Abstract
Simple Summary
This research investigates the adherence and compliance to the ERAS pathway in patients operated for rectal cancer; the results highlights the important role of early postoperative compliance to the postoperative pathway with the development of complications.
Abstract
Early postoperative low compliance to enhanced recovery protocols has been associated with morbidity following colon surgery. The purpose of this study is to evaluate the possible causes of early postoperative low compliance to the enhanced recovery pathway and its relationship with morbidity following rectal surgery for cancer. A total of 439 consecutive patients who underwent elective surgery for rectal cancer have been included in the study. Compliance to enhanced recovery protocol on postoperative day (POD) 2 was evaluated in all patients. Indicators of compliance were naso-gastric tube and urinary catheter removal, recovery of both oral feeding and mobilization, and the stopping of intravenous fluids. Low compliance on POD 2 was defined as non- adherence to two or more items. One-third of patients had low compliance on POD 2. Removal of urinary catheter, intravenous fluids stop, and mobilization were the items with lowest adherence. Advanced age, duration of surgery, open surgery and diverting stoma were predictive factors of low compliance at multivariate analysis. Overall morbidity and major complications were significantly higher (p < 0.001) in patients with low compliance on POD 2. At multivariate analysis, failure to remove urinary catheter on POD 2 (OR = 1.83) was significantly correlated with postoperative complications. Low compliance to enhanced recovery protocol on POD 2 was significantly associated with morbidity. Failure to remove the urinary catheter was the most predictive indicator. Advanced age, long procedure, open surgery and diverting stoma were independent predictive factors of low compliance.
Keywords: rectal surgery, enhanced recovery, overall morbidity, ERAS compliance, low pneumoperitoneum, TAP block
1. Introduction
Enhanced recovery protocols have been associated with a significant improvement of outcome after major surgery for gastrointestinal cancer [1,2,3]. The elderly and patients with multiple comorbidities can be included in the enhanced recovery program, but often require a tailored protocol [4,5].
Early postoperative low compliance to an enhanced recovery protocol has been reported in about one third of patients following elective colonic resection [6,7]. Patients with early low compliance after colonic resection had significant higher morbidity and longer hospital stays [8]. Few data are currently available on both the rate and causes of early low compliance to enhanced recovery protocols following rectal surgery and its relationship with morbidity occurring afterwards.
The first experiences with enhanced recovery protocols were carried out more than 20 years ago. New items reducing perioperative stress and invasiveness of surgery have been subsequently proposed [9,10]. Promising preliminary results have been obtained with low-pressure pneumoperitoneum, multimodal analgesia including abdominal wall blocks, and inferior mesenteric artery preservation in upper rectal cancer surgery [11,12,13,14].
The purpose of this study is to assess which variables can be associated with low compliance to enhanced recovery pathways. The relationship between low compliance and overall postoperative morbidity has also been investigated.
2. Materials and Methods
The present study is performed in accordance with STROBE guidelines [15]. Consecutive patients who underwent elective surgery for rectal cancer in seven Italian hospitals have been included in the study. Patients with combined resections (rectal and other viscera) were excluded. All patients have been prospectively registered in the database of the PeriOperative Italian Society. Each hospital applied a comprehensive ERAS pathway according to the ERAS® Society recommendation in colorectal surgery [16] and followed a pathway implementation program before starting the study [8].
In 35 consecutive patients who underwent surgery for upper rectal cancer at Monza Hospital, Monza, Italy, (Monza subgroup), three operative items have been added to the study protocol: TAP (transversus abdominis plane) block instead thoracic epidural catheter, low pneumoperitoneum (8 mmHg), and inferior mesenteric artery (IMA) sparing.
