Abstract
Purpose
The effect and safety of Semaglutide and Liraglutide on weight loss in people with obesity or overweight were evaluated by a Network Meta-Analysis system to provide an evidence-based reference for clinical treatment.
Methods
Computer searched PubMed, Embase, and Cochrane Library databases to collect Liraglutide and Semaglutide injection monotherapy RCTs until April 2022, using Stata 16 software for Network Meta-Analysis.
Results
Twenty-three RCTs study with 11,545 patients and 4 interventions (semaglutide 2.4mg, semaglutide 1.0mg, liraglutide 3.0mg and liraglutide 1.8 mg) were finally included. In terms of efficacy, semaglutide 2.4mg (−12.47 kg) had the best weight loss, followed by liraglutide 3.0mg (−5.24 kg), semaglutide 1.0mg (−3.74 kg) and liraglutide 1.8mg (−3.29 kg). In terms of decreased HbA1c, semaglutide 2.4mg (MD=−1.48%, 95% CI [−1.93, −1.04]), semaglutide 1.0mg (MD=−1.36%, 95% CI [−1.72, −1.01]), liraglutide 1.8mg (MD=−1.23%, 95%Cl [−1.66, −0.80]) more effective than placebo. In terms of safety, the total incidence of adverse events was semaglutide 2.4mg > liraglutide 3.0mg > liraglutide 1.8mg > semaglutide 1.0mg compare to placebo, the incidence of serious adverse events was liraglutide 3.0mg > liraglutide 1.8mg > semaglutide 2.4mg > semaglutide 1.0mg, the incidence of hypoglycemic events was semaglutide 2.4mg > liraglutide 3.0mg > semaglutide 1.0mg > liraglutide 1.8mg.
Conclusion
This meta-analysis indicates that all GLP-1RAs were more efficacious than placebo in people with obesity or overweight on efficacy. Semaglutide 2.4mg has an absolute advantage in weight loss and decreased HbA1c, but the incidence of total adverse events is also the highest and can cause hypoglycemia. In addition, although liraglutide 3.0mg was less effective than semaglutide 2.4mg, serious adverse events were still the most elevated.
Keywords: glucagon-like peptide-1 receptor agonists, weight loss, systematic review, liraglutide, semaglutide
Introduction
Obesity is a chronic disease with serious health consequences; it can lead to insulin resistance, hypertension, and dyslipidemia, associated with complications such as type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease, and reduce life expectancy.1–4 Recently, obesity has been associated with increased hospitalizations, the need for mechanical ventilation, and death in patients with coronavirus disease 2019 (Covid-19).5,6 Weight loss of 5% to 10% has been shown to reduce obesity-related complications and improve quality of life.7,8 However, it is difficult to maintain weight loss with lifestyle interventions alone.9 Clinical guidelines recommend adjunctive medical therapy, especially for adults with a BMI of 30 or higher or those with comorbidities of 27 or higher.10 glucagon-like peptide-1 receptor agonists (GLP-1RA) activate GLP-1 receptors by mimicking natural GLP-1, enhance insulin secretion, inhibit glucagon secretion in a glucose concentration-dependent manner, and can delay gastric emptying, reducing food intake through central appetite suppression not only has the effect of lowering blood sugar, but also has the effect of weight loss.11 The purpose of this study was based on the 2 GLP-1RA drugs (Liraglutide and Semaglutide) recommended in the diabetes prevention and control guidelines issued by the American Diabetes Association in 202212 and approved by the Food and Drug Administration (FDA), using Network Meta-Analysis (NMA) to objectively evaluate the weight loss effect and safety of subcutaneous injection of liraglutide and semaglutide in people with obesity or overweight, and provide evidence-based medical evidence for clinical practice.
Methods
Registration
The Preferred Reporting Items report this systematic review and NMA for Systematic Reviews and Meta‐Analyses (PRISMA) statement. The study protocol was registered (registration number: CRD42022345166) with the International Prospective Register of Systematic Reviews (PROSPERO).
Data Source
Computer search PubMed, Cochrane Library, Embase database, the search time limit is from the establishment of the database to April 2022, and use the combination of subject headings and free words. Search subject terms include: Weight Loss, Glucagon-Like Peptide 1, Liraglutide, Semaglutide, Randomized Controlled Trial (RCT). Free words include: free words corresponding to subject words in the database. We also searched ClinicalTrials.gov for (unpublished) completed trials, the result of search strategy is shown in Supplementary File 1.
Inclusion and Exclusion Criteria
Inclusion Criteria
The type of study was an RCT, the language was limited to English, the subjects were body mass index (BMI) ≥25, age ≥18, no gender or race, with or without type 2 diabetes, the monotherapy cycle was ≥20 weeks (starting dose + maintenance dose), control group interventions are placebo, other GLP-1RA or other hypoglycemic drugs (such as sitagliptin, glimepiride, etc.), and provide information on any pre-specified primary, secondary and safety endpoints. Exclusion criteria: repeated publications, animal experiments, non-randomized controlled trials, literature for which data could not be extracted or downloaded, conference articles, review, combination or non-single administration of other hypoglycemic drugs, non-subcutaneous injection.
Outcome Indicators
Efficacy outcomes were body weight (primary) and Hemoglobin A1c (HbA1c), change from baseline to study endpoint (drug efficacy = endpoint value - baseline value). Safety outcomes were the number of total adverse events, serious adverse events, and hypoglycemia events.
Literature Screening and Data Extraction
After the literature search, we used Note Express software to eliminate duplicate publications and incomplete documents. The literature was screened according to the title and abstract, and reading the full text to determine the final included literature, extracted the data of the included literature. Two investigators independently performed data according to predetermined criteria for study selection and data extraction, and a third investigator resolved conflicting data. The extracted data includes the following information: (1) Basic information of included studies: title, author, year of publication, gender, age, average body mass index, HbA1c (%). (2) Type and content of the study: duration of treatment, total number of people included in the study. (3) Intervention measures: (the treatment methods of the experimental group and the control group). (4) Outcome indicators (weight change, number of adverse events, number of serious adverse reactions, and amount of hypoglycemia in each treatment group). If a study is published more than once, we will include the most informative and data-complete study.
