Abstract
Production of cytokines including gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) is an important early-stage host response following infection with intracellular pathogens. Development of immunity to these pathogens is determined to a large extent by the timing and relative level of expression of the cytokines. Numerous studies have shown that early cytokine responses involving interleukin-12 (IL-12) and IFN-γ are important for resistance to intracellular pathogens, whereas responses involving IL-4 and IL-10 increase host susceptibility. These often-indistinct early cytokine responses influence the differentiation of naïve CD4+ T helper cells, which later develop into what have commonly been termed Th1- and Th2-type cells. The characterization of CD4+ T-helper-cell responses as Th1 or Th2 type is based largely on the cytokine profiles during the specific phase and has been used in recent years to account for the innate resistance and susceptibility of different inbred strains of mice to several intracellular pathogens. Studies investigating cytokine production in terms of CD4+ T-helper-cell polarization in Burkholderia pseudomallei infection have not been undertaken. In this study, we used semiquantitative reverse transcription-PCR to assess induction of cytokine mRNA in liver and spleen of B. pseudomallei-susceptible BALB/c and relatively resistant C57BL/6 mice following infection with virulent B. pseudomallei. The levels of mRNA for IFN-γ, TNF-α, IL-1β, IL-6, IL-10, and IL-12 increased in both BALB/c and C57BL/6 mice 24 to 36 h after infection. A comparison of BALB/c and C57BL/6 responses revealed the relative levels of expression of mRNA for several of these cytokines, including IFN-γ, were greater in BALB/c mice, suggesting a role for endotoxic shock and cytokine-mediated immunopathology in the development of acute melioidosis. Early induction of mRNA for the cytokines classically associated with development of Th1- and Th2-type responses was absent or minimal, and induction levels in both strains of mice were similar. During the specific phase, cytokine mRNA profiles occurred as a combination of Th1- and Th2-type patterns. Collectively, these results demonstrate that cytokine mRNA responses in BALB/c and C57BL/6 mice following infection with virulent B. pseudomallei do not develop as polarized Th1- or Th2-type profiles. Considering the role of TNF-α and IFN-γ in the processes of endotoxic shock, these results also indicate that selected cytokines, while important for resistance to B. pseudomallei infection, are also potential contributors to immunopathology and the development of acute fulminating disease.
Melioidosis describes a diverse range of glanders-like clinical presentations resulting from infection with the bacterium Burkholderia pseudomallei. Melioidosis affects both humans and animals and was first described in Rangoon in 1911 (52). The most commonly recognized regions of endemicity are Thailand and northern Australia (16, 44). Active cases of melioidosis have also been reported in many areas where the disease is not endemic, including Africa, France, Sri Lanka, and Turkey (33). B. pseudomallei is an opportunistic gram-negative bacterium which exists in the environment as a saprophyte (44, 46). The organism is widely distributed in the soil and surface water of regions of endemicity, including Malaysia (46), Singapore (48), Thailand (16), and northern Australia (3). As a result, there is a high rate of infection in communities that have frequent contact with soil and surface water (16, 24). Examples include rice-farming communities in Thailand, Aboriginal communities in Australia, and Papua New Guinea/Torres Strait Islander populations. Disease becomes particularly prevalent during periods of high rainfall, when it is believed that the organism is physically leached from the soil, temporarily creating a more widespread distribution (24, 46). Infection is thought to occur via ingestion, inhalation, or subcutaneous inoculation of contaminated soil or surface water (24, 33). The potential for transmission of B. pseudomallei via insect vectors including the mosquito Aedes aegypti, which is common in some areas of endemicity, and the rat flea Xenopsylia cheopsis has been described (39). However, there is little supporting evidence for natural vector-borne transmission of B. pseudomallei. Unlike other soil-borne bacteria, such as Clostridium botulinum and Clostridium tetani, for which one factor (the exotoxin) is solely responsible for disease (21), B. pseudomallei has a broad range of virulence determinants that are likely to influence pathogenesis and clinical presentation (44). These include various exotoxins, endotoxin, malleobactin (B. pseudomallei siderophore), flagella, an antiphagocytic capsule, and fimbriae or pili (44, 53). The specific role of each of these virulence determinants in the pathogenesis of melioidosis remains unclear.
There is a wide spectrum of clinical presentations resulting from infection with B. pseudomallei. Classically, three general categories of disease are recognized: acute, subacute, and chronic (33, 44). Symptoms depend partly on where lesions are situated in the body and may include subcutaneous abscesses, pneumonitis, and septic arthritis (33, 44). Diagnosis is confounded by the lack of cardinal symptoms, the possible abrupt onset of clinical signs, and the potential involvement of virtually any organ in the body. This has led to melioidosis being termed “the great imitator” of all infectious diseases (33, 44). Infection may also occur asymptomatically (33, 44). The range of clinical presentations and the potential for asymptomatic infection presumably reflect differences in the route of inoculation, inoculum size, virulence of the infecting strain, and immune competence and genetic predisposition of the host. Acute septicemic melioidosis is the most severe form of disease (11, 33, 44). It is associated with a high mortality rate, often despite intensive antibiotic therapy (11, 12, 36). Acute septicemic melioidosis remains a significant cause of death due to gram-negative sepsis in some areas of endemicity (11, 12). At the opposite end of the spectrum of clinical presentations is the chronic form of disease. Chronic infection can be manifested as a localized infection in almost any organ of the body (36, 44). Similar to asymptomatic infection, chronic infection may persist for many years (12, 44). Both chronic and asymptomatic infection may intensify to an acute form of disease, particularly when the host is immunocompromised (12, 44). The potential for relapse of disease following extended periods of antibiotic therapy has also been recognized (12). Risk factors for disease include alcoholism, renal dysfunction, and diabetes mellitus (12, 33, 36). The ability of B. pseudomallei to lie dormant in the host for prolonged periods has been associated with an ability to survive inside phagocytes including macrophages (25, 29). This has led to the recent description of B. pseudomallei as a facultative intracellular bacterium (29).
