Abstract
The adhesion of Staphylococcus aureus to platelets is a major determinant of virulence in the pathogenesis of endocarditis. Molecular mechanisms mediating S. aureus interactions with platelets, however, are incompletely understood. The present study describes the interaction between S. aureus protein A and gC1qR/p33, a multifunctional, ubiquitously distributed cellular protein, initially described as a binding site for the globular heads of C1q. Suspensions of fixed S. aureus or purified protein A, chemically cross-linked to agarose support beads, were found to capture native gC1qR from whole platelets. Moreover, biotinylated protein A bound specifically to fixed, adherent, human platelets. This interaction was inhibited by unlabeled protein A, soluble recombinant gC1qR (rgC1qR), or anti-gC1qR antibody F(ab′)2 fragments. The interaction between protein A and platelet gC1qR was underscored by studies illustrating preferential recognition of the protein A-bearing S. aureus Cowan I strain by gC1qR compared to recognition of the protein A-deficient Wood 46 strain, as well as inhibition of S. aureus Cowan I strain adhesion to immobilized platelets by soluble protein A. Further characterization of the protein A-gC1qR interaction by solid-phase enzyme-linked immunosorbent assay techniques measuring biotinylated gC1qR binding to immobilized protein A revealed specific binding that was inhibited by soluble protein A with a 50% inhibitory concentration of (3.3 ± 0.7) × 10−7 M (mean ± standard deviation; n = 3). Rabbit immunoglobulin G (IgG) also prevented gC1qR-protein A interactions, and inactivation of protein A tyrosil residues by hyperiodination, previously reported to prevent the binding of IgG Fc, but not Fab, domains to protein A, abrogated gC1qR binding. These results suggest similar protein A structural requirements for gC1qR and IgG Fc binding. Further studies of structure and function using a truncated gC1qR mutant lacking amino acids 74 to 95 demonstrated that the protein A binding domain lies outside of the gC1qR amino-terminal alpha helix, which contains binding sites for the globular heads of C1q. In conclusion, the data implicate the platelet gC1qR as a novel cellular binding site for staphylococcal protein A and suggest an additional mechanism for bacterial cell adhesion to sites of vascular injury and thrombosis.
gC1qR/p33 is a single-chain, multiligand binding protein which migrates with an apparent molecular mass of 33 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) but as a multimer of 97.2 kDa by gel filtration under nondissociating conditions (10). Indeed, recent crystallographic evidence suggests that gC1qR may associate to form a doughnut-shaped ternary complex (21). gC1qR was originally isolated from a membrane preparation of a lymphoblastoid cell line (Raji) but has been shown to have a wide cellular distribution including platelets and endothelial cells (11, 30).
Independent reports from several laboratories demonstrate gC1qR identity with p32, a protein initially copurified with splicing factor SF2 (4, 18, 22), human immunodeficiency virus (HIV) Tat-associated protein (45), and hyaluronic acid binding protein, a member of the “hyaladherins” family of proteins (3, 33). In addition, gC1qR shows 92% sequence homology with YL2, a murine protein which interacts with HIV type 1 Rev (25).
gC1qR is synthesized as a pre-pro protein of 282 amino acids. Enzymatic cleavage results in removal of the N-terminal 73-amino-acid segment and releases the mature form of the molecule. Although gC1qR has been localized predominantly to mitochondria (5, 26), variably low cell surface expression has been observed also (11). On both resting and activated platelets, for example, gC1qR surface expression is poor (29) but is greatly enhanced following platelet adhesion to immobilized fibrinogen or fibronectin (29). Similarly, cell surface expression of gC1qR by endothelial cells is poor unless cells are activated by inflammatory mediators (12). This apparently intrinsic self-regulation of cell surface gC1qR expression may be important physiologically, since the globular domain of C1q is accessible in the circulation, as are other potential gC1qR ligands including high-molecular-weight kininogen, F XII, thrombin, and vitronectin (11).
Staphylococcus aureus is a pathogenic bacterium which causes a variety of infections in humans including endocarditis, osteomyelitis, wound sepsis, skin abscesses, and keratitis (1). At the cardiac valve surface, the interaction between S. aureus and platelets represents a critical event in the induction of infective endocarditis (43). The molecular interactions between platelets and S. aureus are incompletely understood, however.
S. aureus produces an array of potential virulence factors, including protein A (1, 17), a 42-kDa bacterial cell wall component expressed by most strains of S. aureus (6, 7). Protein A binds both the Fcγ region of immunoglobulins (Ig) (6, 20, 24) and the Fab portion of Ig belonging to the VH3+ gene family (13, 19, 20, 24, 35). Previous reports have indicated that the binding of protein A to Ig contributes to its mitogenic activity (35), enhances natural killing activity against lymphoid tumor cell lines (28), and induces Ig secretion by human B cells (34). In addition, protein A has been shown to activate complement via the classical pathway (38). When administered to laboratory animals or tested in vitro, protein A produces a variety of biological effects including hypersensitivity reactions, histamine release from basophils, complement activation, and derepression of the opsonizing activity of serum (8, 24).
The present study describes the specific interaction of staphylococcal protein A with human platelets via gC1qR. This interaction suggests a potential role for gC1qR in S. aureus pathogenesis and a novel mechanism for S. aureus localization to sites of vascular injury and thrombosis.
MATERIALS AND METHODS
Chemicals and reagents.
The following chemicals and reagents were purchased from the sources indicated: protein A (Cowan I), human complement component C1q, bovine serum albumin (BSA), rabbit IgG, p-nitrophenyl phosphate (pNPP), S. aureus Cowan I and Wood 46 strains (formalin-treated cell suspensions), and dimethyl sulfoxide (DMSO), Sigma Chemical Co. (St. Louis, Mo.); alkaline phosphatase-conjugated goat anti-rabbit IgG, Organon Teknika Corp. (West Chester, Pa.); alkaline phosphatase-conjugated streptavidin (AP-STRAV), protein A-agarose, and sulfosuccinimidyl-6-biotinamidohexanoate (NHS-LC-biotin), Pierce Chemical Co. (Rockford, Ill.); Tween 20, J. T. Baker (Phillipsburg, N.J.); PD-10 columns, Pharmacia Biotech (Piscataway, N.J.).