Risk of Bias Assessment and Analysis of Data
The risk of bias for included studies was independently assessed by two investigators using the software Review Manager 5.4.1 according to the criteria of the Cochrane Risk of Bias Assessment Tool. A third investigator resolved the discrepancies when differences in data assessment occurred. Frequentist NMA was performed using the software Stata 16.1. For dichotomous variables, the odds ratio (OR) was used to calculate. For continuous variables, the mean difference (MD) was used to calculate, and each effect size was expressed as a 95% confidence interval (95%-CI). For the continuous variable weight loss(kg) and HbA1c (%), the negative value indicated a reduction with treatment, and the smaller the negative value, the more favorable it was. For the dichotomous variable (number of occurrences of adverse events), a higher value means more occurrences of adverse events and worse results. For each outcome measure, we used the surface under the cumulative ranking (SUCRA) to predict and rank the efficacy or safety of each treatment, and the results were expressed as percentages. Finally, we test the consistency of the entire Network Meta, including the inconsistency test between the overall, local, and closed loops (P < 0.05 indicates a significant difference), draw network plots, funnel plots of outcome indicators, risk of publication bias plots for evaluating the included literature. A sensitivity analysis is necessary if the included studies are high risk.
Results
Inclusion Process and Basic Characteristics of Research Literature
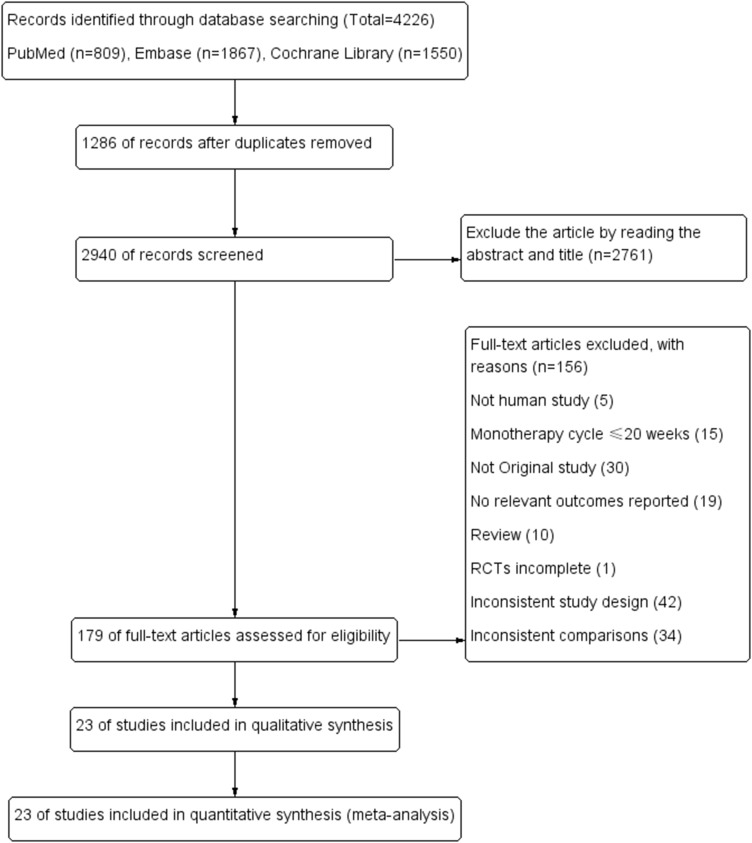
A total of 4226 articles were initially retrieved through the database, and after the screening, literature finally identified 23 studies. There were four interventions included in the 23 studies, of which three were 3-arm studies, 20 were two-arm studies, 6 studies were semaglutide 2.4mg, 3 were semaglutide 1.0mg, 10 were liraglutide 3.0mg, 6 were liraglutide 1.8mg, 20 were placebo, 3 were non-GLP-1RA hypoglycemic drugs. The total number of people in the study was 11,545 included in the study. The selection process and the basic characteristics of the included literature are shown in Figure 1 and Table 1.
Figure 1.
Flow chart of the study selection process.
Table 1.
Basic Characteristics of Included Studies
| Study ID | Year (Mean ± SD) | N | Nmale | BMI | Weeks | Interventions | Δ Weight (kg) | Safety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Mean ±SD | N2 | NTAE | NSAE | NH | |||||||
| 01 NCT03693430 202213 | 47±12 | 152 | 29 | Overweight or obesity | 52 | Semaglutide 2.4mg | 152 | −16.9±10.3 | 152 | 141 | 12 | 16 |
| 47±10 | 152 | 39 | Overweight or obesity | 52 | Placebo | 152 | −3.5±7.4 | 152 | 117 | 18 | 16 | |
| 02 John P H Wilding 202114 | 46±13 | 1306 | 351 | 37.8±6.7 | 68 | Semaglutide 2.4mg | 1306 | −16.1±10.6 | 1306 | 1052 | 128 | 198 |
| 47±12 | 655 | 157 | 38.0±6.5 | 68 | Placebo | 655 | −2.9±7.2 | 655 | 447 | 42 | 80 | |
| 03 Melanie Davies 202115 | 56±10 | 403 | 200 | 35.3±5.9 | 68 | Semaglutide 1.0mg | 403 | −7.1±6.7 | 402 | 261 | 31 | 33 |
| 55±11 | 403 | 213 | 35.9±6.5 | 68 | Placebo | 403 | −3.4±6.2 | 402 | 190 | 37 | 40 | |
| 04 Domenica Rubino 202116 | 47±12 | 535 | 106 | 34.5±6.9 | 48 | Semaglutide 2.4mg | 535 | −7.1±7.5 | 535 | 435 | 41 | 41 |
| 46±12 | 268 | 63 | 34.1±6.7 | 48 | Placebo | 268 | 6.1±7 | 268 | 201 | 15 | 10 | |
| 05 Takashi Kadowaki 202217 | 52±12 | 199 | NA | Overweight or obesity | 68 | Semaglutide 2.4mg | 199 | −13.4±8.6 | 199 | 142 | 10 | 10 |
| 50±9 | 101 | NA | Overweight or obesity | 68 | Placebo | 101 | −2.34±6.2 | 101 | 49 | 7 | 3 | |
| 06 Domenica M Rubino 202218 | 48±14 | 126 | 24 | 37.5±6.8 | 68 | Semaglutide 2.4mg | 126 | −15.8±10.2 | 126 | 115 | 10 | 20 |
| 49±13 | 127 | 30 | 37.5±6.8 | 68 | Liraglutide 3.0mg | 127 | −6.8±9.5 | 127 | 115 | 14 | 18 | |
| 51±12 | 85 | 19 | 37.5±6.8 | 68 | Placebo | 85 | −1.4±9.6 | 85 | 68 | 6 | 10 | |
