Abstract
Natural products have been an invaluable and useful source of anticancer agents over the years. Several compounds have been synthesized from natural products by modifying their structures or by using naturally occurring compounds as building blocks in the synthesis of these compounds for various purposes in different fields, such as biology, medicine, and engineering. Multiple modern and costly treatments have been applied to combat cancer and limit its lethality, but the results are not significantly refreshing. Natural products, which are a significant source of new therapeutic drugs, are currently being investigated as potential cytotoxic agents and have shown a positive trend in preclinical research and have prompted numerous innovative strategies in order to combat cancer and expedite the clinical research. Natural products are becoming increasingly important for drug discovery due to their high molecular diversity and novel biofunctionality. Furthermore, natural products can provide superior efficacy and safety due to their unique molecular properties. The objective of the current review is to provide an overview of the emergence of natural products for the treatment and prevention of cancer, such as chemosensitizers, immunotherapeutics, combinatorial therapies with other anticancer drugs, novel formulations of natural products, and the molecular mechanisms underlying their anticancer properties.
Keywords: natural products, bioactive anti-tumor agents, phytochemicals, anti-cancer plants, chemotherapy, chemoprevention
1. Introduction
Noncommunicable diseases (NCDs) refer to diseases that are not transmitted directly from one individual to another, and account for more than 70% of all deaths worldwide (41 million deaths per year). Cancer is the second leading cause of death among NCDs, after cardiovascular disease [1]. The incidence and mortality of cancer are rapidly increasing throughout the world due to an increase in the aging population. The reasons are complex, including environmental pollution, chemical toxins pollution, ionizing radiation, free radical toxin, microorganisms (bacteria, fungi, viruses, etc.) and their metabolic toxins, genetic characteristics, endocrine imbalance, and immune dysfunction, etc. [2]. Global Cancer Observatory (GLOBOCAN) estimates that 19 million new cancer cases will be reported and 10 million deaths will be caused by this disease in 2020. Cancers in both sexes are most commonly diagnosed in the breast, lung, colon, prostate, and stomach (excluding non-melanoma skin cancer). It is estimated that by 2040, the incidence of cancer in the world population will increase to 30.2 million cases and the mortality rate will increase to 16.3 million cases, respectively [3].
The term “cancer” is used to refer to a large group of diseases (over 277) which are characterized by the uncontrolled growth of abnormal cells and which are caused by a wide range of factors [4]. In fact, any cell in the body is capable of becoming a cancer cell (carcinogenesis) once it has been subjected to a series of successive gene mutations. Specifically, this process occurs in three stages, namely: (a) the initiation stage, when the alteration has already been identified, (b) the promotion stage, where the altered cells are considered totally mutated and then the malignancy of the cells commences, and (c) the progression stage, once the tumor has already grown, and as the cells begin to divide in an accelerated and irreversible manner. Cancer cells may also spread to other parts of the body (metastasis) in addition to growing locally, which is an often cited cause of death from the disease [5].
Cancer research has always been a challenge because of its complexity. Different types of cancer may exhibit substantial differences in relation to genetic alterations, organ involvement, prognosis, and therapeutic management [6]. Even though various treatment options are available, their success relies upon the type and phase of the disease. Among various treatment alternatives, careful surgical removal of malignant tissues/tumor, radiation treatment, chemotherapy, and immunotherapy are generally employed. The effects of surgery and radiotherapy are local, while those of chemotherapy and targeted therapy are systemic. The type and stage of cancer determine whether such therapies are used alone or in combination with other treatments (e.g., radiotherapy and chemotherapy) [7]. Small molecule targeted therapy and chemotherapy are two therapeutic approaches used to treat cancer using chemical compounds. In general, chemotherapy drugs act as cytotoxic agents that interfere with various phases of the cell cycle. The rationale for their application is that cancer cells generally have a faster division rate than normal cells, making them more susceptible to chemotherapeutic agents [1]. Generally, these drugs can be classified into five categories according to their biochemical properties: alkylating agents (for example, cisplatin), antimetabolites (5-fluoroacil), antitumor antibiotics (doxorubicin), topoisomerase inhibitors (topotecan), and tubulin-binding drugs (paclitaxel) [8].
Despite the effectiveness of chemotherapeutic drugs, they can also cause adverse reactions in normal cells, including nausea, vomiting, mucositis, alopecia, neuropathy, alopecia, and myelosuppression. Furthermore, they have been found to be associated with multidrug resistance (MDR), an undesirable phenomenon responsible for more than 90% of the deaths of cancer patients undergoing chemotherapy [9]. Small molecule targeted therapy (SMTT) differs from chemotherapy in that it uses chemicals that target specific molecular targets within cancer cells. It is thought that these targets are modified genetically in cancer and are essential to the development and survival of tumors. The majority of the time, they are implicated in signaling pathways that are dysregulated during cancerous development [10]. The use of target-oriented compounds in clinics includes tyrosine kinases, proteasomes, and poly ADP-ribose polymerase inhibitors such as imatinib, carfilzomib, and ribociclib. The specificity of SMTT drugs is expected to result in a less toxic effect on healthy cells. However, side effects (such as a rash, diarrhea, or hypertension) have been reported [11]. Moreover, they may also trigger mechanisms that lead to the development of drug resistance. Even though these restorative choices are effective in treating different sorts of malignant growths, they have impediments; for example, recurrence of cancer, noncompliance because of extremely unfavorable implications such as fatigue, pain, nausea, anemia, emesis, and baldness, among the symptoms endured by patients. It is important to note that the vast majority of synthetic chemotherapeutic drugs developed before failed to satisfy the needs during clinical trials, despite the higher cost of expenditure. Thus, efforts continue to be made to find better alternatives that balance efficacy and toxicity and comply with drug resistance prevention measures.
The heterogeneous nature of cancer has limited the efficacy of conventional therapies such as radiation and standard chemotherapy in treating and preventing it, as they are likely to kill both normal and cancerous cells in the process, resulting in serious hematological toxicities and damage to the tissues involved [12]. A growing number of patients acquire or develop multiple drug resistance, making chemotherapy a treatment with limited therapeutic benefits. A number of anticancer drugs have also been associated with significant side effects, including cardiotoxicity caused by doxorubicin [13], ototoxicity caused by cisplatin, and cognitive impairment caused by 5-fluorouracil [14]. The adverse effects of chemotherapy on patients, such as kidney damage, gastrointestinal problems, hair loss, and fatigue, compromise adherence to treatment. A negative perception of treatment is also a result of these factors [15].
The majority of the chemotherapeutic drugs used in the clinic today are directed at only one particular target such as specific nucleic acids, particular proteins, or tumorigenic pathways. Platinum drugs, such as oxaliplatin, carboplatin, and cisplatin, are known to inhibit nucleotide synthesis and metabolism along with damaging DNA. Tyrosine kinase inhibitors such as gefitinib, erlotinib, and icotinib are directed at tyrosine kinase. Angiogenesis is inhibited by bevacizumab, sunitinib, and sorafenib [16]. The treatment of cancer has been greatly improved by the development of many drugs, but the disease is still an evolutionary process. Cancer cells are capable of adapting to a given drug treatment. Natural products have been observed to influence multiple oncogenic signaling pathways simultaneously by modulating the activity or expression of their molecular targets. Various natural products affect multiple pathways, including apoptotic cell death, cell proliferation, migration/invasion, angiogenesis, and metastasis. Natural products are capable of generating intracellular signals that trigger events that lead to the death of cancer cells. Chemotherapy can be enhanced by natural products in cases where cancer is resistant to treatment. The high cost of conventional drugs and the rising incidence of cancer have challenged researchers to find more cost-effective and eco-friendly alternatives. In this context, natural products are highly advantageous due to their chemical diversity, low toxicity, safety, and availability, which make them an attractive and affordable alternative to synthetic products [17,18].
In this review, we summarize novel studies and viewpoints on cancer therapy and natural products mostly from 2012 to 2022 (older sources were also cited if necessary) using Web of Science (http://www.webofknowledge.com, accessed on 1 July 2022), Google Scholar (https://scholar.google.com, accessed on 1 April 2022), PubMed (https://www.ncbi.nlm.nih.gov/pubmed, accessed on 1 June 2022), ScienceDirect (https://www.sciencedirect.com, accessed on 1 May 2022), Chinese National Knowledge Infrastructure (CNKI, https://www.cnki.net, accessed on 1 March 2022), Scopus (https://www.scopus.com, accessed on 1 April 2022), and Clinical Trials (https://clinicaltrials.gov, accessed on 1 November 2021). This study provides important insights into the efficacy and mechanism of action of natural products in the treatment of cancer, potentially improving clinical outcomes.
The review provides a comprehensive perspective on the anti-tumor potential of natural products by describing the outcomes of the main in vitro and in vivo experiments that have demonstrated some effects on various forms of cancer. Furthermore, this study summarizes the six directions of their anticancer activity by summarizing the mechanisms by which these agents affect cellular proliferation, differentiation, apoptosis, angiogenesis, and metastasis. In particular, it includes (I) compounds that possess an innate antitumor effect; (II) reverse chemoresistance; (III) inhibit metastasis; (IV) act as cancer immunotherapeutic; and (VI) the development of novel drug delivery systems for natural products. These natural products were chosen due to their potential anticancer properties as well as their substantial evaluation of chemotherapeutic effectiveness. It will provide the reader with an updated perspective on natural therapies and their potential application in cancer treatment in the future. As a result, this article offers a broader range of options for researchers interested in developing new alternatives for treating and preventing cancer, a topic that continues to grow in importance throughout the world.
2. Natural Products (NPs) against Cancer
Natural medicine refers to substances that are produced naturally by living organisms, such as plants, insects, animals, aquatic organisms, and microbes, and possess pharmacological or biological properties [19]. Natural products are precious gifts from nature that can be used for the prevention and treatment of illnesses in humans. Therefore, they are of vital importance and play an irreplaceable role in the development and design of drugs. Since ancient times, natural products have been used to treat human diseases. Recent research indicates that natural products still have the potential to be applied in drug development. The latest review by David J. Newman and Gordon M. Cragg revealed that 32% of all small-molecule drugs approved between January 1981 and September 2019 were natural products and their derivatives [20,21]. Moreover, from 1981 to 2014, 51% of all the 1211 new small molecule drugs approved worldwide were compounds derived from natural products [21]. In addition, a report by Eric Patridge and colleagues, published in 2016, revealed that the US Food and Drug Administration (FDA) approved 547 natural products and their derivatives for use as medications across the years (1827 to 2013) [22]. They are used to treat a number of diseases, primarily cancer, bacterial infections, and hypertension. The same study found that 68% of all 136 small-molecule anticancer drugs available from 1940 to 2014 were natural products based.
One of the most important sources of biologically active compounds is the plant kingdom. Currently, there are more than 350,000 vascular plant species registered in the world, and new species are being added every year [1]. It remains a vast and unexplored field of study that offers many opportunities for drug discovery. Plants can be used therapeutically in a variety of forms, such as tea, extracts, and dyes. In addition, their active compounds may be isolated and used as medicines or as precursors for synthetic and semi-synthetic drugs. There is a large list of phytochemicals (i.e., chemical compounds produced by plants) with therapeutic activity, including terpenes, alkaloids, essential oils, flavonoids, gums, and a variety of primary and secondary metabolic compounds [23]. In the past few decades, phytochemicals have been extensively studied for their potential anticancer properties, with the objective of using them in cancer treatment modalities such as chemotherapy and targeted therapy. For example, berberine is a secondary metabolite produced by plants, commonly found in the roots, rhizomes, and stem barks of Chinese herbs and plants of the Berberis genus. Numerous preclinical and limited human studies have demonstrated that berberine exerts beneficial biological activities against a variety of human diseases, including inflammation, metabolic dysfunction, depression, cardiovascular diseases, neurodegenerative diseases, and different types of cancers [24,25].
Since natural products exhibit unique properties as compared to conventional synthetic molecules, they are both advantageous and challenging in drug discovery. NPs are characterized by a wide range of scaffolds and a high degree of structural complexity [26]. These molecules generally have a higher molecular mass, a higher number of sp3 carbon and oxygen atoms, fewer nitrogen and halogen atoms, higher numbers of H-bond acceptors and donors, a lower calculated octanol–water partition coefficient (cLogP values, which indicate a higher degree of hydrophilicity), and a significantly higher molecular rigidity than synthetic compounds. A number of these differences can be advantageous; for example, the higher rigidity of NPs may be helpful when tackling protein–protein interactions in the drug discovery process. In fact, NPs are one of the most important sources of oral drugs beyond Lipinski’s rule of five [6]. Over the past 20 years, the increasing molecular mass of approved oral drugs illustrates the growing importance of drugs that do not comply with this rule. The structural optimization of NPs has been influenced by evolution to serve specific biological functions, such as regulating endogenous defense mechanisms and interacting (often in competition) with other organisms, which is why they are important for treating diseases such as cancer and infectious diseases. Additionally, the traditional use of these substances may provide insight into their efficacy as well as safety. Furthermore, the NPs pool contains a wide range of ‘bioactive’ compounds compared to typical synthetic small molecule libraries that cover a narrower chemical space [27].
Natural products account for approximately 50% of anti-tumor drugs. They may be derived from plants or can be semi-synthetic chemicals. Currently, many plant-based antitumor drugs are in clinical use, such as taxanes (e.g., Taxol), vinblastine, vincristine, and podophyllotoxin analogs. Robert Noble and Charles Beer discovered vincristine and vinblastine vinca alkaloids in the 1950s from the leaves of Catharanthus roseus (Madagascar periwinkle) [28]. Furthermore, the Taxus genus produces taxane-derived drugs. In 1971, paclitaxel, which is known commercially as Taxol®, was first isolated from Taxus brevifolia Nutt (Pacific yew). This is one of several diterpene taxanes that are used as the main chemotherapeutic agent against several forms of cancer, including ovarian, lung, and breast cancers. During the 1980s, another semi-synthetic taxane drug called docetaxel (Taxotere®) was isolated from Taxus baccata (European yew). Recent developments in chemical modification have focused on podophyllotoxin analogs, particularly teniposide and etoposide, two semi-synthetic antitumor agents. In 1880, Podwyssotzki discovered podophyllotoxin in the North American mayapple Podophyllum peltatum L. Furthermore, podophyllotoxin has also been found in Podophyllum emodi (Indian podophyllum) [29]. In 1966, etoposide was synthesized for the first time and was approved by the United States Food and Drug Administration (FDA) in 1983 as an anticancer agent [30].
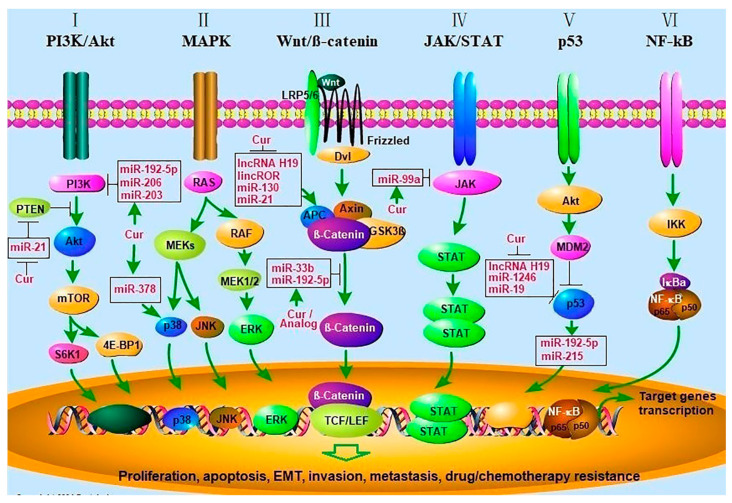
Natural products can be used for chemoprevention and chemotherapy since it effectively suppresses cell proliferation, regulates the cell cycle, and interferes with several tumorigenic signaling pathways, such as phosphoinositide 3-kinase (PI3K), matrix metalloproteinase (MMP), MAPK/ERK known as the Ras-Raf-MEK-ERK pathway, toll-like receptor (TLR) pathway, and AKT pathway. Additionally, natural products may stimulate DNA repairing mechanisms through the action of p21, p27, p51, and/or p53 gene products, such as Bax, Bak, and Bid proteins, which cause the synthesis of protective enzymes such as caspases 3, 7, 8, 9, 10, 12, and modulate antioxidant enzymes such as GST, GSH, and GPx. The chemo-preventive effects occur by inhibiting important events involved in tumor initiation such as ROS removal, enhancing DNA repair, and eradicating transformed cells through immunosurveillance. Furthermore, these effects are exerted through inhibition of proliferation and immunotargeting of altered cells, as well as hindering tumor progression by promoting differentiation, inhibiting angiogenesis, and inducing apoptosis [31]. Curcumin has been one of the most studied phytochemical and its anticancer mechanism is shown in Figure 1.
Figure 1.
Curcumin inhibits cancer progression by regulating many signal pathways. (I) Akt/PI3K/mTOR signaling pathway. PTEN inhibits Akt activation by PI3K, mTOR phosphorylates p70S6K1 (S6K1), and 4E-BP1 which activate cell growth and survival pathways. Curcumin inhibits the Akt/PI3K/mTOR pathway by increasing PTEN expression through decreased miR-21 levels, and by inhibiting PI3K activity by upregulating miR-192-5p, miR-206, and miR-203; (II) MAPK signaling pathway. Signal cascades lead to activation of the MEKs, which subsequently activate the ERK1/2, p38, and JNK cascades and initiate the transcription of genes. Curcumin activates the p38 MAPK pathway by upregulating miR-378, which increases p21/27, cleaves caspase-3,9, and decreases Bcl-2 and MMP2/9. (III) Wnt/β-catenin pathway. The wnt molecule binds to both Frizzled and LRP5/6 receptors to cause the Axin/APC/GSK3β complex to dissociate. As a result, β-catenin is phosphorylated and translocated to the nucleus where it binds TCF/LEF co-transcription factors, which stimulate the transcription of Wnt-response genes. Curcumin inhibits Wnt/ β -catenin signaling through inhibition of lncRNA H19, lincROR, miR-130, 21, and upregulation of miR-192-5p and miR-33b; and (IV) JAK/STAT signaling. This pathway is activated when a ligand binds to a receptor, which communicates signals downstream STATs, whereas STATs are transcription factors that regulate gene expression; (V) p53 signaling pathway. The activation of MDM2 by AKT may inhibit the antitumor activity of p53. Curcumin inhibits lncRNA H19, miR-1246, and miR-19 to increase p53’s anti-tumor activity; (VI) NF-kB signaling pathway. This signaling cascade results in the phosphorylation of IkBα, which is then degraded by the proteasome. This allows the NF-kB/p65/p50 complex to translocate to the nucleus in order to facilitate transcription. This figure was reproduced from [32] (licensed under creative commons license).
2.1. Natural Products against Chemoresistance
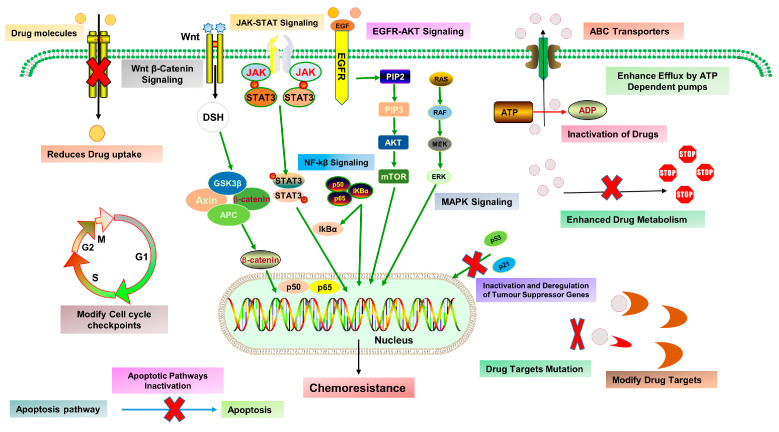
Cancer develops from the abnormality of different molecular paths; therefore, treatment with current standard chemotherapeutic agents still remains less effective due to targeting of one or a few pathways. As a result, higher doses of conventional drugs are required to eliminate the tumor, which evokes harmful effects even worse than the condition itself. Although these agents are understood to kill tumor cells, they also trigger many survival pathways, which eventually induce cancer drug resistance, generally termed chemoresistance, which develops when cancer cells establish the resistance capability against chemotherapy and are categorized into two types: intrinsic (preexistent) and acquired (drugs induced). Chemoresistance development in tumor cells occurs via different molecular paths, e.g., regulation of drug influx and efflux via ATP binding cassette (ABC) transporter, epigenetic factors, hindering cell death, altering metabolism and degradation of drugs, and deactivation of chemotherapeutic drugs, mutations of drug targets, improved DNA repair and modifying growth factors signaling. These factors may act alone or in combination with different signaling pathways (Figure 2) [33].
Figure 2.
The mechanism of chemoresistance in cancer cells may include a variety of molecular mechanisms, such as regulating drug influx and efflux through ABC transporters, inhibiting cell death, altering drug targets, regulating epigenetic factors, inactivating chemotherapeutic agents, inactivating tumor suppressor genes, modifying DNA repair processes, and modulating growth factor signaling (adapted from [33], License Number: 5433490420433, License date: 21 November 2022).
Over the decades, the Food and Drug Administration (FDA) has approved about 300 chemotherapeutic drugs for the treatment of cancer, including taxol, vinca and its derivatives, Platinium analogues, 5-fluorouracil, bevacizumab, erlotinib, nivolumab, ipilimumab, sunitinib, and olaparib, with other different chemotherapeutic drug combinations. However, most of them are highly toxic and induce resistance, which ultimately results in tumor recurrence and metastasis, limiting their anticancer activities due to the development of adaptive resistance by targeted tumor cells and non-specific toxicities towards normal cells [34]. Therefore, better alternatives which are safer and more effective are required to overcome chemoresistance and subsequently sensitize tumor cells to chemotherapeutic agents. Chemosensitization is a widely used approach in which a drug’s activity is augmented using other drugs to suppress chemoresistance. However, the chemosensitizing agent needs to be safe and possess multitargeted inhibition potential of various chemoresistance pathways. The combinatorial chemotherapy produces synergistic effects by suppressing proteins, genes, and pathways involved in chemoresistance and concurrently modulates molecular targets of cancer to improve conventional drug therapy. Intriguingly, in vitro, ex vivo, in vivo, and clinical results suggest that phytochemicals are less toxic, safer, easily available, and highly effective with multi-targeting potential against different malignancies and also reduce the toxicity of conventional chemotherapy by increasing sensitivity of tumor cells [35].
Lupeol has been shown to sensitize cancer therapy-resistant cells by modulating various inflammatory cytokines such as IL-6, TNF-α, IFN-γ and also BAX, BCL-2, FAS, Caspases, Survivin, and PI3K-AKT-mTOR pathways and Cyclins, CDKs, P21, P53, and PCNA molecules, which are involved in cell cycle regulation [36]. Shikonin, a natural naphthoquinone, induces necroptosis in various cancer cell lines such as glioma, osteosarcoma, and hematopoietic cells by enhancing RIP1 and RIP3 and mROS, and also effectively overcomes P-gp, Bcl-2, Bcl-xl, MRP1, and BCRP1 drug resistance [37]. Similarly, Pterostilbene induces apoptosis, autophagy, S-phase cell cycle arrest, and inhibiting MDR1 expression via downregulation of RAGE/P13k/Akt pathways [38]. Epigallocatechin gallate induces G2/M cell cycle arrest and also suppresses MDR levels [39]. Plumbagin inhibits multiple molecular pathways such as cell cycle arrest, antiangiogenic, apoptotic, and autophagic pathways; regulates associated genes such as Akt, STAT3, and Nf-kβ; induces ROS; suppresses glutathione; and causes breakage of DNA strands [40]. Quercetin, a natural flavonoid, has been shown to facilitate cell death and chemosensitivity in pancreatic cells by RAGE/P13/AKT/mTOR axis [41]. Muthusamy et al. showed that ferulic acid reverses the P-gp mediated MDR by inhibiting P13K/AKT/Nf-kβ pathway in a tumor xenograft model [42].
2.2. Natural Products against Metastasis
Metastasis is a highly complicated process that involves primary tumor mass, circulating tumor cells (CTCs), and abnormal tumor microenvironments and is a leading contributor to cancer-linked deaths. The spread of metastasis to a specific organ is determined by various factors: for example, observing patterns of circulation, such as receiving direct blood from the primary site, e.g., hepatic metastasis happens in patients with colorectal cancer. Vascular permeability promotes extravasation at tumor sites, which further increases the probability of metastasis because instead of being autonomous, tumor cells become involved in bidirectional communication with metastatic microenvironments and modify the antitumoral immunity, genomic stability, extracellular milieu, survival signaling, chemoresistance, and proliferation activities. Despite improvements in advanced technologies, metastasis remains a major problem to resolve. The main therapeutic objectives are to prevent metastasis in high-risk patients, reducing advanced lesions, and avoiding additional metastasis with the limited disease [43].
Unlike conventional anticancer therapeutics, phytochemicals modulate multiple pathways during tumor initiation, progression, and metastasis development. Phytochemicals modulate primary epigenetic mechanisms regulating genes in metastasis such as DNA methylation, modification of histones, and/or non-coding RNA (ncRNA) associated multigene silencing. Regarding the complexity of metastatic tumors, multiple drugs can be used, such as the therapeutic approach used against (HIV) virus, because monotherapy can produce some initial responses. However, it lacks durability, so effective and durable therapy is required which minimizes the formation of resistance and improves durability. Phytochemicals can provide a better option due to their compatibility with multiple agents, which needs to be explored in detail. Some of the commonly explored and proven phytochemicals with anticancer and antimetastatic activities are apigenin, allicin, α-carotene, baicalein, and berberine curcumin, wogonin, formononetin, gambojic/ursolic/ellagic acid, papaya pectins, sulforaphane, and isothiocyanates [44].
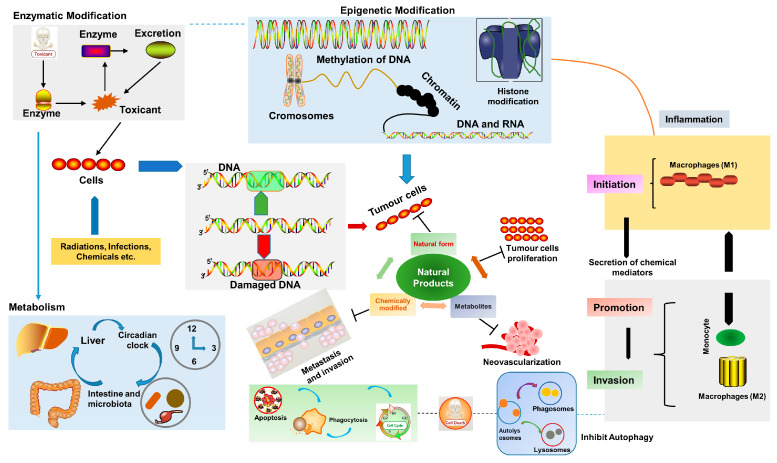
In the recent past, increasing proof of toxicity related to chemotherapeutic agents, even at the smallest therapeutic doses, has paved the way for exploring a nature-based approach and various medicinal plant extracts/phytochemicals have been utilized at different stages of cancer progression [45]; their general mechanisms are shown in Figure 3.
Figure 3.
Several aspects of the development of cancer and the potential role of natural products in cancer prevention and therapy (adapted from [46], licensed under creative common attribution license).
2.3. Natural Products and Cancer Immunotherapy
Tumor immunotherapy is relatively safe and effective, such as immune checkpoint blockade, and is considered a major breakthrough in recent decades. Both types (innate and adaptive) of anti-tumor immune responses can be initiated. Tumor-suppressing natural agents exert their action by upregulating immune responses in the tumor microenvironment, primarily via M1/M2-type macrophages. Recently, Sun et al. found that resveratrol can inhibit the polarization of human monocyte-derived macrophages into M2, and they also observed that lung cancer medium induced polarized M2 macrophages were modified to M1 polarized macrophages after resveratrol treatment by increasing IL-12 and TNF-ɑ and reducing IL-10 levels. Additionally, M2 macrophage markers’ expression was decreased, such as MRC1, chiI3, CCL24, and Retnla [47]. Moreover, Loo et al. successfully increased the immunity in postoperative patients with Rhodiola algida mixture by increasing the expression of IL-2, IL-4, GM-CSF, and mRNA content, thereby decreasing the increased risk of oral cancer [48].
Improving the functional activity of dendritic cells in cancer patients can significantly improve the treatment outcome by activating T cells, as mature dendritic cells secrete IL-12, which acts on T cells and encourage Th1 cell differentiation [49]. Chang et al. showed that the treatment of DCs with Astragalus and Codonopsis polysaccharide could promote the proliferation of CD4+ and CD8+ T cells in mice, and DC-based vaccine increased the anti-metastatic efficacy in 4T1 cells bearing mice by increasing the expression of CD40, CD80, and CD86 levels on the surface of DCs [50]. Similarly, it has been shown that shikonin, combined with B16 tumor cell lysate, could activate DCs to a more mature state with increased surface levels of CD86 and MHC II, which activates Th1 cells and improve its functions. Subsequently, the shikonin-tumor cell load DCs vaccine improved the cytotoxicity of splenocytes to cancer cells, hampered tumor growth, and increased mice’s survival [51].
NK cells help in differentiating stem cells and undifferentiated cancer cells by secreting IFN-γ and TNF-α, which limit the tumor growth by remodeling/regulating the tumor microenvironment. Natural products activate NK cells to stop tumor growth and metastasis [52]. Wu et al. have shown that lupeol can activate NK cells, which further activates the PI3K/Akt and Wnt/β- catenin pathway by increasing the expression of IFN-γ, PFP, and CD107a, as a result, inhibiting the proliferation of gastric cells (N87, HGC27 and BGC823) [53]. In addition, Hou et al. reported that total flavonoids obtained from Hippophae rhamnoides increased the cytotoxicity of NK cells against tumor cells by increasing the expression of NKp44, NKp46, granzymes B, and perforin [54].
MDSCs, a heterogeneous group of cells, promotes tumor progression, angiogenesis, and metastasis to healthy cells/tissues. Recently, Zhou et al. studied the effects of icariin on 4T1-Neu xenograft mice and noted that the number of MDSCs decreased in the spleen of the mice. Furthermore, icariin also restored the secretion IFN-γ by CD8+ T cells in the mice and reduced nitric oxide and ROS levels in MSDCs in vivo, while in vitro treatment differentiated into dendritic cells and macrophages [55]. The Shugan Jianpi formula has recently been reported to effectively regulate tumor microenvironment by suppressing CD8+ cells apoptosis, reducing MDSC proliferation and tumor activity in 4T1 tumor cells bearing mice [56].
Peripheral T lymphocytes CD4+ and CD8+ play a key part in anti-tumor immunity, and CD4+ T cells help in recruiting CD8+ T cells, while CD8+ T cells directly attack and kill tumor cells. CD4+ T cells can be differentiated into many subgroups of cells, such as Th1, Th2, Th9, Th17, Tfh, Treg cells, etc. The Th1/Th2 imbalance plays a vital role in the occurrence of tumors [57], and natural products increase Th1 development, which leads to the suppression of tumors. Wei et al. reported that with the addition of Tetramethylpyrazine phosphate (plant extracts) to the culture of lung cancer patient-derived PBMC cells and healthy subject cells, the expression of IFN-γ and IL-2 was increased, and Th2 cytokines decreased in patient-derived cells; the killing efficiency of macrophages was also improved [58]. Eckol is derived from Ecklonia cava, brown algae, and has shown potent antiproliferative and immunomodulatory activities. In a recent in vivo antitumoral study, the cytotoxic effects of eckol were evaluated by using sarcoma S180 xenograft model. The results stated that all the pro-apoptotic and antiproliferative proteins were upregulated, eckol also stimulated (MPS) and activated dendritic cells, which produced Th1 responses and enhanced CD4+/CD8+ T lymphocyte ratio, as a result cytotoxic T lymphocytes responses were increased [59]. Takei et al. observed that Ginsenoside promotes the transformation of naive T cells into Th1 cells by acting on DCs, increasing IFN-γ level, and in CTL assays, it was found that the Ginsenoside induced more IFN-γ than the mature DCs, which indicates that combined treatment may exert strong immunogenic response [60].
In recent times, cancer immunotherapy has gained some success by engineering antigen-specific T cells, e.g., TCR-T and CAR-T cells. Especially in hematological cancer, the CAR-T targets the CD19 and has been used and allowed for clinical use by the US FDA. Similarly, TCR-T cells have been used to treat cancer; however, MHC-restriction hinders their potential clinical use. There are no reports available which show the use of CAR-T cells and TCR-T cells combined with natural products, but this is an emerging area of research and needs to be explored, and we might obtain some successful outcomes in treating various cancers [61].
Astragalus polysaccharides have been shown to inhibit the proliferation of Treg cells in a dose- and time-dependent manner by reestablishing the cytokine balance and decreasing the expression of Foxp3 in the microenvironment of HCC. Moreover, Astragalus polysaccharides can block the (SDF-1) or its receptor (responsible for recruiting Treg cells into the HCC microenvironment) via the CXCR4/CXCL12 signaling pathway, thus inhibiting Treg cells migration [62]. Astragalus polysaccharides also act as an immunomodulatory agent by activating the TLR4 pathway, which inhibits TGF-β, resulting in a decrease in the number of Treg cells [63]. Similarly, Radix glycyrrhizae polysaccharides have been shown to downregulate the number of Treg cells in the microenvironment of H22 tumor xenograft mice by decreasing the expression of Foxp3 in Treg cells while upregulating the Th1/Th2 ratio in serum; as a result, inhibiting tumor growth [64].
Recently, targeting immune checkpoint molecules (PD-1 and CTLA4) has been considered a promising approach to treat various diseases such as cancer. These checkpoint molecules play an essential role in regulating immune homeostasis [65]. Currently, immune checkpoint molecules include programmed death receptor 1 (PD-1), cytotoxic T-lymphocyte associated antigen 4 (CTLA4), lymphocyte activation gene 3 (LAG3), and T cell immunoglobulin mucin 3 (Tim3). Presently, very little work is done on using natural products to target immune checkpoint molecules, and this research area needs more exploration [66]. Zhang et al. showed that Qiyusanlong decoction suppressed tumor growth in Lewis lung carcinoma-bearing mice by decreasing both mRNA and PD-1/PDL-1 protein levels in the tumor [67]. Similarly, another study revealed that Gegen-qinlian decoction increased the effects of PD-1 blockade in colon cancer via remodeling of intestinal microbiota, which demonstrated that phytochemicals could block PD-1 and thereby could be a possible approach for treating various cancers [68]. Zhao et al. showed that curcumin suppresses Treg cells (CD4+ CD25+) function by decreasing CTLA4 and Foxp3 [69].
2.4. Natural Products in Combination with Other Chemotherapeutic Drugs
Currently, combinatorial therapy has gained importance due to the administration of multiple chemotherapeutics with different biochemical targets, which increases efficacy as well as safety, and this concept is widely applied to different tumors. Many studies have reported the combination of widely used phytochemicals like (Resveratrol, curcumin, and thymoquinone) with other antitumoral drugs and have achieved significant success in preclinical studies [70]. Combination therapy reduces the toxicities associated with traditionally used chemotherapeutics such as doxorubicin (cardiomyopathy) and cisplatin (nephrotoxicity and immunosuppression), etc. The other advantage of combination therapy is overcoming drug resistance, which reduces adverse events by using low doses with the same efficacy and sometimes better pharmacological effects (synergism). Many preclinical studies and clinical trials are conducted to use phytochemicals as adjuvant therapy in various cancers. Chemoresistance has been commonly endured by many traditional chemotherapeutics, which is the main concern and hurdle in tumor therapy along with radiation resistance. Natural products are ideal candidates for chemosensitizing tumor cells and increasing the efficacy of existing drugs [71]. Silybinin has been shown to help doxorubicin in overcoming drug resistance in colorectal cancer by inhibiting GLUT1 expression. Many natural agents act as antioxidants, such as ROS scavenging/antioxidation, thereby reducing the toxicities related to the generation of free radicals. However, drug–drug interaction could be the main disadvantage of combinatorial therapy [72].