Biotinylation.
Proteins to be biotinylated were dialyzed in 1 liter of 0.2 M NaHCO3, pH 8.3. NHS-LC-biotin (60 mg/ml), freshly dissolved in DMSO, was added subsequently. After gentle tumble mixing for 1 to 4 h at room temperature, the reaction was stopped by removal of excess biotin via gel filtration over a PD-10 column. Successful protein biotinylation was determined by immobilizing column fractions on microtiter wells and examining their reactivity with AP-STRAV. AP-STRAV binding was detected as a function of pNPP hydrolysis quantified spectrophotometrically at 405 and 490 nm.
Fixed cell suspensions of Cowan I or Wood 46 strains of S. aureus were diluted in 0.2 M NaHCO3, pH 8.3, to a 2% cell suspension and were labeled by addition of NHS-LC-biotin (60 mg/ml), freshly dissolved in DMSO. After 60 min, bacterial cells were washed extensively in 0.01 mM Tris-buffered 0.15 M NaCl (TBS) and stored at 4°C.
Hyperiodination of protein A.
Hyperiodination of protein A was achieved as described previously (35). Sodium iodide (1 mg/ml) was added to 100 μl of a 1-mg/ml concentration of protein A. Iodination, initiated by the addition of 1.6 mg of chloramine-T/ml, was allowed to proceed at room temperature with gentle tumble mixing for 1 h. The reaction was terminated by the addition of 4.8 mg of sodium metabisulfite/ml. Hyperiodinated protein A was separated from the reaction mixture by passage over a PD-10 column equilibrated with 15 ml of 0.15 M NaCl.
Expression and purification of recombinant gC1qR (rgC1qR).
Construction of plasmid vector pGEX-2T containing the mature form (MF) of gC1qR cDNA corresponding to amino acid residues 74 to 282 of the predicted protein sequence and a deletion mutant expressing amino acids 96 to 282 designated gC1qR (TF) (truncated form) has been described in detail in a previous publication (10). The plasmids were transformed into Escherichia coli strain BL-21 [genotype: E. coli B F− dcm ompT hsdS (rB− mB−) gal; Stratagene, La Jolla, Calif.]. Proteins were expressed as fusion products with glutathione S-transferase (GST). GST fusion proteins were purified on glutathione-Sepharose (Pharmacia Biotech) and gC1qR (TF) or gC1qR (MF) was released by cleavage with thrombin (10).
gC1qR capture by solid-phase protein A.
Native gC1qR was captured from whole platelet lysates (400 to 500 μg of total protein) using protein A-agarose (50 μl) or a suspension of formalin-fixed S. aureus (Cowan I) (100 μl). Platelets were prepared as follows. Whole blood was collected from healthy volunteers by venipuncture after informed consent was obtained. Blood was anticoagulated with 3.2% sodium citrate (1 part anticoagulant to 9 parts whole blood) and centrifuged at 180 × g for 15 min. The resulting platelet-rich plasma (PRP) was collected, and the pH was adjusted to 6.3 to 6.7 with 1 M citric acid. PRP was centrifuged at 1,000 × g for 20 min, and the supernatant was discarded. The platelet pellet was resuspended in 0.01 M HEPES-buffered modified Tyrode's solution. The washed platelets were lysed with 10% Triton X-100–0.15 M NaCl–0.01 M Tris (pH 7.5) containing 100 mM EDTA and 5 mg of leupeptin/ml and exposed to washed suspensions of fixed S. aureus (Cowan I) or protein A-agarose overnight (4°C). After extensive washing, associated proteins were eluted using SDS-sample buffer. Samples were electrophoresed into SDS–10% polyacrylamide gels (23) and evaluated by Western blotting using an anti-gC1qR antibody. Antibody binding was detected by chemiluminescence (ECL; Amersham Corp., Arlington Heights, Ill.).
Similar studies were performed with rgC1qR. Specifically, 50 μl of protein A-agarose was incubated (overnight, 4°C) with 1 ml of 90-μg/ml rgC1qR (MF). The agarose was washed five times with 0.15 M NaCl–3.2 mM NaH2PO4–16.8 mM Na2H2PO4 (pH 7.5) to remove unbound gC1qR. Associated rgC1qR was released by boiling (5 min) in SDS-sample buffer (23) and evaluated by SDS-PAGE and Western blotting, as described above.
rgC1qR binding to immobilized protein A.
Microtiter wells (Corning Laboratory Sciences Co., Corning, N.Y.) were coated (1 h, 37°C) with 60 μl of BSA or protein A (1 μg/ml) in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6). After being blocked (1 h, 37°C) with 200 μl of 1% (wt/vol) fat-free milk in TBS, pH 7.5, wells were incubated with biotinylated gC1qR (1 h, 37°C). Bound biotinylated gC1qR (2 μg/ml) was detected with 60 μl of AP-STRAV (1:1,000 dilution in 0.1% BSA in TBS) and pNPP substrate in 10% diethylamine (1 mg/ml). Color development was measured at dual wavelengths of 405 and 490 nm. Wells were washed between all incubation steps with TBS containing 0.05% Tween 20. The effect of soluble protein A on biotinylated gC1qR binding to immobilized protein A was examined by preincubating (1 h, 37°C) biotinylated gC1qR with increasing concentrations of soluble protein before exposure to microtiter well-immobilized protein A. Biotinylated gC1qR was preincubated also with either the protein A-bearing Cowan I strain or the protein A-deficient Wood 46 strain (1% cell suspension) of S. aureus, and binding to purified, immobilized protein A was evaluated subsequently, as described above. To determine the effect of unlabeled gC1qR or rabbit IgG on gC1qR-protein A interactions, protein A-coated microtiter wells were preincubated (1 h, 37°C) with the soluble competitor before the addition of biotinylated gC1qR. BSA was used throughout as a nonspecific protein competitor control.
