Abstract
Fimbriae are filamentous, cell surface structures which have been proposed to mediate attachment of Bordetella species to respiratory epithelium. Bordetella bronchiseptica has four known fimbrial genes: fim2, fim3, fimX, and fimA. While these genes are unlinked on the chromosome, their protein products are assembled and secreted by a single apparatus encoded by the fimBCD locus. The fimBCD locus is embedded within the fha operon, whose genes encode another putative adhesin, filamentous hemagglutinin (FHA). We have constructed a Fim− B. bronchiseptica strain, RB63, by introducing an in-frame deletion extending from fimB through fimD. Western blot analysis showed that RB63 is unable to synthesize fimbriae but is unaffected for FHA expression. Using this mutant, we assessed the role of fimbriae in pathogenesis in vitro and in vivo in natural animal hosts. Although RB63 was not significantly defective in its ability to adhere to various tissue culture cell lines, including human laryngeal HEp-2 cells, it was considerably altered in its ability to cause respiratory tract infections in rats. The number of ΔfimBCD bacteria recovered from the rat trachea at 10 days postinoculation was significantly decreased compared to that of wild-type B. bronchiseptica and was below the limit of detection at 30 and 60 days postinoculation. The number of bacteria recovered from the nasal cavity and larynx was not significantly different between RB63 and the wild-type strain at any time point. The ability of fimbriae to mediate initial attachment to tracheal tissue was tested in an intratracheal inoculation assay. Significantly fewer RB63 than wild-type bacteria were recovered from the tracheas at 24 h after intratracheal inoculation. These results demonstrate that fimbriae are involved in enhancing the ability of B. bronchiseptica to establish tracheal colonization and are essential for persistent colonization at this site. Interestingly, anti-Bordetella serum immunoglobulin M (IgM) levels were significantly lower in animals infected with RB63 than in animals infected with wild-type B. bronchiseptica at 10 days postinoculation. Even at 30 days postinoculation, RB63-infected animals had lower serum anti-Bordetella antibody titers in general. This disparity in antibody profiles suggests that fimbriae are also important for the induction of a humoral immune response.
Specific attachment to host tissues is a crucial event in the initiation of bacterial infections. For many gram-negative bacteria, attachment has been shown to be mediated by filamentous polymeric protein cell surface structures called fimbriae (27). For instance, type IV pili of Neisseria species and Pseudomonas aeruginosa, as well as type I and pyelonephritis-associated P (Pap) pili of Escherichia coli, have been shown to serve as essential adhesins for colonization (for reviews, see references 1, 10, 22, 34, and 39).
Bordetella pertussis and Bordetella bronchiseptica are small, aerobic, gram-negative bacteria that colonize the respiratory mucosa of humans and other mammals, respectively. Bordetella genome sequence data (http://www.sanger.ac.uk) indicate the existence of at least four fimbrial structural genes, and other studies (7, 15, 28, 35) reveal that Bordetella species express fimbriae of at least four serotypes, Fim2, Fim3, FimX, and FimA, which are encoded by the fim2, fim3, fimX, and fimA genes, respectively. These genes are unlinked on the Bordetella chromosome, and their protein products are 57 to 60% identical at the amino acid level (7, 15). Although results from in vitro and in vivo studies with B. pertussis are consistent with the hypothesis that fimbriae contribute to the adherence of Bordetella to respiratory epithelium (32, 33), and Fim2 and Fim3 have been included as components of current acellular pertussis vaccines (21), the precise role of fimbriae in pathogenesis has not been conclusively established. A major obstacle has been the lack of a natural animal model for this strictly human pathogen.
Like nearly all of the known and suspected colonization and virulence factors expressed by Bordetella, fimbriae are regulated at the transcriptional level by the products of a two-component signal transduction system encoded by the bvgAS locus (11, 43, 46–48, 50). In vitro, BvgAS is active at 37°C (nonmodulating or Bvg+ phase conditions) and can be inactivated by growth at low temperature (22°C) or by the presence of nicotinic acid or MgSO4 (modulating or Bvg− phase conditions) in the culture media. In addition to BvgAS-dependent regulation, the B. pertussis fim2, fim3, and fimX genes are subject to another form of transcriptional control, called phase variation, which has been suggested as a mechanism by which the bacteria escape immune recognition (36, 51).
In B. pertussis and B. bronchiseptica, genes required for secretion and assembly of fimbriae (fimB, fimC, and fimD) are located between fhaB and fhaC, genes required for synthesis and processing of another putative adhesin, filamentous hemagglutinin (FHA) (Fig. 1A). fhaB encodes a 367-kDa precursor protein (FhaB) which is processed by the fhaC gene product to form the mature 220-kDa surface-associated and secreted filamentous molecule, FHA (16, 29). The fimBCD gene cluster and fhaC are transcribed from the fimB promoter and are translationally coupled (30). Based on amino acid similarity to the pap gene products, which are involved in the production of P pili in E. coli, functions have been proposed for the fimBCD gene products (24). FimB resembles the chaperone protein, PapD, which appears to prevent degradation of major fimbrial subunits in the periplasmic space. FimC resembles the usher protein, PapC, which is probably involved in transport of fimbrial subunits across the outer membrane and anchorage of the fimbrial structure (54). FimD is proposed to constitute the adhesive tip of the Bordetella pilus (52).
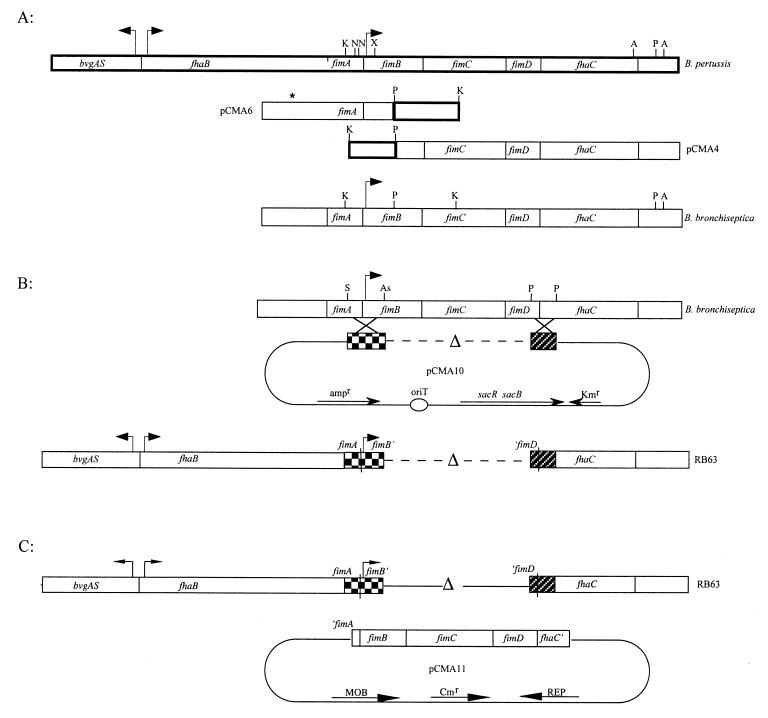
FIG. 1.
(A) Fragments of B. pertussis DNA homologous between B. pertussis and B. bronchiseptica were used to integrate a plasmid into the B. bronchiseptica genome. Flanking regions of B. bronchiseptica DNA were then isolated while the plasmid was excised from the genome. Thick-lined boxes represent the B. pertussis DNA and show the organization of the fim locus; thin-lined boxes represent B. bronchiseptica DNA. Two KpnI-PstI B. pertussis fragments were used to isolate B. bronchiseptica DNA cloned as plasmids pCMA4 and pCMA6. Restriction analysis of B. bronchiseptica DNA revealed differences between B. pertussis and B. bronchiseptica DNA as is represented by the absence of certain restriction sites in B. bronchiseptica. The asterisk represents extra DNA in B. bronchiseptica that does not correspond to B. pertussis sequences in this region. There is evidence that fimA in B. bronchiseptica may be a complete and functional gene (7). A, AlwNI; As, AspI; K, KpnI; N, NspI; P, PstI; S, SmaI; X, XmaI. (B) SalI-AspI and PstI-PstI fragments from B. bronchiseptica were ligated in frame to create the ΔfimBCD mutation. pCMA10 represents the allelic exchange plasmid used to introduce the deletion into the B. bronchiseptica chromosome. RB63 represents the genetic organization of the ΔfimBCD strain. (C) The minimal open reading frame of the fimbrial biogenesis operon (fimBCD) containing the intact fimB promoter was used to complement the Fim− mutant, RB63. pCMA11 represents the complementation plasmid.
