Abstract
Trehalose 6,6′-dimycolate (TDM) plays important roles in the development of granulomatous inflammation during infection with Mycobacterium spp., Rhodococcus spp., etc. To reveal the augmenting effect of TDM on vascular endothelial growth factor (VEGF) production and neovascularization, we investigated murine granulomatous tissue air pouches induced by Rhodococcus sp. strain 4306 TDM dissolved in Freund's incomplete adjuvant (FIA), comparing them to pouches treated with FIA alone. Histologically, granulomatous tissue and new vessel formation, which reached a maximum at day 7, was greatly enhanced by treatment with TDM. At day 1, VEGF-positive neutrophils accumulated in the pouch wall with frequency of 95% of total infiltrating cells, adhering to TDM-containing micelles. By day 3, granulomatous tissue and new vessels started to develop, and VEGF-positive macrophages appeared in a small number and gradually increased in number thereafter. The pouch contents of VEGF, interleukin-1β, tumor necrosis factor alpha, and transforming growth factor β were significantly elevated in TDM-treated pouches, with peaks at days 1, 0.5, 1, and 3, respectively, compared to those of control pouches, while that of basic fibroblast growth factor showed no significant increase. Treatment with anti-VEGF antibody inhibited TDM-induced granulomatous tissue formation and neovascularization, and administration of recombinant VEGF into pouches treated with FIA alone induced neovascularization comparable to that in the TDM-treated pouches. Incubation of neutrophils and macrophages on TDM-coated plastic dishes increased the VEGF release. The present results indicate that TDM augments VEGF production by neutrophils and macrophages and induces neovascularization in the granulomatous tissue.
Trehalose 6,6′-dimycolate (TDM), or cord factor, is a biologically active, cell wall component characteristic of mycolic acid-containing bacteria such as Mycobacterium, Tsukamurella, Gordona, Rhodococcus, Nocardia, and Corynebacterium spp. and plays a central role in pathogenesis during infection. It has immunomodulating functions such as granuloma-forming activity (14, 35), antitumor function (25, 27), and augmentation effect on nonspecific immunity to microbial infection (29). These functions are mediated by various proinflammatory cytokines or mediators such as interleukin-1 (IL-1) (36), IL-12 (28), gamma interferon (IFN-γ) (12, 28), granulocyte-macrophage colony stimulating factor (36), hydrogen peroxide (20), and nitric oxide (12) secreted by activated macrophages.
Infection with these bacteria is pathologically characterized by chronic granulomatous inflammation, which develops based on delayed-type hypersensitivity and accompanying host tissue damage (32). In general, chronic inflammatory diseases involve angiogenesis as a mechanism of repair on inflammation-associated tissue injury (13). Vascular endothelial growth factor (VEGF), a cytokine produced by various types of cells such as vascular endothelial cells (26) and macrophages (24), induces vascular endothelial cell proliferation (7), monocyte migration (6), and increased vascular permeability (16), thus contributing to the development of chronic inflammation. However, involvement of VEGF in the pathogenesis of mycolic acid-containing bacteria is not well understood.
For the study of angiogenesis during wound repair, murine chronic granulomatous tissue air pouches induced by Freund's complete adjuvant (FCA), which includes killed Mycobacterium tuberculosis, have been used as an experimental model (17) in which several cytokines such as platelet-derived growth factor, epidermal growth factor, IL-1β, and tumor necrosis factor alpha (TNF-α) are implicated in the development of granulomatous tissue (1). It has been recently demonstrated that VEGF is involved in neovascularization in the pouch wall (2).
In the present study, to reveal the inducing function of TDM in neovascularization we injected purified TDM dissolved in Freund's incomplete adjuvant (FIA) instead of FCA into the air pouch and investigated angiogenesis and VEGF production in the pouch wall. To identify the cell type responsible for VEGF production, we performed immunohistochemical staining of pouch walls and conducted a biochemical assay of the culture medium of TDM-activated neutrophils and macrophages. We mainly used here TDM purified from Rhodococcus sp. strain 4306 rather than TDM from M. tuberculosis because the former TDM has much shorter mycolic acids (C34 to C38) than the latter (C74 to C86) and is therefore expected to show less toxicity, which would be of great advantage in its pharmacological use.
MATERIALS AND METHODS
Animals.
Male specific-pathogen-free ICR mice, 6 weeks old, were purchased from SLC (Shizuoka, Japan). They were fed standard chow pellets and water ad libitum. Experiments were performed in accordance with the standard guidelines for animal experiments of the Osaka City University Medical School.
Preparation of mycolates.
Several subclasses of mycolates were prepared from Rhodococcus sp. strain 4306 as follows. To obtain TDM, trehalose monomycolate (TMM), and glucose mycolate (GM), bacteria were grown with shaking in a medium containing 1% glucose, 0.5% yeast extract, and 0.5% polypepton for 2 to 4 days at 37°C. For mannose mycolate (MM) and fructose mycolate (FM), Rhodococcus sp. strain 4306 was grown in a medium containing 1% mannose and fructose, respectively, instead of glucose (37). M. tuberculosis Aoyama B was grown in Sauton medium for 5 weeks at 37°C. Lipids were extracted from harvested cells with chloroform-methanol (2:1 [vol/vol]). Each mycolate was purified by developing the lipids on a thin-layer plate of silica gel (Analtech, Inc., Newark, Del.) with chloroform-methanol-acetone-acetic acid (90:10:6:1 [vol/vol/vol/vol]) and subsequently with chloroform-methanol (2:1 [vol/vol]). This procedure was repeated until a single spot was obtained. Purified TDM was analyzed by thin-layer chromatography and gas chromatography-mass spectrometry and revealed to contain C34–38 α-mycolic acids in Rhodococcus sp. strain 4306 and C74–86 α-, methoxy- and keto-mycolic acids in M. tuberculosis.
Treatment of mice.
For induction of an air pouch, mice were injected with 3 ml of air into the dorsal subcutaneous tissue. Twenty-four hours later, a 0.5-ml portion of 300 μg of mycolates in FIA (Difco Laboratories, Detroit, Mich.) or FIA alone as a control was emulsified with saline (1:1 [vol/vol]) to form an oil-in-water emulsion and injected into the air pouch. At 1, 3, 5, 7, 14, 21, and 28 days after injection, mice were sacrificed by an overdose of ether anesthesia. The pouch was taken out, and the wet weight of the pouch walls was measured. Some mice were subjected to an intraperitoneal injection of 20 μg of goat anti-mouse VEGF antibody (R&D Systems, Minneapolis, Minn.) or 20 μg of goat immunoglobulin G (IgG; Southern Biotechnology Associates, Birmingham, United Kingdom) as a control 1 day before the pouch formation. In some FIA-alone-injected pouches, 0.1 or 1 μg of recombinant mouse VEGF (R&D Systems) was injected soon after FIA injection.
