Abstract
Simple Summary
Cancer cells amplify in an uncontrolled fashion. The resulting tumor and metastases need to ensure their survival in the body. To achieve this, cancer cells display increased nutritional needs and an altered metabolism. These metabolic changes start to be targeted for therapeutic interventions in the context of a number of different cancers. Similar to cancer, cells infected with viruses that cause cancer, so-called “oncoviruses”, have altered nutritional needs to support the amplification and spread of new progeny viruses and to ensure the survival of infected cells in the host. Here, we give an update on the similarities between the metabolic alterations observed in many types of cancers and those induced by oncogenic viruses. Furthermore, we discuss the antiviral activities of metabolic inhibitors used for the treatment of cancer.
Abstract
Viruses play an important role in cancer development as about 12% of cancer types are linked to viral infections. Viruses that induce cellular transformation are known as oncoviruses. Although the mechanisms of viral oncogenesis differ between viruses, all oncogenic viruses share the ability to establish persistent chronic infections with no obvious symptoms for years. During these prolonged infections, oncogenic viruses manipulate cell signaling pathways that control cell cycle progression, apoptosis, inflammation, and metabolism. Importantly, it seems that most oncoviruses depend on these changes for their persistence and amplification. Metabolic changes induced by oncoviruses share many common features with cancer metabolism. Indeed, viruses, like proliferating cancer cells, require increased biosynthetic precursors for virion production, need to balance cellular redox homeostasis, and need to ensure host cell survival in a given tissue microenvironment. Thus, like for cancer cells, viral replication and persistence of infected cells frequently depend on metabolic changes. Here, we draw parallels between metabolic changes observed in cancers or induced by oncoviruses, with a focus on pathways involved in the regulation of glucose, lipid, and amino acids. We describe whether and how oncoviruses depend on metabolic changes, with the perspective of targeting them for antiviral and onco-therapeutic approaches in the context of viral infections.
Keywords: oncogenic viruses, metabolism, viral persistence, cancer
1. Introduction
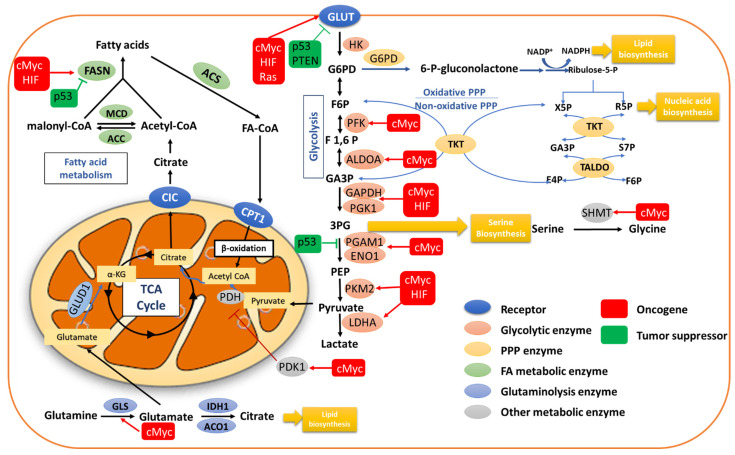
Reprogramming of energy metabolism is considered as a hallmark of cancer cells. Transformed cells adopt metabolic changes to cope with their increased need for fast growth and proliferation [1]. Glucose is a main source of cellular energy and generally converted via glycolysis in the cytoplasm into pyruvate, which is then transferred into mitochondria, where it serves as substrate for the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS). Under hypoxic conditions, OXPHOS is throttled, and cells mainly rely on glycolysis, which produces ATP in an oxygen-independent manner. However, many cancer cells limit glucose utilization to glycolysis as opposed to OXPHOS even under aerobic conditions. This metabolic anomaly was first observed in the thirties of the last century by the German biochemist Otto Warburg and is known as the Warburg effect or aerobic glycolysis [2,3]. As glycolytic ATP production is faster than OXPHOS, glycolysis copes better with the increased need of energy and accelerated growth and proliferation in cancer cells [4]. Indeed, increased transport and utilization of glucose is frequently observed in cancer cells, which then channel glycolytic intermediates into biosynthetic pathways such as the pentose phosphate shunt (PPP) for nucleotide production and regeneration of the reducing equivalent NADPH, which is important for cellular redox homeostasis and the synthesis of lipids and amino acids. Consistently, metabolic reprogramming of lipid and amino acid metabolism is also frequently described in cancer [5,6,7,8,9,10]. A scheme of the metabolic pathways is depicted in Figure 1.
Figure 1.
Reprogramming of cell metabolism in cancer. Metabolic pathways regulating glucose metabolism, including glycolysis, TCA cycle and PPP, fatty acid metabolism, and glutaminolysis, are generally altered in malignant transformation by altered signaling pathways, oncogenes, and tumor suppressor genes. Abbreviations: GLUT, glucose transporter; HK, hexokinase; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F1,6P, fructose 1,6-biphosphate; ALDOA, aldolase A; GA3P, glyceraldehyde 3-phosphate; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PGK1, phosphoglycerokinase 1; PGAM1, phosphoglycerate mutase 1; ENO1, α-enolase; PEP, phosphoenol pyruvate; PKM2, pyruvate kinase isozyme type 2; LDHA, lactate dehydrogenase A; PKD1, pyruvate dehydrogenase kinase 1; PDH, pyruvate dehydrogenase; PPP, pentose phosphate pathway; G6PD, glucose 6-phosphate dehydrogenase; NADPH, nicotinamide adenine dinucleotide phosphate; X5P, xylulose 5-phosphate; R5P, ribose 5-phosphate; S7P, sedoheptulose 7-phosphate; E4P, erythrose 4-phosphate; TKT, transketolase; TALDO, transaldolase; CIC, citrate carrier; CPTI, carnitine palmitoyl transferase 1; MCD, malonyl-coA decarboxylase; ACC, acetyl-coA carboxylase; FASN, fatty acid synthase; ACS, acetyl-coA synthetase; α-KG, α-ketoglutarate; GLS, glutaminase; GLUD1, glutamate dehydrogenase 1; IDH1, isocitrate dehydrogenase 1; Aco1, aconitase 1; 3PG, 3-phosphoglycerate; SHMT, serine hydroxymethyltranferase.
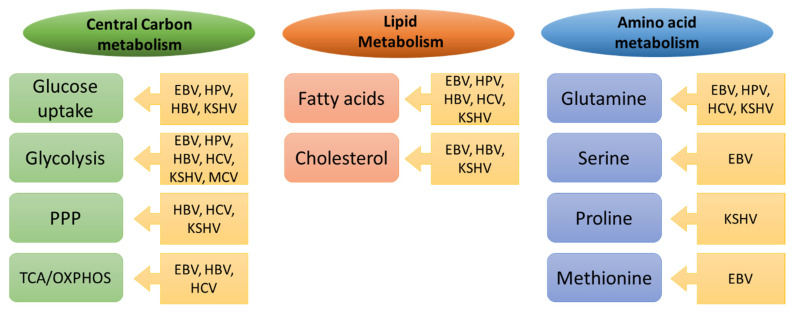
Viruses play an important role in cancer development and about 12% of cancer types are linked to viral infections [11]. Viruses that induce malignant transformation are known as oncoviruses and include, for example, Epstein–Barr virus (EBV), human papillomavirus (HPV), human T cell lymphotropic virus (HTLV), hepatitis B virus (HBV), hepatitis C virus (HCV), Kaposi’s sarcoma herpesvirus (KSHV), and Merkel cell polyomavirus (MCP) [12]. Although the mechanisms of viral oncogenesis differ, all these oncogenic viruses share the ability to establish persistent chronic infections, often with no obvious symptoms for years, during which they manipulate and alter cell signaling pathways that coordinate cell cycle progression, apoptosis, and inflammation [13]. Furthermore, oncogenic viruses introduce into their host cells metabolic adaptations similar to those observed in cancer cells, as shown in Figure 2, and importantly, it seems that they depend on these changes for their persistence and amplification.
Figure 2.
Common metabolic alterations in cancer and oncoviral infections. The key metabolic pathways altered in tumors and targeted by the indicated oncoviruses are depicted. See text for details.
2. Reprogramming of Central Carbon Metabolism in Cancer Cells
Metabolic reprogramming is a vital hallmark of malignant cellular transformation. The highly energy-demanding cancer cells often depend on glycolysis as a fast source for energy production [7,14] and for carbon intermediates as substrates for nucleotide, amino acid, and lipid biosynthesis [15,16]. Concomitantly with the increase in glucose uptake, activities of the mitochondrial TCA cycle and OXPHOS are often preserved in cancer [17,18,19,20,21]. These latter pathways ensure not only a sufficient supply of ATP but also the synthesis of metabolic precursors required for increased biomass in proliferating cells, maintenance of cellular redox homeostasis, and adaptation to the tumor microenvironment. In addition to promoting anabolism in cancer cells, the metabolic shift towards glycolysis also favors cell survival under stressful conditions, increases cell invasiveness, and helps cells overcome immune surveillance [22]. Furthermore, amino acid metabolism and, in particular, glutaminolysis are frequently induced in cancer cells to satisfy amongst others the cellular need for nitrogen for biosynthesis of nucleotides, proteins, lipids, and many other molecules. Furthermore, glutamine is important for the regulation of cellular redox homeostasis [23].
2.1. Glucose Uptake
The transport of glucose across the cellular plasma membrane is a vital step and precedes glycolysis. Expression of cytoplasmic membrane glucose transporters (GLUTs), responsible for glucose uptake, is frequently deregulated in cancer [24]. GLUT1 expression is upregulated in response to the oncogenes such as rat sarcoma oncogene (RAS), c-mylocytomatosis oncogene (cMyc), and hypoxia-inducible factor 1α (HIF1α) [25,26,27] and inhibited by tumor suppressor p53, phosphatase and tensin homolog (PTEN), and microRNA-22 (mir-22) [28,29,30]. The overexpression of GLUT1 was found to be positively correlated with the disease stage in various cancer types [31,32,33]. Additionally, the use of GLUT1 inhibitor STF-31 induces apoptosis in cancer cells [34,35]. Furthermore, the IKK–NF-κB axis, required for cellular transformation, is activated by the loss of p53, which leads to overexpression of GLUT3 and aerobic glycolysis [36].
2.2. Glycolysis
Once across the plasma membrane, glucose is immediately phosphorylated by hexokinases (HKs), which is the first rate-limiting step of glycolysis. Overexpression of HK1 and HK2 in cancer cells has been previously reported, and HK1 overexpression is a poor prognostic marker in colorectal cancer [37,38]. The HK2-mediated Warburg effect is essential for cellular transformation and growth in many cancer types [39]. In prostate cancer cells, loss of PTEN leads to overexpression of HK2 via activation of the Akt/mTORC1/4EBP1 axis and loss of p53 increases the stability of HK2 mRNA by inhibiting the biogenesis of miR-143, which destabilizes HK2 mRNA in cancer cells [39,40]. Importantly, binding of HK1 and 2 to mitochondria also protects cells from apoptosis via retro-translocation of pro-apoptotic Bcl2 family members away from mitochondria, thus inhibiting death receptor-mediated apoptosis [41]. Finally, binding of HK1 to mitochondria reduces glyceraldehyde 3-phosphate dehydrogenase (GAPDH) activity, thus redirecting glycolytic fluxes towards PPP and dampening inflammatory cytokine production [42]. In addition to HK and GAPDH, the expression of many intermediate enzymes of glycolysis, including phosphofrucktokinase (PFK), aldolase (ALODA), phosphoglycerate kinase 1 (PGK1), and alpha enolase (ENO1), is regulated by oncogenes, including cMyc, HIF1α, β-catenin, and tumor suppressor p53. These enzymes are overexpressed in many forms of cancers, which is often associated with poor prognosis and treatment efficacies, and inhibition or knock out of the corresponding genes has been shown to have anti-oncogenic effects [43,44,45,46,47,48,49,50,51].
Pyruvate kinases mediate the final rate-limiting and net ATP-producing step in glycolysis. In cancer cells, the embryonic isoform PKM2 predominates over the adult isoform PKM1 [52]. Switching the expression from PKM2 to PKM1 reverses the Warburg effect, decreases tumorigenicity, and increases oxygen consumption through shunting glycolysis to OXPHOS [53]. In normal cells, the produced pyruvate is converted to acetyl-coA via pyruvate dehydrogenase (PDH), a step that links glycolysis to the TCA cycle and OXPHOS by mediating pyruvate uptake into mitochondria. This reaction is inhibited in cancer cells due to the overexpression of pyruvate dehydrogenase kinases (PDK), a family of PDH complex inhibitors, leading to a reduced acetyl-coA supply to the TCA cycle [54]. It has been shown that knocking down PDK2 in cancer cell lines increases oxygen consumption and reactive oxygen species (ROS) production and induces apoptosis [55]. Noteworthy, HIF1α increases the expression of PDK [54].
In cancer cells, pyruvate produced by aerobic glycolysis is converted to lactate via lactate dehydrogenase (LDH). LDH is a homo- or heterotetramer composed of two subunits, LDHA and LDHB. LDHA expression was found to be upregulated in cancer cells [56]. LDHA inhibitors FX11 and oxamate were found to reduce lactate production and retard the growth of cancer cells [57,58]. Lactate is a key player in the process of carcinogenesis. In many tumors, the level of lactate in cancer cells is elevated up to 40-fold. In cancer cells, the rate of glycolytic fluxes frequently exceeds the capacity of mitochondria to oxidize produced pyruvate due to the limited availability of free CoA molecules and a low NAD+/NADH ratio. Overexpression of LDHA offers a faster alternative compared to the TCA cycle to consume pyruvate and regenerate NAD+ levels through the production of lactate [6]. Elevated intracellular lactate increases the expression of monocarboxylate transporters MCT1 and MCT4, which mediate the efflux of lactate outside the cells [59]. The release of lactate is sufficient to stimulate angiogenesis and consequently carcinogenesis via activation of vascular endothelial growth factor (VEGF) in tumor endothelial cells [60]. Moreover, high levels of lactate are associated with metastasis in different types of cancer [59]. Lactate supports the immune escape of cancer cells via induction of a state of acidosis in the extracellular microenvironment [61] and by its direct inhibitory effect on immune cells, including macrophages [62] and T cells [63].
2.3. Pentose Phosphate Pathway (PPP)
The PPP, a major glucose catabolic pathway, is composed of an oxidative and a non-oxidative phase. The rate-limiting step in the oxidative phase is the dehydrogenation of glucose-6-phosphate (G6P) via glucose-6-phosphate dehydrogenase (G6PD). The products of the oxidative branch of the PPP include not only the nucleoside precursor ribose-5-phosphate (R5P) but also NADPH [64]. The increased metabolic activities in cancer cells are associated with excessive ROS production and increased oxidative stress, which need to be kept in check [65]. In addition to its role as an essential anabolic reducing factor in the biosynthesis of nucleotides and fatty acids (FAs), NADPH is responsible for maintaining cell survival under oxidative stress [66,67]. NADPH is the source of reductive potential used by glutathione reductases (GR) to recycle oxidized glutathione into its reduced form, which is needed for detoxification of ROS [68]. In line with these observations, G6PD activity has been shown to be enhanced in several types of cancer [69,70,71,72]. Several growth factors and oncogenes are capable of increasing the activity of G6PD, including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), phosphoinositide 3-kinase (PI3K), Src, and Ras [73,74,75,76]. On the other hand, the tumor suppressors, p53 and PTEN, reduce the PPP via binding and inhibition of the activity of G6PD [77,78,79].
The reversible non-oxidative phase of the PPP consists of transfer reaction series that result in the production of sugars, including pentoses, for anabolic purposes [80]. This phase of the pathway is under the control of transketolase (TKT) and transaldolase (TALDO), enzymes that link the non-oxidative phase of PPP to glycolysis. TKT catalyzes the reversible conversion of xylulose-5-P and ribose-5-P to glyceraldehyde-3-P and sedoheptulose-7-P and the conversion of xylulose-5-P and erythrose 4-P to glyceraldehyde-3-P and fructose-6-P. TALDO reversibly catalyzes the conversion of glyceraldehyde-3-P and sedoheptulose 7-P to erythrose 4-P and fructose-6-P. Thus, the non-oxidative branch replenishes metabolites of the oxidative branch and regulates the flux of glycolysis by providing fructose 6-P and glyceraldehyde 3-P [81]. Several studies have reported upregulation of TKT and TALDO in several types of cancer [82,83,84]. Moreover, TKT overexpression has been correlated with tumor invasiveness and poor cancer prognosis in lung, prostate, and breast cancer cells [73,85].