Protein A binding to immobilized platelets.
Washed human platelets from outdated concentrates were immobilized and spread on microtiter wells and fixed with glutaraldehyde as described previously (29). The binding of biotinylated protein A was measured after a 60-min incubation at 37°C by enzyme-linked immunosorbent assay using AP-STRAV and pNPP substrate. The reaction was quantified spectrophotometrically at 405 and 490 nm. The specificity of protein A binding was assessed in the presence or absence of unlabeled protein A, rgC1qR (MF), or anti-gC1qR polyclonal antibody F(ab′)2 fragments. BSA was used as a nonspecific protein competitor.
Further studies were performed to evaluate the ability of soluble protein A to inhibit the binding of biotinylated bacterial suspensions of Cowan I and Wood 46 strains of S. aureus to immobilized platelets. For these experiments, binding of biotinylated strains of S. aureus (1% cell suspensions) was examined in the presence of TBS or soluble protein A (0.25 mg/ml), as described above.
RESULTS
gC1qR capture by solid-phase protein A.
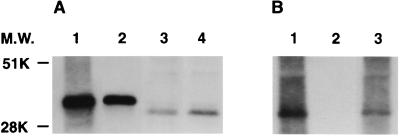
The interaction between gC1qR and protein A was first observed in experiments demonstrating platelet gC1qR capture by protein A-agarose or by formalin-fixed suspensions of S. aureus (Cowan I). Figure 1 demonstrates the ability of both protein A-agarose and fixed suspensions of S. aureus (Cowan I) to capture gC1qR from Triton X-100-lysed platelets as well as from solutions of purified rgC1qR. Captured gC1qR was visualized by Western blot analysis using a polyclonal rabbit anti-gC1qR antibody. In Fig. 1, the relative mobility of platelet-derived gC1qR appears faster than that of the recombinant protein. Although this was not consistently observed, it likely represents proteolytic degradation of gC1qR by platelet proteases during the experimental period. In addition to that of gC1qR, a number of fainter protein bands can be seen in samples precipitated with intact S. aureus (Cowan I), compared to the results of protein A-agarose precipitation. These bands likely represent other platelet adhesive proteins recognizing S. aureus (2, 14–17).
FIG. 1.
Capture of platelet and recombinant gC1qR by solid-phase protein A in the form of protein A-agarose (A) or suspensions of formalin-fixed S. aureus (Cowan I) (B). Platelet suspensions solubilized in Triton X-100 or rgC1qR were incubated with protein A-agarose or a fixed suspension of S. aureus (Cowan I; 15 h, 4°C). After extensive washing, bound material was eluted with SDS-sample buffer and examined by SDS-PAGE and Western blotting using a polyclonal anti-gC1qR antibody detected using enhanced chemiluminescence. Lanes 2 and 3 of panel A depict eluted rgC1qR and platelet gC1qR, respectively; rgC1qR and platelet starting material are shown in lanes 1 and 4. Lane 3 of panel B depicts platelet gC1qR eluted from suspensions of S. aureus (Cowan I); the corresponding whole platelet lysate starting material is shown in lane 1, and lane 2 depicts a control eluate from S. aureus (Cowan I) suspensions processed without exposure to platelets. M.W., molecular weight.
Binding of biotinylated rgC1qR to immobilized protein A.
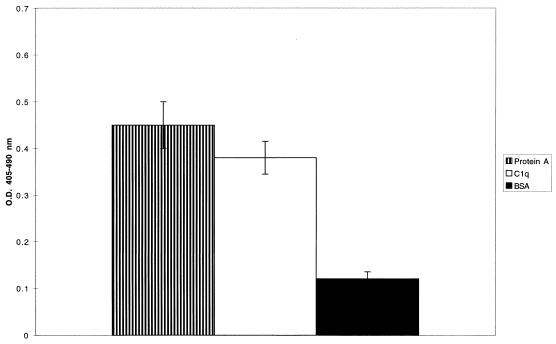
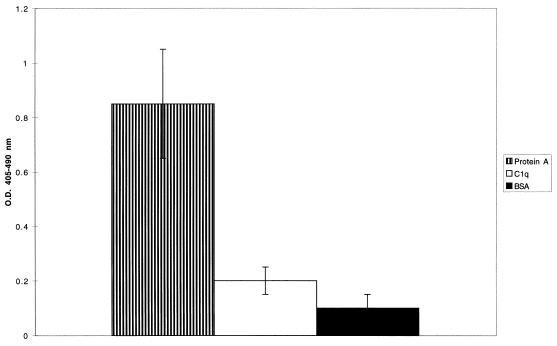
To characterize the interaction between gC1qR and protein A, further studies focused on biotinylated rgC1qR binding to purified protein A, immobilized on microtiter plates. Figure 2 illustrates the binding of biotinylated rgC1qR to immobilized protein A. Protein A binding to immobilized C1q and BSA is presented for comparison as positive and negative controls, respectively. In addition, a truncated form [rgC1qR (TF)] lacking amino-terminal amino acids 74 to 95 also bound to immobilized protein A (Fig. 3). Whereas the binding of gC1qR (TF) to protein A appears increased in comparison to that of gC1qR (MF), its binding to C1q was markedly diminished, as reported previously (10).
FIG. 2.
Binding of biotinylated, full-length rgC1qR (MF) to immobilized protein A. Biotinylated rgC1qR (∼2 μg/ml) was incubated (60 min, 37°C) on protein A-coated microtiter wells. Binding was detected with AP-STRAV and pNPP substrate. C1q- and BSA-coated microtiter wells served as controls. Values represent means ± SD; n = 3. O.D., optical density.
FIG. 3.
Binding of the truncated rgC1qR (TF), lacking amino-terminal residues 74 to 95, to immobilized staphylococcal protein A. Biotinylated rgC1qR (TF) was incubated on protein A-coated microtiter wells for 1 h at 37°C. Binding was detected with AP-STRAV using pNPP substrate. C1q- and BSA-coated microtiter wells served as controls. Values represent means ± SD; n = 6. O.D., optical density.