Histology.
The pouch was fixed in 10% formalin. Paraffin sections were stained with hematoxylin and eosin (H&E). For immunohistochemistry, samples were fixed in a periodate–lysine–3% paraformaldehyde solution overnight at 4°C. Cryosections were cut with a cryostat (Bright Instruments, Huntington, United Kingdom) and immediately air dried. After treatment with 0.05% Triton X-100 in phosphate-buffered saline (PBS), endogenous peroxidase activity was blocked by incubating sections in methanol containing 0.03% hydrogen peroxide for 20 min at room temperature. After washing the mixture with PBS, sections were treated with normal swine serum (Dako, Glostrup, Denmark) for 30 min at room temperature to block nonspecific reactions. Sections were incubated with rabbit anti-human VEGF (V3) antibody (1:100; IBL, Fujioka, Japan), which has been demonstrated to form an immunopositive band of 23 kDa (i.e., the molecular mass of the VEGF monomer) (9), overnight at 4°C. For the negative control, the antibody was absorbed by the synthetic VEGF peptide (IBL). After a washing with PBS, they were incubated with biotinylated swine anti-rabbit IgG antibody (Dako) for 40 min at room temperature, followed by incubation with avidin-biotin peroxidase complex (Vector Laboratories, Peterborough, United Kingdom) for 20 min at room temperature. Immunoreactions were visualized by treating the sections with 0.25 mg of 3,3′-diaminobenzidine tetrahydrochloride per ml in 0.05 M Tris-buffered saline (pH 7.4) in the presence of 0.003% hydrogen peroxide for 3 to 5 min. Counterstaining for nuclei was done with hematoxylin.
For electron microscopy, samples were fixed in 1% Karnovsky's solution overnight at 4°C. They were then postfixed in 1% OsO4 in 0.1 M phosphate buffer (pH 7.4), dehydrated in an ethanol series, and embedded in Polybed (Polysciences, Inc., Warrington, Pa.). Semithin sections were stained with toluidine blue and observed by light microscopy. Thin sections were stained with uranyl acetate and lead citrate and observed under a 1200EX-II electron microscope (JEOL, Tokyo, Japan) at 100 kV.
Enumeration of infiltrating cells.
Numbers of neutrophils and macrophages infiltrating in the pouch wall were counted in toluidine blue-stained sections prepared 1 and 3 days after TDM injection. Five animals were used for each time point. Neutrophils were identified by their lobulated nuclei and dense cytoplasm, and macrophages were identified by their kidney-shaped nuclei and large, pale cytoplasm-bearing projections. Cells were counted at a magnification of ×1,000. Five microscopic fields (0.07 mm2) were examined. The percentages of neutrophils and macrophages versus total infiltrating cells were then calculated.
Carmine dye vascular cast.
At 7 days after TDM or FIA-alone injection, animals were subjected to the intravenous (i.v.) injection of 1 ml of carmine solution (10% carmine red in 5% gelatin solution), which was warmed to 37°C. The pouch wall was refrigerated to solidify the gelatin and form a vascular cast. Samples were fixed in 10% formalin, dehydrated in ethanol, and cleared in methyl benzoate for 2 weeks.
Measurement of cytokine contents in the pouch wall.
The pouch was taken out at 12 h, 1 day, 3 days, 7 days, and 14 days after TDM or FIA-alone injection. By a modification of the method of Appleton et al. (2), samples were homogenized in 0.05% Tween 20 in PBS and centrifuged at 20,000 × g for 10 min. The supernatant was analyzed by using murine enzyme-linked immunosorbent assay (ELISA) kits for IL-1β (Genzyme, Cambridge, Mass.), TNF-α (Genzyme), transforming growth factor-beta (TGF-β; Genzyme), and VEGF (R&D Systems). Basic fibroblast growth factor (bFGF) was measured by a sandwich ELISA assay by using a monoclonal antibody against bFGF (Upstate Biotechnology, Lake Placid, N.Y.).
Isolation and culture of neutrophils and macrophages.
Neutrophils were prepared according to the method of Watt et al. (33). Briefly, after two consecutive intraperitoneal injections of 2 ml of 3% sodium caseinate, peritoneal exudate cells were harvested. Diluted isotonic Percoll was prepared by mixing 9 ml of Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) with 1 ml of 0.2 M phosphate buffer (pH 7.3) containing 1.49 M NaCl and 1 ml of PBS, and then 5 × 107 peritoneal exudate cells per ml of PBS were mixed with 8 ml of diluted isotonic Percoll. Neutrophils were purified by the centrifugation of a continuous density gradient of Percoll at 60,000 × g for 20 min. After washing, they were suspended in RPMI 1640 containing 5% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of streptomycin per liter at a concentration of 5 × 106 cells/500 μl. Viability was 98%, as determined by a trypan blue exclusion test. Neutrophils were identified by their nuclear shape in Giemsa staining, and the purity was 96%.
Macrophages were prepared from the peritoneal exudate cells harvested 4 days after intraperitoneal injection of 2 ml of 10% proteose peptone. Cells were centrifuged at 500 × g for 10 min, washed twice, and suspended in RPMI 1640 containing 5% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml at a concentration of 106 cells/500 μl. The viability was 97% and the purity was 85% as evaluated by a trypan blue exclusion test and the morphology in Giemsa-stained specimens, respectively.
Measurement of cytokine production in culture.
TDM-coated plates were prepared as previously reported (23). Namely, TDM was dissolved in isopropanol, and quantities of 0.01, 0.1, 1, and 10 μg were dispersed into 24-well culture plates. The suspension was allowed to dry in a sterile atmosphere overnight. Noncoated plates were used as controls. Neutrophils (5 × 106 cells) and macrophages (106 cells) were then plated in culture plates and incubated for 3, 6, 24, or 48 h. The supernatants were collected and stored at −80°C. Released cytokines in the supernatants were measured by ELISA kits for VEGF (R&D Systems), IL-1β (Genzyme), and TNF-α (Genzyme).
Toxicities of TDM.
The in vivo toxicity of TDM was measured by the decrease in body weight after i.v. injection of 300 μg of TDM in the form of water-in-oil-in-water (w/o/w) micelles (14). In vitro toxicity was assessed by measuring the growth inhibition of cultured macrophages. Macrophages were harvested as described above and cultured on a 24-well culture plate coated with 0.001, 0.01, 0.1, 1, 10, 100, or 1,000 μg of TDM. At 24 h after incubation, viable cells were measured with the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay, and the 50% inhibitory concentration (IC50) of cell proliferation was calculated.
Statistics.
Data were expressed as mean values ± the standard deviation (SD) or standard error of the mean (SEM). Significant difference was evaluated according to an unpaired Student's t test.
RESULTS
TDM enhances granulomatous tissue formation in the pouch wall.