2.4. Tricarboxylic Acid Cycle (TCA)
The TCA cycle has been recognized as a central hub not only for ATP production but also for the production of biosynthetic precursors. Contrary to the assumption that mitochondrial functions are reduced in cancer cells, many lines of evidence confirm the indispensable activity of the mitochondrial TCA cycle in many different types of cancer. While non-proliferating cells maximize the energy production in the TCA cycle from oxidizable substrates, in cancer cells, the TCA cycle is important for the provision of intermediates for lipid, nucleotide, and amino acid synthesis rather than ATP [6]. This continuous efflux of biosynthetic precursors from the TCA cycle is known as cataplerosis. For cancer cells to preserve TCA cycle activity, cells must provide the necessary substrates. This compensatory supply is known as anaplerosis [86]. Cells use different carbon fuels, including glucose, glutamine, and FA, to maintain anaplerosis [43].
3. Reprogramming of Fatty Acid Metabolism
Compared to normal cells, cancer cells are characterized by lipid accumulation in the form of lipid droplets. The lipid requirement is increased during tumorigenesis for the synthesis of membranes and signaling molecules [8]. De novo synthesis of FA is activated in several cancers via the increased expression of the transport protein citrate carrier (CIC) responsible for transporting the mitochondrial citrate to the cytoplasm [87]. Additionally, ATP citrate lyase (ACLY), the first rate-limiting enzyme in FA de novo synthesis, is upregulated in several types of cancer [88,89,90,91]. Overexpression of ACLY is associated with increased tumorigenesis and gene knockout inhibits tumor growth. The cytosolic form of acetyl-coA carboxylase 1 (ACC1) catalyzes another rate-limiting step of FA synthesis, the conversion of acetyl-coA to malonlyl-coA [92]. The tumor suppressor LKB1 inhibits FA synthesis via activation of the central metabolic sensor, adenosine monophosphate activated protein kinase (AMPK), which inactivates ACC1 [93]. The terminal step of de novo FA synthesis is catalyzed by fatty acid synthase (FASN). Higher activity of FASN supports tumor cell survival and overexpression was reported in different types of cancers [94,95].
4. Reprogramming of Amino Acid Metabolism
Rapidly proliferating cancer cells have increased requirements for amino acids not only for protein synthesis but also as important metabolites and metabolic regulators. Several amino acids were shown to have special importance in cancer metabolism. In particular, glutamine is considered very important, as the most abundant amino acid. Dependence on glutamine metabolism, also referred to as “glutamine addiction”, is a hallmark of cancer cell metabolism.
Glutamine is converted to α-ketoglutarate via glutaminolysis, which fuels the TCA cycle [96]. Conversion of glutamine into citrate by isocitrate dehydrogenase (IDH) can feed into lipogenesis. Cytoplasmic citrate is converted into malate and then pyruvate in an enzymatic process that produces NADPH required for redox homeostasis [97,98]. Additionally, glutamine can serve as a nitrogen donor to support the increased demand for nucleotide synthesis [96,99,100] and contribute to the biosynthesis of uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc), which is needed for protein glycosylation and endoplasmic reticulum stress responses in cancer cells [101]. Glutamine also plays a pivotal role in regulating the increased levels of ROS in tumor cells as it is a precursor for glutathione synthesis [99].
The expression of cancer-specific isoforms of glutaminase (GLS), a key enzyme of glutaminolysis and responsible for converting glutamine into glutamate, is regulated by the oncogene cMyc [102,103]. cMyc was found to induce glutamine conversion into glutamate under normo- and hypoxic conditions. Under glucose deprivation conditions, glutamine was found to be the main precursor for the synthesis of TCA intermediates such as fumarate, malate, and citrate, confirming the ability of glutamine to fuel the TCA cycle [104]. cMyc also induces the expression of enzymes involved in nucleotide biosynthesis pathways, which increase the need for glutamine as a nitrogen donor for nucleotide biosynthesis [100]. Glutamine metabolism was shown to inhibit autophagy in cancer cells through the activation of the mTOR pathway [105]. The highly selective glutaminase inhibitor CB-839 has been tested in clinical trials for cancer treatment. While monotherapies have failed overall, CB-839 is showing promising effects in some combination therapies [106,107,108,109,110].
Several cancer subtypes hyperactivate the anabolic serine and glycine synthesis side-branch of glycolysis, which provide vital precursors for the synthesis of proteins, nucleic acids, and lipids that are essential to cell proliferation. Additionally, serine/glycine biosynthesis influences the cellular anti-oxidative capacity, thus maintaining tumor homeostasis [111]. The glycolytic intermediate 3-phosphoglycerate (3-PG) is the precursor for de novo serine synthesis. Serine can then be converted into glycine, a process mediated by serine hydroxymethyltransferase 1 or 2 (SHMT1/2). Furthermore, serine contributes to the methionine cycle and impacts the availability of S-adenosyl methionine, the donor of methyl groups for epigenetic regulation of RNA and DNA [112].
Proline is a secondary amino acid that is stored in collagen. Proline metabolism is related to ATP production, protein and nucleotide synthesis, and redox homeostasis. Rewiring of proline metabolism has been shown to be tumor type dependent [113,114,115,116,117]. Proline is also a precursor for polyamine synthesis. Polyamines such as spermidine and spermine are polycationic alkylamines [118]. These small molecules are involved in many central processes of cell survival and proliferation, including protein and nucleic acid biosynthesis, chromatin structure stabilization, differentiation, apoptosis, protection against oxidative stress, and intercellular communication [118,119,120,121]. In cancer cells, polyamine metabolism is often dysregulated, and elevated intracellular polyamine levels are needed for transformation and tumor progression [122].
5. Role of Oncogenes, Tumor Suppressors, and Cell Signaling in Regulating Cancer Cell Metabolism
Oncogenes and tumor suppressor genes are key players in the process of carcinogenesis as they regulate cellular proliferation, growth, and/or cell cycle arrest [123]. Many lines of evidence have confirmed and analyzed in depth the roles of these factors in regulating metabolic enzymes and metabolic signaling pathways important for growth, proliferation, and regulation of cellular redox homeostasis [5,124].
5.1. PI3K/Akt/mTOR Pathway
The highly conserved PI3K/Akt/mTOR signaling pathway is commonly deregulated in cancer. Activation of this signaling pathway has important roles in tumorigenesis [125]. The binding of growth factors to its receptors activates PI3K, which in turn activates the downstream serine/threonine kinases Akt and mTOR. Activation of this pathway increases the membrane expression of transporters for glucose, amino acids, and other metabolites [126,127,128,129,130]. Activated Akt was found to stimulate the expression and activity of glycolytic enzymes, leading to increased glycolysis and lactate production [131,132,133]. Moreover, PI3K and Akt increase lipid synthesis via an increase in the expression of lipogenic genes [134,135]. On the other hand, mTOR inhibits autophagy, enhances ribosome biogenesis, and increases protein, FA, and nucleotide synthesis [136,137,138,139]. Additionally, mTOR stimulates aerobic glycolysis via an increase in the expression HIF1α transcript levels and its glycolytic gene targets [137].
In normal cells, PI3K signaling is tightly controlled via the lipid kinase PTEN, which counteracts all metabolic features of the PI3K/Akt/mTOR pathway [140]. Systemic overexpression of PTEN in transgenic mouse models resulted in a metabolic phenotype characterized by diminished aerobic glycolysis and glutaminolysis [141]. The tumor suppressor p53 is a key regulator of metabolic homeostasis [142]. p53 is able to inhibit PI3K signaling via activation of the tumor suppressor genes tuberous sclerosis complexes (TSC) 1 and 2, PTEN, and AMPK [143,144].
5.2. Hypoxia-Inducible Factor (HIF1α)
Hypoxia, or low oxygen tension, significantly affects the process of tumorigenesis. Under normoxic conditions, HIF1α is hydroxylated, which leads to its immediate degradation via the Von Hippel–Lindau (VHL) E3 ubiquitin ligase complex. Under hypoxic conditions, the implied hydroxylases become inactive, thus leading to HIF1α stabilization [145]. HIF1α is a transcription factor complex that is activated in many cancers and its expression is usually associated with a poor prognosis [146]. It regulates and coordinates cellular responses to hypoxia by inducing genes encoding glucose transporters, glycolytic enzymes, and LDHA in order to enhance glycolysis and lactate production while blocking the access of glycolytic products to mitochondria by targeting PDK1 [147,148,149]. Moreover, HIF1α participates in maintaining a functional TCA cycle via glutamine-driven anaplerosis to compensate the limited supply of glycolytic carbon [21]. Furthermore, it activates the reductive carboxylation of glutamine to provide another carbon source for de novo FA synthesis in vitro [97,150].
5.3. cMyc
cMyc, the human homolog of a retroviral gene, is commonly amplified in cancer and plays significant roles in the metabolic adaptation of cancer cells [6]. It can be induced directly or indirectly in response to hormone- or growth-factor-sensitive signal transduction pathways such as PI3K/Akt/mTOR, Wnt/β-catenin, and EGFR/RAS/MAPK [151]. cMyc induces the expression of glycolytic genes, including GLUT1, HK2, and LDHA [27,152]. It promotes the production of the cancer-specific isoform PKM2 by directing the splicing of the pyruvate kinase M pre-mRNA [153]. cMyc is also known to activate glutaminolysis by regulating the expression of GLS isoforms [103,104,154,155] and activate the transcription of enzymes required for nucleotide synthesis [156,157,158]. Finally, cMyc was found to increase the expression of enzymes involved in serine biosynthesis [159]. However, the proliferation of cancer cells was found to mainly depend on the exogenous serine supply via extracellular uptake, with extracellular glycine being unable to compensate for the loss of serine [160]. Intracellular serine may be converted to glycine under the activity of SHMT, which is a direct target for the oncogene cMyc [157].
6. Metabolic Reprogramming in Oncoviruses
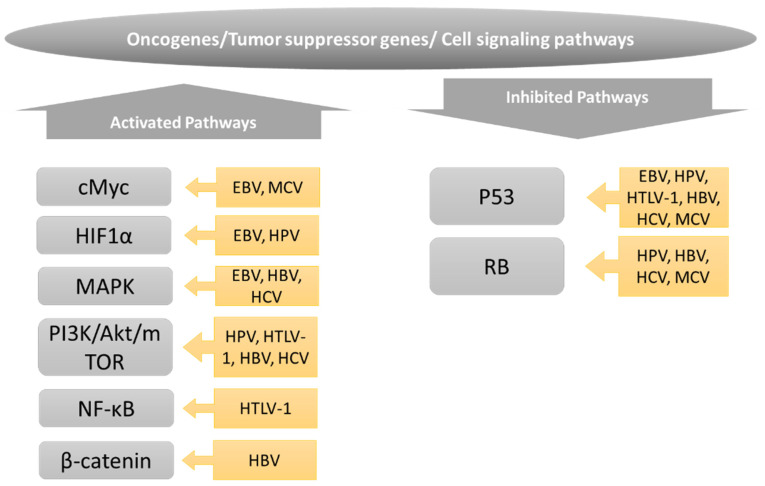
Many similarities have emerged between the metabolic pathways that are modulated in cancer cells versus those altered in cells infected with oncoviruses, as schematically shown in Figure 2. Furthermore, many of the transcription factors, oncogenes, and tumor suppressors that drive metabolic changes in cancer cells are also targeted by oncoviruses, as shown in Figure 3. While virally induced metabolic changes are generally not considered to drive cellular transformation per se, they may facilitate the transformation process in a setting of oxidative stress, genomic instability, and inflammation, as frequently induced by oncoviruses. Importantly, the replication and spread of many oncogenic viruses depend on the metabolic changes in the host cell, which offers important opportunities to target viral replication and the associated cancer using the same therapeutic targets.
Figure 3.
Oncogenes, tumor suppressors, and transcription factors implied in the metabolic alterations associated with oncogenic transformation and oncoviral infections. See text for details.
6.1. Epstein–Barr Virus (EBV)
EBV is a double helix gamma herpes virus that preferentially infects B lymphocytes and epithelial cells [161]. It has been associated with several malignancies [162,163]. After primary infection in B cells, EBV usually starts a latent infection where latent viral proteins stimulate viral DNA replication and cell proliferation. Alternatively, EBV produces infectious virions during the lytic replication phase [164]. The stimulation of lytic reactivation is induced by transcription transactivator proteins ZEBRA and BRLF1 [165]. Several viral genes with oncogenic activity have been identified in the EBV genome, including six nuclear antigens (EBNA1, EBNA2, EBNA3A, EBNA3B, EBNAC, and EBNA-LP) and two latent membrane proteins (LMP1 and LMP2) [166,167].
Early EBV infection induces a transient hyperproliferation period that is suppressed by DNA damage responses and cell cycle arrest. Proliferation-arrested EBV-infected cells showed decreased expression of TCA cycle and OXPHOS enzymes and reduced mitochondrial respiration compared to uninfected B cells. On the other hand, hyperproliferative EBV-infected cells that transform into lymphoblastoid cells showed increases in both glycolysis and OXPHOS [168]. LMP1 overexpression in nasopharyngeal epithelial cells is associated with increased glycolysis and lactate production via increased expression of HK2, PKM2, and LDHA1 [169,170,171]. In concordance with these finding, LMP1 was found to increase HK2 and GLUT1 expression indirectly through decreasing the expression of homeobox gene C8 or, alternatively, by enhancing the degradation of prolyl HIF-hydroxylases, thus stabilizing HIF1α [172]. EBV infection of B lymphocytes directly promoted temporal induction of MCT1 and MCT4 through the viral proteins EBNA2 and LMP1, respectively [173]. Targeting of a recently identified HK isoform, HK domain component 1, has shown therapeutic effects against EBV-induced lymphoma by modulating mitochondrial functions and suppressing EBV replication in mouse models [174]. Glut1-specific chemical inhibitors have been shown to kill NPC cells in vitro [175]. In addition, EBV-encoded microRNAs are highly expressed in EBV-positive tumors and impact glucose metabolism by targeting PTEN/PI3K/Akt, cMyc, and NF-κB and ERK pathways [176,177].
Additionally, EBV infection also affects amino acid metabolism. In addition to enhancing glycolytic enzymes, LMP1 was found to increase glutamine uptake in nasopharyngeal carcinoma cells [178]. Furthermore, an upregulation of GLS1 isoforms was recently shown to stimulate mitochondrial metabolism and cell proliferation in EBV-infected cells [178]. Cell proliferation and the viability of latently EBV-infected cells were sensitive to pharmacological inhibition of GLS1 isoforms [178]. EBV infection was also found to upregulate extracellular serine uptake and de novo serine synthesis [179]. However, methionine restriction, or methionine cycle perturbation, was shown to cause hypomethylation of EBV genomes and de-repress latent membrane protein and lytic gene expression in Burkitt cells. Additionally, methionine metabolism was shown to regulate EBV latency genes required for B cell immortalization [180]. Finally, mTOR inhibitors reduced the capacity of EBV-positive cells to undergo lytic replication in a cell-type-dependent fashion [181].
With respect to nucleotide metabolism, EBV infection of primary B cells was found to upregulate cytidine triphosphate synthases CTPS1 and CTPS2, which is consistent with the observation that purine dNTP biosynthesis is critical in the early stages of EBV-mediated B cell immortalization [182]. Furthermore, EBNA2 and/or EBNA-LP were needed for the induction of CTPS1 in newly infected B cells. Finally, CTPS1 depletion impaired EBV lytic DNA synthesis [183].
The transcriptional factor, EBNA2, was found to induce cMyc expression [184]. EBNA2 and EBNA5 inhibit ubiquitination-induced degradation of HIF1α [185]. The p53 pathway has been found to be inactivated by EBNA3C [186].
EBV also modulates the host cell lipid metabolism. The EBV viral protein BRLF1 expression induces the expression of FASN via activation of the MAPK pathway [187]. Newly EBV-infected B cells are characterized by increased expression of proteins involved in FA and cholesterol metabolism [188]. In EBV-driven cancer cell lines, inhibition of FASN was associated with a decrease in the BRLF1-mediated lytic viral genes, indicating that FA synthesis is required for EBV viral gene expression [187].
6.2. Human Papilloma Virus (HPV)
HPV is a non-enveloped double-stranded DNA virus. Sexually active adolescents are highly susceptible to this infection. The viral genome is composed of three regions: the early region (E), which encodes E1 to E7 proteins necessary for viral replication and cellular transformation; the late region (L), which encodes capsid proteins L1 and L2; and the long control region, which contains the origin of replication and transcription factor binding sites [189]. Low-risk HPV types are associated with formation of benign warts. High-risk HPV types, and in particular HPV 16 and 18, are considered to be the most powerful oncogenic viruses, responsible for about 5% of all cancer cases [190,191,192]. E6 and E7 from high-risk HPV are considered oncoproteins.
The E7 protein of HPV 16 binds to PKM2 and induces a shift from its active tetrameric form to a less active dimeric form in HPV16 E7-transformed 3T3 fibroblasts. The dimeric form is more common in tumors and displays lowered substrate activity [193,194]. High levels of the dimeric form of PKM2 are associated with increased glycolytic intermediates such as fructose biphosphate, essential for amino acid biosynthesis, and increased NADPH biosynthesis, which is needed to regulate ROS levels [195]. To maintain the functionality of the TCA cycle, E7 was shown to promote anaplerotic pathways by activating glutaminolysis [193].