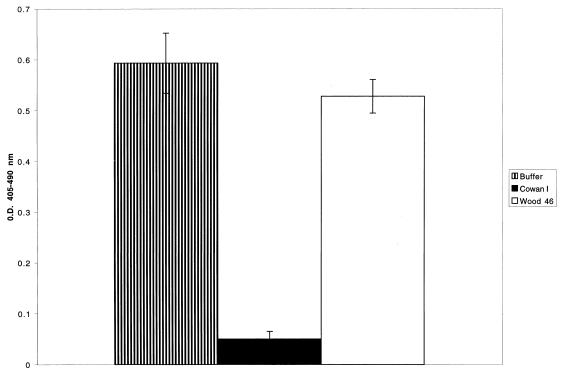
To assess the specificity of rgC1qR-protein A interactions, biotinylated rgC1qR was preincubated with increasing concentrations of soluble protein A. The results are summarized in Table 1. Soluble protein A inhibited the binding of 2 μg of biotinylated rgC1qR/ml with a 50% inhibitory concentration of (3.3 ± 0.7) × 10−7 M (n = 3). BSA was used as a nonspecific protein competitor, and at the highest concentration (80 μg/ml), it reduced biotinylated rgC1qR binding by no more than 20%. In other experiments, immobilized protein A was pretreated with rgC1qR (MF) before exposure to biotinylated rgC1qR. The dose-dependent inhibition of biotinylated ligand binding is illustrated in Table 1. Additional experiments demonstrate the preferential binding of rgC1qR to the protein A-bearing S. aureus Cowan I strain compared to the degree of binding to the Wood 46 strain, which lacks protein A (Fig. 4).
TABLE 1.
Specificity of biotinylated rgC1qR binding to immobilized protein Aa
| Inhibitor/competitor | Dose (μg/ml) | Binding (%) |
|---|---|---|
| None | 100 | |
| Protein A | 2 | 60 ± 17 |
| 6 | 35 ± 9 | |
| 10 | 17 ± 11 | |
| 20 | 8 ± 5 | |
| rgC1qR | 10 | 66 ± 7 |
| 20 | 31 ± 11 | |
| 40 | 20 ± 6 | |
| 80 | 7 ± 5 |
Protein A, immobilized on microtiter wells, was exposed to biotinylated full-length rgC1qR (MF) in the presence or absence of increasing concentrations of soluble protein A or nonbiotinylated rgC1qR. Binding was quantified after a 60-min incubation (37°C). Background rgC1qR binding to immobilized BSA was subtracted from all results. The percent binding of rgC1qR relative to rgC1qR binding in the presence of buffer was calculated. The data represent means ± SD; n = 4.
FIG. 4.
Inhibition of biotinylated gC1qR binding to immobilized protein A by S. aureus Cowan I and Wood 46 strains. Biotinylated rgC1qR (∼2 μg/ml) was incubated (60 min, 37°C) on protein A-coated microtiter wells in the presence or absence of fixed Cowan I or Wood 46 strains of S. aureus (1% cell suspension). Binding was detected with AP-STRAV and pNPP substrate. Values represent means ± SD; n = 3. O.D., optical density.
To evaluate the role of protein A Fc and Fab binding characteristics in gC1qR recognition, studies examined the effect of rabbit IgG on biotinylated rgC1qR binding to immobilized protein A. Preincubation of protein A-coated microtiter wells with increasing concentrations of rabbit IgG inhibited both biotinylated rgC1qR (MF) and rgC1qR (TF) binding in a dose-dependent manner (Table 2). Control wells, preincubated with BSA instead of soluble IgG, showed no significant inhibition (15 ± 6% [mean ± standard deviation {SD}]; n = 3).
TABLE 2.
Inhibition of biotinylated rgC1qR binding to immobilized staphylococcal protein A by rabbit IgGa
| IgG concn (μg/ml) | Inhibition (%) of binding of:
|
|
|---|---|---|
| rgC1qR (MF) | rgC1qR (TF) | |
| 100 | 81 ± 12 | 77 ± 15 |
| 50 | 77 ± 13 | 64 ± 16 |
| 10 | 49 ± 12 | 31 ± 12 |
| 1 | 15 ± 13 | 14 ± 10 |
Protein A, immobilized on microtiter wells, was exposed to increasing concentrations of rabbit IgG for 60 min at 37°C. Biotinylated full-length rgC1qR (MF) or a truncated form lacking N-terminal amino acids 74 to 95 [rgC1qR (TF)] was added subsequently, and binding was quantified after a further 60-min incubation (37°C). Background rgC1qR binding to immobilized BSA was subtracted from all results. The percent inhibition of rgC1qR binding relative to rgC1qR binding to protein A preincubated with buffer instead of IgG was calculated. The data represent means ± SD; n = 3.
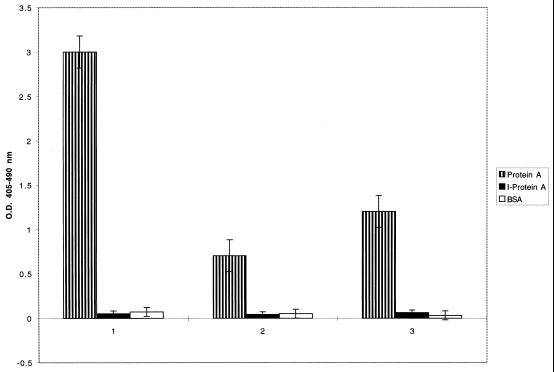
Further studies were performed to compare biotinylated rgC1qR binding to native protein A and protein A whose Ig Fc binding domain had been modified by hyperiodination (35). As shown in Fig. 5, inactivation of tyrosil residues of protein A by hyperiodination resulted in loss of rgC1qR binding. Protein A hyperiodination was verified by demonstrating inhibition of rabbit IgG binding, which occurs exclusively via the IgG Fc domain (35). Microtiter wells coated with BSA were used to assess nonspecific background reactivity.
FIG. 5.