Demographics, perioperative variables, adherence to each item of the protocol, and short-term outcome parameters were prospectively collected in all patients. Indicators of postoperative compliance were naso-gastric tube and urinary catheter removal, recovery of oral feeding and mobilization out of bed, and the stopping of intravenous fluids. Removal of the naso-gastric tube was planned at the end of surgery; and patients were mobilized the day of surgery. The starting of oral feeding and removal of urinary catheter were planned on postoperative day 1. Intravenous fluid infusion was discontinued as early as possible in accordance with the recovery of oral feeding. Low compliance on postoperative day (POD) 2 was defined as non-adherence to two or more items [8].
Criteria to identify each postoperative complication were defined a priori [17] and the Clavien-Dindo classification has been used to grade their severity [18]. Complications graded as IIIb to V were considered as major. Discharge criteria and time to readiness for discharge were defined according to a previous study [19]. Any hospital readmission due to postoperative complications occurring within 30 days after discharge has been registered.
Statistical Analysis
Continuous variables were reported as median along with the interquartile range (IQR) and compared with a Mann-Whitney’s U test, while categorical variables were reported as percentages and compared with the Chi square test. Variables predictive of complications were individuated with uni and multiple logistic regression methods. The analysis of factors associated with low compliance on POD2 was carried out in uneventful patients. Statistics were performed with SPSS 25 (IBM Corp. Released 2017, IBM SPSS Statistics for Windows, Version 25.0. IBM Corp: Armonk, NY, USA).
3. Results
The present analysis includes 439 consecutive cancer patients who underwent elective rectal resection. An (American Society of Anesthesiology) ASA score of 3–4 was found in 141 (32.1%) patients, neoadjuvant chemo-radiotherapy was carried out in 113 (25.7%) patients, and laparoscopic surgery was successfully performed in 373 (82.7%) patients (Table 1).
Table 1.
Patients’ characteristics.
| Variable | Median | IQR | N | % | |
|---|---|---|---|---|---|
| Age | 68.00 | 59.76–76.5 | |||
| Sex | M | 276 | 62.9% | ||
| F | 163 | 37.1% | |||
| BMI | 24.82 | 22.59–27.68 | |||
| BMI class | <25 | 223 | 50.8 | ||
| 25–29 | 164 | 37.4 | |||
| >30 | 52 | 11.8 | |||
| ASA score | 1 | 60 | 13.7% | ||
| 2 | 238 | 54.2% | |||
| 3 | 127 | 28.9% | |||
| 4 | 14 | 3.2% | |||
| Diabetes | 53 | 12.1% | |||
| Preoperative Haemoglobin | 13.40 | 12.2–14.5 | |||
| Neoadjuvant | CT-RT | 113 | 25.7% | ||
| Mechanical bowel preparation | 172 | 39.3% | |||
| Surgery | Anterior resection | 403 | 91.8% | ||
| Abdominoperineal amputation | 36 | 8.2% | |||
| Duration of Surgery (min) | 243 | 191–300 | |||
| Intraoperative inotropes | 23 | 5.3% | |||
| Successful laparoscopy | 363 | 82.7% | |||
| Laparoscopy converted to open surgery | 10 | 2.7% | |||
| Diverting Stoma | 241 | 54.9% | |||
| Drain | 345 | 78.8% | |||
The overall adherence to preoperative and operative items was 81.3%. No patient received oral antibiotics before surgery. Mechanical bowel preparation was carried out in 172 (39.3%) patients, while an abdominal drain was placed in 345 (78.8%). The naso-gastric tube was removed at the end of surgery in 398 (90.8%) patients.
Table 2 shows that a low protocol compliance on POD 2 was found in one-third of patients. The items with the lowest adherence were removal of the urinary catheter, the stopping of intravenous fluids, and mobilization.
Table 2.
Cumulative compliance with postoperative era items.
| Item | POD | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Naso-gastric tube removal | 90.8 | 96.6 | 97.5 | 99.1 | 99.5 |
| Solid Diet | 7.3 | 60.0 | 81.2 | 91.8 | 95.4 |
| Stop IV infusion | 1.6 | 41.2 | 65.0 | 79.2 | 85.4 |
| Urinary Catheter removal | 1.7 | 41.1 | 64.5 | 84.9 | 91.7 |
| Mobilization > 4 h | 8.0 | 43.2 | 65.0 | 74.6 | 81.4 |
Table 3 shows that advanced age, long surgical procedure, open surgery, and diverting stoma were significantly associated to low compliance on POD 2, whereas operative volemia monitoring was associated with high compliance (p = 0.06).