Moreover, E7 degrades the tumor suppressor RB [196] and both E6 and E7 inhibit p53 [197,198,199] and activate the PI3K/Akt/mTOR signaling pathway [200]. HPV16 E6 induces a Warburg effect by interrupting the association between HIF1α and the tumor suppressor von Hippel–Lindau (VHL) and E3 ligase, thus stabilizing HIF1α [201], and has also been reported to interact with cMyc [202,203]. Altered PI3K/Akt signaling was further associated with mitochondrial uncoupling and induction of oxidative stress [204]. In addition to E6 and E7, E5 was also shown to induce glycolysis by stimulating ERK1/2 and Akt signaling [205]. The E2 proteins, negative regulators of E6 and E7, localize to mitochondria, where they induce oxidative stress and modulate OXPHOS [206]. HPV18 E2 was found to localize to several respiratory complexes in mitochondria and this was associated with mitochondrial damage and increased ROS production, which stabilized HIF1α and induced glycolysis [207]. Analysis of the metabolic secretome of one normal and three cancerous cervical cell lines, of which two were HPV positive, showed that the HPV+ cancer cell lines exhibited features of Warburg metabolism, with increased glucose metabolism and upregulated PPP activity. Furthermore, HPV+ cancer cell lines displayed a profile of cysteine/glutathione metabolites that suggested that they differentially deploy glutathione metabolism to maintain a favorable cellular redox balance [208]. Analysis of TCGA data disclosed upregulation of several genes involved in FA metabolism in HPV-positive head and neck squamous cell carcinoma in comparison to HPV-negative cases [209].
6.3. Human T Cell Leukemia Virus 1 (HTLV-1)
HTLV-1, the causative agent of adult T cell leukemia/lymphoma (ATL), is the first retroviral agent known to induce human cancer [210]. In addition to classical retroviral genes, the viral genome encodes two oncoproteins, the transactivator protein Tax and the helix basic zipper proteins, HBZ [211]. After infection and viral entry, reverse transcription of the single-stranded RNA genome takes place in the cytoplasm and the linear double-stranded viral DNA is integrated into the host cell genome. The integrated proviral DNA can be latent or it may be actively transcribed [212]. The virus uses GLUT1 as the entry receptor without clear metabolic alteration after infection [213]. However, hypoxia, frequently encountered by circulating T cells in the lymphoid organs and bone marrow, significantly enhances HTLV-1 reactivation from latency. Furthermore, it has been shown that culturing naturally infected CD4+ T cells in glucose-free medium or inhibition of glycolysis or the mitochondrial electron transport chain strongly suppress HTLV-1 transcription, suggesting that these metabolic processes regulate HTLV-1 proviral latency and reactivation in vivo [214].
Tax and Hbz are two viral oncogenes known to be essential for cellular transformation. Tax is known to activate the PI3K/Akt and NF-κB pathways and inhibits the tumor suppressor p53 [215]. Tax expression is important for the lymphoproliferative and immortalizing effect of HTLV-1 infection [216,217]. Tax increases ROS production, which induces genomic DNA damage and cellular senescence. Additionally, the Tax-dependent activation of NF-κB signaling induces cellular senescence. This effect can be countered through co-expression of HBZ [218]. Both Tax and HBZ induce aberrant cell proliferation and apoptosis [219]. HBZ was shown to activate the mTOR signaling pathway [220]. Recently, the HTLV-1 p30II protein was found to suppress Tax- and HBZ-induced oxidative stress and mitochondrial damage [220]. However, a recent study showed that GR expression and activity and glutathione levels in HTLV-1 patients were reduced compared to healthy control patients, and this reduction was negatively correlated to viral loads [221]. These findings confirm the role of HTLV-1-induced oxidative stress and mitochondrial damage in viral pathogenesis.
Finally, the Hippo signal effector, YAP, known for its implication in the regulation of glutaminolysis, was found to be activated in ATL and its activation to be essential to maintain cellular proliferation of HTLV-1-infected cells. The activation was found to be induced via Tax protein through NF-κB [222].
6.4. Hepatitis B Virus (HBV)
HBV, a hepatotropic, enveloped DNA virus from the family Hepadnaviidae, is a major viral cause of hepatocellular carcinoma [223]. About 300 million people are chronically infected with HBV worldwide. The viral genome harbors four overlapping open reading frames (ORFs) named C, P, S, and X. The ORF C encodes the core protein (HBc) and its related proteins E antigen (HBe) and the precure protein (p22cr), the ORF P encodes the polymerase (pol), the ORF S encodes three types of surface antigens (HBs), and lastly, the ORF X encodes the oncogenic protein X (HBx) [224,225]. After HBV infection, HBV DNA is converted into covalently closed circular DNA (cccDNA), which accumulates in the nucleus as a stable episome, leading to persistent infection [226]. Both immunological and viral factors participate in the development of HBV-induced hepatocellular carcinoma. Chronic infection induces oxidative stress and inflammation that trigger liver fibrosis, characterized by the accumulation of extracellular matrix proteins, including collagen. In the long term, this leads to degradation of the liver microenvironment and cancer development [227]. However, integration of the viral DNA into the host genome can lead to HCC independently of liver damage [228].
The oncogenic viral protein HBx in addition to the pre-S and S polypeptides can activate the PI3/Akt/mTOR, Jak/Stat, Pyk2, Wnt/β-catenin, and the EGFR/RAS/MAPK pathways [229]. HBx also inhibits the tumor suppressors p53 and RB. HBx transgenic mice showed impaired glucose metabolism and increased expression of genes involved in gluconeogenesis [230]. However, in an in vitro model for HBV replication, increased expression of glycolytic factors (FBP aldolase, TPI, PGK1, G6P isomerase) and TCA cycle enzymes (MDH, CS, SDH) and elevation of key glycolytic and TCA metabolites has been reported [231,232]. Increased glycolysis resulted in the activation of nucleotide synthesis [231]. Upregulation of G6PD, the first and rate-limiting enzyme of the PPP, by HBx has been reported [231,233].
Increased glucose uptake, glycolysis, and lactate production were also reported to be induced by a pre-S2 mutant in an mTOR-dependent fashion [234,235]. Using multi-omics analyses to characterize the HBc-transfected hepatoma cell line HepG2, HBc protein was shown to enhance amino acid, lipid, and glucose metabolism in hepatocellular carcinoma [236]. HBc was shown to bind directly and activate enzymes involved in glycine metabolic pathways and phenylalanine degradation. The ability of HBc to modulate glucose and lipid metabolism was found to be regulated via Max-like protein (MLX), which can be recruited to the nucleus in an HBc-dependent manner. MLX binds to and upregulates the glycolytic proteins aldolase C and phosphoenolpyruvate carboxykinase [236]. Knocking down MLX was shown to inhibit Myc-induced metabolic changes and apoptosis, suggesting a role of MLX protein in glycolysis and lipid metabolism in carcinogenesis [237].
In respect to amino acid metabolism, HBV infection was found to significantly increase the expression of glutamine synthetase in liver tissues compared to normal control livers from uninfected patients. Furthermore, in HBV patients with HCC, glutamine synthetase levels were higher than in HBV carriers without HCC [238]. HBx protein has been shown to induce mitochondrial fragmentation via Drp1 stimulation, and mitophagy via parkin, PINK1, and LC3B stimulation [239]. Lipid accumulation, induced by HBV or due to mitochondrial dysfunction, is associated with an increase in HBV gene expression. This process implies induction of lipogenic transcription factors such as sterol regulatory element-binding protein 1 (SREBP-1) and peroxisome proliferator-activated receptor γ [240]. HBV transgenic mice showed increased expression of factors involved in lipid and steroid biosynthesis and metabolism, including retinol-binding protein 1, SREBP2, ATP citrate lyase, and FASN [241,242]. Furthermore, HBV replication and release are sensitive to FASN inhibitors [243,244]. However, HBx has also been reported to stimulate FA oxidation [245]. Finally, HBx overexpression was shown to induce lipid accumulation in HepG2 cells and HBx transgenic mice by the induction of lipogenic transcription factors and FA-binding protein 1 [246,247,248,249] and inhibition of lipid secretion [250], suggesting that it can induce hepatic steatosis. Furthermore, HBV-replicating cells showed elevated levels of enzymes involved in phosphocholine synthesis [231]. In vitro and in HBV-infected humanized mice, elevated levels of factors related to uptake (LDLR), biosynthesis (HMGCR) and transcriptional regulation (SREBP2) of cholesterol [251,252] and hCYP7A1, the rate-limiting enzyme for the conversion of cholesterol into bile acids [251], were detected.
6.5. Hepatitis C Virus (HCV)
HCV, a hepatotropic single-stranded positive-sense RNA virus from the family Flaviviridae, is another important cause of hepatocellular carcinoma [223]. About 1% of the world population are chronic HCV carriers. The viral genome consists of a single large ORF that encodes a single polyprotein that is processed by host and viral proteases to yield the structural proteins core and two envelope glycoproteins as well as seven non-structural proteins (NS) [253].
Similar to HBV infection, chronic HCV infection is associated with oxidative stress and inflammation, which triggers fibrosis and leads to carcinogenesis on the long term [227]. Moreover, chronic HCV infection is generally associated with metabolic disorders such as insulin resistance and steatosis [254,255]. HCV-induced steatosis is associated with increased risk of hepatocellular carcinoma, particularly in the context of genotype 3 [256].
HCV infection was found to activate the N-Ras/PI3K/Akt/mTOR pathway [257] and to inhibit the tumor suppressor p53 [258]. HCV core, NS3, NS5A, and NS5B proteins are responsible for activating PI3/Akt/mTOR and the EGFR/RAS/MAPK pathways and inhibiting the pro-apoptotic proteins p53 and RB [229]. Furthermore, HCV infection in vitro is characterized by increased glucose consumption and lactate production, confirming the activation of glycolytic fluxes, thought to be due to stabilization of HIF1α [259,260,261]. A proteome analysis using an HCV cell culture model showed upregulation of key enzymes involved in glycolysis, the PPP, and the TCA cycle, favoring biosynthetic pathways [262]. NS5A interacts with and activates HK2 in the hepatoma cell line Huh7.5, increasing glycolysis [259]. Additionally, the D2 domain of NS5A is able to activate glucokinase isoenzyme, thus inducing lipogenesis in liver cells. Glucokinase is the predominant isoenzyme of hexokinase in non-cancer cells [263]. Recent studies showed the importance of an active glycolytic pathway for viral replication and the release of virions from infected cells. Dicholoroacetate, a PDK inhibitor that allows reshuttling of pyruvate towards the TCA cycle, suppressed viral replication [264]. Huh7 cells cultured in galactose instead of glucose did not support viral release due to inhibited glycolysis and activation of OXPHOS [265]. However, contradictory data show that HCV reduces GLUT surface expression and induces gluconeogenesis [266].
HCV NS5A was found to affect lipid metabolism by targeting the AMPK/SREBP-1 pathway [267]. Activation of SREBPs was shown to be due to PI3K/Akt signaling [268]. Interestingly, HCV replication is modulated by the interaction between NS5B and FASN, confirming the importance for lipid metabolic reprogramming for HCV persistence [269,270,271]. Furthermore, additional factors and enzymes involved in cholesterol and lipid synthesis, saturation, and secretion have been shown to be essential for HCV replication, virion production, and secretion [272,273,274,275,276,277,278]. Additionally, HCV and, in particular, NS5A promote GLS expression, glutamine uptake, and glutaminolysis in a cMyc-dependent manner [261,279].
Using a combination of metabolic, proteomic, and transcriptomic analyses of HCV-infected cells, increased glucose consumption and metabolism were confirmed. Furthermore, an intracellular accumulation of very-long-chain FAs was detected due to reduced peroxisomal activity [280].
6.6. Kaposi Sarcoma-Associated Herpesvirus (KSHV)
KSHV is a large enveloped double-stranded herpesvirus-8, mainly associated with the development of Kaposi’s sarcoma in untreated HIV patients and other immunocompromised patients [281]. The viral genome harbors about 100 genes [282]. As a herpesvirus, KSHV is capable of latency and lytic replication. In latent infection, the viral genome persists as a multicopy extrachromosomal circular episome. Cancer cells are usually latently infected and express viral oncogenes such as latency-associated nuclear antigen (LANA), viral cyclin (v-cyclin), and Kaposin [283].
KSHV LANA is known to inhibit the tumor suppressors p53 and RB [284,285]. Microvascular endothelial (TIME) cells infected with KSHV show enhanced glucose uptake, glycolysis, and lactate production and reduced oxygen consumption rates, suggesting lower OXPHOS activity [286]. Inhibiting LDH activity with oxamate showed a reduction in KSHV viral production after lytic infection in TIME cells, which indicates the importance of glycolysis for KSHV viral particle production [287]. Furthermore, oxamate reduced the expression of early and late viral genes such as open reading frame (ORF) 45 and ORF 59 (early genes) and ORF26 and K8.1 (late genes) [287]. KSHV infection has been shown to induce glycolytic metabolism by several groups via increased HIF1α expression and stabilization [288,289,290]. The virus-encoded G-protein-coupled receptor (vGPCR) is a direct target of HIF1α and is a major inducer of metabolic changes induced by KSHV infection. Viral mutants lacking vGPCR are unable to induce metabolic changes [291]. PI3K/Akt/mTOR is another signaling pathway stimulated by KSHV that leads to increased glycolysis [292]. Inhibition of PI3K/Akt was found to decrease the glycolytic fluxes in infected cells [293]. KSHV also induced the expression of PPP enzymes, including G6PD, TKT, and TALDO, in a nuclear elongation factor 2 (Nrf2)-dependent fashion. Nrf2 was not only found to be induced in infected cells but also in Kaposi’s sarcoma infection-associated lesions [294].
Mass spectrometric analysis of KSHV-infected TIME cells showed an increase in long-chain FA production. While the precursors of FA synthesis, choline and phosphocholine, were also increased, there was a decrease in the degradation products of phospholipids, glycerophosphorylcholine, and glycerol-3-phosphate. Exposure of infected TIME cells to FASN inhibitors significantly increased cell death [295]. However, FA synthesis in Kaposi’s sarcoma is downregulated and not needed for the expression of early and late KSHV genes [287]. Overproduction of cholesterol esters is shown in latent KSHV infections and inhibition of cholesteryl esterification impairs neo-angiogenesis, suggesting a role of the cholesterol ester biosynthetic pathway in Kaposi’s sarcoma development [296]. Nevertheless, RNA sequencing analysis of Kaposi’s sarcoma biopsies showed a significant downregulation of several lipid metabolic pathways, including FASN, compared to normal tissues [297].
Cells latently infected with KSHV are glutamine addicted and depend on glutaminolysis for survival. Lytic infection also depends on glutaminolysis [287,298]. KSHV infection increases glutamine uptake by upregulating the expression of glutamine receptor SLC1A5. Furthermore, the enhanced glutamine metabolism is due to overexpression of GLS, GDH1, and glutamic-oxalacetate transaminase 2 enzymes, essential for glutaminolysis [299]. Instead of fueling the TCA cycle, glutaminolysis in KSHV-infected cells serves as nitrogen supply for nucleic acid biosynthesis [299]. Moreover, metabolic analysis on three-dimensional cell cultures showed that KSHV infection enhanced nonessential amino acid and in particular proline and the associated polyamine metabolism [300]. Hypusine, a polyamine-derived amino acid, was shown to enhance LANA expression via hypusination of the eukaryotic initiation factor 5 A (eIF5A) [301,302]. Moreover, inhibition of polyamine biosynthesis or eIF5A hypusination decreased LANA expression, reduced viral episomal maintenance, and suppressed viral infection [301,302].
6.7. Merkel Cell Polyomavirus (MCV)
MCV is a non-enveloped, double stranded DNA virus that is thought to be the cause of the majority of Merkel cell carcinoma cases. The viral genome harbors early and late gene regions on opposite strands, with a central non-coding control region containing the origin of replication [303]. After viral infection, the early region is expressed immediately and undergoes splicing into two T antigen oncoproteins, large and small. The late region encodes the viral structural capsid proteins, viral protein 1 (VP1) and viral protein 2 (VP2) [304]. MCV infection is usually persistent and asymptomatic. Nevertheless, VP1 antibodies can be detected in healthy subjects and Merkel cell carcinoma patients but at lower levels [305]. Antibodies against T antigens are hardly detected except in Merkel cell carcinoma patients and can be used to monitor disease progression [306].
Viral T antigens are expressed persistently in Merkel cell carcinoma and participate in the process of tumorigenesis. T antigens were found to inhibit the tumor suppressors RB and p53 and stabilize the cMyc signaling pathway [307,308,309]. Transcriptome analysis of normal human fibroblasts with inducible expression of small T antigen showed upregulation of glycolytic genes and the monocarboxylate lactate transporter SLC16A1. These cells showed increased lactate production, suggesting a reduced OXPHOS activity [310].