In this study, we investigated the role of fimbriae in respiratory pathogenesis in the context of a natural host-pathogen interaction. Using a B. bronchiseptica mutant which is unable to synthesize fimbriae due to a deletion in its fimbrial biogenesis locus, we show that fimbriae are required for efficient establishment and persistent colonization of the trachea. Additionally, we show that fimbriae play an important role in the development of humoral immunity to Bordetella infection.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
Wild-type B. bronchiseptica strain RB50 was isolated in our lab from a naturally infected rabbit (12). All mutant B. bronchiseptica strains used in this work are derivatives of RB50. RB53 contains a 1-bp mutation in bvgS corresponding to the B. pertussis bvgS-C3 allele, which confers a Bvg+ phase constitutive phenotype. RB54 contains an in-frame deletion in bvgS which confers it a Bvg− phase constitutive phenotype. The mutations in the bvgAS loci of these strains were constructed by allelic exchange as described previously (3, 12).
Plasmid pUW1006 was provided by S. Falkow (Stanford University, Stanford, Calif.) and has been previously described (40). pMTL20 and pMTL23 are pUC19 derivatives (9). pEGBR is a PstI deletion derivative of pSS1129 (41, 42) containing the sacBR cartridge from pUM24 (37) and the RP4 origin of transfer, which can be mobilized from E. coli SM10 to confer ampicillin resistance, kanamycin resistance, and sucrose sensitivity. pBBR1MCS is a pBBR1CM derivative containing the pBluescript II IK-lacZα polylinker (26) and replicates autonomously in B. bronchiseptica.
E. coli was grown in Luria-Bertani broth or on Luria-Bertani agar (31). B. bronchiseptica strains were grown in Stainer-Scholte medium or on Bordet-Gengou (BG) agar (Becton Dickinson Microbiology Systems) (6) containing 7.5% defibrinated sheep blood (Mission Laboratories). Relevant antibiotics were used at the following concentrations: streptomycin, 20 μg/ml; kanamycin, 40 μg/ml; chloramphenicol, 50 μg/ml; ampicillin, 100 μg/ml; and gentamicin, 20 μg/ml.
DNA methods.
Isolation of plasmid and chromosomal DNA, restriction enzyme digestions, agarose gel electrophoresis, and DNA ligations were performed by standard methods (38). Restriction enzymes, calf intestinal alkaline phosphatase, Klenow fragment, T4 DNA ligase, and Sequenase were from Promega Corp. (Madison, Wis.), Boehringer Mannheim (Indianapolis, Ind.), New England Biolabs (Beverly, Mass.), or Bethesda Research Laboratories (Gaithersburg, Md.) and were used according to the manufacturers' directions. Plasmid constructions were performed with E. coli DH5α (38).
Cloning and construction of RB63.
B. pertussis fimBCD sequences were obtained from pUW1006 (40). An 892-bp KpnI-PstI DNA fragment encompassing the fimB promoter region and a 1,017-bp PstI-KpnI DNA fragment containing sequences from fimB and fimC were cloned into pMTL20 (Fig. 1). DNA fragments from the resulting plasmids were then regenerated as EcoRI-HindIII fragments, cloned into the suicide plasmid pEG7 (2) to create plasmids pSM5 and pSM6, respectively, and transformed into SM10.λpir. pSM5 and pSM6 were each mobilized into B. bronchiseptica RB50 by conjugation. Cointegrates were selected on medium containing streptomycin (since RB50 is streptomycin resistant) and gentamicin. Chromosomal DNA from the cointegrates was isolated, digested with BamHI (which has a unique site on the plasmid), and religated to generate plasmids pCMA4 and pCMA6 (Fig. 1). In this manner, we cloned flanking regions of B. bronchiseptica DNA. The clones were verified by restriction enzyme analysis.
To construct the ΔfimBCD strain, a 580-bp SalI-AspI DNA fragment from pCMA6 containing the promoter region, translational start site, and signal sequence for fimB was ligated to a 298-bp PstI-PstI DNA fragment from pCMA4 containing the transcriptional start and signal sequence for fhaC and then cloned into our allelic exchange vector, pEGBR, to generate pCMA10. This plasmid confers sucrose sensitivity and kanamycin resistance. The junctions of the two ligated fragments in pCMA10 were sequenced to confirm that the reading frames of the two fragments were undisturbed. pCMA10 was transformed into SM10.λpir and mobilized into RB50 by conjugation. Transconjugates were selected on medium containing streptomycin, to select against E. coli donors, and kanamycin, to select for B. bronchiseptica recipients. Cells in which a second recombination event had occurred resulting in excision of the plasmid were selected on medium containing sucrose. ΔfimBCD candidates were screened by Western blot analysis using anti-Fim3 antibody, and the genotype of RB63 was confirmed by Southern blot analysis (data not shown).
To construct a complementing clone for the fimBCD locus, the minimal open reading frame of the wild-type fimBCD locus was cloned on pBBR1MCS (chloramphenicol resistant) to create plasmid pCMA11. Complementation of the ΔfimBCD mutation by pCMA11 confirmed that the genomic mutation conferred the observed lack of fimbrial expression.
Immunoblot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (27a), using denaturing 10 and 4 to 12% linear gradient gels (acrylamide-bisacrylamide, 29:1). B. bronchiseptica whole cell lysates were transferred to Immobilon P polyvinylidene difluoride (PVDF) membranes (Millipore) and reacted with the antibody of choice. Dilutions used for the primary antibodies were as follows: anti-Fim2 and anti-Fim3 rabbit polyclonal antibody (courtesy of Fritz Mooi), 1:3,000; anti-FHA rabbit polyclonal antibody (courtesy of Lederle-Praxis), 1:4,000; and rat sera, 1:2,000. Antigen-antibody complexes were detected with horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin (Ig) or anti-rat Ig antibodies (Amersham) at a 1:5,000 dilution and visualized by an enhanced chemiluminescence technique (Amersham).
Enzyme-linked immunosorbent assay (ELISA).
Overnight cultures of B. bronchiseptica whole cells were diluted 1:10 in coating buffer (carbonate-bicarbonate buffer, pH 9.6), and 100 μl of suspension was added to each well of a 96-well microtiter plate. The plates were incubated at 37°C for 2 h in a humidified chamber. The wells were washed with phosphate-buffered saline (PBS) plus 1% Tween 20 (PBS-T); then 150 μl of 5% dry instant milk in PBS-T was added to block unbound sites, and the plates were incubated at 37°C for 1 h. Rat antiserum was used as primary antibody; a 100-μl volume of a 1:10 dilution of each serum was added to the first well of the microtiter plate and twofold serially diluted 11 times. Plates were incubated with the primary antibody at 37°C for 2 h, then washed with PBS-T, and incubated at 37°C for 1 h with 100 μl of secondary antibody. For detection of total serum Ig, HRP-conjugated goat anti-rat antibody (Amersham) was used at a 1:5,000 dilution. For detection of serum IgG and IgM, HRP-conjugated goat anti-rat IgG(H+L) (Pierce) and HRP-conjugated goat anti-rat IgM antibodies (Pierce), respectively, were used at a dilution of 1:2,000. For detection of serum IgG2a and IgG2b, a 1:250 dilution of biotin-conjugated mouse anti-rat IgG2a and anti-rat IgG2b (Pharmingen) was used in conjunction with a 1:4,000 dilution of HRP-conjugated streptavidin (Amersham). Absorbance at 402 nm was plotted against dilution, and titers were expressed as the reciprocal of the serum dilution at the x intercept as extrapolated from the linear part of the curve.
