Abstract
Nowadays, cancer disease seems to be the second most common cause of death worldwide. Molecular hybridization is a drug design strategy that has provided promising results against multifactorial diseases, including cancer. In this work, two series of phthalazinone-dithiocarbamate hybrids were described, compounds 6–8, which display the dithiocarbamate scaffold at N2, and compounds 9, in which this moiety was placed at C4. The proposed compounds were successfully synthesized via the corresponding aminoalkyl phthalazinone derivatives and using a one-pot reaction with carbon disulfide, anhydrous H3PO4, and different benzyl or propargyl bromides. The antiproliferative effects of the titled compounds were explored against three human cancer cell lines (A2780, NCI-H460, and MCF-7). The preliminary results revealed significant differences in activity and selectivity depending on the dithiocarbamate moiety location. Thus, in general terms, compounds 6–8 displayed better activity against the A-2780 and MCF-7 cell lines, while most of the analogues of the 9 group were selective toward the NCI-H460 cell line. Compounds 6e, 8e, 6g, 9a–b, 9d, and 9g with IC50 values less than 10 µM were the most promising. The drug-likeness and toxicity properties of the novel phthalazinone-dithiocarbamate hybrids were predicted using Swiss-ADME and ProTox web servers, respectively.
Keywords: phthalazinone, dithiocarbamate, hybridization, one-pot synthesis, antiproliferative activity, drug-likeness, toxicity
1. Introduction
Nowadays, cancer disease seems to be the major cause of death in high-income and upper-middle-income countries, and the second most common cause of death worldwide [1]. It is estimated that almost 10 million people died from cancer in 2020, and 19.3 million new cases were diagnosed [2]. The toxicity and the side effects of current antineoplastic drugs, as well as the appearance of drug resistance, are the main drawbacks of chemotherapy. Therefore, developing new small molecules with fewer side effects, low toxicity and higher efficiency toward cancer is a challenge for researchers [3,4].
The molecular hybridization strategy is based on the combination of pharmacophores from different bioactive molecules in new hybrid compounds. This interesting approach has received significant attention in rational drug design since it would allow derivatives to be obtained with improved affinity and efficacy and often overcome cross-resistance [5]. In addition, in some cases, this approach provided compounds showing modified selectivity profiles, reduced side effects, or even different or dual action mechanisms. Researchers have reported promising results using the molecular hybridization tool against multifactorial diseases, including cancer [6].
Phthalazin-1(2H)-one core is a privileged building block for drug development, since phthalazinone derivatives possess a wide spectrum of pharmacological activities, including anticancer, antidiabetic, antiasthmatic, antihistaminic, antihypertensive, antithrombotic, antidepressant, anti-inflammatory and analgesic effects [7,8,9]. Several 4-substituted phthalazinones, such as olaparib (1) and compound 2 (Figure 1), are recognized antitumor agents acting through poly ADP ribose polymerase (PARP) inhibition [10,11,12]; pyrazole-phthalazinone hybrids, such as compound 3, are also promising drug candidates for cancer treatment, acting as aurora kinase inhibitors [13]. In addition, the anticancer activities of oxadiazol-phthalazinones, such as compound 4, seem to be related to their p38 mitogen-activated protein kinase (MAPK) and topoisomerase II inhibition [14].
Figure 1.
Structure of several phthalazinone derivatives with anticancer properties.
Dithiocarbamate is another chemical entity with a wide range of biological properties, also including anticancer activity [15,16]. The natural product brassinin (5) and related indole-dithiocarbamates are inhibitors of indoleamine 2,3-dioxygenase (IDO), an enzyme involved in tumor immunosuppression (Figure 2) [17,18]. In the last few years, the dithiocarbamate moiety has been incorporated into numerous cores such as benzoxazole [19], triazole [20], 2,4-diarylaminopyrimidine [21], quinazolinone [22,23], chalcone [24], pyridazinone [25] to develop new hybrid compounds displaying in vitro or in vivo antiproliferative activities.
Figure 2.
Brassinin (5) and the novel phthalazinone-dithiocarbamate hybrids (compounds 6–9).
Taking the abovementioned facts into account and that, to our knowledge, no anticancer activity data have been previously reported for compounds containing the phthalazinone and dithiocarbamate scaffolds in the same molecule, we designed a new series of phthalazinone-dithiocarbamate hybrids, compounds 6–9 (Figure 2). The target hybrids could be considered as phthalazinone analogues of brassinin (5), in which the indole nucleus was exchanged for a phthalazinone moiety with different substitution patterns at C4 and the S-methyl group was replaced by unsaturated fragments of different sizes, such as propargyl, benzyl or several p-substituted benzyl groups, some of them present in brassinin analogues and related compounds with potent antiproliferative activity [17,18]. In addition, since the N2 and C4 positions appear to be important for modulating the anticancer activity of the phthalazinone core [7,8,26], we have explored both locations to include the dithiocarbamate scaffold. Herein, we describe the synthesis, in vitro antiproliferative activity, and structure-activity relationships for these novel phthalazinone-dithiocarbamate hybrids 6–9. SwissADME and ProTox-II programs were used to predict the pharmacokinetic properties and toxicity of some of the target compounds.
2. Results and Discussion
2.1. Chemistry
The proposed compounds were synthesized by functionalization of phthalazinone scaffolds 11–14 and via the corresponding alkyl bromides 15–18, using the primary amines 23–26 as key intermediates, as illustrated in Scheme 1 and Scheme 2. Phthalazinones 11–12, both substituted at C4 with a methyl group, were obtained in very good yields from 2-acetyl benzoic acid (10) by refluxing with hydrazine hydrate or methyl hydrazine in ethanol. On the other hand, unsubstituted phthalazinone 13 and its p-tolyl derivative 14 are commercially available.
Scheme 1.
Reagents and conditions: (i) hydrazine hydrate or methyl hydrazine, ethanol reflux, 5 h, 91% (11), 98% (12); (ii) 1,2-dibromoethane, K2CO3, DMF 60 °C, 1.5 h, 50% (15), 75% (16), 45% (17); (iii) NBS, (BzO)2, acetonitrile, reflux, 12 h, 56% (18).
Scheme 2.
Reagents and conditions: (i) potassium phthalimide, DMF reflux, 8 h, 81% (19), 93% (20), 86% (21), 83% (22); (ii) hydrazine hydrate, ethanol reflux, 7 h, 2M HCl, 60 °C 2 h, 90 °C 1 h, Amberlyst A26 (OH), 97% (23), 80% (24), 99% (25), 94% (26); (iii) CS2, K3PO4, DMF, appropriate benzyl bromide or propargyl bromide, DMF, r. t., 3 h, 59% (6a), 70% (6b), 68% (6c), 67% (6d), 76% (6e), 91% (6f), 26% (6g), 55% (7a), 61% (7b), 80% (7c), 74% (7d), 64% (7e), 68% (7f), 54% (7g), 83% (8a), 61% (8b), 59% (8c), 78% (8d), 70% (8e), 59% (8f), 19% (8g), 71% (9a), 66% (9b), 22% (9c), 68% (9d), 85% (9e), 66% (9f), 36% (9g).
The synthesis of 2-(2-bromoethyl)phthalazinones 15–17, precursors of analogues 6–8, was performed by N-alkylation of the adequate phthalazinone with an excess of 1,2-dibromoethane, in DMF at 60 °C, using K2CO3 as a base (Scheme 1). In this reaction, alkyl bromides 15–17 were the major products (45–75% yield), also obtaining as by-products the corresponding N,N′-dialkylation dimers. The 4-bromomethyl phthalazinone 18, a precursor of the 9 analogues, was synthesized from 12 by Wohl–Ziegler’s bromination, with N-bromosuccinimide and benzoyl peroxide and using acetonitrile as an alternative solvent to carbon tetrachloride, to improve the substrate solubility (Scheme 1).
Synthesis of the key intermediates 23–26 was accomplished by adapting Gabriel’s method (Scheme 2). The reaction of bromoalkylphthalazinones 15–18 with potassium phthalimide provided the corresponding isoindolidine-1,3-diones in good yields, which were converted into the amines 23–26 by hydrazinolysis and acidification with 2M HCl followed by treatment with Amberlyst A26 (OH).
Finally, the phthalazinone-dithiocarbamate hybrids 6–9 were successfully obtained by a one-pot reaction, treating the adequate aminoalkyl phthalazinone with carbon disulfide, anhydrous H3PO4, and the different benzyl or propargyl bromides, in DMF, between 0 °C and room temperature (Scheme 2).
2.2. Pharmacology
The target compounds 6–9 were screened in vitro for their antiproliferative effects against three cell cancer lines, A2780 (human ovarian carcinoma), NCI-H460 (human lung carcinoma), and MCF-7 (human breast adenocarcinoma) through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay and using cisplatin as a positive control [27]. The obtained results from this preliminary study are detailed in Table 1 and Table 2.
Table 1.
Antiproliferative activity (IC50 values) of phthalazinone-dithiocarbamate hybrid compounds 6a–g, 7a–g, and 8a–g against A-2780, NCI-H460, and MCF-7 cell lines.

|
IC50 a (µM) | ||||
|---|---|---|---|---|---|
| Compound | R2 | R1 | A-2780 | NCI-H460 | MCF-7 |
| 6a | H |
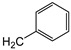
|
88 ± 15 | >100 | 75 ± 1 |
| 7a | p-Tol |