7. Conclusions
This review outlines the similarities of the metabolic reprogramming observed in transformed cells and cells infected with oncogenic viruses. Indeed, viral infection and amplification require the use of the cellular metabolic machinery to synthesize viral proteins, nucleic acids, and lipids, similar to the biosynthetic needs for cell proliferation. Furthermore, redox homeostasis in infected cells needs to be controlled in order to balance its effects on viral replication versus host cell survival and thus viral persistence. Similarly, cancer cells are known to depend on a tight regulation of oxidative stress. Finally, the secretion of certain metabolites (here, we discussed only lactate) is important to ensure cell survival for both cancer and infected cells in an inflammatory microenvironment.
Metabolic reprogramming is not exclusive to oncogenic viruses. Similar changes are often observed in the context of infections with non-oncogenic viruses. The oncovirus-induced metabolic changes outlined here are therefore not sufficient for the induction of carcinogenesis. However, in the context of inflammation and oxidative stress, the presence of “pro-cancerogenic” metabolism is likely to facilitate malignant transformation induced by viruses.
Specific metabolic dependencies in cancer have been the basis for effective therapeutics, including inhibitors that target IDH, and folate and thymidine metabolism [311]. Many of these inhibitors have been tested for their efficacies to inhibit viral replication, as discussed above and detailed in Table 1, suggesting that targeting the host cell metabolism is a therapeutic strategy for viral infections. Furthermore, in some cases, dual therapeutic effects in the context of oncogenic infections have been observed: the inhibition of viral replication and the spread and elimination of transformed cells. These findings warrant further exploration of the dependencies of viruses on host cell metabolism, particularly for viruses where no direct-acting antiviral that specifically targets the virus exists.
Table 1.
Antiviral effects of metabolic inhibitors.
| Metabolic Drug | Target Enzyme | Virus | Model/Cell System | Outcome | Reference |
|---|---|---|---|---|---|
| STF-31 | GLUT1 | EBV | kills NPC cells in vitro | [175] | |
| 2-deoxy-D-Glucose | Hexokinase, PI3K/Akt | HTLV-1 | PBMCs | suppresses viral transcription | [214] |
| HBV | Huh7 | suppresses viral protein production (HBV delivered with adenovirus) | [312] | ||
| oxamate | LDHA | KSHV | TIME | decreases virion production | [287] |
| dichloroacetate | PDK | HCV | Huh7.5 | decreases viral replication | [264] |
| rapamycine | mTOR | EBV | cell type dependent | induces of lytic cycle | [181] |
| CB-839/BPTES | Glutaminase | HCV | Huh7.5 | inhibits replication and infection establishment | [261] |
| KSHV | TIME | decreases virion production | [287] | ||
| difluoromethylornithine | ornithine decarboxylase 1 | KSHV | TIME/BCBL-1 | decreases virion production | [302] |
| N1-guanyl-1,7-diaminoheptane (GC7) | deoxyhypusine synthase | KSHV | TIME/BCBL-1 | decreases virion production | [302] |
| cerulenin | FASN | HCV | Huh7 | decreases viral replication | [313] |
| C75 | FASN | HCV | Huh7 | decreases virion production | [270] |
| GSK1995010 | FASN | HBV | HepG2.2.15.7 | decreases viral replication | [243] |
| 4-(1-(5-(2-cyclopropyl-4-methyl-1H-imidazol-5-yl)- 2,4-dimethylbenzoyl)-3-fluoroazetidin-3- yl)benzonitrile | FASN | HBV | HepG2NTCP | inhibits HBs secretion | [244] |
| LCQ908/pradigastat | diacylglycerol O acyltransferase 1 | HCV | clinical phase trial/Huh7.5 | inhibits replication in vitro but not in vivo | [274] |
| BMS-200150/BMS-20101038 | microsomal transfer protein | HCV | Huh7.5 | decreases virion release | [275,276] |
| 3J [314] | Stearoyl-CoA desaturase | HCV | Huh7.5 | decreases viral replication and assembly | [277] |
| 25-hydroxycholesterol | SREBP | HCV | Huh7 | decreases viral replication | [313] |
| 5-tetradecyloxy-2-furoic acid (TOFA) | acetyl-coA carboxylase | KSHV | TIME | decreases virion production | [287] |
| CP640186 | acetyl-coA carboxylase | HBV | HepG2.2.15.7 | decreases viral replication | [243] |
| 2-(1-((R)-2-(((1s,4S)-4-hydroxycyclohexyl)oxy)-2- (2-methoxyphenyl)ethyl)-5-methyl-6-(oxazol-2-yl)- 2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)- yl)-2-methylpropanoic acid | acetyl-coA carboxylase | HBV | HepG2NTCP | inhibits HBs secretion | [244] |
| (R)-4-((diethylamino)methyl)-N-(2- methoxyphenethyl)-N-(pyrrolidin-3-yl)benzamide | subtilisin kexin isozyme-1/site-1 protease | HBV | HepG2NTCP | inhibits HBs secretion | [244] |
Author Contributions
A.G. original draft preparation, writing; B.B. supervision, writing, review and editing, funding acquisition; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Post-doctoral fellowship from the Agence Nationale de la Recherche sur le Sida et les Hepatites Virales (D22157 to A.G.); DevWeCan Labex (Laboratories of Excellence Network, ANR-LABX-061).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. About the formation of cancer cells. Strahlentherapie. 1956;34:3–13; discussion 41–57. [PubMed] [Google Scholar]
- 3.Warburg O. Origin of cancer cells. Oncologia. 1956;9:75–83. doi: 10.1159/000223920. [DOI] [PubMed] [Google Scholar]
- 4.Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 5.Jones R.G., Thompson C.B. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Hsu P.P., Sabatini D.M. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Swierczynski J., Hebanowska A., Sledzinski T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J. Gastroenterol. 2014;20:2279–2303. doi: 10.3748/wjg.v20.i9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassanein M., Hoeksema M.D., Shiota M., Qian J., Harris B.K., Chen H., Clark J.E., Alborn W.E., Eisenberg R., Massion P.P. Slc1a5 mediates glutamine transport required for lung cancer cell growth and survival. Clin. Cancer Res. 2013;19:560–570. doi: 10.1158/1078-0432.CCR-12-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mates J.M., Segura J.A., Martin-Rufian M., Campos-Sandoval J.A., Alonso F.J., Marquez J. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr. Mol. Med. 2013;13:514–534. doi: 10.2174/1566524011313040005. [DOI] [PubMed] [Google Scholar]
- 11.Taheri F., Goudarzi H., Faghihloo E. Aneuploidy and oncoviruses. Rev. Med. Virol. 2019;29:e2076. doi: 10.1002/rmv.2076. [DOI] [PubMed] [Google Scholar]
- 12.White M.K., Pagano J.S., Khalili K. Viruses and human cancers: A long road of discovery of molecular paradigms. Clin. Microbiol. Rev. 2014;27:463–481. doi: 10.1128/CMR.00124-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiller J.T., Lowy D.R. An introduction to virus infections and human cancer. Recent Results Cancer Res. 2021;217:1–11. doi: 10.1007/978-3-030-57362-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racker E. Bioenergetics and the problem of tumor growth. Am. Sci. 1972;60:56–63. [PubMed] [Google Scholar]
- 15.Vander Heiden M.G., Lunt S.Y., Dayton T.L., Fiske B.P., Israelsen W.J., Mattaini K.R., Vokes N.I., Stephanopoulos G., Cantley L.C., Metallo C.M., et al. Metabolic pathway alterations that support cell proliferation. Cold Spring Harb. Symp. Quant. Biol. 2011;76:325–334. doi: 10.1101/sqb.2012.76.010900. [DOI] [PubMed] [Google Scholar]
- 16.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinhouse S. On respiratory impairment in cancer cells. Science. 1956;124:267–269. doi: 10.1126/science.124.3215.267. [DOI] [PubMed] [Google Scholar]
- 18.Zu X.L., Guppy M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Sanchez R., Rodriguez-Enriquez S., Marin-Hernandez A., Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 20.Fantin V.R., St-Pierre J., Leder P. Attenuation of ldh-a expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Fan J., Kamphorst J.J., Mathew R., Chung M.K., White E., Shlomi T., Rabinowitz J.D. Glutamine-driven oxidative phosphorylation is a major atp source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 2013;9:712. doi: 10.1038/msb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroemer G., Pouyssegur J. Tumor cell metabolism: Cancer’s achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Halama A., Suhre K. Advancing cancer treatment by targeting glutamine metabolism-a roadmap. Cancers. 2022;14:553. doi: 10.3390/cancers14030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adekola K., Rosen S.T., Shanmugam M. Glucose transporters in cancer metabolism. Curr. Opin. Oncol. 2012;24:650–654. doi: 10.1097/CCO.0b013e328356da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Enriquez S., Marin-Hernandez A., Gallardo-Perez J.C., Moreno-Sanchez R. Kinetics of transport and phosphorylation of glucose in cancer cells. J. Cell Physiol. 2009;221:552–559. doi: 10.1002/jcp.21885. [DOI] [PubMed] [Google Scholar]
- 26.Ying H., Kimmelman A.C., Lyssiotis C.A., Hua S., Chu G.C., Fletcher-Sananikone E., Locasale J.W., Son J., Zhang H., Coloff J.L., et al. Oncogenic kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osthus R.C., Shim H., Kim S., Li Q., Reddy R., Mukherjee M., Xu Y., Wonsey D., Lee L.A., Dang C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-myc. J. Biol. Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 28.Boroughs L.K., DeBerardinis R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B., Tang H., Liu X., Liu P., Yang L., Xie X., Ye F., Song C., Xie X., Wei W. Mir-22 as a prognostic factor targets glucose transporter protein type 1 in breast cancer. Cancer Lett. 2015;356:410–417. doi: 10.1016/j.canlet.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Ohnishi T., Kusuyama J., Bandow K., Matsuguchi T. Glut1 expression is increased by p53 reduction to switch metabolism to glycolysis during osteoblast differentiation. Biochem. J. 2020;477:1795–1811. doi: 10.1042/BCJ20190888. [DOI] [PubMed] [Google Scholar]
- 31.Yang J., Wen J., Tian T., Lu Z., Wang Y., Wang Z., Wang X., Yang Y. Glut-1 overexpression as an unfavorable prognostic biomarker in patients with colorectal cancer. Oncotarget. 2017;8:11788–11796. doi: 10.18632/oncotarget.14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu M., Yongzhi H., Chen S., Luo X., Lin Y., Zhou Y., Jin H., Hou B., Deng Y., Tu L., et al. The prognostic value of glut1 in cancers: A systematic review and meta-analysis. Oncotarget. 2017;8:43356–43367. doi: 10.18632/oncotarget.17445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Lu P., Zhou S., Zhang L., Zhao J.H., Tang J.H. Predictive value of glucose transporter-1 and glucose transporter-3 for survival of cancer patients: A meta-analysis. Oncotarget. 2017;8:13206–13213. doi: 10.18632/oncotarget.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan D.A., Sutphin P.D., Nguyen P., Turcotte S., Lai E.W., Banh A., Reynolds G.E., Chi J.T., Wu J., Solow-Cordero D.E., et al. Targeting glut1 and the warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci. Transl. Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraus D., Reckenbeil J., Veit N., Kuerpig S., Meisenheimer M., Beier I., Stark H., Winter J., Probstmeier R. Targeting glucose transport and the nad pathway in tumor cells with stf-31: A re-evaluation. Cell. Oncol. 2018;41:485–494. doi: 10.1007/s13402-018-0385-5. [DOI] [PubMed] [Google Scholar]
- 36.Kawauchi K., Araki K., Tobiume K., Tanaka N. P53 regulates glucose metabolism through an ikk-nf-kappab pathway and inhibits cell transformation. Nat. Cell Biol. 2008;10:611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- 37.He X., Lin X., Cai M., Zheng X., Lian L., Fan D., Wu X., Lan P., Wang J. Overexpression of hexokinase 1 as a poor prognosticator in human colorectal cancer. Tumour. Biol. 2016;37:3887–3895. doi: 10.1007/s13277-015-4255-8. [DOI] [PubMed] [Google Scholar]
- 38.Mathupala S.P., Rempel A., Pedersen P.L. Glucose catabolism in cancer cells: Identification and characterization of a marked activation response of the type ii hexokinase gene to hypoxic conditions. J. Biol. Chem. 2001;276:43407–43412. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- 39.Wang L., Xiong H., Wu F., Zhang Y., Wang J., Zhao L., Guo X., Chang L.J., Zhang Y., You M.J., et al. Hexokinase 2-mediated warburg effect is required for pten- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014;8:1461–1474. doi: 10.1016/j.celrep.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peschiaroli A., Giacobbe A., Formosa A., Markert E.K., Bongiorno-Borbone L., Levine A.J., Candi E., D’Alessandro A., Zolla L., Finazzi Agro A., et al. Mir-143 regulates hexokinase 2 expression in cancer cells. Oncogene. 2013;32:797–802. doi: 10.1038/onc.2012.100. [DOI] [PubMed] [Google Scholar]
- 41.Lauterwasser J., Fimm-Todt F., Oelgeklaus A., Schreiner A., Funk K., Falquez-Medina H., Klesse R., Jahreis G., Zerbes R.M., O’Neill K., et al. Hexokinases inhibit death receptor-dependent apoptosis on the mitochondria. Proc. Natl. Acad. Sci. USA. 2021;118:e2021175118. doi: 10.1073/pnas.2021175118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Jesus A., Keyhani-Nejad F., Pusec C.M., Goodman L., Geier J.A., Stoolman J.S., Stanczyk P.J., Nguyen T., Xu K., Suresh K.V., et al. Hexokinase 1 cellular localization regulates the metabolic fate of glucose. Mol. Cell. 2022;82:1261–1277.e1269. doi: 10.1016/j.molcel.2022.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z., Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell. Mol. Life Sci. 2016;73:377–392. doi: 10.1007/s00018-015-2070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al Hasawi N., Alkandari M.F., Luqmani Y.A. Phosphofructokinase: A mediator of glycolytic flux in cancer progression. Crit. Rev. Oncol. Hematol. 2014;92:312–321. doi: 10.1016/j.critrevonc.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Fu Q., Yu Z. Phosphoglycerate kinase 1 (pgk1) in cancer: A promising target for diagnosis and therapy. Life Sci. 2020;256:117863. doi: 10.1016/j.lfs.2020.117863. [DOI] [PubMed] [Google Scholar]
- 46.Chang Y.C., Chiou J., Yang Y.F., Su C.Y., Lin Y.F., Yang C.N., Lu P.J., Huang M.S., Yang C.J., Hsiao M. Therapeutic targeting of aldolase a interactions inhibits lung cancer metastasis and prolongs survival. Cancer Res. 2019;79:4754–4766. doi: 10.1158/0008-5472.CAN-18-4080. [DOI] [PubMed] [Google Scholar]
- 47.Ji S., Zhang B., Liu J., Qin Y., Liang C., Shi S., Jin K., Liang D., Xu W., Xu H., et al. Aldoa functions as an oncogene in the highly metastatic pancreatic cancer. Cancer Lett. 2016;374:127–135. doi: 10.1016/j.canlet.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 48.Du S., Guan Z., Hao L., Song Y., Wang L., Gong L., Liu L., Qi X., Hou Z., Shao S. Fructose-bisphosphate aldolase a is a potential metastasis-associated marker of lung squamous cell carcinoma and promotes lung cell tumorigenesis and migration. PLoS ONE. 2014;9:e85804. doi: 10.1371/journal.pone.0085804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuang Q., Liang Y., Zhuo Y., Cai Z., Jiang F., Xie J., Zheng Y., Zhong W. The aldoa metabolism pathway as a potential target for regulation of prostate cancer proliferation. Oncol. Targets Ther. 2021;14:3353–3366. doi: 10.2147/OTT.S290284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao Q., Zhu H., Dong L., Shi W., Chen R., Song Z., Huang C., Li J., Dong X., Zhou Y., et al. Integrated proteogenomic characterization of hbv-related hepatocellular carcinoma. Cell. 2019;179:1240. doi: 10.1016/j.cell.2019.10.038. [DOI] [PubMed] [Google Scholar]
- 51.Niu Y., Lin Z., Wan A., Sun L., Yan S., Liang H., Zhan S., Chen D., Bu X., Liu P., et al. Loss-of-function genetic screening identifies aldolase a as an essential driver for liver cancer cell growth under hypoxia. Hepatology. 2021;74:1461–1479. doi: 10.1002/hep.31846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazurek S., Boschek C.B., Hugo F., Eigenbrodt E. Pyruvate kinase type m2 and its role in tumor growth and spreading. Semin. Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Christofk H.R., Vander Heiden M.G., Harris M.H., Ramanathan A., Gerszten R.E., Wei R., Fleming M.D., Schreiber S.L., Cantley L.C. The m2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 54.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. Hif-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Bonnet S., Archer S.L., Allalunis-Turner J., Haromy A., Beaulieu C., Thompson R., Lee C.T., Lopaschuk G.D., Puttagunta L., Bonnet S., et al. A mitochondria-k+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Yao F., Zhao T., Zhong C., Zhu J., Zhao H. Ldha is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumour. Biol. 2013;34:25–31. doi: 10.1007/s13277-012-0506-0. [DOI] [PubMed] [Google Scholar]