Curcumin has been used in combination with many chemotherapeutic drugs for the treatment of various cancers. For example, curcumin sensitizes tumor cells to 5-fluorouracil, which inhibits the tumor growth in HCT116 cells/xenograft model via inhibition of AMPK/UKL1 modulation of the AKT pathway [73]. Similarly, curcumin augmented doxorubicin’s anti-tumor activity by suppressing cell migration and inducing apoptosis in neuroblastoma SH-SY5Y cells via upregulation of p21 p53, and TIMP1 and downregulation of MMP2 [74]. Resveratrol and paclitaxel combination is reported to produce synergistic effects through the stimulation of TRPM2 channel in glioblastoma DBTRG cell line, which resulted in oxidative stress and induced apoptosis, which shows that resveratrol can be used in combination with other chemotherapeutic agents which can enhance the treatment efficiency [75]. Shen et al. reported that noscapine increases the sensitivity of cisplatin by modulating the cell cycle (G2/M phase arrest) and inducing apoptosis (downregulating XIAP and NF-kβ, while upregulating caspase-3) in cisplatin-resistant ovarian SKOV3 cells and xenograft in mice [76]. In addition, Neferine has been shown to increase the antitumor activity of cisplatin in lung cancer A549 cells via G1 phase cell cycle arrest, hypergeneration of ROS, upregulating Bax, BAk, p53, and c-myc levels while downregulating Bcl-2, FAK, VEGF, MMP-2/-9, and loss of membrane potential (ΔΨM) [77]. Wang et al. used cryptotanshinone in combination with paclitaxel against tongue squamous cell carcinoma (CAL 27 and SCC) cell lines, which increased the antitumor activity by inhibiting the migration and proliferation of cells as well as inducing apoptosis due to inhibition of JAK/STAT3 signaling pathway [78].
Similarly, Zhang et al. showed that a combination of formononetin and temozolomide could produce synergistic effects in C6 glioma cells through upregulation of Bax and caspase-3/-9 expression while downregulating Bcl-2 and MMP-2/-9 expressions [79]. Furthermore, Tseng et al. reported that aloe emodin enhanced the cytotoxicity of tamoxifen in different breast cancer cells (MDA-MB 231, MCF-7, HCC1954, and BT-474 cells) via suppression of Ras/ERK and PI3K/mTOR signaling pathway [80]. Similarly, a combination of capsaicin and docetaxel has been shown to have higher cytotoxicity against prostate cancer (LNCaP and PC3) cell lines and tumor xenograft in mice by inhibiting the growth and proliferation via reducing Akt, mTOR, and S6 phosphorylation while increasing (AMPK) phosphorylation [81].
Recently, Saikia et al. reported that combined use of heteronemin with cytarabine increases its sensitivity against leukemia (HL-60) cells by downregulating downstream targets of Ras signaling pathway such as AP-1, NF-kβ, AMPK, and c-myc; as a result it produces synergistic effects, inhibits the growth, and induces apoptosis [82]. Furthermore, the combined use of celastrol and triptolide has been shown to produce synergistic effects against various cancer models in vitro (H1299, H460, SKOV3, OVCAR3, Hela, SIHA, and SW480 cell lines) and in vivo studies (xenograft in mice) by inhibiting growth and proliferation through increasing ROS, G2/M phase arrest, decreasing Akt, surviving, and EGFR expressions [83]. Moreover, the combined use of berberine and sorafenib is effective in liver carcinoma because berberine increases the sensitivity of HCC (HepG2 and SMMC-7721) cells to sorafenib, which ultimately inhibits the growth and proliferation and induces apoptosis by increasing the expression of cleaved PARP and cleaved caspase-3, while decreasing the expression of Bcl-2 and VEGF markers [84]. Desai et al. studied the combined effects of biochanin A and Temozolomide against U-87 and T-98 MG cells and observed that the growth and proliferation of the cells were synergistically inhibited and the expression of p-p53 was increased, while the expression of p-Akt, EGFR, p-ERK, and MT-MMP1 was decreased [85]. Mangiferin increased the sensitivity of oxaliplatin towards MCF-7, Hela, and HT29 cell lines by increasing the expression of caspase-3, S-phase cycle arrest, and reducing Nf-kβ activation, which induced apoptosis [86].
In a recent study, dietary phytochemical fisetin was shown to produce synergistic effects in combination with sorafenib against cervical cancer (Hela) cells by activating DR5-mediated caspase-3/8 activity and mitochondrial apoptotic pathway [87]. In another study, the combined use of ursolic acid and temozolomide has been shown to produce synergistic effects in in vitro and in vivo studies in mice by overcoming temozolomide-resistance due to the downregulation of O6-methylguanine DNA-methyltransferase (MGMT), which resulted in an increase in cytotoxicity and inhibited the proliferation of the tumor cells, which indicates the potential of ursolic acid as a monotherapeutic anticancer agent as well as a chemosensitizer [88]. Similarly, a combination of lipoic acid and other antineoplastic drugs (5-FU and Doxorubicin) resulted in a decrease in p53-mediated stabilization of p21, which resulted in the synergistic killing of colorectal cancer cells [89]. Recently, Huang et al. showed that vitamin D sensitizes oral squamous carcinoma cells to cisplatin by inhibiting lipocalin-2 (LCN2) modulated Nf-kβ signaling pathway via ribosomal protein S3 (RPS3), which partially reversed the cisplatin resistance [90]. Moreover, the combined use of embelin (XIAP inhibitor) and celastrol (NF-kβ inhibitor) in acute myeloid leukemia (HL-60) cell line has been shown to produce synergistic and additive effects by downregulating COX-2 and survivin, which shows that simultaneous inhibition of the two tumor signaling pathways can improve the effectiveness of the chemotherapy [91].
The combination of withaferin A and oxaliplatin produced synergistic effects in pancreatic cancer (in vitro and in vivo studies) by increasing the generation of ROS, which ultimately led to the inhibition of the PI3K/AKT signaling pathway [92]. Yang et al. reported that tectorigenin increases the sensitivity of paclitaxel in paclitaxel-resistant ovarian tumor (SKVO3, MPSC1, and A27800) cell lines by activating caspase-3/-8/-9 and downregulating XIAP, Bcl-2, COX-2, FLIP, IkB, IKK, and Akt, which suggests that it increases the sensitivity and cytotoxicity by inactivating Akt/IkB/IKK/NF-kβ pathway [93]. Moreover, glabridin, a flavonoid, has been shown to reverse the P-gp mediated resistance to the conventional chemotherapeutic drugs (Doxorubicin and paclitaxel) in P-gp overexpressing breast cancer (MDA-MB-231/MDR1 and MCF-7/ADR) cell lines by suppressing P-gp and downregulating the functional activity of P-gp- ATPase and thereby reversing the multidrug resistance [94]. Similarly, Xia et al. showed that gambojic acid promotes gemcitabine’s sensitivity in pancreatic cancer (in vitro and in vivo studies) by inhibiting the ERK/E2F1/RRM2 signaling pathway, which decreased the proliferation and growth of tumors. Moreover, the study also indicated that gambojic acid can be used as a monotherapeutic agent, as well as a chemosensitizer [95]. In addition, the combined use of forbesione with 5-fluorouracil produced synergistic effects in inhibiting growth and proliferation in cholangiocarcinoma cancer (in vitro and in vivo studies using Ham-1 cells), through downregulating Bcl-2 and increasing expression of p53, Bax, Apaf-1, and caspase 3/-9, which induces apoptosis and results in the killing of the tumor cells [96]. Similarly, Su et al. showed that osthole enhances cisplatin’s effects by suppressing NRF2 expression and blocking the PI3K/Akt signaling pathway, thus inhibiting the progression of cisplatin-resistant cervical cancer (SiHa/cDDP) cells in vitro and in an in vivo xenograft model [97].
3. Natural Products against Some Common Types of Cancer
3.1. Lung Cancer
Lung cancer is highly occurring and a standout amongst the most widely recognized human malignancies in both developed and developing nations, with 2.1 million new lung cancer cases and 1.8 million deaths anticipated in 2018, nearly one of every five (18.4%) malignancy deaths [98,99]. Even though various treatment alternatives are available, including surgery, radiotherapy, chemotherapy, and targeted therapy, half of all newly diagnosed cancers are now at an advanced stage, where the effects of treatment are constrained. Treatment in such cases might be restricted to palliative care, leading to a low 5-year survival rate. Lung cancer comprises a couple of subtypes; for example, adenoma-carcinoma (AdCa), the most widely recognized subtype in non-smokers and females; squamous cell carcinoma (SqCC), and small cell lung cancer (SCLC). A few instances of current treatment for malignant lung growth incorporate medical procedures, chemotherapy, and radiotherapy, whereby the choice of treatment relies upon the subtype and stage of lung cancer. Nevertheless, there is a dire need to find other alternative chemotherapeutic agents, either fabricated as a more effective drug or in combination with the current therapeutic agents to have a more prominent and synergistic anticancer activity. Recently, Aucubin has been reported to produce antitumoral effects in lung cancer (A549) cells by inducing cell cycle arrest at G0/G1 phase, inducing p53 activation, and also increasing the activity of the Fas/Fas-ligand system [100]. Similarly, Xu et al. reported that cytisine, a natural alkaloid, produces significant cytotoxic effects against lung cancer A549, NCI-H23, and NCI-H460 cells and in vivo (rat model) by inducing apoptosis via an increase in ROS and loss of membrane potential; increasing BAD, cleaving PARP, and cleaving caspase-3 expressions; and decreasing Bcl-2, pro-PARP, and pro-caspase-3. Moreover, phosphorylation of p38, JNK, and I-kB was increased, while there was a significant decrease in the phosphorylation of ERK, NF-kβ, and STAT3. Furthermore, cytisine arrested the cell cycle at the G2/M phase, which was related to the inhibition of the Akt signaling pathway [101].
In another study, Han et al. showed that L-securinine from dried leaves of Securinega suffruticosa inhibited the proliferation of A549 cancer cells, decreased the expression of DKK1 genes, and promoted the methylation of DKK1 promoter in comparison to 5-azacytidine [102]. Similarly, gedunin isolated from Azadirachta indica induced apoptosis and inhibited the growth and proliferation of lung cancer A549 cells by generating ROS, decreasing the membrane potential, and causing DNA damage via downregulation of PIK3CA, EGFR, AKT, and autophagy. Furthermore, gedunin also disrupted the interaction between Hsp90: Bcl-1:Bcl-2 [103]. In another study, vanillin, the main ingredient of Vanilla planifolia, suppressed the CSC-like behavior of lung cancer NCI-H460 cells by inducing Akt-proteasomal degradation and decreasing downstream CSC transcription factors (Oct4 and Nanog) [104]. Additionally, cepharanthine isolated from Stephania cepharantha inhibited the growth and proliferation of lung cancer H1299 and A549 cells by increasing ROS, loss of membrane potential, and cell cycle arrest [105].
3.2. Breast Cancer
In 2018, there were about 2.1 million newly diagnosed female breast malignant growth cases around the world, representing nearly one of every four tumor cases among women. Breast cancer is the most widely diagnosed cancer by far in most nations (154 of 185) and is also the primary source of cancer-related deaths in more than 100 nations. For malignant breast tumor treatment, numerous alternatives are provided, for example, medical procedures, hormonal treatment, radiation treatment, chemotherapy, and targeted treatment [106]. However, there are also certain limitations (i.e., narrow therapeutic index with non-specific toxic consequences for healthy tissues, increasing chance of infection, etc.), and also the severe side effects persist for months or years, even after treatment completion. Therefore, further studies are required to explicate the underlying fundamental mechanisms of breast cancer to develop new therapeutic strategies. Breast cancer is the collection of abnormal cells, presumably credited to the imbalanced proliferation of cells, apoptosis, and the cluttered autophagy regulation. Various natural products were accounted for as possible anti-cancer agents or thought of as immediate or indirect sources of new chemotherapeutic adjuvants to upgrade the efficacy or enhance the symptoms through autophagy regulation [107]. Higenamine has been demonstrated to increase the anticancer activity (apoptosis and G2/M cell cycle arrest) of cucurbitacin B in breast cancer (T47D and SkBr3) cells by inhibiting Akt and CDK2 [108].
In another study, Citral, isolated from Cymbopogon citrates, suppressed the growth and proliferation of 4T1 breast cancer cells implanted in the nude BALB/c mice [109]. Similarly, euphol inhibited the growth and proliferation of breast cancer (T47D) cells by inducing G1 phase cell cycle arrest via downregulation of cyclin D1 and upregulation of p21 and p27 expressions [110]. In another study, Reddy et al. demonstrated that strophanthidin produces dose-dependent cytotoxic effects against breast (MCF7), liver (HepG2), and lung (A549) cancer cells by attenuating MAPK, Wnt/β-catenin, and PI3K/Akt/mTOR signaling pathways [111]. Moreover, jatrophone isolated from Jatropha isabelli inhibited the proliferation and EMT of triple-negative breast cancer cells by interfering with the cell cycle and Wnt/β-catenin signaling pathways [112]. Deng et al. reported that rotenone induced apoptosis in MCF7 breast cancer cells by producing ROS, chromatin condensation, upregulating Bax, downregulating Bcl2, and cleaving PARP. Furthermore, cJNK and P38 MAPKs were activated, and ERK (1/2) signaling pathways were inactivated [113]. Similarly, Dhandayuthapani et al. demonstrated bromelain to be effective against breast cancer by promoting apoptosis via upregulation of c-JNK, p38 kinase, and activating caspase 3 and 9 [114]. In another study, flavokawain B induced apoptosis and inhibited the proliferation of breast cancer (MCF-7 and MDA-MB231) cells by upregulating various kinases such as p-Akt, p-JNK, p-CREB, p-p53, and p-WNK1 and downregulating Nf-kβ, COX2, MMP-9, GLUT1, and VEGFA [115].
3.3. Ovarian Cancer
Ovarian cancer is one of the leading causes of death associated with the female reproductive system in the Western world. Ovarian cancer is a standout amongst the most deadly gynecological diseases in the female reproductive system, influencing approximately one out of 75 women in the United States. Even though the first-line treatment may profit about 80% of patients with ovarian malignancy, 75% of those patients still experience tumor recurrence, which causes a major worry surrounding the treatment of ovarian cancer in patients. Cisplatin is the most generally utilized chemotherapeutic entity for treating ovarian cancer. Treatment dereliction and death in over 90% of patients with metastatic malady are believed to be brought about by drug resistance. Adverse reactions and resistance developed to platinum-based chemotherapy have turned into an obstacle for ovarian cancer treatment. Therefore, it is important to search for new compounds to treat cancer and reduce the associated side effects of the treatments.
Natural bioactive agents have received an increased consideration in cancer treatment lately [116]. For example, diarylheptanoid hirsutenone potentiates the TRAIL-induced apoptotic activity in ovarian cancer (OVCAR-3 and SKOV-3) cells by enhancing the activation of caspase-8 and BID dependent signaling pathways, which further activates caspase-3/-9, exhibiting the significance of hirsutenone in combination with TRAIL treatment [117]. Paclitaxel, first isolated from Taxus brevifolia, has been used in the management of lung, breast, ovarian, prostate, sarcoma, leukemia, and endometrial cancer; it acts by interfering in spindle function, stabilizing microtubules, inducing apoptosis, cell cycle arrest, and inhibiting the growth of tumor cells [118]. Moreover, barbamine isolated from Berberis amurensis effectively inhibited the growth of cancer in vitro (SKOV3 and Es2 cell lines) and in vivo (SKOV3 xenograft model) by inducing apoptosis and targeting the Wnt/β-catenin pathway [119]. Recently, Nobilietin has also been shown to produce strong antitumor effects in ovarian cancer (HOCCs) cells by increasing the expression of cleaved PARP in a dose-dependent manner and inhibiting proliferation, causing DNA damage and inducing apoptosis. Many in vitro and in vivo studies have demonstrated that various flavonoids produce potent cytotoxic activities by inducing apoptosis, cell cycle arrest, and inhibiting angiogenesis [120].
3.4. Colon Cancer
Colorectal cancer (CRC) is prominent amongst the most occurring tumors worldwide, and as of late, it is the third-highest contributor to cancer-associated deaths in the United States of America and China [121]. It represents over 90% of the malignant tumors of the large bowel, and the other 10% comprises lymphoma and squamous cell carcinoma. The occurrence of colorectal cancer is continually increasing due to weak prognosis in patients possessing widely metastasized tumors, and the fundamental underlying mechanism of metastasis is not completely clear [122]. A few epidemiological investigations have exhibited the relationship of colon tumor growth with dietary propensities, for example, low fiber diet, high fat intake, and low calcium/micronutrient consumption [123]. The inverse correlation between the utilization of vegetables and fruits with different cancers has led researchers to examine the advantages of dietary elements in chemoprevention [124]. Recently, Morin has been shown to induce apoptosis and inhibit the growth of colon cancer (SW480) cells through the generation of ROS and decrease in mitochondrial membrane potential. Furthermore, it increased PARP, Bax, and cleaved caspase-3/-8/-9 expressions [125]. In another study, inflexinol isolated from Isodon excisus has shown the potential to strongly inhibit the growth and proliferation of colon cancer cells by inducing apoptosis through inactivation of NF-kβ in in vitro (SW620 and HCT116 cell lines) and in vivo (SW620 xenograft) rat model [126]. In addition, strychnine from Nux vomica has shown significant antiproliferative effects in vitro (SW480, Lovo, and DLD1) and in vivo (DLD1 xenografted model) by targeting the Wnt/β-catenin pathway by enhancing APC and decreasing β-catenin and c-Myc. Furthermore, the expression of DKK1 was also enhanced, known for negatively regulating the Wnt/β-catenin pathway [127].
In another study, Gao et al. showed that berginin significantly suppressed the proliferation of HCT116 cells by accumulating intracellular ROS, DNA damage, and G1 phase arrest through inhibition of PI3K/AKT/mTOR pathway [128]. Similarly, psoralidin inhibited the viability and proliferation of SW480 cells by inhibiting the Nf-kβ and Bcl-2/Bax signaling pathway [129]. In a recent study, matrine was also shown to produce antitumor effects in vitro against multiple colorectal cancer (LS174T, SW1116, Caco-2 and RKO) cells and in vivo in (LS174T xenografted) mice via G1/G0 phase arrest, reducing Bcl-2 and caspase-3, while upregulating cleaved caspase-3 and Bax expressions [130]. Magnolol isolated from Magnolia officinalis shows significant antiproliferative effects in vitro and in vivo against HT-29 and CT-26 cell lines by inducing apoptosis through intrinsic and extrinsic pathways inhibiting PKCδ/NF-kβ signaling pathway [131]. Similarly, Mi et al. showed that imperatonin isolated from Angelica dahurica suppresses the tumor growth, proliferation, and angiogenesis of human colon cancer (HeLa, Hep3B, and HCT116) cells through HIF-1α targeting through mTOR/p70S6K/4E-BP1 and MAPK signaling pathways [132]. Moreover, aloperinole is an alkaloid extracted from the leaves of Sophora alopecuroides which produces potent cytotoxic effects against colon cancer HCT116 cells by inducing apoptosis and inhibiting the proliferation by G2/M phase cell cycle arrest and increasing the expression of Bax, p21, and p53, while decreasing Bcl-2, CD1, and B1. Furthermore, it also inhibited PI3K/Akt and JAK/Stat3 pathway [133]. Many preclinical and clinical studies have indicated that bioactive components of fruits and vegetables may help in the deterrence of colon cancer.
3.5. Brain Cancer
Brain tumors represent 85–90% of all significant CNS tumors. Glioblastoma (GBM) represents around half of all aggressive brain tumors and is related to low survival. Multimodal treatments, including surgery, pursued by adjuvant chemoradiation therapy (CRT) with temozolomide (TMZ), is a standardized treatment for GBM. The TMZ increases overall survival (OS) from 7.7 to 13.5 months and from 7.9 to 10.0 months in the GBM patients, which is extremely poor [134]. This poor survival is likely due to numerous variables, including systemic toxicity due to higher TMZ dosages, BBB impermeability, CRT resistance, and progression of refractory tumors [135]. Hence, identifying a novel chemotherapeutic agent that can modulate the BBB, restrain tumor development, and stop tumors recurrence is vital for better patient prognosis. In recent studies, ginkgetin extracted from Cephalotaxus fortune has been shown to effectively inhibit the growth, invasion, and proliferation of medulloblastoma (Daoy and D283 MB) cell lines by arresting cell cycle at (G2/M phase), and also inhibit Wnt/β catenin signaling pathway [136]. Similarly, Cao et al. reported that toosendanin shows higher cytotoxic effects in vitro and in vivo against glioblastoma multiforme (U87 and C6 cell lines) by inducing apoptosis via upregulation of ERβ and functional activation of p53 [137]. Recently, Noman et al. isolated prestegane B from Thymelaea microphylla, evaluated it for antiproliferative properties against C6 and Hela cell lines, and found that it shows higher antiproliferative and radical scavenging ability than some of the other known drugs [138]. Extracts of Viscum album are widely used as complementary medicine for cancer therapy, and recently some of the extracts such as aviscumine, iscador Qu, and ML-1 have been shown to regulate the expression of genes associated with the cell migration, invasion, adhesion, and even cell architecture formation in glioma cells by inhibiting TGF-β, SMAD2, and MMP-2/-9. These ingredients have the potential to be used as an adjuvant therapy for the treatment of glioblastoma [139].
3.6. Liver Cancer
Liver cancer was the sixth most commonly reported cancer and the fourth leading cause of cancer-related deaths worldwide in 2018, with around 841,000 new cases and 782,000 deaths every year. Liver cancer is the most widely recognized tumor of the digestive system, with a high mortality rate. Based on conformational evidence, numerous natural dietary compounds are potential hotspots for the prevention and management of liver malignancy [140]. Hepatocellular carcinoma (HCC) is the primary type of malignant liver tumors (70–80%), trailed by intrahepatic cholangiocarcinoma. The major risk factors for liver cancer are hepatitis B/hepatitis C disease, alcohol usage, aflatoxin B1, and metabolic issues. Liver cancer is mostly aggressive with a poor prognosis, having a low five-year survival rate below 9% [141]. Proper interventions such as liver resection, transplantation, and percutaneous removal are considered as the best approaches with curing potential for liver cancer. However, because of various injuries and extrahepatic metastasis, merely 20% of liver cancer patients are appropriate for a medical procedure. On the contrary, chemotherapeutic medications for liver cancer are constrained, and sorafenib is the most well-known medicine. The phase III clinical trials exhibited that sorafenib could increase the overall survival and progression time. However, its clinical gains are modest, and it was noted that sorafenib was only valuable for around 30% of patients, and resistance developed in half a year. Moreover, other issues such as hepatotoxicity, drug resistance, recurrence, and other unwanted side effects exist in present treatments, which compels researchers to look for an alternative therapy [142].
Recently, echinoside A and ds-echinoside, isolated from pearsonothuria graeffei, inhibited tumor growth and proliferation in the HepG2 cells by G0/G1 phase arrest and induced apoptosis via the mitochondrial pathway [143]. In another study, oleuropein has been shown to effectively inhibit the cell viability and proliferation of hepatocellular carcinoma (HepG2) cells by inducing apoptosis through ROS production, increasing Bax, and decreasing Bcl-2. Moreover, oleuropein also targeted the PI3k/Akt signaling pathway [144]. Similarly, crocin isolated from saffron has been shown to produce autophagic apoptosis and inhibit the growth and proliferation of human hepatic cancer (HCCLM3 and HepG2) cells via upregulation of LC3-II and constraining the functional activities of key proteins involved in Akt/mTOR signaling pathway such as p-Akt, p-mTOR, and p-p70S6K [145]. In addition, Alpinia oxyphylla extracts inhibited the proliferation and tumor growth in hepatocellular carcinoma (HepG2, Hep3B, Bel-7402, and SMMC-7721) cell lines and also in vivo Hep3B xenograft by increasing Bax and caspase-3/-9 expressions and decreasing Bcl-2 levels. Furthermore, it also upregulated PTEN and downregulated PI3k and inhibited the phosphorylation of Akt [146]. Alpidine, a cyclic depsipeptide isolated from Aplidium albicans, has produced antiproliferative effects in various tumors such as melanoma, breast and lung cancer via activation of JNK/p38 MAPK pathway and induces apoptosis [147]. Recently, corilagin has been shown to produce significant cytotoxic effects in vitro and in vivo against liver cancer SMMC7721 cells via G2/M phase cycle arrest, and by downregulating p-Akt and cyclin B1/cdc2 and upregulating p-p53 and p-p21Cip1 [148].
3.7. Head and Neck Cancer
Head and neck squamous cell carcinoma (HNSCCs) is among the sixth most common malignancies globally and accounts for 90% of all the head and neck tumors occurring at different anatomical sites. Alcohol consumption, tobacco use, and infection with the human papillomavirus (HPV) are thought to be the oncogenic drivers. Treatment options involve surgical resection, radiotherapy, and targeted chemotherapy, but are mostly ineffective and subsequent relapse frequently occurs due to tumor resistance. Along with treatment failure, these therapies result in higher morbidity and reduced quality of life due to their severely toxic nature [149]. This inadequate therapeutic response can be associated with alterations in intracellular signaling pathways and significant changes in the extracellular tumor microenvironment. Therefore, a better, safe, and effective alternative is needed to address these challenges to improve the treatment standard and reduce the treatment cost. Over the decade, various novel approaches have been considered to understand and target specific disease targets but have fallen short of achieving clinically significant results [150].
Phytochemicals have shown enormous potential in chemosensitizing and inhibiting the growth of HNSCCs in various in vitro and in vivo models. Recently, active constituents of Piper methysticum (kava) such as flavokawain A, flavokawain B, and yangonin have been shown to exhibit antiproliferative effects against OSCC cancer (BICR56 and H400) cells but are non-toxic to normal keratinocytes (OKF6), showing promise for in vivo translation. Additionally, Pycnogenol, isolated from pine bark, inhibited the cell viability and neoplastic transformation in (HSC-3 and TPA-treated JB6) cells by inducing apoptosis through ROS generation, and also increased the expression of cleaved PARP, Caspase-3, and Bax [151]. Moreover, α-mangostin extracted from Garcinia mangostana inhibited the growth and proliferation of human OSSC cells (HSC-2/-3/-4) by inducing apoptosis through a decrease in mitochondrial membrane potential and translocation of cytochrome c and also arrested cell cycle at G1 phase by downregulating (CDks/cyclins) [152]. Thymol isolated from thyme and oregano possesses potent antitumor activity and induces mitochondrial dysfunction and apoptosis in OSCC in vivo model [153]. Polyphenon E, in combination with erlotinib, has been used in clinical trials for the treatment of HSSCs (NCT01116336). Similarly, cucurbitacin B, in combination with other chemotherapeutic drugs, has shown antitumor activity against HSCC and breast cancer [154]. Several in vitro studies have shown that grape seed proanthocyanidins and EGCG produce cytotoxic effects by inducing apoptosis and cell cycle arrest at the G0/G1 phase in HSCC cell lines through various mechanisms [155].
3.8. Prostate Cancer
Prostate cancer is the most commonly diagnosed deadliest cancer in men after lung cancer in the world, having a mortality rate of 3.6% in 2018 and 1.05% increase is expected by 2040 [156]. The estimated rate of prostate cancer diagnosed in men is (one in nine) and mortality (1 in 41), which is quite alarming [157]. The etiological evidence of prostate cancer incidence, progression, and development is not very clear, and various risk factors such as age, race, family history, genetic/somatic mutations, and lifestyle have been linked to it. In particular, age has been a critical factor, and 66 years is the average age of diagnosis [158]. Like other cancers, early diagnosis and treatment are extremely important, but the asymptomatic nature of the disease and complicated diagnostic procedures and treatment expenses make it difficult to manage it properly. Different bioactive compounds have been evaluated and have shown potential to be used for the prevention and treatment of prostate cancer. For example, Shukla et al. tested apigenin against prostate cancer and found that the tumorigenesis in TRAMP mice was suppressed through inhibition of the Nf-kβ pathway [159]. Similarly, afzelin derived from Nymphaea odoratum exhibits anti-tumoral activities against prostate cancer (LNCaP and PC-3) cells by arresting cell cycle at G0 phase and also inhibits the expression of LIM domain kinase-1 [160]. Recently, plectronthoic acid, a novel compound, demonstrated potent significant anti-tumor activities by inhibiting proliferation, induced G0/G1 phase arrest in prostate cancer (PC3, DU145, and CW22Rv1) cells via upregulation of p21/CIP1 and p27/KIP1, and also suppressed mTOR/S6K signaling pathway [161]. In another study, anacardic acid has been shown to inhibit the proliferation and induce apoptosis in human LNCaP cells by autophagy through ER stress/DAPK3/Akt pathway [162]. Delphinidin induces p53 mediated apoptosis by suppressing HDAC function and activating acetylation of p53 in LNCap cells [163]. Moreover, lycorine extracted from Amaryllidaceae plants inhibited the growth, proliferation, migration, and invasion of multiple prostate cancer cells in vitro (LNCaP, PC-3M, DU145, and 22 RV1) and in vivo (PC-3M xenograft) model by abrogating p-STAT3 expression, reversed EMT via STAT3-mediated twist decrease, and inhibited EGF-induced JAK/STAT signaling [164].
Lall et al. established that fisetin effectively inhibits the synthesis of hyaluronan (HA) and increases antiangiogenic high molecular mass-HA and can be used to manage prostate cancer [165]. Similarly, punicalagin, a polyphenol, exhibited apoptotic activity and inhibited the proliferation of PC3 and LNCaP cells via upregulating caspase-3/-8 expressions and decreasing vascular network formation in the CAM model, which also confirmed its antiangiogenic effect [166]. Furthermore, Zeylenone isolated from Uvaria grandiflora Roxb decreased the cell viability, invasion, and metastatic growth of human PCa (DU145) cells by downregulating the Wnt/β-catenin pathway [167]. Moreover, mangiferin has been reported to possess immunomodulatory, apoptotic, antiangiogenic, and gene regulatory effects in vitro, ex vivo, and in vivo in different cancers, especially prostate cancer [168]. Similarly, anethole inhibited the proliferation of prostate cancer PC-3 cells and induced apoptosis by generating ROS, decreasing mitochondrial membrane potential, DNA damage, activating caspase-3 and -9, increasing Bax/Bcl-2 ratio, and leading to G2/M phase cell cycle arrest [169]. In addition, Paeonol inhibited tumor growth in vitro and in vivo by activating intrinsic and extrinsic apoptotic pathways and also inhibited p13k/Akt signaling pathway [170]. Similarly, oleandrin inhibits tumor progression by deregulating multiple pathways such as MAPK, Nf-kβ, and p13k/Akt pathway [171]. Ramamoorthy et al. reported that reserpine isolated from Rauwolfia serpentine induced apoptosis and arrested cell cycle at the G2 phase in prostate cancer (PC3) cells [172].
3.9. Hematological Cancer
Adverse events associated with the use of current therapies such as toxicities, neuropathy, or incessant relapsing have paved the way for using phytochemicals in the treatment of various hematological malignancies such as leukemia, lymphomas, multiple myelomas, Wald Enstrom’s macroglobulinemia, and other hematological diseases such as hemolytic anemia and thrombocytopenia. The frequently used natural products in hematological malignancies are anthracyclines and anthracenediones, vinca alkaloids, isothiocyanates, podophyllotoxin derivatives, polyphenols, and other antioxidants [173,174]. These phytochemicals are also used to sensitize conventional therapeutics such as dexamethasone. Curcumin, agaricus, and neovastat are currently in clinical trials for the treatment of multiple myelomas [175]. Vinca alkaloids such as vincristine and vinblastine are widely used as chemotherapeutic agents for various solid tumors and hematological disorders. Alkaloids such as harringtonine and isoharringtonine, which are isolated from Cephalotaxus harringtonia, act as chemotherapeutic agents and are used in the treatment of acute and chronic myelogenous leukemia and myelodysplastic syndromes. Isothiocyanates are another useful family of electrophilic bioactive compounds such as sinigrin, glucotropaeolin, gluconasturtiin, and glucoraphanin, and are involved in the inhibition of leukemic cells growth and regulate progression and differentiation of tumors, cell cycle, and apoptotic mechanisms. In a recent study, Wu et al. reported that rocaglamide breaks the TRAIL-induced resistance in vitro and in vivo in multiple myeloma and acute leukemia by inhibiting c-FLIP (main factor in TRAIL therapy resistance) expression and thereby increasing the effects of camptothecin, which shows that combined use of rocaglamide can be a suitable therapeutic option [176]. In addition, conophylline, an alkaloid isolated from Tabernaemontana divaricata, suppressed the pancreatic cancer desmoplasia and associated cytokines (IL-6, IL-8, CCL2, and CXCL12) produced by cancer-associated fibroblasts (CAF) and stellate cells. Moreover, the inhibitory and apoptotic effects of conophylline increased more when used in combination with gemcitabine [177]. Most recently, Tubulosine from Alangium salvifolium wang has selectively inhibited JAK3 signals by binding to ATP-binding active site of the kinase (JAK3), thereby reducing the progression and survival of hematopoietic cancer, as shown against HDLM-2, L540, U266, and BKO-84 cancer cells [178].
Similarly, betulinic acid has shown promising cytotoxic effects in various tumors. Phytochemicals provide a wide range of cellular effects by preventing carcinogens from reaching targeted sites and helping in ROS detoxification, increasing immunosurveillance to eliminate transformed cells, activating DNA repair mechanisms, and inhibiting proliferative tumor pathways [31]. In a recent study by Karami et al., gaillardin, a sesquiterpene lactone isolated from Inula ocular-christi, has been shown to produce higher cytotoxic effects in leukemic cells (NALM-6 and MOT-4 with IC50 of 6.1 and 7.3 µM, respectively) by arresting G0/G1 phase and inducing apoptosis, but it shows no cytotoxicity in normal cells [179]. Medicarpin, a natural phytoalexin, has been reported to sensitize myeloid leukemia cells to TRAIL treatment by upregulating pro-apoptotic markers (Cytochrome-c, tBid, Bax, CHOP) and downregulating anti-apoptotic proteins (Bcl-xl, Bcl-2, c-FLIP, XIAP, and survivin). Furthermore, it causes G2/M cell cycle arrest and also increases DR5 expression via activation of the ROS-JNK-CHOP signaling pathway [180]. Furthermore, taxodione isolated from taxodium distichum has been found to induce apoptosis in leukemia k562 cancer cells by generating ROS. Moreover, BCR-ABL, Akt, and STAT5 are sequestered in the mitochondria and are unable to stimulate proliferation [181].
3.10. Miscellaneous Cancer
Many different types of cancers have been affecting and threatening the lives of people, which include skin, stomach, oral cavity, rectum, gastric, gallbladder, pancreas, cervix uteri, penis, kidney, bladder, thyroid, Hodgkin lymphoma, non-Hodgkin lymphoma, leukemia, and osteosarcoma [182]. The interest in using natural products to treat cancer has been rising due to the better efficacy, low toxicity, and lower cost. Recently, Shivamadhu et al. showed that praecetrullus fistulosus lectins inhibited the growth and proliferation of a variety of tumor cells such as Hela, MCF-7, K562, and HT29 cells and also increased the survival of EAC bearing mice by decreasing MMP 2/9 activity and inducing apoptosis in the tumor cells [183]. In another study, oxolane derivatives from Morus alba, such as odisolane, have been proven to inhibit angiogenesis by reducing VEGF, p-ERK effectively, and p-Akt expressions, and can be employed to stop neovascularization in tumors [184]. Recently, hispiloscine and hispidacine alkaloids isolated from Ficus hispida linn showed excellent cytotoxic activity against various cell lines such as breast, lung, and colon, and also demonstrated vasorelaxant activity in rats [185]. Recently, Kim et al. demonstrated that bakuchiol inhibited the viability and EGF-induced neoplastic transformation of skin epithelial carcinoma A431 cell line in vitro and in vivo studies through the inhibition of Blk, Hck, p38MAPK, and Akt/p70S6k pathways [186]. Similarly, cryptolepine inhibited non-melanoma skin cancer proliferation by damaging DNA, S- phase cell cycle arrest, and decreasing membrane potential [187]. Curcubitacin B suppressed the invasion and proliferation of gastric cancer cells through STAT3 inhibition, which also induced apoptosis and furthermore in combination with cisplatin produced increased cytotoxic effects, which indicate that curcibitacin B is a promising STAT3 inhibitor [188]. Phycocyanin is another promising molecule isolated from seaweed, and it exerts its effects by arresting the cell cycle at the G2/M phase in MDA-MB-231, HT29, and A549 cells by decreasing cyclin E and CDk2 expressions and upregulating p21. Furthermore, it activates the mitochondrial apoptotic pathway while inhibiting the proliferative pathways such as PI3K/Akt/mTOR, MAPK, and Nf-kβ pathway [189].