| 07 Christopher Sorli 201719 | 52.7±11.9 | 130 | 80 | Overweight or obesity | 30 | Semaglutide 1.0mg | 130 | −4.67±5.19 | 130 | 47 | 7 | 9 |
| 53.9±11.0 | 129 | 70 | Overweight or obesity | 30 | Placebo | 129 | −0.89±3.46 | 129 | 27 | 5 | 8 | |
| 08 Ayse Dudu Altintas Dogan 202220 | 64.0±8.4 | 20 | 13 | 35.1±3.7 | 40 | Liraglutide 3.0mg | 20 | −9.4 | NA | |||
| 65.3±6.7 | 20 | 11 | 36.6±5.6 | 40 | Placebo | 20 | −1 | |||||
| 09 Julie R Lundgren 202121 | NA | 49 | NA | 32.6±2.9 | 52 | Liraglutide 3.0mg | 49 | −6.8 | 49 | 49 | 6 | 10 |
| NA | 49 | NA | 32.6±2.9 | 52 | Placebo | 49 | 6.1 | 49 | 42 | 2 | 9 | |
| 10 Henrik Gudbergsen 202122 | 59.2 ± 10.8 | 80 | 38 | 32.8 ± 5.5 | 52 | Liraglutide 3.0mg | 80 | −2.8±1.5 | 80 | 77 | 36 | NA |
| 59.3 ± 9.7 | 76 | 27 | 31.3 ± 4.0 | 52 | Placebo | 76 | 1.2±0.5 | 76 | 71 | 27 | NA | |
| 11 Emilie H Zobel 202123 | NA | 15 | NA | 29.5 ± 4.0 | 26 | Liraglutide 1.8mg | 15 | −3 ± 1.75 | NA | |||
| NA | 15 | NA | 28.2 ± 4.7 | 26 | Placebo | 15 | −0.2 ±0.82 | |||||
| 12 Katrine Hygum 201824 | NA | 30 | NA | 33 ± 5.7 | 26 | Liraglutide 1.8mg | 30 | −3.8 | NA | |||
| NA | 30 | NA | 31.3 ± 5.4 | 26 | Placebo | 30 | −0.06 | |||||
| 13 Thomas A Wadden 202025 | 45.4±11.6 | 142 | 23 | 39.3±6.8 | 56 | Liraglutide 3.0mg | 142 | −9.1±10.8 | 142 | 124 | 6 | 20 |
| 49±11.2 | 140 | 24 | 38.7±7.2 | 56 | Placebo | 140 | −4.8±5.3 | 140 | 101 | 2 | 0 | |
| 14 Wen-Huan Feng 201726 | 46.8 ± 1.8 | 29 | NA | 28.1 ± 0.6 | 24 | Liraglutide 1.8mg | 29 | −5.60±0.79 | NA | |||
| 46.3 ± 2.3 | 29 | NA | 26.8 ± 0.7 | 24 | Metformin | 29 | −3.58±0.91 | |||||
| 48.2 ± 2.5 | 27 | NA | 27.5 ± 0.5 | 24 | Grezite | 27 | −0.47±2.76 | |||||
| 15 NCT03480022 202127 | 31.1±6 | 55 | 55 | 42±6.7 | 32 | Liraglutide 3.0mg | 55 | −6.32±0.83 | 55 | 40 | 0 | NA |
| 31.8±5.6 | 27 | 27 | 43.9±7.5 | 32 | Placebo | 27 | −1.67±1.3 | 27 | 8 | 0 | NA | |
| 16 Louise Vedtofte 202028 | 38.8 | 45 | NA | 32.1 | 52 | Liraglutide 1.8mg | 55 | −4.7±1.7 | NA | |||
| 38.3 | 37 | NA | 30.6 | 52 | Placebo | 27 | −1.4±1.2 | |||||
| 17 Xavier Pi-Sunyer 201529 | 45.2±12.1 | 2487 | 530 | 38.3±6.4 | 56 | Liraglutide 3.0mg | 2487 | −8.4±7.3 | 2487 | 2185 | 289 | 400 |
| 45.0±12.0 | 1244 | 273 | 38.3±6.3 | 56 | Placebo | 1244 | −2.8±6.5 | 1244 | 931 | 115 | 182 | |
| 18 A Blackman 201630 | 48.6±9.9 | 180 | 129 | 38.9±6.4 | 32 | Liraglutide 3.0mg | 180 | −6.73±6.59 | 180 | 117 | 6 | 25 |
| 48.4±9.5 | 179 | 129 | 39.4±7.4 | 32 | Placebo | 179 | −1.87±5.44 | 179 | 84 | 6 | 20 | |
| 19 Arne Astrup 200931 | 45.53±10.9 | 90 | 22 | 35.0±2.6 | 20 | Liraglutide 1.8mg | 90 | −5.9±5.0 | 90 | 86 | 10 | 16 |
| 45.91±10.7 | 93 | 23 | 34.8±2.8 | 20 | Liraglutide 3.0mg | 93 | −7.6±4.6 | 93 | 90 | 10 | 22 | |
| 45.86±10.3 | 98 | 24 | 34.9±2.8 | 20 | Placebo | 98 | −3.0±3.3 | 98 | 90 | 6 | 23 | |
| 20 NCT00781937 201132 | 45.9±11.9 | 212 | 34 | 36.0±5.9 | 52 | Liraglutide 3.0mg | 212 | −6.52±0.7 | 212 | 177 | 9 | 27 |
| 46.5±11.0 | 210 | 45 | 35.2±5.9 | 52 | Placebo | 210 | −0.53±0.66 | 210 | 163 | 5 | 26 | |
| 21 Alan Garber 200933 | 52.0±10.8 | 246 | 121 | 32.8±6.3 | 52 | Liraglutide 1.8mg | 246 | −2.45±0.28 | 246 | 183 | 22 | 18 |
| 53.4±10.9 | 248 | 133 | 33.2±5.6 | 52 | Glimepiride | 248 | 1.12±0.27 | 248 | 148 | 20 | 23 | |
| 22 Yutaka Seino 201734 | 58.1±11.6 | 102 | 73 | 26.1±5.2 | 24 | Semaglutide 1.0mg | 102 | −3.9±0.3 | 102 | 73 | 2 | 1 |
| 57.9±10.0 | 103 | 78 | 25.1±3.6 | 24 | Sitagliptin | 103 | 0.0±0.3 | 103 | 68 | 2 | NA | |
| 23 Thomas A Wadden 202135 | 46±13 | 407 | 92 | 38.1±6.7 | 68 | Semaglutide 2.4mg | 407 | −17.5±11.4 | 407 | 379 | 37 | 78 |
| 46±13 | 204 | 24 | 37.8±6.9 | 68 | Placebo | 204 | −6.2±8.6 | 204 | 177 | 6 | 20 | |
Notes: N: The total number of people included in the study; Nmale: total number of males; N1: total number of weight loss; N2: total number of safeties; NTAE: total number of total adverse events; NSAE: total number of serious adverse events; NH: Hypoglycemic events.