In vitro adhesion assay.
HEp-2 human laryngeal epithelial cells (American Type Culture Collection [ATCC]) were grown in minimal essential medium containing 10% fetal calf serum in 25-ml vented culture flasks. Once the cells had reached about 90% confluency, 105 HEp-2 cells were seeded onto coverslips in standard 12-well tissue culture plates and incubated overnight at 37°C. The culture medium was removed and replaced with Stainer-Scholte broth containing various concentrations of bacterial strains to be tested so as to obtain multiplicities of infection (MOIs) of 10, 20, 100, 200, 400, and 500. The plates were spun at 900 rpm for 5 min and then incubated at 37°C for 10 min. The cells were then washed four times with Hanks' balanced salt solution, fixed with methanol, stained with Giemsa stain, and visualized by light microscopy.
Animal experiments. (i) Intranasal inoculations.
Female Wistar rats were obtained at 3 to 4 weeks of age from Charles River Laboratories (Wilmington, Mass.). Rats were briefly and lightly anesthetized by aerosolized halothane and inoculated intranasally with 5 μl of approximately 500 CFU of B. bronchiseptica in sterile PBS. Inocula were grown at 37°C in Stainer-Scholte medium and normalized by optical density at 600 nm (OD600). The number of CFU delivered was determined by plating dilutions on BG plates. Preinfection sera were collected from the tails of the rats prior to inoculation. At the designated time points postinoculation, rats were euthanized by halothane inhalation and serum samples were obtained by cardiac puncture. Colonization levels in the respiratory tract were determined by removing 1 cm of the trachea, the entire larynx, and the nasal septum, homogenizing each tissue sample in 200 μl of PBS, and growing dilutions of the homogenized tissue on BG plates for 2 days.
(ii) Intratracheal inoculations.
Three- to four-week-old female Wistar rats were anesthetized by injecting them in the biceps femoris muscle with 150 μl of a 4:1 mixture of ketamine and xylosine. A small, deep incision was then made in the platysma, and the sternohyoid and sternomastoid muscles were separated to uncover the trachea. Using a 25-gauge needle, 105 bacteria of each Bordetella strain to be tested were injected as a 20-μl volume directly into the trachea. The incision was then sealed using an autoclip. The number of CFU delivered was determined by plating dilutions of the inoculum on BG plates. Colonization levels were determined as described above.
(iii) Measurement of mucosal IgA.
Three- to four-week-old female Wistar rats were inoculated intranasally, as described above, with 500 CFU of wild-type or RB63 bacteria, and infection was monitored for 30 days. Animals were then sacrificed, and the trachea and lungs were removed intact. After bronchioalveolar lavage (BAL) (17) using 8 ml of PBS or nasopharyngeal lavage (4) using 4 ml of PBS, 2 ml of each sample was concentrated 10-fold by lyophilizing the sample and resuspending it in 200 μl of PBS. IgA titers measured by ELISA using either RB50 or RB63 as antigen and a 1:2,000 dilution of HRP-conjugated mouse anti-rat IgA antibody (ICN) for detection yielded titers that were below the level of detection. Therefore, total (versus Bordetella-specific) IgA titers were determined by quantitative Western blot analysis. A 1:375 dilution of BAL and nasal washing samples were blotted onto a PVDF membrane and probed with 1:500 dilution of HRP-conjugated goat anti-rat IgA. The signal detected was compared to a standard curve made using purified rat IgA. Data analysis was performed on a Macintosh computer using the public domain NIH Image program (developed at the National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/).
RESULTS
Construction of a Fim− B. bronchiseptica strain.
Hybridization studies using chromosomal DNA from B. pertussis, B. parapertussis, and B. bronchiseptica, as well as recent data from the Bordetella genome sequencing project (http://www.sanger.ac.uk), have shown that fimB and its flanking sequences are conserved among the three subspecies (54). Further, DNA sequence analysis of the 3′ end of fimC and all of fimD from B. bronchiseptica revealed that the predicted amino acid sequence of FimD of B. bronchiseptica differs from that of B. pertussis by only one amino acid (52). We took advantage of the high degree of sequence similarity between B. pertussis and B. bronchiseptica to clone the fimBCD locus from the chromosome of wild-type B. bronchiseptica strain RB50 (Fig. 1A and Materials and Methods). Compared to B. pertussis, B. bronchiseptica contained an additional 1 kb of DNA between the 3′ end of fhaB and the 5′ end of fimA. Sequence analysis of the B. bronchiseptica fimA locus revealed that unlike B. pertussis, B. bronchiseptica contains an intact fimA gene capable of encoding a 201-amino-acid polypeptide with a molecular mass of approximately 21 kDa (7). Our results confirm this observation. A few differences in the restriction pattern were also observed at and beyond the 3′ end of fhaC (Fig. 1A).
The fimbrial biogenesis apparatus encoded by fimBCD in B. pertussis appears to be responsible for the assembly and surface localization of all fimbrial serotypes, since mutations in any one of these genes results in the inability to detect fimbriae of any serotype (18, 54). Therefore, to analyze the role of fimbriae in Bordetella pathogenesis, we constructed a B. bronchiseptica strain devoid of fimbriae by deleting the fimBCD gene locus (Fig. 1B). Since fhaC, the gene required for processing and localization of FHA, is both transcriptionally and translationally coupled to fimBCD (44, 53, 54), it was important to construct the deletion strain such that FHA expression would not be altered. We therefore constructed a deletion extending from codon 72 of fimB to codon 327 of fimD such that the transcriptional start site for fhaC was left intact. We also predicted that the ability to translate an in-frame fimB-fimD fusion peptide would overcome problems associated with translational coupling between fimD and fhaC (44). The resulting Fim− strain was designated RB63.
In vitro characterization of RB63.
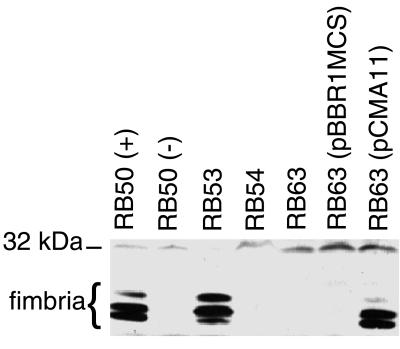
Fimbrial expression in RB63 was examined by Western immunoblot analysis. Whole cell lysates of wild-type and mutant B. bronchiseptica strains were separated by SDS-PAGE on a 10% gel, transferred to a PVDF membrane, and probed with polyclonal antibody generated against the Fim3 subunit of B. pertussis. This antibody recognized a cluster of three bands of molecular masses corresponding to that predicted for the major fimbrial subunits (21 to 24 kDa) as well as other cross-reacting polypeptides (Fig. 2). The 21 to 24-kDa polypeptides were expressed in Bvg+ phase bacteria (RB50 grown under nonmodulating conditions and RB53, a Bvg+ phase-locked strain) but were absent in Bvg− phase bacteria (RB50 grown in the presence of nicotinic acid and RB54, a Bvg− phase-locked strain). These bands were also absent in whole cell lysates of RB63 grown under Bvg+ phase conditions. A similar result was obtained using polyclonal antibodies generated against Fim2 of B. pertussis (data not shown), indicating that RB63 does not express mature fimbrial proteins recognized by either of these antibodies.
FIG. 2.
Western immunoblot analysis of whole-cell lysates of RB50 (+ phase), RB50 (− phase), RB53, RB54, RB63, RB63(pBBR1MCS), and complementation strain RB63(pCMA11). Approximately 25 OD600 units of lysates were loaded per lane and probed with a 1:4,000 dilution of anti-Fim3 antibody. The cluster of bands running at approximately 21 to 24 kDa represents fimbriae. The position of the 32-kDa molecular weight marker is shown on the left.