In another study, Liang et al. demonstrated that isovitexin suppresses the stemness of osteosarcoma (U2OS and MG63) cells and induces apoptosis by disrupting the DNMT1/miR-34a/Bcl-2 nexus [190]. Furthermore, melatonin, a natural hormone produced by the pineal gland of animals, has been reported to inhibit almost all the hallmarks of tumor such as angiogenesis, metastasis, dysregulated metabolism, immune evasion, replicative immortality, and proliferative signaling, and induces apoptosis in various tumors an in vitro and in vivo studies [191]. Furthermore, purpurogallin inhibits the anchorage-dependent/independent growth of the esophageal squamous cell carcinoma in vitro and in vivo through the inhibition of MEK1/2 and ERK1/2 signaling pathways and, moreover, induces cell cycle arrest at S and G2 phases by decreasing Cyclin A1 and cyclin B2 and also activates PARP which induces apoptosis [192]. Xie et al. reported that mahanimbine inhibits the growth, proliferation, and viability of bladder cancer cells by inducing apoptosis with an increase in the expression of Bax and a decrease in Bcl2 levels, and also causes cell cycle arrest at Go/G1 phase. Furthermore it also induced autophagic death by increasing the LC3II and p62 expressions [193]. Similarly, taurine, an important amino acid present in different tissues of the body, has been proven to have antitumor effects in breast, lung, liver, colon, and prostate cancer by suppressing proliferation, invasion, and metastasis and inducing apoptosis [194]. Some of the common natural products which are undergoing different pre-clinical research are presented in Table 1.
Table 1.
Some of the important natural products in managing different types of cancers.
| Sources | Bioactive Constituents | Chemical Formula | Molecular Weight (g/mol) |
Mechanisms | Study Model | Concentrations | Other Cancers | References |
|---|---|---|---|---|---|---|---|---|
| Lung cancer | ||||||||
| Fascaplysinopsis Bergquist | Fascaplysin | C18H11ClN2O | 306.7 | ↓CDK-4, ↑ROS, ↓mTOR, ↓4EBP1, ↓p70S6K1 | A549, H1299, PC-9 and NCI-H526 cell lines | IC50: 0.57–1.15 µM | Liver, ovarian, and colon cancer | [195] |
| Chelidonium majus | Chelidonine | C20H19NO5 | 353.4 | ↓cyclin B1, ↑p21, ↓MMP, ↑ROS/AMPK, ↓p-p70S6K, ↓EGFR, G2/M phase arrest | A549, PC9, H460, H358 cells and H1975 xenograft in mice | IC50: 2.58–20 µM | Breast, liver, head, and neck cancer | [196] |
| Cephaelis Ipecacuanha | Emetine | C29H40N2O4 | 480.6 | ↓MMP-2/-9, ↓ERK1/2, and ↑p38 | A549 and H1299 cell line | IC50: 0.243 and 1 µM | Breast, colon, prostate, and pancreatic cancer | [197] |
| Citrus limon | D-limonene | C10H16 | 136.23 | ↑Bax, ↓Bcl-2, ↑cleaved PARP, ↑Atg5 | H1299 and A549 cells xenograft in Balb/c mice | 0.5–0.75 mM | Skin, liver, breast, and kidney cancer | [198] |
| Artemisia annua | Sesquiterpene (AMDT) | C17H26O2 | 262.39 | ↑caspase 3- 9, G1/S phase arrest | 95-D, HO8910, QGY, and HeLa cells | IC50: 52.44–73.3 µM | Gastric and ovarian cancer | [199] |
| Artemisinin | C15H22O5 | 282.33 | ↓VEGF-C, ↓p38 MAPK | LLC cells and C57BL/6 mice | 5–20 µM | Breast, cervical, gastric, liver, and bladder cancer | [200] | |
| Artemether | C16H26O5 | 298.37 | ↓Bcl-2, ↓cIAP1, ↓cIAP2, ↓CDK1, ↓CDK2, ↓CDK6, ↓cyclin A2, ↓cyclin B1, ↓cyclin D1, ↑p16 | A549, NCI-H1299 cells | 40–80 µM | Brain, breast, and gastric cancer | [201] | |
| Betula platyphylla | Betulin | C30H50O2 | 442.7 | ↑caspase 3- 9 and 8, ↑ Bax, ↑Cyto- C, ↑Bak, ↓PARP | A549, HepG2, Hela, MCF-7 cells | IC50: 10–15 µg/mL | Gastric, renal, colon, and melanoma | [202] |
| Betulinic Acid | C30H48O3 | 456.7 | ↓cyclin A2, ↓Sp1, G2/M phase arrest | A549, Hela, H1299 xenograft mice and Scgb1a1-rtTA/TetO-KrasG12Dmice | ≤40 µM | Colon, breast, prostate, nasopharyngeal, and cervical cancer | [203,204] | |
| Garcinia | Gambogic acid | C38H44O8 | 628.7 | ↑Caspase-3, ↓Bcl2, ↓pI3K, ↓DLL1, ↓DLL3, ↓ DLL4, ↓Jagged1, ↓Jagged2 | SPC-A-1 and A549 cells | 0.5–1 µmol/L | Liver and colorectal | [205] |
| Laminaria japonica | Fucoxanthin | C42H58O6 | 658.9 | ↑p21, ↑p53, ↑puma, ↑Fas, ↓Bcl-2, G0/G1 phase arrest | SPC-A1, A549, H460, H1299 | IC50: <82 µM | Skin, gastric, breast, and colon cancer | [206] |
| Chinese trichosanthes | Trichosanthin | 247 Amino acids | 27 kDa | ↑Caspase-3 and 9, ↑E-cadherin, ↓N-cadherin, ↓Bcl-2, ↑ Bax, ↑DR4, ↑DR5 | NCI-H1299, NCI-H1975, and A549 cells | IC20: 39.5, 61.1 and 49.3 µg/mL | Cervical, gastric, breast, and nasopharyngeal | [207] |
| Hedyotis | Ursolic acid | C30H48O3 | 456.7 | ↓MMP2, ↓MMP 9, ↑E-cadherin, ↓N-cadherin | H1975 cells | IC50: 29.7 nM | Breast, prostate, cervical, colorectal, and liver cancer | [208] |
| Oleanolic acid | C30H48O3 | 456.7 | ↑ROS, ↑Ca2+, ↓Akt, ↓JACk2, ↓STAT3 | A549 cells | IC50: 9.66 µM | Gallbladder, prostate, and gastric cancer | [209] | |
| Scutellaria barbata | Wogonin | C16H12O5 | 284.26 | ↑caspase-3, 8 and 9, ↑ROS | A427, BEAS-2B and A459 cells | 50 µM | Breast, cervical, and nasopharyngeal carcinoma | [210] |
| Luteolin | C15H10O6 | 286.24 | ↑miR-34a-5p, ↑p21, ↑p53, ↓MDM4, ↑caspase-3 and 9 | A549, and H460 xenograft model | IC50: 40 µM | Breast, ovarian, gastric, and prostate cancer | [211] | |
| Polygonum cuspidatum | Polydatin | C20H22O8 | 390.4 | ↓NLRP3, ↓ASC, ↓p-NF-κβ p65 | A549 and H1299 cells | 50 µM | Breast, hepatic, renal, and ovarian cancer | [212] |
| Rheum officinale | Emodin | C15H10O5 | 270.24 | ↑PPARγ, ↑IGFBP1, ↓Sp1 | H1975, and A549 cells xenograft mice | 50 µM | Renal and cervical cancer | [213] |
| Rhizoma zedoariae | β-elemene | C15H24 | 204.35 | ↑caspase-3, ↑ROS, ↑Cyto- C, ↑Bad, ↓Bcl-2 | A549/DDP cells | 20 and 40 µg/mL | Liver, gastric, and bladder cancer | [214] |
| Chansu | Bufalin | C24H34O4 | 386.5 | ↑caspase-3, ↓Akt, ↓ ERK ½, ↓VEGF, ↓cMyc, ↓NF-κβ, ↓p38 MAPK, G1/S phase arrest | A549, NCI-H460 | 2.5–10 µM | Prostate, liver, and ovarian cancer | [215,216] |
| Platycodon grandiflorum | Platycodin-D | C57H92O28 | 1225.3 | ↑Atg-3, ↑Atg-7, ↑Beclin-1, ↑LC3-II, ↓ p-Akt, ↓p-p70S6K, ↓p-4EBP1, ↓ ERK ½ | NCI-H460 and A549 cells | 5–30 µmol/L | Colon, leukemia, and breast cancer | [217] |
| Salvia miltiorrhiza | Tanshinone IIA (TSIIA) | C19H18O3 | 294.34 | ↑Cyto- C, ↓ Bax, ↓MMP, ↑caspase-3 and 9 | A549 cells | IC50: 14.5 µM | Prostate, glioma, leukemia, and hepatoma | [218] |
| Tripterygium wilfordii Hook | Celastrol | C29H38O4 | 450.6 | ↓Bcl-2, ↑ Bax, ↓Akt, ↑Cyto- C, ↓PARP, ↑Fas, ↑FasL | A549 cells | IC50: 2.12 µM | Prostate, brain, and liver cancer | [219] |
| Curcuma longa | Curcumin | C21H20O6 | 368.4 | ↑ROS, ↓ΔΨm, ↓SOD1, ↑caspase-3 and 9, ↑ Bax, G2/M phase arrest | A549 and SPC-A1 cells | 10–25 µM | Pancreatic, gastric, prostate, and colorectal cancer | [220,221] |
| Cinnamomum cassia | Cinnamaldehyde | C9H8O | 132.16 | ↑caspase-3, ↑PARP, ↓Bcl-2,↑Bax,↓Bcl-xL, ↓HIF-1α, ↓β-catenin, ↓MMPs, ↑E-cadherin | NCI-H1299, YTMLC, A549 xenograft model | IC50: 10.5, 32, 41 µg/mL | oral squamous cell carcinoma and colorectal cancer | [222] |
| Coix lacryma-jobi L. | CP-1 polysaccharide | n.d. | n.d. | ↑caspase-3 and 9, ↓ΔΨm, S phase arrest | A549 cells | 10–300 µg/mL | Breast and colon cancer | [223] |
| Ophiopogon japonicus | Ophiopogonin B | C39H62O12 | 722.9 | ↑E-cadherin, ↓N-cadherin, ↓linc00668, ↑miR-432-5p | A549, 293T and THP-1 cells | 5 and 10 µmol/L | Colon, breast, and gastric cancer | [224] |
| Tetrastigma hemsleyanum | Radix Tetrastigma Hemsleyani Flavone | C27H30O10 | 514.5 | ↓MMP 2 and 9, ↓TIMP-1, ↑TIMP-2 | A549 cells | 0.5–10 mg/mL | Liver, leukemia, and stomach cancer | [225] |
| Panax notoginseng | Trilinolein | C57H98O6 | 879.4 | ↓Bcl-2, ↑ Bax, ↑ROS, ↓PARP, ↓Akt, ↑caspase-3, ↑Cyto- C | A549, MKN-45 and A498 cells | 25–100 µg/mL | Liver, colon, and gastric cancer | [226] |
| Fucus vesiculosus | Fucoidan | C7H14O7S | 242.25 | ↑ROS, ↑ATF4, ↑CHOP, ↑TLR4 | A549, CL1-5 and LLC-1 xenograft C57BL/6 mice | 100–800 µg/mL | Breast, liver, and colon cancer | [227] |
| Breast cancer | ||||||||
| Rabdosia rubescens | Oridonin | C20H28O6 | 364.4 | ↓Jagged2, ↓Notch1-4 | 4T1 cells and xenograft BALB/c mice | 0.1–10 mmol/L | Gastric, esophageal, prostate, and pancreatic cancer | [228] |
| Panax notoginseng | Panaxadiol saponins | C30H52O3 | 460.73 | ↓NLR, ↓MPO, ↓G-CSF, ↓PU.1, ↓C/EBPα, ↓EP1, ↓GATA 1 and 2 | 4T1-Luc and xenograft BALB/c mice | 0.1–40 µM | Lung, liver, and colon cancer | [229] |
| Ganoderma lucidum | Ganodermanontriol | C30H48O4 | 472.7 | ↓u-PA, ↓CDC20, ↓survivin | MDA-MB-231 cells | IC50: 11.6 µM | Gastric, prostate, and colon cancer | [230] |
| Polysaccharides | n.d. | n.d. | ↑SOD, ↑CAT, ↑GPx, ↓IL-1β, ↓IL-6, ↓TNF-α | MDA-MB-231 cells, Wistar rats | 0.5–3 mg/mL | Melanoma, cervical, and colorectal cancer | [231,232] | |
| Maclura pomifera | Pomiferin | C25H24O6 | 420.5 | ↑CANX, ↑BCAP31, ↑Mn-SOD,↑ACOX1,↓BMP-7 ↓ID 2 and 3 | MCF-7 and MCF-10A | IC50: 5.2 µM | Liver, cholangiocarcinoma, and colon cancer | [233] |
| Chansu | Bufadienolides | C24H34O2 | 354.52 | ↓STAT3/Mcl-1, ↓Bcl-xL, ↓PARP, ↑caspase-3 | MDA-MB-231, MCF-10A and MCF-7 cells | 0.1–0.5 µM | Liver, leukemia, lung, and gastric cancer | [234] |
| Cimicifuga foetida | Actein | C37H56O11 | 676.8 | ↓VEGF, ↓pERK, ↓pJNK, ↓CD34, ↓CXCR4 | HMEC-1, 4T1 cells xenograft in mice | IC50: 0.065 µM | Gastric, bladder, and lung cancer | [235] |
| Astragalus membranaceus | Formononetin | C16H12O4 | 268.26 | ↓MMP 2, ↓MMP9, ↓PI3K, ↓Akt, ↑TIMP-1 and 2 | MDA-MB-231 and 4T1 cells | 2.5–160 µmol/L | Cervical, bladder, and gastric cancer | [236] |
| Astragulus Polysaccharides | n.d. | n.d. | ↓Bcl-2, ↑ Bax, ↑NO, ↑TNF-α, G1/S phase arrest | MCF-7 cells | 100–1000 µg/mL | Liver, lung, and gastric cancer | [237] | |
| Narcissus L. bulb | Narciclasine | C14H13NO7 | 307.25 | ↑AMPK-ULK1, ↓PRAS40 | MDA-MB-231, MCF-7, MCF-10A, BT483, HCC1937 and xenograft in mice | IC50: 5–100 nM | Brain, liver, and hematological cancers | [238] |
| Cimicifuga racemosa | KHF16 | C37H58O10 | 662.85 | ↓XIAP, Mcl-1, ↓Survivin, ↓Cyclin B1/D1, ↓NF-kβ | MDA-MB-231, MDA-MB-468 and SW527 | IC50: <10 µM | Gastric, bladder, and lung cancer | [239] |
| Pueraria lobata | Puerarin | C21H20O9 | 416.4 | ↓NFκβ, ↑AMPK, ↑ACC, ↑GSK-3β, ↓CREB | MCF-7/Adr cells | 20–100 µM | Bladder, liver, and lung cancer | [240] |
| Gardenia jasminoides Ellis | Genipin | C11H14O5 | 226.23 | ↓Bcl-2, ↑ Bax, ↑caspase-3, ↑JNK, ↑p38 MAPK | MDA-MB-231 cells | IC50: 327 µM | Gastric, multiple myeloma, and bladder cancer | [241] |
| Tetradium ruticarpum | Evodiamine | C19H17N3O | 303.4 | ↓u-PA, ↓MMP 9, Bcl-2, ↑ Bax, ↓CD1, ↓CDK6, G0/G1 arrest | MDA-MB-231 cells and xenograft BALB/c mice | IC50: 90 µM | Bladder, colorectal, pancreatic, and melanoma | [242] |
| Euphorbia prolifera Buch-Ham | Myrsinol diterpene (J196-10-1) |
n.d. | n.d. | ↓MDR, ↓Pgp efflux, ↑ATP hydrolysis | MCF-7/Adr cells | 0.39–50 µM | Gastric, colon and lung cancer | [243] |
| Solanum nigrum L. | α-Solanine | C45H73NO15 | 868.1 | ↑Bax, ↓Bcl-2, ↓Ψm | 4T1 cells and xenograft BALB/c mice | IC50: 17 µM | Pancreatic, esophageal, and prostate cancer | [244] |
| Melonidus suaveolens | Melosuavine I | C41H42N4O6 | 686.79 | ↑caspase 3, ↓Bcl-2, ↑p53 | BT549 cells | IC50: 0.89 µM | Lung, colon, and prostate cancer | [245] |
| Cnidium monnieri (L.) Cusson. | Osthole | C15H16O3 | 244.28 | ↑caspase 9 and 3, ↓PARP, ↑p53 and p23, ↓Cdk2 and CD1 | MDA-MB 435 cells | 20–100 µmol/L | Liver and lung cancer | [246] |
| Ophiopogon japonicus | Ophiopogonin D | C44H70O16 | 855 | ↑Caspase 9 and 8, ↓cyclin B1 and G2/M arrest | MCF-7 cells | 12.5–50 µmol/L | Prostate and laryngeal carcinoma | [247] |
| Panax ginseng C. A. Mey. | Ginsenoside Rg5 | C42H70O12 | 767 | ↑p53, p21 and p15, ↓CD1, CE2 and Cdk4, ↑caspase 6,7,8 and 9, ↓PARP | MCF-7 cells | 25–100 µM | Leukemia, gastric, and cervical cancer | [248] |
| Epimedium brevicornum | Icariside II | C27H30O10 | 514.5 | ↑Cl-Caspase-9,8,7,3, ↑ Cl-PARP, ↑ Bax, ↑Bcl-xL, ↑BimL, ↑Fas, ↓ FasL, ↑FADD, ↓MMP, ↑Cyto- C, ↑AIF | MCF-7 and MDA-MB-231 cells | IC50: 72.73 and 97.14 µM | Lung, melanoma, epidermoid, and prostate cancer | [249] |
| Ovarian cancer | ||||||||
| Tripterygium wilfordii Hook | Triptolide | C20H24O6 | 360.4 | ↓ MMP7 and MMP19, ↑Ecadherin | SKOV3, A2780 cells and SKVO3 xenograft mice | 1.5–150 nM | Colon, renal, and cervical cancer | [250] |
| Panax ginseng | Ginsenoside 20(S)-Rg3 | C42H72O13 | 785 | ↑ Caspase-3 and 9, ↓ PI3K/Akt and IAP | HO-8910 cells | 25–100 µg/mL | Lung, prostate, and breast cancer | [251] |
| Syzygium aromaticum | Kumatakenin | C17H14O6 | 314.29 | ↑ Caspase-3,8 and 9, ↓MCP-1, ↓RANTES, ↓IL-10, ↓VEGF, ↓MMP 2 and 9 | SKOV3 and A2780 cells | <100 µg/mL | Breast, liver, colon, and gastric cancer | [252] |
| Pueraria mirifica | Daidzein | C15H10O4 | 254.24 | ↑Bcl-2, ↑cym-c, ↑cleaved caspase-3/9, G2/M cell arrest, ↓pCdc25c, ↓pCdc2, ↓cyclin B1, ↓MMP-2/9 | SKOV3 cell line | IC50: 20 µM | Breast, colon, bladder, and pancreatic cancer | [253] |
| Thelypteris torresiana (Gaud) | Protoapigenone | C15H10O6 | 286.24 | ↓p-Cdk2, ↓Cdk2, ↓p-Cyclin B1, ↓Cyclin B1, ↑p-Cdc25C, ↓Bcl-xL, ↓Bcl-2, ↑caspase-3 | MDAH-2774 and SKOV3 cells | IC50: 0.69 and 0.78 µM | Lung, breast, and prostate cancer | [254] |
| Epimedium brevicornum | Icariin | C33H40O15 | 676.7 | ↑Caspase-3, ↓miR-21, ↑PTEN and RECK, ↓Bcl-2 | A2780 cells | 13–100 µM | Esophageal, prostate, and liver cancer | [255] |
| Icaritin | C21H20O6 | 368.4 | ↑caspase 3- 9, ↑p53 and ↓Akt/mTOR pathway | OV2002, C13*, A2780cp and PDXs in NOD/SCID mice | 10–50 µM | Endometrial, bladder, colorectal, and prostate cancer | [256] | |
| Gundelia tournefortii | Stigmasterol | C29H48O | 412.7 | ↑ROS, ↑caspase 3 and 9, Bax, ↑BAk, ↑cym-c, ↓VEGFA, ↓MMP 2,9 and 14 | OV90 and ES2 cells | 5–20 µg/mL | Gastric, skin, liver, and lung cancer | [257] |
| Vitex Agnus-castus L. | Casticin | C19H18O8 | 374.3 | ↑FOXO3a, ↓FoxM1, ↓survivin, ↓PLK1, p27KLP1 | SKOV3 and A2780 cells | 2.5–10 µmol/L | Gastric, gallbladder, cervical, and melanoma | [258] |
| Carya cathayensis Sarg | Juglone | C10H6O3 | 174.15 | ↑ROS, ↑p21, ↑Bax, ↑Bad, ↑Cyto c, ↓CDK2, cdc25A, CHK1, and cyclin A, ↓Bcl-2 and Bcl-xL, ↓Cyclin A, S-phase cell cycle arrest | Ishikawa cells | IC50: 20.81 µM | Glioma, lung, and leukemia | [259] |
| Scutellaria baicalensis Georgi | Baicalin and Baicalein | C21H18O11, C15H10O5 | 446.4, 270.24 | ↓VEGF, HIF-1α, cMyc, and NFκB | OVCAR-3, IOSE-364, and CP-70 cells | 5–160 µM | Burkett lymphoma, colorectal, pancreatic, prostate, and osteosarcoma | [260] |
| Potamogeton crispus L. | Luteolin-3’-O-β-D-glucopyranoside | C21H20O11 | 448.4 | ↓MMP-2, ↓MMP-9, G1/S phase arrest | ES-2 cells | 15–240 µg/mL | Colon and breast cancer | [261] |
| Artemisia annua | artesunate | C19H28O8 | 384.4 | ↑ROS, ↑p21, ↓CDKs, ↓Rb, ↓E2F-1, ↓CDC25C, G2/M cell cycle arrest | HEY, IGROV-1, OVCAR8, and OVCAR3 cells, and ID8 xenograft in C57BL/6 mice | IC50: 0.51–31.89 µM | Leukemia, pancreatic, and breast cancer | [262] |
| Arctium lappa | Arctigenin | C21H24O6 | 372.4 | ↓STAT3, ↓survivin, ↓iNOS, ↑caspase3 | OVCAR3 and SKOV3 cells | IC50: 10 µM | Breast, colon, and lung cancer | [263] |
| Asparagus officinalis L. | Asparanin A | C39H64O13 | 740.9 | ↓Bak/Bcl-xl, ↑ROS, ↑Cyto c, ↓Δψm, ↑caspases, G0/G1 cell cycle arrest, ↓PI3K/Akt/mTOR | Ishikawa cells and xenograft BALB/c mice | IC50: 9.34 µM | Liver and pancreatic cancer | [264] |
| Salvia miltiorrhiza | Cryptotanshinone | C19H20O3 | 296.4 | ↑caspase3 and 9, ↑Bax/Bcl-2, ↓MMP 2 and 9 | A2780 cells | 5–30 µM | Leukemia, prostate, and colon cancer | [265] |
| Colon cancer | ||||||||
| Asiatic Moonseed | Dauricine | C38H44N2O6 | 624.8 | ↓cyclin D1, ↓COX2, ↓cMyc, ↓survivin, ↓Bcl-2, ↓IAP1, ↓MMP 9, ↓ICAM1, ↓VEGF, ↓NFκβ | HCT116, HCT8, SW480 and SW260 cells | 5–20 µM | Pancreatic and renal cell carcinoma | [266] |
| Withania somnifera | Withaferin A | C28H38O6 | 470.6 | ↑ROS, ↓ Bcl-2/Bax, ↑caspase3 and 9, ↓ΔΨm | RKO and HCT116 cells | 0.1–10 µM | Lung, breast, and pancreatic cancer | [267] |
| Piper nigrum | Piperine | C17H19NO3 | 285.34 | ↓wnt/β-catenin pathway | SW480 and SW480-pBAR/Renilla, HCT116, DLD1, RKO | 30–100 µM | Lung, liver, breast, and brain cancer | [268] |
| Crocus sativus | Crocetin | C20H24O4 | 328.4 | ↑p53, ↑PIDD, ↑Bax, ↑FAS, ↑caspase-3, -8 and -9 | HCT116 and HT29 cell lines | 100 µM | Prostate, breast, pancreatic, and gastric cancer | [269] |
| Cynanchum paniculatum | Antofine | C23H25NO3 | 363.4 | ↓proliferation, ↑cytotoxicity, G2/M cycle arrest | Col2 and A549 cells | IC50: <9 ng/mL | Breast, lung, and renal carcinoma | [270] |
| Zingiber zerumbet | Zerumbone | C15H22O | 218.33 | ↑ROS, ↓ Bcl-2/Bax, ↑caspase-3/-8/-9, ↓ΔΨm, G2/M cycle arrest | SW480 cell line | IC50: 102 µM | Oral, breast, lung, and prostate cancer | [271] |
| Hymenocallis littoralis | Pancratistatin | C14H15NO8 | 325.27 | ↑LC3II, ↑beclin-1, ↑Bax, ↓cyclin B1, ↓cdc25c, G2/M cycle arrest | SW948, DLD1, HTC15, and HT29 cells | IC50: 15–25 µM | Lymphoma, breast, liver, skin, and teratocarcinoma | [272] |
| Saussurea lappa | Costunolide | C15H20O2 | 232.32 | ↓Survivin, ↓β-catenin, ↓galectin-3, ↓cyclin D1, G2/M cycle arrest | SW480, L-Wnt3a cells | 0.5–5 µM | Breast, prostate, and ovarian cancer | [273] |
| Rehmannia glutinosa | Catalpol | C15H22O10 | 362.33 | ↓VEGF, ↓EGFR2, ↓HIF-1α, ↓IL-1β, ↓IL-6 and 8, ↓iNOS | CT26 cells and xenograft in mice | <40 µM | Gastric, lung, and liver cancer | [274] |
| Laminaria japonica | LJGP | n.d. | n.d. | ↓CDK2, ↓PCNA, ↓E2F-1, ↓cyclin E, ↓cyclin D1, ↓PARP, ↑p27, ↑caspase 9, ↓Bcl-2, G1 phase arrest | HT-29, HepG2 and AGS cells | IC50: 100 µg/mL | Lung, liver, and cervical carcinoma | [275] |
| Rosmarinus officinalis | Rosmarinic acid | C18H16O8 | 360.3 | ↑E-cadherin, ↓N-cadherin, ↓twist, ↓vimentin, ↓MMP 2 and 9, ↓ICAM-1, ↓ITGβ1 | CT26 and HCT116 cells | 50–200 µM | Breast, gastric, leukemia, and cervical cancer | [276] |
| Coptis chinensis | Berberine | C20H18NO4+ | 336.4 | ↑p21, ↓PARP, ↑caspase 8, ↓VEGF, ↓COX2, ↓Bcl-2, G2/M phase arrest | SW480 cells | 0.5–50 µM | Prostate, cervical, esophageal, thyroid, and gastric cancer | [277] |
| Sanguinaria canadensis | Sanguinarine | C20H14NO4+ | 332.3 | ↑ROS, ↓MMP, ↑caspase-3, 8 and 9, ↓Bcl-2, ↓XIAP, ↑Egr-1 | HCT-116 cell line | 0.3–1.2 µM | Breast, prostate, cervical, and pancreatic cancer | [278] |
| Nauclea orientalis | Naucleaoral A and B | C20H20N2O3 | 336.38 | ↑cytotoxicity | Hela and KB cells | IC50: 4.0 and 7.8 µg/mL | Cervical, bladder, and pancreatic cancer | [279] |
| Glycyrrhiza uralensis | Licoricidin | C26H32O5 | 424.5 | ↑caspase-3, 8 and 9, ↓CDK1, ↑AMPK, ↓Akt/mTOR, G1/S phase arrest | SW480 cells and xenograft BALB/c mice | IC50: 7.2 µM | Gastric, lung, prostate, and osteosarcoma | [280] |
| Carpobrotus edulis | Rutin | C27H30O16 | 610.5 | ↑caspase 3, G0/G1 phase arrest | HCT116 and HaCaT cells | 1000 µM IC50: 679 µM |
Breast, lung, and cervical cancer | [281] |
| Scutellaria baicalensis | Baicalin and Baicalein | C21H18O11, C15H10O5 | 446.4, 270.24 | ↓hTERT, ↓MAPK, ↓ERK, ↑p38 | HT-29, SW480 cells and HCT-116 xenograft NSG mice | 10–150 µM | Breast, prostate, and pancreatic cancer | [282] |
| Nicotiana glauca | Scopoletin | C10H8O4 | 192.17 | ↓ERK1, ↓VEGF-A, ↓FGF-2 | HUVEC, CCD18-Co and HCT116 Xenograft mice | IC50: 0.06 µM | Breast, lung, and skin cancer | [283] |
| Morus australis | Morusin | C25H24O6 | 420.5 | ↓ PI3K/Akt, ↓PDK1, ↓XIAP, ↓cMyc, ↓NFκβ, ↑caspase-3, 8 and 9, G1 phase arrest | HT-29 cells | IC50: 6.1–12.7 µM | Breast, ovarian, and prostate cancer | [284] |
| Allium sativum | Diallyl disulphide | C6H10S2 | 146.3 | ↓GSK3β, ↓NFκβ | SW480 cells and AOM/DSS mouse model | 2.5–40 µM | Lung, breast, and gastric cancer | [285] |
| Nandina domestica | Protopine | C20H19NO5 | 353.4 | ↑p53, ↑p21, ↑Bax, ↑caspase-3 and 7, ↑LC3-II | HCT116 cells | 10–40 µM | Prostate, breast, ovarian, and head and neck cancer | [286] |
| Brain cancer | ||||||||
| Nigella sativa | Thymoquinone | C10H12O2 | 164.2 | ↑LC3-II, ↑p62, ↓MMP 2 and 9, ↓FAC, ↓Nf-kβ, ↓ERK, ↓Akt, ↓mTOR | T98MG and U87MG cells | 10–40 µM IC50: 10.3 µM and 8.3 µM |
Breast, liver, colon, and lung cancer | [287,288] |
| Radix Angelica sinensis | Z-ligustilide | C12H14O2 | 190.24 | ↓RhoA, ↓Cdc42, ↓Rac1 | T98MG cells | 2.5–25 µM | Breast, prostate, and colon cancer | [289] |
| Panax ginseng C. A. Mey | Ginsenoside Rh2 | C36H62O8 | 622.9 | ↑miR128, ↑E2F3a, ↑caspase-3 | T98MG, A172, and U251 cells | 12 µg/mL | Breast, ovarian, colon, and prostate cancer | [290] |
| Bolbostemma paniculatum | Tubeimoside-1 | C65H102O29 | 1347.5 | ↓Bcl-2, ↑ Bax, ↑caspase-3, ↑Cyto- C, ↑ROS | U251 and U87 cells | 10–50 µg/mL | Gastric, liver, ovarian, and lung cancer | [291] |
| Thuja occidentalis | α-/β-Thujone | C10H16O | 152.23 | ↓VEGF, ↓Ang-4, ↓CD31 | U87-MG and C6 cells xenograft in mice | LD50: 400 and 300 µg/mL | Melanoma, breast, lung, and colon cancer | [292] |
| Rubia cordifolia L. | Mollugin | C17H16O4 | 284.31 | ↓ Akt, ↓ P70S6K, ↓ mTOR, ↓ ERK ½, ↑JNK, ↑p38 | U87MG, U251 and MKN45 cells | 10–40 µM | Breast, colon, ovarian, and lung cancer | [293] |
| Garcinia brasiliensis | 7-epiclusianone | C33H42O4 | 502.7 | ↓cyclin A, ↑caspase-3, S and G2/M phase arrest | U251MG and U138MG cell lines | IC50: 23 and 18.52 µM | Colon, melanoma, breast, lung, and ovarian cancer | [294] |
| Escherichia coli | Selenocysteine | C3H6NO2Se | 167.06 | ↑ROS, ↑p21waf1/cip1, ↑p53, ↓Akt, ↑p38MAPK, ↑JNK, ↑ERK, S phase cycle arrest | U251 and U87 cell lines | 5–20 µM | Breast, lung, and prostate cancer | [295] |
| Anula helenium | Alantolactone | C15H20O2 | 232.32 | ↑ROS, ↓GSH, ↓Bcl-2, ↑ Bax, ↑p53, ↑Cyto- C, ↑caspase-3/-9, ↓ΔΨm, ↓Nf-kβ | U87, U373 and LN229 cells | IC50: 33–36 µM | Lung, breast, liver, and pancreatic cancer | [296] |
| Carpesium nepalense | Nepalolide A | C20H28O6 | 364.4 | ↓IkB-α, ↓IkB-β, ↓iNOS, ↓NF-kβ | C6 cell line | 2–10 µM | Brain cancer | [297] |
| Buxus microphylla | Cyclovirobuxine D | C26H46N2O | 402.7 | ↑ Bax, ↓Bcl-2, ↑caspase-3, S and G0/G1 phase arrest | T98G and Hs683 cell lines | 15–240 µmol/L | Gastric, colon, breast, and prostate cancer | [298] |
| Euscaphis japonica | Pomolic acid | C30H48O4 | 472.7 | ↑caspase-3 and 9, ↑ROS, ↓MRP1 | A172, GBM-1 and U87 cells | IC50: 8.82, 9.72 and 11.09 µg/mL | Leukemia, melanoma, gastric and uterine cancer | [299] |
| Danshen | Salvianolic Acid B | C36H30O16 | 718.6 | ↑ROS, ↑p53, ↑p38 MAPK | U87 cell line | 1–100 µM | HNSCC, breast, and colon cancer | [300] |
| Glycine max | Soyasapogenol B | C30H50O3 | 458.7 | ↓STAT3, ↓M2 polarization, ↑M1, ↑IL-12 | U373-MG, SaOS2, and LM8 cells xenograft in mice | 1–100 µM | Gastric, breast, colon, and renal cancer | [301] |
| Rosmarinus officinalis | Carnosol | C20H26O4 | 330.4 | ↑p53, ↓twist, ↓Zeb1, ↓slug, ↑miR-200c | U87MG, T98G, U373 MG | IC50: <40 µM | Lymphoma, lung osteosarcoma, and gastric cancer | [302,303] |
| Tillandsia recurvata | HLBT-100 | C19H20O8 | 376.4 | ↑caspase-3, and 7, G1 cell cycle arrest, ↓angiogenesis | NCI60 cell lines (U87-cells) | GI50 values: <0.100 µM IC50 for U87: 0.054 µM |
Breast, prostate, leukemia, and melanoma | [304] |
| Ligusticum chuanxiong hort | Tetramethylpyrazine | C8H12N2 | 136.19 | ↓CXCR4 | C6 cell line | 100 µM | Breast, liver, colon, and lung cancer | [305] |
| Garcinia hanburyi Hook. f | Gambogenic acid | C38H46O8 | 630.8 | ↓cyclin E, ↓cyclin D1, ↓EGFR, ↓Akt, ↓GSK3β, Go/G1 phase arrest | U251 cell line | 0.75–6 µM | Gastric, breast, and lung cancer | [306] |
| Vitis vinifera | Resveratrol | C14H12O3 | 228.24 | ↓Akt, ↑p53 | U87 and patient derived (22,33 and 44 GSC) cells | 5–100 µM | Colon, gastric, and breast cancer | [307] |
| Curcuma aromatica Salisb. | Germacrone | C15H22O | 218.33 | ↑p53, ↑ Bax, ↓Bcl-2, ↑p21, ↓CD1, ↓CDK2, G1 phase arrest | U87 and U251 cells | 50–250 µmol/L | Breast, prostate, and liver cancer | [308] |
| Acori Graminei Rhizoma | Volatile Oil (VOA) | n.d. | n.d. | ↑caspase-3, 8 and 9, ↑ Bax/Bcl-2, ↑LC3-II/I, ↑atg5, ↑beclin1, ↓p62 | U87, U251, 3T3 and A172 cells | 25–250 µg/mL | Pancreatic and breast cancer | [309] |
| Trichosanthes kirilowii Maxim. | Trichosanthin | 247 Amino acids | 27 kDa | ↓LGR5, ↓β-catenin, ↓pGSK-3βSer9, ↓cMyc, ↓CD1 | U87 and U251 cells | IC50: 40 and 51.6 µM | Leukemia and cervical cancer | [310] |
| Streptomyces staurosporeus | Staurosporine | C28H26N4O3 | 466.5 | ↑caspase-3, ↓TDP-43 | U87 cell line | IC50: 5µM | Lung, breast, prostate, and colon cancer | [311] |
| Dysosma versipellis | Deoxypodophyllotoxin | C22H22O7 | 398.4 | ↓Cdc2, ↓CB1, ↓Cdc25C, ↑caspase-8 and 9, ↓Bcl-2, ↓Bcl-xL, G2/M phase arrest | U-87 MG and SF126 cells | IC50: 15.06 and 13.95 nM | Lung, breast, prostate, and colon cancer | [312] |