Assessment of Risk of Bias in Included Studies
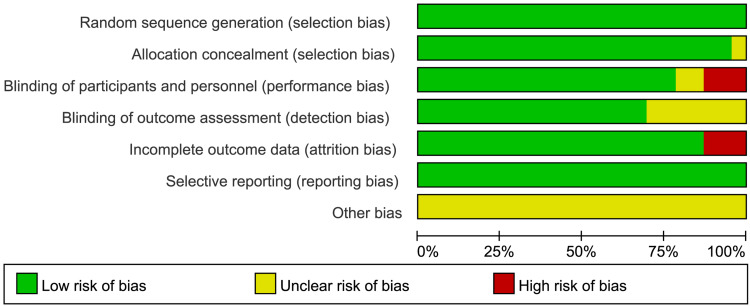
According to the Cochrane Risk of Bias Assessment Tool, in terms of other risks of bias, all studies were rated as unclear risks due to not mentioning in studies. In terms of binding of participants and personnel, two studies did not mention being rated as unclear risk of bias, three studies were open-label and ranked them as high risk of bias. In terms of incomplete outcome data, three studies were rated as high risk of bias due to lacked standard deviation (SD) data. In terms of binding of outcome assessment, seven studies did not mention being rated as unclear risk of bias. The risk of bias assessment plots and risk summary plots are shown in Figures 2 and 3.
Figure 2.
Risk of bias assessment plots.
Figure 3.
Risk of bias summary plots.
Evidence Network
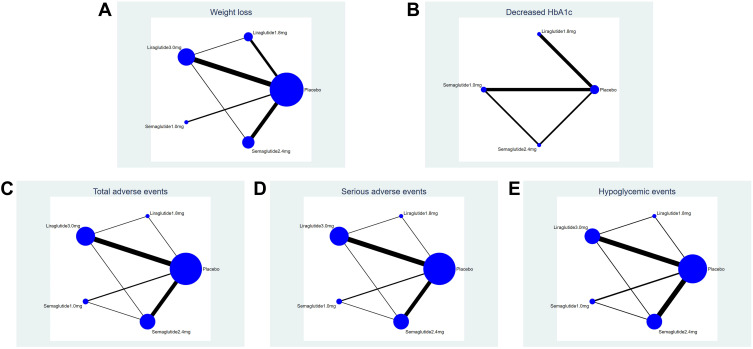
In terms of weight loss, studies (09,10) were excluded because of significant differences (P < 0.05). Twenty-one (91.30%) studies were included, 6 (26.08%) studies were included that reported the decreased HbA1c (%), but the liraglutide 3.0mg was not reported, 18 (78.26%) studies reported the total adverse events and serious adverse events, 16 (69.56%) studies reported the hypoglycemic events. The control group for 3 studies (14,21,22) was non-GLP-1 hypoglycemic drugs (such as sitagliptin, glimepiride and metformin). Each node represents a specific intervention, the node’s size means the total number of people in each study, and the thickness of the line represents the SD or log OR. The evidence network plots for each intervention are shown in Figure 4.
Figure 4.
Network plot. (A) (Weight loss), (B) (decreased HbA1c), (C) (total adverse events), (D) (serious adverse events), (E) (hypoglycemic events).
Note: Each node represents a specific intervention, the size of the nodes corresponds to the number of participants assigned to each treatment.
Inconsistency Check of the Network
We use the I-square to calculate the heterogeneity of each outcome indicator, the results of heterogeneity are shown in Supplementary File 2. Overall inconsistency test on the five outcome indicators of weight loss, decreased HbA1c, total adverse events, serious adverse events, and hypoglycemic events, and the results showed that the chi2 of each outcome indicator was 3.00, 1.04, 1.53, 2.68, and 3.38, respectively, and the five outcome indicators did not show Inconsistency difference, P ≥ 0.05. In the local inconsistency test, the statistical results show that there is no local in each outcome indicator, P ≥ 0.05. In the loop inconsistency analysis, the results showed that the weight loss outcome indicator involved two closed loops, decreased HbA1c involved one closed loop, and the other three outcome indicators involved three closed loops, and the lower bounds of the 95%-CI for all five outcomes included 0 or P ≥ 0.05, indicating a low likelihood of inconsistency between closed loops. The results of inconsistency test and inconsistency of loop-specific approach for efficacy and safety are shown in Tables 2 and 3.
Table 2.
Design-by-Treatment Test
| chi2 | Prob > chi2 | |
|---|---|---|
| Δ Weight(kg) | 3.00 | 0.5581 |
| Δ HbA1c (%) | 1.04 | 0.3087 |
| Total adverse events | 1.53 | 0.9572 |
| Serious adverse events | 2.68 | 0.8478 |
| Hypoglycemic episodes | 3.38 | 0.7592 |
Table 3.
The Inconsistency of Loop-Specific Approach for Efficacy and Safety
| Loop | IF | seIF | z_value | p_value | CI_95 | |
|---|---|---|---|---|---|---|
| Δ Weight(kg) | A-C-E | 1.897 | 1.857 | 1.022 | 0.307 | (0.00,5.54) |
| A-B-C | 0.443 | 1.037 | 0.427 | 0.670 | (0.00,2.48) | |
| Δ HbA1c (%) | A-C-D | 0.360 | 0.181 | 1.987 | 0.047 | (0.00,0.72) |
| Total adverse events | A-D-E | 0.352 | 0.317 | 1.109 | 0.267 | (0.00,0.97) |
| A-C-E | 0.217 | 0.534 | 0.406 | 0.684 | (0.00,1.26) | |
| A-B-C | 0.129 | 1.108 | 0.116 | 0.908 | (0.00,2.30) | |
| Serious adverse events | A-D-E | 0.318 | 0.679 | 0.469 | 0.639 | (0.00,1.65) |
| A-B-C | 0.308 | 0.725 | 0.425 | 0.671 | (0.00,1.73) | |
| A-C-E | 0.235 | 0.510 | 0.460 | 0.645 | (0.00,1.24) | |
| Hypoglycemic events | A-D-E | 0.034 | 0.393 | 0.088 | 0.930 | (0.00,0.81) |
| A-B-C | 0.126 | 0.525 | 0.241 | 0.810 | (0.00,1.15) | |
| A-C-E | 0.051 | 0.376 | 0.136 | 0.892 | (0.00,0.79) |
Notes: A: Placebo; B: Liraglutide 1.8mg; C: Liraglutide 3.0mg; D: Semaglutide 1.0mg; E: Semaglutide 2.4mg.