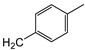
|
10 ± 1 | >100 | 33 ± 1 |
| 8a | Me | 29 ± 3 | >100 | >100 | |
| 6b | H | 78 ± 6 | >100 | 22 ± 2 | |
| 7b | p-Tol |


|
17 ± 1 | >100 | 26 ± 1 |
| 8b | Me | 27 ± 1 | >100 | 37 ± 3 | |
| 6c | H | 61 ± 6 | >100 | 36 ± 6 | |
| 7c | p-Tol |


|
15 ± 1 | >100 | 51 ± 1 |
| 8c | Me | 44 ± 7 | >100 | >100 | |
| 6d | H | 15 ± 1 | >100 | 46 ± 1 | |
| 7d | p-Tol |


|
20 ± 1 | >100 | >100 |
| 8d | Me | 19 ± 1 | >100 | 24 ± 1 | |
| 6e | H | 5.53 ± 0.09 | >100 | 20 ± 1 | |
| 7e | p-Tol |


|
17 ± 1 | >100 | >100 |
| 8e | Me | 7.51 ± 0.13 | >100 | >100 | |
| 6f | H | 55 ± 10 | >100 | 25 ± 1 | |
| 7f | p-Tol |


|
10 ± 1 | >100 | 40 ± 1 |
| 8f | Me | 11 ± 1 | >100 | 14 ± 1 | |
| 6g | H | 5.20 ± 0.13 | >100 | 7.64 ± 0.5 | |
| 7g | p-Tol |
 - |
24 ± 1 | 43 ± 1 | 28 ± 1 |
| 8g | Me | 23 ± 1 | >100 | ND | |
| Cisplatin | - | 0.54 ± 0.01 | 5.54 ± 0.23 | 13 ± 1 |
a Data are expressed as the mean ± standard error from three independent experiments (n = 3).
Table 2.
Antiproliferative activity (IC50 values) of phthalazinone-dithiocarbamate hybrid compounds 9a–g against A-2780, NCI-H460, and MCF-7 cell lines.

|
IC50 a (µM) | |||
|---|---|---|---|---|
| Compound | R1 | A-2780 | NCI-H460 | MCF-7 |
| 9a |