Comparison of biotinylated rgC1qR (MF) (series 2) and rgC1qR (TF) (series 3) binding to microtiter well-immobilized staphylococcal protein A (Protein A) and hyperiodinated protein A (I-Protein A). Biotinylated ligand binding was quantified after 1 h at 37°C using AP-STRAV and pNPP substrate. Biotinylated rabbit IgG binding (series 1) is shown to demonstrate successful protein A hyperiodination. BSA-coated microtiter wells were used to determine background reactivity. Values represent means ± SD; n = 3. O.D., optical density.
Protein A binding to immobilized platelets.
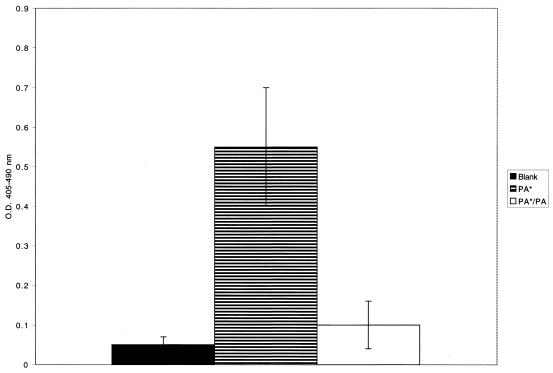
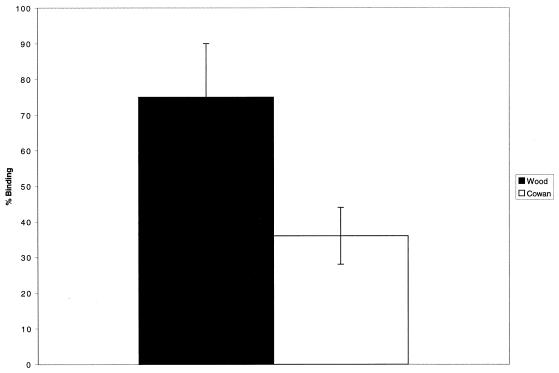
Figure 6 depicts biotinylated protein A binding to fixed, adherent platelets. This binding was inhibited significantly by the presence of excess soluble protein A. Binding was also inhibited by rgC1qR or anti-gC1qR antibody F(ab′)2 fragments (Table 3). Whereas both Cowan I and Wood 46 strains of S. aureus adhered to immobilized platelets, excess soluble protein A preferentially blocked the adhesion of the Cowan I strain (Fig. 7).
FIG. 6.
Biotinylated protein A (PA*) binding to immobilized platelets. Platelets were immobilized on poly-l-lysine-treated microtiter wells and fixed with glutaraldehyde. Biotinylated protein A binding was evaluated after 60 min at 37°C, using AP-STRAV and pNPP substrate. Biotinylated protein A binding was inhibited by unlabeled protein A (10 μg/ml) (PA). Platelet-coated microtiter wells, incubated with buffer instead of biotinylated protein A, were used to assess background reactivity (Blank). Values represent means ± SD; n = 3. O.D., optical density.
TABLE 3.
Inhibition of biotinylated protein A binding to immobilized plateletsa
| Competitor | % Binding |
|---|---|
| Buffer | 100 |
| Anti-gC1qR antibody | 38 ± 9 |
| rgC1qR | 30 ± 12 |
| BSA | 98 ± 16 |
Inhibition of biotinylated protein A binding to immobilized, fixed platelets by anti-gC1qR antibody F(ab′)2 fragments or excess rgC1qR. Platelet-coated microtiter wells were preincubated with 100 μg of anti-gC1qR antibody F(ab′)2 fragments/ml for 60 min at 37°C. Biotinylated protein A was added subsequently, and binding was evaluated using AP-STRAV and pNPP substrate. Parallel microtiter wells were exposed to buffer (0.01 M phosphate-buffered 0.14 M NaCl [pH 7.5]–0.1% NaN3) instead of antibody or biotinylated protein A preincubated for 60 min at 37°C with 90 μg of rgC1qR/ml or BSA. Biotinylated protein A binding in the presence of inhibitors is expressed as a percentage relative to binding in 0.01 M Tris-buffered 0.15 M NaCl. Values are means ± SD; n = 3.
FIG. 7.
Inhibition of biotinylated S. aureus Cowan I and Wood 46 strain (1% cell suspension) adhesion to immobilized, glutaraldehyde-fixed platelets by soluble protein A (0.25 mg/ml). Bacterial cell adhesion was evaluated after 60 min at 37°C, using AP-STRAV and pNPP substrate. The data summarize bacterial cell binding to platelets in the presence of protein A relative to binding in the absence of protein A. Values represent means ± SD; n = 3.
DISCUSSION
S. aureus is a virulent pathogen, responsible for community- and hospital-acquired infections. Infections caused by S. aureus are associated with significant morbidity and mortality. Given the increasing resistance of S. aureus to antibiotics, the prevalence of S. aureus infections will likely continue to rise (42). Specific bacterial and host factors combine to allow the organism to avoid immune surveillance and act as adhesins (9). Protein A may be considered one such virulence factor (1, 9, 17).
The direct binding of S. aureus to platelets is a postulated central mechanism in the pathogenesis of infective endocarditis (39). Indeed, data from several sources (15, 27, 40) indicate that the direct binding of S. aureus to platelets is a major determinant of virulence in the pathogenesis of endocarditis, particularly for pathogenic events occurring after the initial colonization of the valve surface, such as vegetation formation and septic embolization (37). The molecular basis for microbial binding to platelets, however, is unclear.
Fibrinogen and fibrin expressed on the platelet surface may play a role not only in bacterial cell adhesion (15) but also in S. aureus-induced platelet agglutination and aggregation (2, 14). S. aureus also recognizes a variety of other extracellular matrix proteins, including fibronectin, collagen, vitronectin, laminin, and thrombospondin (17), which may be expressed on the surfaces of adherent or aggregated platelets at the sites of vascular lesions. In addition, von Willebrand factor located in the extracellular matrix (14, 16) has been implicated in S. aureus colonization of the subendothelium.