Weight Loss (Δ Weight(Kg))
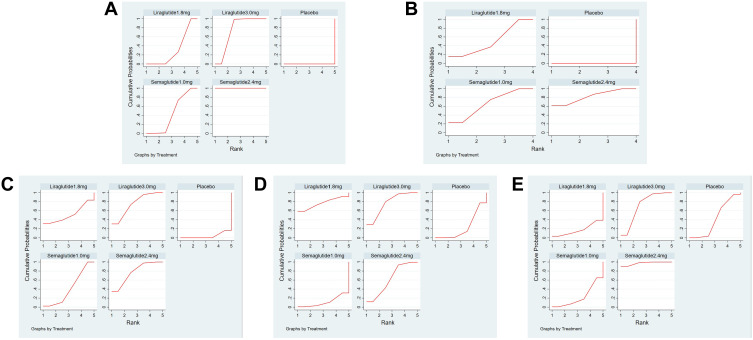
Compared with placebo, all four interventions had some weight loss effect, semaglutide 2.4mg (MD=−12.47kg, 95% CI [−13.25, −11.69]), liraglutide 3.0mg (MD=−5.24kg, 95% CI [−5.82, −4.67]), semaglutide 1.0mg (MD=−3.74kg, 95% CI [−4.87, −2.61]), liraglutide 1.8mg (MD=−3.29kg, 95%Cl [−4.04, −2.53]). In the comparison between interventions, semaglutide 1.0mg (MD=−0.45kg, 95%Cl [−1.81, 0.91]) was not significantly different compared to liraglutide 1.8mg, other comparisons are significant differences. The results of the weight loss of the four interventions are shown in Table 4. The ranking results of the SUCRA showed that semaglutide 2.4mg> liraglutide 3.0mg > semaglutide 1.0mg> liraglutide 1.8mg > placebo, and it means indicated that semaglutide 2.4mg has the best weight loss effect, followed by liraglutide 3.0mg. The results of SUCRA of the four interventions and cumulative probability plots are shown in Table 5 and Figure 5.
Table 4.
Comparisons for the Weight Loss of the Four Interventions
| Semaglutide 2.4mg | ||||
| −7.23 (−8.17, −6.28)* | Liraglutide 3.0mg | |||
| −8.73 (−10.11, −7.36)* | −1.51 (−2.78, −0.24)* | Semaglutide 1.0mg | ||
| −9.19 (−10.27, −8.11)* | −1.96 (−2.87, −1.05)* | −0.45 (−1.81,0.91) | Liraglutide 1.8mg | |
| −12.47 (−13.25, −11.69)* | −5.24 (−5.82, −4.67)* | −3.74 (−4.87, −2.61)* | −3.29 (−4.04, −2.53)* | Placebo |
Note: *Significant difference (P < 0.05).
Table 5.
The SUCRA (%) Results of Network Meta of the 5 Outcome Indicators
| Treatment | Δ Weight(kg) | Δ HbA1c (%) | Total Adverse Events | Serious Adverse Events | Hypoglycemic Events |
|---|---|---|---|---|---|
| Placebo | 0.0 | 0.0 | 4.1 | 23.1 | 41.8 |
| Liraglutide 1.8mg | 31.6 | 50.9 | 51.6 | 76.6 | 17.5 |
| Liraglutide 3.0mg | 74.7 | / | 75.1 | 76.7 | 70.8 |
| Semaglutide 1.0mg | 43.6 | 66.1 | 41.8 | 11.5 | 22.7 |
| Semaglutide 2.4mg | 100.0 | 83.0 | 77.3 | 62.0 | 97.2 |
Figure 5.
Cumulative probability plots. (A) (Weight loss), (B) (decreased HbA1c), (C) (total adverse events), (D) (serious adverse events), (E) (hypoglycemic events).
Decreased HbA1c (%)
Compared with placebo, all three interventions were more effective in decreased HbA1c (%), semaglutide 2.4mg (MD=−1.48%, 95% CI [−1.93, −1.04]), semaglutide 1.0mg (MD=−1.36%, 95% CI [−1.72, −1.01]), liraglutide 1.8mg (MD=−1.23%, 95%Cl [−1.66, −0.80]), There is no significant difference in the comparison between semaglutide 2.4mg and semaglutide 1.0mg and liraglutide 1.8mg. The result of decreased HbA1c (%) is shown in Table 6. The ranking results of the SUCRA showed that semaglutide 2.4mg > semaglutide 1.0mg > liraglutide 1.8mg > placebo, which means that semaglutide 2.4mg has the best-decreased HbA1c (%) effect, followed by semaglutide 1.0mg. The results of SUCRA of the three interventions and cumulative probability plots are shown in Table 5 and Figure 5.
Table 6.
Comparisons for the Δ HbA1c (%) of the Three Interventions
| Semaglutide 2.4mg | |||
| −0.12 (−0.56,0.33) | Semaglutide 1.0mg | ||
| −0.25 (−0.87,0.37) | −0.13 (−0.69,0.42) | Liraglutide 1.8mg | |
| −1.48 (−1.93, −1.04)* | −1.36 (−1.72, −1.01)* | −1.23 (−1.66, −0.80)* | Placebo |
Note: *Significant difference (P < 0.05).
Total Adverse Events
Compared with placebo, there was a significant difference in the incidence of total adverse events with semaglutide 2.4 mg (OR = 2.36, 95%Cl [1.84, 3.03], P < 0.05), Liraglutide 3.0 mg (OR = 2.35, 95%Cl [1.82, 3.02], P < 0.05), semaglutide 1.0 mg (OR = 1.82, 95%Cl [1.29, 2.56], P < 0.05). Liraglutide 1.8mg (OR=1.84, 95%Cl [0.54, 6.30] compared with placebo and other pairwise comparisons were no significant difference. The results are shown in Table 7. The ranking results of the SUCRA showed that semaglutide 2.4mg> liraglutide 3.0mg> liraglutide 1.8mg> semaglutide 1.0mg> placebo, which means been demonstrated that semaglutide 1.0 mg had the lowest incidence of total adverse events, semaglutide 2.4mg had the highest incidence of total adverse events. The results of SUCRA of the four interventions and cumulative probability plots are shown in Table 5 and Figure 5.
Table 7.