|
12 ± 1 | 7.36 ± 0.08 | 12 ± 1 |
| 9b |

|
32 ± 3 | 8.49 ± 0.25 | 10 ± 1 |
| 9c |

|
43 ± 4 | 12 ± 1 | 84 ± 15 |
| 9d |

|
34 ± 5 | 7.77 ± 0.17 | 39 ± 4 |
| 9e |

|
24 ± 1 | 12 ± 1 | 33 ± 2 |
| 9f |

|
12 ± 1 | 25 ± 1 | 62 ± 2 |
| 9g |

|
6.75 ± 0.12 | 34 ± 1 | 29 ± 2 |
| Cisplatin | - | 0.54 ± 0.01 | 5.54 ± 0.23 | 13 ± 1 |
a Data are expressed as the mean ± standard error from three independent experiments (n = 3).
As can be observed, significant differences in antiproliferative effects were detected for the target compounds depending on the dithiocarbamate scaffold location. Thus, all compounds displaying the dithiocarbamate fragment at N2 (compounds 6–8) were active against the A2780 cell line, with the IC50 values ranging from 5.20 to 88 µM, most of them were also active against the MCF-7 cell line, and only one of them displayed moderate activity toward the NCI-H460 cell line (compound 7g, IC50 = 43 ± 1 µM).
However, the compounds 9, containing the dithiocarbamate moiety at C4, exhibited moderate to good antiproliferative activity versus the three cancer cell lines studied. In addition, the selectivity displayed by almost all of them to the NCI-H460 cell line is noteworthy. Particularly, the analogues 9a, 9b, and 9d, containing a benzyl, 4-methylbenzyl, or 4-chlorobenzyl group linked to the sulfur atom, respectively, were the most potent compounds against this lung cancer cell line, with IC50 values below 10 µM and close to that of cisplatin (IC50 = 5.54 ± 0.23 µM), the reference drug.
In the case of compounds 6–8 containing the dithiocarbamate scaffold at N2 and different substitution pattern at C4, regarding the A-2780 ovarian cancer cell line, the best results were achieved with the p-bromobenzyl derivatives 6e and 8e, and the propargyl derivative 6g, with IC50 values of 5.53 ± 0.09, 7.51 ± 0.13 and 5.20 ± 0.13 µM, respectively.
In addition, the bromine atom substitution in compounds 6e and 8e (C4 methyl analogue) by other halogens, such as fluorine and chlorine, nitro or methyl groups, or even its removal, caused a decrease in activity. The replacement bromine-chlorine in the series of S-benzyl derivatives 6a–f appeared to be the best tolerated, while a better result was achieved with the bromine-nitro substitution in the case of their C4 methyl analogues 8a–f. This seems to indicate that the magnitude and electronegativity of the substituent in both series significantly affected the activity. However, no significant differences in the antiproliferative activity were observed within the series of S-benzyl analogues 7a–f containing a p-tolyl group at C4, which displayed IC50 values between 10 and 20 µM.
Concerning to MCF-7 cell line, most of the S-benzyl analogues containing the dithiocarbamate fragment at N2 (compounds 6–8a–f) exhibited moderate inhibitory activity against this cell line, specifically when the substituent at the para position was a bromine, chlorine, nitro or methyl group. However, the replacement of the benzyl moiety in compounds 6a–f by a propargyl group significantly improved the antiproliferative activity against the MCF-7 cell line. Thus, compound 6g, with an IC50 value of 7.64 ± 0.5, was 1.7 times more potent than cisplatin (IC50 = 13 ± 1) on the same cancer cell line.
The results obtained for compounds 6–8 revealed analogues 6e, 8e, and 6g as the most interesting, compound 6g being the most promising because of its potency and selectivity versus the A-2780 and MCF-7 cell lines.
On the other hand, as was previously mentioned, most of the analogues displaying the dithiocarbamate moiety at C4 (compounds 9), were active toward the NCI-H460 cell line, these being 9a, 9b, and 9d, with IC50 values of 7.36 ± 0.08, 8.49 ± 0.25 and 7.77 ± 0.17, respectively, the most potent and selective. Interestingly, the propargyl group of the 9 compounds also provided the best result against the A-2780 cell line, since the compound 9g, with an IC50 value of 6.75 ± 0.12, was the most active derivative against this cell line.
2.3. Drug-Like and Toxicity Properties Prediction
To estimate the potential of target compounds as drugs in vivo, several physicochemical properties related to ADME (absorption, distribution, metabolism, and excretion) processes, pharmacokinetic and drug-likeness aspects, were calculated for the most active compounds (6e, 8e, 6g, 9a–b, 9d, and 9g) using the SwissADME web tool [28]. Table 3 includes the computed values for simple physicochemical and lipophilicity descriptors, among them the n-octanol/water partition coefficient (log Po/w) and topological polar surface area (TPSA), two factors affecting the bioavailability.
Table 3.
Physicochemical and lipophilicity properties.
| Compound | Molecular Weight (g/mol) |
Heavy Atoms | Arom. Heavy Atoms | Fraction Csp3 | Rotable Bonds | H-Bond Aceptors | H-Bond Donors | Molar Refractivity | TPSA (Å2) | Log Po/w |
|---|---|---|---|---|---|---|---|---|---|---|
| 6e | 434.37 | 25 | 16 | 0.17 | 7 | 2 | 1 | 112.06 | 104.31 | 3.92 |
| 8e | 448.40 | 26 | 16 | 0.21 | 7 | 2 | 1 | 117.02 | 104.31 | 4.24 |
| 6g | 303.40 | 20 | 10 | 0.21 | 6 | 2 | 1 | 87.65 | 104.31 | 2.28 |
| 9a | 355.48 | 24 | 16 | 0.17 | 6 | 2 | 1 | 104.52 | 104.31 | 3.36 |
| 9b | 369.50 | 25 | 16 | 0.21 | 6 | 2 | 1 | 109.48 | 104.31 | 3.73 |
| 9d | 389.92 | 25 | 16 | 0.17 | 6 | 2 | 1 | 109.53 | 104.31 | 3.85 |
| 9g | 288.39 | 19 | 10 | 0.31 | 4 | 2 | 0 | 85.00 | 92.28 | 2.94 |
As can be seen in Table 3, all the studied compounds showed good lipophilicity properties (consensus log Po/w from 2.28 to 4.24). The TPSA value for most of them (6e, 8e, 6g, 9a–b, and 9d) was 104.31 Å2, that for compound 9g was 92.28 Å2. Compounds with TPSA values ranging between 20 and 130 Å2 are predicted as favorable for passive diffusion across the gastrointestinal wall [28]. On the other hand, moderate polar molecules (TPSA < 79 Å2) and those that are acceptably lipophilic (log Po/w from 0.4 to 6.0) have a high probability for crossing the blood–brain barrier (BBB) [29], which is not desirable when only peripheral effects are of interest.
In connection to this, the brain or intestinal estimated permeation method (BOILED-egg), an accurate predictive model for the evaluation of human intestinal absorption (HIA) and brain access by computing lipophilicity and polarity, showed all compounds in the white area (Figure 3), the physicochemical space of molecules with a high probability of absorption after oral administration, and none of them in the space of molecules with high probability to cross the BBB (yellow area or yolk). The prediction reported an interesting pharmacokinetic profile for these compounds, regarding the oral absorption and the absence of effects on the central nervous system (CNS). Figure 3 also reveals that none of the seven studied compounds was a substrate for P-glycoprotein (PGP), with a key role in the active efflux of drugs across biological membranes, including the gastrointestinal wall [30]. In addition, PGP, due to its overexpression in some tumor cells, is related to multidrug resistance in cancers [31].
Figure 3.
Predicted BOILED-egg model for compounds 6e, 8e, 6g, 9a–b, 9d, and 9g.
Regarding the interaction of these compounds with isoenzymes of the CYP450 superfamily, and in particular, with the five most significant isoforms (CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4) for drug metabolism, S-propargyl analogues 6g and 9g were predicted as inhibitors of CYP1A2, CYP2C19, and CYP2C9, while S-benzyl derivatives 6e, 8e, 9a–b and 9d were predicted as inhibitors of four (CYP1A2, CYP2C19, CYP2C9, and CYP3A4) of the five major CYP450 isoenzymes and, consequently, they would be more resistant to metabolism (Table S1).
The bioavailability radar for the selected analogues, which includes the water solubility, unsaturation degree, flexibility, lipophilicity, size, and polarity parameters (Figure 4) showed that all compounds fell entirely in the pink area for five of the six physicochemical properties computed, all of them being outside of it for the unsaturation descriptor in this radar plot. The seven compounds fulfilled five of these six properties and passed the Lipinski [32], Ghose [33], Veber [34], Egan [35], and Muegge [36] filters associated with a good prediction of drug-likeness.
Figure 4.
Bioavailability radar: (a) compound 6e; (b) compound 8e; (c) compound 6g; (d) compound 9a; (e) compound 9b; (f) compound 9d; (g) compound 9g.
The toxicity of the seven selected compounds was calculated by using the ProTox-II server [37], a web platform which integrates 33 prediction models built from in vitro and in vivo assay data. This web server provides information about acute toxicity (DL50, mg/kg), hepatotoxicity, and several toxicity endpoints, including carcinogenicity, mutagenicity, immunotoxicity and cytotoxicity to human cells. In Table 4, are detailed the predicted DL50 values, toxicity category, as well as the percentages of molecular similarity and prediction accuracy.
Table 4.
