Abstract
Histoplasma capsulatum induces a cell-mediated immune response in lungs and lymphoid organs of mammals. Resolution of primary infection in mice depends on interleukin-12 (IL-12), since neutralization of this monokine increases susceptibility to infection. The present study was designed to determine if blockade of IL-12 disrupts the protective immune response by altering the influx of lineage-specific cells into infected lungs and the numbers of cells expressing CD80, CD86, CD119, and major histocompatibility complex class II (MHC II) molecules. In mice given anti-IL-12, there was a 2.5-fold decrease in total numbers of T cells on days 3 to 10 of infection and a 4-fold increase in Mac-1/Gr-1+ cells on days 7 and 10 compared to infected controls. CD80+ lung cells from anti-IL-12-treated mice were 2- to 3-fold greater than those from controls on days 7 and 10, whereas the total numbers of CD86+ cells were 2- to 3-fold less and MHC II+ cells were 1.5- to 2-fold less on days 3 and 5. Cells expressing CD119 were reduced 1.5-fold on day 5. Treatment with monoclonal antibodies (MAb) to CD80, CD86, or both reduced the fungal burden slightly compared to that in rat immunoglobulin G-treated controls, whereas after IL-12 neutralization, blocking of CD80 reduced the tissue burden by 2.5-fold and this correlated with a decrease in IL-4. Regardless, mortality was not altered by treatment with MAb to CD80 or CD86. We conclude that (i) IL-12 neutralization alters the nature of the inflammatory response in lungs and the expression of CD80 and CD86 on lineage-specific cells, (ii) the immune response during infection with H. capsulatum is controlled via mechanisms independent of the CD80 and CD86 costimulatory pathways, and (iii) decreased expression of CD86 and MHC II may modulate generation of optimal protective immunity.
Histoplasma capsulatum is an intracellular pathogenic fungus that is responsible for mild disease in immunocompetent hosts and a progressive and fatal disease if untreated in immunocompromised hosts (7). The initial site of infection is the lung, where yeast cells, produced from inhaled microconidia, are ingested by alveolar macrophages (Mφ) via an interaction between the CD11/CD18 family of adhesion molecules and yeast cell wall components (4). Phagocytosis of yeast cells by Mφ results in a permissive environment for survival and replication of yeasts. Resistance to H. capsulatum infection in mammals is primarily dependent on a cellular immune response mediated by T cells and phagocytes. Resolution of infection in mice requires the production of cytokines, especially gamma interferon (IFN-γ) (1, 30, 33), and release of this cytokine by NK and T cells is dependent on the pathogen-induced release of the monokine interleukin-12 (IL-12) (26). H. capsulatum infection of mice with a genetic absence of IFN-γ or those given antibodies (Ab) to IL-12 results in an uncontrollable and fatal fungal burden (1, 2, 33). IL-12 release is necessary for Mφ to kill yeasts before day 5 of infection, since animals depleted of IL-12 beyond this point survive the infection (1).
The purpose of this study was to determine if neutralization of IL-12 and subsequent IFN-γ depletion altered the expression of cell surface molecules involved in the generation of protective cell-mediated immunity. The molecules CD80, CD86, major histocompatibility complex class II (MHC II), and CD119 (IFN-γ receptor) were chosen for analysis because of their potential contribution to an effector cell-mediated immune response to H. capsulatum. We assessed if blockade of IL-12 altered the number of cells that express these molecules and their surface density.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and maintained in the animal facility at the University of Cincinnati. All experiments used animals that were 6 to 12 weeks of age. Control and infected mice were housed in laminar flow units. Athymic nude mice were purchased from the National Cancer Institute (Frederick, Md.).
Preparation of yeast cultures and i.n. infection of mice.
H. capsulatum yeast cells (strain G217B) were grown in 50 ml of Ham's F-12 medium supplemented with glucose (18.2 g/liter), glutamic acid (1 g/liter), HEPES (6 g/liter), and cysteine (8.4 mg/liter) for 48 h at 37°C. Cell suspensions were prepared by two washes with Hanks' balanced salt solution (HBSS) containing 0.2 M HEPES and 0.5% bovine serum albumin (BSA) followed by a third wash at 100 × g. The final volume was adjusted to equal 2.5 × 106 or 0.6 × 106 yeast cells per 50 μl of buffer. Mice anesthetized with Metophane (Pitman-Moore, Mundelein, Ill.) were infected intranasally (i.n.) with 50 μl of yeast suspension. Control animals were given equal volumes of buffer alone.
Enumeration of yeast burden in infected tissues.
Spleens and lungs from mice were removed at selected intervals during infection and homogenized in 10 ml HBSS, and 100-μl portions of serial dilutions were streaked onto 150-mm-diameter plates containing 3.7% brain heart infusion (Difco, Detroit, Mich.), 1% glucose, 0.01% cysteine hydrochloride (Sigma, St. Louis, Mo.), 2% agar (Difco), 5 mg of gentamicin (Sigma) per ml, and 5% defibrinated sheep erythrocytes (Colorado Serum Co., Denver, Colo.). Plates were incubated at 30°C, and CFU were enumerated after 7 days.
Ab and immunofluorescent reagents.
Fluorescein isothiocyanate (FITC)-conjugated monoclonal Ab (MAb) to CD45 (clone 30F11.1), CD45R/B220 (clone RA3-6B2), CD90.2 (Thy-1, clone 30-H12), and Ly-6G/Gr-1 (clone RB6-8C5); biotin-conjugated MAb to CD119 (IFN-γ receptor, clone GR20), CD86 (clone GL-1), and anti-mouse I-Ab (clone AF6-120.1); phycoerythrin-conjugated CD80 (clone IG-10); and streptavidin-phycoerythrin (SAv-PE) were purchased from Pharmingen (San Diego, Calif.). Ab produced by the anti-Mac-1 hybridoma (anti-CR3, clone M1/70) was purified from tissue culture supernatant with a protein G affinity column (Pharmacia, Piscataway, N.J.) and conjugated to FITC as previously reported (13).