Comparisons for the Total Adverse Events of the Four Interventions
| Semaglutide 2.4mg | ||||
| 1.00 (0.71,1.42) | Liraglutide 3.0mg | |||
| 1.28 (0.37,4.50) | 1.28 (0.37,4.42) | Liraglutide 1.8mg | ||
| 1.30 (0.92,1.82) | 1.29 (0.85,1.96) | 1.01 (0.28,3.62) | Semaglutide 1.0mg | |
| 2.36 (1.84,3.03)* | 2.35 (1.82,3.02)* | 1.84 (0.54,6.30) | 1.82 (1.29,2.56)* | Placebo |
Note: *Significant difference (P < 0.05).
Serious Adverse Events
Compared with placebo, there was a significant difference in the incidence of serious adverse events with liraglutide 3.0mg (OR = 1.47, 95%Cl [1.07, 2.02], P < 0.05). Liraglutide 1.8mg (OR = 1.67, 95%Cl [0.68,4.09], P > 0.05), semaglutide 2.4mg (OR = 1.29, 95%Cl [0.97,1.71], P > 0.05) and semaglutide 1.0mg (OR = 0.87, 95%Cl [0.54,1.39], P > 0.05) had no significant difference. There was no significant difference between the interventions (P ≥ 0.05). The results are shown in Table 8. The ranking results of the SUCRA showed that the incidence of serious adverse events of the four interventions from high to low was liraglutide 3.0mg> liraglutide 1.8mg> semaglutide 2.4mg> placebo> semaglutide 1.0mg, it suggested that semaglutide 1.0 mg had the lowest incidence of serious adverse events, liraglutide 3.0 mg had the highest incidence of adverse events. The results of SUCRA of the four interventions and cumulative probability plots are shown in Table 5 and Figure 5.
Table 8.
Comparisons for the Serious Adverse Events of the Four Interventions
| Liraglutide 3.0mg | ||||
| 0.88 (0.36,2.13) | Liraglutide 1.8mg | |||
| 1.14 (0.74,1.74) | 1.29 (0.50,3.32) | Semaglutide 2.4mg | ||
| 1.47 (1.07,2.02)* | 1.67 (0.68,4.09) | 1.29 (0.97,1.71) | Placebo | |
| 1.69 (0.95,3.03) | 1.92 (0.69,5.33) | 1.49 (0.93,2.38) | 1.15 (0.72,1.85) | Semaglutide 1.0mg |
Note: *Significant difference (P < 0.05).
Hypoglycemic Events
Compared with placebo, semaglutide 2.4mg (OR = 1.38, 95%Cl [1.14,1.67], P < 0.05) had a significant difference in the incidence of hypoglycemic events, and the result show that semaglutide 2.4mg can cause hypoglycemia. Liraglutide 3.0mg (OR = 1.14, 95%Cl [0.97,1.33], P > 0.05), semaglutide 1.0mg (OR = 0.85, 95%Cl [0.57,1.25], P > 0.05) and liraglutide 1.8mg (OR = 0.75, 95%Cl [0.39,1.42], P > 0.05) had no significant difference in the incidence of hypoglycemic events, and its result show that these interventions do not cause hypoglycemia. There was a significant difference between semaglutide 2.4mg and semaglutide 1.0mg (OR = 1.63, 95%Cl [1.10,2.41], P < 0.05), and there was no statistical significance in other groups (P≥ 0.05), the results are shown in Table 9. The ranking results of the SUCRA showed that the incidence of hypoglycemic events from high to low is semaglutide 2.4mg>liraglutide 3.0mg>placebo>semaglutide 1.0mg>liraglutide 1.8mg, it means shown that liraglutide 1.8mg had the lowest incidence of hypoglycemic events, semaglutide 2.4mg had the highest incidence of hypoglycemic events. The results of SUCRA of the four interventions and cumulative probability plots are shown in Table 5 and Figure 5.
Table 9.
Comparisons for the Hypoglycemic Episodes of the Four Interventions
| Semaglutide 2.4mg | ||||
| 1.21 (0.95,1.54) | Liraglutide 3.0mg | |||
| 1.38 (1.14,1.67)* | 1.14 (0.97,1.33) | Placebo | ||
| 1.63 (1.10,2.41)* | 1.35 (0.89,2.05) | 1.18 (0.80,1.75) | Semaglutide 1.0mg | |
| 1.84 (0.95,3.59) | 1.52 (0.80,2.89) | 1.34 (0.71,2.54) | 1.13 (0.53,2.39) | Liraglutide 1.8mg |
Note: *Significant difference (P < 0.05).
Sensitivity Analysis
Six high-risk studies (06, 08, 09, 12, 14, 22) were excluded, and sensitivity analysis was performed on weight loss as the primary outcome indicator. The results showed that the network meta-analysis did not change significantly, which suggests that the network meta-analysis results were reliable. The result of sensitivity analysis is shown in Table 10.
Table 10.
Comparisons for the Weight Loss of the Four Interventions After 6 High-Risk Studies Were Excluded
| Semaglutide 2.4mg | ||||
| −7.03 (−8.08, −5.98)* | Liraglutide 3.0mg | |||
| −8.52 (−9.97, −7.07)* | −1.49 (−2.81, −0.16)* | Semaglutide1.0mg | ||
| −9.15 (−10.40, −7.89)* | −2.12 (−3.18, −1.05)* | −0.63 (−2.12,0.86) | Liraglutide 1.8mg | |
| −12.25 (−13.11, −11.40)* | −5.22 (−5.85, −4.60)* | −3.74 (−4.91, −2.57)* | −3.11 (−4.02, −2.19)* | Placebo |
Note: *Significant difference (P < 0.05).
Publication Bias Analysis
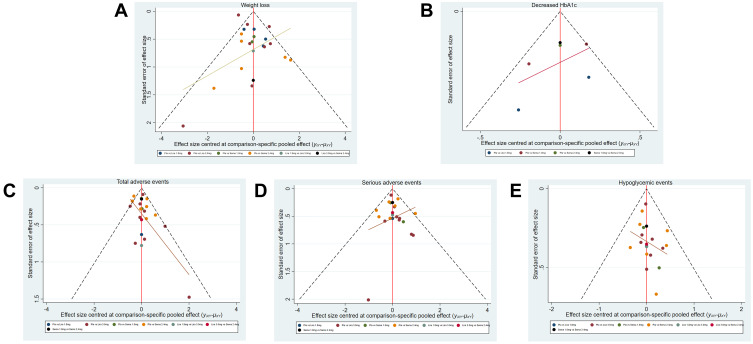
Stata Software drew inverted funnel plots for weight loss, Δ HbA1c (%), total adverse events, serious adverse events, and incidence of hypoglycemic events. The results show that, in the five funnel charts, most of the research scatter points are located above the inverted funnel chart and have a biased distribution and a small number of scatter points are located at the bottom of the inverted funnel chart, it suggests that the above results have a certain publication bias and may be affected by the small sample effect. The comparison-adjusted funnel plots for efficacy and safety are shown in Figure 6.