Table 3.
Variables associated with low compliance on postoperative day 2.
| Variable | Postoperative Compliance | Univariate Analysis | Multivariate Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High Compliance | Low Compliance | OR | 95% CI | Sign. | OR | 95% CI | Sign. | ||||||
| N/Median | %/IQR | N/Median | %/IQR | ||||||||||
| Men | 67 | 38.1% | 36 | 40.9% | 1.126 | 0.668 | 1.899 | 0.656 | |||||
| Age (years) | 67 | 58.22–75 | 71.95 | 61–77.74 | 1.032 | 1.007 | 1.057 | 0.011 | 1.036 | 1.006 | 1.067 | 0.018 | |
| BMI | 24.82 | 22.2–27.39 | 24.10 | 22.12–27.14 | 0.992 | 0.926 | 1.062 | 0.808 | |||||
| BMI class | <25 | 89 | 51.4% | 51 | 58.0% | 1 (ref) | |||||||
| 25–29 | 65 | 37.6% | 27 | 30.7% | 0.725 | 0.412 | 1.276 | 0.265 | |||||
| >30 | 19 | 11.0% | 10 | 11.4% | 0.918 | 0.397 | 2.127 | 0.843 | |||||
| ASA score | 1 | 27 | 15.3% | 10 | 11.4% | 1 (ref) | |||||||
| 2 | 99 | 56.3% | 48 | 54.5% | 1.309 | 0.586 | 2.923 | 0.511 | |||||
| 3 | 42 | 23.9% | 29 | 33.0% | 1.864 | 0.784 | 4.433 | 0.159 | |||||
| 4 | 8 | 4.5% | 1 | 1.1% | 0.338 | 0.037 | 3.052 | 0.334 | |||||
| Diabetes | 15 | 8.5% | 14 | 15.9% | 2.031 | 0.932 | 4.424 | 0.075 | |||||
| Haemoglobin (g/dL) | 13.70 | 12.8–14.6 | 13.10 | 12.3–14.3 | 0.829 | 0.696 | 0.989 | 0.037 | 0.913 | 0.742 | 1.124 | 0.392 | |
| Neoadjuvant CT/RT | 36 | 20.5% | 19 | 21.6% | 1.071 | 0.573 | 2.003 | 0.830 | |||||
| Mechanical bowel preparation | 71 | 40.6% | 33 | 37.5% | 0.879 | 0.519 | 1.488 | 0.631 | |||||
| Preoperative glucidic drink | 108 | 61.4% | 59 | 67.0% | 1.281 | 0.748 | 2.194 | 0.367 | |||||
| Epidural catheter | 47 | 26.9% | 26 | 29.5% | 1.142 | 0.648 | 2.013 | 0.646 | |||||
| Intraoperative advanced volemia monitoring | 59 | 33.7% | 13 | 14.8% | 0.341 | 0.175 | 0.664 | 0.002 | 0.48 | 0.222 | 1.036 | 0.062 | |
| Operative inotropes | 5 | 2.9% | 4 | 4.5% | 1.619 | 0.424 | 6.186 | 0.481 | |||||
| Operative warming | 172 | 98.3% | 88 | 100.0% | 1.760 | 0.769 | 2.356 | 0.897 | |||||
| Duration of Surgery | 215 | 175.5–275 | 263 | 210–317.5 | 1.006 | 1.003 | 1.009 | 0.000 | 1.006 | 1.002 | 1.010 | 0.002 | |
| Open Surgery | 15 | 8.5% | 20 | 22.7% | 3.157 | 1.526 | 6.531 | 0.002 | 2.732 | 1.173 | 6.362 | 0.02 | |
| Abdominoperineal amputation | 12 | 6.8% | 12 | 13.6% | 1 (ref) | ||||||||
| Anterior resection | 164 | 93.2% | 76 | 86.4% | 0.463 | 0.199 | 1.079 | 0.074 | |||||
| Diverting stoma | 70 | 39.8% | 58 | 65.9% | 2.928 | 1.716 | 4.995 | 0.000 | 1.907 | 1.033 | 3.518 | 0.039 | |
| Drain | 141 | 80.1% | 76 | 86.4% | 1.572 | 0.771 | 3.206 | 0.213 | 1.293 | 0.573 | 2.916 | 0.536 | |
ref: reference.