Acute toxicity results obtained from ProTox-II web platform.
| Compound | DL50 (mg/kg) | Toxicity Class | Average Similarity (%) | Prediction Accuracy (%) |
|---|---|---|---|---|
| 6e | 350 | 4 | 47.19 | 54.26 |
| 8e | 350 | 4 | 53.95 | 67.38 |
| 6g | 350 | 4 | 46.36 | 54.26 |
| 9a | 500 | 4 | 47.02 | 54.26 |
| 9b | 500 | 4 | 47.63 | 54.26 |
| 9d | 500 | 4 | 49.72 | 54.26 |
| 9g | 500 | 4 | 47.18 | 54.26 |
The program establishes six toxicity categories, from the most to the least toxic, considering the DL50 thresholds. In this case, all compounds were classified as toxics of class 4 for acute oral toxicity (harmful if swallowed), with DL50 values of 350 mg/kg (6e, 8e, and 6g) or 500 mg/kg (9a–b, 9d, and 9g), considering an average of molecular similarity around 50% and a prediction accuracy higher than 50% (Table 4). In addition, all compounds were classified as lacking hepatotoxicity, immunotoxicity, mutagenicity and cytotoxicity, with probability values ranging from 0.51 to 0.99. However, four of them, specifically compounds 6g, 9a–b, and 9g were predicted as potentially carcinogenic with a probability value of 0.53 (Table S2). Overall, three of the seven selected compounds (6e, 8e, and 9d) displayed favorable toxicological profiles in accordance with the ProTox-II webserver.
3. Materials and Methods
3.1. Materials and Instrumentation
Air-sensitive reactions were performed under an Ar atmosphere. Solvents were dried before use following standard procedures. Reactions were supervised by qualitative thin-layer chromatography (TLC) using silica gel plates (Merck 60 F254, 0.25 mm). Flash column chromatography was performed on silica gel Merck 60 (230–400 mesh) under pressure. 1H NMR, 13C NMR, and DEPT spectra were registered on Bruker DPX 400 and Bruker ARX 400 spectrometers, in CDCl3, CD3OD, or DMSO-d6 with TMS as an internal standard. Chemical shifts (δ) are given in parts per million (ppm) and coupling constants (J) in Hertz (Hz). High-resolution mass spectra (HRMS) were recorded on a VG Autospec M and Bruker FTMS APEX XIII spectrometers. Melting points were measured in a Stuart Scientific apparatus. Synthesis of phthalazinones 11–12 and 4-bromomethyl phthalazinone 18 was performed as previously described [38,39]. Synthesis of 2-(2-bromoethyl) phthalazinones 15–17, which was accomplished by adapting procedures previously reported, as detailed in the Supplementary Materials Section.
3.2. Chemical Synthesis
3.2.1. General Procedure for the Preparation of 2-(Phthalazinylalkyl)isoindoline-1,3-Diones (19–22)
To a solution of phthalazinone 15–18 (0.47 mmol) in DMF (7 mL) was added phthalimide potassium salt (0.62 mmol). The reaction mixture was stirred at reflux for 8 h and then at r.t. overnight. After the addition of H2O (10 mL), the white precipitate formed was filtered and dried in vacuum affording the desired compound.
2-(2-(1-Oxophthalazin-2(1H)-yl)ethyl)isoindoline-1,3-dione (19). White solid; yield: 81%; Rf = 0.2 (hexane/EtOAc, 1:1); 1H NMR (CDCl3): δ = 8.35 (d, 1H, J = 7.8 Hz, H8 phthalazine), 7.98 (s, 1H, H4 phthalazine), 7.80–7.71 (m, 4H, Ar), 7.68–7.61 (m, 3H, Ar), 4.53 (t, 2H, J = 5.5 Hz, H2′), 4.18 (t, 2H, J = 5.5 Hz, H1′); 13C NMR (CDCl3): δ = 168.3 (CO), 159.8 (C1 phthalazine), 138.0 (C4 phthalazine), 134.0, 133.2, 132.1, 131.8, 129.7, 127.8, 126.8, 126.2, 123.4, 49.7 (C2′), 36.7 (C1′); HRMS (ESI): m/z [M+H]+ calcd for C18H14N3O3: 320.10297, found: 320.10340.
2-(2-(1-Oxo-4-p-tolylphthalazin-2(1H)-yl)ethyl)isoindoline-1,3-dione (20). White solid; yield: 93%; Rf = 0.2 (hexane/EtOAc, 1:1); 1H NMR (CDCl3): δ = 8.46–8.42 (m, 1H, H8 phthalazine), 7.75–7.68 (m, 4H, Ar), 7.66–7.62 (m, 3H, Ar), 7.15–7.08 (m, 4H, Ar), 4.59 (t, 2H, J = 5.2 Hz, H2′), 4.22 (t, 2H, J = 5.2 Hz, H1′), 2.36 (s, 3H, CH3); 13C NMR (CDCl3): δ = 168.3 (CO), 159.6 (C1 phthalazine), 147.5, 138.9, 133.9, 132.9, 132.2, 132.1, 131.5, 129.3, 129.2, 129.1, 128.1, 127.2, 126.9, 123.3, 49.2 (C2′), 36.9 (C1′), 21.4 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C25H20N3O3: 410.14992, found: 410.14979.
2-(2-(4-Methyl-1-oxophthalazin-2(1H)-yl)ethyl)isoindoline-1,3-dione (21). White solid; yield: 86%; Rf = 0.2 (hexane/EtOAc, 1:1); 1H NMR (CDCl3): δ = 8.33 (d, 1H J = 7.6 Hz, H8 phthalazine), 7.75–7.70 (m, 3H, H5, H6, H7 phthalazine), 7.69–7.61 (m, 4H, Ar), 4.46 (t, 2H, J = 5.2 Hz, H2′), 4.13 (t, 2H, J = 5.2 Hz, H1′), 2.23 (s, 3H, CH3); 13C NMR (CDCl3): δ = 168.2 (CO), 159.6 (C1 phthalazine), 143.7 (C4 phthalazine), 133.8, 132.9, 132.1, 131.3, 129.7, 127.5, 127.0 (C8 phthalazine), 124.8, 123.1, 48.6 (C2′), 36.8 (C1′), 18.4 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C19H16N3O3: 334.11862, found: 334.11899.
2-((3-Methyl-4-oxo-3,4-dihydrophthalazin-1-yl)methyl)isoindoline-1,3-dione (22). White solid; yield: 83%; Rf = 0.4 (hexane/EtOAc, 1:2); 1H NMR (CDCl3): δ = 8.47 (d, 1H, J = 7.8 Hz, H5 phthalazine), 7.94–7.90 (m, 3H, H6, H7, H8 phthalazine), 7.87–7.76 (m, 4H, Ar), 5.19 (s, 2H, CH2), 3.68 (s, 3H, CH3); 13C NMR (CDCl3): δ = 168.2 (CO), 159.7 (C4 phthalazine), 138.6 (C1 phthalazine), 134.3, 133.2, 132.3, 131.8, 128.3, 128.0, 127.6 (C5 phthalazine), 123.7, 123.4, 39.8 (CH3), 38.6 (CH2); HRMS (ESI): m/z [M+H]+ calcd for C18H14N3O3: 320.10297, found: 320.10307.
3.2.2. General Procedure for the Preparation of Aminoalkyl Phthalazin-1(2H)-Ones (23–26)
To a solution of compound 19–22 (0.74 mmol) in EtOH (11 mL) was added hydrazine hydrate (1.10 mmol) and the reaction mixture was refluxed for 7 h. The solvent was evaporated, 2M HCl (20.6 mmol) was added and the mixture was stirred for 2 h at 60 °C and then for 1 h at 90 °C, followed by the addition of H2O (11 mL) at r.t. The white precipitate formed was filtered off, washed with H2O and the solvent was removed under vacuum. The residue obtained was dissolved in MeOH (4 mL) and treated with Amberlyst A26 (OH) to afford the desired compound.
2-(2-Aminoethyl)phthalazin-1(2H)-one (23). Yellowish oil; yield: 97%; Rf = 0.2 (CH2Cl2/MeOH/NH3, 90/9.5/0.5); 1H NMR (CD3OD): δ = 8.37 (s, 1H, H4), 8.33 (d, 1H, J = 7.9 Hz, H8), 7.93–7.83 (m, 3H, H5, H6, H7), 4.38 (t, 2H, J = 6.1 Hz, H1′), 3.29–3.13 (m, 2H, H2′); 13C NMR (CD3OD): δ = 161.6 (C1), 140.5 (C4), 134.8, 133.2, 131.2, 128.7, 127.9, 127.1, 52.6 (C1′), 40.7 (C2′); HRMS (ESI): m/z [M+H]+ calcd for C10H12N3O: 190.09749, found: 190.09747.
2-(2-Aminoethyl)-4-p-tolylphthalazin-1(2H)-one (24). Yellowish oil; yield: 80%; Rf = 0.2 (CH2Cl2/MeOH/NH3, 90/9.5/0.5); 1H NMR (CD3OD): δ = 8.38–8.30 (m, 1H, H8), 7.80–7.72 (m, 2H, Ar), 7.70–7.63 (m, 1H, Ar), 7.40 (d, 2H, J = 8.0 Hz, Ar), 7.28 (d, 2H, J = 8.0 Hz, Ar), 4.32 (t, 2H, J = 6.2 Hz, H1′), 3.20–3.10 (m, 2H, H2′), 2.39 (s, 3H, CH3); 13C NMR (CD3OD): δ = 160.8 (C1), 148.9, 140.4, 134.3, 133.3, 132.7, 130.6, 130.5, 130.1, 129.0, 127.9, 127.6, 54.3 (C1′), 41.3 (C2′), 21.4 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C17H18N3O: 280.14444, found: 280.14445.
2-(2-Aminoethyl)-4-methylphthalazin-1(2H)-one (25). Yellowish oil; yield: 99%; Rf = 0.2 (CH2Cl2/MeOH/NH3, 90/9.5/0.5); 1H NMR (CD3OD): δ = 8.17 (d, 1H, J = 7.3 Hz, H8), 7.83–7.64 (m, 3H, H5, H6, H7), 4.27–4.11 (m, 2H, H1′), 3.15–2.95 (m, 2H, H2′), 2.47 (s, 3H, CH3); 13C NMR (CD3OD): δ =161.1 (C1), 146.0 (C4), 134.4, 132.7, 130.7, 128.2, 127.3, 126.3, 53.9 (C1′), 41.2 (C2′), 18.9 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C11H14N3O: 204.11314, found: 204.11314.
4-(Aminomethyl)-2-methylphthalazin-1(2H)-one (26). Yellowish oil; yield: 94%; Rf = 0.2 (CH2Cl2/MeOH/NH3, 90/9.5/0.5); 1H NMR (CD3OD): δ = 8.25–8.16 (m, 1H, H8), 7.88–7.79 (m, 2H, Ar), 7.78–7.70 (m, 1H, Ar), 4.12 (s, 2H, CH2), 3.75 (s, 3H, CH3); 13C NMR (CD3OD): δ = 160.8 (C1), 147.1 (C4), 134.4, 132.7, 129.4, 128.0, 127.3 (C8), 125.1, 42.7 (CH2), 39.7 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C10H12N3O: 190.09749, found: 190.09746.
3.2.3. General Procedure for the Preparation of Phthalazinyle Alkyl Dithiocarbamates (6–9)
To a solution of compound 23–26 (0.15 mmol) in DMF (1.5 mL) at 0 °C was added K3PO4 (0.30 mmol) and dropwise CS2 (0.37 mmol) and the reaction mixture was stirred for 1 h at the same temperature. A solution of the appropriate alkyl bromide (0.15 mmol) in DMF (0.4 mL) was added and stirring was continued for 3h, allowing the reaction mixture to gradually reach r.t. The solvent was evaporated and the residue was purified by column chromatography on silica gel (hexane/EtOAc, 4:1) affording the desired compound.
Benzyl N-(2-(1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (6a). White solid; yield: 59%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.55 (br s, 1H, NH), 8.41 (d, 1H, J = 7.8 Hz, H8 phthalazine), 8.19 (s, 1H, H4 phthalazine), 7.86–7.75 (m, 2H, H6, H7 phthalazine), 7.70 (d, 2H, J = 7.8 Hz, H5 phthalazine), 7.34 (d, 2H, J = 7.0 Hz, Ar), 7.29–7.21 (m, 3H, Ar), 4.57–4.53 (m, 2H, H2′), 4.49 (s, 2H, CH2S), 4.20–4.10 (m, 2H, H1′); 13C NMR (CDCl3): δ = 197.7 (CS), 161.0 (C1 phthalazine), 139.0 (C4 phthalazine), 136.4, 133.7, 132.2, 129.7, 129.2, 128.6, 127.6, 127.5, 126.8, 126.4, 50.2 (C2′), 48.2 (C1′), 39.8 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H18N3OS2: 356.08858, found: 356.08830.