Figure 6.
Funnel plots. (A) (Weight loss), (B) (decreased HbA1c), (C) (total adverse events), (D) (serious adverse events), (E) (hypoglycemic events).
Discussion
The 23 studies included in this study are all RCTs. The results of the NMA showed that in terms of weight loss and decreased HbA1c (%), the best weight loss effect is semaglutide 2.4mg, which can reach 12.47kg, followed by liraglutide 3.0 mg is 5.24kg. The best decreased HbA1c effect is semaglutide 2.4mg, which can get 1.48%, followed by semaglutide 1.0mg 1.36%, liraglutide 1.23%. This result shows that semaglutide 2.4mg has a complete weight loss and decreased HbA1c advantage. In terms of total adverse events, compared with placebo, except for liraglutide 1.8mg, which was not significantly different (which may be related to the small sample size of the included studies), the other three interventions were significantly different, and the incidence of semaglutide 2.4mg is the largest. In terms of serious adverse reactions, only liraglutide 3.0 mg was significantly different compared to placebo, and others were not statistically significant. In terms of hypoglycemic events, compared with placebo and semaglutide 1.0mg, except for semaglutide 2.4mg, which was significantly different, other pairwise comparisons had no statistical significance. However, this result is different from that of Lin Xia36 et al reported liraglutide 1.8mg and semaglutide 1.0mg in the hypoglycemic events and weight loss. The final result shows that the more weight loss, the greater the incidence of adverse events. In clinical practice, we need to find a balance, pay more attention to the adverse reactions of drugs, and monitor blood sugar at all times to find out, under the premise of preventing adverse reactions, how to maximize weight loss and choose an appropriate program is essential for the people with obesity or overweight.
All study data were from the literature, 3 studies were open-label, and 3 were missing data; the results were less likely to affect weight loss but may have a more significant impact on safety results. In Sensitivity analysis, the results of the network meta-analysis did not change significantly and were reliable. Overall, the quality of the included literature was good, the risk was low, and the results were reliable. The results of the publication bias analysis showed that this study might have particular publication bias and be affected by minor sample effects. The results of the inconsistency test between the overall, local and closed-loop included in the study and heterogeneity showed that there was no statistically significant inconsistency (P ≥ 0.05 or CI_95 including 0) for each outcome indicator, the final result indicated that the consistency test results of the network are reliable. However, Study 2032 showed a significant difference when using I-square to test for heterogeneity because all the experimental subjects were Asian people and induced to lose 5% of their body weight by daily diet before using the intervention drug.
People with obesity or overweight have a severe impact on our physical health and lead to an increased incidence of various diseases, especially people with obesity or overweight and type 2 diabetes, which is often accompanied by complications such as cardiovascular disease.1–4 GLP-1RA can bring us weight loss and achieve the effect of lowering blood sugar, reducing the death rate of Covid-19,5 and has a protective effect on our cardiovascular.11 The primary purpose of this study is to compare the efficacy and safety of two GLP-1RA for weight management (liraglutide and Semaglutide) that the FDA has approved and recommended in the 2022 American Diabetes Association’s standards for diabetes care and provide evidence for individualized medication management in clinical practice.12 However, this study also has limitations: (1) There is no differentiated discussion of specific regions or races because there may be differences in the physical quality of different ethnic groups. (2) In this network Meta study, the cycle of monotherapy in the RCT study ranged from 20 to 68 weeks, with a wide span, some 24 weeks can also achieve the same weight loss effect, and the change in weight between 24 and 68 weeks is small, so it is possible that the follow-up treatment cycle is to prevent weight rebound, but there may be a significant impact on safety. (3) Some RCT studies have underlying diseases patients, while some are healthy obese or overweight people. (4) The sample size of the RCT studies included in some interventions is small, it may have influenced the results, and a larger sample size may be needed to support this study.
Conclusion
This meta-analysis indicates that all GLP-1RAs were more efficacious than placebo in people with obesity or overweight on efficacy. Semaglutide 2.4mg has an absolute advantage in weight loss and decreased HbA1c, but the incidence of total adverse reactions is also the highest and can cause hypoglycemia. In addition, although liraglutide 3.0mg was less effective than Semaglutide 2.4mg, serious adverse events were still the most elevated.
Acknowledgments
This study was supported by National Key Clinical Specialty Construction Project (Clinical Pharmacy) and High-Level Clinical Key Specialty (Clinical Pharmacy) in Guangdong Province.
Funding Statement
This study was supported by the National Key Specialty Construction Project (Clinical Pharmacy) and the High-level Clinical Key Specialty of Guangdong Province, and the funders were the central finance subsidy fund for the improvement of medical services and guarantee capacity, code Z155080000004; the Guangzhou Minsheng Science and Technology Research Program Project, code 201803010096.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. Took part in drafting, revising or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nestor JJ, Parkes D, Feigh M., et al. Effects of ALT-801, a GLP-1 and glucagon receptor dual agonist, in a translational mouse model of non-alcoholic steatohepatitis. Sci Rep. 2022;12:1. doi: 10.1038/s41598-022-10577-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao F, Zhou Q, Cong Z, et al. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nat Commun. 2022;13:1. doi: 10.1038/s41467-021-27699-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Ruiten CC, Smits MM, Kok MD, et al. Mechanisms underlying the blood pressure lowering effects of dapagliflozin, exenatide, and their combination in people with type 2 diabetes: a secondary analysis of a randomized trial. Cardiovasc Diabetol. 2022;21:1. doi: 10.1186/s12933-022-01492-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley CL, McMillin SM, Hwang AY, et al. High-dose once-weekly semaglutide: a new option for obesity management. Ann Pharmacother. 2022;56(8):941–950. doi: 10.1177/10600280211053867 [DOI] [PubMed] [Google Scholar]
- 5.Berkovic MC, Rezic T, Bilic-Curcic I, et al. Semaglutide might be a key for breaking the vicious cycle of metabolically associated fatty liver disease spectrum? World J Clin Cases. 2022;10(20):6759–6768. doi: 10.12998/wjcc.v10.i20.6759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y-H, Hung H-Y. Recent advances in natural anti-obesity compounds and derivatives based on in vivo evidence: a mini-review. Eur J Med Chem. 2022;237:114405. doi: 10.1016/j.ejmech.2022.114405 [DOI] [PubMed] [Google Scholar]
- 7.Fabrizio Muratori F, Di Sacco VG, Di Sacco G, et al. Efficacy of liraglutide 3.0 mg treatment on weight loss in patients with weight regain after bariatric surgery. Eating and Weight Disorders - Studies on Anorexia. Bulimia Obesity. 2022;1:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barboza JJ, Huamán MR, Melgar B, et al. Efficacy of liraglutide in non-diabetic obese adults: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2022;11:2998. doi: 10.3390/jcm11112998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alabduljabbar K, Al-Najim W, Carel W. The Impact Once-Weekly Semaglutide 2.4 mg Will Have on Clinical Practice: a Focus on the STEP Trials. Nutrients. 2022;14:2217. doi: 10.3390/nu14112217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875–E891. doi: 10.1503/cmaj.191707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Jing D. Research progress of GLP-1 receptor agonists in the treatment of type 2 diabetes mellitus. Geriatrics Res. 2021;2(03):55–60. [Google Scholar]
- 12.American Diabetes Association. Introduction: standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S1–S2. doi: 10.2337/dc22-Sint [DOI] [PubMed] [Google Scholar]
- 13.Two-year Research Study Investigating How Well Semaglutide Works in People Suffering from Overweight or Obesity (STEP 5). Available from: https://clinicaltrials.gov/ct2/show/NCT03693430?term=03693430&draw=2&rank=1. Accessed November 25, 2022.