Purification of Ab for depletion experiments.
Rat hybridomas producing MAb to murine CD80 (B7-1, clone IG-10) and CD86 (B7-2, clone GL-1) were purchased from the American Type Culture Collection, Rockville, Md. Purification was accomplished by passing tissue culture supernatants over an anti-bovine immunoglobulin (Ig) Sepharose column in tandem with a protein G (Pharmacia) affinity column, followed by elution with 0.1 M glycine-HCl, pH 2.8. After concentration, the Ab was dialyzed against HBSS and filter sterilized. Alternatively, hybridoma cells were injected into pristane-primed nude mice, and ascites fluid was collected and purified by the capryllic acid-ammonium sulfate method (16). Purified Ab was dialyzed against HBSS, filter sterilized, and stored at −20°C until use. Ab against murine IL-12 (clones 15.1 and 15.6) were prepared as previously described (1).
Treatment of mice with MAb.
For IL-12 neutralization studies, mice were injected intraperitoneally with a mixture of 250 μg each of MAb 15.1 and 15.6 1 day before infection and on days 1, 3, 7, and 14 of infection as previously described (1). For experiments designed to examine the effects of CD80 and CD86 blockade, animals were injected i.n. with 200 μg of either anti-CD80 or anti-CD86 or a mixture of both in a final volume of 50 μl 1 day before infection and on days 1, 3, 5, 7, 10, 14, and 17 of infection. This dose of MAb has been reported to block function (27).
Determination of cytokine levels in infected lungs.
Tissue homogenates or single-cell suspensions from lungs of infected animals used for CFU analyses were centrifuged at 700 × g for 10 min, and supernatants were collected and stored at −70°C until use. Enzyme-linked immunosorbent assay kits for the detection of IFN-γ, IL-4, granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor alpha (TNF-α) were purchased from Endogen (Woburn, Mass.), and analysis was performed according to the manufacturer's instructions.
Preparation of single-cell suspension from lung tissue.
Lungs from infected animals were removed on days 3, 5, 7, 10, 14, and 21 of infection, initially crushed with a 10-ml syringe plunger, teased apart with forceps, and suspended in RPMI medium containing glutamine (0.29 mg/ml), penicillin, streptomycin (100 U/ml, 100 mg/ml), and 10% fetal bovine serum. The organs were homogenized into single-cell suspensions by sequential passage through 16-, 18-, and 20-gauge needles. The mononuclear fraction was isolated by separation on 40 to 70% Percoll gradients (Pharmacia). For surface phenotyping, cells were resuspended in phosphate-buffered saline (pH 7.3) containing 1% BSA and 0.1% azide.
Cell surface phenotype.
Cells isolated from lungs were pelleted (1 × 105 to 5 × 105) at 350 × g and incubated with a saturating amount of Ab for 15 min at 4°C. Cells were washed twice with phosphate-buffered saline containing 1% BSA and 0.1% azide before addition of SAv-PE for biotinylated reagents followed by incubation and washing as before. For two-color analyses, cells were incubated with FITC-conjugated lineage-specific MAb, washed, and then incubated with phycoerythrin-conjugated CD80 or CD86-biotin and SAv-PE and then washed as before. All samples were resuspended in a 1% paraformaldehyde solution before analysis on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, Calif.). Flow cytometry data, reported as percent positive cells and mean fluorescence intensity (MFI), were determined with Cell Quest analysis software. Changes in the density of surface antigens can be determined by flow cytometry by measuring MFI values. In order to compare data from several experiments, these values were calculated as the fold increase in fluorescence intensity of the positive population over the MFI of the negative cells. This analysis corrects for variations in baseline values that fluctuate because of autofluorescence or nonspecific MAb interactions.
The absolute number of cells expressing each surface marker was calculated by multiplying the percent positive cells of each phenotype by the total number of cells derived from the Percoll gradients. Since cell types other than blood lineage cells were present within analysis gates that contained the total population of live cells, the data were normalized to represent hemopoietic lineage cells within the gate by multiplying by the percentage of CD45+ cells. This Ab (clone 30F11.1) recognizes all blood cells except erythrocytes (22).
Statistical analyses.
Student's t test was used to analyze differences in total numbers of cells expressing a certain phenotype, fungal burdens, and cytokine levels.
RESULTS
Enumeration of cells isolated from infected lungs.
The total cell numbers in lungs of anti-IL-12-treated mice that were infected with 2.5 × 106 yeasts were significantly greater than those in control animals on days 7 and 10 of infection (P ≤ 0.01 and 0.02, respectively) (Table 1). The total numbers of cells from anti-IL-12-treated animals after day 10 were not available, since 100% of these animals succumbed to infection and died before day 14. The mean survival time for these mice was 13.1 ± 0.9 days.
TABLE 1.
Total numbers of cells isolated from lungs of mice infected with H. capsulatum
| Day of infection | Total cell number (106)a in mice given:
|
||
|---|---|---|---|
| Rat IgG and 2.5 × 106 yeasts | Anti-IL-12 and 2.5 × 106 yeasts | Anti-IL-12 and 0.6 × 106 yeasts | |
| 0b | 1.79 ± 0.3 | —c | — |
| 3 | 8.50 ± 1.1 | 5.50 ± 0.8 | 2.06 ± 0.2 |
| 5 | 12.38 ± 1.3 | 7.70 ± 1.5 | 3.09 ± 0.6 |
| 7 | 17.57 ± 1.6 | 27.92 ± 4.2 | 7.62 ± 1.4 |
| 10 | 17.76 ± 1.4 | 28.12 ± 5.4 | 15.97 ± 4.9 |
| 14 | 16.19 ± 1.8 | — | 17.00 ± 3.1 |
| 21 | 10.78 ± 1.3 | — | 15.20 ± 1.1 |
Values are means ± standard errors from a minimum of five mice per group.
