomycin, dexamethasone, or AG on fever response and levels of cytokines in supernatant fluids from SEA-stimulated PBMC.
In this series of experiments, PBMC (107 cells/ml) was incubated with SEA (1 ng/ml) plus vehicle, anisomycin (0.4 μg/ml), dexamethasone (4 μg/ml), or AG (100 μg/ml) for 72 h. As shown in Table 3, either anisomycin, dexamethasone, or AG attenuated the ability of SEA to induce the febrile response in rabbits and the pyrogenic-cytokine synthesis in supernatant fluids.
TABLE 3.
Effects of incubation of human PBMC (107 cells/ml) with SEA (1 ng/ml) plus vehicle, AG, dexamethasone, or anisomycin for 72 h on both the cytokine contents in the supernatant fluids and on the FI in rabbitsa
| Treatment (μg/ml) | Cytokine production (U/ml, mean ± SEM)
|
FI (°C/2 h) (n) | ||||
|---|---|---|---|---|---|---|
| IL-1 | IL-2 | IL-6 | TNF | IFN | ||
| AIM-V medium | 10 ± 2.1 | 2 ± 1.2 | 1 ± 0.3 | 4 ± 1.6 | 11 ± 1.4 | 0.14 ± 0.04 (8) |
| SEA plus vehicle | 2,312 ± 70.3b | 48 ± 3.2b | 38 ± 3.2b | 170 ± 3.2b | 278 ± 15.1b | 1.33 ± 0.12b (6) |
| SEA plus AG (100) | 593 ± 39.2c | 10 ± 1.2c | 2 ± 0.4c | 22 ± 2.1c | 85 ± 12.3c | 0.57 ± 0.08c (6) |
| SEA plus Dex (4) | 306 ± 34.2c | 4 ± 0.6c | 5 ± 0.6c | 17 ± 3.2c | 100 ± 14.8c | 0.42 ± 0.10c (6) |
| SEA plus Ani (0.4) | 501 ± 62.1c | 7 ± 0.7c | 16 ± 2.7c | 24 ± 2.8c | 110 ± 14.2c | 0.56 ± 0.08c (6) |
Data represent the means (±SEM) of results of triplicate cultures and are representative of results in the three separate experiments. Dex, dexamethasone; Ani, anisomycin.
Significantly different from the corresponding control values (AIM-V medium group): P < 0.05 (ANOVA).
Significantly different from the corresponding control values (SEA plus vehicle group): P < 0.05 (ANOVA).
n, number of rabbits tested.
DISCUSSION
The data presented here demonstrate that the pyrogenic response to supernatant fluids from SEA-stimulated human PBMC was characteristic of a response to endogenous pyrogen in that it was brief and monophasic and was destroyed by heating supernatant fluids at 70°C for 30 min. The i.v. administration of supernatant fluids from SEA-treated human PBMC caused a dose-, time-, and cell number-dependent fever in rabbits. This fever pattern is unlikely to be due to the residue SEA present in supernatant fluids, since we have previously demonstrated that such a low dose of SEA is below the pyrogenic threshold (10). Our preliminary studies have also shown that adding SEA antibody to supernatant fluids obtained from human PBMC treated with SEA failed to inhibit the ability of supernatant fluids to induce fever in rabbits. Moreover, the present results showed that addition of polymyxin B (an antibiotic inhibiting many of biological activities of endotoxin) into supernatant fluids did not reduce the fever response in rabbits to supernatant fluids obtained from human PBMC treated with SEA. These data suggest that the fever induced by supernatant fluids obtained from PBMC treated with SEA is brought about by some certain kinds of endogenous pyrogenic substances released rather than SEA itself or endotoxin contamination.
Indeed, as shown in our previous results (10), the febrile responses were associated with the circulating levels of IFN, TNF, and IL-2 after the i.v. administration of SEA. Both body temperature and levels of IFN, TNF, and IL-2 in serum simultaneously started to rise at 1 to 2 h and reached their peak values at 3 to 5 h after SEA injection. The SEA-induced fever and elevated levels of these cytokines in serum were attenuated by pretreatment with systemic administration of anisomycin (a protein synthesis inhibitor) or dexamethasone (an effective anti-inflammatory and immunosuppressive agent) in rabbits. In the present study, the febrile responses in rabbits to supernatant fluids from SEA-stimulated human PBMC were also in parallel with levels of IL-1, TNF, IFN-γ, IL-2, and IL-6 in supernatant fluids. It was found that both the body temperature and the levels of IL-1, TNF, IFN-γ, and IL-6 in supernatant fluids began to rise at 6 h and reached their peak levels at 72 to 96 h after the start of SEA-PBMC incubation. On the other hand, the level of IL-2 in supernatant fluids rose at 6 h, peaked at 18 to 24 h, and returned to a normal level at 96 h after SEA-PBMC incubation. Both the fever in rabbits to supernatant fluids and the increased levels of IL-1, TNF, IFN-γ, IL-2, and IL-6 in supernatant fluids from the SEA-stimulated PBMC were decreased by incubation of the SEA-stimulated human PBMC with either anisomycin or dexamethasone. The results have been confirmed by our recent study in which the rabbit PBMC were treated with SEA and then the concentrations of cytokines in the supernatant fluids and the pyrogenic responses to the supernatant fluids were checked in vitro and in vivo, respectively (S.-J. Won et al., unpublished data). Apparently, there is no discrepancy between human and rabbit PBMC in terms of pyrogenic cytokine production responses to SEA. The present results further showed that adding anti-IL-1β MAb, but not adding anti-TNF-α or anti-IFN-γ MAb, into supernatant fluids from PBMC treated with SEA almost completely abolished the pyrogenic activity exerted by the supernatant fluids in vivo. The antipyretic action of MAb to IL-1β was greater than that exerted by MAb to TNF-α or IFN-γ. The data are consistent with the concept that IL-1β represents an important mediator for fever induced by lipopolysaccharide (29) or SEA.