In the past two decades there have been many studies on the epidemiology (3, 16), bacteriology (44), and antibiotic susceptibility (15) of B. pseudomallei. There have been relatively few studies on the immunopathogenesis of melioidosis, in part because of the lack of a suitably defined animal model. As a result, the interactions between B. pseudomallei and the host, with particular reference to the immunopathogenesis of acute versus chronic infection, remain poorly characterized. BALB/c and C57BL/6 mice have been used extensively to investigate the immunopathogenesis of several intracellular infections including listeriosis (13), leishmaniasis (23, 42), yersiniosis (4, 7), and mycobacterial infection (2, 8). In many of these BALB/c-C57BL/6 models, BALB/c mice are innately susceptible to infection whereas C57BL/6 mice are relatively resistant. Previous studies in our laboratory have demonstrated that BALB/c mice are also highly susceptible to infection with virulent B. pseudomallei, while C57BL/6 mice are relatively resistant (24, 32). Following intravenous (i.v.) infection of BALB/c mice with as few as 37 CFU of virulent B. pseudomallei, substantial bacterial growth occurs in liver and spleen, followed by an overwhelming bacteremia to which the mice succumb within 72 to 96 h (32). C57BL/6 mice do not develop bacteremia; however, bacterial growth in liver and spleen that eventually leads to a fatal outcome demonstrates incomplete resistance (32). The BALB/c-C57BL/6 model is considered to represent an excellent model for the acute and chronic forms of human melioidosis (24, 32). Recent studies by Hoppe et al. have confirmed these observations (25). The availability of this model provides an opportunity to investigate the immunopathogenesis of melioidosis in vivo and, in particular, the mechanisms that lead to the development of either acute or chronic disease.
Cytokines have been extensively studied and characterized in recent years. It is now known that selected cytokines are critical immunoregulatory determinants of disease pathogenesis and progression. Studies on a broad range of intracellular pathogens including Listeria (19, 27), Leishmania (23, 42, 43), and Yersinia (4, 7) spp. and mycobacteria (8) have demonstrated that gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and several interleukins (IL) are crucial in determining the level of innate host resistance. These cytokines, which may be produced within minutes of infection (19, 51a), regulate the antimicrobial activity of macrophages and influence the interactions between macrophages and lymphocytes that have been shown to be important for effective anti-B. pseudomallei activity (50). These studies and others (34, 40) have resulted in the general classification of cytokine responses following intracellular infection as either Th1 or Th2 type, based on the activity of CD4+ T helper cells and the cytokine profiles induced. In this classification, the Th1-type cytokine profile typically involves IFN-γ, IL-2, and TNF-β, resulting in increased macrophage activation and subsequent host resistance to intracellular infection. On the other hand, a preferential Th2-type cytokine response involves IL-5, IL-6, IL-10, and IL-13, expression of which decreases macrophage activation, leading to increased host susceptibility. While divergent Th1- and Th2-type cytokine profiles typically coincide with development of T-cell specificity 4 to 5 days postinfection, it is the cytokines present during the early stages of infection that influence and ultimately determine the differentiation pathway of naïve CD4+ T helper cells (34, 42, 43). Early IL-12 is known to induce a predominantly Th1-type cellular response (23, 27, 30, 42, 43). In contrast, early IL-4 has been associated with increased Th2-type cell development (23, 27, 30, 42, 43). Distinct induction of polarized Th1- and Th2-type cytokine profiles was first demonstrated by Mosmann et al. in vitro (34) and later by Scott in vivo using a murine model of Leishmania major infection (43). The two periods of immune activation following infection, both of which are ultimately aimed at activating macrophages for killing intracellular pathogens, have been referred to as the T-cell-independent pathway (early) and the T-cell-dependent pathway (late) (2, 5, 5a). The importance of early cytokine production by natural killer (NK) cells, macrophages, and other cells such as mast cells in the former pathway has been demonstrated (5, 5a, 27, 42). It is now known that the combination of cytokines produced in the early nonspecific T-cell-independent phase, which influences the differentiation pathway of T lymphocytes in the later specific T-cell-dependent phase, is perhaps the most crucial factor that dictates the level of innate host resistance to intracellular pathogens.
Innate resistance of mouse strains such as C57BL/6 mice to numerous intracellular pathogens has been associated with increased activity of Th1-type cells and induction of a strong Th1-type cytokine response (4, 8, 23, 42). In contrast, the innate susceptibility of BALB/c mice to numerous intracellular pathogens has been linked to hypoproduction of IFN-γ and a preferential Th2-type cytokine response involving increased activity of Th2-type cells. This is because Th1-type cytokines typically stimulate cell-mediated immunity, which is important for resistance to intracellular pathogens (4, 27). Cytokines produced in the Th2-type profile are usually regarded as anti-inflammatory and are involved in humoral immunity, which is considered important for resistance to extracellular pathogens (4, 27, 42). Since the concept of CD4+ T-helper-cell subsets was first introduced, distinct induction of Th1- and Th2-type cytokine profiles has been demonstrated in numerous models of intracellular infection (23, 27, 42, 43). However, many studies have since described induction of nondistinct (i.e., nonpolarized Th1- and Th2-type) cytokine profiles following intracellular infection (7, 37). Recent reviews on cytokine responses and intracellular pathogens have suggested that, in terms of cytokine responses, exceptions to the polarized Th1- and Th2-type patterns are more common than the classical paradigm predicts (1, 30). A comprehensive study investigating cytokine induction in melioidosis has not previously been undertaken.
In the present study, we used the highly specific and sensitive technique of reverse transcription-PCR (RT-PCR) to assess cytokine gene expression in BALB/c and C57BL/6 mice following infection with a virulent strain of B. pseudomallei. Specifically, we compared the levels of induction of mRNA for IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-10, and IL-12 in liver and spleen of mice following infection. The cytokines assessed covered both classical Th1- and Th2-type cytokines as well as a range of proinflammatory and anti-inflammatory cytokines.
MATERIALS AND METHODS
Animals.
Male and female C57BL/6 and BALB/c mice (8 to 16 weeks of age; purchased from James Cook University Small Animal Breeding Unit, Townsville, Queensland, Australia) were used. Mice were maintained in a positive-pressure environment at 23°C and were fed a pelleted protein-enriched diet, and water was provided ad libitum. Experiments were conducted according to National Health and Medical Research Council guidelines.
Bacteria.
The virulent strain of B. pseudomallei used in this study was isolated from clinical material from a fatal case of pneumonia at the Townsville General Hospital. The isolate was cultured on Ashdown agar and was identified by colonial morphology and API 20NE (bioMeriéux, La Balme, France). The bacteria were grown in brain heart infusion broth (Oxoid, Hampshire, England) at 37°C for 18 h and were stored at −80°C. The number of viable bacteria was determined by plating serial dilutions of the suspension onto Ashdown agar and counting the colonies after 24 to 48 h of incubation at 37°C. This strain of B. pseudomallei has previously been characterized in terms of virulence, with a 10-day 50% lethal dose of <10 CFU in BALB/c mice (32).
Infection of mice and preparation of organs.