Data from noninfected animals.
—, not determined.
Analysis of cells expressing phenotypic markers in lungs of control and anti-IL-12-treated mice.
Lungs from infected mice treated with anti-IL-12 or rat IgG were removed on days 3, 5, 7, 10, 14, and 21 of infection for controls (rat IgG) or through day 10 for IL-12-neutralized animals and analyzed by flow cytometry for expression of surface markers. The lymphocyte populations of cells were reduced in numbers after IL-12 neutralization, whereas myeloid lineage cell numbers increased (Fig. 1). A comparison of the absolute numbers of T cells in lungs from rat Ig- and anti-IL-12-treated mice revealed that this population of cells was decreased 1.5- to 2.5-fold in the latter group. These reduced numbers of T cells were significantly different from those for rat IgG controls on all days analyzed (days 3, 5, 7, and 10; P ≤ 0.027, 0.008, 0.014, and 0.022, respectively). B cells also were significantly less after IL-12 neutralization on day 5 (2.5-fold; P ≤ 0.046) and day 7 (3-fold; P ≤ 0.046) (data not shown). After IL-12 neutralization a threefold increase in numbers of myeloid-lineage cells in lungs was observed on days 7 and 10 of infection (P ≤ 0.001 and P ≤ 0.033, respectively), as well as a fourfold increase in Gr-1+ cells on day 7 (P ≤ 0.008) and fivefold greater numbers on day 10 (P ≤ 0.05).
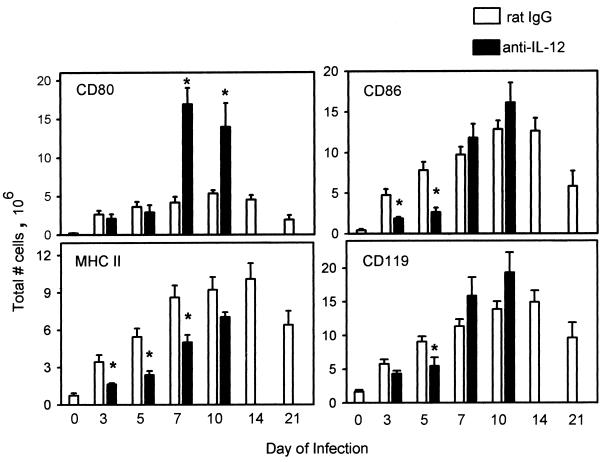
FIG. 1.
Analysis of the modulation of lineage-specific cells after IL-12 neutralization. Flow cytometry data for the percentages of positive cells were corrected for the percentage of CD45+ cells within the analysis gate. The data from two experiments were expressed as the mean of the total number of cells expressing each marker (± standard error) for five to nine animals per group. Asterisks indicate data that were significantly different for control and anti-IL-12-treated mice (P ≤ 0.05). Data for day 0 were from uninfected (naive) mice (n = 5).
The effect of IL-12 neutralization on the numbers of cells expressing the accessory molecules CD80 and CD86 differed in that the numbers of CD80+ cells increased on day 7 (3.5-fold; P ≤ 0.001) and day 10 (2.5-fold; P ≤ 0.004), whereas the numbers of CD86+ cells were depressed by approximately threefold on days 3 (P ≤ 0.01) and 5 (P ≤ 0.006) but were similar to control values by day 7 (Fig. 2).
FIG. 2.
Modulation of the number of lung cells expressing CD80, CD86, MHC II, and CD119 after administration of anti-IL-12. The total number of cells was calculated from the percent-positive data for each marker on days 3, 5, 7, 10, 14, and 21. Data for day 0 were from uninfected (naive) mice (n = 5). The data from two experiments were expressed as the mean ± standard error for five to nine mice per group. Asterisks indicate data that were significantly different for control and anti-IL-12-treated mice as determined by Student's t test (P ≤ 0.05).
The absolute numbers of CD119+ cells were similar in lungs of control and IL-12-depleted animals, except on day 5, when the latter group displayed a significant decrease of 1.5-fold (P ≤ 0.024). The number of cells expressing MHC II molecules increased over those for noninfected mice in both rat IgG- and anti-IL-12-treated mice as infection progressed, but the MHC II+ cells observed after IL-12 neutralization were significantly decreased compared to those for controls on days 3 (2-fold; P ≤ 0.039), 5 (2.5-fold; P ≤ 0.010), and 7 (1.7-fold; P ≤ 0.028).
Changes in cell surface densities of MHC II, CD80, and CD86 molecules.
Initially we determined if infection with H. capsulatum altered the relative densities of surface MHC II, CD80, CD86, and Mac-1 molecules. To accomplish this, MFI values were compared between naive (noninfected) and rat IgG-treated mice on days 3, 5, 7, 10, 14, and 21 of infection. The densities of surface CD80 and Mac-1 molecules were similar in the two groups through day 7, whereas CD86 expression was increased approximately 2.5-fold on days 3 and 5 (P ≤ 0.05). The MFI for MHC II expression decreased three- to fourfold in the rat IgG-treated group when compared to noninfected (naive) animals (P ≤ 0.02) on all days analyzed. Therefore, no changes in surface expression of CD80 and Mac-1 were observed, CD86 expression increased, and MHC II expression decreased during the first week.