Table 4 reports short-term outcomes. Postoperative morbidity occurred in 149 (32.6%) patients and major complications occurred in 27 (6.2%) patients. Twenty-four (5.5%) patients underwent reoperation. Median time to readiness for discharge and length of hospital stay were 5 (4–8) and 6 (5–8) days. The readmission rate was 3.0% (13 patients).
Table 4.
Patient’s outcomes.
| Variable | Median | IQR | N | % | |
|---|---|---|---|---|---|
| Postoperative Pain (NRS) | POD 1 | 2 | 1–4 | ||
| POD 2 | 2 | 0–3 | |||
| POD 3 | 1 | 0–2 | |||
| POD 4 | 0 | 0–1 | |||
| Overall morbidity | 149 | 32.6% | |||
| Major complication | 27 | 6.2% | |||
| Clavien-Dindo grade | 0 | 290 | 67.0% | ||
| 1 | 54 | 12.5% | |||
| 2 | 44 | 10.2% | |||
| IIIa | 18 | 4.2% | |||
| IIIb | 22 | 5.1% | |||
| Iva | 3 | 0.7% | |||
| Ivb | 2 | 0.5% | |||
| V | 0 | 0.0% | |||
| Anastomotic leak | 27 | 6.2% | |||
| Abdominal abscess | 8 | 1.8% | |||
| Respiratory complication | 11 | 2.5% | |||
| Wound infection | 16 | 3.7% | |||
| Urinary infection | 12 | 2.8% | |||
| Reoperation | 24 | 5.5% | |||
| Readmission | 13 | 3.0% | |||
| Day Fit for Discharge | 5 | 4–8 | |||
| Length of stay | 6 | 5–8 | |||
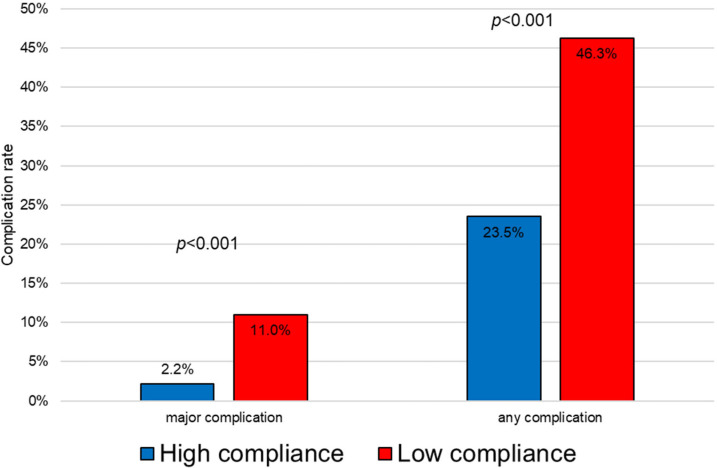
Figure 1 shows that patients with low compliance on POD 2 had higher overall morbidity and major complications. At multivariate analysis, failure to remove the urinary catheter on POD 2 was significantly correlated with postoperative complications (Table 5).
Figure 1.
Correlation between morbidity and compliance on postoperative day 2.
Table 5.