| Anemone taipaiensis | Saponin B | C48H78O17 | 927.1 | ↑Fas-l, ↑caspase-3, ↓Bcl-2, G1/S phase arrest | U87MG cells | IC50: 6.7 µmol/L | Leukemia and breast cancer | [313] |
| Liver cancer | ||||||||
| Strychnos nux-vomica Linn | Brucine | C23H26N2O4 | 394.5 | ↓HIF-1, ↓MMP-2, ↓FN, ↓LOX, ↓CD | SMMC-7721, HepG2, and HCC in Male Kunming mice | 20–150 µM | Colon and breast cancer | [314] |
| Azadirachta indica | Nimbolide | C27H30O7 | 466.5 | ↑caspase-3,7 and 9, ↑ Bax, ↓Bcl-2, ↓Mcl-1, ↓XIAP, ↓ c-IAP1, ↓c-IAP2, G2/M phase arrest | PLC/PRF/5 and Huh-7 cells xenograft Balb/c mice | 1–5 µM | Pancreatic, breast, lung, and colon cancer | [315] |
| Gardenia jasminoides ellis | Geniposide | C17H24O10 | 388.4 | ↓miR-224, ↓wnt/βcatenin, ↓Akt | HepG2 and Huh7 cells | 100–500 µM | Brain, oral, skin, and colon cancer | [316] |
| Solanum nigrum L. | Solamargine | C45H73NO15 | 868.1 | ↓pcna, ↓Ki67↓Bcl-2, ↑ Bax, ↑caspase-3 and 9, G2/M phase arrest | SMMC-7721 and HepG2 cells | IC50: 12.17 and 20 µM | Colon, lung, prostate, and breast cancer | [317] |
| Matricaria recutita | α- bisabolol | C15H26O | 222.37 | ↑caspase-3,-8 and -9, ↑Fas, ↓Bcl-2, ↑p53, ↑Nf-kβ, | HepG2, ECa109, PC-3 and Hela cells | 1–20 µM | Colon, brain, endometrial, and prostate cancer | [318] |
| Ganoderma lucidum | Ganoderic acid A | C30H44O7 | 516.7 | ↓cyclin D1, ↑p21, ↑cleaved caspase 3, G0/G1 phase arrest | HepG2 and SMMC-7721 cells | IC50: 187.6 and 158.9 µmol/L | Prostate, breast, lung, and meningioma | [319] |
| Thalictrum glandulosissimum | Hernandezine | C39H44N2O7 | 652.8 | ↑AMPK, ↑Atg-7 | HepG2 and Hep3B cells | IC50: 7.42 and 6.71 µM | Lung, prostate, breast, and cervical cancer | [320] |
| Angelica gigas Nakai | Decursin | C19H20O5 | 328.4 | ↑LATS1, ↑βTRCP, ↑p-YAP, ↑cleaved caspase 3, ↑cleaved PARP, G1 phase cell cycle arrest | HepG2, Huh-7 cells and tumor xenograft in mice | 5–80 µM | Gastric, lung, prostate, and lymphoma | [321] |
| Lavandula officinalis | Linalool | C10H18O | 154.25 | ↓cyclin A, ↓CDK4, ↑p21, ↑p27, ↑ROS, ↑caspase-3, ↓Ras, ↓Akt, ↓mTOR, G0/G1 phase arrest | HepG2 cell line | 0–2.5 mM | Leukemia, breast, prostate, and ovarian cancer | [322] |
| Tylophora indica | Tylophorine | C24H27NO4 | 393.5 | ↓cyclin A2, G1 phase cell cycle arrest | HepG2, HONE-1, and NUGC-3 cells | 2 µM | Breast, stomach, nasopharyngeal, and colon cancer | [323] |
| Patrinia scabra Bunge | Lariciresinol | C20H24O6 | 360.4 | ↓ΔΨm, ↑Cyto- C, ↑caspase-3 and 9, ↑PARP, ↓Bcl-2/Bax | HepG2 cells | IC50: 208 µg/mL | Leukemia, breast, and prostate cancer | [324] |
| Oroxylum indicum | Oroxin B | C27H30O15 | 594.5 | ↑PTEN, ↓COX-2, ↓VEGF, ↓p-Akt, ↓PI3K | SMMC-7721 | 0.34–1.68 µM | Breast, lung, and lymphoma | [325] |
| Stephania tetrandra | Tetrandrine | C38H42N2O6 | 622.7 | ↓wnt/β-catenin, ↓MTA1, ↑E-cadherin, ↑occludin, ↓Vimentin | Huh7, Hep3B and HCCLM9 xenograft Balb/c mice | 0.5–4 µM | Colon, esophageal, and pancreatic cancer | [326] |
| Scutellaria lateriflora | Scutellarein | C15H10O6 | 286.24 | ↓HIF-1α, ↓Flt-1, ↓VEGFA, ↓MMP 2 and 9, ↑caspase-3 | HepG2, MCF-7, EAC, A549 and liver carcinoma and ascites lymphoma model in mice | IC50: <13.8 µM | Gastric, colon, lung, and fibrosarcoma | [327] |
| Toddalia asiatica Lam | Chelerythrine | C21H18NO4+ | 348.4 | ↓MMP 2 and 9, ↓p-FAK ↓PI3K, ↓Akt, ↓mTOR, ↓c-JNK, ↓ERK | Hep3B Cell line | 0.625–5 µM | Breast, prostate, renal, and lung cancer | [328] |
| Astragalus complanatus R.Br. | (FAC) flavonoids | n.d. | n.d. | ↑caspase-3 and 8, ↑ Bax, ↑p21, ↑p27, ↓CDK1, CDK4, ↓cyclin B1, ↓cyclin D1, G0/G1 and S phase arrest | SMMC-7721 and HepG2 cells | IC50: 48 and 53 µg/mL | Breast and nasopharyngeal | [329] |
| Quercus Iberica | Quercetin | C15H10O7 | 302.23 | ↑E-cadherin, ↓MMP9, ↑LC3, ↓Vimentin, ↓Jak2, ↓STAT3, | LM3 cells and xenograft mice model | 20–200 µM | Breast, colon, ovarian, and pancreatic cancer | [330] |
| Trichosanthis Radix | Cucurbitacin B | C32H46O8 | 558.7 | ↓cdc2, ↓cyclin D1, ↓c-Raf, S phase arrest | BEL-7402 cells and xenograft mice | IC50: 0.32 µM | Brest, pancreatic, and laryngeal cancer | [331] |
| Colchium speciosum | Colchicine | C22H25NO6 | 399.4 | ↑AKAP12, ↑TGFB2, ↑MX1, ↓APOH, ↑GDF15, ↑IL32 | HCC24 and HCC38/KMUH, F28 and F59/KMUH cell lines and Balb/c-nu xenograft | 2 and 6 ng/mL | Thyroid, oropharyngeal, and breast cancer | [332] |
| Head and Neck cancer | ||||||||
| Wilkstroemia elliptica Merr. | Umbelliferone | C9H6O3 | 162.14 | ↑ROS, ↓MMP, G0/G1 cycle arrest | HOC KB cells | IC50: 200 µM | Renal prostate, lung, and breast cancer | [333] |
| Dioscorea nipponica | Dioscin | C45H72O16 | 869 | ↑p53, ↓Cyclin A, ↓CDK2, ↓p-ERK, ↓Bcl-2, ↑p-JNK, ↑p-p38, ↑Bax, ↑cleaved caspase-3/-9 | NP69, Hep-2 and TU-212 cells | IC50: <5 µg/mL | Gastric, breast, lung, ovarian, and colorectal cancer | [334] |
| Maclura pomifera | Osajin | C25H24O5 | 404.5 | ↑Bax, ↓Bcl-2, ↑Fasl, ↑cym-c, ↓GRP78, ↑caspase-3,4,8 and -9 | TW076, TW04 and CG-1 cells | IC50: 5 µM | Kidney, prostate, breast, and colon cancer | [335] |
| Cichorium intybus | Esculetin | C9H6O4 | 178.14 | ↓pJAK1/2, ↑ROS, ↓STAT3, G1/S cycle arrest | TU-212, M4e, Hep-2 xenograft in mice | IC50: 2.969, 12.88 and 1.958 µM | Pancreatic, prostate, colon, and lung cancer | [336] |
| Serratia marcescens | Prodigiosin | C20H25N3O | 323.4 | ↓Cyclin D1, ↑beclin-1, ↓mTOR, ↓PI3K/Akt, G0/G1 phase arrest | OECM1 and SAS cell lines | IC50: 1.59 and 3.25 µM | Breast, gastric, colon, and hematopoietic cancer | [337] |
| Albatrellus confluens | Neoalbaconol | C22H34O3 | 346.5 | ↓PDK1, ↓PI3K/AKT HK-2, ↑RIP1, ↑RIP3 | NP69, k562,MCF-7 and A549 and C666-1 xenograft in mice | IC50: ≤18 µM | Lung, breast, colon, and gastric cancer | [338] |
| Haematococcus pluvilis | Astaxanthin | C40H52O4 | 596.8 | ↓PI3k/Akt, ↓STAT3, ↓Nf-kβ, ↓miR-21, ↓HOTAIR | SCC131 and SCC4 cells | IC50: 720 and 700 µM | Pancreatic, breast, colon, and melanoma | [339] |
| Geranium thunbergii | Geraniin | C41H28O27 | 952.6 | ↓MMP2, ↓Fak, ↓Src, ↓ERK ½ | SCC-9 and SCC-14 cells | 20–80 µM | Brain, breast, colon, ovarian, bladder, and osteosarcoma | [340] |
| Forsythia suspensa | Phillygenin | C21H24O6 | 372.4 | ↑Caspase-3/-9, ↑Bax, ↑ROS, ↓Bcl-2, G2/M phase arrest, ↓Nf-kβ | SH-1-V1 cell line and xenograft in mice | IC50: 6 µM | Lung, liver, and pancreatic cancer | [341] |
| Enicosanthellum phalcrum | Liriodenine | C17H9NO3 | 275.26 | ↑Bax, ↑Caspase-3, ↓Bcl-2, G2/M phase arrest | ECA-109 cell line | 0.1–20 µM | Breast, prostate, and gastric cancer | [342] |
| Halichondria sp. | Ilimaquinone | C22H30O4 | 358.5 | ↑ROS, ↑LC3B-II, ↑Atg5, ↓p-pAkt, ↓p-p38, ↓HIF-1α, ↓Mcl-1, ↓Bcl-2 | SCC4 and SCC2095 cells | IC50: <9 µM | Prostate, lung, and colon cancer | [343] |
| Tabernaemontana catharinensis | Coronaridine | C21H26N2O2 | 338.4 | ↑apoptosis, ↑cytotoxicity | Hep-2 cell line | IC50: 54.47 µg/mL | Leukemia, breast, colon, and gastric cancer | [344] |
| Chelidonium majus | Ukrain | C66H75N6O18PS | 1303.4 | ↓EGFR, ↓AKT2, ↓STAT3, JAK1, ↓β-catenin, ↑CYP1A1, ↑CYP1B1 | FaDu, HlaC78 cells | EC50: <11 µg/mL | Lung and prostate cancer | [345] |
| Prostate Cancer | ||||||||
| Cruciferae | Indole-3-carbinol | C9H9NO | 147.17 | ↓CDK6, ↑Bax, ↓Bcl-2, ↑p21, ↑p27, G1 phase arrest | PC-3 cells | 30–100 µM | Melanoma, colon, breast, and endometrial | [346] |
| Genista tinctoria | Genistein | C15H10O5 | 270.24 | ↓HOTAIR, miR-34a↓NFκβ, ↓Akt | PC3 and Du145 cells | 25 µM | Lung and breast cancer | [347,348] |
| Punica granatum | Ellagic acid | C14H6O8 | 302.19 | ↑IL-6, ↓STAT3, ↓Akt, ↓ERK | LNCaP and PC-3 cells | 20–100 µM | Breast and ovarian cancer | [349,350] |
| Stephania tetrandra | Fangchinoline | C37H40N2O6 | 608.7 | ↓NR4A1, ↓survivin, ↑ROS, ↑caspase 3 and 8 | MiaPaCa-2 and Panc-1 cells | IC50: 11.1 and 17 µM | Gastric, bladder, breast, and colon cancer | [351] |
| Melodinus khasianus | Khasuanine A | C21H24N2O3 | 352.43 | ↑Caspase-3, ↑p53, ↓Bcl-2 | PC3 cell line | IC50: 0.45 µM | Breast, lung, and colon cancer | [352] |
| Camptotheca acuminate | Camptothecin | C20H16N2O4 | 348.4 | ↑ROS, ↑c-Myc, ↑sp1, ↑PI3k/Akt, ↑hTERT, G2/M cycle arrest | LNCaP cells | 1–5 µM | Leukemia, breast, colon, and lung cancer | [353] |
| Andrographis paniculata | Andrographolide | C20H30O5 | 350.4 | ↓MMP11, ↑γH2AX, ↑ Caspase-3, and 7, G2/M phase arrest | PC3, LNCaP, and 22RV1 SCID orthotopic model | GI50: 26.2, 28.1 and 24.2 µM | Glioblastoma, renal, colon, and ovarian cancer | [354] |
| Dioscorea nipponica | Diosgenin | C27H42O3 | 414.6 | ↓Bcl-2, ↓beclin-1, ↑caspase-9, ↓ PI3K, ↓Akt, ↓mTOR, | DU145 cell line | IC50: 6.757 µg/mL | Breast, liver, and gastric cancer | [355] |
| Murraya koenigii | Mahanine | C23H25NO2 | 347.4 | ↓DNMT1, ↓DNMT3B, ↓PDK1, ↓Akt, ↑RASSF1A | LNCaP and PC-3 cells | 10–20 µM | Colon, brain, and lung cancer | [356] |
| Quercus petraea | Procyanidin | C30H26O13 | 594.5 | ↓ΔΨm, ↑ apoptosis, and necrosis | PC-3 cell line | 100–300 µg/mL | Breast, lung, stomach, and colon cancer | [357] |
| Santalum album | α-santalol | C15H24O | 220.35 | ↓Survivin, ↓p-Akt, ↑ Caspase-3, ↑cleaved PARP | LNCaP and PC-3 cells | 20 and 40 µM | Breast, colon, and skin cancer | [358] |
| Goniothalamus spp. | Altholactone | C13H12O4 | 232.23 | ↓p65, ↓NF-kβ, ↓STAT3, ↓survivin, ↓Bcl-2, ↑Bax, S phase arrest | DU145, PC3, and LNCap cells | 20 and 40 µM IC50: 38.5 µM |
Colon, leukemia, and bladder cancer | [359] |
| Nauclea subdita | Subditine | C20H15N3O2 | 330.1237 | ↑ROS, ↓MMP, ↑cym-c, ↑caspase-3,7 and 9, ↓Bcl-2 | LNCaP and PC-3 cells | IC50: <14 µM | Breast, lung, and colon cancer | [360] |
| Isodon eriocalyx | Eriocalyxin B | C20H24O5 | 344.4 | ↑cleaved caspase 3 and 8, ↑cleaved PARP, ↑LC3B-II, ↓Akt/mTOR | PC3 and 22RV1 cells | IC50: <4 µM | Breast, colon, lung, and bladder cancer | [361] |
| Garcinia indica | Garcinol | C38H50O6 | 602.8 | ↑ Bax/Bcl2, ↑caspase-3 and 9, ↓PARP, ↑PI3K, ↑Akt, ↑mTOR | PC-3 cells and xenograft mice | 30 µM | Breast, lung, leukemia, and bladder cancer | [362] |
| Allium atroviolaceum | Tricin | C17H14O7 | 330.29 | ↓MiR-21 | PC-3 cell line | IC50: 117.5 µM | Breast, colon, and liver cancer | [363] |
| Sinomenium acutum | Sinomenine | C19H23NO4 | 329.4 | ↓miR-23a, ↓CD1, ↓CDk4, ↓Bcl-2, ↓MMP-2 and 9, ↓PI3K/Akt, ↓JAK/STAT | PC-3 cells | 0.25–1 mM | Gastric, ovarian, breast, and lung cancer | [364] |
| Hematological Cancer | ||||||||
| Artemisia annua | Dihydroartemisinin | C15H24O5 | 284.35 | ↓VEGF, ↓ERK1/2 | RPMI18226 MM cells | IC50: 30.24 µmol/L | Brain, melanoma, and ovarian | [365] |
| Artemisia absinthum | Cardamonin | C16H14O4 | 270.28 | ↑ROS, ↑Ca2+, ↑ Caspase-3, -8 and-9, ↓Bcl-2, ↑Bax, ↑ cyto-c, ↑AIF, ↑GRP78, ↑FasL, ↑DAP, | WEHI-3 cell line | 2–10 µM | Gastric, nasopharyngeal, prostate, and colon cancer | [366] |
| Myristica fragrans | Myristicin | C11H12O3 | 192.21 | ↑Caspase-3, ↑Cyto- C, ↓PARP, ↓ERCC1, ↓RAD50, 51, ↓ATM | K562 cells | IC50: 368 µM | Stomach, lung, and ovarian cancer | [367] |
| Ganggui luhui wan | Meisoindigo | C17H12N2O2 | 276.29 | ↑Caspase-3,8 and 9, ↑Bax, ↑Cyto- C, ↑PARP, ↓Bcl-2, | HL60 cell line | 20 µmol/L | Colon, breast, and lung cancer | [368] |
| Cananga odorata | Sampangine | C15H8N2O | 232.24 | ↑ROS, ↓ΔΨm, ↑Caspase-3, G1 phase arrest | HL60 cell line | 1–20 µM | Lung, head, and neck cancer | [369] |
| Ambrosia maritoma | Damsin | C15H20O3 | 248.32 | ↓c-Src, ↓AKT, STAT5, ↓NFkβ | CEM/ADR5000, CCRF CEM cells | IC50: 4.8 µM | Colon, breast, and lung cancer | [370] |
| Forsythia suspensa | Pinoresinol | C20H22O6 | 358.4 | ↑p21 WAF1/Cip1, G0/G1 phase arrest | HL60, HL60R, and K562 cells | IC50: 8 and 32 µM | Breast, prostate, and colon cancer | [371] |
| Danggui longhui wan | Indirubins | C16H10N2O2 | 262.26 | ↓c-Src, ↓Abl kinase, ↓Gsk3β | KCL-22 and T315I mutant KCL-22 cells | IC50: 0.3–0.9 µM | Colon, breast, renal, and pancreatic cancer | [372] |
| Arnica chamissonis | Helenalin | C15H18O4 | 262.3 | ↓NFkβ, ↑ROS | Jurkat T (J16) and Neo jurkat or Bcl-2 jurkat cells | 2–20 µM | Breast, prostate, and colon cancer | [373] |
| Cinnamosma fragrans | Capsicodendrin | C34H48O10 | 616.7 | DNA damage and proapoptotic | K562 and HL-60 cells | IC50: <0.5 µM | Breast, lung, and colon cancer | [374] |
| Cephalotaxus fortunei | Homoharringtonine | C29H39NO9 | 545.6 | ↓SP1/TET1/5hmC/FLT3/MYC | MA9.3ITD, MA9.3RAS, MONOMAC 6, Kasumi-1 and xenograft in mice | IC50: 9.2–36.7 nM | Colon, breast, and gastric cancer | [375] |
| Papaver somniferum | Noscapine | C22H23NO7 | 413.4 | ↓Bcl-2, ↓XIAP, ↓Mcl-1, ↓Bcl-xl, ↑Bax, ↓TRAF1, ↓IKK, ↓NFkβ, | KBM-5 and U266 cells | IC50: 84.4 and 155 µmol/L | Colon, breast, lung, and ovarian cancer | [376] |
| Streptomyces cinnamonensis | Monensin | C36H62O11 | 670.9 | ↓MyB, ↓MyB-NFIB | HEK-Luc, HEK-MyB-Luc, and MyB-NFIB ACC cells | 0.3–3 µM | Prostate, ovarian, colon, and pancreatic cancer | [377] |
| Brucea antidysenteriea | Bruceantin | C28H36O11 | 548.6 | ↑NOTCH1, ↑HES1, G1 phase arrest | MM-CSCs cells | IC50: 77 nM | Breast, colon, osteosarcoma, and lung cancer | [378] |
| Aglaia foveolata | Silvestrol | C34H38O13 | 654.7 | ↓FLT3, ↓miR155, ↓ p65 | Mv4-11, THP-1, AML blasts from patients and Mv4-11 xenograft murine model | IC50: 2.7, 3.8 and 12 nM | Liver, colon, and melanoma | [379] |
| Miscellaneous Cancer | ||||||||
| Cynanchum otophyllum | Paeoniflorin | C23H28O11 | 480.5 | ↑HTRA3, ↑Bax | Capan-1 and MIAPaCa-2 cells | IC50: 862.7, and 489.5 µM | Pancreatic, glioma, lung, and colon cancer | [380] |
| Periploca sepium bunge | Baohuoside-I | C27H30O10 | 514.5 | ↓β-catenin, ↓survivin, ↓cyclin D1 | Eca109 cells and Eca109-Luc xenograft in mice | 12.5–50 µg/mL IC50:24.8 µg/mL |
Esophageal, breast, and leukemia | [381] |
| Ochrosia elliptica | Ellipticine | C17H14N2 | 246.31 | ↑AIF, ↑Cyto- C, ↑ROS, ↑caspase-3, 7 and 9, ↑ERK, ↑JNK, G2/M cycle arrest | RL95-2 cells | 1–10 µM | Endometrial, ovarian, thyroid, and breast cancer | [382] |
| Ilex hainanensis | Ilexgenin A | C30H46O6 | 502.7 | ↓IL-6, ↑TNF-αG0/G1 cycle arrest | RAW 264.7 and B16-F10 xenograft in mice | IC50: 66.57 and 27.34 µM | Melanoma, colon, breast, and cervical cancer | [383] |
| Dysoxylum binectariferum | Flavopiridol | C21H20ClNO5 | 401.8 | ↑caspase-3, 8 and 9, ↓Mcl-1 ↑cyclin-B1, G2/M cycle arrest | KKU-055, -100, -214 cells and KKU-213 xenograft in Balb/c mice | IC50: 40–213 nM | Osteosarcoma, leukemia, lung, and breast cancer | [384] |
| Crinum asiaticum | Crinamine | C17H19NO4 | 301.34 | ↓Snail1, ↓vimentin, ↓VEGF-A, ↓CDK4, ↓RHOA, ↓PLK1, ↓BCL2L1, ↓Akt1 | SiHa and C33a cell lines | IC50: 23.52 and 60.89 µM | Cervical, prostate, gastric, and colon cancer | [385] |
| Alpinia katsumadai | Alpinetin | C16H14O4 | 270.28 | ↓Bcl-2, ↓Bcl-xL, ↑ Bax, ↑Cyto- C, ↑XIAP, ↑caspase-3, 8 and 9 | BxPC-3, PANC1, and AsPC-1 cells | 20–80 µg/mL | Hepatoma, leukemia, colon, lung, and breast cancer | [386] |
| Combretum caffrum | Combretastatin A4 | C18H20O5 | 316.3 | ↓N-cadherin, ↓Vimentin, ↓slug, ↓snail1, ↓zeb1, ↓p-PI3K, ↓p-Akt | TPC1 cell line | 5 and 10 µM | Thyroid, breast, colon, and lung cancer | [387] |
| Heliotropium Indicum Linn | Indicine N-oxide | C15H25NO6 | 315.36 | ↑p53, cleave PARP, cleave DNA | Hela, MCF-7, PC3, and SiHa cell lines | IC50: 46–91 µM | Colon, breast, prostate, and head and oral cancer | [388] |
| Arnebia euchroma | Alkannin | C16H16O5 | 288.29 | ↑ROS, ↓ΔΨm, ↑p38 MAPK, ↑JNK | MDA-MB-231, HCT116, A549, Huh7, HepG2, MCF cells | IC50: 5–12 µM | Breast, colon, lung, liver, and glioma | [389] |
| Artemisia princeps | Jaceosidin | C17H14O7 | 330.29 | ↑pCdc25C, ↑p21, ↑ROS, ↓ERk ½, ↑ATM-Chk1/2, G2/M phase arrest | HES, HESC, Hec1, and KLE cell line | IC50: 52.68–147.14 µM | Endometrial, ovarian, glioblastoma, and oral cancer | [390] |
| Coccinia grandis | Kaempferol | C15H10O6 | 286.24 | ↑LC3-II, ↓p62, ↓G9a, ↑IRE1-JNK-CHOP | AGS, NCI-N87, MKN-74, SNU-216, and SNU-638 | IC50: 50 µM | Gastric, colon, breast, prostate, and pancreatic cancer | [391] |
| Daphne odora | Daphnetin | C9H6O4 | 178.14 | ↓RhoA, ↓Cdc42 | LM8 OS cells | 1–30 µM | Osteosarcoma, breast, lung, and colon cancer | [392] |
| Cerbera odollam | Cerberin | C32H48O9 | 576.7 | ↓cMyc, ↑Caspase 3 and 7, ↑ROS ↓Bcl-2, ↓Mcl-1, ↓STAT-3, ↓PLK-1, G2/M phase arrest | PANC-1, MIA Paca2, A549, HepG2, HT29 cells and mice model | GI50: <90 nM | Pancreatic, liver, colon, and breast cancer | [393] |
| Asiatic toad | Cinobufagin | C26H34O6 | 442.5 | ↓Bcl-2, ↓CB1, ↑p21, ↓CDC2, ↑puma, ↑caspase 3, G2/M phase arrest | EC-109, Kyse-150, and Kyse-520 cells | IC50: 0.91, 0.66 and 0.62 µM | Gastric, liver, colon, and melanoma | [394] |
| Astragalus membranaceus | Swainsonine | C8H15NO3 | 173.21 | ↑E-cadherin,↓N-cadherin, ↓Vimentin, ↓slug, ↓snail1, ↓zeb1, ↓p-PI3K, ↓p-Akt, ↓Twist1 | EC9706 and 293T cells | 10–100 µg/mL | Esophageal, colon, liver, and glioma | [395] |
| Magnolia officinalis | Honokiol | C18H18O2 | 266.3 | ↓cyclin D1, ↓CDK2 and 4, ↓Akt/mTOR, ↓p38, ↓ERK, G0/G1 phase arrest | WRO, SW579 cells, and ARO cells xenograft in Balb/c mice | IC50: 37.7, 19.9, and 36.3 µM | Thyroid, leukemia, prostate, and colon cancer | [396] |
| Polymethoxyflavone | Tangeritin | C20H20O7 | 372.4 | ↓ MMP, ↑ Caspase-3, -8 and -9, ↑ Bax, ↑ Bid, ↑ tBid, ↑ p53, ↑ p21/cip1, ↑ Fas and ↑ FasL | AGS cell line | 5–240 µM | Gastric, colon, and lung cancer | [397] |
| Amoora rohituka | Aphanin | C35H54O4 | 538.80 | ↓K-Ras, ↓p-AKT, ↓cMyc, ↓cyclin D1, ↓STAT3, G0/G1 cycle arrest | HPAF-II, BxPC3, and HPAC cells | IC50: 12.80, 15.68 and 17.26 µM | Pancreatic, lung, colon, liver, and skin cancer | [398] |
| Eucalyptus globulus | Caffeic acid | C9H8O4 | 180.16 | ↑ Caspase-1, 3, and 8, G0/G1 cycle arrest | SK-Mel-28 cells | 25–200 µM | Melanoma, breast, colon, gastric, and ovarian cancer | [399] |
| Zingiber officinale | 6-gingerol | C17H26O4 | 294.4 | ↑ROS, ↑Bax/Bcl-2, ↑Caspase-3, 9, ↑cym-c, G2/M phase arrest | AGS cell line | IC50: 250 µM | Liver, lung, breast, and retinoblastoma | [400] |
| Humulus lupulus | Xanthohumol | C21H22O5 | 354.4 | ↑caspase-3, 8 and 9, ↑PARP, ↑p53, ↑AIF, ↓Bcl2, ↓XIAP, S phase cycle arrest | Ca Ski cell line | IC50: 20 µM | Cervical, breast, and mammary adenocarcinoma | [401] |
| Trollius chinensis | Orientin and Vitexin | C21H20O11, C21H20O10 | 448.4, 432.4 |
↑p53, and ↓Bcl2 | EC-109 cells | 5–80 µM | Esophageal, breast, colon, and lung cancer | [402] |
| Rhodiola rosea | Salidroside | C14H20O7 | 300.3 | ↓p-MAPK, ↓p-ERK, ↓p-PI3K, ↓p-AKT, ↓miR99a, ↑p21, ↓Bcl-2 | GES-1, NU-216 and MGC803 cells | 0.8–8000 µM | Gastric and bladder cancer | [403] |
| Syzgium aromaticum | Eugenol | C10H12O2 | 164.2 | ↑ROS, ↑Bax, ↓PCNA, ↓ΔΨm, G2/M cycle arrest | SIHA, SK-MEL-28, A2058, MCF-7, MDA-MB-231 | IC50: <23 µM | Cervical, breast, colon, and lung cancer | [404] |
| Uapaca togoensis | Arborinine | C16H15NO4 | 285.29 | ↓MMP, ↑ROS, G0/G1 and S phase arrest | CCRF-SEM, MDA-MB-231, HCT119, U87 MG, HepG2 cells | IC50: <10 µM | Leukemia, brain, breast, and pancreatic cancer | [405] |
| Peganum harmala | Harmine | C13H12N2O | 212.25 | ↑beclin-1, ↑LC3-II, ↓Bcl-2, ↑Bax, ↓Akt/mTOR, ↓p70S6k | MGC-803 and SGC-7901 cells | IC50: <59 µM | Gastric, breast, ovarian, and pancreatic cancer | [406] |
| Artemisia vestita | Hispidulin | C16H12O6 | 300.26 | ↓VEGFR2, ↓Akt, ↓mTOR, ↓S6 kinase, ↓PI3K, ↓Bcl-2 | PANC-28, BxPC-3, HUVEC, PANC-1 xenograft BALB/c mice | IC50: 20–200 µmol/L | Pancreatic, glioblastoma, and ovarian cancer | [407] |
| Didemnum cuculiferum | Vitilevuamide | C77H114N14O21S | 1603.9 | ↓tubulin polymerization, G2/M phase arrest | A498, HCT116, A5249, and SK-MEL-5 cells and p388 xenograft in mice | LC50: 6–311 nM | Kidney, colon, prostate, ovarian, and pancreatic cancer | [408] |
| Piper longum | Piperlongumine | C17H19NO5 | 317.34 | ↑p-elF2α, ↓ΔΨm, ↑ATF4, ↑CHOP, ↓TrxR1 | SGC-7901, BGC-823, KATO III cell lines | 0.625–20 µM | Gastric, breast, lung, and colon cancer | [409] |
| Chrysanthemum parthenium | Parthenolide | C15H20O3 | 248.32 | ↓NFκβ, ↓VEGF, ↓c-jun, ↓c-Fos | KYSE510, Het-1A, and Eca109 xenograft BALB/c mice | IC50: 13.3, 21.54, and 10.3 µM | Esophageal carcinoma, colon, and prostate cancer | [410] |
| Solanum nigrum L. | Degalactotigonin | C50H82O22 | 1035.2 | ↓Hedgehog Gli1, ↓GSK3β | U2OS, U2OS/MTX, ZOS-M, and ZOS xenograft in mice | IC50: 12.91 –31.46 µmol/L | Osteosarcoma, colon, and pancreatic cancer | [411] |
Note: Bold font chemical compounds represent the compounds with good anticancer potential against the selected cancer model (lower IC50 values).
4. Novel Formulations of Natural Products for Chemotherapy
The major concern of traditional therapies is their poor accessibility to the tumor site; therefore, higher doses are required to achieve a desired pharmacological response, which causes adverse drug reactions. The advancement of innovative nanotechnologies in medicines can significantly improve the treatment at clinical settings by overcoming the existing limitations associated with the diagnosis and treatment of various fatal diseases. Despite a wide range of antitumor effects exhibited by natural compounds in vitro preclinical studies, various hurdles still exist in translating these promising results into in vivo or clinical trials, resulting in failure and expectations. Despite remarkable health benefits, natural products’ full clinical potential has not yet been unlocked due to low aqueous solubility, poor absorption, and lower bioavailability, and shorter retention time in the biological environment. After administration, natural agents need to interact with various physico-chemical barriers that can alter their structure and affect their antitumoral activity. Therefore, novel formulation strategies are adopted to prevent the degradation of the natural compound and their parent structure, which helps retain their chemopreventive and chemotherapeutic activities [412].
Nanobased formulations in the size range of 30–100 nm have a higher surface area and can easily pass through the membranes and be absorbed. These unique characteristics of nanoparticles make them significantly attractive because it can easily overcome the inherent poor solubility and absorption problem of many natural compounds and other chemical entities. Various nano-based formulations have been employed for drug delivery in order to achieve targeted delivery, improve aqueous solubility and bioavailability, and also enhance the retention time, thereby minimizing the adverse effects and toxicities and also protecting the drug molecules from the detrimental effects of the bio environment such as enzymatic attack, pH fluctuation, and biochemical degradation [413]. Nanoencapsulation can protect and deliver the natural agents in their natural structural form and target it to the body’s specific tissues. Various nanostructures are designed and developed for drug delivery applications such as polymeric nanoparticles, liposomes, micelles, nanogels, solid lipid nanoparticles, dendrimers, nanocapsules, nanoemulsion, metallic, and ceramic nanoparticles.
Recently, Muosa et al. prepared ellagic acid (EA) or diindolylmethane (DIM) loaded PEG-PLGA nanoparticles, tested them against pancreatic cancer SUIT2-luciferase cells, and observed that the nanoformulation of the bioactive compounds produced more significant effects than the compounds alone by inhibiting the viability, angiogenesis, and growth of the pancreatic tumor cells [414]. Similarly, β-sterol loaded PLGA nanoparticles showed better antitumoral activity against breast cancer MDA-MB-231 and MCF-7 cell lines by inhibiting the proliferation and growth of the cells [415]. Recently, Feng et al. demonstrated that sequential delivery of α-mangostin and triptolide loaded polymeric micelles can improve the permeation and therapeutic efficacy in pancreatic cancer by inactivating cancer-associated fibroblast triggered by TGF-β and, as a result, can increase the perfusion at the tumor site, induce apoptosis, and inhibit proliferation in the orthotopic model of pancreatic ductal adenocarcinoma [416]. In a recent study, PEGylated betulinic acid loaded liposomes were developed and were shown to have better tumor inhibitory effects in in vitro (HepG2 and Hela cells) and in vivo (U14 cells xenograft) cancer models [417]. Recently, Liu et al. prepared a coordination nanoassembly of luteolin and ferric ions, which increased the solubility, stability, absorption, and efficacy, and acted as a chemotherapeutic agent as well as photothermal agent, which shows that it can overcome the problem of stability and increase its therapeutic efficacy [418].