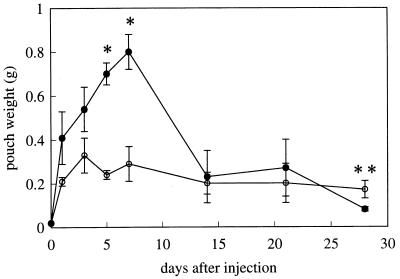
The extent of granulomatous tissue formation was evaluated by the wet weight of pouch walls. While the pouches treated with FIA alone slightly increased in weight to 0.330 ± 0.078 g at day 3 and maintained similar levels until day 28, those treated with TDM from Rhodococcus sp. strain 4306 went up considerably, reaching a peak (0.803 ± 0.076 g) at day 7, and then decreased to nearly the control level (0.080 ± 0.014 g) at day 28 (Fig. 1). The values and kinetics for M. tuberculosis TDM-treated pouches were comparable (0.753 ± 0.085 g at day 7 and 0.124 ± 0.046 g at day 28) to those of Rhodococcus sp. strain 4306 TDM-treated ones.
FIG. 1.
Time course of the wet weight of pouch walls from control (○) and Rhodococcus sp. strain 4306 TDM-treated (●) mice after injection of either FIA alone or 300 μg of TDM in FIA, respectively. Data represent the means ± the SD (n = three to five mice). ∗, P < 0.01; ∗∗, P < 0.05 (versus control).
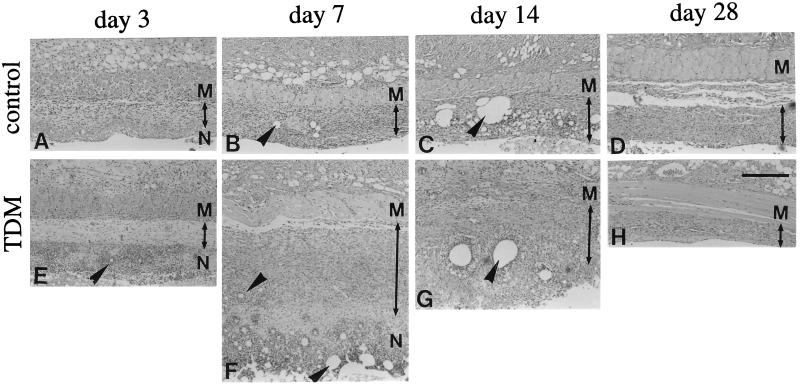
Granulomatous tissue development in the pouch wall, as revealed by histological analysis, paralleled the increase in pouch weight described above (Fig. 2). The pouch wall consisted of the necrotic tissue facing the cavity and the surrounding granulomatous tissue, both of which included micelles within them (Fig. 2B, C, F, and G). TDM-treated pouches exhibited thicker granulomatous tissue than control (FIA-alone-treated) ones at day 7 and more prompt healing at day 28, as indicated by a smaller pouch weight (Fig. 1) and less fluid in the cavity (data not shown).
FIG. 2.
Time course of the histology of pouch walls from control (upper panels) and Rhodococcus sp. strain 4306 TDM-treated (lower panels) mice at 3, 7, 14, and 28 days after injection of either FIA alone or 300 μg of TDM in FIA, respectively. While control pouches showed no appreciable increase in the thickness of granulomatous tissue (as indicated by arrowed bars) during experimental periods, TDM-treated pouches significantly increased in thickness at day 7 and then decreased at day 14. Various sizes of oil droplets (arrowheads) were localized in the necrotic tissue and granulomatous tissue. M, dermal muscular layer; N, necrotic tissue. Images were H&E stained. Bar, 200 μm.
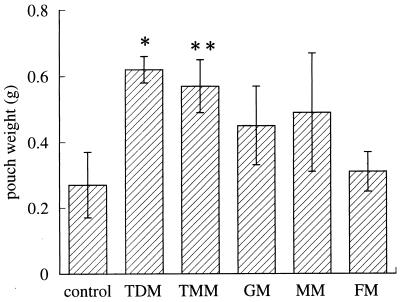
Other mycolates also exhibited granulomatogenic activity that was not so high as that of TDM, as represented by the increase in pouch weight; TMM significantly increased the pouch weight, while with GM and MM there was a slight but not significant increase and FM showed only a slight effect (Fig. 3). Histologically, granulomatous tissue formation and neovascularization were prominent in the pouch walls with an increased weight (data not shown).
FIG. 3.
Comparison of granulomatogenic activity among various mycolates from Rhodococcus sp. strain 4306 as evaluated by the wet weight of pouch walls at 7 days after injection. FIA alone (control) or 300 μg of Rhodococcus sp. strain 4306 TDM, TMM, GM, MM, or FM was injected into the pouch cavity. Data represent the means ± the SD (n = three to five mice). ∗, P < 0.01; ∗∗, P < 0.05 (versus control).
TDM enhances neovascularization in the pouch wall.