Variables associated with any complication.
| Variables | Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Sign. | OR | 95% CI | Sign. | ||||
| ASA score 3–4 | 1.211 | 0.793 | 1.849 | 0.375 | |||||
| Age | 1.000 | 0.983 | 1.018 | 0.968 | |||||
| Men | 1.513 | 0.990 | 2.312 | 0.056 | |||||
| Diabetes | 1.420 | 0.787 | 2.564 | 0.245 | |||||
| BMI < 25 | 1 (ref) | ||||||||
| BMI 25–29 | 1.142 | 0.735 | 1.774 | 0.555 | |||||
| BMI ≥ 30 | 1.707 | 0.918 | 3.175 | 0.091 | |||||
| Neadjuvant CT/RT | 1.388 | 0.888 | 2.170 | 0.150 | |||||
| Mechanical bowel preparation | 1.037 | 0.690 | 1.561 | 0.860 | |||||
| Surgery | Anterior resection | 1 (ref) | |||||||
| Abdominoperineal amputation | 1.107 | 0.529 | 2.318 | 0.787 | |||||
| Successful_laparoscopy | 0.693 | 0.416 | 1.155 | 0.159 | |||||
| Failure to remove NG tube on POD2 | 1.784 | 0.535 | 5.949 | 0.346 | |||||
| Failure to have solid diet on POD2 | 2.113 | 1.293 | 3.452 | 0.003 | 1.357 | 0.737 | 2.498 | 0.327 | |
| Failure to stop IV fluids on POD2 | 2.191 | 1.445 | 3.321 | 0.000 | 1.518 | 0.895 | 2.574 | 0.122 | |
| Failure to remove urinary catehter on POD2 | 2.359 | 1.550 | 3.591 | 0.000 | 1.806 | 1.133 | 2.878 | 0.013 | |
| Failure to mobilize >4 h on POD 2 | 1.835 | 1.206 | 2.793 | 0.005 | 1.466 | 0.936 | 2.295 | 0.095 | |
| Poorly controlled pain on POD2 (NRS > 3) | 1.882 | 1.142 | 3.101 | 0.013 | 1.430 | 0.839 | 2.438 | 0.189 | |
ref: reference.
Table 6 reports data on patients of the Monza subgroup who have a higher rate of ASA 3 compared to the overall series. The TAP block and IMA sparing technique were successfully performed in all patients, while low pneumoperitoneum failed in 5 (14.2%) patients who needed an increase up to 12 mmHg. Lymph-node collection and postoperative pain score were similar to the overall series, while early mobilization was observed in 32 (91.4%) patients. No anastomotic leak occurred.
Table 6.
Monza subgroup patients’ characteristics.
| Variable | Value | Median | IQR | N | % |
|---|---|---|---|---|---|
| Men | 18 | 51 | |||
| Age | 71 | 61.5–80 | |||
| BMI class | <25 | 16 | 46 | ||
| 25–29 | 11 | 31 | |||
| ≥30 | 8 | 23 | |||
| ASA score | 1 | 2 | 5.7 | ||
| 2 | 13 | 37 | |||
| 3 | 19 | 54 | |||
| 4 | 2 | 5.7 | |||
| IMA sparing | 35 | 100 | |||
| TAP-block | 35 | 100 | |||
| Failure Low Pneumop. | 5 | 8.6 | |||
| Successful laparoscopy | 35 | 100 | |||
| Lymph nodes harvested | 15 | 12–21 | |||
| NRS | POD 1 | 2 | 2–5 | ||
| POD 2 | 2 | 2–5 | |||
| POD 3 | 1 | 1–3 | |||
| POD 4 | 0 | 0–2 | |||
| Mobilization > 4 h POD2 | 32 | 91 | |||
| Clavien Dindo | 0 | 21 | 60 | ||
| 1 | 4 | 11 | |||
| 2 | 6 | 17 | |||
| 3a | 1 | 2.8 | |||
| 3b | 1 | 2.8 | |||
| 4 | 0 | 0 | |||
| 5 | 0 | 0 | |||
| Anastomotic leak | 0 | 0 | |||
| Fit for discharge, d | 6 | 4–7 | |||
| LOS, d | 7 | 5–8 | |||
| Readmission | 3 | 8.6 |
4. Discussion
Low compliance to an enhanced recovery protocol was found in about one- third of patients after rectal surgery. Patients with low compliance on POD 2 had higher overall morbidity and major complications. Variables associated with early low compliance were advanced age, long procedure, open surgery, and diverting stoma. Upon multivariate analysis, failure to remove the urinary catheter on POD 2 was significantly correlated with postoperative complications.