4-Methylbenzyl N-(2-(1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (6b). White solid; yield: 70%; Rf = 0.3 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.50 (br s, 1H, NH), 8.41 (d, 1H, J = 7.7 Hz, H8 phthalazine), 8.19 (s, 1H, H4 phthalazine), 7.87–7.76 (m, 2H, H6, H7 phthalazine), 7.72 (d, 1H, J = 7.7 Hz, H5 phthalazine), 7.23 (d, 2H, J = 7.8 Hz, Ar), 7.07 (d, 2H, J = 7.8 Hz, Ar), 4.59–4.53 (m, 2H, H2′), 4.45 (s, 2H, CH2S), 4.19–4.11 (m, 2H, H1′), 2.30 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.9 (CS), 161.0 (C1 phthalazine), 139.1 (C4 phthalazine), 137.2, 133.7, 133.2, 132.2, 129.7, 129.3, 129.1, 127.6, 126.8 (C8 phthalazine), 126.4 (C5 phthalazine), 50.2 (C2′), 48.3 (C1′), 39.6 (CH2S), 21.2(CH3); HRMS (ESI): m/z [M+H]+ calcd for C19H20N3OS2: 370.10423, found: 370.10389.
4-Fluorobenzyl N-(2-(1-oxophthalazin-2(1H)-yl)ethyl) dithiocarbamate (6c). White solid; yield: 68%; Rf = 0.2(hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.55 (br s, 1H, NH), 8.41 (d, 2H, J = 7.5 Hz, H8 phthalazine), 8.20 (s, 1H, H4 phthalazine), 7.88–7.75 (m, 2H, H6, H7 phthalazine), 7.72 (d, 2H, J = 7.5 Hz, H5 phthalazine), 7.35–7.27 (m, 2H, Ar), 6.97–6.90 (m, 2H, CHCF), 4.59–4.53 (m, 2H, H2′), 4.46 (s, 2H, CH2S), 4.21–4.08 (m, 2H, H1′); 13C NMR (CDCl3): δ = 197.5 (CS), 163.4 (CF), 161.1 (C1 phthalazine), 139.1 (C4 phthalazine), 133.8, 132.5, 132.3, 130.8, 130.7, 129.8, 127.6, 126.9 (C8 phthalazine), 126.4 (C5 phthalazine), 115.6 (CHCF), 115.4 (CHCF), 50.3 (C2′), 48.5 (C1′), 39.0 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H17FN3OS2: 374.07916, found: 374.07859.
4-Chlorobenzyl N-(2-(1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (6d). White solid; yield: 67%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.55 (br s, 1H, NH), 8.42 (d, 1H, J = 7.8 Hz, H8 phthalazine), 8.20 (s, 1H, H4 phthalazine), 7.88–7.78 (m, 2H, H6, H7 phthalazine), 7.73 (d, 1H, J = 7.8 Hz, H5 phthalazine), 7.28 (d, 2H, J = 8.4 Hz, Ar), 7.22 (d, 2H, J = 8.4 Hz, Ar), 4.59–4.54 (m, 2H, H2′), 4.46 (s, 2H, CH2S), 4.18–4.10 (m, 2H, H1′); 13C NMR (CDCl3): δ = 197.3 (CS), 161.1 (C1 phthalazine), 139.1 (C4 phthalazine), 135.4, 133.8, 133.3, 132.3, 130.5, 129.7, 128.8, 127.6, 126.9, 126.4, 50.3 (C2′), 48.6 (C1′), 39.0 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H17ClN3OS2: 390.04961, found: 390.04983.
4-Bromobenzyl N-(2-(1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (6e). White solid; yield: 76%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.57 (br s, 1H, NH), 8.40 (d, 1H, J = 7.7 Hz, H8 phthalazine), 8.19 (s, 1H, H4 phthalazine), 7.88–7.76 (m, 2H, H6, H7 phthalazine), 7.71 (d, 1H, J = 7.7 Hz, H5 phthalazine), 7.36 (d, 2H, J = 8.2 Hz, Ar), 7.21 (d, 2H, J = 8.2 Hz, Ar), 4.58–4.52 (m, 2H, H2′), 4.44 (s, 2H, CH2S), 4.20–4.10 (m, 2H, H1′); 13C NMR (CDCl3): δ = 197.2 (CS), 161.1 (C1 phthalazine), 139.1 (C4 phthalazine), 136.0, 133.8, 132.3, 131.7, 130.9, 129.7, 127.6, 126.9 (C8 phthalazine), 126.4 (C5 phthalazine), 121.4 (CBr), 50.3 (C2′), 48.5 (C1′), 39.0 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H17BrN3OS2: 433.99909, found: 433.99854.
4-Nitrobenzyl N-(2-(1-oxophthalazin-2(1H)-yl)ethyl) dithiocarbamate (6f). White solid; yield: 91%; Rf = 0.1 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.66 (br s, 1H, NH), 8.45 (d, 2H, J = 7.8 Hz, H8 phthalazine), 8.24 (s, 1H, H4 phthalazine), 8.13 (d, 2H, J = 8.6 Hz, CHCNO2), 7.91–7.82 (m, 2H, H6, H7 phthalazine), 7.76 (d, 1H, J = 7.8 Hz, H5 phthalazine), 7.54 (d, 2H, J = 8.6 Hz, Ar), 4.62 (s, 2H, CH2S), 4.61–4.57 (m, 2H, H2′), 4.21–4.14 (m, 2H, H1′); 13C NMR (DMSO): δ = 195.9 (CS), 158.7 (C1 phthalazine), 146.4, 146.1, 137.9 (C4 phthalazine), 133.4, 131.9, 129.9, 129.4, 127.1, 126.7, 125.7 (C8 phthalazine), 123.4 (CHCNO2), 48.8 (C2′), 44.8 (C1′), 37.1 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H17N4O3S2: 401.07366, found: 401.07363.
Prop-2-ynyl N-(2-(1-oxophthalazin-2(1H)-yl)ethyl) dithiocarbamate (6g). White solid; yield: 26%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.61 (br s, 1H, NH), 8.45 (d, 2H, J = 7.6 Hz, H8 phthalazine), 8.23 (s, 1H, H4 phthalazine), 7.88–7.80 (m, 2H, H6, H7 phthalazine), 7.74 (d, 2H, J = 7.6 Hz, H5 phthalazine), 4.60–4.54 (m, 2H, H2′), 4.19–4.12 (m, 2H, H1′), 4.00 (d, 2H, J = 2.6 Hz, CH2S), 2.18 (t, 1H, J = 2.6 Hz, HC≡); 13C NMR (CDCl3): δ = 195.8 (CS), 161.2 (C1 phthalazine), 139.2 (C4 phthalazine), 133.9, 132.3, 129.8, 127.7, 127.0 (C8 phthalazine), 126.4 (C5 phthalazine), 78.6 (C≡), 71.8 (HC≡), 50.3 (C2′), 48.7 (C1′), 24.0 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C14H14N3OS2: 304.05728, found: 304.05715.
Benzyl N-(2-(1-oxo-4-p-tolylphthalazin-2(1H)-yl)ethyl)dithiocarbamate (7a). White solid; yield: 55%; Rf = 0.3(hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.70 (br s, 1H, NH), 8.54–8.50 (m, 1H, H8 phthalazine), 7.84–7.74 (m, 3H, H5, H6, H7 phthalazine), 7.48 (d, J = 8.0 Hz, 2H, Ar), 7.36–7.31 (m, 4H, Ar), 7.28–7.22 (m, 3H, Ar), 4.68–4.60 (m, 2H, H2′), 4.45 (s, 2H, CH2S), 4.22–4.14 (m, 2H, H1′), 2.44 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.5 (CS), 160.6 (C1 phthalazine), 148.5, 139.6, 136.2, 133.3, 131.8, 131.7, 129.4, 129.3, 129.1, 128.5, 127.9, 127.4, 127.2, 127.1, 50.1 (C2′), 48.6 (C1′), 39.7 (CH2S), 21.4 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C25H24N3OS2: 446.13553, found: 446.13492.
4-Methylbenzyl N-(2-(1-oxo-4-p-tolylphthalazin-2(1H)-yl)ethyl)dithiocarbamate (7b). White solid; yield: 61%; Rf = 0.3 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.70 (br s, 1H, NH), 8.53–8.49 (m, 1H, H8 phthalazine), 7.83–7.77 (m, 3H, H5, H6, H7 phthalazine), 7.48 (d, 2H, J = 7.9 Hz, Ar), 7.33 (d, 2H, J = 7.9 Hz, Ar), 7.22 (d, 2H, J = 7.9 Hz, Ar), 7.07 (d, 2H, J = 7.9 Hz, Ar), 4.65–4.60 (m, 2H, H2′), 4.41 (s, 2H, CH2S), 4.19–4.14 (m, 2H, H1′), 2.44 (s, 3H, CH3), 2.30 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.7 (CS), 160.7 (C1 phthalazine), 148.6, 139.6, 137.2, 133.4, 133.1, 131.9, 131.8, 129.5, 129.4, 129.3, 129.3, 129.1, 128.0, 127.3, 127.2, 50.1 (C2′), 48.6 (C1′), 39.5 (CH2S), 21.5 (CH3), 21.2 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C26H26N3OS2: 460.15118, found: 460.15043.
4-Fluorobenzyl N-(2-(1-oxo-4-p-tolylphthalazin-2(1H)-yl)ethyl)dithiocarbamate (7c). White solid; yield: 80%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.74 (br s, 1H, NH), 8.54–8.49 (m, 1H, H8 phthalazine), 7.83–7.76 (m, 3H, H5, H6, H7 phthalazine), 7.48 (d, 2H, J = 7.9 Hz, Ar), 7.35–7.27 (m, 4H, Ar), 6.98–6.90 (m, 2H, CHCF), 4.66–4.59 (m, 2H, H2′), 4.44 (s, 2H, CH2S), 4.22–4.13 (m, 2H, H1′), 2.45 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.3 (CS), 163.4 (CF), 160.7 (C1 phthalazine), 148.6, 139.7, 133.4, 131.9, 131.8, 130.8, 130.8, 129.5, 129.4, 128.0, 127.3, 127.2, 115.6 (CHCF), 115.4 (CHCF), 50.2 (C2′), 48.7 (C1′), 38.9 (CH2S), 21.5 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C25H23FN3OS2: 464.12611, found: 464.12534.
4-Chlorobenzyl N-(2-(1-oxo-4-p-tolylphthalazin-2(1H)-yl)ethyl)dithiocarbamate (7d). White solid; yield: 74%; Rf = 0.2(hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.75 (br s, 1H, NH), 8.53–8.49 (m, 1H, H8 phthalazine), 7.83–7.77 (m, 3H, H5, H6, H7 phthalazine), 7.47 (d, 2H, J = 8.0 Hz, Ar), 7.33 (d, 2H, J = 8.0 Hz, Ar), 7.27 (d, 2H, J = 8.4 Hz, Ar), 7.21 (d, 2H, J = 8.4 Hz, Ar), 4.65–4.60 (m, 2H, H2′), 4.43 (s, 2H, CH2S), 4.21–4.13 (m, 2H, H1′), 2.45 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.0 (CS), 160.7 (C1 phthalazine), 148.6, 139.7, 135.3, 133.4, 133.2, 131.9, 131.8, 130.5, 129.5, 129.4, 129.3, 128.7, 127.9, 127.3, 127.2, 50.2 (C2′), 48.7 (C1′), 38.9 (CH2S), 21.5 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C25H23ClN3OS2: 480.09656, found: 480.09582.
4-Bromobenzyl N-(2-(1-oxo-4-p-tolylphthalazin-2(1H)-yl)ethyl)dithiocarbamate (7e). White solid; yield: 64%; Rf = 0.3 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.75 (br s, 1H, NH), 8.52–8.49 (m, 1H, H8 phthalazine), 7.81–7.76 (m, 3H, H5, H6, H7 phthalazine), 7.47 (d, 2H, J = 7.9 Hz, Ar), 7.38–7.32 (m, 4H, Ar), 7.20 (d, 2H, J = 8.2 Hz, Ar), 4.65–4.59 (m, 2H, H2′), 4.41 (s, 2H, CH2S), 4.20–4.12 (m, 2H, H1′), 2.45 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.0 (CS), 160.7 (C1 phthalazine), 148.6, 139.7, 135.9, 133.4, 131.9, 131.8, 131.7, 130.9, 129.5, 129.4, 128.0, 127.3, 127.2, 121.3 (CBr), 50.2 (C2′), 48.8 (C1′), 38.9 (CH2S), 21.5 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C25H23BrN3OS2: 524.04604, found: 524.04536.
4-Nitrobenzyl N-(2-(1-oxo-4-p-tolylphthalazin-2(1H)-yl)ethyl)dithiocarbamate (7f). White solid; yield: 68%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3, δ): 8.84 (br s, 1H, NH), 8.52–8.48 (m, 1H, H8 phthalazine), 8.08 (d, 2H, J = 8.8 Hz, CHCNO2), 7.83–7.77 (m, 3H, H5, H6, H7 phthalazine), 7.52–7.45 (m, 4H, Ar), 7.33 (d, 2H, J = 7.8 Hz, Ar), 4.64–4.61 (m, 2H, H2′), 4.58 (s, 2H, CH2S), 4.21–4.13 (m, 2H, H1′), 2.45 (s, 3H, CH3); 13C NMR (CDCl3, δ): 196.2 (CS), 160.9 (C1 phthalazine), 148.7, 147.2, 145.2, 139.7, 133.5, 131.9, 131.8, 130.0, 129.5, 129.4, 127.9, 127.3, 127.2, 123.7 (CHCNO2), 50.4 (C2′), 49.1 (C1′), 38.7 (CH2S), 21.5 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C25H23N4O3S2: 491.12061, found: 491.12036.