The individual identities of the three peptide species were not determined. It is possible that they represent different fimbrial subunits (Fim2, Fim3, FimX, and FimA) or degradation products of a single fimbrial subunit type. Probing with a panel of antifimbrial antibodies, however, indicates that deletion of the fimBCD locus eliminates all detectable fimbrial subunits. It is perhaps surprising that fimbrial subunits were not detected in whole cell lysates of RB63 since expression of fim2, fim3, and fimX should not be affected by the ΔfimBCD mutation. It is likely that accumulation of unassembled major pilus subunits signals their degradation in the absence of the FimB chaperone.
To verify that the absence of fimbrial expression in RB63 was due to deletion of the fimBCD genes, the fimBCD gene cluster was cloned into plasmid pBBR1MCS (26) to construct pCMA11, which was then electroporated into RB63 (Fig. 1C). Immunoblot analysis of B. bronchiseptica whole cell lysates probed with anti-Fim3 antibody revealed that the cluster of bands migrating at approximately 21 to 24 kDa that was absent in RB63 reappeared in the complemented strain, RB63(pCMA11) (Fig. 2). Lack of fimbrial expression in RB63 was, therefore, due to the deletion of the fimbrial biogenesis genes.
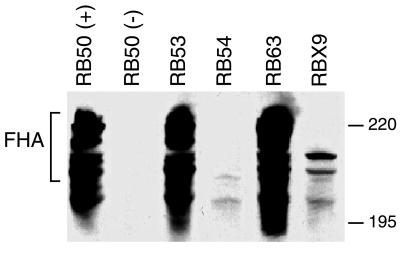
Since fhaC is required for FHA expression, and since FHA itself serves as a putative adhesin for Bordetella attachment, it was important that FHA expression in RB63 was not disrupted by the ΔfimBCD mutation. To examine the effect of the fimBCD deletion on FHA expression, whole cell lysates of wild-type and mutant B. bronchiseptica strains were probed with polyclonal antibody generated against FHA from B. pertussis (Fig. 3). A cluster of bands migrating at approximately 220 kDa, the predicted size for FHA, was detected in lysates of RB50 grown under nonmodulating conditions and of RB53 but was absent in lysates of RB50 grown under modulating conditions, a ΔfhaB strain (RBX9), and RB54. RB63 grown under Bvg+ phase conditions expressed this cluster of bands, indicating that FHA expression in RB63 was unaltered. Secretion of FHA was apparently also unaffected by the ΔfimBCD mutation as FHA was detected in the supernatants of RB63 at levels similar to the wild-type level (data not shown).
FIG. 3.
Western immunoblot analysis of whole-cell lysates of RB50 (+ phase), RB50 (− phase), RB53, RB54, RB63, and RBX9 probed with anti-FHA antibody. The cluster of bands running at approximately 220 kDa represents FHA. Positions of molecular weight markers (220 and 195 kDa) are shown on the right.
Finally, no differences were seen when the growth rates of RB50 and RB63 in supplemented Stainer-Scholte medium were compared, indicating that deletion of the fimbrial biogenesis operon did not alter growth in vitro.
In vitro adherence by fimbrial mutants.
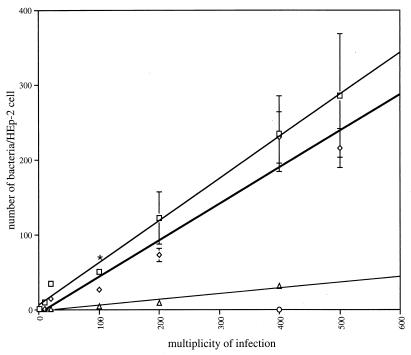
To explore the possibility that fimbriae function as adhesins in vitro, we compared RB63 and wild-type B. bronchiseptica for the ability to adhere to various tissue culture cell lines, including L2 rat lung epithelial cells (ATCC CCL 149), J774 mouse macrophages (ATCC TIB 67), HEp-2 human laryngeal epithelial cells (ATCC CCL 23), Caco-2 human intestinal epithelial cells (ATCC HTB 37), and Intestine 407 human intestinal epithelial cells (ATCC CCL 6). Although B. bronchiseptica efficiently bound all of these cell lines in a Bvg+ phase-dependent manner, a role for fimbriae was not evident. A representative assay with HEp-2 cells, to which B. pertussis has been reported to bind by a fimbria-dependent mechanism (49), is shown in Fig. 4. Briefly, wild-type or mutant B. bronchiseptica strains were added to confluent monolayers of HEp-2 cells at various MOIs and the number of attached bacteria per HEp-2 cell after incubation was determined by counting Giemsa-stained bacteria under a light microscope. As expected, Bvg+ phase bacteria (RB50 grown under nonmodulating conditions) were able to adhere to HEp-2 cells whereas Bvg− phase bacteria (RB54) were not (Fig. 4). The number of RB63 bacteria associated with HEp-2 cells was significantly less than for RB50 when used at an MOI of 100, the same MOI at which van den Berg et al. observed a difference in Fim+ and Fim− B. pertussis binding to these cells (49). However, at all other MOIs tested (10 to 500), RB63 and RB50 bound with nearly equal efficiency. For all other cell lines tested as well, the number of bound RB63 and RB50 was not significantly different at any MOI. Thus, while we propose that fimbriae do play a role in adherence to respiratory epithelium in vivo (see below), they do not appear to mediate adherence to the nonciliated tissue culture cells used in these assays under the conditions employed. An FHA− mutant, in contrast, was dramatically impaired in its ability to bind HEp-2 and other cells at all MOIs tested (Fig. 4 and data not shown). This result confirms a role for FHA in mediating adherence to epithelial cells in vitro and also demonstrates that FHA function was not disrupted by the ΔfimBCD mutation.
FIG. 4.
In vitro analysis of adhesive functions of different strains of B. bronchiseptica. HEp-2 cells infected with RB50, RB63, RB54, or RBX9 at an MOI of 10, 20, 100, 200, 400, or 500 were washed, and the bacteria adhering to 30 representative epithelial cells were counted and averaged after staining with Giemsa stain. The asterisk represents significantly different attachment levels between RB50 and RB63 with a P value of ≤0.0002. Error bars represent the standard error from the mean. Bordetella strains used for this assay were the wild-type strain RB50 (open squares), Fim− strain RB63 (open diamonds), FHA− strain RBX9 (open triangles), and constitutively Bvg− strain RB54 (open circle).
Fimbriae are required for efficient and persistent colonization of the trachea in a rat respiratory infection model.
To investigate the role of fimbriae in vivo, the ability of RB63 to establish infection and to persist at various sites in the respiratory tract was compared with that of RB50 in a rat model (2). This model is extremely sensitive (50% infectious dose of <20 CFU [13]) and allows the role of bacterial virulence factors to be investigated during the course of natural infection (2). In contrast to murine models, in which large numbers of bacteria (104 to 106 CFU of either B. pertussis or B. bronchiseptica) must be delivered in a large volume (25 to 50 μl) to reproducibly observe colonization of the lower respiratory tract, the rat trachea consistently and reproducibly becomes colonized with B. bronchiseptica within 10 days after inoculation with as few as 100 CFU delivered in a 5-μl droplet to the external nares. Moreover, tracheal colonization persists for the life of the animal, allowing us to investigate mechanisms of persistence as well as of initial establishment of infection.