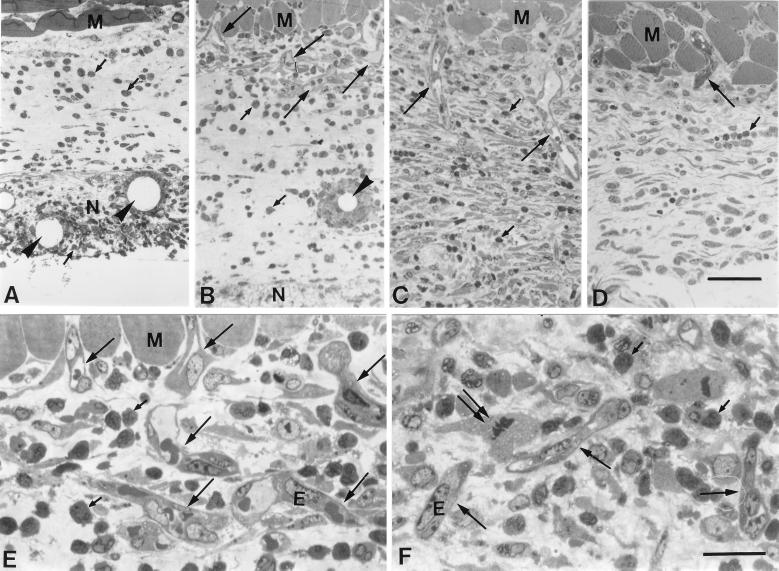
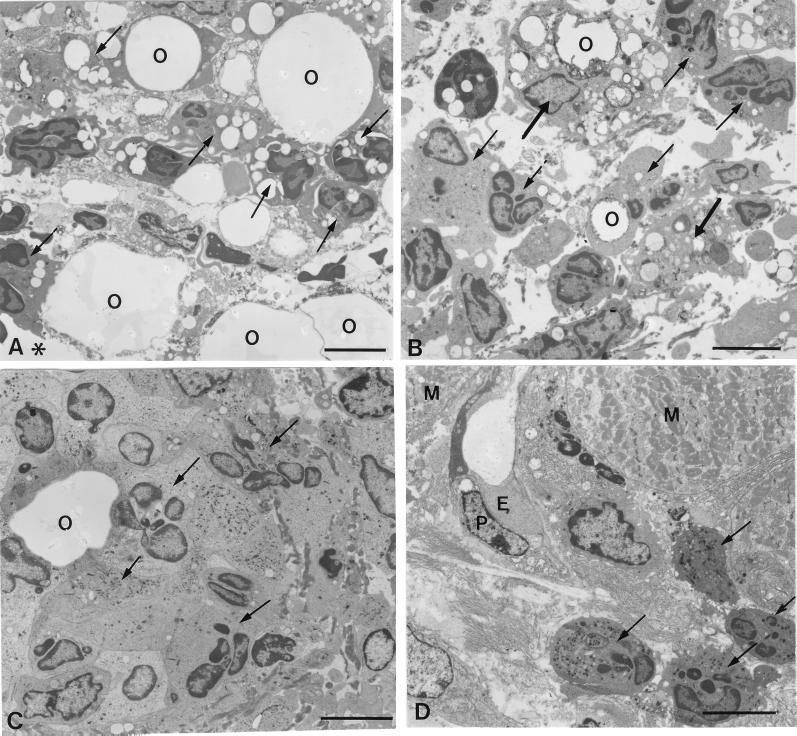
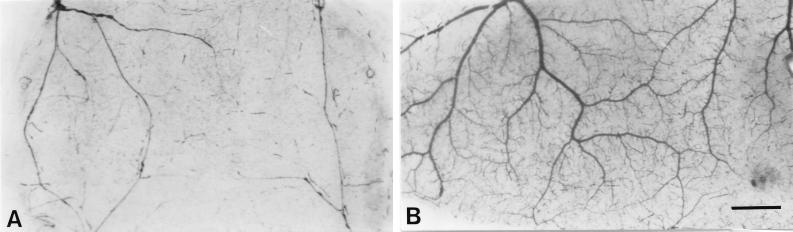
At day 1, neither granulomatous tissue nor neovascularization was induced in the connective tissue area between the necrotic tissue and a dermal muscular layer in TDM-treated pouches (Fig. 4A). More than 95% of the inflammatory cells infiltrating into the necrotic tissue were neutrophils, as identified by their lobulated nuclei. As seen electron microscopically, they phagocytosed many small oil droplets or closely adhered to large droplets (Fig. 5A). They were also found in the connective tissue (Fig. 4A). At day 3, granulomatous tissue, which was characterized by myofibroblastic cells, appeared in the connective tissue, and new vessels began to invade there from the muscular layer in TDM-treated pouches (Fig. 4B), while they were not detected in FIA-alone-treated pouches (data not shown). Although macrophages appeared in a small number, neutrophils were still predominant both in the necrotic tissue and in the granulomatous tissue (Fig. 4B): neutrophils were 92%, and monocytes/macrophages were 8%. Many neutrophils surrounded the oil droplets in the granulomatous tissue (Fig. 4B and 5C), and macrophages intermingling in the neutrophil infiltrate also phagocytosed the oil droplets (Fig. 5B). New vessels which entered the granulomatous tissue were associated with the neutrophil infiltrate (Fig. 4E and 5D). Endothelial cell mitosis was common in new vessels (Fig. 4F). At day 7, granulomatous tissue continued to develop, and new vessels extended deeper into the granulomatous tissue (Fig. 4C). In the control pouches, on the other hand, both neutrophil infiltration and neovascularization were not prominent (Fig. 4D). Carmine dye vascular cast prepared at day 7 clearly demonstrated that neovascularization was more prominent in the TDM-treated pouches (Fig. 6).
FIG. 4.
Neovascularization in the granulomatous tissue in Rhodococcus sp. strain 4306 TDM-treated pouches at days 1 (A), 3 (B, E, and F), and 7 (C) and in FIA-alone-treated pouches at day 7 (D). In TDM-treated pouches, new vessels (large arrows) started to elongate from the muscular layer into the granulomatous tissue at day 3 (B) and deeply extended into the tissue at day 7 (C). Both necrotic tissue and granulomatous tissue were infiltrated by many neutrophils (small arrows), which were aggregated around the oil droplets (arrowheads) in some places. In FIA-alone-treated pouches, on the other hand, the extension of new vessels (large arrows) into the connective tissue was only slight, and the number of infiltrating neutrophils was much smaller at day 7 (D). Neovascular endothelial cells with a mitotic figure (double arrows) were seen, being associated with a prominent neutrophil infiltrate (small arrows) (E and F). Toluidine blue staining was used. E, endothelial cells; M, muscular layer. (A to D) Bar, 50 μm. (E and F) Bar, 20 μm.
FIG. 5.
Electron micrographs of infiltrating cells in the pouch wall treated with Rhodococcus sp. strain 4306 TDM at 1 (A) or 3 (B to D) days after injection. At day 1, the necrotic tissue facing the pouch cavity (asterisks) contained many neutrophils (arrows). (A) Neutrophils were attached to the oil droplets and phagocytosed small droplets. (B) At day 3, a few macrophages (thick arrows), which phagocytosed the oil droplets, joined the neutrophil infiltrate (arrows) in the necrotic tissue. (C) In the granulomatous tissue, neutrophils accumulated around the oil droplets. (D) Beneath the muscular layer, new vessels extended, being accompanied by neutrophil infiltrate (arrows). E, endothelial cells; P, pericytes; O, oil droplets; M, muscular layer. Bar, 5 μm.
FIG. 6.
Vascular development in the wall of FIA-alone-treated (A) and Rhodococcus sp. strain 4306 TDM-treated (B) pouches at 7 days, as demonstrated by carmine dye vascular casts. Neovascularization is prominent in the latter pouches. Bar, 1 mm.
Contents of VEGF and other cytokines in the pouch wall.
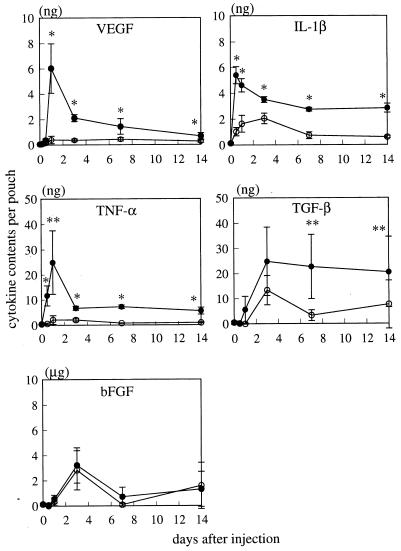
The contents of proinflammatory cytokines in the pouch wall were measured at various time intervals after TDM or FIA-alone injection (Fig. 7). In TDM-treated pouches, the amount of VEGF strikingly increased 1 day after injection and then decreased by day 7, showing significantly larger values than those for control pouches during this time period. IL-1β and TNF-α showed kinetics similar to those of VEGF, exhibiting a peak at days 0.5 and 1, respectively, and maintained significantly higher levels than the controls by day 14. On the other hand, TGF-β showed significantly higher levels than the controls after day 3, and bFGF showed no significant increase at any time points compared to the control pouches.