Prop-2-ynyl N-(2-(1-oxo-4-p-tolylphthalazin-2(1H)-yl)ethyl)dithiocarbamate (7g). White solid; yield: 54%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.80 (br s, 1H, NH), 8.53 (d, 2H, J = 7.4 Hz, H8 phthalazine), 7.84–7.77 (m, 3H, H5, H6, H7 phthalazine), 7.48 (d, 2H, J = 7.9 Hz, Ar), 7.35 (d, 2H, J = 7.9 Hz, Ar), 4.66–4.61 (m, 2H, H2′), 4.21–4.13 (m, 2H, H1′), 3.97 (d, 2H, J = 2.6 Hz, CH2S), 2.46 (s, 3H, CH3), 2.18 (t, 1H, J = 2.6 Hz, HC≡); 13C NMR (CDCl3): δ = 195.6 (CS), 160.8 (C1 phthalazine), 148.7, 139.7, 133.5, 131.9, 131.9, 129.6, 129.4, 128.0, 127.4, 127.2, 78.6 (C≡), 71.8 (HC≡), 50.2 (C2′), 48.9 (C1′), 24.0 (CH2S), 21.5 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C21H20N3OS2: 394.10423, found: 394.10354.
Benzyl N-(2-(4-methyl-1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (8a). White solid; yield: 83%; Rf = 0.2 (hexane/EtOAc, 4:1); 1H NMR (CDCl3): δ = 8.73 (br s, 1H, NH), 8.44 (d, 2H, J = 8.3 Hz, H8 phthalazine), 7.87–7.81 (m, 1H, Ar), 7.81–7.74 (m, 2H, Ar), 7.34 (d, 2H, J = 7.3 Hz, Ar), 7.29–7.21 (m, 3H, Ar), 4.55–4.50 (m, 2H, H2′), 4.49 (s, 2H, CH2S), 4.17–4.09 (m, 2H, H1′), 2.59 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.6 (CS), 161.0 (C1 phthalazine), 145.1 (C4 phthalazine), 136.5, 133.5, 131.8, 129.9, 129.2, 128.6, 127.5, 127.4, 127.3, 125.1, 49.9 (C2′), 48.8 (C1′), 39.8 (CH2S), 18.9 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C19H20N3OS2: 370.10423, found: 370.10393.
4-Methylbenzyl N-(2-(4-methyl-1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (8b). White solid; yield: 61%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.71 (br s, 1H, NH), 8.46–8.42 (m, 1H, H8 phthalazine), 7.88–7.82 (m, 1H, Ar), 7.81–7.76 (m, 2H, Ar), 7.23 (d, 2H, J = 7.8 Hz, Ar), 7.07 (d, 2H, J = 7.8 Hz, Ar), 4.54–4.51 (m, 2H, H2′), 4.44 (s, 2H, CH2S), 4.16–4.09 (m, 2H, H1′), 2.59 (s, 3H, CH3C = N), 2.30 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.7 (CS), 161.0 (C1 phthalazine), 145.1 (C4 phthalazine), 137.2, 133.5, 133.2, 131.8, 129.9, 129.3, 129.1, 127.4, 127.2 (C8 phthalazine), 125.1, 49.9 (C2′), 48.7 (C1′), 39.5 (CH2S), 21.2 (CH3), 18.9 (CH3C = N); HRMS (ESI): m/z [M+H]+ calcd for C20H22N3OS2: 384.11988, found: 384.11941.
4-Fluorobenzyl N-(2-(4-methyl-1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (8c). White solid; yield: 59%; Rf = 0.3 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.78 (br s, 1H, NH), 8.45–8.41 (m, 1H, H8 phthalazine), 7.89–7.83 (m, 1H, Ar), 7.81–7.76 (m, 2H, Ar), 7.33–7.29 (m, 2H, Ar), 6.96–6.91 (m, 2H, CHCF), 4.54–4.51 (m, 2H, H2′), 4.46 (s, 2H, CH2S), 4.15–4.08 (m, 2H, H1′), 2.59 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.3 (CS), 163.4 (CF), 161.1 (C1 phthalazine), 145.2 (C4 phthalazine), 133.6, 132.5, 131.9, 130.8, 130.7, 129.9, 127.4, 127.3, 125.1, 115.6 (CHCF), 115.4 (CHCF), 50.0 (C2′), 48.9 (C1′), 38.9 (CH2S), 18.9 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C19H19FN3OS2: 388.09481, found: 388.09433.
4-Chlorobenzyl N-(2-(4-methyl-1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (8d). White solid; yield: 78%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.77 (br s, 1H, NH), 8.42 (d, 2H, J = 7.5 Hz, H8 phthalazine), 7.86–7.81 (m, 1H, Ar), 7.80–7.73 (m, 2H, Ar), 7.27 (d, 2H, J = 8.2 Hz, Ar), 7.20 (d, 2H, J = 8.2 Hz, Ar), 4.53–4.49 (m, 2H, H2′), 4.45 (s, 2H, CH2S), 4.14–4.08 (m, 2H, H1′), 2.58 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.0 (CS), 161.1 (C1 phthalazine), 145.2 (C4 phthalazine), 135.4, 133.6, 133.2, 131.8, 130.5, 129.9, 128.7, 127.3, 127.2, 125.1, 50.0 (C2′), 48.9 (C1′), 38.9 (CH2S), 18.9 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C19H19ClN3OS2: 404.06526, found: 404.06483.
4-Bromobenzyl N-(2-(4-methyl-1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (8e). White solid; yield: 70%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.76 (br s, 1H, NH), 8.46–8.43 (m, 1H, H8 phthalazine), 7.88–7.83 (m, 1H, Ar), 7.82–7.77 (m, 2H, Ar), 7.37 (d, 2H, J = 8.2 Hz, Ar), 7.22 (d, 2H, J = 8.2 Hz, Ar), 4.54–4.50 (m, 2H, H2′), 4.44 (s, 2H, CH2S), 4.15–4.08 (m, 2H, H1′), 2.60 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.0 (CS), 161.1 (C1 phthalazine), 145.2 (C4 phthalazine), 136.0, 133.6, 131.9, 131.7, 130.9, 129.9, 127.5, 127.3 (C8 phthalazine), 125.1, 121.4 (CBr), 50.0 (C2′), 49.0 (C1′), 39.0 (CH2S), 19.0 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C19H19BrN3OS2: 448.01474, found: 448.01421.
4-Nitrobenzyl N-(2-(4-methyl-1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (8f). White solid; yield: 59%; Rf = 0.1 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.90 (br s, 1H, NH), 8.46–8.41 (m, 1H, H8 phthalazine), 8.09 (d, 2H, J = 8.7 Hz, CHCNO2), 7.89–7.84 (m, 1H, Ar), 7.82–7.77 (m, 2H, Ar), 7.51 (d, 2H, J = 8.7 Hz, Ar), 4.58 (s, 2H, CH2S), 4.55–4.50 (m, 2H, H2′), 4.15–4.07 (m, 2H, H1′), 2.59 (s, 3H, CH3); 13C NMR (CDCl3): δ = 196.1 (CS), 161.2 (C1 phthalazine), 147.2 (CNO2), 145.3, 145.2, 133.7, 131.9, 130.0, 129.9, 127.4, 127.2, 125.2, 123.8 (CHCNO2), 50.2 (C2′), 49.3 (C1′), 38.7 (CH2S), 19.0 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C19H19N4O3S2: 415.08931, found: 415.08914.
Prop-2-ynyl N-(2-(4-methyl-1-oxophthalazin-2(1H)-yl)ethyl)dithiocarbamate (8g). White solid; yield: 19%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.81 (br s, 1H, NH), 8.49–8.45 (m, 1H, H8 phthalazine), 7.89–7.78 (m, 3H, H5, H6, H7 phthalazine), 4.57–4.52 (m, 2H, H2′), 4.16–4.10 (m, 2H, H1′), 3.99 (d, 2H, J = 2.6 Hz, CH2S), 2.61 (s, 3H, CH3), 2.18 (t, 2H, J = 2.6 Hz, HC≡); 13C NMR (CDCl3): δ = 195.6 (CS), 161.2 (C1 phthalazine), 145.3 (C4 phthalazine), 133.6, 131.9, 130.0, 127.5, 127.4 (C8 phthalazine), 125.1, 78.6 (C≡), 71.8 (HC≡), 50.0 (C2′), 49.1 (C1′), 24.0 (CH2S), 19.0 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C15H16N3OS2: 318.07293, found: 318.07273.
Benzyl N-((3-methyl-4-oxo-3,4-dihydrophthalazin-1-yl)methyl)dithiocarbamate (9a). White solid; yield: 71%; Rf = 0.3 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.42–8.31 (m, 2H, NH, H5 phthalazine), 7.83–7.77 (m, 2H, Ar), 7.77–7.68 (m, 1H, Ar), 7.41 (d, 2H, J = 7.2 Hz, Ar), 7.34–7.26 (m, 3H, Ar), 5.19 (d, 2H, J = 4.2 Hz, CH2), 4.62 (s, 2H, CH2S), 3.76 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.8 (CS), 159.5 (C4 phthalazine), 139.9, 136.5, 133.6, 132.2, 129.2, 128.8, 128.0, 127.7, 127.6, 127.4, 123.7, 47.5 (CH2), 40.2 (CH2S), 39.5 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C18H18N3OS2: 356.08858, found: 356.08825.
4-Methylbenzyl N-((3-methyl-4-oxo-3,4-dihydrophthalazin-1-yl)methyl)dithiocarbamate (9b). White solid; yield: 66%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.45 (br s, 1H, NH), 8.32 (d, 2H, J = 7.9 Hz, H5 phthalazine), 7.80–7.77 (m, 2H, Ar), 7.74–7.68 (m, 1H, Ar), 7.29 (d, 2H, J = 7.9 Hz, Ar), 7.11 (d, 2H, J = 7.9 Hz, Ar), 5.18 (d, 2H, J = 4.3 Hz, CH2), 4.57 (s, 2H, CH2S), 3.72 (s, 3H, CH3N), 2.32 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.9 (CS), 159.5 (C4 phthalazine), 140.1, 137.4, 133.6, 133.3, 132.2, 129.4, 129.1, 128.0, 127.5, 127.3, 123.7, 47.5 (CH2), 39.9 (CH2S), 39.5 (CH3N), 21.3 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C19H20N3OS2: 370.10423, found: 370.10397.
4-Fluorobenzyl N-((3-methyl-4-oxo-3,4-dihydrophthalazin-1-yl)methyl)dithiocarbamate (9c). White solid; yield: 22%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.46 (br s, 1H, NH), 8.33 (d, 1H, J = 8.0 Hz, H5 phthalazine), 7.82–7.77 (m, 2H, Ar), 7.74–7.68 (m, 1H, Ar), 7.42–7.35 (m, 2H, Ar), 7.03–6.95 (m, 2H, CHCF), 5.18 (d, 2H, J = 4.3 Hz, CH2), 4.60 (s, 2H, CH2S), 3.73 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.5 (CS), 163.5 (CF), 159.5 (C4 phthalazine), 139.8, 133.6, 132.5, 132.3, 130.9, 130.8, 128.0, 127.7, 127.5 (C5 phthalazine), 123.6, 115.7 (CHCF), 115.5 (CHCF), 47.5 (CH2), 39.5 (CH3), 39.4 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H17FN3OS2: 374.07916, found: 374.07870.
4-Chlorobenzyl N-((3-methyl-4-oxo-3,4-dihydrophthalazin-1-yl)methyl)dithiocarbamate (9d). White solid; yield: 68%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.43 (d, 2H, J = 7.7 Hz, H5 phthalazine), 8.23 (br s, 1H, NH), 7.85–7.77 (m, 3H, H6, H7, H8 phthalazine), 7.35 (d, 2H, J = 8.4 Hz, Ar), 7.28 (d, 2H, J = 8.4 Hz, Ar), 5.19 (d, 2H, J = 4.2 Hz, CH2), 4.59 (s, 2H, CH2S), 3.81 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.2 (CS), 159.4 (C4 phthalazine), 139.7, 135.3, 133.5, 133.4, 132.2, 130.4, 128.7, 127.8, 127.5, 127.3, 123.5, 47.5 (CH2), 39.4 (CH3), 39.2 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H17ClN3OS2: 390.04961, found: 390.04919.