A potential clinically effective therapeutic approach is the combinatorial delivery of bioactive molecules, producing synergistic or additive effects at lower doses, and minimizing the associated toxicities. Ahmadi et al. co-encapsulated hydroxystyrol and doxorubicin in PLGA-co-Acrylic acid nanoparticles and delivered them to HT-29 colon cancer cells, which resulted in higher apoptosis and cell cycle arrest and regulated the genes expression better than the single-loaded formulations and free drugs [419]. Similarly, the liquid crystalline nanoparticle of resveratrol and pemetrexed was developed for the management of lung cancer and tested against A549 lung cancer cells and urethane induced lung cancer model in mice, and the results indicated that the cytotoxicity, cellular uptake, and antitumoral activity was significantly improved compared to the drugs alone [420]. Furthermore, paclitaxel loaded artesunate-phospholipid liposomes have been tested against MCF-7, HepG2, and A549 cell lines, and it showed better cellular internalization and synergistically enhanced the in vitro antitumoral efficacy against all the tested cell lines [421]. Moreover, Wang et al. developed a hyaluronic acid-coated PEI-PLGA nanosystem for co-delivery of gambogic acid and pTRAIL for triple-negative breast cancer (TNBC) therapy. They observed that combinatorial nanosystem significantly inhibited tumor growth and proliferation by inducing apoptosis in vitro and in vivo tumor xenograft model of TNBC [422].
Similarly, Bian et al. investigated the combinatorial anticancer effects of curcumin and sorafenib-loaded lactosylated nanoparticles against the hepatocellular carcinoma HepG2 cell line HCC tumor xenograft model. They found that the combinatorial nanosystem even reduced the smallest tumor volume and exhibited the strongest inhibitory potential with lower systemic toxicity [423]. In addition, Wang et al. developed a novel PB@MIL-100 (Fe) dual metal-organic framework loaded with artemisinin for dual photothermal/chemotherapeutic purpose and evaluated in vitro and in vivo studies and found that the d-MOFs system produced synergistic effects with lower toxicity and can be a good option to be utilized as a photothermal-chemotherapeutic nanomedicine for the cancer therapy [424].
Nanocrystals are an important and emerging carrier system for delivering therapeutics. Recently, Wang et al. synthesized hyaluronic acid-coated camptothecin-loaded nanocrystals, evaluated them against various cancer cell lines such as MDA-MB-231, MCF-7, HepG2, and IMR-90 cells, and demonstrated that HA decorated CP nanocrystals improved the cytotoxicity, specificity, anti-migration activity, and antiproliferative activity [425]. Gupta et al. conjugated/encapsulated berberine in PAMAM dendrimers and evaluated them against MCF-7 and MDA-MB468 cell lines and in vivo pharmacokinetic studies in albino rats. They found that the conjugated berberine produced more significant and improved anticancer effects than the berberine encapsulated dendrimers [426]. Ding et al. reported a novel nanogel carrier system for co-delivery of EGCG and siRNA for drug-resistant breast cancer therapy and demonstrated that the multicomponent nanogel carrier has more pronounced cytotoxic effects and showed improved anticancer activity in in vitro MDA-MB231 cell line and in vivo tumor xenograft model [427]. Nanoparticles can specifically target cancer cells and enhance the specificity and efficacy of therapeutic modalities, resulting in improved patient compliance, response, and survival. However, the nanomaterial used for drug delivery applications must be biocompatible and biodegradable so that the unloaded material can be degraded into non-toxic metabolites and removed from the body via circulation. Various nanomaterials for cancer treatment are at the preliminary stages of research, and more significant in vivo data with excellent clinical efficacy are required in order to bring it to clinical settings for commercial use.
Moreover, various new natural compounds of different structures, isolated from various plants, have been deemed as models, leads, or heads of series, and their structural alteration has resulted in compounds with pharmacological action and remarkable therapeutic potential outcomes (Figure 4). This research area, which is persistently growing and is of tremendous current interest, investigates new natural products originating from various sources. The significance of natural compounds in anticancer medication exploration could go past molecular diversity and novelty in structures (Table 2). The discovery of new natural compound structures with critical biological significance and new mechanisms of activity could likewise pioneer innovative research.
Figure 4.
Approved marketed anticancer drugs from 1950 to 2014 which are directly obtained or derived from natural products or imitate them (adopted from [428], licensed under creative common attribution license).
Table 2.
Natural products undergoing clinical trials for the prevention/treatment of various cancers.
| Cancer | Bioactives | Class/Family | Combination With Other Drugs |
Clinical trial Status | Results | Purpose | References |
|---|---|---|---|---|---|---|---|
| Lung | Curcumin | Phenol | Gefitinib/Erlotinib | Unknown | Not reported | Safety and Tolerability | NCT02321293 * |
| - | - | Lovaza | Ongoing | - | Prevention | NCT03598309 * | |
| Epigallocatechin | Catechin | mLDG | - | - | Treatment | NCT02577393 * | |
| Sulforaphane | Organosulfur | - | - | Prevention | NCT03232138 * | ||
| Gossypol | Phenolic aldehyde | DTX, CIS | Unknown | Not reported | Treatment | NCT01977209* | |
| Breast | ATRA | Retinoid | Anastrozole | Ongoing | - | - | NCT04113863 * |
| Curcumin | Phenol | - | - | - | NCT03980509 * | ||
| - | - | Paclitaxel | Completed | Not posted | - | NCT03072992 * | |
| Genistein | Isoflavone | Gemcitabine | - | Efficacious | Treatment/Prevention | NCT00244933 * | |
| 7-hydroxystaurosporine | Staurosporine derivative | - | Not posted | Treatment | NCT00001444 * | ||
| Emodin | Resin | Unknown | Not posted | Observational | NCT01287468 * | ||
| Cognutrin | Fatty acid + Phenols |
Completed | Efficacious | Treatment | NCT01823991 * | ||
| Cervical/Ovarian | Curcumin | Phenol | Cyclophosphamide, Lansoprazole, Aspirin, Vitamin D, Pembrolizumab | Ongoing | - | - | NCT03192059 * |
| OPT-821 | Saponin | Antigen-KLH conjugate Vaccine | Completed | Efficacious | Treatment | NCT00857545 * | |
| Colon | Curcumin | Phenol | 5-FU | Ongoing | - | - | NCT02724202 * |
| - | - | Irinotecan | Completed | Not posted | Toxicity and Pharmacokinetics | NCT01859858 * | |
| - | - | Avastin/FOLFIRI | Completed | Not reported | Treatment | NCT02439385 * | |
| - | - | FOLFOX | Completed | - | - | NCT01490996 * | |
| - | - | Celecoxib | Unknown | - | - | NCT00295035 * | |
| Andrographolide | Diterenoid | Capecitabine | Terminated | Low accrual rate | Treatment | NCT01993472 * | |
| Berberine | Benzylisoquinoline alkaloid | Ongoing | Not posted | Prevention | NCT03281096 * | ||
| Aquamin | Multi-mineral complex | Calcium carbonate | Active | - | - | NCT02647671 * | |
| Cyanidin-3-glucoside | Anthocyanin | Curcumin | Unknown | - | Prevention | NCT01948661 * | |
| Ellagic Acid | Phenol | Completed | - | Treatment | NCT01916239 * | ||
| Silymarin | Flavonolignan | Completed | Unknown | - | NCT03130634 * | ||
| Brain | Chlorogenic acid | Phenol | - | Not posted | Treatment | NCT02728349 * | |
| Perillyl alcohol | Terpenes | Ongoing | - | Treatment | NCT02704858 * | ||
| Head and Neck | Combretastatin A4 Phosphate | Phenol derivative | Completed | - | Treatment | NCT00060242 * | |
| β-Carotene | Terpenoid | α-Tocopherol | - | - | Prevention | NCT00169845 * | |
| Capsaicin | Capsaicinoid | Radiation therapy | - | - | Supportive care | NCT00003610 * | |
| Liver | Silybin | Flavonoid | - | - | Treatment | NCT01129570 * | |
| Prostate | Curcumin | Phenol | - | - | Supportive | NCT01917890 * | |
| - | - | Taxotere | Terminated | No Significant outcome | - | NCT02095717 * | |
| Lycopene | Carotenoid | Vit D3 and E, Selenium, green tea extract | Completed | Not posted | Treatment | NCT00844792 * | |
| Polyphenon E | polyphenol | EGCG | - | Efficacious | Treatment/prevention | NCT00676780 * | |
| Cholecalciferol | Vitamin D | - | Effective | - | NCT00524680 * | ||
| Hematological | Bioperine | Alkaloid | Curcumin | - | - | - | NCT00113841 * |
| Plitidepsin | Depsipeptide | Bortezomib and dexamethasone | - | Not posted | - | NCT02100657 * | |
| Bryostatin 1 | polyketide | - | - | - | NCT00003171 * | ||
| Homoharringtonine | Alkaloid | - | - | - | NCT00006364 * | ||
| Skin cancer | Ingenol Mebutate | Diterpene ester | - | - | - | NCT01325688 * | |
| Advanced solid tumors | Elisidepsin | Depsipeptide | Erlotinib | - | Not posted | - | NCT00884845 * |
| Discodermolide | Polyketide | - | unknown | - | [429] | ||
| Tongue | Luteolin | Flavonoid | Unknown | Not posted | Treatment | NCT03288298 * | |
| GI Tumors | Resveratrol | Phenol | Completed | Not posted | Treatment | NCT01476592 * | |
| Pancreatic | Etoposide | Podophyllotoxin derivative | Gemcitabine | - | - | - | NCT00202800 * |
* represent the clinical trial number obtained from https://clinicaltrials.gov (accessed on 1 June 2022).
The increasing trend in natural products research shows that natural products have sustained their credibility as a viable and fertile area for drug discovery and advancement. Otherwise, there would have been a substantial therapeutic deficit in many most important clinical areas, such as cancer, cardiovascular disease, and neurodegenerative diseases. The most encouraging part is the continuous introduction of new natural product chemotypes with intriguing structures and biological activities, which are highly diverse and respond to much wider screening opportunities provided by a plethora of newly discovered targets. Natural products can provide a basic structural unit, and many lead molecules can be built upon by providing some useful input from the synthetic chemical libraries and utilizing modern computational and high throughput allied technologies.
5. Conclusions and Future Perspectives
In summary, the development of cancer is a complex process that is influenced by many factors. The present review highlights the importance of natural molecules in cancer management, since natural products provide inexhaustible sources of compounds with unique structures and new mechanisms of action. Moreover, natural compounds, either used alone or in combination, can be beneficial in the treatment and prevention of cancer. However, further studies are needed to describe the effect of natural compounds on cancer progression. In addition, it is evident that there is still much to be explored based on the natural diversity of the world, and because of technological advancements there are several new prototypes for pharmacologically active compounds appearing in screening programs, which will accelerate the exploration of natural compounds for different diseases such as cancer.
The growing incidence of cancer and limitations of conventional therapy such as higher costs, toxicities due to non-specific targeting, increased chemoresistance, and metastasis has led to tumor recurrence and posed a serious challenge to design and develop an alternative biocompatible, eco-friendly, and cost-effective strategy to combat cancer. Primary prevention is a better way to control cancer, benefiting both present and future generations, and estimates suggest that 30 to 50% of incident cancers can be prevented by translating prior knowledge of causes into effective interventions. Under this scenario, natural products are expected to revolutionize cancer treatment in the next decade due to their higher efficacy, biodegradability, and biocompatibility. Though the clinical applications of natural compounds are limited due to their poor aqueous solubility, rapid catabolism, targeting specificity, poor intestinal absorption, and reduced bioavailability, although nanotechnology-based formulations are used to overcome these limitations. Moreover, there is a lack of insight into the molecular interactions of natural compounds with various signaling molecules at the preclinical stage; for this purpose, in silico approaches such as molecular docking are needed to understand the interactions of natural products in different signaling pathways and identify their associated carcinogenesis biomarkers which can be further confirmed by many in vitro and in vivo models. Additionally, methodological flaws in various clinical studies such as smaller sample size, lack of placebo or control groups, and shorter trial duration have been observed. Thus, for many natural products, it is important to perform large-scale and well-controlled clinical trials to confirm and validate their anticancer activities, adverse effects, and safeties prior to their usage as anticancer drugs/adjuvants. Furthermore, extensive standardization based on modern approaches by evaluating their efficacy, composition, dosage-regimen, safety, quality, bioavailability, manufacturing practices, and regulatory and approval procedures need to be performed on the promising natural products to meet the quality and criteria of the international standard. Interestingly, pharmaceutical industries have vast experience and knowledge about drug development, and, therefore, combining the advantages of modern and traditional medicines can increase the development and availability of natural compounds for patient use.
A novel integrated approach for drug discovery, taking advantage of the ethnopharmacological knowledge validated and confirmed through the interdisciplinary efforts involving natural products chemistry, pharmacology, medicinal chemistry, biochemistry, cellular and molecular biology, is required to harvest the true potential of natural products. The higher biofunctionality and biodiversity of natural products make them attractive and unique from a drug discovery perspective, which needs to be further explored using modern screening techniques, which can provide various new prototypes that are pharmacologically active, thus increasing the speed of exploitation of natural compounds. Moreover, advancements in analytical techniques and computational chemistry coupled with artificial intelligence can help in the facilitation and identification of new natural active molecules for pharmacological evaluation. Further research on natural products and their limitations may lead to the discovery of new potent anticancer therapeutics with enhanced efficacy, safety, and quality for chemoprevention and chemotherapy.
In summary, this review is intended to increase scientists’ and researchers’ awareness of natural products’ diverse benefits, which can be utilized to develop safer and newer cancer treatments, as well as provide a solid foundation for future studies on natural compounds in cancer therapy. In the future, further studies are needed to investigate combinations of natural products comprising more than two natural products or combinations of existing chemotherapeutic drugs with less frequently utilized natural products. Moreover, many of these products were tested on limited cancer types, so their spectrum of activity should be expanded. Bioavailability limits the effectiveness of naturally occurring compounds. Thus, researchers must focus not only on the efficacy of the compound, which is of high interest, but also on drug delivery systems able to overcome pharmacokinetic issues, in addition to studying derivatives with a high degree of biological efficacy and availability.
Acknowledgments
We are thankful to Jiangxi University of Chinese Medicine, Nanchang, China for providing digital resources.
Author Contributions
Conceptualization, A.N.; visualization, P.H.; methodology, A.N.: software, Y.L.; investigation, A.N.; formal analysis, J.Z.; data curation, A.N.; writing—original draft preparation, A.N.; validation, W.Z.; resources, Q.Z.; funding acquisition, Q.Z.; supervision, Q.Z. and M.Y.; project administration, Q.Z. and M.Y.; and writing—review and editing, A.N., Q.Z., and M.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Funding Statement
This research was funded by National Natural Science Foundation of China, grant number 82060719, and Jiangxi University of Chinese Medicine (Traditional Chinese Medicine Preparation Technology and Equipment Innovation Team, funding number CXTD22006).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cadoná F.C., Dantas R.F., de Mello G.H., Silva F.P., Jr. Natural products targeting into cancer hallmarks: An update on caffeine, theobromine, and (+)-catechin. Crit. Rev. Food Sci. Nutr. 2021;62:7222–7241. doi: 10.1080/10408398.2021.1913091. [DOI] [PubMed] [Google Scholar]
- 2.Brennan P., Davey-Smith G. Identifying Novel Causes of Cancers to Enhance Cancer Prevention: New Strategies Are Needed. JNCI J. Natl. Cancer Inst. 2022;114:353–360. doi: 10.1093/jnci/djab204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Hassanpour S.H., Dehghani M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017;4:127–129. doi: 10.1016/j.jcrpr.2017.07.001. [DOI] [Google Scholar]
- 5.Rahman M.M., Sarker M.T., Tumpa M.A.A., Yamin M., Islam T., Park M.N., Islam M.R., Rauf A., Sharma R., Cavalu S. Exploring the recent trends in perturbing the cellular signaling pathways in cancer by natural products. Front. Pharmacol. 2022;13:950109. doi: 10.3389/fphar.2022.950109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang B., Zhang Y. Teaching an old dog new tricks: Drug discovery by repositioning natural products and their derivatives. Drug Discov. Today. 2022;27:1936–1944. doi: 10.1016/j.drudis.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhammad N., Usmani D., Tarique M., Naz H., Ashraf M., Raliya R., Tabrez S., Zughaibi T.A., Alsaieedi A., Hakeem I.J., et al. The role of natural products and their multitargeted approach to treat solid cancer. Cells. 2022;11:2209. doi: 10.3390/cells11142209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickens E., Ahmed S. Principles of cancer treatment by chemotherapy. Surgery. 2018;36:134–138. [Google Scholar]
- 9.Liu S., Khan A.R., Yang X., Dong B., Ji J., Zhai G. The reversal of chemotherapy-induced multidrug resistance by nanomedicine for cancer therapy. J. Control. Release. 2021;335:1–20. doi: 10.1016/j.jconrel.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Lee S., Rauch J., Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020;21:1102. doi: 10.3390/ijms21031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bashraheel S.S., Domling A., Goda S.K. Update on targeted cancer therapies, single or in combination, and their fine tuning for precision medicine. Biomed. Pharmacother. 2020;125:110009. doi: 10.1016/j.biopha.2020.110009. [DOI] [PubMed] [Google Scholar]
- 12.Sharifi-Rad J., Quispe C., Patra J.K., Singh Y.D., Panda M.K., Das G., Adetunji C.O., Michael O.S., Sytar O., Polito L., et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxidative Med. Cell. Longev. 2021;2021:3687700. doi: 10.1155/2021/3687700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nonnekens J., Hoeijmakers J.H. After surviving cancer, what about late life effects of the cure? EMBO Mol. Med. 2017;9:4–6. doi: 10.15252/emmm.201607062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wigmore P.M., Mustafa S., El-Beltagy M., Lyons L., Umka J., Bennett G. Chemo Fog. Springer; New York, NY, USA: 2010. Effects of 5-FU; pp. 157–164. [DOI] [PubMed] [Google Scholar]
- 15.Cardona-Mendoza A., Olivares-Niño G., Díaz-Báez D., Lafaurie G.I., Perdomo S.J. Chemopreventive and Anti-tumor Potential of Natural Products in Oral Cancer. Nutr. Cancer. 2022;74:779–795. doi: 10.1080/01635581.2021.1931698. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q., Feng J., Liu W., Wen C., Wu Y., Liao Q., Zou L., Sui X., Xie T., Zhang J., et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv. Drug Deliv. Rev. 2022;188:114445. doi: 10.1016/j.addr.2022.114445. [DOI] [PubMed] [Google Scholar]
- 17.Pepper J.W., Findlay C.S., Kassen R., Spencer S.L., Maley C.C. Synthesis: Cancer research meets evolutionary biology. Evol. Appl. 2009;2:62–70. doi: 10.1111/j.1752-4571.2008.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yagüe E., Arance A., Kubitza L., O’Hare M., Jat P., Ogilvie C.M., Hart I.R., Higgins C.F., Raguz S. Ability to acquire drug resistance arises early during the tumorigenesis process. Cancer Res. 2007;67:1130–1137. doi: 10.1158/0008-5472.CAN-06-2574. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y., Ouyang Z., Du H., Wang M., Wang J., Sun H., Kong L., Xu Q., Ma H., Sun Y. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sin. B. 2022;12:4011–4039. doi: 10.1016/j.apsb.2022.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 21.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from January 1981 to September 2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 22.Patridge E., Gareiss P., Kinch M.S., Hoyer D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Priya S., Satheeshkumar P.K. 5-Natural Products From Plants: Recent Developments in Phytochemicals, Phytopharmaceuticals, and Plant-Based Neutraceuticals as Anticancer Agents. In: Prakash B., editor. Functional and Preservative Properties of Phytochemicals. Academic Press; Cambridge, MA, USA: 2020. pp. 145–163. [Google Scholar]
- 24.Šudomová M., Berchová-Bímová K., Marzocco S., Liskova A., Kubatka P., Hassan S.T. Berberine in human oncogenic herpesvirus infections and their linked cancers. Viruses. 2021;13:1014. doi: 10.3390/v13061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liskova A., Samec M., Koklesova L., Brockmueller A., Zhai K., Abdellatif B., Siddiqui M., Biringer K., Kudela E., Pec M., et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021;12:155–176. doi: 10.1007/s13167-021-00242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atanasov A.G., Zotchev S.B., Dirsch V.M., The International Natural Product Sciences Taskforce. Supuran C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lachance H., Wetzel S., Kumar K., Waldmann H. Charting, navigating, and populating natural product chemical space for drug discovery. J. Med. Chem. 2012;55:5989–6001. doi: 10.1021/jm300288g. [DOI] [PubMed] [Google Scholar]
- 28.Dey P., Kundu A., Kumar A., Gupta M., Lee B.M., Bhakta T., Dash S., Kim H.S. Recent Advances in Natural Products Analysis. Elsevier; Amsterdam, The Netherlands: 2020. Analysis of Alkaloids (Indole Alkaloids, Isoquinoline Alkaloids, Tropane Alkaloids) pp. 505–567. [Google Scholar]
- 29.Ramos A.C., Peláez R., López J.L., Caballero E., Medarde M., San Feliciano A. Heterolignanolides. Furo-and thieno-analogues of podophyllotoxin and thuriferic acid. Tetrahedron. 2001;57:3963–3977. doi: 10.1016/S0040-4020(01)00271-X. [DOI] [Google Scholar]
- 30.Talib W.H., Daoud S., Mahmod A.I., Hamed R.A., Awajan D., Abuarab S.F., Odeh L.H., Khater S., Al Kury L.T. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules. 2022;27:4818. doi: 10.3390/molecules27154818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Găman A.M., Egbuna C., Găman M.-A. Phytochemicals as Lead Compounds for New Drug Discovery. Elsevier; Amsterdam, The Netherlands: 2020. Natural Bioactive Lead Compounds Effective Against Haematological Malignancies; pp. 95–115. [Google Scholar]
- 32.Wang H., Zhang K., Liu J., Yang J., Tian Y., Yang C., Li Y., Shao M., Su W., Song N. Curcumin regulates cancer progression: Focus on ncRNAs and molecular signaling pathways. Front. Oncol. 2021;11:660712. doi: 10.3389/fonc.2021.660712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khatoon E., Banik K., Harsha C., Sailo B.L., Thakur K.K., Khwairakpam A.D., Vikkurthi R., Devi T.B., Gupta S.C., Kunnumakkara A.B. Seminars in Cancer Biology. Academic Press; Cambridge, MA, USA: 2020. Phytochemicals in Cancer Cell Chemosensitization: Current Knowledge and Future Perspectives. [DOI] [PubMed] [Google Scholar]
- 34.Bordoloi D., Roy N.K., Monisha J., Padmavathi G., Kunnumakkara A.B. Multi-targeted agents in cancer cell chemosensitization: What we learnt from curcumin thus far. Recent Pat. Anti-Cancer Drug Discov. 2016;11:67–97. doi: 10.2174/1574892810666151020101706. [DOI] [PubMed] [Google Scholar]
- 35.Monisha J., Jaiswal A., Banik K., Choudhary H., Singh A.K., Bordoloi D., Kunnumakkara A.B. Cancer Cell Chemoresistance and Chemosensitization. World Scientific; Singapore: 2018. Cancer Cell Chemoresistance: A Prime Obstacle in Cancer Therapy; pp. 15–49. [Google Scholar]
- 36.Maurya S.K., Shadab G., Siddique H.R. Chemosensitization of Therapy Resistant Tumors: Targeting Multiple Cell Signaling Pathways by Lupeol, A Pentacyclic Triterpene. Curr. Pharm. Des. 2020;26:455–465. doi: 10.2174/1381612826666200122122804. [DOI] [PubMed] [Google Scholar]
- 37.Yu J., Zhong B., Chen X. Drug Resistance in Colorectal Cancer: Molecular Mechanisms and Therapeutic Strategies. Elsevier; Amsterdam, The Netherlands: 2020. Induction of Programmed Necrosis by Phytochemicals in Colorectal Cancer; pp. 117–133. [Google Scholar]
- 38.Hsu Y.-H., Chen S.-Y., Wang S.-Y., Lin J.-A., Yen G.-C. Pterostilbene Enhances Cytotoxicity and Chemosensitivity in Human Pancreatic Cancer Cells. Biomolecules. 2020;10:709. doi: 10.3390/biom10050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Datta S., Sinha D. EGCG maintained Nrf2-mediated redox homeostasis and minimized etoposide resistance in lung cancer cells. J. Funct. Foods. 2019;62:103553. doi: 10.1016/j.jff.2019.103553. [DOI] [Google Scholar]
- 40.Tripathi S.K., Panda M., Biswal B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019;125:566–582. doi: 10.1016/j.fct.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Lan C.-Y., Chen S.-Y., Kuo C.-W., Lu C.-C., Yen G.-C. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 2019;27:887–896. doi: 10.1016/j.jfda.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muthusamy G., Gunaseelan S., Prasad N.R. Ferulic acid reverses P-glycoprotein-mediated multidrug resistance via inhibition of PI3K/Akt/NF-κB signaling pathway. J. Nutr. Biochem. 2019;63:62–71. doi: 10.1016/j.jnutbio.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 43.Irani S. Emerging insights into the biology of metastasis: A review article. Iran. J. Basic Med. Sci. 2019;22:833–847. doi: 10.22038/ijbms.2019.32786.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapinova A., Kubatka P., Liskova A., Baranenko D., Kruzliak P., Matta M., Büsselberg D., Malicherova B., Zulli A., Kwon T.K., et al. Controlling metastatic cancer: The role of phytochemicals in cell signaling. J. Cancer Res. Clin. Oncol. 2019;145:1087–1109. doi: 10.1007/s00432-019-02892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang P., Jiang Y., Pan Y., Ding X., Rhea P., Ding J., Hawke D.H., Felsher D., Narla G., Lu Z., et al. Mistletoe extract Fraxini inhibits the proliferation of liver cancer by down-regulating c-Myc expression. Sci. Rep. 2019;9:6428. doi: 10.1038/s41598-019-41444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh Y.-C., Ho C.-T., Pan M.-H. Recent advances in cancer chemoprevention with phytochemicals. J. Food Drug Anal. 2020;28:14–37. doi: 10.1016/j.jfda.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Sun L., Chen B., Jiang R., Li J., Wang B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell. Immunol. 2017;311:86–93. doi: 10.1016/j.cellimm.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Loo W.T., Jin L., Chow L.W., Cheung M.N., Wang M. Rhodiola algida improves chemotherapy-induced oral mucositis in breast cancer patients. Expert Opin. Investig. Drugs. 2010;19:S91–S100. doi: 10.1517/13543781003727057. [DOI] [PubMed] [Google Scholar]
- 49.Galluzzi L., Senovilla L., Vacchelli E., Eggermont A., Fridman W.H., Galon J., Sautès-Fridman C., Tartour E., Zitvogel L., Kroemer G. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1:1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang W.T., Lai T.H., Chyan Y.J., Yin S.Y., Chen Y.H., Wei W.C., Yang N.-S. Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PLoS ONE. 2015;10:e0122374. doi: 10.1371/journal.pone.0122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang H.F., Zeng Z., Chen M.Q. Roles of Kupffer cells in liver transplantation. Hepato-Gastroenterology. 2012;59:1251–1257. doi: 10.5754/hge12046. [DOI] [PubMed] [Google Scholar]
- 52.Fang F., Xiao W., Tian Z. Seminars in Immunology. Academic Press; Cambridge, MA, USA: 2017. NK Cell-Based Immunotherapy for Cancer; pp. 37–54. [DOI] [PubMed] [Google Scholar]
- 53.Wu X.-T., Liu J.-Q., Lu X.-T., Chen F.-X., Zhou Z.-H., Wang T., Zhu S.-P., Fei S.-J. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int. Immunopharmacol. 2013;16:332–340. doi: 10.1016/j.intimp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Hou D., Wang D., Ma X., Chen W., Guo S., Guan H. Effects of total flavonoids of sea buckthorn (Hippophae rhamnoides L.) on cytotoxicity of NK92-MI cells. Int. J. Immunopathol. Pharmacol. 2017;30:353–361. doi: 10.1177/0394632017736673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J., Wu J., Chen X., Fortenbery N., Eksioglu E., Kodumudi K.N., Epling-Burnette P.K., Dong J., Djeu J.Y., Wei S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 2011;11:890–898. doi: 10.1016/j.intimp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y.-T., Li J., Qi X., Pei Y.-x., Shi W.-G., Lin H.-S. Effects of Shugan Jianpi Formula (疏肝健脾方) on myeloid-derived suppression cells-mediated depression breast cancer mice. Chin. J. Integr. Med. 2017;23:453–460. doi: 10.1007/s11655-016-2734-4. [DOI] [PubMed] [Google Scholar]
- 57.Murakami H., Ogawara H., Hiroshi H. Th1/Th2 cells in patients with multiple myeloma. Hematology. 2004;9:41–45. doi: 10.1080/10245330310001652437. [DOI] [PubMed] [Google Scholar]
- 58.Wei H., Sun R., Xiao W., Feng J., Zhen C., Xu X., Tian Z. Type two cytokines predominance of human lung cancer and its reverse by traditional Chinese medicine TTMP. Cell. Mol. Immunol. 2004;1:63–70. [PubMed] [Google Scholar]
- 59.Zhang M.-Y., Guo J., Hu X.-M., Zhao S.-Q., Li S.-L., Wang J. An in vivo anti-tumor effect of eckol from marine brown algae by improving the immune response. Food Funct. 2019;10:4361–4371. doi: 10.1039/C9FO00865A. [DOI] [PubMed] [Google Scholar]
- 60.Takei M., Tachikawa E., Hasegawa H., Lee J.-J. Dendritic cells maturation promoted by M1 and M4, end products of steroidal ginseng saponins metabolized in digestive tracts, drive a potent Th1 polarization. Biochem. Pharmacol. 2004;68:441–452. doi: 10.1016/j.bcp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Zhang Q., Chen Y., Liang C.-L., Liu H., Qiu F., Dai Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 2020;121:109570. doi: 10.1016/j.biopha.2019.109570. [DOI] [PubMed] [Google Scholar]
- 62.Li Q., Bao J.-M., Li X.-L., Zhang T., Shen X.-H. Inhibiting effect of Astragalus polysaccharides on the functions of CD4+ CD25highTreg cells in the tumor microenvironment of human hepatocellular carcinoma. Chin. Med. J. 2012;125:786–793. [PubMed] [Google Scholar]
- 63.Du X., Chen X., Zhao B., Lv Y., Zhang H., Liu H., Chen Z., Chen Y., Zeng X. Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factor β and the frequency of regulatory T cells. FEMS Immunol. Med. Microbiol. 2011;63:228–235. doi: 10.1111/j.1574-695X.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 64.He X., Li X., Liu B., Xu L., Zhao H., Lu A. Down-regulation of Treg cells and up-regulation of TH1/TH2 cytokine ratio were induced by polysaccharide from Radix Glycyrrhizae in H22 hepatocarcinoma bearing mice. Molecules. 2011;16:8343–8352. doi: 10.3390/molecules16108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasagi S., Kawano S., Kumagai S. PD-1 and autoimmunity. Crit. Rev. Immunol. 2011;31:265–295. doi: 10.1615/critrevimmunol.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- 66.Chikuma S., Kanamori M., Mise-Omata S., Yoshimura A. Suppressors of cytokine signaling: Potential immune checkpoint molecules for cancer immunotherapy. Cancer Sci. 2017;108:574–580. doi: 10.1111/cas.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X., Tong J., Li Z. Qiyusanlong decoction inhibits the level of PD-1/PD-L1 in mice bearing Lewis lung carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2016;32:770–774. [PubMed] [Google Scholar]
- 68.Lv J., Jia Y., Li J., Kuai W., Li Y., Guo F., Xu X., Zhao Z., Lv J., Li Z. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10:415. doi: 10.1038/s41419-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao G.-J., Lu Z.-Q., Tang L.-M., Wu Z.-S., Wang D.-W., Zheng J.-Y., Qiu Q.-M. Curcumin inhibits suppressive capacity of naturally occurring CD4+ CD25+ regulatory T cells in mice in vitro. Int. Immunopharmacol. 2012;14:99–106. doi: 10.1016/j.intimp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 70.Lin S.R., Chang C.H., Hsu C.F., Tsai M.J., Cheng H., Leong M.K., Sung P.J., Chen J.C., Weng C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020;177:1409–1423. doi: 10.1111/bph.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bishayee A., Sethi G. Seminars in Cancer Biology. Elsevier; Amsterdam, The Netherlands: 2016. Bioactive Natural Products in Cancer Prevention and Therapy: Progress and Promise; pp. 1–3. [DOI] [PubMed] [Google Scholar]