Mice were inoculated i.v. with 2.5 × 102 CFU of virulent B. pseudomallei in 200 μl of phosphate-buffered saline (PBS) via the lateral tail vein. Control mice received PBS only. At 0, 24, 36, 48, and 72 h following infection, three mice were euthanized and the livers and spleens were aseptically excised. Samples were also prepared from C57BL/6 mice at 96 h, 7 days, and 14 days. Samples were immediately wrapped in aluminum foil, submerged in liquid nitrogen to prevent RNA degradation, and stored at −80°C until use.
RNA extraction and RT.
Total RNA was extracted using Trizol reagent (Gibco-BRL, Life Technologies), in accordance with the manufacturer's instructions and chloroform-isoamyl alcohol purification based on methods previously described (14, 51). Ground-glass Dounce tissue homogenizers (Quantum Scientific) were used. Following precipitation with isopropanol and subsequent ethanol washes, the resultant RNA pellets were resuspended in diethyl pyrocarbonate-treated water. RNase-free DNase (Promega) was used to remove genomic DNA based on methods described by Dilworth and McCarrey (18). Total RNA was quantified using spectrophotometry at 260 nm, and the quality of the RNA was determined by the ratio of optical density at 260 nm to that at 280 nm. Ratios of greater than 1.8 were considered acceptable. Three micrograms of total RNA was reverse transcribed in a total volume of 20 μl containing 0.5 μg of oligo(dT)15 (Promega) as the primer, 1× First Strand buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2; Gibco-BRL, Life Technologies), 10 mM dithiothreitol (Gibco-BRL, Life Technologies), 500 μM deoxynucleoside triphosphate (dNTP) mixture (Promega), and 200 U of Superscript II reverse transcriptase (Gibco-BRL, Life Technologies) based on methods previously described (7, 19, 22a, 51). This included an initial step of incubation at 42°C for 2 min without Superscript II, followed by the addition of Superscript II and incubation at 42°C for 50 min and then at 95°C for 4 min. After RT, 1.5 U of RNase H (Promega) was added and the mixture was incubated at 37°C for 20 min to remove any residual RNA from the reaction product. Heating cycles for RT-PCR were performed in an automated DNA thermal cycler (Bresatec; MJ Research Inc.; PTC-100). The resultant cDNA was stored at −20°C until PCR amplification.
PCR procedure.
The final PCR mixture contained 2 μl of cDNA, 1× PCR buffer (1 mM Tris-HCl [pH 9.0], 5 mM KCl, 0.01% Triton X-100 [Promega]), 1 to 4.5 mM MgCl2 (Promega), 200 μM dNTP mixture (Promega), 1 U of Taq DNA polymerase (Promega), 1 μM concentrations of (each) sense and antisense primers, and sterile water to 100 μl. Cytokine-specific primers were synthesized by Gibco-BRL, Life Technologies, according to sequence designs previously described (7, 19, 35, 40) (Table 1). The optimal MgCl2 concentration for each primer pair was determined experimentally. The thermal cycling parameters were as follows: 95°C for 2 min, followed by 30 cycles each of denaturation at 94°C for 50 s, annealing of primer and fragment at 60°C for 50 s, and primer extension at 72°C for 1 min. A final extension of 72°C for 4 min was included. In preliminary experiments, amplification for 30 cycles was shown to lie in the linear portion of the curve for the amount of PCR product produced. PCR products were visualized by UV light after electrophoresis through a 2.0 to 2.5% agarose gel containing 0.5 μg of ethidium bromide/ml. As the DNA size marker, a 123-bp ladder (Gibco-BRL, Life Technologies) was used. Analysis was performed using a GELDOC 1000 system and Molecular Analyst software. PCR using primers for β-actin was performed on each individual sample as an internal positive-control standard. As a negative control, PCR omitting cDNA (water as the substitute) was run concurrently. PCR using primers for β-actin was also performed using non-reverse-transcribed total RNA for each sample to confirm that amplification was based solely on cDNA. PCR-assisted mRNA amplification was repeated twice for three separately prepared cDNA samples. Results shown are representative of triplicate cDNA samples.
TABLE 1.
Cytokine-specific primer pair sequences used in PCR
| Cytokine | Oligonucleotide sequence (5′–3′)a | Size (bp)b | Reference |
|---|---|---|---|
| β-Actin | TGG AAT CCT GTG GCA TCC ATG AAA C | 348 | 35 |
| TAA AAC GCA GCT CAG TAA CAG TCC G | |||
| IFN-γ | AGC GGC TGA CTG AAC TCA GAT TGT AG | 243 | 35 |
| GTC ACA GTT TTC AGC TGT ATA GGG | |||
| TNF-α | GGC AGG TCT ACT TTG GAG TCA TTG C | 307 | 35 |
| ACA TTC GAG GCT CCA GTG AAT TCG G | |||
| IL-1β | TCA TGG GAT GAT GAT GAT AAC CTG CT | 502 | 19 |
| CCC ATA CTT TAG GAA GAC ACG GAT T | |||
| IL-2 | TGA TGG ACC TAC AGG AGC TCC TGA G | 167 | 35 |
| GAG TCA AAT CCA GAA CAT GCC GCA G | |||
| IL-4 | CGA AGA ACA CCA CAG AGA GTG AGC T | 180 | 35 |
| GAC TCA TTC ATG GTG CAG CTT ATC G | |||
| IL-6 | CTG GTG ACA ACC ACG GCC TTC CCT A | 600 | 19 |
| ATG CTT AGG CAT AAC GCA CTA GGT T | |||
| IL-10 | ACC TGG TAG AAG TGA TGC CCC AGG CA | 237 | 7 |
| CTA TGC AGT TGA TGA AGA TGT CAA A | |||
| IL-12(p35) | GCA AGA GAC ACA GTC CTG GG | 618 | 40 |
| TGC ATC AGC TCA TCG ATG GC | |||
| IL-12(p40) | GAG GTG GAC TGG ACT CCC GA | 618 | 40 |
| CAA GTT CTT GGG CGG GTC TG |
For each primer pair, the sense sequence is shown above the antisense sequence.
Predicted size of the PCR product.