Thus, mechanisms contributing to the adhesion of S. aureus to platelets are complex and multimodal. Quantitative analyses of S. aureus binding to platelets using flow cytometry suggest the involvement of carbohydrate-rich and platelet microbicidal proteins as well as platelet Fc receptors (14, 44). Earlier studies further suggested an indirect role for S. aureus protein A in microbially induced human platelet aggregation in plasma requiring IgG and platelet Fc receptors (14). Results from the present study describe a novel interaction between staphylococcal protein A and gC1qR, a ubiquitously expressed cellular protein (10, 11). The interaction between immobilized protein A and gC1qR is specific and can be inhibited by both unlabeled gC1qR and soluble protein A. Studies using an rgC1qR mutant lacking N-terminal amino acid residues 74 to 95 indicate that the protein A binding site is likely to reside outside of the gC1qR N-terminal alpha-helical domain responsible in part for C1q binding (10). In fact, consistently increased binding of gC1qR (TF) compared to that of gC1qR (MF) was observed. Whether this represents increased biotinylation of gC1qR (TF) relative to gC1qR (MF) or conformational rearrangements remains to be determined.
Staphylococcal protein A is a 45-kDa bacterial membrane protein that can interact with either the Fcγ domain of IgG or the Fab domain, which also mediates conventional antigen binding of the VH3+ family of Ig heavy-chain variable gene products (20, 35, 36). Recent studies suggest that all individual domains of staphylococcal protein A (E, D, A, B, and C) bind both IgG Fc fragments and human Fab (20). Nevertheless, inactivation of tyrosil residues of protein A results in selective loss of Fcγ binding, thus implying distinct structural requirements for Fc and Fab binding (35). In the present study, inactivation of protein A tyrosil residues by hyperiodination not only abolished rabbit IgG binding, which occurs exclusively via the immunoglobulin Fc domain (35), as expected, but also abrogated rgC1qR binding. These findings suggest similar structural requirements for gC1qR and IgG Fc binding to protein A.
Although gC1qR is a predominantly intracellular protein (5, 26), it is also expressed on the cell surface (4, 11, 30) and has been detected extracellularly in cell culture medium, as well as in body fluids including plasma, serum, cerebrospinal fluid, saliva, and tears (31). Thus, staphylococcal protein A interactions with gC1qR may serve to localize S. aureus microorganisms to cell surfaces. Furthermore, inhibition of gC1qR binding to protein A by IgG, whether competitive or mediated by steric hindrance effects, suggests a mechanism for preventing protein A interactions with IgG and offers a potential survival advantage for the organism by providing protection from host defenses.
Whereas previous studies implicated protein A in the adherence of S. aureus to human mesothelial cell monolayers (32), a direct role for protein A in platelet interactions with S. aureus has not been recognized heretofore (41, 44). Since gC1qR is not readily expressed on platelets in suspension (29), it is likely that studies examining S. aureus interactions with platelet suspensions would have missed contributions by protein A. In the present work, platelet interactions with protein A were demonstrated after platelet immobilization and spreading on poly-l-lysine to induce surface gC1qR expression (29). Participation of gC1qR was confirmed by studies showing that both fluid-phase gC1qR and anti-gC1qR antibody F(ab′)2 fragments were inhibitory. Moreover, gC1qR interaction with intact microorganisms is supported by studies demonstrating the predilection of gC1qR for the protein A-bearing Cowan I strain of S. aureus over the protein A-deficient Wood 46 strain.
Additional studies performed with biotinylated Cowan I and Wood 46 strains of S. aureus demonstrate the preferential inhibition of Cowan I adhesion to immobilized platelets by exogenous, soluble protein A. Inhibition of adhesion, however, was incomplete, likely due to redundant adhesive mechanisms (2, 14–17). Such mechanisms are also likely to contribute to the adhesion of the protein A-deficient Wood 46 strain of S. aureus to platelets. Indeed, SDS-PAGE analysis of solid-phase precipitation experiments of whole, lysed platelets with intact S. aureus (Cowan I) demonstrates a number other protein bands in addition to the prominent gC1qR band.
Interestingly, adhesion of the Wood 46 strain to platelets was also partially inhibited by exogenous protein A. However, protein A-induced inhibition of Cowan I strain adhesion to platelets consistently exceeded inhibition of Wood 46 strain adhesion by at least twofold. These results may suggest steric inhibition of Wood 46 binding to the platelet surface in the presence of protein A and/or the ability of gC1qR to recognize additional S. aureus proteins. The latter hypothesis may be supported by the slight but consistently observed inhibition of biotinylated gC1qR binding that occurred in the presence of S. aureus Wood 46.
Since platelets adhere and become activated on an abnormal cardiac valve endothelium (15, 27, 39, 40), data from the present study support the hypothesis that protein A interactions with exposed platelet gC1qR may contribute to bacterial cell adhesion and localization to sites of vascular injury and thrombosis. Interactions between native gC1qR and bacterial surface protein A are supported by solid-phase platelet gC1qR precipitation studies using formalin-fixed S. aureus Cowan I, studies illustrating inhibition of protein A binding to intact platelets by anti-gC1qR antibodies, and experiments showing the preferential inhibition of the binding of the protein A-expressing Cowan I strain of S. aureus to platelets by soluble protein A.
In conclusion, S. aureus is an important human pathogen which has been described as interacting with platelets (15, 27, 39, 40). The adherence of bacteria to platelets at the surface of an abnormal valvular endothelium may constitute the primary event for localizing S. aureus to sites of vascular injury and thrombosis. The present study presents evidence for a novel recognition mechanism between S. aureus and human blood platelets involving staphylococcal protein A and the platelet gC1qR, a 33-kDa ubiquitously distributed cell protein originally identified for its ability to interact with the globular head domain of C1q (11). In vitro evidence suggests that gC1qR would be expressed on platelets following adhesion to sites of vascular injury and inflammation (30). In addition, gC1qR is expressed by vascular endothelial cells, and expression is upregulated by inflammatory cytokines (12). Thus, gC1qR may serve as an additional potential binding site for microbial interaction with the vasculature. Moreover, the observed inhibition of protein A binding to platelets by fluid-phase rgC1qR may suggest a new avenue for therapeutic intervention.