- 57.Le A., Cooper C.R., Gouw A.M., Dinavahi R., Maitra A., Deck L.M., Royer R.E., Vander Jagt D.L., Semenza G.L., Dang C.V. Inhibition of lactate dehydrogenase a induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X., Yang Z., Chen Z., Chen R., Zhao D., Zhou Y., Qiao L. Effects of the suppression of lactate dehydrogenase a on the growth and invasion of human gastric cancer cells. Oncol. Rep. 2015;33:157–162. doi: 10.3892/or.2014.3600. [DOI] [PubMed] [Google Scholar]
- 59.San-Millan I., Brooks G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the warburg effect. Carcinogenesis. 2017;38:119–133. doi: 10.1093/carcin/bgw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vegran F., Boidot R., Michiels C., Sonveaux P., Feron O. Lactate influx through the endothelial cell monocarboxylate transporter mct1 supports an nf-kappab/il-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 61.Choi S.Y., Collins C.C., Gout P.W., Wang Y. Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J. Pathol. 2013;230:350–355. doi: 10.1002/path.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goetze K., Walenta S., Ksiazkiewicz M., Kunz-Schughart L.A., Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol. 2011;39:453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 63.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., et al. Inhibitory effect of tumor cell-derived lactic acid on human t cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 64.Jiang P., Du W., Wu M. Regulation of the pentose phosphate pathway in cancer. Protein. Cell. 2014;5:592–602. doi: 10.1007/s13238-014-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nogueira V., Hay N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013;19:4309–4314. doi: 10.1158/1078-0432.CCR-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kowalik M.A., Columbano A., Perra A. Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Front. Oncol. 2017;7:87. doi: 10.3389/fonc.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandez-Marcos P.J., Nobrega-Pereira S. Nadph: New oxygen for the ros theory of aging. Oncotarget. 2016;7:50814–50815. doi: 10.18632/oncotarget.10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen M., Shen M., Li Y., Liu C., Zhou K., Hu W., Xu B., Xia Y., Tang W. Gc-ms-based metabolomic analysis of human papillary thyroid carcinoma tissue. Int. J. Mol. Med. 2015;36:1607–1614. doi: 10.3892/ijmm.2015.2368. [DOI] [PubMed] [Google Scholar]
- 70.Langbein S., Frederiks W.M., zur Hausen A., Popa J., Lehmann J., Weiss C., Alken P., Coy J.F. Metastasis is promoted by a bioenergetic switch: New targets for progressive renal cell cancer. Int. J. Cancer. 2008;122:2422–2428. doi: 10.1002/ijc.23403. [DOI] [PubMed] [Google Scholar]
- 71.Zampella E.J., Bradley E.L., Jr., Pretlow T.G., 2nd Glucose-6-phosphate dehydrogenase: A possible clinical indicator for prostatic carcinoma. Cancer. 1982;49:384–387. doi: 10.1002/1097-0142(19820115)49:2<384::AID-CNCR2820490229>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 72.Kuo W., Lin J., Tang T.K. Human glucose-6-phosphate dehydrogenase (g6pd) gene transforms nih 3t3 cells and induces tumors in nude mice. Int. J. Cancer. 2000;85:857–864. doi: 10.1002/(SICI)1097-0215(20000315)85:6<857::AID-IJC20>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 73.Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stanton R.C., Seifter J.L., Boxer D.C., Zimmerman E., Cantley L.C. Rapid release of bound glucose-6-phosphate dehydrogenase by growth factors. Correlation with increased enzymatic activity. J. Biol. Chem. 1991;266:12442–12448. doi: 10.1016/S0021-9258(18)98918-0. [DOI] [PubMed] [Google Scholar]
- 75.Tian W.N., Pignatare J.N., Stanton R.C. Signal transduction proteins that associate with the platelet-derived growth factor (pdgf) receptor mediate the pdgf-induced release of glucose-6-phosphate dehydrogenase from permeabilized cells. J. Biol. Chem. 1994;269:14798–14805. doi: 10.1016/S0021-9258(17)36695-4. [DOI] [PubMed] [Google Scholar]
- 76.Pan S., World C.J., Kovacs C.J., Berk B.C. Glucose 6-phosphate dehydrogenase is regulated through c-src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009;29:895–901. doi: 10.1161/ATVBAHA.109.184812. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X., Zhang X., Li Y., Shao Y., Xiao J., Zhu G., Li F. Pak4 regulates g6pd activity by p53 degradation involving colon cancer cell growth. Cell Death Dis. 2017;8:e2820. doi: 10.1038/cddis.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang P., Du W., Wang X., Mancuso A., Gao X., Wu M., Yang X. P53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011;13:310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hong X., Song R., Song H., Zheng T., Wang J., Liang Y., Qi S., Lu Z., Song X., Jiang H., et al. Pten antagonises tcl1/hnrnpk-mediated g6pd pre-mrna splicing which contributes to hepatocarcinogenesis. Gut. 2014;63:1635–1647. doi: 10.1136/gutjnl-2013-305302. [DOI] [PubMed] [Google Scholar]
- 80.Ge T., Yang J., Zhou S., Wang Y., Li Y., Tong X. The role of the pentose phosphate pathway in diabetes and cancer. Front. Endocrinol. 2020;11:365. doi: 10.3389/fendo.2020.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stincone A., Prigione A., Cramer T., Wamelink M.M., Campbell K., Cheung E., Olin-Sandoval V., Gruning N.M., Kruger A., Tauqeer Alam M., et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015;90:927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin L., Zhou Y. Crucial role of the pentose phosphate pathway in malignant tumors. Oncol. Lett. 2019;17:4213–4221. doi: 10.3892/ol.2019.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basta P.V., Bensen J.T., Tse C.K., Perou C.M., Sullivan P.F., Olshan A.F. Genetic variation in transaldolase 1 and risk of squamous cell carcinoma of the head and neck. Cancer Detect Prev. 2008;32:200–208. doi: 10.1016/j.cdp.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C., Guo K., Gao D., Kang X., Jiang K., Li Y., Sun L., Zhang S., Sun C., Liu X., et al. Identification of transaldolase as a novel serum biomarker for hepatocellular carcinoma metastasis using xenografted mouse model and clinic samples. Cancer Lett. 2011;313:154–166. doi: 10.1016/j.canlet.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 85.Langbein S., Zerilli M., Zur Hausen A., Staiger W., Rensch-Boschert K., Lukan N., Popa J., Ternullo M.P., Steidler A., Weiss C., et al. Expression of transketolase tktl1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br. J. Cancer. 2006;94:578–585. doi: 10.1038/sj.bjc.6602962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Owen O.E., Kalhan S.C., Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 87.Catalina-Rodriguez O., Kolukula V.K., Tomita Y., Preet A., Palmieri F., Wellstein A., Byers S., Giaccia A.J., Glasgow E., Albanese C., et al. The mitochondrial citrate transporter, cic, is essential for mitochondrial homeostasis. Oncotarget. 2012;3:1220–1235. doi: 10.18632/oncotarget.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Y., Bollu L.R., Tozzi F., Ye X., Bhattacharya R., Gao G., Dupre E., Xia L., Lu J., Fan F., et al. Atp citrate lyase mediates resistance of colorectal cancer cells to sn38. Mol. Cancer Ther. 2013;12:2782–2791. doi: 10.1158/1535-7163.MCT-13-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beckner M.E., Fellows-Mayle W., Zhang Z., Agostino N.R., Kant J.A., Day B.W., Pollack I.F. Identification of atp citrate lyase as a positive regulator of glycolytic function in glioblastomas. Int. J. Cancer. 2010;126:2282–2295. doi: 10.1002/ijc.24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szutowicz A., Kwiatkowski J., Angielski S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Br. J. Cancer. 1979;39:681–687. doi: 10.1038/bjc.1979.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Wang Y., Shen L., Pang Y., Qiao Z., Liu P. Prognostic and therapeutic implications of increased atp citrate lyase expression in human epithelial ovarian cancer. Oncol. Rep. 2012;27:1156–1162. doi: 10.3892/or.2012.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bourbeau M.P., Bartberger M.D. Recent advances in the development of acetyl-coa carboxylase (acc) inhibitors for the treatment of metabolic disease. J. Med. Chem. 2015;58:525–536. doi: 10.1021/jm500695e. [DOI] [PubMed] [Google Scholar]
- 93.Carling D., Zammit V.A., Hardie D.G. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 94.Menendez J.A., Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 95.Yang Y.A., Han W.F., Morin P.J., Chrest F.J., Pizer E.S. Activation of fatty acid synthesis during neoplastic transformation: Role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp. Cell Res. 2002;279:80–90. doi: 10.1006/excr.2002.5600. [DOI] [PubMed] [Google Scholar]
- 96.Jin L., Alesi G.N., Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619–3625. doi: 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Metallo C.M., Gameiro P.A., Bell E.L., Mattaini K.R., Yang J., Hiller K., Jewell C.M., Johnson Z.R., Irvine D.J., Guarente L., et al. Reductive glutamine metabolism by idh1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang H., Zhou L., Shi Q., Zhao Y., Lin H., Zhang M., Zhao S., Yang Y., Ling Z.Q., Guan K.L., et al. Sirt3-dependent got2 acetylation status affects the malate-aspartate nadh shuttle activity and pancreatic tumor growth. EMBO J. 2015;34:1110–1125. doi: 10.15252/embj.201591041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang J., Pavlova N.N., Thompson C.B. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J. 2017;36:1302–1315. doi: 10.15252/embj.201696151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Altman B.J., Stine Z.E., Dang C.V. From krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer. 2016;16:619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao P., Tchernyshyov I., Chang T.C., Lee Y.S., Kita K., Ochi T., Zeller K.I., De Marzo A.M., Van Eyk J.E., Mendell J.T., et al. C-myc suppression of mir-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wise D.R., DeBerardinis R.J., Mancuso A., Sayed N., Zhang X.Y., Pfeiffer H.K., Nissim I., Daikhin E., Yudkoff M., McMahon S.B., et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le A., Lane A.N., Hamaker M., Bose S., Gouw A., Barbi J., Tsukamoto T., Rojas C.J., Slusher B.S., Zhang H., et al. Glucose-independent glutamine metabolism via tca cycling for proliferation and survival in b cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., et al. Bidirectional transport of amino acids regulates mtor and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang W.H., Qiu Y., Stamatatos O., Janowitz T., Lukey M.J. Enhancing the efficacy of glutamine metabolism inhibitors in cancer therapy. Trends Cancer. 2021;7:790–804. doi: 10.1016/j.trecan.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qu X., Sun J., Zhang Y., Li J., Hu J., Li K., Gao L., Shen L. C-myc-driven glycolysis via txnip suppression is dependent on glutaminase-mondoa axis in prostate cancer. Biochem. Biophys. Res. Commun. 2018;504:415–421. doi: 10.1016/j.bbrc.2018.08.069. [DOI] [PubMed] [Google Scholar]
- 108.Lampa M., Arlt H., He T., Ospina B., Reeves J., Zhang B., Murtie J., Deng G., Barberis C., Hoffmann D., et al. Glutaminase is essential for the growth of triple-negative breast cancer cells with a deregulated glutamine metabolism pathway and its suppression synergizes with mtor inhibition. PLoS ONE. 2017;12:e0185092. doi: 10.1371/journal.pone.0185092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mates J.M., Campos-Sandoval J.A., Santos-Jimenez J.L., Marquez J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett. 2019;467:29–39. doi: 10.1016/j.canlet.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 110.Tannir N.M., Agarwal N., Porta C., Lawrence N.J., Motzer R., McGregor B., Lee R.J., Jain R.K., Davis N., Appleman L.J., et al. Efficacy and safety of telaglenastat plus cabozantinib vs placebo plus cabozantinib in patients with advanced renal cell carcinoma: The cantata randomized clinical trial. JAMA Oncol. 2022;8:1411–1418. doi: 10.1001/jamaoncol.2022.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amelio I., Cutruzzola F., Antonov A., Agostini M., Melino G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maddocks O.D., Labuschagne C.F., Adams P.D., Vousden K.H. Serine metabolism supports the methionine cycle and DNA/rna methylation through de novo atp synthesis in cancer cells. Mol. Cell. 2016;61:210–221. doi: 10.1016/j.molcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sahu N., Dela Cruz D., Gao M., Sandoval W., Haverty P.M., Liu J., Stephan J.P., Haley B., Classon M., Hatzivassiliou G., et al. Proline starvation induces unresolved er stress and hinders mtorc1-dependent tumorigenesis. Cell Metab. 2016;24:753–761. doi: 10.1016/j.cmet.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 114.Tang L., Zeng J., Geng P., Fang C., Wang Y., Sun M., Wang C., Wang J., Yin P., Hu C., et al. Global metabolic profiling identifies a pivotal role of proline and hydroxyproline metabolism in supporting hypoxic response in hepatocellular carcinoma. Clin. Cancer Res. 2018;24:474–485. doi: 10.1158/1078-0432.CCR-17-1707. [DOI] [PubMed] [Google Scholar]
- 115.Togashi Y., Arao T., Kato H., Matsumoto K., Terashima M., Hayashi H., de Velasco M.A., Fujita Y., Kimura H., Yasuda T., et al. Frequent amplification of oraov1 gene in esophageal squamous cell cancer promotes an aggressive phenotype via proline metabolism and ros production. Oncotarget. 2014;5:2962–2973. doi: 10.18632/oncotarget.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Elia I., Broekaert D., Christen S., Boon R., Radaelli E., Orth M.F., Verfaillie C., Grunewald T.G.P., Fendt S.M. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 2017;8:15267. doi: 10.1038/ncomms15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu W., Hancock C.N., Fischer J.W., Harman M., Phang J.M. Proline biosynthesis augments tumor cell growth and aerobic glycolysis: Involvement of pyridine nucleotides. Sci. Rep. 2015;5:17206. doi: 10.1038/srep17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pegg A.E., Casero R.A., Jr. Current status of the polyamine research field. Methods Mol. Biol. 2011;720:3–35. doi: 10.1007/978-1-61779-034-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terui Y., Yoshida T., Sakamoto A., Saito D., Oshima T., Kawazoe M., Yokoyama S., Igarashi K., Kashiwagi K. Polyamines protect nucleic acids against depurination. Int. J. Biochem. Cell Biol. 2018;99:147–153. doi: 10.1016/j.biocel.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 120.Kurata H.T., Akrouh A., Li J.B., Marton L.J., Nichols C.G. Scanning the topography of polyamine blocker binding in an inwardly rectifying potassium channel. J. Biol. Chem. 2013;288:6591–6601. doi: 10.1074/jbc.M112.383794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rao J.N., Rathor N., Zhuang R., Zou T., Liu L., Xiao L., Turner D.J., Wang J.Y. Polyamines regulate intestinal epithelial restitution through trpc1-mediated Ca2+ signaling by differentially modulating stim1 and stim2. Am. J. Physiol. Cell Physiol. 2012;303:C308–C317. doi: 10.1152/ajpcell.00120.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murray-Stewart T.R., Woster P.M., Casero R.A., Jr. Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 2016;473:2937–2953. doi: 10.1042/BCJ20160383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iurlaro R., Leon-Annicchiarico C.L., Munoz-Pinedo C. Regulation of cancer metabolism by oncogenes and tumor suppressors. Methods Enzymol. 2014;542:59–80. doi: 10.1016/B978-0-12-416618-9.00003-0. [DOI] [PubMed] [Google Scholar]