To determine the effect of reduced IL-12 levels on the relative density of surface molecules, the fold increase in MFI values between rat IgG- and anti-IL-12-treated mice was compared (Fig. 3). After IL-12 neutralization, the density of CD80 on lung cells was similar to that in rat IgG-treated mice on days 3 and 5 but was decreased 1.5- to 2.0-fold on days 7 and 10 (P ≤ 0.02). CD86 densities were significantly decreased on lung cells isolated from anti-IL-12-treated mice on days 5, 7, and 10 (P ≤ 0.03), whereas surface expression of Mac-1 was increased on all days analyzed (P ≤ 0.05). Although the density measurements for MHC II differed between rat IgG- and anti-IL-12-treated animals, none of these differences was significant (P ≤ 0.05).
FIG. 3.
Alteration in surface antigen expression after IL-12 neutralization. The density of expression for each marker was measured by MFI, and the fold increase in these values over those for the negative population was calculated. The data from two experiments were expressed as the mean ± standard error for five to nine mice per group. Asterisks indicate data that were significantly different for control and anti-IL-12-treated mice as determined by Student's t test (P ≤ 0.05).
Cellular phenotype during reduced fungal burden.
It was possible that the changes observed in cell surface phenotype after IL-12 neutralization were caused by an increased fungal burden. To address this hypothesis, mice treated with anti-IL-12 were infected with an inoculum of H. capsulatum (0.6 × 106) that approximated the burden in control mice during days 7 to 14. We did not include a group of rat IgG-treated animals injected with 0.6 × 106 yeasts, since the purpose of this experiment was to ascertain if the fungal burden or anti-IL-12 influenced the inflammatory response. The lungs of mice that received the reduced number of yeasts and anti-IL-12 contained 1.91 × 106 ± 0.70 × 106 CFU on day 10 of infection. This value was approximately the same (P ≤ 0.05) as that observed in controls on day 7 (2.4 × 106 ± 0.45 × 106).
When the fungal burden was matched, the absolute numbers of cells infiltrating lungs in control mice and anti-IL-12-treated mice infected with 0.6 × 106 yeasts were comparable (Table 1). The total numbers of Mac-1+ and Thy-1+ cells were similar in lungs of control mice on day 7 and in those infected with 0.6 × 106 yeasts on day 10 (Fig. 4). The number of T cells from mice treated with anti-IL-12 and exposed to 2.5 × 106 yeasts on day 10 were also the same, although the fungal burden was increased fourfold over that in controls.
FIG. 4.
Comparison of the total numbers of Mac-1+ and Thy-1+ lung cells in mice given anti-IL-12 and infected with different numbers of yeasts. The total numbers of lineage-specific cells in lungs of mice infected with 2.5 × 106 yeasts and treated with rat IgG or anti-IL-12 and mice administered anti-IL-12 and infected with 0.6 × 106 yeast cells were determined by flow cytometry. The data from two experiments were expressed as the mean (± standard error) of the numbers of cells expressing each marker for five to nine mice per group. Data for day 0 were from uninfected mice (n = 5). Asterisks indicate data that were significantly different for control and anti-IL-12-treated groups receiving 2.5 × 106 yeasts as determined by Student's t test (P ≤ 0.05).
The pattern of increased cell numbers for CD80, CD86, CD119, and MHC II observed in control mice infected with 2.5 × 106 yeasts (Fig. 1) was similar in anti-IL-12-treated mice infected with 0.6 × 106 yeasts (data not shown). The numbers of CD80-, CD86-, and CD119-bearing cells were comparable in anti-IL-12-treated mice infected with 2.5 × 106 or 0.6 × 106 yeasts on days 7 and 10, respectively, but MHC II+ cells were reduced in the animals infected with the reduced inoculum (P ≤ 0.045).
Distribution of CD80+ and CD86+ cells among different cell lineages.
Since CD80 and CD86 are expressed by multiple cell types, data from two-color immunofluorescent analyses of surface expression of these molecules versus the lineage markers Thy-1 (T cells), B220 (B cells), Mac-1 (myeloid cells), and Gr-1 (granulocytes) were calculated as the total number of lineage-specific cells that express these markers during histoplasmosis in control and IL-12-neutralized animals (Fig. 5). The majority of CD80+ cells on day 3 of infection were Mac-1+, and most coexpressed the granulocyte marker Gr-1. This myeloid cell dominance for CD80+ cells continued through day 7 and then declined as the number of CD80+ T cells increased on days 10 and 14 of infection (Fig. 5A). The distribution of CD80+ cells in IL-12-depleted mice was dramatically different (Fig. 5B). The large increase in Mac-1+ and Gr-1+ cells that expressed CD80 was delayed until after day 5 and peaked on day 7, when 25% of all Mac-1+ cells in lungs coexpressed CD80. In contrast, cells expressing both markers in controls on days 3 and 5 represented 10% of the total Mac-1+ cells. Although the total T-cell population on day 10 was increased after IL-12 neutralization, the number of CD80+ T cells was greatly diminished (ninefold less than controls) (Fig. 5B).
FIG. 5.
Lineage distribution of CD80+ and CD86+ lung cells. The expression of CD80 and CD86 on myeloid-lineage cells (Mac-1+), T cells (Thy-1+), and B cells (B220+) was determined by two-color immunofluorescence analysis. The data from two experiments were expressed as the mean of the total number of lineage-specific cells that coexpress CD80 or CD86 from lungs of naive mice (n = 5) and infected mice from days 3 to 21 of infection (n ≥ 6 per group). CD80+ lineage+ data (A, B, and C) and CD86+ lineage+ values (D, E, and F) were determined for rat IgG-treated mice infected with 2.5 × 106 yeasts (A and D), mice given anti-IL-12 and 2.5 × 106 yeasts (B and E), and mice given anti-IL-12 and 0.6 × 106 (C and F).