- 72.Wang L., Sun J., Gao P., Su K., Wu H., Li J., Lou W. Wnt1-inducible signaling protein 1 regulates laryngeal squamous cell carcinoma glycolysis and chemoresistance via the YAP1/TEAD1/GLUT1 pathway. J. Cell. Physiol. 2019;234:15941–15950. doi: 10.1002/jcp.28253. [DOI] [PubMed] [Google Scholar]
- 73.Zhang P., Lai Z.-L., Chen H.-F., Zhang M., Wang A., Jia T., Sun W.-Q., Zhu X.-M., Chen X.-F., Zhao Z., et al. Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice. J. Exp. Clin. Cancer Res. 2017;36:190. doi: 10.1186/s13046-017-0661-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Namkaew J., Jaroonwitchawan T., Rujanapun N., Saelee J., Noisa P. Combined effects of curcumin and doxorubicin on cell death and cell migration of SH-SY5Y human neuroblastoma cells. Vitr. Cell. Dev. Biol.-Anim. 2018;54:629–639. doi: 10.1007/s11626-018-0288-9. [DOI] [PubMed] [Google Scholar]
- 75.Öztürk Y., Günaydın C., Yalçın F., Nazıroğlu M., Braidy N. Resveratrol enhances apoptotic and oxidant effects of paclitaxel through TRPM2 channel activation in DBTRG glioblastoma cells. Oxidative Med. Cell. Longev. 2019;2019:4619865. doi: 10.1155/2019/4619865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen W., Liang B., Yin J., Li X., Cheng J. Noscapine Increases the Sensitivity of Drug-Resistant Ovarian Cancer Cell Line SKOV3/DDP to Cisplatin by Regulating Cell Cycle and Activating Apoptotic Pathways. Cell Biochem. Biophys. 2015;72:203–213. doi: 10.1007/s12013-014-0438-y. [DOI] [PubMed] [Google Scholar]
- 77.Sivalingam K.S., Paramasivan P., Weng C.F., Viswanadha V.p. Neferine Potentiates the Antitumor Effect of Cisplatin in Human Lung Adenocarcinoma Cells Via a Mitochondria-Mediated Apoptosis Pathway. J. Cell. Biochem. 2017;118:2865–2876. doi: 10.1002/jcb.25937. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y., Lu H.-L., Liu Y.-D., Yang L.-Y., Jiang Q.-K., Zhu X.-J., Fan H.-N., Qian Y. Cryptotanshinone sensitizes antitumor effect of paclitaxel on tongue squamous cell carcinoma growth by inhibiting the JAK/STAT3 signaling pathway. Biomed. Pharmacother. 2017;95:1388–1396. doi: 10.1016/j.biopha.2017.09.062. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X., Ni Q., Wang Y., Fan H.-w., Li Y. Synergistic anticancer effects of formononetin and temozolomide on glioma C6 cells. Biol. Pharm. Bull. 2018;8:1194–1202. doi: 10.1248/bpb.b18-00002. [DOI] [PubMed] [Google Scholar]
- 80.Tseng H.-S., Wang Y.-F., Tzeng Y.-M., Chen D.-R., Liao Y.-F., Chiu H.-Y., Hsieh W.-T. Aloe-Emodin Enhances Tamoxifen Cytotoxicity by Suppressing Ras/ERK and PI3K/mTOR in Breast Cancer Cells. Am. J. Chin. Med. 2017;45:337–350. doi: 10.1142/S0192415X17500215. [DOI] [PubMed] [Google Scholar]
- 81.Sánchez B.G., Bort A., Mateos-Gómez P.A., Rodríguez-Henche N., Díaz-Laviada I. Combination of the natural product capsaicin and docetaxel synergistically kills human prostate cancer cells through the metabolic regulator AMP-activated kinase. Cancer Cell Int. 2019;19:54. doi: 10.1186/s12935-019-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saikia M., Retnakumari A.P., Anwar S., Anto N.P., Mittal R., Shah S., Pillai K.S., Balachandran V.S., Peter V., Thomas R., et al. Heteronemin, a marine natural product, sensitizes acute myeloid leukemia cells towards cytarabine chemotherapy by regulating farnesylation of Ras. Oncotarget. 2018;9:18115–18127. doi: 10.18632/oncotarget.24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang Q.-W., Cheng K.-J., Mei X.-L., Qiu J.-G., Zhang W.-J., Xue Y.-Q., Qin W.-M., Yang Y., Zheng D.-W., Chen Y., et al. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget. 2015;6:32790–32804. doi: 10.18632/oncotarget.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Y., Wang K., Gu C., Yu G., Zhao D., Mai W., Zhong Y., Liu S., Nie Y., Yang H. Berberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenib. Oncol. Rep. 2018;40:1525–1532. doi: 10.3892/or.2018.6552. [DOI] [PubMed] [Google Scholar]
- 85.Desai V., Jain A., Shaghaghi H., Summer R., Lai J.C.K., Bhushan A. Combination of biochanin A and temozolomide impairs tumor growth by modulating cell metabolism in glioblastoma multiforme. Anticancer Res. 2019;39:57–66. doi: 10.21873/anticanres.13079. [DOI] [PubMed] [Google Scholar]
- 86.Du Plessis-Stoman D., Du Preez J.G.H., van de Venter M. Combination treatment with oxaliplatin and mangiferin causes increased apoptosis and downregulation of NFκB in cancer cell lines. Afr. J. Tradit. Complement. Altern. Med. 2011;8:177–184. doi: 10.4314/ajtcam.v8i2.63206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin M.-T., Lin C.-L., Lin T.-Y., Cheng C.-W., Yang S.-F., Lin C.-L., Wu C.-C., Hsieh Y.-H., Tsai J.-P. Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumor Biol. 2016;37:6987–6996. doi: 10.1007/s13277-015-4526-4. [DOI] [PubMed] [Google Scholar]
- 88.Zhu Z., Du S., Ding F., Guo S., Ying G., Yan Z. Ursolic acid attenuates temozolomide resistance in glioblastoma cells by downregulating O(6)-methylguanine-DNA methyltransferase (MGMT) expression. Am. J. Transl. Res. 2016;8:3299–3308. [PMC free article] [PubMed] [Google Scholar]
- 89.Neitzel C., Seiwert N., Göder A., Diehl E., Weber C., Nagel G., Stroh S., Rasenberger B., Christmann M., Fahrer J. Lipoic Acid Synergizes with Antineoplastic Drugs in Colorectal Cancer by Targeting p53 for Proteasomal Degradation. Cells. 2019;8:794. doi: 10.3390/cells8080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang Z., Zhang Y., Li H., Zhou Y., Zhang Q., Chen R., Jin T., Hu K., Li S., Wang Y., et al. Vitamin D promotes the cisplatin sensitivity of oral squamous cell carcinoma by inhibiting LCN2-modulated NF-κB pathway activation through RPS3. Cell Death Dis. 2019;10:936. doi: 10.1038/s41419-019-2177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pazhang Y., Jaliani H.Z., Imani M., Dariushnejad H. Synergism between NF-kappa B inhibitor, celastrol, and XIAP inhibitor, embelin, in an acute myeloid leukemia cell line, HL-60. J. Cancer Res. Ther. 2016;12:155. doi: 10.4103/0973-1482.150407. [DOI] [PubMed] [Google Scholar]
- 92.Li X., Zhu F., Jiang J., Sun C., Wang X., Shen M., Tian R., Shi C., Xu M., Peng F., et al. Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer Lett. 2015;357:219–230. doi: 10.1016/j.canlet.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 93.Yang Y.-I., Lee K.-T., Park H.-J., Kim T.J., Choi Y.S., Shih I.-M., Choi J.-H. Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer cells through downregulation of the Akt and NFκB pathway. Carcinogenesis. 2012;33:2488–2498. doi: 10.1093/carcin/bgs302. [DOI] [PubMed] [Google Scholar]
- 94.Qian J., Xia M., Liu W., Li L., Yang J., Mei Y., Meng Q., Xie Y. Glabridin resensitizes p-glycoprotein-overexpressing multidrug-resistant cancer cells to conventional chemotherapeutic agents. Eur. J. Pharmacol. 2019;852:231–243. doi: 10.1016/j.ejphar.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Xia G., Wang H., Song Z., Meng Q., Huang X., Huang X. Gambogic acid sensitizes gemcitabine efficacy in pancreatic cancer by reducing the expression of ribonucleotide reductase subunit-M2 (RRM2) J. Exp. Clin. Cancer Res. 2017;36:107. doi: 10.1186/s13046-017-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boueroy P., Hahnvajanawong C., Boonmars T., Saensa-ard S., Wattanawongdon W., Kongsanthia C., Salao K., Wongwajana S., Anantachoke N., Reutrakul V. Synergistic Effect of Forbesione From Garcinia hanburyi in Combination with 5-Fluorouracil on Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2017;18:3343–3351. doi: 10.22034/APJCP.2017.18.12.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su J., Zhang F., Li X., Liu Z. Osthole promotes the suppressive effects of cisplatin on NRF2 expression to prevent drug-resistant cervical cancer progression. Biochem. Biophys. Res. Commun. 2019;514:510–517. doi: 10.1016/j.bbrc.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 98.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 99.Ye W., Sun W., Chen R., Wang Z., Cui X., Zhang H., Qian S., Zheng Q., Zhou Y., Wan J., et al. Pharmacokinetics in rat plasma and tissue distribution in mice of galangin determined by UHPLC–MS/MS. Acta Chromatogr. 2019;31:120–125. doi: 10.1556/1326.2017.00389. [DOI] [Google Scholar]
- 100.Hung J.-Y., Yang C.-J., Tsai Y.-M., Huang H.-W., Huang M.-S. Antiproliferative activity of aucubin is through cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Clin. Exp. Pharmacol. Physiol. 2008;35:995–1001. doi: 10.1111/j.1440-1681.2008.04935.x. [DOI] [PubMed] [Google Scholar]
- 101.Xu W.-T., Li T.-Z., Li S.-M., Wang C., Wang H., Luo Y.-H., Piao X.-J., Wang J.-R., Zhang Y., Zhang T., et al. Cytisine exerts anti-tumour effects on lung cancer cells by modulating reactive oxygen species-mediated signalling pathways. Artif. Cells Nanomed. Biotechnol. 2020;48:84–95. doi: 10.1080/21691401.2019.1699813. [DOI] [PubMed] [Google Scholar]
- 102.Han S., Yang X., Pan Y., Qi Q., Shen J., Fang H., Ji Z. L-securinine inhibits the proliferation of A549 lung cancer cells and promotes DKK1 promoter methylation. Oncol. Lett. 2017;14:4243–4248. doi: 10.3892/ol.2017.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hasan A., Haque E., Hameed R., Maier P.N., Irfan S., Kamil M., Nazir A., Mir S.S. Hsp90 inhibitor gedunin causes apoptosis in A549 lung cancer cells by disrupting Hsp90:Beclin-1:Bcl-2 interaction and downregulating autophagy. Life Sci. 2020;256:118000. doi: 10.1016/j.lfs.2020.118000. [DOI] [PubMed] [Google Scholar]
- 104.Srinual S., Chanvorachote P., Pongrakhananon V. Suppression of cancer stem-like phenotypes in NCI-H460 lung cancer cells by vanillin through an Akt-dependent pathway. Int. J. Oncol. 2017;50:1341–1351. doi: 10.3892/ijo.2017.3879. [DOI] [PubMed] [Google Scholar]
- 105.Hua P., Sun M., Zhang G., Zhang Y., Tian X., Li X., Cui R., Zhang X. Cepharanthine induces apoptosis through reactive oxygen species and mitochondrial dysfunction in human non-small-cell lung cancer cells. Biochem. Biophys. Res. Commun. 2015;460:136–142. doi: 10.1016/j.bbrc.2015.02.131. [DOI] [PubMed] [Google Scholar]
- 106.Hutchinson L. Breast cancer: Challenges, controversies, breakthroughs. Nat. Rev. Clin. Oncol. 2010;7:669–670. doi: 10.1038/nrclinonc.2010.192. [DOI] [PubMed] [Google Scholar]
- 107.Zhou X., Yue G.G.-L., Tsui S.K.-W., Pu J., Fung K.-P., Lau C.B.-S. Elaborating the role of natural products on the regulation of autophagy and their potentials in breast cancer therapy. Curr. Cancer Drug Targets. 2018;18:239–255. doi: 10.2174/1568009617666170330124819. [DOI] [PubMed] [Google Scholar]
- 108.Jin Z.-Q., Hao J., Yang X., He J.-H., Liang J., Yuan J.-W., Mao Y., Liu D., Cao R., Wu X.-Z., et al. Higenamine enhances the antitumor effects of cucurbitacin B in breast cancer by inhibiting the interaction of AKT and CDK2. Oncol. Rep. 2018;40:2127–2136. doi: 10.3892/or.2018.6629. [DOI] [PubMed] [Google Scholar]
- 109.Nigjeh S.E., Yeap S.K., Nordin N., Rahman H., Rosli R. In vivo anti-tumor effects of citral on 4T1 breast cancer cells via induction of apoptosis and downregulation of aldehyde dehydrogenase activity. Molecules. 2019;24:3241. doi: 10.3390/molecules24183241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang L., Wang G., Yang D., Guo X., Xu Y., Feng B., Kang J. Euphol arrests breast cancer cells at the G1 phase through the modulation of cyclin D1, p21 and p27 expression. Mol. Med. Rep. 2013;8:1279–1285. doi: 10.3892/mmr.2013.1650. [DOI] [PubMed] [Google Scholar]
- 111.Reddy D., Ghosh P., Kumavath R. Strophanthidin Attenuates MAPK, PI3K/AKT/mTOR, and Wnt/β-Catenin Signaling Pathways in Human Cancers. Front. Oncol. 2020;9:1469. doi: 10.3389/fonc.2019.01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fatima I., El-Ayachi I., Taotao L., Lillo M.A., Krutilina R., Seagroves T.N., Radaszkiewicz T.W., Hutnan M., Bryja V., Krum S.A., et al. The natural compound Jatrophone interferes with Wnt/β-catenin signaling and inhibits proliferation and EMT in human triple-negative breast cancer. PLoS ONE. 2017;12:e0189864. doi: 10.1371/journal.pone.0189864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deng Y.-T., Huang H.-C., Lin J.-K. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010;49:141–151. doi: 10.1002/mc.20583. [DOI] [PubMed] [Google Scholar]
- 114.Dhandayuthapani S., Perez H.D., Paroulek A., Chinnakkannu P., Kandalam U., Jaffe M., Rathinavelu A. Bromelain-Induced Apoptosis in GI-101A Breast Cancer Cells. J. Med. Food. 2012;15:344–349. doi: 10.1089/jmf.2011.0145. [DOI] [PubMed] [Google Scholar]
- 115.Abu N., Akhtar M.N., Yeap S.K., Lim K.L., Ho W.Y., Abdullah M.P., Ho C.L., Omar A.R., Ismail J., Alitheen N.B. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases in vitro. BMC Complement. Altern. Med. 2016;16:86. doi: 10.1186/s12906-016-1046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stewart B., Wild C.P. World Cancer Report 2014. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 117.Lee C.S., Jang E.-R., Kim Y.J., Myung S.C., Kim W., Lee M.W. Diarylheptanoid hirsutenone enhances apoptotic effect of TRAIL on epithelial ovarian carcinoma cell lines via activation of death receptor and mitochondrial pathway. Investig. New Drugs. 2012;30:548–557. doi: 10.1007/s10637-010-9601-5. [DOI] [PubMed] [Google Scholar]
- 118.Weaver B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell. 2014;25:2677–2681. doi: 10.1091/mbc.e14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H., Jiao Y., Shi C., Song X., Chang Y., Ren Y., Shi X. Berbamine suppresses cell proliferation and promotes apoptosis in ovarian cancer partially via the inhibition of Wnt/β-catenin signaling. Acta Biochim. Biophys. Sin. 2018;50:532–539. doi: 10.1093/abbs/gmy036. [DOI] [PubMed] [Google Scholar]
- 120.Chen T.G., Li L.Y., Wei Y.R., Zhang L.W. Screening, identification and activity evaluation of pancreatic lipase inhibition in Prunella vulgaris. China J. Chin. Mater. Med. 2018;43:4665–4671. doi: 10.19540/j.cnki.cjcmm.2018.0123. [DOI] [PubMed] [Google Scholar]
- 121.Siegel R.L., Miller K.D., Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970–2014. JAMA. 2017;318:572–574. doi: 10.1001/jama.2017.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun J., Ding C., Yang Z., Liu T., Zhang X., Zhao C., Wang J. The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial-mesenchymal transition. J. Transl. Med. 2016;14:42. doi: 10.1186/s12967-016-0786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Imai H., Sawada K., Sato A., Nishi K., Sasaki T., Takahashi T., Ohori H. Complete resection of liver metastases of colorectal cancer after high efficacy bevacizumab, S-1, and CPT-11 combination chemotherapy. Gan Kagaku Ryoho Cancer Chemother. 2015;42:101–104. [PubMed] [Google Scholar]
- 124.Murthy K.C., Jayaprakasha G., Patil B.S. Obacunone and obacunone glucoside inhibit human colon cancer (SW480) cells by the induction of apoptosis. Food Chem. Toxicol. 2011;49:1616–1625. doi: 10.1016/j.fct.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 125.Sithara T., Arun K., Syama H., Reshmitha T., Nisha P. Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of warburg effect. Front. Pharmacol. 2017;8:640. doi: 10.3389/fphar.2017.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ban J.O., Oh J.H., Hwang B.Y., Moon D.C., Jeong H.-S., Lee S., Kim S., Lee H., Kim K.-B., Han S.B., et al. Inflexinol inhibits colon cancer cell growth through inhibition of nuclear factor-κB activity via direct interaction with p50. Mol. Cancer Ther. 2009;8:1613–1624. doi: 10.1158/1535-7163.MCT-08-0694. [DOI] [PubMed] [Google Scholar]
- 127.Ren H., Zhao J., Fan D., Wang Z., Zhao T., Li Y., Zhao Y., Adelson D., Hao H. Alkaloids from nux vomica suppresses colon cancer cell growth through Wnt/β-catenin signaling pathway. Phytother. Res. 2019;33:1570–1578. doi: 10.1002/ptr.6347. [DOI] [PubMed] [Google Scholar]
- 128.Gao X., Wang Y., Zhang J., Lin L., Yao Q., Xiang G. Bergenin suppresses the growth of colorectal cancer cells by inhibiting PI3K/AKT/mTOR signaling pathway. Trop. J. Pharm. Res. 2017;16:2307–2313. doi: 10.4314/tjpr.v16i10.1. [DOI] [Google Scholar]
- 129.Jin Z., Yan W., Jin H., Ge C., Xu Y. Differential effect of psoralidin in enhancing apoptosis of colon cancer cells via nuclear factor-κB and B-cell lymphoma-2/B-cell lymphoma-2-associated X protein signaling pathways. Oncol. Lett. 2016;11:267–272. doi: 10.3892/ol.2015.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gu Y.-Y., Chen M.-H., May B.H., Liao X.-Z., Liu J.-H., Tao L.-T., Man-yuen Sze D., Zhang A.L., Mo S.-L. Matrine induces apoptosis in multiple colorectal cancer cell lines in vitro and inhibits tumour growth with minimum side effects in vivo via Bcl-2 and caspase-3. Phytomedicine. 2018;51:214–225. doi: 10.1016/j.phymed.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Su C.-M., Weng Y.-S., Kuan L.-Y., Chen J.-H., Hsu F.-T. Suppression of PKCδ/NF-κB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo. Int. J. Mol. Sci. 2020;21:3527. doi: 10.3390/ijms21103527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mi C., Ma J., Wang K.S., Zuo H.X., Wang Z., Li M.Y., Piao L.X., Xu G.H., Li X., Quan Z.S., et al. Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J. Ethnopharmacol. 2017;203:27–38. doi: 10.1016/j.jep.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 133.Zhang L., Zheng Y., Deng H., Liang L., Peng J. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int. J. Mol. Med. 2014;33:1613–1620. doi: 10.3892/ijmm.2014.1718. [DOI] [PubMed] [Google Scholar]
- 134.McNeill K.A. Epidemiology of brain tumors. Neurol. Clin. 2016;34:981–998. doi: 10.1016/j.ncl.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 135.Vengoji R., Macha M.A., Batra S.K., Shonka N.A. Natural products: A hope for glioblastoma patients. Oncotarget. 2018;9:22194–22219. doi: 10.18632/oncotarget.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ye Z.-N., Yu M.-Y., Kong L.-M., Wang W.-H., Yang Y.-F., Liu J.-Q., Qiu M.-H., Li Y. Biflavone ginkgetin, a novel Wnt inhibitor, suppresses the growth of medulloblastoma. Nat. Prod. Bioprospect. 2015;5:91–97. doi: 10.1007/s13659-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cao L., Qu D., Wang H., Zhang S., Jia C., Shi Z., Wang Z., Zhang J., Ma J. Toosendanin exerts an anti-cancer effect in glioblastoma by inducing estrogen receptor β- and p53-mediated apoptosis. Int. J. Mol. Sci. 2016;17:1928. doi: 10.3390/ijms17111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Noman L., Oke-Altuntas F., Zellagui A., Sahin Yaglioglu A., Demirtas I., Cardoso S.M., Akkal S., Gherraf N., Rhouati S. A novel benzimidazole and other constituents with antiproliferative and antioxidant properties from Thymelaea microphylla Coss. et Dur. Nat. Prod. Res. 2017;31:2032–2041. doi: 10.1080/14786419.2016.1274888. [DOI] [PubMed] [Google Scholar]
- 139.Schötterl S., Hübner M., Armento A., Veninga V., Wirsik N.M., Bernatz S., Lentzen H., Mittelbronn M., Naumann U. Viscumins functionally modulate cell motility-associated gene expression. Int. J. Oncol. 2017;50:684–696. doi: 10.3892/ijo.2017.3838. [DOI] [PubMed] [Google Scholar]
- 140.Meng X., Li Y., Li S., Gan R.-Y., Li H.-B. Natural Products for Prevention and Treatment of Chemical-Induced Liver Injuries. Compr. Rev. Food Sci. Food Saf. 2018;17:472–495. doi: 10.1111/1541-4337.12335. [DOI] [PubMed] [Google Scholar]
- 141.Kotecha R., Takami A., Espinoza J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget. 2016;7:52517–52529. doi: 10.18632/oncotarget.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou Y., Li Y., Zhou T., Zheng J., Li S., Li H.-B. Dietary natural products for prevention and treatment of liver cancer. Nutrients. 2016;8:156. doi: 10.3390/nu8030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao Q., Xue Y., Wang J.-F., Li H., Long T.-T., Li Z., Wang Y.-M., Dong P., Xue C.-H. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearsonothuria graeffei. J. Sci. Food Agric. 2012;92:965–974. doi: 10.1002/jsfa.4678. [DOI] [PubMed] [Google Scholar]
- 144.Yan C.-M., Chai E.-Q., Cai H.-Y., Miao G.-Y., Ma W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015;11:4617–4624. doi: 10.3892/mmr.2015.3266. [DOI] [PubMed] [Google Scholar]
- 145.Yao C., Liu B.-B., Qian X.-D., Li L.-Q., Cao H.-B., Guo Q.-S., Zhou G.-F. Crocin induces autophagic apoptosis in hepatocellular carcinoma by inhibiting Akt/mTOR activity. OncoTargets Ther. 2018;11:2017–2028. doi: 10.2147/OTT.S154586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hui F., Qin X., Zhang Q., Li R., Liu M., Ren T., Zhao M., Zhao Q. Alpinia oxyphylla oil induces apoptosis of hepatocellular carcinoma cells via PI3K/Akt pathway in vitro and in vivo. Biomed. Pharmacother. 2019;109:2365–2374. doi: 10.1016/j.biopha.2018.11.124. [DOI] [PubMed] [Google Scholar]
- 147.García-Fernández L.F., Losada A., Alcaide V., Álvarez A.M., Cuadrado A., González L., Nakayama K., Nakayama K.I., Fernández-Sousa J.M., Muñoz A., et al. Aplidin™ induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C δ. Oncogene. 2002;21:7533–7544. doi: 10.1038/sj.onc.1205972. [DOI] [PubMed] [Google Scholar]
- 148.Ming Y., Zheng Z., Chen L., Zheng G., Liu S., Yu Y., Tong Q. Corilagin inhibits hepatocellular carcinoma cell proliferation by inducing G2/M phase arrest. Cell Biol. Int. 2013;37:1046–1054. doi: 10.1002/cbin.10132. [DOI] [PubMed] [Google Scholar]
- 149.Alsahafi E., Begg K., Amelio I., Raulf N., Lucarelli P., Sauter T., Tavassoli M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cramer J.D., Burtness B., Le Q.T., Ferris R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019;16:669–683. doi: 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 151.Yang I.-H., Shin J.-A., Kim L.-H., Kwon K.H., Cho S.-D. The caspase 3-dependent apoptotic effect of pycnogenol in human oral squamous cell carcinoma HSC-3 cells. J. Clin. Biochem. Nutr. 2016;58:40–47. doi: 10.3164/jcbn.15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kwak H.-H., Kim I.-R., Kim H.-J., Park B.-S., Yu S.-B. α-Mangostin Induces Apoptosis and Cell Cycle Arrest in Oral Squamous Cell Carcinoma Cell. Evid.-Based Complement. Altern. Med. 2016;2016:9060649. doi: 10.1155/2016/5352412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.De La Chapa J.J., Singha P.K., Lee D.R., Gonzales C.B. Thymol inhibits oral squamous cell carcinoma growth via mitochondria-mediated apoptosis. J. Oral Pathol. Med. 2018;47:674–682. doi: 10.1111/jop.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chattopadhyay I. Pharmacotherapeutic Botanicals for Cancer Chemoprevention. Springer; Singapore: 2020. Role of Nutrigenetics and Nutrigenomics in Cancer Chemoprevention; pp. 167–188. [Google Scholar]
- 155.Katiyar S.K. Emerging phytochemicals for the prevention and treatment of head and neck cancer. Molecules. 2016;21:1610. doi: 10.3390/molecules21121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rawla P. Epidemiology of prostate cancer. World J. Oncol. 2019;10:63. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Salehi B., Fokou P.V.T., Yamthe L.R.T., Tali B.T., Adetunji C.O., Rahavian A., Mudau F.N., Martorell M., Setzer W.N., Rodrigues C.F., et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients. 2019;11:1483. doi: 10.3390/nu11071483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zaidi S., Gandhi J., Joshi G., Smith N.L., Khan S.A. The anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis. 2019;22:351–361. doi: 10.1038/s41391-018-0085-2. [DOI] [PubMed] [Google Scholar]
- 159.Shukla S., Shankar E., Fu P., MacLennan G.T., Gupta S. Suppression of NF-κB and NF-κB-regulated gene expression by apigenin through IκBα and IKK pathway in TRAMP mice. PLoS ONE. 2015;10:e0138710. doi: 10.1371/journal.pone.0138710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhu K.-C., Sun J.-M., Shen J.-G., Jin J.-Z., Liu F., Xu X.-L., Chen L., Liu L.-T., Lv J.-J. Afzelin exhibits anti-cancer activity against androgen-sensitive LNCaP and androgen-independent PC-3 prostate cancer cells through the inhibition of LIM domain kinase 1. Oncol. Lett. 2015;10:2359–2365. doi: 10.3892/ol.2015.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Akhtar N., Syed D.N., Khan M.I., Adhami V.M., Mirza B., Mukhtar H. The pentacyclic triterpenoid, plectranthoic acid, a novel activator of AMPK induces apoptotic death in prostate cancer cells. Oncotarget. 2016;7:3819–3831. doi: 10.18632/oncotarget.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tan J., Jiang X., Yin G., He L., Liu J., Long Z., Jiang Z., Yao K. Anacardic acid induces cell apoptosis of prostatic cancer through autophagy by ER stress/DAPK3/Akt signaling pathway. Oncol. Rep. 2017;38:1373–1382. doi: 10.3892/or.2017.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jeong M.-H., Ko H., Jeon H., Sung G.-J., Park S.-Y., Jun W.J., Lee Y.-H., Lee J., Lee S.-w., Yoon H.-G., et al. Delphinidin induces apoptosis via cleaved HDAC3-mediated p53 acetylation and oligomerization in prostate cancer cells. Oncotarget. 2016;7:56767–56780. doi: 10.18632/oncotarget.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hu M., Peng S., He Y., Qin M., Cong X., Xing Y., Liu M., Yi Z. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget. 2015;6:15348–15361. doi: 10.18632/oncotarget.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lall R.K., Syed D.N., Khan M.I., Adhami V.M., Gong Y., Lucey J.A., Mukhtar H. Dietary flavonoid fisetin increases abundance of high-molecular-mass hyaluronan conferring resistance to prostate oncogenesis. Carcinogenesis. 2016;37:918–928. doi: 10.1093/carcin/bgw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Adaramoye O., Erguen B., Nitzsche B., Höpfner M., Jung K., Rabien A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC-3 and LNCaP cells. Chem.-Biol. Interact. 2017;274:100–106. doi: 10.1016/j.cbi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 167.Zeng S., Zhu B., Zeng J., Wu W., Jiang C. Zeylenone represses the progress of human prostate cancer by downregulating the Wnt/β-catenin pathway. Mol. Med. Rep. 2018;18:5572–5578. doi: 10.3892/mmr.2018.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Núñez Selles A.J., Daglia M., Rastrelli L. The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. BioFactors. 2016;42:475–491. doi: 10.1002/biof.1299. [DOI] [PubMed] [Google Scholar]
- 169.Elkady A.I. Anethole inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and apoptosis. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.-Anti-Cancer Agents. 2018;18:216–236. doi: 10.2174/1871520617666170725165717. [DOI] [PubMed] [Google Scholar]
- 170.Xu Y., Zhu J.-Y., Lei Z.-M., Wan L.-J., Zhu X.-W., Ye F., Tong Y.-Y. Anti-proliferative effects of paeonol on human prostate cancer cell lines DU145 and PC-3. J. Physiol. Biochem. 2017;73:157–165. doi: 10.1007/s13105-016-0537-x. [DOI] [PubMed] [Google Scholar]
- 171.Kanwal N., Rasul A., Hussain G., Anwar H., Shah M.A., Sarfraz I., Riaz A., Batool R., Shahbaz M., Hussain A., et al. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem. Toxicol. 2020;143:111570. doi: 10.1016/j.fct.2020.111570. [DOI] [PubMed] [Google Scholar]
- 172.Ramamoorthy M.D., Kumar A., Ayyavu M., Dhiraviam K.N., Ayyau M. Reserpine induces apoptosis and cell cycle arrest in hormone independent prostate cancer cells through mitochondrial membrane potential failure. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.-Anti-Cancer Agents. 2018;18:1313–1322. doi: 10.2174/1871520618666180209152215. [DOI] [PubMed] [Google Scholar]
- 173.Royston K.J., Tollefsbol T.O. The epigenetic impact of cruciferous vegetables on cancer prevention. Curr. Pharmacol. Rep. 2015;1:46–51. doi: 10.1007/s40495-014-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Doll R., Peto R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. JNCI J. Natl. Cancer Inst. 1981;66:66–1192. doi: 10.1093/jnci/66.6.1192. [DOI] [PubMed] [Google Scholar]
- 175.Kang B., Park H., Kim B. Anticancer Activity and Underlying Mechanism of Phytochemicals against Multiple Myeloma. Int. J. Mol. Sci. 2019;20:2302. doi: 10.3390/ijms20092302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu Y., Giaisi M., Köhler R., Chen W.-M., Krammer P.H., Li-Weber M. Rocaglamide breaks TRAIL-resistance in human multiple myeloma and acute T-cell leukemia in vivo in a mouse xenogtraft model. Cancer Lett. 2017;389:70–77. doi: 10.1016/j.canlet.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 177.Ishii N., Araki K., Yokobori T., Hagiwara K., Gantumur D., Yamanaka T., Handa T., Tsukagoshi M., Igarashi T., Watanabe A., et al. Conophylline suppresses pancreatic cancer desmoplasia and cancer-promoting cytokines produced by cancer-associated fibroblasts. Cancer Sci. 2019;110:334–344. doi: 10.1111/cas.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim B.-H., Yi E.H., Jee J.-G., Jeong A.J., Sandoval C., Park I.-C., Baeg G.H., Ye S.-K. Tubulosine selectively inhibits JAK3 signalling by binding to the ATP-binding site of the kinase of JAK3. J. Cell. Mol. Med. 2020;24:7427–7438. doi: 10.1111/jcmm.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Karami A., Hamzeloo-Moghadam M., Yami A., Barzegar M., Mashati P., Gharehbaghian A. Antiproliferative Effect of Gaillardin from Inula oculus-christi in Human Leukemic Cells. Nutr. Cancer. 2020;72:1043–1056. doi: 10.1080/01635581.2019.1665188. [DOI] [PubMed] [Google Scholar]
- 180.Trivedi R., Maurya R.P., Mishra D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014;5:e1465. doi: 10.1038/cddis.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Uchihara Y., Tago K., Funakoshi-Tago M. The mechanisms of taxodione-induced apoptosis in BCR-ABL-positive leukemia cells. Nihon Yakurigaku Zasshi Folia Pharmacol. Jpn. 2019;153:147–154. doi: 10.1254/fpj.153.147. [DOI] [PubMed] [Google Scholar]
- 182.Wu C., Li M., Meng H., Liu Y., Niu W., Zhou Y., Zhao R., Duan Y., Zeng Z., Li X., et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci. China Life Sci. 2019;62:640–647. doi: 10.1007/s11427-018-9461-5. [DOI] [PubMed] [Google Scholar]
- 183.Shivamadhu M.C., Srinivas B.K., Jayarama S., Chandrashekaraiah S.A. Anti-cancer and anti-angiogenic effects of partially purified lectin from Praecitrullus fistulosus fruit on in vitro and in vivo model. Biomed. Pharmacother. 2017;96:1299–1309. doi: 10.1016/j.biopha.2017.11.082. [DOI] [PubMed] [Google Scholar]
- 184.Lee S.R., Park J.Y., Yu J.S., Lee S.O., Ryu J.-Y., Choi S.-Z., Kang K.S., Yamabe N., Kim K.H. Odisolane, a Novel Oxolane Derivative, and Antiangiogenic Constituents from the Fruits of Mulberry (Morus alba L.) J. Agric. Food Chem. 2016;64:3804–3809. doi: 10.1021/acs.jafc.6b01461. [DOI] [PubMed] [Google Scholar]
- 185.Yap V.A., Loong B.-J., Ting K.-N., Loh S.H.-S., Yong K.-T., Low Y.-Y., Kam T.-S., Lim K.-H. Hispidacine, an unusual 8,4′-oxyneolignan-alkaloid with vasorelaxant activity, and hispiloscine, an antiproliferative phenanthroindolizidine alkaloid, from Ficus hispida Linn. Phytochemistry. 2015;109:96–102. doi: 10.1016/j.phytochem.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 186.Kim J.-E., Kim J.H., Lee Y., Yang H., Heo Y.-S., Bode A.M., Lee K.W., Dong Z. Bakuchiol suppresses proliferation of skin cancer cells by directly targeting Hck, Blk, and p38 MAP kinase. Oncotarget. 2016;7:14616–14627. doi: 10.18632/oncotarget.7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pal H.C., Katiyar S.K. Cryptolepine, a plant alkaloid, inhibits the growth of non-melanoma skin cancer cells through inhibition of topoisomerase and induction of DNA damage. Molecules. 2016;21:1758. doi: 10.3390/molecules21121758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu J., Chen Y., Yang R., Zhou T., Ke W., Si Y., Yang S., Zhang T., Liu X., Zhang L., et al. Cucurbitacin B inhibits gastric cancer progression by suppressing STAT3 activity. Arch. Biochem. Biophys. 2020;684:108314. doi: 10.1016/j.abb.2020.108314. [DOI] [PubMed] [Google Scholar]
- 189.Jiang L., Wang Y., Yin Q., Liu G., Liu H., Huang Y., Li B. Phycocyanin: A potential drug for cancer treatment. J. Cancer. 2017;8:3416–3429. doi: 10.7150/jca.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liang X., Xu C., Cao X., Wang W. Isovitexin Suppresses Cancer Stemness Property And Induces Apoptosis Of Osteosarcoma Cells by Disruption of The DNMT1/miR-34a/Bcl-2 Axis. Cancer Manag. Res. 2019;11:8923. doi: 10.2147/CMAR.S222708. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Talib W.H. Melatonin and cancer hallmarks. Molecules. 2018;23:518. doi: 10.3390/molecules23030518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xie X., Zu X., Liu F., Wang T., Wang X., Chen H., Liu K., Wang P., Liu F., Zheng Y., et al. Purpurogallin is a novel mitogen-activated protein kinase kinase 1/2 inhibitor that suppresses esophageal squamous cell carcinoma growth in vitro and in vivo. Mol. Carcinog. 2019;58:1248–1259. doi: 10.1002/mc.23007. [DOI] [PubMed] [Google Scholar]
- 193.Xie H., Zhang T., Yang N., Li Z., Liu Y. Anticancer effects of Mahanimbine alkaloid on the human bladder cancer cells are due to the induction of G0/G1 cell cycle arrest, apoptosis and autophagy. J. BUON. 2020;25:1166–1171. [PubMed] [Google Scholar]