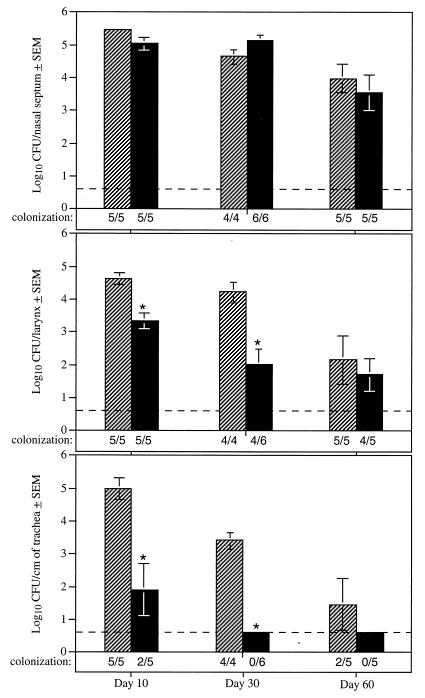
Groups of Wistar rats were inoculated intranasally with approximately 500 CFU of either RB50 or RB63. Animals were sacrificed 10, 30, and 60 days postinoculation, and levels of B. bronchiseptica adhering to the nasal septum, larynx, and trachea were determined. At each of these time points, the numbers of CFU recovered from the nasal cavities of both wild-type- and RB63-inoculated animals were similar, with a mean colonization level of 104 to 105 (Fig. 5). In contrast, at 10 days postinoculation, while B. bronchiseptica were recovered from the tracheas of all animals infected with RB50, only two of the five animals showed evidence of tracheal colonization by RB63. At 30 days postinoculation, the difference between RB50 and RB63 was even more striking: RB50 was recovered from the tracheas of all infected animals, while RB63 was not detected in the tracheas of any of the animals at this time point. At 60 days postinoculation, B. bronchiseptica was recovered from the tracheas of some of the animals infected with RB50 but not from the tracheas of any of the animals infected with RB63. B. bronchiseptica strains lacking fimbriae were, therefore, defective in the ability to colonize the trachea but relatively unaffected in the ability to colonize the nasal cavity. Results were similar for Lewis rats, an in-bred strain derived from Wistar rats.
FIG. 5.
Histograms showing mean colonization levels in the noses, larynxes, and tracheas of 4-week-old, female Wistar rats inoculated intranasally with B. bronchiseptica. Rats were inoculated with the wild-type strain RB50 (hatched bars) and the ΔfimBCD strain RB63 (solid bars) and sacrificed after 10, 30, and 60 days. The lower limit of detection was four bacteria and is represented by the dashed line. Significantly different colonization levels are designated by ∗ for P values of ≤0.005. Error bars represent the standard error from the mean (SEM).
Fimbriae are required for efficient establishment of tracheal colonization.
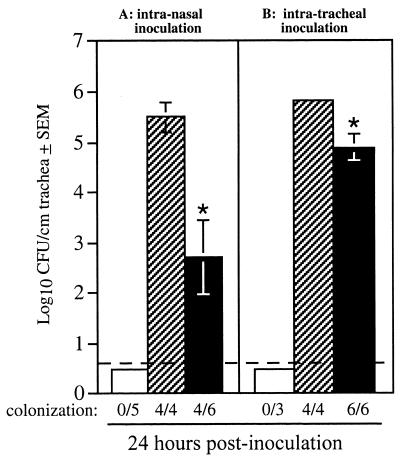
Time course studies of rat respiratory tract colonization following a small-volume (5-μl) intranasal inoculation with B. bronchiseptica have shown that the nasal cavity becomes colonized first, followed by colonization of the trachea (2, 55). In this experimental model of infection, bacteria in the nasal cavity apparently serve as a reservoir from which the trachea is seeded. As such, the tracheal colonization defect displayed by RB63 could be due to a defect in its ability to progress from the nasal cavity to the trachea, an inability to attach specifically to tracheal epithelium, and/or an inability to survive the innate or induced antibacterial defense mechanisms operative in the trachea. To address these possibilities, we performed two types of experiments. In the first, we inoculated rats intranasally with a large volume (50 μl) containing 106 CFU of either wild-type bacteria or RB63. Previous experiments indicate that approximately 10% of the initial inoculum reaches the trachea when administered in this manner. Inoculation by this route, therefore, eliminates the requirement that bacteria be able to translocate from the nasal cavity to the trachea. At 24 h postinoculation, 105 to 106 Bvg+ phase bacteria (wild-type strain RB50 grown under nonmodulating conditions) but not Bvg− phase bacteria (RB54) were recovered per cm of trachea. For RB63, B. bronchiseptica was recovered from the tracheas of only four of the six animals inoculated by this route, and the number of bacteria recovered from those animals was significantly decreased compared to RB50 (103 versus 105 to 106; P < 0.05) (Fig. 6A).
FIG. 6.
Histograms showing mean colonization levels in the tracheas of 4-week-old, female Wistar rats inoculated intranasally with 106 CFU (A) and intratracheally with 105 CFU (B) of B. bronchiseptica. Rats were inoculated with wild-type strain RB50 (hatched bars), the Bvg− phase-locked strain RB54 (open bars), and the ΔfimBCD strain RB63 (solid bars) and sacrificed after 24 h and 5 days. The lower limit of detection was four bacteria and is represented by the dashed line; ∗ indicates that colonization levels for RB63 were significantly lower than those of RB50 with a P value of ≤0.05. Error bars represent the standard error from the mean (SEM).
In the second type of experiment, we inoculated rats intratracheally with 105 CFU delivered in a 20-μl volume and determined the number of CFU remaining in 1 cm of trachea 24 h postinoculation (Fig. 6B). This method also overcomes the requirement for the progression of infection from the nose to the trachea. This procedure, however, requires administration of general anesthesia to the animal, which can result in decreased airway protection by reducing respiratory rate, suppressing gag and coughing reflexes, and inhibiting mucociliary clearance (25). Therefore, this method not only bypasses the requirement for bacterial translocation from the nasal cavity to the trachea but also bypasses several innate host defense mechanisms. In previous experiments, we have shown that general anesthesia can mask the contribution made by virulence factors required for tracheal colonization; for example, general anesthesia nearly eliminates the requirement for FHA (14). Thus, as expected, the tracheal colonization defect displayed by Fim− bacteria was less pronounced than in unanesthetized animals. The fact that Fim− bacteria were defective compared with the wild type in this assay, however, suggests a role for fimbriae even in a compromised host. This could reflect a role for fimbriae to enhance efficiency of adherence to tracheal epithelium, a role that is partially overcome when bacteria are delivered directly to the trachea by intratracheal inoculation or when the host defense mechanisms are overcome by anesthesia.
Taken together, the above results indicate that at least one role for fimbriae during infection is to enhance the efficiency of B. bronchiseptica to colonize the tracheal epithelium.
Role of fimbriae in development of a humoral immune response.
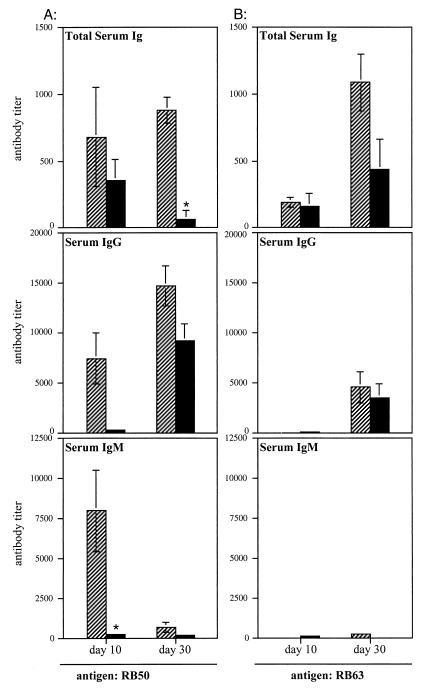
Rabbits and rats infected with wild-type B. bronchiseptica generate a strong antibody response directed against numerous Bvg+ phase factors as well as polypeptides not regulated by Bvg (2, 12). To investigate the role of fimbriae in the development of anti-Bordetella humoral immunity, we quantified anti-Bordetella antibody levels in sera from rats infected with RB50 or RB63 by ELISA using RB50 whole cells as the antigen (Fig. 7A). For these experiments we used Lewis rats, an in-bred strain of Wistar rats, to minimize animal to animal variations. As mentioned earlier, colonization of Lewis rats by RB50 and RB63 was similar to that of Wistar rats. RB50-infected Lewis rats had higher total anti-Bordetella serum antibody levels than RB63-infected rats, and this difference was significant at 30 days postinoculation. Analysis of specific antibody isotypes revealed that although IgG levels were higher in RB50-infected rats than in RB63-infected rats, especially at 10 days postinoculation, differences in IgM levels were even more dramatic: RB50-infected rats generated significantly higher anti-Bordetella serum IgM titers than RB63- infected rats at 10 days postinoculation. By 30 days postinoculation, the difference in IgM titers between RB50- and RB63-infected animals was much less apparent. It is likely that most of the early IgM response had undergone isotype switching by day 30. It must be noted that since the individual ELISAs use different secondary and sometimes tertiary antibodies, comparisons between antibody titers generated in wild-type- and RB63-infected animals can be made only within one class of antibody (i.e., IgM titers cannot be directly compared with IgG or total Ig titers, and the sum of IgG plus IgM titers cannot be expected to equal total Ig titers).