The distribution of CD86 on lineage-specific cells was similar to that of CD80. The initial increase in cells expressing this marker from days 3 to 7 was in cells of myeloid lineage, followed by an increase in Thy-1+ CD86+ cells between days 7 and 14 (Fig. 5D). The difference in expression of CD80 and CD86 was that 1.5-fold more myeloid-lineage cells and 2- to 3-fold more T cells expressed CD86. After IL-12 neutralization, the Mac-1/GR-1+ CD86+ subset increased between days 5 and 7 (Fig. 5E), but fewer cells expressed both markers than cells that coexpressed CD80. Numbers of Thy-1+ CD86+ cells were slightly greater than those of CD80+ T cells on day 10 after IL-12 neutralization, but both populations were approximately ninefold less than those in lungs of control animals.
A phenotypic analysis of lung cells isolated from mice infected with a reduced yeast burden (0.6 × 106) revealed a different cellular distribution of CD80 and CD86 than that observed in animals infected with 2.5 × 106 yeasts. The dominant populations expressing both markers, Mac-1+ and Gr-1+, were observed between days 5 and 10 of infection, declined between days 10 to 14, and increased on days 14 and 21 (Fig. 5C and F). A major difference in lung phenotype was observed in the T-cell populations. Thirteen percent of the total number of T cells isolated from animals given 0.6 × 106 yeasts expressed CD80 and 30% expressed CD86 during the peak on day 21, whereas 3 to 5% of T cells in lungs from animals receiving 2.5 × 106 yeasts expressed these markers on day 10.
Effect of blocking CD80 and CD86 on fungal burden.
Since both CD80 and CD86 were upregulated during infection with H. capsulatum and expression of these molecules was altered in animals treated with anti-IL-12, we determined if blocking of either or both of these CD28 ligands would alter the outcome of disease. Lungs and spleens were removed from animals treated with rat IgG or MAb to CD80, CD86, or both on days 7, 14, and 21 of infection, and the fungal burden was determined by CFU analysis. Although there was a slight decrease in the burden on day 7 in both spleens and lungs of all groups, none of these values were significantly different from those for controls treated with rat IgG (P ≤ 0.05) (Table 2).
TABLE 2.
Analysis of fungal burden in lungs and spleens of mice infected with H. capsulatum
| Ab treatment | Lung CFU (105)a on day:
|
Spleen CFU (104)a on day:
|
||||
|---|---|---|---|---|---|---|
| 7 | 14 | 21 | 7 | 14 | 21 | |
| Rat IgG | 24.8 ± 4.2 | 1.41 ± 2.8 | 0.02 ± 0.01 | 8.2 ± 1.8 | 2.15 ± 0.24 | 0.11 ± 0.04 |
| Anti-CD80 | 16.2 ± 2.4 | 1.11 ± 3.5 | 0.04 ± 0.01 | 6.9 ± 1.3 | 2.13 ± 0.41 | 0.14 ± 0.03 |
| Anti-CD86 | 18.8 ± 3.8 | 1.17 ± 4.1 | 0.02 ± 0.01 | 4.9 ± 1.2 | 2.62 ± 0.57 | 0.08 ± 0.02 |
| Anti-CD80+ anti-CD86 | 18.3 ± 4.0 | 1.16 ± 2.9 | 0.02 ± 0.01 | 4.8 ± 1.2 | 2.13 ± 0.53 | 0.05 ± 0.01 |
| Anti-IL-12 | 149.4 ± 27b | —d | — | 80.9 ± 27b | — | — |
| Anti-IL-12+ anti-CD80 | 59.6 ± 1.3bc | — | — | 69.7 ± 16b | — | — |
Five to 10 animals inoculated with 2.5 × 106 yeasts were analyzed in each group. Values are means ± standard errors.
Significantly different from value for rat IgG-treated control mice (P ≤ 0.02).
Significantly different from value for mice treated with anti-IL-12 alone (P ≤ 0.01).
—, not determined.
The fungal burden in lungs and spleens of mice after IL-12 neutralization was significantly increased over control levels. Since the number of CD80-bearing cells was increased after IL-12 neutralization, we determined if administration of anti-CD80 and anti-IL-12 modulated the fungal burden. The CFU were significantly decreased (P ≤ 0.01), by 2.5-fold, when CD80 was blocked (Table 2).
To determine if modulation of cytokine levels correlated with the reduction in CFU, supernatants from lungs of mice treated with rat IgG, anti-IL-12, anti-CD80, or both anti-IL-12 and anti-CD80 were analyzed for the amounts of IFN-γ, IL-4, TNF-α, and GM-CSF (Table 3). TNF-α levels in the lungs were similar among all groups. GM-CSF levels in all three experimental groups were significantly decreased compared to those in infected controls (P ≤ 0.005), but the values for anti-CD80-treated, IL-12-neutralized, and anti-CD80- and anti-IL-12-treated animals were comparable to each other. IL-4 levels in lungs from control animals and IL-12-neutralized mice were not significantly different, whereas treatment with anti-CD80 reduced IL-4 levels in both infected controls given rat IgG and mice given anti-IL-12 (P ≤ 0.01 and P ≤ 0.03, respectively). In addition, IL-4 levels in animals treated with both antibodies were reduced compared with those in mice treated with IL-12 alone (P ≤ 0.006). IFN-γ was slightly reduced when CD80 was blocked (P ≤ 0.04), whereas cytokine levels in the experimental groups given anti-IL-12 were four- to fivefold decreased (P ≤ 0.007) compared to those in the rat IgG-treated controls. The ratios of IFN-γ to IL-4 were 1,030 and 1,070 for the control and anti-CD80-treated groups, respectively, whereas these ratios were greatly reduced after IL-12 neutralization (to 284 and to 330) when MAb to both IL-12 and CD80 were administered.
TABLE 3.