- 14.Wilding JPH, Batterham RL, Calanna S, et al; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 15.Davies M, Færch L, Jeppesen OK, et al; STEP 2 Study Group. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, Phase 3 trial. Lancet. 2021;397(10278):971–984. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 16.Rubino D, Abrahamsson N, Davies M, et al; STEP 4 Investigators. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: the STEP 4 Randomized Clinical Trial. JAMA. 2021;325(14):1414–1425. doi: 10.1001/jama.2021.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadowaki T, Isendahl J, Khalid U, et al; STEP 6 investigators. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193–206. doi: 10.1016/S2213-8587(22)00008-0 [DOI] [PubMed] [Google Scholar]
- 18.Rubino DM, Greenway FL, Khalid U, et al; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity Without Diabetes: the STEP 8 Randomized Clinical Trial. JAMA. 2022;327(2):138–150. doi: 10.1001/jama.2021.23619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–260. doi: 10.1016/S2213-8587(17)30013-X [DOI] [PubMed] [Google Scholar]
- 20.Altintas Dogan AD, Hilberg O, Hess S, et al. Respiratory Effects of Treatment with a Glucagon-Like Peptide-1 Receptor Agonist in Patients Suffering from Obesity and Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2022;17:405–414. doi: 10.2147/COPD.S350133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundgren JR, Janus C, Jensen SBK, et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N Engl J Med. 2021;384(18):1719–1730. doi: 10.1056/NEJMoa2028198 [DOI] [PubMed] [Google Scholar]
- 22.Gudbergsen H, Overgaard A, Henriksen M, et al. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: a randomized controlled trial. Am J Clin Nutr. 2021;113(2):314–323. doi: 10.1093/ajcn/nqaa328 [DOI] [PubMed] [Google Scholar]
- 23.Zobel EH, Ripa RS, von Scholten BJ, et al. Effect of Liraglutide on Vascular Inflammation Evaluated by [64Cu] DOTATATE. Diagnostics. 2021;11(8):1431. doi: 10.3390/diagnostics11081431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hygum K, Harsløf T, Jørgensen NR, et al. Bone resorption is unchanged by liraglutide in type 2 diabetes patients: a randomised controlled trial. Bone. 2020;132:115197. doi: 10.1016/j.bone.2019.115197 [DOI] [PubMed] [Google Scholar]
- 25.Wadden TA, Tronieri JS, Sugimoto D, et al. Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: the SCALE IBT Randomized Controlled Trial. Obesity. 2020;28(3):529–536. doi: 10.1002/oby.22726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng W, Gao C, Bi Y, et al. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9(8):800–809. doi: 10.1111/1753-0407.12555 [DOI] [PubMed] [Google Scholar]
- 27.Liraglutide 3mg (Saxenda) on Weight, Body Composition, Hormonal and Metabolic Parameters in Obese Women with PCOS - Full Text View, Available from: https://clinicaltrials.gov/ct2/show/NCT03480022?term=03480022&draw=2&rank=1. Accessed November 25, 2022.
- 28.Vedtofte L, Bahne E, Foghsgaard S, et al. One Year’s Treatment with the Glucagon-Like Peptide 1 Receptor Agonist Liraglutide Decreases Hepatic Fat Content in Women with Nonalcoholic Fatty Liver Disease and Prior Gestational Diabetes Mellitus in a Randomized, Placebo-Controlled Trial. J Clin Med. 2020;9(10):3213. doi: 10.3390/jcm9103213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pi-Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 30.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes. 2016;40(8):1310–1319. doi: 10.1038/ijo.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astrup A, Rössner S, Van Gaal L, et al; NN8022-1807 Study Group. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–1616. doi: 10.1016/S0140-6736(09)61375-1 [DOI] [PubMed] [Google Scholar]
- 32.Liraglutide improves weight maintenance and weight loss in obese adults without diabetes after diet-induced weight loss: the SCALE™ 56-week randomized study. Available from: https://clinicaltrials.gov/ct2/show/NCT00781937?term=00781937&draw=2&rank=1. Accessed November 25, 2022.
- 33.Garber A, Henry R, Ratner R, et al; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, Phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. doi: 10.1016/S0140-6736(08)61246-5 [DOI] [PubMed] [Google Scholar]
- 34.Seino Y, Terauchi Y, Osonoi T, et al. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):378–388. doi: 10.1111/dom.13082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadden TA, Bailey TS, Billings LK, et al; STEP 3 Investigators. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults with Overweight or Obesity: the STEP 3 Randomized Clinical Trial. JAMA. 2021;325(14):1403–1413. doi: 10.1001/jama.2021.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia L, Shen T, Dong W, et al. Comparative efficacy and safety of 8 GLP-1RAs in patients with type 2 diabetes: a network meta-analysis. Diabetes Res Clin Pract. 2021;177:108904. doi: 10.1016/j.diabres.2021.108904 [DOI] [PubMed] [Google Scholar]