ACKNOWLEDGMENTS
This work was supported in part by grant HL 50291 from the National Institutes of Health, National Heart, Lung, and Blood Institute (E.I.B.P. and B.G.), and grant IM771 (B.G.) from the American Cancer Society.
We are grateful to Tara Murphy for technical assistance.
REFERENCES
- 1.Archer G L. Staphylococcus aureus: a well armed pathogen. Clin Infect Dis. 1998;26:1179–1181. doi: 10.1086/520289. [DOI] [PubMed] [Google Scholar]
- 2.Bayer A S, Sullam P M, Ramos M, Li C, Cheung A L, Yeaman M R. Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect Immun. 1995;63:3634–3641. doi: 10.1128/iai.63.9.3634-3641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das S, Deb T B, Kumar R, Datta K. Multifunctional activities of human fibroblast 34-kDa hyaluronic acid binding protein. Gene. 1997;190:223–225. doi: 10.1016/s0378-1119(97)00035-8. [DOI] [PubMed] [Google Scholar]
- 4.Deb T, Datta K. Molecular cloning of human fibroblast hyaluronic acid binding protein confirms its identity with p32, a protein co-purified with splicing factor SF-2. J Biol Chem. 1996;271:2206–2212. doi: 10.1074/jbc.271.4.2206. [DOI] [PubMed] [Google Scholar]
- 5.Dedio J, Jahnen-Dechent W, Bachmann M, Muller-Esterl W. The multiligand-binding protein gC1qR, putative C1q receptor, is a mitochondrial protein. J Immunol. 1998;160:3534–3542. [PubMed] [Google Scholar]
- 6.Deisenhofer J. Crystallographic refinement and atomic models of human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- 7.Easmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. 1 and 2. London, United Kingdom: Academic Press, Ltd.; 1983. [Google Scholar]
- 8.Fosgren A, Ghetie V, Lindmar R, Sjoquist J. Protein A and its exploitation. In: Easmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. Vol. 2. London, United Kingdom: Academic Press, Ltd.; 1983. pp. 429–478. [Google Scholar]
- 9.Foster T J, McDevitt D. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol Lett. 1994;118:199–205. doi: 10.1111/j.1574-6968.1994.tb06828.x. [DOI] [PubMed] [Google Scholar]
- 10.Ghebrehiwet B, Lim B L, Peerschke E I B, Willis A C, Reid K B M. Isolation cDNA cloning, and overexpression of a 33-kDa cell surface glycoprotein that binds to the globular ‘heads’ of C1q. J Exp Med. 1994;179:1809–1821. doi: 10.1084/jem.179.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghebrehiwet B, Peerschke E I B. Structure and function of gC1q-R; a multiligand binding cellular protein. Immunobiology. 1998;199:225–238. doi: 10.1016/S0171-2985(98)80029-6. [DOI] [PubMed] [Google Scholar]
- 12.Guo W-X, Ghebrehiwet B, Weksler B, Schweitzer K, Peerschke E I B. Upregulation of endothelial cell binding proteins/receptors for complement component C1q by inflammatory cytokines. J Lab Clin Med. 1999;133:541–550. doi: 10.1016/s0022-2143(99)90183-x. [DOI] [PubMed] [Google Scholar]
- 13.Harboe M, Foelling I. Recognition of two distinct groups of human IgM and IgA based on different binding to staphylococci. Scand J Immunol. 1974;3:471–482. doi: 10.1111/j.1365-3083.1974.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 14.Hawiger J, Steckley S, Hammond D, Cheng C. Staphylococci-induced human platelet injury mediated by protein A and immunoglobulin G Fc fragment receptor. J Clin Investig. 1979;64:931–937. doi: 10.1172/JCI109559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann M, Lai Q J, Albrecht R M, Mosher D F, Proctor R A. Adhesion of Staphylococcus aureus to surface-bound platelets: role of fibrinogen/fibrin and platelet integrins. J Infect Dis. 1993;167:312–322. doi: 10.1093/infdis/167.2.312. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann M, Hartleib J, Kehrel B, Montgomery R R, Sixma J J, Peters G. Interaction of von Willebrand factor with Staphylococcus aureus. J Infect Dis. 1997;176:984–991. doi: 10.1086/516502. [DOI] [PubMed] [Google Scholar]
- 17.Hogevik H, Soderquist B, Tung H S, Olaison L, Westberg A, Ryden C, Tarkowski A, Andersson R. Virulence factors of Staphylococcus aureus strains causing infective endocarditis—a comparison with strains from skin infections. APMIS. 1998;106:901–908. [PubMed] [Google Scholar]
- 18.Honore B, Madsen P, Rasmusen H H, Vandekerckhove J, Celis J E, Lefferts H. Cloning and expression of a cDNA covering the complete coding sequence of p32 subunit of human pre-mRNA splicing factor SF-2. Gene. 1993;134:283–287. doi: 10.1016/0378-1119(93)90108-f. [DOI] [PubMed] [Google Scholar]
- 19.Inganas M. Comparison of mechanisms of interaction between protein A from Staphylococcus aureus and human monoclonal IgG, IgA, and IgM in relation to the classical Fc and the alternative F(ab′)2 protein A epsilon interactions. Scand J Immunol. 1981;13:343–352. doi: 10.1111/j.1365-3083.1981.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 20.Jansson B, Uhlen M, Nygren P A. All individual domains of staphylococcal protein A show Fab binding. FEMS Immunol Med Microbiol. 1998;20:69–78. doi: 10.1111/j.1574-695X.1998.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Zhang Y, Krainer A R, Xu R M. Crystal structure of human p32, a doughnut shaped acidic mitochondrial matrix protein. Proc Natl Acad Sci USA. 1999;96:3572–3577. doi: 10.1073/pnas.96.7.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krainer A R, Mayeda A, Kozak D, Binns G. Functional expression of cloned human splicing factor SF-2: homology to RNA binding proteins, U170k, and drosophila splicing regulators. Cell. 1991;66:383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Langone J J. Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- 25.Luo Y, Yu H, Peterlin B M. Cellular protein modulates effects of human immunodeficiency virus type 1. Rev J Virol. 1994;68:3850–3856. doi: 10.1128/jvi.68.6.3850-3856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muta T, Kang D, Kitajima S, Fujiwara T, Hamasaki N. p32 protein, a splicing factor 2-associated protein, is localized in mitochondria matrix and is functionally important in maintaining oxidative phosphorylation. J Biol Chem. 1997;272:24363–24370. doi: 10.1074/jbc.272.39.24363. [DOI] [PubMed] [Google Scholar]