RESULTS
Cytokine mRNA levels for IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-10, and IL-12 in liver of BALB/c and C57BL/6 mice following infection with virulent B. pseudomallei are shown in Fig. 1. Results for spleen are shown in Fig. 2. The top panel of each figure shows bright, size-specific bands (348 bp) of constant intensity for the anticipated β-actin-specific cDNA product. The β-actin gene is expressed at a relatively constant level in cells and is commonly used in semiquantitative RT-PCR systems to assess the relative efficiency of each individual PCR. These results confirm the semiquantitative nature of the method and allow comparison of the kinetics of cytokine mRNA production over time. The absence of PCR product from PCR using primers for β-actin and water instead of cDNA, which were concurrently processed with each PCR run, ensured a lack of contaminating exogenous DNA. Since DNA polymerase amplifies both cDNA and genomic DNA with equal efficiencies, the absence of PCR product from PCR using non-reverse-transcribed total RNA and primers for β-actin confirmed that the amplification was based solely on cDNA (18).
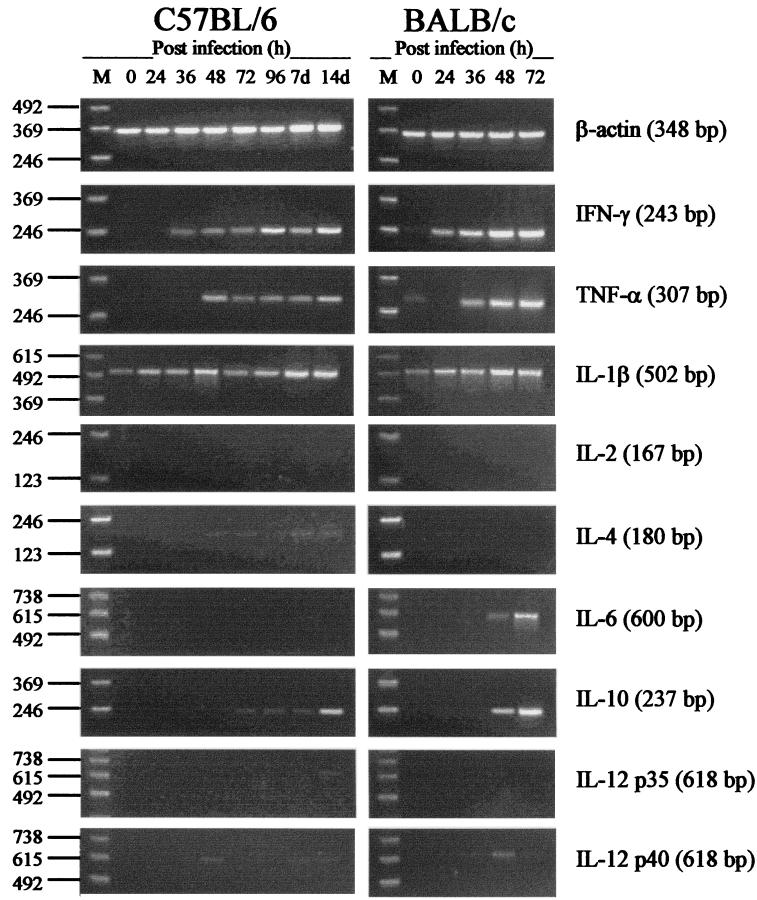
FIG. 1.
Cytokine mRNA responses in liver of C57BL/6 and BALB/c mice infected i.v. with 2.5 × 102 CFU of virulent B. pseudomallei. At various times after infection (0 to 96 h and 7 and 14 days), liver was excised and total RNA was extracted. Genomic DNA was removed, and cDNA was subjected to PCR using cytokine-specific primers. Molecular weight markers (M) are shown in the left lane of each gel. Data are representative of three mice at each time point.
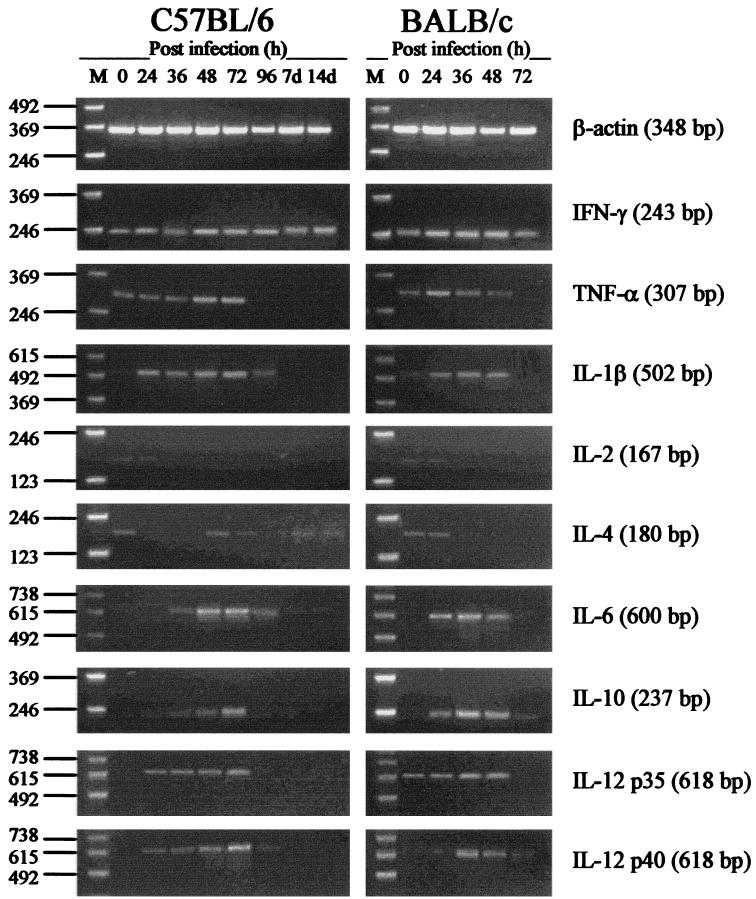
FIG. 2.
Cytokine mRNA responses in spleen of C57BL/6 and BALB/c mice infected i.v. with 2.5 × 102 CFU of virulent B. pseudomallei. At various times after infection (0 to 96 h and 7 and 14 days), spleen was excised and total RNA was extracted. Genomic DNA was removed, and cDNA was subjected to PCR using cytokine-specific primers. Molecular weight markers (M) are shown in the left lane of each gel. Data are representative of three mice at each time point.