4-Bromobenzyl N-((3-methyl-4-oxo-3,4-dihydrophthalazin-1-yl)methyl)dithiocarbamate (9e). White solid; yield: 85%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.48–8.20 (m, 2H, NH, H5 phthalazine), 7.84–7.71 (m, 3H, H6, H7, H8 phthalazine), 7.43 (d, 2H, J = 8.2 Hz, Ar), 7.29 (d, 2H, J = 8.2 Hz, Ar), 5.18 (d, 2H, J = 4.0 Hz, CH2), 4.57 (s, 2H, CH2S), 3.78 (s, 3H, CH3); 13C NMR (CDCl3): δ = 197.2 (CS), 159.5 (C4 phthalazine), 139.8, 136.0, 133.6, 132.3, 131.8, 130.9, 128.0, 127.7, 127.4 (C5 phthalazine), 123.6, 121.6 (CBr), 47.6 (CH2), 39.5 (CH3), 39.4 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H17BrN3OS2: 433.99909, found: 433.99869.
4-Nitrobenzyl N-((3-methyl-4-oxo-3,4-dihydrophthalazin-1-yl)methyl)dithiocarbamate (9f). White solid; yield: 66%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (DMSO-d6): δ = 10.54 (br s, 1H, NH), 8.32–8.28 (m, 1H, H5 phthalazine), 8.15 (d, 2H, J = 8.7 Hz, CHCNO2), 7.94–7.85 (m, 3H, H6, H7, H8 phthalazine), 7.63 (d, 2H, J = 8.7 Hz, Ar), 5.13 (s, 2H, CH2), 4.69 (s, 2H, CH2S), 3.71 (s, 3H, CH3); 13C NMR (DMSO): δ = 195.9 (CS), 158.5 (C4 phthalazine), 146.5, 146.0, 140.8, 133.4, 132.0, 130.0, 128.3, 127.0, 126.3 (C5 phthalazine), 124.5, 123.4 (CHCNO2), 48.1 (CH2), 39.0 (CH3), 37.3 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C18H17N4O3S2: 401.07366, found: 401.07356.
Prop-2-ynyl N-((3-methyl-4-oxo-3,4-dihydrophthalazin-1-yl)methyl)dithiocarbamate (9g). White solid; yield: 36%; Rf = 0.2 (hexane/EtOAc, 2:1); 1H NMR (CDCl3): δ = 8.48–8.39 (m, 2H, NH, H5 phthalazine), 7.86–7.76 (m, 3H, H6, H7, H8 phthalazine), 5.19 (d, 2H, J = 4.2 Hz, CH2), 4.10 (d, 2H, J = 2.6 Hz, CH2S), 3.81 (s, 3H, CH3), 2.6 (t, 1H, J = 2.6 Hz, HC≡); 13C NMR (CDCl3): δ = 195.7 (CS), 159.5 (C4 phthalazine), 139.4, 133.6, 132.4, 127.5 (C5 phthalazine), 123.5, 78.4 (C≡), 72.2 (HC≡), 47.5 (CH2), 39.5 (CH3), 24.3 (CH2S); HRMS (ESI): m/z [M+H]+ calcd for C14H14N3OS2: 304.05728, found: 304.05719.
3.3. Antiproliferative Activity Studies
Antiproliferative effects of titled compounds on NCI-H460, A2780, and MCF-7 cells were studied using the colorimetric MTT assay [40,41,42]. All the cell lines were acquired from ATCC. Cells (passages between 15 and 18) were seeded in 96-well plates (15,000 cells per well for the NCI-H460 cell line, 4000 for the A2780 cell line, and 10,000 for the MCF-7 cell line), incubated for 24 h in the culture medium (RPMI1640 supplemented with 10% FBS and 2 mM L-glutamine for both NCI-H460 and A2780 cell lines and EMEM supplemented with 10% FBS and 0.01 mg/mL of bovine insulin for MCF-7 cell line), and treated at 37 °C for 96 h (A2780 and MCF-7) and 48 h (NCI-H460) with concentrations in the range of 0.1–100 µM of target compounds and the reference drug (cisplatin) dissolved in DMSO. Three wells were used for each of the variants tested. After the incubation time, aliquots of MTT solution in phosphate-buffered saline (10 μL) were added to each well and incubated for 4 h. The color formed was quantified by a spectrophotometric plate reader (Tecan Ultra evolution) at 595 nm wavelength. In all experiments, DMSO controls were included. The percentage of inhibition of cell viability was calculated by the formula % inhibition = 100 − ((AO × 100)/AT), where AO is the absorbance observed in the treated wells and AT is the absorbance observed in the DMSO control wells.
The antiproliferative potency of the studied compounds was determined from concentration–effect curves, by using GraphPad Prism software (version 2.01), and was expressed as 50% inhibitory concentrations (IC50). Correlation coefficients (r2) were higher than 0.995 for all the compounds tested.
3.4. Drug-Like and Toxicity Properties Prediction
Physicochemical properties, drug-likeness, and ADME of new phthalazinone-dithiocarbamate hybrids were predicted by using the free web-server SwissADME [43]. The 2D structure of compounds was drawn using ChemDraw Professional 18.2.0.48 to obtain the corresponding SMILE code, which was inserted into the SwissADME website. The software computes many parameters affecting pharmacokinetics, interaction with carriers and CYP450 enzymes, and drug-like properties. In addition, it provides two kinds of diagrams, the bioavailability radar for a quick drug-likeness appraisal, and the BOILED-Egg tool for graphical estimation of HIA and BBB permeation.
Acute toxicity, organ toxicity (hepatotoxicity), and toxicity endpoints (carcinogenicity, immunotoxicity, mutagenicity, and cytotoxicity) were computed using the webserver ProTox-II [44]. This free virtual lab incorporates molecular similarity for DL50 prediction and pharmacophore-based models as well as fragment propensities and machine learning models for the prediction of hepatotoxicity and several toxicity endpoints. The website takes the 2D structure to estimate the toxicity of small molecules.
4. Conclusions
Two novel series of phthalazinone-dithiocarbamate hybrids were successfully obtained and characterized, compounds 6–8 containing the dithiocarbamate group at N2 and compounds 9 displaying this fragment at C4. The antiproliferative activity of all synthesized compounds was evaluated against three human cancer cell lines (A-2780, NCI-H460, MCF-7). Among compounds 6–8, the 4-bromobenzyl derivatives 6e, 8e, and the propargyl analogue 6g had good activity versus the A-2780 cell line, with IC50 values of 5.53 ± 0.09 µM, 7.51 ± 0.13 µM, and 5.20 ± 0.13 µM, respectively. Compound 6g also showed good antiproliferative potential against the MCF-7 cell line (IC50 = 7.64 ± 0.5 µM).
On the other hand, most of compounds 9 had good activity and selectivity versus the NCI-H460 cell line, with IC50 values below or around 10 µM, the benzyl analogues 9a–b and 9d were the most promising. Interestingly, the propargyl group in compounds 9 also provided the best result against the A-2780 cell line, compound 9g with an IC50 value of 6.75 ± 0.12 µM was the most active derivative against this ovarian cancer cell line A-2780.
Drug-like and toxicity predictions suggested interesting properties for the studied phthalazinone-dithiocarbamate hybrids, specifically regarding oral absorption, bioavailability, absence of effects on the CNS, and the toxicological profile. All of this seems to indicate that compounds 6e, 8e, 6g, 9a–b, 9d, and 9g could have good potential for the future development of anticancer agents.
Acknowledgments
N.V. thanks the Universidade de Vigo for the PhD fellowship.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238115/s1, Table S1: CYP450 inhibition profile of compounds 6e, 8e, 6g, 9a–b, 9d, and 9g; Table S2: Toxicity model predicted by ProTox-II for compounds 6e, 8e, 6g, 9a–b, 9d, and 9g; synthesis of compounds 15–17; copies of 1H NMR and 13C NMR of compounds 6–9.
Author Contributions
Conceptualization, C.T.; methodology, N.V., C.T, P.B. and J.B.; validation, P.B. and J.B.; formal analysis, C.T., N.V. and P.B.; investigation, C.T., N.V. and P.B.; resources, C.T., P.B. and M.I.L.; writing—original draft preparation, C.T and P.B.; writing—review and editing, C.T., P.B., J.B. and M.I.L.; project administration, C.T.; funding acquisition, C.T., P.B., J.B. and M.I.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
This research was supported with funding from Universidade de Vigo, Xunta de Galicia (ED431C 2022/20 and ED431G 2019/02) and European Regional Development Fund (ERDF).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dagenais G.R., Leong D.P., Rangarajan S., Lanas F., Lopez-Jaramillo P., Gupta R., Diaz R., Avezum A., Oliveira G.B.F., Wielgosz A., et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet. 2020;395:785–794. doi: 10.1016/S0140-6736(19)32007-0. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Ward R.A., Fawell S., Floc’h N., Flemington V., McKerrecher D., Smith P.D. Challenges and Opportunities in Cancer Drug Resistance. Chem. Rev. 2021;121:3297–3351. doi: 10.1021/acs.chemrev.0c00383. [DOI] [PubMed] [Google Scholar]
- 4.Kamal A., Azeeza S., Bharathi E.V., Malik M.S., Shetti R.V. Search for new and novel chemotherapeutics for the treatment of human malignancies. Mini Rev. Med. Chem. 2010;10:405–435. doi: 10.2174/138955710791330918. [DOI] [PubMed] [Google Scholar]
- 5.De Oliveira Pedrosa M., Duarte da Cruz R.M., de Oliveira Viana J., de Moura R.O., Ishiki H.M., Barbosa Filho J.M., Diniz M.F., Scotti M.T., Scotti L., Bezerra Mendonca F.J. Hybrid compounds as direct multitarget ligands: A review. Curr. Top. Med. Chem. 2017;17:1044–1079. doi: 10.2174/1568026616666160927160620. [DOI] [PubMed] [Google Scholar]
- 6.Nepali K., Sharma S., Sharma M., Bedi P.M., Dhar K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014;77:422–487. doi: 10.1016/j.ejmech.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Singh J., Suryan A., Kumar S., Sharma S. Phthalazinone scaffold: Emerging tool in the development of target based novel anticancer agents. Anticancer Agents Med. Chem. 2020;20:2228–2245. doi: 10.2174/1871520620666200807220146. [DOI] [PubMed] [Google Scholar]