- 124.Nagarajan A., Malvi P., Wajapeyee N. Oncogene-directed alterations in cancer cell metabolism. Trends Cancer. 2016;2:365–377. doi: 10.1016/j.trecan.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shaw R.J., Cantley L.C. Ras, pi(3)k and mtor signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 126.Barata J.T., Silva A., Brandao J.G., Nadler L.M., Cardoso A.A., Boussiotis V.A. Activation of pi3k is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of t cell acute lymphoblastic leukemia cells. J. Exp. Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Edinger A.L., Thompson C.B. Akt maintains cell size and survival by increasing mtor-dependent nutrient uptake. Mol. Biol. Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roos S., Jansson N., Palmberg I., Saljo K., Powell T.L., Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007;582:449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wieman H.L., Wofford J.A., Rathmell J.C. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/akt regulation of glut1 activity and trafficking. Mol. Biol. Cell. 2007;18:1437–1446. doi: 10.1091/mbc.e06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu R.H., Pelicano H., Zhang H., Giles F.J., Keating M.J., Huang P. Synergistic effect of targeting mtor by rapamycin and depleting atp by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005;19:2153–2158. doi: 10.1038/sj.leu.2403968. [DOI] [PubMed] [Google Scholar]
- 131.Elstrom R.L., Bauer D.E., Buzzai M., Karnauskas R., Harris M.H., Plas D.R., Zhuang H., Cinalli R.M., Alavi A., Rudin C.M., et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 132.Plas D.R., Talapatra S., Edinger A.L., Rathmell J.C., Thompson C.B. Akt and bcl-xl promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 133.Rathmell J.C., Fox C.J., Plas D.R., Hammerman P.S., Cinalli R.M., Thompson C.B. Akt-directed glucose metabolism can prevent bax conformation change and promote growth factor-independent survival. Mol. Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bauer D.E., Hatzivassiliou G., Zhao F., Andreadis C., Thompson C.B. Atp citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 135.Chang Y., Wang J., Lu X., Thewke D.P., Mason R.J. Kgf induces lipogenic genes through a pi3k and jnk/srebp-1 pathway in h292 cells. J. Lipid. Res. 2005;46:2624–2635. doi: 10.1194/jlr.M500154-JLR200. [DOI] [PubMed] [Google Scholar]
- 136.Gingras A.C., Raught B., Sonenberg N. Regulation of translation initiation by frap/mtor. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 137.Duvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., Triantafellow E., Ma Q., Gorski R., Cleaver S., et al. Activation of a metabolic gene regulatory network downstream of mtor complex 1. Mol. Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peterson T.R., Sengupta S.S., Harris T.E., Carmack A.E., Kang S.A., Balderas E., Guertin D.A., Madden K.L., Carpenter A.E., Finck B.N., et al. Mtor complex 1 regulates lipin 1 localization to control the srebp pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ben-Sahra I., Howell J.J., Asara J.M., Manning B.D. Stimulation of de novo pyrimidine synthesis by growth signaling through mtor and s6k1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Song M.S., Salmena L., Pandolfi P.P. The functions and regulation of the pten tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 141.Garcia-Cao I., Song M.S., Hobbs R.M., Laurent G., Giorgi C., de Boer V.C., Anastasiou D., Ito K., Sasaki A.T., Rameh L., et al. Systemic elevation of pten induces a tumor-suppressive metabolic state. Cell. 2012;149:49–62. doi: 10.1016/j.cell.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Berkers C.R., Maddocks O.D., Cheung E.C., Mor I., Vousden K.H. Metabolic regulation by p53 family members. Cell Metab. 2013;18:617–633. doi: 10.1016/j.cmet.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vousden K.H., Ryan K.M. P53 and metabolism. Nat. Rev. Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 145.Gatenby R.A., Smallbone K., Maini P.K., Rose F., Averill J., Nagle R.B., Worrall L., Gillies R.J. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br. J. Cancer. 2007;97:646–653. doi: 10.1038/sj.bjc.6603922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Keith B., Johnson R.S., Simon M.C. Hif1alpha and hif2alpha: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.O’Rourke J.F., Pugh C.W., Bartlett S.M., Ratcliffe P.J. Identification of hypoxically inducible mrnas in hela cells using differential-display pcr. Role of hypoxia-inducible factor-1. Eur. J. Biochem. 1996;241:403–410. doi: 10.1111/j.1432-1033.1996.00403.x. [DOI] [PubMed] [Google Scholar]
- 148.Semenza G.L., Roth P.H., Fang H.M., Wang G.L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994;269:23757–23763. doi: 10.1016/S0021-9258(17)31580-6. [DOI] [PubMed] [Google Scholar]
- 149.Schodel J., Oikonomopoulos S., Ragoussis J., Pugh C.W., Ratcliffe P.J., Mole D.R. High-resolution genome-wide mapping of hif-binding sites by chip-seq. Blood. 2011;117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wise D.R., Ward P.S., Shay J.E., Cross J.R., Gruber J.J., Sachdeva U.M., Platt J.M., DeMatteo R.G., Simon M.C., Thompson C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wierstra I., Alves J. The c-myc promoter: Still mystery and challenge. Adv. Cancer Res. 2008;99:113–333. doi: 10.1016/S0065-230X(07)99004-1. [DOI] [PubMed] [Google Scholar]
- 152.Kim J.W., Zeller K.I., Wang Y., Jegga A.G., Aronow B.J., O’Donnell K.A., Dang C.V. Evaluation of myc e-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol. Cell Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.David C.J., Chen M., Assanah M., Canoll P., Manley J.L. Hnrnp proteins controlled by c-myc deregulate pyruvate kinase mrna splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yuneva M., Zamboni N., Oefner P., Sachidanandam R., Lazebnik Y. Deficiency in glutamine but not glucose induces myc-dependent apoptosis in human cells. J. Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang R., Dillon C.P., Shi L.Z., Milasta S., Carter R., Finkelstein D., McCormick L.L., Fitzgerald P., Chi H., Munger J., et al. The transcription factor myc controls metabolic reprogramming upon t lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo Q.M., Malek R.L., Kim S., Chiao C., He M., Ruffy M., Sanka K., Lee N.H., Dang C.V., Liu E.T. Identification of c-myc responsive genes using rat cdna microarray. Cancer Res. 2000;60:5922–5928. [PubMed] [Google Scholar]
- 157.Nikiforov M.A., Chandriani S., O’Connell B., Petrenko O., Kotenko I., Beavis A., Sedivy J.M., Cole M.D. A functional screen for myc-responsive genes reveals serine hydroxymethyltransferase, a major source of the one-carbon unit for cell metabolism. Mol. Cell Biol. 2002;22:5793–5800. doi: 10.1128/MCB.22.16.5793-5800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.O’Connell B.C., Cheung A.F., Simkevich C.P., Tam W., Ren X., Mateyak M.K., Sedivy J.M. A large scale genetic analysis of c-myc-regulated gene expression patterns. J. Biol. Chem. 2003;278:12563–12573. doi: 10.1074/jbc.M210462200. [DOI] [PubMed] [Google Scholar]
- 159.Sun L., Song L., Wan Q., Wu G., Li X., Wang Y., Wang J., Liu Z., Zhong X., He X., et al. Cmyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015;25:429–444. doi: 10.1038/cr.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Labuschagne C.F., van den Broek N.J., Mackay G.M., Vousden K.H., Maddocks O.D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014;7:1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 161.Wang X., Kenyon W.J., Li Q., Mullberg J., Hutt-Fletcher L.M. Epstein-barr virus uses different complexes of glycoproteins gh and gl to infect b lymphocytes and epithelial cells. J. Virol. 1998;72:5552–5558. doi: 10.1128/JVI.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cao Y., Xie L., Shi F., Tang M., Li Y., Hu J., Zhao L., Zhao L., Yu X., Luo X., et al. Targeting the signaling in epstein-barr virus-associated diseases: Mechanism, regulation, and clinical study. Signal Transduct. Target Ther. 2021;6:15. doi: 10.1038/s41392-020-00376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cao Y. Ebv based cancer prevention and therapy in nasopharyngeal carcinoma. NPJ Precis. Oncol. 2017;1:10. doi: 10.1038/s41698-017-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Murata T., Tsurumi T. Switching of ebv cycles between latent and lytic states. Rev. Med. Virol. 2014;24:142–153. doi: 10.1002/rmv.1780. [DOI] [PubMed] [Google Scholar]
- 165.Germini D., Sall F.B., Shmakova A., Wiels J., Dokudovskaya S., Drouet E., Vassetzky Y. Oncogenic properties of the ebv zebra protein. Cancers. 2020;12:1479. doi: 10.3390/cancers12061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Young L.S., Yap L.F., Murray P.G. Epstein-barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer. 2016;16:789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]
- 167.Munz C. Latency and lytic replication in epstein-barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019;17:691–700. doi: 10.1038/s41579-019-0249-7. [DOI] [PubMed] [Google Scholar]
- 168.McFadden K., Hafez A.Y., Kishton R., Messinger J.E., Nikitin P.A., Rathmell J.C., Luftig M.A. Metabolic stress is a barrier to epstein-barr virus-mediated b-cell immortalization. Proc. Natl. Acad. Sci. USA. 2016;113:E782–E790. doi: 10.1073/pnas.1517141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang J., Jia L., Liu T., Yip Y.L., Tang W.C., Lin W., Deng W., Lo K.W., You C., Lung M.L., et al. Mtorc2-mediated pdhe1alpha nuclear translocation links ebv-lmp1 reprogrammed glucose metabolism to cancer metastasis in nasopharyngeal carcinoma. Oncogene. 2019;38:4669–4684. doi: 10.1038/s41388-019-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lo A.K., Dawson C.W., Young L.S., Ko C.W., Hau P.M., Lo K.W. Activation of the fgfr1 signalling pathway by the epstein-barr virus-encoded lmp1 promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J. Pathol. 2015;237:238–248. doi: 10.1002/path.4575. [DOI] [PubMed] [Google Scholar]
- 171.Xiao L., Hu Z.Y., Dong X., Tan Z., Li W., Tang M., Chen L., Yang L., Tao Y., Jiang Y., et al. Targeting epstein-barr virus oncoprotein lmp1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene. 2014;33:4568–4578. doi: 10.1038/onc.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jiang Y., Yan B., Lai W., Shi Y., Xiao D., Jia J., Liu S., Li H., Lu J., Li Z., et al. Repression of hox genes by lmp1 in nasopharyngeal carcinoma and modulation of glycolytic pathway genes by hoxc8. Oncogene. 2015;34:6079–6091. doi: 10.1038/onc.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bonglack E.N., Messinger J.E., Cable J.M., Ch’ng J., Parnell K.M., Reinoso-Vizcaino N.M., Barry A.P., Russell V.S., Dave S.S., Christofk H.R., et al. Monocarboxylate transporter antagonism reveals metabolic vulnerabilities of viral-driven lymphomas. Proc. Natl. Acad. Sci. USA. 2021;118:e2022495118. doi: 10.1073/pnas.2022495118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen Q., Feng J., Wu J., Yu Z., Zhang W., Chen Y., Yao P., Zhang H. Hkdc1 c-terminal based peptides inhibit extranodal natural killer/t-cell lymphoma by modulation of mitochondrial function and ebv suppression. Leukemia. 2020;34:2736–2748. doi: 10.1038/s41375-020-0801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang J., Jia L., Tsang C.M., Tsao S.W. Ebv infection and glucose metabolism in nasopharyngeal carcinoma. Adv. Exp. Med. Biol. 2017;1018:75–90. doi: 10.1007/978-981-10-5765-6_6. [DOI] [PubMed] [Google Scholar]
- 176.Cai L.M., Lyu X.M., Luo W.R., Cui X.F., Ye Y.F., Yuan C.C., Peng Q.X., Wu D.H., Liu T.F., Wang E., et al. Ebv-mir-bart7-3p promotes the emt and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor pten. Oncogene. 2015;34:2156–2166. doi: 10.1038/onc.2014.341. [DOI] [PubMed] [Google Scholar]
- 177.Lyu X., Wang J., Guo X., Wu G., Jiao Y., Faleti O.D., Liu P., Liu T., Long Y., Chong T., et al. Ebv-mir-bart1-5p activates ampk/mtor/hif1 pathway via a pten independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog. 2018;14:e1007484. doi: 10.1371/journal.ppat.1007484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krishna G., Soman Pillai V., Valiya Veettil M. Upregulation of gls1 isoforms kga and gac facilitates mitochondrial metabolism and cell proliferation in epstein-barr virus infected cells. Viruses. 2020;12:811. doi: 10.3390/v12080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang L.W., Shen H., Nobre L., Ersing I., Paulo J.A., Trudeau S., Wang Z., Smith N.A., Ma Y., Reinstadler B., et al. Epstein-barr-virus-induced one-carbon metabolism drives b cell transformation. Cell Metab. 2019;30:539–555.e511. doi: 10.1016/j.cmet.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guo R., Liang J.H., Zhang Y., Lutchenkov M., Li Z., Wang Y., Trujillo-Alonso V., Puri R., Giulino-Roth L., Gewurz B.E. Methionine metabolism controls the b cell ebv epigenome and viral latency. Cell Metab. 2022;34:1280–1297.e1289. doi: 10.1016/j.cmet.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Adamson A.L., Le B.T., Siedenburg B.D. Inhibition of mtorc1 inhibits lytic replication of epstein-barr virus in a cell-type specific manner. Virol. J. 2014;11:110. doi: 10.1186/1743-422X-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hafez A.Y., Messinger J.E., McFadden K., Fenyofalvi G., Shepard C.N., Lenzi G.M., Kim B., Luftig M.A. Limited nucleotide pools restrict epstein-barr virus-mediated b-cell immortalization. Oncogenesis. 2017;6:e349. doi: 10.1038/oncsis.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liang J.H., Wang C., Yiu S.P.T., Zhao B., Guo R., Gewurz B.E. Epstein-barr virus induced cytidine metabolism roles in transformed b-cell growth and survival. mBio. 2021;12:e0153021. doi: 10.1128/mBio.01530-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Young L.S., Rickinson A.B. Epstein-barr virus: 40 years on. Nat. Rev. Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 185.Darekar S., Georgiou K., Yurchenko M., Yenamandra S.P., Chachami G., Simos G., Klein G., Kashuba E. Epstein-barr virus immortalization of human b-cells leads to stabilization of hypoxia-induced factor 1 alpha, congruent with the warburg effect. PLoS ONE. 2012;7:e42072. doi: 10.1371/journal.pone.0042072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chatterjee K., Das P., Chattopadhyay N.R., Mal S., Choudhuri T. The interplay between epstein-bar virus (ebv) with the p53 and its homologs during ebv associated malignancies. Heliyon. 2019;5:e02624. doi: 10.1016/j.heliyon.2019.e02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li Y., Webster-Cyriaque J., Tomlinson C.C., Yohe M., Kenney S. Fatty acid synthase expression is induced by the epstein-barr virus immediate-early protein brlf1 and is required for lytic viral gene expression. J. Virol. 2004;78:4197–4206. doi: 10.1128/JVI.78.8.4197-4206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang L.W., Wang Z., Ersing I., Nobre L., Guo R., Jiang S., Trudeau S., Zhao B., Weekes M.P., Gewurz B.E. Epstein-barr virus subverts mevalonate and fatty acid pathways to promote infected b-cell proliferation and survival. PLoS Pathog. 2019;15:e1008030. doi: 10.1371/journal.ppat.1008030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Doorbar J., Quint W., Banks L., Bravo I.G., Stoler M., Broker T.R., Stanley M.A. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30((Suppl. 5)):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 190.Crosbie E.J., Einstein M.H., Franceschi S., Kitchener H.C. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–899. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 191.Satterwhite C.L., Torrone E., Meites E., Dunne E.F., Mahajan R., Ocfemia M.C., Su J., Xu F., Weinstock H. Sexually transmitted infections among us women and men: Prevalence and incidence estimates, 2008. Sex Transm. Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 192.de Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to hpv by site, country and hpv type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mazurek S., Zwerschke W., Jansen-Durr P., Eigenbrodt E. Effects of the human papilloma virus hpv-16 e7 oncoprotein on glycolysis and glutaminolysis: Role of pyruvate kinase type m2 and the glycolytic-enzyme complex. Biochem. J. 2001;356:247–256. doi: 10.1042/bj3560247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Crook T., Morgenstern J.P., Crawford L., Banks L. Continued expression of hpv-16 e7 protein is required for maintenance of the transformed phenotype of cells co-transformed by hpv-16 plus ej-ras. EMBO J. 1989;8:513–519. doi: 10.1002/j.1460-2075.1989.tb03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., et al. Inhibition of pyruvate kinase m2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Darnell G.A., Schroder W.A., Antalis T.M., Lambley E., Major L., Gardner J., Birrell G., Cid-Arregui A., Suhrbier A. Human papillomavirus e7 requires the protease calpain to degrade the retinoblastoma protein. J. Biol. Chem. 2007;282:37492–37500. doi: 10.1074/jbc.M706860200. [DOI] [PubMed] [Google Scholar]