FIG. 7.
Anti-Bordetella antibody titers in sera collected from RB50- and RB63-infected animals were measured by ELISA using RB50 (A) or RB63 (B) whole cells as the antigen. Specifically, total serum antibody, IgM, and IgG titers were determined. Sera collected from Lewis rats sacrificed at 10 and 30 days postinoculation were tested. Hatched bars represent RB50-infected animals; solid bars represent RB63-infected animals; ∗ indicates that antibody titers for RB63-infected animals were significantly lower than those for RB50-infected animals, with a P value of ≤0.02. Error bars represent 1 standard error from the mean. The lower limit of detection of antibody titer is 10 U. Mock (PBS)-infected animals had antibody titers below the level of detection.
To determine if the early IgM response generated in RB50-infected animals was directed against fimbriae, a similar ELISA was conducted with RB63 whole cells, which lack fimbriae, as antigen. Switching from RB50 to RB63 as the antigen eliminated the early IgM and IgG reactivity in sera from RB50-infected animals (Fig. 7B). This result suggests that the majority of the antibody, particularly IgM, induced early during B. bronchiseptica infection may be directed against fimbriae.
The decreased serum IgM response in RB63-infected rats could be due to the absence of fimbrial antigens or the decreased number of bacteria colonizing the trachea. Previous studies from our laboratory showed that although FHA− B. bronchiseptica bacteria could not colonize the rat trachea, they induced a serum IgM response that is similar to that induced by wild-type B. bronchiseptica (reference 14 and data not shown), indicating that lack of tracheal colonization does not a priori result in a decreased IgM response. The decreased serum IgM response in RB63-infected rats, therefore, appears to be directly related to the lack of fimbrial antigens.
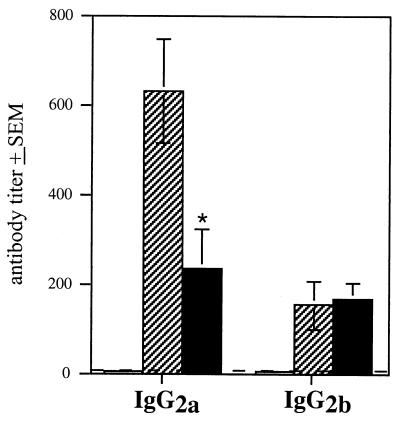
Since the generation of IgG isotypes can reflect whether a response is primarily Th1 or Th2 mediated, we measured relative anti-Bordetella IgG isotype levels in sera from RB50- and RB63-infected animals. Quantitative Western blot analysis as well as ELISAs using wild-type whole cells as antigen were used to determine IgG2a and IgG2b titers. Similar levels of IgG2b titers were induced in both wild-type- and RB63-infected Lewis rats (Fig. 8). However, IgG2a titers generated in wild-type-infected animals were significantly higher than those generated in RB63-infected animals (Fig. 8). These results suggest that fimbriae specifically enhance the induction of IgG2a antibodies.
FIG. 8.
Anti-Bordetella IgG2b titers in sera collected from RB50-, RB63-, and mock-infected Lewis rats were measured by ELISA using RB50 whole cells as antigen. Sera collected from animals sacrificed at 30 days postinoculation were tested. Open bars represent PBS-treated (mock-infected) animals; hatched bars represent RB50-infected animals; solid bars represent RB63-infected animals; ∗ indicates that antibody titers for RB63-infected animals were significantly lower than those for RB50-infected animals, with a P value of ≤0.05. The dashed line represents the lower limit of detection, i.e., 10 U. Error bars indicate standard error from the mean (SEM).
Serum IgA antibodies could not be detected in any of the animals infected with either RB50 or RB63. To measure mucosal IgA levels, we inoculated rats intranasally with 500 CFU of either the wild-type or mutant strain and monitored infection for 30 days. Mucosal IgA titers in BAL fluid and in nasal washings were determined using ELISA and quantitative Western blot analysis. Surprisingly, mucosal IgA titers could not be detected in either wild-type- or RB63-infected animals (data not shown).
DISCUSSION
The existence of several unlinked fimbrial genes on the Bordetella chromosome and the lack of a relevant animal model of infection for B. pertussis have impeded progress in understanding the role of fimbriae in Bordetella respiratory infection. In an attempt to overcome these obstacles, we created a B. bronchiseptica Fim− strain by deleting the fimBCD biogenesis operon and assessed its pathogenicity in a rat model of respiratory infection. As natural hosts for B. bronchiseptica, rats provide a sensitive model for investigating virulence gene function (2). In contrast to wild-type B. bronchiseptica, fimBCD mutant bacteria colonized the trachea only sporadically at early time points and were not recovered from the trachea at or beyond 30 days postinoculation. At each of these time points, however, the fimbrial mutant was able to persist in the nasal cavity at levels similar to wild-type levels. These results confirm and extend previous studies in which it was shown that Fim− B. pertussis strains were defective in tracheal colonization in mice (18, 32). That B. bronchiseptica fimbriae, like B. pertussis fimbriae, contribute to tracheal colonization is further supported by the observation that Fim− bacteria showed a decreased level of tracheal colonization even in anesthetized animals. When a large-volume inoculation of Fim− bacteria was injected directly into the trachea, the Fim− mutant was recovered from the trachea at levels significantly lower than the wild-type level. Together these data demonstrate that fimbriae play an important role in initial establishment of colonization and an essential role in long-term persistence in the trachea.
A role for fimbriae in adherence to epithelial cells was not, however, reflected in our in vitro analyses using various tissue culture cell lines: the binding efficiencies of wild-type and Fim− B. bronchiseptica strains did not differ over a large range of MOIs in any of the cell lines tested, including HEp-2 cells. Although van den Berg et al. reported a role for B. pertussis fimbriae in binding to HEp-2 cells (49), the conditions of their assay differed significantly from ours and the number of wild-type B. pertussis cells binding to HEp-2 cells (∼0.5 bacterium/cell) in their assay was much lower than the number of wild-type B. bronchiseptica (Fig. 4) or B. pertussis (data not shown) binding to HEp-2 cells in our assay (∼50 bacteria/cell) when used at the same MOI of 100. Because a difference in binding specificity for B. pertussis versus B. bronchiseptica fimbriae could have important implications with regard to host range specificity, we are currently constructing Fim− B. pertussis strains to determine if B. pertussis fimbriae affect HEp-2 cell binding in our assay and to establish a basis for comparative analyses.