- 8.Vila N., Besada P., Costas T., Costas-Lago M.C., Terán C. Phthalazin-1(2H)-one as a remarkable scaffold in drug Discovery. Eur. J. Med. Chem. 2015;97:462–482. doi: 10.1016/j.ejmech.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Terán C., Besada P., Vila N., Costas-Lago M.C. Recent advances in the synthesis of phthalazin-1(2H)-one core as a relevant pharmacophore in medicinal chemistry. Eur. J. Med. Chem. 2019;161:468–478. doi: 10.1016/j.ejmech.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Menear K.A., Adcock C., Boulter R., Cockcroft X.L., Copsey L., Cranston A., Dillon K.J., Drzewiecki J., Garman S., Gomez S., et al. 4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: A Novel Bioavailable Inhibitor of Poly(ADP-ribose) Polymerase-1. J. Med. Chem. 2008;51:6581–6591. doi: 10.1021/jm8001263. [DOI] [PubMed] [Google Scholar]
- 11.Almahli H., Hadchity E., Jaballah M.Y., Daher R., Ghabbour H.A., Kabil M.M., Al-shakliah N.S., Eldehna W.M. Development of novel synthesized phthalazinone-based PARP-1 inhibitors with apoptosis inducing mechanism in lung cancer. Bioorg. Chem. 2018;77:443–456. doi: 10.1016/j.bioorg.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Xin M., Sun J., Huang W., Tang F., Liu Z., Jin Q., Wang J. Design and synthesis of novel phthalazinone derivatives as potent poly(ADP-ribose)polymerase 1 inhibitors. Future Med. Chem. 2020;12:1691–1707. doi: 10.4155/fmc-2020-0009. [DOI] [PubMed] [Google Scholar]
- 13.Prime M.E., Courtney S.M., Brookfield F.A., Marston R.W., Walker V., Warne J., Boyd A.E., Kairies N.A., von der Saal W., Limberg A., et al. Phthalazinone pyrazoles as potent, selective, and orally bioavailable inhibitors of Aurora-A kinase. J. Med. Chem. 2011;54:312–319. doi: 10.1021/jm101346r. [DOI] [PubMed] [Google Scholar]
- 14.Hekal M.H., El-Naggar A.M., Abu El-Azm F.S.M., El-Sayed W.M. Synthesis of new oxadiazol-phthalazinone derivatives with anti-proliferative activity; molecular docking, pro-apoptotic, and enzyme inhibition profile. RSC Adv. 2020;10:3675–3688. doi: 10.1039/C9RA09016A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaul L., Süss R., Zannettino A., Richter K. The revival of dithiocarbamates: From pesticides to innovative medical treatments. iScience. 2021;24:102092. doi: 10.1016/j.isci.2021.102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinde N.D., Sakla A.P., Shankaraiah M. An insight into medicinal attributes of dithiocarbamates: Bird’s eye view. Bioorg. Chem. 2020;105:104347. doi: 10.1016/j.bioorg.2020.104346. [DOI] [PubMed] [Google Scholar]
- 17.Gaspari P., Banerjee T., Malachowski W.P., Muller A.J., Prendergast G.C., DuHadaway J., Bennett S., Donovan A.M. Structure-activity study of brassinin derivatives as indoleamine 2,3-dioxygenase inhibitors. J. Med. Chem. 2006;49:684–692. doi: 10.1021/jm0508888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee T., Duhadaway J.B., Gaspari P., Sutanto-Ward E., Munn D.H., Mellor A.L., Malachowski W.P., Prendergast G.C., Muller A.J. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene. 2008;27:2851–2857. doi: 10.1038/sj.onc.1210939. [DOI] [PubMed] [Google Scholar]
- 19.Omar A.M.E., AboulWafa O.M., El-Shoukrofy M.S., Amr M.E. Benzoxazole derivatives as new generation of anti-breast cancer agents. Bioorg. Chem. 2020;96:103593. doi: 10.1016/j.bioorg.2020.103593. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y.C., Duan Y.C., Ma J.L., Xu R.M., Zi X., Lv W.L., Wang M.M., Ye X.W., Zhu S., Mobley D., et al. Triazole-dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J. Med. Chem. 2013;56:8543–8560. doi: 10.1021/jm401002r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su Y., Li R., Ning X., Lin Z., Zhao X., Zhou J., Liu J., Jin Y., Yin Y. Discovery of 2,4-diarylaminopyrimidine derivatives bearing dithiocarbamate moiety as novel FAK inhibitors with antitumor and anti-angiogenesis activities. Eur. J. Med. Chem. 2019;177:32–46. doi: 10.1016/j.ejmech.2019.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Ding P.P., Gao M., Mao B.B., Cao S.L., Liu C.H., Yang C.R., Li Z.F., Liao J., Zhao H., Li Z., et al. Synthesis and biological evaluation of quinazolin-4(3H)-one derivatives bearing dithiocarbamate side chain at C2-position as potential antitumor agents. Eur. J. Med. Chem. 2016;108:364–373. doi: 10.1016/j.ejmech.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Cao S.L., Wang Y., Zhu L., Liao J., Guo Y.W., Chen L.L., Liu H.Q. Synthesis and cytotoxic activity of N-((2-methyl-4(3H)-quinazolinon-6-yl)methyl)dithiocarbamates. Eur. J. Med. Chem. 2010;45:3850–3857. doi: 10.1016/j.ejmech.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Fu D.J., Zhang S.Y., Liu Y.C., Zhang L., Liu J.J., Song J., Zhao R.H., Li F., Sun H.H., Liu H.M., et al. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate-chalcone derivatives. Bioorg. Med. Chem. Lett. 2016;26:3918–3922. doi: 10.1016/j.bmcl.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Terán C., Besada P., Costas T., Vila N., Costas-Lago M.C. Compuestos de Estructura Híbrida Piridaziona Ditiocarbamato Con Actividad Antineoplásica. ES2469990B1. Spanish Patent. 2015 January 27;
- 26.Abo-elmagd N.E., George R.F., Ezzat M.A., Arafa R.K. New 1-phthalazinone scaffold based compounds: Design, cytotoxicity and protein kinase inhibition activity. Mini Rev. Med. Chem. 2018;18:1759–1774. doi: 10.2174/1389557518666180903153254. [DOI] [PubMed] [Google Scholar]
- 27.Costas-Lago M.C., Vila N., Rahman A., Besada P., Rozas I., Brea P., Loza M.I., González-Romero E., Terán C. Novel pyridazin-3(2H)-one-based guanidine derivatives as potential DNA minor groove binders with anticancer activity. ACS Med. Chem. Lett. 2022;13:463–469. doi: 10.1021/acsmedchemlett.1c00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daina A., Zoete V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montarani F., Ecker G.F. Prediction of drug-ABC-transporter interaction-recent advances and future challenges. Adv. Drug Deliv. Rev. 2015;86:17–26. doi: 10.1016/j.addr.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharom F.J. ABC multidrug transporters: Structure, function and role in chemoresistence. Pharmacohenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 32.Lipinski C.A., Lombardo F., Dominy B.W., Feney P.J. Experimental and computational approaches to estimate solubility and premeabilty in drug discoveryand development settings. Adv. Drug. Deliv. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 33.Ghose A.K., Viswanadhan V.N., Wendoloski J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb. Chem. 1999;1:55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 34.Veber D.F., Johnson S.R., Cheng H.Y., Smith B.R., Ward K.W., Kopple K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 35.Egan W.J., Merz K.M., Baldwin J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000;43:3867–3877. doi: 10.1021/jm000292e. [DOI] [PubMed] [Google Scholar]
- 36.Muegge I., Heald S.L., Brittelli D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001;44:1841–1846. doi: 10.1021/jm015507e. [DOI] [PubMed] [Google Scholar]
- 37.Banerjee P., Eckert A.O., Schrey A.K., Preissner R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46:W257–W263. doi: 10.1093/nar/gky318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vila N., Besada P., Viña D., Sturlese M., Moro S., Terán C. Synthesis, biological evaluation and molecular modeling studies of phthalazin-1(2H)-one derivatives as novel cholinesterase inhibitors. RSC Adv. 2016;6:46170–46185. doi: 10.1039/C6RA03841G. [DOI] [Google Scholar]
- 39.Besada P., Viña D., Costas T., Costas-Lago M.C., Vila N., Torres-Terán I., Sturlese M., Moro S., Terán C. Pyridazinones containing dithiocarbamoyl moieties as a new class of selective MAO-B inhibitors. Bioorg. Chem. 2021;115:105203. doi: 10.1016/j.bioorg.2021.105203. [DOI] [PubMed] [Google Scholar]
- 40.Descôteaux C., Provencher-Mandeville J., Mathieu I., Perron V., Mandal S.K., Asselin E., Berube G. Synthesis of 17-beta-estradiol platinum(II) complexes: Biological evaluation on breast cancer cell lines. Bioorg. Med. Chem. Lett. 2003;13:3927–3931. doi: 10.1016/j.bmcl.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Reithofer M.R., Valiahdi S.M., Jakupec M.A., Arion V.B., Egger A., Galanski M., Keppler B.K. Novel di- and tetracarboxylatoplatinum(IV) complexes. synthesis, characterization, cytotoxic activity, and DNA platination. J. Med. Chem. 2007;50:6692–6699. doi: 10.1021/jm070897b. [DOI] [PubMed] [Google Scholar]
- 42.Martinez A., Lorenzo J., Prieto M.J., Llorens R., Font-Bardia M., Solans X., Avilés F.X., Moreno V. Synthesis, characterization and biological activity of trans-platinum(II) and trans-platinum(IV) complexes with 4-hydroxymethylpyridine. ChemBioChem. 2005;6:2068–2077. doi: 10.1002/cbic.200500108. [DOI] [PubMed] [Google Scholar]
- 43.SwissADME. [(accessed on 26 September 2022)]. Available online: http://www.swissadme.ch.
- 44.ProTox-II. [(accessed on 27 September 2022)]. Available online: https://tox-new.charite.de/protox_II/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article or Supplementary Material.