In liver, baseline levels of mRNA for the cytokines assayed were minimal. The only cytokine mRNA detected in uninfected mice was low levels of mRNA for IL-1β and, in BALB/c mice, very low levels of mRNA for TNF-α. Increases in the amounts of mRNA for several cytokines were detected in liver of both BALB/c and C57BL/6 mice 24 to 36 h after infection. In BALB/c mice, production of large amounts of mRNA was observed for both IFN-γ and TNF-α. Induction of mRNA for these cytokines was rapid, beginning at 24 h after infection and peaking at 48 to 72 h, prior to host death. Increases in the amounts of mRNA for IL-1β and IL-6 were also observed in BALB/c mice 36 to 48 h after infection. Among the other cytokines assayed, only an increase in IL-10 mRNA at 48 to 72 h in BALB/c mice was detected. No increase in the amount of mRNA for IL-12, IL-4, or IL-2 was detected in BALB/c mice. In liver of C57BL/6 mice, increases in the amounts of mRNA for IFN-γ, TNF-α, and IL-1β were observed 48 to 72 h after infection, though the increases were less marked than BALB/c responses. Low levels of mRNA for IL-10 were also detected during the later stages of infection in C57BL/6 mice. No mRNA for the other cytokines assayed was detected in liver of C57BL/6 mice at any time point.
In spleen, low baseline levels of mRNA for IFN-γ and TNF-α were detected in both BALB/c and C57BL/6 mice. Very low baseline levels of mRNA for IL-1β and IL-4 were also detected in both strains of mice. Detectable levels of mRNA for IL-12p35 were observed in spleen of uninfected BALB/c mice. Increases in mRNA for IFN-γ were observed in spleen of both BALB/c and C57BL/6 mice 24 to 36 h following infection. These increases peaked at 24 to 48 h in BALB/c mice and at 48 to 72 h in C57BL/6 mice. In C57BL/6 mice, a moderate increase in the level of mRNA for IFN-γ persisted in spleen for the duration of the assay period (14 days). Minor-to-moderate increases in the levels of mRNA for TNF-α, IL-1β, IL-6, IL-12, and IL-10 were detected 24 to 48 h after infection in both mouse strains. The majority of these mRNA increases peaked at 24 to 48 h in BALB/c mice and at 48 to 72 h in C57BL/6 mice. The levels of mRNA for most of these cytokines were minimal or undetectable at 96 h and thereafter. No increase in the level of mRNA for IL-2 was detected in spleen of either BALB/c or C57BL/6 mice at any time point. Low levels of mRNA for IL-4 were detected in C57BL/6 mice during the later stages of infection.
DISCUSSION
B. pseudomallei is able to survive and multiply within mammalian cells (25, 29). However, the role of intracellular parasitism in the immunopathogenesis of melioidosis remains unknown. In recent years, BALB/c-C57BL/6 murine models have been used extensively to investigate the immunopathogenesis of several intracellular infections, including listeriosis (13), leishmaniasis (23, 42), yersiniosis (4, 7), and mycobacterial infection (2, 8). These studies and others (19, 27, 34, 40, 43) have demonstrated that production of selected cytokines and development of distinct Th1- and Th2-type cytokine profiles are critical in determining the pathway of disease progression and the level of innate host resistance to intracellular pathogens. More-extensive studies on a broader range of intracellular pathogens have revealed that cytokine cascades induced by intracellular organisms are highly specific for the infecting agent and often do not develop as classical polarized Th1- and Th2-type patterns (1, 7, 30, 37). As is the case for numerous other intracellular pathogens, BALB/c mice have been shown to be highly susceptible to infection with virulent B. pseudomallei while C57BL/6 mice are relatively resistant (24, 32). The BALB/c-C57BL/6 murine model has been partially characterized in terms of the level of innate host resistance to virulent B. pseudomallei and is becoming increasingly popular as a means of investigating the pathogenesis of melioidosis in vivo (24, 25, 32, 51). The purpose of this study was to investigate the cytokine cascades induced by virulent B. pseudomallei in BALB/c and C57BL/6 mice to determine the role of early cytokine production and CD4+ T-helper-cell polarization in determining the pathway of disease progression in this model. We conducted a comprehensive PCR-assisted mRNA analysis of cytokine induction in liver and spleen of BALB/c and C57BL/6 mice in both the early and later stages of infection with virulent B. pseudomallei. We assessed a broad range of cytokines, IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-10, and IL-12, to determine the roles of both classical Th1- and Th2-type cytokines and a range of proinflammatory and anti-inflammatory cytokines in the development of acute versus chronic murine melioidosis.
Preliminary studies in our laboratory correlated high levels of mRNA for the proinflammatory cytokines TNF-α, IL-1β, and IL-6 in liver during the early stages of infection with development of acute disease in BALB/c mice (51). These studies demonstrated minor increases in the levels of mRNA for TNF-α and IL-1β in C57BL/6 mice. The results of the present study confirm the association between high levels of mRNA for TNF-α, IL-1β, and IL-6 in liver and development of acute B. pseudomallei infection in BALB/c mice (Fig. 1). This study also demonstrates moderate increases in the levels of mRNA for TNF-α and IL-1β in liver during the later stages of infection in C57BL/6 mice (Fig. 1). Similar trends in the production of IFN-γ were observed in liver, where mRNA for IFN-γ was detected at much higher levels in BALB/c mice in the early stages of infection than in C57BL/6 mice (Fig. 1). The moderate increase in the level of mRNA for IFN-γ that was detected during the early stages of infection in C57BL/6 mice persisted for the duration of the assay period (14 days; Fig. 1). Several studies have demonstrated a genetic predisposition of BALB/c and C57BL/6 mice to be low- and high-IFN-γ responders, respectively (8, 17, 28). The general classification of BALB/c and C57BL/6 mice as low- and high-IFN-γ responders has been used in recent years to account for the innate susceptibility and resistance of these mouse strains to numerous intracellular pathogens (4, 7, 8, 17). The results of the present study demonstrate that this is not the case for B. pseudomallei infection. Instead, this study indicates that production of IFN-γ, TNF-α, and other cytokines in melioidosis represents a critical balance between responses that are important for host resistance and responses that can contribute heavily to immunopathology, depending on the timing and relative level of expression of cytokines. While sufficient cytokine responses involving IFN-γ appear to be important for resistance to B. pseudomallei (C57BL/6 mice; Fig. 1), hyperproduction of selected cytokines is associated with development of acute fulminating disease (BALB/c mice; Fig. 1).