Operative fluid overload and inadequate pain control can be determinants of postoperative low compliance to enhanced recovery protocol [20,21,22]; however, low compliance can also be considered an early sign for underlying complications. In a series of colon cancer patients, the failure to remove the urinary catheter and to stop intravenous fluids on POD 2 was a predictive indicator of morbidity [8]. To detect an early low compliance might yield to identify patients with higher risk to develop complications afterwards. These patients could benefit from proper diagnostics and the early treatment of complications. This is very important, especially in patients with advanced age and multiple comorbidities.
Previous studies found that minimally invasive colorectal surgery had an independent role to favor early postoperative recovery, to reduce overall morbidity, and to shorten the hospital stay [9,10,23,24]. In the present series, successful laparoscopic surgery was widely performed and conversion to laparotomy was necessary in only 2.7% of patients. A multivariate analysis showed that open surgery was the most important variable associated with low compliance to enhanced recovery protocol on POD 2. Our data also suggest that the elderly and patients who were given a long surgical procedure or diverting stoma had a lower compliance rate. Therefore, a tailored approach with a tight postoperative monitoring should be performed in these patients.
The rate of low compliance on POD 2 was similar to that reported following colonic surgery [8]. The lowest protocol adherence was found for removal of the urinary catheter and the stopping of intravenous fluids, whereas the highest adherence was found for naso-gastric tube removal and oral feeding recovery. Early low compliance to postoperative protocol was significantly associated with overall morbidity and major complications. In particular, failure to remove the urinary catheter on POD 2 played an independent role to favor postoperative morbidity. A delayed removal of urinary catheter increases urinary tract infections, and reduces the patient’s mobilization favoring respiratory complications [25].
The impact of the three additional operative items was positive. Both TAP block and IMA sparing were successfully performed in all patients, allowing good pain control, no anastomotic leak, and a lymph-node collection comparable to the overall series. A failure of low pneumoperitoneum was recorded in 14% of patients. These promising results and previous reports [20,21,22] should encourage the incorporation of a TAP block, low pneumoperitoneum, and IMA sparing in the enhanced recovery protocols.
A possible limitation of the present study is that participating hospitals could differ in the degree of enhanced recovery pathway implementation. However, the high adherence to preoperative and operative items indicates that the vast majority of patients followed a comprehensive protocol. The wide range of patients’ age and ASA score suggests a small likelihood of selection bias.
5. Conclusions
In conclusion, early low compliance to postoperative enhanced recovery protocols was associated with overall morbidity and major complications following rectal surgery. Variables associated with early low compliance were advanced age, long procedure, open surgery and diverting stoma, suggesting a tailored and careful approach in these patients.
Author Contributions
Conceptualization, M.C., F.F. and M.B.; Data curation, M.C., C.P., L.P., A.M., F.F., R.P., M.S., M.T., N.T. and L.R.; Investigation, M.C. and M.B.; Supervision, M.B.; Writing—original draft, M.C., C.P. and M.B.; Writing—review & editing, M.C., L.P., A.M., F.F., R.P., M.S., M.T., N.T. and L.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of ASST Monza (protocol code 0012747 17 April 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study; data were collected anonymously without any identifying information.