FIG. 7.
Time course of the contents of various cytokines in the pouch wall treated with FIA alone (○) and Rhodococcus sp. strain 4306 TDM (●). The value was expressed as the content per pouch. Data represent the means ± the SD (n = three to five mice). ∗, P < 0.01; ∗∗, P < 0.05 versus control.
Localization of VEGF in the pouch wall.
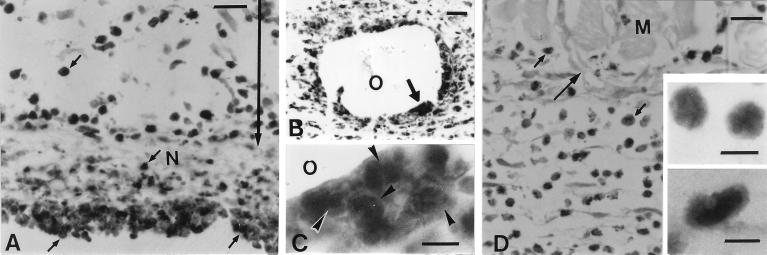
Immunohistochemistry demonstrated that infiltrating cells in the necrotic tissue and granulomatous tissue in TDM-treated pouches exhibited intense staining for VEGF at day 1 (Fig. 8A and B). They were morphologically identified as neutrophils by their round cell contour and their lobulated nuclei (Fig. 8C). At day 3, VEGF-positive neutrophils were associated with the neovasculature in the granulomatous tissue (Fig. 8D). A small number of VEGF-positive macrophages were also found among the neutrophil infiltrate (Fig. 8D, inset).
FIG. 8.
Immunohistochemistry for VEGF of the pouch wall treated with Rhodococcus sp. strain 4306 TDM at days 1 (A to C) and 3 (D). At day 1, VEGF-positive neutrophils (small arrows) distributed in the necrotic tissue and granulomatous tissue (indicated by arrowed bar). They were abundant in the area facing the pouch cavity (A) and oil droplets (B). (C) A higher magnification of the area indicated by a thick arrow in panel B revealed that infiltrating cells had the lobulated nuclei (arrowheads) indicative of neutrophils. At 3 days, new vessels (arrows) extended into the granulomatous tissue, where VEGF-positive cells (small arrows) were abundant. At a higher magnification, most of the VEGF-positive cells were seen to have a small, round shape with the lobulated nuclei indicative of neutrophils (upper inset); a few positive cells were larger and had the kidney-shaped nuclei indicative of macrophages (lower inset). M, muscular layer; N, necrotic tissue; O, oil droplets. (A and B) Bar, 20 μm. (C) Bar, 5 μm. (D) Bar, 20 μm. (Insets) Bar, 5 μm.
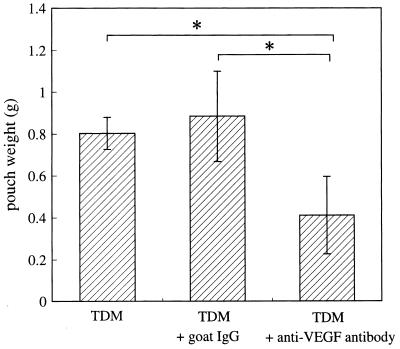
Suppression of TDM-induced granulomatous tissue formation and neovascularization by treatment with anti-VEGF antibody.
To demonstrate the involvement of VEGF in granulomatous tissue formation and neovascularization in TDM-treated pouches, we injected anti-VEGF antibody intraperitoneally 1 day before TDM injection. Compared to IgG injection as a control, anti-VEGF antibody injection significantly decreased the weight of the pouches (Fig. 9). Consistent with these data, histological analysis demonstrated a decrease in the thickness of pouch wall and a lower frequency of new vessels in anti-VEGF antibody-treated mice compared to IgG-treated mice (see Fig. 11A and B).
FIG. 9.
Suppression of the Rhodococcus sp. strain 4306 TDM-induced increase in pouch weight by treatment with anti-VEGF antibody. Anti-VEGF antibody or goat IgG as a control was intraperitoneally injected 1 day before pouch formation, and the pouch weight as an index of granulomatous tissue formation was measured at day 7. Data represent the means ± the SD (n = three mice). ∗, P < 0.05 versus TDM or TDM plus goat IgG.
FIG. 11.
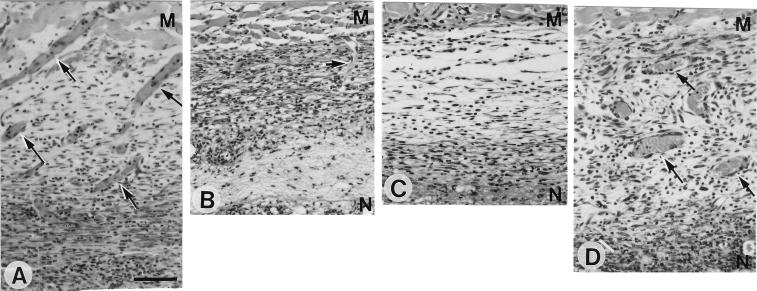
Histology of TDM-treated pouch walls at 7 days after intraperitoneal injection of 20 μg of goat IgG (A) or 20 μg of anti-VEGF antibody (B) and histology of FIA-alone-treated pouch walls at 7 days without (C) or with (D) 1 μg of recombinant VEGF injected into the pouch cavity. (A and B) Many new vessels (arrows) filled with erythrocytes extend from the dermal muscular layer in TDM-treated pouches treated with IgG (A), while they decrease in number with treatment with anti-VEGF antibody (B). (C and D) Administration of recombinant VEGF into the pouch cavity induces new vessels (arrows) in the granulomatous tissue of FIA-alone-treated pouches. H&E staining was used. M, muscular layer; N, necrotic tissue facing on the pouch cavity. (A to D) Bar, 50 μm.
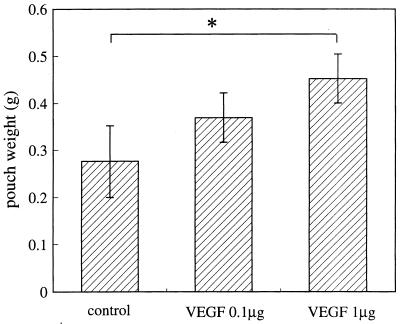
Induction of granulomatous tissue development and neovascularization in the pouch wall by administration of VEGF into FIA-alone-treated pouches.