Cytokine levels in lungs of H. capsulatum-infected mice treated with MAb to CD80, IL-12, or botha
| Treatment | Total amount measured per lung (mean ± SE)b
|
|||
|---|---|---|---|---|
| IFN-γ (ng) | IL-4 (pg) | TNF-α (ng) | GM-CSF (ng) | |
| Rat IgG | 309.9 ± 56 | 301.1 ± 44 | 29.8 ± 3.0 | 5.8 ± 0.3 |
| Anti-CD80 | 177.8 ± 17 | 166.5 ± 11 | 24.3 ± 2.5 | 4.4 ± 0.3 |
| Anti-IL-12 | 76.1 ± 18 | 268.0 ± 18 | 23.2 ± 2.2 | 3.5 ± 0.3 |
| Anti-IL-12 + anti CD80 | 63.3 ± 14 | 191.6 ± 0.8 | 20.4 ± 1.7 | 3.0 ± 0.2 |
Individual lung supernatants from homogenates assayed for CFU analysis on day 7 of infection were analyzed by enzyme-linked immunosorbent assay for cytokine or monokine levels (n = 6).
The total amounts of cytokines per lung in uninfected mice were 5.1 ± 0.2 ng of IFN-γ, 47.5 ± 3.0 pg of IL-4, 0.8 ± 0.1 ng of TNF-α, and 0.6 ± 0.1 ng of GM-CSF (n = 6).
Regardless of the decreased fungal burden and reduced IL-4 levels after anti-CD80 treatment in IL-12-neutralized mice, seven of seven anti-IL-12-treated mice and seven of eight mice treated with anti-IL-12 and anti-CD80 succumbed to infection by day 16. The mean survival times were 13.1 ± 0.9 days for the anti-IL-12 treated group and 12.8 ± 0.6 days for animals given both MAb.
DISCUSSION
The principal goal of this study was to further explore the effects of IL-12 neutralization in lungs of mice infected with H. capsulatum. Emphasis was placed on determining alterations in the surface expression of molecules responsible for interactions between T cells and antigen-presenting cells, namely, CD80, CD86, and MHC II, as well as the IFN-γ receptor (CD119). After IL-12 neutralization, there were perturbations in the influx of myeloid and T cells and reductions in the total numbers of cells that express CD86 and MHC II.
In association with the increased fungal burden in mice given MAb to IL-12, there was a dramatically different inflammatory response. Two major alterations in the lineage-cell distribution in lungs of IL-12-depleted mice were (i) the large increase of myeloid cells, especially polymorphonuclear leukocytes, into the lung after day 5 of infection and (ii) the almost complete absence of T-cell infiltration during the first week. The lack of endogenous IL-12 clearly modulated the evolution of the inflammatory response. That IL-12 influences pulmonary inflammation has been demonstrated in a model of cryptococcosis. Administration of exogenous IL-12 increases both mononuclear leukocyte infiltration into lungs of mice and the levels of the chemoattractants macrophage inflammatory protein-1 alpha and monocyte chemotactic protein 1 (14).
The T-cell influx in lungs of infected controls peaks during the second week of infection, while the numbers of myeloid cells decrease (5). After IL-12 neutralization, the expected rise in the number of T cells in lungs was not observed. The failure of these cells to migrate into infected lungs could have been caused by a disrupted chemokine response, since T-cell influx into inflammatory sites can be controlled by multiple chemokines and their receptors (29). Although evidence directly linking endogenous IL-12 generation and T-cell chemotaxis is scarce, one study reported that IL-12 upregulates E- and P-selectin ligands on T cells, thus facilitating recruitment into inflamed tissue (31).
IL-12 neutralization affected cells expressing CD80 and CD86 differently. The numbers of CD80+ cells were fourfold greater on days 7 and 10, but the surface density was twofold less. The differences in CD86+ cells were observed on days 3 and 5, when a threefold reduction in total numbers and a significant decrease in surface density were detected after IL-12 neutralization. Since there was a decrease in both the numbers of CD86+ cells and their surface density, the availability of CD86 molecules for binding to CD28 was definitely less than in controls. This reduction during the first week indicated that expression of this molecule was influenced by the IFN-γ deficiency induced by administration of MAb to IL-12.
Since IFN-γ is a potent stimulator of MHC II surface expression (9, 12, 21), we tested the effect of IL-12 neutralization on the number of cells that expressed MHC II and the density of expression. H. capsulatum infection produced a downregulation in surface MHC II molecules on lung cells isolated from rat Ig- and anti-IL-12-treated mice compared to noninfected controls. Although the number of MHC II-bearing cells in infected anti-IL-12-treated mice was markedly less than that in infected controls on days 3 to 7, there were no significant differences in MFI values between the two groups. Therefore, decreased levels of IFN-γ affected the numbers of MHC II+ cells but not the density per cell. These data strongly suggest that IFN-γ is not the sole regulator of MHC II expression in H. capsulatum-infected mice. TNF-α and GM-CSF, two other cytokines that stimulate MHC II expression, may have compensated for the IFN-γ deficiency (12, 21).
An important consideration for these studies was to determine if the altered inflammatory response in mice administered anti-IL-12 and injected with 2.5 × 106 yeasts was simply a consequence of an increased fungal burden or the lack of IL-12 and IFN-γ. To address these issues, we designed an experiment in which the CFU in lungs of infected controls would be commensurate with those in mice receiving MAb to IL-12 during the early phases of infection (≤10 days). An inoculum size of 0.6 × 106 was selected and given to mice treated with anti-IL-12. The marked elevations in myeloid cells that were observed in mice infected with 2.5 × 106 yeasts and given anti-IL-12 were not apparent in the group injected with anti-IL-12 and the lower inoculum. Likewise, the variation in the number of CD80+ and CD86+ cells was no longer detected. For these cell populations, the fungal burden stimulated the fluctuations. On the other hand, the MHC II+ cell numbers were significantly lower in the group receiving anti-IL-12 and the lower inoculum of H. capsulatum. The decline in this cell population was likely to be modified by the deficiency in IL-12 and IFN-γ rather than by a change in the number of yeasts recovered from lungs.