Data Availability Statement
Data can be obtained under request to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fearon K.C.H., Ljungqvist O., Von Meyenfeldt M., Revhaug A., Dejong C.H.C., Lassen K., Nygren J., Hausel J., Soop M., Andersen J., et al. Enhanced Recovery after Surgery: A Consensus Review of Clinical Care for Patients Undergoing Colonic Resection. Clin. Nutr. 2005;24:466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Spanjersberg W.R., Reurings J., Keus F., van Laarhoven C.J. Fast Track Surgery versus Conventional Recovery Strategies for Colorectal Surgery. Cochrane Database Syst. Rev. 2011;2:CD007635. doi: 10.1002/14651858.CD007635.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Varadhan K.K., Neal K.R., Dejong C.H.C., Fearon K.C.H., Ljungqvist O., Lobo D.N. The Enhanced Recovery after Surgery (ERAS) Pathway for Patients Undergoing Major Elective Open Colorectal Surgery: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2010;29:434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Launay-Savary M.-V., Mathonnet M., Theissen A., Ostermann S., Raynaud-Simon A., Slim K. Are Enhanced Recovery Programs in Colorectal Surgery Feasible and Useful in the Elderly? A Systematic Review of the Literature. J. Visc. Surg. 2017;154:29–35. doi: 10.1016/j.jviscsurg.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Braga M., Pecorelli N., Scatizzi M., Borghi F., Missana G., Radrizzani D. Enhanced Recovery Program in High-Risk Patients Undergoing Colorectal Surgery: Results from the PeriOperative Italian Society Registry. World J. Surg. 2017;41:860–867. doi: 10.1007/s00268-016-3766-9. [DOI] [PubMed] [Google Scholar]
- 6.Currie A., Burch J., Jenkins J.T., Faiz O., Kennedy R.H., Ljungqvist O., Demartines N., Hjern F., Norderval S., Lassen K., et al. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection. Ann. Surg. 2015;261:1153–1159. doi: 10.1097/SLA.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 7.Gianotti L., Beretta S., Luperto M., Bernasconi D., Valsecchi M.G., Braga M. Enhanced Recovery Strategies in Colorectal Surgery: Is the Compliance with the Whole Program Required to Achieve the Target? Int. J. Color. Dis. 2014;29:329–341. doi: 10.1007/s00384-013-1802-x. [DOI] [PubMed] [Google Scholar]
- 8.Ceresoli M., Pedrazzani C., Pellegrino L., Ficari F., Braga M., Muratore A., Tamini N., Beretta L., Azzola M., Radrizzani D., et al. Early Non Compliance to Enhanced Recovery Pathway Might Be an Alert for Underlying Complications Following Colon Surgery. Eur. J. Surg. Oncol. 2022 doi: 10.1016/j.ejso.2022.06.033. in press . [DOI] [PubMed] [Google Scholar]
- 9.Vlug M.S., Wind J., Hollmann M.W., Ubbink D.T., Cense H.A., Engel A.F., Gerhards M.F., van Wagensveld B.A., van der Zaag E.S., van Geloven A.A.W., et al. Laparoscopy in Combination with Fast Track Multimodal Management Is the Best Perioperative Strategy in Patients Undergoing Colonic Surgery. Ann. Surg. 2011;254:868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 10.Braga M., Scatizzi M., Borghi F., Missana G., Radrizzani D., Gemma M., Beretta L., Bona S., Monzani R., Azzola M., et al. Identification of Core Items in the Enhanced Recovery Pathway. Clin. Nutr. ESPEN. 2018;25:139–144. doi: 10.1016/j.clnesp.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Özdemir-van Brunschot D.M.D., van Laarhoven K.C.J.H.M., Scheffer G.J., Pouwels S., Wever K.E., Warlé M.C. What Is the Evidence for the Use of Low-Pressure Pneumoperitoneum? A Systematic Review. Surg. Endosc. 