To demonstrate whether exogenous VEGF can induce granulomatous tissue development and neovascularization as seen with TDM treatment, we injected 0.1 or 1 μg of VEGF into FIA-alone-treated pouches. The pouch weight increased to significantly higher values with 1 μg of VEGF (Fig. 10). Histologically, granulomatous tissue increased in thickness and neovascularization was more prominent (Fig. 11D) than in the pouches without VEGF administration (Fig. 11C).
FIG. 10.
Increase in pouch weight caused by administration of exogenous VEGF into FIA-alone-treated pouches. Recombinant VEGF was injected immediately after FIA injection, and the pouch weight was measured at day 7. Data represent the means ± the SD (n = three mice). ∗, P < 0.05 versus control.
TDM stimulates in vitro production of VEGF and other cytokines by neutrophils and macrophages.
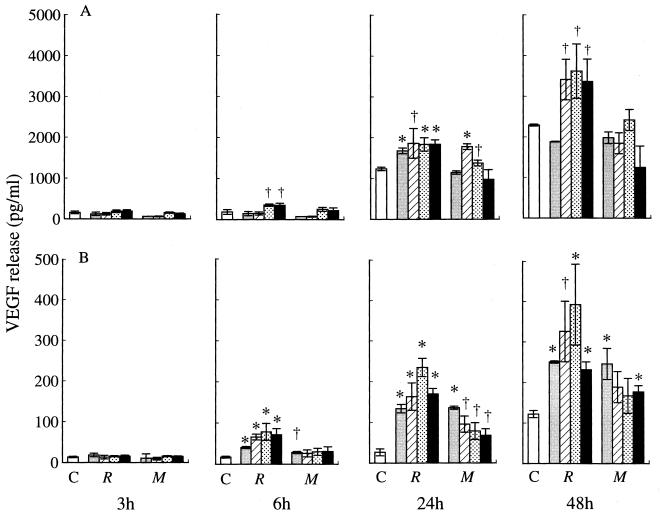
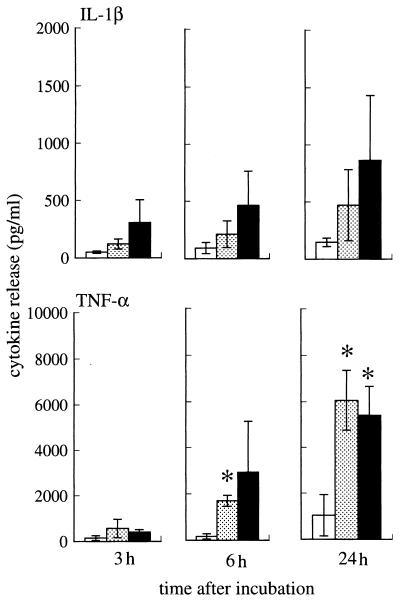
With incubation on Rhodococcus sp. strain 4306 TDM-coated plastic dishes, both neutrophils and macrophages produced significantly larger amounts of VEGF than those incubated on noncoated dishes at between 6 and 48 h (Fig. 12). There was dose dependency at between 0.01 and 1 μg of TDM per well, although 10 μg of TDM induced less VEGF production than did 1 μg. M. tuberculosis TDM also augmented VEGF production by macrophages but failed to stimulate the production by neutrophils (Fig. 12). Neutrophils incubated with 1 or 10 μg of Rhodococcus sp. strain 4306 TDM also produced significantly larger amounts of TNF-α after 6 h, a result similar to that with VEGF (Fig. 13). IL-1β production was also raised by treatment with Rhodococcus sp. strain 4306 TDM, but a significant difference was not obtained (Fig. 13).
FIG. 12.
VEGF production by neutrophils (A) and macrophages (B) after incubation on noncoated (□) dishes or Rhodococcus sp. strain 4306 TDM (R)- or M. tuberculosis TDM (M)-coated dishes in doses of 0.01 ( ), 0.1 (▨), 1 (
), 0.1 (▨), 1 (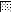 ), or 10 (■) μg. Data represent the means ± the SEM (n = 3). ∗, P < 0.01; †, P < 0.05 (versus incubation on noncoated dishes).
), or 10 (■) μg. Data represent the means ± the SEM (n = 3). ∗, P < 0.01; †, P < 0.05 (versus incubation on noncoated dishes).
FIG. 13.
IL-1β and TNF-α production by neutrophils after incubation on noncoated (□) dishes and Rhodococcus sp. strain 4306 TDM-coated dishes with doses of 1 (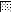 ) or 10 (■) μg. Data represent the means ± the SEM (n = 3). ∗, P < 0.01 (versus noncoated dishes).
) or 10 (■) μg. Data represent the means ± the SEM (n = 3). ∗, P < 0.01 (versus noncoated dishes).
In vivo and in vitro toxicities of TDM.
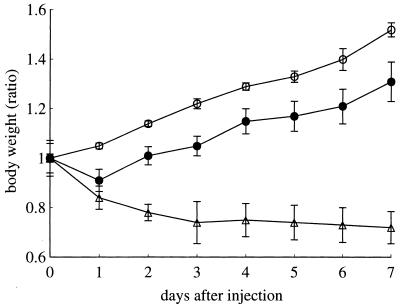
In vivo toxicity was evaluated by measuring the decrease of body weight. When Rhodococcus sp. strain 4306 TDM was injected i.v., mice showed a steady increase in body weight until day 7, although to a smaller degree than with mice treated with w/o/w micelles alone. In contrast, mice treated with M. tuberculosis TDM continuously decreased in body weight (Fig. 14).
FIG. 14.
Body weights of mice at various time intervals after i.v. injection of w/o/w micelles alone (○), Rhodococcus sp. strain 4306 TDM (●), or M. tuberculosis TDM (▵).
The in vitro toxicity was represented by the IC50 of macrophages after 24 h of incubation with TDM. The IC50 for Rhodococcus sp. strain 4306 TDM was 25 μg and that for M. tuberculosis TDM was 0.6 μg.
DISCUSSION
It is known that TDM exerts a wide variety of biological functions, such as granulomatogenic and antitumor effects, by inducing the secretion of various cytokines or mediators from activated macrophages. The present study has demonstrated that TDM also enhances the production of VEGF and thereby promotes angiogenesis in the granulomatous tissue.
By treatment with TDM, new vessels started to grow in the pouch wall at day 3 concurrently with granulomatous tissue formation. Preceding this event, the VEGF content in the pouch wall remarkably increased by day 1, and many VEGF-positive cells accumulated in the lesion, suggesting an inducing role for VEGF in angiogenesis during TDM-elicited granulomatous inflammation. This interpretation was supported by the results that treatment with anti-VEGF antibody suppressed TDM-induced granulomatous tissue formation and neovascularization and, moreover, that administration of recombinant VEGF induced these two events in FIA-alone-treated pouches.