The influx of T cells appeared to be dependent on the fungal burden, since their numbers were similar when the burdens were matched (day 7 in infected controls and day 10 for mice anti-IL-12 treated and inoculated with 0.6 × 106 yeasts). Yet, the lungs of anti-IL-treated mice infected with 2.5 × 106 yeasts also contained similar numbers of T cells on day 10, when the CFU were fourfold greater. However, the subset distribution of T cells in this group of mice was altered. CD86+ Thy-1+ cells were decreased compared to those in infected controls and mice given anti-IL-12 and infected with 0.6 × 106 yeasts. It is possible that the large cellular influx triggered on day 10 in mice IL-12 neutralized and infected with 2.5 × 106 yeasts resulted in a transient migration of T cells and that their numbers are not as important as their phenotype.
There are conflicting data on the function of CD80 and CD86 in promoting differentiation of a T-helper cell type 1 (Th1) versus a Th2 response. In experimental allergic encephalomyelitis (15) and bronchial asthma (27) the interaction between CD80 and CD28 triggers differentiation of Th1 cells that produce IFN-γ, and CD86 is responsible for generation of Th2 cells and IL-4 production. Subsequent data revealed that CD86-transfected CHO cells preferentially induce IL-4-producing cells (11) or that there is no skewing of T cells to a Th1 or Th2 phenotype by either CD80 or CD86 (19).
Studies have shown that induction of Th1 or Th2 cytokines in animals given MAb to CD80 or CD86 varies with the model of infection. Blocking of CD86 reduces IL-4 levels and the burden in mice infected with either Leishmania major (3, 6) or Candida albicans (17), whereas blocking of both CD80 and CD86 results in reduced IFN-γ levels but has no effect on the burden of Listeria monocytogenes (32). In experimental schistosomiasis (28) and borreliosis (23) CD86 blockade reduces IL-4 levels, but it reduces the burden only during schistosomiasis. A common factor in most of these disease models is that inhibition of CD86 appears to reduce IL-4 levels. However, administration of anti-CD86 increases the numbers of IL-4- and IFN-γ-secreting cells in mice infected with Leishmania donovani (18). In contrast, anti-CD80 treatment reduced IL-4 levels in control and anti-IL-12-treated mice infected with H. capsulatum. Thus, the effects of CD80 and CD86 on cytokine production differ from those in other experimental models.
To determine if CD80 and CD86 influenced the course of disease, groups of infected mice were treated i.n. with MAb to these molecules that have been reported to block effector cell function (27). Treatment with MAb to CD80, CD86, or both reduced the burden only slightly compared to that in rat Ig-treated controls. Thus, blockade of CD80 and CD86 did not enhance an efficient host resistance mechanism. A significant decrease in burden was observed in animals treated with anti-CD80 and anti-IL-12. Under these conditions, the reduction in fungal burden was more dramatic, because host control of histoplasmosis was blunted in the presence of anti-IL-12, and the beneficial effect of CD80 blockade was observed only in a model where the host response is impaired. A similar result was reported for resistant mice infected with L. major (6). In this study the burden was slightly reduced after administration of CTLA4-Ig, a reagent that blocks both CD80 and CD86, but the greater effect was in the significant reduction of burden by the same treatment in susceptible mice.
Disease progression may be controlled by altering the relative levels of cytokines that are required for healing and those that exacerbate infection (8, 10, 24, 25). That the balance between Th1 and Th2 cytokines, rather than the absolute values, is important for disease amelioration has been shown in a model of leishmaniasis (20). Susceptable BALB/c mice that lack the IL-4 receptor alpha chain do not increase IFN-γ levels over those in wild-type animals, yet infection is partially controlled. This study concludes that even low levels of IFN-γ can have a healing effect in the absence of IL-4. During histoplasmosis, anti-IL-12 treatment changed the ratio of IFN-γ to IL-4 but not the absolute levels of the latter. The reduction in IL-4 caused by anti-CD80 treatment slightly improved the balance between the two cytokines and, as a consequence, partially restored host resistance. Regardless of any reduction in burden observed, the outcome of disease was the same. In the presence or absence of anti-CD80, anti-IL-12 recipients died with comparable mean survival times. Therefore, the impact of CD80 blockade was transient and failed to completely restore protective immunity.
The present study demonstrated that IL-12 neutralization and infection with H. capsulatum altered the inflammatory response by greatly enhancing the myeloid cell influx into mouse lungs and greatly reducing the T-cell population, the numbers of cells that express MHC II molecules, and the numbers and density of the costimulatory molecule CD86. Alterations in these two surface antigens, which function in antigen presentation and as a second signal for activation of T cells, respectively, were observed early in infection when the effects of anti-IL-12 treatment are detrimental. The CD86 costimulatory molecule was not entirely responsible for protective immunity but may be part of a mechanism of T-cell activation and recruitment necessary for establishing an adaptive immune response.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-42747 and AI-34361 and by a Merit Review Award from the Department of Veterans Affairs.
We thank George Brunner for excellent technical assistance and Simon Newman, Carlos Subauste, and Francisco Gomez for their critical review of the manuscript.