- 27.Nicolau D P, Freeman C D, Nightingale C H, Quintiliani R, Coe C J, Maderazo E G, Cooper B W. Reduction of bacterial titers by low-dose aspirin in experimental aortic valve endocarditis. Infect Immun. 1993;61:1593–1595. doi: 10.1128/iai.61.4.1593-1595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel P C, Stefanescu-Soare I, Menezes J. Staphylococcal protein A enhances natural killing activity against lymphoid tumor cell lines. Int J Cancer. 1981;28:277–284. doi: 10.1002/ijc.2910280305. [DOI] [PubMed] [Google Scholar]
- 29.Peerschke E I B, Reid K B M, Ghebrehiwet B. Identification of a novel 33-kDa C1q-binding site on human blood platelets. J Immunol. 1994;152:5896–5901. [PubMed] [Google Scholar]
- 30.Peerschke E I B, Ghebrehiwet B. Platelet receptors for the complement component C1q: implications for hemostasis and thrombosis. Immunobiology. 1998;199:239–249. doi: 10.1016/S0171-2985(98)80030-2. [DOI] [PubMed] [Google Scholar]
- 31.Peterson K L, Zhang W, Lu P D, Keilbaugh S A, Peerschke E I B, Ghebrehiwet B. The C1q-binding cell membrane proteins cC1q-R and gC1q-R are released from activated cells: subcellular distribution and immunochemical characterization. Clin Immunol Immunopathol. 1997;84:17–26. doi: 10.1006/clin.1997.4374. [DOI] [PubMed] [Google Scholar]
- 32.Poston S M, Glancey G R, Wyatt J E, Hogan T, Foster T J. Co-elimination of mec and spa genes in Staphylococcus aureus and the effect of agr and protein A production on bacterial adherence to cell monolayers. J Med Microbiol. 1993;39:422–428. doi: 10.1099/00222615-39-6-422. [DOI] [PubMed] [Google Scholar]
- 33.Rao C M, Deb T B, Gupta S, Datta K. Regulation of cellular phosphorylation of hyaluronan binding protein and its role in the formation of second messenger. Biochim Biophys Acta. 1997;1336:387–393. doi: 10.1016/s0304-4165(97)00049-4. [DOI] [PubMed] [Google Scholar]
- 34.Ringden O. Induction of immunoglobulin secretion by protein A from Staphylococcus aureus in human blood and bone marrow B cells. Scand J Immunol. 1985;22:17–26. doi: 10.1111/j.1365-3083.1985.tb01855.x. [DOI] [PubMed] [Google Scholar]
- 35.Romagnani S, Giudizi M G, Del Prete G, Maggi E, Biagiotti R, Almerigogna F, Ricci M. Demonstration on protein A of two distinct immunoglobulin binding sites and their role in the mitogenic activity of Staphylococcus aureus Cowan I on human B cells. J Immunol. 1982;129:596–602. [PubMed] [Google Scholar]
- 36.Sasso E H, Silverman G J, Mannik M. Human IgA and IgG F(ab′)2 that bind to staphylococcal protein A belong to the VHIII subgroup. J Immunol. 1991;147:1877–1883. [PubMed] [Google Scholar]
- 37.Scheld W M, Zak O, Vosbeck K, Sande M A. Bacterial adhesion in the pathogenesis of infective endocarditis: interaction of bacterial dextran, platelets and fibrin. J Clin Investig. 1978;61:1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stalenheim G, Gotze O, Cooper N R, Sjoquist J, Muller-Eberhard H J. Consumption of human complement components by complexes of IgG with protein A of Staphylococcus aureus. Immunochemistry. 1973;10:501–507. doi: 10.1016/0019-2791(73)90221-8. [DOI] [PubMed] [Google Scholar]
- 39.Sullam P M. Host-pathogen interactions in the development of bacterial endocarditis. Curr Opin Infect Dis. 1994;7:304–309. [Google Scholar]
- 40.Sullam P M, Bayer A S, Foss W M, Cheung A L. Diminished platelet binding in vitro by Staphylococcus aureus is associated with reduced virulence in a rabbit model of infective endocarditis. Infect Immun. 1996;64:4915–4921. doi: 10.1128/iai.64.12.4915-4921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usui Y, Ohshima Y, Ichiman Y, Ohtomo T, Shimada H. Some biochemical properties of the components of Staphylococcus aureus binding to human platelets. Zentbl Bakteriol. 1997;286:56–62. doi: 10.1016/s0934-8840(97)80075-8. [DOI] [PubMed] [Google Scholar]
- 42.Voss A, Doebbeling B N. The worldwide prevalence of methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 1995;5:101–106. doi: 10.1016/0924-8579(94)00036-t. [DOI] [PubMed] [Google Scholar]
- 43.Yeaman M R, Norman D C, Bayer A S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeaman M R, Sullam P M, Dazin P F, Norman D C, Bayer A S. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J Infect Dis. 1992;166:65–73. doi: 10.1093/infdis/166.1.65. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, Loewenstein P M, Zhang Z, Green M. In vitro interaction of the human immunodeficiency virus type 1 Tat transactivator and the general transcription factor TFIIB with the cellular protein TAP. J Virol. 1995;69:3017–3023. doi: 10.1128/jvi.69.5.3017-3023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]