- 194.Tu S., Zhang X.L., Wan H.F., Xia Y.Q., Liu Z.Q., Yang X.H., Wan F.S. Effect of taurine on cell proliferation and apoptosis human lung cancer A549 cells. Oncol. Lett. 2018;15:5473–5480. doi: 10.3892/ol.2018.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rath B., Hochmair M., Plangger A., Hamilton G. Anticancer activity of fascaplysin against lung cancer cell and small cell lung cancer circulating tumor cell lines. Mar. Drugs. 2018;16:383. doi: 10.3390/md16100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xie Y.-J., Gao W.-N., Wu Q.-B., Yao X.-J., Jiang Z.-B., Wang Y.-W., Wang W.-J., Li W., Hussain S., Liu L., et al. Chelidonine selectively inhibits the growth of gefitinib-resistant non-small cell lung cancer cells through the EGFR-AMPK pathway. Pharmacol. Res. 2020;159:104934. doi: 10.1016/j.phrs.2020.104934. [DOI] [PubMed] [Google Scholar]
- 197.Kim J.H., Cho E.B., Lee J., Jung O., Ryu B.J., Kim S.H., Cho J.Y., Ryou C., Lee S.Y. Emetine inhibits migration and invasion of human non-small-cell lung cancer cells via regulation of ERK and p38 signaling pathways. Chem.-Biol. Interact. 2015;242:25–33. doi: 10.1016/j.cbi.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 198.Yu X., Lin H., Wang Y., Lv W., Zhang S., Qian Y., Deng X., Feng N., Yu H., Qian B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018;11:1833–1847. doi: 10.2147/OTT.S155716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhai D.-D., Supaibulwatana K., Zhong J.-J. Inhibition of tumor cell proliferation and induction of apoptosis in human lung carcinoma 95-D cells by a new sesquiterpene from hairy root cultures of Artemisia annua. Phytomedicine. 2010;17:856–861. doi: 10.1016/j.phymed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 200.Wang J., Zhang B., Guo Y., Li G., Xie Q., Zhu B., Gao J., Chen Z. Artemisinin Inhibits Tumor Lymphangiogenesis by Suppression of Vascular Endothelial Growth Factor C. Pharmacology. 2008;82:148–155. doi: 10.1159/000148261. [DOI] [PubMed] [Google Scholar]
- 201.Chen J., Huang X., Tao C., Xiao T., Li X., Zeng Q., Ma M., Wu Z. Artemether attenuates the progression of non-small cell lung cancer by inducing apoptosis, cell cycle arrest and promoting cellular senescence. Biol. Pharm. Bull. 2019;42:1720–1725. doi: 10.1248/bpb.b19-00391. [DOI] [PubMed] [Google Scholar]
- 202.Li Y., He K., Huang Y., Zheng D., Gao C., Cui L., Jin Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010;49:630–640. doi: 10.1002/mc.20638. [DOI] [PubMed] [Google Scholar]
- 203.Mertens-Talcott S.U., Noratto G.D., Li X., Angel-Morales G., Bertoldi M.C., Safe S. Betulinic acid decreases ER-negative breast cancer cell growth in vitro and in vivo: Role of Sp transcription factors and microRNA-27a: ZBTB10. Mol. Carcinog. 2012;52:591–602. doi: 10.1002/mc.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hsu T.-I., Wang M.-C., Chen S.-Y., Huang S.-T., Yeh Y.-M., Su W.-C., Chang W.-C., Hung J.-J. Betulinic acid decreases specificity protein 1 (Sp1) level via increasing the sumoylation of sp1 to inhibit lung cancer growth. Mol. Pharmacol. 2012;82:1115–1128. doi: 10.1124/mol.112.078485. [DOI] [PubMed] [Google Scholar]
- 205.Zhu M., Wang M., Jiang Y., Wu H., Lu G., Shi W., Cong D., Song S., Liu K., Wang H. Gambogic Acid Induces Apoptosis of Non-Small Cell Lung Cancer (NSCLC) Cells by Suppressing Notch Signaling. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:7146–7151. doi: 10.12659/MSM.912563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mei C., Zhou S., Zhu L., Ming J., Zeng F., Xu R. Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar. Drugs. 2017;15:39. doi: 10.3390/md15020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.You C., Sun Y., Zhang S., Tang G., Zhang N., Li C., Tian X., Ma S., Luo Y., Sun W., et al. Trichosanthin enhances sensitivity of non-small cell lung cancer (NSCLC) TRAIL-resistance cells. Int. J. Biol. Sci. 2018;14:217–227. doi: 10.7150/ijbs.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ruan J.S., Zhou H., Yang L., Wang L., Jiang Z.S., Sun H., Wang S.M. Ursolic Acid Attenuates TGF-β1-Induced Epithelial-Mesenchymal Transition in NSCLC by Targeting Integrin αVβ5/MMPs Signaling. Oncol. Res. 2019;27:593–600. doi: 10.3727/096504017X15051723858706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Song Y., Kong L., Sun B., Gao L., Chu P., Ahsan A., Qaed E., Lin Y., Peng J., Ma X., et al. Induction of autophagy by an oleanolic acid derivative, SZC017, promotes ROS-dependent apoptosis through Akt and JAK2/STAT3 signaling pathway in human lung cancer cells. Cell Biol. Int. 2017;41:1367–1378. doi: 10.1002/cbin.10868. [DOI] [PubMed] [Google Scholar]
- 210.Wang C., Cui C. Inhibition of lung cancer proliferation by wogonin is associated with the activation of apoptosis and generation of reactive oxygen species. Balk. Med. J. 2020;37:29–33. doi: 10.4274/balkanmedj.galenos.2019.2019.7.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jiang Z.-Q., Li M.-H., Qin Y.-M., Jiang H.-Y., Zhang X., Wu M.-H. Luteolin inhibits tumorigenesis and induces apoptosis of non-small cell lung cancer cells via regulation of MicroRNA-34a-5p. Int. J. Mol. Sci. 2018;19:447. doi: 10.3390/ijms19020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zou J., Yang Y., Yang Y., Liu X. Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-κB pathway. Biomed. Pharmacother. 2018;108:130–136. doi: 10.1016/j.biopha.2018.09.051. [DOI] [PubMed] [Google Scholar]
- 213.Tang Q., Wu J., Zheng F., Chen Y., Hann S.S. Emodin increases expression of insulin-like growth factor binding protein 1 through activation of MEK/ERK/AMPKα and interaction of PPARγ and Sp1 in lung cancer. Cell. Physiol. Biochem. 2017;41:339–357. doi: 10.1159/000456281. [DOI] [PubMed] [Google Scholar]
- 214.Yao C.-C., Tu Y.-R., Jiang J., Ye S.-F., Du H.-X., Zhang Y. β-elemene reverses the drug resistance of lung cancer A549/DDP cells via the mitochondrial apoptosis pathway. Oncol. Rep. 2014;31:2131–2138. doi: 10.3892/or.2014.3083. [DOI] [PubMed] [Google Scholar]
- 215.Jiang Y., Zhang Y., Luan J., Duan H., Zhang F., Yagasaki K., Zhang G. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology. 2010;62:573–583. doi: 10.1007/s10616-010-9310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Huang A.-C., Yang M.-D., Hsiao Y.-T., Lin T.-S., Ma Y.-S., Peng S.-F., Hsia T.-C., Cheng Y.-D., Kuo C.-L., Chung J.-G. Bufalin inhibits gefitinib resistant NCI-H460 human lung cancer cell migration and invasion in vitro. J. Ethnopharmacol. 2016;194:1043–1050. doi: 10.1016/j.jep.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 217.Zhao R., Chen M., Jiang Z., Zhao F., Xi B., Zhang X., Fu H., Zhou K. Platycodin-D Induced Autophagy in Non-Small Cell Lung Cancer Cells via PI3K/Akt/mTOR and MAPK Signaling Pathways. J. Cancer. 2015;6:623–631. doi: 10.7150/jca.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang J., Wang J., Jiang J.-Y., Liu S.-D., Fu K., Liu H.-Y. Tanshinone IIA induces cytochrome c-mediated caspase cascade apoptosis in A549 human lung cancer cells via the JNK pathway. Int. J. Oncol. 2014;45:683–690. doi: 10.3892/ijo.2014.2471. [DOI] [PubMed] [Google Scholar]
- 219.Mou H., Zheng Y., Zhao P., Bao H., Fang W., Xu N. Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria-and Fas/FasL-mediated pathways. Toxicol. Vitr. 2011;25:1027–1032. doi: 10.1016/j.tiv.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 220.Piantino C.B., Salvadori F.A., Ayres P.P., Kato R.B., Srougi V., Leite K.R., Srougi M. An evaluation of the anti-neoplastic activity of curcumin in prostate cancer cell lines. Int. Braz. J. Urol. 2009;35:354–361. doi: 10.1590/S1677-55382009000300012. [DOI] [PubMed] [Google Scholar]
- 221.Wang C., Song X., Shang M., Zou W., Zhang M., Wei H., Shao H. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non-small-cell lung cancer cells. Future Oncol. 2019;15:1243–1253. doi: 10.2217/fon-2018-0708. [DOI] [PubMed] [Google Scholar]
- 222.Wu C., Zhuang Y., Jiang S., Tian F., Teng Y., Chen X., Zheng P., Liu S., Zhou J., Wu J., et al. Cinnamaldehyde induces apoptosis and reverses epithelial-mesenchymal transition through inhibition of Wnt/β-catenin pathway in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 2017;84:58–74. doi: 10.1016/j.biocel.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 223.Lu X., Liu W., Wu J., Li M., Wang J., Wu J., Luo C. A polysaccharide fraction of adlay seed (Coix lachryma-jobi L.) induces apoptosis in human non-small cell lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2013;430:846–851. doi: 10.1016/j.bbrc.2012.11.058. [DOI] [PubMed] [Google Scholar]
- 224.Hu C., Jiang R., Cheng Z., Lu Y., Gu L., Li H., Li L., Gao Q., Chen M., Zhang X. Ophiopogonin-B suppresses epithelial-mesenchymal transition in human lung adenocarcinoma cells via the Linc00668/miR-432-5p/EMT axis. J. Cancer. 2019;10:2849–2856. doi: 10.7150/jca.31338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhong L.-R., Zheng J., Sun Q., Wei K., Hu Y. Radix Tetrastigma hemsleyani flavone inhibits proliferation, migration, and invasion of human lung carcinoma A549 cells. OncoTargets Ther. 2016;9:635–641. doi: 10.2147/OTT.S92707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chou P.-Y., Huang G.-J., Pan C.-H., Chien Y.-C., Chen Y.-Y., Wu C.-H., Sheu M.-J., Cheng H.-C. Trilinolein inhibits proliferation of human non-small cell lung carcinoma A549 through the modulation of pi3k/akt pathway. Am. J. Chin. Med. 2011;39:803–815. doi: 10.1142/S0192415X11009214. [DOI] [PubMed] [Google Scholar]
- 227.Hsu H.-Y., Lin T.-Y., Lu M.-K., Leng P.-J., Tsao S.-M., Wu Y.-C. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci. Rep. 2017;7:srep44990. doi: 10.1038/srep44990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xia S., Zhang X., Li C., Guan H. Oridonin inhibits breast cancer growth and metastasis through blocking the Notch signaling. Saudi Pharm. J. 2017;25:638–643. doi: 10.1016/j.jsps.2017.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guo W.-Q., Chen Y.-G., Shi R.-Z., He K., Wang J.-F., Shao J.-H., Wan J.-B., Gao J.-L. 20(S)-Protopanaxdiol Suppresses the Abnormal Granule-Monocyte Differentiation of Hematopoietic Stem Cells in 4T1 Breast Cancer-Bearing Mouse. Evid.-Based Complement. Altern. Med. 2020;2020:8747023. doi: 10.1155/2020/8747023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jiang J., Jedinak A., Sliva D. Ganodermanontriol (GDNT) exerts its effect on growth and invasiveness of breast cancer cells through the down-regulation of CDC20 and uPA. Biochem. Biophys. Res. Commun. 2011;415:325–329. doi: 10.1016/j.bbrc.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 231.Xiaoping C., Yan C., Shuibing L., Youguo C., Jianyun L., Lanping L. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009;77:389–393. doi: 10.1016/j.carbpol.2009.01.009. [DOI] [Google Scholar]
- 232.Zhao L., Dong Y., Chen G., Hu Q. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2010;80:783–789. doi: 10.1016/j.carbpol.2009.12.029. [DOI] [Google Scholar]
- 233.Yang R., Hanwell H., Zhang J., Tsao R., Meckling K.A. Antiproliferative Activity of Pomiferin in Normal (MCF-10A) and Transformed (MCF-7) Breast Epithelial Cells. J. Agric. Food Chem. 2011;59:13328–13336. doi: 10.1021/jf202898g. [DOI] [PubMed] [Google Scholar]
- 234.Dong Y., Yin S., Li J., Jiang C., Ye M., Hu H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis. 2011;16:394–403. doi: 10.1007/s10495-011-0573-5. [DOI] [PubMed] [Google Scholar]
- 235.Yue G.G.-L., Xie S., Lee J.K.-M., Kwok H.-F., Gao S., Nian Y., Wu X.-X., Wong C.-K., Qiu M.-H., Lau C.B.-S. New potential beneficial effects of actein, a triterpene glycoside isolated from Cimicifuga species, in breast cancer treatment. Sci. Rep. 2016;6:35263. doi: 10.1038/srep35263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhou R., Xu L., Ye M., Liao M., Du H., Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm. Metab. Res. 2014;46:753–760. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]
- 237.Li W., Song K., Wang S., Zhang C., Zhuang M., Wang Y., Liu T. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C. 2019;98:685–695. doi: 10.1016/j.msec.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 238.Cao C., Huang W., Zhang N., Wu F., Xu T., Pan X., Peng C., Han B. Narciclasine induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis. Cell Prolif. 2018;51:e12518. doi: 10.1111/cpr.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kong Y., Li F., Nian Y., Zhou Z., Yang R., Qiu M.-H., Chen C. KHF16 is a Leading Structure from Cimicifuga foetida that Suppresses Breast Cancer Partially by Inhibiting the NF-κB Signaling Pathway. Theranostics. 2016;6:875–886. doi: 10.7150/thno.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hien T.T., Kim H.G., Han E.H., Kang K.W., Jeong H.G. Molecular mechanism of suppression of MDR1 by puerarin from Pueraria lobata via NF-κB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP-activated protein kinase in breast cancer MCF-7/adr cells. Mol. Nutr. Food Res. 2010;54:918–928. doi: 10.1002/mnfr.200900146. [DOI] [PubMed] [Google Scholar]
- 241.Moon A., Kim E.-S., Jeong C.-S. Genipin, a constituent of Gardenia jasminoides Ellis, induces apoptosis and inhibits invasion in MDA-MB-231 breast cancer cells. Oncol. Rep. 2012;27:567–572. doi: 10.3892/or.2011.1508. [DOI] [PubMed] [Google Scholar]
- 242.Du J., Wang X.-F., Zhou Q.-M., Zhang T.-L., Lu Y.-Y., Zhang H., Su S.-B. Evodiamine induces apoptosis and inhibits metastasis in MDA-MB-231 human breast cancer cells in vitro and in vivo. Oncol. Rep. 2013;30:685–694. doi: 10.3892/or.2013.2498. [DOI] [PubMed] [Google Scholar]
- 243.Wang H., Chen X., Li T., Xu J., Ma Y. A myrsinol diterpene isolated from a traditional herbal medicine, LANGDU reverses multidrug resistance in breast cancer cells. J. Ethnopharmacol. 2016;194:1–5. doi: 10.1016/j.jep.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 244.Mohsenikia M., Alizadeh A.M., Khodayari S., Khodayari H., Kouhpayeh S.A., Karimi A., Zamani M., Azizian S., Mohagheghi M.A. The protective and therapeutic effects of alpha-solanine on mice breast cancer. Eur. J. Pharmacol. 2013;718:1–9. doi: 10.1016/j.ejphar.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 245.Fang Z.-Y., Ren Y.-D., Du S.-Y., Zhang M., Wang Y.-S., Fang L., Zhang H. Melosuavine I, an apoptosis-inducing bisindole alkaloid from Melodinus suaveolens. Fitoterapia. 2019;133:175–179. doi: 10.1016/j.fitote.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 246.Wang L., Peng Y., Shi K., Wang H., Lu J., Li Y., Ma C. Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis. J. Biomed. Res. 2015;29:132–138. doi: 10.7555/JBR.27.20120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yan Z., Liu G., Liang M., Xu Y. Ophiopogonin D inhibits cell proliferation and induces apoptosis of human laryngocarcinoma through downregulation of cyclin B1 and MMP-9 and upregulation of p38-MAPK signaling. Oncol. Lett. 2019;17:1877–1882. doi: 10.3892/ol.2018.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kim S.-J., Kim A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015;39:125–134. doi: 10.1016/j.jgr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Huang C., Chen X., Guo B., Huang W., Shen T., Sun X., Xiao P., Zhou Q. Induction of apoptosis by Icariside II through extrinsic and intrinsic signaling pathways in human breast cancer MCF7 cells. Biosci. Biotechnol. Biochem. 2012;76:1322–1328. doi: 10.1271/bbb.120077. [DOI] [PubMed] [Google Scholar]
- 250.Zhao H., Yang Z., Wang X., Zhang X., Wang M., Wang Y., Mei Q., Wang Z. Triptolide inhibits ovarian cancer cell invasion by repression of matrix metalloproteinase 7 and 19 and upregulation of E-cadherin. Exp. Mol. Med. 2012;44:633–641. doi: 10.3858/emm.2012.44.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang J.-H., Nao J.-F., Zhang M., He P. 20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumor Biol. 2014;35:11985–11994. doi: 10.1007/s13277-014-2497-5. [DOI] [PubMed] [Google Scholar]
- 252.Woo J.-H., Ahn J.-H., Jang D.S., Lee K.-T., Choi J.-H. Effect of kumatakenin isolated from cloves on the apoptosis of cancer cells and the alternative activation of tumor-associated macrophages. J. Agric. Food Chem. 2017;65:7893–7899. doi: 10.1021/acs.jafc.7b01543. [DOI] [PubMed] [Google Scholar]
- 253.Hua F., Li C.-H., Chen X.-G., Liu X.-P. Daidzein exerts anticancer activity towards SKOV3 human ovarian cancer cells by inducing apoptosis and cell cycle arrest, and inhibiting the Raf/MEK/ERK cascade. Int. J. Mol. Med. 2018;41:3485–3492. doi: 10.3892/ijmm.2018.3531. [DOI] [PubMed] [Google Scholar]
- 254.Chang H.-L., Su J.-H., Yeh Y.-T., Lee Y.-C., Chen H.-M., Wu Y.-C., Yuan S.-S.F. Protoapigenone, a novel flavonoid, inhibits ovarian cancer cell growth in vitro and in vivo. Cancer Lett. 2008;267:85–95. doi: 10.1016/j.canlet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 255.Li J., Jiang K., Zhao F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015;33:2829–2836. doi: 10.3892/or.2015.3891. [DOI] [PubMed] [Google Scholar]
- 256.Gao L., Chen M., Ouyang Y., Li R., Zhang X., Gao X., Lin S., Wang X. Icaritin induces ovarian cancer cell apoptosis through activation of p53 and inhibition of Akt/mTOR pathway. Life Sci. 2018;202:188–194. doi: 10.1016/j.lfs.2018.03.059. [DOI] [PubMed] [Google Scholar]
- 257.Bae H., Song G., Lim W. Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction. Pharmaceutics. 2020;12:488. doi: 10.3390/pharmaceutics12060488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jiang L., Cao X.-C., Cao J.-G., Liu F., Quan M.-F., Sheng X.-F., Ren K.-Q. Casticin induces ovarian cancer cell apoptosis by repressing FoxM1 through the activation of FOXO3a. Oncol. Lett. 2013;5:1605–1610. doi: 10.3892/ol.2013.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang Y.-Y., Zhang F., Zhang Y.-S., Thakur K., Zhang J.-G., Liu Y., Kan H., Wei Z.-J. Mechanism of juglone-induced cell cycle arrest and apoptosis in Ishikawa Human endometrial cancer cells. J. Agric. Food Chem. 2019;67:7378–7389. doi: 10.1021/acs.jafc.9b02759. [DOI] [PubMed] [Google Scholar]
- 260.Chen J., Li Z., Chen A.Y., Ye X., Luo H., Rankin G.O., Chen Y.C. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int. J. Mol. Sci. 2013;14:6012–6025. doi: 10.3390/ijms14036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Du Y., Feng J., Wang R., Zhang H., Liu J. Effects of flavonoids from Potamogeton crispus L. on proliferation, migration, and invasion of human ovarian cancer cells. PLoS ONE. 2015;10:e0130685. doi: 10.1371/journal.pone.0130685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Greenshields A.L., Shepherd T.G., Hoskin D.W. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol. Carcinog. 2017;56:75–93. doi: 10.1002/mc.22474. [DOI] [PubMed] [Google Scholar]
- 263.Huang K., Li L.A., Meng Y.G., You Y.Q., Fu X.Y., Song L. Arctigenin promotes apoptosis in ovarian cancer cells via the iNOS/NO/STAT3/survivin signalling. Basic Clin. Pharmacol. Toxicol. 2014;115:507–511. doi: 10.1111/bcpt.12270. [DOI] [PubMed] [Google Scholar]
- 264.Zhang F., Zhang Y.-Y., Sun Y.-S., Ma R.-H., Thakur K., Zhang J.-G., Wei Z.-J. Asparanin A from Asparagus officinalis L. Induces G0/G1 Cell Cycle Arrest and Apoptosis in Human Endometrial Carcinoma Ishikawa Cells via Mitochondrial and PI3K/AKT Signaling Pathways. J. Agric. Food Chem. 2020;68:213–224. doi: 10.1021/acs.jafc.9b07103. [DOI] [PubMed] [Google Scholar]
- 265.Jiang G., Liu J., Ren B., Zhang L., Owusu L., Liu L., Zhang J., Tang Y., Li W. Anti-tumor and chemosensitization effects of Cryptotanshinone extracted from Salvia miltiorrhiza Bge. on ovarian cancer cells in vitro. J. Ethnopharmacol. 2017;205:33–40. doi: 10.1016/j.jep.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 266.Yang Z., Li C., Wang X., Zhai C., Yi Z., Wang L., Liu B., Du B., Wu H., Guo X., et al. Dauricine induces apoptosis, inhibits proliferation and invasion through inhibiting NF-κB signaling pathway in colon cancer cells. J. Cell. Physiol. 2010;225:266–275. doi: 10.1002/jcp.22261. [DOI] [PubMed] [Google Scholar]
- 267.Xia S., Miao Y., Liu S. Withaferin A induces apoptosis by ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2018;503:2363–2369. doi: 10.1016/j.bbrc.2018.06.162. [DOI] [PubMed] [Google Scholar]
- 268.De Almeida G.C., Oliveira L.F.S., Predes D., Fokoue H.H., Kuster R.M., Oliveira F.L., Mendes F.A., Abreu J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020;10:11681. doi: 10.1038/s41598-020-68574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ray P., Guha D., Chakraborty J., Banerjee S., Adhikary A., Chakraborty S., Das T., Sa G. Crocetin exploits p53-induced death domain (PIDD) and FAS-associated death domain (FADD) proteins to induce apoptosis in colorectal cancer. Sci. Rep. 2016;6:32979. doi: 10.1038/srep32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lee S.K., Nam K.-A., Heo Y.-H. Cytotoxic activity and G2/M cell cycle arrest mediated by antofine, a phenanthroindolizidine alkaloid isolated from Cynanchum paniculatum. Planta Med. 2003;69:21–25. doi: 10.1055/s-2003-37021. [DOI] [PubMed] [Google Scholar]
- 271.Sithara T., Dhanya B.P., Arun K.B., Sini S., Dan M., Kokkuvayil Vasu R., Nisha P. Zerumbone, a cyclic sesquiterpene from Zingiber zerumbet induces apoptosis, cell cycle arrest, and antimigratory effects in SW480 colorectal cancer cells. J. Agric. Food Chem. 2018;66:602–612. doi: 10.1021/acs.jafc.7b04472. [DOI] [PubMed] [Google Scholar]
- 272.Xiong Y., Xiong Y.-J., Liu D.-Y., Shen R.-R. Pancratistatin Inhibits the Growth of Colorectal Cancer Cells by Inducing Apoptosis, Autophagy, and G2/M Cell Cycle Arrest. Med. Sci. Monit. 2019;25:6015–6022. doi: 10.12659/MSM.916116. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 273.Dong G.-Z., Shim A.-R., Hyeon J.S., Lee H.J., Ryu J.-H. Inhibition of Wnt/β-catenin pathway by dehydrocostus lactone and costunolide in colon cancer cells. Phytother. Res. 2015;29:680–686. doi: 10.1002/ptr.5299. [DOI] [PubMed] [Google Scholar]
- 274.Zhu P., Wu Y., Yang A., Fu X., Mao M., Liu Z. Catalpol suppressed proliferation, growth and invasion of CT26 colon cancer by inhibiting inflammation and tumor angiogenesis. Biomed. Pharmacother. 2017;95:68–76. doi: 10.1016/j.biopha.2017.08.049. [DOI] [PubMed] [Google Scholar]
- 275.Go H., Hwang H.-J., Nam T.-J. A glycoprotein from Laminaria japonica induces apoptosis in HT-29 colon cancer cells. Toxicol. Vitr. 2010;24:1546–1553. doi: 10.1016/j.tiv.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 276.Han Y.-H., Kee J.-Y., Hong S.-H. Rosmarinic acid activates AMPK to inhibit metastasis of colorectal cancer. Front. Pharmacol. 2018;9:68. doi: 10.3389/fphar.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Murthy K.N.C., Jayaprakasha G.K., Patil B.S. The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. Eur. J. Pharmacol. 2012;688:14–21. doi: 10.1016/j.ejphar.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 278.Han M.H., Kim G.-Y., Yoo Y.H., Choi Y.H. Sanguinarine induces apoptosis in human colorectal cancer HCT-116 cells through ROS-mediated Egr-1 activation and mitochondrial dysfunction. Toxicol. Lett. 2013;220:157–166. doi: 10.1016/j.toxlet.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 279.Sichaem J., Surapinit S., Siripong P., Khumkratok S., Jong-aramruang J., Tip-pyang S. Two new cytotoxic isomeric indole alkaloids from the roots of Nauclea orientalis. Fitoterapia. 2010;81:830–833. doi: 10.1016/j.fitote.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 280.Ji S., Tang S., Li K., Li Z., Liang W., Qiao X., Wang Q., Yu S., Ye M. Licoricidin inhibits the growth of SW480 human colorectal adenocarcinoma cells in vitro and in vivo by inducing cycle arrest, apoptosis and autophagy. Toxicol. Appl. Pharmacol. 2017;326:25–33. doi: 10.1016/j.taap.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 281.Jayameena P., Sivakumarik A.K., Rajesh S. Rutin: A potential anticancer drug against human colon cancer (Hct116) cells. Int. J. Biol. Pharm. Allied Sci. 2018;7:1731–1745. [Google Scholar]
- 282.Dou J., Wang Z., Ma L., Peng B., Mao K., Li C., Su M., Zhou C., Peng G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget. 2018;9:20089–20102. doi: 10.18632/oncotarget.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tabana Y.M., Hassan L.E.A., Ahamed M.B.K., Dahham S.S., Iqbal M.A., Saeed M.A.A., Khan S.S., Sandai D., Majid A.S.A., Oon C.E., et al. Scopoletin, an active principle of tree tobacco (Nicotiana glauca) inhibits human tumor vascularization in xenograft models and modulates ERK1, VEGF-A, and FGF-2 in computer model. Microvasc. Res. 2016;107:17–33. doi: 10.1016/j.mvr.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 284.Lee J.-C., Won S.-J., Chao C.-L., Wu F.-L., Liu H.-S., Ling P., Lin C.-N., Su C.-L. Morusin induces apoptosis and suppresses NF-κB activity in human colorectal cancer HT-29 cells. Biochem. Biophys. Res. Commun. 2008;372:236–242. doi: 10.1016/j.bbrc.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 285.Saud S.M., Li W., Gray Z., Matter M.S., Colburn N.H., Young M.R., Kim Y.S. Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-κB and prevents colitis-induced colorectal cancer by inhibiting GSK-3β. Cancer Prev. Res. 2016;9:607–615. doi: 10.1158/1940-6207.CAPR-16-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Son Y., An Y., Jung J., Shin S., Park I., Gwak J., Ju B.G., Chung Y.-H., Na M., Oh S. Protopine isolated from Nandina domestica induces apoptosis and autophagy in colon cancer cells by stabilizing p53. Phytother. Res. 2019;33:1689–1696. doi: 10.1002/ptr.6357. [DOI] [PubMed] [Google Scholar]
- 287.Racoma I.O., Meisen W.H., Wang Q.-E., Kaur B., Wani A.A. Thymoquinone Inhibits Autophagy and Induces Cathepsin-Mediated, Caspase-Independent Cell Death in Glioblastoma Cells. PLoS ONE. 2013;8:e72882. doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chowdhury F.A., Hossain M.K., Mostofa A.G.M., Akbor M.M., Bin Sayeed M.S. Therapeutic potential of thymoquinone in glioblastoma treatment: Targeting major gliomagenesis signaling pathways. BioMed Res. Int. 2018;2018:4010629. doi: 10.1155/2018/4010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yin J., Wang C., Mody A., Bao L., Hung S.-H., Svoronos S.A., Tseng Y. The Effect of Z-Ligustilide on the Mobility of Human Glioblastoma T98G Cells. PLoS ONE. 2013;8:e66598. doi: 10.1371/journal.pone.0066598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wu N., Wu G.-c., Hu R., Li M., Feng H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol. Sin. 2011;32:345–353. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yang Y., Jia G., Wang Q., Wang R., Deng D., Xue L., Shao N., Zhang Y., Xia X., Zhi F. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome C/Caspase-3 pathway. OncoTargets Ther. 2015;8:303–311. doi: 10.2147/OTT.S76063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pudełek M., Catapano J., Kochanowski P., Mrowiec K., Janik-Olchawa N., Czyż J., Ryszawy D. Therapeutic potential of monoterpene α-thujone, the main compound of Thuja occidentalis L. essential oil, against malignant glioblastoma multiforme cells in vitro. Fitoterapia. 2019;134:172–181. doi: 10.1016/j.fitote.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 293.Zhang L., Wang H., Zhu J., Xu J., Ding K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2014;450:247–254. doi: 10.1016/j.bbrc.2014.05.101. [DOI] [PubMed] [Google Scholar]
- 294.Sales L., Pezuk J.A., Borges K.S., Brassesco M.S., Scrideli C.A., Tone L.G., dos Santos M.H., Ionta M., de Oliveira J.C. Anticancer activity of 7-epiclusianone, a benzophenone from Garcinia brasiliensis, in glioblastoma. BMC Complement. Altern. Med. 2015;15:393. doi: 10.1186/s12906-015-0911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wang K., Fu X.-T., Li Y., Hou Y.-J., Yang M.-F., Sun J.-Y., Yi S.-Y., Fan C.-D., Fu X.-Y., Zhai J., et al. Induction of S-Phase Arrest in Human Glioma Cells by Selenocysteine, a Natural Selenium-Containing Agent Via Triggering Reactive Oxygen Species-Mediated DNA Damage and Modulating MAPKs and AKT Pathways. Neurochem. Res. 2016;41:1439–1447. doi: 10.1007/s11064-016-1854-8. [DOI] [PubMed] [Google Scholar]
- 296.Khan M., Yi F., Rasul A., Li T., Wang N., Gao H., Gao R., Ma T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life. 2012;64:783–794. doi: 10.1002/iub.1068. [DOI] [PubMed] [Google Scholar]
- 297.Wang C.-N., Shiao Y.-J., Lin Y.-L., Chen C.-F. Nepalolide A inhibits the expression of inducible nitric oxide synthase by modulating the degradation of IκB-α and IκB-β in C6 glioma cells and rat primary astrocytes. Br. J. Pharmacol. 1999;128:345–356. doi: 10.1038/sj.bjp.0702785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhou L., Tang H., Wang F., Ou S., Wu T., Fang Y., Xu J., Guo K. Cyclovirobuxine D inhibits cell proliferation and migration and induces apoptosis in human glioblastoma multiforme and low-grade glioma. Oncol. Rep. 2020;43:807–816. doi: 10.3892/or.2020.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Guimarães L.P.T.P., da Graça Rocha G., Queiroz R.M., Martins C.A., Takiya C.M., Gattass C.R. Pomolic acid induces apoptosis and inhibits multidrug resistance protein MRP1 and migration in glioblastoma cells. Oncol. Rep. 2017;38:2525–2534. doi: 10.3892/or.2017.5895. [DOI] [PubMed] [Google Scholar]
- 300.Wang Z.-S., Luo P., Dai S.-H., Liu Z.-B., Zheng X.-R., Chen T. Salvianolic acid B induces apoptosis in human glioma U87 cells through p38-mediated ROS generation. Cell. Mol. Neurobiol. 2013;33:921–928. doi: 10.1007/s10571-013-9958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Fujiwara Y., Shiraishi D., Yoshitomi M., Ikeda T., Mizuta H., Takeya M., Komohara Y. Soyasapogenols contained in soybeans suppress tumour progression by regulating macrophage differentiation into the protumoural phenotype. J. Funct. Foods. 2015;19:594–605. doi: 10.1016/j.jff.2015.09.055. [DOI] [Google Scholar]
- 302.Giacomelli C., Daniele S., Natali L., Iofrida C., Flamini G., Braca A., Trincavelli M.L., Martini C. Carnosol controls the human glioblastoma stemness features through the epithelial-mesenchymal transition modulation and the induction of cancer stem cell apoptosis. Sci. Rep. 2017;7:15174. doi: 10.1038/s41598-017-15360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Giacomelli C., Natali L., Trincavelli M.L., Daniele S., Bertoli A., Flamini G., Braca A., Martini C. New insights into the anticancer activity of carnosol: p53 reactivation in the U87MG human glioblastoma cell line. Int. J. Biochem. Cell Biol. 2016;74:95–108. doi: 10.1016/j.biocel.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 304.Lowe H.I.C., Toyang N.J., Watson C.T., Ayeah K.N., Bryant J. HLBT-100: A highly potent anti-cancer flavanone from Tillandsia recurvata (L.) L. Cancer Cell Int. 2017;17:38. doi: 10.1186/s12935-017-0404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chen Z., Pan X., Georgakilas A.G., Chen P., Hu H., Yang Y., Tian S., Xia L., Zhang J., Cai X., et al. Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits glioma by down regulating chemokine receptor CXCR4 expression. Cancer Lett. 2013;336:281–289. doi: 10.1016/j.canlet.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 306.Chen H.-B., Zhou L.-Z., Mei L., Shi X.-J., Wang X.-S., Li Q.-L., Huang L. Gambogenic acid-induced time- and dose-dependent growth inhibition and apoptosis involving Akt pathway inactivation in U251 glioblastoma cells. J. Nat. Med. 2012;66:62–69. doi: 10.1007/s11418-011-0553-7. [DOI] [PubMed] [Google Scholar]
- 307.Clark P.A., Bhattacharya S., Elmayan A., Darjatmoko S.R., Thuro B.A., Yan M.B., van Ginkel P.R., Polans A.S., Kuo J.S. Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration. J. Neurosurg. 2017;126:1448–1460. doi: 10.3171/2016.1.JNS152077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Liu B., Gao Y.-Q., Wang X.-M., Wang Y.-C., Fu L.-Q. Germacrone inhibits the proliferation of glioma cells by promoting apoptosis and inducing cell cycle arrest. Mol. Med. Rep. 2014;10:1046–1050. doi: 10.3892/mmr.2014.2290. [DOI] [PubMed] [Google Scholar]
- 309.Chen L., Jiang Z., Ma H., Ning L., Chen H., Li L., Qi H. Volatile Oil of Acori Graminei Rhizoma-Induced Apoptosis and Autophagy are dependent on p53 Status in Human Glioma Cells. Sci. Rep. 2016;6:21148. doi: 10.1038/srep21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Miao J., Jiang Y., Wang D., Zhou J., Fan C., Jiao F., Liu B., Zhang J., Wang Y., Zhang Q. Trichosanthin suppresses the proliferation of glioma cells by inhibiting LGR5 expression and the Wnt/β-catenin signaling pathway. Oncol. Rep. 2015;34:2845–2852. doi: 10.3892/or.2015.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Nan Y.-N., Zhu J.-Y., Tan Y., Zhang Q., Jia W., Hua Q. Staurosporine induced apoptosis rapidly downregulates TDP-43 in glioma cells. Asian Pac. J. Cancer Prev. 2014;15:3575–3579. doi: 10.7314/APJCP.2014.15.8.3575. [DOI] [PubMed] [Google Scholar]
- 312.Guerram M., Jiang Z.-Z., Sun L., Zhu X., Zhang L.-Y. Antineoplastic effects of deoxypodophyllotoxin, a potent cytotoxic agent of plant origin, on glioblastoma U-87 MG and SF126 cells. Pharmacol. Rep. 2015;67:245–252. doi: 10.1016/j.pharep.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 313.Wang Y., Tang H., Zhang Y., Li J., Li B., Gao Z., Wang X., Cheng G., Fei Z. Saponin B, a novel cytostatic compound purified from Anemone taipaiensis, induces apoptosis in a human glioblastoma cell line. Int. J. Mol. Med. 2013;32:1077–1084. doi: 10.3892/ijmm.2013.1500. [DOI] [PubMed] [Google Scholar]