- 197.Spangle J.M., Munger K. The human papillomavirus type 16 e6 oncoprotein activates mtorc1 signaling and increases protein synthesis. J. Virol. 2010;84:9398–9407. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pim D., Massimi P., Dilworth S.M., Banks L. Activation of the protein kinase b pathway by the hpv-16 e7 oncoprotein occurs through a mechanism involving interaction with pp2a. Oncogene. 2005;24:7830–7838. doi: 10.1038/sj.onc.1208935. [DOI] [PubMed] [Google Scholar]
- 199.Fischer M., Uxa S., Stanko C., Magin T.M., Engeland K. Human papilloma virus e7 oncoprotein abrogates the p53-p21-dream pathway. Sci. Rep. 2017;7:2603. doi: 10.1038/s41598-017-02831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang L., Wu J., Ling M.T., Zhao L., Zhao K.N. The role of the pi3k/akt/mtor signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer. 2015;14:87. doi: 10.1186/s12943-015-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Guo Y., Meng X., Ma J., Zheng Y., Wang Q., Wang Y., Shang H. Human papillomavirus 16 e6 contributes hif-1alpha induced warburg effect by attenuating the vhl-hif-1alpha interaction. Int. J. Mol. Sci. 2014;15:7974–7986. doi: 10.3390/ijms15057974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Veldman T., Liu X., Yuan H., Schlegel R. Human papillomavirus e6 and myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. USA. 2003;100:8211–8216. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dang C.V., O’Donnell K.A., Zeller K.I., Nguyen T., Osthus R.C., Li F. The c-myc target gene network. Semin. Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 204.Cruz-Gregorio A., Aranda-Rivera A.K., Aparicio-Trejo O.E., Coronado-Martinez I., Pedraza-Chaverri J., Lizano M. E6 oncoproteins from high-risk human papillomavirus induce mitochondrial metabolism in a head and neck squamous cell carcinoma model. Biomolecules. 2019;9:351. doi: 10.3390/biom9080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Crusius K., Auvinen E., Alonso A. Enhancement of egf- and pma-mediated map kinase activation in cells expressing the human papillomavirus type 16 e5 protein. Oncogene. 1997;15:1437–1444. doi: 10.1038/sj.onc.1201312. [DOI] [PubMed] [Google Scholar]
- 206.Romanczuk H., Howley P.M. Disruption of either the e1 or the e2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc. Natl. Acad. Sci. USA. 1992;89:3159–3163. doi: 10.1073/pnas.89.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lai D., Tan C.L., Gunaratne J., Quek L.S., Nei W., Thierry F., Bellanger S. Localization of hpv-18 e2 at mitochondrial membranes induces ros release and modulates host cell metabolism. PLoS ONE. 2013;8:e75625. doi: 10.1371/journal.pone.0075625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pappa K.I., Daskalakis G., Anagnou N.P. Metabolic rewiring is associated with hpv-specific profiles in cervical cancer cell lines. Sci. Rep. 2021;11:17718. doi: 10.1038/s41598-021-96038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Prusinkiewicz M.A., Gameiro S.F., Ghasemi F., Dodge M.J., Zeng P.Y.F., Maekebay H., Barrett J.W., Nichols A.C., Mymryk J.S. Survival-associated metabolic genes in human papillomavirus-positive head and neck cancers. Cancers. 2020;12:253. doi: 10.3390/cancers12010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Poiesz B.J., Ruscetti F.W., Gazdar A.F., Bunn P.A., Minna J.D., Gallo R.C. Detection and isolation of type c retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous t-cell lymphoma. Proc. Natl. Acad. Sci. USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ratner L. Molecular biology of human t cell leukemia virus. Semin. Diagn. Pathol. 2020;37:104–109. doi: 10.1053/j.semdp.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kulkarni A., Bangham C.R.M. Htlv-1: Regulating the balance between proviral latency and reactivation. Front. Microbiol. 2018;9:449. doi: 10.3389/fmicb.2018.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Manel N., Kim F.J., Kinet S., Taylor N., Sitbon M., Battini J.L. The ubiquitous glucose transporter glut-1 is a receptor for htlv. Cell. 2003;115:449–459. doi: 10.1016/S0092-8674(03)00881-X. [DOI] [PubMed] [Google Scholar]
- 214.Kulkarni A., Mateus M., Thinnes C.C., McCullagh J.S., Schofield C.J., Taylor G.P., Bangham C.R.M. Glucose metabolism and oxygen availability govern reactivation of the latent human retrovirus htlv-1. Cell Chem. Biol. 2017;24:1377–1387.e1373. doi: 10.1016/j.chembiol.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Matsuoka M., Jeang K.T. Human t-cell leukemia virus type 1 (htlv-1) and leukemic transformation: Viral infectivity, tax, hbz and therapy. Oncogene. 2011;30:1379–1389. doi: 10.1038/onc.2010.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mahgoub M., Yasunaga J.I., Iwami S., Nakaoka S., Koizumi Y., Shimura K., Matsuoka M. Sporadic on/off switching of htlv-1 tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. USA. 2018;115:E1269–E1278. doi: 10.1073/pnas.1715724115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhong W., Cao X., Pan G., Niu Q., Feng X., Xu M., Li M., Huang Y., Yi Q., Yan D. Orp4l is a prerequisite for the induction of t-cell leukemogenesis associated with human t-cell leukemia virus 1. Blood. 2022;139:1052–1065. doi: 10.1182/blood.2021013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ho Y.K., Zhi H., DeBiaso D., Philip S., Shih H.M., Giam C.Z. Htlv-1 tax-induced rapid senescence is driven by the transcriptional activity of nf-kappab and depends on chronically activated ikkalpha and p65/rela. J. Virol. 2012;86:9474–9483. doi: 10.1128/JVI.00158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kawatsuki A., Yasunaga J.I., Mitobe Y., Green P.L., Matsuoka M. Htlv-1 bzip factor protein targets the rb/e2f-1 pathway to promote proliferation and apoptosis of primary CD4+ t cells. Oncogene. 2016;35:4509–4517. doi: 10.1038/onc.2015.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hutchison T., Malu A., Yapindi L., Bergeson R., Peck K., Romeo M., Harrod C., Pope J., Smitherman L., Gwinn W., et al. The tp53-induced glycolysis and apoptosis regulator mediates cooperation between htlv-1 p30(ii) and the retroviral oncoproteins tax and hbz and is highly expressed in an in vivo xenograft model of htlv-1-induced lymphoma. Virology. 2018;520:39–58. doi: 10.1016/j.virol.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ehtiati S., Youssefi M., Rafatpanah H., Mashkani B., Khadem-Rezaiyan M., Zahedi Avval F. Glutathione reductase system changes in htlv-1 infected patients. Virusdisease. 2022;33:32–38. doi: 10.1007/s13337-022-00758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao T., Wang Z., Fang J., Cheng W., Zhang Y., Huang J., Xu L., Gou H., Zeng L., Jin Z., et al. Htlv-1 activates yap via nf-kappab/p65 to promote oncogenesis. Proc. Natl. Acad. Sci. USA. 2022;119:e2115316119. doi: 10.1073/pnas.2115316119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.El-Serag H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1261. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tsukuda S., Watashi K. Hepatitis b virus biology and life cycle. Antiviral Res. 2020;182:104925. doi: 10.1016/j.antiviral.2020.104925. [DOI] [PubMed] [Google Scholar]
- 225.Karayiannis P. Hepatitis b virus: Virology, molecular biology, life cycle and intrahepatic spread. Hepatol. Int. 2017;11:500–508. doi: 10.1007/s12072-017-9829-7. [DOI] [PubMed] [Google Scholar]
- 226.Levrero M., Zucman-Rossi J. Mechanisms of hbv-induced hepatocellular carcinoma. J. Hepatol. 2016;64:S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 227.D’Souza S., Lau K.C., Coffin C.S., Patel T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020;26:5759–5783. doi: 10.3748/wjg.v26.i38.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xie Y. Hepatitis b virus-associated hepatocellular carcinoma. Adv. Exp. Med. Biol. 2017;1018:11–21. doi: 10.1007/978-981-10-5765-6_2. [DOI] [PubMed] [Google Scholar]
- 229.Arzumanyan A., Reis H.M., Feitelson M.A. Pathogenic mechanisms in hbv- and hcv-associated hepatocellular carcinoma. Nat. Rev. Cancer. 2013;13:123–135. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 230.Shin H.J., Park Y.H., Kim S.U., Moon H.B., Park D.S., Han Y.H., Lee C.H., Lee D.S., Song I.S., Lee D.H., et al. Hepatitis b virus x protein regulates hepatic glucose homeostasis via activation of inducible nitric oxide synthase. J. Biol. Chem. 2011;286:29872–29881. doi: 10.1074/jbc.M111.259978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li H., Zhu W., Zhang L., Lei H., Wu X., Guo L., Chen X., Wang Y., Tang H. The metabolic responses to hepatitis b virus infection shed new light on pathogenesis and targets for treatment. Sci. Rep. 2015;5:8421. doi: 10.1038/srep08421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen Y.Y., Wang W.H., Che L., Lan Y., Zhang L.Y., Zhan D.L., Huang Z.Y., Lin Z.N., Lin Y.C. Bnip3l-dependent mitophagy promotes hbx-induced cancer stemness of hepatocellular carcinoma cells via glycolysis metabolism reprogramming. Cancers. 2020;12:655. doi: 10.3390/cancers12030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu B., Fang M., He Z., Cui D., Jia S., Lin X., Xu X., Zhou T., Liu W. Hepatitis b virus stimulates g6pd expression through hbx-mediated nrf2 activation. Cell Death Dis. 2015;6:e1980. doi: 10.1038/cddis.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Teng C.F., Hsieh W.C., Wu H.C., Lin Y.J., Tsai H.W., Huang W., Su I.J. Hepatitis b virus pre-s2 mutant induces aerobic glycolysis through mammalian target of rapamycin signal cascade. PLoS ONE. 2015;10:e0122373. doi: 10.1371/journal.pone.0122373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Teng C.F., Wu H.C., Hsieh W.C., Tsai H.W., Su I.J. Activation of atp citrate lyase by mtor signal induces disturbed lipid metabolism in hepatitis b virus pre-s2 mutant tumorigenesis. J. Virol. 2015;89:605–614. doi: 10.1128/JVI.02363-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xie Q., Fan F., Wei W., Liu Y., Xu Z., Zhai L., Qi Y., Ye B., Zhang Y., Basu S., et al. Multi-omics analyses reveal metabolic alterations regulated by hepatitis b virus core protein in hepatocellular carcinoma cells. Sci. Rep. 2017;7:41089. doi: 10.1038/srep41089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carroll P.A., Diolaiti D., McFerrin L., Gu H., Djukovic D., Du J., Cheng P.F., Anderson S., Ulrich M., Hurley J.B., et al. Deregulated myc requires mondoa/mlx for metabolic reprogramming and tumorigenesis. Cancer Cell. 2015;27:271–285. doi: 10.1016/j.ccell.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Moudi B., Heidari Z., Mahmoudzadeh-Sagheb H., Alavian S.M., Lankarani K.B., Farrokh P., Randel Nyengaard J. Concomitant use of heat-shock protein 70, glutamine synthetase and glypican-3 is useful in diagnosis of hbv-related hepatocellular carcinoma with higher specificity and sensitivity. Eur. J. Histochem. 2018;62:2859. doi: 10.4081/ejh.2018.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kim S.J., Khan M., Quan J., Till A., Subramani S., Siddiqui A. Hepatitis b virus disrupts mitochondrial dynamics: Induces fission and mitophagy to attenuate apoptosis. PLoS Pathog. 2013;9:e1003722. doi: 10.1371/journal.ppat.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hwang K.B., Kyaw Y.Y., Kang H.R., Seong M.S., Cheong J. Mitochondrial dysfunction stimulates hbv gene expression through lipogenic transcription factor activation. Virus Res. 2020;277:197842. doi: 10.1016/j.virusres.2019.197842. [DOI] [PubMed] [Google Scholar]
- 241.Hajjou M., Norel R., Carver R., Marion P., Cullen J., Rogler L.E., Rogler C.E. Cdna microarray analysis of hbv transgenic mouse liver identifies genes in lipid biosynthetic and growth control pathways affected by hbv. J. Med. Virol. 2005;77:57–65. doi: 10.1002/jmv.20427. [DOI] [PubMed] [Google Scholar]
- 242.Yang F., Yan S., He Y., Wang F., Song S., Guo Y., Zhou Q., Wang Y., Lin Z., Yang Y., et al. Expression of hepatitis b virus proteins in transgenic mice alters lipid metabolism and induces oxidative stress in the liver. J. Hepatol. 2008;48:12–19. doi: 10.1016/j.jhep.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 243.Okamura H., Nio Y., Akahori Y., Kim S., Watashi K., Wakita T., Hijikata M. Fatty acid biosynthesis is involved in the production of hepatitis b virus particles. Biochem. Biophys. Res. Commun. 2016;475:87–92. doi: 10.1016/j.bbrc.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 244.Hyrina A., Burdette D., Song Z., Ramirez R., Okesli-Armlovich A., Vijayakumar A., Bates J., Trevaskis J.L., Fletcher S.P., Lee W.A., et al. Targeting lipid biosynthesis pathways for hepatitis b virus cure. PLoS ONE. 2022;17:e0270273. doi: 10.1371/journal.pone.0270273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wang M.D., Wu H., Huang S., Zhang H.L., Qin C.J., Zhao L.H., Fu G.B., Zhou X., Wang X.M., Tang L., et al. Hbx regulates fatty acid oxidation to promote hepatocellular carcinoma survival during metabolic stress. Oncotarget. 2016;7:6711–6726. doi: 10.18632/oncotarget.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wu Y.L., Peng X.E., Zhu Y.B., Yan X.L., Chen W.N., Lin X. Hepatitis b virus x protein induces hepatic steatosis by enhancing the expression of liver fatty acid binding protein. J. Virol. 2016;90:1729–1740. doi: 10.1128/JVI.02604-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen G., Liang G., Ou J., Goldstein J.L., Brown M.S. Central role for liver x receptor in insulin-mediated activation of srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl. Acad. Sci. USA. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Seo J.B., Moon H.M., Kim W.S., Lee Y.S., Jeong H.W., Yoo E.J., Ham J., Kang H., Park M.G., Steffensen K.R., et al. Activated liver x receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression. Mol. Cell Biol. 2004;24:3430–3444. doi: 10.1128/MCB.24.8.3430-3444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kim K.H., Shin H.J., Kim K., Choi H.M., Rhee S.H., Moon H.B., Kim H.H., Yang U.S., Yu D.Y., Cheong J. Hepatitis b virus x protein induces hepatic steatosis via transcriptional activation of srebp1 and ppargamma. Gastroenterology. 2007;132:1955–1967. doi: 10.1053/j.gastro.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 250.Kang S.K., Chung T.W., Lee J.Y., Lee Y.C., Morton R.E., Kim C.H. The hepatitis b virus x protein inhibits secretion of apolipoprotein b by enhancing the expression of n-acetylglucosaminyltransferase iii. J. Biol. Chem. 2004;279:28106–28112. doi: 10.1074/jbc.M403176200. [DOI] [PubMed] [Google Scholar]
- 251.Oehler N., Volz T., Bhadra O.D., Kah J., Allweiss L., Giersch K., Bierwolf J., Riecken K., Pollok J.M., Lohse A.W., et al. Binding of hepatitis b virus to its cellular receptor alters the expression profile of genes of bile acid metabolism. Hepatology. 2014;60:1483–1493. doi: 10.1002/hep.27159. [DOI] [PubMed] [Google Scholar]
- 252.Li Y.J., Zhu P., Liang Y., Yin W.G., Xiao J.H. Hepatitis b virus induces expression of cholesterol metabolism-related genes via tlr2 in hepg2 cells. World J. Gastroenterol. 2013;19:2262–2269. doi: 10.3748/wjg.v19.i14.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dubuisson J. Hepatitis c virus proteins. World J. Gastroenterol. 2007;13:2406–2415. doi: 10.3748/wjg.v13.i17.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Negro F. Abnormalities of lipid metabolism in hepatitis c virus infection. Gut. 2010;59:1279–1287. doi: 10.1136/gut.2009.192732. [DOI] [PubMed] [Google Scholar]