Neutrophils are considered to be the major source of VEGF in the pouch wall at day 1 because they were positively stained for VEGF and exceeded 95% of infiltrating cells. The capacity of neutrophils for producing VEGF has been demonstrated both in vivo (30) and in vitro (11, 34). In the pouch wall, neutrophils adhered to and phagocytosed TDM-dissolved oil droplets. When incubated on the surface of coated TDM, neutrophils secreted large amounts of VEGF into the culture medium, indicating that TDM directly acts on neutrophils and induces VEGF release. Macrophages are also assumed to function as the source of VEGF from their positive staining for VEGF and in vitro VEGF production by the stimuli of TDM. Their contribution in the very early phase of granulomatous tissue formation seems to be low, however, because their proportion to total infiltrating leukocytes at days 1 to 3 was too small to account for a prominent increase in VEGF during this time period. Macrophages increased in number after day 3 and should be engaged in VEGF production in the later phases of inflammation.
Recently, an initiating role of neutrophils in granulomatous inflammation during mycobacterial infection has been proposed because they are among the first cells to arrive at the site of mycobacterial infection (4), are able to kill mycobacterial bacilli (5, 15), and produce chemokines for monocyte migration (15). The present finding that neovascularization is enhanced by TDM-activated neutrophils via VEGF secretion provides a new insight into the contribution of neutrophils in the progression of diseases during the infection of mycolic acid-containing bacteria.
Other angiogenic cytokines, such as TGF-β and TNF-α (10), were also generated in TDM-treated pouches, a finding consistent with the previous result with FCA-treated pouches (1) (bFGF was also elevated in TDM-treated pouches but was not significantly higher than the controls). TNF-α, which peaked at day 1, was possibly secreted by neutrophils because these cells predominantly were distributed in the pouch wall at this time point and are reported to produce TNF-α (8, 21, 31). In contrast, TGF-β levels increased after day 3, suggesting its role in the later phase of inflammation. Although these cytokines may also participate in angiogenesis in the pouch wall, the essential role of VEGF is clearly demonstrated by the present experiments with anti-VEGF antibody and recombinant VEGF.
Because VEGF increases vascular permeability (16) and enhances monocyte migration (6), this cytokine is considered to be central to the acute-phase inflammatory response to injury (34). Consistent with this view, neutrophils activated by TNF-α release VEGF (11, 34) and other tissue elements, such as cardiac myocytes, synovial fibroblasts, and piliosebaceous cells, also express VEGF, being stimulated by proinflammatory cytokines such as IL-1β (19, 22), TNF-α (3), and TGF-β (3). In the present study, incubation with TDM induced neutrophil activation, as indicated by TNF-α and IL-1β generation. The VEGF release seen here might also be a representative feature of TDM-induced activation of neutrophils. Whether IL-1β and TNF-α secreted by activated neutrophils further act on themselves and enhance VEGF production remains unclear because such a positive feedback mechanism has not been elucidated for VEGF.
TDM has toxicities and biological activities, both of which are closely related to the acyl number and structure of the mycolic acid moiety (37). In this study, TDM from Rhodococcus sp. strain 4306, which has much shorter carbon chain length of mycolic acids than that from M. tuberculosis, exhibited lower toxicities both in vivo and in vitro. Although mycobacterial TDM similarly induced VEGF production in vitro, the amount was less than that produced by Rhodococcus sp. strain 4306 TDM, presumably due to the higher toxicities to cells, indicating that it may be superior to M. tuberculosis TDM for pharmacological use.
It is reported that there was a difference in the granuloma-forming activity of mycolates by their sugar moieties; namely, TDM and GM from Rhodococcus ruber (formerly termed Nocardia rubra) had high activity, while its MM and FM showed low activity (36). We found here a similar difference in granulomatous tissue-forming activity among mycolates, from low (FM) to high (TMM and TDM).
In conclusion, TDM enhances VEGF production by activated neutrophils and macrophages and thereby contributes to the development of granulomatous inflammation.
ACKNOWLEDGMENT
We thank Liying Fan, Department of Anatomy, Kanazawa University Medical School, Kanazawa, Japan, for advice on VEGF immunohistochemistry.
REFERENCES
- 1.Appleton I, Tomlinson A, Colville-Nash P R, Willoughby D A. Temporal and spatial immunolocalization of cytokines in murine chronic granulomatous tissue. Lab Investig. 1993;69:405–414. [PubMed] [Google Scholar]
- 2.Appleton I, Brown N J, Willis D, Colville-Nash P R, Alam C, Brown J R, Willoughby D A. The role of vascular endothelial growth factor in a murine chronic granulomatous tissue air pouch model of angiogenesis. J Pathol. 1996;180:90–94. doi: 10.1002/(SICI)1096-9896(199609)180:1<90::AID-PATH615>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Berse B, Hunt J A, Diegel R J, Morganelli P, Yeo K, Brown F, Fava R A. Hypoxia augments cytokine (transforming growth factor-beta [TGF-β] and IL-1)-induced vascular endothelial growth factor secretion by human synovial fibroblasts. Clin Exp Immunol. 1999;115:176–182. doi: 10.1046/j.1365-2249.1999.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch H. The relationship between phagocytic cells and human tubercle bacilli. Am Rev Tuberc. 1948;58:662–670. doi: 10.1164/art.1948.58.6.662. [DOI] [PubMed] [Google Scholar]
- 5.Brown A E, Holzer T J, Anderson B R. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156:985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- 6.Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti P C, Pan Y-C E, Olander J V, Connolly D T, Stern D. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly D T, Heuvelman D M, Nelson R, Olander J V, Eppley B L, Delfino J J, Siegel N R, Leimgruber R M, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Investig. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djeu J Y, Serbousek D, Blanchard D K. Release of tumor necrosis factor by human polymorphonuclear leukocytes. Blood. 1990;76:1405–1409. [PubMed] [Google Scholar]
- 9.Fan L, Iseki S. Immunohistochemical localization of vascular endothelial growth factor in the endocrine glands of the rat. Arch Histol Cytol. 1998;61:17–28. doi: 10.1679/aohc.61.17. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J, Brem H. Angiogenesis and inflammation. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 2nd ed. New York, N.Y: Raven Press; 1992. pp. 821–839. [Google Scholar]