- 314.Shu G., Mi X., Cai J., Zhang X., Yin W., Yang X., Li Y., Chen L., Deng X. Brucine, an alkaloid from seeds of Strychnos nux-vomica Linn., represses hepatocellular carcinoma cell migration and metastasis: The role of hypoxia inducible factor 1 pathway. Toxicol. Lett. 2013;222:91–101. doi: 10.1016/j.toxlet.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 315.Hsueh K.-C., Lin C.-L., Tung J.-N., Yang S.-F., Hsieh Y.-H. Nimbolide induced apoptosis by activating ERK-mediated inhibition of c-IAP1 expression in human hepatocellular carcinoma cells. Environ. Toxicol. 2018;33:913–922. doi: 10.1002/tox.22576. [DOI] [PubMed] [Google Scholar]
- 316.Yu X., Wang Y., Tao S., Sun S. Geniposide plays anti-tumor effects by down-regulation of microRNA-224 in HepG2 and Huh7 cell lines. Exp. Mol. Pathol. 2020;112:104349. doi: 10.1016/j.yexmp.2019.104349. [DOI] [PubMed] [Google Scholar]
- 317.Xie X., Zhu H., Yang H., Huang W., Wu Y., Wang Y., Luo Y., Wang D., Shao G. Solamargine triggers hepatoma cell death through apoptosis. Oncol. Lett. 2015;10:168–174. doi: 10.3892/ol.2015.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chen W., Hou J., Yin Y., Jang J., Zheng Z., Fan H., Zou G. α-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFκB. Biochem. Pharmacol. 2010;80:247–254. doi: 10.1016/j.bcp.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 319.Wang X., Sun D., Tai J., Wang L. Ganoderic acid A inhibits proliferation and invasion, and promotes apoptosis in human hepatocellular carcinoma cells. Mol. Med. Rep. 2017;16:3894–3900. doi: 10.3892/mmr.2017.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Law B.Y.K., Mok S.W.F., Chan W.K., Xu S.W., Wu A.G., Yao X.J., Wang J.R., Liu L., Wong V.K.W. Hernandezine, a novel AMPK activator induces autophagic cell death in drug-resistant cancers. Oncotarget. 2016;7:8090–8104. doi: 10.18632/oncotarget.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Li J., Wang H., Wang L., Tan R., Zhu M., Zhong X., Zhang Y., Chen B., Wang L. Decursin inhibits the growth of HepG2 hepatocellular carcinoma cells via Hippo/YAP signaling pathway. Phytother. Res. 2018;32:2456–2465. doi: 10.1002/ptr.6184. [DOI] [PubMed] [Google Scholar]
- 322.Rodenak-Kladniew B., Castro A., Stärkel P., de Saeger C., de Bravo M.G., Crespo R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018;199:48–59. doi: 10.1016/j.lfs.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 323.Wu C.-M., Yang C.-W., Lee Y.-Z., Chuang T.-H., Wu P.-L., Chao Y.-S., Lee S.-J. Tylophorine arrests carcinoma cells at G1 phase by downregulating cyclin A2 expression. Biochem. Biophys. Res. Commun. 2009;386:140–145. doi: 10.1016/j.bbrc.2009.05.138. [DOI] [PubMed] [Google Scholar]
- 324.Ma Z.-J., Lu L., Yang J.-J., Wang X.-X., Su G., Wang Z.-l., Chen G.-h., Sun H.-m., Wang M.-y., Yang Y. Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. Eur. J. Pharmacol. 2018;821:1–10. doi: 10.1016/j.ejphar.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 325.Li N.-N., Meng X.-S., Bao Y.-R., Wang S., Li T.-J. Evidence for the involvement of COX-2/VEGF and PTEN/Pl3K/AKT pathway the mechanism of oroxin B treated liver cancer. Pharmacogn. Mag. 2018;14:207–213. doi: 10.4103/pm.pm_119_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhang Z., Liu T., Yu M., Li K., Li W. The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells. J. Exp. Clin. Cancer Res. 2018;37:7. doi: 10.1186/s13046-018-0678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Thirusangu P., Vigneshwaran V., Avin B.V., Rakesh H., Vikas H.M., Prabhakar B.T. Scutellarein antagonizes the tumorigenesis by modulating cytokine VEGF mediated neoangiogenesis and DFF-40 actuated nucleosomal degradation. Biochem. Biophys. Res. Commun. 2017;484:85–92. doi: 10.1016/j.bbrc.2017.01.067. [DOI] [PubMed] [Google Scholar]
- 328.Zhu Y., Pan Y., Zhang G., Wu Y., Zhong W., Chu C., Qian Y., Zhu G. Chelerythrine inhibits human hepatocellular carcinoma metastasis in vitro. Biol. Pharm. Bull. 2018;41:36–46. doi: 10.1248/bpb.b17-00451. [DOI] [PubMed] [Google Scholar]
- 329.Hu Y.-W., Liu C.-Y., Du C.-M., Zhang J., Wu W.-Q., Gu Z.-L. Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by flavonoids from Astragalus complanatus. J. Ethnopharmacol. 2009;123:293–301. doi: 10.1016/j.jep.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 330.Wu L., Li J., Liu T., Li S., Feng J., Yu Q., Zhang J., Chen J., Zhou Y., Ji J., et al. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019;8:4806–4820. doi: 10.1002/cam4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Chan K.T., Meng F.Y., Li Q., Ho C.Y., Lam T.S., To Y., Lee W.H., Li M., Chu K.H., Toh M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010;294:118–124. doi: 10.1016/j.canlet.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 332.Lin Z.-Y., Wu C.-C., Chuang Y.-H., Chuang W.-L. Anti-cancer mechanisms of clinically acceptable colchicine concentrations on hepatocellular carcinoma. Life Sci. 2013;93:323–328. doi: 10.1016/j.lfs.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 333.Vijayalakshmi A., Sindhu G. Umbelliferone arrest cell cycle at G0/G1 phase and induces apoptosis in human oral carcinoma (KB) cells possibly via oxidative DNA damage. Biomed. Pharmacother. 2017;92:661–671. doi: 10.1016/j.biopha.2017.05.128. [DOI] [PubMed] [Google Scholar]
- 334.Si L., Zheng L., Xu L., Yin L., Han X., Qi Y., Xu Y., Wang C., Peng J. Dioscin suppresses human laryngeal cancer cells growth via induction of cell-cycle arrest and MAPK-mediated mitochondrial-derived apoptosis and inhibition of tumor invasion. Eur. J. Pharmacol. 2016;774:105–117. doi: 10.1016/j.ejphar.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 335.Huang T.-T., Liu F.-G., Wei C.-F., Lu C.-C., Chen C.-C., Lin H.-C., Ojcius D.M., Lai H.-C. Activation of Multiple Apoptotic Pathways in Human Nasopharyngeal Carcinoma Cells by the Prenylated Isoflavone, Osajin. PLoS ONE. 2011;6:e18308. doi: 10.1371/journal.pone.0018308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhang G., Xu Y., Zhou H.-F. Esculetin Inhibits Proliferation, Invasion, and Migration of Laryngeal Cancer In Vitro and In Vivo by Inhibiting Janus Kinas (JAK)-Signal Transducer and Activator of Transcription-3 (STAT3) Activation. Med. Sci. Monit. 2019;25:7853–7863. doi: 10.12659/MSM.916246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Cheng M.-F., Lin C.-S., Chen Y.-H., Sung P.-J., Lin S.-R., Tong Y.-W., Weng C.-F. Inhibitory growth of oral squamous cell carcinoma cancer via bacterial prodigiosin. Mar. Drugs. 2017;15:224. doi: 10.3390/md15070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Deng Q., Yu X., Xiao L., Hu Z., Luo X., Tao Y., Yang L., Liu X., Chen H., Ding Z., et al. Neoalbaconol induces energy depletion and multiple cell death in cancer cells by targeting PDK1-PI3-K/Akt signaling pathway. Cell Death Dis. 2013;4:e804. doi: 10.1038/cddis.2013.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kowshik J., Nivetha R., Ranjani S., Venkatesan P., Selvamuthukumar S., Veeravarmal V., Nagini S. Astaxanthin inhibits hallmarks of cancer by targeting the PI3K/NF-κΒ/STAT3 signalling axis in oral squamous cell carcinoma models. IUBMB Life. 2019;71:1595–1610. doi: 10.1002/iub.2104. [DOI] [PubMed] [Google Scholar]
- 340.Yeh C.-M., Hsieh M.-J., Yang J.-S., Yang S.-F., Chuang Y.-T., Su S.-C., Liang M.-Y., Chen M.-K., Lin C.-W. Geraniin inhibits oral cancer cell migration by suppressing matrix metalloproteinase-2 activation through the FAK/Src and ERK pathways. Environ. Toxicol. 2019;34:1085–1093. doi: 10.1002/tox.22809. [DOI] [PubMed] [Google Scholar]
- 341.He J., Wei W., Yang Q., Wang Y. Phillygenin Exerts In Vitro and In Vivo Antitumor Effects in Drug-Resistant Human Esophageal Cancer Cells by Inducing Mitochondrial-Mediated Apoptosis, ROS Generation, and Inhibition of the Nuclear Factor kappa B NF-κB Signalling Pathway. Med. Sci. Monit. 2019;25:739–745. doi: 10.12659/MSM.913138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 342.Wu G., Chen G., Zhou J., Zhu H., Chu J., Zhang F. Liriodenine enhances radiosensitivity in esophageal cancer ECA-109 cells by inducing apoptosis and G2/M arrest. Oncol. Lett. 2018;16:5020–5026. doi: 10.3892/ol.2018.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lin C.-W., Bai L.-Y., Su J.-H., Chiu C.-F., Lin W.-Y., Huang W.-T., Shih M.-C., Huang Y.-T., Hu J.-L., Weng J.-R. Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells. Biomedicines. 2020;8:296. doi: 10.3390/biomedicines8090296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rizo W.F., Ferreira L.E., Colnaghi V., Martins J.S., Franchi L.P., Takahashi C.S., Beleboni R.O., Marins M., Pereira P.S., Fachin A.L. Cytotoxicity and genotoxicity of coronaridine from Tabernaemontana catharinensis A. DC in a human laryngeal epithelial carcinoma cell line (Hep-2) Genet. Mol. Biol. 2013;36:105–110. doi: 10.1590/S1415-47572013005000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Herrmann R., Skaf J., Roller J., Polednik C., Holzgrabe U., Schmidt M. Anticancer effects of NSC-631570 (Ukrain) in head and neck cancer cells: In vitro analysis of growth, invasion, angiogenesis and gene expression. Oncol. Rep. 2020;43:282–295. doi: 10.3892/or.2019.7416. [DOI] [PubMed] [Google Scholar]
- 346.Chinni S.R., Li Y., Upadhyay S., Koppolu P.K., Sarkar F.H. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 347.Banerjee S., Li Y., Wang Z., Sarkar F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Chiyomaru T., Yamamura S., Fukuhara S., Yoshino H., Kinoshita T., Majid S., Saini S., Chang I., Tanaka Y., Enokida H., et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS ONE. 2013;8:e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Bell C., Hawthorne S. Ellagic acid, pomegranate and prostate cancer—A mini review. J. Pharm. Pharmacol. 2008;60:139–144. doi: 10.1211/jpp.60.2.0001. [DOI] [PubMed] [Google Scholar]
- 350.Eskandari E., Heidarian E., Amini S., Saffari-Chaleshtori J. Evaluating the effects of ellagic acid on pSTAT3, pAKT, and pERK1/2 signaling pathways in prostate cancer PC3 cells. J. Cancer Res. Ther. 2016;12:1266–1271. doi: 10.4103/0973-1482.165873. [DOI] [PubMed] [Google Scholar]
- 351.Lee H.-S., Safe S., Lee S.-O. Inactivation of the orphan nuclear receptor NR4A1 contributes to apoptosis induction by fangchinoline in pancreatic cancer cells. Toxicol. Appl. Pharmacol. 2017;332:32–39. doi: 10.1016/j.taap.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 352.Zhou J., Feng J.-H., Fang L. A novel monoterpenoid indole alkaloid with anticancer activity from Melodinus khasianus. Bioorg. Med. Chem. Lett. 2017;27:893–896. doi: 10.1016/j.bmcl.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 353.Dilshara M.G., Jayasooriya R.G.P.T., Choi Y.H., Kim G.-Y. Camptothecin induces c-Myc- and Sp1-mediated hTERT expression in LNCaP cells: Involvement of reactive oxygen species and PI3K/Akt. Food Chem. Toxicol. 2019;127:53–60. doi: 10.1016/j.fct.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 354.Forestier-Román I.S., López-Rivas A., Sánchez-Vázquez M.M., Rohena-Rivera K., Nieves-Burgos G., Ortiz-Zuazaga H., Torres-Ramos C.A., Martínez-Ferrer M. Andrographolide induces DNA damage in prostate cancer cells. Oncotarget. 2019;10:1085–1101. doi: 10.18632/oncotarget.26628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Nie C., Zhou J., Qin X., Shi X., Zeng Q., Liu J., Yan S., Zhang L. Diosgenin-induced autophagy and apoptosis in a human prostate cancer cell line. Mol. Med. Rep. 2016;14:4349–4359. doi: 10.3892/mmr.2016.5750. [DOI] [PubMed] [Google Scholar]
- 356.Agarwal S., Amin K.S., Jagadeesh S., Baishay G., Rao P.G., Barua N.C., Bhattacharya S., Banerjee P.P. Mahanine restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in prostate cancer cells. Mol. Cancer. 2013;12:99. doi: 10.1186/1476-4598-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Shang X.J., Yao G., Ge J.P., Sun Y., Teng W.H., Huang Y.F. Procyanidin Induces Apoptosis and Necrosis of Prostate Cancer Cell Line PC-3 in a Mitochondrion-Dependent Manner. J. Androl. 2009;30:122–126. doi: 10.2164/jandrol.108.005629. [DOI] [PubMed] [Google Scholar]
- 358.Bommareddy A., McGlynn D., Lewis M., Lockus L., Seward J., Hong K.L., VanWert A.L., Dwivedi C. Akt/survivin pathway inhibition enhances the apoptotic cell death-induced by alpha-santalol in human prostate cancer cells. Fitoterapia. 2020;143:104552. doi: 10.1016/j.fitote.2020.104552. [DOI] [PubMed] [Google Scholar]
- 359.Jiang C., Masood M., Rasul A., Wei W., Wang Y., Ali M., Mustaqeem M., Li J., Li X. Altholactone Inhibits NF-κB and STAT3 Activation and Induces Reactive Oxygen Species-Mediated Apoptosis in Prostate Cancer DU145 Cells. Molecules. 2017;22:240. doi: 10.3390/molecules22020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Liew S.Y., Looi C.Y., Paydar M., Cheah F.K., Leong K.H., Wong W.F., Mustafa M.R., Litaudon M., Awang K. Subditine, a New Monoterpenoid Indole Alkaloid from Bark of Nauclea subdita (Korth.) Steud. Induces Apoptosis in Human Prostate Cancer Cells. PLoS ONE. 2014;9:e87286. doi: 10.1371/journal.pone.0087286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Yu Z., Chen Y., Liang C. Eriocalyxin B induces apoptosis and autophagy involving akt/mammalian target of rapamycin (mTOR) pathway in prostate cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019;25:8534–8543. doi: 10.12659/MSM.917333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wang Y., Tsai M.-L., Chiou L.-Y., Ho C.-T., Pan M.-H. Antitumor Activity of Garcinol in Human Prostate Cancer Cells and Xenograft Mice. J. Agric. Food Chem. 2015;63:9047–9052. doi: 10.1021/acs.jafc.5b03851. [DOI] [PubMed] [Google Scholar]
- 363.Ghasemi S., Lorigooini Z., Wibowo J., Amini-khoei H. Tricin isolated from Allium atroviolaceum potentiated the effect of docetaxel on PC3 cell proliferation: Role of miR-21. Nat. Prod. Res. 2019;33:1828–1831. doi: 10.1080/14786419.2018.1437439. [DOI] [PubMed] [Google Scholar]
- 364.Xu F., Li Q., Wang Z., Cao X. Sinomenine inhibits proliferation, migration, invasion and promotes apoptosis of prostate cancer cells by regulation of miR-23a. Biomed. Pharmacother. 2019;112:108592. doi: 10.1016/j.biopha.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 365.Wu X.-H., Zhou H.-J., Lee J. Dihydroartemisinin inhibits angiogenesis induced by multiple myeloma RPMI8226 cells under hypoxic conditions via downregulation of vascular endothelial growth factor expression and suppression of vascular endothelial growth factor secretion. Anti-Cancer Drugs. 2006;17:839–848. doi: 10.1097/01.cad.0000224443.85834.32. [DOI] [PubMed] [Google Scholar]
- 366.Liao N.-C., Shih Y.-L., Chou J.-S., Chen K.-W., Chen Y.-L., Lee M.-H., Peng S.-F., Leu S.-J., Chung J.-G. Cardamonin induces cell cycle arrest, apoptosis and alters apoptosis associated gene expression in WEHI-3 mouse leukemia cells. Am. J. Chin. Med. 2019;47:635–656. doi: 10.1142/S0192415X19500332. [DOI] [PubMed] [Google Scholar]
- 367.Martins C., Doran C., Silva I.C., Miranda C., Rueff J., Rodrigues A.S. Myristicin from nutmeg induces apoptosis via the mitochondrial pathway and down regulates genes of the DNA damage response pathways in human leukaemia K562 cells. Chem.-Biol. Interact. 2014;218:1–9. doi: 10.1016/j.cbi.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 368.Wang Y., Zhu X.-F., Xiao Z.-J., Wang H.-H., Zhou J.-M., Mei Y.-P., Deng R., Jiang W.-Q., Liu Z.-C. Inducement effect of Meisoindigo on apoptosis of leukemia cell line HL-60 and its mechanism. Ai Zheng Chin. J. Cancer. 2005;24:1464–1468. [PubMed] [Google Scholar]
- 369.Kluza J., Mazinghien R., Degardin K., Lansiaux A., Bailly C. Induction of apoptosis by the plant alkaloid sampangine in human HL-60 leukemia cells is mediated by reactive oxygen species. Eur. J. Pharmacol. 2005;525:32–40. doi: 10.1016/j.ejphar.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 370.Saeed M., Jacob S., Sandjo L.P., Sugimoto Y., Khalid H.E., Opatz T., Thines E., Efferth T. Cytotoxicity of the Sesquiterpene Lactones Neoambrosin and Damsin from Ambrosia maritima Against Multidrug-Resistant Cancer Cells. Front. Pharmacol. 2015;6:267. doi: 10.3389/fphar.2015.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Sepporta M.V., Mazza T., Morozzi G., Fabiani R. Pinoresinol Inhibits Proliferation and Induces Differentiation on Human HL60 Leukemia Cells. Nutr. Cancer. 2013;65:1208–1218. doi: 10.1080/01635581.2013.828089. [DOI] [PubMed] [Google Scholar]
- 372.Gaboriaud-Kolar N., Myrianthopoulos V., Vougogiannopoulou K., Gerolymatos P., Horne D.A., Jove R., Mikros E., Nam S., Skaltsounis A.-L. Natural-based indirubins display potent cytotoxicity toward wild-type and t315i-resistant leukemia cell lines. J. Nat. Prod. 2016;79:2464–2471. doi: 10.1021/acs.jnatprod.6b00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hoffmann R., von Schwarzenberg K., López-Antón N., Rudy A., Wanner G., Dirsch V.M., Vollmar A.M. Helenalin bypasses Bcl-2-mediated cell death resistance by inhibiting NF-κB and promoting reactive oxygen species generation. Biochem. Pharmacol. 2011;82:453–463. doi: 10.1016/j.bcp.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 374.Karmahapatra S., Kientz C., Shetty S., Yalowich J.C., Rakotondraibe L.H. Capsicodendrin from Cinnamosma fragrans Exhibits Antiproliferative and Cytotoxic Activity in Human Leukemia Cells: Modulation by Glutathione. J. Nat. Prod. 2018;81:625–629. doi: 10.1021/acs.jnatprod.7b00887. [DOI] [PubMed] [Google Scholar]
- 375.Li C., Dong L., Su R., Bi Y., Qing Y., Deng X., Zhou Y., Hu C., Yu M., Huang H., et al. Homoharringtonine exhibits potent anti-tumor effect and modulates DNA epigenome in acute myeloid leukemia by targeting SP1/TET1/5hmC. Haematolgica. 2020;105:148–160. doi: 10.3324/haematol.2018.208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Sung B., Ahn K.S., Aggarwal B.B. Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-κB signaling pathway. Cancer Res. 2010;70:3259–3268. doi: 10.1158/0008-5472.CAN-09-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Yusenko M.V., Trentmann A., Andersson M.K., Ghani L.A., Jakobs A., Arteaga Paz M.-F.A., Mikesch J.-H., von Kries J.P., Stenman G., Klempnauer K.-H. Monensin, a novel potent MYB inhibitor, suppresses proliferation of acute myeloid leukemia and adenoid cystic carcinoma cells. Cancer Lett. 2020;479:61–70. doi: 10.1016/j.canlet.2020.01.039. [DOI] [PubMed] [Google Scholar]
- 378.Issa M.E., Berndt S., Carpentier G., Pezzuto J.M., Cuendet M. Bruceantin inhibits multiple myeloma cancer stem cell proliferation. Cancer Biol. Ther. 2016;17:966–975. doi: 10.1080/15384047.2016.1210737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Alachkar H., Santhanam R., Harb J.G., Lucas D.M., Oaks J.J., Hickey C.J., Pan L., Kinghorn A.D., Caligiuri M.A., Perrotti D., et al. Silvestrol exhibits significant in vivo and in vitro antileukemic activities and inhibits FLT3 and miR-155 expressions in acute myeloid leukemia. J. Hematol. Oncol. 2013;6:21. doi: 10.1186/1756-8722-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Li Y., Gong L., Qi R., Sun Q., Xia X., He H., Ren J., Zhu O., Zhuo D. Paeoniflorin suppresses pancreatic cancer cell growth by upregulating HTRA3 expression. Drug Des. Dev. Ther. 2017;11:2481–2491. doi: 10.2147/DDDT.S134518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Shan B., Wang L., Lu A., Liu X., Sang M., Meng F., Cao Q., Ji X. The flavonoid Baohuoside-I inhibits cell growth and downregulates survivin and cyclin D1 expression in esophageal carcinoma via β-catenin-dependent signaling. Oncol. Rep. 2011;26:1149–1156. doi: 10.3892/or.2011.1400. [DOI] [PubMed] [Google Scholar]
- 382.Kim J.Y., Lee S.G., Chung J.-Y., Kim Y.-J., Park J.-E., Koh H., Han M.S., Park Y.C., Yoo Y.H., Kim J.-M. Ellipticine induces apoptosis in human endometrial cancer cells: The potential involvement of reactive oxygen species and mitogen-activated protein kinases. Toxicology. 2011;289:91–102. doi: 10.1016/j.tox.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 383.Yang H., Liu C., Zhang Y.-q., Ge L.-t., Chen J., Jia X.-q., Gu R.-x., Sun Y., Sun W.-d. Ilexgenin A induces B16-F10 melanoma cell G1/S arrest in vitro and reduces tumor growth in vivo. Int. Immunopharmacol. 2015;24:423–431. doi: 10.1016/j.intimp.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 384.Saisomboon S., Kariya R., Vaeteewoottacharn K., Wongkham S., Sawanyawisuth K., Okada S. Antitumor effects of flavopiridol, a cyclin-dependent kinase inhibitor, on human cholangiocarcinoma in vitro and in an in vivo xenograft model. Heliyon. 2019;5:e01675. doi: 10.1016/j.heliyon.2019.e01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Khumkhrong P., Piboonprai K., Chaichompoo W., Pimtong W., Khongkow M., Namdee K., Jantimaporn A., Japrung D., Asawapirom U., Suksamrarn A., et al. Crinamine Induces Apoptosis and Inhibits Proliferation, Migration, and Angiogenesis in Cervical Cancer SiHa Cells. Biomolecules. 2019;9:494. doi: 10.3390/biom9090494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Du J., Tang B., Wang J., Sui H., Jin X., Wang L., Wang Z. Antiproliferative effect of alpinetin in BxPC-3 pancreatic cancer cells. Int. J. Mol. Med. 2012;29:607–612. doi: 10.3892/ijmm.2012.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Liang W., Lai Y., Zhu M., Huang S., Feng W., Gu X. Combretastatin A4 Regulates Proliferation, Migration, Invasion, and Apoptosis of Thyroid Cancer Cells via PI3K/Akt Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016;22:4911–4917. doi: 10.12659/MSM.898545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Appadurai P., Rathinasamy K. Indicine N-oxide binds to tubulin at a distinct site and inhibits the assembly of microtubules: A mechanism for its cytotoxic activity. Toxicol. Lett. 2014;225:66–77. doi: 10.1016/j.toxlet.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 389.Zheng Q., Li Q., Zhao G., Zhang J., Yuan H., Gong D., Guo Y., Liu X., Li K., Lin P. Alkannin induces cytotoxic autophagy and apoptosis by promoting ROS-mediated mitochondrial dysfunction and activation of JNK pathway. Biochem. Pharmacol. 2020;180:114167. doi: 10.1016/j.bcp.2020.114167. [DOI] [PubMed] [Google Scholar]
- 390.Lee J.-G., Kim J.-H., Ahn J.-H., Lee K.-T., Baek N.-I., Choi J.-H. Jaceosidin, isolated from dietary mugwort (Artemisia princeps), induces G2/M cell cycle arrest by inactivating cdc25C-cdc2 via ATM-Chk1/2 activation. Food Chem. Toxicol. 2013;55:214–221. doi: 10.1016/j.fct.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 391.Kim T.W., Lee S.Y., Kim M., Cheon C., Ko S.-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9:875. doi: 10.1038/s41419-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Fukuda H., Nakamura S., Chisaki Y., Takada T., Toda Y., Murata H., Itoh K., Yano Y., Takata K., Ashihara E. Daphnetin inhibits invasion and migration of LM8 murine osteosarcoma cells by decreasing RhoA and Cdc42 expression. Biochem. Biophys. Res. Commun. 2016;471:63–67. doi: 10.1016/j.bbrc.2016.01.179. [DOI] [PubMed] [Google Scholar]
- 393.Hossan M.S., Chan Z.-Y., Collins H.M., Shipton F.N., Butler M.S., Rahmatullah M., Lee J.B., Gershkovich P., Kagan L., Khoo T.-J., et al. Cardiac glycoside cerberin exerts anticancer activity through PI3K/AKT/mTOR signal transduction inhibition. Cancer Lett. 2019;453:57–73. doi: 10.1016/j.canlet.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 394.Deng X., Sheng J., Liu H., Wang N., Dai C., Wang Z., Zhang J., Zhao J., Dai E. Cinobufagin Promotes Cell Cycle Arrest and Apoptosis to Block Human Esophageal Squamous Cell Carcinoma Cells Growth via the p73 Signalling Pathway. Biol. Pharm. Bull. 2019;42:1500–1509. doi: 10.1248/bpb.b19-00174. [DOI] [PubMed] [Google Scholar]
- 395.Ma J., Wang L., Li J., Zhang G., Tao H., Li X., Sun D., Hu Y. Swainsonine Inhibits Invasion and the EMT Process in Esophageal Carcinoma Cells by Targeting Twist1. Oncol. Res. 2018;26:1207–1213. doi: 10.3727/096504017X15046134836575. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 396.Lu C.-H., Chen S.-H., Chang Y.-S., Liu Y.-W., Wu J.-Y., Lim Y.-P., Yu H.-I., Lee Y.-R. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol. Res. 2017;115:288–298. doi: 10.1016/j.phrs.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 397.Dong Y., Cao A., Shi J., Yin P., Wang L., Ji G., Xie J., Wu D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014;31:1788–1794. doi: 10.3892/or.2014.3034. [DOI] [PubMed] [Google Scholar]
- 398.Rabi T., Catapano C.V. Aphanin, a triterpenoid from Amoora rohituka inhibits K-Ras mutant activity and STAT3 in pancreatic carcinoma cells. Tumor Biol. 2016;37:12455–12464. doi: 10.1007/s13277-016-5102-2. [DOI] [PubMed] [Google Scholar]
- 399.Pelinson L.P., Assmann C.E., Palma T.V., da Cruz I.B.M., Pillat M.M., Mânica A., Stefanello N., Weis G.C.C., de Oliveira Alves A., de Andrade C.M. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019;46:2085–2092. doi: 10.1007/s11033-019-04658-1. [DOI] [PubMed] [Google Scholar]
- 400.Mansingh D.P., OJ S., Sali V.K., Vasanthi H.R. [6]-Gingerol–induced cell cycle arrest, reactive oxygen species generation, and disruption of mitochondrial membrane potential are associated with apoptosis in human gastric cancer (AGS) cells. J. Biochem. Mol. Toxicol. 2018;32:e22206. doi: 10.1002/jbt.22206. [DOI] [PubMed] [Google Scholar]
- 401.Yong W.K., Abd Malek S.N. Xanthohumol Induces Growth Inhibition and Apoptosis in Ca Ski Human Cervical Cancer Cells. Evid.-Based Complement. Altern. Med. 2015;2015:921306. doi: 10.1155/2015/921306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.An F., Wang S., Tian Q., Zhu D. Effects of orientin and vitexin from Trollius chinensis on the growth and apoptosis of esophageal cancer EC-109 cells. Oncol. Lett. 2015;10:2627–2633. doi: 10.3892/ol.2015.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Yang L., Yu Y., Zhang Q., Li X., Zhang C., Mao T., Liu S., Tian Z. Anti-gastric cancer effect of Salidroside through elevating miR-99a expression. Artif. Cells Nanomed. Biotechnol. 2019;47:3500–3510. doi: 10.1080/21691401.2019.1652626. [DOI] [PubMed] [Google Scholar]
- 404.Júnior P.L.d.S., Câmara D.A.D., Costa A.S., Ruiz J.L.M., Levy D., Azevedo R.A., Pasqualoto K.F.M., de Oliveira C.F., de Melo T.C., Pessoa N.D.S., et al. Apoptotic effect of eugenol envolves G2/M phase abrogation accompanied by mitochondrial damage and clastogenic effect on cancer cell in vitro. Phytomedicine. 2016;23:725–735. doi: 10.1016/j.phymed.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 405.Kuete V., Sandjo L.P., Seukep J.A., Zeino M., Mbaveng A.T., Ngadjui B., Efferth T. Cytotoxic compounds from the fruits of Uapaca togoensis towards multifactorial drug-resistant cancer cells. Planta Med. 2015;81:32–38. doi: 10.1055/s-0034-1383362. [DOI] [PubMed] [Google Scholar]
- 406.Li C., Wang Y., Wang C., Yi X., Li M., He X. Anticancer activities of harmine by inducing a pro-death autophagy and apoptosis in human gastric cancer cells. Phytomedicine. 2017;28:10–18. doi: 10.1016/j.phymed.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 407.He L., Wu Y., Lin L., Wang J., Wu Y., Chen Y., Yi Z., Liu M., Pang X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011;102:219–225. doi: 10.1111/j.1349-7006.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 408.Edler M.C., Fernandez A.M., Lassota P., Ireland C.M., Barrows L.R. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the vinca alkaloids, and dolastatin 10. Biochem. Pharmacol. 2002;63:707–715. doi: 10.1016/S0006-2952(01)00898-X. [DOI] [PubMed] [Google Scholar]
- 409.Zou P., Xia Y., Ji J., Chen W., Zhang J., Chen X., Rajamanickam V., Chen G., Wang Z., Chen L., et al. Piperlongumine as a direct TrxR1 inhibitor with suppressive activity against gastric cancer. Cancer Lett. 2016;375:114–126. doi: 10.1016/j.canlet.2016.02.058. [DOI] [PubMed] [Google Scholar]
- 410.Tian B., Xiao Y., Ma J., Ou W., Wang H., Wu J., Tang J., Zhang B., Liao X., Yang D., et al. Parthenolide Inhibits Angiogenesis in Esophageal Squamous Cell Carcinoma Through Suppression of VEGF. OncoTargets Ther. 2020;13:7447–7458. doi: 10.2147/OTT.S256291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zhao Z., Jia Q., Wu M.-S., Xie X., Wang Y., Song G., Zou C.-Y., Tang Q., Lu J., Huang G., et al. Degalactotigonin, a natural compound from Solanum nigrum L., inhibits growth and metastasis of osteosarcoma through GSK3β inactivation—Mediated repression of the Hedgehog/Gli1 pathway. Clin. Cancer Res. 2018;24:130–144. doi: 10.1158/1078-0432.CCR-17-0692. [DOI] [PubMed] [Google Scholar]
- 412.Kashyap D., Tuli H.S., Yerer M.B., Sharma A., Sak K., Srivastava S., Pandey A., Garg V.K., Sethi G., Bishayee A. Seminars in Cancer Biology. Academic Press; Cambridge, MA, USA: 2019. Natural Product-Based Nanoformulations for Cancer Therapy: Opportunities and Challenges. [DOI] [PubMed] [Google Scholar]
- 413.Srinivasan M., Rajabi M., Mousa S.A. Nanobiomaterials in Cancer Therapy. Elsevier; Amsterdam, The Netherlands: 2016. Nanobiomaterials in Cancer Therapy; pp. 57–89. [Google Scholar]
- 414.Mousa D.S., El-Far A.H., Saddiq A.A., Sudha T., Mousa S.A. Nanoformulated Bioactive Compounds Derived from Different Natural Products Combat Pancreatic Cancer Cell Proliferation. Int. J. Nanomed. 2020;15:2259–2268. doi: 10.2147/IJN.S238256. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 415.Andima M., Costabile G., Isert L., Ndakala A.J., Derese S., Merkel O.M. Evaluation of β-Sitosterol loaded PLGA and PEG-PLA nanoparticles for effective treatment of breast cancer: Preparation, physicochemical characterization, and antitumor activity. Pharmaceutics. 2018;10:232. doi: 10.3390/pharmaceutics10040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Feng J., Xu M., Wang J., Zhou S., Liu Y., Liu S., Huang Y., Chen Y., Chen L., Song Q., et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials. 2020;241:119907. doi: 10.1016/j.biomaterials.2020.119907. [DOI] [PubMed] [Google Scholar]
- 417.Liu Y., Gao D., Zhang X., Liu Z., Dai K., Ji B., Wang Q., Luo L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C. 2016;64:124–132. doi: 10.1016/j.msec.2016.03.080. [DOI] [PubMed] [Google Scholar]
- 418.Liu Y., Zhao L., Shen G., Chang R., Zhang Y., Yan X. Coordination self-assembly of natural flavonoids into robust nanoparticles for enhanced in vitro chemo and photothermal cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020;598:124805. doi: 10.1016/j.colsurfa.2020.124805. [DOI] [Google Scholar]
- 419.Ahmadi E., Zarghami N., Jafarabadi M.A., Alizadeh L., Khojastehfard M., Yamchi M.R., Salehi R. Enhanced anticancer potency by combination chemotherapy of HT-29 cells with biodegradable, pH-sensitive nanoparticles for co-delivery of hydroxytyrosol and doxorubicin. J. Drug Deliv. Sci. Technol. 2019;51:721–735. doi: 10.1016/j.jddst.2019.03.003. [DOI] [Google Scholar]
- 420.Abdelaziz H.M., Elzoghby A.O., Helmy M.W., Samaha M.W., Fang J.-Y., Freag M.S. Liquid crystalline assembly for potential combinatorial chemo–herbal drug delivery to lung cancer cells. Int. J. Nanomed. 2019;14:499–517. doi: 10.2147/IJN.S188335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Xia Q., Ling L., Ismail M., Du Y., He W., Zhou W., Yao C., Li X. Paclitaxel encapsulated in artesunate-phospholipid liposomes for combinatorial delivery. J. Drug Deliv. Sci. Technol. 2019;51:372–382. doi: 10.1016/j.jddst.2019.03.010. [DOI] [Google Scholar]
- 422.Wang S., Shao M., Zhong Z., Wang A., Cao J., Lu Y., Wang Y., Zhang J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017;24:1791–1800. doi: 10.1080/10717544.2017.1406558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Bian Y., Guo D. Targeted Therapy for Hepatocellular Carcinoma: Co-Delivery of Sorafenib and Curcumin Using Lactosylated pH-Responsive Nanoparticles. Drug Des. Dev. Ther. 2020;14:647. doi: 10.2147/DDDT.S238955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wang D., Zhou J., Chen R., Shi R., Zhao G., Xia G., Li R., Liu Z., Tian J., Wang H., et al. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials. 2016;100:27–40. doi: 10.1016/j.biomaterials.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 425.Wang J., Muhammad N., Li T., Wang H., Liu Y., Liu B., Zhan H. Hyaluronic Acid-Coated Camptothecin Nanocrystals for Targeted Drug Delivery to Enhance Anticancer Efficacy. Mol. Pharm. 2020;17:2411–2425. doi: 10.1021/acs.molpharmaceut.0c00161. [DOI] [PubMed] [Google Scholar]
- 426.Gupta L., Sharma A.K., Gothwal A., Khan M.S., Khinchi M.P., Qayum A., Singh S.K., Gupta U. Dendrimer encapsulated and conjugated delivery of berberine: A novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int. J. Pharm. 2017;528:88–99. doi: 10.1016/j.ijpharm.2017.04.073. [DOI] [PubMed] [Google Scholar]
- 427.Ding J., Liang T., Min Q., Jiang L.-P., Zhu J.-J. “Stealth and Fully-Laden” Drug Carriers: Self-Assembled Nanogels Encapsulated with Epigallocatechin Gallate and siRNA for Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces. 2018;10:9938–9948. doi: 10.1021/acsami.7b19577. [DOI] [PubMed] [Google Scholar]
- 428.Amaral R., dos Santos S.A., Andrade L.N., Severino P., Carvalho A.A. Natural Products as Treatment against Cancer: A Historical and Current Vision. Clin. Oncol. 2019;4:1562. [Google Scholar]
- 429.Mita A., Lockhart A.C., Chen T.-L., Bochinski K., Curtright J., Cooper W., Hammond L., Rothenberg M., Rowinsky E., Sharma S. A phase I pharmacokinetic (PK) trial of XAA296A (Discodermolide) administered every 3 wks to adult patients with advanced solid malignancies. J. Clin. Oncol. 2004;22:2025. doi: 10.1200/jco.2004.22.90140.2025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.