- 255.Sheridan D.A., Shawa I.T., Thomas E.L., Felmlee D.J., Bridge S.H., Neely D., Cobbold J.F., Holmes E., Bassendine M.F., Taylor-Robinson S.D. Infection with the hepatitis c virus causes viral genotype-specific differences in cholesterol metabolism and hepatic steatosis. Sci. Rep. 2022;12:5562. doi: 10.1038/s41598-022-09588-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Negro F. Facts and fictions of hcv and comorbidities: Steatosis, diabetes mellitus, and cardiovascular diseases. J. Hepatol. 2014;61:S69–S78. doi: 10.1016/j.jhep.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 257.Mannova P., Beretta L. Activation of the n-ras-pi3k-akt-mtor pathway by hepatitis c virus: Control of cell survival and viral replication. J. Virol. 2005;79:8742–8749. doi: 10.1128/JVI.79.14.8742-8749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mitchell J.K., Midkiff B.R., Israelow B., Evans M.J., Lanford R.E., Walker C.M., Lemon S.M., McGivern D.R. Hepatitis c virus indirectly disrupts DNA damage-induced p53 responses by activating protein kinase r. mBio. 2017;8:e00121-17. doi: 10.1128/mBio.00121-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ramiere C., Rodriguez J., Enache L.S., Lotteau V., Andre P., Diaz O. Activity of hexokinase is increased by its interaction with hepatitis c virus protein ns5a. J. Virol. 2014;88:3246–3254. doi: 10.1128/JVI.02862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ripoli M., D’Aprile A., Quarato G., Sarasin-Filipowicz M., Gouttenoire J., Scrima R., Cela O., Boffoli D., Heim M.H., Moradpour D., et al. Hepatitis c virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J. Virol. 2010;84:647–660. doi: 10.1128/JVI.00769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Levy P.L., Duponchel S., Eischeid H., Molle J., Michelet M., Diserens G., Vermathen M., Vermathen P., Dufour J.F., Dienes H.P., et al. Hepatitis c virus infection triggers a tumor-like glutamine metabolism. Hepatology. 2017;65:789–803. doi: 10.1002/hep.28949. [DOI] [PubMed] [Google Scholar]
- 262.Diamond D.L., Syder A.J., Jacobs J.M., Sorensen C.M., Walters K.A., Proll S.C., McDermott J.E., Gritsenko M.A., Zhang Q., Zhao R., et al. Temporal proteome and lipidome profiles reveal hepatitis c virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010;6:e1000719. doi: 10.1371/journal.ppat.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Perrin-Cocon L., Kundlacz C., Jacquemin C., Hanoulle X., Aublin-Gex A., Figl M., Manteca J., Andre P., Vidalain P.O., Lotteau V., et al. Domain 2 of hepatitis c virus protein ns5a activates glucokinase and induces lipogenesis in hepatocytes. Int. J. Mol. Sci. 2022;23:919. doi: 10.3390/ijms23020919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jung G.S., Jeon J.H., Choi Y.K., Jang S.Y., Park S.Y., Kim S.W., Byun J.K., Kim M.K., Lee S., Shin E.C., et al. Pyruvate dehydrogenase kinase regulates hepatitis c virus replication. Sci. Rep. 2016;6:30846. doi: 10.1038/srep30846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yu T., Yang Q., Tian F., Chang H., Hu Z., Yu B., Han L., Xing Y., Jiu Y., He Y., et al. Glycometabolism regulates hepatitis c virus release. PLoS Pathog. 2021;17:e1009746. doi: 10.1371/journal.ppat.1009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kasai D., Adachi T., Deng L., Nagano-Fujii M., Sada K., Ikeda M., Kato N., Ide Y.H., Shoji I., Hotta H. Hcv replication suppresses cellular glucose uptake through down-regulation of cell surface expression of glucose transporters. J. Hepatol. 2009;50:883–894. doi: 10.1016/j.jhep.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 267.Meng Z., Liu Q., Sun F., Qiao L. Hepatitis c virus nonstructural protein 5a perturbs lipid metabolism by modulating ampk/srebp-1c signaling. Lipids. Health Dis. 2019;18:191. doi: 10.1186/s12944-019-1136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shi Q., Hoffman B., Liu Q. Pi3k-akt signaling pathway upregulates hepatitis c virus rna translation through the activation of srebps. Virology. 2016;490:99–108. doi: 10.1016/j.virol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 269.Huang J.T., Tseng C.P., Liao M.H., Lu S.C., Yeh W.Z., Sakamoto N., Chen C.M., Cheng J.C. Hepatitis c virus replication is modulated by the interaction of nonstructural protein ns5b and fatty acid synthase. J. Virol. 2013;87:4994–5004. doi: 10.1128/JVI.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yang W., Hood B.L., Chadwick S.L., Liu S., Watkins S.C., Luo G., Conrads T.P., Wang T. Fatty acid synthase is up-regulated during hepatitis c virus infection and regulates hepatitis c virus entry and production. Hepatology. 2008;48:1396–1403. doi: 10.1002/hep.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nio Y., Hasegawa H., Okamura H., Miyayama Y., Akahori Y., Hijikata M. Liver-specific mono-unsaturated fatty acid synthase-1 inhibitor for anti-hepatitis c treatment. Antiviral Res. 2016;132:262–267. doi: 10.1016/j.antiviral.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 272.Sarhan M.A., Abdel-Hakeem M.S., Mason A.L., Tyrrell D.L., Houghton M. Glycogen synthase kinase 3beta inhibitors prevent hepatitis c virus release/assembly through perturbation of lipid metabolism. Sci. Rep. 2017;7:2495. doi: 10.1038/s41598-017-02648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zapatero-Belinchon F.J., Otjengerdes R., Sheldon J., Schulte B., Carriqui-Madronal B., Brogden G., Arroyo-Fernandez L.M., Vondran F.W.R., Maasoumy B., von Hahn T., et al. Interdependent impact of lipoprotein receptors and lipid-lowering drugs on hcv infectivity. Cells. 2021;10:1626. doi: 10.3390/cells10071626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gane E., Stedman C., Dole K., Chen J., Meyers C.D., Wiedmann B., Zhang J., Raman P., Colvin R.A. A diacylglycerol transferase 1 inhibitor is a potent hepatitis c antiviral in vitro but not in patients in a randomized clinical trial. ACS Infect. Dis. 2017;3:144–151. doi: 10.1021/acsinfecdis.6b00138. [DOI] [PubMed] [Google Scholar]
- 275.Gastaminza P., Cheng G., Wieland S., Zhong J., Liao W., Chisari F.V. Cellular determinants of hepatitis c virus assembly, maturation, degradation, and secretion. J. Virol. 2008;82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Huang H., Sun F., Owen D.M., Li W., Chen Y., Gale M., Jr., Ye J. Hepatitis c virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. USA. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lyn R.K., Singaravelu R., Kargman S., O’Hara S., Chan H., Oballa R., Huang Z., Jones D.M., Ridsdale A., Russell R.S., et al. Stearoyl-coa desaturase inhibition blocks formation of hepatitis c virus-induced specialized membranes. Sci. Rep. 2014;4:4549. doi: 10.1038/srep04549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Dharancy S., Malapel M., Perlemuter G., Roskams T., Cheng Y., Dubuquoy L., Podevin P., Conti F., Canva V., Philippe D., et al. Impaired expression of the peroxisome proliferator-activated receptor alpha during hepatitis c virus infection. Gastroenterology. 2005;128:334–342. doi: 10.1053/j.gastro.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 279.Higgs M.R., Lerat H., Pawlotsky J.M. Hepatitis c virus-induced activation of beta-catenin promotes c-myc expression and a cascade of pro-carcinogenetic events. Oncogene. 2013;32:4683–4693. doi: 10.1038/onc.2012.484. [DOI] [PubMed] [Google Scholar]
- 280.Lupberger J., Croonenborghs T., Roca Suarez A.A., Van Renne N., Juhling F., Oudot M.A., Virzi A., Bandiera S., Jamey C., Meszaros G., et al. Combined analysis of metabolomes, proteomes, and transcriptomes of hepatitis c virus-infected cells and liver to identify pathways associated with disease development. Gastroenterology. 2019;157:537–551.e539. doi: 10.1053/j.gastro.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Uldrick T.S., Whitby D. Update on kshv epidemiology, kaposi sarcoma pathogenesis, and treatment of kaposi sarcoma. Cancer Lett. 2011;305:150–162. doi: 10.1016/j.canlet.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Juillard F., Tan M., Li S., Kaye K.M. Kaposi’s sarcoma herpesvirus genome persistence. Front. Microbiol. 2016;7:1149. doi: 10.3389/fmicb.2016.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Liu N., Shi F., Yang L., Liao W., Cao Y. Oncogenic viral infection and amino acid metabolism in cancer progression: Molecular insights and clinical implications. Biochim. Biophys. Acta Rev. Cancer. 2022;1877:188724. doi: 10.1016/j.bbcan.2022.188724. [DOI] [PubMed] [Google Scholar]
- 284.Friborg J., Jr., Kong W., Hottiger M.O., Nabel G.J. P53 inhibition by the lana protein of kshv protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 285.Radkov S.A., Kellam P., Boshoff C. The latent nuclear antigen of kaposi sarcoma-associated herpesvirus targets the retinoblastoma-e2f pathway and with the oncogene hras transforms primary rat cells. Nat. Med. 2000;6:1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 286.Delgado T., Carroll P.A., Punjabi A.S., Margineantu D., Hockenbery D.M., Lagunoff M. Induction of the warburg effect by kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. USA. 2010;107:10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sanchez E.L., Pulliam T.H., Dimaio T.A., Thalhofer A.B., Delgado T., Lagunoff M. Glycolysis, glutaminolysis, and fatty acid synthesis are required for distinct stages of kaposi’s sarcoma-associated herpesvirus lytic replication. J. Virol. 2017;91:e02237-16. doi: 10.1128/JVI.02237-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shin Y.C., Joo C.H., Gack M.U., Lee H.R., Jung J.U. Kaposi’s sarcoma-associated herpesvirus viral ifn regulatory factor 3 stabilizes hypoxia-inducible factor-1 alpha to induce vascular endothelial growth factor expression. Cancer Res. 2008;68:1751–1759. doi: 10.1158/0008-5472.CAN-07-2766. [DOI] [PubMed] [Google Scholar]
- 289.Carroll P.A., Kenerson H.L., Yeung R.S., Lagunoff M. Latent kaposi’s sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J. Virol. 2006;80:10802–10812. doi: 10.1128/JVI.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cai Q., Murakami M., Si H., Robertson E.S. A potential alpha-helix motif in the amino terminus of lana encoded by kaposi’s sarcoma-associated herpesvirus is critical for nuclear accumulation of hif-1alpha in normoxia. J. Virol. 2007;81:10413–10423. doi: 10.1128/JVI.00611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Singh R.K., Lang F., Pei Y., Jha H.C., Robertson E.S. Metabolic reprogramming of kaposi’s sarcoma associated herpes virus infected b-cells in hypoxia. PLoS Pathog. 2018;14:e1007062. doi: 10.1371/journal.ppat.1007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bhatt A.P., Damania B. Aktivation of pi3k/akt/mtor signaling pathway by kshv. Front. Immunol. 2012;3:401. doi: 10.3389/fimmu.2012.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bhatt A.P., Jacobs S.R., Freemerman A.J., Makowski L., Rathmell J.C., Dittmer D.P., Damania B. Dysregulation of fatty acid synthesis and glycolysis in non-hodgkin lymphoma. Proc. Natl. Acad. Sci. USA. 2012;109:11818–11823. doi: 10.1073/pnas.1205995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gjyshi O., Bottero V., Veettil M.V., Dutta S., Singh V.V., Chikoti L., Chandran B. Kaposi’s sarcoma-associated herpesvirus induces nrf2 during de novo infection of endothelial cells to create a microenvironment conducive to infection. PLoS Pathog. 2014;10:e1004460. doi: 10.1371/journal.ppat.1004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Delgado T., Sanchez E.L., Camarda R., Lagunoff M. Global metabolic profiling of infection by an oncogenic virus: Kshv induces and requires lipogenesis for survival of latent infection. PLoS Pathog. 2012;8:e1002866. doi: 10.1371/journal.ppat.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Angius F., Uda S., Piras E., Spolitu S., Ingianni A., Batetta B., Pompei R. Neutral lipid alterations in human herpesvirus 8-infected huvec cells and their possible involvement in neo-angiogenesis. BMC Microbiol. 2015;15:74. doi: 10.1186/s12866-015-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tso F.Y., Kossenkov A.V., Lidenge S.J., Ngalamika O., Ngowi J.R., Mwaiselage J., Wickramasinghe J., Kwon E.H., West J.T., Lieberman P.M., et al. Rna-seq of kaposi’s sarcoma reveals alterations in glucose and lipid metabolism. PLoS Pathog. 2018;14:e1006844. doi: 10.1371/journal.ppat.1006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sanchez E.L., Carroll P.A., Thalhofer A.B., Lagunoff M. Latent kshv infected endothelial cells are glutamine addicted and require glutaminolysis for survival. PLoS Pathog. 2015;11:e1005052. doi: 10.1371/journal.ppat.1005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhu Y., Li T., Ramos da Silva S., Lee J.J., Lu C., Eoh H., Jung J.U., Gao S.J. A critical role of glutamine and asparagine gamma-nitrogen in nucleotide biosynthesis in cancer cells hijacked by an oncogenic virus. mBio. 2017;8:e01179-17. doi: 10.1128/mBio.01179-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Choi U.Y., Lee J.J., Park A., Zhu W., Lee H.R., Choi Y.J., Yoo J.S., Yu C., Feng P., Gao S.J., et al. Oncogenic human herpesvirus hijacks proline metabolism for tumorigenesis. Proc. Natl. Acad. Sci. USA. 2020;117:8083–8093. doi: 10.1073/pnas.1918607117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Fiches G.N., Wu Z., Zhou D., Biswas A., Li T.W., Kong W., Jean M., Santoso N.G., Zhu J. Polyamine biosynthesis and eif5a hypusination are modulated by the DNA tumor virus kshv and promote kshv viral infection. PLoS Pathog. 2022;18:e1010503. doi: 10.1371/journal.ppat.1010503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Choi U.Y., Lee J.J., Park A., Jung K.L., Lee S.A., Choi Y.J., Lee H.R., Lai C.J., Eoh H., Jung J.U. Herpesvirus-induced spermidine synthesis and eif5a hypusination for viral episomal maintenance. Cell Rep. 2022;40:111234. doi: 10.1016/j.celrep.2022.111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.DeCaprio J.A. Molecular pathogenesis of merkel cell carcinoma. Annu. Rev. Pathol. 2021;16:69–91. doi: 10.1146/annurev-pathmechdis-012419-032817. [DOI] [PubMed] [Google Scholar]
- 305.Pastrana D.V., Tolstov Y.L., Becker J.C., Moore P.S., Chang Y., Buck C.B. Quantitation of human seroresponsiveness to merkel cell polyomavirus. PLoS Pathog. 2009;5:e1000578. doi: 10.1371/journal.ppat.1000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Paulson K.G., Lewis C.W., Redman M.W., Simonson W.T., Lisberg A., Ritter D., Morishima C., Hutchinson K., Mudgistratova L., Blom A., et al. Viral oncoprotein antibodies as a marker for recurrence of merkel cell carcinoma: A prospective validation study. Cancer. 2017;123:1464–1474. doi: 10.1002/cncr.30475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Houben R., Shuda M., Weinkam R., Schrama D., Feng H., Chang Y., Moore P.S., Becker J.C. Merkel cell polyomavirus-infected merkel cell carcinoma cells require expression of viral t antigens. J. Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kwun H.J., Shuda M., Feng H., Camacho C.J., Moore P.S., Chang Y. Merkel cell polyomavirus small t antigen controls viral replication and oncoprotein expression by targeting the cellular ubiquitin ligase scffbw7. Cell Host Microbe. 2013;14:125–135. doi: 10.1016/j.chom.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Borchert S., Czech-Sioli M., Neumann F., Schmidt C., Wimmer P., Dobner T., Grundhoff A., Fischer N. High-affinity rb binding, p53 inhibition, subcellular localization, and transformation by wild-type or tumor-derived shortened merkel cell polyomavirus large t antigens. J. Virol. 2014;88:3144–3160. doi: 10.1128/JVI.02916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Berrios C., Padi M., Keibler M.A., Park D.E., Molla V., Cheng J., Lee S.M., Stephanopoulos G., Quackenbush J., DeCaprio J.A. Merkel cell polyomavirus small t antigen promotes pro-glycolytic metabolic perturbations required for transformation. PLoS Pathog. 2016;12:e1006020. doi: 10.1371/journal.ppat.1006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Li H., Ning S., Ghandi M., Kryukov G.V., Gopal S., Deik A., Souza A., Pierce K., Keskula P., Hernandez D., et al. The landscape of cancer cell line metabolism. Nat. Med. 2019;25:850–860. doi: 10.1038/s41591-019-0404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wu Y.H., Yang Y., Chen C.H., Hsiao C.J., Li T.N., Liao K.J., Watashi K., Chen B.S., Wang L.H. Aerobic glycolysis supports hepatitis b virus protein synthesis through interaction between viral surface antigen and pyruvate kinase isoform m2. PLoS Pathog. 2021;17:e1008866. doi: 10.1371/journal.ppat.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Su A.I., Pezacki J.P., Wodicka L., Brideau A.D., Supekova L., Thimme R., Wieland S., Bukh J., Purcell R.H., Schultz P.G., et al. Genomic analysis of the host response to hepatitis c virus infection. Proc. Natl. Acad. Sci. USA. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ramtohul Y.K., Black C., Chan C.C., Crane S., Guay J., Guiral S., Huang Z., Oballa R., Xu L.J., Zhang L., et al. Sar and optimization of thiazole analogs as potent stearoyl-coa desaturase inhibitors. Bioorg. Med. Chem. Lett. 2010;20:1593–1597. doi: 10.1016/j.bmcl.2010.01.083. [DOI] [PubMed] [Google Scholar]