In this study, cytokine mRNA responses in spleen were less marked than responses in liver. Increases in the levels of mRNA for IFN-γ, TNF-α, IL-1β, and IL-6 were observed in spleen of both BALB/c and C57BL/6 mice during the early stages of infection (Fig. 2). The levels of induction of mRNA for these cytokines in spleen of both BALB/c and C57BL/6 mice were similar, although peak levels generally occurred earlier in BALB/c mice (24 to 36 h) than in C57BL/6 mice (48 to 72 h; Fig. 2). The results of the present study confirm production of mRNA for IL-6 in liver of BALB/c mice but not C57BL/6 mice (Fig. 1). The potential contribution of hepatocytes to IL-6 production in melioidosis is an interesting possibility, particularly when considered in terms of the potential intracellular interactions between B. pseudomallei and the host cell. Increased levels of mRNA for IL-6 were observed in spleen of both mouse strains (Fig. 2). These results indicate that the importance of IL-6 as a synergistic factor in determining the progression of acute disease in BALB/c mice, as suggested in a previous study (51), may be limited. However, it is important to consider the amount of IL-6 produced in toto. The results of the present study suggest that this is greater in BALB/c mice. Hence, the potential contribution of IL-6 to the development of acute disease in BALB/c mice cannot be completely excluded.
IL-12, produced by macrophages after interaction with bacteria or parasites, is a heterodimer consisting of the subunits p35 (35 kDa) and p40 (40 kDa) (4a). Although p35 and p40 are encoded by separate genes and are independently regulated, cells must express both subunits to generate the bioactive form of IL-12 (4a). Minor increases in the levels of mRNA for IL-12 (both p35 and p40 subunits) were observed in spleen of both BALB/c and C57BL/6 mice during the early stages of infection with virulent B. pseudomallei (Fig. 2). Because these responses were minimal and similar in both strains of mice, it is reasonable to suggest a limited role for IL-12 in determining the pathway of disease progression in this model. However, reduction of IL-12 bioactivity by monoclonal antibodies in a recent study on murine melioidosis by Santanirand et al. (41a) resulted in increased host susceptibility to B. pseudomallei infection, suggesting an important role for IL-12 in melioidosis. It should be noted, however, that these studies were based on the use of a dissimilar murine model and used a strain of B. pseudomallei different from the one used in this study. Furthermore, the authors did not assess cytokine responses per se. The potency of IL-12 and the known complexity in the regulation of cytokine genes, particularly for IL-12 (4a), must be considered when interpreting results of cytokine transcriptional studies.
Moderate increases in the levels of mRNA for IL-10 were observed in spleen of both BALB/c and C57BL/6 mice during the early stages of infection (Fig. 2). As observed with other cytokines assayed, levels of mRNA for IL-10 peaked in BALB/c mice earlier than in C57BL/6 mice (Fig. 2). Increased levels of mRNA for IL-10 were also detected in liver of both mouse strains during the later stages of infection (Fig. 1). Because IL-10 is a potent anti-inflammatory cytokine, it is possible that IL-10 has an important suppressive immunoregulatory role in the early stages of B. pseudomallei infection. Among its many activities, IL-10 inhibits the generation of nitrogen radicals in macrophages and suppresses proinflammatory cytokine production (37, 45). In view of the results of the present study, it may be argued that the earlier appearance of IL-10 in spleen of BALB/c mice could lead to a preferential Th2-type cytokine response, diminished cell-mediated immunity, and increased host susceptibility. However, concurrent up-regulation of IL-12, IFN-γ, and TNF-α combined with a lack of IL-4 during the same period argues against the development of classical polarized Th1- and Th2-type profiles. Simultaneous increases in the levels of mRNA for IL-10 and IFN-γ, as observed in this study, are consistent with recent reports on cytokine responses to other intracellular pathogens (7, 19, 37). Such studies have suggested a potential beneficial role of IL-10 for increased host resistance during intracellular infection, despite the known anti-inflammatory actions of this cytokine (45). A cytokine pattern involving high levels of IL-10 and IFN-γ without an increase in IL-2 or IL-4 has recently been coined the Tr1-type profile (22, 30). While the results of the present study do appear to “fit” this pattern more closely than the classical polarized Th1- and Th2-type profiles, we suggest that such a classification could serve to oversimplify the complex nature of T-cell responses occurring in vivo. Description of other prominent cytokine patterns and T-cell subsets (e.g., Th3 profile [reviewed in reference 30]; Th0 cells [reviewed in reference 29a]) adds to the difficulty in interpreting cytokine profiles that are not polarized, particularly when considering such a broad range of individual cytokines. As recently suggested by Kelso, T-cell cytokine profiles following infection most likely form a continuous spectrum in both levels and combinations, in which polarized Th1- and Th2-type profiles merely represent possible extreme poles of the spectrum (29a, 30).
It is important to consider the role of virulence in the determination of cytokine profiles (31, 38). In this study, we used a highly virulent strain of B. pseudomallei, as determined by the 50% lethal dose in BALB/c mice (32). Other studies have demonstrated that cytokine production in vivo is regulated to a large extent by the virulence of the infecting strain of pathogen (31, 38). Highly virulent Listeria monocytogenes and nonvirulent L. monocytogenes invoke different cytokine profiles (31, 38). There is increasing evidence to suggest that particular bacterial virulence factors such as exotoxins are potent modulators of cytokine responses. Examples include pneumolysin of Streptococcus pneumoniae (26) and listeriolysin O of L. monocytogenes (31). Virulence factors such as these may help to explain the link between strain virulence and induction of distinct cytokine profiles. The role of virulence in determining cytokine profiles in melioidosis has yet to be investigated.
In terms of cytokine responses in patients infected with B. pseudomallei, few studies have been conducted. Initial studies by Brown et al. correlated high levels of urinary neopterin and serum IFN-γ with acute clinical presentation and fatal outcome in melioidosis (9, 10). These studies suggested that severe melioidosis involves impairment of specific T-cell-mediated immunity and induction of nonspecific and ineffective T-cell responses generated predominantly by CD4+ T cells. It is interesting to consider these implications in relation to the known inhibitory actions of the Tr1-type cytokine profile on the generation of specific T-cell-mediated immunity (22). A potential role for TNF-α in the development of acute melioidosis was later demonstrated by Suputtamongkol et al. (47). The description of IL-6 as a potential predictor of mortality in B. pseudomallei sepsis by Friedland et al. (20) is another demonstration of the importance of proinflammatory cytokine hyperproduction in melioidosis. While these studies have investigated production of cytokines on a limited basis, the present study represents the first comprehensive analysis of the role of a broad range of cytokines in melioidosis. The results of this study demonstrate that the trends in cytokine production occurring in the BALB/c-C57BL/6 murine model parallel the few known cytokine responses occurring in human melioidosis. High levels of mRNA for IFN-γ, TNF-α, and IL-6 coincide with the development of acute disease in BALB/c mice. In C57BL/6 mice, moderate increases in the levels of mRNA for these cytokines correlate with development of chronic disease. Response trends observed in BALB/c mice are similar to those reported in patients acutely infected with B. pseudomallei and demonstrate the importance of cytokine hyperproduction in acute melioidosis. The cytokine responses observed in C57BL/6 mice demonstrate the importance of production of selected cytokines including IFN-γ at sufficient levels in chronic melioidosis. These findings are in accordance with those of Santanirand et al. (41, 41a), who recently demonstrated an important role for IFN-γ in resistance to B. pseudomallei in a murine model. However, the reduction of cytokine bioactivity by monoclonal antibodies in previous studies could provide little insight into the actual immune mechanisms (responses) operating in acute versus chronic melioidosis. Finally, the results of the present study demonstrate that early cytokine responses in the BALB/c-C57BL/6 murine model following infection with virulent B. pseudomallei do not develop as polarized Th1- and Th2-type profiles. These results are consistent with recent reviews on the complex nature of cytokine responses following infection with intracellular pathogens. While not unexpected, the results of the present study are inconsistent with preliminary suggestions by others of skewed Th1- and Th2-type profiles in melioidosis (25). It is important to note however, that the previous implication of CD4+ T-helper-cell switching in melioidosis was based on antibody isotype responses and not cytokine profiles per se (25). Furthermore, the previous study used a strain of B. pseudomallei of lower virulence than the strain used in this study, which may influence Th1- and Th2-type cytokine profiles.