REFERENCES
- 1.Allendoerfer R, Boivin G P, Deepe G S., Jr Modulation of immune responses in murine pulmonary histoplasmosis. J Infect Dis. 1997;175:905–914. doi: 10.1086/513989. [DOI] [PubMed] [Google Scholar]
- 2.Allendoerfer R, Deepe G S., Jr Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect Immun. 1997;65:2564–2569. doi: 10.1128/iai.65.7.2564-2569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown J A, Titus R G, Nabavi N, Glimcher L H. Blockade of CD86 ameliorated Leishmania major infection by down-regulating the Th2 response. J Infect Dis. 1996;174:1303–1308. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- 4.Bullock W E, Wright S D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987;165:195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cain J A, Deepe G S., Jr Evolution of the primary immune response to Histoplasma capsulatum in murine lungs. Infect Immun. 1998;66:1473–1481. doi: 10.1128/iai.66.4.1473-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corry D B, Reiner S L, Linsley P S, Lockley R M. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 7.Deepe G S., Jr The immune response to Histoplasma capsulatum: unearthing its secrets. J Lab Clin Med. 1994;123:201–205. [PubMed] [Google Scholar]
- 8.Deepe G S, Jr, Seder R A. Molecular and cellular determinants of immunity to Histoplasma capsulatum. Res Immunol. 1998;149:397–406. doi: 10.1016/s0923-2494(98)80763-3. [DOI] [PubMed] [Google Scholar]
- 9.Ding L, Linsley P S, Huang L, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 10.Erb K J, Blank C, Moll H. Susceptibility to Leishmania major in IL-4 transgenic mice is not correlated with the lack of a Th1 immune response. Immunol Cell Biol. 1996;74:239–244. doi: 10.1038/icb.1996.43. [DOI] [PubMed] [Google Scholar]
- 11.Freeman G J, Boussiotis V A, Anumanthan A, Bernstein G M, Ke X, Rennert P D, Gray G S, Gribben J G, Nadler L M. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 12.Glimcher L H, Kara C J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 13.Goding J W. Conjugation of antibodies with fluorochromes: modification to the standard methods. J Immunol Methods. 1976;13:215–225. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami K, Qureshi M H, Zhang T, Koguchi Y, Shibuya K, Naoe S, Saito A. Interferon-gamma (IFN-gamma)-dependent protection and synthesis of chemoattractants for mononuclear leucocytes caused by IL-12 in the lungs of mice infected with Cryptococcus neoformans. Clin Exp Immunol. 1999;117:113–122. doi: 10.1046/j.1365-2249.1999.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L M. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 16.McKinney M M, Parkinson A. A simple non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods. 1987;96:271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- 17.Mencacci A, Cenci E, Del Sero G, d'Ostiani C F, Mosci P, Trinchieri G, Adorini L, Romani L. IL-10 is required for development of protective Th1 responses in IL-12-deficient mice upon Candida albicans infection. J Immunol. 1998;161:6228–6237. [PubMed] [Google Scholar]
- 18.Murphy M L, Engwerda C R, Gorak P M A, Kaye P M. B7-2 blockade enhances T cell responses to Leishmania donovani. J Immunol. 1997;159:4460–4466. [PubMed] [Google Scholar]
- 19.Natesan M, Razi-Wolf Z, Reiser H. Costimulation of IL-4 production by murine B7-1 and B7-2 molecules. J Immunol. 1996;156:2783–2791. [PubMed] [Google Scholar]
- 20.Noben-Trauth N, Paul W E, Sacks D L. IL-4 and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J Immunol. 1999;162:6132–6140. [PubMed] [Google Scholar]
- 21.Panek R B, Benveniste N. Class II MHC gene expression in microglia. Regulation by the cytokines IFN-γ, TNF-α, and TGF-β. J Immunol. 1995;154:2846–2854. [PubMed] [Google Scholar]
- 22.Scheid M P, Triglia D. Further description of the Ly-5 system. Immunogenetics. 1979;9:423–432. [Google Scholar]
- 23.Shanafelt M C, Kang I, Barthold S W, Bockenstedt L K. Modulation of murine Lyme borreliosis by interruption of the B7/CD28 T-cell costimulatory pathway. Infect Immun. 1998;66:266–271. doi: 10.1128/iai.66.1.266-271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szalay G, Ladel C H, Blum C, Kaufmann S H E. IL-4 neutralization or TNF-α treatment ameliorate disease by an intracellular pathogen in IFN-γ receptor-deficient mice. J Immunol. 1996;157:4746–4750. [PubMed] [Google Scholar]
- 25.Tonnetti L, Spaccepelo R, Cenci E, Mencacci A, Puccetti P, Coffman R L, Bistoni F, Romani R. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol. 1995;25:1559–1565. doi: 10.1002/eji.1830250614. [DOI] [PubMed] [Google Scholar]
- 26.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 27.Tsuyuki S, Tsuyuki J, Einsle K, Kopf M, Coyle A J. Costimulation through B7-2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J Exp Med. 1997;185:1671–1679. doi: 10.1084/jem.185.9.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villanueva P O F, Reiser H, Stadecker M J. Regulation of T helper cell responses in experimental murine schistosomiasis by IL-10. J Immunol. 1994;153:5190–5199. [PubMed] [Google Scholar]
- 29.Ward S G, Westwick J. Chemokines: understanding their role in T-lymphocyte biology. Biochem J. 1998;333:457–470. doi: 10.1042/bj3330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu-Hsieh B. Relative susceptibilities of inbred mouse strains C57BL/6 and A/J to infection with Histoplasma capsulatum. Infect Immun. 1989;57:3788–3792. doi: 10.1128/iai.57.12.3788-3792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie H, Lim Y C, Luscinskas F W, Lichtman A H. Acquisition of selectin binding and peripheral homing properties by CD4(+) and CD8(+) T cells. J Exp Med. 1999;189:1765–1776. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhan Y, Cheers C. Either B7-1 or B7-2 is required for Listeria monocytogenes-specific production of gamma interferon and interleukin-2. Infect Immun. 1996;64:5349–5441. doi: 10.1128/iai.64.12.5439-5441.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P, Sieve M C, Bennett J, Kwon-Chung K J, Tewari R P, Gazzinelli R T, Sher A, Seder R A. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]