- 11.Gaudry M, Brégerie O, Andriew V, Benna J E, Pocidalo M-A, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90:4153–4161. [PubMed] [Google Scholar]
- 12.Guillemard E, Geniteau-Legendre M, Kergot R, Lemaire G, Gessani S, Labarre C, Quero A M. Simultaneous production of IFN-gamma, IFN-alpha/beta and nitric oxide in peritoneal macrophages from TDM-treated mice. J Biol Regul Homeostatic Agents. 1998;12:106–111. [PubMed] [Google Scholar]
- 13.Jackson R J, Seed M P, Kircher C H, Willoughby D A, Winkler J D. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- 14.Kaneda K, Sumi Y, Kurano F, Kato Y, Yano I. Granuloma formation and hemopoiesis induced by C36–48-mycolic acid-containing glycolipids from Nocardia rubra. Infect Immun. 1986;54:869–875. doi: 10.1128/iai.54.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasahara K, Sato I, Ogura K, Takeuchi H, Kobayashi K, Adachi M. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J Infect Dis. 1998;178:127–137. doi: 10.1086/515585. [DOI] [PubMed] [Google Scholar]
- 16.Keck P J, Hauser S D, Krivi G, Sanzo K, Warren T, Feder J, Connolly D T. Vascular permeability factor and endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 17.Kimura M, Suzuki J, Amemiya K. Mouse granuloma pouch induced by Freund's complete adjuvant with croton oil. J Pharmacobio-Dyn. 1985;8:393–400. doi: 10.1248/bpb1978.8.393. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S, Inaba K, Kimura I, Kimura M. Inhibitory effects of tetrandrine on angiogenesis in adjuvant-induced chronic inflammation and tube formation of vascular endothelial cells. Biol Pharm Bull. 1998;21:346–349. doi: 10.1248/bpb.21.346. [DOI] [PubMed] [Google Scholar]
- 19.Kozlowska U, Blume-Peytave U, Kodelja V, Sommer C, Goerdt S, Jablonska S, Orfanos C E. Vascular endothelial growth factor expression induced by proinflammatory cytokines (interleukin 1 alpha, beta) in cells of the human pilosebaceous unit. Dermatology. 1998;196:89–92. doi: 10.1159/000017878. [DOI] [PubMed] [Google Scholar]
- 20.Lepoivre M, Tenu J P, Petit J F. Transmembrane potential variations accompanying the PMA-triggered O2 and H2O2 release by mouse peritoneal macrophages. FEBS Lett. 1982;149:233–239. doi: 10.1016/0014-5793(82)81107-1. [DOI] [PubMed] [Google Scholar]
- 21.Lord P C, Wilmoth L M G, Mizel S B, McCall C E. Expression of interleukin 1a and b genes by human blood polymorphonuclear leukocytes. J Clin Investig. 1991;87:1312–1321. doi: 10.1172/JCI115134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama K, Mori Y, Murasawa S, Masaki H, Takahashi N, Tsutsumi Y, Moriguchi Y, Shibazaki Y, Tanaka Y, Shibuya M, Inada M, Matsubara H, Iwasaka T. Interleukin-1 beta upregulates cardiac expression of vascular endothelial growth factor and its receptor KDR/flk-1 via activation of protein tyrosine kinases. J Mol Cell Cardiol. 1999;31:607–617. doi: 10.1006/jmcc.1998.0895. [DOI] [PubMed] [Google Scholar]
- 23.Matsunaga I, Oka S, Yano I. Mycoloyl glycolipids stimulate macrophages to release a chemotactic factor. FEMS Microbiol Lett. 1990;67:49–54. doi: 10.1016/0378-1097(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 24.McLaren J, Prentice A, Charnock-Jones D S, Millican S A, Muller K H, Sharkey A M, Smith S K. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Investig. 1996;98:482–489. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natsuhara Y, Oka S, Kaneda K, Kato Y, Yano I. Parallel antitumor, granuloma-forming and tumor-necrosis-factor-priming activities of mycoloyl glycolipids from Nocardia rubra that differ in carbohydrate moiety: structure-activity relationships. Cancer Immunol Immunother. 1990;31:99–106. doi: 10.1007/BF01742373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomura M, Yamaguchi S, Harada S, Hayashi Y, Yamashita T, Yamashita J, Yamamoto H. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270:28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- 27.Orbach-Arbouys S, Tenu J-P, Petit J-F. Enhancement of in vitro and in vivo antitumor activity by cord factor (6-6′-dimycolate of trehalose) administered suspended in saline. Int Arch Allergy Appl Immunol. 1983;71:67–73. doi: 10.1159/000233364. [DOI] [PubMed] [Google Scholar]
- 28.Oswald I P, Dozois C M, Petit J-F, Lemaire G. Interleukin-12 synthesis is a required step in trehalose dimycolate-induced activation of mouse peritoneal macrophages. Infect Immun. 1997;65:1364–1369. doi: 10.1128/iai.65.4.1364-1369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parant M, Parant F, Chedid L, Drapier J C, Petit J F, Wietzerbin J, Lederer E. Enhancement of nonspecific immunity to bacterial infection by cord factor (6,6′-trehalose dimycolate) J Infect Dis. 1977;135:771–777. doi: 10.1093/infdis/135.5.771. [DOI] [PubMed] [Google Scholar]
- 30.Taichman N S, Young S, Cruchley A T, Taylor P, Paleolog E. Human neutrophils secrete vascular endothelial growth factor. J Leukoc Biol. 1997;62:397–400. doi: 10.1002/jlb.62.3.397. [DOI] [PubMed] [Google Scholar]
- 31.Tiku K, Tiku M L, Skosey J L. Interleukin-1 production by human polymorphonuclear neutrophils. J Immunol. 1986;136:3677–3685. [PubMed] [Google Scholar]
- 32.Vermeulen M W, Fenton M J. Immunopathology of tuberculosis. In: Kradin R L, Robinson B W S, editors. Immunopathology of lung disease. Boston, Mass: Butterworth-Heinemann; 1996. pp. 231–266. [Google Scholar]
- 33.Watt S M, Burgess A W, Metcarf D. Isolation and surface labeling of murine polymorphonuclear neutrophils. J Cell Physiol. 1979;100:1–22. doi: 10.1002/jcp.1041000102. [DOI] [PubMed] [Google Scholar]
- 34.Webb N J A, Myers C R, Watson C J, Bottomley M J, Brenchley P E C. Activated human neutrophils express vascular endothelial growth factor (VEGF) Cytokine. 1998;10:254–257. doi: 10.1006/cyto.1997.0297. [DOI] [PubMed] [Google Scholar]
- 35.Yano I, Tomiyasu I, Kitabatake S, Kaneda K. Granuloma-forming activity of mycolic acid-containing glycolipids in Nocardia and related taxa. Acta Leprol. 1984;2:341–349. [PubMed] [Google Scholar]
- 36.Yano I, Oka S, Natsuhara Y, Kato Y, Tomiyasu I, Kaneda K. Molecular structure and immunopharmacological activities of the new glycolipids containing mycolic acids in actinomycetales. In: Okami Y, Beppu T, Ogawara H, editors. Biology of actinomycetes. Tokyo, Japan: Japan Scientific Societies Press; 1988. pp. 469–477. [Google Scholar]
- 37.Yano I. Structure analysis and granulomagenic activities of mycolic acid-containing glycolipids in acid-fast bacteria. Kekkaku. 1988;63:191–204. . (In Japanese with English abstract.) [PubMed] [Google Scholar]
- 38.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]