In several animal models of endotoxemia, monoclonal antibodies to specific cytokines have been used to reduce cytokine-mediated immunopathology (6, 49). Similar approaches for control of cytokine hyperproduction in BALB/c mice during infection with virulent B. pseudomallei may prove efficacious. However, because an increase in the transcriptional activity of cytokine genes does not necessarily translate into release of functional cytokine gene products, investigation into the bioactivity and levels of specific cytokines in serum in the BALB/c-C57BL/6 murine model is required prior to any attempts of immunomodulation. The use of immunotherapy for melioidosis as an adjunct to antibiotic therapy, particularly for the acute septicemic form of disease, remains a possibility.
ACKNOWLEDGMENTS
These studies were supported with funding from a Merit Research Grant of James Cook University.
We gratefully acknowledge the contribution of Catriona McElnea for advice on molecular biology and techniques. We also thank Justin LaBrooy for critical review of the manuscript.
REFERENCES
- 1.Allen J E, Maizels R M. Th1-Th2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–392. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg R, Castro A G, Pedrosa J, Silva R A, Orme I M, Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashdown L. Epidemiological aspects of melioidosis in Australia. Communicable Dis Intelligence. 1991;15:272–273. [Google Scholar]
- 4.Autenrieth I B, Beer M, Bohn E, Kaufmann S H E, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Babik J M, Adams E, Tone Y, Fairchild P J, Tone M, Waldmann H. Expression of murine IL-12 is regulated by translational control of the p35 subunit. J Immunol. 1999;162:4069–4078. [PubMed] [Google Scholar]
- 5.Bancroft G J, Schreiber R D, Bosma G C, Bosma M J, Unanue E R. A T cell-independent mechanism of macrophage activation by interferon-γ. J Immunol. 1987;139:1104–1107. [PubMed] [Google Scholar]
- 5a.Bancroft G J, Sheehan K C F, Schreiber R D, Unanue E R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–130. [PubMed] [Google Scholar]
- 6.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 7.Bohn E, Heesemann J, Ehlers S, Autenrieth I B. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect Immun. 1994;62:3027–3032. doi: 10.1128/iai.62.7.3027-3032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brett S J, Butler R. Resistance to Mycobacterium lepraemurium is correlated with the capacity to generate macrophage activating factor(s) in response to mycobacterial antigens in vitro. Immunology. 1986;59:339–345. [PMC free article] [PubMed] [Google Scholar]
- 9.Brown A E, Dance D A B, Chaowagul W, Webster H K, White N J. Activation of cellular immune responses in melioidosis patients as assessed by urinary neopterin. Trans R Soc Trop Med Hyg. 1990;84:583–584. doi: 10.1016/0035-9203(90)90049-k. [DOI] [PubMed] [Google Scholar]
- 10.Brown A E, Dance D A B, Suputtamongkol Y, Chaowagul W, Kongchareon S, Webster H K, White N J. Immune cell activation in melioidosis: increased serum levels of interferon-γ and soluble interleukin-2 receptors without change in soluble CD8 protein. J Infect Dis. 1991;163:1145–1148. doi: 10.1093/infdis/163.5.1145. [DOI] [PubMed] [Google Scholar]
- 11.Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M E, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community-acquired septicaemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 12.Chaowagul W, Suputtamongkol Y, Dance D A B, Rajchanuvong A, Pattara-arechachai J, White N J. Relapse in melioidosis: incidence and risk factors. J Infect Dis. 1993;168:1181–1185. [PubMed] [Google Scholar]
- 13.Cheers C, Haigh A M, Kelso A, Metcalf D, Stanley E R, Young A M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988;56:247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Dance D A B, Wuthiekanun V, Chaowagul W, White N J. The antimicrobial susceptibility of Pseudomonas pseudomallei. Emergence of resistance in vitro and during treatment. J Antimicrob Chemother. 1989;24:295–309. doi: 10.1093/jac/24.3.295. [DOI] [PubMed] [Google Scholar]
- 16.Dance D A B. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Gee A L W, Sonnenfeld G, Mansfield J M. Genetics of resistance to the African trypanosomes. V. Qualitative and quantitative differences in interferon production among susceptible and resistant mouse strains. J Immunol. 1985;134:2723–2726. [PubMed] [Google Scholar]
- 18.Dilworth D D, McCarrey J R. Single-step elimination of contaminating DNA prior to reverse transcriptase PCR. PCR Methods Appl. 1992;1:279–282. doi: 10.1101/gr.1.4.279. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers S, Mielke M E A, Blankenstein T, Hahn H. Kinetic analysis of cytokine gene expression in the livers of naïve and immune mice infected with Listeria monocytogenes. J Immunol. 1992;149:3016–3022. [PubMed] [Google Scholar]
- 20.Friedland J S, Suputtamongkol Y, Remick D G, Chaowagul W, Strieter R M, Kunkel S L, White N J, Griffin G E. Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect Immun. 1992;60:2402–2408. doi: 10.1128/iai.60.6.2402-2408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorbach S L. Clostridia. In: Schaechter M, Medoff G, Eisenstein B I, editors. Mechanisms of microbial disease. 2nd ed. Baltimore, Md: Williams and Wilkins; 1993. pp. 299–306. [Google Scholar]
- 22.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J E, Roncarolo M G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 22a.Havell E A, Moldawer L L, Helfgott D, Kilian P L, Sehgal P B. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992;148:1486–1492. [PubMed] [Google Scholar]
- 23.Heinzel F P, Rerko R M, Hujer A M. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell Immunol. 1998;184:129–142. doi: 10.1006/cimm.1998.1267. [DOI] [PubMed] [Google Scholar]