An interesting effect of fimbrial expression elucidated from this study was their role in the induction of a serum antibody response. Lack of fimbriae resulted in decreased induction of Bordetella-specific IgM antibodies. The fact that the high levels of anti-Bordetella IgM antibodies in sera from RB50-infected animals were detected in ELISAs using RB50 as the antigen but not in ELISAs using RB63 (Fim−) as the antigen suggests that the majority of these antibodies were directed against fimbriae. The fact that an FHA− strain, which is also defective in tracheal colonization, induces an IgM response that is not different from that induced by wild-type B. bronchiseptica suggests that the decreased IgM response seen in animals infected with the Fim− strain is a reflection of fimbrial antigenicity rather than lack of tracheal colonization. B cells can be induced to secrete IgM in response to binding large polymeric antigens. Our results suggest that, perhaps due to their polymeric nature, B. bronchiseptica fimbriae may serve as T-independent antigens that are important for the induction of an early host IgM response.
The specific IgG subtype induced can reflect whether the host immune response is primarily antibody mediated or cell mediated. In rats, IgG1 and IgG2a are considered to be controlled by Th2 cytokines whereas IgG2b and IgG2c are controlled by Th1 cytokines (5, 8, 19). Our analysis showed that while fimbrial mutants were defective in inducing a strong IgG2a response, their ability to induce IgG2b titers was indistinguishable from that of the wild type. However, compared to mock-infected animals, both wild-type- and RB63-infected animals showed an upregulation of both IgG2a and IgG2b titers. These observations suggest that while Bordetella infection induces both Th1 and Th2 responses in the host, fimbriae are important for inducing an immune response that is predominantly Th2 mediated.
Mucosal immune responses form an important defense against respiratory pathogens. Fimbriae have been implicated as important inducers of mucosal responses (23, 45). While Gueirard et al. have reported detection of low levels of IgA in BALB/c mice infected with B. bronchiseptica (20), Bordetella-specific IgA could not be detected in any of the wild-type- or RB63-infected rats in our study. Although consistent with previous studies using B. bronchiseptica in rabbits (12), this result was somewhat surprising. Recent data from our lab suggests that the inability of B. bronchiseptica to induce a mucosal IgA response may be due to the action of toxins secreted by the recently discovered type III secretion system in Bordetella (57). Sera from mice infected with type III-deficient B. bronchiseptica contained high titers of anti-Bordetella IgA, while anti-Bordetella IgA was nearly undetectable in sera from mice infected with wild-type B. bronchiseptica (56). We are currently exploring potential synergystic and/or antagonistic effects of fimbriae and type III secretion in modulation of host immune responses.
Our results suggest two roles for fimbriae: as adhesins enhancing attachment of Bordetella to respiratory epithelium in the trachea, and as immunomodulators influencing the development of the humoral immune response to Bordetella infection. Are these two functions related? If recognition of pilus-mediated attachment by the host is subject to different levels of sensitivity based on the particular niche in the respiratory tract that becomes colonized, then the answer may be yes. If so, the niches distinguished by fimbriae must be present in the nasopharynx, since FHA− bacteria, which are also defective specifically in tracheal colonization (14), induce a serum antibody response that is qualitatively indistinguishable from that induced by wild-type B. bronchiseptica. This hypothesis suggests that fimbriae may mediate attachment to specific cell types within the nasopharynx that influence the subsequent humoral immune response that develops. Alternatively, Fim+ and Fim− bacteria may adhere to the same population of cells, but fimbriae may mediate binding to a specific receptor(s) involved in controlling the cytokine pattern induced and hence the characteristics of the immune response that ultimately develops. To investigate these hypotheses, we are currently developing in vivo and ex vivo approaches to identify specific cells in the respiratory tract to which Bordetella bind, the specific receptor(s) involved, and the cytokine responses that result.
It is important to recognize, however, that although our data support the hypothesis that Bordetella fimbriae mediate adherence to tracheal epithelium and, like others, we are tempted to propose that they do indeed function as epithelial cell adhesins, this role has not yet been definitively proven. Lack of efficient establishment and persistence of tracheal colonization by Fim− bacteria could result solely from altered interactions between B. bronchiseptica and components of the host's innate or adaptive immune systems. We are hopeful that the development of relevant respiratory tissue models will allow us to definitively determine the exact functions of these important structures in Bordetella pathogenesis.
ACKNOWLEDGMENTS
We thank members of our laboratory for helpful discussions and comments on the manuscript, and we thank F. R. Mooi and Lederle-Praxis for antibodies.
We are supported by grants from NIH (AI43986 to P.A.C. and AI38417 to J.F.M.) and a research training grant from the American Lung Association of California to S.M.
REFERENCES
- 1.Abraham S N, Jonsson A B, Normark S. Fimbriae-mediated host-pathogen cross-talk. Curr Opin Microbiol. 1998;1:75–81. doi: 10.1016/s1369-5274(98)80145-8. [DOI] [PubMed] [Google Scholar]
- 2.Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 3.Akerley B J, Monack D M, Falkow S, Miller J F. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol. 1992;174:980–990. doi: 10.1128/jb.174.3.980-990.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asakura K, Saito H, Hata M, Kataura A. Antigen specific immunoglobulin production by NALT lymphocytes in rats. Acta Oto-Laryngol Suppl. 1996;523:80–83. [PubMed] [Google Scholar]
- 5.Binder J, Graser E, Hancock W W, Wasowska B, Sayegh M H, Volk H D, Kupiec-Weglinski J W. Downregulation of intragraft IFN-gamma expression correlates with increased IgG1 alloantibody response following intrathymic immunomodulation of sensitized rat recipients. Transplantation. 1995;60:1516–1524. doi: 10.1097/00007890-199560120-00025. [DOI] [PubMed] [Google Scholar]
- 6.Bordet J, Gengou O. L'endotoxine coquelucheuse. Ann Inst Pasteur. 1909;23:415–419. [Google Scholar]
- 7.Boschwitz J S, van der Heide H G J, Mooi F R, Relman D A. Bordetella bronchiseptica expresses the fimbrial structural subunit gene fimA. J Bacteriol. 1997;179:7882–7885. doi: 10.1128/jb.179.24.7882-7885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cetre C, Pierrot C, Cocude C, Lafitte S, Capron A, Capron M, Khalife J. Profiles of Th1 and Th2 cytokines after primary and secondary infection by Schistosoma mansoni in the semipermissive rat host. Infect Immun. 1999;67:2713–2719. doi: 10.1128/iai.67.6.2713-2719.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers S P, Prior S E, Barstow D A, Minton N P. The pMTL nic- cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene. 1988;68:139–149. doi: 10.1016/0378-1119(88)90606-3. [DOI] [PubMed] [Google Scholar]
- 10.Connell H, Hedlund M, Agace W, Svanborg C. Bacterial attachment to uro-epithelial cells: mechanisms and consequences. Adv Dent Res. 1997;11:50–58. doi: 10.1177/08959374970110011701. [DOI] [PubMed] [Google Scholar]
- 11.Cotter P A, Miller J F. BvgAS dependent phenotypic modulation of Bordetella species. In: Rappuoli R, Scarlato V, Arico B, editors. Signal transduction and bacterial virulence. R. G. Austin, Tex: Landes; 1995. pp. 21–42. [Google Scholar]
- 12.Cotter P A, Miller J F. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter P A, Miller J F. Genetic analysis of the Bordetella-host interaction. Ann N Y Acad Sci. 1996;797:65–76. doi: 10.1111/j.1749-6632.1996.tb52950.x. [DOI] [PubMed] [Google Scholar]