It has been shown that NO is likely to function both as a direct effector and as an immunoregulatory molecule (25, 32). NO is a short-lived biological mediator produced by NO synthase in a wide variety of mammalian cells (6, 22). NO is able to inhibit the production of IFN-γ and IL-1β by Th1 cells (20, 32) and is likely to be an essential mediator for superantigen-induced cytokine production of human cells (18, 31). Preliminary experiments (S.-J. Won and M.-T. Lin, unpublished data) found that levels of pyrogenic cytokines and NO products in supernatant fluids obtained from rabbit or human PBMC treated with SEA were simultaneously increased. The increased levels of cytokines and NO products in supernatant fluids were decreased by incubation of the SEA-stimulated PBMC with either anisomycin, dexamethasone (an inhibitor of NOS) (27), or AG (a selective inhibitor of inducible NOS) (8) in vitro. These observations prompted us to think that NO mediates SEA-induced pyrogenic cytokines synthesis in PBMC. In fact, staphylococcal enterotoxins are a group of proteins produced by S. aureus that cause fever, food poisoning, or septic shock in humans (1, 15). Excess production of NO has also been implicated in the pathogenesis of septic shock (13), inflammatory and immunologically mediated diseases (21), and complications of diabetes (4). Thus, it appears that AG or dexamethasone inhibits the NO synthase, which appears to be responsible for the excess production of NO linked to pyrogenic cytokine production and these disease states (such as fever, septic shock, or inflammatory and immunologically mediated diseases).
In summary, the present results show that the febrile response to supernatant fluids from SEA-stimulated human PBMC was associated with the levels of IL-1, TNF, IFN-γ, IL-2, and IL-6 in the supernatant fluids. Both the febrile responses and the increased levels of IL-1, TNF, IFN-γ, IL-2, and IL-6 in the supernatant fluids were decreased by adding either anisomycin, dexamethasone, or AG into SEA-treated PBMC. Furthermore, adding anti-IL-1β MAb, anti-IFN-γ MAb, or anti-TNF-α MAb to supernatant fluids significantly decreased the pyrogenic response to supernatant fluids from SEA-stimulated PBMC. The antipyretic actions exerted by anti-IL-1β MAb were greater than those exerted by anti-TNF-α MAb or anti-IFN-γ MAb. The data suggest that SEA acts through the NO synthase mechanisms in PBMC to stimulate the synthesis or release of pyrogenic cytokines (in particular, IL-1β).
ACKNOWLEDGMENTS
This study was supported by National Science Council (Taipei, Taiwan, Republic of China) grants NSC 87-2314-B-006-063 and NSC 89-2316-B-010-014 and by the Veterans' General Hospital-National Yang-Ming University joint research program (VTY 89-P5-37), Tsou's Foundation, Taipei, Taiwan, Republic of China.
S.J.W. and W.T.H. contributed equally to this study.