- 24.Hirst R G, Indriana J, Cocciolone R A. An introduction to melioidosis. Innate resistance and acquired immunity to Pseudomonas pseudomallei. Aust Biol. 1992;5:203–213. [Google Scholar]
- 25.Hoppe I, Brenneke B, Rohde M, Kreft A, Häubler S, Reganzerowski A, Steinmetz I. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect Immun. 1999;67:2891–2900. doi: 10.1128/iai.67.6.2891-2900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 28.Huygen K, Palfliet K. Strain variation in interferon γ production of BCG-sensitized mice challenged with PPD. I. CBA/Ca mice are low producers in vivo, but high producers in vitro. Cell Immunol. 1983;80:329–334. doi: 10.1016/0008-8749(83)90121-1. [DOI] [PubMed] [Google Scholar]
- 29.Jones A L, Beveridge T J, Woods D E. Intracellular survival of Burkholderia pseudomallei. Infect Immun. 1996;64:782–790. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today. 1995;16:374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 30.Kelso A. Cytokines: principles and prospects. Immunol Cell Biol. 1998;76:300–317. doi: 10.1046/j.1440-1711.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn M, Goebel W. Induction of cytokines in phagocytic mammalian cells infected with virulent and avirulent Listeria strains. Infect Immun. 1994;62:348–356. doi: 10.1128/iai.62.2.348-356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leakey A, Ulett G C, Hirst R G. BALB/c and C57BL/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animals models for the acute and chronic forms of human melioidosis. Microb Pathog. 1998;24:269–275. doi: 10.1006/mpat.1997.0179. [DOI] [PubMed] [Google Scholar]
- 33.Leelarasamee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 35.Murray L J, Lee R, Martens C. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur J Immunol. 1990;20:163–170. doi: 10.1002/eji.1830200124. [DOI] [PubMed] [Google Scholar]
- 36.Norton R, Roberts B, Freeman M, Wilson M, Ashhurst-Smith C, Lock W, Brookes D, La Brooy J. Characterisation and molecular typing of Burkholderia pseudomallei: are disease presentations of melioidosis clonally related? FEMS Immunol Med Microbiol. 1998;20:37–44. doi: 10.1111/j.1574-695X.1998.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 37.Pie S, Bernard P M, Bachi P T, Nauciel C. Gamma interferon and interleukin-10 gene expression in innately susceptible and resistant mice during the early phase of Salmonella typhimurium infection. Infect Immun. 1996;64:849–854. doi: 10.1128/iai.64.3.849-854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poston R M, Kurlander R J. Cytokine expression in vivo during murine listeriosis. Infection with live, virulent bacteria is required for monokine and lymphokine messenger RNA accumulation in the spleen. J Immunol. 1992;149:3040–3044. [PubMed] [Google Scholar]
- 39.Redfearn M S, Palleroni N J. Glanders and melioidosis. In: Hubbert W T, McCulloch W F, Schnurrengerger P R, editors. Diseases transmitted from animals to man. 6th ed. Springfield, Ill: Charles C. Thomas; 1975. pp. 110–128. [Google Scholar]
- 40.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf S F, Bistoni F. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J Immunol. 1994;152:5167–5175. [PubMed] [Google Scholar]
- 41.Santanirand P, Harley V S, Dance D A B, Raynes J G, Drasnar B S, Bancroft G J. Interferon-γ mediates host resistance in a murine model of melioidosis. Biochem Soc Trans. 1997;25:287S. doi: 10.1042/bst025287s. [DOI] [PubMed] [Google Scholar]
- 41a.Santanirand P, Harley V S, Dance D A B, Drasnar B S, Bancroft G J. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect Immun. 1999;67:3593–3600. doi: 10.1128/iai.67.7.3593-3600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharton T M, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 44.Smith C J, Allen J C, Noor Embi M, Othman O, Razak N, Ismail G. Human melioidosis: an emerging medical problem. MIRCEN J Appl Microbiol Biotechnol. 1987;3:343–366. [Google Scholar]
- 45.Smith S R, Terminelli C, Kenworthy-Bott L, Calzetta A, Donkin J. The cooperative effects of TNF-α and IFN-γ are determining factors in the ability of IL-10 to protect mice from lethal endotoxemia. J Leukoc Biol. 1994;55:711–718. doi: 10.1002/jlb.55.6.711. [DOI] [PubMed] [Google Scholar]
- 46.Strauss J M, Groves M G, Mariappan M, Ellison D W. Melioidosis in Malaysia. II. Distribution of Pseudomonas pseudomallei in soil and surface water. Am J Trop Med Hyg. 1969;18:698–702. [PubMed] [Google Scholar]
- 47.Suputtamongkol Y, Kwiatkowski D, Dance D A B, Chaowagul W, White N J. Tumor necrosis factor in septicemic melioidosis. J Infect Dis. 1992;165:561–564. doi: 10.1093/infdis/165.3.561. [DOI] [PubMed] [Google Scholar]
- 48.Thin R N T. Pseudomonas pseudomallei in the surface water of Singapore. Singap Med J. 1971;12:181–182. [PubMed] [Google Scholar]
- 49.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 50.Ulett G C, Ketheesan N, Hirst R G. Macrophage-lymphocyte interactions mediate anti-Burkholderia pseudomallei activity. FEMS Immunol Med Microbiol. 1998;21:283–286. doi: 10.1111/j.1574-695X.1998.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 51.Ulett, G. C., N. Ketheesan, and R. G. Hirst. Proinflammatory cytokine mRNA responses in experimental Burkholderia pseudomallei infection in mice. Acta Trop. 74:229–234. [DOI] [PubMed]
- 51a.Ulich T R, Guo K, Remick D, Del Castillo J, Yin S. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991;146:2316–2323. [PubMed] [Google Scholar]
- 52.Whitmore A, Krishnaswami C S. An account of the discovery of a hitherto undescribed infective disease occurring among the population of Rangoon. Indian Med Gaz. 1912;92:262–267. [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H, Chaowagul W, Sokol P A. Siderophore production by Pseudomonas pseudomallei. Infect Immun. 1991;59:776–780. doi: 10.1128/iai.59.3.776-780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]