- 14.Cotter P A, Yuk M H, Mattoo S, Akerley B J, Boschwitz J, Relman D A, Miller J F. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun. 1998;66:5921–5929. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuzzoni A, Pedroni P, Riboli B, Grandi G, de Ferra F. Nucleotide sequence of the fim3 gene from Bordetella pertussis and homology to fim2 and fimX gene products. Nucleic Acids Res. 1990;18:1640. doi: 10.1093/nar/18.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domenighini M, Relman D, Capiau C, Falkow S, Prugnola A, Scarlato V, Rappuoli R. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol Microbiol. 1990;4:787–800. doi: 10.1111/j.1365-2958.1990.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 17.Ganz T, Sherman M P, Selsted M E, Lehrer R I. Newborn rabbit alveolar macrophages are deficient in two microbicidal cationic peptides, MCP-1 and MCP-2. Am Rev Respir Dis. 1985;132:901–904. doi: 10.1164/arrd.1985.132.4.901. [DOI] [PubMed] [Google Scholar]
- 18.Geuijen C A, Willems R J, Bongaerts M, Top J, Gielen H, Mooi F R. Role of the Bordetella pertussis minor fimbrial subunit, FimD, in colonization of the mouse respiratory tract. Infect Immun. 1997;65:4222–4228. doi: 10.1128/iai.65.10.4222-4228.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gracie J A, Bradley J A. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26:1217–1221. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 20.Gueirard P, Minoprio P, Guiso N. Intranasal inoculation of Bordetella bronchiseptica in mice induces long-lasting antibody and T-cell mediated immune responses. Scand J Immunol. 1996;43:181–192. doi: 10.1046/j.1365-3083.1996.d01-30.x. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson L, Hallander H O, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. 1996;334:349–355. doi: 10.1056/NEJM199602083340602. . (Erratum, 334:1207.) [DOI] [PubMed] [Google Scholar]
- 22.Hahn H P. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene. 1997;192:99–108. doi: 10.1016/s0378-1119(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 23.Hedlund M, Svensson M, Nilsson A, Duan R D, Svanborg C. Role of the ceramide-signaling pathway in cytokine responses to P-fimbriated Escherichia coli. J Exp Med. 1996;183:1037–1044. doi: 10.1084/jem.183.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hultgren S J, Abraham S, Caparon M, Falk P, de St. Geme J W, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 25.Konrad F, Marx T, Schrag M, Kilian J. Combination anesthesia and bronchial transport velocity. Effects of anesthesia with isoflurane, fentanyl, vecuronium, and oxygen-nitrous oxide breathing on bronchial mucus transport. Anaesthesist. 1997;46:403–407. doi: 10.1007/s001010050417. [DOI] [PubMed] [Google Scholar]
- 26.Kovach M E, Phillips R W, Elzer P H, Roop II R M, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 27.Kuehn M J. Establishing communication via Gram-negative bacterial pili. Trends Microbiol. 1997;5:130–132. doi: 10.1016/S0966-842X(96)30045-0. [DOI] [PubMed] [Google Scholar]
- 27a.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Livey I, Duggleby C J, Robinson A. Cloning and nucleotide sequence analysis of the serotype 2 fimbrial subunit gene of Bordetella pertussis. Mol Microbiol. 1987;1:203–209. doi: 10.1111/j.1365-2958.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- 29.Locht C, Bertin P, Menozzi F D, Renauld G. The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Mol Microbiol. 1993;9:653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 30.Locht C, Geoffroy M C, Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992;11:3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniatis T, Weintraub H. Gene expression and differentiation [editorial] Curr Opin Genet Dev. 1992;2:197–198. [PubMed] [Google Scholar]
- 32.Mooi F R, Jansen W H, Brunings H, Gielen H, van der Heide H G, Walvoort H C, Guinee P A. Construction and analysis of Bordetella pertussis mutants defective in the production of fimbriae. Microb Pathog. 1992;12:127–135. doi: 10.1016/0882-4010(92)90115-5. [DOI] [PubMed] [Google Scholar]
- 33.Musser J M, Hewlett E L, Peppler M S, Selander R K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986;166:230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassif X, Marceau M, Pujol C, Pron B, Beretti J L, Taha M K. Type-4 pili and meningococcal adhesiveness. Gene. 1997;192:149–53. doi: 10.1016/s0378-1119(96)00802-5. [DOI] [PubMed] [Google Scholar]
- 35.Pedroni P, Riboli B, de Ferra F, Grandi G, Toma S, Arico B, Rappuoli R. Cloning of a novel pilin-like gene from Bordetella pertussis: homology to the fim2 gene. Mol Microbiol. 1988;2:539–543. doi: 10.1111/j.1365-2958.1988.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 36.Preston N W, Timewell R M, Carter E J. Experimental pertussis infection in the rabbit: similarities with infection in primates. J Infect. 1980;2:227–235. doi: 10.1016/s0163-4453(80)90650-7. [DOI] [PubMed] [Google Scholar]
- 37.Ried J L, Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in Gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57:239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Soto G E, Hultgren S J. Bacterial adhesins: common themes and variations in architecture and assembly. J Bacteriol. 1999;181:1059–1071. doi: 10.1128/jb.181.4.1059-1071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stibitz S, Weiss A A, Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988;170:2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stibitz S, Yang M S. Genetic and immunological studies on polypeptides encoded by the vir locus of Bordetella pertussis. Dev Biol Stand. 1991;73:87–91. [PubMed] [Google Scholar]
- 42.Stibitz S, Yang M S. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stock J B, Surette M G, Levit M G, Park P. Two-component signal transduction systems: structure-function relationships and mechanisms of catalysis. In: Hoch J, Silhavy T, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 25–51. [Google Scholar]
- 44.Stormo G D, Schneider T D, Gold L M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982;10:2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svanborg C, Hedlund M, Connell H, Agace W, Duan R D, Nilsson A, Wullt B. Bacterial adherence and mucosal cytokine responses. Receptors and transmembrane signaling. Ann NY Acad Sci. 1996;797:177–190. doi: 10.1111/j.1749-6632.1996.tb52959.x. [DOI] [PubMed] [Google Scholar]
- 46.Uhl M A, Miller J F. The Bordetella BvgAS signal transduction system. In: Silhavy T, Hoch J A, editors. Signal transducing genetic switches. Washington, D.C.: ASM Press; 1995. pp. 333–349. [Google Scholar]
- 47.Uhl M A, Miller J F. Central role of the BvgS receiver as a phosphorylated intermediate in a complex two-component phosphorelay. J Biol Chem. 1996;271:33176–33180. doi: 10.1074/jbc.271.52.33176. [DOI] [PubMed] [Google Scholar]
- 48.Uhl M A, Miller J F. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 49.van den Berg B M, Beekhuizen H, Willems R J, Mooi F R, van Furth R. Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infect Immun. 1999;67:1056–1062. doi: 10.1128/iai.67.3.1056-1062.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss A A, Hewlett E L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- 51.Willems R, Paul A, van der Heide H G, ter Avest A R, Mooi F R. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990;9:2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willems R J, Geuijen C, van der Heide H G, Matheson M, Robinson A, Versluis L F, Ebberink R, Theelen J, Mooi F R. Isolation of a putative fimbrial adhesin from Bordetella pertussis and the identification of its gene. Mol Microbiol. 1993;9:623–634. doi: 10.1111/j.1365-2958.1993.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 53.Willems R J, Geuijen C, van der Heide H G, Renauld G, Bertin P, van den Akker W M, Locht C, Mooi F R. Mutational analysis of the Bordetella pertussis fim/fha gene cluster: identification of a gene with sequence similarities to haemolysin accessory genes involved in export of FHA. Mol Microbiol. 1994;11:337–347. doi: 10.1111/j.1365-2958.1994.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 54.Willems R J, van der Heide H G, Mooi F R. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol Microbiol. 1992;6:2661–2671. doi: 10.1111/j.1365-2958.1992.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 55.Yoda H, Nakayama K, Nakagawa M. Experimental infection of Bordetella bronchiseptica to rabbits. Jikken Dobutsu. 1982;31:113–118. [PubMed] [Google Scholar]
- 56.Yuk, M. H., E. T. Harvill, P. A. Cotter, and J. F. Miller. Disruption of type III secretion in Bordetella bronchiseptica leads to increased clearance from the tracheas of immunocompetent mice but increased virulence in immunodeficient mice. Mol. Microbiol., in press.
- 57.Yuk M H, Harvill E T, Miller J F. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]