2016;30:2049–2065. doi: 10.1007/s00464-015-4454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albers K.I., Polat F., Helder L., Panhuizen I.F., Snoeck M.M.J., Polle S.B.W., de Vries H., Dias E.M., Slooter G.D., de Boer H.D., et al. Quality of Recovery and Innate Immune Homeostasis in Patients Undergoing Low- Versus Standard Pressure Pneumoperitoneum During Laparoscopic Colorectal Surgery (RECOVER) Ann. Surg. 2022;276:e664–e673. doi: 10.1097/SLA.0000000000005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaibu Z., Chen Z., Theophilus A., Mzee S.A.S. Preservation of the Arterial Arc Formed by Left Colic Artery, Proximal Inferior Mesenteric Artery, and the First Branch of Sigmoid Arteries in Anus Saving Treatment of Low Rectal Cancer. Am. Surg. 2021;87:1956–1964. doi: 10.1177/0003134820983188. [DOI] [PubMed] [Google Scholar]
- 14.Liu K.-Y., Lu Y.-J., Lin Y.-C., Wei P.-L., Kang Y.-N. Transversus Abdominis Plane Block for Laparoscopic Colorectal Surgery: A Meta-Analysis of Randomised Controlled Trials. Int. J. Surg. 2022;104:106825. doi: 10.1016/j.ijsu.2022.106825. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson U.O., Scott M.J., Hubner M., Nygren J., Demartines N., Francis N., Rockall T.A., Young-Fadok T.M., Hill A.G., Soop M., et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019;43:659–695. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 17.Bozzetti F., Braga M., Gianotti L., Gavazzi C., Mariani L. Postoperative Enteral versus Parenteral Nutrition in Malnourished Patients with Gastrointestinal Cancer: A Randomised Multicentre Trial. Lancet. 2001;358:1487–1492. doi: 10.1016/S0140-6736(01)06578-3. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D., Demartines N., Clavien P.-A. Classification of Surgical Complications. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiore J.F., Faragher I.G., Bialocerkowski A., Browning L., Denehy L. Time to Readiness for Discharge Is a Valid and Reliable Measure of Short-Term Recovery After Colorectal Surgery. World J. Surg. 2013;37:2927–2934. doi: 10.1007/s00268-013-2208-1. [DOI] [PubMed] [Google Scholar]
- 20.Myles P.S., Andrews S., Nicholson J., Lobo D.N., Mythen M. Contemporary Approaches to Perioperative IV Fluid Therapy. World J. Surg. 2017;41:2457–2463. doi: 10.1007/s00268-017-4055-y. [DOI] [PubMed] [Google Scholar]
- 21.Bragg D., El-Sharkawy A.M., Psaltis E., Maxwell-Armstrong C.A., Lobo D.N. Postoperative Ileus: Recent Developments in Pathophysiology and Management. Clin. Nutr. 2015;34:367–376. doi: 10.1016/j.clnu.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Guay J., Nishimori M., Kopp S.L. Epidural Local Anesthetics Versus Opioid-Based Analgesic Regimens for Postoperative Gastrointestinal Paralysis, Vomiting, and Pain After Abdominal Surgery. Anesth. Analg. 2016;123:1591–1602. doi: 10.1213/ANE.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy R.H., Francis E.A., Wharton R., Blazeby J.M., Quirke P., West N.P., Dutton S.J. Multicenter Randomized Controlled Trial of Conventional Versus Laparoscopic Surgery for Colorectal Cancer Within an Enhanced Recovery Programme: EnROL. J. Clin. Oncol. 2014;32:1804–1811. doi: 10.1200/JCO.2013.54.3694. [DOI] [PubMed] [Google Scholar]
- 24.Braga M., Frasson M., Zuliani W., Vignali A., Pecorelli N., Di Carlo V. Randomized Clinical Trial of Laparoscopic versus Open Left Colonic Resection. Br. J. Surg. 2010;97:1180–1186. doi: 10.1002/bjs.7094. [DOI] [PubMed] [Google Scholar]
- 25.Zaouter C., Kaneva P., Carli F. Less Urinary Tract Infection by Earlier Removal of Bladder Catheter in Surgical Patients Receiving Thoracic Epidural Analgesia. Reg. Anesth. Pain Med. 2009;34:542–548. doi: 10.1097/AAP.0b013e3181ae9fac. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained under request to the corresponding author.