REFERENCES
- 1.Alouf J E, Knoll H, Kohler W. The family of mitogenic, shock-inducing and superantigenic toxins from staphylococci and streptococci. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. San Diego, Calif: Academic Press; 1991. pp. 367–414. [Google Scholar]
- 2.Baker P E, Gillis S, Smith K A. Monoclonal cytolytic T-cell lines. J Exp Med. 1979;149:273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark W G, Page J S. Pyrogenic responses to staphylococcal enterotoxins A and B in cats. J Bacteriol. 1968;96:1940–1946. doi: 10.1128/jb.96.6.1940-1946.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbet J A, Tilton R G, Chang K, Hasan K S, Ido Y, Wang J L, Sweetland M A, Lancaster J R, Jr, Williamson J R, McDaniel M U. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevent diabetic vascular dysfunction. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- 5.Fleischer B, Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988;167:1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Föstermann U, Schmidt H H H W, Pollock J S, Sheng H, Mitchell J A, Warner T D, Nakane M, Murad F. Isoforms of nitric oxide synthase, characterization and purification from different cell types. Biochem Pharmacol. 1991;42:1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- 7.Fraser J D. High affinity binding sites of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989;339:221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths M J D, Messent M, MacAllister R J, Evans T W. Aminoguanidine selectively inhibits inducible nitric oxide synthetase. Br J Pharmacol. 1993;110:963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodoval L F, Morris E L, Crawley G J, Beisel W R. Pathogenesis of lethal shock after intravenous staphylococcal enterotoxin B in monkeys. Appl Microbiol. 1968;16:187–192. doi: 10.21236/ad0666852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang W T, Lin M T, Won S J. Staphylococcal enterotoxin A-induced fever is associated with increased circulating levels of cytokines in rabbits. Infect Immun. 1997;65:2656–2662. doi: 10.1128/iai.65.7.2656-2662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W T, Lin M T, Won S J. Mechanisms and sites of pyrogenic action exerted by staphylococcal enterotoxin A in rabbits. Neurosci Lett. 1997;236:53–56. doi: 10.1016/s0304-3940(97)00759-3. [DOI] [PubMed] [Google Scholar]
- 12.Kappler J, Kotzin B, Herron L, Gelfand E W, Bigler R D, Boylston A, Carrel S, Posnett D N, Choi Y, Marrack P. Vβ-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 13.Kilbourn R G, Jubran A, Gross S S, Griffith O W, Levi R, Adams J, Lodato R F. Reversal of endotoxin-mediated shock by NG-methyl-l-arginine, an inhibitor of inducible nitric oxide synthase. Biochem Biophys Res Commun. 1990;172:1132–1135. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- 14.Kotb M. Bacterial pyrogenic exotoxins as superantigens. Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotzin B L, Leung D Y M, Kappler J, Marrack P. Superantigens and human disease. Adv Immunol. 1993;54:99–146. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 16.Lin M T, Chern Y F, Chen S Y. Depletion of noradrenalin in the hypothalamus reduces the febrile response induced by prostaglandin E2, thyrotropin-releasing hormone and β-endorphin. Neuropharmacology. 1985;24:1039–1042. doi: 10.1016/0028-3908(85)90188-1. [DOI] [PubMed] [Google Scholar]
- 17.Liu C T, Sanders R P. Modification of lethality induced by staphylococcal enterotoxin B in dutch rabbits. Am J Vet Res. 1980;41:399–404. [PubMed] [Google Scholar]
- 18.Marcinkiewicz J, Chain B M. Differential regulation of cytokine production by nitric oxide. Immunology. 1993;80:146–159. [PMC free article] [PubMed] [Google Scholar]
- 19.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 20.Moncada S, Palmer R M J, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 21.Mulligan M S, Palmer R M J, Higgs E A. Tissue injury caused by deposition of immune complexes is l-arginine dependent. Proc Natl Acad Sci USA. 1991;88:638–644. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan C F. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 23.Normann S J, Jaeger R F, Johnsey R T. Pathology of experimental enterotoxemia. The in vivo localization of staphylococcal enterotoxin B. Lab Investig. 1969;20:17–25. [PubMed] [Google Scholar]
- 24.Nussler A K, Billiar T R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- 25.Palmer R M J, Ferrige A G, Moncada S. Nitric oxide release accounts for biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 26.Pettit G W, Elwell M R, Jahrling P B. Possible endotoxemia in rabbits after intravenous injection of Staphylococcus aureus enterotoxin B. J Infect Dis. 1977;135:646–648. doi: 10.1093/infdis/135.4.646. [DOI] [PubMed] [Google Scholar]
- 27.Raddomski M W, Palmer R M J, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA. 1990;87:10043–10049. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rameshwar P, Gascon P. Release of interleukin-1 and interleukin-6 from human monocytes by antithymocyte globulin: requirement for de novo synthesis. Blood. 1992;80:2531–2538. [PubMed] [Google Scholar]
- 29.Rothwell N J. Function and mechanisms of interleukin-1 in the brain. Trends Pharmacol Sci. 1991;12:430–436. doi: 10.1016/0165-6147(91)90623-z. [DOI] [PubMed] [Google Scholar]
- 30.Spero L, Johnson-Winergar A, Schmidt J J. Enterotoxins of staphylococci. In: Hardegree M C, Tu A T, editors. Handbook of natural toxin. New York, N.Y: Marcel Dekker, Inc.; 1988. pp. 131–164. [Google Scholar]
- 31.Sriskandan S, Evans J, Cohen J. Bacterial superantigen-induced human lymphocyte responses are nitric oxide dependent and mediated by IL-12 and IFN-γ. J Immunol. 1996;156:2430–2435. [PubMed] [Google Scholar]
- 32.Taylor-Robinson A W, Liew F Y, Severn A, Xu D, McSorley S, Garside P, Padron J, Phillips R S. Regulation of the immune response by nitric oxide differentially produced by T-helper type 1 and T-helper type 2 cells. Eur J Immunol. 1994;24:980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 33.Vink A, Coulie P G, Wauters P, Nordan R P, Snick J V. B cell growth and differentiation activity of interleukin-HP1 and related murine plasmacytoma growth factor. Synergy with interleukin 1. Eur J Immunol. 1988;18:607–612. doi: 10.1002/eji.1830180418. [DOI] [PubMed] [Google Scholar]